1. Introduction
The treatment of breast cancer has evolved significantly over the past decades, with an increasing emphasis on personalized care models that optimize both oncologic and cosmetic outcomes. [
1,
2] Traditionally, the focus of breast cancer treatment has been on achieving optimal oncologic control, often with little regard for the aesthetic and psychological well-being of patients. However, with increasing awareness of the importance of an integrated and holistic approach to patient care, personalized treatment strategies have become increasingly important. [
3] These approaches not only prioritize cancer removal but also aim to preserve or improve quality of life particularly in terms of body image and emotional recovery.
At the “Fondazione Policlinico Universitario Agostino Gemelli IRCCS” in Rome, Italy, an innovative multidisciplinary model for the management of breast cancer, called ROME (Radiological and Oncoplastic Multidisciplinary Evaluation), has been developed. This innovative model integrates the expertise of multiple specialists into a unified, patient-centered approach.
The ROME framework brings together radiologists, surgeons, oncoplastic specialists, oncologists, radiation oncologists, geneticists, and psychologists to ensure a comprehensive assessment and personalized treatment plan for each patient. The ROME model begins with a thorough radiological assessment using advanced imaging techniques, followed by an oncoplastic assessment to identify surgical approaches that achieve optimal oncologic resection while maintaining or improving breast aesthetics.
This combination of assessments allows the team to customize treatment strategies based on the patient’s individual clinical profile, including tumor characteristics, genetic factors, and aesthetic preferences. By integrating evidence-based guidelines with real-time multidisciplinary input, ROME ensures that treatment plans balance the need for effective cancer control with the desire for an optimal aesthetic outcome. The success of the ROME model has led to significant improvements in both oncologic and aesthetic outcomes, contributing to increased patient satisfaction and quality of life. In this article, we describe the ROME model in detail, analyzing the various phases of our diagnostic-therapeutic path and illustrating how this multidisciplinary, patient-centered approach, may represent a new standard for personalized breast cancer care in breast units. (
Table 1.)
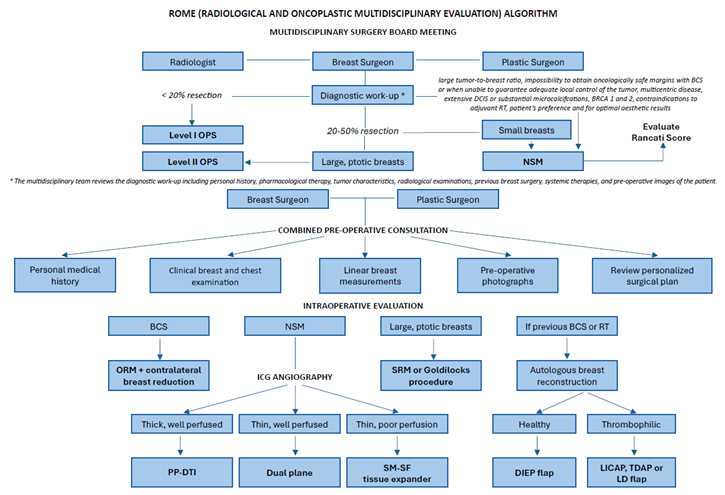
2. Multidisciplinary Surgery Board Meeting
The multidisciplinary surgery board meeting is held weekly to allow all specialists to discuss the diagnosis, staging, management and complementary therapies for new cases, complex patients and complications and employ evidence-based recommendations to construct an individual treatment plan. Additional cases requiring major or minor revisional surgery following complications are also discussed at this time. The multidisciplinary team reviews the diagnostic work-up including personal history, pharmacological therapy, tumor characteristics, radiological examinations, previous breast surgery and systemic therapies, and pre-operative images of the patient to select the best surgical plan. Integrating the best clinical and scientific evidence, centered around consensus-based guidelines and EBM, is essential to develop personalized treatment plans. [
4]
Tumor characteristics such as size and location, tumoral resection, breast size, shape, and glandular density, previous breast surgery, and the patient’s desires are major determining factors in surgical planning. Patients with operable breast cancer may undergo breast conserving surgery (BCS) or conservative mastectomy with immediate breast reconstruction (IBR). [
5] BCS has largely become the standard of care for low tumor-to-breast volume and early stage breast cancer. [
6,
7] When < 20% of breast volume is removed, Level I oncoplastic surgery (OPS) techniques are applied with the use of simple advancement or rotation flaps to fill the resected area. [
8] II level OPS is most frequent indicated in breast cancer with large and/or ptotic breasts for which a standard conserving surgery with safe margins may result in a breast deformity or unsuccessful surgery, a high tumor-to-breast volume ratio, and multifocal breast cancer [
8]. Defects following BCS can be repaired using volume displacement or volume replacement techniques with contralateral symmetry surgery as appropriate. [
9] OPS has extended the indications for BCS and allows from simple glandular reshaping to breast tissue mobilization without compromising the natural shape of the breast. Factors involved in selecting OPS are the tumor size to breast volume rate, tumor location, degree of mammary ptosis/ macromastia and pre-existing breast asymmetry. [
8] When 20-50% of the breast volume is resected in patients with large and ptotic breasts, II level OPS techniques are applied. [
10] However, patients with small breasts undergo mastectomy to preserve breast aesthetics. The authors typically use volume displacement techniques, such as oncoplastic reduction mammaplasty (ORM) with contralateral symmetrization, to maintain breast aesthetics. About therapeutic mammaplasty, we preferably apply J-scar mammaplasty technique over inverted-T pattern mammaplasty as it guarantees a reduced complication rate. [
11] OPS has not only proven to further expand breast conservative surgery with wider resection and lower recurrence rates but has also determined superior esthetic results (
Figure 1).
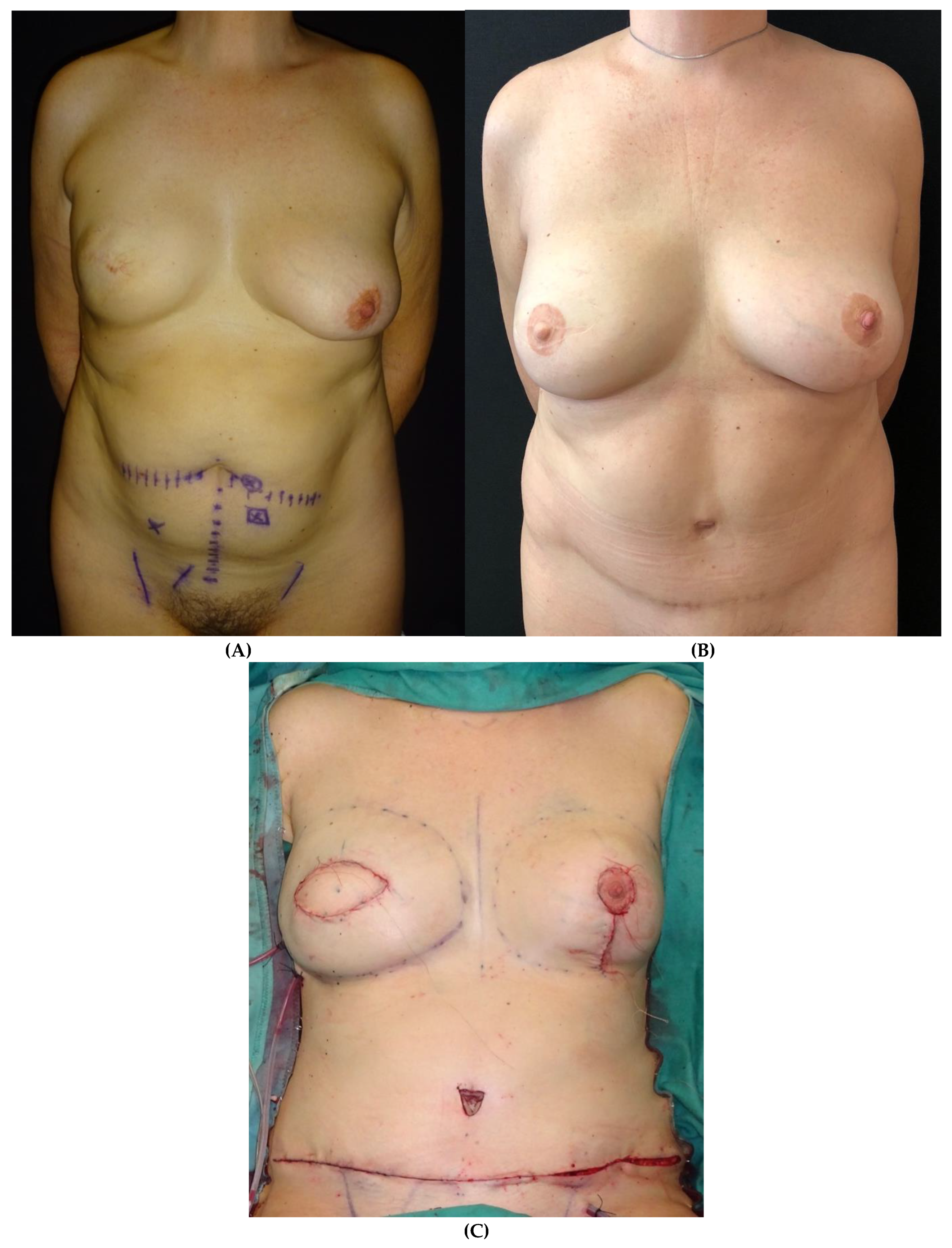
Clinical case 1
Figure 1.
– Patient with right breast cancer undergoing oncoplastic redcution mammaplasty (ORM) with J-scar (A) Preoperative view. (B) 2 year postoperative view after adjuvant radiotherapy of the right breast. (C) Intraoperative view following right supero-external quadrantectomy with specimen weight of 80 grams. (D) Immediate intraoperative result.
Figure 1.
– Patient with right breast cancer undergoing oncoplastic redcution mammaplasty (ORM) with J-scar (A) Preoperative view. (B) 2 year postoperative view after adjuvant radiotherapy of the right breast. (C) Intraoperative view following right supero-external quadrantectomy with specimen weight of 80 grams. (D) Immediate intraoperative result.
Common indications for nipple-sparing mastectomy (NSM) include a large tumor-to-breast ratio, the impossibility to obtain oncologically safe margins with BCS or when unable to guarantee adequate local control of the tumor, multicentric disease, extensive ductal carcinoma in situ (DCIS) or substantial microcalcifcations, inherited high-risk gene mutations such as BRCA 1 and 2, contraindications to adjuvant radiotherapy, patient’s preference and for optimal aesthetic results; absolute contraindications for NSM include inflammatory breast cancer and locally advance tumor involving the skin or nipple-areola complex (NAC). [
12,
13] For those undergoing mastectomy, immediate breast reconstruction (IBR) is considered an oncologically safe technique for selected patients that permits enhanced quality of life and esthetic outcomes.
Mastectomy flap thickness (MFT) is evaluated within digital mammography according to
Rancati score to determine the patients who are candidates for prepectoral (PP) breast reconstruction. [
14] We perform PP implant-based reconstruction, mainly Direct-To-Implant (DTI), in over 90% of the conservative mastectomies in accordance with the Italian data related to the Italian Senology Centers belonging to Senonetwork. [
15] Thanks to the innovation of PP-DTI, the cost-effective technique demonstrates a reduction in operative time and complications related to submuscular breast implant placement, a reduction in contralateral breast symmetrization in unilateral cases, excellent esthetic outcomes and a superior quality of life. [
16,
17,
18]
Patients with thick mastectomy flaps (Rancati score 2 and 3, i.e. MFT equal to or greater than 1 cm) undergo PP-DTI breast reconstruction. In case of thin mastectomy flaps (MFT between 0.7cm and 1cm measured at the site of incision) and well vascularized at ICG angiography, patients undergo PP-DTI (
Figure 2 and
Figure 3). Patients with thin mastectomy flaps (0.5-0,7mm measured at the site of incision) that are well vascularized at ICG angiography, preferably in bilateral cases, undergo renewed dual plane DTI breast reconstruction. [
19] Thin patients with poorly vascularized mastectomy flaps at ICG angiography undergo submuscular-subfascial tissue expander placement. [
20]
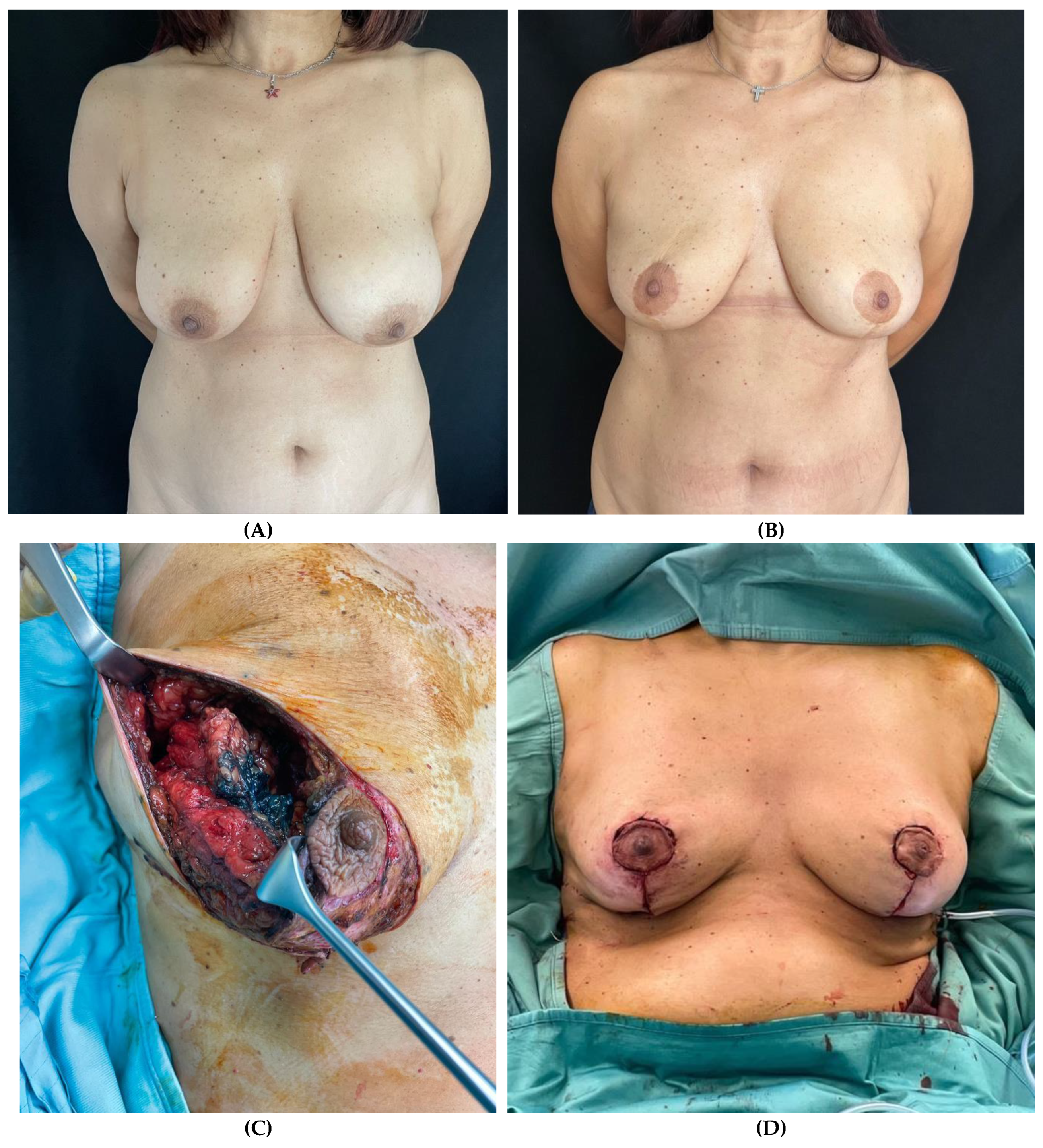
Clinical case 2
Figure 2.
– Patient undergoing right nipple-sparing mastectomy (NSM) and immediate prepectoral breast reconstruction. (A) Pre-operative view. (B) Post-operative view after a 2-year follow-up. (C) Intra-operative view of the mastectomy flap thickness of the right breast at the incision site. (D) Immediate intraoperative view after prepectoral placement of short height, high projection, polyurethane-covered 115 cc anatomical implant.
Figure 2.
– Patient undergoing right nipple-sparing mastectomy (NSM) and immediate prepectoral breast reconstruction. (A) Pre-operative view. (B) Post-operative view after a 2-year follow-up. (C) Intra-operative view of the mastectomy flap thickness of the right breast at the incision site. (D) Immediate intraoperative view after prepectoral placement of short height, high projection, polyurethane-covered 115 cc anatomical implant.
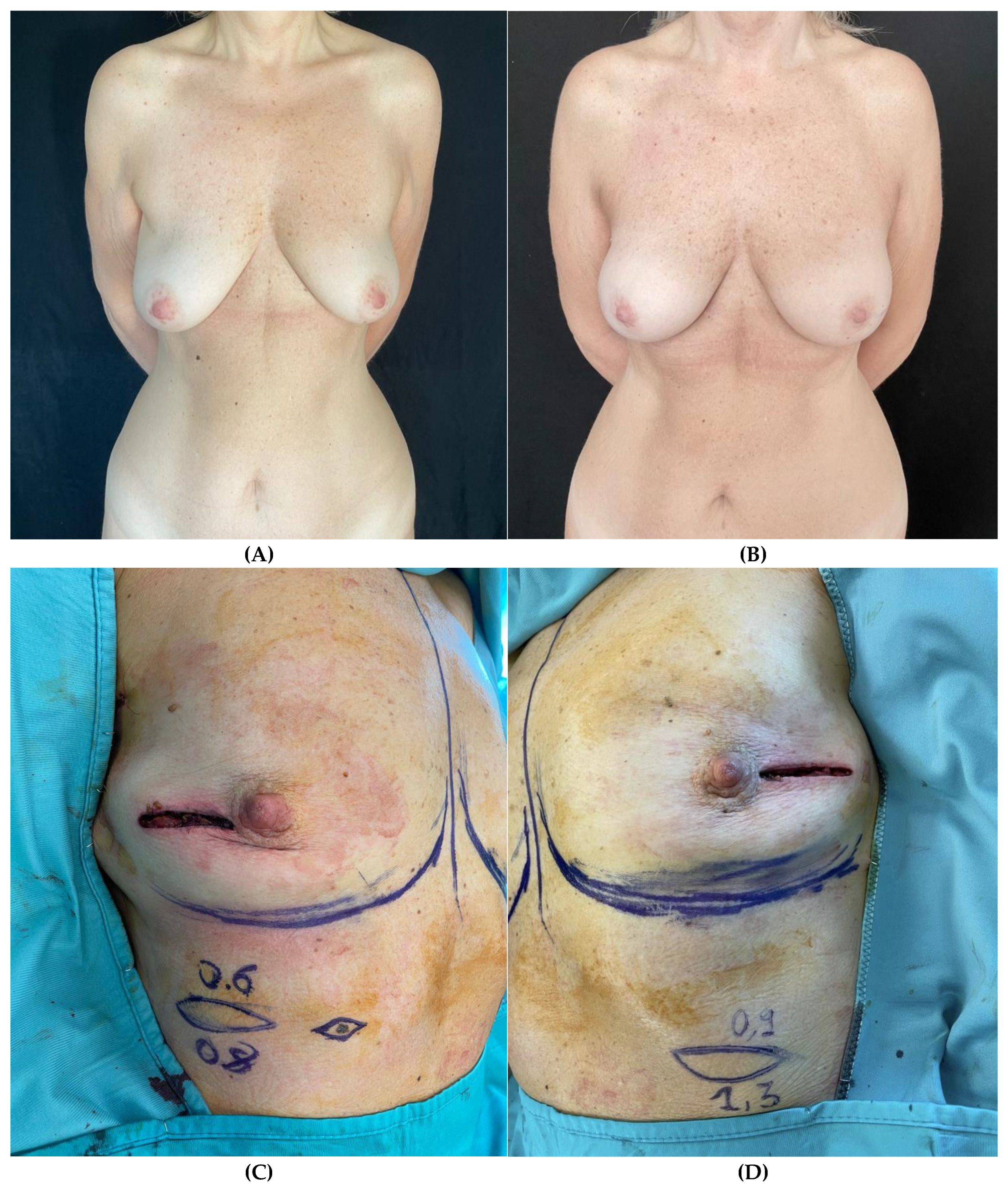
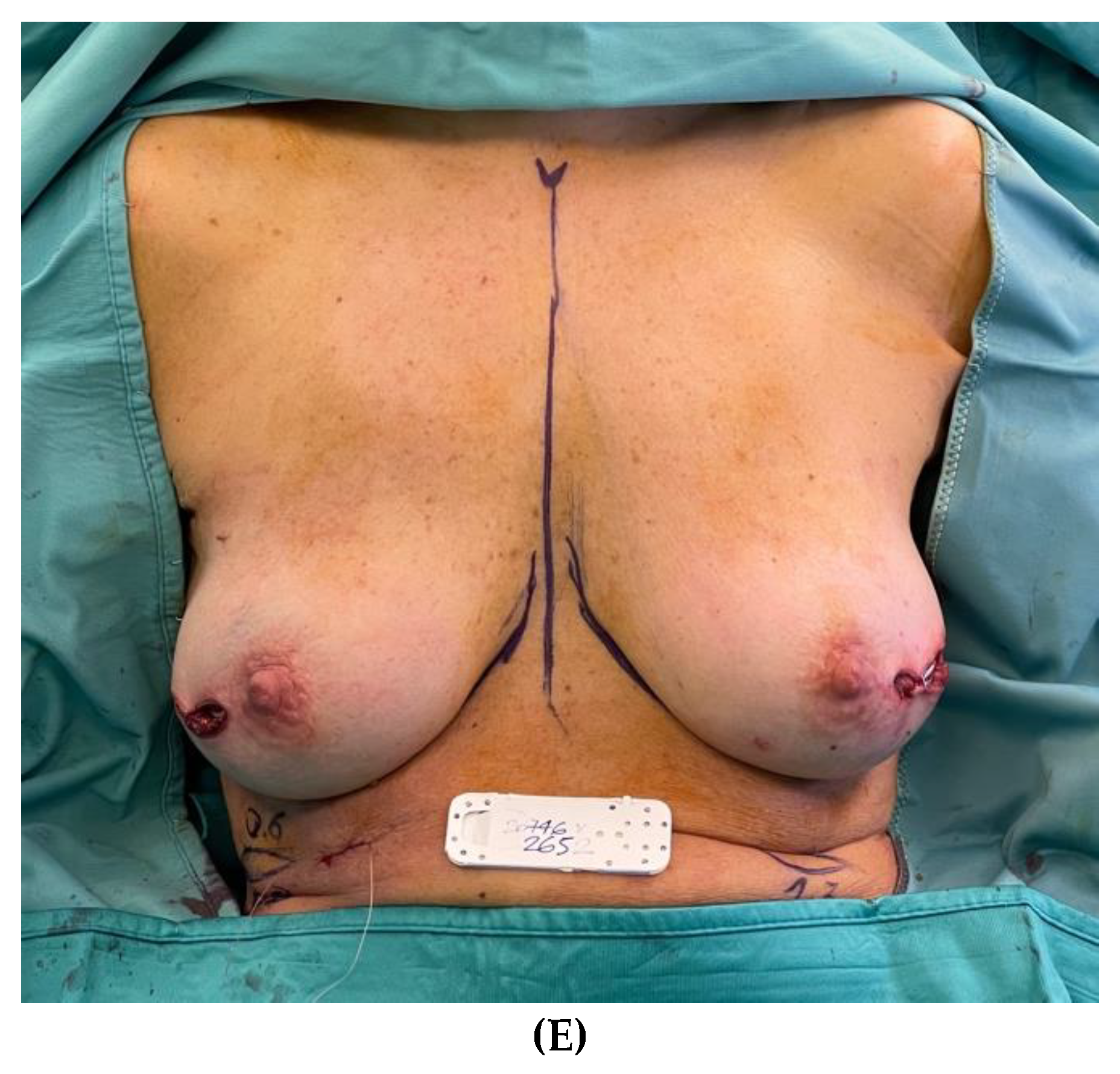
Clinical case 3
Figure 3.
– Patient with right breast cancer undergoing bilateral NSM due to BRCA mutation and immediate prepectoral breast reconstruction bilaterally. (A) Preoperative view with noticeable breast asymmetry. (B) Postoperative view after 3-year follow-up. (C, D) Intraoperative view of the thickness measurements of the mastectomy flaps of both breasts at the incision site. (E) Immediate intraoperative view after prepectoral placement of short height, high projection, polyurethane-covered 265 cc anatomical implants bilaterally.
Figure 3.
– Patient with right breast cancer undergoing bilateral NSM due to BRCA mutation and immediate prepectoral breast reconstruction bilaterally. (A) Preoperative view with noticeable breast asymmetry. (B) Postoperative view after 3-year follow-up. (C, D) Intraoperative view of the thickness measurements of the mastectomy flaps of both breasts at the incision site. (E) Immediate intraoperative view after prepectoral placement of short height, high projection, polyurethane-covered 265 cc anatomical implants bilaterally.
Patients with very large and ptotic breasts who are poor candidates for NSM may undergo skin-reducing mastectomy (SRM) with J-scar pattern or inverted T pattern. PP DTI reconstruction is performed in case of thick mastectomy flaps (Rancati score 2 and 3). Patients with Rancati score 1 undergo submuscular-subfascial tissue expander placement.
Patients who are obese or who are poor candidates for implant-based reconstruction may undergo Goldilocks mastectomy, a simple mastectomy without implant reconstruction that uses redundant mastectomy flap tissue alone to create a breast mound. [
21]
If the patient has a history of breast augmentation, a thorough evaluation is performed, and surgery is planned according to the oncological resection required and MFT. In case of BCS and abundant residual gland, the implants are removed, and the remaining gland is remodeled. In patients undergoing mastectomy, our preferred reconstruction is PP DTI (Rancati score 2 and 3), and submuscular DTI reconstruction in Rancati score 1 patients. [
22]
Generally, in patients who have undergone previous radiotherapy of the breast as for BCS and radiation treatment (RT), given the high incidence of complications, the authors opt for autologous-based reconstruction with flaps. Autologous breast reconstruction is planned at the joined consultation where the plastic surgeons together with the breast surgeon, select the most indicated flap according to the patient’s somatotype, coagulative status, and personal wishes. All patients undergo a pre-operative screening to identify asymptomatic or unidentified coagulation disorders or prothrombotic status. According to the literature, the DIEP flap is our gold standard, and the PAP flap is considered in patient without excess abdominal skin or previous abdominoplasty(
Figure 4). [
23,
24] In pro-thrombotic patients, a lateral intercostal artery perforator (LICAP) flap, Thoracodorsal artery perforator (TDAP) flap or pedicled latissimus dorsi (LD) flap are planned (
Figure 5).
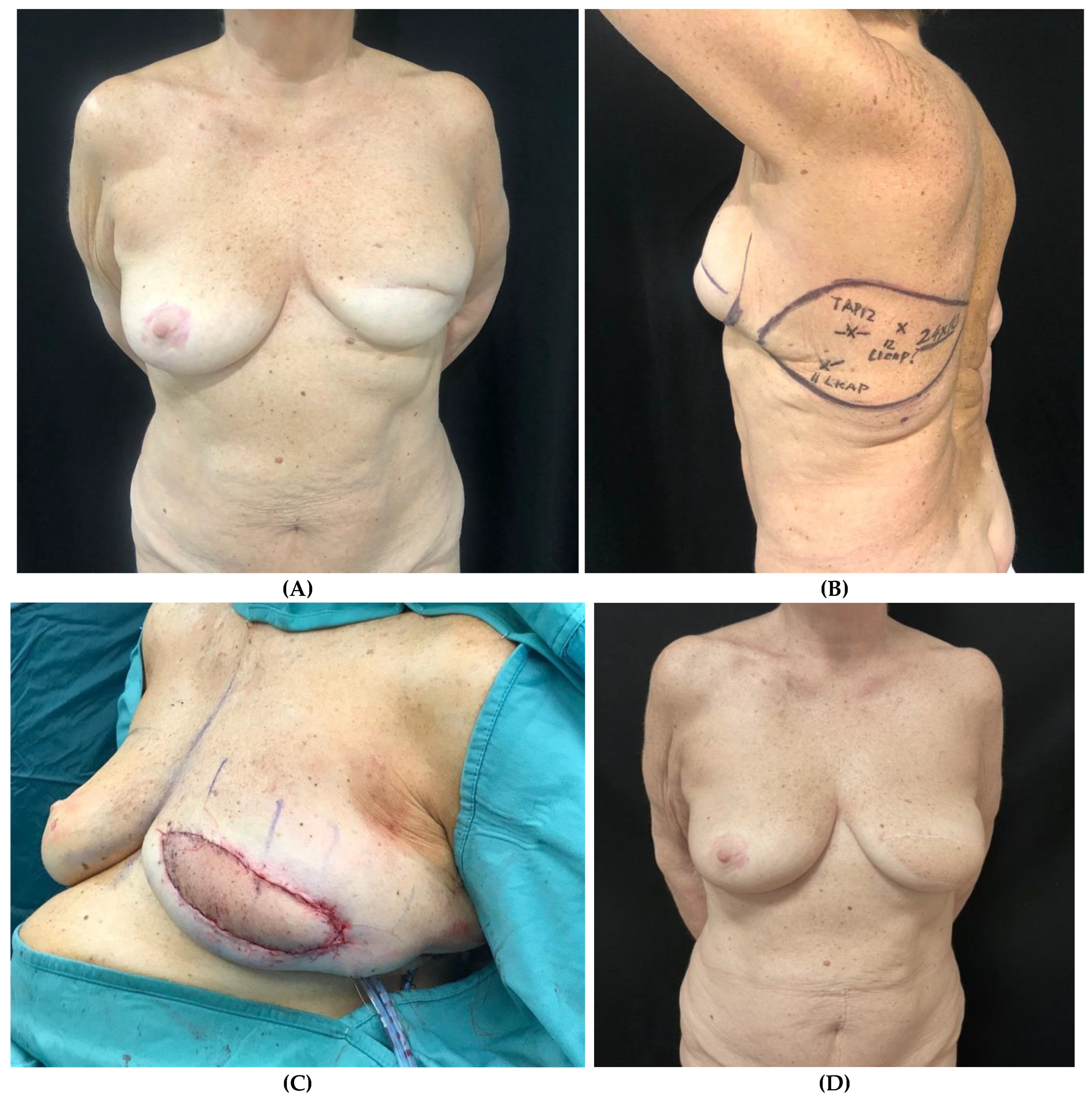
Clinical case 4
Figure 4.
– Patient planned for delayed left breast reconstruction with TDAP flap following previous skin sparing mastectomy (SSM) and submuscular tissue expander placement. (A) Preoperative view. (B) Preoperative markings of the skin paddle and TDAP perforators identified at ultrasound (C) Intraoperative view following flap inset. (D) Post-operative view after 3-year follow-up.
Figure 4.
– Patient planned for delayed left breast reconstruction with TDAP flap following previous skin sparing mastectomy (SSM) and submuscular tissue expander placement. (A) Preoperative view. (B) Preoperative markings of the skin paddle and TDAP perforators identified at ultrasound (C) Intraoperative view following flap inset. (D) Post-operative view after 3-year follow-up.
Clinical case 5
Figure 5.
– Patient who underwent delayed right breast reconstruction with DIEP flap following previous skin sparing mastectomy (SSM) and submuscular tissue expander placement. (A) Preoperative view. (B) Postoperative view after 2-year follow-up. (C) Intraoperative view following flap inset and contralateral mastopexy.
Figure 5.
– Patient who underwent delayed right breast reconstruction with DIEP flap following previous skin sparing mastectomy (SSM) and submuscular tissue expander placement. (A) Preoperative view. (B) Postoperative view after 2-year follow-up. (C) Intraoperative view following flap inset and contralateral mastopexy.
3. Combined Pre-Operative Consultation
At the time of the weekly surgery board meeting, a consultation with both the breast surgeon and plastic surgeon is programmed in order to perform a joined assessment of the patient. Any additional appointments are programmed at this time if deemed necessary. When possible, the multidisciplinary consultation is programmed the same day as the pre-operative evaluation to limit patient discomfort in returning several times to the hospital. A pre-operative psychological evaluation is also provided for all patients at this time.
The plastic surgeon and breast surgeon together perform a detailed evaluation of the patient that includes:
Demographic variables (age, weight, height, BMI) and medical history, current pharmacological therapy, type of breast cancer and location, neoadjuvant chemotherapy, previous radiotherapy, and previous aesthetic or reconstructive breast surgery.
Clinical breast and chest examination to determine breast shape and volume, the presence of breast anomalies or chest malformations, the presence of scars from previous breast surgery, signs of previous radiotherapy including fibrosis or skin alterations, presence of implants from previous breast augmentation, collect linear breast measurements (sternal notch-to-nipple distance, nipple-to-inframammary fold distance, nipple-to-nipple distance, and chest, waist and hip circumference). The plastic surgeon and the breast surgeon decide together when to perform an autologous or implant-based reconstruction. In case of implant-based reconstruction, the position of the implant (whether prepectoral or retropectoral) is chosen based on mammograms and clinical evaluation, including MFT and based on the Rancati score for the classification of breast tissue coverage (BTCC). [
14]
Skin incision is also planned. In patients undergoing mastectomy and IBR, the skin incision may be radial lateral, at the inframammary fold, or planned as a skin-reducing mastectomy (SRM) in case of very large and ptotic breasts. The radial incision provides the best access for performing mastectomy while also maintaining maximal vascularization of the NAC. [
25,
26] In case of nipple resection for tumor infiltration after intraoperative frozen section, the radial incision may be extended to include the NAC. The mastectomy may also be performed through the IMF incision when the breast is small and without ptosis. We prefer the lateral IMF approach as it provides adequate exposure, eliminates visible scars on the anterior surface of the breast and preserves the anterior intercostal artery perforator (AICAP), an important neurovascular pedicle for the NAC. [
27] Previous breast scars represent a further skin access, to be preferred as to avoid devascularization of the NAC, particularly in patients who have previously undergone breast reduction surgery.
Photographic documentation of the patient’s breasts in the frontal, oblique and lateral projections.
Review and discussion of the personalized surgical plan and post-operative indications with the patient and their family.
Patients undergoing II level OPS with displacement technique, such as a oncoplastic reduction mammaplasty (ORM), are marked bilaterally for a J-scar mammaplasty by the plastic surgeon following tumor localization with pre-operative breast ultrasound and/or mammogram. [
11]
4. Intraoperative Evaluation
In case of ORM with the J-scar mammaplasty, partial breast resection is performed by the breast surgeon through the planned incisions marked by the plastic surgeon. Following tumor resection, the plastic surgeon appropriately mobilizes the residual parenchyma by creating a dermo-glandular flap to fill the defect. The vascularization of the flap may be
superomedial for defects of the external breast quadrants,
superolateral for defects of the inferointernal quadrants, and
inferior for defects of the superior quadrants. [
28] The resected gland is weighted to perform an analogous resection of the inferior quadrants of the contralateral breast reduction and maintain breast symmetry.
The surgical treatment of axillary lymph nodes is performed according to the latest NCCN guidelines. [
29]
For patients undergoing mastectomy, the mastectomy flap thickness is intraoperatively assessed at the margins of the surgical incision. We perform an intraoperative ICG angiography on all patients to determine whether or not PP-DTI is feasible and if the NAC may be preserved. If the mastectomy flaps are well vascularized, PP-DTI is deemed appropriate and performed. The mastectomy specimen is weighted and, depending on its weight, implant sizers are used to select the breast implant that best fills the prepectoral pocket. The volume of the breast implant is usually equal to or 20% greater than the weight of the mastectomy specimen. Patients undergo skin-sparing mastectomy (SSM) in case of tumor involvement of the NAC. In case of implant-based IBR the implant is slightly smaller than the mastectomy specimen. A contralateral breast reduction is usually performed to obtain symmetry.
Nowadays, in our center, all patients receive polyurethane-coated, anatomical, short or medium height-breast implants. We prefer polyurethane-coated implants for the stability provided and the integration into the surrounding tissues which reduces the dead space and the incidence of post-operative seromas. [
30,
31]
In cases of suboptimal vascularization of the mastectomy flaps, a submuscular-subfascial tissue expander is placed. [
20]
Immediately upon skin closure, a single-use negative pressure wound therapy (sNPWT) care system is applied to the surgical wound and maintained for 7 days post-operatively. The sNPWT care system is systematically used in high-risk patients such as smokers, obese, and patients with thin mastectomy flaps undergoing PP or renewed dual plane DTI.
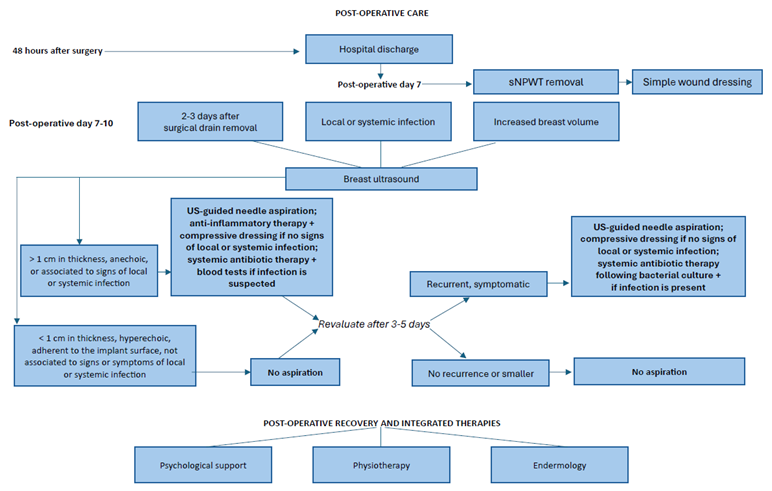
5. Post-Operative Care
Proper patient education is fundamental to avoid early post-operative complications. At the time of hospital discharge, usually after 2 days, every patient is instructed on how to perform surgical drain care every 24 hours and maintain arm and shoulder rest to avoid tension on surgical incisions and fluid accumulation. Patients without sNPWT are also instructed on how to perform proper wound care.
Post-operative check-ups are performed at the out-patient breast unit clinic at the FPG. At the first scheduled post-operative check-up on post-operative day 7, the sNPWT care system is removed from the surgical wound. Upon dressing removal, we evaluate the surgical wound and determine if there is any delay in wound healing. In such cases, patients undergo surgical wound revision in a dedicated outpatient clinic setting. Patients are instructed to maintain the surgical wounds clean and perform wound dressing changes every 24 hours. Surgical drains are assessed at every post-operative evaluation and removed when fluid collection is less than 20 mL and stable in the preceding 24 hours.
Additionally, attentive post-operative care following breast surgery and reconstruction is important to identify the early signs of complications. Proper wound and surgical drain management are fundamental for a successful implant-based breast reconstruction. The addition of breast ultrasound (US) may identify fluid collections and reduce the risk of infection, would dehiscence and implant loss. [
32]
Breast US is a vital tool for breast surgery. US is useful in the initial evaluation of patients presenting with signs and symptoms of fluid accumulation in the axillary cavity or in the Implant pocket. During the post-operative check-ups, patients routinely undergo a breast US with the aid of a color-coded duplex sonography 2-3 days after surgical removal of the drain to check that there is no fluid around the implant. US evaluation is of course performed if signs or symptoms of clinical or subclinical infection are present, or in case of a sudden increase in breast volume.
In case of significant fluid accumulation in the axillary cavity, a US-guided needle aspiration may be performed. After aspiration, a compressive dressing is applied to the area of the aspirated area to reduced further fluid accumulation. The patient is re-evaluated 3-5 days following fluid aspiration. A fluid aspiration may be repeated in cases of large fluid accumulations. Repeated aspirations are avoided if the fluid accumulation is significant smaller.
In case of fluid collection around the implant, the size and characteristics are determined through US evaluation. If the fluid collection is small, less than 1 cm in thickness, hyperechoic, adherent to the implant surface, or not associated to signs or symptoms of local or systemic infection, fluid aspiration is avoided, and the patient is monitored by US the following week. If the fluid collection is > 1 cm in thickness, anechoic, or associated to signs of local or systemic infection, US-guided needle aspiration is performed followed by fluid analysis with culture and gram stain. An anti-inflammatory therapy and compressive dressing is applied if no signs of local or systemic infection are present. A broad-spectrum antibiotic therapy is prescribed for patients with signs and symptoms that suggest an infection and is continued until the causative agent is revealed, then the appropriate antibiotic is selected. Blood tests including a complete blood count and indicators of inflammation are prescribed prior to and throughout the course of antibiotic therapy. Patients are revaluated 3 to 5 days later. In the absence of fluid accumulations or presence of small fluid accumulations, no aspiration is performed. In the presence of recurrent and symptomatic fluid accumulations, US-guided need aspiration is repeated, and the treatment proceeds as previously described.
If after surgical drain removal no fluid accumulations have occurred, patients are instructed to gradually begin arm and shoulder movement to regain mobility and functionality. Simple exercises may be performed a few times a day for 5-10 minutes. In case of limited range of motion, patients are advised to undergo physical rehabilitation with a physiotherapist.
After complete wound healing, all patients are prescribed a silicone-based gel to improve final scar appearance. A post-operative evaluation is planned at 3, 6 and 12 months.
In case of adjuvant radiotherapy, secondary surgical interventions, such as lipofilling, are discussed with the patient.
6. Postoperative Functional Recovery Through Integrated Therapies
The postoperative recovery following breast cancer surgery is a process that requires more than just physical healing; it also involves emotional, psychological and aesthetic rehabilitation. In the context of the ROME model, the inclusion of integrated therapies plays a pivotal role in enhancing functional recovery, preventing complications such as lymphedema and improving aesthetic outcomes.
Psychological well-being is a cornerstone of the recovery process after breast cancer surgery. The emotional toll of the diagnosis and treatment can significantly impact patients’ mental health, self-esteem and body image. Within the ROME framework, psychological support is integrated as part of the multidisciplinary care approach providing patients with professional counseling and therapeutic support to navigate the emotional complexities of their recovery journey.
Psychological counseling helps patients address feelings of anxiety, depression and body image issues that may arise post-surgery. Support groups and individual therapy sessions are offered to foster emotional resilience, encourage coping strategies and enhance overall mental well-being. This holistic support system not only accelerates emotional healing but also improves physical recovery by empowering patients to take an active role in their rehabilitation and by enhancing their overall quality of life.
Physiotherapy is an essential part of postoperative care in breast cancer patients focusing on both functional recovery and the prevention of complications like lymphedema. Following surgery, patients often experience limitations in arm mobility, strength and range of motion that may impair daily activities and prolong the recovery process. A tailored physiotherapy program designed to restore upper body function is crucial to regain mobility and independence.
One of the key concerns in postoperative breast cancer patients is lymphedema, a common complication resulting from the removal of lymph nodes during surgery. Lymphedema leads to fluid retention, swelling and discomfort in the arm that can significantly impact quality of life. In the ROME model, physiotherapists work proactively to prevent the onset of lymphedema through manual lymphatic drainage and specific exercises to promote lymphatic flow and education on self-care techniques. These methods help patients reduce the risk of lymphedema, improve arm function and prevent long-term complications.
Endermology, a non-invasive treatment using mechanical massage, is increasingly employed in postoperative breast cancer care to enhance aesthetic outcomes. The procedure stimulates the skin and underlying tissue promoting lymphatic drainage, improving blood circulation and encouraging tissue regeneration. For breast cancer patients, endermology offers significant benefits in improving skin elasticity reducing the appearance of scars and enhancing overall breast aesthetics after surgery.
Endermology can be particularly useful for patients undergoing breast-conserving surgery and autologous reconstruction, as it helps to refine and smooth the contours of the breast contributing to more natural-looking results. In conjunction with other therapies, such as physiotherapy and psychological support, endermology helps restore the aesthetic appearance of the breast, addressing both functional and emotional recovery needs.
7. Conclusion
The ROME (Radiological and Oncoplastic Multidisciplinary Evaluation) model represents a major step forward in the personalized care of breast cancer patients. By bringing together the expertise of multiple specialists within a collaborative, evidence-based framework, ROME ensures that treatment plans are meticulously tailored to meet each patient’s unique clinical and aesthetic needs.
The success of this model is based on its comprehensive approach, combining detailed radiological assessments, oncoplastic evaluations and continuous multidisciplinary input throughout every stage of the treatment process - from preoperative planning to postoperative care through Integrated Therapies. The multidisciplinary surgery board meetings and combined preoperative consultations play a crucial role in developing individualized treatment strategies that effectively balance oncological outcomes with the preservation of breast aesthetics.
This collaborative approach continues into the operating room and post-operative care, ensuring the highest standard of care and the best possible outcomes in both oncological control and cosmetic results. ROME model may not only enhance patient satisfaction but also contribute to a better quality of life for breast cancer patients. The integration of clinical, radiological, and aesthetic considerations through a patient-centered approach is setting a new benchmark for breast cancer care. Looking forward, ROME represents a pioneering model for personalized breast cancer surgery and reinforces the need for a holistic, multidisciplinary approach to oncology, marking a new era in the standard of care for breast cancer patients.
References
- Krzyszczyk P, Acevedo A, Davidoff EJ, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018;6(3-4):79-100. [CrossRef]
- Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018;109(6):952-963. [CrossRef]
- Alzeer J. Integrating medicine with lifestyle for personalized and holistic healthcare. Uzh. January 2023.
- https://www.academia.edu/111394498/Integrating_medicine_with_lifestyle_for_personalized_and_holistic_healthcare.
- Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ. 2018;363:k4245. Published 2018 Dec 10. [CrossRef]
- Mason EJ, Di Leone A, Franco A, D'Archi S, Rianna C, Sanchez AM, Murando F, Accetta C, Scardina L, Terribile DA, Masetti R, Franceschini G. Oncoplastic Breast Surgery versus Conservative Mastectomy in the Management of Large Ductal Carcinoma In Situ (DCIS): Surgical, Oncological, and Patient-Reported Outcomes. Cancers (Basel). 2022 Nov 16;14(22):5624. [CrossRef] [PubMed] [PubMed Central]
- Franceschini G, Mason EJ, Grippo C, et al. Image-Guided Localization Techniques for Surgical Excision of Non-Palpable Breast Lesions: An Overview of Current Literature and Our Experience with Preoperative Skin Tattoo. J Pers Med. 2021;11(2):99. Published 2021 Feb 4. [CrossRef]
- Nardone L, Valentini V, Marino L, et al. A feasibility study of neo-adjuvant low-dose fractionated radiotherapy with two different concurrent anthracycline-docetaxel schedules in stage IIA/B-IIIA breast cancer. Tumori. 2012;98(1):79-85. [CrossRef]
- Mason EJ, Di Leone A, Franco A, D'Archi S, Rianna C, Sanchez AM, Murando F, Accetta C, Scardina L, Terribile DA, Masetti R, Franceschini G. Oncoplastic Breast Surgery versus Conservative Mastectomy in the Management of Large Ductal Carcinoma In Situ (DCIS): Surgical, Oncological, and Patient-Reported Outcomes. Cancers (Basel). 2022 Nov 16;14(22):5624. [CrossRef] [PubMed] [PubMed Central]
- Chu CK, Hanson SE, Hwang RF, Wu LC. Oncoplastic partial breast reconstruction: concepts and techniques. Gland Surg. 2021;10(1):398-410. [CrossRef]
- Vindigni V, Marena F, Zanettin C, Bassetto F. Breast Reconstruction: The Oncoplastic Approach. J Clin Med. 2024;13(16):4718. Published 2024 Aug 12. [CrossRef]
- Gasperoni C, Salgarello M, Gasperoni P. A personal technique: mammaplasty with J scar. Ann Plast Surg. 2002;48(2):124-130. [CrossRef]
- Scardina L, Di Leone A, Biondi E, Carnassale B, Sanchez AM, D'Archi S, Franco A, Moschella F, Magno S, Terribile D, Gentile D, Fabi A, D'Angelo A, Barone Adesi L, Visconti G, Salgarello M, Masetti R, Franceschini G. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J Pers Med. 2022 Sep 19;12(9):1533. [CrossRef] [PubMed] [PubMed Central]
- Franceschini G. Editorial: Methods in breast cancer. Front Oncol. 2023;13:1304941. Published 2023 Nov 10. [CrossRef]
- Rancati A, Angrigiani C, Hammond D, et al. Preoperative digital mammography imaging in conservative mastectomy and immediate reconstruction. Gland Surg. 2016;5(1):9-14. [CrossRef]
- Ghilli M, Lisa AVE, Salgarello M, et al. Oncoplastic and reconstructive surgery in SENONETWORK Italian breast centers: lights and shadows. Breast. 2024;73:103601. [CrossRef]
- Salgarello M, Visconti G, Barone-Adesi L, Franceschini G, Masetti R. Contralateral breast symmetrisation in immediate prosthetic breast reconstruction after unilateral nipple-sparing mastectomy: the tailored reduction/augmentation mammaplasty. Arch Plast Surg. 2015;42(3):302-308. [CrossRef]
- Scardina L, Di Leone A, Biondi E, et al. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J Pers Med. 2022;12(9):1533. Published 2022 Sep 19. [CrossRef]
- Nava MB, Catanuto G, Rocco N. How to optimize aesthetic outcomes in implant-based breast reconstruction. Arch Plast Surg. 2018 Jan;45(1):4-13. [CrossRef] [PubMed] [PubMed Central]
- Salgarello M. Ricostruzione mammaria: perché autologa, perché eterologa. Presented as part of Finalmente Mammella! 2024; November 8, 2034; Santa Margherita Ligure, Italy.
- Salgarello M, Visconti G, Barone-Adesi L. Use of the subpectoral fascia flap for expander coverage in postmastectomy breast reconstruction. Plast Reconstr Surg. 2011;127(2):1010-1011. [CrossRef]
- Richardson H, Ma G. The Goldilocks mastectomy. Int J Surg. 2012;10(9):522-526. [CrossRef]
- Salgarello M, Fabbri M, Visconti G, Barone Adesi L. Implant-Based Breast Reconstruction After Nipple-Sparing and Skin-Sparing Mastectomy in Breast-Augmented Patients: Prepectoral or Submuscular Direct-to-Implant Reconstruction?. Aesthet Surg J. 2024;44(5):503-515. [CrossRef]
- Haddock NT, Teotia SS. Efficient DIEP Flap: Bilateral Breast Reconstruction in Less Than Four Hours. Plast Reconstr Surg Glob Open. 2021;9(9):e3801. Published 2021 Sep 7. [CrossRef]
- Clarke-Pearson EM, Chadha M, Dayan E, et al. Comparison of irradiated versus nonirradiated DIEP flaps in patients undergoing immediate bilateral DIEP reconstruction with unilateral postmastectomy radiation therapy (PMRT). Ann Plast Surg. 2013;71(3):250-254. [CrossRef]
- Munhoz AM, Montag E, Filassi JR, Gemperli R. Immediate nipple-areola-sparing mastectomy reconstruction: An update on oncological and reconstruction techniques. World J Clin Oncol. 2014;5(3):478-494. [CrossRef]
- Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg. 2011;19(4):129-133. [CrossRef]
- Nahabedian MY, Angrigiani C, Rancati A, Irigo M, Acquaviva J, Rancati A. The Importance of Fifth Anterior Intercostal Vessels following Nipple-Sparing Mastectomy. Plast Reconstr Surg. 2022;149(3):559-566. [CrossRef]
- Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol. 2010;17(5):1375-1391. [CrossRef]
- National Comprehensive Cancer Network. Breast Cancer (version 4.2022). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419.
- Salgarello M, Pagliara D, Barone Adesi L, Visconti G, Wild JB, Matey P. Direct to Implant Breast Reconstruction With Prepectoral Micropolyurethane Foam-Coated Implant: Analysis of Patient Satisfaction. Clin Breast Cancer. 2021;21(4):e454-e461. [CrossRef]
- de Vita R, Buccheri EM, Villanucci A, Pozzi M. Breast Reconstruction Actualized in Nipple-sparing Mastectomy and Direct-to-implant, Prepectoral Polyurethane Positioning: Early Experience and Preliminary Results. Clin Breast Cancer. 2019;19(2):e358-e363. [CrossRef]
- Banuelos J, Esquer-Garrigos Z, Sohail MR, et al. Diagnosis of Infectious Fluid Collections in Implant-Based Breast Reconstruction: The Role of Ultrasound. J Breast Imaging. 2019;1(4):310-315. [CrossRef]
Table 1.
The five fundamental steps of ROME (Radiological and Oncoplastic Multidisciplinary Evaluation).
Table 1.
The five fundamental steps of ROME (Radiological and Oncoplastic Multidisciplinary Evaluation).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).