5. Experimental Section
General Information. Unless otherwise specified, the starting reagents and deuterated solvent were purchased from commercial sources and used without further purification (Sigma-Aldrich, Fisher scientific, TCI). All solvents were dried and freshly distilled before use, taking precautions to exclude moisture by refluxing over CaH2. All reactions were performed under an argon-inert atmosphere. Thin layer chromatography (TLC) was performed on precoated sheets of silica gel 60 with fluorescent indicator UV254 (Merck). Detection was accomplished by exposure to a UV lamp and by heating after exposure to an ethanolic solution of p-anisaldehyde. Chromatographic separations were achieved on silica gel columns (Kieselgel 60, 40−63 μm, Merck) using a cyclohexane/ethyl acetate eluent system. Flash chromatography purifications were performed on Interchim Puriflash (Puriflash columns 50 μ) using a cyclohexane/ethyl acetate eluent system. In all cases, distilled solvents were used as eluents for column chromatography. NMR spectra were recorded on a Bruker AvanceTM 300 spectrometer. 1H NMR spectra were recorded at 300 MHz and data are reported as chemical shift (δ) in ppm, multiplicity (s = singlet, d = doublet, t = triplet, b = broad, m = multiplet), coupling constants J in Hz and integration. 13C NMR spectra were recorded at 75 MHz using broadband proton decoupling and the data were reported as chemical shift (δ) in ppm. High-resolution mass spectra (HRMS) were measured on Agilent 6530 Q-Tof MS system. The Q-TOF MS instrument was operated under the following conditions: Ion source ESI+ Agilent Jet Stream or APCI in positive ionization mode. FTIR spectra were recorded with a PerkinElmer Frontier.
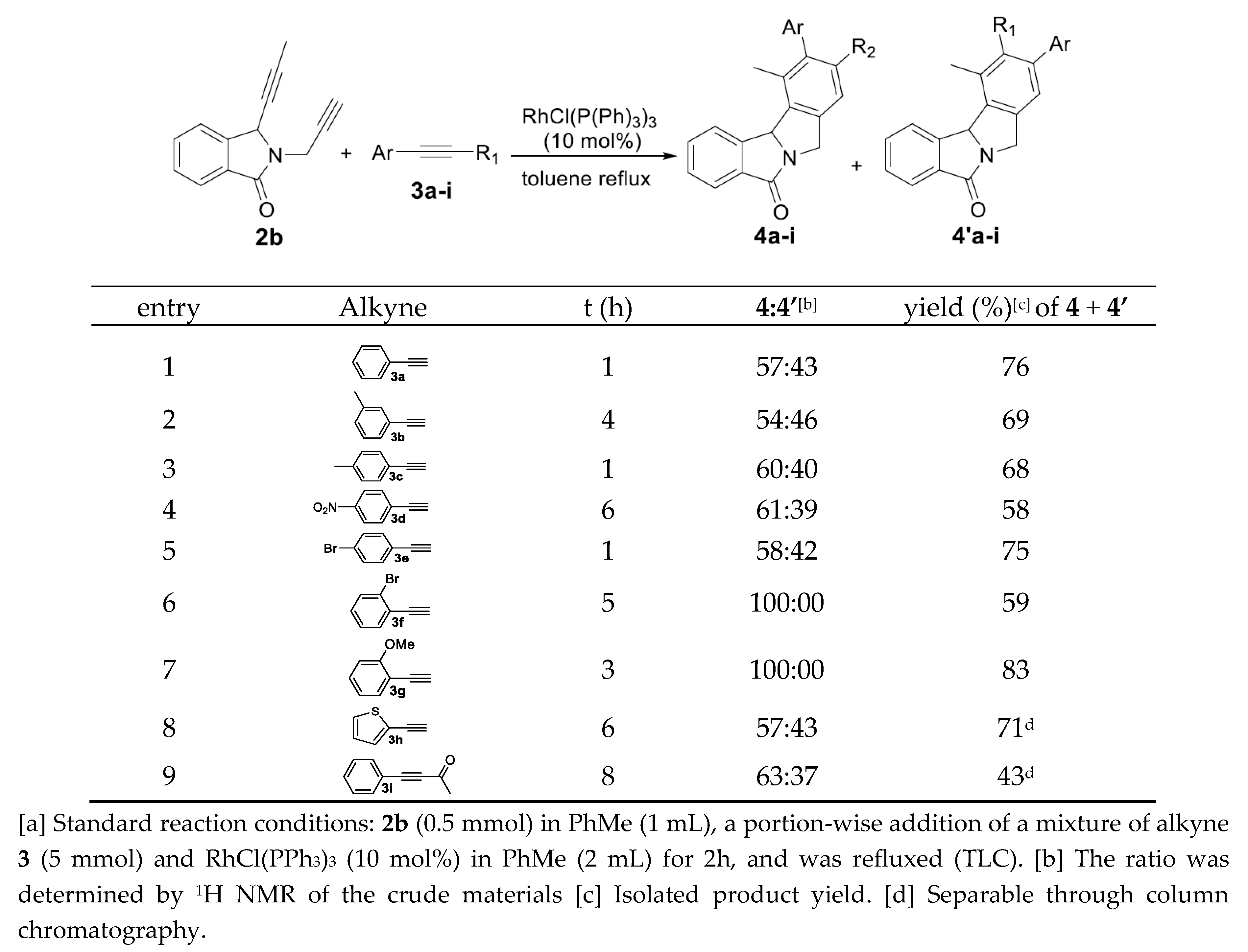
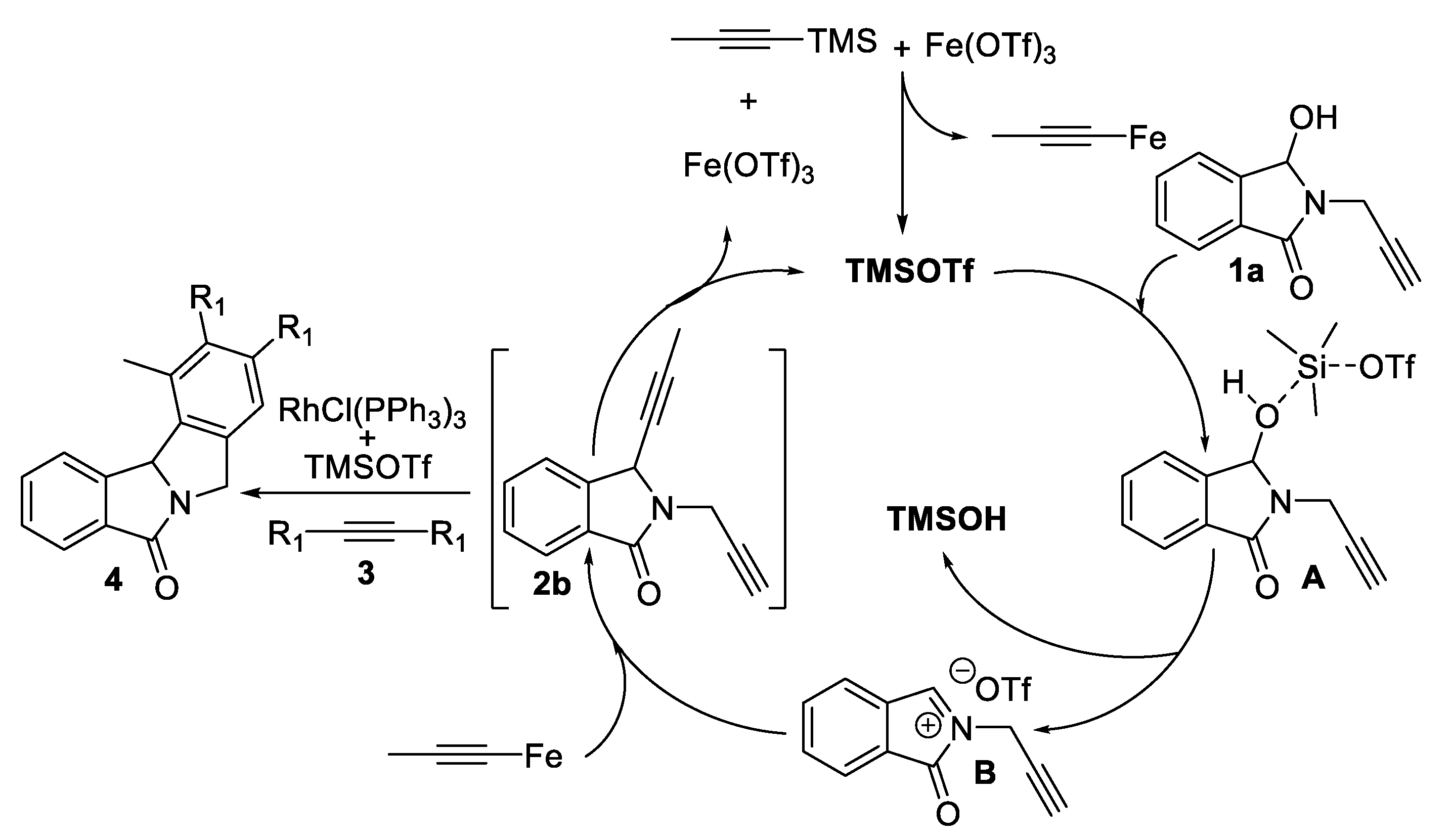
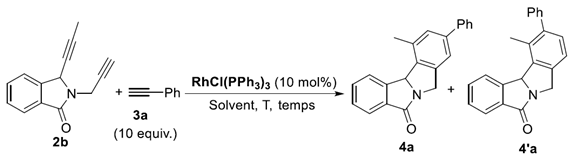
Representative Procedure for the Rhodium(I)-Catalyzed Cyclotrimerization of Dialkynes: To a solution of alkynes 3a-w (2.3 mmol, 10 eq) and RhCl(PPh3)3 (0.023 mmol, 0.1 eq) in toluene (4 mL) we slowly add the substrate 2b (0.23 mmol, 1 equiv.), for 2 h, under argon. The reaction mixture was heated under reflux and monitored by TLC. After the total conversion of the starting material, the solvent was removed under reduced pressure. The crude product was then purified by flash chromatography on a silica gel column using a mixture of cyclohexane/AcOEt as the eluent or DCM/AcOEt to give the desired compounds 4.
Synthesis and characterization of compounds 4a/4a‘.
These products were obtained as a mixture of separable two regioisomers in 76% global yield, with a ratio of 4a:4a’: 60:40.
11-Methyl-9-phenyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4a).
Major regioisomer-4a: This product was isolated as a white solid, Rf (cyclohexane/AcOEt: 9/1) = 0.24; m.p. = 174-176 °C; IR (νmax cm-1): 1687;1H NMR (300 MHz, CDCl3): δH 7.99-7.89 (m, 1H, Haro), 7.89 (d, J = 7.5 Hz, 1H, Haro), 7.63 (td, J = 7.6, 1.3 Hz, 1H, Haro), 7.56-7.49 (m, 3H, Haro), 7.46-7.34 (m, 3H, Haro), 7.33 (s, 1H, Haro), 7.30 (s, 1H, Haro), 6.14 (s, 1H, CH), 5.29 (d, J = 15.0 Hz, 1H, CH2), 4.52 (d, J = 15.0 Hz, 1H, CH2), 2.73 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.2 (Cqaro), 142.7 (Cqaro), 142.1 (Cqaro), 140.6 (Cqaro), 135.9 (Cqaro), 133.8 (Cqaro), 133.7 (Cqaro), 132.2 (CHaro), 129.0 (2 x CHaro), 128.8 (CHaro), 128.8 (CHaro), 127.7 (CHaro), 127.3 (2 x CHaro), 125.2 (CHaro), 124.8 (CHaro), 119.7 (CHaro), 69.5 (CH), 49.5 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C22H17NO [M+H]+: 312.1383, found 312.1404.
11-Methyl-10-phenyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4a’).
Minor regioisomer-4a’: This product was isolated as a white solid, Rf (cyclohexane/AcOEt: 9/1) = 0.23; m.p. = 173-175 °C; IR (νmax cm-1): 1687; 1H NMR (300 MHz, CDCl3): δH 7.97-7.93 (m, 1H, Haro), 7.90 (d, J = 7.3 Hz, 1H, Haro), 7.61 (td, J = 7.5, 1.3 Hz, 1H, Haro), 7.51 (t, J = 7.4 Hz, 1H, Haro), 7.42-7.35 (m, 3H, Haro), 7.28 (d, J = 1.8 Hz, 2H, Haro), 7.19 (d, J = 4.3 Hz, 2H, Haro), 6.17 (s, 1H), 5.29 (d, J = 15.0 Hz, 1H, CH2), 4.51 (d, J = 14.9 Hz, 1H, CH2), 2.57 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.5 (Cqaro), 142.3 (Cqaro), 141.3 (Cqaro), 140.9 (Cqaro), 138.0 (Cqaro), 133.8 (Cqaro), 132.2 (CHaro), 131.3 (Cqaro), 130.5 (CHaro), 129.5 (2 x CHaro), 128.8 (CHaro), 128.3 (2 x CHaro), 127.2 (CHaro), 125.4 (CHaro), 124.7 (CHaro), 120.8 (CHaro), 70.0 (CH), 49.5 (CH2), 20.2 (CH3) ppm. HRMS (+ESI) calculated for C22H17NO [M+H]+: 312.1383, found 312.1404.
Synthesis and characterization of compounds 4b/4b‘.
This product was obtained as a mixture of inseparable two regioisomers and was isolated as a yellow oil in 69% global yield, with a ratio of 4b/4b’: 54/46, Rf (cyclohexane/AcOEt: 7/3) = 0.22; IR (νmax cm-1): 1705.
11-Methyl-9-(m-tolyl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4b).
Major regioisomer-4b: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.93 (d, J = 5.5 Hz, 1H, Haro), 7.88 (d, J = 3.8 Hz, 1H, Haro), 7.54-7.47 (m, 2H, Haro), 7.37-7.27 (m, 6H, Haro), 6.16 (s, 1H, CH), 5.29 (d, J = 14.9 Hz, 1H, CH2), 4.51 (d, J = 14.9 Hz, 1H, CH2), 2.58 (s, 3H, CH3), 2.40 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 145.4 (Cqaro), 142.3 (Cqaro), 141.1 (Cqaro), 140.4 (Cqaro), 137.8 (Cqaro), 135.6 (Cqaro), 133.5 (Cqaro), 132.0 (CHaro), 131.2 (Cqaro), 130.3 (CHaro), 128.7 (CHaro), 128.6 (CHaro), 128.1 (CHaro), 127.8 (CHaro), 126.5 (CHaro), 125.1 (CHaro), 124.3 (CHaro), 120.6 (CHaro), 69.9 (CH), 49.3 (CH2), 21.6 (CH3), 21.5 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO [M+H]+: 326.1539, found 326.1560.
11-Methyl-10-(m-tolyl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4b’).
Minor regioisomer-4b’: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.96 (d, J = 5.3 Hz, 1H, Haro), 7.91 (d, J = 3.7 Hz, 1H, Haro), 7.66-7.58 (m, 2H, Haro), 7.22-7.14 (m, 4H, Haro), 7.10-7.05 (m, 2H, Haro), 6.13 (s, 1H, CH), 5.29 (d, J = 14.9 Hz, 1H, CH2), 4.51 (d, J = 14.9 Hz, 1H, CH2), 2.72 (s, 3H, CH3), 2.42 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 74.0 (C=O), 145.1 (Cqaro), 142.5 (Cqaro), 142.1 (Cqaro), 140.6 (Cqaro), 138.4 (Cqaro), 137.8 (Cqaro), 133.6 (Cqaro), 133.6 (Cqaro), 132.0 (CHaro), 130.1 (CHaro), 128.7 (2 x CHaro), 128.3 (CHaro), 127.9 (CHaro), 125.3 (CHaro), 124.6 (CHaro), 124.6 (CHaro), 119.6 (CHaro) 69.4 (CH), 49.3 (CH2), 21.7 (CH3), 20.1 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO [M+H]+: 326.1539, found 326.1560.
Synthesis and characterization of compounds 4c/4c‘.
These products were obtained as a mixture of inseparable two regioisomers and were isolated as a yellow oil in 68% global yield, with a ratio of 4c/4c’: 60/40, Rf (cyclohexane/AcOEt: 8/2) = 0.23; IR (νmax cm-1): 1697.
11-Methyl-9-(p-tolyl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4c).
Major regioisomer-4c: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.98-7.86 (m, 2H, Haro), 7.62 (t, J = 7.5 Hz, 1H, Haro), 7.53-7.42 (m, 2H, Haro), 7.32 (s, 1H, Haro), 7.29 (s, 1H, Haro), 7.26-7.13 (m, 3H, Haro), 6.13 (s, 1H, CH), 5.28 (d, J = 14.9 Hz, 1H, CH2), 4.51 (d, J = 15.0 Hz, 1H, CH2), 2.72 (s, 3H, CH3), 2.39 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1(C=O), 145.2 (Cqaro), 142.6 (Cqaro), 142.0 (Cqaro), 137.7 (Cqaro), 137.5 (Cqaro), 135.6 (Cqaro), 133.7 (Cqaro), 133.7 (Cqaro), 132.1 (CHaro), 129.7 (2 x CHaro), 128.8 (CHaro), 128.5 (CHaro), 127.1 (2 x CHaro), 125.2 (CHaro), 124.7 (CHaro), 119.5 (CHaro), 69.5 (CH), 49.5 (CH2), 21.8 (CH3), 21.2 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO [M+H]+: 326.1539, found 326.1565.
11-Methyl-10-(p-tolyl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4c’).
Minor regioisomer-4c’e: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.95 (d, J = 7.7 Hz, 1H, Haro), 7.90 (d, J = 7.5 Hz, 1H, Haro), 7.61 (t, J = 7.5 Hz, 1H, Haro), 7.50 (t, J = 7.5 Hz, 1H, Haro), 7.25-7.14 (m, 6H, Haro), 6.16 (s, 1H, CH), 5.28 (d, J = 14.5 Hz, 1H, CH2), 4.51 (d, J = 15.0 Hz, 1H, CH2), 2.58 (s, 3H, CH3), 2.41 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.6 (Cqaro), 142.2 (Cqaro), 140.7 (Cqaro), 138.3 (Cqaro), 138.0 (Cqaro), 136.9 (Cqaro), 133.8 (Cqaro), 132.2 (CHaro), 131.3 (Cqaro), 130.5 (CHaro), 129.4 (2 x CHaro), 129.0 (2 x CHaro), 128.8 (CHaro), 125.4 (CHaro), 124.7 (CHaro), 120.7 (CHaro), 70.0 (CH), 49.5 (CH2), 21.3 (CH3), 20.2 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO [M+H]+: 326.1539, found 326.1565.
Synthesis and characterization of compounds 4d/4d‘.
These products were obtained as a mixture of inseparable two regioisomers and were isolated as a yellow oil in 58% global yield, with a ratio of 4d/4d’: 61/39, Rf (cyclohexane/AcOEt: 7/3) = 0.34; IR (νmax cm-1): 1690, 1511, 1339.
11-Methyl-9-(4-nitrophenyl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4d).
Major regioisomer-4d: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 8.30-8.24 (m, 2H, Haro), 7.96-7.86 (m, 2H, Haro), 7.71-7.60 (m, 2H, Haro), 7.54-7.43 (m, 2H, Haro), 7.36 (s, 1H, Haro), 7.33 (s, 1H, Haro), 6.17 (s, 1H, CH), 5.30 (d, J = 15.2, 2.6 Hz, 1H, CH2), 4.51 (d, J = 15.3, 2.1 Hz, 1H, CH2), 2.57 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 148.2 (Cqaro), 147.1 (Cqaro), 145.1 (Cqaro), 142.2 (Cqaro), 139.9 (Cqaro), 138.5 (Cqaro), 133.6 (Cqaro), 132.3 (CHaro), 131.1 (Cqaro), 130.4 (CHaro), 130.1 (CHaro), 128.9 (CHaro), 128.0 (CHaro), 125.3 (CHaro), 124.8 (CHaro), 124.2 (CHaro), 123.6 (CHaro), 121.2 (CHaro), 69.8 (CH), 49.4 (CH2), 20.1 (CH3) ppm. HRMS (+ESI) calculated for C22H16N2O3 [M+H]+: 357.1234, found 357.1254.
11-Methyl-10-(4-nitrophenyl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4d’).
Minor regioisomer-4d’: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 8.30-8.24 (m, 2H, CHaro), 7.96-7.86 (m, 2H, CHaro), 7.71-7.60 (m, 2H, CHaro), 7.54-7.43 (m, 2H, CHaro), 7.24-7.16 (m, 2H, CHaro), 6.14 (s, 1H, CH), 5.30 (d, J = 15.2, 2.6 Hz, 1H, CH2), 4.51 (d, J = 15.3, 2.1 Hz, 1H, CH2), 2.75 (s, 1H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 147.3 (Cqaro), 147.0 (Cqaro), 144.8 (Cqaro), 143.2 (Cqaro), 139.5 (Cqaro), 137.7 (Cqaro), 134.4 (Cqaro), 133.5 (Cqaro), 132.3 (CHaro), 130.4 (2 x CHaro), 128.9 (2 x CHaro), 125.2 (CHaro), 124.8 (CHaro), 123.6 (2 x CHaro), 119.9 (CHaro), 69.4 (CH), 49.3 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C22H16N2O3 [M+H]+: 357.1234, found 357.1254.
Synthesis and characterization of compounds 4e/4e‘.
This product was obtained as a mixture of inseparable two regioisomers and was isolated as a yellow oil, in 75% global yield, with a ratio of 4e/4e’: 58/42, Rf (cyclohexane/AcOEt: 7/3) = 0.26; IR (νmax cm-1): 1688.
9-(4-Bromophenyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4e):
Major regioisomer-4e: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 8.04 (d, J = 7.9 Hz, 1H, Haro), 8.00 (d, J = 7.7 Hz, 1H, Haro), 7.71 (t, J = 7.6 and1.4 Hz, 1H, Haro), 7.66-7.58 (m, 3H, Haro), 7.36 (s, 2H, Haro), 7.23 (d, J = 1.9 Hz, 2H, Haro), 6.26 (s, 1H, CH), 5.38 (d, J = 15.1 Hz, 1H, CH2), 4.60 (d, J = 15.1 and 1.6 Hz, 1H, CH2), 2.65 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.4 (Cqaro), 141.3 (2 x Cqaro), 141.0 (Cqaro), 140.2 (Cqaro), 138.2 (Cqaro), 133.7 (Cqaro), 132.2 (CHaro), 131.5 (2 x CHaro), 131.2 (2 x CHaro), 130.3 (CHaro), 128.9 (CHaro), 125.3 (CHaro), 124.8 (CHaro), 121.5 (Cqaro), 120.9 (CHaro), 69.9 (CH), 49.4 (CH2), 20.1 (CH3) ppm. HRMS (+ESI) calculated for C22H16BrNO [M+H]+: 390.0488, found 390.0508.
10-(4-Methoxyphenyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4e’).
Minor regioisomer-4e’: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 8.06-7.96 (m, 2H, Haro), 7.75-7.68 (m, 1H, Haro), 7.67 -7.57 (m, 2H, Haro), 7.50 (d, J = 8.5 Hz, 2H, Haro), 7.40-7.32 (m, 3H, Haro), 6.22 (s, 1H, CH), 5.38 (d, J = 15.1 Hz, 1H, CH2), 4.60 (d, J = 15.1 Hz, 1H, CH2), 2.82 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.1 (Cqaro), 142.9 (Cqaro), 140.8 (Cqaro), 139.5 (Cqaro), 136.3 (Cqaro), 134.1 (Cqaro), 133.6 (Cqaro), 132.2 (CHaro), 132.1 (2 x CHaro), 128.9 (2 x CHaro), 128.5 (CHaro), 125.2 (CHaro), 124.8 (CHaro), 122.0 (Cqaro), 120.9 (CHaro), 119.5 (CHaro), 69.5 (CH), 49.4 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C22H16BrNO [M+H]+: 390.0488, found 390.0508.
Synthesis and characterization of compound 4f.
10-(2-Bromophenyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4f).
This product was obtained as a single regioisomer in 59% yield.
This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 7/3) = 0.24; IR (νmax cm-1): 1696; 1H NMR (300 MHz, CDCl3): δH 7.95 (d, J = 7.7 Hz, 1H, Haro), 7.90 (d, J = 8.4 Hz, 1H, Haro), 7.68-7.60 (m, 2H, Haro), 7.51 (t, J = 7.4 Hz, 1H, Haro), 7.34 (td, J = 7.5, 1.3 Hz, 1H, Haro), 7.23 (dd, J = 3.8, 1.9 Hz, 1H, Haro), 7.21-7.15 (m, 2H, Haro), 7.12 (s, 1H, Haro), 6.15 (s, 1H, CH), 5.28 (d, J = 15.0 Hz, 1H, CH2), 4.52 (d, J = 15.0 Hz, 1H, CH2), 2.72 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.1 (Cqaro), 141.9 (Cqaro), 141.9 (Cqaro), 141.7 (Cqaro), 136.2 (Cqaro), 133.7 (Cqaro), 133.3 (CHaro), 133.2 (Cqaro), 132.2 (CHaro), 131.3 (CHaro), 130.8 (CHaro), 129.1 (CHaro), 128.8 (CHaro), 127.6 (CHaro), 125.2 (CHaro), 124.8 (CHaro), 122.6 (Cqaro), 122.0 (CHaro), 69.6 (CH), 49.4 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C22H16BrNO [M+H]+: 390.0488, found 390.0482.
Synthesis and characterization of compound 4g.
10-(2-Methoxyphenyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4g).
This product was obtained as a single regioisomer in 83% yield.
This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 7/3) = 0.23; IR (νmax cm-1): 1696;1H NMR (300 MHz, CDCl3): δH 7.93 (d, J = 7.8 Hz, 1H, Haro), 7.88 (d, J = 7.5 Hz, 1H, Haro), 7.62 (t, J = 7.5 Hz, 1H, Haro), 7.49 (t, J = 7.4 Hz, 1H, Haro), 7.38-7.28 (m, 2H, Haro), 7.24-7.15 (m, 2H, Haro), 7.08-6.91 (m, 2H, Haro), 6.13 (s, 1H, CH), 5.27 (d, J = 14.9 Hz, 1H, CH2), 4.51 (d, J = 14.8 Hz, 1H, CH2), 3.80 (s, 3H, O-CH3), 2.70 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 156.5 (Cqaro), 145.3 (Cqaro), 141.8 (Cqaro), 139.2 (Cqaro), 135.5 (Cqaro), 133.7 (Cqaro), 133.1 (Cqaro), 132.1 (CHaro), 130.9 (CHaro), 130.9 (CHaro), 130.0 (Cqaro), 129.0 (CHaro), 128.7 (CHaro), 125.2 (CHaro), 124.7 (CHaro), 122.2 (CHaro), 121.0 (CHaro), 111.2 (CHaro), 69.6 (CH), 55.7 (O-CH3), 49.5 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO2 [M+H]+: 342.1489, found 342.1513.
Synthesis and characterization of compounds 4h/4h‘.
This product was obtained as a mixture of separable two regioisomers in 71% global yield, with a ratio of 4h/4h’: 57/43.
11-Methyl-9-(thiophen-2-yl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4h).
Major regioisomer-4h: This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 8/2) = 0.25; IR (νmax cm-1): 1687;1H NMR (300 MHz, CDCl3): δH 7.91 (d, J = 8.9 Hz, 1H, Haro), 7.88 (d, J = 7.2 Hz, 1H, Haro), 7.62 (t, J = 7.4 Hz, 1H, Haro), 7.49 (t, J = 7.4 Hz, 1H, Haro), 7.35 (s, 1H, Haro), 7.32 (s, 1H, Haro), 7.29-7.27 (m, 2H, Haro), 7.08-7.06 (m, 1H, Haro), 6.10 (s, 1H, CH), 5.26 (d, J = 15.0 Hz, 1H, CH2), 4.48 (d, J = 14.9 Hz, 1H, CH2), 2.69 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 145.1 (Cqaro), 143.6 (Cqaro), 142.8 (Cqaro), 136.1 (Cqaro), 135.0 (Cqaro), 134.0 (Cqaro), 133.6 (Cqaro), 132.2 (CHaro), 128.8 (CHaro), 128.2 (CHaro), 127.5 (CHaro), 125.3 (CHaro), 125.2 (CHaro), 124.8 (CHaro), 123.6 (CHaro), 118.4 (CHaro), 69.5 (CH), 49.4 (CH2), 21.7 (CH3) ppm. HRMS (+ESI) calculated for C20H15NOS [M+H]+: 318.0947, found 318.0977.
11-Methyl-10-(thiophen-2-yl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4h’).
Minor regioisomer-4h’: This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 8/2) = 0.21; IR (νmax cm-1): 1687;1H NMR (300 MHz, CDCl3): δH 7.96 (d, J = 8.7 Hz, 1H, Haro), 7.90 (d, J = 7.2 Hz, 1H, Haro), 7.62 (td, J = 7.5, 1.3 Hz, 1H, Haro), 7.51 (t, J = 7.4 Hz, 1H, Haro), 7.37-7.31 (m, 2H, Haro), 7.14 (d, J = 7.8 Hz, 1H, Haro), 7.09 (dd, J = 5.1, 3.5 Hz, 1H, Haro), 6.99 (dd, J = 3.5, 1.2 Hz, 1H, Haro), 6.16 (s, 1H, CH), 5.27 (d, J = 15.2 Hz, 1H, CH2), 4.50 (d, J = 15.1 Hz, 1H, CH2), 2.71 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.2 (C=O), 145.5 (Cqaro), 142.3 (Cqaro), 141.5 (Cqaro), 138.4 (Cqaro), 134.5 (Cqaro), 133.7 (Cqaro), 132.3 (Cqaro), 132.3 (CHaro), 131.3 (CHaro), 128.9 (CHaro), 127.3 (CHaro), 127.1 (CHaro), 125.7 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 120.8 (CHaro), 69.9 (CH), 49.5 (CH2), 20.4 (CH3) ppm. HRMS (+ESI) calculated for C20H15NOS [M+H]+: 318.0947, found 318.0977.
Synthesis and characterization of compounds 4i/4i‘.
This product was obtained as a mixture of separable two regioisomers in 43% global yield, with a ratio of 4i/4i’: 63/37.
10-Acetyl-11-methyl-9-phenyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4i).
Major regioisomer-4i: This product was isolated as a white solid, Rf (cyclohexane/AcOEt: 7/3) = 0.19; m.p. = 198-200 °C; IR (νmax cm-1): 1691;1H NMR (300 MHz, CDCl3): δH 7.97-7.86 (m, 2H, Haro), 7.68-7.56 (m, 1H, Haro), 7.52 (t, J = 7.5 Hz, 1H, Haro), 7.48-7.36 (m, 3H, Haro), 7.31 (s, 1H, Haro), 7.25-7.18 (m, 1H, Haro), 7.17-7.08 (m, 1H, Haro), 6.17 (s, 1H, CH), 5.31 (d, J = 15.2 Hz, 1H, CH2), 4.51 (d, J = 15.1 Hz, 1H, CH2), 2.48 (s, 3H, CH3), 1.79 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 204.5 (C=O), 174.1 (C=O), 145.1 (Cqaro), 142.7 (Cqaro), 141.0 (Cqaro), 140.3 (Cqaro), 139.6 (Cqaro), 138.9 (Cqaro), 133.6 (Cqaro), 132.5 (Cqaro), 132.4 (CHaro), 130.1 (CHaro), 129.9 (CHaro), 129.0 (CHaro), 129.0 (CHaro), 128.5 (CHaro), 128.2 (CHaro), 125.2 (CHaro), 124.9 (CHaro), 119.9 (CHaro), 69.8 (CH), 49.4 (CH2), 30.4 (CH3), 20.1 (CH3) ppm. HRMS (+ESI) calculated for C24H19NO2 [M+H]+: 354.1489, found 354.1515.
9-Acetyl-11-methyl-10-phenyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4i’).
Minor regioisomer-4i’: This product was isolated as a white solid, Rf (cyclohexane/AcOEt: 7/3) = 0.23; m.p. = 197-199 °C; IR (νmax cm-1): 1691; 1H NMR (300 MHz, CDCl3): δH 7.94 (d, J = 7.9 Hz, 1H, Haro), 7.89 (d, J = 7.7 Hz, 1H, Haro), 7.63 (t, 1H, Haro), 7.52 (t, J = 7.4 Hz, 1H, Haro), 7.43-7.34 (m, 3H, Haro), 7.30 (m, J = 2.8 Hz, 2H, , Haro), 7.15 (s, 1H, Haro), 6.17 (s, 1H, CH), 5.30 (d, J = 15.3 Hz, 1H, CH2), 4.51 (d, J = 15.2 Hz, 1H, CH2), 2.63 (s, 3H, CH3), 1.92 (s, 3H, CH3) ppm.13C NMR (75 MHz, CDCl3): δC 207.5 (C=O), 174.2 (C=O), 145.1 (Cqaro), 142.5 (Cqaro), 141.7 (Cqaro), 139.8 (Cqaro), 139.5 (Cqaro), 137.2 (Cqaro), 133.5 (Cqaro), 132.4 (CHaro), 129.6 (Cqaro), 129.0 (CHaro), 129.0 (2 x CHaro), 128.9 (2 x CHaro), 128.2 (CHaro), 125.3 (CHaro), 124.8 (CHaro), 122.5 (CHaro), 69.6 (CH), 49.5 (CH2), 32.5 (CH3), 19.0 (CH3) ppm. HRMS (+ESI) calculated for C24H19NO2 [M+H]+: 354.1489, found 354.1515.
Synthesis and characterization of compounds 4j/4j‘.
These products were obtained as a mixture of inseparable two regioisomers and were isolated as a yellow oil in 51% global yield, with a ratio of 4j/4j’: 60/40, Rf (cyclohexane/AcOEt: 8/2) = 0.22; IR (νmax cm-1): 1691.
9-Benzyl-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4j).
Major regioisomer-4j: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.94-7.84 (m, 2H, Haro), 7.64-7.54 (m, 1H, Haro), 7.47 (t, J = 7.5 Hz, 1H, Haro), 7.32-7.26 (m, 2H, Haro), 7.23-7.15 (m, 3H, Haro), 6.95 (s, 1H, Haro), 6.92 (s, 1H, Haro), 6.05 (s, 1H, CH), 5.32-5.12 (m, 1H, CH2), 4.44 (t, J = 14.8 Hz, 1H, CH2), 3.93 (s, 2H, CH2), 2.62 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 145.3 (Cqaro), 142.3 (Cqaro), 140.8 (Cqaro), 138.7 (Cqaro), 134.6 (Cqaro), 133.6 (Cqaro), 133.4 (Cqaro), 132.0 (CHaro), 130.3 (CHaro), 128.9 (3 x CHaro), 128.6 (CHaro), 128.6 (CHaro), 126.3 (CHaro), 125.1 (CHaro), 124.6 (CHaro), 121.4 (CHaro), 69.4 (CH), 49.3 (CH2), 41.6 (CH2), 21.6 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO [M+H]+: 326.1539, found 326.1562.
Synthesis and characterization of compounds 4k/4k‘.
These products were obtained as a mixture of inseparable two regioisomers and were isolated as a yellow oil in 40% global yield, with a ratio of 4k /4k’: 73/27, Rf (cyclohexane/AcOEt: 7/3) = 0.42; IR (νmax cm-1): 1688.
9-(tert-Butyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4k).
Major regioisomer-4k: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.93-7.83 (m, 2H, CHaro), 7.60 (t, J = 7.5 Hz, 1H, CHaro), 7.51-7.43 (m, 1H, CHaro), 7.14 (s, 1H, CHaro), 7.10 (s, 1H, CHaro), 6.06 (s, 1H, CH), 5.22 (d, J = 14.9 Hz, 1H, CH2), 4.44 (dd, J = 14.8, 7.8 Hz, 1H, CH2), 2.66 (s, 3H, CH3), 1.30 (s, 9H, 3 x CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 152.2 (Cqaro), 145.4 (Cqaro), 142.0 (Cqaro), 134.0 (Cqaro), 133.7 (Cqaro), 132.9 (Cqaro), 132.0 (CHaro), 128.6 (CHaro), 126.8 (CHaro), 125.1 (CHaro), 124.6 (CHaro), 117.9 (CHaro), 69.5 (CH), 49.6 (CH2), 34.7 (Cq), 31.5 (3 x CH3), 21.9 (CH3) ppm. HRMS (+ESI) calculated for C20H21NO [M+H]+: 292.1696, found 292.1714.
10-(tert-Butyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4k’).
Minor regioisomer-4k’: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.98 (d, J = 7.9 Hz, 1H, Haro), 7.93-7.83 (m, 1H, Haro), 7.60 (t, J = 7.5 Hz, 1H, Haro), 7.51-7.43 (m, 1H, Haro), 7.36 (d, J = 8.1 Hz, 1H, Haro), 7.05 (d, J = 8.1 Hz, 1H, Haro), 6.16 (s, 1H, CH), 5.22 (d, J = 14.9 Hz, 1H, CH2), 4.44 (dd, J = 14.8, 7.8 Hz, 1H, CH2), 2.87 (s, 3H, CH3), 1.42 (s, 9H, 3 x CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 147.7 (Cqaro), 145.7 (Cqaro), 139.5 (Cqaro), 139.3 (Cqaro), 133.9 (Cqaro), 132.7 (Cqaro), 132.0 (CHaro), 128.7 (CHaro), 126.8 (CHaro), 125.7 (CHaro), 124.6 (CHaro), 120.5 (CHaro), 70.4 (CH), 49.2 (CH2), 36.0 (Cq), 31.4 (3 x CH3), 22.3 (CH3) ppm. HRMS (+ESI) calculated for C20H21NO [M+H]+: 292.1696, found 292.1714.
Synthesis and characterization of compounds 4l/4l‘.
These products were obtained as a mixture of separable two regioisomers in 54% global yield, with a ratio of 4l/4l’: 55/45.
Methyl 1-methyl-7-oxo-7, 11b-dihydro-5H-isoindolo [1,2-a]isoindole-3-carboxylate (4l).
Major regioisomer-4l: This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 8/2) = 0.14; IR (νmax cm-1): 1703 (2 x C=O);1H NMR (300 MHz, CDCl3): δH 7.92 (d, J = 7.9 Hz, 1H, Haro), 7.88 (d, J = 7.7 Hz, 1H, Haro), 7.78 (s, 2H, Haro), 7.63 (td, J = 7.6, 1.3 Hz, 1H, Haro), 7.54-7.47 (m, 1H, Haro), 6.11 (s, 1H, CH), 5.26 (d, J = 15.2 Hz, 1H, CH2), 4.47 (d, J = 15.2 Hz, 1H, CH2), 3.90 (s, 3H , CH3), 2.71 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 173.9 (C=O), 166.7 (C=O), 144.4 (Cqaro), 142.4 (Cqaro), 141.7 (Cqaro), 133.8 (Cqaro), 133.6 (Cqaro), 132.3 (CHaro), 131.1 (CHaro), 130.7 (Cqaro), 129.0 (CHaro), 125.3 (CHaro), 124.8 (CHaro), 122.2 (CHaro), 69.6 (CH), 52.4 (O-CH3), 49.2 (CH2), 21.6 (CH3) ppm. HRMS (+ESI) calculated for C18H15NO3 [M+H]+: 294.1125, found 294.1147.
Methyl 1-methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindole-2-carboxylate (4l’).
Minor regioisomer-4l’: This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 8/2) = 0.12; IR (νmax cm-1): 1703 (2 x C=O). 1H NMR (300 MHz, CDCl3): δH 7.98 (d, J = 7.8 Hz, 1H, Haro), 7.87 (d, J = 7.6 Hz, 1H, Haro), 7.83 (d, J = 7.9 Hz, 1H, Haro), 7.62 (t, J = 7.5 Hz, 1H, Haro), 7.50 (t, J = 7.4 Hz, 1H, Haro), 7.16 (d, J = 7.9 Hz, 1H, Haro), 6.17 (s, 1H, CH), 5.27 (d, J = 15.6 Hz, 1H , CH2), 4.48 (d, J = 15.6 Hz, 1H, CH2), 3.89 (s, 3H, CH3), 2.90 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.3 (C=O), 168.0 (C=O), 145.4 (Cqaro), 145.4 (Cqaro), 139.1 (Cqaro), 135.9 (Cqaro), 133.4 (Cqaro), 132.5 (CHaro), 131.2 (CHaro), 130.2 (Cqaro), 129.0 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 120.7 (CHaro), 69.7 (CH), 52.2 (O-CH3), 49.7 (CH2), 20.6 (CH3) ppm. HRMS (+ESI) calculated for C18H15NO3 [M+H]+: 294.1125, found 294.1147.
Synthesis and characterization of compound 4m.
Dimethyl 1-methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindole-2,3-dicarboxylate (4m).
This product was obtained as a single regioisomer in a 45% yield.
This product was isolated as a white solid in 45% yield, Rf (cyclohexane/AcOEt: 7/3) = 0.38; m.p. = 196-198 °C; IR (νmax cm-1): 1736, 1719, 1692;1H NMR (300 MHz, CDCl3): δH 7.95-7.84 (m, 2H, Haro), 7.77 (s, 1H, Haro), 7.62 (t, J = 7.6, 1.4 Hz, 1H, Haro), 7.51 (t, J = 7.4 Hz, 1H, Haro), 6.13 (s, 1H, CH), 5.28 (d, J = 15.4 Hz, 1H, CH2), 4.47 (d, J = 15.3 Hz, 1H , CH2), 3.94 (s, 3H, CH3), 3.88 (s, 3H, CH3), 2.64 (s, 3H, CH3) ppm.13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 169.5 (C=O), 165.8 (C=O), 144.2 (Cqaro), 142.8 (Cqaro), 142.4 (Cqaro), 135.9 (Cqaro), 133.4 (Cqaro), 132.5 (CHaro), 131.5 (Cqaro), 129.2 (CHaro), 128.5 (Cqaro), 125.3 (CHaro), 124.9 (CHaro), 122.7 (CHaro), 69.6 (CH), 52.9 (CH3), 52.8 (CH3), 49.3 (CH2), 18.7 (CH3) ppm. HRMS (+ESI) calculated for C24H19NO2 [M+H]+: 352.1179, found 352.1203.
Synthesis and characterization of compounds 4n/4n‘.
These products were obtained as a mixture of separable two regioisomers in 88% global yield, with a ratio of 4n/4n’: 60/40.
2-((1-Methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-3-yl)methyl)isoindoline-1,3-dione (4n).
Major regioisomer-4n: This product was isolated as a white solid, Rf (DCM/AcOEt: 9/1) = 0.24; m.p. = 174-176 °C; IR (νmax cm-1): 1687;1H NMR (300 MHz, CDCl3): δH 7.89-7.80 (m, 4H, Haro), 7.73-7.68 (m, 2H, Haro), 7.58 (t, J = 7.5 Hz, 1H, Haro), 7.46 (t, J = 7.4 Hz, 1H, Haro), 7.18 (s, 1H, Haro), 7.14 (s, 1H, Haro), 6.03 (s, 1H, CH), 5.18 (d, J = 15.1 Hz, 1H, CH2), 4.79 (s, 2H, CH2), 4.41 (d, J = 15.1 Hz, 1H, CH2), 2.63 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 168.1 (2 x C=O), 145.0 (Cqaro), 142.6 (Cqaro), 137.1 (Cqaro), 136.5 (Cqaro), 134.2 (2 x CHaro), 133.9 (Cqaro), 133.6 (Cqaro), 132.2 (2 x Cqaro), 132.1 (CHaro), 130.0 (CHaro), 128.8 (CHaro), 125.1 (CHaro), 124.7 (CHaro), 123.5 (2 x CHaro), 121.2 (CHaro), 69.4 (CH), 49.3 (CH2), 41.3 (CH2), 21.6 (CH3) ppm. HRMS (+ESI) calculated for C25H18N2O3 [M+H]+: 395.1390, found 395.1395.
2-((1-Methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-2-yl)methyl)isoindoline-1,3-dione (4n’).
Minor regioisomer-4n’: This product was isolated as a white solid, Rf (DCM/AcOEt: 9/1) = 0.19; m.p. = 208-210 °C; IR (νmax cm-1): 1687;1H NMR (300 MHz, CDCl3): δH 7.95 (d, J = 7.8 Hz, 1H, Haro), 7.88-7.79 (m, 3H, Haro), 7.74-7.69 (m, 2H, Haro), 7.59 (t, J = 7.4 Hz, 1H, Haro), 7.47 (t, J = 7.5 Hz, 1H, Haro), 7.32 (d, J = 7.8 Hz, 1H, Haro), 7.07 (d, J = 7.8 Hz, 1H, Haro), 6.13 (s, 1H, CH), 5.19 (d, J = 15.1 Hz, 1H, CH2), 4.98-4.75 (m, 2H, CH2), 4.41 (d, J = 15.0 Hz, 1H, CH2), 2.82 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 168.2 (2 x C=O), 145.5 (Cqaro), 141.3 (Cqaro), 138.1 (Cqaro), 134.2 (2 x CHaro), 134.2 (Cqaro), 134.1 (Cqaro), 133.6 (Cqaro), 132.2 (CHaro), 132.2 (Cqaro), 132.1 (CHaro), 129.9 (Cqaro), 128.8 (CHaro), 125.2 (CHaro), 124.7 (CHaro), 123.5 (2 x CHaro), 121.0 (CHaro), 69.8 (CH), 49.5 (CH2), 39.3 (CH2), 18.5 (CH3) ppm. HRMS (+ESI) calculated for C25H18N2O3 [M+H]+: 395.1390, found 395.1395.
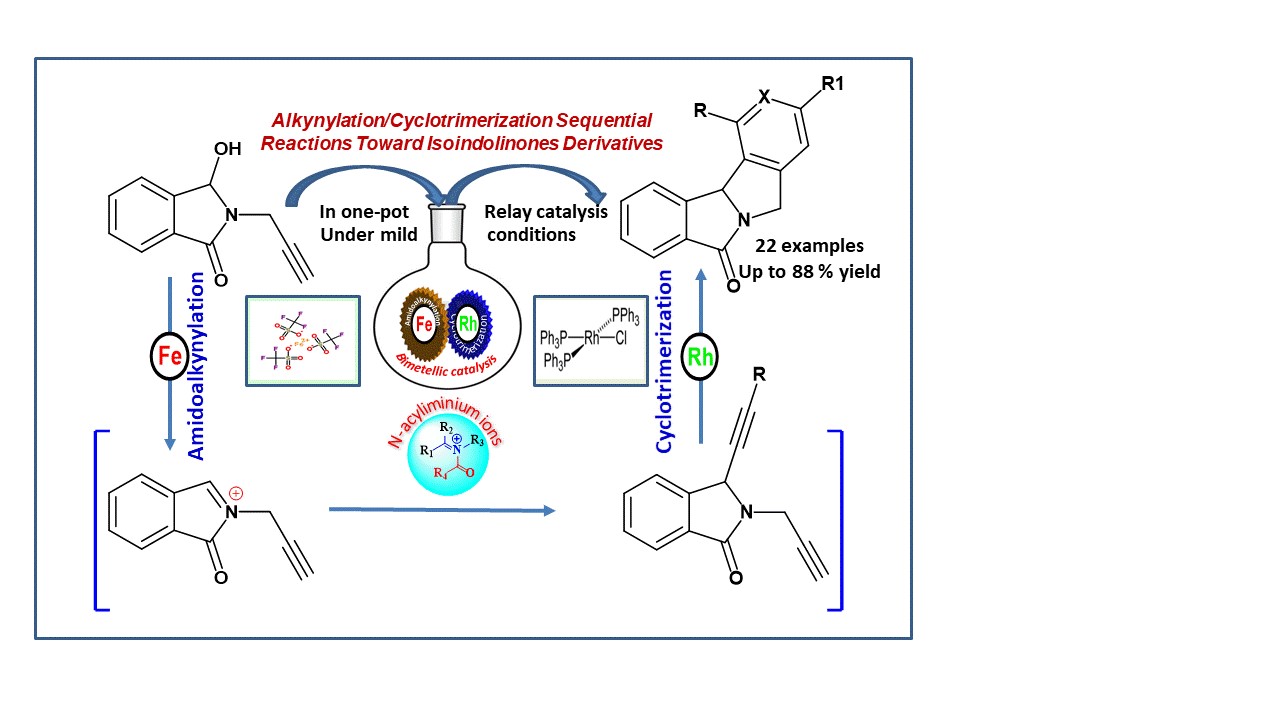
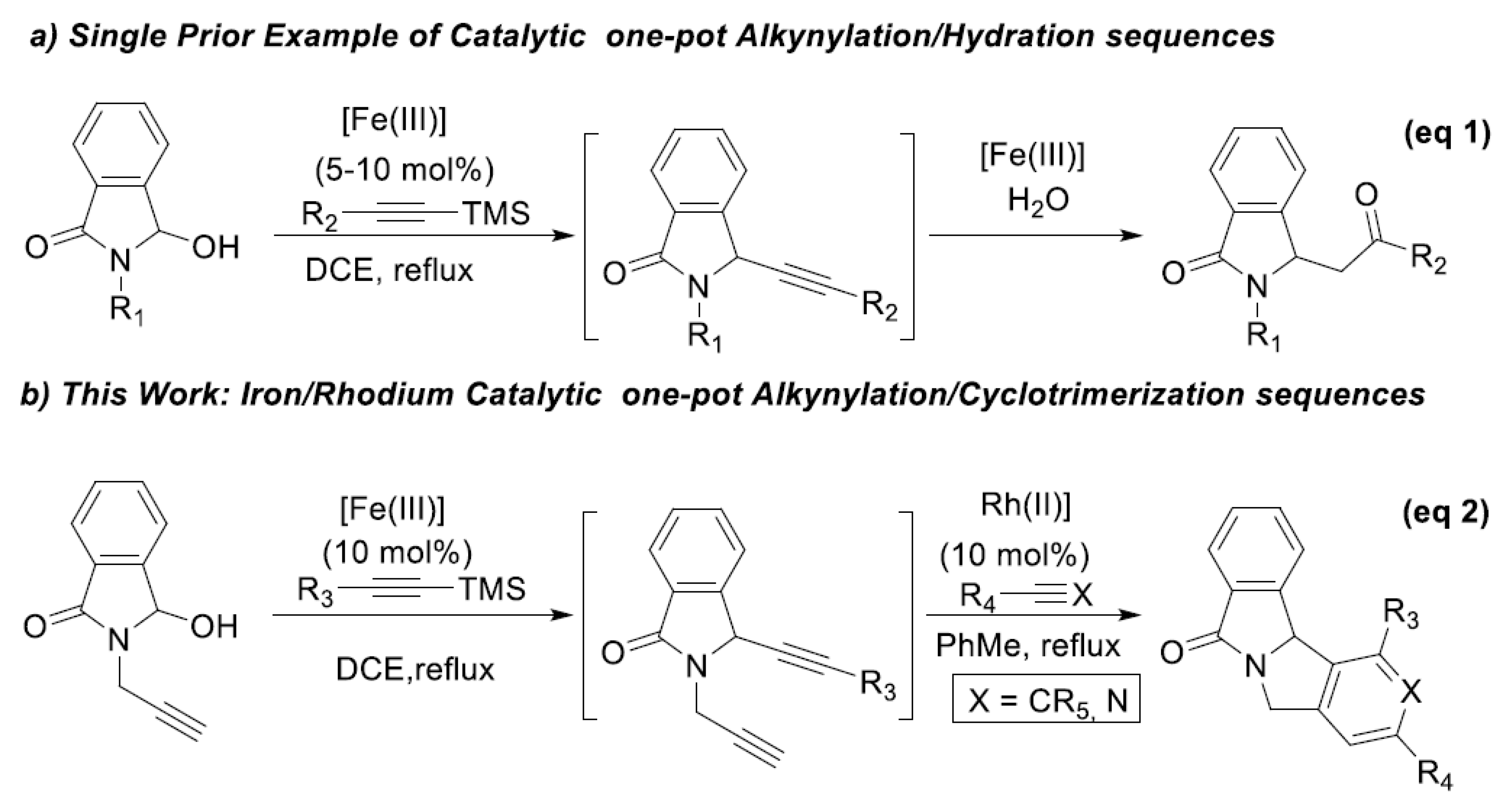
Representative Procedure for the Iron(III)/Rhodium(I)-Catalyzed One-Pot Alkynylation/Cyclotrimerization of N,O-Acetals: Commercially available Fe(OTf)3 (Aldrich, 5 mol%) was added to a solution of hydroxylactam 1 (0.5 mmol) and propyne-TMS 2 (1.1 equiv.) in 1,2-dichloroethane (2 mL) under argon. The mixture was placed in a pre-heated oil bath at reflux and magnetically stirred. The progress of the reaction was monitored by TLC. The solvent was evaporated under reduced pressure. The intermediate 2b was solubilized without purification in 2 mL of toluene and was added dropwise over 2 h to a solution of 3a-t alkynes (10 equiv.) and RhCl(PPh3)3 (10 mol%) in toluene (4 mL). The reaction mixture was heated under reflux. After the total conversion of the starting material as monitored by TLC, the solvent was removed under reduced pressure. The crude products were purified by flash chromatography (silica gel column, cyclohexane/AcOEt, or DCM/AcOEt as eluents).
Synthesis and characterization of compounds 4a/4a‘.
These products were obtained as a mixture of separable two regioisomers in 60% global yield, with a ratio of 4a/4a’: 60/40.
Data analyses were identical in all respects with cyclotrimerization step data.
Synthesis and characterization of compounds 4e/4e‘.
These products were obtained as a mixture of separable two regioisomers in 51% global yield, with a ratio of 4e/4e’: 60/40.
Data analyses were identical in all respects with cyclotrimerization step data.
Synthesis and characterization of compounds 4f.
This product was obtained as a single regioisomer in 51% global yield.
Data analyses were identical in all respects with cyclotrimerization step data.
Synthesis and characterization of compounds 4g.
This product was obtained as a single regioisomer in 73% global yield.
Data analyses were identical in all respects with cyclotrimerization step data.
Synthesis and characterization of compounds 4m.
This product was obtained as a single regioisomer in 35% global yield.
Data analyses were identical in all respects with cyclotrimerization step data.
Synthesis and characterization of compounds 4n/4n‘.
These products were obtained as a mixture of separable two regioisomers in 70% global yield, with a ratio of 4n/4n’: 60/40.
Data analyses were identical in all respects with cyclotrimerization step data.
Synthesis and characterization of compounds 4o/4o‘.
These products were obtained as a mixture of separable two regioisomers in 59% global yield, with a ratio of 4o/4o’: 58/42.
11-Methyl-9-(thiophen-3-yl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4o).
Major regioisomer-4o: This compound was isolated as a white solid, Rf (cyclohexane/AcOEt: 8/2) = 0.21; m.p. = 193-195 °C; IR (νmax cm-1): 1683; 1H NMR (300 MHz, CDCl3): δH 7.95-7.85 (m, 2H, Haro), 7.68-7.57 (m, 1H, Haro), 7.55-7.44 (m, 1H, Haro), 7.41 (s, 1H, Haro), 7.39-7.32 (m, 3H, Haro), 7.31 (s, 1H, Haro), 6.11 (s, 1H, CH), 5.27 (d, J = 15.7 Hz, 1H, CH2), 4.49 (d, J = 15.0 Hz, 1H, CH2), 2.70 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 145.2 (Cqaro), 142.7 (Cqaro), 141.7 (Cqaro), 136.5 (Cqaro), 135.7 (Cqaro), 133.9 (Cqaro), 133.7 (Cqaro), 132.2 (CHaro), 128.8 (CHaro), 128.0 (CHaro), 126.5 (CHaro), 126.4 (CHaro), 125.2 (CHaro), 124.7 (CHaro), 120.8 (CHaro), 119.0 (CHaro), 69.5 (CH), 49.4 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C20H15NOS [M+H]+: 318.0947, found 318.0947.
11-Methyl-10-(thiophen-3-yl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4o’).
Minor regioisomer-4o’: This compound was isolated as a white solid, Rf (cyclohexane/AcOEt: 8/2) = 0.18; m.p. = 191-193 °C; IR (νmax cm-1): 1696; 1H NMR (300 MHz, CDCl3): δH 7.96 (d, J = 7.7 Hz, 1H, Haro), 7.90 (d, J = 7.6 Hz, 1H, Haro), 7.62 (t, J = 7.1 Hz, 1H, Haro), 7.50 (t, J = 7.4 Hz, 1H, Haro), 7.42-7.33 (m, 1H, Haro), 7.27-7.25 (m, 1H, Haro), 7.20-7.10 (m, 2H, Haro), 7.12 -7.04 (m, 1H, Haro), 6.16 (s, 1H, CH), 5.27 (d, J = 15.2 Hz, 1H, CH2), 4.50 (d, J = 15.0 Hz, 1H, CH2), 2.63 (s, 2H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.5 (Cqaro), 141.5 (Cqaro), 140.9 (Cqaro), 138.1 (Cqaro), 136.9 (Cqaro), 133.7 (Cqaro), 132.2 (CHaro), 131.7 (Cqaro), 130.4 (CHaro), 129.1 (CHaro), 128.8 (CHaro), 125.4 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 123.2 (CHaro), 120.8 (CHaro), 69.9 (CH), 49.5 (CH2), 20.2 (CH3) ppm. HRMS (+ESI) calculated for C20H15NOS [M+H]+: 318.0947, found 318.0947.
Synthesis and characterization of compounds 4p/4p‘.
These products were obtained as a mixture of inseparable two regioisomers and were isolated as a yellow oil in 70% global yield, with a ratio of 4p/4p’: 51/49, Rf (cyclohexane/AcOEt: 7/3) = 0.35; IR (νmax cm-1): 1695.
11-Methyl-9-(naphthalen-2-yl)-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4p).
The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 8.01-7.84 (m, 10H, Haro) (4p or 4p’), 7.72 (dd, J = 9.1, 1.8 Hz, 2H, Haro) (4p or 4p’), 7.68-7.60 (m, 3H, Haro) (4p or 4p’), 7.54-7.46 (m, 7H, Haro) (4p or 4p’), 7.44-7.39 (m, 2H, Haro) (4p or 4p’), 7.30 (d, J = 7.8 Hz, 1H, Haro) (4p or 4p’), 7.21 (d, J = 7.8 Hz, 1H, Haro) (44p or 4p’), 6.16 (s, 1H, CH), 5.32 (dd, J = 15.1, 3.5 Hz, 1H, CH2), 4.54 (d, J = 15.0 Hz, 1H, CH2), 2.76 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.5 (Cqaro) (4p or 4p’), 145.2 (Cqaro) (4p or 4p’), 142.8 (Cqaro) (4p or 4p’), 142.2 (Cqaro) (4p or 4p’), 142.0 (Cqaro) (4p or 4p’), 141.0 (Cqaro) (4p or 4p’), 138.9 (Cqaro) (4p or 4p’), 138.1 (Cqaro) (4p or 4p’), 137.9 (Cqaro) (4p or 4p’), 136.0 (Cqaro) (4p or 4p’), 133.9 (Cqaro) (4p or 4p’), 133.8 (Cqaro) (4p or 4p’), 133.7 (Cqaro) (4p or 4p’), 133.7 (Cqaro) (4p or 4p’), 133.4 (Cqaro) (4p or 4p’), 132.9 (Cqaro) (4p or 4p’), 132.5 (Cqaro) (4p or 4p’), 132.2 (CHaro) (4p or 4p’), 132.2 (CHaro) (4p or 4p’), 131.5 (Cqaro) (4p or 4p’), 130.7 (CHaro) (4p or 4p’), 129.0 (CHaro) (4p or 4p’), 128.8 (2 x CHaro) (4p or 4p’), 128.7 (CHaro) (4p or 4p’), 128.3 (CHaro) (4p or 4p’), 128.2 (CHaro) (4p or 4p’), 128.1 (CHaro) (4p or 4p’), 127.9 (3 x CHaro) (4p or 4p’), 127.8 (CHaro) (4p or 4p’), 126.6 (CHaro) (4p or 4p’), 126.5 (CHaro) (4p or 4p’), 126.2 (CHaro) (4p or 4p’), 126.2 (CHaro) (4p or 4p’), 126.0 (CHaro) (4p or 4p’), 125.6 (CHaro) (4p or 4p’), 125.4 (CHaro) (4p or 4p’), 125.2 (CHaro) (4p or 4p’), 124.8 (2 x CHaro) (4p or 4p’), 120.8 (CHaro) (4p or 4p’), 120.0 (CHaro) (4p or 4p’), 69.5 (CH), 49.5 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C26H19NO [M+H]+: 362.1539, found 362.1562.
Synthesis and characterization of compounds 4q/4q‘.
This product was obtained as a mixture of separable two regioisomers in 68% global yield, with a ratio of 4q/4q’: 53/47.
9-(4-Methoxyphenyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4q).
Major regioisomer-4q: This product was isolated as a white solid, Rf (cyclohexane/AcOEt: 7/3) = 0.24; m.p. = 181-183 °C; IR (νmax cm-1): 1691; 1H NMR (300 MHz, CDCl3): δH 7.93 (d, J = 7.8 Hz, 1H, Haro), 7.88 (d, J = 7.5 Hz, 1H, Haro), 7.62 (t, J = 7.2 Hz, 1H, Haro), 7.53-7.44 (m, 3H, Haro), 7.27 (d, J = 6.7 Hz, 2H, Haro), 6.96 (d, J = 8.7 Hz, 2H, Haro), 6.11 (s, 1H, CH), 5.27 (d, J = 15.0 Hz, 1H, CH2), 4.50 (d, J = 14.9 Hz, 1H, CH2), 3.84 (s, 3H, O-CH3), 2.71 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δc 174.1 (C=O), 159.5 (Cqaro), 145.3 (Cqaro), 142.7 (Cqaro), 141.6 (Cqaro), 135.2 (Cqaro), 133.7 (Cqaro), 133.6 (Cqaro), 133.1 (Cqaro), 132.1 (CHaro), 128.8 (CHaro), 128.3 (3 x CHaro), 125.2 (CHaro), 124.7 (CHaro), 119.2 (CHaro), 114.4 (2 x CHaro), 69.5 (CH), 55.5 (O-CH3), 49.5 (CH2), 21.8 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO2 [M+H]+: 342.1489, found 342.1510.
10-(4-Methoxyphenyl)-11-methyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4q’).
Minor regioisomer-4q’: This product was isolated as a white solid, Rf (cyclohexane/AcOEt: 7/3) = 0.37; m.p. = 180-182 °C IR (νmax cm-1): 1691;1H NMR (300 MHz, CDCl3): δH 7.95 (d, J = 7.8 Hz, 1H, Haro), 7.90 (d, J = 7.5 Hz, 1H, Haro), 7.61 (t, J = 7.6, 1.3 Hz, 1H, Haro), 7.52 (t, J = 7.5 Hz, 1H, Haro), 7.24-7.13 (m, J = 8.6, 6.3 Hz, 4H, Haro), 7.01-6.90 (d, 2H, Haro), 6.16 (s, 1H, CH), 5.28 (d, J = 15.0 Hz, 1H, CH2 ), 4.51 (d, J = 15.0 Hz, 1H, CH2), 3.86 (s, 3H, O-CH3), 2.58 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 158.9 (Cqaro), 145.6 (Cqaro), 141.9 (Cqaro), 140.6 (Cqaro), 138.0 (Cqaro), 133.8 (Cqaro), 133.6 (Cqaro), 132.2 (CHaro), 131.4 (Cqaro), 130.6 (2 x CHaro), 130.5 (CHaro), 128.8 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 120.7 (CHaro), 113.7 (2 x CHaro), 70.0 (CH), 55.5 (O-CH3), 49.5 (CH2), 20.3 (CH3). HRMS (+ESI) calculated for C23H19NO2 [M+H]+: 342.1489, found 342.1510.
Synthesis and characterization of compounds 4r/4r‘.
These products were obtained as a mixture of separable two regioisomers in 69% global yield, with a ratio of 4r/4r’: 70/30.
10-(Hydroxymethyl)-11-methyl-9-phenyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4r).
Major regioisomer-4r: This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 5/5) = 0.11; IR (νmax cm-1): 3383, 1678;1H NMR (300 MHz, CDCl3): δH 7.89 (d, J = 7.6 Hz, 2H, Haro), 7.62-7.55 (m, 1H, Haro), 7.53-7.37 (m, 4H, Haro), 7.35 (s, 1H, Haro), 7.19 (d, J = 7.4 Hz, 1H, Haro), 7.05 (dd, J = 5.7, 3.3 Hz, 1H, Haro), 6.16 (s, 1H, CH), 5.30 (d, J = 15.0 Hz, 1H, CH2), 4.51 (d, J = 15.0 Hz, 1H, CH2), 4.33 (s, J = 2.2 Hz, 2H, CH2), 2.34 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.2 (C=O), 145.5 (Cqaro), 141.1 (Cqaro), 140.6 (Cqaro), 139.8 (Cqaro), 139.1 (Cqaro), 136.7 (Cqaro), 133.7 (Cqaro), 132.2 (CHaro), 132.1 (Cqaro), 129.5 (CHaro), 129.2 (CHaro), 128.9 (CHaro), 128.8 (CHaro), 128.8 (CHaro), 127.6 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 119.9 (CHaro), 69.9 (CH), 63.5 (CH2OH), 49.6 (CH2), 19.9 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO2 [M+H]+: 342.1489, found 342.1513.
9-(Hydroxymethyl)-11-methyl-10-phenyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4r’).
Minor regioisomer-4r’: This compound was isolated as a yellow oil, Rf (cyclohexane/AcOEt: 5/5) = 0.13; IR (νmax cm-1): 3383, 1678; 1H NMR (300 MHz, CDCl3): δH 7.98 (d, J = 7.8 Hz, 1H, Haro), 7.86 (d, J = 7.5 Hz, 1H, Haro), 7.61 (t, J = 7.6, 1.3 Hz, 1H, Haro), 7.48 (t, J = 7.5 Hz, 1H, Haro), 7.45-7.27 (m, 5H, Haro), 7.04 (s, 1H, Haro), 6.15 (s, 1H, CH), 5.22 (d, J = 15.1 Hz, 1H, CH2), 4.59 (s, 2H, CH2), 4.44 (d, J = 15.1 Hz, 1H, CH2), 2.85 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.2 (C=O), 145.5 (Cqaro), 143.9 (Cqaro), 141.2 (Cqaro), 141.1 (Cqaro), 137.3 (Cqaro), 135.7 (Cqaro), 134.3 (Cqaro), 133.6 (Cqaro), 132.3 (CHaro), 129.3 (2 x CHaro), 128.8 (CHaro), 128.3 (2 x CHaro), 127.5 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 122.7 (CHaro), 69.8 (CH), 59.8 (CH2OH), 49.5 (CH2), 18.6 (CH3) ppm. HRMS (+ESI) calculated for C23H19NO2 [M+H]+: 342.1489, found 342.1513.
Synthesis and characterization of compounds 4s/4s‘.
These products were obtained as a mixture of inseparable two regioisomers and were isolated as a yellow oil in 55% global yield, with a ratio of 4s/4s’: 63/37, Rf (cyclohexane/AcOEt: 7/3) = 0.28; IR (νmax cm-1): 1694.
11-Methyl-9-propyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4s).
Major regioisomer-4s: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.98-7.84 (m, 2H, Haro), 7.60 (t, J = 7.5 Hz, 1H, Haro), 7.47 (t, J = 7.4 Hz, 1H, Haro), 6.93 (s, 1H, Haro), 6.88 (s, 1H, Haro), 6.05 (s, 1H, CH), 5.20 (d, J = 14.6 Hz, 1H, CH2), 4.43 (d, J = 14.8 Hz, 1H, CH2), 2.63 (s, 3H, CH3), 2.58-2.49 (m, 2H, CH2), 1.64-1.52 (m, 2H, CH2), 0.99 -0.88 (m, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.4 (Cqaro), 143.5 (Cqaro), 142.0 (Cqaro), 134.1 (Cqaro), 133.7 (Cqaro), 133.1 (Cqaro), 132.0 (CHaro), 129.9 (CHaro), 128.6 (CHaro), 125.1 (CHaro), 124.6 (CHaro), 120.9 (CHaro), 69.5 (CH), 49.4 (CH2), 37.7 (CH2), 24.8 (CH2), 21.6 (CH3), 13.9 (CH3) ppm. HRMS (+ESI) calculated for C19H19NO [M+H]+: 278.1539, found 278.1556.
11-Methyl-10-propyl-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-5-one (4s’).
Minor regioisomer-4s’: The NMR characteristics of this product were extracted from the spectrum of the mixture; 1H NMR (300 MHz, CDCl3): δH 7.98-7.84 (m, 2H, CHaro), 7.60 (t, J = 7.5 Hz, 1H, CHaro), 7.47 (t, J = 7.4 Hz, 1H, CHaro), 7.09 (d, J = 7.7 Hz, 1H, CHaro), 7.03 (d, J = 7.8 Hz, 1H, CHaro), 6.13 (s, 1H, CH), 5.20 (d, J = 14.6 Hz, 1H, CH2), 4.43 (d, J = 14.8 Hz, 1H, CH2), 2.63 (s, 3H), 2.58-2.49 (m, 2H, CH2), 1.64-1.52 (m, 2H, CH2), 0.99-0.88 (m, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 145.8 (Cqaro), 140.7 (Cqaro), 139.2 (Cqaro), 137.6 (Cqaro), 133.8 (Cqaro), 132.1 (CHaro), 131.6 (Cqaro), 129.7 (CHaro), 128.6 (CHaro), 125.1 (CHaro), 125.2 (CHaro), 120.6 (CHaro), 69.9 (CH), 49.4 (CH2), 35.5 (CH2), 23.9 (CH2), 18.2 (CH3), 14.2 (CH3) ppm. HRMS (+ESI) calculated for C19H19NO [M+H]+: 278.1539, found 278.1556.
Synthesis and characterization of compounds 4t/4t‘.
These products were obtained as a mixture of separable two regioisomers in 82% global yield, with a ratio of 4t/4t’: 52/48.
2-(2-(1-Methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-3-yl)ethyl)isoindoline-1,3-dione (4t)
Major regioisomer-4t: This product was isolated as a white solid, Rf (DCM/AcOEt: 9/1) = 0.22; m.p. = 179-181 °C; IR (νmax cm-1): 1690; 1H NMR (300 MHz, CDCl3): δH 7.91-7.80 (m, 4H, Haro), 7.74-7.68 (m, 2H, Haro), 7.60 (t, J = 7.4 Hz, 1H, Haro), 7.48 (t, J = 7.4 Hz, 1H, Haro), 7.02 (s, 1H, Haro), 6.99 (s, 1H, Haro), 6.05 (s, 1H, CH), 5.17 (d, J = 15.0 Hz, 1H, CH2), 4.41 (d, J = 14.9 Hz, 1H, CH2), 3.913.79 (m, 2H, CH2), 2.98-2.87 (m, 2H, CH2), 2.62 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.0 (C=O), 168.3 (2 x C=O), 145.3 (Cqaro), 142.4 (Cqaro), 138.8 (Cqaro), 135.3 (Cqaro), 134.1 (2 x CHaro), 133.7 (Cqaro), 133.7 (Cqaro), 132.2 (2 x Cqaro), 132.1 (CHaro), 130.3 (CHaro), 128.7 (CHaro), 125.2 (CHaro), 124.7 (CHaro), 123.4 (2 x CHaro), 121.4 (CHaro), 69.5 (CH), 49.3 (CH2), 39.3 (CH2), 34.3 (CH2), 21.6 (CH3) ppm. HRMS (+ESI) calculated for C26H20N2O3 [M+H]+: 409.1547, found 409.1548.
2-(2-(1-Methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-2-yl)ethyl)isoindoline-1,3-dione (4t’).
Minor regioisomer-4t’: This product was isolated as a white solid, Rf (DCM/AcOEt: 9/1) = 0.15; m.p. = 178-180 °C; IR (νmax cm-1): 1690;1H NMR (300 MHz, CDCl3): δH 7.96 (d, J = 7.8 Hz, 1H, Haro), 7.88-7.79 (m, 3H, Haro), 7.75-7.66 (m, 2H, Haro), 7.60 (t, J = 7.7 Hz, 1H, Haro), 7.48 (t, J = 7.4 Hz, 1H, Haro), 7.17 (d, J = 7.7 Hz, 1H, Haro), 7.04 (d, J = 7.7 Hz, 1H, Haro), 6.13 (s, 1H, CH), 5.20 (d, J = 14.9 Hz, 1H, CH2), 4.42 (d, J = 14.9 Hz, 1H, CH2), 3.81 (t, J = 8.2 Hz, 2H, CH2), 3.00 (t, J = 8.3 Hz, 2H, CH2), 2.77 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.1 (C=O), 168.3 (2 x C=O), 145.6 (Cqaro), 140.4 (Cqaro), 138.1 (Cqaro), 136.0 (Cqaro), 134.1 (2 x CHaro), 133.7 (Cqaro), 132.4 (Cqaro), 132.2 (2 x Cqaro), 132.2 (CHaro), 130.3 (CHaro), 128.7 (CHaro), 125.3 (CHaro), 124.6 (CHaro), 123.4 (2 x CHaro), 121.0 (CHaro), 69.9 (CH), 49.4 (CH2), 38.3 (CH2), 32.3 (CH2), 18.2 (CH3) ppm. HRMS (+ESI) calculated for C26H20N2O3 [M+H]+: 409.1547, found 409.1548.
Synthesis and characterization of compounds 4u/4u‘.
These products were obtained as a mixture of separable two regioisomers in 72% global yield, with a ratio of 4u/4u’: 67/33.
1-((1-Methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-3-yl)methyl)pyrrolidine 2,5-dione (4u).
Major regioisomer-4u: This product was isolated as a white solid, Rf (DCM/AcOEt: 9/1) = 0.22; m.p. = 208-210 °C; IR (νmax cm-1): 1705; 1H NMR (300 MHz, CDCl3): δH 7.86 (t, J = 8.4 Hz, 2H, Haro), 7.59 (t, J = 7.5 Hz, 1H, Haro), 7.47 (t, J = 7.4 Hz, 1H, Haro), 7.14 (s, 1H, Haro), 7.10 (s, 1H, Haro), 6.04 (s, 1H, CH), 5.19 (d, J = 15.1 Hz, 1H, CH2), 4.60 (d, J = 2.3 Hz, 2H, CH2), 4.41 (d, J = 15.1 Hz, 1H, CH2), 2.69 (s, 4H, 2 x CH2), 2.63 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 176.9 (2 x C=O), 170.0 (C=O), 145.0 (Cqaro), 142.6 (Cqaro), 136.7 (Cqaro), 136.4 (Cqaro), 133.9 (Cqaro), 133.6 (Cqaro), 132.1 (CHaro), 130.3 (CHaro), 128.8 (CHaro), 125.2 (CHaro), 124.7 (CHaro), 121.5 (CHaro), 69.4 (CH), 49.3 (CH2), 42.1 (CH2), 28.3 (2 x CH2), 21.6 (CH3) ppm. HRMS (+ESI) calculated for C21H18N2O3 [M+H]+: 347.1390, found 347.1410.
1-((1-Methyl-7-oxo-7,11b-dihydro-5H-isoindolo[1,2-a]isoindol-2-yl)methyl)pyrrolidine-2,5-dione (4u’).
Minor regioisomer-4u’: This product was isolated as a white solid, Rf (DCM/AcOEt: 9/1) = 0.17; m.p. = 197-199 °C; IR (νmax cm-1): 1705; 1H NMR (300 MHz, CDCl3): δH 7.95 (d, J = 7.8 Hz, 1H, Haro), 7.86 (d, J = 7.5 Hz, 1H, Haro), 7.60 (t, J = 7.5 Hz, 1H, Haro), 7.48 (t, J = 7.4 Hz, 1H, Haro), 7.27 (d, J = 8.6 Hz, 1H, Haro), 7.07 (d, J = 7.9 Hz, 1H, Haro), 6.13 (s, 1H, CH), 5.20 (d, J = 15.0 Hz, 1H, CH2), 4.71 (d, J = 16.2 Hz, 2H, CH2), 4.42 (d, J = 14.6 Hz, 1H, CH2), 2.80-2.74 (m, 4H, 2 x CH2), 2.72 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 177.1 (2 x C=O), 174.2 (C=O), 145.6 (Cqaro), 141.5 (Cqaro), 138.1 (Cqaro), 133.6 (Cqaro), 133.5 (Cqaro), 132.5 (Cqaro), 132.3 (CHaro), 130.1 (CHaro), 128.8 (CHaro), 125.3 (CHaro), 124.7 (CHaro), 121.0 (CHaro), 69.8 (CH), 49.5 (CH2), 39.9 (CH2), 28.3 (2 x CH2), 18.6 (CH3) ppm. HRMS (+ESI) calculated for C21H18N2O3 [M+H]+: 347.1390, found 347.1410.
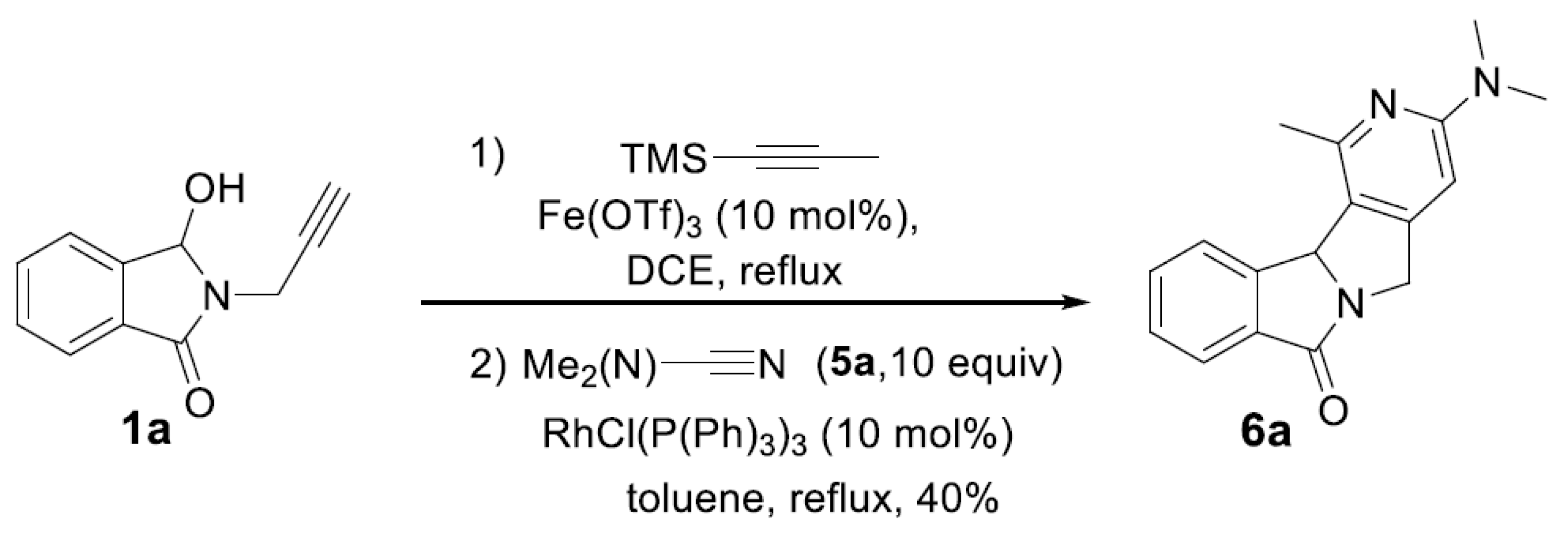
Synthesis and characterization of compound 6a.
3-(Dimethylamino)-1-methyl-5,11b-dihydro-7H-pyrido[3’,4’:3,4]pyrrolo[2,1-a]isoindol-7-one (6a).
This product was isolated as a yellow oil in 40% yield, Rf (cyclohexane/AcOEt: 7/3) = 0.5; IR (νmax cm-1): 1695;1H NMR (300 MHz, CDCl3): δH 7.92-7.80 (m, 2H, Haro), 7.61 (td, J = 7.5 and 1.4 Hz, 1H, Haro), 7.48 (t, J = 7.4 Hz, 1H, Haro), 6.23 (s, 1H, Haro), 5.96 (s, 1H, CH), 5.12 (d, J = 15.6 Hz, 1H, CH2), 4.34 (d, J = 15.5 Hz, 1H, CH2), 3.05 (s, 6H, 2 x CH3), 2.68 (s, 3H, CH3) ppm. 13C NMR (75 MHz, CDCl3): δC 174.2 (C=O), 159.1 (Cqaro), 152.8 (Cqaro), 151.2 (Cqaro), 145.9 (Cqaro), 133.3 (Cqaro), 132.3 (CHaro), 128.7 (CHaro), 124.7 (CHaro), 124.6 (CHaro), 120.1 (Cqaro), 97.3 (CHaro), 67.8 (CH), 49.3 (CH2), 38.3 (2 x CH3), 24.7 (CH3) ppm. HRMS (+ESI) calculated for C17H17N3O [M+H]+: 280,1450, found 280.1448.