Submitted:
28 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patients and Methods
2.1. Study Design and Patients
2.2. Endpoints
2.3. Statistical Methods
3. Results
3.1. Patients
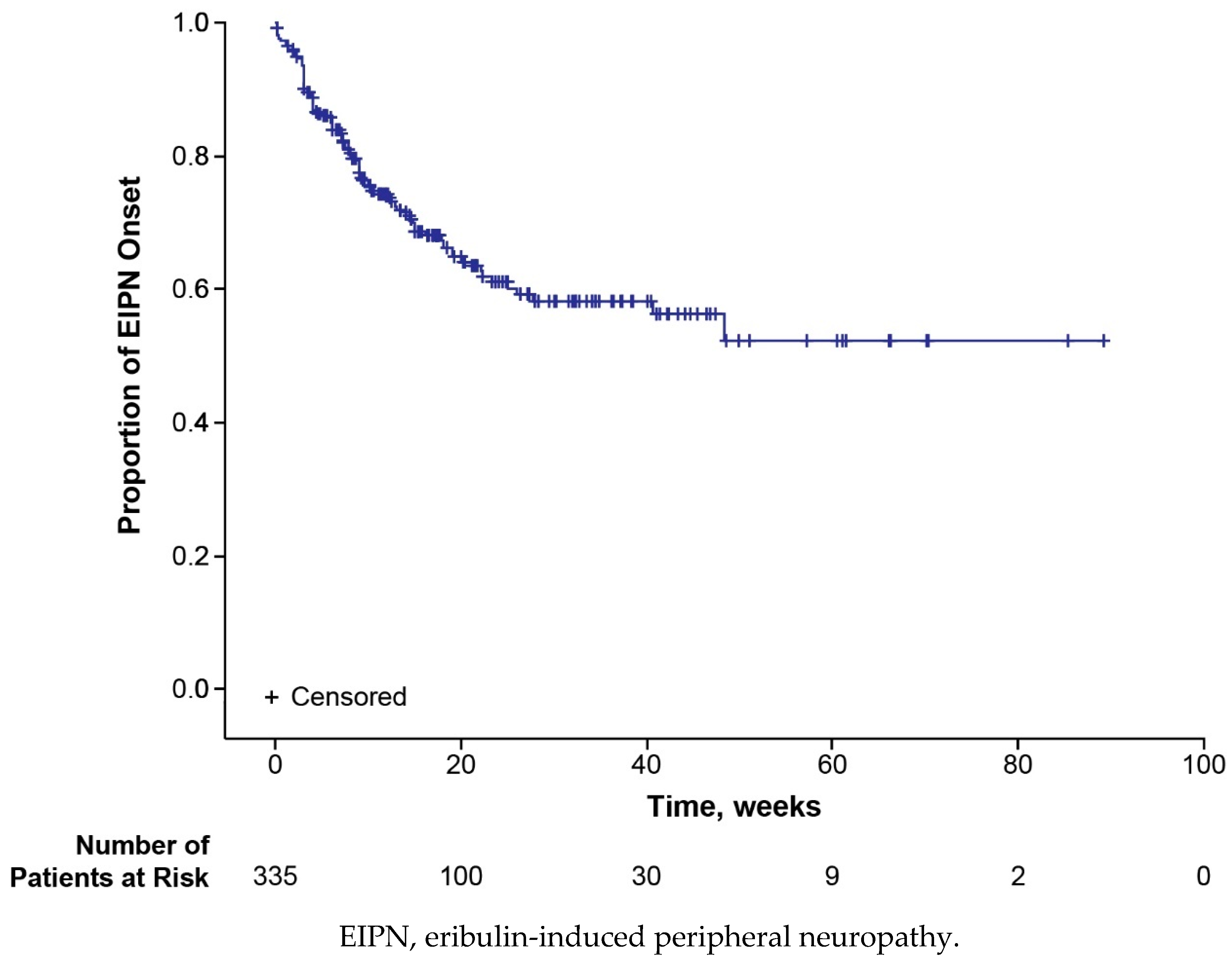
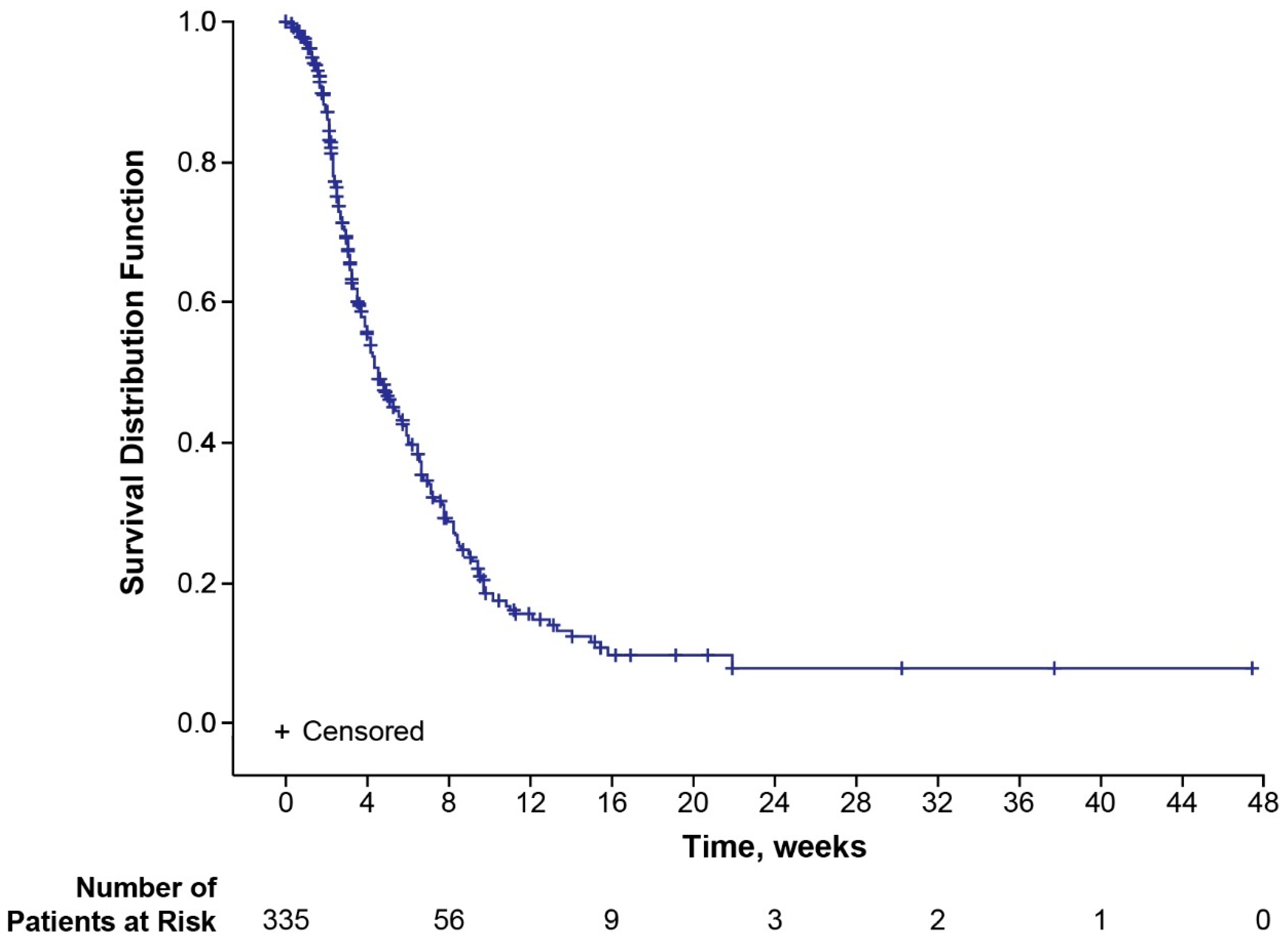
3.2. Time to Disease Progression
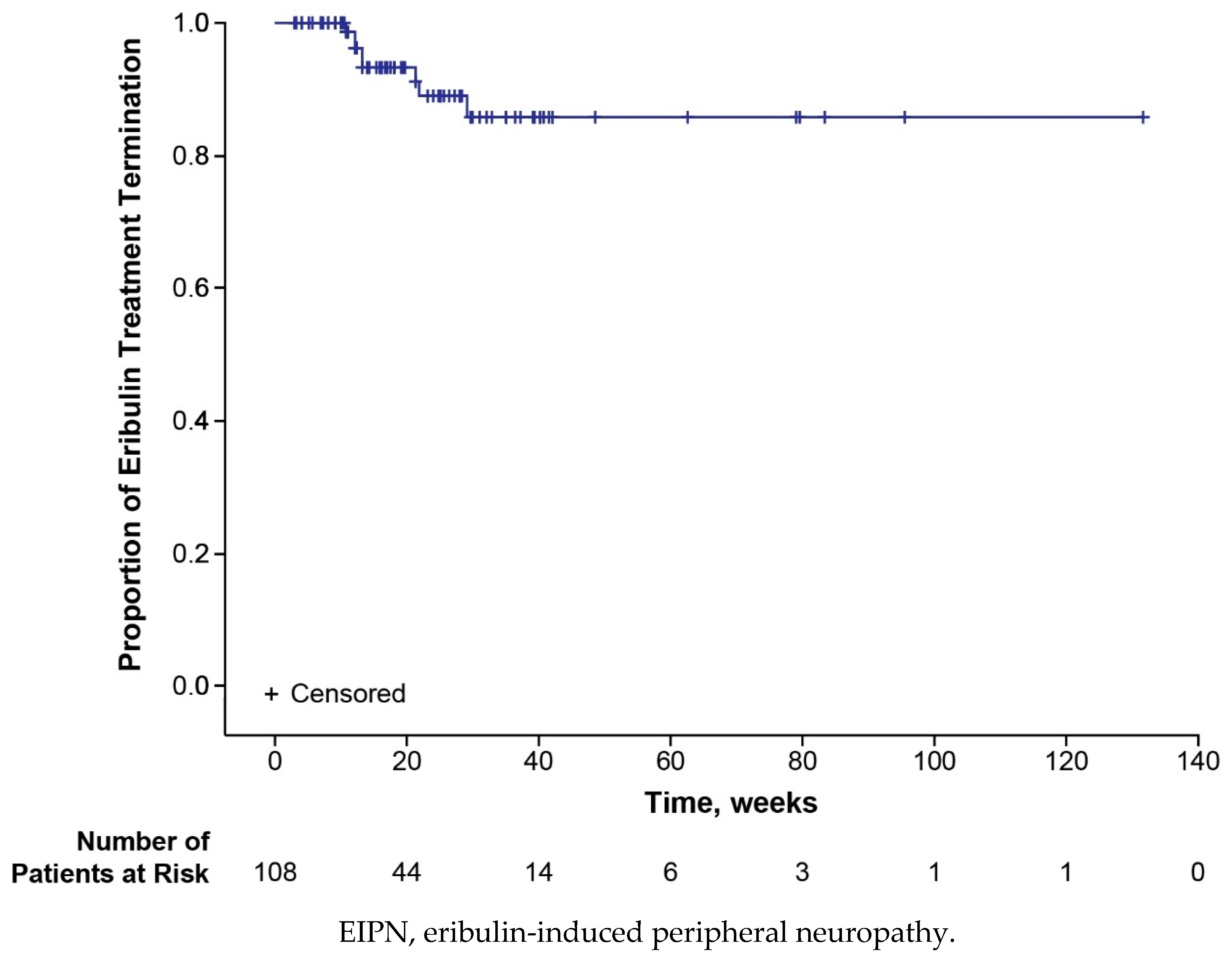
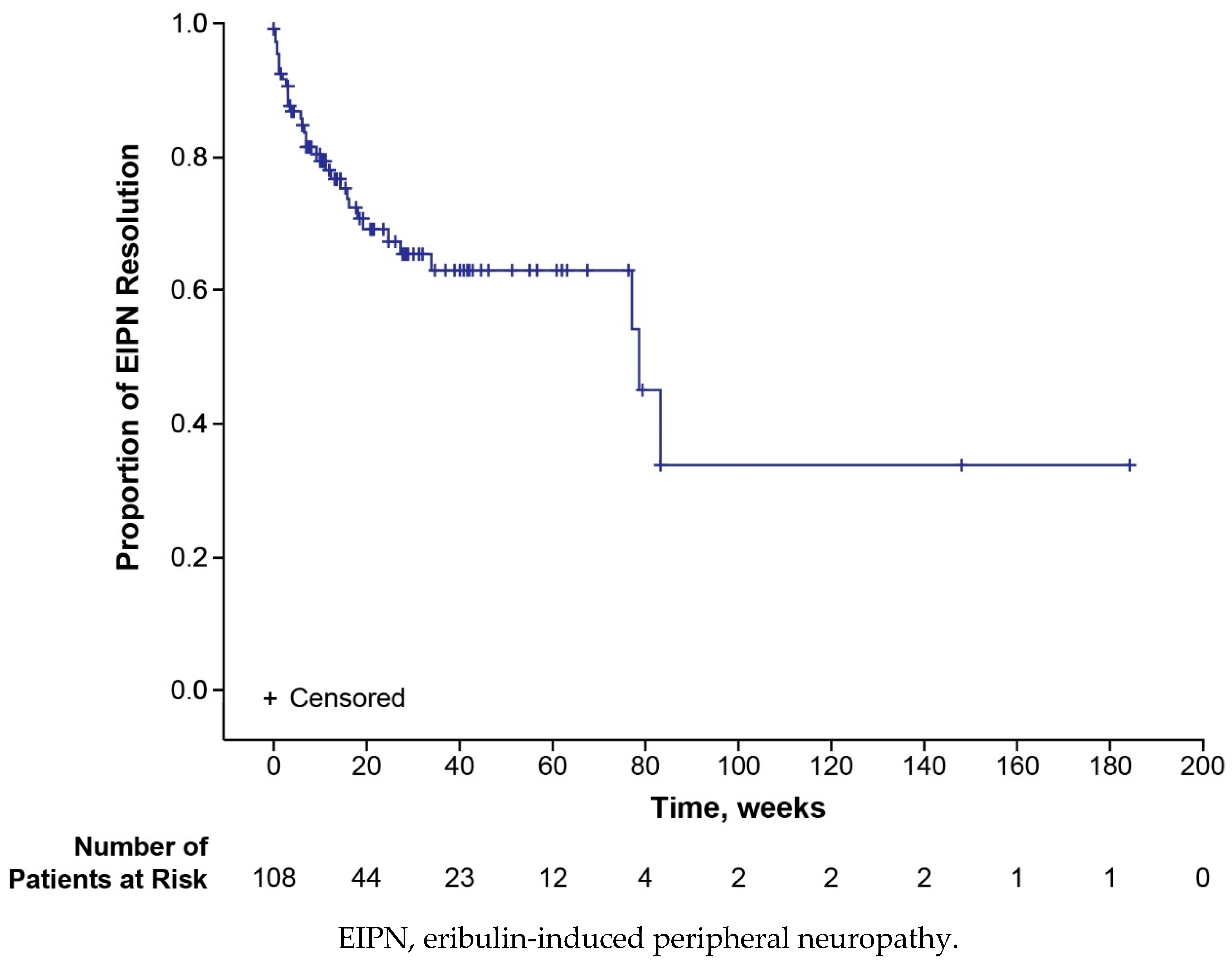
3.3. Safety
3.4. Patient-Reported Outcomes
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L. , et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, L.T.; Garcia, A.A.; Vogel, C.; Pellegrino, C.; Lindquist, D.L.; Iannotti, N.; Gopalakrishna, P.; Sparano, J.A. Eribulin mesylate versus ixabepilone in patients with metastatic breast cancer: a randomized Phase II study comparing the incidence of peripheral neuropathy. Breast Cancer Res Treat 2013, 140, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A. , et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Version 6.2024. Availabe online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. (accessed on 14 November 2024).
- Peng, L.; Hong, Y.; Ye, X.; Shi, P.; Zhang, J.; Wang, Y.; Zhao, Q. Incidence and relative risk of peripheral neuropathy in cancer patients treated with eribulin: a meta-analysis. Oncotarget 2017, 8, 112076–112084. [Google Scholar] [CrossRef] [PubMed]
- Lück, H.J.; Schmidt, M.; Hesse, T.; Hoffmann, O.; Heinrich, B.J.; Park-Simon, T.W.; Grischke, E.M.; Weide, R.; Müller-Huesmann, H.; Lüdtke-Heckenkamp, K. , et al. Incidence and Resolution of Eribulin-Induced Peripheral Neuropathy (IRENE) in locally advanced or metastatic breast cancer: prospective cohort study. Oncologist 2023, 28, e1152–e1159. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; O'Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Diéras, V.; Delozier, T. , et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.A.; Awada, A.; Twelves, C.; Yelle, L.; Perez, E.A.; Velikova, G.; Olivo, M.S.; He, Y.; Dutcus, C.E.; Cortes, J. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015, 33, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, J.; Sakata, Y.; Matsuoka, T. Chemotherapy-induced peripheral neuropathy in breast cancer patients treated with eribulin: interim data from a post-marketing observational study. Breast Cancer 2019, 26, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Halaven (eribulin mesylate) [package insert]. Eisai Inc.: Nutley, NJ, 2022.
- Halaven 0.44 mg/ml [Fachinformation]. Eisai Europe Ltd.: Hatfield, Hertfordshire, UK, 2017.
- European Medicines Agency. Halaven: EPAR assessment report. EMA/441074/2014. Procedure No.: EMEA/H/C/002084/II/0011. Availabe online: https://www.ema.europa.eu/en/documents/variation-report/halaven-h-c-2084-ii-0011-epar-assessment-report-variation_en.pdf. (accessed on 26 February 2024).
| Characteristic | Patientsa (N = 335) |
|---|---|
| Age, years Median (range) Mean (SD) |
60.0 (32.0, 83.0) 60.0 (11.2) |
| Sex, n (%) Female Male |
334 (99.7) 1 (0.3) |
| Mean body weight, kg (SD)b | 71.2 (16.4) |
| Locally advanced breast cancer, n (%) | 17 (5.1) |
| Metastatic sites, n (%) Bone Brain Lung Liver Other Missing |
318 (94.9) 219 (65.4) 28 (8.4) 134 (40.0) 178 (53.1) 148 (44.2) 0 |
| Subtype, n (%) Luminal A Luminal B HER2 enriched Basal-like Missing |
56 (16.7) 86 (25.7) 39 (11.6) 46 (13.7) 108 (32.2) |
| Patients with ≥ 1 previous anticancer treatment, n (%) 1 2 ≥3 |
330 (98.5) 15 (4.5) 26 (7.8) 289 (86.3) |
| Type of previous anticancer treatment, n (%)c Paclitaxel Cyclophosphamide Epirubicin Bevacizumab Fulvestrant Capecitabine Letrozole Tamoxifen Docetaxel Palbociclib |
330 (98.5) 228 (68.1) 177 (52.8) 153 (45.7) 131 (39.1) 104 (31.0) 99 (29.6) 92 (27.5) 83 (24.8) 82 (24.5) 75 (22.4) |
| Previous neurotoxic anticancer treatment, n (%) Taxanes Platin derivatives Vinca alkaloids Other |
301 (89.9) 294 (87.8) 68 (20.3) 19 (5.7) 19 (5.7) |
| Predisposition for peripheral neuropathy, n (%) Hypothyreosis Diabetes mellitus type 1 or 2 Renal impairment Inflammatory diseases Herpes zoster Alcohol abuse Other |
113 (33.7) 58 (17.3) 33 (9.9) 15 (4.5) 11 (3.3) 7 (2.1) 1 (0.3) 9 (2.7) |
| Peripheral neuropathy ongoing at baseline, n (%) Maximum CTCAE grade, nd 1 2 3 |
147 (43.9) 108 (32.2) 69 (20.6) 6 (1.8) |
| Incidence, n (%) | Total (N = 335) |
Age < 65 Years (n = 214) |
Age ≥ 65 Years (n = 121) |
|---|---|---|---|
| Any EIPN eventa Worsening of pre-existing peripheral neuropathy New-onset EIPN |
108 (32.2) 53 (15.8) 82 (24.5) |
64 (29.9) 31 (14.5) 51 (23.8) |
44 (36.4) 22 (18.2) 31 (25.6) |
| Any EIPN event of grade ≥3 | 18 (5.4) | 12 (5.6) | 6 (5.0) |
| Resolution of all EIPN eventsb,c | 34 (31.5) |
24 (37.5) |
10 (22.7) |
| Any therapeutic intervention for EIPNc,d | 21 (19.4) |
13 (20.3) |
8 (18.2) |
| Any EIPN event leading to dose modificatione | 13 (3.9) |
6 (2.8) |
7 (5.8) |
| Any EIPN event leading to dose delayf | 5 (1.5) | 2 (0.9) | 3 (2.5) |
| Any EIPN event leading to eribulin termination | 8 (2.4) | 6 (2.8) | 2 (1.7) |
| TEAEs, n (%) | Total (N = 335) |
Age < 65 Years (n = 214) |
Age ≥ 65 Years (n = 121) |
|---|---|---|---|
| Any TEAE | 322 (96.1) | 208 (97.2) | 114 (94.2) |
| Eribulin-related TEAE | 238 (71.0) | 149 (69.6) | 89 (73.6) |
| TEAE grade ≥ 3 | 214 (63.9) | 141 (65.9) | 73 (60.3) |
| Serious TEAE Nonfatal Fatala,b |
185 (55.2) 139 (41.5) 81 (24.2) |
122 (57.0) 93 (43.5) 54 (25.2) |
63 (52.1) 46 (38.0) 27 (22.3) |
| Serious eribulin-related TEAE | 57 (17.0) | 37 (17.3) | 20 (16.5) |
| Eribulin-related TEAEs leading to dose modification | 52 (15.5) | 34 (15.9) | 18 (14.9) |
| Eribulin-related TEAEs leading to eribulin termination | 16 (4.8) | 9 (4.2) | 7 (5.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





