Introduction-
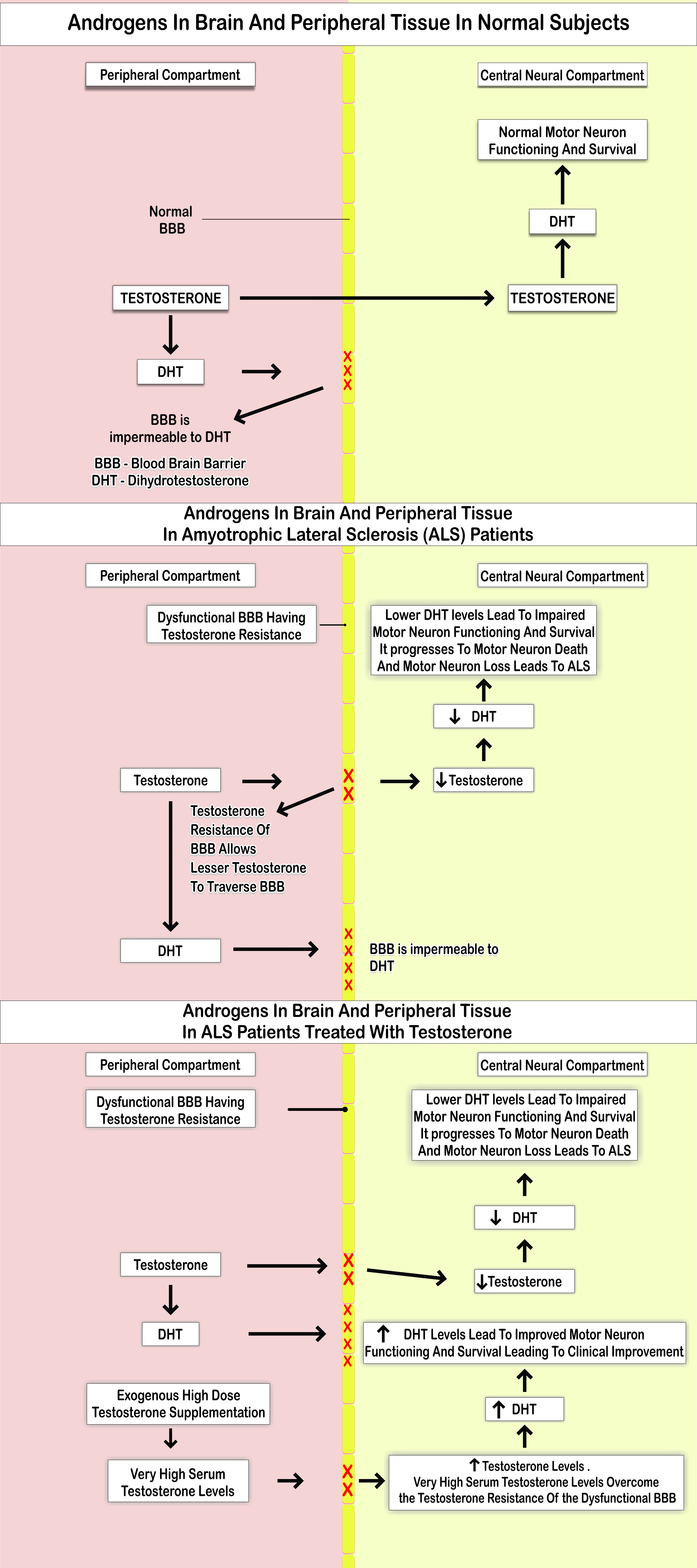
Testosterone is implicated by some studies in Amyotrophic lateral Sclerosis
(ALS) pathogenesis. A recent paper [
1] found markedly low CSF dihydrotestosterone (DHT) levels in ALS patients as compared to normal controls and postulated that deficiency of DHT in brain leads to ALS. Since DHT has very poor blood brain barrier (BBB) penetration, hence almost the entire DHT found in brain is formed from testosterone in the brain. The paper [
1] postulated that ALS patients have a BBB which has a low permeability to testosterone ( a sort of testosterone resistance) and this model explains the peripheral hyper-androgenic phenotype found in many Amyotrophic lateral Sclerosis
/Motor Neuron disease (ALS/MND) patients.
Ou et al [
2] in their study using a two sample Mendelian randomization (MR) approach found that higher levels of serum sex hormone binding globulin were associated with an increased risk of ALS. They confirmed this causal effect using sensitivity analyses, including the MR-Egger , weighted median , and weighted mode methods. Higher levels of Sex hormone binding globulin (SHBG) are associated with a decrease in amount of biologically active free testosterone which is the fraction which traverses the Blood brain barrier and is the main contributor to intra-cerebral and cerebrospinal fluid testosterone .
Celotti et al [
3] in their study found that maximum concentration of the enzyme 5-alpha reductase were found in the corticospinal tracts amongst all white matter structures of the brain. This leads to the corticospinal tracts having maximum dihydrotestosterone (DHT) concentrations amongst all white matter structures of the brain thereby giving support to the hypotheses proposed by Sawal et al that DHT is essential for proper functioning and survival of the motor cortex and corticospinal tracts .
Castelli et al [
4] in their study on rat brains found that in the cerebral cortex in layers II, III and V , 5α reductase 2 immunoreactivity was predominantly expressed on the cell bodies of larger pyramidal neurons again demonstrating that DHT is essential for proper functioning and survival of the motor cortex and corticospinal tracts .
Gargiulo-Monachelli et al [
5]in their paper evaluated gonadal hormone levels in both male and female ALS patients and controls. They found that both male and female ALS patients had higher serum testosterone levels than age matched controls with the difference between female ALS patients and female controls being statistically significant. There was no significant difference in serum levels of Sex hormone binding globulin (SHBG ) between patients and control subjects. They also found that ALS patients showing higher testosterone levels presented with a more rapid worsening of the monthly forced vital capacity (FVC) values indicating rapid disease progression. They found that levels of total testosterone ( TT) and free testosterone (FT )were significantly higher in ALS patients with a monthly FVC decline ≥ 2.5 % than in ALS patients with a monthly FVC decline< 2.5 % ( p values being < 0.05 for both TT and FT).
Based on these studies , we can assume that decreased brain DHT may play a causative role in ALS and replenishing brain DHT levels may have a therapeutic role in ALS. Since brain DHT levels can only be increased by administration of testosterone which traverses the BBB, it is plausible that testosterone may have a therapeutic effect in ALS.
Methods
9 patients diagnosed with ALS were included in the study. Inclusion criteria were that patients had to fulfil the diagnosis of clinically definite ALS and clinically probable ALS as per the El Escorial Criteria (EEC) (Brooks, Miller, Swash, & Munsat, 2000) [
6]. Exclusion criteria were history of prostate cancer, symptomatic benign prostatic hyperplasia, serum prostate specific antigen (PSA)values >4.0 ng/ml, male breast cancer, cardiac failure, severe liver or renal dysfunction and active anti-coagulant use. Required clearances from Institutional Ethics Committee was obtained (ECR/28/Inst/PB/2013/RR-19-IEC/OAS/05). Written informed consent was taken from patients for the study. Patients and their caretakers were informed in detail about the possible side-effects of testosterone and of the doses to be used. Patients were administered testosterone enanthate injections in the ventrogluteal hip region by a trained nurse under aseptic conditions. Testosterone enanthate injections were initiated at a dose of 250 mg per week and then increased accordingly as detailed in
Table 2. Initial assessment of disease severity and subsequent assessments were done using the ALS Functional rating scale-Revised (ALSFRS-R) by a trained neurologist. Patients were monitored and their serum biochemistry , hemogram, packed cell volume, haematocrit and serum prostate specific antigen (PSA) were measured regularly every fortnight.
Results
Study group comprised of 7 male and 2 female ALS patients. Mean age of patients in study group was 61 years . Patients reported varying degree of improvement in fasciculations with testosterone . Improvement in fasciculations was noticed usually after 3-4 weeks of testosterone therapy . Improvement was seen only with higher doses of testosterone . Minimal response to therapy was seen in bulbar symptoms. Stabilization or increments of ALSFRS-R scores were seen in few patients. Side effects of testosterone observed in the study cohort were self-limiting and non-life threatening. Pain and swelling at the injection site was the most common adverse effect.
Table 1.
Clinical details of study patients .
Table 1.
Clinical details of study patients .
Serial Number
Gender,Age |
Occupation,Handedness |
Clinical presentation |
Comorbidities, medications being taken. Substance abuse. |
Investigations |
1.
M,50 y.
AM
|
Pharmacist.Right handed |
Voice change for 4 months, dysphagia for 3 months, right upper shoulder fasciculations for 2 months, distal right arm weakness for 1 month.
Total duration of symptoms at presentation – 4 months
|
DM+, on OHA’s for 4 years. Dyslipidemia – on atorvastatin. |
MRI Brain/Cervical spine normal.
EPS – preganglionic neurogenic localization.
Hormonal profile – Normal.
|
2.
F,67.
LD
|
Housewife.
Right handed.
|
Right Lower limb weakness x 12 months, left Lower limb weakness for 10 months. Florid fasciculations present with atrophy of thighs. Bilateral lower limb spasticity present with upgoing plantars. Distal right arm weakness for 5 months. Left arm weakness for 3 months.
Total duration of symptoms at presentation – 12 months
|
None |
MRI Brain normal.
MRI Cervical spine -Minor PIVD C6-C7 without any cord compression.
EPS – preganglionic neurogenic localization.
|
| 3. M,73.DM |
Retired Bank Manager.
Right handed.
|
Difficulty in holding pen and writing/ signatures with right hand since 11 months. Right forearm fasciculations since 11 months. Right thigh fasciculations since 9 months followed by right distal leg weakness in form of slipping of slippers . Atrophy of right hand muscles , right distal leg muscles present. Left hand weakness since 6 months followed by left distal leg weakness with wasting. Bulbar symptoms in form of speech dysfunction, dysphagia, drooling of saliva present since past 3-4 months. Widespread fasciculations present. |
HTN +,DM+-on OHA’s.
Dylipedemia + - on statins.
|
MRI Brain – Fazekas grade I hypertensive changes.
MRI Spine -normal.
EPS – preganglionic neurogenic localization.
|
4.
M,70.AK
|
Retired Policeman.
Right handed.
|
Speech deficits for 1 year. Initially relatives noticed difficulty in understanding speech on telephonic conversations. Gradually progressed. Right proximal upper limb fasciculations for 6 months . Distal right upper limb weakness affecting only the thumb and index finger for 5 months. Thumb/index finger movements, especially opposition affected. Right first dorsal interosseus wasting present. Fasciculations present in right first dorsal interosseus.
Total duration of symptoms at presentation – 12 months
|
HTN. |
MRI Brain – Normal.
|
5.
M,61.AK
|
Tea shop owner. Right handed. |
Right upper limb weakness ( distal followed by proximal ) for one and a half year, left upper limb weakness for 1 year Left lower limb distal weakness for 8 months followed by right lower limb distal weakness for 6 months. Atrophy present in all 4 limbs, more marked in distal upper limb musculature. Bulbar symptoms in form of occasional choking for 3 months. Fasciculations present.
Flail arm variant with right hand demonstrating split hand syndrome with pronounced wasting of right thumb/index finger musculature.
Total duration of symptoms at presentation – 18 months
|
DM for 6 years, on OHA’s. Reformed cigarette smoker. |
MRI Brain/Cervical spine normal.
EPS – preganglionic neurogenic localization.
Hormonal profile – Normal.
USG-abdomen- Multiple hepatic cysts, renal cortical cysts present.
Paraneoplastic work-up – Normal.
|
6.
M,61.
RC
|
Lorry driver. Right handed. |
Proximal right upper limb weakness followed by proximal left upper limb weakness followed by distal right upper limb weakness for 6 months. Proximal right lower limb weakness for 4 months. Thinning and atrophy present in both upper limbs – proximally as well as distally as well as right proximal lower limb. Fasciculations present. No bulbar symptoms.
Total duration of symptoms at presentation – 6 months
|
DM x 3 years , on OHA’s. H/O Bell’s palsy 8 years back which improved over 3-4 months. Small left forearm lipoma present. |
MRI Brain-mild frontal atrophy.
EPS – preganglionic neurogenic localization.
|
7.
F,38.
KD
|
Housewife.
Right handed.
|
Right proximal and distal upper limb fasciculations for 5 months followed by right upper limb distal weakness f/b left UL distal weakness f/b right upper limb proximal weakness f/b weakness of bilateral lower limbs as well as increase in weakness in upper limbs. Atrophy present in distal upper limb muscles bilaterally. Deep tendon reflexes very brisk. No bulbar symptoms. Widespread fasciculations present over all 4 limbs.
Total duration of symptoms at presentation – 5 months
|
No Co-morbidity.
Family history negative for ALS/MND.
|
MRI Brain normal.
|
8.
M,62.
RK
|
Retired army veteran. Later set up an hardware shop. Right handed. |
Bulbar onset of symptoms. Relatives noticed difficulty in understanding patient’s speech on phone which progressed over time. 2 months after onset of speech difficulty, patient developed dysphagia – initially with liquids and later with solid foods. Patient also developed fasciculations over proximal left upper limb f/b fasciculations over right upper limb f/b generalised fasciculations. This was followed by mild left upper limb weakness f/b right upper limb weakness noticed by patients while lifting very heavy iron implements and machine parts at his hardware shop.
Total duration of symptoms at presentation – 6 months
|
None |
MRI Brain normal.
EPS – preganglionic neurogenic localization
|
9.
M,70 VD
|
Retired Pharmacist.
Right handed.
|
Right hand weakness followed by fasciculations in right first dorsal interosseus (FDI) f/b left upper limb distal weakness f/b minor proximal weakness in bilateral lower limbs with fasciculations present over all 4 limbs . Atrophy seen in distal limb muscles , hands >> foot/leg muscles. No Dysphagia. Total duration of symptoms at presentation – 15 months |
HTN for 5 years. On anti-hypertensive drugs. |
MRI Brain – normal.
EPS – preganglionic neurogenic localization
|
Table 2.
Evolution of disease in study patients receiving testosterone using revised ALS Functional Rating Scale (ALS-FRS-R).
Table 2.
Evolution of disease in study patients receiving testosterone using revised ALS Functional Rating Scale (ALS-FRS-R).
| Serial Number |
ALS Functional Rating Scale (ALS-FRS-R) at presentation
|
Details of the therapeutic intervention and duration for which patient received testosterone at our centre. |
ALS Functional Rating Scale (ALS-FRS-R) after treatment. |
1. M,50 y.
|
41 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week , increased after 1 week to twice and then after 1 week to thrice a week and after 1 week to 4 times a week . PSA, serum biochemical and haematological parameters monitored.
Duration – 2 months.
|
41.
After 3-4 weeks , patient noticed 60 % decrease in right upper arm fasciculations . No improvement in salivation/drooling. Patient reported 10% improvement in clarity of voice and 10% improvement of dysphagia but not enough to improve ALS-FRS-R score . Patient discontinued follow up at our centre 2 months after initiation of testosterone injections.
|
2.
F,67.
LD
|
33/44.
As patient was illiterate , item 4 of ALS-FRS-R could not be assessed and was excluded from scoring.
|
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week , increased after 1 week to twice and then after 1 week to thrice a week . Serum biochemical and haematological parameters monitored.
Duration – 3 months.
|
33/44.
After 4 weeks at a dose of 250 mg thrice a week , patient noticed 30-40 % decrease in fasciculations. No other benefit seen. Patient discontinued follow up at our centre 3 months after initiation of testosterone injections
|
3.
M,73.DM
|
15 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing and frequency gradually increased. Patient reached dose of 350 mg i.m daily. PSA, Serum biochemical and haematological parameters monitored.
Patient was also taking riluzole 50 mg BD since past 10 months.
Duration – 2 months.
|
15.
Fasciculations decreased by 50% at dose of 250 mg i.m. daily and by 70% at dose of 350 mg i.m daily. Patient reported further subjective improvement at a dose of 500 mg i.m. daily but no further improvement in fasciculations . No improvement on ALS-FRS-R score.
However subsequently patient’s PSA increased to 12ng/ml. Multiparametric MRI-prostate was negative for malignancy. Patient also developed hyperbilirubinemia owing to which both testosterone and riluzole were stopped. Fasciculations re-appeared 3 weeks post testosterone cessation.
|
4.
M,70.AK
|
43 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing gradually increased. Patient reached dose of 350 mg i.m once a week. PSA, serum biochemical and haematological parameters monitored.
Duration – 2 months.
|
43
At dose of 350 mg once a week, patient noticed 50% reduction in fasciculations. Patient also had some improvement in right thumb and index finger range of motion and in muscle power . Patient could oppose his right thumb and index finger and could make a pincer grasp and do activities involving pincer grasp which he was unable to do earlier. However improvement was not enough to improve ALS-FRS-R score . No improvement in speech dysfunction Patient stopped follow-up at our centre after 2 months.
|
5.
M,61.AK
|
31 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing and frequency gradually increased. Patient reached dose of 500 mg i.m daily. PSA, Serum biochemical and haematological parameters monitored.
Duration – 8 months.
|
32.
At dose of 350 mg i.m daily , fasciculations reduced by 50-60%. At dose of 500 mg i.m daily , ALS-FRS-R became 32 as dysphagia improved. Patient’s walking improved but not enough to improve his ALS-FRS-R score on item 8 . Patient maintained his improved ALS-FRS-R score for 3 months . However patient was administered Vit D3 60,000 IU for 2 weeks . His fasciculations worsened and his muscle power in affected muscles deteriorated. ALS-FRS-R score became 30. Vitamin D3 was stopped, testosterone dose was subsequently increased gradually to 700 mg i.m daily leading to improvement in his symptoms with the ALS-FRS-R score improving to 32.
|
6.
M,61.
RC
|
43 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing and frequency gradually increased. Patient currently on dose of 600 mg i.m daily. PSA, Serum biochemical and haematological parameters monitored.
Duration -9 months.
|
45.
At dose of 350 mg i.m daily , fasciculations decreased by 25%. At dose of 500 mg i. daily , fasciculations decreased by 50%. Handwriting improved , patient gained 1 point on item 4 , gained 1 point on 5(a), could button and unbutton his clothes ( excepting the top collar button)- therefore scored as not having gained one point on item 6 , improved on item 9 concerning climbing stairs but not enough to gain one point. At dose of 600 mg i.m daily , fasciculations decreased by 60-65% . No further other gain on ALS-FRS-R.
|
7.
F,38.
KD
|
36 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing and frequency gradually increased. Patient currently on dose of 500 mg i.m every 3rd day ( gap of 2 days between injections). Serum biochemical and haematological parameters monitored.
Duration – 16 months.
|
48.
At dose of 250 mg i.m every 3rd day , fasciculations decreased by 40%. At dose of 350 mg every 3rd day , fasciculations decreased by 70% and at dose of 500 mg every 3rd day, patient was having very occasional fasciculations only in right shoulder region once in 4-5 days lasting only for 1-2 seconds.
At dose of 350 mg i.m every 3rd day , patient started having improvement in muscle power and after 4-5 weeks at a dose of 500 mg i.m every 3rd day , all items of ALS-FRS-R were scored at 4 leading to a total score of 48. The activity which was last to recover was eating steamed rice-dal ( lentils in curry). One has to mix the lentil curry with rice using fingers, mix it and then eat it with fingers/hand. It is a dextrous task and patient noticed improvement in this task only at a dose of 500 mg i.m every 3rd day and this deficit was the last to recover.
Patient currently says that although she has no clinical deficit but when she does her household work, after 2-3 hours , she has to take rest for 15-20 minutes which was not the case prior to onset of the illness. However there is no household task which she cannot do with pre-illness speed and efficiency .
|
8.
M,62.
RK
|
38 |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing and frequency gradually increased. Patient currently on dose of 700 mg i.m daily. PSA, Serum biochemical and haematological parameters monitored.
Duration – 8 months.
|
41.
At dose of 250 mg i.m daily, there was no improvement. At dose of 350 mg daily , fasciculations decreased by 10% , at dose of 500 mg daily, fasciculations decreased by 20% and at dose of 700 mg daily, fasciculations decreased by 40% .
At dose of 500 mg daily , patient noticed improvement in swallowing and decreased salivation. The improvement increased at a dose of 700 mg daily . Drooling of saliva decreased by 60% and patient had an improvement of 2 points on item 2 of ALS-FRS-R. Swallowing improved and prior to initiation of testosterone , patient had to eat soft food and take sips of water after every bite but post testosterone injections , patient experienced only mild symptoms . Patient explained that he could now eat 4-5 rotis ( a flatbread made of wheat ) with dal ( lentils in curry) or vegetables and experienced some mild difficulty only at the end of the meal. This lead to improvement of one point on item no 3 of ALS-FRS-R.
However there was no improvement in speech at any dose of testosterone.
Though not a component of ALS-FRS-R scale , another thing noticed by patient was he had progressive improvement in muscular strength at doses of 500 mg and 700 mg testosterone injections daily . He noticed that earlier he had some difficulty in lifting very heavy iron implements and machine parts at his hardware shop but it lessened with increasing dose of testosterone injections .
|
9.
M,70 VD
|
41. |
Testosterone enanthate injections initiated at dose of 250 mg i.m. once a week . Dosing and frequency gradually increased. Patient currently on dose of 500 mg i.m alternate day ( gap of one day between injections) . PSA, Serum biochemical and haematological parameters monitored.
Duration – 9 months.
|
45.
At dose of 250 mg i.m alternate day ( gap of one day between injections) , fasciculations decreased by 10%. At dose of 350 mg i.m alternate day , fasciculations decreased by 25% and at dose of 500 mg i.m alternate day, fasciculations decreased by 50-60%.
No improvement in muscle power at 250 mg i.m alternate day. Patient noticed improvement of muscle power in affected muscles at dose of 350 mg i.m alternate day which further increased at a dose of 500 mg i.m alternate day. Patients hand writing improved gradually with alphabets a and r improving in the last ( patient specifically had difficulty in writing these 2 alphabets and had to write them frequently since his name included these 2 alphabets). His ability to break roti to dump it in dal to make a morsel improved gradually. Ability to unbutton clothes was regained earlier than ability to button clothes which he could do independently but slowly and with extreme difficulty with the top collar button. He could post testosterone dose at 500 mg alternate day climb stairs at a normal pre-illness speed. Scores on items 4,5(a),6 and 9 of ALS-FRS-R improved.
|
Table 3.
– dverse effects of testosterone therapy in our study group.
Table 3.
– dverse effects of testosterone therapy in our study group.
Serial Number
Gender, Age. |
Adverse effect |
1.
M,50 y.
AM
|
Pain and swelling at Injection site. |
2.
F,67.
LD
|
Pain at Injection site. Hirsutism. Irritability. Voice change.
|
3.
M,73.
DM
|
Pain at Injection site. Slight elevation of serum alkaline phosphatase on testosterone initiation which was clinically asymptomatic and did not increase further on serial monitoring . |
4.
M,70.
AK
|
Pain at Injection site. |
5.
M,61.
AK
|
Pain at Injection site. Increased irritability, insomnia, anger issues . However these were not disabling and in view of motor improvement, patient chose to continue therapy. |
6.
M,61.
RC
|
Pain at Injection site. One episode of superficial tissue infection at injection site which improved with antibiotics/anti-inflammatory agents. Increased anger, irritability. |
7.
F,38.
KD
|
Pain at Injection site. Hirsutism. Oligomenorrhoea later evolving to amenorrhoea, voice change , skin hyper-pigmentation with acne requiring topical treatment with anti-seborrheic lotions, shampoos. Increased sexual desire, development of snoring . Patient also developed pica where she felt the strong desire to eat mud/chalk and ate these items to satisfy her craving. At dose of 500 mg every 3rd day , patient also developed secondary polycythaemia. Haemoglobin was 16.8 gm/dl and packed cell volume rose to 56%. Haematology consult was taken and patient initiated on monthly phlebotomy. |
8.
M,62.
RK
|
Pain and swelling at Injection site. Increased irritability, stubborn behaviour. |
9.
M,70 VD
|
Pain at Injection site. Increased libido. Patient also reported better , sustained penile erections. |
Discussion-
Results of our study summarised above show that testosterone may provide some degree of therapeutic benefit in ALS/MND. Treatment with testosterone leads to increase in serum testosterone levels with resultant increase in brain testosterone levels. This increased brain testosterone is converted partially to DHT leading to increase in brain DHT levels and this likely resulted in some degree of clinical improvement in our small cohort . There are other studies which indirectly support this hypothesis -
Role of Connexins in ALS -
Studies [
7,
8,
9,
10] have found that Connexin 43 in astrocytes contributes to motor neuron toxicity in ALS with increasing levels of connexin 43 leading to elevated intracellular calcium levels and cell damage.
Other studies [
7,
8,
9,
10] have found that Connexin 43 hemichannels mediate spatial and temporal disease spread in amyotrophic lateral sclerosis.
Connexins are multimeric proteins that form gap junctions which are communicating junctions between adjacent cells for inter-cellular exchange of metabolites and ions This inter-cellular communication or Gap junctional intercellular communication (GJIC) mediated via gap junctions plays an important role in cellular homeostasis.
It has been found that expression of connexin 43 is upregulated in the motor cortex and spinal cord of patients with ALS . This upregulated expression of connexin 43 is accompanied by increased hemichannel activity and gap junction coupling leading to elevated concentration of intracellular calcium which leads to motor neuron damage.
Androgens regulate a variety of tissues and cell-specific genes including connexins . Castration is associated with a dramatic increase in Connexin 43 mRNA and protein expression, and this coincides with induction of cellular apoptosis. Treatment with testosterone or dihydrotestosterone ( DHT) abolishes castration induced Cx43 expression and prevented apoptosis in male Sprague–Dawley rats. Thus deficiency of DHT in the brain of ALS patients probably leads to over-production of connexion 43 leading to neuronal death and resultant clinical symptoms.
Explaining the Vitamin D Paradox in ALS Through the Connexin Pathway
Blasco et al [
11] in a survival analyses of ALS patients found a deleterious effect of higher vitamin D concentrations on the prognosis, independently of ALSFRS-R score at diagnosis and BMI, suggestive of the fact that this relation may be direct and independent of the nutritional status.
Cortese et al [
12] in their study group comprising of 71 ALS patients and 151 healthy controls also found that higher serum vitamin D levels co-related positively with a higher ALS severity score using ALSFRS-R implicating a negative effect of high vitamin D serum concentrations on ALS severity.
Lee et al [
13] in their study found that 1-alpha 25-Dihydroxy Vitamin D3 reversed testosterone-induced down-regulation of Connexin 43 in rat testes granulosa cells. Connexin 43 protein expression was markedly decreased when cells were treated with a high dose of testosterone but this effect was blocked by pre-treatment with 1-alpha 25 -Dihydroxy Vitamin D3.
Clairmont et al [
14] in their study found that Vitamin D receptor dependent increase in connexin 43 protein levels and connexin 43 mRNA levels. An increase in Vitamin D3 levels increased both Connexin 43 mRNA levels as well as connexin 43 protein concentrations. They postulated that the activated Vitamin D receptor may activate the expression of genes involved in regulation of connexion 43 transcription or of genes involved in regulation of the post-transcriptional stability of connexion 43 mRNA.
Thus vitamin D3 interferes with androgen suppression of connexin 43 levels and also increases connexin 43 levels independently. Increased connexin 43 levels are deleterious in ALS thus explaining this paradox.
Juvenile ALS with mutated Sigma -1 receptor and possible role of dihydrotestosterone.
Al-Saif et al [
15] in their study on juvenile ALS ( ALS with age of onset <25 years) found mutated Sigma-1 receptor to be the causative mechanism of ALS in their patients in Saudi Arabia with an autosomal recessive mode of inheritance Sigma-1 receptor (Sig-1R) is an endoplasmic reticulum (ER) chaperone that binds a wide range of ligands, including neurosteroids.
Thomas et al [
16] demonstrated that treatment with a Sigma-1 inhibitor prevented 5α-dihydrotestosterone (DHT)-mediated nuclear translocation of the cytoplasmic androgen receptor (AR) , induced ubiquitin proteasome mediated degradation of androgen receptor (AR) and suppressed the transcriptional activity and levels of androgen receptor.
We postulate that in the juvenile ALS cases reported by Al-Saif et al with the sigma -1 receptor mutation , the mutation renders the sigma-1 receptor ineffective. This leads to cessation of process of 5α-dihydrotestosterone (DHT)-mediated nuclear translocation of the cytoplasmic androgen receptor (AR) , increased ubiquitin proteasome mediated degradation of androgen receptor (AR) and decreased transcriptional activity and decreased levels of androgen receptor. This leads to neuronal death in susceptible groups leading to juvenile ALS .
Role of Neanderthal-Denisovan DNA introgression in ALS/MND
Oceanian individuals ( which includes Near Oceania, which includes New Guinea, the Bismarck archipelago and the Solomon Islands and the Remote Oceania which includes Micronesia, Santa Cruz, Vanuatu, New Caledonia, Fiji and Polynesia) have the highest levels of combined Neanderthal and Denisovan ancestry worldwide [
17,
18].
Various studies have found that incidence of ALS/MND in Australasia/Oceania is second highest in the world [
19,
20].
Also there is high-incidence of ALS in the Western Pacific among the Auyu- and Jakai-speaking people of West New Guinea, among the Chamorros of the Mariana Islands and among Japanese in the Kii Peninsula of Japan which no hypotheses proposed till date including the cycad toxin Beta – N-Methyl-Amino L alanine (BMAA) have been able to explain [
21]. We hypothesise that increased incidence of ALS in these isolated island communities is owing to higher amounts of Neanderthal-Denisovan genes in them . We further propose that higher amounts of Neanderthal-Denisovan genes predispose to ALS/MND via the androgen pathway through the presence of a blood brain barrier which has lesser permeability to testosterone as compared to blood brain barrier present in normal individuals . Our hypothesis is supported by the following facts .
Neanderthals Compared to Anatomically Modern Humans – Masculine Body: Feminine Brains !
Neanderthals – Higher Peripheral Androgens, More Masculine Phenotype.
Cieri et al [
22] proposed that the fossil evidence reflects a significant reduction in androgen-mediated craniofacial masculinity between the modern humans and Neanderthals coincident with archeologically visible increases in human population size and density and with a markedly increased rate of cultural evolution. Craniofacial feminization appears to have contributed to modern human population growth and cumulative cultural evolution.
Other studies [
23] have also shown that Neanderthals were more strongly built and more heavily muscled than modern humans.
Another study [
24] postulated that Neanderthals/Denisovans would have higher steroid hormone levels ( including testosterone) than modern humans.
Nelson et al [
25] in their study on digit ratio found that Neanderthals have lower 2D:4D digit ratios than most contemporary human populations indicating increased androgenization and higher testosterone levels. 2D:4D digit ratio is a commonly used measure which is a surrogate marker for intrauterine testosterone exposure [
26].
Thus we see that data from fossil osseus , genetic, anthropological sources indicate that Neanderthals were more heavily built and had higher serum testosterone levels than anatomically modern humans.
Regional Brain Volume Differences in Males and Females in Anatomically Modern Humans.
Allen et al [
27] found using high resolution MRI scans with automated tissue segmentation that females have more grey and total volumes of the left occipital lobe and grey volumes of the right occipital lobe as compared to male subjects.
Smaers et al [
28] in their study evaluated prefrontal volumes of preserved brains and found males had higher right pre-frontal cortical volumes as compared to females.
Raz et al [
29] using high resolution MRI scans found that males had higher right cerebellar hemisphere volumes than females.
Escalona et al [
30] using MRI imaging also found that males had significantly higher cerebellar volumes than females.
Schutter et al [
31] in their study on 149 healthy male and female volunteers between 10-27 years found that the endogenous testosterone levels in males were positively associated with grey matter volume of the right cerebellar hemisphere.
Pearlson et al [
32] used MRI based volumetric evaluation and found that men have higher left inferior parietal lobule volumes than women.
Differences in Brains of Neanderthals and Anatomically Modern Humans
Kochiyama et al [
33] in their study found that Neanderthals had smaller right cerebellar hemisphere volumes than modern humans . They also found that Neanderthals had smaller parietal region volumes than anatomically modern humans(AMH) . However Neanderthals had larger occipital regions than modern humans.( Details of fossil samples used in this study - Four Neanderthals -Amud 1- adult male 25 years old , La Chapelle-aux-Saints 1 – mature male , La Ferrassie 1 - adult male around 45 years , Forbes’ Quarry 1 – adult female. Four AMH’s – Qafzeh 9– likely young male , Skhul 5 – adult male , Mladec 1– likely young female , Cro-magnon 1 – mature male. )
Pearce et al [
34] had also demonstrated that Neanderthals had larger orbits and larger visual cortices than modern humans. Richards et al [
35] found that Neanderthals had smaller dorsolateral prefrontal cortices than modern humans .
Thus we see that the Neanderthal brain resembles the brain of an anatomically modern human female more than it resembles the brain of an anatomically modern human male.
Brain Atrophy Patterns in ALS/MND and the Neanderthal Link
Agosta et al [
36] in their MRI based volumetric study of 25 ALS patients( 14 males, 11 females) without any signs of fronto-temporal dementia found that patients had significant reduction of grey matter density in the right precentral gyrus.
Tan et al [
37] their MRI based volumetric study of 23 ALS patients without any symptoms of fronto-temporal dementia found that ALS patients had marked atrophy of the inferior lobules of the right cerebellum ( lobules VII B, VIII A , VIII B,IX ).
Thorns et al [
38] found that ALS patients had significant atrophy of the right (but not left) precentral gyrus .
Kim et al [
39] in their MRI study employing voxel based morphometry found that ALS patients had atrophy affecting the left orbitofrontal , bilateral superior frontal and left inferior parietal regions.
Thus we see that the brain regions preferentially affected in ALS are the ones which are less developed in Neanderthals as compared to anatomically modern humans. These preferentially affected brain regions are recent acquisitions from an evolutionary viewpoint.
Many of these brain regions are also less developed in female sex of anatomically modern humans as compared to males. This hints that this better development of these brain regions in males is because of effect of testosterone or dihydrotestosterone. Since Neanderthals have brains more like brains of female sex of anatomically modern humans , it is likely that Neanderthals had lower amounts of brain testosterone and brain dihydrotestosterone as compared to anatomically modern humans .We postulate that patients with ALS/MND have more Neanderthal genes as compared to normal individuals. Thus they have higher peripheral blood testosterone levels but lower brain testosterone/dihydrotestosterone levels . These lower brain testosterone/dihydrotestosterone levels predispose them to ALS/MND.
Since Sawal et al [
1] found normal testosterone levels but markedly reduced dihydrotestosterone levels in ALS patients as compared to controls , we postulate that dihydrotestosterone is possibly the androgen responsible for better development and survival of these evolutionary recent acquisitions of the brain. These DHT dependent brain structures atrophy in ALS/MND as DHT levels decrease . The split-hand syndrome found in ALS/MND and the differing thumb joint architecture of Neanderthals and anatomically modern humans also supports this.
The Split Hand Syndrome in ALS-
It refers to a dissociated pattern of muscle atrophy seen in the hands in ALS , which preferentially affects the musculature around the thenar eminence , mainly the abductor pollicis brevis (APB) and first dorsal interosseous muscle (FDI), with relative preservation of the hypothenar muscles , particularly the abductor digit minimi (ADM). Loss of precise movements involving the thumb and index finger is an early characteristic of split hand syndrome. It is postulated that corticomotoneuronal input to the thenar complex is preferentially affected in ALS.
Corcia et al [
40] postulated that the dying forward hypothesis potentially explains that occurrence of the split-hand sign in ALS. The thumb/first finger muscles (APB/FDI) exhibit a greater anatomical and function cortical representation, in part related to recent evolution of specialised activity of these muscles.
Consequently, the corticomotoneuronal projections can effect a greater degree of cortical hyperexcitability and thereby predisposition for neurodegeneration of the spinal motor neurons innervating these hand muscle groups of muscles via an anterograde glutamatergic mechanism. They also found that more evidence for a dying forward hypothesis mechanism was provided by clinical observation of relative sparing of the oculomotor and Onuf’s nuclei in ALS/MND , which do not directly synapse with the corticospinal tract.
Also studies [
41] employing transcranial magnetic stimulation have established cortical hyperexcitability as an early and specific feature of ALS, correlating with patterns of disease spread and the split-hand sign.
The Neanderthal Thumb and the Explanation of the Split Hand Syndrome
Bardo et al [
42] in their work on Neanderthal thumbs found that the Neanderthals possessed trapezial carpometacarpal joints that were flatter and more transversely oriented with extension of their radial and ulnar borders, a trapezial-Mc1 (proximal joint of first metacarpal) joint that was orthogonal relative to the transverse plane, and a small trapezial-trapezoid joint surface. Presence of these features favoured transmission of axial force from the thumb across the radial side of the hand, favouring more extended and adducted thumb movements . These anatomical features favour extended thumb movements, associated with axially/parasagitally-oriented joints. They found that this morphology is consistent with habitual use of a transverse power squeeze grip, in which an object is held transversely across the palm of the hand with strongly flexed fingers and the thumb is extended and adducted to brace against the object.
They also found that modern humans have a trapezio-metacarpal (TMc) complex morphology that favors thumb abduction and that this movement, combined with axial pronation and flexion of the thumb, comprises thumb opposition. An opposed thumb is habitually used by modern humans in strong precision “pad-to-pad” grips, in which the thumb pad opposes the index finger pad, and the joints of the TMc complex are oriented obliquely relative to the transverse plane.
Thus we can infer from these that during evolution , modern humans developed thumb morphology that favoured development of muscles involved in thumb abduction and opposition and for development of strong “pad—to-pad “ grips , muscles like first dorsal interossei (FDI) also likely developed better in modern humans as FDI besides causing abduction of the index finger also rotates the index finger at the metacarpophalangeal joint favouring “pad—to-pad “ precision grips.
These muscles , being recent phylogenetic acquisitions in human evolution , are preferentially affected in ALS/MND. We propose that the cortical areas sub-serving cortical representation of these muscle groups have been acquired by modern humans relatively recently during evolution. These cortical areas are likely dihydrotestosterone (DHT) dependent for their survival and proper functioning. As ALS sets in , DHT concentrations drop and these areas being sensitive to DHT concentrations get affected and owing to cortical neuronal loss, the entire cortico-motoneuronal circuit is affected leading to the split hand syndrome.
Neanderthal Genes , Impulse Control Disorders in ALS, Autism, ADHD, Evolution of Response Inhibition and Impulse Control – Are Brain Androgens the Key ?
Impulse control disorders are frequently documented in ALS/MND. Neuro-imaging studies have documented right inferior frontal gyrus atrophy in ALS/MND [
43,
44] . Reduced levels of 5-HIAA in spinal cord tissue samples have been documented in several post-mortem studies on ALS/MND patients [
45,
46,
47]
Cucala et al [
48] found that introgressed Neanderthal alleles are enriched in ADHD risk variants . Thus people inheriting a larger amount of Neanderthal genes in their genomes have a higher propensity to ADHD. Pauly et al [
49] found that Neanderthal single nucleotide polymorphisms (SNP) are significantly enriched in autistic probands compared to race-matched controls. They also found 25 rare and common Neanderthal SNPs that are significantly enriched in autism .
Imaging studies have shown reduced size and reduced functional activity of the right prefrontal cortex (PFC) in patients with ADHD [
50]. In humans, the right inferior PFC is specialized for controlled behaviour, more specifically for response inhibition and also for attention.
Various studies [
51,
52] have found lower 2D:4D ratios in ADHD, autism and autism spectrum disorder (ASD) as compared to controls indirectly implicating higher serum testosterone levels in these patients. Studies have also found higher serum testosterone levels in ASD and ADHD patients [
53,
54,
55].
Studies have found elevated whole blood serotonin in more than 25% of ASD patients [
56]. Also probands with ASD from multiplex families who had more than one affected child were found to have higher whole blood 5-HT levels than probands from simplex families who had a single affected child.
Low levels of the serotonin end metabolite 5-hydroxyindolacetic acid (5-HIAA) in cerebrospinal fluid in autism and ASD are suggestive of reduced serotonin pathway function have been documented in several studies. Studies have found that subjects with low CSF 5-HIAA levels have impaired impulse control leading to impulse control disorder characterised by substance abuse, suicide attempts, inappropriate and excessive aggression and violence [
57,
58,
59] . Psychotropic drugs which increase serotonin and 5-HIAA levels lead to improvement of these symptoms further confirming this causal relationship.
Studies have found that serotonin receptor 5-HT
4R binding is inversely linked to serotonin levels . Haahr et al [
60] found global reduction in cerebral 5-HT
4R binding in healthy volunteers exposed to a three-week intervention with selective serotonin reuptake inhibitors. Perfalk et al [
61] in their study on 41 healthy men found that testosterone levels co-related negatively with global 5-HT
4R levels suggesting that higher testosterone levels co-relate with higher serotonin levels.
This explains the autism paradox wherein autism patients have higher whole blood serotonin levels but lower CSF 5-HIAA levels. We postulate that the blood brain barrier in autism patients has some degree of “testosterone resistance “, that is , allows lesser penetration of testosterone thus leading to lower brain and CSF testosterone levels and resultant lower brain DHT levels. This will lead to higher LH levels and consequently higher blood testosterone levels. Higher blood testosterone leads to higher blood serotonin levels and lower brain and CSF testosterone levels lead to lower levels of serotonin and 5-HIAA in the brain and CSF .
We propose that this “testosterone resistant “ BBB is a Neanderthal trait which causes abnormalities in language, communication, social interaction and social development and plays a role in pathogenesis of autism, autism spectrum disorder and ADHD . Our hypotheses is supported by the fact that impaired hand dexterity is seen in autism on tasks like pegboard and line drawing [
62,
63]. This is because of poorer fine motor control and co-ordination especially in tasks involving thumb and index finger. As already explained above , this is probably because of persistence of an Neanderthal trait affecting the thumb muscles and is an androgen dependent phenomenon.
Social Cognitive Deficits and Loss of Sympathy/Empathy in Behaviour of ALS/MND Patients – Pointers from Social Behaviour of Neanderthals , Early Modern Humans (Qafzeh Hominids) and Modern Humans .
2D:4D ratio of Neanderthals , Early modern humans( Qafzeh Hominids) and Modern Humans have been calculated around 0.928, 0.935 and 0.957 respectively [
25]. Early modern humans( Qafzeh Hominids) have been found to have thumb structure, musculature and biomechanics similar to modern humans rather than Neanderthals [
64]. Also fossil studies of Early modern humans ( Qafzeh hominids) manifest archaeological signatures of modern human behaviour. Clues to acquisition of higher cognitive abilities include long-distance procurement and exchange of raw materials, intensification of resource extraction, especially aquatic and vegetable resources, and group and individual self-identification through artefact styling , regional artefact styles, self adornment, e.g., beads and ornaments, and use of ochre pigment are all reflected in the shells and ochre present at Qafzeh Cave [
65]. The physical traits of Qafzeh fossils show reduction of androgen mediated masculine physical and osseus traits . Low 2D:4D ratio means exposure to higher testosterone levels. Also modern humans have a lower 2D:4D ratio than Qafzeh hominids . Sawal et al postulate that higher serum testosterone levels are the result of lower DHT levels in the brain leading to higher LH levels because of decreased feedback inhibition . Cortical areas involved in thumb control, abstract thinking, higher cognitive tasks, response inhibition etc have been acquired by modern humans relatively recently during evolution. These cortical areas are likely dihydrotestosterone(DHT) dependent for their survival and proper functioning. These cortical areas sub-serve functions which are essential to normal social functioning as per norms practised in modern human societies.
ALS/MND patients show deficits in social cognition and loss of sympathy/empathy in their behaviour which are essential components of normal societal behaviour [
66,
67] .
Since DHT has minimal penetration across the blood brain barrier in modern humans , higher DHT levels in brain likely resulted from a blood brain barrier which was more permeable to testosterone which in turn led to higher brain DHT levels which led to LH suppression, lower LH levels, resultant lower serum testosterone levels, higher brain serotonin and 5-HIAA levels, lesser aggression and better social functioning and evolution of modern societies. This again supports that Neanderthals had a blood brain barrier which was less permeable to testosterone which resulted in lower brain testosterone and consequently lower brain DHT , lesser LH suppression and higher serum testosterone levels. Reduced social interactions in autism also support this .
Language Deficits in ALS/MND and the Evidence from Vertebrate Evolution
Language abnormalities are increasingly being recognised in ALS/MND patients , even in those who do not have an overlapping fronto-temporal dementia (FTD) component [
68]. Many language abnormalities like deficits in naming ability, syntactic comprehension and verbal fluency have been documented [
69]. Deficits in language syntactic structure and complexity like reduced mean sentence length, reduced number of words in a sentence and increased syntactic and semantic errors have been noticed in ALS/MND patients [
70].
Role of Androgens in Vertebrate Nervous System Evolution.
Okafor et al [
71] found that the androgen receptor first appeared in the vertebrate lineage 450 million years ago in Chondrichthyes (or cartilaginous fish). The androgen receptor is activated by DHT and testosterone in mammals and 11- ketotestosterone in fishes. They further found that during the course of evolution, a threonine substitution instead of leucine in AncAR1 ( the oldest steroid receptor member) had occurred as the largest effect mutation which enabled DHT binding and significantly weakened progesterone binding to the receptor.
Appearance of androgen receptor in cartilaginous fishes coincides with remarkable developments in their nervous system like appearance of true cerebellum for the first time in vertebrate evolution and appearance of true myelin for the first time during evolution [
72,
73] . Also cartilaginous fishes demonstrate increased brain to body weight ratios [
74] than their evolutionary predecessors and a more complex nervous system likely because of androgen action.
Evolution of Language – Role of Androgens.
Studies on Campbell’s monkeys have revealed an unrivalled degree of vocal complexity. Adult males produced six different loud call types, which they combined into various structured sequences in highly context-specific ways. that can function as carriers of meaning. The male Campbell’s monkey call system may be the most complex example of ‘proto-syntax’ in animal communication known to date [
75]. Female Campbell monkeys also produce different calls but not as complex and structured as the males do pointing to a possible role of androgens in evolution of language [
76].
The Songbird Evidence for Role of Androgens in Development of Language Circuits of Brain .
The function of birdsong is to attract mates and repel rival males .
Predominantly it’s the male birds who sing. Castration leads to reduction or cessation of song in males and administration of testosterone to female birds causes them to sing. Testosterone affects singing behaviour by controlling the forebrain song nuclei. The anterior forebrain pathway and area X, an anterior forebrain nucleus , are essential for a normal birdsong. Area X lesions lead to dramatic deterioration of song syntax. It has also been found that females prefer males who sing phonologically and syntactically complex songs. Studies have found that androgen receptor expression increases in the song nuclei in the breeding season a result of increase in testosterone levels [
77,
78,
79].
The Bengalese finch (BF) is a domesticated strain of the wild white-rumped munia (WRM) . BF’s have been domesticated and bred for around 250 years. The BF sings syntactically complicated songs compared to those of its ancestral strain WRM. This greater song complexity has resulted from a increase in song learning ability of BF’s [
80]. BF’s are less aggressive and have a lesser beak bite force than the WRM’s. This is attributed to the fact that when WRM’s were imported from China, calm birds were chosen to prevent any losses during transportation. Thus the initial WRM population in Japan consisted of birds which were less aggressive and had a stronger paternal/group instinct. Later in-breeding of these birds ( which already constituted a small gene pool with very less genetic diversity) led to positive amplification of these traits – a phenomenon which has been labelled by some as the domestication syndrome and emergence of the strain known as BF’s.
Wada et al [
81]found that BF’s have a significantly higher expression of androgen receptors in area X as compared to WRM’s. Takahasi et al [
82] found upon volumetric analysis that song nuclei were overall larger in BF’s as compared to WRM’s but the biggest difference in size was in the area X nuclei size. Oddly WRM’s have higher circulating serum testosterone levels than BF’s although the difference does not reach statistical significance. However since studies by Soma et al and Fraley et al have shown that androgen receptors in song nuclei increase as a result of increase in testosterone levels, it is likely that BF have lower serum testosterone levels but higher brain testosterone levels than WRM. It is also likely that increased brain testosterone levels are responsible for production of syntactically complex songs in BF’s. Thus we may surmise that area X , responsible for production of complex birdsongs , has evolved in BF’s owing to higher brain testosterone levels in BF’s.
The example of BF’s/WRM’s can be compared to the case scenario of early modern humans /Neanderthals . This offers a clue to evolution of syntactically and phonologically complex language in early modern humans which allowed better communication and formation of bigger social groups with more genetic diversity, higher diversity of ideas and larger, complex societies. This was one of the key reasons behind early modern humans continuing survival while the Neanderthals became extinct.
Table 4.
– Differences between Neanderthals and modern humans compared to differences between White rumped munia and Bengalese finches.
Table 4.
– Differences between Neanderthals and modern humans compared to differences between White rumped munia and Bengalese finches.
| Trait |
Neanderthals |
Modern Humans |
| Body structure |
More muscular |
Less Muscular |
| Language |
Less developed |
More developed |
| Androgen dependent brain structures |
Lesser developed |
More developed |
| Serum testosterone levels |
Higher |
Lower than Neanderthals |
| |
White Rumped Munia |
Bengalese Finch |
| Bite response |
Aggressive biting response |
Docile biting response |
| Biting force |
Higher |
Lower |
| Aggressiveness |
More aggressive |
Less aggressive |
| Ability to incubate eggs and rear chicks of other birds. |
Absent. |
Present |
| |
|
|
Reduced Verbal Fluency in ALS, Role of Androgens in Evolution of Prefrontal Cortex in Ashkenazi Jews and Parsis of India – Similar Evolutionary Mechanisms at Work as in the Bengalese Finch ?
Studies have found that Ashkenazi Jews have a higher intelligence quotient amongst all ethnic groups but also that they do not show any marked advantage on visual-spatial tasks while they excel at verbal fluency and mathematics [
83]. As detailed earlier, Neanderthals have larger visual cortices than modern humans whereas modern humans have more developed prefrontal cortical areas related to verbal fluency and mathematical skills. Thus we may surmise that Ashkenazi Jews have lesser Neanderthal genes as compared to other ethnic groups. Ashkenazi Jews had a strict endogamous society having very little inward gene inflow and gene studies have shown that Ashkenazi Jews have descended form a very small genetic pool [
84]. Studies of Jewish history have shown that through medieval and early modern times, great majority of the Ashkenazi Jews had managerial and financial jobs and these were jobs of high complexity requiring good verbal and mathematical skills. Jews were very poorly represented in manual professions like farming and craftsmanship which require muscular effort. Since managerial/financial/mercantile jobs offered greater money and social standing , Jews who were particularly good at these jobs enjoyed increased reproductive success. Richer Jews and their progeny ( as long as they stayed rich) lived healthier and longer as they had access to better , nutritious food, lived in larger, houses which avoided over-crowding, could afford fuel and better heated houses especially in older times when medicine was primitive and epidemics of contagious diseases were common . A larger number of children of Jews who worked in managerial/financial/mercantile jobs which were better paying survived and reached adulthood as compared to children of Jews who were farmers/craftsmen/did manual jobs and were poor. This gradually tilted the scales in favour of gene pool favouring a neural phenotype which was better suited to managerial/financial/mercantile jobs. Early twentieth century studies described Jew males having a less masculine built [
85]. This can be explained scientifically by our Neanderthal/modern human theory . Ashkenazi Jews have higher brain testosterone and dihydrotestosterone leading to better development of cortical areas like the prefrontal cortex but have lower circulating testosterone leading to lesser muscular bodies. Thus they self select in occupations where verbal fluency or mathematical abilities are required. A similar mechanism explains a greater proportion of population doing cognitively complex jobs in case of the Parsis ( ethnic group originating from Persia and practising Zoroastrianism) .
Various studies have found that ALS/MND patients show deficits both in verbal fluency and executive dysfunction suggestive of impaired function of the prefrontal cortices [
86,
87]. This prefrontal cortical dysfunction is likely because of reduced brain DHT levels in ALS/MND patients.
Why 5α-Reductase 2 Deficient Pseudo-Hermaphrodites Do Not Have a Higher Propensity to ALS/MND ?
Sawal et al [
1] found very significant reductions in CSF DHT levels in both male and female ALS patients as compared to controls and postulated that a deficiency of DHT in the central nervous system because of a dysfunctional “testosterone impermeable ” BBB lead to neuronal death leading to ALS/MND. However described cases of 5α-Reductase 2 deficient pseudo-hermaphrodites do not appear to have elevated risk of ALS/MND and this appears to be a strong evidence against the pathogenic mechanism postulated by Sawal et al.
However a detailed analysis reveals that it is not so . Evaluation of clusters of 5α-Reductase 2 deficient pseudo-hermaphrodites found in Dominician Republic, Papua New Guinea, Turkey and Oman has revealed that the affected individuals have a reduction in amount of 5α-reductase 2 rather than a complete absence of the enzyme [
88,
89,
90,
91]. Affected individuals possess a mutated 5α-reductase 2 enzyme which has decreased activity and also requires a much higher amount of the cofactor NADPH than the normal enzyme. Imperato-McGinley et al [
92] found that in the affected individuals , the percentage conversion of testosterone to dihydrotestosterone was one sixth of the normal individuals.
Also serum analysis of affected individuals shows decreased DHT levels, normal to increased serum testosterone levels and increased Luteinising hormone (LH) levels. Since only Testosterone can cross the BBB and most of the transfer across the BBB occurs when testosterone levels are at their peak , normal to increased serum testosterone concentrations coupled with a normal BBB allow adequate testosterone to cross the BBB and enter the CSF of these individuals . This creates a scenario of normal to increased substrate availability with decreased catalytic efficacy of the mutated 5α-reductase 2 enzyme and probably these individuals attain CSF DHT levels which despite being lower than CSF DHT levels in normal individuals can still sustain the DHT dependent cortical areas, motor neurons and tracts. This is the reason that a higher incidence of ALS in these individuals has not been described. CSF testosterone, CSF DHT and CSF 5α-reductase 2 enzyme level measurements in these individuals can clarify this further.
Conclusions
Our study demonstrates that testosterone may have a therapeutic role in ALS/MND . However more data from randomised controlled trials and meta-analyses evaluating testosterone in ALS/MND with longer follow up periods would be required before reaching any further conclusion
Statement of Contribution
Dr Nishit Sawal. D.M.(Neurology). Consultant , Department of Neurology, Fortis Hospital, Mohali. Punjab. India drnishitsawal@gmail.com. Corresponding Author . Conceived the entire idea along with the co-author , recruited all patients , planned and supervised the therapeutic intervention, wrote the manuscript and uploaded it.
Dr Aditi Panwar. MBBS, DMRD ( Post Graduate Diploma in Radiodiagnosis) Pro Bono Research Assistant, Sai Diagnostic Centre .Chandigarh.India . draditipanwar@gmail.com. Conceived the entire idea along with the corresponding author and wrote the manuscript .
There is no conflict of interest for any of the authors.
The authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research; full access to all of the data; and the right to publish any and all data.
None of the Authors has any disclosures to make.
There is no conflict of interest for any of the authors.
Both authors have approved the manuscript and agree to its submission to Biomedicines.
References
- Sawal N, Kaur J, Kaur K, Gombar S: Dihydrotestosterone in Amyotrophic lateral sclerosis—The missing link? Brain Behav. 2020, 10. [CrossRef]
- Ou Y-N, Yang L, Wu B-S, Tan L, Yu J-T: Causal effects of serum sex hormone binding protein levels on the risk of amyotrophic lateral sclerosis: a mendelian randomization study. Ann Transl Med. 2022, 10:1054–1054. [CrossRef]
- Celotti F, Melcangi RC, Negri-Cesi P, Poletti A: Testosterone metabolism in brain cells and membranes. J Steroid Biochem Mol Biol. 1991, 40:673–8. [CrossRef]
- Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, Ennas MG: Regional distribution of 5α-reductase type 2 in the adult rat brain: An immunohistochemical analysis. Psychoneuroendocrinology. 2013, 38:281–93. [CrossRef]
- Gargiulo-Monachelli G, Sivori M, Meyer M, Sica R, De Nicola A, Gonzalez-Deniselle M: Circulating Gonadal and Adrenal Steroids in Amyotrophic Lateral Sclerosis: Possible Markers of Susceptibility and Outcome. Hormone and Metabolic Research. 2014, 46:433–9. [CrossRef]
- Brooks BR, Miller RG, Swash M, Munsat TL: El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000, 1:293–9. [CrossRef]
- Almad AA, Doreswamy A, Gross SK, Richard J, Huo Y, Haughey N, Maragakis NJ: Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia. 2016, 64:1154–69. [CrossRef]
- Almad AA, Taga A, Joseph J, et al.: Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS. Proceedings of the National Academy of Sciences. 2022, 119:. [CrossRef]
- Huang X, Su Y, Wang N, et al.: Astroglial Connexins in Neurodegenerative Diseases. Front Mol Neurosci. 2021, 14:. [CrossRef]
- Huynh H, Alpert L, Laird D, Batist G, Chalifour L, Alaoui-Jamali M: Regulation of the gap junction connexin 43 gene by androgens in the prostate. J Mol Endocrinol. 2001, 26:1–10. [CrossRef]
- Blasco H, Madji Hounoum B, Dufour-Rainfray D, et al.: Vitamin D is Not a Protective Factor in ALS. CNS Neurosci Ther. 2015, 21:651–6. [CrossRef]
- Cortese R, D’Errico E, Introna A, et al.: Vitamin D Levels in Serum of Amyotrophic Lateral Sclerosis Patients. (P2.069). Neurology. 2015, 84:. [CrossRef]
- Lee C-T, Wang J-Y, Chou K-Y, Hsu M-I: 1,25-Dihydroxyvitamin D3 increases testosterone-induced 17beta-estradiol secretion and reverses testosterone-reduced connexin 43 in rat granulosa cells. Reproductive Biology and Endocrinology. 2014, 12:90. [CrossRef]
- Clairmont A, Tessmann D, Stock A, Nicolai S, Stahi W, Sies H: SHORT COMMUNICATION: Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear vitamin D receptor. Carcinogenesis. 1996, 17:1389–91. [CrossRef]
- Al-Saif A, Al-Mohanna F, Bohlega S: A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011, 70:913–9. [CrossRef]
- Thomas JD, Longen CG, Oyer HM, et al.: Sigma1 Targeting to Suppress Aberrant Androgen Receptor Signaling in Prostate Cancer. Cancer Res. 2017, 77:2439–52. [CrossRef]
- Choin J, Mendoza-Revilla J, Arauna LR, et al.: Genomic insights into population history and biological adaptation in Oceania. Nature. 2021, 592:583–9. [CrossRef]
- Akkuratov EE, Gelfand MS, Khrameeva EE: Neanderthal and Denisovan ancestry in Papuans: A functional study. J Bioinform Comput Biol. 2018, 16:1840011. [CrossRef]
- Marin B, Boumédiene F, Logroscino G, et al.: Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol. 2016, dyw061. [CrossRef]
- Logroscino G, Piccininni M, Marin B, et al.: Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17:1083–97. [CrossRef]
- Garruto RM, Yanagihara R: Contributions of isolated Pacific populations to understanding neurodegenerative diseases. Folia Neuropathol. 2009, 47:149–70.
- Cieri RL, Churchill SE, Franciscus RG, Tan J, Hare B: Craniofacial Feminization, Social Tolerance, and the Origins of Behavioral Modernity. Curr Anthropol. 2014, 55:419–43. [CrossRef]
- Churchill S, Rhodes J: How strong were the Neandertals? Leverage and muscularity at the shoulder and elbow in mousterian foragers. Period Biol. 2006, 108:457–70.
- Wang P-Y, Yang Y, Shi X-Q, et al.: Distilling functional variations for human UGT2B4 upstream region based on selection signals and implications for phenotypes of Neanderthal and Denisovan. Sci Rep. 2023, 13:3134. [CrossRef]
- Nelson E, Rolian C, Cashmore L, Shultz S: Digit ratios predict polygyny in early apes, Ardipithecus, Neanderthals and early modern humans but not in Australopithecus. Proceedings of the Royal Society B: Biological Sciences. 2011, 278:1556–63. [CrossRef]
- Vivekananda U, Manjalay Z-R, Ganesalingam J, et al.: Low index-to-ring finger length ratio in sporadic ALS supports prenatally defined motor neuronal vulnerability. J Neurol Neurosurg Psychiatry. Published Online First:. 1 January 2011. [CrossRef]
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W: Sexual dimorphism and asymmetries in the gray–white composition of the human cerebrum. Neuroimage. 2003, 18:880–94. [CrossRef]
- Smaers JB, Mulvaney PI, Soligo C, Zilles K, Amunts K: Sexual Dimorphism and Laterality in the Evolution of the Primate Prefrontal Cortex. Brain Behav Evol. 2012, 79:205–12. [CrossRef]
- Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD: Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. AJNR Am J Neuroradiol. 1998, 19:65–71.
- Escalona PR, McDonald WM, Doraiswamy PM, et al.: In vivo stereological assessment of human cerebellar volume: effects of gender and age. AJNR Am J Neuroradiol. 1991, 12:927–9.
- Schutter DJLG, Meuwese R, Bos MGN, Crone EA, Peper JS: Exploring the role of testosterone in the cerebellum link to neuroticism: From adolescence to early adulthood. Psychoneuroendocrinology. 2017, 78:203–12. [CrossRef]
- Frederikse ME: Sex Differences in the Inferior Parietal Lobule. Cerebral Cortex. 1999, 9:896–901. [CrossRef]
- Kochiyama T, Ogihara N, Tanabe HC, et al.: Reconstructing the Neanderthal brain using computational anatomy. Sci Rep. 2018, 8:6296. [CrossRef]
- Pearce E, Stringer C, Dunbar RIM: New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proceedings of the Royal Society B: Biological Sciences. 2013, 280:20130168. [CrossRef]
- Richards GD, Jabbour RS, Guipert G, Defleur A: Endocranial anatomy of the Guercy 1 early Neanderthal from Baume Moula-Guercy (Soyons, Ardèche, France). Anat Rec. 2023, 306:564–93. [CrossRef]
- Agosta F, Pagani E, Rocca MA, et al.: Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp. 2007, 28:1430–8. [CrossRef]
- Tan RH, Devenney E, Dobson-Stone C, et al.: Cerebellar Integrity in the Amyotrophic Lateral Sclerosis - Frontotemporal Dementia Continuum. PLoS One. 2014, 9:e105632. [CrossRef]
- Thorns J, Jansma H, Peschel T, Grosskreutz J, Mohammadi B, Dengler R, Münte TF: Extent of cortical involvement in amyotrophic lateral sclerosis – an analysis based on cortical thickness. BMC Neurol. 2013, 13:148. [CrossRef]
- Kim H-J, de Leon M, Wang X, Kim HY, Lee Y-J, Kim Y-H, Kim SH: Relationship between Clinical Parameters and Brain Structure in Sporadic Amyotrophic Lateral Sclerosis Patients According to Onset Type: A Voxel-Based Morphometric Study. PLoS One. 2017, 12:e0168424. [CrossRef]
- Corcia P, Bede P, Pradat P-F, Couratier P, Vucic S, de Carvalho M: Split-hand and split-limb phenomena in amyotrophic lateral sclerosis: pathophysiology, electrophysiology and clinical manifestations. J Neurol Neurosurg Psychiatry. 2021, 92:1126–30. [CrossRef]
- Bae JS, Menon P, Mioshi E, Kiernan MC, Vucic S: Cortical hyperexcitability and the split-hand plus phenomenon: Pathophysiological insights in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014, 15:250–6. [CrossRef]
- Bardo A, Moncel M-H, Dunmore CJ, Kivell TL, Pouydebat E, Cornette R: The implications of thumb movements for Neanderthal and modern human manipulation. Sci Rep. 2020, 10:19323. [CrossRef]
- Rusina R, Vandenberghe R, Bruffaerts R: Cognitive and Behavioral Manifestations in ALS: Beyond Motor System Involvement. Diagnostics. 2021, 11:624. [CrossRef]
- Mezzapesa DM, Ceccarelli A, Dicuonzo F, et al.: Whole-brain and regional brain atrophy in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2007, 28:255–9.
- Banki CM, Molnár G: Cerebrospinal fluid 5-hydroxyindoleacetic acid as an index of central serotonergic processes. Psychiatry Res. 1981, 5:23–32. [CrossRef]
- Ohsugi K, Adachi K, Mukoyama M, Ando K: Lack of change in indoleamine metabolism in spinal cord of patients with amyotrophic lateral sclerosis. Neurosci Lett. 1987, 79:351–4. [CrossRef]
- Sofic E, Riederer P, Gsell W, Gavranovic M, Schmidtke A, Jellinger K: Biogenic amines and metabolites in spinal cord of patients with Parkinson’s disease and amyotrophic lateral sclerosis. J Neural Transm Park Dis Dement Sect. 1991, 3:133–42. [CrossRef]
- Esteller-Cucala P, Maceda I, Børglum AD, Demontis D, Faraone S V., Cormand B, Lao O: Genomic analysis of the natural history of attention-deficit/hyperactivity disorder using Neanderthal and ancient Homo sapiens samples. Sci Rep. 2020, 10:8622. [CrossRef]
- Pauly R, Johnson L, Feltus FA, Casanova EL: Enrichment of a subset of Neanderthal polymorphisms in autistic probands and siblings. Mol Psychiatry. 2024, 29:3452–61. [CrossRef]
- Arnsten AFT: ADHD and the Prefrontal Cortex. J Pediatr. 2009, 154:I-S43. [CrossRef]
- Fusar-Poli L, Rodolico A, Sturiale S, et al.: Second-to-Fourth Digit Ratio (2D:4D) in Psychiatric Disorders: A Systematic Review of Case-control Studies. Clinical Psychopharmacology and Neuroscience. 2021, 19:26–45. [CrossRef]
- Işık Ü, Kılıç F, Aktepe E, Tanrıtanır B: The Relationship between Second-to-Fourth Digit Ratios, Attention-Deficit/Hyperactivity Disorder Symptoms, Aggression, and Intelligence Levels in Boys with Attention-Deficit/Hyperactivity Disorder. Psychiatry Investig. 2020, 17:596–602. [CrossRef]
- Artık A, Çengel Kültür SE, Portakal O, Karaboncuk AY: The association between autistic traits and serum testosterone, oxytocin, and androstenedione levels in prepubertal male drug naive children with attention-deficit/hyperactivity disorder. International Journal of Developmental Neuroscience. 2023, 83:98–107. [CrossRef]
- Wang Z, Zhang B, Mu C, et al.: Androgen levels in autism spectrum disorders: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2024, 15:. [CrossRef]
- SCERBO AS, KOLKO DJ: Salivary Testosterone and Cortisol in Disruptive Children: Relationship to Aggressive, Hyperactive, and Internalizing Behaviors. J Am Acad Child Adolesc Psychiatry. 1994, 33:1174–84. [CrossRef]
- Muller CL, Anacker AMJ, Veenstra-VanderWeele J: The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016, 321:24–41. [CrossRef]
- Adamsen D, Meili D, Blau N, Thöny B, Ramaekers V: Autism associated with low 5-hydroxyindolacetic acid in CSF and the heterozygous SLC6A4 gene Gly56Ala plus 5-HTTLPR L/L promoter variants. Mol Genet Metab. 2011, 102:368–73. [CrossRef]
- De Grandis E, Serrano M, Pérez-Dueñas B, et al.: Cerebrospinal fluid alterations of the serotonin product, 5-hydroxyindolacetic acid, in neurological disorders. J Inherit Metab Dis. 2010, 33:803–9. [CrossRef]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M: CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996, 40:1067–82. [CrossRef]
- Haahr ME, Fisher PM, Jensen CG, et al.: Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry. 2014, 19:427–32. [CrossRef]
- Perfalk E, Cunha-Bang S da, Holst KK, Keller S, Svarer C, Knudsen GM, Frokjaer VG: Testosterone levels in healthy men correlate negatively with serotonin 4 receptor binding. Psychoneuroendocrinology. 2017, 81:22–8. [CrossRef]
- Lidstone DE, Miah FZ, Poston B, Beasley JF, Dufek JS: Manual dexterity in children with autism spectrum disorder: A cross-syndrome approach. Res Autism Spectr Disord. 2020, 73:101546. [CrossRef]
- Yamaguchi K, Sano M, Fukatsu R: Hand and Finger Functions and Characteristics of Line Drawing Movement in Preschool Children with Autism Spectrum Disorder: Preliminary Study. Asian J Occup Ther. 2019, 15:77–83. [CrossRef]
- Niewoehner WA: Behavioral inferences from the Skhul/Qafzeh early modern human hand remains. Proceedings of the National Academy of Sciences. 2001, 98:2979–84. [CrossRef]
- Hovers E, Ilani S, Bar-Yosef O, Vandermeersch B: An Early Case of Color Symbolism. Curr Anthropol. 2003, 44:491–522. [CrossRef]
- Beeldman E, Raaphorst J, Klein Twennaar M, et al.: The cognitive profile of behavioural variant FTD and its similarities with ALS: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2018, 89:995–1002. [CrossRef]
- Crockford C, Newton J, Lonergan K, et al.: ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology. 2018, 91:. [CrossRef]
- Ceslis A, Argall R, Henderson RD, McCombe PA, Robinson GA: The spectrum of language impairments in amyotrophic lateral sclerosis. Cortex. 2020, 132:349–60. [CrossRef]
- Tsermentseli S, Leigh PN, Taylor LJ, Radunovic A, Catani M, Goldstein LH: Syntactic processing as a marker for cognitive impairment in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016, 17:69–76. [CrossRef]
- Yoshizawa K, Yasuda N, Fukuda M, et al.: Syntactic Comprehension in Patients with Amyotrophic Lateral Sclerosis. Behavioural Neurology. 2014, 2014:1–8. [CrossRef]
- Denise Okafor C, Colucci JK, Cato ML, et al.: Evolutionary tuning of a key helix drove androgen selectivity. 2021. [CrossRef]
- Montgomery JC, Bodznick D, Yopak KE: The Cerebellum and Cerebellum-Like Structures of Cartilaginous Fishes. Brain Behav Evol. 2012, 80:152–65. [CrossRef]
- de Bellard ME: Myelin in cartilaginous fish. Brain Res. 2016, 1641:34–42. [CrossRef]
- Smeets WJAJ, Nieuwenhuys R, Roberts BL: The Central Nervous System of Cartilaginous Fishes. Springer Berlin Heidelberg: Berlin, Heidelberg; 1983. [CrossRef]
- Ouattara K, Lemasson A, Zuberbühler K: Campbell’s monkeys concatenate vocalizations into context-specific call sequences. Proceedings of the National Academy of Sciences. 2009, 106:22026–31. [CrossRef]
- Ouattara K, Zuberbühler K, N’goran EK, Gombert J-E, Lemasson A: The alarm call system of female Campbell’s monkeys. Anim Behav. 2009, 78:35–44. [CrossRef]
- Kobayashi K, Uno H, Okanoya K: Partial lesions in the anterior forebrain pathway affect song production in adult Bengalese finches. Neuroreport. 2001, 12:353–8. [CrossRef]
- Soma KK, Hartman VN, Wingfield JC, Brenowitz EA: Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol. 1999, 409:224–36. [CrossRef]
- Fraley GS, Steiner RA, Lent KL, Brenowitz EA: Seasonal changes in androgen receptor mRNA in the brain of the white-crowned sparrow. Gen Comp Endocrinol. 2010, 166:66–71. [CrossRef]
- Okanoya K: Evolution of song complexity in Bengalese finches could mirror the emergence of human language. J Ornithol. 2015, 156:65–72. [CrossRef]
- Wada K, Hayase S, Imai R, et al.: Differential androgen receptor expression and DNA methylation state in striatum song nucleus Area X between wild and domesticated songbird strains. European Journal of Neuroscience. 2013, 38:2600–10. [CrossRef]
- Takahashi M: Innate constraints in birdsong learning : comparative neuroethological studies between domesticated and wild strains of Bengalese finches. 2006.
- COCHRAN G, HARDY J, HARPENDING H: NATURAL HISTORY OF ASHKENAZI INTELLIGENCE. J Biosoc Sci. 2006, 38:659–93. [CrossRef]
- Carmi S, Hui KY, Kochav E, et al.: Sequencing an Ashkenazi reference panel supports population-targeted personal genomics and illuminates Jewish and European origins. Nat Commun. 2014, 5:4835. [CrossRef]
- Byrne R: Re-Masculinizing the Jew: Gender and Zionism Until the First World War. Gnovis. 2011, 1:1–10.
- De Marchi F, Carrarini C, De Martino A, et al.: Cognitive dysfunction in amyotrophic lateral sclerosis: can we predict it? Neurological Sciences. 2021, 42:2211–22. [CrossRef]
- Barker MS, Ceslis A, Argall R, McCombe P, Henderson RD, Robinson GA: Verbal and nonverbal fluency in amyotrophic lateral sclerosis. J Neuropsychol. 2024, 18:265–85. [CrossRef]
- Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE: Steroid 5α-Reductase Deficiency in Man: An Inherited Form of Male Pseudohermaphroditism. Science (1979). 1974, 186:1213–5. [CrossRef]
- Imperato-McGinley J, Miller M, Wilson JD, Peterson RE, Shackleton C, Gajdusek DC: A cluster of male pseudohermaphrodites with 5α-reductase deficiency in Papua New Guinea. Clin Endocrinol (Oxf). 1991, 34:293–8. [CrossRef]
- Akgun S, Ertel NH, Imperato-Mcginley J, Sayli BS, Shackleton C: Familial male pseudohermaphroditism due to 5-alpha-reductase deficiency in a Turkish Village. Am J Med. 1986, 81:267–74. [CrossRef]
- al-Attia HM: Male pseudohermaphroditism due to 5 alpha-reductase-2 deficiency in an Arab kindred. Postgrad Med J. 1997, 73:802–7. [CrossRef]
- Thigpen AE, Davis DL, Gautier T, Imperato-McGinley J, Russell DW: The Molecular Basis of Steroid 5α-Reductase Deficiency in a Large Dominican Kindred. New England Journal of Medicine. 1992, 327:1216–9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).