1. Introduction
According to the World Health Organization (WHO), there were approximately 249 million malaria cases in the 85 countries where the disease is endemic and an estimated 608,000 deaths in 2022 [
1]. The WHO has supported efforts to reduce the global burden of malaria by developing a framework for working toward elimination. The current Global technical strategy for malaria 2016–2030 (GTS) target is reducing malaria incidence and mortality rates by 90% and eliminating malaria in at least 35 countries by 2030 [
2]. Between 2011 and 2021, there was an annual average reduction of malaria incidence of 25.4% [
3]. However, this decrease was heterogeneous both within and across countries, and there was a 5% global increase in malaria incidence rates between 2019 and 2020, likely due in part to disruptions during the COVID-19 pandemic [
4].
With global efforts to reduce the burden of malaria, several African countries reached the pre-elimination stages of malaria transmission [
5,
6,
7,
8]. The pre-elimination status is defined by a population with either a rapid diagnostic test (RDT) positivity rate below 5% annually or a parasite positivity rate lower than 5% among those with a fever [
9]. The decrease in global malaria cases has been largely attributed in part to vector control that utilizes tools such as insecticide-treated bed nets (ITN) and indoor residual spraying (IRS), which target indoor-foraging (endophagic) and indoor-resting (endophilic) mosquitoes [
10,
11]. As a result of the implementation of these control methods, many of the principal vector species of malaria, such as
Anopheles gambiae sensu lato (s.l.), have been declining in abundance in some countries [
12,
13,
14].
Identification of the knowledge gaps must be done to adequately assess vector-borne pathogens across the African continent. There is a need for improved infrastructure, an understanding of the ecology of vector species and the environmental conditions that impact them, to assess their role in disease transmission [
15,
16,
17]. For example, in Zambia, the need for vector ecology studies could be alleviated by improving the understanding of human-vector contact frequency, which is pivotal to assessing disease transmission in vector-borne systems and planning mitigation measures [
15]. To adequately assess trends in vector-borne diseases in Africa, there must be an effort to increase the capacity for research on vector-borne zoonotic diseases by enhancing the interdisciplinary focus of research and building stronger communications between countries [
18].
Despite these achievements and the identification of areas for improvement, the final elimination of malaria from many of these locations (e.g., southern Zambia, northeastern South Africa, Botswana, and Namibia) is now proving to be frustratingly difficult, and such locations remain static in the pre-elimination stage. Tenacious residual malaria infections persist in these pre-elimination zones despite access to rapid diagnostics and treatment, comprehensive IRS campaigns, and the widespread distribution of ITNs [
9]. Despite the prolonged extirpation or decline of major vector species, persistent residual malaria has prompted many involved in malaria control to suspect that understudied secondary vector species contribute to the remaining malaria transmission in pre-elimination zones [
19,
20]. These residual malaria cases highlight that existing vector control tools that have focused on the major vector species alone are not sufficient to eliminate malaria from some regions [
21].
Species often considered secondary vectors have been implicated in malaria transmission in pre-elimination zones and are often understudied. Examples include
An. vaneedeni and
An. parensis (members of the
An. funestus group) in southern Africa and
An. coustani and
An. ziemanni in central Africa [
22,
23,
24,
25]. An expanding body of work has implicated
An. squamosus, An. rufipes, and
An. coustani in malaria transmission in Zambia, especially in pre-elimination settings [
12,
20,
26,
27,
28].
Anopheles collections in Zambia revealed morphological misidentification and underrepresentation of many anopheline species in sequence databases, which confounds efforts to confirm the identity of potential malaria vector species [
29]. Therefore, continued development of methodologies that allow more accurate species identification of these understudied malaria vector populations is critical for understanding malaria transmission in the pre-elimination setting and elucidating the potential role of the understudied secondary vectors in residual malaria cases.
Studies of malaria transmission dynamics in pre-elimination settings are particularly relevant to discussions of gene drive and other novel vector control strategies. For example, the ITN, one of the most successful malaria control tools, was devised with the understanding that the primary malaria vector bite humans at night indoors while people are sleeping [
30]. Other
Anopheles species that bite humans outdoors could circumvent control measures like ITNs. Other regions in Africa will inevitably reach the malaria pre-elimination phase at some future time through the implementation of available and perhaps new control strategies. Understanding the behavior and ecology of understudied malaria vectors will be critical in advancing comprehensive malaria control strategies targeting multiple vector species that can lead to malaria elimination.
Anopheles squamosus is a secondary vector species of malaria that can be found across sub-Saharan Africa [
31]. This species is currently understudied because the species is believed to forage outdoors (exophagic) and to be primarily associated with non-human animal hosts (zoophilic) [
20]. These are based on limited studies and created a perception that it does not pose a threat to public health. However, studies have shown that An. squamosus will opportunistically feed on humans and can be present around human dwellings [
17,
20]. Moreover, this species has been found naturally infected with Plasmodium falciparum, a causative agent of human malaria, and is abundantly collected during mosquito surveys [
26,
32].
Another barrier to studying
An. squamosus is the morphological identification and separation of this species from other difficult to distinguish African anophelines. Many other anophelines, like
An. gambiae s.l., exist as complexes of morphologically similar species with members exhibiting variable vector capability [
26,
33], insecticide resistance [
34], and host association [
35,
36]. Therefore, the ability to distinguish exact species within an anopheline group is critical for assessing malaria risk associated and planning effective interventions targeted to the particular species of concern. As adults,
An. squamosus is morphologically identical to
An. cydippis [
37]
, and there are at least five chromosomal forms based on chromosome that may correspond to cryptic species under the name
An. squamosus [
58]. Unless genetic methods are used to distinguish these taxa, any study of these species is based on an uncertain amalgamation of these cryptic species.
Creating further challenges to study this and other understudied malaria vectors is the diversity and morphological similarity among Afrotropical anophelines [
58]. Distinguishing African anophelines from one another often requires specimens with morphological characters intact, which can challenging because of the methods and logistics of field collections [
26,
33,
34,
35,
36].
Existing knowledge of An. squamosus is scattered across multiple articles published over decades, some of which are not digitized or available online, which posed a challenge to accessing and compiling what is known about this species. As of September 2024, only 53 results are found when searching "Anopheles squamosus" in PubMed. The objective of this review is to present a summary of the literature recovered on the identification, distribution, ecology and biology of this species. In addition, it aims to shed light on the knowledge gaps regarding An. squamosus as an understudied malaria vector and to inspire future research on this and other understudied Anopheles species in Africa.
2. Materials and Methods
We conducted literature searches for
An. squamosus using online search engines including PubMed, Science Direct, Google Scholar, and the National Center for Biotechnology Information (NCBI) nucleotide database. Terms that were searched ranged from broad terms, such as “
Anopheles,” to specific terms such as “
Anopheles squamosus.” In each search engine, the following search terms were used: “
Anopheles,” “
Anopheles squamosus,” “
Anopheles squamosus AND
Plasmodium falciparum,” along with other variations to find instances of where
An. squamosus was mentioned. We investigated the literature and extracted all available records of
An. squamosus and other
Anopheles spp. found together with
An. squamosus in an Excel spreadsheet (See Supplementary Material
Table S1). Additional species occurrence records were also retrieved from the Africa Vector Database [
38]. For categorizing each country to subregions in a continent, we used the geographic regional groups according to UN M49 Standard country and area codes for statistical use [
39]. For example, UN M49 Standard classifies Africa into 4 subgroups, namely Eastern, Middle, Southern, and Western Africa.
The distribution map of
An. squamosus was created using QGIS software version 3.30.2 [
40]. The basemap of African continent topography in the public domain was obtained from Natural Earth Tiles [
41].
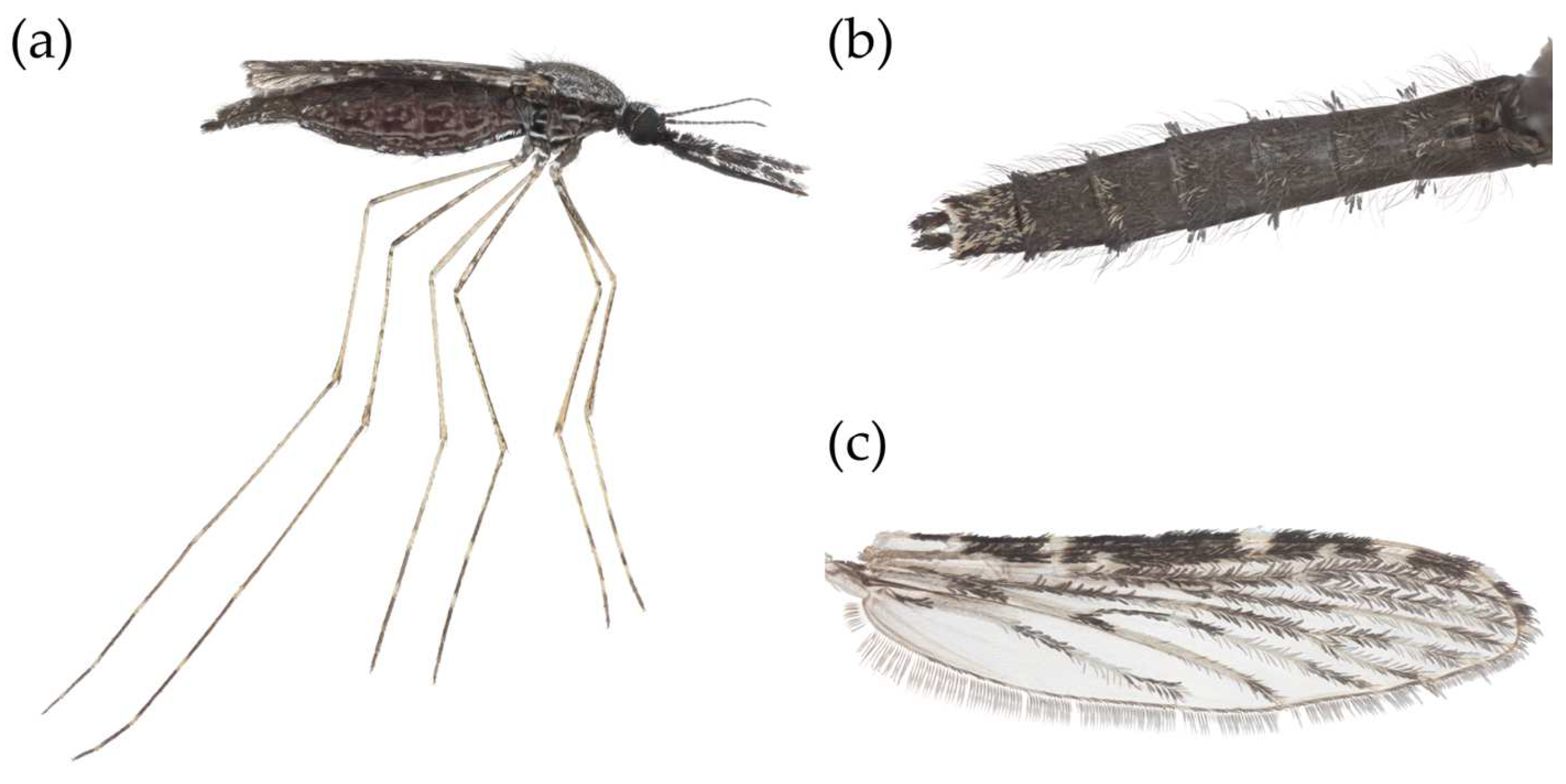
Representative images of An. squamosus morphology were created using adult female specimens collected along the outer perimeter of a goat pen using mouth aspirations at the Hanatanga Village in the Ngolwe zone of southern Zambia (16° 12' 26.766" S; 27° 0' 1.554" E) in January 2024. Collected An. squamous were frozen at 0 °C overnight and mounted to a glass microscope slide within 24 hours. The mounted mosquito was photographed with a Canon 5D SR digital SLR camera using a focus-stacking system that consisted of a 5x or 10x Mitutoyo infinity-corrected microscope objective attached to a Canon 200mm L prime lens. The camera and lens were mounted onto an automated StackShot rail (Cognisys Inc., Traverse City, MI). The camera was moved so that the mosquito specimen was just out of focus, then the camera was moved in steps of either 7µm (5x) or 15µm (10x), with one photograph taken between each step, until the mosquito was out of focus in the opposite direction. The raw images were stacked (i.e., the in-focus areas of each image were digitally merged to create one image with the entire specimen in focus) using Helicon Focus (v 8.2.13) program and then edited to clean the background using Adobe Photoshop (v 25.5.1).
3. Results
3.1. Anopheles Squamosus Identification
In 1901, Theobald described
An. squamosus as "a very pronounced scaly species, not like any other
Anopheles I have ever seen" [
31]. As an adult,
An. squamosus can be identified by several key features from other
Anopheles, except for
An. cydippis, as described in several morphological identification keys [
42,
43,
44]. The first distinguishing feature is the presence of laterally projecting tufts of abdominal scales (
Figure 1B).
Anopheles squamosus is described by Evans [
42] as being predominantly black with contrasting pure white scales, females having shaggy palpi with four narrow bands that are white, black, or bronzy-brown dorsal scales, and their last dorsal segment having numerous white scales (
Figure 1A).
A study to assess the accuracy of identifying anopheline species found that only 37% of adult
An. squamosus were identified correctly by morphology [
24]. Distinguishing this species must be done in their 4th instar larval stage or through molecular tools. However, utilizing larval keys to differentiate
An. squamosus from
An. cydippis is challenging.
Currently,
An, squamosus makes up only 0.6% of the literature on anopheline species when searching PubMed, Science Direct, and Google Scholar. In addition, there is a major lack of genomic resources for this species within NCBI, with only 210 genetic sequences as of September 2024. Furthermore, no cytochrome C oxidase subunit I (COI) sequences of
An. cydippis are available, and the internal transcribed spacer 2 (ITS2) assay used by many researchers to distinguish cryptic anopheline species does not reliably amplify
An. squamosus/An. cydippis DNA [
32].
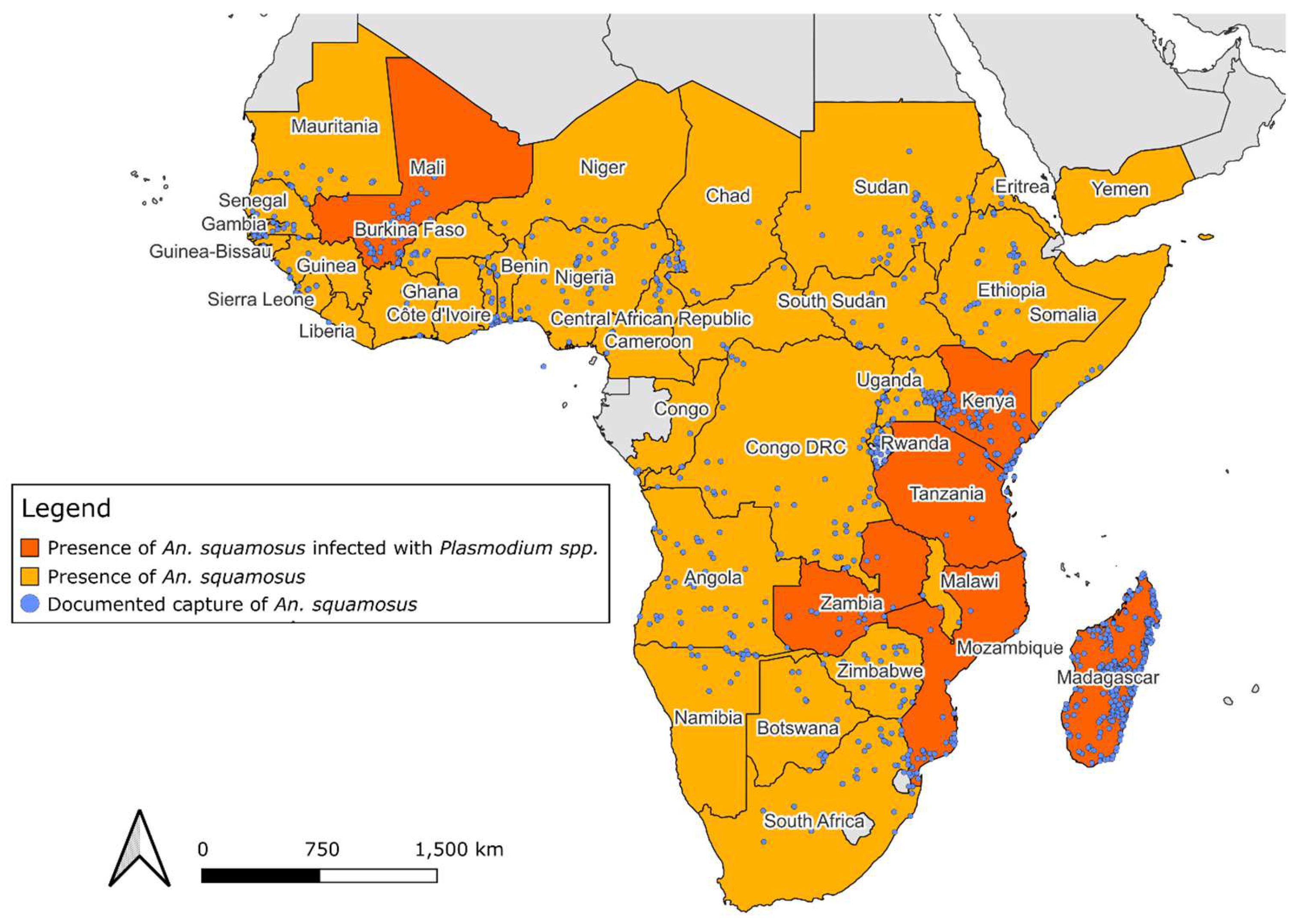
3.2. Anopheles Squamosus Distribution
In 1901 The current literature describing the distribution of these species varies throughout historical documentation. Theobald [
31] describes this species as being native to the Middle African region in 1907. Evans [
42] states that the distribution of
An. squamosus is broad, covering Western, Eastern, and Southern Africa in 1927. Similarly, De Meillon [
45] describes its distribution as widespread and practically across the whole continent in 1951. The literature review from this study suggested that
An. squamosus is widespread across the African continent, consistent with the findings of De Meillon [
45]. This species has been documented in 41 African countries across entomological surveys since 1898, as described in the Africa Vector Database and by other anopheline distribution descriptions (
Table 1) [
38]. The Africa Vector Database contains an inventory of anopheline species in Africa between 1898 and 2016 created by Kenyan Medical Research Institute and Wellcome Trust collaborators to document anopheline species occurrence. The data compiled from this database in addition to literature published after 2016, identified 1,331 unique geographic coordinate points where
An. squamosus presence has been reported [
12,
17,
26,
27,
28,
38,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55] (
Figure 2).
The first documented capture of this species occurred in 1898 in Sierra Leone (Western Africa). Two years later, it was documented in eastern Africa, 5,765 km away. By 1904,
An. squamosus has been documented in Africa's Eastern, Middle, Southern, and Western regions. Identifying this species at distance points over a short period indicates that this species was widespread throughout Africa before 1898. The known distribution of
An. squamosus based on these data reaches its northern limit at Adrar, Mauritania (20.511°N, -13.049°E) [
38] and extends as far south as Northern Cape, South Africa (-30.452°S, 21.228°E) [
33]. Its most eastern occurrence has been documented in the Sava region of Madagascar (-15.2428°S, 50.4434°W) [
38] and the furthest western report was from Thies, Senegal (14.801°N, -16.926°E) [
38].
Kuznetsov [
56] described the only detection of
An. squamosus outside of the African continent in the country of Yemen. This observation occurred in 1965 during larval sampling in the coastal plain of Tihama in quick-drying pools that are not ideal for the complete lifecycle of anopheline species. A total of 380 larvae were documented in this survey, with one larva being identified as
An. squamosus. Since this documentation, there has not been another published occurrence of
An. squamosus outside of Africa. It remains to be determined if this species is reproductively established in Yemen and other western Asian countries.
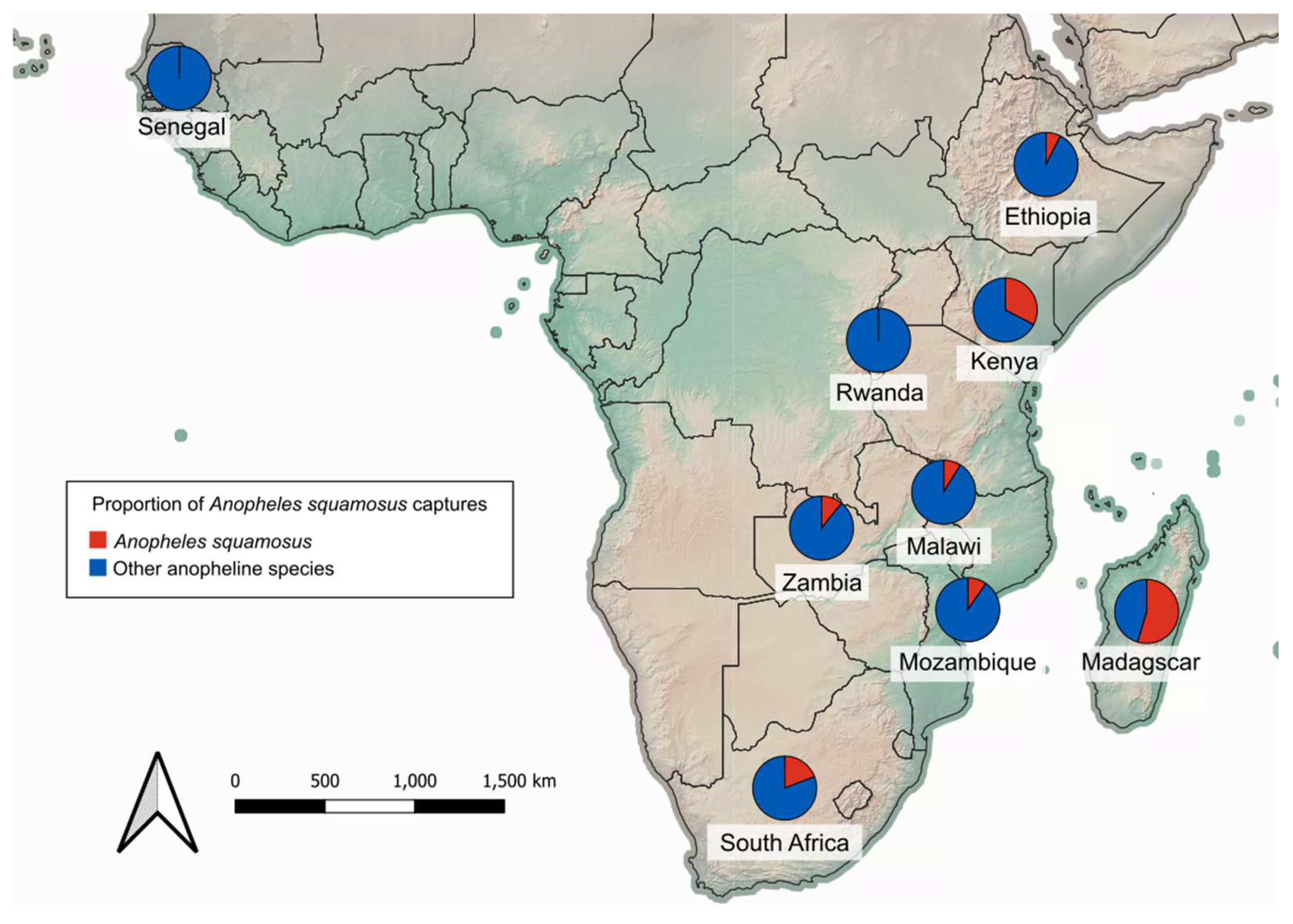
Anopheles squamosus can be found in abundance throughout much of the known distribution. Anopheline surveillance was informative at providing a snapshot of the community composition of potential vectors inhabiting a given location. Across 25 studies in nine countries (Ethiopia, Kenya, Madagascar, Malawi, Mozambique, Rwanda, Senegal, South Africa, and Zambia) primarily in eastern and southern Africa,
An. squamosus comprised as low as 0.1% and as high as 68.0% of adult anopheline trap captures (
Figure 3,
Table 2) [
17,
20,
23,
24,
27,
32,
33,
35,
36,
46,
48,
49,
51,
52,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66]. These studies consisted of both indoor and outdoor surveys using various methods of collection (human-baited traps, animal-baited traps, CDC light traps).
The widespread distribution of
An. squamosus is similar to that of the primary malaria vectors in Africa, such as those in the
An. gambiae and
An. funestus groups [
38]. Based on the data in the African Vectors Database,
An. squamosus was captured most often alongside species in the
An. gambiae complex,
An. coustani, and
An. funestus complex [
38]. The occurrence of
An. squamosus in regions where other vectors actively transmit malaria suggests that this vector has potential exposure to circulating parasites and may sustain malaria transmission when primary vectors are reduced or eliminated.
3.2.1. Larval Biology of Anopheles squamosus
Identification of larval habitats for
An. squamosus is essential, as they can be morphologically distinguished from
An. cydippis in this life stage [
32]. In addition, control of malaria vectors in the larval stage can be more effective in certain settings due to their inability to escape the habitats [
54,
69,
70]. Since indoor targeted malaria control for adult anophelines is ineffective against exophagic vectors, targeting these species' larval stages could be an effective alternative control strategy.
Anopheles squamosus larvae were recorded in naturally occurring (ponds, rivers, and lagoons) and human-created (rice fields, irrigation drains, and tire tracks) bodies of water [
65]. They were often associated with six other African anopheline species in these larval habitats (
An. gambiae s.l.
, An. funestus s.l.
, An. coustani, An. cinereus,
An. demeilloni, and
An. pharoensis) [
48,
71]. In swamps of Ethiopia, they have been found to coexist in high numbers with
An. pharoensis [
59]. The highest contributing factors for the presence of
An. squamosus larvae are high vegetation and algae [
59]. There is also a positive correlation of
An. squamosus with shallow depths of water [
48]. In Ethiopia, Kenea et al. [
59] found that
An. squamosus larvae were located further away from human dwellings. In contrast, Adugna et al. [
48] reported a positive correlation of
An. squamosus larval habitats being closer to human dwellings. The studies on larval habitats and species associations were spatio-temporally sparse and only completed in eastern Africa. Therefore, the reported findings and any conclusions drawn from them are extremely limited.
There is very little known on the microbial tolerance of
An. squamosus. Anopheles squamosus larvae have been observed to be more tolerant than
An. gambiae complex when exposed to fungal biocontrol agents such as
Coelomomyces [
71].
Coelomomyces fungus can infect mosquito larvae and prevent pupation while other organisms remain unaffected [
72]. Muspratt found that
Coelomomyces fungus was highly effective for controlling
An. gambiae larvae (>95% mortality) [
71]. However, when
An. squamosus was exposed to the same
Coelomomyces fungus, there was a very low percentage (8% infection rate) of larvae infected with the biocontrol agent [
71]. This suggests that
An. squamosus may behave differently from principal malaria vector species when exposed to such biological control agents, and studies of immune response based on the well-studied principal malaria vectors may not translate to other anopheline species. There are no other studies testing additional microbial larvicides on
An. squamosus reported as of September 2024.
3.2.2. Adult Anopheles squamosus Behavior
Understanding vector behavior is critical for disease control, as these vector behaviors can be interrupted to effectively reduce pathogen transmission [
73,
74]. Approximately 30 to 40 of the 430 described
Anopheles species are currently known to be vectors of human pathogens [
75]. A key behavioral trait that primary human malaria vectors exhibit is the propensity to feed on people indoors at night [
73,
76]. Targeting this behavior using ITN and IRS campaigns has drastically reduced malaria cases in many areas [
23,
30]. However, this has caused changes in the behaviors of primary vectors in some countries and shifts in the anopheline community composition in others [
12,
77,
78,
79].
Knowledge on the behavior of adult
An. squamosus is scarce and can be attributed to the lack of research focused on this species. This species is commonly associated with livestock and considered zoophilic, which has made it less of a concern for human health initiatives [
20]. In low transmission areas of Mozambique,
An. squamosus will predominantly exhibit exophagic behavior [
23], and they were reported foraging in the early evening in one study in Zambia [
20].
Despite the notion that
An. squamosus adults express exophagic behavior, this species has been recorded in indoor settings. In high transmission areas in Mozambique, up to 68.4% of
An. squamosus were captured host-seeking indoors, predominantly in the evening before human occupants went to bed and while they were in bed [
23]. The close proximity of
An. squamosus to humans can increase instances of malaria transmission due to their potential ability to vector human
Plasmodium parasites [
80]. This species has been captured using CDC light traps, human landing catches (HLC), human-baited traps, and livestock-baited traps. Seven trapping studies were identified during our literature review that collected
An. squamous or
An. cydippis indoors. Across these studies, up to 41% of trap captures consisted of species identified as
An. squamosus [
23,
27,
32,
47,
48,
57,
58] with an average of 14.7% of indoor trap captures identified as
An. squamosus.
Historically, this species has been considered zoophilic [
31], as
Anopheles squamosus has demonstrated a preference for feeding on non-human hosts if they are present [
20]. However, there is evidence that feeding behavior for
An. squamosus varies depending on its location. Across several studies, there were observed differences in HLC in different countries, which may be attributed to the availability of alternate hosts [
17,
24,
47,
58,
66]. The recorded host species for
An. squamosus include sheep, cow, pigeon, chicken, goat, dog, pig, and human [
20,
26,
27,
33,
48,
49]. From five host association studies between 2011 and 2022, where 670 specimens were collected, the host most often found for
An. squamosus was cow (n=250), followed by goat (n=158), non-human (n=65), pig (n=60), cow and goat (50), cow and human (n=40), human (n=26), cow and pig (n=12), dog (n=5), human and animal (n=3), and chicken (n=1) (
Table 3) [
20,
26,
27,
48,
49]. From these studies, a total of 10.3% of the blood meals contained human blood, indicating that this species readily feeds on humans across habitats. In Macha, Zambia,
An. squamosus had a higher rate of reported anthropophily when compared to Cameroon, Kenya, and Senegal based on indoor CDC light trap collections [
20].
3.3. Anopheles squamosus Contribution to Pathogen Transmission
Anopheles squamosus has been implicated as a potential pathogen vector in Zambia, Madagascar, Mozambique, Namibia, Mali, Kenya, and Tanzania (
Table 4) [
20,
23,
38,
60,
61,
65,
81,
82]. Though it is considered a secondary vector species of certain human diseases, such as malaria, secondary vector species may become primary vectors under certain conditions [
83]. Distinguishing features of a primary malaria vector include their relative abundance, a high propensity to feed on humans, and sporozoite rate [
84]. Most of the published studies on
An. squamosus is focused on Zambia and Madagascar. Despite being identified across sub-Saharan Africa, pathogen transmission is unique to a location, as environmental and socio-economic factors impact transmission, which can vary from country to country [
85].
Anopheles squamosus has been found infected with human
Plasmodium sporozoites in Kenya, Mali, Mozambique, Madagascar, Tanzania, and Zambia [
23,
26,
27,
38,
78]. Reported infection rates are variable due to sample size, however, these reports verify that
An. squamosus feeds on humans frequently enough to be infected with infectious human-only parasites. The first detection of
Plasmodium sporozoites in
An. squamosus was in Tanzania in 1964, where a single mosquito was infected with the parasite [
87]. In a study in Mozambique,
An. squamosus had the highest
Plasmodium sporozoite infection rate of 5.8%, more than
An. parensis, An. arabiensis, and
An. funestus [
23]. In a trapping study in Zambia, all three captured
An. squamosus samples were found to be infected with
P. falciparum [
27]. In Madagascar, two distinct strains of
P. vivax, another causative agent of human malaria, were identified in
An. squamosus [
49].
Rift Valley fever virus (RVFV) is a virus that causes a mosquito-borne viral zoonosis affecting humans and livestock. RVFV can cause severe disease in animals, while severe symptoms in humans are rare [
90,
91].
Aedes mosquitoes are recognized as the primary vector for RVFV, however,
Anopheles and
Culex mosquitoes are considered secondary vectors [
90]. Some anopheline species can maintain RVFV and transmit it to their offspring [
60] and in Madagascar, RVFV was detected in five pools of
An. squamosus, suggesting this species could be a host of RVFV [
60].
Bluetongue disease, caused by bluetongue virus (BTV), is transmitted predominantly by
Culicoides biting midges among ruminant animals. It causes a wide array of symptoms for ruminant animals but distinctly causes severe swelling of the tongue [
92]. A survey of mosquitoes in Madagascar identified a pool of
An. squamosus that tested positive for BTV [
89]. Though it does not indicate that this species is a competent vector of BTV it demonstrates that these mosquitoes can acquire the virus from infected animals. Further research will be needed to determine any role for
An. squamosus in maintenance and transmission of BTV.
4. Discussion
Anopheles squamosus has been documented in 43 of the 54 currently recognized African countries, with many studies reporting a robust number of this species. These collection records indicate that this species was likely widespread and well-established long before their first documentation in the early 1900s. The broad distribution of
An. squamosus is similar to other primary malaria vectors in Africa, such as
An. gambiae, An. arabiensis, and
An. funestus, suggesting that
An. squamosus has a reasonable chance of coming into contact with human hosts and infectious parasites. In Zambia, this species exhibited a high human blood index, even in the presence of livestock [
20]. In addition, they have been found infected with human
Plasmodium sporozoites [
28,
88]. These combinations of factors suggest that
An. squamosus may play a more significant role in malaria transmission than previously recognized.
Anopheles squamosus poses a challenge for malaria transmission [
19,
87,
93]. due to its exophilic nature, which allows them to evade indoor-based malaria intervention methods such as ITN and IRS. As a result, humans are more likely to encounter
An. squamosus and other secondary vector species in outdoor settings when outdoor-based interventions are not put in place [
30,
77]. For example, in Mozambique, there was an increase in outdoor
An. squamosus population numbers [
23] after an increase in IRS campaigns. This suggests a potential behavioral adaptation or shift in mosquito community populations in response to indoor malaria control. Therefore, targeted control of primary endophagic vectors through ITN and IRS has the potential to shift vector status in favor of exophagic vectors like
An. squamosus [
94]. This highlights the need for comprehensive control approaches that address both indoor and outdoor transmission risks.
The public health significance of An. squamosus cannot be understated, as it has also been identified as a potential vector of RVFV, and BTV due to zoophilic behavior. For An. squamosus, records indicate a high vector abundance, a propensity to feed on humans, and positive Plasmodium sporozoite rates, thus indicating that this species is a competent vector for malaria.
Despite its significant role in malaria transmission, there is a lack of reliable molecular identification tools such as internal transcribed spacer (ITS) polymerase chain reaction (PCR) for
An. squamosus. The current molecular identification of
An. squamosus based on Sanger sequencing of Cytochrome Oxidase I is not ideal for routine surveillance. Since
An. squamosus is morphologically identical to
An. cydippis in their adult life stages, distinguishing between these cryptic species becomes challenging without robust molecular species identification tools [
95]. This represents the key barrier to gaining further understanding of the biology and behavior of this species. Therefore, there is a pressing research need for building more genomic resources to improve our understanding of and enhance surveillance for
An. squamosus.
Past studies that identify
An. squamosus on morphology alone may not be accurate [
29]. In our effort to create a reliable distribution map, we attempted to separate records verified only by morphological examination from records confirmed by both morphological and molecular methods. However, the lack of detailed metadate for many of the records and publications prevented accurate categorization. This further emphasizes the need for cost-effective tools that will help enhance species identification, allowing further investigations to inform the role of
An. squamosus in pathogen transmission and development of disease mitigation strategies.
5. Conclusion
Anopheles squamosus is a widespread mosquito species found abundantly across the African continent. Despite implication in malaria transmission and potentially vectoring other diseases impacting human and animal health, research and information on this species remains scarce. Historically, An. squamosus was regarded as an exophagic and zoophilic species and as a result, they were not viewed as a threat to public health. However, our review indicates that this species occupies the same range and exhibits similar behaviors to primary malaria vectors, as they have been implicated on feeding on humans in indoor settings and found to be infected with human Plasmodium parasites. Anopheles squamosus continues to be a cryptic species that has circumvented disease control measures. Addressing these challenges requires further research to gain more information on their biology and behavior, ultimately enabling development of comprehensive vector control strategy that will lead to malaria elimination.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: Records of
Anopheles squamosus occurrences.
Author Contributions
Conceptualization, V.T.N. and Y.L.; investigation, V.T.N., D.D.S., B.B.A., T.A., T.P., M.E.G, D.E.N., K.S., L.S., E.S., L.E.R., and Y.L.; data curation, V.T.N., D.D.S., B.B.A., T.A., and T.P.; formal analysis, V.T.N.; resources, E.S., D.E.N., and Y.L.; validation, D.K.M., P.T., L.E.R., A.R., M.E.G., K.S., E.S., R.A., D.E.N., and Y.L.; writing—original draft preparation, V.T.N. and Y.L..; writing—review and editing, V.T.N., D.K.M., P.T., L.E.R., R.A., M.E.G., K.S., E.S., D.E.N., and Y.L.; visualization, V.T.N..; supervision, D.K.M, P.T., and Y.L.; project administration, Y.L.; funding acquisition, V.T.N. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the United States National Institute of Health as part of the International Centers of Excellence for Malaria Research (2U19AI089680), Small Grant Program (R03AI178041), and T32 Institutional Training Grant (T32AI0074717). Additional support was provided by the United States Department of Agriculture National Institute of Food and Agriculture multistate Hatch project (1025565), the Bloomberg Philanthropies, the Johns Hopkins Malaria Research Institute, the College of Agricultural and Life Sciences Dean’s Award at the University of Florida, and Global Fellows Program at the University of Florida International Center.
Data Availability Statement
No new data were created in this study. The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank Dr. Phillimon Ndubani, as well as his incredible staff members at the Macha Research Trust for facilitating our mosquito collection to obtain high quality images of our study species. We thank Dr. Ana L. Romero-Weaver at the University of Florida for reviewing our manuscript. We thank Dr. Liliana Cano and Dr. Bianca Burini at the University of Florida for serving as faculty advisors on our committee.
Conflicts of Interest
The authors declare no conflicts of interest. The funders did not have a specific role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the funding agencies.
References
- World Health Organization World Malaria Report 2023; World Health Organization: Geneva, 2023; ISBN 978-92-4-008617-3.
- World Health Organization Global Technical Strategy for Malaria 2016-2030; World Health Organization, 2015; ISBN 978-92-4-156499-1.
- Chalageri, V.H.; Marinaik, S.B.; Nath, S.N.; Singhal, R.; Rawat, S.; Ravikumar, K.; Shariff, M.; Eapen, A. Malaria Control – Lessons Learned from Trends of Malaria Indices over Three Decades in Karnataka, India. Malar J 2023, 22, 353. [CrossRef]
- Gao, L.; Shi, Q.; Liu, Z.; Li, Z.; Dong, X. Impact of the COVID-19 Pandemic on Malaria Control in Africa: A Preliminary Analysis. Trop Med Infect Dis 2023, 8, 67. [CrossRef]
- World Health Organization World Malaria Report 2017; World Health Organization: Geneva, 2017; ISBN 978-92-4-156552-3.
- DePina, A.J.; Niang, E.H.A.; Barbosa Andrade, A.J.; Dia, A.K.; Moreira, A.; Faye, O.; Seck, I. Achievement of Malaria Pre-Elimination in Cape Verde According to the Data Collected from 2010 to 2016. Malar J 2018, 17, 236. [CrossRef]
- Lee, P.-W.; Liu, C.-T.; Rampao, H.S.; do Rosario, V.E.; Shaio, M.-F. Pre-Elimination of Malaria on the Island of Príncipe. Malar J 2010, 9, 26. [CrossRef]
- Kobayashi, T.; Kurani, S.; Hamapumbu, H.; Stevenson, J.C.; Thuma, P.E.; Moss, W.J. Prevalence of Glucose-6-Phosphate Dehydrogenase Deficiency and Gametocytemia in a Pre-Elimination, Low Malaria Transmission Setting in Southern Zambia. Am J Trop Med Hyg 2021, 104, 1000–1002. [CrossRef]
- Moonasar, D.; Nuthulaganti, T.; Kruger, P.S.; Mabuza, A.; Rasiswi, E.S.; Benson, F.G.; Maharaj, R. Malaria Control in South Africa 2000-2010: Beyond MDG6. Malar J 2012, 11, 1–7. [CrossRef]
- World Health Organization Malaria Vector Control Available online: https://www.who.int/teams/global-malaria-programme/prevention/vector-control (accessed on 3 May 2023).
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The Effect of Malaria Control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015 526:7572 2015, 526, 207–211. [CrossRef]
- Cross, D.E.; Thomas, C.; McKeown, N.; Siaziyu, V.; Healey, A.; Willis, T.; Singini, D.; Liywalii, F.; Silumesii, A.; Sakala, J.; et al. Geographically Extensive Larval Surveys Reveal an Unexpected Scarcity of Primary Vector Mosquitoes in a Region of Persistent Malaria Transmission in Western Zambia. Parasit Vectors 2021, 14, 91. [CrossRef]
- Bayoh, M.N.; Mathias, D.K.; Odiere, M.R.; Mutuku, F.M.; Kamau, L.; Gimnig, J.E.; Vulule, J.M.; Hawley, W.A.; Hamel, M.J.; Walker, E.D. Anopheles gambiae: Historical Population Decline Associated with Regional Distribution of Insecticide-Treated Bed Nets in Western Nyanza Province, Kenya. Malar J 2010, 9, 62. [CrossRef]
- O’Loughlin, S.M.; Magesa, S.M.; Mbogo, C.; Mosha, F.; Midega, J.; Burt, A. Genomic Signatures of Population Decline in the Malaria Mosquito Anopheles gambiae. Malar J 2016, 15, 182. [CrossRef]
- Mubemba, B.; Mburu, M.M.; Changula, K.; Muleya, W.; Moonga, L.C.; Chambaro, H.M.; Kajihara, M.; Qiu, Y.; Orba, Y.; Hayashida, K.; et al. Current Knowledge of Vector-Borne Zoonotic Pathogens in Zambia: A Clarion Call to Scaling-up “One Health” Research in the Wake of Emerging and Re-Emerging Infectious Diseases. PLoS Negl Trop Dis 2022, 16, e0010193. [CrossRef]
- Okoro, O.J.; Deme, G.G.; Okoye, C.O.; Eze, S.C.; Odii, E.C.; Gbadegesin, J.T.; Okeke, E.S.; Oyejobi, G.K.; Nyaruaba, R.; Ebido, C.C. Understanding Key Vectors and Vector-Borne Diseases Associated with Freshwater Ecosystem across Africa: Implications for Public Health. Sci Total Environ 2023, 862, 160732. [CrossRef]
- Degefa, T.; Githeko, A.K.; Lee, M.-C.; Yan, G.; Yewhalaw, D. Patterns of Human Exposure to Early Evening and Outdoor Biting Mosquitoes and Residual Malaria Transmission in Ethiopia. Acta Trop 2021, 216, 105837. [CrossRef]
- Swei, A.; Couper, L.I.; Coffey, L.L.; Kapan, D.; Bennett, S. Patterns, Drivers, and Challenges of Vector-Borne Disease Emergence. Vector Borne Zoonotic Dis 2020, 20, 159–170. [CrossRef]
- Antonio-Nkondjio, C.; Kerah, C.H.; Simard, F.; Awono-Ambene, P.; Chouaibou, M.; Tchuinkam, T.; Fontenille, D. Complexity of the Malaria Vectorial System in Cameroon: Contribution of Secondary Vectors to Malaria Transmission. J Med Entomol 2006, 43, 1215–1221. [CrossRef]
- Fornadel, C.M.; Norris, L.C.; Franco, V.; Norris, D.E. Unexpected Anthropophily in the Potential Secondary Malaria Vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis 2011, 11, 1173. [CrossRef]
- Rodriguez, M.H. Residual Malaria: Limitations of Current Vector Control Strategies to Eliminate Transmission in Residual Foci. J Infect Dis 2021, 223, S55–S60. [CrossRef]
- Tabue, R.N.; Nem, T.; Atangana, J.; Bigoga, J.D.; Patchoke, S.; Tchouine, F.; Fodjo, B.Y.; Leke, R.G.; Fondjo, E. Anopheles ziemanni a Locally Important Malaria Vector in Ndop Health District, North West Region of Cameroon. Parasit Vectors 2014, 7, 262. [CrossRef]
- Montoya, L.F.; Alafo, C.; Martí-Soler, H.; Máquina, M.; Comiche, K.; Cuamba, I.; Munguambe, K.; Cator, L.; Aide, P.; Galatas, B.; et al. Overlaying Human and Mosquito Behavioral Data to Estimate Residual Exposure to Host-Seeking Mosquitoes and the Protection of Bednets in a Malaria Elimination Setting Where Indoor Residual Spraying and Nets Were Deployed Together. PLoS One 2022, 17, e0270882. [CrossRef]
- Lobo, N.F.; Laurent, B.S.; Sikaala, C.H.; Hamainza, B.; Chanda, J.; Chinula, D.; Krishnankutty, S.M.; Mueller, J.D.; Deason, N.A.; Hoang, Q.T.; et al. Unexpected Diversity of Anopheles Species in Eastern Zambia: Implications for Evaluating Vector Behavior and Interventions Using Molecular Tools. Sci Rep 2015, 5, 17952. [CrossRef]
- Chinula, D.; Hamainza, B.; Chizema, E.; Kavishe, D.R.; Sikaala, C.H.; Killeen, G.F. Proportional Decline of Anopheles quadriannulatus and Increased Contribution of An. arabiensis to the An. gambiae Complex Following Introduction of Indoor Residual Spraying with Pirimiphos-Methyl: An Observational, Retrospective Secondary Analysis of Pre-Existing Data from South-East Zambia. Parasit Vectors 2018, 11, 544. [CrossRef]
- Stevenson, J.C.; Pinchoff, J.; Muleba, M.; Lupiya, J.; Chilusu, H.; Mwelwa, I.; Mbewe, D.; Simubali, L.; Jones, C.M.; Chaponda, M.; et al. Spatio-Temporal Heterogeneity of Malaria Vectors in Northern Zambia: Implications for Vector Control. Parasit Vectors 2016, 9, 1–15. [CrossRef]
- Gebhardt, M.E.; Searle, K.M.; Kobayashi, T.; Shields, T.M.; Hamapumbu, H.; Simubali, L.; Mudenda, T.; Thuma, P.E.; Stevenson, J.C.; Moss, W.J.; et al. Understudied Anopheline Contribute to Malaria Transmission in a Low-Transmission Setting in the Choma District, Southern Province, Zambia. Am J Trop Med Hyg 2022, 106, 1406–1413. [CrossRef]
- Saili, K.; de Jager, C.; Sangoro, O.P.; Nkya, T.E.; Masaninga, F.; Mwenya, M.; Sinyolo, A.; Hamainza, B.; Chanda, E.; Fillinger, U.; et al. Anopheles rufipes Implicated in Malaria Transmission Both Indoors and Outdoors alongside Anopheles funestus and Anopheles arabiensis in Rural South-East Zambia. Malar J 2023, 22, 95. [CrossRef]
- Jones, C.M.; Ciubotariu, I.I.; Muleba, M.; Lupiya, J.; Mbewe, D.; Simubali, L.; Mudenda, T.; Gebhardt, M.E.; Carpi, G.; Malcolm, A.N.; et al. Multiple Novel Clades of Anopheline Mosquitoes Caught Outdoors in Northern Zambia. Front Trop Dis 2021, 2.
- Govella, N.J.; Okumu, F.O.; Killeen, G.F. Insecticide-Treated Nets Can Reduce Malaria Transmission by Mosquitoes Which Feed Outdoors. Am J Trop Med Hyg 2010, 82, 415–419. [CrossRef]
- Theobald, Fred.V. A Monograph of the Culicidae, or Mosquitoes. Mainly Compiled from the Collections Received at the British Museum from Various Parts of the World in Connection with the Investigation into the Cause of Malaria Conducted by the Colonial Office and the Royal Society; Printed by order of the Trustees: London, 1907; Vol. IV, pp. 1–700;.
- Hoffman, J.E.; Ciubotariu, I.I.; Simubali, L.; Mudenda, T.; Moss, W.J.; Carpi, G.; Norris, D.E.; Stevenson, J.C.; Southern And Central Africa International Centers Of Excellence For Malaria Research. Phylogenetic Complexity of Morphologically Identified Anopheles squamosus in Southern Zambia. Insects 2021, 12, 146. [CrossRef]
- Jupp, P.G.; McIntosh, B.M.; Nevill, E.M. A Survey of the Mosquito and Culicoides Faunas at Two Localities in the Karoo Region of South Africa with Some Observations of Bionomics. Onderstepoort J Vet Res 1980, 47, 1–6.
- Main, B.J.; Lee, Y.; Collier, T.C.; Norris, L.C.; Brisco, K.; Fofana, A.; Cornel, A.J.; Lanzaro, G.C. Complex Genome Evolution in Anopheles coluzzii Associated with Increased Insecticide Usage in Mali. Mol Ecol 2015, 24, 5145–5157. [CrossRef]
- Logan, T.M.; Linthicum, K.J.; Thande, P.C.; Wagateh, J.N.; Roberts, C.R. Mosquito Species Collected from a Marsh in Western Kenya during the Long Rains. J Am Mosq Control Assoc 1991, 7, 395–399.
- Konate, L.; Diagne, N.; Brahimi, K.; Faye, O.; Legros, F.; Rogier, C.; Petrarca, V.; Trape, J.F. Biology of the Vectors and Transmission of Plasmodium falciparum, P. malariae and P. ovale in a Village in the Savanna of West Africa (Dielmo, Senegal). Parasite 1994, 1, 325–333. [CrossRef]
- Gillies, M.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region).; 1987.
- Kyalo, D.; Amratia, P.; Mundia, C.W.; Mbogo, C.M.; Coetzee, M.; Snow, R.W. A Geo-Coded Inventory of Anophelines in the Afrotropical Region South of the Sahara: 1898-2016. Wellcome Open Res 2017, 2, 57. [CrossRef]
- UNSD — Methodology Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 21 February 2024).
- QGIS Association QGIS Geographic Information System Available online: https://www.qgis.org/ (accessed on 21 February 2024).
- Natural Earth Tiles Natural Earth Vector and Raster Tiles Available online: http://naturalearthtiles.org/ (accessed on 22 February 2024).
- Evans, A.M. A Short Illustrated Guide to the Anophelines of Tropical and South Africa; Liverpool: University Press Ltd. London: Hodder and Stoughton Ltd., 1927;
- Glick, J.I. Illustrated Key to the Female Anopheles of Southwestern Asia and Egypt (Diptera: Culicidae); Walter Reed Biosystematics Unit, 1992;
- Coetzee, M. Key to the Females of Afrotropical Anopheles Mosquitoes (Diptera: Culicidae). Malar J 2020, 19, 70. [CrossRef]
- de Meillon, B. Species and Varieties of Malaria Vectors in Africa and Their Bionomics. Bull World Health Organ 1951, 4, 419–441.
- Cornel, A.J.; Lee, Y.; Almeida, A.P.G.; Johnson, T.; Mouatcho, J.; Venter, M.; de Jager, C.; Braack, L. Mosquito Community Composition in South Africa and Some Neighboring Countries. Parasit Vectors 2018, 11, 331. [CrossRef]
- Degefa, T.; Yewhalaw, D.; Zhou, G.; Atieli, H.; Githeko, A.K.; Yan, G. Evaluation of Human-Baited Double Net Trap and Human-Odour-Baited CDC Light Trap for Outdoor Host-Seeking Malaria Vector Surveillance in Kenya and Ethiopia. Malar J 2020, 19, 174. [CrossRef]
- Adugna, T.; Yewhelew, D.; Getu, E. Bloodmeal Sources and Feeding Behavior of Anopheline Mosquitoes in Bure District, Northwestern Ethiopia. Parasit Vectors 2021, 14, 166. [CrossRef]
- Finney, M.; McKenzie, B.A.; Rabaovola, B.; Sutcliffe, A.; Dotson, E.; Zohdy, S. Widespread Zoophagy and Detection of Plasmodium Spp. in Anopheles Mosquitoes in Southeastern Madagascar. Malar J 2021, 20, 25. [CrossRef]
- Guarido, M.M.; Govender, K.; Riddin, M.A.; Schrama, M.; Gorsich, E.E.; Brooke, B.D.; Almeida, A.P.G.; Venter, M. Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa. Viruses 2021, 13, 2148. [CrossRef]
- Haileselassie, W.; Zemene, E.; Lee, M.-C.; Zhong, D.; Zhou, G.; Taye, B.; Dagne, A.; Deressa, W.; Kazura, J.W.; Yan, G.; et al. The Effect of Irrigation on Malaria Vector Bionomics and Transmission Intensity in Western Ethiopia. Parasit Vectors 2021, 14, 516. [CrossRef]
- Maekawa, Y.; Pemba, D.; Kumala, J.; Gowelo, S.; Higa, Y.; Futami, K.; Sawabe, K.; Tsuda, Y. DNA Barcoding of Mosquitoes Collected through a Nationwide Survey in 2011 and 2012 in Malawi, Southeast Africa. Acta Trop 2021, 213, 105742. [CrossRef]
- Membere, O.; Bawo, D.D.S.; Onwuteaka, J.; Ugbomeh, A.P.; Nwosu, O.R. Abundance and Diversity of Insects Associated with Rhizophora Mangle and Avicennia Germinans in Bundu-Ama Mangrove Ecosystem of the Niger Delta, Nigeria. Sci Afr 2021, 14, e01058. [CrossRef]
- Nicholas, K.; Bernard, G.; Bryson, N.; Mukabane, K.; Kilongosi, M.; Ayuya, S.; Mulama, D.H. Abundance and Distribution of Malaria Vectors in Various Aquatic Habitats and Land Use Types in Kakamega County, Highlands of Western Kenya. Ethiop J Health Sci 2021, 31. [CrossRef]
- Zemene, E.; Belay, D.B.; Tiruneh, A.; Lee, M.-C.; Yewhalaw, D.; Yan, G. Malaria Vector Dynamics and Utilization of Insecticide-Treated Nets in Low-Transmission Setting in Southwest Ethiopia: Implications for Residual Transmission. BMC Infect Dis 2021, 21, 882. [CrossRef]
- Kuznetsov, R.L. Detection of Anopheles caustani Laveran, 1900 and Anopheles squamosus Theobald, 1901 on the Territory of the Yemen Arab Republic. Med Parazitol (Mosk) 1971, 40, 441–443.
- Fontenille, D.; Rakotoarivony, I.; Rajaonarivelo, E.; Lepers, J. p Etude des Culicidae dans le Firaisam-pokontany d’Ambohimanjaka aux Environs de Tananarive: Résultats d’une Enquête Longitudinale, en Particulier sur la Transmission Vectorielle du Paludisme. Arch Inst Pasteur Madagascar 1988, 55, 231–243.
- Andrianaivolambo, L.; Domarle, O.; Randrianarivelojosia, M.; Ratovonjato, J.; Le Goff, G.; Talman, A.; Ariey, F.; Robert, V. Anthropophilic Mosquitoes and Malaria Transmission in the Eastern Foothills of the Central Highlands of Madagascar. Acta Trop 2010, 116, 240–245. [CrossRef]
- Kenea, O.; Balkew, M.; Gebre-Michael, T. Environmental Factors Associated with Larval Habitats of Anopheline Mosquitoes (Diptera: Culicidae) in Irrigation and Major Drainage Areas in the Middle Course of the Rift Valley, Central Ethiopia. J Vector Borne Dis 2011, 48, 85–92.
- Ratovonjato, J.; Olive, M.-M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reynes, J.-M.; Elissa, N. Detection, Isolation, and Genetic Characterization of Rift Valley Fever Virus from Anopheles (Anopheles) coustani, Anopheles (Anopheles) squamosus, and Culex (Culex) antennatus of the Haute Matsiatra Region, Madagascar. Vector Borne Zoonotic Dis 2011, 11, 753–759. [CrossRef]
- Masaninga, F.; Muleba, M.; Masendu, H.; Songolo, P.; Mweene-Ndumba, I.; Mazaba-Liwewe, M.; Kamuliwo, M.; Ameneshewa, B.; Siziya, S.; Babaniyi, O. Distribution of Yellow Fever Vectors in Northwestern and Western Provinces, Zambia. Asian Pac J Trop Med 2014, 7, S88–S92. [CrossRef]
- Munhenga, G.; Brooke, B.D.; Spillings, B.; Essop, L.; Hunt, R.H.; Midzi, S.; Govender, D.; Braack, L.; Koekemoer, L.L. Field Study Site Selection, Species Abundance and Monthly Distribution of Anopheline Mosquitoes in the Northern Kruger National Park, South Africa. Malar J 2014, 13, 27. [CrossRef]
- Nepomichene, T.N.J.J.; Tata, E.; Boyer, S. Malaria Case in Madagascar, Probable Implication of a New Vector, Anopheles Coustani. Malar J 2015, 14, 475. [CrossRef]
- St. Laurent, B.; Cooke, M.; Krishnankutty, S.M.; Asih, P.; Mueller, J.D.; Kahindi, S.; Ayoma, E.; Oriango, R.M.; Thumloup, J.; Drakeley, C.; et al. Molecular Characterization Reveals Diverse and Unknown Malaria Vectors in the Western Kenyan Highlands. Am J Trop Med Hyg 2016, 94, 327–335. [CrossRef]
- Tantely, M.L.; Le Goff, G.; Boyer, S.; Fontenille, D. An Updated Checklist of Mosquito Species (Diptera: Culicidae) from Madagascar. Parasite 2016, 23, 20. [CrossRef]
- Hakizimana, E.; Karema, C.; Munyakanage, D.; Githure, J.; Mazarati, J.; Tongren, J.; Takken, W.; Binagwaho, A.; Koenraadt, S. Spatio-Temporal Distribution of Mosquitoes and Risk of Malaria Infection in Rwanda. Acta Trop 2018, 182. [CrossRef]
- Sang, R.; Arum, S.; Chepkorir, E.; Mosomtai, G.; Tigoi, C.; Sigei, F.; Lwande, O.W.; Landmann, T.; Affognon, H.; Ahlm, C.; et al. Distribution and Abundance of Key Vectors of Rift Valley Fever and Other Arboviruses in Two Ecologically Distinct Counties in Kenya. PLoS Negl Trop Dis 2017, 11, e0005341. [CrossRef]
- Fontenille, D.; Rakotoarivony, I. Reappearance of Anopheles funestus as a Malaria Vector in the Antananarivo Region, Madagascar. Trans R Soc Trop Med Hyg 1988, 82, 644–645. [CrossRef]
- Killeen, G.F.; Fillinger, U.; Knols, B.G. Advantages of Larval Control for African Malaria Vectors: Low Mobility and Behavioural Responsiveness of Immature Mosquito Stages Allow High Effective Coverage. Malar J 2002, 1, 1–7. [CrossRef]
- Foster, W.A.; Walker, E.D. Mosquitoes (Culicidae). In Medical and Veterinary Entomology (Third Edition); Mullen, G.R., Durden, L.A., Eds.; Academic Press, 2019; pp. 261–325 ISBN 978-0-12-814043-7.
- Muspratt, J. Destruction of the Larvae of Anopheles gambiae Giles by a Coelomomyces Fungus. Bull World Health Organ 1963, 29, 81–86.
- Couch, J.N. Mass Production of Coelomomyces, a Fungus That Kills Mosquitoes. Proc Natl Acad Sci U S A 1972, 69, 2043–2047. [CrossRef]
- Elliott, R. The Influence of Vector Behavior on Malaria Transmission. Am J Trop Med Hyg 1972, 21, 755–763. [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl Trop Dis 2020, 14, e0007831. [CrossRef]
- CDC Anopheles Species Mosquitos Available online: https://www.cdc.gov/mosquitoes/about/life-cycles/anopheles.html (accessed on 26 January 2022).
- Russell, T.L.; Beebe, N.W.; Cooper, R.D.; Lobo, N.F.; Burkot, T.R. Successful Malaria Elimination Strategies Require Interventions That Target Changing Vector Behaviours. Malar J 2013, 12, 56. [CrossRef]
- Jamet, H.; Curtis, C. Mosquito Behavior and Vector Control. Annu Rev Entomol 2005, 50, 53–70. [CrossRef]
- Kibret, S.; Wilson, G.G. Increased Outdoor Biting Tendency of Anopheles arabiensis and Its Challenge for Malaria Control in Central Ethiopia. Public Health 2016, 141, 143–145. [CrossRef]
- Msugupakulya, B.J.; Urio, N.H.; Jumanne, M.; Ngowo, H.S.; Selvaraj, P.; Okumu, F.O.; Wilson, A.L. Changes in Contributions of Different Anopheles Vector Species to Malaria Transmission in East and Southern Africa from 2000 to 2022. Parasit Vectors 2023, 16, 408. [CrossRef]
- Midega, J.T.; Smith, D.L.; Olotu, A.; Mwangangi, J.M.; Nzovu, J.G.; Wambua, J.; Nyangweso, G.; Mbogo, C.M.; Christophides, G.K.; Marsh, K.; et al. Wind Direction and Proximity to Larval Sites Determines Malaria Risk in Kilifi District in Kenya. Nat Commun 2012, 3, 674. [CrossRef]
- Huestis, D.L.; Dao, A.; Diallo, M.; Sanogo, Z.L.; Samake, D.; Yaro, A.S.; Ousman, Y.; Linton, Y.-M.; Krishna, A.; Veru, L.; et al. Windborne Long-Distance Migration of Malaria Mosquitoes in the Sahel. Parasit Vectors 2019, 574, 404–408. [CrossRef]
- Lukubwe, O.; Mwema, T.; Joseph, R.; Maliti, D.; Iitula, I.; Katokele, S.; Uusiku, P.; Walusimbi, D.; Ogoma, S.B.; Gueye, C.S.; et al. Baseline Characterization of Entomological Drivers of Malaria Transmission in Namibia: A Targeted Operational Entomological Surveillance Strategy. Parasit Vectors 2023, 16, 220. [CrossRef]
- Oaks, S.C.; Mitchell, V.S.; Pearson, G.W.; Carpenter, C.C.J. Vector Biology, Ecology, and Control. In Malaria: Obstacles and Opportunities; National Academies Press (US), 1991 ISBN 0-309-54389-4.
- Graumans, W.; Jacobs, E.; Bousema, T.; Sinnis, P. When Is a Plasmodium-Infected Mosquito an Infectious Mosquito? Trends Parasitol 2020, 36, 705–716. [CrossRef]
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Hemming, D.; Agusto, F.; Evans, K.J.; Fefferman, N.; Gaff, H.; Gumel, A.; LaDeau, S.; et al. Climate, Environmental and Socio-Economic Change: Weighing up the Balance in Vector-Borne Disease Transmission. Philos Trans R Soc Lond B Biol Sci 2015, 370, 20130551. [CrossRef]
- Mwema, T.; Lukubwe, O.; Joseph, R.; Maliti, D.; Iitula, I.; Katokele, S.; Uusiku, P.; Walusimbi, D.; Ogoma, S.B.; Tambo, M.; et al. Human and Vector Behaviors Determine Exposure to Anopheles in Namibia. Parasit Vectors 2022, 15, 436. [CrossRef]
- Gillies, M.T. The Role of Secondary Vectors of Malaria in North-East Tanganyika. Trans R Soc Trop Med Hyg 1964, 58, 154–158. [CrossRef]
- Stevenson, J.C.; Simubali, L.; Mbambara, S.; Musonda, M.; Mweetwa, S.; Mudenda, T.; Pringle, J.C.; Jones, C.M.; Norris, D.E. Detection of Plasmodium falciparum Infection in Anopheles squamosus (Diptera: Culicidae) in an Area Targeted for Malaria Elimination, Southern Zambia. J Med Entomol 2016, 53, 1482–1487. [CrossRef]
- Andriamandimby, S.F.; Viarouge, C.; Ravalohery, J.-P.; Reynes, J.-M.; Sailleau, C.; Tantely, M.L.; Elissa, N.; Cardinale, E.; Sall, A.A.; Zientara, S.; et al. Detection in and Circulation of Bluetongue Virus among Domestic Ruminants in Madagascar. Vet Microbiol 2015, 176, 268–273. [CrossRef]
- Hartman, A. Rift Valley Fever. Clin Lab Med 2017, 37, 285–301. [CrossRef]
- CDC Rift Valley Fever Available online: https://www.cdc.gov/vhf/rvf/index.html (accessed on 11 March 2024).
- USDA Bluetongue Available online: https://www.aphis.usda.gov/wcm/connect/APHIS_Content_Library/SA_Our_Focus/SA_Animal_Health/SA_Animal_Disease_Information/Cattle/bluetongue-disease-info/ (accessed on 24 August 2023).
- Sougoufara, S.; Ottih, E.C.; Tripet, F. The Need for New Vector Control Approaches Targeting Outdoor Biting Anopheline Malaria Vector Communities. Parasit Vectors 2020, 13, 295. [CrossRef]
- Afrane, Y.A.; Mweresa, N.G.; Wanjala, C.L.; Gilbreath III, T.M.; Zhou, G.; Lee, M.-C.; Githeko, A.K.; Yan, G. Evaluation of Long-Lasting Microbial Larvicide for Malaria Vector Control in Kenya. Malar J 2016, 15, 577. [CrossRef]
- Stevenson, J.C.; Norris, D.E. Implicating Cryptic and Novel Anophelines as Malaria Vectors in Africa. Insects 2016, 8, 1. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).