1. Introduction
Acute gastroenteritis (AGE) due to norovirus infection represents in a significant global health burden, with high levels of morbidity across all ages, and the possibility of mortality in at-risk groups such as the very young, very old and the immunocompromised [
1]. Prophylactic vaccination is a potential strategy for preventing norovirus AGE, and several candidate vaccines are in clinical development [
2,
3]. The most advanced candidate is HIL-214 (formerly TAK-214), a bivalent virus-like particle (VLP) vaccine containing two VLPs targeting the predominant norovirus genogroups responsible for most human disease, GI.1 and GII.4 [
4]. As antigenic drift has resulted in multiple variants of GII.4, the vaccine GII.4 VLP consists of a consensus sequence (GII.4c) constructed from three different naturally occurring GII.4 genotype variants, 2002 (Houston), 2006a (Yerseke), and 2006b (Den Haag); the construct is intended to induce a broad immunity against diverse variants within the GII.4 genotype [
4,
5,
6].
Several clinical trials have demonstrated immunogenicity and an acceptable safety profile in adults and older-adult volunteers for various compositions of HIL-214, which contain different ratios of the two VLP antigens, and adjuvanted with aluminum hydroxide (Al(OH)
3) with or without monophosphoryl lipid A (MPL) [
7,
8,
9,
10,
11]. From those trials, a single-dose composition, comprising 15 μg GI.1 and 50 μg GII.4c VLPs with 0.5 mg Al(OH)
3, was selected for adults. No added benefit to immunogenicity was observed from a second dose or from the inclusion of MPL in the vaccine composition [
10,
11]. This single-dose composition was subsequently evaluated in a proof-of-concept study involving US Navy recruits, where the vaccine efficacy against moderate-to-severe AGE associated with any norovirus genotype was estimated to be 61.8% (95.01% CI, 20.8–81.6) [
12].
The current Phase 2 study was designed to assess the long-term immunogenicity of HIL-214 in adult participants pooled from three previous clinical trials, up to five years after vaccination. In this report, the immunogenicity analysis focuses on the 15/50/0 composition (i.e. dose quantities in µg of GI.1 VLP/GII.4c VLP/MPL) administered as a single dose, because these VLP quantities and this regimen were selected for further clinical development. The analyses of the 1-dose 15/50/15 regimen and the 2-dose 15/50/0 regimen were conducted to support the analysis of the 1-dose 15/50/0 regimen; and as with the 1-dose 15/50/0 regimen, both regimens were used in at least two of the previous trials.
2. Methods
2.1. Study Design
This long-term follow-up Phase 2 study, designated as NOR-213, is registered on ClinicalTrials.gov (NCT03039790) and EudraCT (2016-004288-37). The study population consisted of adults who had previously participated in one of three trials (NOR-107, NOR-204, and NOR-210) conducted at 11 trial sites - two in Belgium and nine in the United States - between February 21, 2017 and July 22, 2021. All participants completed and signed a new informed consent for this study. The protocol was approved by the ethics committees of the respective study sites and conducted according to current guidelines of the ICH and GCP. The three initial trials were:
NOR-107 (
NCT02038907 [
10]) was conducted in two sites in Belgium between 28 March 2014 and 19 June 2015 in 420 adults aged 18 to 64 years. Participants received one or two doses of 11 different compositions of GI.1 and GII.4c with or without 15 µg or 50 µg of MPL and/or 167 µg or 500 µg Al(OH)
3 as adjuvants – see
Table 1. Participants from NOR-107 entered the present study during their third year after vaccination.
NOR-204 (NCT02661490 [
11]) was conducted in 10 sites in the US mainly involving 294 older adults aged ≥ 60 years, with a control group of 26 adults aged 18 to 49 years, who received one or two doses of 15 µg GI.1 and 50 µg GII.4c with 500 µg Al(OH)
3 with or without 15 µg MPL – see
Table 1. Participants from NOR-204 entered the present study during their second year after vaccination.
NOR-210 (NCT02475278 [
9]) was conducted at one site in the US in 50 adults aged 18 to 49 years, who received one dose of 15 µg GI.1 and 50 µg GII.4c with 500 µg Al(OH)
3. Participants from NOR-210 entered the present study during their second year after vaccination.
For inclusion in the present study, male or female volunteers aged over 18 years must have previously received at least one intramuscular dose of HIL-214 in one of the previously mentioned trials and must not have received any investigational product from any other clinical trial within 30 days prior to the start of this study. Acceptance into the study was also contingent on the investigator’s assessment that the volunteer would complete the follow up, provide the required blood samples, and had no behavioral or cognitive impairment or psychiatric condition that might interfere with their participation.
2.2. Objectives
Primary and secondary objectives were to evaluate the humoral immune response annually for up to five years after at least one dose of HIL-214 as measured by histoblood-group-binding–antigen (HBGA) blocking and pan-Ig assays, respectively. An assessment of antibody-secreting cell (ASC) frequency was included as an exploratory objective.
2.3. Primary Endpoints
The primary immunogenicity endpoints were the geometric mean titers (GMTs) of GI.1-specific and GII.4c-specific HBGA-blocking antibodies. Secondary endpoints were GMTs of of GI.1-specific and GII.4c-specific pan-Ig antibodies. Exploratory endpoints included the frequency of GI.1-specific and GII.4c-specific ASCs. All primary, secondary, and exploratory evaluations were performed at yearly intervals up to 5 years after primary vaccination.
2.4. Procedures
At the initial trial visit, a baseline blood sample was taken from each participant, with further blood draws occurring on Day 28 or 56 (one month after the first or second dose) and at 6 months (Day 208 or 211 depending on the study). In this extension study, blood samples were drawn at 2 years and then at one-year intervals until the fifth anniversary of their first HIL-214 dose. At each study visit, participants were interviewed to determine if any serious adverse events (SAEs) had occurred since the previous visit. These SAEs were assessed by the investigator for severity and potential causality related to the vaccine. Participants were instructed to report any SAE immediately or within 24 hours to the investigator for further investigation. A data monitoring committee, which was already in place to oversee the risks, benefits and scientific validity of the entire HIL-214 development program, had oversight of the present study.
2.5. Immunogenicity Assays
Sera were prepared immediately from blood draws at each study visit and shipped at -20ºC to the PPD Bioanalytical Laboratory (Richmond, VA, USA). HBGA-blocking antibodies were measured as described previously [
13,
14]. Pan-Ig antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Both assays were performed with 2-fold dilutions of serum samples where a titer represents the reciprocal of the dilution. ASCs were identified in an ELISpot assay of cultured peripheral-blood mononuclear cells (PBMCs), largely based on a previously published method [
15] and performed as a service by CEVAC - Ghent University (Ghent, Belgium). Briefly, memory B cells were induced to differentiate into plasma cells by culturing PBMCs with R848 (InvivoGen) and recombinant human IL-2 (R&D Systems) for 5 days at 37°C/5%CO
2. Cells were then transferred into (96-plate) culture wells coated with 100 µL of GI.1 or GII.4c VLPs at 10 µg/mL or with 100 µl goat–anti-human IgG at 50 µg/mL (Affinipure, Jackson Laboratories) to enumerate GI.1- or GII.4c-specific antibody or IgG secreting plasma cells, respectively. The antibody/antigen spots formed were detected by a conventional immunoenzymatic procedure. The results were expressed as frequencies of GI.1- and GII.4c-specific memory B cells per million of IgG-producing memory B cells.
2.6. Statistics
Data from participants who received a given HIL-214 composition were pooled for each time-point for the analysis (see
Table 1). For this trial, there were two analysis sets defined: the Full Analysis set (FAS) which consisted of all participants with data from at least one follow-up time point, and the Per-Protocol set (PPS) which included all participants in the FAS with no major protocol deviations that could potentially confound the primary (immunogenicity) endpoint. An additional post-hoc analysis of the immunogenicity data was performed, excluding data from participants considered to have had a natural norovirus infection from the time point after the infection occurred. Natural infection was indicated by a ≥4-fold increase in titer, over the most recent previously collected titer, for either antibody assay (HBGA-blocking or pan-Ig), or either GI.1 or GII.4c-specificity. A third sub-set, the ASC subset, consisted of participants originally included in the cell-mediated immunity (CMI) subsets from the NOR-107 and NOR-204 trials and was analyzed descriptively for ASC frequencies. Safety data, including the occurrence of serious adverse events or deaths, are presented descriptively. All statistical analyses were generated using SAS Version 9.4 (SAS Institute Inc. NC, USA).
3. Results
3.1. Disposition and Demographics
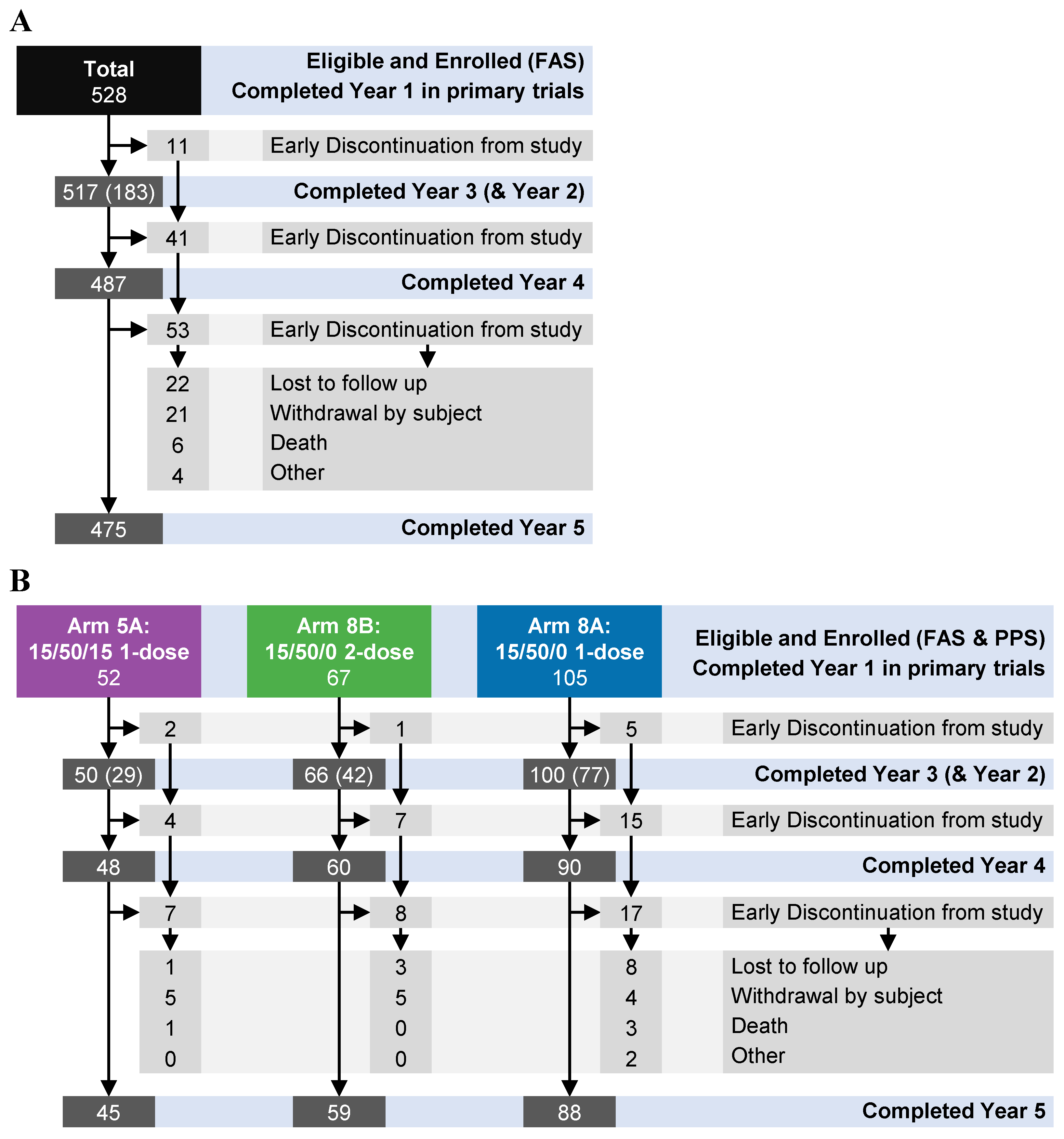
In total, 528 individuals were enrolled into this long-term follow-up study comprising participants from NOR-107, NOR-210, or NOR-204 clinical trials (
Figure 1;
Table 1). Fifty-three participants discontinued the study, including 22 lost to follow-up, 21 who withdrew, 6 who died and 4 who discontinued for unspecified reasons. In both the FAS and the PPS, 105 participants were 1-dose 15/50/0 recipients (Arm 8A), 67 were 2-dose 15/50/0 recipients (Arm 8B), and 52 were 1-dose 15/50/15 recipients (Arm 5A). A subset analyzed for ASCs consisted of 234 participants exclusively from NOR-107 and NOR-204, including 34 recipients from Arm 8A who received a single dose of 15/50/0.
Overall, the mean age was 54 years, with the majority of participants (77%) aged between 18 to 64 years (
Supplementary Table S1). Sixty-one percent of participants were female. In terms of race, most participants identified as White (97%). In terms of ethnicity, most participants (65%) did not report a category, and 32% reported as being not Hispanic or Latino. For recipients of the 15/50 GI.1/GII.4c compositions, mean ages differed: in Arm 8A, the mean age was 54 years, compared with 64 and 62 years in Arms 8B and 5A, respectively (
Table 2). Additionally, fewer participants were female in Arm 8A compared with Arms 8B and 5A (48% versus 57% and 65%, respectively).
3.2. Immunogenicity by HBGA-Blocking Antibodies (Primary Objective)
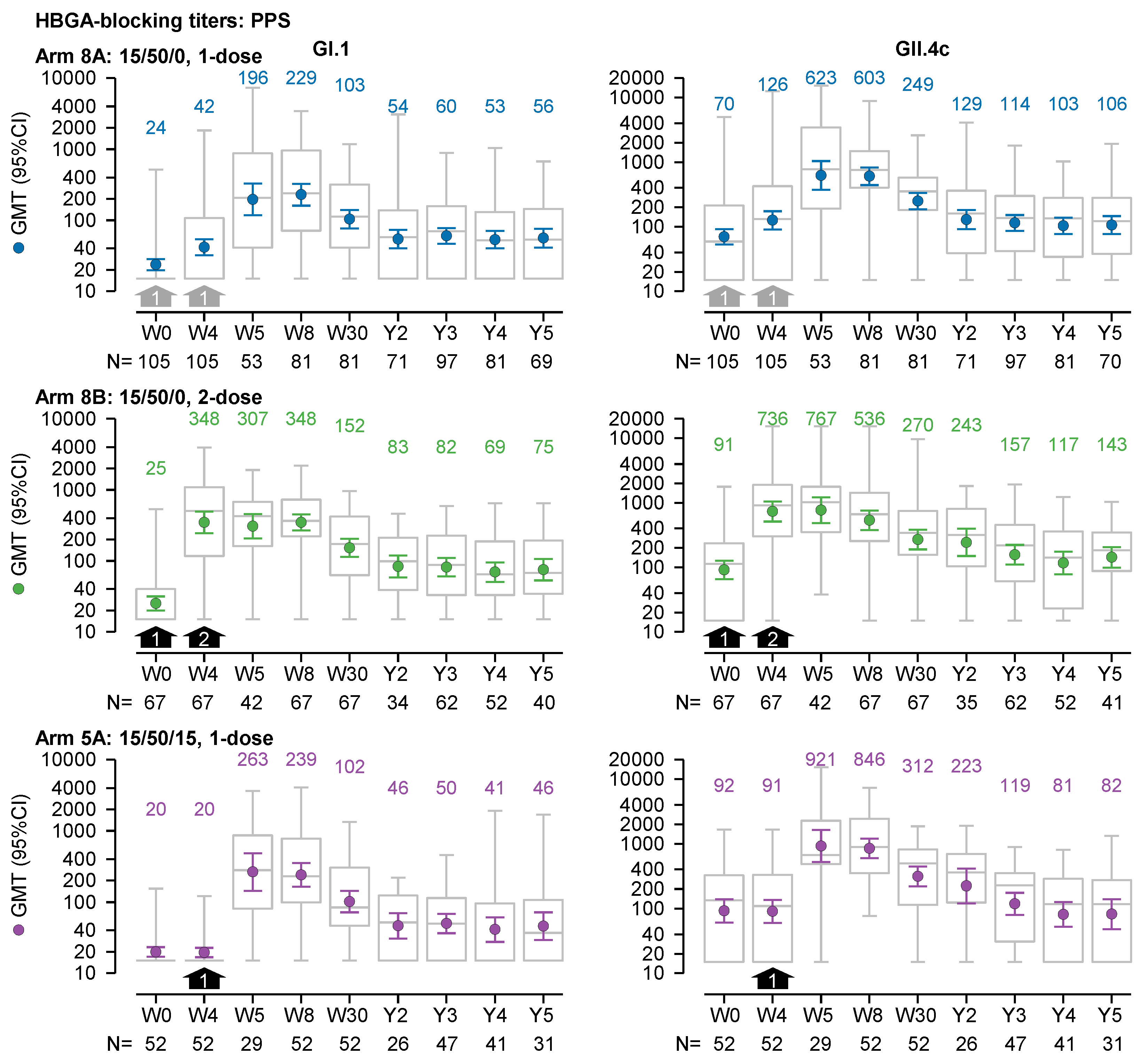
In the 1-dose 15/50/0 recipients (Arm 8A), GI.1-specific and GII.4c-specific HBGA-blocking antibodies appeared to persist up to 5 years post-vaccination. These antibodies peaked at 4 to 8 weeks, plateaued above baseline after 3 years (
Figure 2), and showed greater fold changes compared with baseline for GI.1 GMTs than for GII.4c GMTs. This difference stemmed from lower baseline GI.1 GMTs, likely reflecting less recent immune exposure and lower prevalence of GI.1 genotypes compared with GII.4 genotypes. Hence, from 3 to 5 years, GI.1 GMTs ranged between 53 (95% confidence interval [95%CI], 40–71) and 60 (95%CI, 46–77; N=69–97), remaining below the peak GMT of 229 [95%CI, 161–326]; N=81), but approximately 2-fold higher than baseline GMTs of 24 [95%CI, 20–28]; N=105). GII.4c GMTs ranged between 103 (95%CI, 77–138) and 114 (95%CI, 86–152; N=70–97), below the peak GMT of 623 [95%CI, 372–1042]; N=53), yet consistently above baseline, but by less than 2-fold (70 [95%CI, 53–92]; N=105). After 3 to 5 years, Median GI.1-specific titers (122–136) and GII.4c-specific titers (52–70) after 3 to 5 years were also higher compared with their respective baseline median titers of 59 and 15.
The HBGA-blocking GMTs at 3 to 5 years were also higher than baseline when excluding data from vaccine recipients considered to have had a natural norovirus infection between 2 to 5 years after vaccination (i.e. ≤19% of 1-dose 15/50/0 recipients had either a GI.1-like or GII.4-like infection;
Supplementary Figure S1). For recipients of the 1-dose 15/50/0 regimen (Arm 8A), the trends in the HBGA-blocking GMTs remained consistent when analyzed by initial trial (i.e., NOR-107, NOR-210, and NOR 204) and by age within NOR-204 (
Supplementary Figure S2). All GI.1-specific HBGA-blocking GMTs at 3 to 5 years were higher than those at baseline, and except for one GMT (in the 60- to 94-year age group from NOR-204), and GII.4c-specific HBGA-blocking GMTs at 3 to 5 years were also higher than those at baseline.
The trends in the HBGA-blocking GMTs of recipients of the 2-dose 15/50/0 (Arm 8B) and the 1-dose 15/50/15 (Arm 5A) regimen were similar to those of the 1-dose 15/50/0 recipients (
Figure 2). In recipients of the 2-dose 15/50/0 regimen, GI.1-specific and GII.4c-specific HBGA-blocking GMTs measured 3 to 5 years after dosing, were higher than at baseline. For GI.1, GMTs ranged between 69 [95%CI, 51–95] and 82 [95%CI, 60–110; N=40–62] after 3 to 5 years, compared with 25 [95%CI, 20–32; N=67] at baseline; and for GII.4c, GMTs ranged between 117 [95%CI, 78–174] and 157 [95%CI, 110–223; N=41–62] after 3 to 5 years versus 91 [95%CI, 66–127; N=67]) at baseline. In recipients of the 1-dose 15/50/15 regimen (Arm 5A), GI.1 GMTs 3 to 5 years after dosing were higher than at baseline (41 [95%CI, 28–61] to 50 [95%CI, 37–69; N=31–47] versus 20 [N=52]. GII.4c GMTs were higher than baseline only at 3 years within the 3-to-5-year period (119 [95%CI, 80–176; N=31–47] versus 92 [95%CI, 61–141; N=52]).
3.3. Immunogenicity by Pan-Ig Antibody Binding Assay (Secondary Objective)
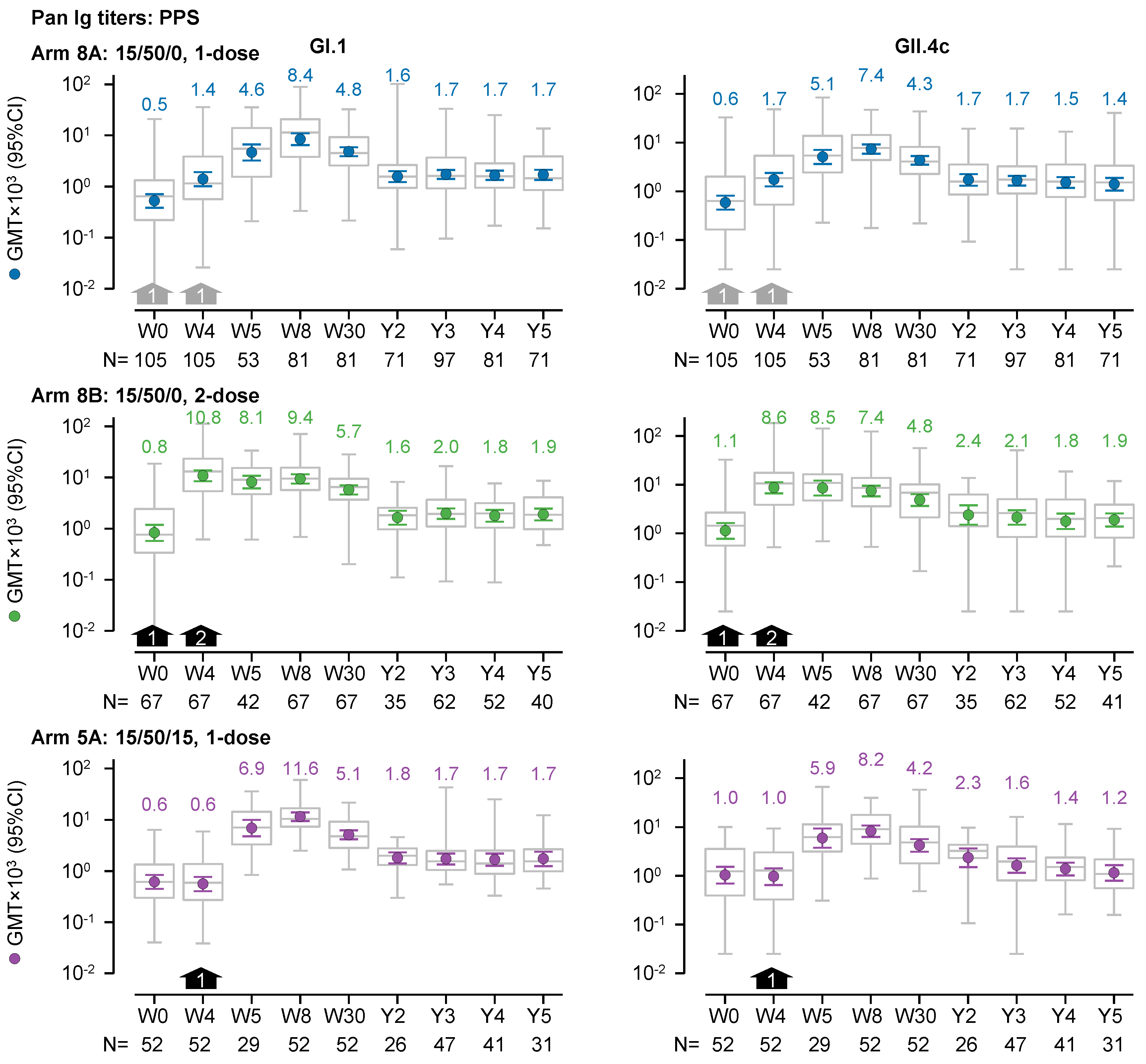
GI.1- and GII.4c-specific pan-Ig binding antibodies also appeared to persist up to 5-years after vaccination, similar to what was observed with HBGA-blocking antibodies (
Figure 3). In recipients of the 1-dose 15/50/0 regimen (Arm 8A), the magnitude of the fold change after 3 to 5 years compared with baseline was greater than 2-fold for both GI.1 and GII.4c GMTs. For GI.1, GMTs after 3 to 5 years were 1.7×10
3 (N=71–97), whereas the GMT at baseline was 0.5×10
3 (N=105). For GII.4c, GMTs after 3 to 5 years ranged between 1.4 and 1.7×10
3 (N=71–97), whereas the GMT at baseline was 0.6×10
3 (N=105).
For recipients of the 1-dose 15/50/0 regimen (Arm 8A), the trends in the pan-Ig GMTs remained consistent when analyzed by the initial trial and by age in NOR-204. All GI.1 GMTs at 3 to 5 years were higher than those at baseline, and except for one GMT (in the 18- to 49-year group from NOR 204) all 3- to 5-year GII.4c GMTs were higher than those at baseline (
Supplementary Figure S3).
The trends in the pan-Ig GMTs were similar when excluding data from vaccine recipients who were considered to have had a natural norovirus infection between 2 years to 5 years after vaccination (data not shown).
The trends in the pan-Ig GMTs of recipients of the 2-dose 15/50/0 regimen (Arm 8B) and the 1-dose 15/50/15 regiment(Arm 5A) were similar to those of the 1-dose 15/50/0 recipients (
Figure 3). In recipients of the 2-dose 15/50/0 regimen (Arm 8B) and the 1-dose 15/50/15 regimen (Arm 5A), all GI.1 GMTs were approximately 2-fold higher and all GII.4 GMTs were less than 2-fold higher, respectively, 3 to 5 years after dosing compared with baseline.
3.4. Antibody-Secreting–Cell Responses (Exploratory Objective)
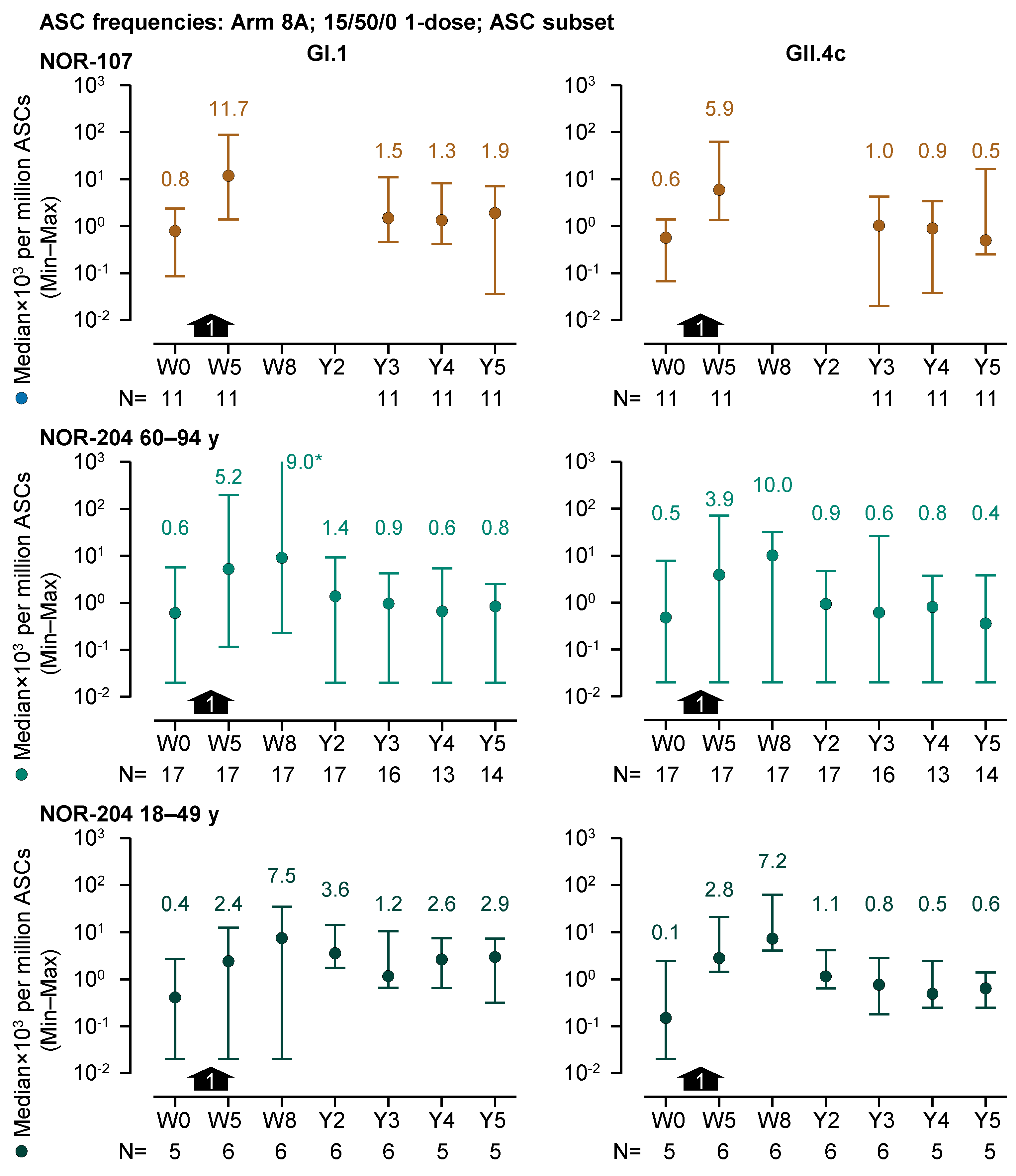
There was evidence of B-cell memory to HIL-214 vaccination 3 to 5 years after dosing (
Figure 4). For the 1-dose 15/50/0 recipients (Arm 8A) in the ASC subset, stratified by former trial, all 3- to 5-year GI.1-specific median ASC frequencies, except one median (from the 60- to 94-year group from NOR-204), were greater than those at baseline. Similarly, all 3- to 5-year GII.4c-specific ASC frequencies, except two medians (from NOR-107 and 60- to 94-year group from NOR-204), were greater than those at baseline. From 3 to 5 years, GI.1 median ASC frequencies ranged between 0.6 and 2.9×10
3 per million ASCs (N=5–16), compared with baseline median frequencies ranging between 0.4 and 0.8×10
3 per million ASCs (N=5–17). GII.4c median ASC frequencies ranged between 0.4 and 1.0×10
3 per million ASCs (N=5–16), whereas baseline median frequencies ranged between 0.1 and 0.6×10
3 per million ASCs (N=5–17).
3.5. Safety
Although there was no safety objective in the study, life-threatening and fatal SAEs, potential vaccine-related SAEs, potential immune–mediated events, events of medical significance and any procedure-related medical occurrences were monitored during the follow-up period (1–5 years after vaccination;
Table 3). Overall, there were 26 adverse events (AEs) reported by 18 participants (3.4%), including 21 SAEs reported by 16 participants, including 6 deaths. One SAE (metastatic renal cell carcinoma led to the withdrawal of one participant, and it was ultimately fatal. The investigators did not consider that any of the AEs, including SAEs and deaths, were related to the study vaccinations administered in the previous trials. However, one AE was causally related to the blood draw in this trial, and this was reported as a mild-intensity post-procedural complication.
4. Discussion
This 2- to 5-year follow-up Phase 2 study of adult HIL-214 recipients from 3 previous clinical trials (NOR-107, NOR-210, and NOR-204) identified evidence that norovirus vaccine immunogenicity persisted up to 5 years, notably marked by HBGA-blocking titers, pan-Ig titers and ASC frequencies remaining above pre-vaccination baseline values. The analysis was descriptive and focused on the 1-dose 15/50/0 arm, which comprised 105 of the 528 participants recruited. This dosage was selected as the preferred one for adults in two previous trials (NOR-107 and NOR-204). Participants, enrolled at 2 sites in Belgium and 9 sites in the USA, were considered immune experienced to norovirus. The mean age at vaccination was 54 years, with 77% of participants aged between 18 and 64 years, 97% identifying as White, and 61% being female. SAEs reported during follow-up were considered unrelated to vaccination and consistent with expectations based on the demographics and prior medical conditions of the study population.
The evidence supporting persistent vaccine immunogenicity was derived from primary and secondary analyses of serum HBGA-blocking titers and pan-Ig titers, respectively. Although no inferential statistics were applied and the GMTs from 3 to 5 years post-vaccination were only approximately 2-fold or less than 2-fold greater than baseline (pre-vaccination), several aspects of the analyses reinforced the study’s main conclusion. These aspects included: (i) the GMT relationship identified at the 3-, 4- and 5-year timepoints, suggesting that GMTs had plateaued at a steady-state level higher than the pre-vaccination baseline; (ii) the GMT relationship generally held when analyzed with respect to subgroups based on previous trial allocation; (iii) the GMT relationship persisted when data from putatively infected participants were excluded; and (iv) similar GMT relationships were observed with related dosages (2-dose 15/50/0 and 1-dose 15/50/15). Additionally, the analysis of a smaller ASC subset suggested that circulating B cells in peripheral blood may have contributed to the sustained production of serum antibodies through 5 years.
The absence of a placebo control group for monitoring long-term changes from baseline titers due to natural infections during the study was a limitation of the analysis. Estimates suggest a norovirus disease rate of approximately 5% of the adult population per year [
16,
17,
18]. The baseline GMTs prior to vaccination reflected immune experience to norovirus, particularly to GII.4-specific over GI.1-specific genotype norovirus strains. The mitigation step to remove data by the final year from up to 19% of vaccine-recipients with putative natural GI.1-specific or GII.4-specific norovirus infections, inevitably resulted in lower post-vaccination GMTs, as a putative infection was defined by an increase in a GI.1- or GII.4c-specific titer. This mitigation may have biased the results towards underestimating the true difference from baseline, especially with GII.4c-specific GMTs. Nevertheless, as stated above, the adjusted GMTs generally remained above the respective pre-vaccination baselines.
Another limitation was that antibody titers and ASC frequencies (in a smaller subset) in peripheral blood provided only a narrow view of anti-norovirus immunity and an indirect assessment of cellular immune memory [
19]. Nevertheless, it is reasonable to assume that both factors would play a role in an anamnestic response to norovirus infection. Moreover, HBGA-blocking antibodies (the primary endpoint measure) may be clinically relevant because these antibodies potentially neutralize norovirus infection of enterocytes in the gut, and the incidence of norovirus gastroenteritis is lower in individuals with mutations that prevent the synthesis of HBGA [
20,
21,
22,
23,
24].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, JC and AB; Methodology, GLR, RA, JC and AB; Formal Analysis, IE and AB.; Investigation, GLR and RA; Resources, JC, IE and AB; Data Curation, IE and AB; Writing – Original Draft Preparation, AB; Writing – Review & Editing, all authors; Supervision, JC and AB; Project Administration, JC, IE and AB; Funding Acquisition, JC and AB.
Funding
The trial was sponsored and funded by Takeda Vaccines, Inc. (MA, USA). Scientific writing in the preparation of the manuscript was funded by HilleVax, Inc. (Seattle, WA 98101, USA).
Institutional Review Board Statement
The trial protocol, protocol amendments, informed consent form (ICF), and other information that required approval were reviewed and approved by the institutional review board (IRB) of each clinical site.
Informed Consent Statement
Written informed consent was obtained from each participant prior to performing any trial-specific procedures.
Data Availability Statement
The clinical-study report (NOR-213) and associated data on which this manuscript is based are available upon request from Takeda Pharmaceuticals International AG, Switzerland.
Acknowledgments
The authors thank the participants of the NOR-213 clinical study. The authors thank, Frank Baehner and Taisei Masuda (former employees of Takeda, Switzerland at the time NOR-213 was underway), Nancy Le Cam (consultant for Takeda at the time NOR-213 was underway) and John Treanor (the principal investigator of NOR-204 at the Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA), for their contributions to the design and conduct of the clinical trials feeding NOR-213. The authors also thank Frances Chen (Takeda Vaccines Inc. USA) for data management and statistical support in completing the NOR-213 clinical-study report on which this manuscript is based. Matthew Morgan (MG Science Communications, Belgium) provided scientific writing services.
Conflicts of Interest
At the time of this report, GLR is (or has been in the past 3 years) a consultant for Curevac, Hillevax, ICON Genetics, Minervax, Novavax, Osivax, Quantoom, and Sumitomo Pharma, Virometix. RA has received research support from Takeda Vaccines, Inc. and Hillevax, Inc., and at the time of this report serves as a consultant to Hillevax, Inc. At the time of this report, IE is a full-time employee of Takeda Vaccines and holds Takeda stock. At the initiation and running of the study, JK and AB were full-time employees of Takeda Vaccines; and at the time of this report, AB is a full-time employees of HilleVax Inc.
References
- Patel, M.M.; Widdowson, M.A.; Glass, R.I.; Akazawa, K.; Vinje, J.; Parashar, U.D. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008, 14, 1224–1231. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, M.; Hu, Q. Advances in Human Norovirus Vaccine Research. Vaccines (Basel). 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Waerlop, G.; Janssens, Y.; Jacobs, B.; Jarczowski, F.; Diessner, A.; Leroux-Roels, G.; et al. Immune responses in healthy adults elicited by a bivalent norovirus vaccine candidate composed of GI.4 and GII.4 VLPs without adjuvant. Front Immunol. 2023, 14, 1188431. [Google Scholar] [CrossRef] [PubMed]
- Hoa Tran, T.N.; Trainor, E.; Nakagomi, T.; Cunliffe, N.A.; Nakagomi, O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol. 2013, 56, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Atmar, R.L.; Frey, S.E.; Gormley, R.; Chen, W.H.; Ferreira, J.; et al. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate--reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis. 2014, 210, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I.; Bok, K.; Taylor, R.; Haynes, J.R.; Sosnovtsev, S.V.; Richardson, C.; et al. Immunogenicity and specificity of norovirus Consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 2012, 30, 3580–3586. [Google Scholar] [CrossRef]
- Atmar, R.L.; Baehner, F.; Cramer, J.P.; Lloyd, E.; Sherwood, J.; Borkowski, A.; et al. Persistence of Antibodies to 2 Virus-Like Particle Norovirus Vaccine Candidate Formulations in Healthy Adults: 1-Year Follow-up With Memory Probe Vaccination. J Infect Dis. 2019, 220, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Baehner, F.; Cramer, J.P.; Song, E.; Borkowski, A.; Mendelman, P.M.; et al. Rapid Responses to 2 Virus-Like Particle Norovirus Vaccine Candidate Formulations in Healthy Adults: A Randomized Controlled Trial. J Infect Dis. 2016, 214, 845–853. [Google Scholar] [CrossRef]
- Atmar, R.L.; Cramer, J.P.; Baehner, F.; Han, C.; Borkowski, A.; Mendelman, P.M. An Exploratory Study of the Salivary Immunoglobulin A Responses to 1 Dose of a Norovirus Virus-Like Particle Candidate Vaccine in Healthy Adults. J Infect Dis. 2019, 219, 410–414. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Cramer, J.P.; Mendelman, P.M.; Sherwood, J.; Clemens, R.; Aerssens, A.; et al. Safety and Immunogenicity of Different Formulations of Norovirus Vaccine Candidate in Healthy Adults: A Randomized, Controlled, Double-Blind Clinical Trial. J Infect Dis. 2018, 217, 597–607. [Google Scholar] [CrossRef]
- Treanor, J.; Sherwood, J.; Cramer, J.P.; Le Cam Bouveret, N.; Lin, S.; Baehner, F.; et al. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine. 2020, 38, 5842–5850. [Google Scholar] [CrossRef]
- Sherwood, J.; Mendelman, P.M.; Lloyd, E.; Liu, M.; Boslego, J.; Borkowski, A.; et al. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine 2020, 38, 6442–6449. [Google Scholar] [CrossRef]
- Reeck, A.; Kavanagh, O.; Estes, M.K.; Opekun, A.R.; Gilger, M.A.; Graham, D.Y.; et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010, 202, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Saez-Llorens, X.; Blazevic, V.; Lopez, P.; Lopez, E.; Masuda, T.; et al. Immunogenicity of a bivalent virus-like particle norovirus vaccine in children from 1 to 8 years of age: A phase 2 randomized, double-blind study. Vaccine. 2022, 40, 3588–3596. [Google Scholar] [CrossRef]
- Crotty, S.; Aubert, R.D.; Glidewell, J.; Ahmed, R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004, 286, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.; Gambhir, M.; Leon, J.; Lopman, B. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis. 2013, 19, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Lopman, B.A.; Hall, A.J.; Parashar, U.D.; Lee, B.Y. The potential economic value of a human norovirus vaccine for the United States. Vaccine. 2012, 30, 7097–7104. [Google Scholar] [CrossRef] [PubMed]
- CDC. Norovirus Burden and Trends. 2023. https://www.cdc.gov/norovirus/burden.html (accessed on 22 April 2024).
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.C.; Boshier, F.A.T.; Brewer-Jensen, P.D.; Roy, S.; Costantini, V.; Mallory, M.L.; et al. Immune Imprinting Drives Human Norovirus Potential for Global Spread. mBio. 2022, 13, e0186122. [Google Scholar] [CrossRef]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J.; Svensson, L. Genetic Susceptibility to Human Norovirus Infection: An Update. Viruses. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Tohma, K.; Lepore, C.J.; Gao, Y.; Ford-Siltz, L.A.; Parra, G.I. Population Genomics of GII.4 Noroviruses Reveal Complex Diversification and New Antigenic Sites Involved in the Emergence of Pandemic Strains. mBio 2019, 10. [Google Scholar] [CrossRef]
- Haga, K.; Ettayebi, K.; Tenge, V.R.; Karandikar, U.C.; Lewis, M.A.; Lin, S.C.; et al. Genetic Manipulation of Human Intestinal Enteroids Demonstrates the Necessity of a Functional Fucosyltransferase 2 Gene for Secretor-Dependent Human Norovirus Infection. mBio. 2020, 11. [Google Scholar] [CrossRef]
Figure 1.
Schematic description of participant disposition for all eligible and enrolled participants entered the follow-up study from the initial trials NOR-107, NOR-210, and NOR-204. Participants from NOR-210 and NOR-204 entered the follow-up study at the beginning of Year 2 (i.e., 1-year after vaccination), whereas participants from NOR-207 entered at the beginning of Year 3. The disposition is shown for (A) the full-analysis set (FAS) for the entire study population; and (B) for the FAS for Arms 5A, 8B and 8A. The light grey boxes indicate participants who discontinued, and the reasons for discontinuation. The numbers in the light-grey boxes represent cumulative totals in accordance with the directions of the arrows. PPS=per-protocol set.
Figure 1.
Schematic description of participant disposition for all eligible and enrolled participants entered the follow-up study from the initial trials NOR-107, NOR-210, and NOR-204. Participants from NOR-210 and NOR-204 entered the follow-up study at the beginning of Year 2 (i.e., 1-year after vaccination), whereas participants from NOR-207 entered at the beginning of Year 3. The disposition is shown for (A) the full-analysis set (FAS) for the entire study population; and (B) for the FAS for Arms 5A, 8B and 8A. The light grey boxes indicate participants who discontinued, and the reasons for discontinuation. The numbers in the light-grey boxes represent cumulative totals in accordance with the directions of the arrows. PPS=per-protocol set.
Figure 2.
GI.1-specific and GII.4c-specific HBGA-blocking geometric mean titers (GMTs; left and right graphs, respectively) over a 5-year period from prior to dosing (Week 0; W0) in the preceding trials to the end of the present follow-up study (Year 5, Y5) in the per-protocol set (PPS) without data from participants putatively infected with norovirus (with either a GI.1-like or GII.4-like genotype and identified by ≥4-fold increase in antibody titer at a subsequent visit during the follow-up period). Three dosage regimens are shown: (i) 1 dose of 15/50/0 (either at W0 or W4; grey arrows); (ii) 2 doses of 15/50 (at W0 and W4; black arrows); and 1 dose of 15/50/15 at W4 (black arrows). GMTs and 95% confidence intervals are represented by colored round symbols (and value above the plot) and error bars, respectively. Medians, 25 and 75 percentiles, minimum and maximum values are represented by grey box and whisker plots.
Figure 2.
GI.1-specific and GII.4c-specific HBGA-blocking geometric mean titers (GMTs; left and right graphs, respectively) over a 5-year period from prior to dosing (Week 0; W0) in the preceding trials to the end of the present follow-up study (Year 5, Y5) in the per-protocol set (PPS) without data from participants putatively infected with norovirus (with either a GI.1-like or GII.4-like genotype and identified by ≥4-fold increase in antibody titer at a subsequent visit during the follow-up period). Three dosage regimens are shown: (i) 1 dose of 15/50/0 (either at W0 or W4; grey arrows); (ii) 2 doses of 15/50 (at W0 and W4; black arrows); and 1 dose of 15/50/15 at W4 (black arrows). GMTs and 95% confidence intervals are represented by colored round symbols (and value above the plot) and error bars, respectively. Medians, 25 and 75 percentiles, minimum and maximum values are represented by grey box and whisker plots.
Figure 3.
GI.1-specific and GII.4c-specific pan-Ig geometric mean titers (GMTs; left and right graphs, respectively) over a 5-year period from prior to dosing (Week 0; W0) in the preceding trials to the end of the present follow-up study (Year 5, Y5) in the per-protocol set (PPS). Three dosage regimens are shown: (i) 1 dose of 15/50/0 (either at W0 or W4; grey arrows); (ii) 2 doses of 15/50 (at W0 and W4; black arrows); and 1 dose of 15/50/15 at W4 (black arrows). GMTs and 95% confidence intervals are represented by colored round symbols (and value above the plot) and error bars, respectively. Medians, 25 and 75 percentiles, minimum and maximum values are represented by grey box and whisker plots. Note, the GMTs in the figure are expressed in a scientific notation that includes a 103 multiplier.
Figure 3.
GI.1-specific and GII.4c-specific pan-Ig geometric mean titers (GMTs; left and right graphs, respectively) over a 5-year period from prior to dosing (Week 0; W0) in the preceding trials to the end of the present follow-up study (Year 5, Y5) in the per-protocol set (PPS). Three dosage regimens are shown: (i) 1 dose of 15/50/0 (either at W0 or W4; grey arrows); (ii) 2 doses of 15/50 (at W0 and W4; black arrows); and 1 dose of 15/50/15 at W4 (black arrows). GMTs and 95% confidence intervals are represented by colored round symbols (and value above the plot) and error bars, respectively. Medians, 25 and 75 percentiles, minimum and maximum values are represented by grey box and whisker plots. Note, the GMTs in the figure are expressed in a scientific notation that includes a 103 multiplier.
Figure 4.
GI.1-specific and GII.4c-specific median antibody -secreting cell (ASC) frequencies per million antigen-secreting cells (ASCs) (left and right graphs, respectively) over a 5-year period from prior to dosing (Week 0; W0) in the preceding trials to the end of the present follow-up study (Year 5, Y5) for recipients of a single dose of 15/50/0 (Arm 8A) at W4 (black arrows) in the ASC subset. Medians, minimum and maximum values are represented by colored round symbols (and value above the plot) and error bars, respectively. *The maximum value reported at this time point was 2.3×106 and hence potentially erroneous (>million GI.1-specifc ASCs per million ASCs). Note, the median ASC frequencies in the figure are expressed in a scientific notation that includes a 103 multiplier.
Figure 4.
GI.1-specific and GII.4c-specific median antibody -secreting cell (ASC) frequencies per million antigen-secreting cells (ASCs) (left and right graphs, respectively) over a 5-year period from prior to dosing (Week 0; W0) in the preceding trials to the end of the present follow-up study (Year 5, Y5) for recipients of a single dose of 15/50/0 (Arm 8A) at W4 (black arrows) in the ASC subset. Medians, minimum and maximum values are represented by colored round symbols (and value above the plot) and error bars, respectively. *The maximum value reported at this time point was 2.3×106 and hence potentially erroneous (>million GI.1-specifc ASCs per million ASCs). Note, the median ASC frequencies in the figure are expressed in a scientific notation that includes a 103 multiplier.
Table 1.
Arm assignment by former trial, HIL-214 composition and doses.
Table 1.
Arm assignment by former trial, HIL-214 composition and doses.
| Arm |
HIL-214 components (µg) |
Doses |
Number of participants from former trialb
|
Total Number of Participants |
| GI.1 |
GII.4c |
Al(OH)3
|
MPL |
NOR-107 |
NOR-210 |
NOR-204 |
| 1A |
15 |
15 |
500 |
50 |
1 |
25 |
|
|
25 |
| 2A |
15 |
50 |
500 |
50 |
1 |
19 |
|
|
19 |
| 3A |
50 |
50 |
500 |
50 |
1 |
27 |
|
|
27 |
| 4A |
15 |
15 |
500 |
15 |
1 |
27 |
|
|
27 |
| 5Aa |
15 |
50 |
500 |
15 |
1 |
23 |
|
29 |
52 |
| 6A |
50 |
50 |
500 |
15 |
1 |
27 |
|
|
27 |
| 7A |
15 |
15 |
500 |
0 |
1 |
25 |
|
|
25 |
| 8Aa |
15 |
50 |
500 |
0 |
1 |
28 |
24 |
53 |
105 |
| 9A |
50 |
50 |
500 |
0 |
1 |
22 |
|
|
22 |
| 10A |
50 |
150 |
500 |
0 |
1 |
28 |
|
|
28 |
| 11A |
15 |
50 |
167 |
0 |
1 |
21 |
|
|
21 |
| 5B |
15 |
50 |
500 |
15 |
2 |
|
|
35 |
35 |
| 8Ba |
15 |
50 |
500 |
0 |
2 |
25 |
|
42 |
67 |
| 10B |
50 |
150 |
500 |
0 |
2 |
24 |
|
|
24 |
| 11B |
15 |
50 |
167 |
0 |
2 |
24 |
|
|
24 |
Table 2.
Demographic Characteristics in Arms 5A, 8B and 8A of the PPS.
Table 2.
Demographic Characteristics in Arms 5A, 8B and 8A of the PPS.
| Category |
5A: 15/50/15,
1-Dose |
8B: 15/50/0,
2-Dose |
8A: 15/50/0,
1-Dose |
| |
|
N=52 |
N=67 |
N=105 |
| |
|
Mean (SD) |
| Period from 1st Dose to Last Visit (years) |
4.9 (0.5) |
4.9 (0.4) |
4.9 (0.4) |
| Age (years) |
Overall |
62.3 (16.8) |
63.6 (19.4) |
53.9 (21.2) |
| |
|
|
n (%) |
|
| Age range: |
18–64 |
30 (57.7) |
28 (41.8) |
72 (68.6) |
| (years) |
65–84 |
17 (32.7) |
29 (43.3) |
22 (21.0) |
| |
≥85 |
5 (9.6) |
10 (14.9) |
11 (10.5) |
| Female |
34 (65.4) |
38 (56.7) |
50 (47.6) |
| Ethnicity: |
Not Reported |
23 (44.2) |
25 (37.3) |
28 (26.7) |
| |
Not Hispanic or Latino |
28 (53.8) |
39 (58.2) |
69 (65.7) |
| |
Hispanic or Latino |
1 (1.9) |
3 (4.5) |
8 (7.6) |
| Race |
White |
51 (98.1) |
64 (95.5) |
94 (89.5) |
| |
Black or African American |
1 (1.9) |
3 (4.5) |
8 (7.6) |
| |
Asian |
0 (-) |
0 (-) |
1 (1.0) |
| |
Native Hawaiian or Other Pacific Islander |
0 (-) |
0 (-) |
1 (1.0) |
| |
Multiraciala
|
0 (-) |
0 (-) |
1 (1.0) |
Table 3.
AEs During Year 2 to Year 5 Post Primary Vaccination.
Table 3.
AEs During Year 2 to Year 5 Post Primary Vaccination.
| |
FAS (N=528) |
| |
Events ne
|
Participants n (%) |
| Any Adverse Event (AE) |
26 |
18 (3.4) |
| Related to Trial Procedure |
1 |
1 (0.2) |
| Related to Prior Trial Vaccine |
0 |
0 (-) |
| Mild |
1 |
1 (0.2) |
| Moderate |
6 |
3 (0.6) |
| Severe |
19 |
14 (2.7) |
| Leading to Study Withdrawal |
1 |
1 (0.2) |
| Potential Immune-Mediated Event |
6 |
5 (0.9) |
| Related to Prior Trial Vaccine |
0 |
0 (-) |
| Leading to Study Withdrawal |
0 |
0 (-) |
| Serious Adverse Events (SAEs) |
21 |
15 (2.8) |
| Related to Prior Trial Vaccine |
0 |
0 (-) |
| Leading to Study Withdrawal |
1 |
1 (0.2) |
| Deaths |
6 |
6 (1.1) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).