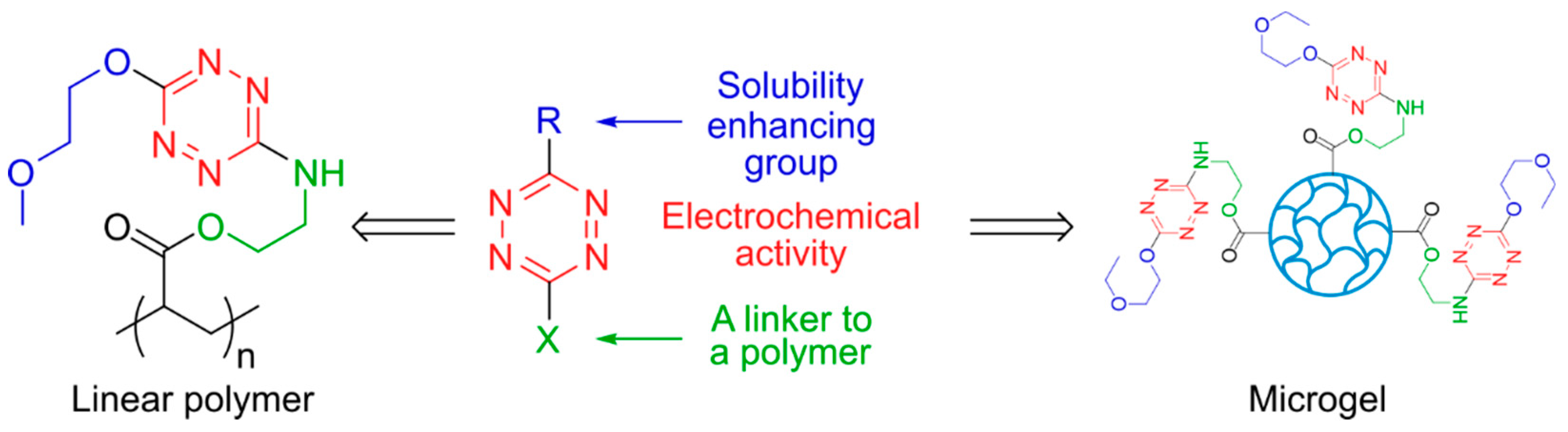
Introduction
Attempts to make batteries more environmentally friendly have led to an increased interest in organic redox-active materials, which can be used in both combined and all-organic devices. These organic molecules are cheaper and more sustainable, as their cost is not limited by the production or availability of rare elements [
1]. The structural diversity of organic redox-active materials is very wide; it includes organosulfur derivatives [
2,
3,
4], carbonyl compounds [
5,
6], free radical systems [
7,
8], viologens [
9] and some others. Nevertheless, new tailor-made organic molecules with targeted electrochemical and physical properties (such as solubility, redox potential values, and the possibility of multi-electron transfer) are currently in high demand.
One of the promising and "greener" alternatives to Li-ion batteries, which currently occupy a significant part of the market, are redox-flow batteries (RFBs). RFBs have the unique feature of allowing for independent scaling up of their power (determined by the electrode surface area) and capacity (related to the amount of redox-active compounds in the tanks) [
10]. From this point, RFBs, which are suitable for large-scale energy storage technologies, can be seen as a potential complement to Li-ion batteries.
RFBs using water-soluble organic redox-active materials are of particular interest [
11]. They are safer and cheaper than their non-aqueous and inorganic counterparts and are in line with sustainable chemistry requirements. Commercial RFBs all use aqueous supporting electrolytes, highlighting the need for water-soluble redox-active materials. Despite some progress in this area, [
12,
13] the search for new water-soluble organic redox-active materials remains a challenging task.
One of the main trends is the development of polymer-based redox-active materials for aqueous organic RFBs. Polymers with densely populated redox sites feature high charge-transport and charge-storage capacities [
14,
15]. The incorporation of a redox unit into the polymer chain often improves cycling stability and helps overcome technical issues related to cell design. More importantly, it prevents the movement of redox species across the membrane, which can lead to severe capacity loss and make the cell more demanding on the type of membrane separating the anode and cathode compartments [
16,
17]. For example, the popular organic redox-active compound TEMPO, in its low molecular weight form, is prone to cross over even through the most expensive ion-selective membranes. Polymers, on the other hand, can be separated using relatively inexpensive size-exclusion (dialysis) membranes and microporous separators. There are several publications where this approach was used for ferrocene tetramer and a linear viologen polymer in organic solvent [
18], viologen and ferrocene polymers in organic solvent [
19], polymers embedded with TEMPO and viologen groups in water [
20]. For reviews, one can also see [
13,
21]. Also, the addition of a polymer moiety can enhance the solubility of the redox-active group in a targeted solvent and prevent precipitation. However, there are still a very limited number of groups available for inclusion in polymers: mainly TEMPO, viologens, and ferrocenes. Water-soluble anode-type compounds are particularly scarce [
13].
Considering this, we focused on developing new types of water-soluble polymers that contain s-tetrazine (1,2,4,5-tetrazines) as a redox-active functional group. The s-tetrazines are one of the smallest organic molecules capable of reversible two-electron reduction in the protic media [
22] and can be used as an anolyte. Among other important benefits of the s-tetrazines, high solubility, and exceptional chemical stability in combination with rather rich chemistry allowing targeted structural modification should be mentioned. The examples of the application of tetrazines in energy conversion and storage systems are relatively rare. They have been used in combination with Li anode [
23,
24]. Recently, the first testing of the low-weight tetrazines in a lab-scale non-aqueous redox flow battery has been performed and gave promising results [
25]. Application of the tetrazine-containing water-soluble polymers as the anolyte in aqueous RFBs has not been reported as yet.
From the structural point of view, both linear and branched (crosslinked) polymers can be considered as the carriers for redox-active groups. Most of the studies are focused on polymers with linear architecture [
26,
27,
28,
29] due to the variability of the synthetic procedures and ease in control of the properties of the material. Recently, a few works appeared that deal with more complicated polymer structures such as microgels, i.e., the weakly cross-linked submicron-size polymer meshes [
30]. The branched systems possess a lower viscosity than the linear polymers of the similar molecular weight [
31], while the concentration of the redox-active centers can be as high as that for the linear polymer-based systems, which favorable for use in RFBs. The examples of redox-active water-soluble microgels are rare; the microgels on the base of poly(hydroquinone) [
32,
33] and TEMPO (4-amino,2,2,6,6-tetramethylpiperydine-N-oxyl) [
34,
35] have been reported. Aqueous dispersions of such microgels can be treated as the new branch in a promising class of materials based on redox-active colloids (RACs) [
36].
In this paper, we report the synthesis of various water-soluble s-tetrazine derivatives, which can be used for further integration into polymer chains. Two approaches were tested for the synthesis of redox-active polymers: polymerization of tetrazine-containing monomers and incorporation of tetrazine fragments into already prepared polymers; the latter one was successful yielding a new water-soluble redox polymer. Besides the polymer, the first example of a tetrazine-based microgel is reported. The results of electrochemical testing of new tetrazine-containing monomers and new water-soluble redox polymers will be discussed.
Results and Discussion
1. Synthesis of the water-soluble low-weight s-tetrazine derivatives
Our first goal was to synthesize the water-soluble form of the s-tetrazine molecule and equip it with the functional group responsible for the incorporation in the polymer chain (
Scheme 1).
Tetrazines containing the leaving groups in 3,6 positions are prone to nucleophilic aromatic substitution which provides a convenient route to their structural modification [
42]. Easily available 3,6-dipyrazolyltetrazine
1 was used as the starting compound (
Scheme 2). The presence of two leaving groups was required for the stepwise functionalization of the tetrazine core with the hydrophilicity enhancing moiety (unsubstituted tetrazines are only very weakly soluble in water) as well as with the other structural motif responsible for the follow-up formation of the redox-active polymer. This synthetic strategy was used for the synthesis of the tetrazine-containing monomers as well as for the grafting of the tetrazine moiety into the already prepared polyme.
The ethylcellosolve and morpholine fragments were chosen as the substituents increasing the hydrophilicity of the tetrazines. The reaction of 1 with ethyl cellosolve was carried out in mild conditions at room temperature in the acetonitrile solution to prevent the substitution of both pyrazole rings. The targeted compound 2 was isolated in 72 % yield. If the reaction is performed using ethyl cellosolve as the solvent, both pyrazole rings were replaced with the ethyl cellosolve fragments (3). The replacement of the pyrazole ring for the morpholine fragment yielded tetrazine 4 in 44 %. Unexpectedly, the morpholine-containing tetrazine 4 turned out to be poorly soluble in water. Consequently, it is not perspective for the microgel formation and further research was performed with the tetrazines modified with the ethyl cellosolve group.
There are two possible ways for the incorporation of the tetrazine fragments into the polymer chain: (1) formation of the tetrazine-containing monomer capable of further (co)polymerization; (2) synthesis of the functionalized low molecular weight tetrazine suitable for grafting on the pre-prepared polymer (preferably, by easy click-chemistry-like reaction).
Scheme 2.
Synthesis of the tetrazine derivatives.
Scheme 2.
Synthesis of the tetrazine derivatives.
Within the first approach, compounds
2 and
3 were used for the synthesis of the acrylate-containing monomers
5 and
6. The reaction of
2 with 2-hydroxyethyl acrylate gave the targeted mono-acrylate derivative in a low yield (20 %) due to the competing replacement of both leaving groups for the 2-hydroxyethyl acrylate. Additionally, a significant amount of tetrazine
3 containing two ethyl cellosolve groups was also present in the reaction mixture decreasing the yield of the targeted derivative
5. It was shown that the nucleophilic substitution in tetrazines is a dynamic and reversible process: the presence of two different leaving groups and an external nucleophile may yield a complicated mixture of the products [
43]. The exchange would be selective only if one of the reagents is a much better leaving group. Meanwhile, that was not the case for the combination of pyrazole and ethyl cellosolve groups. Additionally, the leaving group abilities of the ethyl cellosolve and 2-hydroxyethyl acrylate fragments are also similar, yielding the inseparable mixture with a low content of the targeted monomer
5.
To overcome the problem, an alternative approach to the insertion of the polymerizable acrylate fragment was also tested, taking 3 as the starting compound. The nucleophilicity of N-3-aminopropyl methacrylamide is higher and it forms a poor leaving group as compared to 2-hydroxyethyl acrylate. The reaction with the methacrylamide derivative was successfully performed in mild conditions: the targeted tetrazine 6 with a polymerizable acrylate group was isolated in a practical 52 % yield.
An alternative route to the tetrazine-containing polymers was also tested, based on the insertion of the redox-active moiety in the already prepared polymer chain. The reaction of
3 with the poly(acrylamide) containing 3-aminopropyl pendant groups gave a cross-linked polymer soluble neither in water nor in organic solvents. More likely, the substitution of both ethyl cellosolve groups takes place. To overcome the problem,
3 was converted to derivative
7 (70%) with the N-containing poor leaving group. Thus, modified s-tetrazine
7 was used as a redox-active unit to be further grafted to the carboxylic groups of the chosen polymers. For the comparison, the model low-weight tetrazine derivative
9 was also prepared. All new tetrazine derivatives
2-7 and
9 were completely characterized using HRMS and NMR spectroscopy (
Figures S1-S18 in SI). The
13C NMR spectra of all compounds contain the signals in the 160–166 ppm range inherent to the tetrazine carbon atoms [
44] (see SI for the details).
2. Preparation and characterization of the water-soluble polymers decorated with tetrazine groups.
Polymerization of the tetrazine monomers
The acrylate-containing monomers
5 and
6 were subjected to the free-radical copolymerization with the N-isopropylacrylamide monomers (see Experimental section for the details) to synthesize small cross-linked polymer particles, the microgels [
45]. To our disappointment, the characteristic orange color indicating the presence of the tetrazine fragment has completely disappeared during the synthesis, indicating its destruction. The control experiments with monomer
7 showed that the characteristic absorption band at 417 nm inherent to the tetrazine unit completely disappears after half an hour heating (80 ºC) in the presence of the polymerization initiator (K
2S
2O
8), see
Figure S20 in SI. A plausible mechanism of decomposition may involve the addition of the sulphate radicals to the tetrazine units. Thus, the commonly used protocol for the radical polymerization [
46] turned out to be inapplicable in our case. Moreover, it seems that any type of radical polymerization (including polymerization in bulk under vacuum or the use of controlled polymerization methods) will lead to the decomposition of the tetrazine fragments due to the presence of active radicals. Thus, we had to abandon this strategy of the tetrazine-containing polymer preparation and concentrated on the grafting approach.
Water soluble linear PAA with the s-tetrazine pending groups (10)
The modification of a polymer with pendant carboxylic groups using the tetrazine-containing alcohol
7 can be performed via the esterification process. First, the experimental procedure was optimized using propionic acid as a model. Activation of the carboxy groups with carbonyldiimidazole (CDI) [
47] was performed. However, the follow-up reaction with tetrazine-containing alcohol
7 in the presence of a strong base (1,8-diazabicyclo(5.4.0)undec-7-ene, DBU) resulted in the partial decomposition of the tetrazine moiety. Since the preliminary experiments showed that tetrazine moiety did not withstand the presence of a base, it was excluded, whereas the reaction temperature was increased (from 50 to 70 °C) as well as the reaction time. Thus, the optimized procedure was applied to the modification of the polymers.
In the first experiment, the s-tetrazine pending groups from
7 were used for the decoration of the linear polymer containing carboxylic groups, i.e., the polyacrylic acid (PAA) with M
w = 12500. The resulting solution was thoroughly purified by dialysis and filtering, to remove the residual chemicals. The aqueous solution of the tetrazine-modified PAA (PAA-TZ)
10 turned out to be transparent; its bright orange color was typical for the tetrazine groups (See
Figure 1a).
Dynamic light scattering (DLS) investigation was performed to study the behavior of the polymer before and after modification in both acidic and basic conditions. PAA is known to be a pH-sensitive polymer, the degree of its monomer ionization, and, consequently, the size of a polymer coil, is dependent on the solution pH with pKa=4.25 [
48].
Table 1 summarizes the results; the size distributions were very narrow in all cases (
Figure S21 in SI). It is clearly seen that the modified polymer
10 possesses a much bigger hydrodynamic radius R
h compared to the starting one, both below and above pKa of acrylic acid. That may be due to either the substantial increase of its effective hydrodynamic radius (as a result of the attachment of many bulky TZ pending groups forming the “brush-like” structure) or the partial aggregation of the chains into stable nanosized aggregates. PAA-TZ did not precipitate in an aqueous medium, the solution remained homogeneous for several weeks.
Hydrophilic s-tetrazine-modified poly(N-isopropylacrylamide-co-acrylic acid) microgel (8)
The cross-linked polymer particles, namely poly(N-isopropylacrylamide-co-acrylic acid) microgels (
MG), with M
w = 1.7 × 10
9 Da molecular weight, 27 mol% of AA [
40] and hydrodynamic radius R
h equal to 350 nm at pH 6.5 and T = 23 °C (
Table 1) were also tested as the possible s-tetrazine carriers. The initial microgel particles can be easily prepared
via the one-step synthesis using free-radical precipitation polymerization; these microgels are known to form stable dispersions in aqueous conditions [
49].
Table 1.
The hydrodynamic radii (Rh) and the radii of gyration (Rg) of PAA, PAA-TZ 10, the MG-TZ and MG in water at different pH and temperatures.
Table 1.
The hydrodynamic radii (Rh) and the radii of gyration (Rg) of PAA, PAA-TZ 10, the MG-TZ and MG in water at different pH and temperatures.
| Code |
pH |
T = 23 °C |
T = 50 °C |
| Rh, nm |
Rg, nm |
Rg/Rh
|
Rh, nm |
Rg, nm |
Rg/Rh
|
| PAA |
2.4 |
4 ± 1 |
– |
– |
3 ± 1 |
– |
– |
| 6.5 |
7 ± 1 |
– |
– |
7 ± 1 |
– |
– |
| 9.1 |
8 ± 1 |
– |
– |
7 ± 1 |
– |
– |
| PAA-TZ |
2.4 |
13 ± 2 |
– |
– |
12 ± 2 |
– |
– |
| 6.5 |
34 ± 3 |
– |
– |
34 ± 3 |
– |
– |
| 9.1 |
35 ± 3 |
– |
– |
36 ± 3 |
– |
– |
| MG |
2.4 |
159 ± 2 |
98 ± 2 |
0.62 ± 0.02 |
–* |
–* |
–* |
| 6.5 |
356 ± 7 |
140 ± 5 |
0.39 ± 0.02 |
286 ± 7 |
163 ± 8 |
0.57 ± 0.03 |
| 9.1 |
388 ± 5 |
145 ± 7 |
0.37 ± 0.02 |
341 ± 5 |
140 ± 8 |
0.41 ± 0.02 |
| MG-TZ |
2.4 |
206 ± 8* |
–* |
–* |
–* |
–* |
–* |
| 6.5 |
120 ± 5 |
105 ± 9 |
0.87 ± 0.02 |
122 ± 6 |
109 ± 11 |
0.89 ± 0.02 |
| 9.1 |
118 ± 5 |
107 ± 10 |
0.90 ± 0.02 |
119 ± 5 |
107 ± 10 |
0.89 ± 0.02 |
The microgels with grafted tetrazine groups (
MG-TZ) retained the ability to form dispersions in water without aggregation and precipitation in a wide range of conditions, producing a semi-opaque liquid with a typical tetrazine orange hue. The dispersion image and the AFM image of the dried MG-TZ are presented in
Figure 1c. The distinct spherical monodisperse microgel particles are clearly seen in the AFM microphotograph.
In detail, the properties of MG-TZ dispersions were analyzed using dynamic and static light scattering methods (DLS/SLS). We found that MG-TZ typically appears to be spheres with R
h ~120 nm in water, indicating that the grafting process did not lead to the inter-crosslinking of the gel particles (
Table 1). The significant reduction of the size of the MG-TZ compared to the initial microgel size can be attributed to the reduction of the number of carboxylic groups during the modification process. The shape factor of the MG-TZ particles (R
g/R
h value where R
g is the radius of gyration) is inherent to the collapsed microgels, corresponding to a homogeneous sphere shape factor. It was found that the MG-TZ aqueous dispersions are stable in a wide pH range (from ~4.5 to 10) at all the temperatures measured (See
Figure S22 in SI). At low pH, the microgels form aggregates and gradually precipitate with time; the effect is promoted with the temperature increase. Here it should be noted that the initial microgels are endowed by both thermo- and pH-sensitivity due to the nature of the comprising polymers [
50,
51].
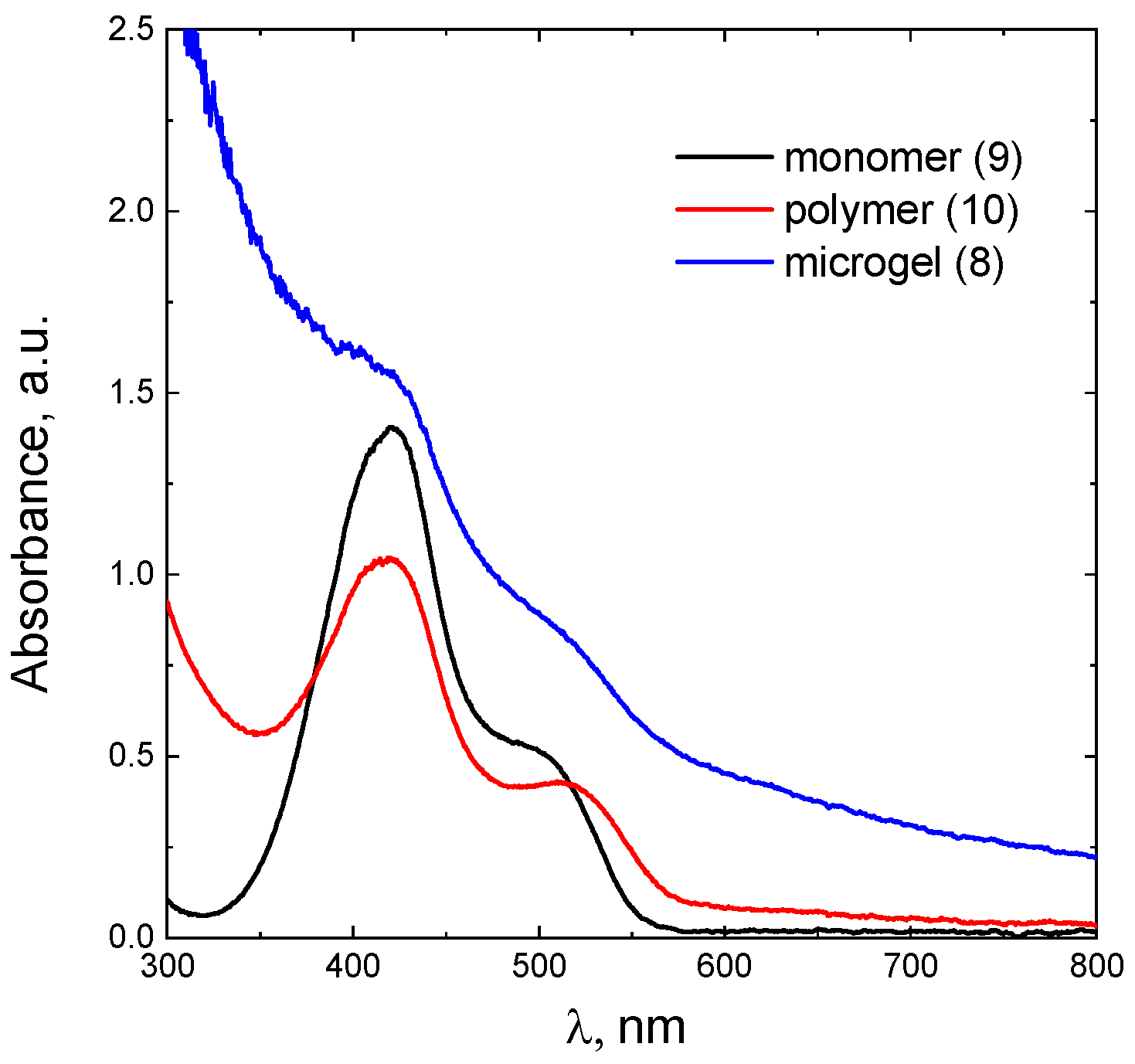
To confirm that the tetrazine units were successfully grafted into the polymer matrix, a comparative study of the electronic spectra of the low-weight derivative
9 as well as polymers
8 and
10 was performed. As follows from
Figure 2, the characteristic absorbance at 417 and 503 nm typical for the tetrazine moiety[
52] was observed for
8-10 in aqueous solution. That signifies that the process of the Tz-groups attachment to both the linear polymer chain and the cross-linked microgel species proceeded successfully. In the case of microgel
8 and polymer
10, the tetrazine’s absorbance is superimposed to the smooth downward curve corresponding to the light scattering. This was taken into account as background and the absorption at 417 nm was determined. Assuming that the molar absorption of the tetrazine group is not affected by the binding to the polymer, the molar absorption for tetrazine
7 (see
Figure S19) was used to determine the concentration of the tetrazine fragments in the modified polymers
8 and
10 and the degree of modification. It was shown that 10 and 3 % of the monomer units were modified with the tetrazine moiety in
10 and
8, respectively. The lower grafting in the microgel is a consequence of higher sterical blocking of the carboxylic groups. It should be additionally emphasized that the samples were extensively purified by dialysis and filtration prior to all experiments assuming the absence non-bonded to polymer tetrazines.
The polymer and microgel compounds under study swell in water at room temperature without precipitation since it is good solvent for them. The viscosity of the polymer (or polymer microgel) solution increases with the concentration increase due to the formation of the entanglements between polymer chains. In case of our species, the apparent viscosity increases sharply at concentrations above 3 wt %, which means that it passes from diluted to semi-diluted mode [
53,
54]. Tetrazine
7 is hygroscopic, it dissolves in very small quantities of water. Since in redox flow batteries the reagents are pumped through the electrochemical cell, meaning they should not be too viscous, this concentration value can be taken as a solubility threshold.
3. Electrochemical properties of the water-soluble tetrazine derivatives
Redox-activity of the low-weight tetrazines in aqueous solution
To test the suitability of new water-soluble tetrazine derivatives as the redox active pendant groups in polymers, with a further focus on use in energy conversion and storage area, their voltammetric behavior was investigated. Noteworthy, electrochemical properties of s-tetrazines in organic solvents have been widely explored (see, e.g., refs. in the review [
52]). Thus, a reversible one-electron reduction at around –1.25 – –1.55 V vs. Fc
+/Fc can be observed for the tetrazines substituted with the NR
2 and OR groups that are structurally similar to the derivatives described herein[
52]. In contrast, the data on the tetrazines behavior in aqueous solutions are scarce, due to a limited solubility of the pristine s-tetrazine in water [
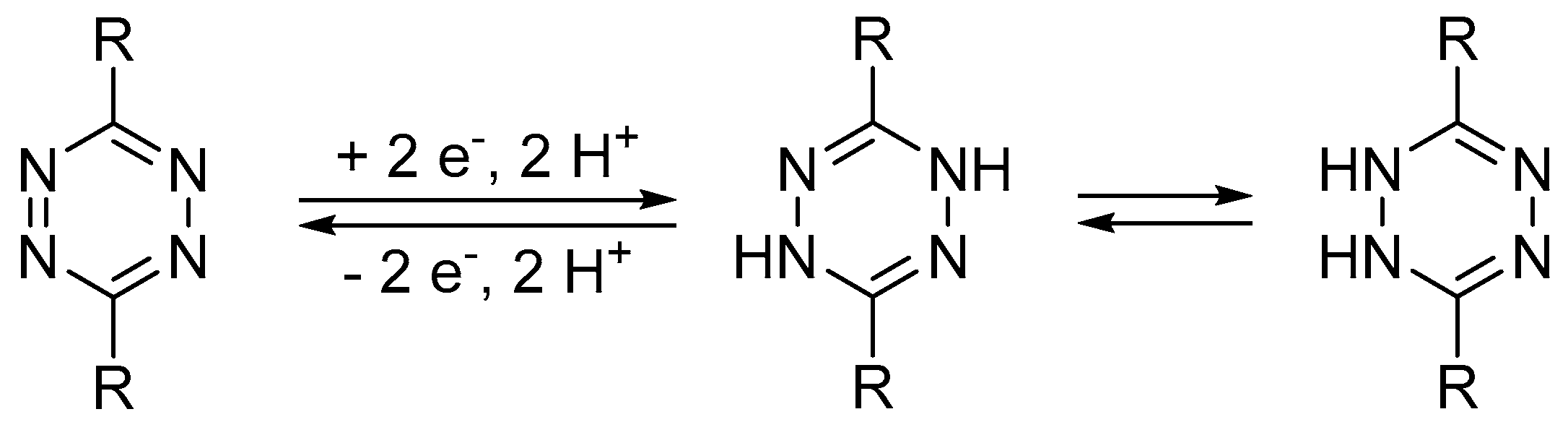
22]. It was shown that the tetrazine fragment undergoes a two-electron two-proton one-step redox process at a potential of around –0.2 V vs. Ag/AgCl/KCl
sat. at pH 7 in an aqueous solution [
22] (
Scheme 3).
For energy conversion systems, two key points are important, i.e., the reversibility of the process and the electrochemical reaction rate. As follows from
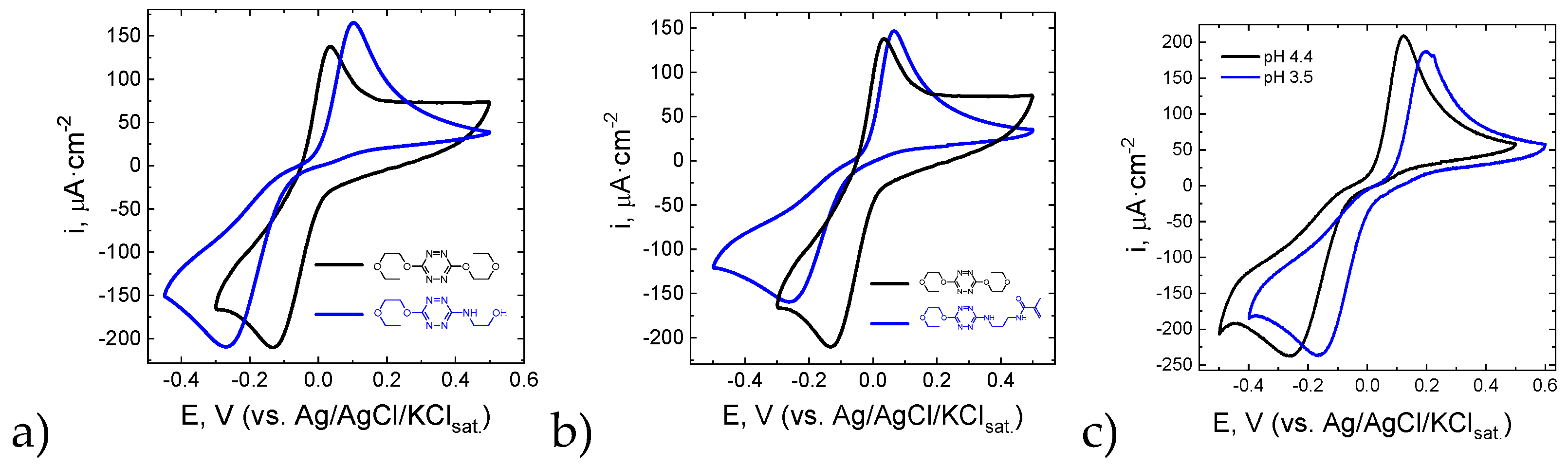
Scheme 3, the tetrazine reduction is coupled with the protonation. Thus, the buffering properties of the solution are important to accelerate the electrochemical reaction and to provide reversibility of the redox process. The voltammetric curves measured for tetrazines
3, 6, and
7 are given in
Figure 3a,b. One can see that the reduction is quasi reversible (the peak currents ratio is close to unity), but the direct-reverse peak separation exceeds 160 mV even in the acetate buffer. The comparison of
3-7 and
3-6 couples showed that the electron donating and bulky substituents slow down the electrochemical reaction. In contrast, an increase in the acidity of the medium (from pH 4.4 to 3.5) slightly increased the electrochemical reaction rate (
Figure 3c).
The potential values measured for tetrazines
3,
6,
7, and
9 at a Pt electrode in an acetate buffer solution are given in
Table 2. Varying the substituents in
3 and
6 positions allows tuning of the potential values as well as increasing the rate of the redox process.
Electron transfer (ET) kinetics is known to be influenced by the working electrode material. In search of a way to improve the ET kinetics of terazines reduction, various electrode materials were tested. Application of glassy carbon electrode taking tetrazine
7 as a model did not improve the situation (
Figure S24): relatively slow kinetics (peak-to-peak separation ~400 mV) was observed. Afterwards, carbon paper electrode (Toray TGP-H-90), one of the common electrodes for flow cell studies [
55,
56] was tested. As it has been previously shown, carbon electrodes preliminary annealed in air demonstrated enhanced performance [
57,
58] in a flow cell; therefore, cyclic voltammetry responses of tetrazine
7 on pristine and annealed (400 °C, 30 h) carbon paper were compared (
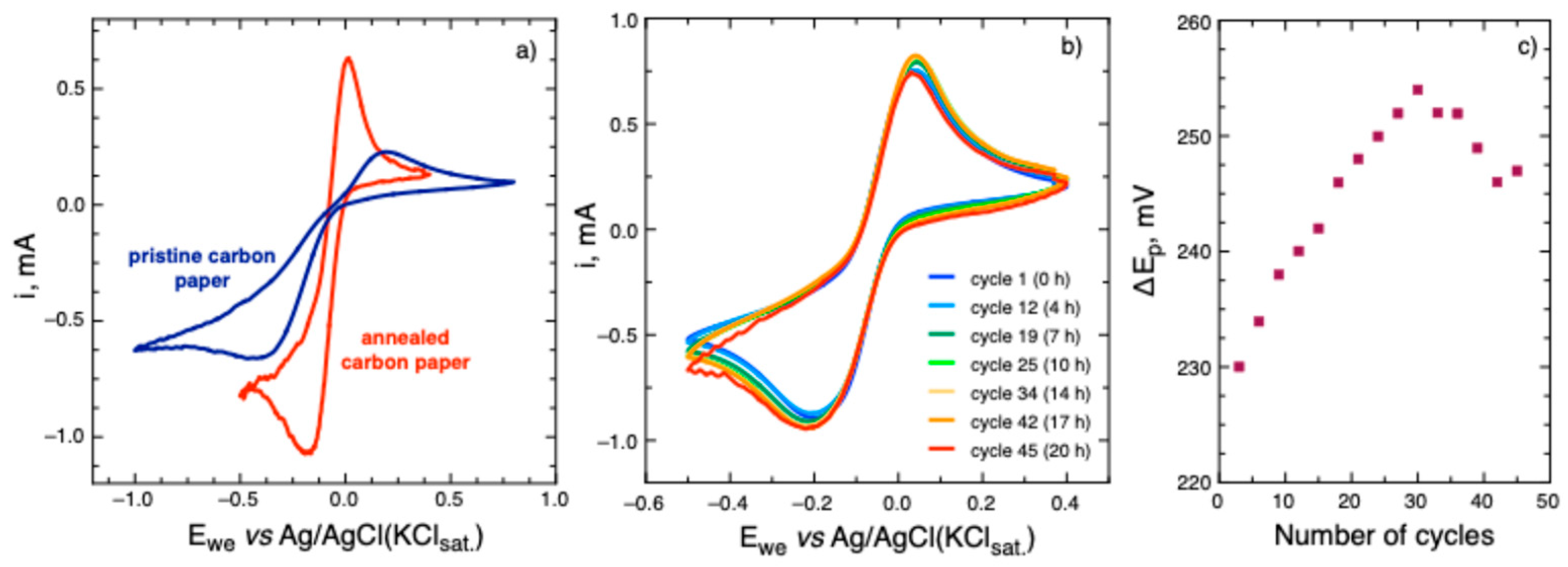
Figure 4a). Slow scan rate (5 mV/s) was used to reduce the capacitive currents due to the high surface area of the electrode.
Indeed, reaction kinetics and reversibility of tetrazine
7 reduction was significantly enhanced (
Figure 4a) as compared to the results obtained at the pristine electrode. Tetrazine
7 at the pristine electrode demonstrates higher peak-to-peak separation, i.e. slower reaction rate and lower reversibility. The possible reason can be a higher amount of oxygen groups formed on the carbon paper surface upon annealing in air. They may enhance the electrode process by participating in protonation steps involved in the tetrazine reduction reaction.
The long-term cycling of tetrazine
7 on the annealed carbon paper electrode was also performed. Groups of three consecutive cycles, each group followed by an OCP period of 1 hour, were measured for 20 hours. It can be seen that tetrazine
7 demonstrates decent cycling stability (
Figure 4b). The peak-to-peak separation increases during first 30 cycles and then there is a stabilization (and even reduction) of Δ
Ep (
Figure 4c). The change in peak-to-peak separation may be attributed to slow changing in the surface conditions of carbon paper upon cycling in acetate buffer solution.
Thus, the proper selection of the working electrode material allows the significant improvement of the new tetrazines reduction kinetics. Though their application in fast-operating systems such as electrochemical capacitors is still under question, these compounds may be considered of the potential interest as possible anolyte reagents for RFBs.
Table 2.
The peak potentials, peak separation, and half-wave potentials of tetrazines 3, 6, 7, and 9 vs. Ag/AgCl/KClsat. in a 0.2 M acetate buffer solution pH 4.4 at a Pt electrode at a scan rate of 25 mV/s.
Table 2.
The peak potentials, peak separation, and half-wave potentials of tetrazines 3, 6, 7, and 9 vs. Ag/AgCl/KClsat. in a 0.2 M acetate buffer solution pH 4.4 at a Pt electrode at a scan rate of 25 mV/s.
| № |
Structure |
Ep,c, V |
Ep,a, V |
(Ep,a – Ep,c), V |
(Ep,c + Ep,a)/2 , V |
| 3 |
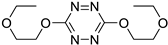 |
–0.131 |
0.034 |
0.165 |
–0.049 |
| 7 |
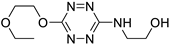 |
–0.270 |
0.103 |
0.373 |
–0.084 |
| 6 |
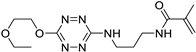 |
–0.261 |
0.066 |
0.327 |
–0.098 |
| 9 |
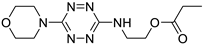 |
–0.222 |
0.062 |
0.284 |
–0.080 |
Figure 4.
(a) Cyclic voltammograms of 7 at the pristine and annealed carbon paper electrodes in the 0.2 M acetate buffer solution at pH 4.4 (5 mV/s scan rate); (b) Cyclic voltammograms of 7 in a 0.2 M acetic buffer solution at pH 4.4 at annealed carbon paper electrode at a potential scan rate of 5 mV/s recorded over time period of 20 hours; (c) the evolution of cathodic peak current with time during repetitive cycling of tetrazine 7 on the annealed carbon paper electrode.
Figure 4.
(a) Cyclic voltammograms of 7 at the pristine and annealed carbon paper electrodes in the 0.2 M acetate buffer solution at pH 4.4 (5 mV/s scan rate); (b) Cyclic voltammograms of 7 in a 0.2 M acetic buffer solution at pH 4.4 at annealed carbon paper electrode at a potential scan rate of 5 mV/s recorded over time period of 20 hours; (c) the evolution of cathodic peak current with time during repetitive cycling of tetrazine 7 on the annealed carbon paper electrode.
Electrochemical characterization of the tetrazine-containing polymers and microgels
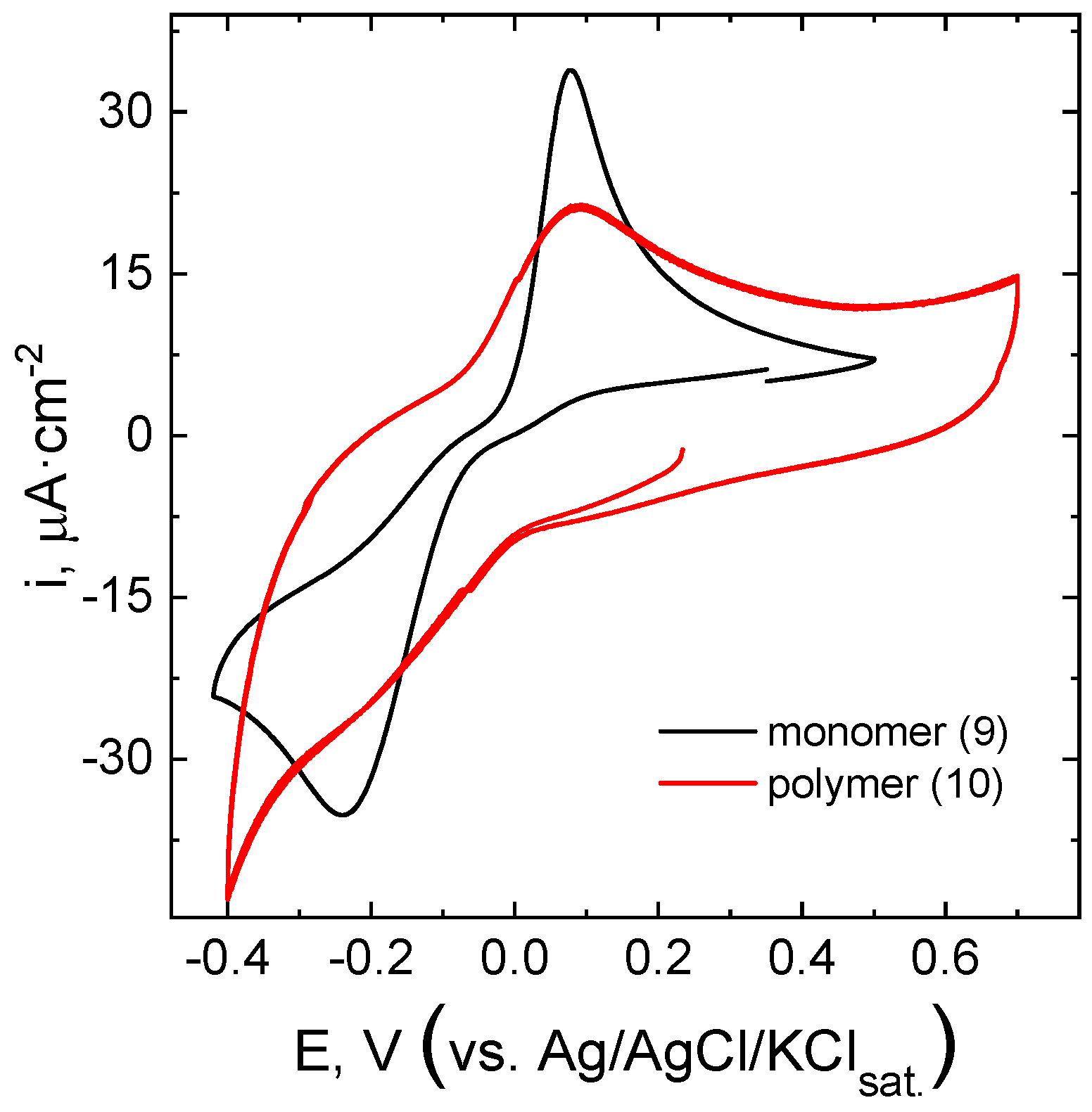
To estimate the suitability of polymers
8 and
10 for energy storage applications, their redox activity in aqueous solution was studied using cyclic voltammetry. The voltammetry curves measured for the linear polymer
10 and for its low-weight analog
9 on glassy carbon electrode are given in
Figure 5. One can see that the direct and reverse peak potential values for
9 and
10 coincide well. The direct-reverse peak separation is rather significant in both cases; this indicates that the electrochemical reaction is relatively slow. However, the voltammograms measured for polymer
10 at the annealed carbon paper electrode demonstrated good reversibility (
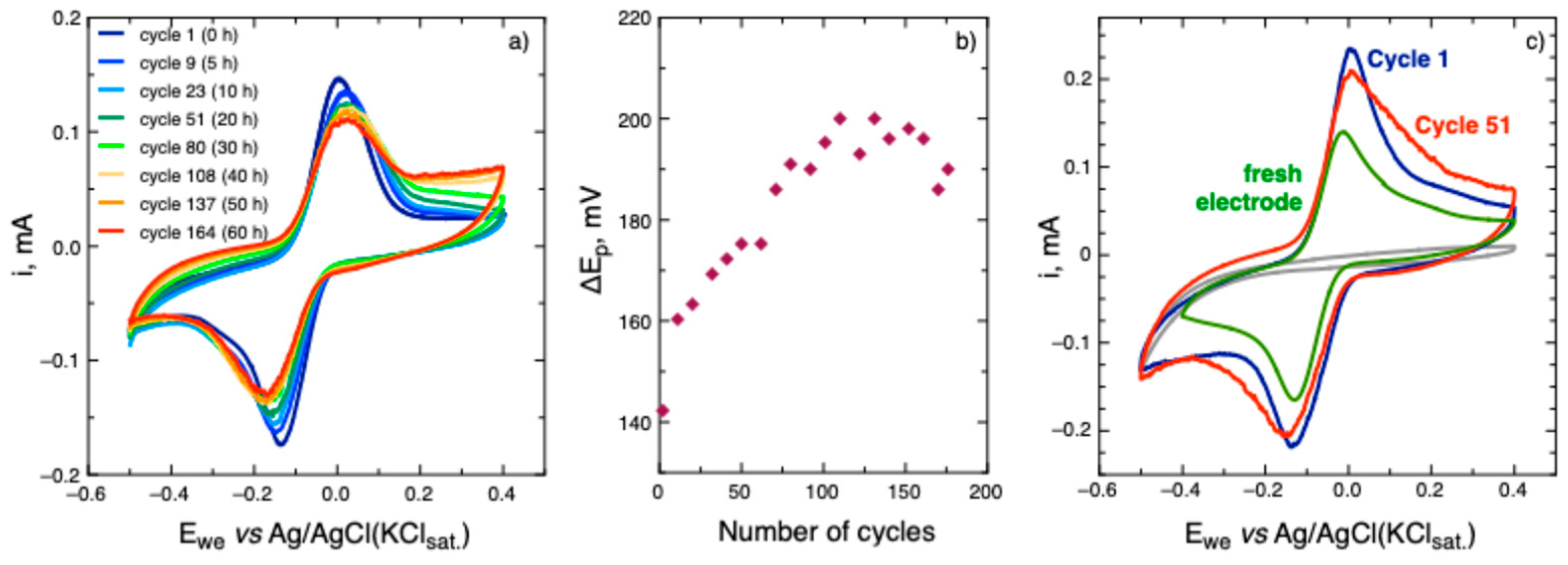
Figure 6a) and much lower peak separation (∼140 mV), similar to what have been observed in the case of tetrazine
7.
During the long-term cycling (cyclic voltammograms were recorded by groups of three cycles, each group followed by OCP period of 1 hour) a steady increase of peak-to-peak separation (
Figure 6b) was observed up to 100 cycles, and after that there is stabilization at ∼200 mV, i.e. polymer
10 demonstrated quite slow but relatively stable reaction kinetics.
Figure 6.
(a) Cyclic voltammograms of polymer 10 in 0.1 M acetic buffer solution at pH 4.4 at annealed carbon paper electrode at scan rate of 25 mV/s, recorded over time period of 60 hours; (b) the evolution of cathodic peak current with time during repetitive cycling of of polymer 10 on the annealed carbon paper electrode. (c) 1st (blue) and 51st (red) cyclic voltammogram recorded on the annealed carbon paper electrode, and cyclic voltammogram (green) of a fresh carbon paper electrode in the same polymer 10 solution after long-term cycling. Grey curve represents background voltammogram.
Figure 6.
(a) Cyclic voltammograms of polymer 10 in 0.1 M acetic buffer solution at pH 4.4 at annealed carbon paper electrode at scan rate of 25 mV/s, recorded over time period of 60 hours; (b) the evolution of cathodic peak current with time during repetitive cycling of of polymer 10 on the annealed carbon paper electrode. (c) 1st (blue) and 51st (red) cyclic voltammogram recorded on the annealed carbon paper electrode, and cyclic voltammogram (green) of a fresh carbon paper electrode in the same polymer 10 solution after long-term cycling. Grey curve represents background voltammogram.
To determine whether the increase of peak-to-peak separation is caused by surface changes of the carbon paper electrode or degradation of the polymer solution, the carbon paper electrode was removed from the cell after long-term cycling and replaced with a fresh electrode. We observed that peak-to-peak separation on the fresh electrode returned to its initial value (
Figure 6c). Thus its increase is attributed to the change in carbon paper surface conditions rather than degradation of the polymer solution. The reduction currents observed in the background voltammogram (
Figure 6c) at the potentials less than –0.3 V
vs Ag/AgCl may correspond to the electrochemical reduction of oxygen-containing functional groups on the carbon electrode surface; that may affect the reaction kinetics.
Apparent self-diffusion coefficients D for the tetrazine-modified polymers and microgels were evaluated using dynamic light method (see Materials and Methods section for the details). They were found to be equal to 6.7∙10
-8 cm
2/s for PAA-TZ and 1.8∙10
-8 cm
2/s for MG-TZ in water at pH 6.5, which is typical for such systems [
35]. Determination of the ET rate constant is challenging since electrochemical reduction of tetrazines is a ECEC process including consecutive electrochemical (E) and chemical (C) steps [
22]. The classical Nicolson method [
59] is not applicable in this case. Theoretically, the desired value could be obtained by computational modeling of CV series at different potential sweep rates. However, this is a complicated task since many independent parameters (two electron transfer constants, two reduction potentials, rates and equilibrium constants for two chemical steps, diffusion coefficient) must be fitted.
The slow protonation step might be a possible reason why we were unable to detect the redox activity of the microgel
8 on glassy carbon electrode, despite that it contains tetrazine fragments, as confirmed by UV-Vis data. However, we were able to observe the electrochemical response of microgel
8 on the annealed carbon paper electrode (
Figure 7). The voltammograms demonstrate prominent adsorption peak of reduced tetrazine fragments, which gradually decreases upon long-term cycling. Similar to the case of tetrazine-grafted linear polymers, it is probably caused by reduction of oxygen-containing groups on carbon electrode surface.
Slow in itself, the electrochemical reaction in microgels becomes even more impeded. The “network mesh structure” and relatively large size of the cross-linked microgel impedes penetration of the protons required for the reduction. Based on these experimental results, we can speculate that the use of relatively small branched star polymers [
60], or hyperbranched polymers[
61] could be more effective carriers for these redox-active species, both from the point of the ET kinetics and the choice of separator membrane for the RFB cell. This study is in progress now. At present, the tetrazine-modified polymer
10 with well-pronounced redox activity may be considered as the first step towards fabrication of a novel type of water-soluble anodic materials.
Theoretical capacity of the presented species can be estimated as 234 mAh·g–1 for low molecular weight tetrazine 7 and as 58 mAh·g–1 for the linear polymer compound and 173 mAh·L–1 for its aqueous solution, taking into account the solubility and viscosity increase. However, there is still room for improvement, as only a small number of carboxylic groups have been modified with tetrazine units.
It would be interesting to compare new tetrazine derivatives with the previously reported electrolyte materials. Noteworthy, the number of water-soluble organic molecules suitable for the performance as anodic active materials is still rather limited (see reviews [
12,
13,
62] ). The mostly investigated species are antraquinones. Anthraquinone-2-sulfonic acid demonstrates one-electron redox process at E
0 = –0.07 vs. AgCl/Ag [
63]); reduction of 3,4-dihydroxy-9,10-anthraquinone-2-sulfonic acid is a two-electron process with E
0 = –0.12 V vs. AgCl/Ag [
64]. Thus, the reduction potentials for antraquinones and tetrazines are similar whereas, as concerns water solubility, the tetrazines have advantages over antraquinones. Methyl viologen derivatives can be considered as the other popular candidate for redox-active anodic material. They have higher water solubility as compared to tetrazines and show more cathodic reduction potential [
65]. Unfortunately, they are toxic to human beings and animals and are banned in many countries. Their redox activity produces superoxide anions which are linked to the development of Parkinson's disease [
66]. Also, naphthalene diimides demonstrate promising negolyte qualities, but yet as low molecular weight compounds [
67]. Thus, the search for the optimal water-soluble anodic material is still a challenge, and tetrazine derivatives may be considered as perspective ones.