1. Introduction
Landfill leachate is a liquid produced by concentration and storage of the high polluted liquid originated from solid wastes deposited in the landfill, and is considered a complex liquid with high content of organic matter, ammonia, sulphate, chloride and metals [
1,
2]. This liquid can be treated by biological treatment to reduce organic matter and toxicity [
3,
4] or by other treatment processes like flocculation, chemical oxidation or reverse osmosis [
5,
6,
7]. The nature and concentration of the organic matter in landfill leachate are the main factors affecting the successful treatment of this liquid.
Concentration of organic matter can be very different in landfill leachates and normally when COD value is low, landfill leachate can be processed by biological treatment [
1,
2,
8,
9]. In high organic loaded leachates, reduction in COD is normally not well performed [
10,
11,
12]. Organic matter present in these high organic loaded leachates, most of the times, has the origin in composting of organics from solid wastes (composting leachate) [
13,
14,
15].
Biological treatment combined with flocculation or oxidation is accepted nowadays as the most effective treatment of landfill leachates [
16,
17,
18,
19]. Flocculation is economically sustainable, compared to other processes like ozonation or Fenton oxidation [
20,
21,
22]. If biological treatment is planned to be applied, the biodegradability of the organic compounds present in the leachate has to be considered [
23].
In landfill leachates, the presence of humic substances (HSs) has been described in literature [
24,
25,
26]. Specially in composting leachate, the odour which is proximate to wood soil and the dark yellow and black colours of most landfill leachates are produced by HSs [
27,
28,
29]. HSs are known to be recalcitrant compounds [
30,
31] and conduct to low reduction of COD in the biological treatment. They have been cited to be abundant in landfill leachates, reaching sometimes 60% of the total organic matter [
30,
32].
HSs are divided into two fractions: humic and fulvic acids (HAs and FAs), with different solubility attending pH [
33]. HAs are insoluble at low pH and FAs are soluble at low, medium and high pH [
24,
32,
34,
35]. The insolubility of HAs when the pH is low is due to the protonation under acidic conditions of the functional groups which are present in these substances: carboxylic acids and phenolic alcohols. The difference in the pH-dependent forms of the carboxylic acids and phenols produces Zeta potencial variation due to ionization-protonation [
36]. In addition, protonation of the functional groups of the HAs at low pH can facilitate the formation of H-bonds among different molecules and to induce flocculation of the intermolecular structures formed. At higher pH (slightly acid or neutral), carboxylic acids and phenolic alcohols are ionized and Zeta potential increases because of the formation of positive ions in the HA surface. This increase in the Zeta potential absolute value as a consequence of ionization of the functional groups of HAs can maintain in suspension HAs because of electrostatic repulsion between different molecules. In literature, it is reported ionization of carboxylic acids at pH over 3.5-4.0 and phenolic groups over 5.5-6.0 [
37]. In accordance with these considerations, flocculation of HAs from landfill leachate has to be performed at low pH, because at slightly acid, neutral and high pH, HAs will be maintained in suspension [
38].
In this article, we prove an important presence of HAs in landfill leachate, which procced from composting leachate, a frequent situation in high COD leachates. These recalcitrant organics are flocculated at low pH in accordance with their chemical properties and stability of aqueous suspensions. Flocculation at low pH is the main treatment for the reduction of recalcitrant compounds (HAs) and the explanation of this novel procedure is focused in the chemical properties of HAs at different pH values. Flocculation at low pH reduces significantly COD value of landfill leachates, specially with high organic load, and is considered an effective and sustainable treatment of this polluted liquid. Presence of HAs in landfill leachate was proved by chemical analysis (CHNS) and infrared spectrometry (FT-IR), and the evolution of the molecular size of the HAs and aggregates are monitored by the analysis of mean diameter in the flocculation at low pH.
2. Materials and Methods
2.1. Composition of Landfill Leachate
Landfill leachate was characterized in the laboratory analysing the samples collected in the leachate deposit (March-June) of the Waste Treatment Centre (WTC) of Salamanca, Spain. In the characterization of this high polluted liquid, pH, organic load (chemical oxygen demand, COD), colour, solids (total solids, TS and volatile solids, VS), nitrogen compounds (organic nitrogen, ammonia, nitrates and nitrites), phosphate, sulfate, chloride and metals were measured by the standard methods described in literature [
39]. pH was measured using a pHmeter with precision 0.1 pH units (CRISON MicropH 2000).
COD and colour were chosen for monitoring the effectivity in the treatment of landfill leachate by flocculation at low pH. COD was measured in triplicate by the colorimetric closed reflux method (5220D, [
39]). Because of the high concentration of organics in leachate (high COD value), samples were diluted 8 times and a pattern calibration line was used for COD values in the range 2000-20000 mg/L (potassium hydrogen phthalate). Because of the presence of HSs in the landfill leachate which can interfere in colour analysis, the spectrophotometric method was selected for measuring colour, detecting maximum absorbance wavelength (chromatic method, method 2120C, [
39]).
Solids were analysed by the standard methods described in literature for total and volatile solids, based in thermal treatment at 105 °C and 550 °C (TS: 2540B and VS: 2540E, [
39]).
Organic nitrogen (N
org) and ammonia (NH
4+) were analysed by the standard method (Kjeldahl, 4500-NH
3 B and E, [
39]), after filtration by Millipore filters (0.45 μm). N
org analysis was performed by a previous digestion in H
2SO
4 before Kjeldahl distillation (4500-N
org B, [
39]).
Samples were also filtered by Millipore filters (0.45 μm) for NO3-, NO2-, SO42-, PO43- and Cl- analysis, and measured by ionic chromatography (ICS 2000 ISO 10304-1, Dionex). ICS 2000 measurement conditions for 10 μL injection volume were: cell temperature 35 °C, column temperature 30 °C, eluent flow rate 1.00 mL/min (35 mM KOH) and 50 mA of applied current. The column used was 4x250 mm IonPac AS19 and the guard 4x50 mm IonPac AG19. The software Chromeleon of Dionex was employed for calibration and measurement of all these anions together in a unique chromatogram.
Metals present in leachate were analysed by ICP-MS (Agilent 7800, Chemical Analysis Service of the University of Salamanca). Because of the high organic content in landfill leachate, the samples were treated with concentrated nitric acid in a closed Teflon glass (50 mL final sample volume). The ICP-MS spectrometer was calibrated with two multi-element patterns prepared from certificated Panreac standard solutions (1000 mg/L). Measurement flux conditions of the ICP-MS were: plasma gas 15 L/min, auxiliary gas 0.9 L/min, nebulization gas 0.99 L/min and cell gas (Helio) 4.5 mL/min.
2.2. Analysis of Humic Acids
HAs analysis in literature is based in a suggested method with two variants, depending in concentration (5510, [
39]). In natural waters, with low HSs content, the method proposed is a concentration of HSs by using ionic exchange columns and the measurement of dissolved organic carbon (DOC) [
40]. In liquids with high HSs content, HAs have to be precipitated at acid pH, attending the chemical properties of HAs by the method described by Christensen et al., 1998 [
30,
34]. Following the method of Christensen et al., 200 mL leachate samples were acidified with HCl concentrated to pH = 1.0 and placed for 24 hours. The precipitate after this time was purified twice during 48 hours in total with 100 mL acid solution (0.5 % HF v/v and 0.5 % HCl v/v). The resulting precipitate, filtered by Millipore filters (0.45 μm), was purified 3 times with HCl acid solution (pH 2.0), separated in small portions and placed for a further analysis in an incubator (35 °C, 5 days).
HAs obtained by the procedure described before were characterized by CHNS analysis using a LECO CHNS-932 analyzer (Elemental Microanalysis Service of the UCM, Madrid University) and infrared spectrometry (FT-IR, Perkin Elmer SpectrumTwo). Infrared spectra were obtained from samples prepared in pellets of the HAs from leachate and commercial AHA (Aldrich humic acid) in KBr, measuring the absorbance in the range of wavenumber 4000-400 cm-1.
2.3. Reduction of pH
The reduction of pH in landfill leachate was performed using H2SO4 9 M for acidification. Samples were collected during pH reduction separated by 0.5 units of pH and COD, colour and Z potential were quantified in triplicate to monitor leachate treatment related to the chemical properties of the HAs.
Zeta potential and mean diameter were analysed in samples using the same equipment (Malvern Zetasizer Nano ZS).
3. Results and Discussion
3.1. Composition of Landfill Leachate
Composition of landfill leachate is shown in
Table 1 where most important parameters are recorded (pH, organic load, solids, colour and nutrients). COD value is very high: 42000 ± 2516 mg/L, in view of other data from literature [
1,
2,
4], but is coincident with values for leachates with high content of organic matter [
24,
41]. The high value of organic matter concentration is due to the characteristics of this liquid, produced from the solid wastes deposited in the landfill and the liquid originated from them, and mixed with the fluid residue proceed from composting of the organic fraction of these solids (composting leachate) [
42]. As a consequence of aerobic oxidation of organic matter, composting process produces the formation of HSs, conducing to the formation of alcohols (phenols) and specially carboxylic acids [
43].
Value of solids is high in this liquid (total solids, TS: 16410 ± 486 mg/L), with high proportion of organic solids: more than 60 % of TS are VS (VS = 10076 ± 620 mg/L,
Table 1).
Colour in landfill leachate has been analyzed frequently in literature by the method of the Pt-Co scale [
44,
45], which compare the tonality of the liquid with prepared standard solutions of K
2PtCl
6 with CoCl
3. This method does not reflect the real colour of this liquid because the maximum wavelength absorbed is not measured.
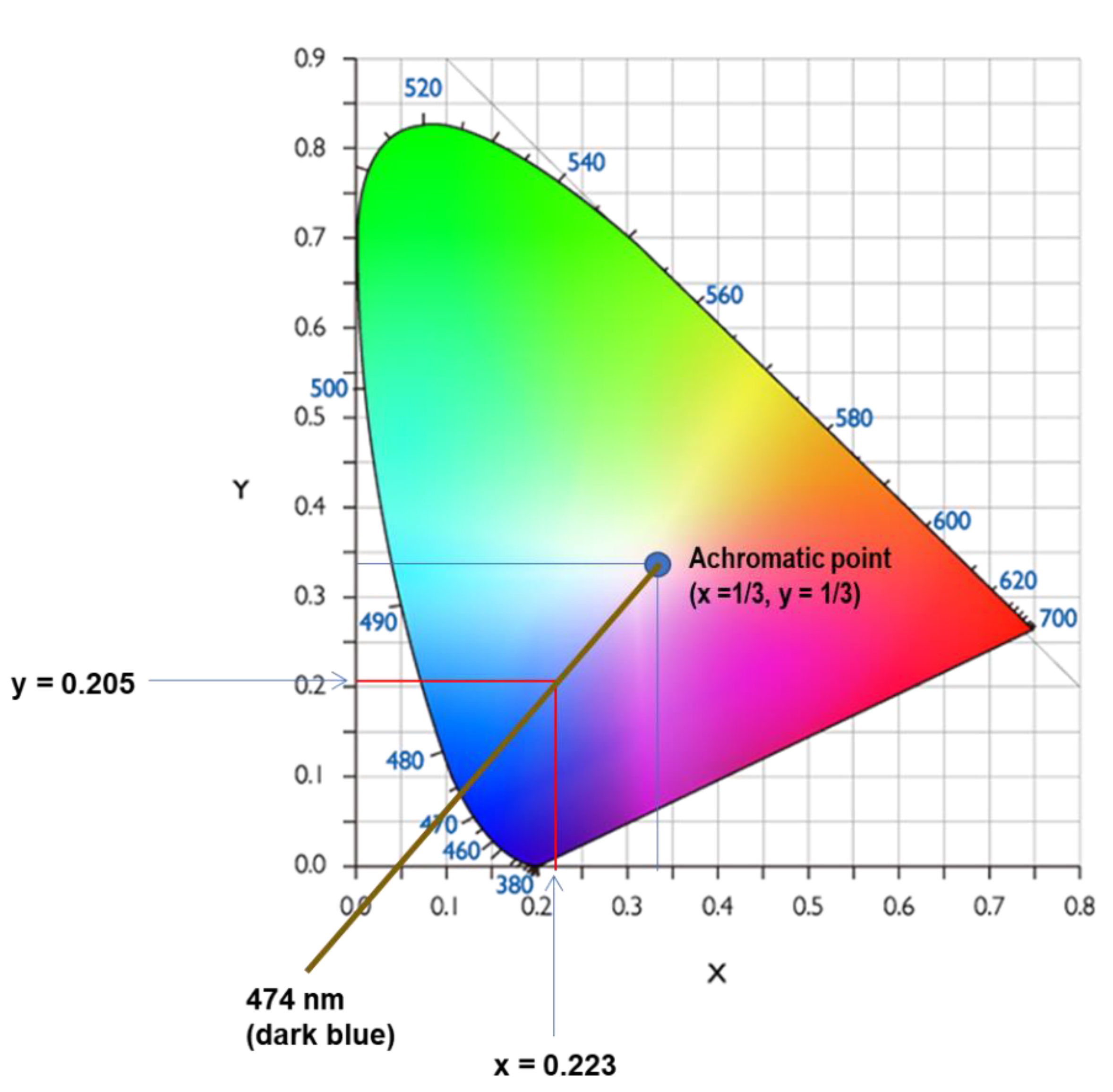
The analysis of colour in the leachate was performed by the chromatic method described in section 2.1, which obtains the predominant wavelength absorbed by this liquid, shown in
Figure 1. This predominant wavelength was 474 nm, which is assigned in the chromaticity diagram to dark blue tonalities. The quantitative value of colour obtained is high in
Table 1 (ABS = 0.537) and the external appearance of this liquid is dense with a dark brown colour and a blue-yellowish iridescence.
The concentrations of nutrients reported in
Table 1 show high content of Cl
-, NH
4+, NO
3- and SO
42-, the most abundant negative ions in this liquid. Cl
- is extremely abundant, reaching a concentration close to 12 g/L, also ammonia is highly abundant (2.5 g/L) and PO
43- and NO
2- are present in low concentration [
46]. These concentrations for nutrients are consistent with the values obtained by other authors [
24,
41,
47,
48].
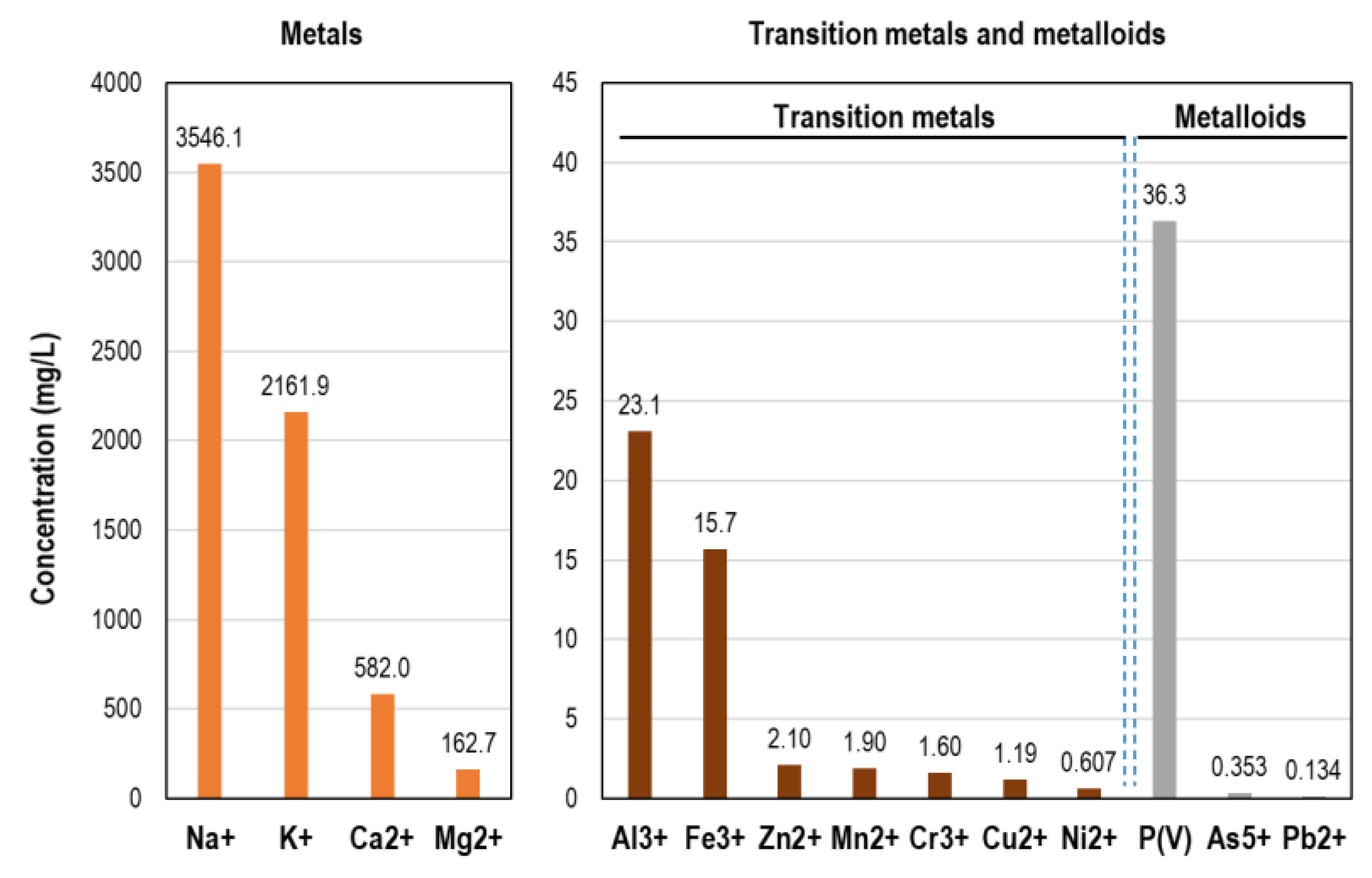
Metal ions content in landfill leachate shows high concentrations of alkali metals Na
+ and K
+, and abundance of alkaline earth metals Ca
2+ and Mg
2+ (
Figure 2). P(V), Al
3+ and Fe
3+ appear in this liquid with visible concentration. Zn
2+, Mn
2+, Cr
3+ and Cu
2+ contribute in very low concentration (1.2-2.1 mg/L) and other cations (Ni
2+, As
5+ and Pb
2+) below 1 mg/L. The content of metal ions in this landfill leachate can be considered normal in accordance with data from literature [
41,
48,
49].
3.2. Flocculation of Landfill Leachate at Low pH
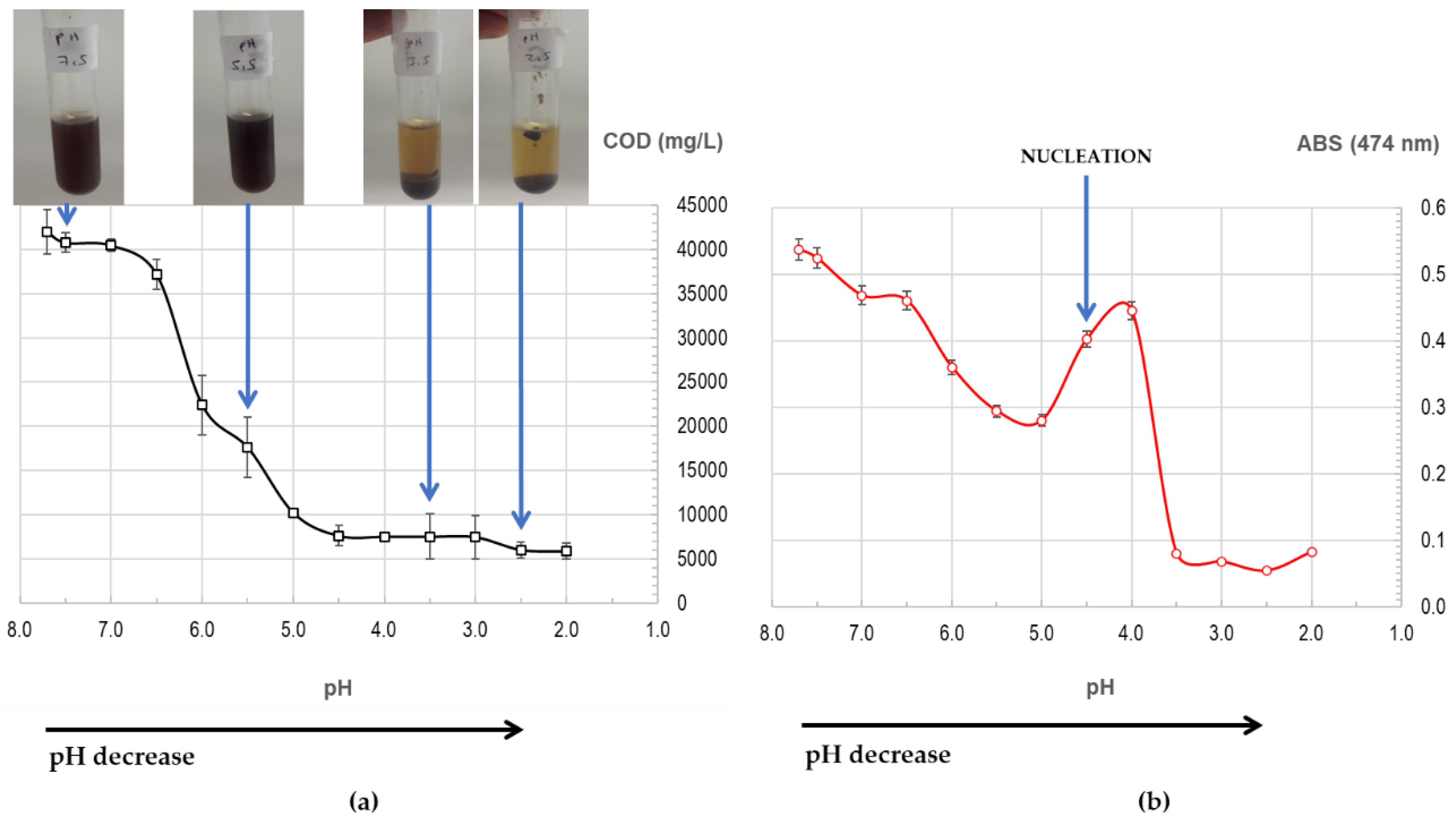
Flocculation of landfill leachate at low pH was performed by using H
2SO
4 9 M for a gradual acidification. This liquid flocculates at pH 5.0 (
Figure 3a) and COD is reduced from an initial value of 42000 ± 2516 mg/L to a final value of 5850 ± 923 mg/L at pH 2.0 (86.1 % reduction, numerical values in SM,
Table S1).
Colour reduction is observed during flocculation at low pH in
Figure 3b from initial ABS
474nm = 0.537 ± 0.000 (pH 7.7) to 0.082 ± 0.000 (pH 2.0, 84.7 % reduction, numerical values in SM,
Table S1). ABS increases at pH 4.5 and more visible at pH 4.0, and this is explained by nucleation, the beginning of flocculation, when the incipient formed flocs increase ABS and precipitation starts. This flocculation experiment was repeated and the behaviour observed was the same, producing clearly flocculation and precipitation with formation of an important quantity of foam.
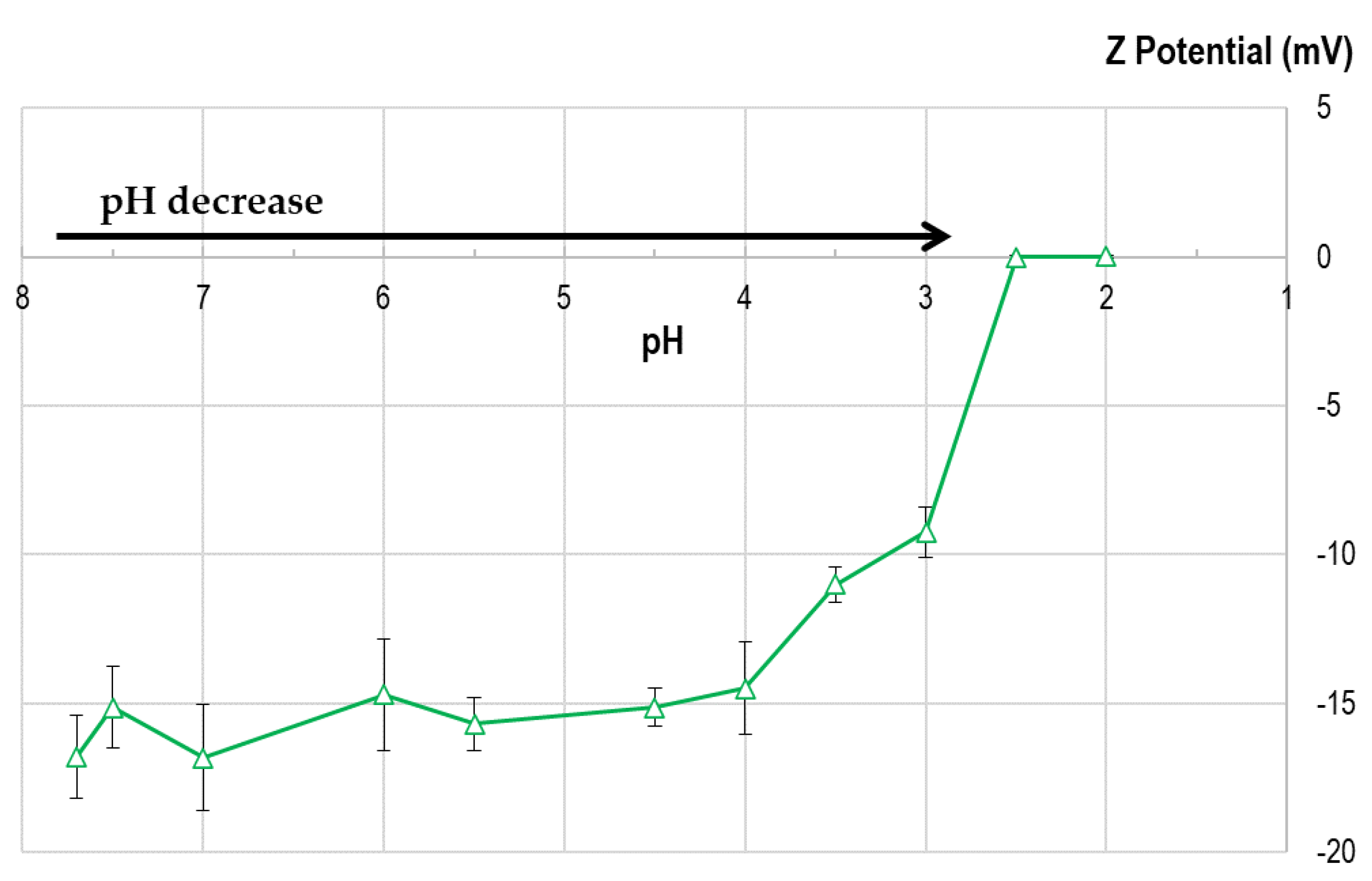
During flocculation at low pH, Z potential value was analyzed (
Figure 4). The initial value of Z potential in landfill leachate is -16.8 ± 1.4 mV at pH 7.7 and when the pH value decreases below 5.0, Z potential diminish quickly to zero (
Figure 4 and numerical values in SM,
Table S1). The decay in absolute value in Z potential is highly evident at pH below 3.5. This initial value of Z potential in leachate (-16.8 ± 1.4 mV), in the range -15 to -25 mV, is in accordance with the value obtained recently by other authors [
45,
50].
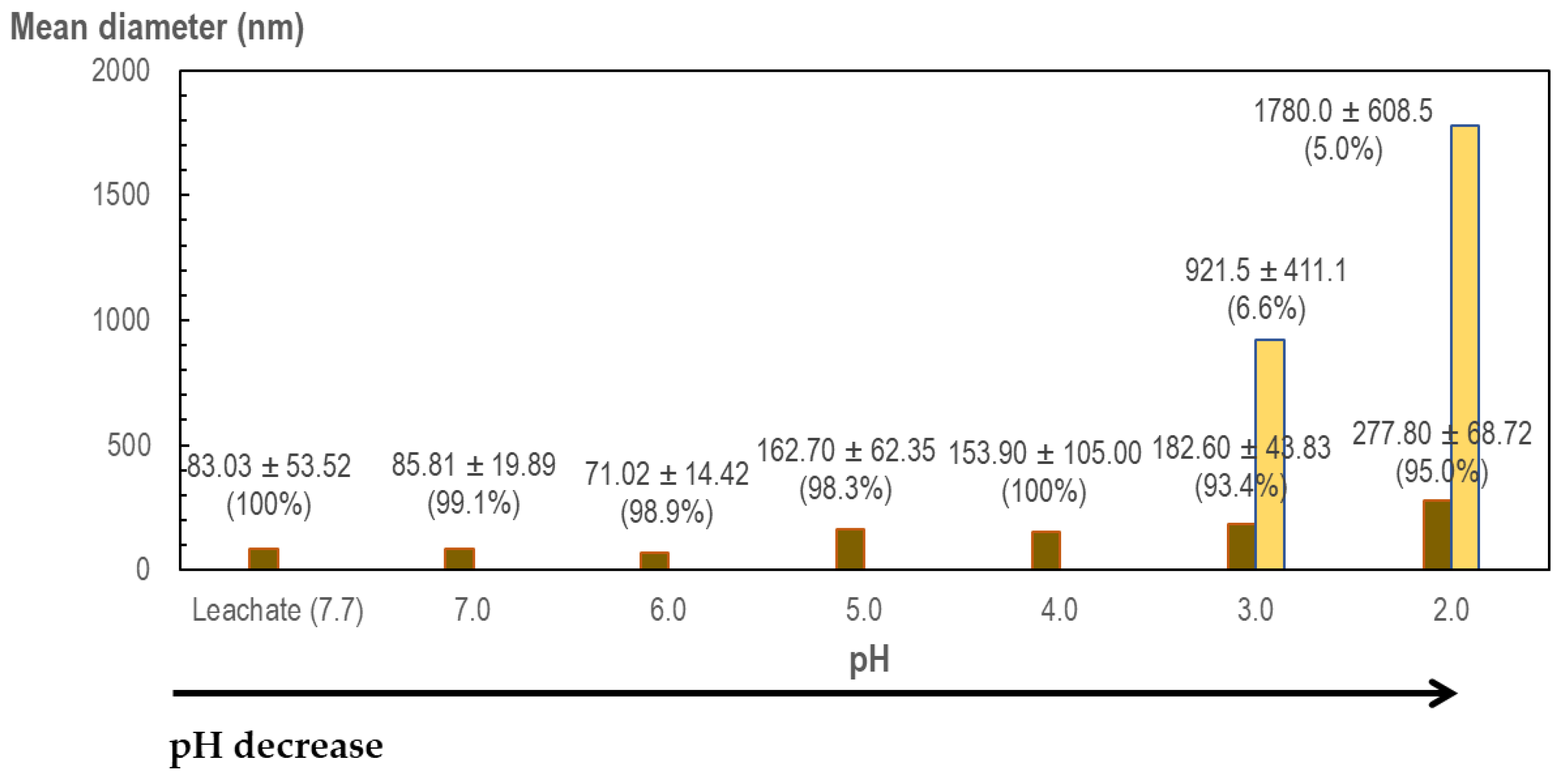
Mean diameter was measured during flocculation at low pH to follow particle size evolution in landfill leachate. The initial distribution of particles in this liquid is not very diverse attending mean ± SD values in leachate in
Figure 5 (83.03 ± 53.52 nm). During flocculation at low pH procedure, particle size increases at pH = 5.0 and big particles are formed at pH 3.0 and 2.0 in
Figure 5. The explanation of the formation of these big aggregates is assumed to be the intermolecular interaction of different molecules of HAs. Mean diameter was measured in the precipitate, resulting in a solid composed by much bigger particles: 5590 ± 579.8 nm (100%). This value in the size of the particles (5.6 μm) is coincident with the observed value in literature for the aggregates of natural HAs, several micrometers [
51].
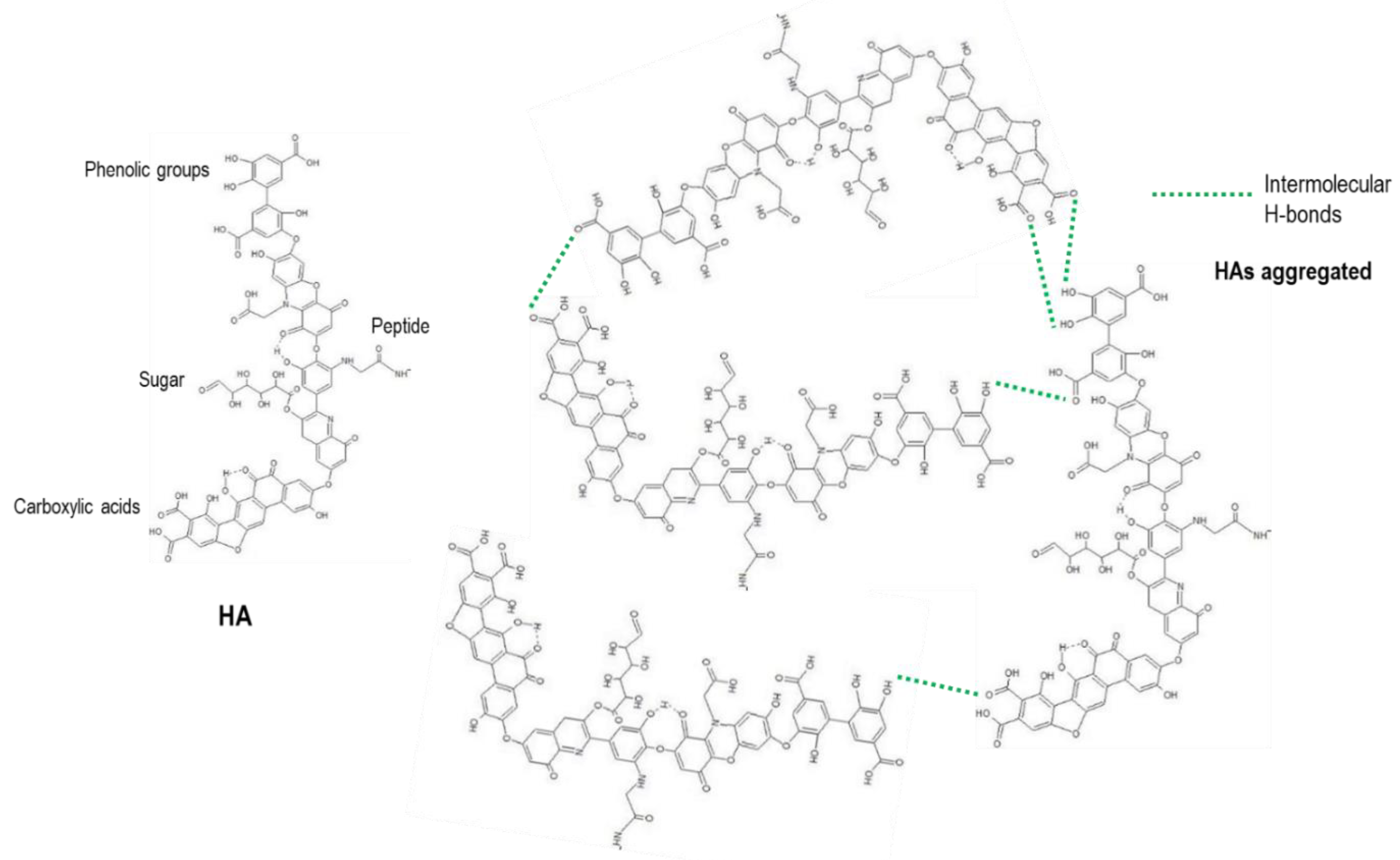
Flocculation of landfill leachate with the decrease of pH is explained because of the presence of HAs in this residue, with an important fraction of the liquid produced in composting of organic matter from solid wastes. The typical molecular structure of a HA is a long aromatic chain with some ramifications in which the functional groups are located. These functional groups are carboxylic acids normally situated at the end of the aromatic chain, phenolic groups distributed in the molecule and also few sugar and peptides located in the central part of the molecule (
Figure 6, HA). When the carboxylic acids and phenolic alcohols are protonated at low pH, the number of negative charges decreases in the external part of the molecule and in consequence, Zeta potential absolute value drops. The abundance of protons in the surface of the HA molecules favours the aggregation by interaction of intermolecular H-bonds [
37,
52].
In accordance with the chemical properties of HAs, in the gradual decrease of pH, phenolic alcohols are firstly protonated at pH below 5.5 and carboxylic acids lately at pH below 3.5 [
37]. When the pH reaches 2.0, all the functional groups (phenols and carboxylic acids) are protonated, Zeta potential value drops to zero and H-bonds are formed between HA molecules, with the resulting increment in the molecular size and weight of the aggregates and conducting to precipitation. While the precipitation process of HAs is producing, other organic molecules are dragged and the reduction of the global COD value can be highly increased (86.1%). This high reduction in the COD value is much more than the expected in accordance with the percentage of HAs in the total organic matter [
30].
The visual appearance of landfill leachate changes significantly after flocculation at low pH. Clarification is produced from dark brown, initial colour observed in leachate, to light yellowish-brown colour of a cleaner liquid (
Figure 3 a)). During precipitation, the formation of foam is observed and is attributed to the formation of HA aggregates.
3.3. Humic Acids Characterization
Samples collected from the precipitate and washed in HF-HCl acid medium (section 2.2) were analysed after flocculation at low pH, in which HCl was used to avoid the interference of H2SO4 in CHNS chemical analysis. These samples were analysed by CHNS analysis and FT-IR.
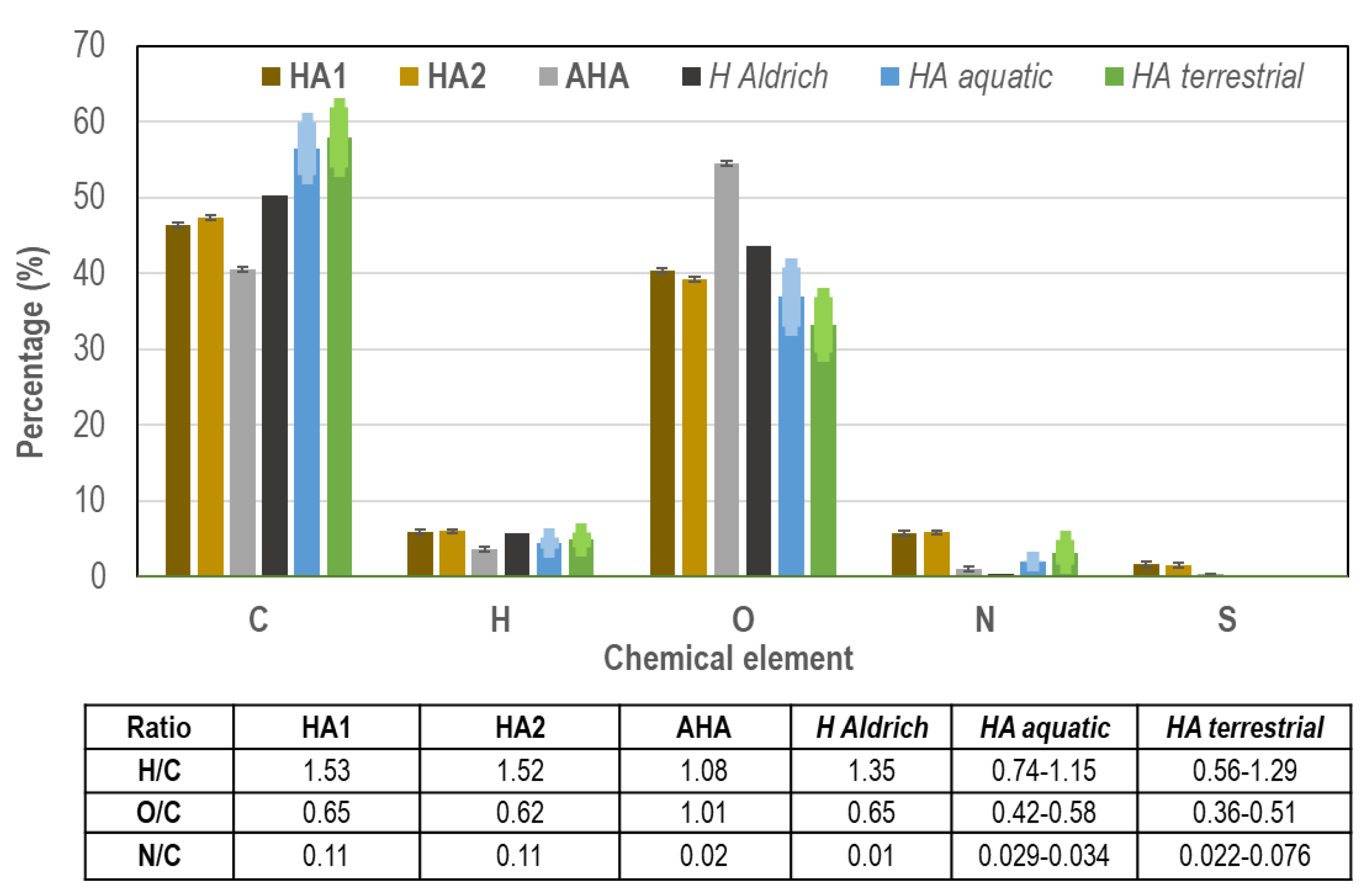
Figure 7 records CHNS elemental chemical analysis, where two independent samples of the precipitate (HA1 and HA2) and HA from Aldrich (AHA), for comparison, were analysed and also compared with data of commercial (H Aldrich) and natural (HA aquatic and HA terrestrial) humic acids from literature. The results were normalized to 100 % of total percentage of organic components, and oxygen (O) was obtained by the difference to 100 % of the sum of the other four elements in percentage (C, H, N and S). The ratios of hydrogen, oxygen and nitrogen to carbon (H/C, O/C and N/C) were obtained from the results of element analysis values. The carbon percentage in HAs from landfill leachate is in between the percentage of aquatic and terrestrial HAs and AHA (commercial), and close to those values and the value of H Aldrich from literature [
24]. The percentage of nitrogen in HAs is higher than the other references (natural and commercial HAs), due to amines and amides present in HA molecules and the important concentration of N-ammonia in the samples of landfill leachate (
Table 1), which are coincident with literature [
24,
53]. Oxygen, present in the main functional groups of the HAs (carboxylic acids and phenols), is very similar in percentage in HAs from landfill leachate and natural HAs, aquatic and terrestrial, specially aquatic. Sulphur percentage reference value has not been found in data from literature and the result measured from commercial HA (AHA) is below detection limit in
Figure 7 (numerical values in SM,
Table S2). The presence of S in the samples of HAs from landfill leachate (1.60 ± 0.35 %, SM,
Table S2) can be attributed to sulphate, which is abundant in leachate (
Table 1).
With regards to element ratios in
Figure 7, H/C ratio value close to 1.0 is indicative of a chemical structure with abundance of aromatic rings (AHA and natural HAs). When the value of H/C is over 1.0 (HA1 and HA2, landfill leachate), this is indicative of the abundance of aliphatic groups. O/C ratios in HAs from landfill leachate are higher related to natural humic acids (abundance of carboxylic acids and phenolic groups), but coincident with H Aldrich value from literature and lower compared to AHA analysed value. N/C ratios are higher in HAs from leachate compared to the other values from natural and commercial humic acids, as commented before (ammonia in leachate,
Table 1).
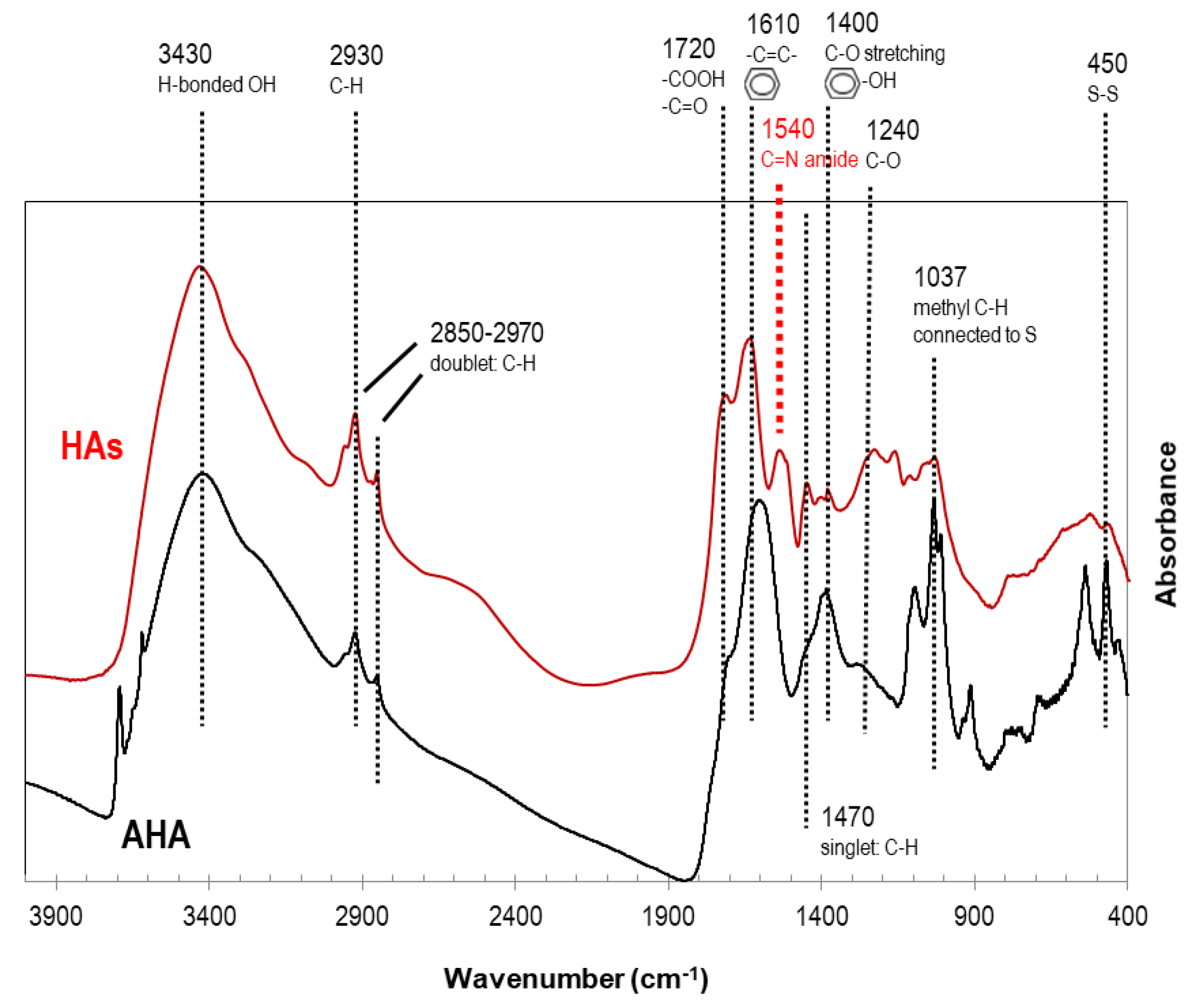
The result of FT-IR spectrometric characterization of HAs in the precipitate of landfill leachate after pH reduction treatment is shown in
Figure 8, where FT-IR spectra of HAs (red line) and Aldrich humic acid (black line) are recorded.
The most representative signals of HAs are assigned and explained in detail in accordance with literature data [
24,
30,
54]. There is a wide band (3400-3000 cm
-1) with the maximum at 3430 cm
-1 which is assigned to H-bonds formed between different molecules (aggregation of HAs) [
30] and also this signal is indicative of phenol, hydroxyl and carboxyl functional groups (O-H stretching). There are two other significative signals appearing in HAs and AHA spectra and assigned to aliphatic groups (C-H) of organic structures: the singlet at 1470 cm
-1 and specially the doublet at 2970-2850 cm
-1. In the FT-IR spectra of HAs in leachate and AHA, the characteristic peaks of C=O bonds (1720 cm
-1) in ketonic and protonated carboxylic acid groups are visible and also the signals of stretching of double carbon bonds (C=C) are highly visible (1610 cm
-1), typical of aromatic rings. There is a signal at 1540 cm
-1 only visible in HAs and not AHA attributed to amide groups (C=N stretching), which is not visible in AHA due to a lower content of N (
Figure 7). The peak at 1400 cm
-1 is assigned to phenolic OH (C-O stretching and deformation of O-H). The slight signal located at 1240 cm
-1 is attributed to the C-O bond (stretching). The peak at 1037 cm
-1, present specially in AHA and also visible in HAs, is assigned to rocking vibrations of aliphatic groups (C-H) connected to S. The S content observed in CHNS analysis in AHA was below detection limit and was detected in HAs (
Figure 7). Finally the peak at 450 cm
-1 has been assigned to S-S bonds [
30], slightly visible in HAs from leachate and much more visible in AHA, with much lower S content.
5. Conclusions
In this article we present a new method for the treatment of high polluted landfill leachate for a sustainable use and treatment of this residue, consisting in the flocculation at low pH. High polluted landfill leachate has a high proportion of composting leachate (composting of solid wastes), which means the abundance of humic acids in this liquid. In view of the chemical properties in aqueous solutions of these HAs, which are protonated at low pH and are ionized at medium-high pH, landfill leachate is effectively flocculated at pH 2.0.
Under acidic conditions, phenolic alcohols present in HAs are protonated at pH below 5.5 and carboxylic acids at pH below 3.5. The protonation of the functional groups of the HA molecules reduces highly Zeta potential absolute value to zero and conducts to the formation of intermolecular H-bonds, resulting in big aggregates which are unstable in solution and precipitate. During the precipitation process of HAs, other organic molecules are dragged and COD and colour are reduced in high extent (84-86 %).
Mean diameter of the molecules has been measured during flocculation at low pH and the formation of big aggregates has been detected at pH 3.0 and 2.0 and also in the precipitate. This precipitate was analysed by CHNS chemical analysis and infrared spectrometry (FT-IR), resulting very closed in chemical composition to commercial and natural HAs and showing in the FT-IR spectrum significative signals coincident with commercial HA, specially with carboxylic acids and phenolic alcohols which are characteristic of the chemical structure of a humic acid. The formation of intermolecular H-bonds between HAs also has been detected by FT-IR spectrometry.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
C.C.: conceptualization, methodology, validation, formal analysis, data curation, supervision, funding acquisition, writing—original draft preparation, writing—review and editing. M.L.P. and B.D.R.: methodology, software, formal analysis, investigation and data curation. All authors have read and agreed to the final version of the manuscript.
Funding
The funding sources of this research were “Junta de Castilla y León (ADE)” (project 04/09/VA/0010), “Laboratorio Castilla y León, Valladolid Labaqua” and University of Salamanca, Strategic Plan of Research and Knowledge Transfer, C1 Research Projects 2021, project 18KA5N/463AC01.
Data Availability Statement
All data used are reported in the manuscript and Supplementary Material (SM). Additional data, if necessary, will be purchased under request.
Acknowledgments
We acknowledge specially the staff of the WTC of Salamanca: Roberto Hernández and Javier Vázquez, for their valuable help in facilitate landfill leachate sample collection and solve technical questions. Milena A. Vega, from the University of Salamanca, is also gratefully acknowledged for technical support and analysis performance of FT-IR spectrometry, Z potential and mean diameter. Elemental Microanalysis Service of Madrid University (UCM) is acknowledge for assistance and performance of CHNS elemental chemical analysis and the Chemical Analysis Service of the University of Salamanca (Emilio Romero) is also acknowledged for metal analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J.V. Landfill leachate treatment methods: a review. Environ. Chem. Let. 2006, 4, 51–61. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, Y.; Wang, S.; Zheng, S.; Gou, J. Organic matter and concentrated nitrogen removal by shortcut nitrification and denitrification from municipal landfill leachate. J. Environ. Sci. 2007, 19, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, S.; Karimi-Jashni, A.; Sartaj, M. Treatment of municipal landfill leachate using a combined anaerobic digester and activated sludge system. Waste Manag. 2010, 30, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, S.; Sun, Y.; Feng, P.; Li, J. Advanced treatment of landfill leachate by a new combination process in a full-scale plant. J. Hazard. Mat. 2009, 172, 408–415. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Ye, X.; Chen, D.; Zheng, K.; Qin, F. , Zhou, S., Ye, X., Chen, D., Zheng, K., Qin, F., 2011. Transformation of pollutants in landfill leachate treated by a combined sequence batch reactor, coagulation, Fenton oxidation and biological aerated filter technology. Proc. Safety and Environ. Protec. 89, 112-120. Proc. Safety and Environ. Protec. 2011, 89, 112–120. [Google Scholar] [CrossRef]
- Mahmud, K.; Hossain, M.D.; Shams, S. Different treatment strategies for highly polluted landfill leachate in developing countries. Waste Manag. 2012, 32, 2096–2105. [Google Scholar] [CrossRef]
- Ding, A.; Zhang, Z.; Fu, J.; Cheng, L. Biological control of leachate from municipal landfills. Chemosphere 2001, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, H.; Kaushal, P. Comparative study of different biological processes for non-segregated municipal solid waste (MSW) leachate treatment. Environ. Tech. Innovat. 2018, 9, 134–139. [Google Scholar] [CrossRef]
- Pi, K.W.; Li, Z.; Wan, D.J.; Gao, L.X. Pretreatment of municipal landfill leachate by a combined process. Proc. Safety and Environ. Protec. 2009, 87, 191–196. [Google Scholar] [CrossRef]
- Cotman, M.; Gotvajn, A.Z. Comparison of different physico-chemical methods for the removal of toxicants from landfill leachate. J. Hazard. Mat. 2010, 178, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.L.; Fernandes, H.; Costa, R.H.R. Landfill leachate treatment as measured by nitrogen transformations in stabilization ponds. Bioresour. Technol. 2013, 147, 562–568. [Google Scholar] [CrossRef]
- Tamrat, M.; Costa, C.; Márquez, M.C. Biological treatment of leachate from solid wastes: kinetic study and simulation. Biochem. Eng. J. 2012, 66, 46–51. [Google Scholar] [CrossRef]
- Costa, C.; Domínguez, J.; Autrán, B.; Márquez, M.C. Dynamic Modeling of biological treatment of leachates from solid wastes. Environ. Model. Assess. 2018, 23, 165–173. [Google Scholar] [CrossRef]
- Domínguez, J.; Costa, C.; Autrán, B.; Márquez, M.C. Characterization and biological stabilization of leachates from solid wastes in north-centre Spain for agricultural application. Waste Biomass Valor. 2019, 10, 167–178. [Google Scholar] [CrossRef]
- Marañón, E.; Castrillón, L.; Fernández-Nava, Y.; Fernández-Méndez, A.; Fernández-Sánchez, A. Coagulation-flocculation as a pretreatment Process at a landfill leachate nitrification-denitrification plant. J. Hazard. Mat. 2008, 156, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Salem, Z.; Hamouri, K.; Djemaa, R.; Allia, K. Evaluation of landfill leachate pollution and treatment. Desalination 2008, 220, 108–114. [Google Scholar] [CrossRef]
- Castrillón, L.; Fernández-Nava, Y.; Ulmanu, M.; Anger, I.; Marañón, E. Physico-chemical and biological treatment of MSW landfill leachate. Waste Manag. 2010, 30, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Chemlal, R.; Azzouz, L.; Kernani, R.; Abdi, N.; Lounici, H.; Grib, H.; Mameri, N.; Drouiche, N. Combination of advanced oxidation and biological processes for the leachate treatment. Ecol. Eng. 2014, 73, 281–289. [Google Scholar] [CrossRef]
- Marañón, E.; Castrillón, L.; Fernández-Nava, Y.; Fernández-Méndez, A.; Fernández-Sánchez, A. Colour, turbidity and COD removal from old landfill leachate by coagulation-flocculation treatment. Waste Manag. Res. 2010, 28, 731–737. [Google Scholar] [CrossRef]
- De Torres-Socías, E.; Prieto-Rodríguez, L.; Zapata, A.; Fernández-Calderero, I.; Oller, I.; Malato, S. Detailed treatment line for a specific landfill leachate remediation. Brief economic assessment. Chem. Eng. Journal 2015, 261, 60–66. [Google Scholar] [CrossRef]
- Oloibiri, V.; Chys, M.; De Wandel, S.; Demeestere, K.; Van Hulle, S.W.H. Removal of organic matter and ammonium from landfill leachate through different scenarios: Operational cost evaluation in a full-scale case study of Flemish landfill. J. Environ. Manag. 2017, 203, 774–781. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, H.; Sun, L.; Shi, S. Integrated assessment for aerobic biodegradability of organic substances. Chemosphere 2002, 48, 133–138. [Google Scholar] [CrossRef]
- Kang, K.-H.; Shin, H.S.; Park, H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications. Water Res. 2002, 36, 4023–4032. [Google Scholar] [CrossRef]
- Sír, M.; Podhola, M.; Patocka, T.; Honzajkova, Z.; Kocurek, P.; Kubal, M.; Kuras, M. The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate. J. Hazard. Mat. 2012, 207-208, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and conventional technologies for landfill leachate treatment: A review. Sustainability 2017, 9, 9. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Chai, X.-L.; Katsoyiannis, I.A. The application of bioflocculant for the removal of humic acids from stabilized landfill leachates. J. Environ. Manag. 2004, 70, 35–41. [Google Scholar] [CrossRef]
- Tong, H.; Yin, K.; Ge, L.; Giannis, A.; Chuan, V.W.L.; Wang, J.-Y. Monitoring transitory profiles of leachate humic substances in landfill aeration reactors in mesophilic and thermophilic conditions. J. Hazard. Mat. 2015, 287, 342–348. [Google Scholar] [CrossRef]
- Oulego, P.; Collado, S.; Laca, A.; Díaz, M. Impact of leachate composition on the advanced oxidation treatment. Wat. Res. 2016, 88, 389–402. [Google Scholar] [CrossRef]
- Guangxia, Q.I.; Dongbei, Y.U.E.; Yongfeng, N.I.E. Characterization of humic substances in bio-treated municipal solid waste landfill leachate. Front. Environ. Sci. Eng. 2012, 6, 711–716. [Google Scholar] [CrossRef]
- Zhang, D.; Vahala, R.; Wang, Y.; Smets, B.F. Microbes in biological processes for municipal landfill leachate treatment: Community, function and interaction. Internat. Biodet. and Biodeg. 2016, 113, 88–96. [Google Scholar] [CrossRef]
- Christensen, J. B.; Jensen, D. L.; Grøn, C.; Filip, Z.; Christensen, T. H. Characterization of the dissolved organic carbon in landfill leachate polluted groundwater. Wat. Res. 1998, 32, 125–135. [Google Scholar] [CrossRef]
- Graber, E.R.; Rudich, Y. Atmospheric HULIS: How humic-like are they? A comprehensive and critical review. Atmospheric Chem. Phys. 2006, 6, 729–753. [Google Scholar] [CrossRef]
- Gomes de Melo, B.A.; Lopes Motta, F.; Andrade Santana, M.H. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mat. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Dia, O.; Drogui, P.; Buelna, G.; Dubé, R. Hybrid process, electrocoagulation-biofiltration for landfill leachate treatment. Waste Manag. 2018, 75, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.L.; Melo, B.A.G.; Santana, M.H.A. Deprotonation and protonation of humic acids as a strategy for the technological development of pH-responsive nanoparticles with fungicidal potential. N Biotechnol. 2016, 33, 773–780. [Google Scholar] [CrossRef]
- Álvarez-Puebla, R.A.; Garrido, J.J. Effect of pH on the aggregation of a gray humic acid in colloidal and solid states. Chemosphere 2005, 57, 659–667. [Google Scholar] [CrossRef]
- Souza, F. de; Roca Bragança, S. Humic Acid as Dispersant of an Alumina Suspension and its Rheological Behaviour. Mater. Res. 2018, 21, e20170759. [Google Scholar] [CrossRef]
- APHA, AWWA, WEF. Standard methods for the examination of water and wastewater; American Public Health Association, 23 rd edition: Washington DC, USA, 2017. [Google Scholar]
- Abbt-Braun, G.; Lankes, U.; Frimmel, F.H. Structural characterization of aquatic humic substances –The need for a multiple method approach. Aquat. Sci. 2004, 66, 151–170. [Google Scholar] [CrossRef]
- Ziyang, L.; Bin, D.; Xiaoli, C.; Yu, S.; Youcai, Z.; Nanwen, Z. Characterization of refuse Landfill leachates of three different stages in landfill stabilization process. J. Environ. Sci. 2009, 21, 1309–1314. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Elbl, J.; Koda, E.; Adamcová, D.; Bilgin, A.; Lukas, V.; Podlasek; A. ; Kintl, A.; Wdowska, M.; Brtnický, M. Chemical Composition and Hazardous Effects of Leachate from the Active Municipal Solid Waste Landfill Surrounded by Farmlands. Sustainability 2020, 12, 4531. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Tejera, J. , Miranda, R., Hermosilla, D., Urra, I., Negro, C., Blanco, A. Treatment of a Mature Landfill Leachate: Comparison between Homogeneous and Heterogeneous Photo-Fenton with Different Pretreatments. Water 2019, 11, 1849. [Google Scholar] [CrossRef]
- Aziz, H.A. , Rahim, N.A., Ramli, S.F., Alazaiza, M.Y.D., Omar, F.M., Hung, Y-T. Potential Use of Dimocarpus longan Seeds as a Flocculant in Landfill Leachate Treatment. Water 2018, 10, 1672. [Google Scholar] [CrossRef]
- Matovelle, C.; Quinteros, M.; Heras, D. Machine Learning Techniques in Dosing Coagulants and Biopolymers for Treating Leachate Generated in Landfills. Water 2023, 15, 4200. [Google Scholar] [CrossRef]
- Kamal, A.; Makhatova, A.; Yergali, B.; Baidullayeva, A.; Satayeva, A.; Kim, J.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Biological Treatment, Advanced Oxidation and Membrane Separation for Landfill Leachate Treatment: A Review. Sustainability 2022, 14, 14427. [Google Scholar] [CrossRef]
- Remmas, N.; Manfe, N.; Zerva, I.; Melidis, P.; Raga, R.; Ntougias, S. A Critical Review on the Microbial Ecology of Landfill Leachate Treatment Systems. Sustainability 2023, 15, 949. [Google Scholar] [CrossRef]
- Wdowczyk, A.; Szyma´nska-Pulikowska, A. Differences in the Composition of Leachate from Active and Non-Operational Municipal Waste Landfills in Poland. Water 2020, 12, 3129. [Google Scholar] [CrossRef]
- Ramli, S.F.; Aziz, H.A.; Omar, F.M.; Yusoff, M.S.; Halim, H.; Kamaruddin, M.A.; Ariffin, K.S.; Hung, Y.-T. Reduction of COD and Highly Coloured Mature Landfill Leachate by Tin Tetrachloride with Rubber Seed and Polyacrylamide. Water 2021, 13, 3062. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Le Coustumer, P. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloids and Surfaces A: Physicochem. Eng. Aspects 2006, 272, 48–55. [Google Scholar] [CrossRef]
- Pinedo, L.M.; Riascos, B.D.; Quintero, X.E.; Costa, C. Mechanism of pH sensitive flocculation for organic load and colour reduction in landfill leachate. Waste Manag. 2022, 144, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.L. de; Santos, R.F. dos; Souza, J.C. de; Izário, H.J. Characterization of controlled landfill leachate from the city of Guaratinguetá - SP, Brazil. Ambiente e Agua - An Interdisciplinary Journal of Applied Science 2018, 13, 1. [Google Scholar] [CrossRef]
- Tanaka, T. Functional groups and reactivity of size-fractionated Aldrich humic acid. Thermochim. Acta 2012, 532, 60–64. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).