Submitted:
08 December 2024
Posted:
10 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Metformin in Viral Infections and Its Therapeutic Applications Across Multiple Pathogens
2.1. Metformin's Antiviral Potential Against Influenza: Mechanisms and Therapeutic Insight
2.2. Metformin in the Context of COVID-19: Mechanisms of Action and Its Potential as a Therapeutic Agent Against SARS-CoV-2
2.3. Metformin and HIV: Exploring Its Potential in Modulating Immune Responses and Enhancing Treatment Outcomes
2.4. Metformin in Hepatitis C: Potential Therapeutic Effects on Viral Replication, Inflammation, and Hepatic Fibrosis
2.5. Metformin in Hepatitis B: Targeting Insulin Resistance, Inflammation, and Fibrosis in Chronic Liver Disease
2.6. Metformin as an Antiviral: Potential Applications Against Cytomegalovirus, Herpes Simplex Virus, Zika Virus, Dengue Virus, Epstein-Barr Virus, Human Papillomavirus, and Others
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug design, development and therapy 2023, 17, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Amengual-Cladera, E.; Morla-Barcelo, P.M.; Morán-Costoya, A.; Sastre-Serra, J.; Pons, D.G.; Valle, A.; Roca, P.; Nadal-Serrano, M. Metformin: From Diabetes to Cancer-Unveiling Molecular Mechanisms and Therapeutic Strategies. Biology 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Redkva, O.V.; Babinets, L.S.; Halabitska, I.M. EVALUATION OF PARAMETERS OF ACTUAL TYPICAL PATHOGENETIC SYNDROMES IN COMORBIDITY OF TYPE 2 DIABETES MELLITUS AND CHRONIC PANCREATITIS. Wiadomosci lekarskie (Warsaw, Poland : 1960) 2021, 74, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Du, M.R.; Gao, Q.Y.; Liu, C.L.; Bai, L.Y.; Li, T.; Wei, F.L. Exploring the Pharmacological Potential of Metformin for Neurodegenerative Diseases. Frontiers in aging neuroscience 2022, 14, 838173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.E.; Kamyshnyi, O. The interplay of gut microbiota, obesity, and depression: insights and interventions. Cellular and molecular life sciences : CMLS 2024, 81, 443. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, F.; Li, S.; Jiang, N.; Yu, C.; Zhu, X.; Qin, Y.; Hui, J.; Meng, L.; Song, C.; et al. Metformin promotes innate immunity through a conserved PMK-1/p38 MAPK pathway. Virulence 2020, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Sarker, A.; Ayaz, M.; Shatabdy, A.R.; Haque, N.; Jalouli, M.; Rahman, M.D.H.; Mou, T.J.; Dey, S.K.; Hoque Apu, E.; et al. An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Naicker, N.; Sigal, A.; Naidoo, K. Metformin as Host-Directed Therapy for TB Treatment: Scoping Review. Frontiers in microbiology 2020, 11, 435. [Google Scholar] [CrossRef]
- Bilyi, A.K.; Antypenko, L.M.; Ivchuk, V.V.; Kamyshnyi, O.M.; Polishchuk, N.M.; Kovalenko, S.I. 2-Heteroaryl-[1,2,4]triazolo[1,5-c]quinazoline-5(6 H)-thiones and Their S-Substituted Derivatives: Synthesis, Spectroscopic Data, and Biological Activity. ChemPlusChem 2015, 80, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. International journal of molecular sciences 2024, 25. [Google Scholar] [CrossRef]
- Nosulenko, I.S.; Voskoboynik, O.Y.; Berest, G.G.; Safronyuk, S.L.; Kovalenko, S.I.; Kamyshnyi, O.M.; Polishchuk, N.M.; Sinyak, R.S.; Katsev, A.V. Synthesis and Antimicrobial Activity of 6-Thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]-quinazolin-2-one Derivatives. Scientia pharmaceutica 2014, 82, 483–500. [Google Scholar] [CrossRef]
- Froldi, G. View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences. Pharmaceuticals (Basel, Switzerland) 2024, 17. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. Journal of molecular endocrinology 2019, 63, R17–r35. [Google Scholar] [CrossRef]
- Kamyshna, II.; Pavlovych, L.B.; Maslyanko, V.A.; Kamyshnyi, A.M. Analysis of the transcriptional activity of genes of neuropeptides and their receptors in the blood of patients with thyroid pathology. Journal of medicine and life 2021, 14, 243–249. [Google Scholar] [CrossRef]
- Lyubomirskaya, E.S.; Kamyshnyi, A.M.; Krut, Y.Y.; Smiianov, V.A.; Fedoniuk, L.Y.; Romanyuk, L.B.; Kravets, N.Y.; Mochulska, O.M. SNPs and transcriptional activity of genes of innate and adaptive immunity at the maternal-fetal interface in woman with preterm labour, associated with preterm premature rupture of membranes. Wiadomosci lekarskie (Warsaw, Poland : 1960) 2020, 73, 25–30. [Google Scholar]
- Kamyshna, II.; Pavlovych, L.B.; Sydorchuk, L.P.; Malyk, I.V.; Kamyshnyi, A.M. BDNF blood serum linkage with BDNF gene polymorphism (rs6265) in thyroid pathology patients in the West-Ukrainian population. Endocrine regulations 2021, 55, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Babinets, L. Different consequences of the treatment of osteoarthritis in gastrointestinal comorbidity with exocrine pancreatic insufficiency. Family Medicine & Primary Care Review 2021, 23, 422–428. [Google Scholar] [CrossRef]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Therapeutic advances in endocrinology and metabolism 2021, 12, 2042018820980225. [Google Scholar] [CrossRef] [PubMed]
- Siavash Dastjerdi, M.; Tabbakhian, M.; Sabzghabaee, A.M.; Razavi, N. Severity of Gastrointestinal Side Effects of Metformin Tablet Compared to Metformin Capsule in Type 2 Diabetes Mellitus Patients. Journal of Research in Pharmacy Practice 2017, 6, 73. [Google Scholar] [CrossRef]
- Zemlyak, O.S.; Babinets, L.S.; Halabitska, I.M. THE ROLE OF ENDOTOXICOSIS AND INFLAMMATION IN DEEPENING THE PANCREATIC FUNCTIONAL INSUFFICIENCY IN CHRONIC PANCREATITIS IN COMBINATION WITH TYPE 2 DIABETES. Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego 2023, 51, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Oksenych, V.; Kamyshnyi, O. Exploring the Efficacy of Alpha-Lipoic Acid in Comorbid Osteoarthritis and Type 2 Diabetes Mellitus. 2024. [Google Scholar] [CrossRef]
- Lee, H.S.; Noh, J.Y.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Metformin reduces the risk of developing influenza A virus related cardiovascular disease. Heliyon 2023, 9, e20284. [Google Scholar] [CrossRef]
- Ventura-López, C.; Cervantes-Luevano, K.; Aguirre-Sánchez, J.S.; Flores-Caballero, J.C.; Alvarez-Delgado, C.; Bernaldez-Sarabia, J.; Sánchez-Campos, N.; Lugo-Sánchez, L.A.; Rodríguez-Vázquez, I.C.; Sander-Padilla, J.G.; et al. Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2022, 152, 113223. [Google Scholar] [CrossRef]
- Bhutta, M.S.; Gallo, E.S.; Borenstein, R. Multifaceted Role of AMPK in Viral Infections. Cells 2021, 10. [Google Scholar] [CrossRef]
- Parthasarathy, H.; Tandel, D.; Siddiqui, A.H.; Harshan, K.H. Metformin suppresses SARS-CoV-2 in cell culture. Virus research 2023, 323, 199010. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ao, H.; Guo, G.; Liu, M. The Role and Mechanism of Metformin in Inflammatory Diseases. Journal of inflammation research 2023, 16, 5545–5564. [Google Scholar] [CrossRef]
- Dziedzic, A.; Saluk-Bijak, J.; Miller, E.; Bijak, M. Metformin as a Potential Agent in the Treatment of Multiple Sclerosis. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef]
- Babinets, L.; Migenko, B.; Borovyk, I.; Halabitska, I.; Lobanets, N.; Onyskiv, O. The role of cytocin imbalance in the development of man infertility. Wiadomosci lekarskie (Warsaw, Poland : 1960) 2020, 73, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Sorrenti, V.; Buriani, A.; Fortinguerra, S.; Scapagnini, G.; Zella, D. Resveratrol, Rapamycin and Metformin as Modulators of Antiviral Pathways. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Plowman, T.J.; Christensen, H.; Aiges, M.; Fernandez, E.; Shah, M.H.; Ramana, K.V. Anti-Inflammatory Potential of the Anti-Diabetic Drug Metformin in the Prevention of Inflammatory Complications and Infectious Diseases Including COVID-19: A Narrative Review. International journal of molecular sciences 2024, 25. [Google Scholar] [CrossRef]
- Martin, D.E.; Cadar, A.N.; Bartley, J.M. Old drug, new tricks: the utility of metformin in infection and vaccination responses to influenza and SARS-CoV-2 in older adults. Frontiers in aging 2023, 4, 1272336. [Google Scholar] [CrossRef]
- Khodadadi, M.; Jafari-Gharabaghlou, D.; Zarghami, N. An update on mode of action of metformin in modulation of meta-inflammation and inflammaging. Pharmacological Reports 2022, 74. [Google Scholar] [CrossRef] [PubMed]
- Nojima, I.; Wada, J. Metformin and Its Immune-Mediated Effects in Various Diseases. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Babinets, L.S.; Halabitska, I.M.; Kotsaba, Y.Y.; Borovyk, I.O.; Migenko, B.O.; Ryabokon, S.S.; Tsybulska, L.S. The effect of the proteolisis' system activity for the trophological status of patients with osteoarthrosis and excretory insufficiency of pancreas. Wiadomosci lekarskie (Warsaw, Poland : 1960) 2018, 71, 273–276. [Google Scholar]
- Suardi, C.; Cazzaniga, E.; Graci, S.; Dongo, D.; Palestini, P. Link between Viral Infections, Immune System, Inflammation and Diet. International journal of environmental research and public health 2021, 18. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunological reviews 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Tropical medicine and infectious disease 2024, 9. [Google Scholar] [CrossRef]
- Sutter, A.; Landis, D.; Nugent, K. Metformin has immunomodulatory effects which support its potential use as adjunctive therapy in tuberculosis. The Indian journal of tuberculosis 2024, 71, 89–95. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: update on mechanisms of action and repurposing potential. Nature reviews. Endocrinology 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Nagendra, L.; Bhattacharya, S.; Kalra, S.; Kapoor, N. Metformin in COVID-19: Is There a Role Beyond Glycemic Control? International journal of endocrinology and metabolism 2023, 21, e132965. [Google Scholar] [CrossRef]
- Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin-The Good Old Drug. Pharmaceuticals (Basel, Switzerland) 2021, 14. [Google Scholar] [CrossRef]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Frontiers in endocrinology 2021, 12, 718942. [Google Scholar] [CrossRef]
- Nogales, A.; Martínez-Sobrido, L. Reverse Genetics Approaches for the Development of Influenza Vaccines. International journal of molecular sciences 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Raza, M.A.; Amjad, M.N.; Ud Din, G.; Yue, L.; Shen, B.; Chen, L.; Dong, W.; Xu, H.; Hu, Y. A comprehensive review of influenza B virus, its biological and clinical aspects. Frontiers in microbiology 2024, 15, 1467029. [Google Scholar] [CrossRef]
- Yen, F.S.; Wei, J.C.; Shih, Y.H.; Hsu, C.Y.; Hsu, C.C.; Hwu, C.M. Metformin Use before Influenza Vaccination May Lower the Risks of Influenza and Related Complications. Vaccines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Cummings, T.H.; Magagnoli, J.; Hardin, J.W.; Sutton, S.S. Patients with Obesity and a History of Metformin Treatment Have Lower Influenza Mortality: A Retrospective Cohort Study. Pathogens (Basel, Switzerland) 2022, 11. [Google Scholar] [CrossRef]
- Greene, E.; Green, C.L.; Hurst, J.; MacIver, N.J. Metformin use associated with lower rate of hospitalization for influenza in individuals with diabetes. Diabetes, obesity & metabolism 2024, 26, 3281–3289. [Google Scholar] [CrossRef]
- Thom, R.E.; D'Elia, R.V. Future applications of host direct therapies for infectious disease treatment. Frontiers in immunology 2024, 15, 1436557. [Google Scholar] [CrossRef] [PubMed]
- Brandi, P.; Conejero, L.; Cueto, F.J.; Martínez-Cano, S.; Dunphy, G.; Gómez, M.J.; Relaño, C.; Saz-Leal, P.; Enamorado, M.; Quintas, A.; et al. Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections. Cell reports 2022, 38, 110184. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Metformin: Activation of 5' AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Frontiers in genetics 2022, 13, 1022739. [Google Scholar] [CrossRef]
- Martin, D.E.; Cadar, A.N.; Panier, H.; Torrance, B.L.; Kuchel, G.A.; Bartley, J.M. The effect of metformin on influenza vaccine responses in nondiabetic older adults: a pilot trial. Immunity & ageing : I & A 2023, 20, 18. [Google Scholar] [CrossRef]
- Xia, C.; Wang, T.; Hahm, B. Triggering Degradation of Host Cellular Proteins for Robust Propagation of Influenza Viruses. International journal of molecular sciences 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Role of metformin in various pathologies: state-of-the-art microcapsules for improving its pharmacokinetics. Therapeutic delivery 2020, 11, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Choe, J.Y.; Park, S.H. Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. The Korean journal of internal medicine 2022, 37, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Chavez, V.; Hunter, R.L. Morphoproteomic Features of Pulmonary Influenza A (H1N1) with Therapeutic Implications: A Case Study. Annals of clinical and laboratory science 2022, 52, 991–995. [Google Scholar]
- Yang, S.; Wang, L.; Pan, X.; Liang, Y.; Zhang, Y.; Li, J.; Zhou, B. 5-Methoxyflavone-induced AMPKα activation inhibits NF-κB and P38 MAPK signaling to attenuate influenza A virus-mediated inflammation and lung injury in vitro and in vivo. Cellular & molecular biology letters 2022, 27, 82. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, J.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. Activation of AMP-Activated Protein Kinase Attenuates Tumor Necrosis Factor-α-Induced Lipolysis via Protection of Perilipin in 3T3-L1 Adipocytes. Endocrinology and metabolism (Seoul, Korea) 2014, 29, 553–560. [Google Scholar] [CrossRef]
- Uddin, M.A.; Akhter, M.S.; Kubra, K.T.; Siejka, A.; Barabutis, N. Metformin in acute respiratory distress syndrome: An opinion. Experimental gerontology 2021, 145, 111197. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Y.; Zhao, Z.C.; Liu, X.L.; Peng, Y.G.; Zhu, H.M.; Yan, S.F.; Liu, Y.J.; Xie, Q.; Jiang, Y.; Zeng, S.Z. Metformin Mitigates Sepsis-Induced Acute Lung Injury and Inflammation in Young Mice by Suppressing the S100A8/A9-NLRP3-IL-1β Signaling Pathway. Journal of inflammation research 2024, 17, 3785–3799. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8. [Google Scholar] [CrossRef]
- Tao, S.; Drexler, I. Targeting Autophagy in Innate Immune Cells: Angel or Demon During Infection and Vaccination? Frontiers in immunology 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Pehote, G.; Vij, N. Autophagy Augmentation to Alleviate Immune Response Dysfunction, and Resolve Respiratory and COVID-19 Exacerbations. Cells 2020, 9. [Google Scholar] [CrossRef]
- Chen, T.; Tu, S.; Ding, L.; Jin, M.; Chen, H.; Zhou, H. The role of autophagy in viral infections. Journal of biomedical science 2023, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, D.H.; Thapa, S.; Abdulrahman, B.; Vankuppeveld, L.; Schatzl, H.M. Metformin reduces prion infection in neuronal cells by enhancing autophagy. Biochemical and biophysical research communications 2020, 523, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zou, W.; Leng, Y.; Mu, Z.; Zhan, L. Neuroprotective Effects of Metformin on Cerebral Ischemia-Reperfusion Injury: Modulation of JNK and p38 MAP Kinase Signaling Pathways. Cell biochemistry and biophysics 2024, 82, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Rothenburg, S.; Brennan, G. Species-Specific Host-Virus Interactions: Implications for Viral Host Range and Virulence. Trends in microbiology 2020, 28, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, M.D.; Fradet-Turcotte, A. Virus DNA Replication and the Host DNA Damage Response. Annual review of virology 2018, 5, 141–164. [Google Scholar] [CrossRef]
- Shaikh, S.R.; MacIver, N.J.; Beck, M.A. Obesity Dysregulates the Immune Response to Influenza Infection and Vaccination Through Metabolic and Inflammatory Mechanisms. Annual review of nutrition 2022, 42, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Hulme, K.D.; Noye, E.C.; Short, K.R.; Labzin, L.I. Dysregulated Inflammation During Obesity: Driving Disease Severity in Influenza Virus and SARS-CoV-2 Infections. Frontiers in immunology 2021, 12, 770066. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Qiu, L.; Zhang, C.; Deng, Q.; Leng, Q. Immunomodulatory and Antiviral Activity of Metformin and Its Potential Implications in Treating Coronavirus Disease 2019 and Lung Injury. Frontiers in immunology 2020, 11, 2056. [Google Scholar] [CrossRef]
- Moreira, D.; Silvestre, R.; Cordeiro-da-Silva, A.; Estaquier, J.; Foretz, M.; Viollet, B. AMP-activated Protein Kinase As a Target For Pathogens: Friends Or Foes? Current drug targets 2016, 17, 942–953. [Google Scholar] [CrossRef]
- Prantner, D.; Perkins, D.J.; Vogel, S.N. AMP-activated Kinase (AMPK) Promotes Innate Immunity and Antiviral Defense through Modulation of Stimulator of Interferon Genes (STING) Signaling. The Journal of biological chemistry 2017, 292, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Qin, G.; Zhao, J.; Sun, Y.; Zhang, B.; Li, D.; Wang, B.; Jin, X.; Wu, H. Metformin activates the STING/IRF3/IFN-β pathway by inhibiting AKT phosphorylation in pancreatic cancer. American journal of cancer research 2020, 10, 2851–2864. [Google Scholar] [PubMed]
- Pereira, G.; Leão, A.; Erustes, A.G.; Morais, I.B.M.; Vrechi, T.A.M.; Zamarioli, L.D.S.; Pereira, C.A.S.; Marchioro, L.O.; Sperandio, L.P.; Lins Í, V.F.; et al. Pharmacological Modulators of Autophagy as a Potential Strategy for the Treatment of COVID-19. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. Journal of inflammation research 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Yi, J. Biomaterials Designed to Modulate Reactive Oxygen Species for Enhanced Bone Regeneration in Diabetic Conditions. Journal of functional biomaterials 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C. , et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9. [Google Scholar] [CrossRef]
- Saenwongsa, W.; Nithichanon, A.; Chittaganpitch, M.; Buayai, K.; Kewcharoenwong, C.; Thumrongwilainet, B.; Butta, P.; Palaga, T.; Takahashi, Y.; Ato, M. , et al. Metformin-induced suppression of IFN-α via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Scientific reports 2020, 10, 3229. [Google Scholar] [CrossRef]
- Luo, P.; Qiu, L.; Liu, Y.; Liu, X.L.; Zheng, J.L.; Xue, H.Y.; Liu, W.H.; Liu, D.; Li, J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. The American journal of tropical medicine and hygiene 2020, 103, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Torunoglu, S.T.; Zajda, A.; Tampio, J.; Markowicz-Piasecka, M.; Huttunen, K.M. Metformin derivatives - Researchers' friends or foes? Biochemical pharmacology 2023, 215, 115743. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. Metformin Enhances B Cell Function and Antibody Responses of Elderly Individuals With Type-2 Diabetes Mellitus. Frontiers in aging 2021, 2, 715981. [Google Scholar] [CrossRef] [PubMed]
- Bartee, E.; McFadden, G. Cytokine synergy: an underappreciated contributor to innate anti-viral immunity. Cytokine 2013, 63, 237–240. [Google Scholar] [CrossRef]
- Halabitska, I.; Babinets, L.; Kotsaba, Y. PATHOGENETIC FEATURES OF COMORBIDITY OF PRIMARY OSTEOARTHRITIS AND DISEASES WITH EXOCRINE PANCREATIC INSUFFICIENCY. Georgian medical news 2021, 57–62. [Google Scholar]
- Hao, W.R.; Yang, T.L.; Lai, Y.H.; Lin, K.J.; Fang, Y.A.; Chen, M.Y.; Hsu, M.H.; Chiu, C.C.; Yang, T.Y.; Chen, C.C. , et al. The Association between Influenza Vaccine and Risk of Chronic Kidney Disease/Dialysis in Patients with Hypertension. Vaccines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Hsin, P.Y.; Hsiao, Y.C.; Chen, B.J.; Zhuang, Z.Y.; Lee, C.W.; Lee, W.J.; Vo, T.T.T.; Tseng, C.F.; Tseng, S.F. , et al. A Narrative Review: Repurposing Metformin as a Potential Therapeutic Agent for Oral Cancer. Cancers 2024, 16. [Google Scholar] [CrossRef]
- Fert, A.; Richard, J.; Raymond Marchand, L.; Planas, D.; Routy, J.P.; Chomont, N.; Finzi, A.; Ancuta, P. Metformin facilitates viral reservoir reactivation and their recognition by anti-HIV-1 envelope antibodies. iScience 2024, 27, 110670. [Google Scholar] [CrossRef]
- Yang, A.; Shi, M.; Wu, H.; Lau, E.S.H.; Ma, R.C.W.; Kong, A.P.S.; So, W.Y.; Luk, A.O.Y.; Chan, J.C.N.; Chow, E. Long-term metformin use and risk of pneumonia and related death in type 2 diabetes: a registry-based cohort study. Diabetologia 2021, 64, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, M.; Boguszewska, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The role of AMPK in metabolism and its influence on DNA damage repair. Molecular biology reports 2020, 47, 9075–9086. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Díaz-Pozo, P.; Canet, F.; Díaz-Morales, N.; Abad-Jiménez, Z.; López-Domènech, S.; Vezza, T.; Apostolova, N.; Morillas, C.; Rocha, M. , et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox biology 2022, 53, 102342. [Google Scholar] [CrossRef]
- Jing, W.; Liu, C.; Su, C.; Liu, L.; Chen, P.; Li, X.; Zhang, X.; Yuan, B.; Wang, H.; Du, X. Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs. Frontiers in immunology 2023, 14, 1107670. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants (Basel, Switzerland) 2024, 13. [Google Scholar] [CrossRef]
- Chow, E.; Yang, A.; Chung, C.H.L.; Chan, J.C.N. A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer. Pharmaceuticals (Basel, Switzerland) 2022, 15. [Google Scholar] [CrossRef]
- Justice, J.N.; Gubbi, S.; Kulkarni, A.S.; Bartley, J.M.; Kuchel, G.A.; Barzilai, N. A geroscience perspective on immune resilience and infectious diseases: a potential case for metformin. GeroScience 2021, 43, 1093–1112. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Diabetes and coronavirus (SARS-CoV-2): Molecular mechanism of Metformin intervention and the scientific basis of drug repurposing. PLoS pathogens 2021, 17, e1009634. [Google Scholar] [CrossRef]
- Samuel, S.M.; Varghese, E.; Büsselberg, D. Therapeutic Potential of Metformin in COVID-19: Reasoning for Its Protective Role. Trends in microbiology 2021, 29, 894–907. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Vorobets, I.; Halabitska, I.; Kamyshnyi, O. Modulatory Roles of AHR, FFAR2, FXR, and TGR5 Gene Expression in Metabolic-Associated Fatty Liver Disease and COVID-19 Outcomes. Viruses 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Budarna, O.; Halabitska, I.; Petakh, P.; Kamyshnyi, O. Genomic insight into COVID-19 severity in MAFLD patients: a single-center prospective cohort study. Frontiers in genetics 2024, 15, 1460318. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Vari, S.G.; Kamyshnyi, A. Genetic Predictors of Comorbid Course of COVID-19 and MAFLD: A Comprehensive Analysis. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Pedrosa, A.R.; Martins, D.C.; Rizzo, M.; Silva-Nunes, J. Metformin in SARS-CoV-2 infection: A hidden path - from altered inflammation to reduced mortality. A review from the literature. Journal of diabetes and its complications 2023, 37, 108391. [Google Scholar] [CrossRef]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J.; et al. Metformin reduces SARS-CoV-2 in a Phase 3 Randomized Placebo Controlled Clinical Trial. medRxiv : the preprint server for health sciences, 2023. [Google Scholar] [CrossRef]
- Zhao, M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. International journal of antimicrobial agents 2020, 55, 105982. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, C.; Fei, Y.; Shen, M.; Zhou, Y.; Zhang, Y.; Zeng, X.; Zhang, S. Anti-inflammatory and immune therapy in severe coronavirus disease 2019 (COVID-19) patients: An update. Clinical immunology (Orlando, Fla.) 2022, 239, 109022. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Oksenych, V.; Kamyshnyi, O. Predictive analysis of osteoarthritis and chronic pancreatitis comorbidity: complications and risk factors. Frontiers in endocrinology 2024, 15, 1492741. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Putilin, D.A.; Evchenko, S.Y.; Fedoniuk, L.Y.; Tokarskyy, O.S.; Kamyshny, O.M.; Migenko, L.M.; Andreychyn, S.M.; Hanberher, I.I.; Bezruk, T.O. The Influence of Metformin to the Transcriptional Activity of the mTOR and FOX3 Genes in Parapancreatic Adipose Tissue of Streptozotocin-Induced Diabetic Rats. Journal of medicine and life 2020, 13, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J. , et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. The Lancet. Infectious diseases 2023, 23, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Kamyshnyi, O.; Matskevych, V.; Lenchuk, T.; Strilbytska, O.; Storey, K.; Lushchak, O. Metformin to decrease COVID-19 severity and mortality: Molecular mechanisms and therapeutic potential. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2021, 144, 112230. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Del Ángel, R.M.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Rodríguez-Carlos, A.; Trujillo-Paez, J.V.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; Rivas-Santiago, B. , et al. A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin. Microorganisms 2024, 12. [Google Scholar] [CrossRef]
- Wiernsperger, N.; Al-Salameh, A.; Cariou, B.; Lalau, J.D. Protection by metformin against severe Covid-19: An in-depth mechanistic analysis. Diabetes & metabolism 2022, 48, 101359. [Google Scholar] [CrossRef]
- Petrelli, F.; Grappasonni, I.; Nguyen, C.T.T.; Tesauro, M.; Pantanetti, P.; Xhafa, S.; Cangelosi, G. Metformin and Covid-19: a systematic review of systematic reviews with meta-analysis. Acta bio-medica : Atenei Parmensis 2023, 94, e2023138. [Google Scholar] [CrossRef]
- To, E.E.; Erlich, J.R.; Liong, F.; Luong, R.; Liong, S.; Esaq, F.; Oseghale, O.; Anthony, D.; McQualter, J.; Bozinovski, S. , et al. Mitochondrial Reactive Oxygen Species Contribute to Pathological Inflammation During Influenza A Virus Infection in Mice. Antioxidants & redox signaling 2020, 32, 929–942. [Google Scholar] [CrossRef]
- Silverii, G.A.; Fumagalli, C.; Rozzini, R.; Milani, M.; Mannucci, E.; Marchionni, N. Is Metformin Use Associated with a More Favorable COVID-19 Course in People with Diabetes? Journal of clinical medicine 2024, 13. [Google Scholar] [CrossRef]
- Somasundaram, M.; Mathew, S.K.; Paul, S.; Kurian, S.J.; Kunhikatta, V.; Karanth, S.; Shetty, S.; Kudru, C.U.; Manu, M.K.; Saravu, K. , et al. Metformin use and its association with various outcomes in COVID-19 patients with diabetes mellitus: a retrospective cohort study in a tertiary care facility. Annals of medicine 2024, 56, 2425829. [Google Scholar] [CrossRef]
- Lalau, J.D.; Al-Salameh, A.; Hadjadj, S.; Goronflot, T.; Wiernsperger, N.; Pichelin, M.; Allix, I.; Amadou, C.; Bourron, O.; Duriez, T. , et al. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes & metabolism 2021, 47, 101216. [Google Scholar] [CrossRef]
- Petakh, P.; Isevych, V.; Mohammed, I.; Loshak, K.; Poliak, I.; Kamyshnyi, O. Association between Use of Metformin and Insulin with Hematological Parameters in COVID-19 Patients with Type 2 Diabetes: A Single Center, Cross-Sectional Study. Clinical Diabetology 2022, 11, 432–433. [Google Scholar] [CrossRef]
- Boulware, D.R.; Murray, T.A.; Proper, J.L.; Tignanelli, C.J.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Cohen, K.; Puskarich, M.A.; Belani, H.K. , et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination and Booster on Coronavirus Disease 2019 (COVID-19) Symptom Severity Over Time in the COVID-OUT Trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2023, 76, e1–e9. [Google Scholar] [CrossRef]
- Deaton, A.; Cartwright, N. Understanding and misunderstanding randomized controlled trials. Social science & medicine (1982) 2018, 210, 2–21. [Google Scholar] [CrossRef]
- Petakh, P.; Griga, V.; Mohammed, I.B.; Loshak, K.; Poliak, I.; Kamyshnyiy, A. Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes. Medical archives (Sarajevo, Bosnia and Herzegovina) 2022, 76, 329–332. [Google Scholar] [CrossRef]
- Malhotra, A.; Hepokoski, M.; McCowen, K.C.; J, Y.J.S. ACE2, Metformin, and COVID-19. iScience 2020, 23, 101425. [Google Scholar] [CrossRef]
- Wihandani, D.M.; Purwanta, M.L.A.; Mulyani, W.R.W.; Putra, I.; Supadmanaba, I.G.P. New-onset diabetes in COVID-19: The molecular pathogenesis. BioMedicine 2023, 13, 3–12. [Google Scholar] [CrossRef]
- Samuel, S.; Varghese, E.; Büsselberg, D. Therapeutic Potential of Metformin in COVID-19: Reasoning for Its Protective Role. Trends in microbiology 2021, 29. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, Z.; Wang, Q.; Chen, Y.; Lu, D.; Wu, W. COVID-19 and Diabetes: A Comprehensive Review of Angiotensin Converting Enzyme 2, Mutual Effects and Pharmacotherapy. Frontiers in endocrinology 2021, 12, 772865. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Kamyshna, I.; Oksenych, V.; Zavidniuk, N.; Kamyshnyi, A. The Intersection of COVID-19 and Metabolic-Associated Fatty Liver Disease: An Overview of the Current Evidence. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes. Viruses 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Miguel, V.; Rey-Serra, C.; Tituaña, J.; Sirera, B.; Alcalde-Estévez, E.; Herrero, J.I.; Ranz, I.; Fernández, L.; Castillo, C.; Sevilla, L. , et al. Enhanced fatty acid oxidation through metformin and baicalin as therapy for COVID-19 and associated inflammatory states in lung and kidney. Redox biology 2023, 68, 102957. [Google Scholar] [CrossRef]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacology & therapeutics 2018, 192, 150–162. [Google Scholar] [CrossRef]
- Sydorchuk, L.; Dzhuryak, V.; Sydorchuk, A.; Levytska, S.; Petrynych, V.; Knut, R.; Kshanovska, A.; Iftoda, O.; Tkachuk, O.; Kyfiak, P. , et al. The cytochrome 11B2 aldosterone synthase gene rs1799998 single nucleotide polymorphism determines elevated aldosterone, higher blood pressure, and reduced glomerular filtration, especially in diabetic female patients. Endocrine regulations 2020, 54, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Repchuk, Y.; Sydorchuk, L.P.; Sydorchuk, A.R.; Fedonyuk, L.Y.; Kamyshnyi, O.; Korovenkova, O.; Plehutsa, I.M.; Dzhuryak, V.S.; Myshkovskii, Y.M.; Iftoda, O.M. , et al. Linkage of blood pressure, obesity and diabetes mellitus with angiotensinogen gene (AGT 704T>C/rs699) polymorphism in hypertensive patients. Bratislavske lekarske listy 2021, 122, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes & metabolism 2020, 46, 423–426. [Google Scholar] [CrossRef]
- Petakh, P.; Oksenych, V.; Kamyshnyi, A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2023, 163, 114892. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Nykyforuk, A.; Yao, R.; Imbery, J.F.; Oksenych, V.; Korda, M.; Kamyshnyi, A. Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.; Kamyshnyi, A. Metformin Therapy Changes Gut Microbiota Alpha-Diversity in COVID-19 Patients with Type 2 Diabetes: The Role of SARS-CoV-2 Variants and Antibiotic Treatment. Pharmaceuticals (Basel, Switzerland) 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Kobyliak, N.; Kamyshnyi, A. Gut microbiota in patients with COVID-19 and type 2 diabetes: A culture-based method. Frontiers in cellular and infection microbiology 2023, 13, 1142578. [Google Scholar] [CrossRef] [PubMed]
- Asar, T.; Al-Abbasi, F.; Sheikh, R.; Zeyadi, M.; Nadeem, M.; Naqvi, S.; Kumar, V.; Anwar, F. Metformin’s dual impact on Gut microbiota and cardiovascular health: A comprehensive analysis. Biomedicine & Pharmacotherapy 2024, 178, 117128. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamyshnyi, A. Unveiling the potential pleiotropic effects of metformin in treating COVID-19: a comprehensive review. Frontiers in molecular biosciences 2023, 10, 1260633. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamyshnyi, A. Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: impact on inflammation markers. Inflammopharmacology 2024, 32, 885–891. [Google Scholar] [CrossRef]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J. , et al. Favorable Antiviral Effect of Metformin on SARS-CoV-2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of COVID-19. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2024, 79, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, Z.; Xiang, B.; Liu, J.; Geng, L.; Xu, D.; Zhan, M.; Xu, Y.; Zhang, B. Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism. Scientific reports 2024, 14, 12406. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; El Kholy, A.A.; El-Khateeb, E.; Alexiou, A.; Papadakis, M.; Elekhnawy, E.; Alsubaie, N.; Hamad, R.S.; Batiha, G.E. The potential therapeutic effect of metformin in type 2 diabetic patients with severe COVID-19. European review for medical and pharmacological sciences 2023, 27, 11445–11456. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Kamyshna, I.; Lyubomirskaya, K.; Moshynets, O.; Kobyliak, N.; Oksenych, V.; Kamyshnyi, A. Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: A meta-analysis. Frontiers in immunology 2023, 14, 1069894. [Google Scholar] [CrossRef] [PubMed]
- Kamyshnyi, A.; Koval, H.; Kobevko, O.; Buchynskyi, M.; Oksenych, V.; Kainov, D.; Lyubomirskaya, K.; Kamyshna, I.; Potters, G.; Moshynets, O. Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Buchynskyi, M.; Oksenych, V.; Kamyshna, I.; Kamyshnyi, O. Exploring Paxlovid Efficacy in COVID-19 Patients with MAFLD: Insights from a Single-Center Prospective Cohort Study. Viruses 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Huling, J.D.; Tignanelli, C.J.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Cohen, K.; Puskarich, M.A.; Belani, H.K.; Proper, J.L. , et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. The New England journal of medicine 2022, 387, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Lowe, J.R.; Bramante, C.T.; Shah, S.; Klatt, N.R.; Sherwood, N.; Aronne, L.; Puskarich, M.; Tamariz, L.; Palacio, A. , et al. Metformin and Covid-19: Focused Review of Mechanisms and Current Literature Suggesting Benefit. Frontiers in endocrinology 2021, 12, 587801. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadian, M.; Nejadghaderi, S.A.; Zahmatkesh, M.M.; Hajikhani, B.; Mirsaeidi, M.; Nasiri, M.J. The Efficacy and Potential Mechanisms of Metformin in the Treatment of COVID-19 in the Diabetics: A Systematic Review. Frontiers in endocrinology 2021, 12, 645194. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Randall, M.D. Does metformin affect outcomes in COVID-19 patients with new or pre-existing diabetes mellitus? A systematic review and meta-analysis. British journal of clinical pharmacology 2022, 88, 2642–2656. [Google Scholar] [CrossRef]
- Sardu, C.; Marfella, R.; Prattichizzo, F.; La Grotta, R.; Paolisso, G.; Ceriello, A. Effect of Hyperglycemia on COVID-19 Outcomes: Vaccination Efficacy, Disease Severity, and Molecular Mechanisms. Journal of clinical medicine 2022, 11. [Google Scholar] [CrossRef]
- Han, K.; Fyles, A.; Shek, T.; Croke, J.; Dhani, N.; D'Souza, D.; Lee, T.Y.; Chaudary, N.; Bruce, J.; Pintilie, M. , et al. A Phase II Randomized Trial of Chemoradiation with or without Metformin in Locally Advanced Cervical Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2022, 28, 5263–5271. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Hubbard, A.E.; Gutiérrez, J.P.; Pimpale, G.; Juárez-Flores, A.; Ghosh, R.; de Jesús Ascencio-Montiel, I.; Bertozzi, S.M. Estimating the effect of realistic improvements of metformin adherence on COVID-19 mortality using targeted machine learning. Global epidemiology 2024, 7, 100142. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, T.D. Coordination chemistry suggests that independently observed benefits of metformin and Zn(2+) against COVID-19 are not independent. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 2024, 37, 983–1022. [Google Scholar] [CrossRef]
- Harmon, D.C.; Levene, J.A.; Rutlen, C.L.; White, E.S.; Freeman, I.R.; Lapidus, J.A. Preadmission Metformin Use Is Associated with Reduced Mortality in Patients with Diabetes Mellitus Hospitalized with COVID-19. Journal of general internal medicine, 2024. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Bronowicka-Szydełko, A.; Rabczyński, M.; Bednarska-Chabowska, D.; Adamiec-Mroczek, J.; Doroszko, A.; Trocha, M.; Kujawa, K.; Matera-Witkiewicz, A.; Kuźnik, E. , et al. Insulin and Metformin Administration: Unravelling the Multifaceted Association with Mortality across Various Clinical Settings Considering Type 2 Diabetes Mellitus and COVID-19. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Zaongo, S.D.; Chen, Y. Metformin may be a viable adjunctive therapeutic option to potentially enhance immune reconstitution in HIV-positive immunological non-responders. Chinese medical journal 2023, 136, 2147–2155. [Google Scholar] [CrossRef]
- Babinets, L.; Halabitska, I. Chronic inflammatory process and bone tissue changes in patients with osteoarthritis and exocrine pancreatic insufficiency. Lekarsky Obzor 2020, 69, 7–10. [Google Scholar]
- Chew, G.M.; Padua, A.J.P.; Chow, D.C.; Souza, S.A.; Clements, D.M.; Corley, M.J.; Pang, A.P.S.; Alejandria, M.M.; Gerschenson, M.; Shikuma, C.M. , et al. Effects of Brief Adjunctive Metformin Therapy in Virologically Suppressed HIV-Infected Adults on Polyfunctional HIV-Specific CD8 T Cell Responses to PD-L1 Blockade. AIDS research and human retroviruses 2021, 37, 24–33. [Google Scholar] [CrossRef]
- Pollak, M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia 2017, 60, 1662–1667. [Google Scholar] [CrossRef]
- Hasanvand, A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: a new perspective for treatment and prevention of diseases. Inflammopharmacology 2022, 30, 775–788. [Google Scholar] [CrossRef]
- Choi, Y.K.; Park, K.G. Metabolic roles of AMPK and metformin in cancer cells. Molecules and cells 2013, 36, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Woldeyohanis, A.E.; Demeke, C.A. A review on protective roles and potential mechanisms of metformin in diabetic patients diagnosed with COVID-19. Metabolism open 2021, 12, 100137. [Google Scholar] [CrossRef]
- Schuiveling, M.; Vazirpanah, N.; Radstake, T.; Zimmermann, M.; Broen, J. Metformin, A New Era for an Old Drug in the Treatment of Immune Mediated Disease? Current drug targets 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ding, Y.; Yu, X.; Ma, D.; Yang, B.; Li, Y.; Huang, L.; Chen, Z.; Zheng, J.; Yang, C. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. American journal of translational research 2019, 11, 2393–2402. [Google Scholar] [PubMed]
- Smigiel, K.; Srivastava, S.; Stolley, J.; Campbell, D. Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunological reviews 2014, 259. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, M.A.; Ancuta, P.; Gilmore, N.; Routy, J.P. Regulatory T cells in HIV infection: can immunotherapy regulate the regulator? Clinical & developmental immunology 2012, 2012, 908314. [Google Scholar] [CrossRef]
- Esmail Nia, G.; Mohammadi, M.; Sharifizadeh, M.; Ghalamfarsa, G.; Bolhassani, A. The role of T regulatory cells in the immunopathogenesis of HIV: Clinical implications. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases 2024, 28, 103866. [Google Scholar] [CrossRef]
- Bai, B.; Chen, H. Metformin: A Novel Weapon Against Inflammation. Frontiers in pharmacology 2021, 12, 622262. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Hu, K.; Tang, Y.; Zeng, X.; Liu, J.; Xu, J. Effects of metformin treatment on serum levels of C-reactive protein and interleukin-6 in women with polycystic ovary syndrome: a meta-analysis: A PRISMA-compliant article. Medicine 2017, 96, e8183. [Google Scholar] [CrossRef] [PubMed]
- Obare, L.M.; Temu, T.; Mallal, S.A.; Wanjalla, C.N. Inflammation in HIV and Its Impact on Atherosclerotic Cardiovascular Disease. Circulation research 2024, 134, 1515–1545. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Cao, W.; Li, T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. Journal of immunology research 2021, 2021, 7316456. [Google Scholar] [CrossRef]
- Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Marette, A.; Routy, B.; Chen, Y.; Routy, J.P. Metformin effect on gut microbiota: insights for HIV-related inflammation. AIDS research and therapy 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. The lancet. HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef]
- Topol, I.; Kamyshny, A. Study of expression of TLR2, TLR4 and transckription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian medical news 2013, 115–122. [Google Scholar]
- MacCann, R.; Landay, A.L.; Mallon, P.W.G. HIV and comorbidities - the importance of gut inflammation and the kynurenine pathway. Current opinion in HIV and AIDS 2023, 18, 102–110. [Google Scholar] [CrossRef]
- Ponte, R.; Mehraj, V.; Ghali, P.; Couëdel-Courteille, A.; Cheynier, R.; Routy, J.P. Reversing Gut Damage in HIV Infection: Using Non-Human Primate Models to Instruct Clinical Research. EBioMedicine 2016, 4, 40–49. [Google Scholar] [CrossRef]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Topol, I.A.; Kamyshny, A.M.; Abramov, A.V.; Kolesnik, Y.M. Expression of XBP1 in lymphocytes of the small intestine in rats under chronic social stress and modulation of intestinal microflora composition. Fiziolohichnyi zhurnal (Kiev, Ukraine : 1994) 2014, 60, 38–44. [Google Scholar] [CrossRef]
- Rezaei, S.; Timani, K.; He, J. Metformin Treatment Leads to Increased HIV Transcription and Gene Expression through Increased CREB Phosphorylation and Recruitment to the HIV LTR Promoter. Aging and disease 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Mazzuti, L.; Turriziani, O.; Mezzaroma, I. The Many Faces of Immune Activation in HIV-1 Infection: A Multifactorial Interconnection. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Patankar, V.; Kitchen, S.; Zhen, A. Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection. Viruses 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences 2014, 19, 658–664. [Google Scholar]
- Routy, J.-P.; Isnard, S.; Mehraj, V.; Ostrowski, M.; Chomont, N.; Ancuta, P.; Ponte, R.; Planas, D.; Dupuy, F.; Angel, J. Effect of metformin on the size of the HIV reservoir in non-diabetic ART-treated individuals: Single-arm non-randomised Lilac pilot study protocol. BMJ Open 2019, 9, e028444. [Google Scholar] [CrossRef]
- Nelson, A.G.; Zhang, X.; Ganapathi, U.; Szekely, Z.; Flexner, C.W.; Owen, A.; Sinko, P.J. Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. Journal of controlled release : official journal of the Controlled Release Society 2015, 219, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.R.; Adams, J.L.; Kashuba, A.D. HIV PrEP Trials: The Road to Success. Clinical investigation 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.; Lambotte, O.; Lamplough, R.; Ndung'u, T.; Sugarman, J. , et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nature medicine 2021, 27, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Kristófi, R.; Eriksson, J. Metformin as an anti-inflammatory agent: A short review. Journal of Endocrinology 2021, 251. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Babinets, L.; Oksenych, V.; Kamyshnyi, O. Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- McCabe, L.; Burns, J.E.; Latifoltojar, A.; Post, F.A.; Fox, J.; Pool, E.; Waters, A.; Santana, B.; Garvey, L.; Johnson, M. , et al. MAVMET trial: maraviroc and/or metformin for metabolic dysfunction associated fatty liver disease in adults with suppressed HIV. AIDS (London, England) 2024, 38, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- McCrea, J.B.; Patel, M.; Liu, Y.; Vargo, R.; Witter, R.; Litovsky, A.; Stoch, S.A.; Iwamoto, M.; Matthews, R.P. Pharmacokinetics of Atorvastatin and Metformin after Coadministration with Islatravir in Healthy Adults. Journal of clinical pharmacology, 2024. [Google Scholar] [CrossRef]
- Corley, M.J.; Pang, A.P.S.; Shikuma, C.M.; Ndhlovu, L.C. Cell-type specific impact of metformin on monocyte epigenetic age reversal in virally suppressed older people living with HIV. Aging cell 2024, 23, e13926. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Miao, X.; Taskar, K.; Magee, M.; Gorycki, P.; Moore, K.; Tai, G. No dose adjustment of metformin or substrates of organic cation transporters (OCT)1 and OCT2 and multidrug and toxin extrusion protein (MATE)1/2K with fostemsavir coadministration based on modeling approaches. Pharmacology research & perspectives 2024, 12, e1238. [Google Scholar] [CrossRef]
- Mhlanga, N.L.; Netangaheni, T.R. Interventions for Type 2 Diabetes reduction among older people living with HIV in Harare. South African family practice : official journal of the South African Academy of Family Practice/Primary Care 2024, 66, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Hurbans, N.; Naidoo, P. Comorbidity and concomitant medication use in an integrase strand transfer inhibitor naïve cohort on first-line dolutegravir-based antiretroviral therapy. The Pan African medical journal 2024, 47, 137. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Omata, M. Hepatitis C virus and hepatocellular carcinoma. Biology 2013, 2, 304–316. [Google Scholar] [CrossRef]
- Coppola, N.; Vatiero, L.M.; Sagnelli, E. HCV genotype 2 as a risk factor for reactivation of chronic HCV infection. Gut 2005, 54, 1207. [Google Scholar] [CrossRef]
- Babinets, L.S.; Shaihen, O.R.; Homyn, H.O.; Halabitska, I.M. Specific aspects of clinical course in case of combination of chronic pancreatitis and concomitant viral hepatitis C. Wiadomosci lekarskie (Warsaw, Poland : 1960) 2019, 72, 595–599. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Ferraro, D.; Carbone, G.; Frampton, A.E.; Vennarecci, G.; Kykalos, S.; Schizas, D.; Theocharis, S.; Machairas, N. The Emerging Role of Metformin in the Treatment of Hepatocellular Carcinoma: Is There Any Value in Repurposing Metformin for HCC Immunotherapy? Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y. Metformin and statins and their role in reducing hepatocellular carcinoma risk: Randomized trials are needed: Editorial on "Metformin and statins reduce hepatocellular carcinoma risk in chronic hepatitis C patients with failed antiviral therapy". Clinical and molecular hepatology 2024, 30, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.; Sutter, A.; Khemka, S.; Songtanin, B.; Nichols, J.; Nugent, K. Metformin as adjuvant treatment in hepatitis C virus infections and associated complications. The American journal of the medical sciences 2024, 368, 90–98. [Google Scholar] [CrossRef]
- Leslie, J.; Geh, D.; Elsharkawy, A.M.; Mann, D.A.; Vacca, M. Metabolic dysfunction and cancer in HCV: Shared pathways and mutual interactions. Journal of hepatology 2022, 77, 219–236. [Google Scholar] [CrossRef]
- Stephenne, X.; Foretz, M.; Taleux, N.; van der Zon, G.C.; Sokal, E.; Hue, L.; Viollet, B.; Guigas, B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 2011, 54, 3101–3110. [Google Scholar] [CrossRef]
- Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Marchand-Brustel, Y.; Auberger, P.; Tanti, J.-F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer research 2011, 71, 4366–4372. [Google Scholar] [CrossRef]
- Suhail, M.; Sohrab, S.S.; Kamal, M.A.; Azhar, E.I. Role of hepatitis c virus in hepatocellular carcinoma and neurological disorders: an overview. Frontiers in oncology 2022, 12, 913231. [Google Scholar] [CrossRef] [PubMed]
- Ferrín, G.; Guerrero, M.; Amado, V.; Rodríguez-Perálvarez, M.; De la Mata, M. Activation of mTOR Signaling Pathway in Hepatocellular Carcinoma. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J. , et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell metabolism 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, W.; Jin, H.; Wang, X. mTOR in viral hepatitis and hepatocellular carcinoma: function and treatment. BioMed research international 2014, 2014, 735672. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World journal of gastroenterology 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Maiers, J.L.; Chakraborty, S. The Cellular, Molecular, and Pathologic Consequences of Stress on the Liver. The American journal of pathology 2023, 193, 1353–1354. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M.; Wroński, S.; Tarasiuk, J.; Maredziak, M. Metformin Decreases Reactive Oxygen Species, Enhances Osteogenic Properties of Adipose-Derived Multipotent Mesenchymal Stem Cells In Vitro, and Increases Bone Density In Vivo. Oxidative medicine and cellular longevity 2016, 2016, 9785890. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell death & disease 2022, 13, 29. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Hammerstad, S.S.; Grock, S.F.; Lee, H.J.; Hasham, A.; Sundaram, N.; Tomer, Y. Diabetes and Hepatitis C: A Two-Way Association. Frontiers in endocrinology 2015, 6, 134. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Guaraldi, G.; Nascimbeni, F.; Romagnoli, D.; Zona, S.; Targher, G. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease - Evidence from three different disease models: NAFLD, HCV and HIV. World journal of gastroenterology 2016, 22, 9674–9693. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Rinaldi, L.; Guerrera, B.; Restivo, L.; Marrone, A.; Giordano, M.; Zampino, R. NAFLD and NASH in HCV Infection: Prevalence and Significance in Hepatic and Extrahepatic Manifestations. International journal of molecular sciences 2016, 17. [Google Scholar] [CrossRef]
- Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D'Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F. , et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14. [Google Scholar] [CrossRef]
- Hyun, B.; Shin, S.; Lee, A.; Lee, S.; Song, Y.; Ha, N.J.; Cho, K.H.; Kim, K. Metformin Down-regulates TNF-α Secretion via Suppression of Scavenger Receptors in Macrophages. Immune network 2013, 13, 123–132. [Google Scholar] [CrossRef]
- Hegazy, W.A.H.; Rajab, A.A.H.; Abu Lila, A.S.; Abbas, H.A. Anti-diabetics and antimicrobials: Harmony of mutual interplay. World journal of diabetes 2021, 12, 1832–1855. [Google Scholar] [CrossRef]
- Liu, X.; Yu, P.; Xu, Y.; Wang, Y.; Chen, J.; Tang, F.; Hu, Z.; Zhou, J.; Liu, L.; Qiu, W. , et al. Metformin induces tolerogenicity of dendritic cells by promoting metabolic reprogramming. Cellular and molecular life sciences : CMLS 2023, 80, 283. [Google Scholar] [CrossRef]
- Urbanowicz, A.; Zagożdżon, R.; Ciszek, M. Modulation of the Immune System in Chronic Hepatitis C and During Antiviral Interferon-Free Therapy. Archivum immunologiae et therapiae experimentalis 2019, 67, 79–88. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Q.; Ling, L.P.; Wu, Y.; Meng, D.X.; Li, X.; Zhang, C.Q. Metformin attenuates motility, contraction, and fibrogenic response of hepatic stellate cells in vivo and in vitro by activating AMP-activated protein kinase. World journal of gastroenterology 2018, 24, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Liu, S.; Zhang, Y.; Shen, D.; Wang, P.; Dang, X. Metformin ameliorates liver fibrosis induced by congestive hepatopathy via the mTOR/HIF-1α signaling pathway. Annals of hepatology 2023, 28, 101135. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ma, J.; Dong, L.; Zhu, C.; Zhang, J.; Li, J. Metformin exerts anti-liver fibrosis effect based on the regulation of gut microbiota homeostasis and multi-target synergy. Heliyon 2024, 10, e24610. [Google Scholar] [CrossRef]
- Tsai, P.C.; Kuo, H.T.; Hung, C.H.; Tseng, K.C.; Lai, H.C.; Peng, C.Y.; Wang, J.H.; Chen, J.J.; Lee, P.L.; Chien, R.N. , et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. Journal of hepatology 2023, 78, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Fofana, I.; Fafi-Kremer, S.; Baumert, T.F. Hepatitis C virus entry into hepatocytes: molecular mechanisms and targets for antiviral therapies. Journal of hepatology 2011, 54, 566–576. [Google Scholar] [CrossRef]
- Tsai, W.L.; Chang, T.H.; Sun, W.C.; Chan, H.H.; Wu, C.C.; Hsu, P.I.; Cheng, J.S.; Yu, M.L. Metformin activates type I interferon signaling against HCV via activation of adenosine monophosphate-activated protein kinase. Oncotarget 2017, 8, 91928–91937. [Google Scholar] [CrossRef] [PubMed]
- Shojaeian, A.; Nakhaie, M.; Amjad, Z.; Boroujeni, A.; Shokri, S.; Mahmoudvand, S. Leveraging metformin to combat hepatocellular carcinoma: its therapeutic promise against hepatitis viral infections. Journal of Cancer Metastasis and Treatment 2024, 10. [Google Scholar] [CrossRef]
- Zheng, J.; Woo, S.L.; Hu, X.; Botchlett, R.; Chen, L.; Huo, Y.; Wu, C. Metformin and metabolic diseases: a focus on hepatic aspects. Frontiers of medicine 2015, 9, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Guan, Q.; Shi, J.S.; Xu, Z.H.; Geng, Y. Metformin alleviates liver fibrosis in mice by enriching Lactobacillus sp. MF-1 in the gut microbiota. Biochimica et biophysica acta. Molecular basis of disease 2023, 1869, 166664. [Google Scholar] [CrossRef] [PubMed]
- Pavlo, P.; Kamyshna, I.; Kamyshnyi, A. Effects of metformin on the gut microbiota: A systematic review. Molecular metabolism 2023, 77, 101805. [Google Scholar] [CrossRef]
- Padilha, M.D.M.; Melo, F.T.V.; Laurentino, R.V.; da Silva, A.; Feitosa, R.N.M. Dysregulation in the microbiota by HBV and HCV infection induces an altered cytokine profile in the pathobiome of infection. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases 2024, 29, 104468. [Google Scholar] [CrossRef]
- Schwenger, K.J.; Clermont-Dejean, N.; Allard, J.P. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP reports : innovation in hepatology 2019, 1, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Song, H.; Zhang, X.; Song, G.; Wang, Y.; Ding, X.; Duan, X.; Li, L.; Sun, T.; Kan, Q. Metformin attenuated sepsis-related liver injury by modulating gut microbiota. Emerging microbes & infections 2022, 11, 815–828. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Internal and emergency medicine 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing intestinal barrier efficiency: A novel metabolic diseases therapy. Frontiers in nutrition 2023, 10, 1120168. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Cong, B. Advances in the mechanism of metformin with wide-ranging effects on regulation of the intestinal microbiota. Frontiers in microbiology 2024, 15, 1396031. [Google Scholar] [CrossRef]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert opinion on drug safety 2013, 12, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.; Ribaldone, D.G.; Saracco, G.M. Metformin and Hepatocellular Carcinoma Risk Reduction in Diabetic Patients with Chronic Hepatitis C: Fact or Fiction? Viruses 2023, 15. [Google Scholar] [CrossRef]
- Lin, D.; Reddy, V.; Osman, H.; Lopez, A.; Koksal, A.R.; Rhadhi, S.M.; Dash, S.; Aydin, Y. Additional Inhibition of Wnt/β-Catenin Signaling by Metformin in DAA Treatments as a Novel Therapeutic Strategy for HCV-Infected Patients. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Shahbaz, A.; Umair, M.; Sachmechi, I. Treatment of Hepatitis C with Sofosbuvir, Velpatasvir and Voxilaprevir Decreases Hemoglobin A1c and Dependence on Anti-Glycemic Medications. Biomedical Journal of Scientific & Technical Research 2018, 09. [Google Scholar] [CrossRef]
- Rosenthal, E.S.; Kottilil, S.; Polis, M.A. Sofosbuvir and ledipasvir for HIV/HCV co-infected patients. Expert opinion on pharmacotherapy 2016, 17, 743–749. [Google Scholar] [CrossRef]
- Zhou, J.; Ke, Y.; Lei, X.; Wu, T.; Li, Y.; Bao, T.; Tang, H.; Zhang, C.; Wu, X.; Wang, G. , et al. Meta-analysis: The efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Annals of hepatology 2020, 19, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Iwama, H.; Miyoshi, H.; Tani, J.; Oura, K.; Tadokoro, T.; Sakamoto, T.; Nomura, T.; Morishita, A.; Yoneyama, H. , et al. Diabetes mellitus and metformin in hepatocellular carcinoma. World journal of gastroenterology 2016, 22, 6100–6113. [Google Scholar] [CrossRef]
- Shimada, S.; Kamiyama, T.; Orimo, T.; Nagatsu, A.; Kamachi, H.; Taketomi, A. High HbA1c is a risk factor for complications after hepatectomy and influences for hepatocellular carcinoma without HBV and HCV infection. Hepatobiliary surgery and nutrition 2021, 10, 454–463. [Google Scholar] [CrossRef]
- Berk, J.; Lorigiano, T.J.; Sulkowski, M.; Mixter, S. REPLACING INSULIN WITH ANTI-VIRALS: A CLINICAL VIGNETTE ON DIABETES AND HCV TREATMENT. AACE clinical case reports 2020, 6, e59–e61. [Google Scholar] [CrossRef]
- Abdel Monem, M.S.; Farid, S.F.; Abbassi, M.M.; Youssry, I.; Andraues, N.G.; Hassany, M.; Selim, Y.M.M.; El-Sayed, M.H. The potential hepatoprotective effect of metformin in hepatitis C virus-infected adolescent patients with beta thalassemia major: Randomised clinical trial. International journal of clinical practice 2021, 75, e14104. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Pelusi, S.; Aghemo, A.; Gritti, S.; Pasulo, L.; Bianco, C.; Iegri, C.; Cologni, G.; Degasperi, E.; D'Ambrosio, R. , et al. Dysmetabolism, Diabetes and Clinical Outcomes in Patients Cured of Chronic Hepatitis C: A Real-Life Cohort Study. Hepatology communications 2022, 6, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escaja, C.; C, Á.N.; González-Diéguez, L.; Cadahía, V.; Varela, M.; de Jorge, M.; Castaño-García, A.; Rodríguez, M. Diabetes is not associated with an increased risk of hepatocellular carcinoma in patients with alcoholic or hepatitis C virus cirrhosis. Revista espanola de enfermedades digestivas 2021, 113, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, M.L.; Vieira, C.P.; Caris, J.A.; Marques, M.P.; Rocha, A.; Paz, T.A.; Rezende, R.E.F.; Lanchote, V.L. Liver Fibrosis Stages Affect Organic Cation Transporter 1/2 Activities in Hepatitis C Virus-Infected Patients. Pharmaceuticals (Basel, Switzerland) 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Wong, K.; Ravindranayagam, N.; Tang, L.; Grace, J.; Wong, D.; Con, D.; Sinclair, M.; Majumdar, A.; Kutaiba, N. , et al. Statin, aspirin and metformin use and risk of hepatocellular carcinoma related outcomes following liver transplantation: A retrospective study. World journal of transplantation 2024, 14, 94914. [Google Scholar] [CrossRef]
- Campbell, C.; Wang, T.; McNaughton, A.L.; Barnes, E.; Matthews, P.C. Risk factors for the development of hepatocellular carcinoma (HCC) in chronic hepatitis B virus (HBV) infection: a systematic review and meta-analysis. Journal of viral hepatitis 2021, 28, 493–507. [Google Scholar] [CrossRef]
- Zhou, S.N.; Zhang, N.; Liu, H.H.; Xia, P.; Zhang, C.; Song, J.W.; Fan, X.; Shi, M.; Jin, L.; Zhang, J.Y. , et al. Skewed CD39/CD73/adenosine pathway contributes to B-cell hyperactivation and disease progression in patients with chronic hepatitis B. Gastroenterology report 2021, 9, 49–58. [Google Scholar] [CrossRef]
- Arbuthnot, P.; Kew, M. Hepatitis B virus and hepatocellular carcinoma. International journal of experimental pathology 2001, 82, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Aloysius, M.M.; Sharma, N.R.; Pallav, K. Advances in treatment and prevention of hepatitis B. World journal of gastrointestinal pharmacology and therapeutics 2021, 12, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.H.; Zhang, Y.J.; Pan, Q.C.; Mao, R.C.; Qin, Y.L.; Liu, H.Y.; Zhang, Y.M.; Yu, Y.S.; Tang, Z.H.; Lu, M.J. , et al. Metformin inhibits hepatitis B virus protein production and replication in human hepatoma cells. Journal of viral hepatitis 2014, 21, 597–603. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, S.Y.; Seki, E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Seminars in liver disease 2019, 39, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Xiao, H. Metformin Actions on the Liver: Protection Mechanisms Emerging in Hepatocytes and Immune Cells against NASH-Related HCC. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Wu, T.; Wu, Y.; Shao, C.; Li, F.; Lin, Y.; Feng, S.; Wang, W.; Zhong, B. Insulin resistance exhibits varied metabolic abnormalities in nonalcoholic fatty liver disease, chronic hepatitis B and the combination of the two: a cross-sectional study. Diabetology & metabolic syndrome 2019, 11, 45. [Google Scholar] [CrossRef]
- Akter, S. Non-alcoholic Fatty Liver Disease and Steatohepatitis: Risk Factors and Pathophysiology. Middle East journal of digestive diseases 2022, 14, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Taniguchi, E.; Itou, M.; Sakata, M.; Sumie, S.; Sata, M. Insulin resistance and chronic liver disease. World journal of hepatology 2011, 3, 99–107. [Google Scholar] [CrossRef]
- García-Compeán, D.; Orsi, E.; Kumar, R.; Gundling, F.; Nishida, T.; Villarreal-Pérez, J.Z.; Del Cueto-Aguilera Á, N.; González-González, J.A.; Pugliese, G. Clinical implications of diabetes in chronic liver disease: Diagnosis, outcomes and management, current and future perspectives. World journal of gastroenterology 2022, 28, 775–793. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Leerapun, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut and liver 2021, 15, 827–840. [Google Scholar] [CrossRef]
- de Oliveira, S.; Houseright, R.A.; Graves, A.L.; Golenberg, N.; Korte, B.G.; Miskolci, V.; Huttenlocher, A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. Journal of hepatology 2019, 70, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, A.; Shokri-Naei, S.; Karbasforoushan, S.; Bahari, H.; Baradaran Rahimi, V.; Heidari, R.; Askari, V.R. Metformin beyond an anti-diabetic agent: A comprehensive and mechanistic review on its effects against natural and chemical toxins. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2023, 165, 115263. [Google Scholar] [CrossRef]
- Nevola, R.; Beccia, D.; Rosato, V.; Ruocco, R.; Mastrocinque, D.; Villani, A.; Perillo, P.; Imbriani, S.; Delle Femine, A.; Criscuolo, L. , et al. HBV Infection and Host Interactions: The Role in Viral Persistence and Oncogenesis. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef]
- Zheng, P.; Dou, Y.; Wang, Q. Immune response and treatment targets of chronic hepatitis B virus infection: innate and adaptive immunity. Frontiers in cellular and infection microbiology 2023, 13, 1206720. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Chen, Y.; Soong, L.; Chen, Y.; Cai, J.; Liang, Y.; Sun, J. Metformin Modulates T Cell Function and Alleviates Liver Injury Through Bioenergetic Regulation in Viral Hepatitis. Frontiers in immunology 2021, 12, 638575. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.; Park, S.Y.; Le, C.T.; Park, W.S.; Choi, D.H.; Cho, E.H. Metformin ameliorates activation of hepatic stellate cells and hepatic fibrosis by succinate and GPR91 inhibition. Biochemical and biophysical research communications 2018, 495, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lu, S.; Hou, C.; Ren, K.; Wang, M.; Liu, X.; Zhao, S.; Liu, X. Mitigation of liver fibrosis via hepatic stellate cells mitochondrial apoptosis induced by metformin. International immunopharmacology 2022, 108, 108683. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hou, C.; Wang, M.; Ren, K.; Zhou, D.; Liu, X.; Zhao, S.; Liu, X. Metformin induces mitochondrial fission and reduces energy metabolism by targeting respiratory chain complex I in hepatic stellate cells to reverse liver fibrosis. The international journal of biochemistry & cell biology 2023, 157, 106375. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Gao, P. Diabetes Mellitus and Risk of Hepatocellular Carcinoma. BioMed research international 2017, 2017, 5202684. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Ryan, J.D.; Tsochatzis, E.A. Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Annals of translational medicine 2017, 5, 40. [Google Scholar] [CrossRef]
- Yang, K.; Song, M. New Insights into the Pathogenesis of Metabolic-Associated Fatty Liver Disease (MAFLD): Gut-Liver-Heart Crosstalk. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Ouyang, J.; Zaongo, S.D.; Zhang, X.; Qi, M.; Hu, A.; Wu, H.; Chen, Y. Microbiota-Meditated Immunity Abnormalities Facilitate Hepatitis B Virus Co-Infection in People Living With HIV: A Review. Frontiers in immunology 2021, 12, 755890. [Google Scholar] [CrossRef]
- Rosell-Díaz, M.; Petit-Gay, A.; Molas-Prat, C.; Gallardo-Nuell, L.; Ramió-Torrentà, L.; Garre-Olmo, J.; Pérez-Brocal, V.; Moya, A.; Jové, M.; Pamplona, R. , et al. Metformin-induced changes in the gut microbiome and plasma metabolome are associated with cognition in men. Metabolism: clinical and experimental 2024, 157, 155941. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Díaz, M.; Fernández-Real, J.M. Metformin, Cognitive Function, and Changes in the Gut Microbiome. Endocrine reviews 2024, 45, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatology communications 2019, 3, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Lee, C.H.; Cheng, Y.D.; Gau, S.Y.; Tsai, T.H.; Chung, N.J.; Lee, C.Y. Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: A real-world cohort study. Frontiers in endocrinology 2022, 13, 1027484. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Zheng, Y.X.; Zhou, P.C.; Xiao, Y.N.; Tan, H.Z. Metformin use improves survival of diabetic liver cancer patients: systematic review and meta-analysis. Oncotarget 2016, 7, 66202–66211. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Koufakis, T.; Kalopitas, G.; Papadakos, S.P.; Kotsa, K.; Germanidis, G. Management of type 2 diabetes in patients with compensated liver cirrhosis: Short of evidence, plenty of potential. Diabetes & metabolic syndrome 2024, 18, 102935. [Google Scholar] [CrossRef]
- Huang, S.C.; Kao, J.-H. The interplay between chronic hepatitis B and diabetes mellitus: A narrative and concise review. The Kaohsiung journal of medical sciences 2023, 40. [Google Scholar] [CrossRef] [PubMed]
- Farfan Morales, C.; Cordero, C.; Osuna-Ramos, J.; Monroy Muñoz, I.; De Jesús-González, L.; Muñoz-Medina, J.; Hurtado Monzón, A.; Reyes-Ruiz, J.; Del Angel, R. The antiviral effect of metformin on zika and dengue virus infection. Scientific reports 2021, 11. [Google Scholar] [CrossRef]
- Poglitsch, M.; Weichhart, T.; Hecking, M.; Werzowa, J.; Katholnig, K.; Antlanger, M.; Krmpotic, A.; Jonjic, S.; Hörl, W.H.; Zlabinger, G.J. , et al. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2012, 12, 1458–1468. [Google Scholar] [CrossRef]
- Rampersad, S.; Tennant, P. Replication and Expression Strategies of Viruses. Viruses 2018, 55–82. [Google Scholar] [CrossRef]
- Combs, J.A.; Monk, C.H.; Harrison, M.A.A.; Norton, E.B.; Morris, C.A.; Sullivan, D.E.; Zwezdaryk, K.J. Inhibiting cytomegalovirus replication through targeting the host electron transport chain. Antiviral research 2021, 194, 105159. [Google Scholar] [CrossRef] [PubMed]
- Wong, M. Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomedical journal 2013, 36, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bachman, L.O.; Zwezdaryk, K.J. Targeting the Host Mitochondria as a Novel Human Cytomegalovirus Antiviral Strategy. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Li, H.; Ning, X.; Liu, H.; Chen, Y.; Ding, X.; Zhang, H.; Leng, S. Metformin suppressed human cytomegalovirus (hCMV) replication and its potential molecular mechanisms in human fibroblasts. The Journal of Immunology 2017, 198, 158.123–158.123. [Google Scholar] [CrossRef]
- Saavedra, D.; Añé-Kourí, A.L.; Barzilai, N.; Caruso, C.; Cho, K.H.; Fontana, L.; Franceschi, C.; Frasca, D.; Ledón, N.; Niedernhofer, L.J. , et al. Aging and chronic inflammation: highlights from a multidisciplinary workshop. Immunity & ageing : I & A 2023, 20, 25. [Google Scholar] [CrossRef]
- Nojima, I.; Eikawa, S.; Tomonobu, N.; Hada, Y.; Kajitani, N.; Teshigawara, S.; Miyamoto, S.; Tone, A.; Uchida, H.A.; Nakatsuka, A. , et al. Dysfunction of CD8 + PD-1 + T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Scientific reports 2020, 10, 14928. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, L.A.; Neiderhiser, L.; DeLuca, N.A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. Journal of virology 1998, 72, 3307–3320. [Google Scholar] [CrossRef]
- Movaqar, A.; Abdoli, A.; Aryan, E.; Jazaeri, E.O.; Meshkat, Z. Metformin promotes autophagy activity and constrains HSV-1 replication in neuroblastoma cells. Gene Reports 2021. [Google Scholar] [CrossRef]
- Vink, E.I.; Smiley, J.R.; Mohr, I. Subversion of Host Responses to Energy Insufficiency by Us3 Supports Herpes Simplex Virus 1 Replication during Stress. Journal of virology 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Berber, E.; Rouse, B.T. Controlling Herpes Simplex Virus-Induced Immunoinflammatory Lesions Using Metabolic Therapy: a Comparison of 2-Deoxy-d-Glucose with Metformin. Journal of virology 2022, 96, e0068822. [Google Scholar] [CrossRef]
- Nadhan, R.; Patra, D.; Krishnan, N.; Rajan, A.; Gopala, S.; Ravi, D.; Srinivas, P. Perspectives on mechanistic implications of ROS inducers for targeting viral infections. European journal of pharmacology 2021, 890, 173621. [Google Scholar] [CrossRef] [PubMed]
- Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Adamska, A. Examining the clinical relevance of metformin as an antioxidant intervention. Frontiers in pharmacology 2024, 15, 1330797. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Chanh, H.Q.; Tam, D.T.H.; Vuong, N.L.; Chau, N.T.X.; Chau, N.V.V.; Phong, N.T.; Trieu, H.T.; Luong Thi Hue, T.; Cao Thi, T. , et al. Metformin as adjunctive therapy for dengue in overweight and obese patients: a protocol for an open-label clinical trial (MeDO). Wellcome open research 2020, 5, 160. [Google Scholar] [CrossRef]
- Cheang, N.; Ting, H.; Koh, H.; Alonso, S. In vitro and in vivo efficacy of Metformin against dengue. Antiviral research 2021, 195, 105186. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X. , et al. Targeting the signaling in Epstein-Barr virus-associated diseases: mechanism, regulation, and clinical study. Signal transduction and targeted therapy 2021, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pablos, M.; Paiva, B.; Zabaleta, A. Epstein-Barr virus-acquired immunodeficiency in myalgic encephalomyelitis-Is it present in long COVID? Journal of translational medicine 2023, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Afzali, B.; Kazemian, M. EBV-associated diseases: Current therapeutics and emerging technologies. Frontiers in immunology 2022, 13, 1059133. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; You, C.; Meng, S.; Lai, Z.; Ai, W.; Zhang, J. EBV Infection and Its Regulated Metabolic Reprogramming in Nasopharyngeal Tumorigenesis. Frontiers in cellular and infection microbiology 2022, 12, 935205. [Google Scholar] [CrossRef]
- Adamson, A.L.; Le, B.T.; Siedenburg, B.D. Inhibition of mTORC1 inhibits lytic replication of Epstein-Barr virus in a cell-type specific manner. Virology journal 2014, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, C.; He, Z.; Cai, Y.; Chen, J. Metformin inhibits cervical cancer cell proliferation by modulating PI3K/Akt-induced major histocompatibility complex class I-related chain A gene expression. Journal of experimental & clinical cancer research : CR 2020, 39, 127. [Google Scholar] [CrossRef]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: shedding new lights on therapeutic repurposing. Journal of translational medicine 2023, 21, 403. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, M.J.; Song, S.O. Metformin and Cervical Cancer Risk in Patients with Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea. Endocrinology and metabolism (Seoul, Korea) 2022, 37, 929–937. [Google Scholar] [CrossRef]
- Curry, J.; Alnemri, A.; Philips, R.; Fiorella, M.; Sussman, S.; Stapp, R.; Solomides, C.; Harshyne, L.; South, A.; Luginbuhl, A. , et al. CD8+ and FoxP3+ T-Cell Cellular Density and Spatial Distribution After Programmed Death-Ligand 1 Check Point Inhibition. The Laryngoscope 2023, 133, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Rethinking about Metformin: Promising Potentials. Korean journal of family medicine 2024, 45, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Yoshida, K.; Suzuki, M.; Saito, I.; Kanegae, Y. Adenovirus-encoding virus-associated RNAs suppress HDGF gene expression to support efficient viral replication. PloS one 2014, 9, e108627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gomez-Manzano, C.; Rivera-Molina, Y.; Lang, F.F.; Conrad, C.A.; Fueyo, J. Oncolytic adenovirus research evolution: from cell-cycle checkpoints to immune checkpoints. Current opinion in virology 2015, 13, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Prusinkiewicz, M.; Tu, J.; Dodge, M.; MacNeil, K.; Radko-Juettner, S.; Fonseca, G.; Pelka, P.; Mymryk, J. Differential Effects of Human Adenovirus E1A Protein Isoforms on Aerobic Glycolysis in A549 Human Lung Epithelial Cells. Viruses 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clinical microbiology reviews 2014, 27, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; He, M.; Jung, J.U.; Lu, C.; Gao, S.J. Suppression of Kaposi's Sarcoma-Associated Herpesvirus Infection and Replication by 5'-AMP-Activated Protein Kinase. Journal of virology 2016, 90, 6515–6525. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.C.; Shao, S.L.; Jiang, X.J.; Xie, P.Y.; Sun, W.S.; Yu, T.F. Interactions between Autophagy and DNA Viruses. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, J.; Chen, H.; Liang, J.; Xie, C.; Gu, X.; Wang, R.; Mao, Z.; Zhang, Y.; Li, Q. , et al. Bmi-1-RING1B prevents GATA4-dependent senescence-associated pathological cardiac hypertrophy by promoting autophagic degradation of GATA4. Clinical and translational medicine 2022, 12, e574. [Google Scholar] [CrossRef] [PubMed]
- Poorghobadi, S.; Hosseini, S.Y.; Sadat, S.M.; Abdoli, A.; Irani, S.; Baesi, K. The Combinatorial Effect of Ad-IL-24 and Ad-HSV-tk/GCV on Tumor Size, Autophagy, and UPR Mechanisms in Multiple Myeloma Mouse Model. Biochemical genetics 2024. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Osuna-Ramos, J.F.; Monroy-Muñoz, I.E.; De Jesús-González, L.A.; Muñoz-Medina, J.E.; Hurtado-Monzón, A.M.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The antiviral effect of metformin on zika and dengue virus infection. Scientific reports 2021, 11, 8743. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Yi, P.; Baker, C.; Casey, G.; Xie, X.; Luo, H.; Cai, J.; Fan, X.; Soong, L. , et al. Metformin restrains ZIKV replication and alleviates virus-induced inflammatory responses in microglia. International immunopharmacology 2023, 121, 110512. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Suhail, H.; Arumugaswami, V.; Pellett, P.E.; Giri, S.; Kumar, A. AMP-Activated Protein Kinase Restricts Zika Virus Replication in Endothelial Cells by Potentiating Innate Antiviral Responses and Inhibiting Glycolysis. Journal of immunology (Baltimore, Md. : 1950) 2020, 204, 1810–1824. [Google Scholar] [CrossRef]
- Velazquez-Cervantes, M.A.; López-Ortega, O.; Cruz-Holguín, V.J.; Herrera Moro-Huitron, L.; Flores-Pliego, A.; Lara-Hernandez, I.; Comas-García, M.; Villavicencio-Carrisoza, O.; Helguera-Reppeto, A.C.; Arévalo-Romero, H. , et al. Metformin Inhibits Zika Virus Infection in Trophoblast Cell Line. Current microbiology 2024, 81, 133. [Google Scholar] [CrossRef] [PubMed]
- Cheang, Y.Z.N.; Ting, H.R.D.; Koh, H.Q.V.; Alonso, S. In vitro and in vivo efficacy of Metformin against dengue. Antiviral research 2021, 195, 105186. [Google Scholar] [CrossRef] [PubMed]
- Bonglack, E.N.; Messinger, J.E.; Cable, J.M.; Ch'ng, J.; Parnell, K.M.; Reinoso-Vizcaíno, N.M.; Barry, A.P.; Russell, V.S.; Dave, S.S.; Christofk, H.R. , et al. Monocarboxylate transporter antagonism reveals metabolic vulnerabilities of viral-driven lymphomas. Proceedings of the National Academy of Sciences of the United States of America 2021, 118. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Herrmann, A.L.; Däschle, A.; Kuhn, B.J.; Strobel, T.D.; Lohrey, C.; Bulkescher, J.; Krijgsveld, J.; Hoppe-Seyler, F. Effects of Metformin on the virus/host cell crosstalk in human papillomavirus-positive cancer cells. International journal of cancer 2021, 149, 1137–1149. [Google Scholar] [CrossRef]
- Hsu, A.T.; Hung, Y.C.; Fang, S.H.; D'Adamo, C.R.; Mavanur, A.A.; Svoboda, S.M.; Wolf, J.H. Metformin use and the risk of anal intraepithelial neoplasia in type II diabetic patients. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland 2021, 23, 3220–3226. [Google Scholar] [CrossRef] [PubMed]
- Veeramachaneni, R.; Yu, W.; Newton, J.M.; Kemnade, J.O.; Skinner, H.D.; Sikora, A.G.; Sandulache, V.C. Metformin generates profound alterations in systemic and tumor immunity with associated antitumor effects. Journal for immunotherapy of cancer 2021, 9. [Google Scholar] [CrossRef]
- Wilkie, M.D.; Anaam, E.A.; Lau, A.S.; Rubbi, C.P.; Vlatkovic, N.; Jones, T.M.; Boyd, M.T. Metabolic Plasticity and Combinatorial Radiosensitisation Strategies in Human Papillomavirus-Positive Squamous Cell Carcinoma of the Head and Neck Cell Lines. Cancers 2021, 13. [Google Scholar] [CrossRef]
- Sharma, S.; Munger, K. KDM6A-Mediated Expression of the Long Noncoding RNA DINO Causes TP53 Tumor Suppressor Stabilization in Human Papillomavirus 16 E7-Expressing Cells. Journal of virology 2020, 94. [Google Scholar] [CrossRef] [PubMed]
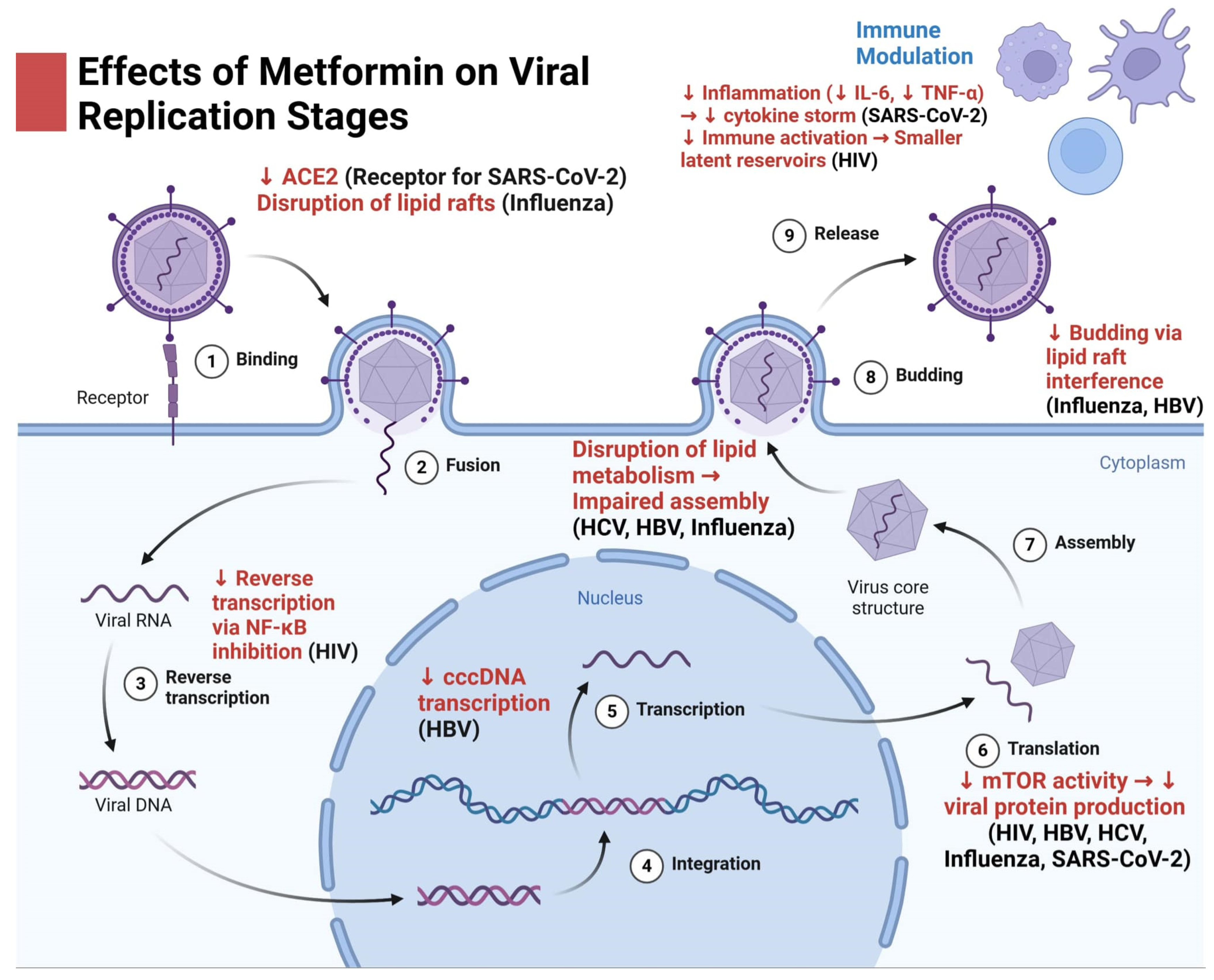
| Author and Year | Type of study | Key Findings |
| Fu-Shun Yen et al., 2022 [46] | Cohort Study | Pre-influenza vaccination metformin use in older adults with T2DM significantly reduced the risks of severe influenza-related complications and mortality, with greater benefits observed with longer usage. |
| Han Sol Lee et al., 2023 [23] | Experimental and Statistical Analysis | Metformin reduced Influenza A Virus -related cardiovascular risks by inhibiting viral replication and cytokine expression (MCP-1, IP-10) through AKT/MAPK signaling regulation. |
| Dominique E. Martin et al., 2023 [52] | Pilot Double-Blinded Placebo-Controlled Trial | Metformin may enhance immune resilience in older adults by improving specific flu vaccine responses and reducing markers of T cell exhaustion. |
| Elizabeth Greene et al., 2024 [48] | Retrospective Observational Study | Metformin use in diabetic patients significantly reduces the likelihood of hospitalization following an emergency department visit for influenza. |
| Tammy H. Cummings et al., 2022 [47] | Retrospective Cohort Study | Metformin use is associated with reduced influenza mortality in patients with obesity, likely due to its effects on T-cell function and immune response. |
| Paola Brandi et al., 2022 [50] | Experimental Study (Mouse Model) | The inactivated mucosal vaccine MV130 induces trained immunity, offering protection against viral respiratory infections, but this protection is negated by metformin. |
| Robert E. Brown et al., 2022 [56] | Case Study with Morphoproteomics | Morphoproteomic analysis suggests metformin and vitamin D3 could serve as adjunctive therapies to improve immune response and prevent severe outcomes in pulmonary H1N1 influenza. |
| Daniela Frasca et al., 2021 [82] | Experimental Study | Metformin improves B cell function and enhances antibody responses in elderly individuals with T2DM, supporting its potential as an anti-aging agent for immune function. |
| Wen-Rui Hao et al., 2023 [85] | Retrospective Study | Influenza vaccination reduces the risk of chronic kidney disease and the need for dialysis in patients with hypertension, with a dose-dependent protective effect observed across both influenza and non-influenza seasons. |
| Wipawee Saenwongsa et al., 2020 [79] | Observational Study | Metformin treatment in T2DM impairs the antibody response and interferon-alpha (IFN-α) expression following seasonal influenza vaccination, potentially hindering long-term protection. This finding suggests that the standard influenza vaccine may not be fully effective for T2DM patients and highlights the need for improved vaccine strategies for this group. |
| Aimin Yang et al., 2021 [88] | Cohort Study (Registry-based) | Long-term metformin use in T2DM individuals is associated with a lower risk of pneumonia hospitalisation and related mortality. |
| Author and Year | Type of study | Key Findings |
| Malhotra et al., 2020 [120] | Preclinical/Clinical | Metformin may enhance ACE2 expression, potentially offering cardiopulmonary protection in COVID-19 by regulating the renin-angiotensin-aldosterone system (RAAS). |
| Bramante et al., 2022 [144] | Randomized, placebo-controlled trial | No significant reduction in primary composite endpoint (hypoxemia, ED visit, hospitalization, or death) for metformin (OR 0.84, P = 0.19), ivermectin (OR 1.05, P = 0.78), or fluvoxamine (OR 0.94, P = 0.75). Secondary analysis showed metformin reduced ED visits, hospitalization, or death (OR 0.58, P = 0.02), but not significantly for ivermectin or fluvoxamine. |
| Pavlo Petakh et al., 2023 [133] | Observational Study | COVID-19 patients with T2DM have reduced gut microbiota alpha-diversity. |
| Jean-Daniel Lalau, Abdallah Al-Salameh, Samy Hadjadj, et al., 2021 [115] | Observational Study | Metformin use in patients with T2DM hospitalized for COVID-19 was associated with a lower 28-day mortality rate (16.0% vs 28.6%, P < 0.0001) and reduced odds of death (OR 0.710, 95% CI [0.537−0.938]) compared to non-users. |
| Pavlo Petakh et al., 2024 [137] | Single-center prospective observational study | Metformin therapy was associated with reduced expression of key genes (PRKAA1, SLC2A1, MTOR) involved in Th1/Th17 cell differentiation and inflammatory pathways. |
| Carolyn T Bramante et al., 2023 [107] | Randomised Phase 3 Trial | Metformin reduced long COVID incidence by 41% compared to placebo, with the greatest effect when started early. |
| Carolyn T Bramante et al., 2024 [138] | Randomised Clinical Trial | Metformin reduced SARS-CoV-2 viral load by 3.6-fold, hospitalizations by 58%, and long COVID by 42%. |
| Kathy Han et al., 2022 [149] | Phase II Randomised Trial | Metformin reduced cervical tumor hypoxia by 10.2% and improved 2-year disease-free survival to 67%. |
| David R Boulware et al., 2023 [117] | Secondary Analysis of RCT Data | Vaccine-boosted participants experienced the least severe and shortest-lasting COVID-19 symptoms (p < 0.001). |
| Pavlo Petakh et al., 2022 | Retrospective study | COVID-19 patients with T2DM who used metformin before hospitalization had significantly lower CRP levels, suggesting anti-inflammatory benefits.[119] |
| Claudia Ventura-López et al., 2022 [24] | In vitro study & Phase IIb RCT | Metformin glycinate inhibited viral replication in vitro without cytotoxicity and reduced viral load and oxygen needs in vivo. |
| Fabio Petrelli et al., 2023 [111] | Meta-analysis | Metformin use in diabetic patients with COVID-19 reduced the risk of severity, complications, and mortality compared to other treatments. |
| Giovanni Antonio Silverii et al., 2024 [113] | Retrospective Study | Metformin use was associated with a reduction in in-hospital mortality in people with diabetes, but the effect did not persist after adjusting for confounding factors using the COVID-19 Mortality Risk Score. |
| Pavlo Petakh et al., 2023 [131] | Observational study | The Firmicutes/Bacteroidetes (F/B) ratio in gut microbiota was higher in patients with both T2D and COVID-19. F/B ratio positively correlated with CRP levels, and metformin treatment modified this relationship. The F/B ratio may serve as a biomarker for inflammation. |
| Verónica Miguel et al., 2023 [126] | Experimental Study | Metformin and baicalin enhanced fatty acid oxidation, improving mitochondrial function, reducing inflammation, fibrosis, and improving outcomes in COVID-19 patients and animal models with lung and kidney damage. |
| Pavlo Petakh et al., 2023 [134] | Observational Study | T2D patients with COVID-19 showed increased Clostridium and Candida, and decreased Bifidobacterium and Lactobacillus. Metformin use without antibiotics increased Bacteroides and Lactobacillus, while decreasing Enterococcus and Clostridium. |
| Yongwang Hou et al., 2024 [139] | Bioinformatics and Preclinical Study | Metformin may treat COVID-19/LUAD by regulating glucose metabolism and key signaling pathways like AMPK and mTOR, inhibiting cell proliferation. |
| H M Al-Kuraishy et al., 2023 [140] | Prospective Cohort Study | Metformin was more effective than other diabetic treatments in reducing inflammation, oxidative stress, and improving radiological and clinical outcomes in T2DM patients with COVID-19. |
| Pavlo Petakh et al., 2024 [125] | Observational Study | Metformin modulates T-cell mRNA expression: FOXP3 (Treg marker) upregulated 1.96-fold, RORC (Th17 marker) downregulated 1.84-fold, and TBX21 (Th1 marker) downregulated 11.4-fold. Patients not using metformin showed dysregulated immune profiles. |
| Muhilvannan Somasundaram et al., 2024 [114] | Retrospective Cohort Study | Metformin use was associated with shorter hospitalization, reduced mortality risk, and improved levels of LDH, CRP, and D-dimer in COVID-19 patients with diabetes. |
| Sky Qiu et al., 2024 [150] | Retrospective Cohort Study | Improved adherence to metformin (by 5% or 10%) was associated with a reduction in mortality risk from COVID-19, with a 1.26% absolute decrease in risk for a 10% adherence increase. |
| Thomas D Lockwood, 2024 [151] | Coordination Chemistry Analysis | Metformin and Zn²⁺ are suggested to have a mechanistic relationship in improving COVID-19 outcomes. Metformin enhances Zn²⁺ bioavailability and coordination, which may synergistically inhibit viral proteases and reduce inflammation, potentially improving outcomes when used together. |
| David C Harmon et al., 2024 [152] | Retrospective cohort study | Pre-admission metformin use was associated with reduced in-hospital mortality, lower risk of ICU admission, and less need for mechanical ventilation in hospitalized COVID-19 patients with diabetes. The effect was particularly notable in reducing mortality from respiratory causes. |
| Łukasz Lewandowski et al., 2024 [153] | Retrospective cohort study | Insulin and metformin showed weak associations with mortality, but their interactions with other treatments and factors like remdesivir, low-molecular-weight heparin, age, and hsCRP influenced death risk. RDW-SD was strongly associated with mortality, with a significant increase in death risk with higher RDW-SD. |
| Author and Year | Type of study | Key Findings |
| Fert et al., 2024 [87] | Experimental study | Metformin decreased virion release, increased productively infected CD4lowHIV-p24+ T cells, enhanced tetherin and Bcl-2 expression, and improved recognition of infected cells by HIV-1 antibodies. |
| McCabe et al., 2024 [187] | Open-label, randomized trial | Neither maraviroc (MVC), metformin, nor their combination significantly reduced liver fat compared to ART alone in PWH with MAFLD. |
| Rezaei et al., 2024 | Experimental study | Metformin increased HIV transcription, gene expression, and production via CREB phosphorylation and recruitment to the HIV LTR promoter. |
| McCrea et al., 2024 [188] | Phase 1, open-label study | Coadministration of islatravir with atorvastatin and metformin did not have a clinically meaningful effect on the pharmacokinetics of either drug. |
| Corley et al., 2024 [189] | Retrospective analysis, randomized and single-arm trials | Metformin reduced epigenetic age in monocytes but not in CD8+ T cells, suggesting cell-type-specific effects. Larger studies are needed to validate findings. |
| Nguyen et al., 2024 [190] | Physiologically based pharmacokinetic (PBPK) modeling study | Fostemsavir (a gp120-directed attachment inhibitor) and its active moiety temsavir showed no clinically relevant impact on metformin concentrations or inhibition of OCT1, OCT2, MATE1/2K transporters. PBPK modeling confirmed no significant drug-drug interaction, supporting that no dose adjustment of metformin is required during coadministration with fostemsavir, despite initial in vitro data indicating potential transporter inhibition. |
| Mhlanga et al.,, 2024 [191] | Qualitative multi-method study | The study identified key interventions to reduce T2DM among older people living with HIV in Harare, including improved screening and health education. It also highlighted the use of metformin as a pharmacological intervention when lifestyle changes fail. |
| Hurbans et al., 2024 [192] | Prospective cohort study | Dolutegravir was generally safe and effective, but concomitant use of metformin led to increased blood glucose levels. Drug interactions were minimal, with only 0.7% of participants discontinuing dolutegravir due to interactions with supplements and antacids. Further investigation into dolutegravir-induced hyperglycemia is needed. |
| Author and Year | Type of study | Virus | Key Findings |
| Tsai et al., 2023 [222] | Cohort Study | HCV | Metformin significantly HCC risk in patients with diabetes and chronic hepatitis C after successful antiviral therapy. The 5-year cumulative HCC incidence was 10.9% in non-metformin users vs. 2.6% in metformin users. A risk model identified cirrhosis and T2DM non-metformin use as the most critical factors for HCC prediction. Metformin also reduced liver-related complications. |
| Shimada et al., 2021 [242] | Cohort Study | HCV | Patients with high HbA1c (≥7.0%) had worse overall survival (55% vs. 71%) and relapse-free survival (13 vs. 26 months) in NBNC-HCC. High HbA1c was also associated with increased postoperative complications. Metformin use was linked to better survival and recurrence outcomes. |
| Lin et al., 2021 [237] | Experimental study | HCV | Metformin inhibited Wnt/β-catenin signaling in chronic HCV-infected cells after DAA treatment, leading to decreased proliferation, increased apoptosis, and reversal of HCV-induced HCC. |
| Berk et al., 2020 [243] | Case study | HCV | Successful treatment of HCV led to significant improvement in glycemic control in a patient with uncontrolled T2DM, with HbA1c dropping from 11.6% to 5.7% without any other interventions, suggesting potential benefits of HCV treatment on insulin sensitivity. |
| Abdel Monem et al., 2021 [244] | Randomized clinical trial | HCV | Metformin used in HCV-infected adolescents with beta thalassemia major led to significant improvement in oxidative stress markers, liver fibrosis, and liver enzyme levels, suggesting its potential as a hepatoprotective agent. |
| Valenti et al., 2022 [245] | Cohort Study | HCV | In patients treated with direct-acting antivirals for HCV, higher BMI and diabetes were linked to advanced fibrosis. Diabetes was also associated with poor liver stiffness improvement and increased risk of de novo HCC and cardiovascular events. Statin use was protective, and metformin showed a protective association against HCC. |
| Rodríguez-Escaja et al., 2021 [246] | Cohort Study | HCV | In patients with alcoholic or HCV cirrhosis, diabetes was not a risk factor for developing HCC. No significant differences in HCC incidence were found between diabetic and non-diabetic patients, even after adjusting for co-factors and excluding metformin use. |
| Thomaz et al., 2024 [247] | Clinical Pharmacology Study | HCV | Liver fibrosis stages affected the in vivo activity of organic cation transporters (OCT1/2) in HCV-infected patients. Advanced fibrosis and cirrhosis were associated with a 25% reduction in OCT1/2 activity after achieving sustained virologic response. No significant changes were observed in the early stages of treatment. |
| Chung et al., 2024 [248] | Retrospective Study | HBV | In a retrospective study of liver transplant recipients for HCC, statin, aspirin, and metformin use did not show a statistically significant association with improved HCC-related outcomes (recurrence or mortality). The study suggests no benefit for these drugs in post-LT HCC recurrence prevention, indicating the need for further prospective, multicenter studies to clarify any potential benefit. |
| Campbell et al., 2021 [249] | Meta-Analysis | HBV | This meta-analysis found that T2DM is a significant risk factor for HCC in individuals with chronic HBV infection, increasing the hazard of HCC by over 25%. The association was weakened in studies adjusted for metformin use, suggesting that further research on the impact of antidiabetic drugs and glycemic control is needed. Enhanced screening for HCC in individuals with HBV and diabetes is recommended. |
| Zhou et al., 2020 [250] | Experimental Study | HBV | CD39 and CD73 expression on B-cells was reduced in chronic hepatitis B patients with high HBV DNA, HBeAg positivity, and active liver inflammation. This was linked to B-cell hyperactivation. Metformin reduced activation markers by regulating AMPK. Targeting the CD39/CD73/adenosine pathway using metformin could help reverse HBV-induced immune dysfunction. |
| Author and Year | Type of study | Virus | Key Findings |
| Chen et al., 2022 [313] | Experimental study on mice and human myocardium | CMV | Bmi-1-RING1B prevents GATA4-dependent senescence-associated pathological cardiac hypertrophy (SA-PCH) by promoting selective autophagic degradation of GATA4. Autophagy activators like metformin or rapamycin may serve as therapeutic options to prevent SA-PCH and cardiac dysfunction. |
| Combs et al., 2021 [283] | In vitro experimental study | CMV | CMV replication depends on functional host mitochondria, and drugs targeting the electron transport chain, such as metformin, inhibit viral replication. Repurposing metformin as an antiviral is promising due to its established safety profile and ability to reduce CMV titers. |
| Nojima et al., 2020 [288] | Experimental study | CMV | T2DM impairs the multifunctionality of CD8⁺ PD-1⁺ T cells and links metabolic dysfunction to immune suppression. Metformin restores CD8⁺ T cell function by enhancing glycolysis, improving cytokine production, and reducing tumor growth and viral susceptibility. |
| Poorghobadi et al., 2024 [314] | Mouse model experimental study | Herpes simplex virus 1 (HSV-1) | Ad-HSV-tk/GCV reduced tumor size and increased LC3B expression, promoting autophagy in multiple myeloma. Ad-IL-24 enhanced UPR gene expression but had a less pronounced effect on tumor reduction, and co-administration of Ad-HSV-tk and Ad-IL-24 showed no synergistic effect. |
| Berber & Rouse, 2022 [292] | Experimental study on HSV-1 in ocular infection | HSV-1 | Metformin and 2-deoxy-d-glucose (2DG) reduced herpetic stromal keratitis (HSK) severity, but 2DG increased the risk of herpetic encephalitis due to enhanced HSV reactivation. Metformin was safer, maintaining inflammatory cell functionality, including IFN-γ-producing Th1 and CD8 T cells in the trigeminal ganglion. |
| Farfan-Morales et al., 2021 [315] | In vitro and in vivo studies on DENV and ZIKV | Dengue virus (DENV), Zika virus (ZIKV), Yellow fever virus (YFV) | Metformin inhibited in vitro replication of DENV, ZIKV, and YFV, showing the strongest effect on DENV. MET reduced disease severity and increased survival in DENV-infected mice but failed to protect immunodeficient mice against ZIKV in vivo. |
| Wang et al., 2023 [316] | In vitro study on ZIKV infection in microglia | ZIKV | Metformin reduced ZIKV replication in microglia in a dose- and time-dependent manner. It modulated inflammatory responses, upregulating type I and III interferons (IFNα2, IFNβ1, IFNλ3) and downregulating ISGs like GBP4, OAS1, MX1, and ISG15. The findings suggest metformin may have therapeutic potential for ZIKV infection in microglia. |
| Singh et al., 2020 [317] | In vitro study on endothelial cells | ZIKV | The study explores how AMPK restricts ZIKV replication in endothelial cells. AMPK activation (via metformin or other activators) potentiates innate antiviral responses (e.g., IFNs, OAS2, ISG15) and inhibits glycolysis, which reduces viral replication. In contrast, inhibition of AMPK or increased glycolysis promoted virus replication. |
| Velazquez-Cervantes et al., 2024 [318] | In vitro study on trophoblast cell line | ZIKV | The study investigates the effects of metformin on ZIKV infection in a trophoblast cell line (JEG3). Metformin reduces viral replication and protein synthesis, reverses cytoskeletal changes, and reduces lipid droplet formation associated with the infection, suggesting metformin as a potential antiviral agent for ZIKV. |
| Cheang et al., 2021 [319] | In vitro and in vivo study | DENV | Metformin showed poor anti-DENV activity in vitro, with pro-DENV effects observed in certain cell lines (Vero cells). In vivo, oral administration of metformin did not reduce viral titers or improve disease severity in mouse models, and high doses worsened the outcome (higher viremia, mortality, and hyper-inflammation). The study suggests AMPK activation could be a potential host target. |
| Bonglack et al., 2021 [320] | In vitro study | Epstein-Barr Virus (EBV) | EBV infection upregulates MCT1 and MCT4, supporting glycolysis. Dual inhibition of both transporters halts cell growth, causes lactate accumulation, decreases oxygen consumption, depletes glutathione, and enhances sensitivity to phenformin and metformin. |
| Hoppe-Seyler et al., 2021 [321] | In vitro study | Human Papillomavirus (HPV) | Metformin downregulates E6/E7 oncogene expression in HPV-positive cervical and head/neck cancer cells through glucose and PI3K pathways. Despite E6/E7 repression, Metformin causes a reversible proliferative stop and prevents senescence induced by E6/E7 inhibition or chemotherapy, suggesting potential for repurposing Metformin in cancer therapy. |
| Hsu et al., 2021 [322] | Nested case-control study | HPV | Metformin use was associated with a 56% lower likelihood of anal intraepithelial neoplasia (AIN) in type 2 diabetic patients. This suggests that Metformin may offer protective effects against AIN, a precursor to anal cancer, potentially due to its influence on HPV-related pathways. |
| Veeramachaneni et al., 2021 [323] | Preclinical mouse model study | HPV | Long-term metformin treatment significantly reduced tumor growth, increased CD8+ T-cells, and upregulated immune responses in head and neck cancer models. Acute metformin exposure, however, had limited antitumor effects. Combinatorial approaches with immune checkpoint inhibitors (ICIs) may enhance its therapeutic potential. |
| Wilkie et al., 2021 [324] | In vitro study on HPV-positive SCCHN | HPV | HPV-positive head and neck cancer cells exhibited a metabolically diverse phenotype. Sensitization to ionizing radiation (IR) required a combination of 2-deoxy-D-glucose and metformin, targeting both mitochondrial respiration and glycolysis. This approach could reduce radiation doses and minimize treatment impact on long-term function. |
| Sharma and Munger, 2020 [325] | In vitro study on HPV16 E7-expressing cells | Human Papillomavirus 16 (HPV-16) | HPV16 E7 stabilizes the tumor suppressor TP53 via the long noncoding RNA (lncRNA) DINO, which is regulated by KDM6A. DINO levels increase in HPV16 E7-expressing cells and further stabilize TP53. Cells are sensitized to metabolic stress (e.g., by metformin) and chemotherapy (e.g., doxorubicin) in a DINO-dependent manner, linking DINO to TP53 activation and cell death response. |
| Curry et al., 2023 [305] | Clinical trial, analysis of tumor samples | HPV | After treatment with durvalumab and metformin, significant changes were observed in CD8+ and FoxP3+ T-cell densities and spatial distributions in head and neck squamous cell carcinoma (HNSCC). HPV-positive tumors had greater intercellular distances (ID) than HPV-negative ones. Pathologic responders showed higher CD8+ density and ID. These findings suggest that T-cell distribution patterns may predict response to immune checkpoint inhibitors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





