Submitted:
06 February 2024
Posted:
07 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
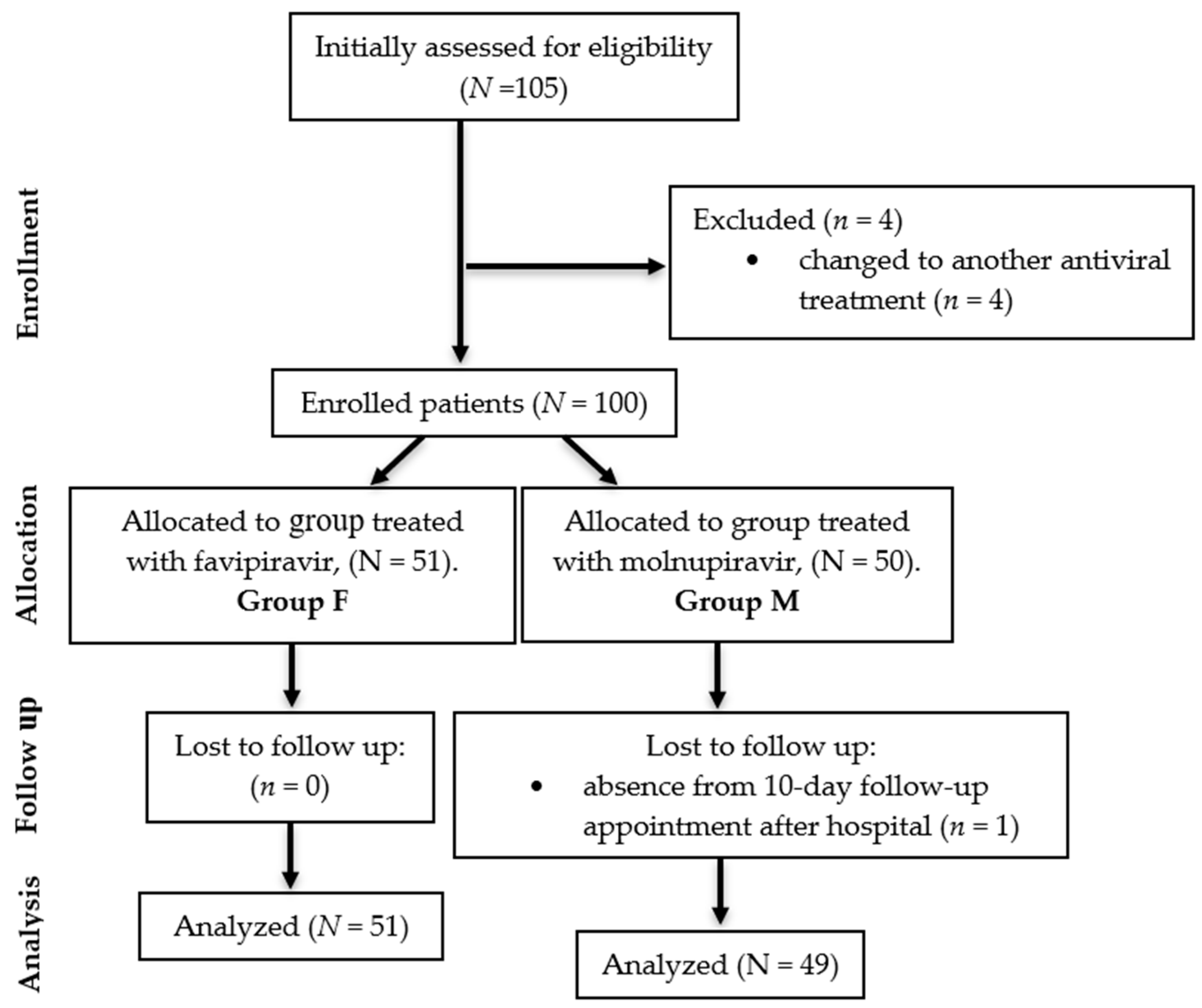
2.1. Study design
- Being aged above 18 years;
- Pre-existing diagnosis of type II diabetes prior to inclusion in the study;
- Positive test for the identification of SARS-CoV-2 viral antigen or RNA;
- No history of previous antiviral therapy.
- The exclusion criteria from the study were:
- Pregnancy;
- Death before completing the antiviral treatment period;
- Absence from the 10-day follow-up appointment after hospital admission, within the hospital outpatient clinic.
- The need to change to another antiviral treatment.
2.2. Data Collection
2.3. Statistical analysis
- p: the probability of the phenomenon, where 0 ≤ p ≤ 1,
- q: the complementary probability, where q = 1 - p,
- t: the probability factor,
- Δx: the permissible margin of error,
- N: the population size.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, A.; Manjunath, K.; Ranjan, R.K.; Kaushik, S.; Kumar, S.; Verma, V. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020, 16, e1008762. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Pöhlmann, S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004, 12, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Vennema, H.; Godeke, G.J.; Rossen, J.W.; Voorhout, W.F.; Horzinek, M.C.; Opstelten, D.J.; Rottier, P.J. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 1996, 15, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.L.; Teoh, K.T.; Lo, J.; Chan, C.M.; Kien, F.; Escriou, N.; Tsao, S.W.; Nicholls, J.M.; Altmeyer, R.; Peiris, J.S.M.; et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008, 82, 11318–11330. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science (80-.). 2020, 368, 779–782. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; Farias de Oliveira, T.; Campos Alcântara, R.; Monteiro Arnozo, G.; Rodrigues da Silva Filho, E.; Galdino Dos Santos, A.G.; Oliveira da Cunha, E.J.; Salgueiro de Aquino, S.H.; et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien. Klin. Wochenschr. 2021, 133, 377–382. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Anwer, M.K.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Bhatia, S.; et al. There is nothing exempt from the peril of mutation – The Omicron spike. Biomed. Pharmacother. 2022, 148, 112756. [Google Scholar] [CrossRef]
- Nistor-Cseppento, C.D.; Moga, T.D.; Bungau, A.F.; Tit, D.M.; Negrut, N.; Pasca, B.; Bochis, C.F.; Ghitea, T.C.; Jurcau, A.; Purza, A.L.; et al. The Contribution of Diet Therapy and Probiotics in the Treatment of Sarcopenia Induced by Prolonged Immobilization Caused by the COVID-19 Pandemic. Nutrients 2022, 14, 4701. [Google Scholar] [CrossRef]
- Moga, T.D.; Nistor-Cseppento, C.D.; Bungau, S.G.; Tit, D.M.; Sabau, A.M.; Behl, T.; Nechifor, A.C.; Bungau, A.F.; Negrut, N. The Effects of the “Catabolic Crisis” on Patients’ Prolonged Immobility after COVID-19 Infection. Medicina (B. Aires). 2022, 58, 828. [Google Scholar] [CrossRef] [PubMed]
- Negrut, N.; Codrean, A.; Hodisan, I.; Bungau, S.; Tit, D.M.; Marin, R.; Behl, T.; Banica, F.; Diaconu, C.C.; Nistor-Cseppento, D.C. Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 2021, 21, 648. [Google Scholar] [CrossRef] [PubMed]
- Eloy, P.; Le Grand, R.; Malvy, D.; Guedj, J. Combined treatment of molnupiravir and favipiravir against SARS-CoV-2 infection: One + zero equals two? EBioMedicine 2021, 74, 103663. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin. Infect. Dis. 2023, 76, e342–e349. [Google Scholar] [CrossRef] [PubMed]
- Negru, P.A.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Endres, L.; Stoicescu, M.; Pasca, B.; Tit, D.M.; et al. Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir. Biomed. Pharmacother. 2022, 147, 112700. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Adhikari, N.K.J.; Kwon, H.Y.; Teo, K.; Siemieniuk, R.; Lamontagne, F.; Chan, A.; Mishra, S.; Murthy, S.; Kiiza, P.; et al. Anti-Ebola therapy for patients with Ebola virus disease: a systematic review. BMC Infect. Dis. 2019, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Baranovich, T.; Wong, S.-S.; Armstrong, J.; Marjuki, H.; Webby, R.J.; Webster, R.G.; Govorkova, E.A. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013, 87, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Smith, L.K.; Rajwanshi, V.K.; Kim, B.; Deval, J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5’-triphosphate towards influenza A virus polymerase. PLoS One 2013, 8, e68347. [Google Scholar] [CrossRef]
- Vanderlinden, E.; Vrancken, B.; Van Houdt, J.; Rajwanshi, V.K.; Gillemot, S.; Andrei, G.; Lemey, P.; Naesens, L. Distinct Effects of T-705 (Favipiravir) and Ribavirin on Influenza Virus Replication and Viral RNA Synthesis. Antimicrob. Agents Chemother. 2016, 60, 6679–6691. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Singh, P.; Barkate, H.; Patil, S.; Rangwala, S.; Pendse, A.; Kadam, J.; Wu, W.; Caracta, C.F.; Tandon, M. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int. J. Infect. Dis. 2021, 103, 62–71. [Google Scholar] [CrossRef]
- Pilkington, V.; Pepperrell, T.; Hill, A. A review of the safety of favipiravir – a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020, 6, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Kumar Arora, M.; Asdaq, S.M.B.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq Ali, A.; Al-Shammeri, A.M.; et al. Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L. Decoding molnupiravir-induced mutagenesis in SARS-CoV-2. J. Biol. Chem. 2021, 297, 100867. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Pruijssers, A.J.; Chappell, J.D.; Gribble, J.; Lu, X.; Andres, E.L.; Bluemling, G.R.; Lockwood, M.A.; Sheahan, T.P.; Sims, A.C.; et al. Small-Molecule Antiviral β-d-N(4)-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance. J. Virol. 2019, 93, e01348-19. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.A. 2nd; Eron, J.J.J.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022, 14, eabl7430. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ 2021, 375, n2422. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Fitero, A.; Bungau, S.G.; Tit, D.M.; Endres, L.; Khan, S.A.; Bungau, A.F.; Romanul, I.; Vesa, C.M.; Radu, A.-F.; Tarce, A.G.; et al. Comorbidities, Associated Diseases, and Risk Assessment in COVID-19—A Systematic Review. Int. J. Clin. Pract. 2022, 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Allard, R.; Leclerc, P.; Tremblay, C.; Tannenbaum, T.-N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 2010, 33, 1491–1493. [Google Scholar] [CrossRef]
- Cortes Garcia, M.; Sierra Moros, M.J.; Santa-Olalla Peralta, P.; Hernandez-Barrera, V.; Jimenez-Garcia, R.; Pachon, I. Clinical characteristics and outcomes of diabetic patients who were hospitalised with 2009 pandemic influenza A H1N1 infection. J. Infect. 2012, 64, 218–224. [Google Scholar] [CrossRef]
- Gorricho, J.; Garjón, J.; Alonso, A.; Celaya, M.C.; Saiz, L.C.; Erviti, J.; López, A. Use of oral antidiabetic agents and risk of community-acquired pneumonia: a nested case-control study. Br. J. Clin. Pharmacol. 2017, 83, 2034–2044. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.-Z.; Liu, B.; Wu, R.; Yang, Y.-Y.; Xiao, X.-Q.; Zhang, X. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. ScientificWorldJournal. 2014, 2014, 603409. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kumar, K.; Singh, S.; Sehgal, A.; Sachdeva, M.; Bhatia, S.; Al-Harrasi, A.; Buhas, C.; Teodora Judea-Pusta, C.; Negrut, N.; et al. Unveiling the role of polyphenols in diabetic retinopathy. J. Funct. Foods 2021, 85, 104608. [Google Scholar] [CrossRef]
- Popa, A.; Chereji, A.-I.; Dodu, M.A.; Chereji, I.; Fitero, A.; Daina, C.M.; Daina, L.G.; Badau, D.; Neculoiu, D.C.; Domnariu, C. The Impact of Changes regarding Working Circumstances during COVID-19 Pandemic upon Patients Evaluated for Thyroid Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 9856. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, A.; Salmasi, M.; Soltaninejad, F.; Salahi, M.; Javanmard, S.H.; Amra, B. Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Adv. Respir. Med. 2023, 91, 18–25. [Google Scholar] [CrossRef] [PubMed]
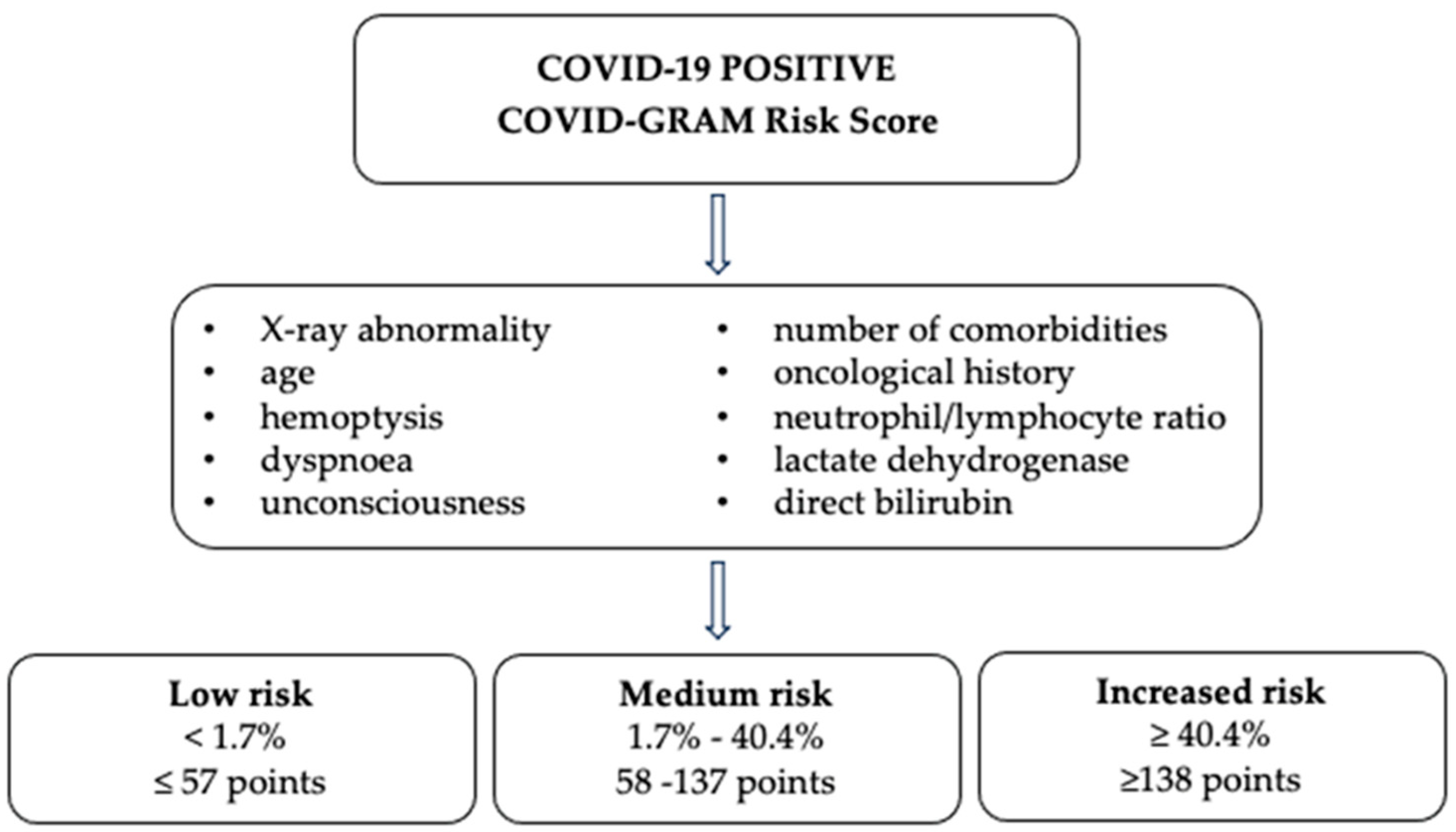
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- COVID-GRAM Critical Illness Risk Score. Available online: https://www.mdcalc.com/calc/10303/covid-gram-critical-illness-risk-score (accessed on 7 August 2023).
- World Health Organization. COVID-19 - Tests. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-resources/science-in-5/episode-14---covid-19---tests?gclid=Cj0KCQjwiIOmBhDjARIsAP6YhSUhh1k3L0EYckHvj8sW- lsJnYh7lAeUloiWlhJ9axHC3vnksU2z5VoaAijuEALw_wcB (accessed on 7 August 2023).
- Elliott, A.C.; Woodward, W.A. Statistical analysis quick reference guidebook : with SPSS examples, 1st ed.; Sage Publications: Thousand Oaks, CA, USA, 2007; ISBN 1-4129-2560-6. [Google Scholar]
- Prajapati, G.; Das, A.; Sun, Y.; Fonseca, E. Hospitalization Among Patients Treated With Molnupiravir: A Retrospective Study of Administrative Data. Clinical Therapeutics 2023. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Berry, K.; Streja, E.; Rajeevan, N.; Li, Y.; Yan, L.; Cunningham, F.; Hynes, D.M.; Rowneki, M.; Bohnert, A.; et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes. medRxiv 2022, 2022.2012.2005.22283134. [Google Scholar] [CrossRef]
- Gentry, C.A.; Nguyen, P.; Thind, S.K.; Kurdgelashvili, G.; Williams, R.J. Characteristics and outcomes of US Veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. Journal of Infection 2023, 86, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records. BMJ 2023, 380, e072705. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis. Clinical Infectious Diseases 2022, 76, 453–460. [Google Scholar] [CrossRef]
- Wai, A.K.-C.; Chan, C.Y.; Cheung, A.W.-L.; Wang, K.; Chan, S.C.-L.; Lee, T.T.-L.; Luk, L.Y.-F.; Yip, E.T.-F.; Ho, J.W.-K.; Tsui, O.W.-K.; et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. The Lancet Regional Health – Western Pacific 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. The Lancet 2023, 401, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.L.; Orton, C.M.; Grinsztejn, B.; Donaldson, G.C.; Crabtree Ramírez, B.; Tonkin, J.; Santos, B.R.; Cardoso, S.W.; Ritchie, A.I.; Conway, F.; et al. Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care. The Lancet Respiratory Medicine 2023, 11, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhsen, S.; Al-Numair, N.S.; Saheb Sharif-Askari, N.; Basamh, R.; Alyounes, B.; Jabaan, A.; Saheb Sharif-Askari, F.; Alosaimi, M.F.; Alsohime, F.; Halwani, R.; et al. Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia. Front Med (Lausanne) 2022, 9, 826247. [Google Scholar] [CrossRef] [PubMed]
- Özlüşen, B.; Kozan, Ş.; Akcan, R.E.; Kalender, M.; Yaprak, D.; Peltek, İ.B.; Keske, Ş.; Gönen, M.; Ergönül, Ö. Effectiveness of favipiravir in COVID-19: a live systematic review. European Journal of Clinical Microbiology & Infectious Diseases 2021, 40, 2575–2583. [Google Scholar] [CrossRef]
- Nemec, H.M.; Ferenczy, A.; Christie, B.D. 3rd; Ashley, D.W.; Montgomery, A. Correlation of D-dimer and Outcomes in COVID-19 Patients. Am. Surg. 2022, 88, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, X.; Chen, J.; Ouyang, M.; Zhang, H.; Zhao, X.; Tang, L.; Luo, Q.; Xu, M.; Yang, L.; et al. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J. Thromb. Thrombolysis 2020, 50, 548–557. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Hayıroğlu, M.İ.; Çınar, T.; Tekkeşin, A.İ. Fibrinogen and D-dimer variances and anticoagulation recommendations in Covid-19: current literature review. Rev. Assoc. Med. Bras. 2020, 66, 842–848. [Google Scholar] [CrossRef]
- Mutair, A. Al; Shamou, J.; Alhumaid, S.; Layqah, L.; Ahmed, G.Y.; Thoyaja, K.; Mohaini, M. Al; Almahmoud, S.; Barry, M.; Khan, A.; et al. Overview of clinical outcome and therapeutic effectiveness of Favipiravir in patients with COVID-19 admitted to intensive care unit, Riyadh, Saudi Arabia. J. Infect. Public Health 2022, 15, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, C.; Zhang, Q.; Wu, F.; Yu, B.; Lv, J.; Li, Y.; Li, T.; Zhang, S.; Wu, C.; et al. C-Reactive Protein Level May Predict the Risk of COVID-19 Aggravation. Open Forum Infect Dis 2020, 7, ofaa153. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Puenpatom, A.; Moncada, P.A.; Burgess, L.; Duke, E.R.; Ohmagari, N.; Wolf, T.; Bassetti, M.; Bhagani, S.; Ghosn, J.; et al. Effect of Molnupiravir on Biomarkers, Respiratory Interventions, and Medical Services in COVID-19 : A Randomized, Placebo-Controlled Trial. Ann Intern Med 2022, 175, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Pontolillo, M.; Ucciferri, C.; Borrelli, P.; Di Nicola, M.; Vecchiet, J.; Falasca, K. Molnupiravir as an Early Treatment for COVID-19: A Real Life Study. Pathogens 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Ishida, K.; Muranaka, E.; Sasazawa, H.; Mito, H.; Yano, Y.; Hase, R. A Favipiravir-induced Fever in a Patient with COVID-19. Intern Med 2020, 59, 2951–2953. [Google Scholar] [CrossRef]
- Bely, P. A.; Krasheninnikov, A. E.; Matveev, A. V.; Zaslavskaya, K. Y. FAVIPIRAVIR IN THE TREATMENT OF MILD CORONAVIRUS INFECTION: RESULTS OF A MULTICENTER OPEN-LABEL, POST-REGISTRATION, NON-INTERVENTIONAL STUDY. Eksperimental'naya i Klinicheskaya Farmakologiya 2023, 86, 18-27, covidwho-2312974. [Google Scholar]
- United States Food and Drug Administration. Title of Site. Available online: https://www.fda.gov/media/155053/download (accessed on 18 October 2023).
- Santi Laurini, G.; Montanaro, N.; Motola, D. Safety Profile of Molnupiravir in the Treatment of COVID-19: A Descriptive Study Based on FAERS Data. J Clin Med 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ergür, F.; Yıldız, M.; Şener, M.U.; Kavurgacı, S.; Ozturk, A. Adverse effects associated with favipiravir in patients with COVID-19 pneumonia: a retrospective study. Sao Paulo Med J 2022, 140, 372–377. [Google Scholar] [CrossRef]
- Manole, F.; Marian, P.; Mekeres, G.M.; Voiţă-Mekereş, F. Systematic Review of the Effect of Aging on Health Costs. Archives of Pharmacy Practice 2023, 14, 58–61. [Google Scholar] [CrossRef]


| Parameter | Group F (n=51) | Group M (n=49) | P-value |
|---|---|---|---|
| DD | |||
| Age, years, mean ± SD | 71.16 ± 12.50 | 65.84 ± 16.66 | 0.075a |
| Male gender, n (%) | 17 (33.33) | 22 (44.90) | 0.643b |
| Urban residence, n (%) | 16 (31.37) | 21 (42.86) | 0.411b |
| VHC, n (%) | 2 (3.92) | 1 (2.04) | 0.563b |
| Clinical data | |||
| Stomatitis, n (%) | 0 (0) | 1 (2) | 0.317b |
| Diarrhea, n (%) | 3 (5.88) | 4 (8.16) | 0.705b |
| Abdominal pain, n (%) | 4 (7.84) | 3 (6.12) | 0.705b |
| Nausea, n (%) | 6 (11.76) | 3(6.12) | 0.317b |
| Vomiting, n (%) | 3 (5.88) | 0 (0) | 0.083b |
| Dizziness, n (%) | 2 (3.92) | 1 (2) | 0.563b |
| SpO2, M ± SD | 92.29 ± 6.59 | 92.67 ± 7.14 | 0.783a |
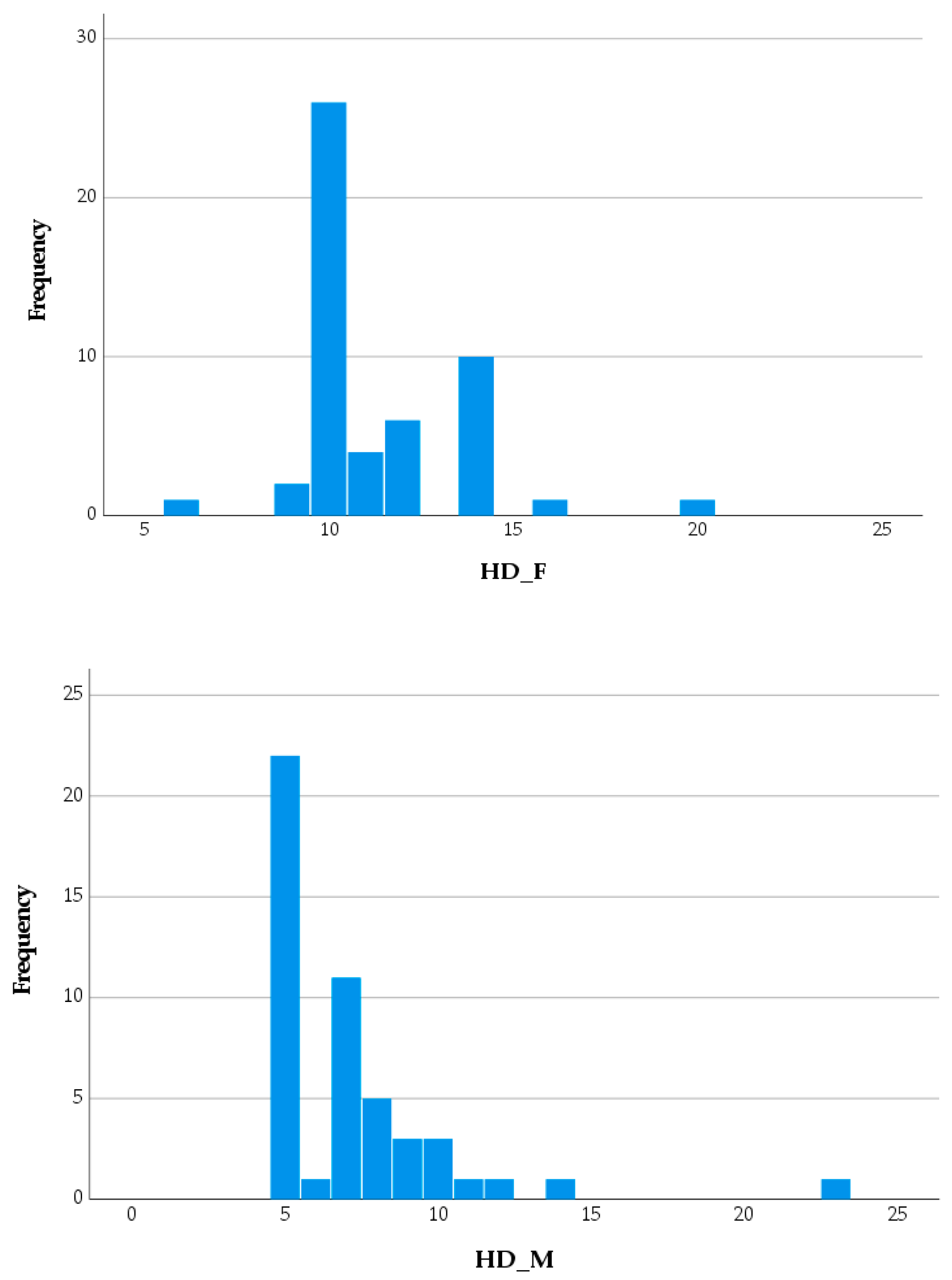
| POH, M ± SD | 4.31 ± 1.35 | 4.55 ± 1.57 | 0.420a |
| BMI, M ± SD | 28.91 ± 3.98 | 28.26 ± 3.52 | 0.390a |
| PMH | |||
| CVC, n (%) | 40 (78.43) | 33 (67.35) | 0.412b |
| CKD, n (%) | 11 (21.57) | 12 (24.49) | 0.834b |
| N, n (%) | 2 (3.92) | 1 (2.04) | 0.563b |
| CPD, n (%) | 1 (1.96) | 2 (4.08) | 0.563b |
| CVD, n (%) | 8 (15.69) | 5 (10.20) | 0.692b |
| Hep B, n (%) | 1 (1.96) | 0 (0.00) | 0.317b |
| ID, n (%) | 1 (1.96) | 2 (4.08) | 0.563b |
| Paraclinical investigations, mean ± SD | |||
| Ferritin, (ng/mL) | 1314.06 ± 1266.20 | 843.53 ± 1160.23 | 0.061a |
| HbA1c, (mg%) | 7.24 ± 0.57 | 7.25 ± 0.65 | 0.924a |
| D-dimer, (ng/mL) | 1039.18 ± 801.80 | 1106.02 ±1439.30 | 0.776a |
| ALAT (mg%) | 33.78 ± 15.24 | 32.91±15.99 | 0.781a |
| CRP (mg/L) | 92.02 ± 90.96 | 60.92 ± 73.41 | 0.062a |
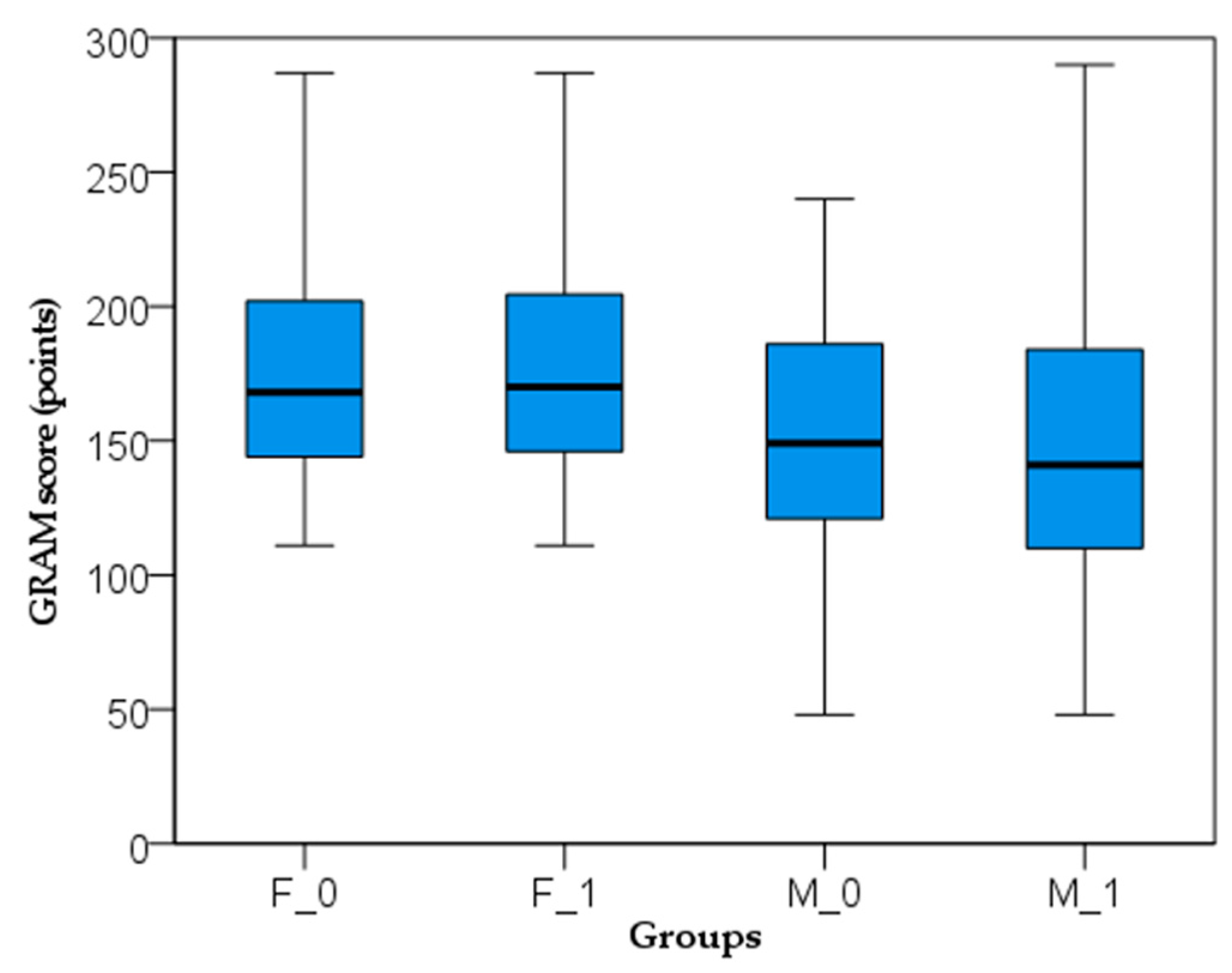
| COVID-GRAM risk score | 178.14 ± 43.78 | 160.59 ± 59.41 | 0.129a |
| Risk of critical illness | t0 | t1 | P-value |
|---|---|---|---|
| Group F | |||
| Low, n (%) | 0 (0%) | 0 (0%) | - |
| Medium, n (%) | 9 (17.65%) | 10 (19.61%) | 0.818a |
| High, n (%) | 42 (82.35%) | 41 (80.39%) | 0.912a |
| Group M | |||
| Low, n (%) | 1 (2.04%) | 1 (2.04%) | 1.000a |
| Medium, n (%) | 19 (38.78%) | 22 (44.90%) | 0.639a |
| High, n (%) | 29 (59.18%) | 26 (53.06%) | 0.685a |
| Adverse effects, n (%) | M group | F group | P-valuea |
|---|---|---|---|
| Stomatitis | 8 (15.69) | 7 (14.29) | 0.796 |
| Diarrhea | 1 (1.96) | 9 (18.37) | 0.011 |
| Abdominal pain | 2 (3.92) | 3 (6.12) | 0.654 |
| Nausea | 8 (15.69) | 14 (28.57) | 0.2 |
| Vomiting | 1 (1.96) | 4 (8.16) | 0.179 |
| Dizziness | 5 (9.80) | 9 (18.37) | 0.285 |
| ALAT elevated | 1 (1.96) | 3 (4.08) | 0.317 |
| Total | 26 (50.98) | 48 (97.96) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





