1. Introduction
Cancer is a leading cause of mortality worldwide according to the WHO [
1], and its early detection is critical for improving the survival rates [
1]. Advances in diagnostic technologies aim to identify the disease at its earliest stages [
2,
3,
4]. Biomarkers are being investigated and are associated to specific biological or chemical changes associated with cancer [
5,
6,
7,
8,
9]. Surface Acoustic Wave (SAW)[
10] sensors[
11], operating through the propagation of acoustic waves on piezoelectric materials, have shown great potential for this purpose. Their high sensitivity to changes in mass and viscosity properties at the sensor surface makes them ideal candidates for detecting cancer biomarkers [
12,
13,
14].
SAW sensors are versatile and can detect a wide range of biomarkers, from volatile organic compounds (VOCs) in exhaled breath to larger biomolecules such as proteins and DNA found in body fluids. They offer several advantages such as label-free detection, real-time operation, and compatibility with miniaturized and portable systems, making them promising for non-invasive early diagnose.
Numerous reviews exist on the development and application of SAW sensors [
11,
13], primarily focusing on their general principles, device configurations, and material properties. These reviews provide an extensive foundation for understanding the underlying mechanisms of SAW. In the biosensing domain, reviews often highlight SAW sensors sensitivity to detecting biomolecules like proteins, DNA, and cells [
14,
15,
16], but these works typically doesn’t focus into the cancer specific domain.
This paper aims to address this gap by analyzing the potential of SAW technology in detecting cancer biomarkers. The paper discusses the fundamental principles of SAW technology, detailing the types of devices, piezoelectric materials, and guiding layers that optimize their performance. In addition, recent advancements in the functionalization of sensor surfaces to improve specificity and sensitivity are highlighted. Through a review of key studies, the current challenges and opportunities for using SAW sensors in cancer diagnostics are analyzed, emphasizing their potential for integration into clinical diagnostic platforms.
2. SAW Sensor Devices and their Key Features
Surface Acoustic Wave (SAW) devices operate based on the propagation of acoustic waves along the surface of a piezoelectric material, such as quartz or lithium niobate, when an oscillating electric field is applied. This technology has versatile applications; one of the most common uses is as SAW filters in telecommunications. SAW filters leverage carefully designed interdigitated transducers (IDTs)[
17] to selectively transmit or block specific frequencies, making them essential components in wireless communication systems where precise signal filtering is required. SAW sensor devices are specifically designed to exploit the surface sensitivity of SAW technology. By applying a chemically selective layer on the device surface, SAW sensors detect target analytes, such as gases or biological markers, by capturing them on this coating. When these analytes bind to the surface, they cause detectable shifts in the wave properties, such as frequency or amplitude, based on changes in surface mass, viscosity, or electrical characteristics. These shifts enable SAW sensors to identify and quantify specific analytes, making them ideal for applications in environmental monitoring and medical diagnostics, where their high sensitivity to trace compounds is a significant advantage.
2.1. Types of SAW Devices
The biomarkers for cancer cover a wide range, from small molecules, such as volatile organic compounds (VOCs)[
18,
19], to whole cells [
20,
21], as well as proteins [
22], RNA [
23], and DNA [
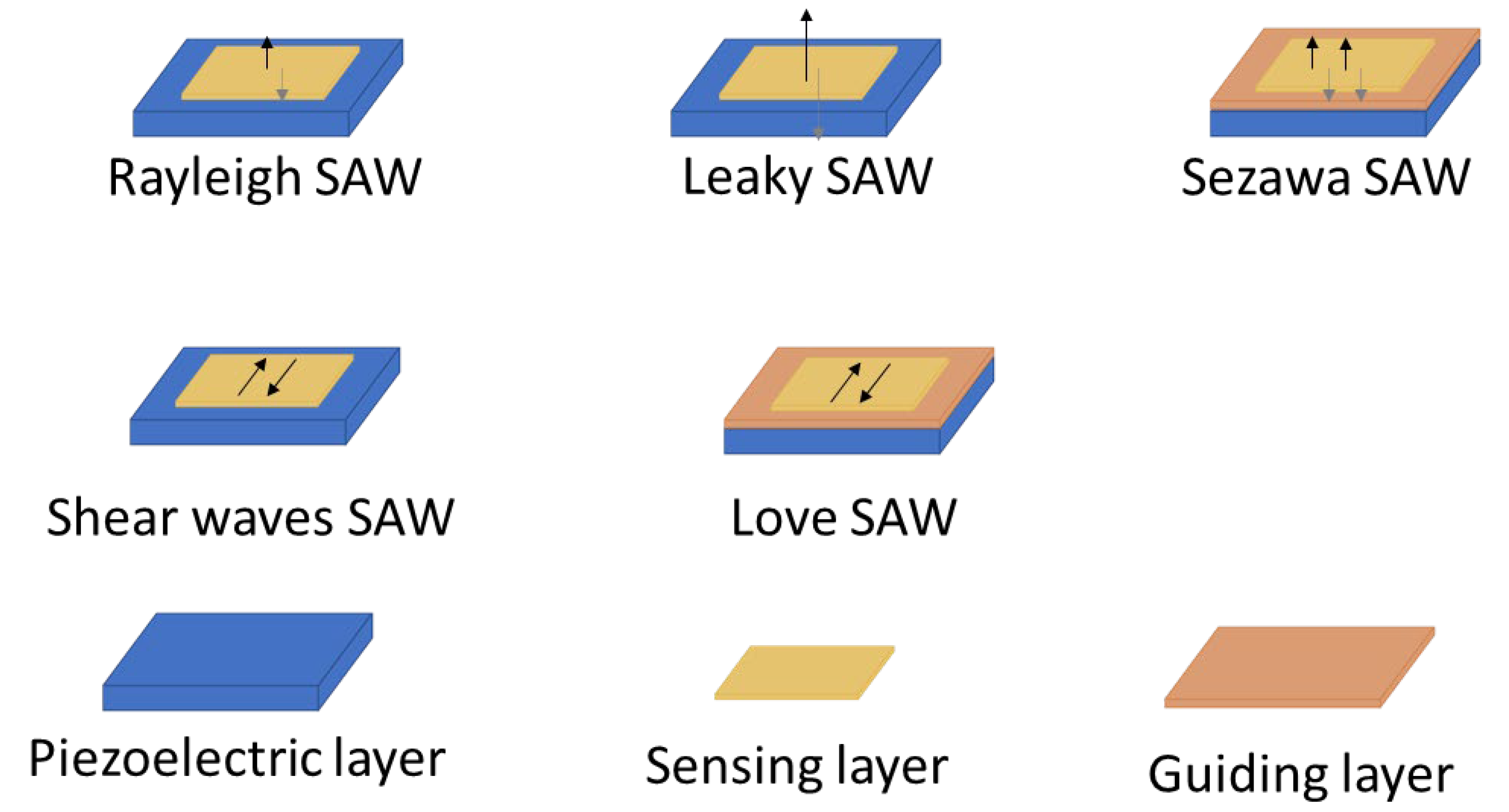
24] molecules found in both air and liquid samples. Considering this diversity, the sensory characteristics required for SAW biosensing must be versatile and precisely tuned to detect these varied targets. Regarding the types of Surface Acoustic Wave (SAW) several kinds of devices are employed in biosensing, each with distinct wave propagation modes that improves sensitivity and selectivity for different biomarker types [
25] (
Figure 1).
Two main wave propagation modes are employed in SAW sensor devices. Rayleigh waves are the most traditional type [
22,
26], producing surface-confined waves with both vertical and horizontal displacement components, which makes them highly sensitive to changes in surface mass and viscosity. Due to their high sensitivity to surface loading, Rayleigh SAW devices experience increased signal losses when used in liquid environments, making them more suitable for sensing in air. By carefully design of the device, selecting the appropriate cut, piezoelectric material and IDT configuration parallel to the wave propagation, Shear Horizontal SAW (SH-SAW) devices generate waves that move horizontally, parallel to the surface, which minimizes their sensitivity to liquid loading. This makes SH-SAW devices particularly suited for biosensing in liquid environments [
27,
28,
29], such as blood or saliva analysis.
Other advanced SAW configurations can be considered subtypes of these two main wave modes. Among Rayleigh-type waves, there are Leaky-SAW devices, which are a variant of Rayleigh waves where part of the wave energy “leaks” into the surrounding [
21,
30]. This is caused by strong piezoelectric materials cut in the appropriated angles, that makes waves to leaky energy into the medium as it travels. This characteristic makes them especially useful in liquid sensing despite the increased damping. Another Rayleigh-based subtype is Sezawa waves [
31], which are higher-order Rayleigh waves that require a layered structure, that helps to confine the energy wave closer to the surface, typically with a thin guiding layer on the substrate, leading to enhanced sensitivity and higher operational frequencies, but higher levels of noise, damping and technical complexities .
The Love-mode SAW devices, a subtype of the shear SAW devices is by far the most common SAW type used in biomarker cancer detection[
23,
26,
32,
33]. Love-mode SAW devices guide SH-SAW waves within a thin overlay layer on the piezoelectric substrate. This structure traps wave energy near the surface, enhancing sensitivity to surface-bound analytes and making Love-mode devices particularly effective for detecting biomolecules in difficult liquid samples (
Table 1).
Lamb Waves and Flexural Plate Waves (FPW) were not included in this review, as they are distinct from SAWs in that they propagate through the entire thickness of a thin membrane or plate rather than being confined to the surface. Additionally, while certain Bulk Acoustic Waves (BAWs) have a leaky mode that allows a fraction of their energy to interact with the surface, they were excluded from this review because BAWs fundamentally differ from surface waves, as they propagate through the entire volume of the material rather than being limited to the surface.
2.2. Structural Characteristics of the SAW Devices
Regarding the piezoelectric material, quartz, lithium niobate, lithium tantalate, and zinc oxide are the most commonly used piezoelectric materials in SAW devices due to their complementary properties (
Table 1). Quartz has exceptional thermal stability and cost-effectiveness, making it ideal when temperature stability is crucial. Lithium niobate, has a strong piezoelectric coupling, so it has a high sensitivity. Lithium tantalate displays a balance between thermal stability and moderate coupling, making it versatile for sensor applications that requires both stability and sensitivity. Finally, zinc oxide (ZnO) adds further versatility due to its strong piezoelectric response and ability to be deposited as a thin film. This makes ZnO especially valuable in wearable sensors where flexibility of the surface acoustic waves is essential.
Also, wave guiding layers play a key role in SAW’s device characteristics. Non-piezoelectric guiding layers, such as silicon dioxide (SiO₂) or polymethyl methacrylate (PMMA), are commonly used to confine wave energy close to the surface Depending on theirs wave propagation properties such as attenuation and wave velocity. Non-piezoelectric layers are also cost-effective and easier to deposit, making them practical for general-purpose SAW applications. On the other hand, piezoelectric guiding layers, such as zinc oxide (ZnO), aluminum nitride (AlN)[
44], enable coupling between electric fields and acoustic waves, which allow additional tuning. But they are more difficult to deposit as they need to respect the crystallization to have piezoelectric properties.
Interdigitated transducer (IDT) configurations are essential in SAW device design, as modifications to finger spacing, orientation, and the number of electrodes directly influence wave characteristics, such as frequency, amplitude, and propagation mode. Narrower finger spacing enables higher-frequency wave generation, thereby increasing device sensitivity. Custom IDT configurations are also critical for generating shear-horizontal (SH) waves, where both the piezoelectric substrate and proper alignment of the IDT in the correct direction are crucial for effective shear wave generation. In Sezawa and leaky SAW devices, the IDT configuration controls the wave frequency, where accuracy is essential for proper wave excitation.
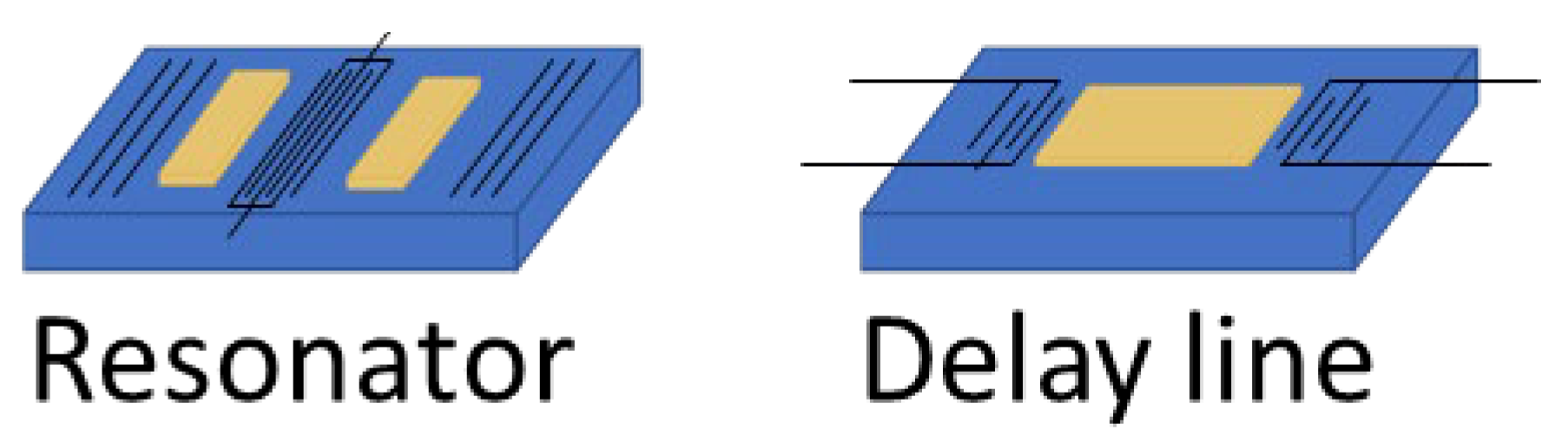
2.3. Delay Lines Versus Resonator
In Surface Acoustic Wave (SAW) sensors, there are two main configurations: delay lines and resonators
Figure 2. Each has specific advantages and limitations, which can make them better suited for detecting different types of cancer biomarkers, depending on their size and the surrounding environment (gas or liquid).
Delay lines consist of two interdigital transducers (IDTs), one to send the acoustic wave and one to receive it, with a set path in between. This design provides a broad frequency response, which is useful for detecting various changes, such as shifts in mass, viscosity, or temperature. Delay lines are simpler to manufacture and work well for continuous monitoring, especially in gas environments. In gases, where there is minimal damping of the wave, delay lines are very sensitive to small molecules, such as volatile organic compounds (VOCs) that can be linked to cancers (like in breath analysis for lung cancer). Delay lines can also work in liquid environments, where they are better suited for detecting larger biomarkers, such as proteins or even whole cells, because their broad frequency range captures the large shifts caused by these bigger molecules. However, delay lines have lower frequency stability, which may cause signal drift over time, particularly in liquids where there is more damping.
Resonators, on the other hand, use reflective gratings to trap waves between two IDTs, creating a stable standing wave at a specific resonant frequency. Reflectors are typically used to induce resonance in SAW devices. In some designs, negative reflectors, created, for example, by alternating open and shorted strips to shift the wave phase by 180 degrees, can be employed to better confine acoustic energy within the resonator [
45]. Resonators are very sensitive to small frequency shifts, making them ideal for detecting tiny changes in mass, which is useful for sensing small cancer biomarkers like metabolites or proteins in liquid samples. Resonators are also less affected by liquid damping, which allows them to keep stable, precise measurements even in viscous liquids like blood. However, resonators have a narrower frequency response and are more complex to fabricate, which makes them less adaptable for real-time, general sensing or for detecting large biomarkers like cells.
In summary, delay lines are better for general-purpose sensing, especially in gases or for larger biomarkers like cells and proteins. Resonators are best for applications that need high stability and sensitivity, especially in liquids, and are ideal for detecting small molecules or proteins.
2.3. Sensing Layers in SAW
The sensitive layer in Surface Acoustic Wave (SAW) biosensors is fundamental for improving the device performance since it interacts with the specific analytes. This layer is sometimes functionalized with biomolecules like antibodies[
32,
39,
46,
47,
48], aptamers[
21,
38], or DNA probes [
23,
49] that have a high affinity for some biomarkers of interest. But other sensitive materials are also employed to detect VOCs in air or cell such as polymers [
18,
37], Molecular-imprinted Polymers [
19,
33,
50,
51,
52], carbon structures [
26,
53] or even uncoated [
54,
55] when selectivity can be obtained by other methods. When the target analyte binds or is adsorbed to the sensitive layer, it causes the detectable change in the mass or mechanical properties.
For the functionalization the more straightforward approach is the direct immobilization of sensing molecules, such as antibodies or proteins directly onto the surface of the SAW device [
24,
39,
40,
45]. This is usually achieved through methods like physical adsorption and covalent bonding techniques. Physical adsorption relies on non-covalent interactions, such as electrostatic forces, hydrophobic interactions, or van der Waals forces, to hold the biomolecules onto the sensor surface. This method is relatively straightforward but may result in weak and reversible attachments. On the other hand, covalent bonding techniques involve forming strong chemical bonds between functional groups on the sensor surface and reactive groups on the capture molecules. Common strategies include using cross-linking agents or activating the surface with chemical treatments to introduce reactive groups. Covalent attachment provides a more stable and durable linkage, which is advantageous for long-term sensor applications.
However, direct immobilization has certain limitations. The random orientation of immobilized biomolecules can affect their binding sites' accessibility, potentially reducing the sensor's sensitivity. Additionally, the activity of the immobilized molecules may be compromised if their active sites are obstructed or if they undergo conformational changes upon attachment. Non-specific binding of other substances present in the sample can also interfere with the sensor's selectivity, leading to false signals or increased background noise.
To overcome the challenges associated with direct immobilization a more common approach is used. Intermediate layer modifications are introduced as a mediating layer between the SAW surface and the capture molecules. They create an environment that enhances the specific capture of analytes while minimizing non-specific interactions, although they can lower the sensitivity.
Incorporating a gold layer [
34,
36] onto the sensor surface acts as an interface between the device substrate and the capture molecules, facilitating efficient biomolecule immobilization. Gold allows for the formation of self-assembled monolayers (SAMs) through strong thiol-gold bonds, enabling the covalent and oriented attachment of biomolecules like antibodies or DNA probes. This controlled immobilization preserves the biological activity of the capture molecules and enhances the sensitivity and selectivity of the sensor. Additionally, the gold layer provides chemical stability and reduces non-specific adsorption, leading to improved sensor performance in applications such as medical diagnostics. Molecules like dextran or GPTES [
23,
24] provide binding points to the surface that can reduce non-specific adsorption. They also offer functional groups for the covalent attachment of biomolecules in a controlled orientation, preserving their biological activity. Polymers or hydrogels [
30,
38], composed of networks, create three-dimensional matrixes that can immobilize a high density of capture molecules. Their porous nature facilitates the diffusion of analytes to the binding sites while maintaining the structural integrity of the immobilized biomolecules. This results in enhanced steric stability and preserves the functionality of the capture molecules.
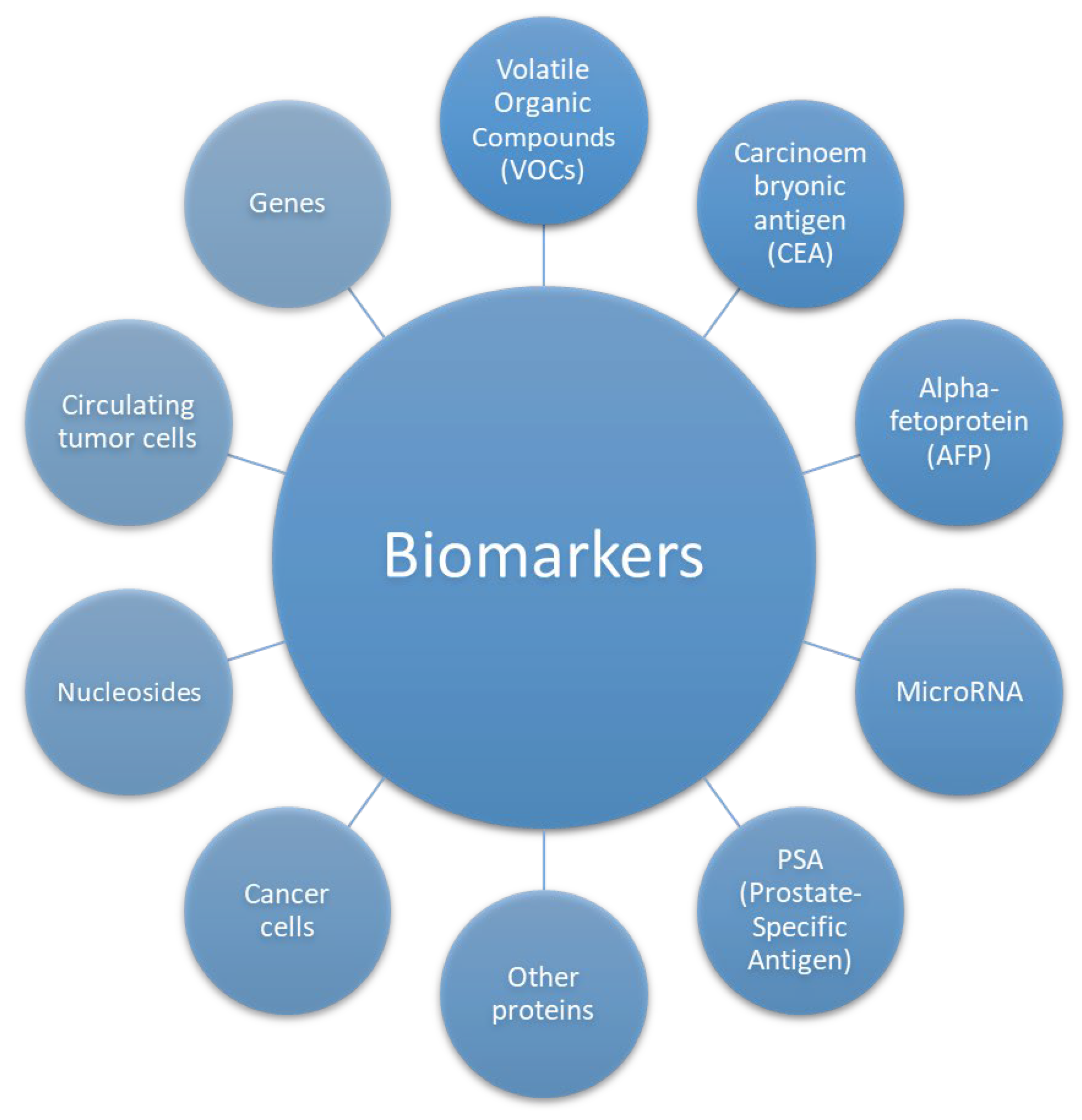
3. Biomarkers
Cancer biomarkers are molecules, genes, proteins, or other substances found in the body that can indicate the presence, progression, or response to cancer treatments. They have become increasingly significant in recent years due to their role in diagnosis, prognosis, monitoring and personalized treatment. A recent review of the main cancer biomarkers can be found in [
8]. In general, the biomarkers can be categorized based on their clinical applications and molecular characteristics. From a clinical perspective, diagnostic biomarkers are used to identify the presence of cancer, such as prostate-specific antigen (PSA) for prostate cancer. Prognostic biomarkers provide insights into the likely disease progression or risk of recurrence, exemplified by HER2/neu in breast cancer. Predictive biomarkers assess the likelihood of a patient's response to specific therapies, such as EGFR mutations in non-small cell lung cancer for targeted treatments. Monitoring biomarkers track treatment responses or detect disease recurrence, with CA-125 being a notable example in ovarian cancer.
From a molecular standpoint, biomarkers include a range of types. Protein-based biomarkers, such as tumor antigens and tumor-related proteins, include examples like alpha-fetoprotein (AFP) for liver cancer. Genetic biomarkers involve detecting specific gene mutations or alterations, such as BRCA1 and BRCA2 mutations in breast and ovarian cancer. RNA-based biomarkers, including microRNAs, can indicate abnormal gene expression associated with cancer, such as microRNAs detected in liquid biopsies for lung cancer. Volatile organic compounds (VOCs), detected non-invasively in breath, may also indicate cancer presence, such as lung cancer. Metabolite biomarkers reflect tumor-related metabolic activity, as seen with 2-hydroxyglutarate in IDH-mutant tumors.
Additionally, biomarkers can be classified based on their origin. Circulating biomarkers, such as circulating tumor cells (CTCs) and tumor-derived free DNA, are detectable in blood or other bodily fluids. Tissue biomarkers, on the other hand, are identified in biopsy samples, with Ki-67 serving as a marker for cell proliferation in tumor tissues. This multifaceted classification highlights the diverse utility of biomarkers in cancer diagnosis, prognosis, and treatment monitoring.
This review provides a comprehensive compilation mostly of recent studies (2015–2024) focusing on cancer biomarkers detected using various types of surface acoustic wave (SAW) biosensors,
Figure 3.
3.1. VOCs
This review highlights the potential of VOCs as biomarkers for early, non-invasive cancer detection. For over five decades, research has focused on volatile organic compounds (VOCs) emitted by the human body, with early work by Linus Pauling in 1971 identifying breath as containing around 250 VOCs. By 1999, studies by Phillips and colleagues expanded this number to over 3,400 compounds. These VOCs, produced through metabolic processes, are carried to the lungs via the bloodstream and exhaled. As such, changes in breath composition can be linked to diseases, including cancer. Works on lung cancer detecting VOCs with SAW biosensors are the predominant in literature due surely to that it is often asymptomatic in its early stages, with symptoms like coughing, chest pain, and weight loss commonly overlooked. As a result, 85% of cases are diagnosed at advanced stages, leading to a low 5-year survival rate of around 10–15%. Despite advancements in diagnostic methods like CT scans and biopsies, the late-stage detection limits treatment options. Besides, lung cancer produces 1.6 million deaths annually, surpassing the total number of deaths due to colon, prostate and breast cancers. Detecting VOCs in breath with gas sensors, as SAW biosensors, is of the most interesting strategies to be able to diagnose this cancer at the beginning of the disease and in non-invasive mode [
56,
57,
58].
Also, gastrointestinal cancer accounts for 22.2% of global cancer-related deaths, with histological biopsy under endoscopy being the primary diagnostic method. However, endoscopy has limitations, including cost, invasiveness, and low diagnostic rates for early-stage cancer due to nonspecific symptoms. Non-invasive, cost-effective diagnostic methods are urgently needed. Common alternatives like fecal occult blood tests, serum biomarkers (e.g., CEA, CA199), and gastrointestinal barium angiography also have drawbacks, including high false positive/negative rates, low accuracy, and procedural challenges. The need for reliable non-invasive biomarkers is critical, with VOCs emerging as promising candidates due to their link to oxidative reactions in cancer cells, which spread through the blood to the respiratory system [
59].
Gas sensors have shown significant promise in detecting lung cancer through the analysis of volatile organic compounds (VOCs) in exhaled breath. Arrays de MOS sensors and advanced nanosensors are commonly used for lung cancer detection due to their high sensitivity and sensitivity, robustness, and low cost. They detect VOCs like acetone, toluene, and benzene, toluene and formaldehyde in exhaled breath, which are associated with metabolic changes in lung cancer cells. These portable systems are integrated with machine learning algorithms for improved accuracy in lung cancer diagnosis and to provide a non-invasive, real-time diagnostic option [
60].
SAW sensors demonstrate significant potential for the detection of VOC biomarkers associated with cancer, owing to their exceptional sensitivity and selectivity, rapid real-time response capabilities, low costs, portability, as well as their ability to operate at room temperature and facilitate label-free detection. However, their application in this field remains relatively limited when compared to other technologies such as Metal Oxide Semiconductor (MOS) sensors or gas chromatography-mass spectrometry (GC-MS) [
58]. The lower use of SAW sensors may be attributed to challenges in optimizing their design for specific VOCs and the dominance of alternative, more established methods in cancer biomarker detection. This highlights the need for further research to develop SAW sensors, particularly for their potential integration into compact, point-of-care diagnostic device for clinical applications.
The following studies use SAW sensor technology for the diagnosis of lung cancer through the detection of volatile organic compounds (VOCs) in breath samples. Thus, in [
18] a Love-mode Surface Acoustic Wave (Love-SAW) sensor was developed incorporating silver-modified polypyrrole (Ag/PPy) nanoparticles to detect volatile organic compounds (VOCs) at room temperature. The sensor, built on an ST-cut quartz substrate, detects acetone, ethanol, and toluene with detection limits of 3 ppb, 5 ppb, and 20 ppb, respectively. The Ag/PPy sensor showed higher sensitivity than a PPy-only sensor, likely due to silver's catalytic effect. Sensitivity values were 910 Hz/ppm for acetone, 742 Hz/ppm for ethanol, and 340 Hz/ppm for toluene, with fast response times. The sensor exhibited low cross-sensitivity to other gases and moderate performance in humid conditions.The study suggests that Ag/PPy-modified Love-SAW sensors exhibit significant potential for the detection of VOCs at parts-per-billion (ppb) concentrations, making them highly suitable for breath analysis applications. Also, in [
61] the same authors developed a Love-SAW sensors operating at 160 MHz with a dual-layer structure, including an SiO₂ guiding layer and a sensitive polypyrrole (PPy) layer modified with gold nanoparticles (Au NPs). These sensors detect ammonia and ethylene, which are biomarkers for cancer. The addition of Au NPs significantly improved sensor sensitivity, lowering the detection limits to 67 ppb for ammonia and 87 ppb for ethylene. While the sensors showed high sensitivity in dry air, response stability decreased with humidity, suggesting the need for humidity control. The Au-modified PPy sensors offer enhanced selectivity, reproducibility, and are promising for breath analysis and monitoring low-concentration VOCs.
In [
53], a filter-based Surface Acoustic Wave (SAW) sensor operating at 433.92 MHz was developed for the detection of decane, a volatile organic compound (VOC) associated with lung cancer. The sensor incorporates a thin oxidized graphene (GO) film as the sensing layer, applied to the SAW device with a thickness of 150–200 nm. This GO layer confers high sensitivity, achieving a detection limit of 0.2 ppm and exhibiting rapid response times. The device demonstrated stable performance with minimal variations in insertion loss. The non-conductive nature of the GO film mitigates electromechanical coupling effects, enhancing its efficacy in detecting non-polar VOCs such as decane. These characteristics suggest the sensor's potential for early lung cancer diagnosis through breath analysis.
Other studies highlight the potential of integrating Surface Acoustic Wave (SAW) sensors with complementary technologies, such as chromatography and Metal Oxide Semiconductors (MOS) sensors, which, as previously noted, are widely employed for the detection of such biomarkers. For instance, in [
55], a Surface Acoustic Wave (SAW) gas chromatography system was developed for the early screening of lung cancer through the analysis of exhaled breath. This system combines a 500 MHz SAW sensor with a gas chromatography (GC) module, enabling rapid and sensitive detection of lung cancer biomarkers, with a detection limit of 1 picogram (pg). By analyzing breath samples from 19 lung cancer patients and 19 healthy controls, the system successfully identified key volatile organic compounds (VOCs), such as alkanes and benzene derivatives, which distinguish between the two groups. This non-invasive diagnostic approach demonstrates high sensitivity and holds significant potential for clinical point-of-care applications. In [
54], a hybrid electronic nose system (HENS) is presented, combining metal oxide semiconductor (MOS) and surface acoustic wave (SAW) sensors for the detection of lung cancer biomarkers in breath samples. The system utilizes nine MOS sensors to detect low-molecular-weight volatile organic compounds (VOCs), such as benzene and hexane, and uncoated SAW resonators for the detection of high-molecular-weight VOCs, such as tridecane. The SAW sensors, operating at 250 MHz on ST-cut quartz substrates, provide improved stability and are uncoated to minimize environmental interference. With temperature control and a differential structure, the system achieves high sensitivity (93.62%) and selectivity (83.37%) using an artificial neural network (ANN) model, demonstrating its potential as a promising tool for non-invasive lung cancer screening.
For gastrointestinal cancer detection, in [
19], a Love wave sensor with a molecularly imprinted polymer (MIP) thin film was developed for the detection of ethanol and toluene, targeting colorectal cancer diagnostics. Built on an AT-cut quartz substrate with a SiO₂ guiding layer, the sensor operates with shear-horizontal waves at a 40 μm wavelength. The MIP layer, imprinted with adenosine monophosphate (AMP), enhances the sensor's selectivity, providing up to four times the sensitivity of non-imprinted polymer (NIP) sensors and faster response times. The sensor exhibits high specificity, indicating its potential for colorectal cancer biomarker detection.
3.2. Carcinoembryonic Antigen (CEA)
Carcinoembryonic antigen (CEA) is a well-established biomarker primarily associated with colorectal cancer, though it may also be elevated in other malignancies such as pancreatic, gastric, and lung cancers. In colorectal cancer, CEA is most often used to monitor disease progression, recurrence, and response to treatment rather than for initial diagnosis, as not all patients with colorectal cancer exhibit elevated levels. Elevated CEA levels after surgery, for example, may indicate cancer recurrence, making it a valuable tool in follow-up care [
62].
3.2.1. CEA Detection in Exhaled Breath Condensate (EBC)
This research [
40] presents a portable Love-wave sensor developed for the detection of carcinoembryonic antigen (CEA) in exhaled breath condensate (EBC) for early lung cancer diagnosis. The sensor employs a CEA-specific aptamer immobilized on a gold-coated silicon dioxide layer, operating at a frequency of 164 MHz. It demonstrates high sensitivity (0.577°/(ng/mL)) and a detection limit of 1.04 ng/mL, with stable performance for up to 10 days. The sensor exhibits high specificity for CEA, with minimal interference from other lung cancer biomarkers such as NSE and SCC. This approach integrates aptamer technology with a dual-channel Love-wave sensor, enabling label-free, real-time detection. Another study [
47] introduces a Love Wave Surface Acoustic Wave (SAW) immunosensor constructed on an ST-cut quartz substrate with a 3 µm SiO₂ guiding layer, operating at 160 MHz. The sensor utilizes a gold staining amplification method to enhance sensitivity and employs a sandwich immunoassay with gold nanoparticle (AuNP) conjugates. The sensor offers a detection range of 1 to 16 ng/mL and a detection limit of 1 ng/mL. It demonstrates high selectivity against interfering agents such as NSE and SCC, and its performance shows a high correlation (0.999) with clinical chemiluminescent immunoassays. This device provides a rapid, non-invasive tool for early lung cancer screening in high-risk populations. In [
46], a point-of-care diagnostic system is developed using a Love-wave Surface Acoustic Wave (SAW) immunosensor with immunogold staining to detect lung cancer biomarkers. The sensor, built on an ST-cut quartz substrate with a SiO₂ guiding layer, operates at 165 MHz with gold interdigitated transducers (IDTs) and utilizes a dual-SAW setup to enhance specificity and stability. It detects CEA, neuron-specific enolase (NSE), and squamous cell carcinoma antigen (SCC) with detection limits of 0.967 ng/mL, 1.598 ng/mL, and 0.663 ng/mL, respectively. The immunogold staining method increases sensitivity by up to 20 times. The system, tested with samples from lung cancer patients and healthy controls, shows a strong correlation with commercial chemiluminescence assays, highlighting its potential for non-invasive, early-stage lung cancer detection. In another study [
63], a miniaturized Love-wave Surface Acoustic Wave (SAW) immunosensor is developed for the detection of CEA as part of a lung cancer screening method. The sensor is fabricated on a 36° rotated Y-cut LiTaO₃ substrate with a 2 µm SiO₂ guiding layer, operating at 165 MHz. The detection method utilizes a gold nanoparticle (AuNP)-enhanced immunoassay, where CEA antibodies are conjugated with AuNPs and undergo mass enhancement via gold staining. This approach achieves a detection limit of 1.25 ng/mL and demonstrates a strong correlation (r = 0.999) with clinical chemiluminescence assays, indicating its potential for high-accuracy, non-invasive early lung cancer detection.
3.2.2. CEA Detection in Non-Exhaled Breath Condensate
A Love-mode Surface Acoustic Wave (Love-SAW) sensor based on an ST-cut quartz substrate operating at 120 MHz is presented in [
36]. The sensor uses Au-coated interdigital transducers and a self-assembled monolayer (SAM) of anti-CEA antibodies for detecting carcinoembryonic antigen (CEA). The sensor demonstrates a sensitivity of 0.31 ng/mL and shows strong selectivity against other tumor markers like AFP and CA125. Integrated with a microfluidic chamber, the system allows real-time analysis within 30-40 minutes. Stability tests over 30 days revealed only an 8% decrease in performance, indicating reliable long-term functionality. In the two following references [
32,
37] nanocomposite layers of polymidine doped with nanoparticles are used as sensitive layers to detect CEA by Love-mode surface acoustic wave (SAW) biosensors, and in both cases the biosensor exhibited high selectivity against other tumor markers (AFP, CA125). In [
37] the biosensor is fabricated on an At-cut quartz substrate operating at 230 MHz and uses a polyimide/MXene-Au nanoparticle (PI/MXene-AuNP) nanocomposite layer, functionalized with anti-CEA antibodies through a thioglycolic acid linker. It demonstrates an impressive detection limit of 0.001 ng/mL for CEA, a sensitivity of 83 Hz/ng/mL and maintains stability for up to 75 days under periodic testing. In [
32] the biosensor is fabricated on an ST-cut quartz substrate operating at 120 MHz and uses a polyimide (PI) nanocomposite layer, doped with gold nanoparticles (AuNP), molybdenum disulfide (MoS₂), and reduced graphene oxide (rGO). The biosensor uses the nanocomposite thin film as a bioreceptor base, enhanced by high-density AuNP to support anti-CEA antibody immobilization via thioglycolic acid. Experimental results reveal a low detection limit of 0.084 ng/mL for CEA and stability for up to 80 days. The study highlights the PI/AuNP–MoS₂–rGO composite’s ability to support stable acoustic wave transmission with minimal signal loss, offering a highly sensitive and durable platform for CEA detection in clinical diagnostics. In [
39] the Love mode surface acoustic wave (SAW) immunosensor is built on an ST-cut 90° X quartz substrate with a SiO
2 wave-guiding layer of 1000 nm thickness and operates at a resonant frequency of 196 MHz Its surface was functionalized through CEA antibody immobilization. Gold nanoparticles (AuNPs) were bound with CEA antibodies to amplify the mass loading sensitivity. The detection range was between 0.2 and 5 ng/mL, with a limit of detection of 0.2 ng/mL. The response times were around 10 to 2 minutes. Furthermore, the sensor demonstrated low interference from nonspecific adsorption of molecules like L-tryptophan and alpha-fetoprotein. No data about stability or reproducibility were shown.
3.3. Alpha-Fetoprotein (AFP)
Alpha-fetoprotein (AFP) is a glycoprotein primarily synthesized during fetal development by the liver and yolk sac. In adults, it is typically produced at low levels, but its expression increases in certain cancers, particularly hepatocellular carcinoma (HCC), making it a key biomarker for this type of liver cancer. AFP is often used in clinical settings to diagnose and monitor liver cancer progression. High levels of AFP in the bloodstream are indicative of malignancy in liver tissue, although some HCC patients may still have normal AFP levels, which can complicate diagnosis [
64].
In [
38], the development of a novel Love-mode surface acoustic wave (Love-SAW) aptasensor, fabricated on a ST-90° X cut quartz with dummy fingers and based on a molybdenum disulfide (MoS₂)/gold nanoparticles (Au NPs) monolayer for the detection of alpha-fetoprotein (AFP) is presented. The sensor is capable of detecting biomarkers in serum with a dynamic range of 0.01 ng/mL to 100 ng/mL and an impressive detection limit of 4.79 pg/mL. It achieves a response time of approximately 15 minutes and exhibits high selectivity for alpha-fetoprotein (AFP), a biomarker associated with liver cancer, while effectively discriminating against other biomarkers such as prostate-specific antigen (PSA) and carcinoembryonic antigen (CEA). Additionally, the sensor retains 85% of its initial performance after 20 days and demonstrates consistent reproducibility across multiple usage cycles. In [
30], a leaky surface acoustic wave immunosensor for ultrasensitive detection of alpha-fetoprotein (AFP) is developed. The device operates at a frequency of 175 MHz on a 36° YX-cut lithium tantalate (LiTaO₃) substrate, with Ti/Au-coated interdigital transducers (IDTs). The sensor uses a sandwich-type assay with MoS₂@Cu₂O-Au nanoparticles conjugated to secondary antibodies and employs gold staining to amplify the signal. The leaky surface acoustic wave sensor achieves detection limits of 5.5 pg/mL with gold staining and 25 pg/mL without staining, effectively detecting AFP in human serum and saliva samples. The immunosensor shows high selectivity and long-term stability of about 5 weeks when stored at 4°C.
3.4. MicroRNAs
MicroRNAs (miRNAs) are small RNA molecules, typically 18–24 nucleotides long, that regulate gene expression by binding to messenger RNAs (mRNAs), leading to either degradation of the mRNA or inhibition of its translation. This process allows miRNAs to control various biological functions, including cell growth, differentiation, and apoptosis. Specific miRNAs are often upregulated or downregulated in cancer cells, and their expression profiles can serve as biomarkers for cancer detection and prognosis [
65]. miR-21 is commonly overexpressed in cancers such as breast, lung, and liver cancer, where it promotes cell survival and tumor growth. miR-34, a known tumor suppressor, is frequently downregulated in many cancers, contributing to uncontrolled cell proliferation and resistance to cell death. miR-155 is elevated in cancers like lymphoma and breast cancer, and plays a role in immune response modulation and tumor progression. These miRNAs can be detected in bodily fluids such as blood, saliva, and urine, offering the potential for non-invasive cancer diagnostics.
In [
23] a Love-mode Surface Acoustic Wave (SAW) biosensor array based on a lithium tantalate (LiTaO₃) substrate with a silicon dioxide (SiO₂) guiding layer and operating at 200 MHz is developed. The experiments were performed with human serum spiked with three exomal miRNAs (miR-21, miR-106b and miR-155). The sensor uses a sandwich hybridization assay with titanium dioxide (TiO₂) nanoparticles conjugated to probes, followed by photocatalytic silver staining for signal enhancement. Detection limits were as low as 0.012 pM for synthetic miRNAs and 0.21 pg/mL for exosomal miRNAs derived from MCF-7 breast cancer cells. A reference sensor was included to improve reproducibility by normalizing sensor responses, reducing variation due to environmental factors, and compensating for noise introduced by the high frequency. This configuration demonstrated high sensitivity, selectivity, and reproducibility, making it suitable for cancer biomarker detection in liquid samples.
3.5. PSA (Prostate-Specific Antigen)
PSA is a protein produced by the prostate gland, primarily used as a biomarker for prostate cancer. Elevated levels of PSA in the blood may suggest prostate cancer, though they can also indicate benign conditions like benign prostatic hyperplasia or prostatitis. PSA testing is commonly employed for screening and monitoring prostate cancer, but it has limitations, including false positives and negatives. Hence, it is often used in conjunction with other diagnostic tests to improve accuracy [
66].
Different studies have been carried out with SAW sensors to detect this biomarker. Thus, a Love wave sensor utilizing hydrophilic molecularly-imprinted polymer (MIP) layers to detect prostate-specific membrane antigen (PSMA) is fabricated. The MIP is synthesized via reversible addition-fragmentation chain transfer (RAFT) polymerization, enhancing sensor specificity and minimizing non-specific protein interactions. A 160 MHz Love wave is generated on a piezoelectric quartz substrate with a SiO₂ guiding layer to concentrate acoustic energy and to improve the sensitivity. The sensor detects PSMA with a limit of 0.013 ng/mL and shows high stability and reversibility, comparable to ELISA assays. This approach enables detection in complex media like mouse serum, making it suitable for early prostate cancer diagnostics [
33]. In [
41], other cost-effective surface acoustic wave (SAW) biosensor is designed for early prostate cancer detection by quantifying prostate-specific antigen (PSA) levels in biological samples. The device utilizes shear-horizontal (SH) surface acoustic waves on an ST-cut quartz substrate to detect mass loading changes caused by PSA binding, with phase detection as the primary measurement method. A simplified driver circuit generates a 16.9 MHz square wave, while phase shifts are extracted using a low-frequency down-sampling technique (100 kHz), reducing hardware complexity and costs. Despite using a 1-bit analog-to-digital converter (ADC), this down-sampling method ensures phase accuracy with errors as low as 1% and a minimum phase delay of 0.3 ns. This streamlined design demonstrates that accurate PSA quantification is achievable with sufficient samples, making it suitable for single-chip integration and potential point-of-care applications. In [
43], the device operates on a ZnO-coated Si substrate and utilizes both Rayleigh and Sezawa wave modes. When the ZnO film is thinner than 2.8 µm, the Rayleigh wave dominates, while thicker films (>2.8 µm) facilitate the Sezawa wave mode. The biosensor, operating around 130 MHz in Sezawa mode, uses a cystamine self-assembled monolayer (SAM) and anti-PSA antibody on gold electrodes to achieve linear frequency shifts for PSA concentrations ranging from 2 to 20,000 ng/mL. By combining SAW streaming effects with the advantages of Sezawa waves, the system enables efficient, label-free PSA detection with minimal sample volume, offering a real-time, portable tool for prostate cancer diagnostics.
3.6. Other Protein Biomarkers
Numerous proteins serving as cancer biomarkers have been successfully detected using SAW sensors, including:
3.6.1. Epidermal Growth Factor (EGF)
Epidermal Growth Factor (EGF) is a key protein involved in the regulation of cell growth, proliferation, and survival. It binds to the Epidermal Growth Factor Receptor (EGFR), which is a receptor tyrosine kinase. EGFR is commonly overexpressed or mutated in many cancers, including non-small-cell lung cancer, colorectal cancer, glioblastoma, and breast cancer, where it promotes oncogenic signaling pathways that facilitate cancer cell proliferation. As biomarker of cancer, EGF and EGFR have significant roles. Overexpression or mutations of EGFR are commonly associated with several types of cancer, including non-small cell lung cancer, colorectal cancer, and glioblastomas. These mutations often lead to the continuous activation of signaling pathways that promote cancer cell proliferation and resistance to apoptosis (programmed cell death) [
67].
In [
27], a shear-horizontal surface acoustic wave (SH-SAW) biosensor was developed for the detection of epidermal growth factor (EGF), utilizing 3-aminopropyltriethoxysilane (APTES) and glutaraldehyde films as functional layers. The sensor operates at a frequency of 121.6 MHz on a 36° Y-X lithium tantalate (LiTaO₃) substrate with a silicon dioxide (SiO₂) guiding layer. EGF is captured through surface functionalization with either APTES or glutaraldehyde. Experimental findings reveal frequency shifts proportional to EGF concentrations ranging from 0.2 to 5 ng/mL, with the glutaraldehyde film demonstrating superior sensitivity at 1.709 kHz/(ng/mL), compared to 0.641 kHz/(ng/mL) for the APTES film.[
27]A semi-empirical model, modified from the Sauerbrey equation, enables direct conversion of frequency shift to analyte concentration. The biosensor shows promise for real-time, label-free cancer biomarker detection in biofluids.
3.6.2. C-Reactive Protein (CRP), Lipoprotein (a) (Lp(a)), Apolipoprotein B (ApoB)
In [
29], a Shear Horizontal Surface Acoustic Wave (SH-SAW) sensor system operating at 250 MHz was constructed using a dual-channel delay line configuration on a 36° Y-cut, 90° X-propagated quartz substrate with a thickness of 0.5 mm. This SH-SAW sensor demonstrated the capability to detect biological markers, including C-reactive protein (CRP), lipoprotein (a) (Lp(a)), and apolipoprotein B (ApoB), which are associated with inflammatory processes linked to cancer. The sensor achieved high sensitivity, with detection limits for CRP spanning from 1.9 to 118 µg/mL and a limit of detection (LoD) of 390 ng/mL. For Lp(a), it exhibited a detection range of 83 to 1402 µg/mL. The system also provided rapid responses, with detection times as short as 3 minutes.
3.6.3. hMAM
hMAM ("Human Mammaglobin") is a protein primarily found in human breast tissue. It is known as a molecular marker widely used in oncology, particularly for the detection and monitoring of breast cancer.
In [
34], a CMOS-integrated SAW (147-150 MHz Rayleigh waves) biosensor is fabricated for the detection of mammaglobin (hMAM), a breast cancer biomarker, using a streptavidin/biotin-based immunoassay. The SAW device operates on a 0.5 μm CMOS platform with ZnO as the piezoelectric layer and a gold coating for functionalization, enabling the binding of hMAM-specific antibodies. Testing demonstrated a frequency sensitivity of 8.704 pg/Hz and a mass sensitivity of 2810.25 m²/kg. Selectivity was validated against bovine serum albumin, confirming specific hMAM detection. The device shows potential for low-cost, scalable cancer biomarker detection with high sensitivity in a miniaturized format.
3.6.4. CA125
CA125 is a glycoprotein found on the surface of many cells, and its levels are often elevated in the blood of patients with ovarian cancer. However, it is important to note that elevated CA125 levels are not exclusive to ovarian cancer and can be found in conditions such as: Endometriosis, pelvic inflammatory disease (PID), menstruation, liver disease and other cancers, like lung, breast, and pancreatic cancer. It is most commonly used to track the effectiveness of treatment in patients with known ovarian cancer, particularly after surgery or chemotherapy and in conjunction with imaging and other tests to assess the possibility of ovarian cancer.
In [
26], this paper presents a Rayleigh-mode surface acoustic wave (SAW fabricated with ST-cut quartz) immunosensor for detecting carcinoma antigen 125 (CA125), an ovarian cancer biomarker. It operates at 203.5 MHz in a delay-line configuration. The sensor employs a chemically vapor-deposited graphite tube decorated with gold nanoparticles in the inside as both the fluidic channel and sensing element. The graphene-AuNP structure enhances sensitivity by trapping CA125 antibodies within the fluidic channel. demonstrated high sensitivity with a wide linear response from 0.01 to 300 mU/mL and a detection limit of 0.00371 mU/mL. The device showed excellent specificity against other biomarkers and retained stability over extended use, supporting its potential for biomedical diagnostics.
3.6.5. Bcl-2
Bcl-2 ("B-cell lymphoma 2") Bcl-2 protein (short for "B-cell lymphoma 2") is a key regulator of apoptosis (programmed cell death). It belongs to a family of proteins that control the balance between cell survival and death, playing a crucial role in maintaining cellular homeostasis by deciding whether a cell should live or die.
In [
28], a shear-horizontal surface acoustic wave (SH-SAW) biosensor was developed on an ST-cut quartz substrate for the detection of Bcl-2 protein, a urinary biomarker associated with early ovarian cancer. Operating at a frequency of 16.8 MHz, the sensor employs a delay line structure with microfabricated interdigital transducers (IDTs) and a functionalized delay path. The detection mechanism relies on an anti-Bcl-2 monoclonal antibody layer to capture Bcl-2. The sensor achieves a sensitivity of 0.5 ng/mL, exhibiting a linear frequency response to increasing Bcl-2 concentrations. To minimize non-specific adsorption, the surface is functionalized with self-assembled monolayers (SAMs), Protein A/G, and Pluronic F127. The sensor demonstrates selective and repeatable detection over up to 10 cycles, highlighting its potential as a non-invasive tool for ovarian cancer screening.
3.6.6. Streptavidin
It is a protein sourced from the bacterium Streptomyces avidinii, known for its exceptionally high and specific affinity for biotin (vitamin B7). This interaction represents one of the strongest non-covalent bonds found in nature. While it is not a cancer biomarker itself, it plays a crucial role in cancer biomarker detection when used in conjunction with biotin-labeled probes.
In [
35], A Rayleigh surface acoustic wave (SAW) microfluidics-based lab-on-a-chip (LoC) device was developed for biosensing applications, specifically tailored for the detection of biomolecules in complex media.. The device incorporates four nanoscale surface acoustic wave (SAW) resonators operating at 1.2 GHz, along with a microfluidic system that facilitates multiplexed measurements and fluid mixing. It is constructed on 128° X-rotated Y-cut lithium niobate (LN) wafers. A biotin-polyethylene glycol (bPEG) coating is applied to the surface to prevent fouling and improve selectivity by inhibiting non-specific binding. While streptavidin (SA) is not directly related to cancer, its stable, specific binding to biotin makes it a valuable component in biosensing. When combined with biotin-labeled probes, SA can effectively assist in detecting cancer biomarkers. The device achieved a sensitivity threshold of 290 pM for SA, even in the presence of high concentrations of bovine serum albumin (BSA), a crucial characteristic for applications in biological fluids where interfering proteins are prevalent. In [
45], a Rayleigh surface acoustic wave (SAW) resonator biosensor was developed, utilizing both positive and negative reflectors to improve biomolecule detection in liquid samples after drying. Operating at a high frequency of 1.285 GHz on a lithium niobate (LiNbO₃) substrate, the device incorporates interdigital transducers (IDTs) without a guiding layer, enabling direct binding of molecules to the resonant surface to optimize sensitivity. The sensor demonstrates a limit of detection (LoD) of 104 pM for biotin-streptavidin and a normalized mass sensitivity of -296 m²/kg. The inclusion of positive and negative reflectors helps to concentrate wave energy within the sensitive area, enhancing both detection stability and the dynamic range of the sensor.
3.6.7. Alfa-Glycosidase
Alpha-glycosidase is an enzyme that plays a key role in the digestion of carbohydrates. It is involved in the breakdown of complex carbohydrates (such as starch and disaccharides) into simpler sugars like glucose. This enzyme is found primarily in the small intestine and is essential for the absorption of sugars from the digestive tract. Such enzymes could participate in processes related to carbohydrate metabolism, oxidative stress, or glycation product breakdown. α-Glycosidase is an enzyme involved in carbohydrate metabolism, and while not a primary cancer biomarker, its altered activity may reflect metabolic changes associated with certain cancers.
In [
22] a high-frequency Rayleigh Surface Acoustic Wave (SAW) Lab-on-Chip (LoC) biosensor operating at 740 MHz for the detection of active α-glycosidase is developed. The device, fabricated on a lithium niobate (LiNbO₃) substrate, is functionalized with a specially designed probe molecule, 7-mercapto-1-eptyl-D-maltoside, which mimics a substrate for α-glycosidase. The sensor was tested in aqueous samples with α-glycosidase concentrations from 0.15 to 150 U/mL, achieving a detectable signal within this range and showing strong sensitivity to active enzyme concentrations up to 15 U/mL. To ensure specificity, the sensor was also tested with acarbose, an enzyme inhibitor, resulting in an 80% signal decrease in its presence.
3.7. Cancerous Cells
Cancer cells are cells that grow and divide uncontrollably, ignoring the normal regulatory mechanisms of the body. Unlike healthy cells, they can invade surrounding tissues and spread to other parts of the body (a process known as metastasis). These cells often result from genetic mutations that disrupt normal cell functions, such as growth, repair, and programmed cell death (apoptosis).
Currently, methods such as MTT assay, flow cytometry, and Ki67 staining are used to assess cell growth or proliferation in 2D cultures. In 3D cultures, measurements typically involve endpoint procedures like trypsinization, trypan blue staining, and quantification. However, there is a significant need for non-invasive, contact-free techniques to monitor cell growth or proliferation over time, particularly for 3D tumor spheroid models and stem cell regeneration studies. Using SAW) sensors could provide an innovative alternative to these existing approaches [
42].In [
42], a Shear Horizontal Surface Acoustic Wave (SH-SAW) biosensor was developed for quantifying cell growth in both 2D and 3D cultures. The SH-SAW device, built on a 36° Y-cut lithium tantalate (LiTaO₃) substrate with a 200 nm ZnO coating, operates at 14.05 MHz and incorporates interdigital transducers (IDTs) and a polydimethylsiloxane (PDMS) well to support cell cultures. The ZnO layer enhances the sensor's sensitivity by amplifying frequency shifts caused by cell mass loading, providing up to four times greater sensitivity compared to an uncoated sensor. The device successfully detected cell concentrations ranging from 6,250 to 50,000 cells per 100 µL for both cancerous (A549) and non-cancerous (RAW264.7) cells. Additionally, the SH-SAW biosensor monitored cell growth over eight days in A549 spheroids cultured on a 3D nanofiber scaffold, showing a linear frequency shift that correlated with tumor spheroid expansion over time. This SH-SAW biosensor provides a real-time, non-invasive tool for studying cell proliferation in 3D models, making it a valuable asset for cancer research and regenerative medicine applications. In [
68], a surface acoustic wave (SAW) biosensor configured as a delay line was developed on an X-cut lithium niobate (LiNbO₃) substrate, operating at a center frequency of 10.3 MHz, for the detection and differentiation of primary (HT-29) and metastatic (SW-48) colon cancer cells. Significant resonance peak shifts were observed, especially with SW-48 cells, which exhibited higher negative surface charges compared to HT-29 cells. This variation primarily influenced the wave through electroacoustic interactions. These findings highlight the potential for distinguishing cell types based on their electroacoustic profiles, offering a promising approach for diagnosing cancer progression. In [
48], a shear horizontal surface acoustic wave (SH-SAW) biosensor was developed on a Y36°-X lithium tantalate (LiTaO₃) substrate for detecting antigen-specific cells, such as T cells, in liquid environments. Operating at 122.5 MHz with a SiO₂ waveguiding layer, the sensor was functionalized with CD3 antibodies to selectively capture Jurkat cells from a mixed-cell suspension. A liquid microchamber integrated with a peristaltic pump facilitated continuous flow, enabling rapid, label-free cell detection, with a sensitivity of 1,000 cells/mL and a frequency shift of 280 Hz. This SH-SAW platform offers a rapid and efficient method for cell detection, making it highly relevant for monitoring immune and cancer cells. In [
69], an SH-SAW (Shear Horizontal Surface Acoustic Wave) sensor was developed for the rapid detection and purification of tumor cells (TCs), specifically colorectal adenocarcinoma HCT-8 cells, from biofluids. The sensor operates at 122.5 MHz on a 36° Y-X LiTaO₃ substrate, incorporating a 4 µm SiO₂ guiding layer to facilitate wave propagation in liquid environments. The device is equipped with Au/Cr interdigital transducers (IDTs), fabricated using photolithography with 50 finger pairs and a 34 µm periodicity. It demonstrates a mass sensitivity of 4 Hz/ng, enabling selective detection of TCs through a microfluidic system. Stability testing reveals minimal frequency drift under varying humidity and temperature conditions, while a dual-delay line configuration enhances selectivity by reducing acoustoelectric interference. This SH-SAW biosensor, combined with a micro-incubation feature, supports non-invasive, label-free detection of cancer cells in clinical applications. Additionally, in [
20], a hybrid sensor integrating surface acoustic waves (SAWs) and dielectrophoresis (DEP) was developed for trapping and detecting cells, including White Blood Cells (WBC), RPMI, and U87 cells. The device utilizes a Love wave-based SAW mechanism on a lithium niobate (LiNbO₃) substrate with a zinc oxide (ZnO) waveguide layer, operating at approximately 142 MHz. The DEP force, used in place of a sacrificial layer, traps cells, making the sensor reusable and versatile for cell detection based on dielectric properties. This configuration enhances sensitivity to 5.6e−5 dB/cell/mL (or 2.13 Hz/cell/mL), providing stable and selective detection. Changes in resonance frequency and amplitude allow differentiation between natural and cancerous cells from the brain, intestine, and breast, offering a reusable platform for detecting and releasing various cell types.
3.8. Nucleosides
Nucleosides are organic molecules consisting of two components: a nitrogenous base (which can be a purine or pyrimidine) and a sugar molecule (either ribose or deoxyribose). They are the building blocks of nucleotides, which are essential for the formation of DNA and RNA. Unlike nucleotides, nucleosides do not have phosphate groups attached to them.
Nucleosides can serve as cancer biomarkers due to their altered levels in biological fluids (e.g., blood or urine) under pathological conditions, including cancer. Changes in nucleoside metabolism often occur in cancer cells due to increased proliferation, DNA synthesis, and turnover, making them potential indicators of cancer activity [
70].
In [
50], a Love-wave Surface Acoustic Wave (SAW) sensor is described that utilizes molecularly imprinted polymers (MIPs) for the detection of nucleosides, such as adenosine-5'-monophosphate (5'-AMP) and pseudouridine (Pseu), which serve as cancer biomarkers. The sensor, developed on an AT-cut quartz substrate with a 4 µm SiO₂ guiding layer, operates at 118 MHz. The MIP layer is specifically imprinted to bind 5'-AMP and Pseu, enabling selective interaction with these nucleosides. The device is integrated with a microfluidic system for real-time monitoring. The sensor achieves a detection limit of 5 ppm for 5'-AMP, with frequency shifts of about 150 Hz per injection, highlighting its potential for non-invasive cancer biomarker analysis in liquid samples for monitoring treatment efficacy. Similarly, in [
51], another Love-wave sensor coated with a molecularly imprinted polymer (MIP) is developed to detect adenosine monophosphate (AMP) as a representative nucleoside linked to cancer biomarkers. This sensor operates on an AT-cut quartz substrate at 117 MHz with a 4 µm SiO₂ guiding layer and uses a porous AMP-imprinted polymer coating for selective AMP binding. In aqueous media, the sensor demonstrates a frequency shift of approximately 137 Hz per ppm for AMP concentrations as low as 6 ppm, proving its high sensitivity. The sensor's reproducibility across measurements has been confirmed, with plans to extend its application to detect specific cancer-related nucleosides. In [
52], a Love-wave Surface Acoustic Wave (SAW) sensor is presented, coated with a molecularly imprinted polymer (MIP) layer, designed for detecting adenosine monophosphate (AMP) as a model for colorectal cancer biomarkers. The sensor, built on an AT-cut quartz substrate with a 4.5 µm SiO₂ guiding layer, operates at 117 MHz. The MIP layer, specifically designed to recognize AMP, demonstrated selectivity and sensitivity, with frequency shifts of up to -2.5 kHz in response to 2126 ppm ethanol vapor, observed when comparing pre- and post-template extraction stages. The response was found to increase with thicker MIP films (200 nm vs. 600 nm), indicating the sensor's potential for sensitive and selective biomarker detection in gas-phase sensing applications relevant to cancer monitoring.
This body of literature demonstrates the development of similar devices for detecting AMP, underscoring their potential in cancer diagnostics.
3.9. Circulating Tumor Cells
Circulating tumor cells (CTCs) are cancer cells that break away from the primary tumor and travel through the bloodstream. These cells play an essential role in metastasis, which is the process by which cancer spreads to other organs. While most CTCs are unable to survive in the blood due to the hostile environment, a small fraction can evade immune defenses and form secondary tumors in distant sites. Detecting and analyzing these cells provides crucial information about cancer's progression, its metastatic capabilities, and its response to treatments [
71].
In [
21], a leaky surface acoustic wave (SAW) aptasensor array is presented, designed for the label-free, high-sensitivity detection of circulating tumor cells (CTCs), specifically MCF-7 breast cancer cells. The sensor operates on a 36° YX-cut lithium tantalate (LiTaO₃) substrate at 100 MHz, with aptamers targeting MUC1 proteins on the cell surface for the capture of MCF-7 cells. The array consists of five detection sensors and one reference sensor, each with independent oscillation circuits to ensure stability. This setup offers a broad linear detection range from 100 to 10⁷ cells/mL, with a detection limit as low as 32 cells/mL. The sensor also demonstrates excellent specificity, distinguishing MCF-7 cells from non-target cells, and retains 90% of its performance after ten regeneration cycles, highlighting its potential for clinical cancer diagnostics.
3.10. Genes
HER2/neu (also known as Human Epidermal Growth Factor Receptor 2) is a gene that plays a key role in the development of certain types of cancer, especially breast cancer. It encodes a protein that is involved in cell growth and division. When the HER2 gene is amplified (i.e., produces too many copies) or the HER2 protein is overexpressed on the surface of cells, it can promote cancerous growth. This is commonly seen in some breast cancers, referred to as HER2-positive breast cancer.
In [
72], a label-free SH-SAW biosensor for detecting HER-2/neu, a breast cancer biomarker, is presented. The sensor is based on a 36° YX-LiTaO₃ substrate operating at 428.5 MHz. It employs neutravidin and biotinylated protein A linkers for site-directed immobilization of anti-HER-2 antibodies, enhancing signal response and achieving a detection limit of 10 ng/mL. The sensor is fabricated with a parylene C layer to ensure chemical homogeneity and is integrated with a microfluidic flow cell, enabling real-time detection under low-flow conditions. Stability tests using PBS and selectivity evaluations against bovine serum albumin demonstrate its strong potential for non-invasive clinical breast cancer diagnostics.[
72]BRCA1, ptch, and p53 BRCA1, PTCH, and p53 are all crucial genes that play significant roles in cell regulation, DNA repair, and tumor suppression, and mutations in these genes are associated with various types of cancer. Mutations in BRCA1 are most famously linked to hereditary breast and ovarian cancers, significantly increasing the risk of developing these cancers. Mutations in PTCH are associated with Basal Cell Nevus Syndrome (Gorlin syndrome), which increases the risk of developing basal cell carcinoma and other tumors like medulloblastoma. Mutations in p53 are among the most common genetic alterations found in human cancers, including lung, colon, and breast cancers. In [
24], a Love-wave surface acoustic wave (SAW) sensor is presented for the detection of single-nucleotide mutations in cancer-associated genes, including BRCA1, PTCH, and p53. Operating at 148 MHz on an ST-cut quartz substrate with a carboxymethylated dextran (CMD) layer, the sensor utilizes a streptavidin-biotin system for the immobilization of DNA probes, enabling hybridization assays. The sensor differentiates between mutant and wild-type sequences by analyzing mass and viscosity changes, utilizing association/dissociation kinetics, with a detection sensitivity of 10 nM DNA concentrations. The system allows for multiple binding cycles with minimal signal degradation, providing a rapid, label-free method for mutation detection, which holds potential for clinical genetic cancer screening applications.
4. Future Trends
From the perspective of traditional biomarker detection methods, techniques such as enzyme-linked immunosorbent assays (ELISA) and polymerase chain reaction (PCR) are constrained by significant technological limitations, including slow detection times and the high cost of reagents for each assay. These methods are also labor-intensive and not conducive to continuous patient monitoring during treatment. Additionally, given that cancer is a multifactorial disease involving complex cellular events and multiple molecular interactions, the simultaneous detection of various biomarkers is essential for accurate diagnosis and prognosis. Moreover, the detection of biomarkers such as volatile organic compounds (VOCs) by traditional techniques, such as gas chromatography-mass spectrometry (GC-MS), is also subject to similar limitations as those observed with ELISA and PCR, including long analysis times and high operational costs.
The same happens for the cancerous cell detection by image techniques (CT, SPECT, MRI, and PET) and biopsies, which in addition are very expensive as in operational costs as in acquisition costs.
Due to the limitations of the aforementioned techniques the development of advanced biosensing devices for clinical applications has become a growing area of research to detect cancer cells and their biomarkers. This has served as the driving motivation behind this review on acoustic biosensors for cancer detection, with the objective of assessing the current advancements in the field and exploring potential avenues for enhancement in future research. The selection of SAW biosensors is based on the advantages they offer for potential clinical applications, such as, label-free detection, real-time operation, and compatibility with miniaturized and portable systems, making them promising tools for non-invasive cancer diagnosis.
As emphasized in the review, despite the significant amount of research on SAW biosensors, much of it has been directed towards environmental monitoring and food safety, rather than cancer diagnostics. Translating advancements in SAW biosensor technology and in nanomaterial technology into the realm of cancer diagnostics could unlock significant innovations and breakthroughs. Thus, in addition to their established role in detecting complex biomolecules, SAW sensors are also capable of detecting small volatile organic compounds (VOCs) in the breath, which are increasingly recognized as potential biomarkers for a variety of diseases, including cancer in early stages. However, as pointed out in the paper, this area remains underexplored in the context of cancer detection. The limited literature available on the detection of VOCs as biomarkers using SAW sensors is primarily focused on lung cancer diagnostics. For detecting them, SAWs sensors can incorporate diverse sensing materials such as metal oxides [
73], stationary phases [
74], room temperature ionic liquids (RTILs) [
75], or nanostructured materials [
76]. Stationary phases, commonly used in gas chromatography, can be integrated into SAW devices as sensitive layers, improving their specificity for particular VOCs. Polymers like polydimethylsiloxane (PDMS), polyethylene glycol (PEG), and other materials can be coated onto the SAW sensor surface, where they interact with VOC molecules through absorption or adsorption processes. By selecting or engineering polymers with specific chemical affinities, the sensitivity and selectivity of SAW sensors can be significantly enhanced for targeted VOCs. Additionally, the incorporation of lipid bilayers, which replicate the natural environment of cell membranes, can facilitate the integration of membrane proteins or receptors into the sensor surface. While this strategy has been successfully applied in QCM sensors, it remains relatively unexplored in SAW devices.
Another line of innovation arises from the advancement of more sophisticated systems, including flexible SAW sensors [
77,
78], which can be incorporated into wearable devices for continuous, real-time health monitoring. These sensors offer the potential for non-invasive detection and analysis, enabling applications such as early disease diagnosis, personalized health tracking, and the monitoring of environmental exposure to harmful substances.
And finally, an additional cutting-edge direction for future research is the SAW sensor integration with microfluidic technology. These devices are particularly well-suited for integration with microfluidic systems, creating powerful platforms for cancer diagnostics. Their ability to manipulate fluids and particles at the microscale enables precise control over sample handling, essential for lab-on-a-chip technologies. While some studies have begun exploring this integration [
79,
80,
81], there is significant potential for further development. Advancing this field could lead to highly sensitive, rapid, and portable diagnostic tools, enhancing early cancer detection and personalized medicine.
5. Conclusions
Surface Acoustic Wave (SAW) sensors have evolved into a promising tool for cancer biomarker detection, combining high sensitivity, real-time operation, label-free detection, and versatility in detecting a wide range of analytes. This review has outlined how specific configurations, such as Love-wave and SH-SAW devices, are particularly suited to the challenges of detecting biomarkers in both gaseous and liquid environments, including VOCs in exhaled breath and biomolecules in bodily fluids.
Nevertheless, significant challenges must be addressed before SAW sensors can become a standard tool in cancer diagnostics. Current limitations include optimizing sensitivity for specific biomarkers, and mitigating the effects of environmental variables like humidity, and validating their clinical performance across diverse patient populations, primarily for VOC biomarkers. Furthermore, while recent advancements in piezoelectric materials, guiding layers, and functionalization techniques have improved performance, these innovations often increase manufacturing complexity and cost.
Future research should prioritize the integration of SAW sensors into hybrid diagnostic systems that combine complementary technologies, such as gas chromatography or MOS sensors technology for VOC sensing, to enhance detection specificity, sensitivity for low concentrations, stability, reproducibility and robustness. Additionally, efforts to miniaturize and standardize SAW devices for widespread clinical use will be essential to bridge the gap between laboratory research and real-world applications.
In conclusion, while SAW sensors still face technical and practical hurdles, their potential to revolutionize cancer diagnostics through non-invasive, scalable, and real-time detection methods is undeniable. Further innovation in sensor design and validation in clinical settings will determine their ultimate impact in addressing the global burden of cancer.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organiation Cancer Key Facts.
- Manhas, N.; Kumar, L.S.; Adimule, V. Early-Stage Diagnosis of Breast Cancer: Amelioration in Approaches. In Drug and Therapy Development for Triple Negative Breast Cancer; Kendrekar, P., Adimule, V., Hurst, T., Eds.; Wiley, 2023; pp. 1–34 ISBN 978-3-527-35175-6.
- Kokabi, M.; Tahir, M.N.; Singh, D.; Javanmard, M. Advancing Healthcare: Synergizing Biosensors and Machine Learning for Early Cancer Diagnosis. Biosensors 2023, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Azab, M.Y.; Hameed, M.F.O.; Obayya, S.S.A. Overview of Optical Biosensors for Early Cancer Detection: Fundamentals, Applications and Future Perspectives. Biology 2023, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer Biomarkers and Their Biosensors: A Comprehensive Review. TrAC Trends in Analytical Chemistry 2023, 158, 116813. [Google Scholar] [CrossRef]
- Sarkar, S.; Hazra, S.; Patra, S.; Gogoi, M. Biosensors for Cancer Detection: A Review. TrAC Trends in Analytical Chemistry 2024, 180, 117978. [Google Scholar] [CrossRef]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2024, 24, 37. [Google Scholar] [CrossRef]
- Abdul Wahab, M.R.; Palaniyandi, T.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Sugumaran, A.; Sivaji, A.; Kaliamoorthy, S.; Kumarasamy, S. Biomarker-Specific Biosensors Revolutionise Breast Cancer Diagnosis. Clinica Chimica Acta 2024, 555, 117792. [Google Scholar] [CrossRef]
- Rayleigh, Lord On Waves Propagated along the Plane Surface of an Elastic Solid. Proceedings of the London Mathematical Society 1885, s1-17, 4–11. [CrossRef]
- Tang, Z.; Wu, W.; Yang, P.; Luo, J.; Fu, C.; Han, J.-C.; Zhou, Y.; Wang, L.; Wu, Y.; Huang, Y. A Review of Surface Acoustic Wave Sensors: Mechanisms, Stability and Future Prospects. SR 2024, 44, 249–266. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent Advances in Acoustic Wave Biosensors for the Detection of Disease-Related Biomarkers: A Review. Analytica Chimica Acta 2021, 1164, 338321. [Google Scholar] [CrossRef]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Zida, S.I.; Lin, Y.; Khung, Y.L. Current Trends on Surface Acoustic Wave Biosensors. Adv Materials Technologies 2021, 6, 2001018. [Google Scholar] [CrossRef]
- Fourati, N.; Attia, G.; Khaoulani, S.; Zerrouki, C. Applications and Recent Trends in Surface Acoustic Wave Biosensors. In Piezoelectric Sensors; Lieberzeit, P., Ed.; Springer International Publishing: Cham, 2024; pp. 225–251. ISBN 978-3-031-53785-1. [Google Scholar]
- Gouda, M.; Ghazzawy, H.S.; Alqahtani, N.; Li, X. The Recent Development of Acoustic Sensors as Effective Chemical Detecting Tools for Biological Cells and Their Bioactivities. Molecules 2023, 28, 4855. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Voltmer, F.W. Direct Piezoelectric Coupling to Surface Elastic Waves. Applied Physics Letters 1965, 7, 314–316. [Google Scholar] [CrossRef]
- Šetka, M.; Bahos, F.A.; Matatagui, D.; Gràcia, I.; Figueras, E.; Drbohlavová, J.; Vallejos, S. Love Wave Sensors with Silver Modified Polypyrrole Nanoparticles for VOCs Monitoring. Sensors 2020, 20, 1432. [Google Scholar] [CrossRef]
- Hallil, H.; Omar Aouled, N.; Plano, B.; Delépée, R.; Agrofoglio, L.; Dejous, C.; Rebière, D. Love Wave Sensor Based on Thin Film Molecularly Imprinted Polymer: Study of VOCs Adsorption. JICS 2020, 9, 118–122. [Google Scholar] [CrossRef]
- Ghayour, R.; Hojjat, Y.; Karafi, M.R.; Sadeghiyan, H. Development of a Hybrid DEP-SAW Device for Trapping/Sensing Target Cells. Applied Acoustics 2018, 141, 355–361. [Google Scholar] [CrossRef]
- Chang, K.; Pi, Y.; Lu, W.; Wang, F.; Pan, F.; Li, F.; Jia, S.; Shi, J.; Deng, S.; Chen, M. Label-Free and High-Sensitive Detection of Human Breast Cancer Cells by Aptamer-Based Leaky Surface Acoustic Wave Biosensor Array. Biosensors and Bioelectronics 2014, 60, 318–324. [Google Scholar] [CrossRef]
- Gagliardi, M.; Agostini, M.; Lunardelli, F.; Miranda, A.; Luminare, A.G.; Cervelli, F.; Gambineri, F.; Cecchini, M. A Surface Acoustic Wave (SAW)-Based Lab-on-Chip for the Detection of Active α-Glycosidase. Biosensors 2022, 12, 1010. [Google Scholar] [CrossRef]
- Han, S.B.; Lee, S.S. Simultaneous Detection of Exosomal microRNAs Isolated from Cancer Cells Using Surface Acoustic Wave Sensor Array with High Sensitivity and Reproducibility. Micromachines 2024, 15, 249. [Google Scholar] [CrossRef]
- Gronewold, T.M.A.; Baumgartner, A.; Quandt, E.; Famulok, M. Discrimination of Single Mutations in Cancer-Related Gene Fragments with a Surface Acoustic Wave Sensor. Anal. Chem. 2006, 78, 4865–4871. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Das, P.Kr.; Bhethanabotla, V.R. Surface Acoustic Waves in Biosensing Applications. Sensors and Actuators Reports 2021, 3, 100041. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Li, M.; Qian, L.; Wang, L.; Li, H. Surface Acoustic Wave Immunosensor Based on Au-Nanoparticles-Decorated Graphene Fluidic Channel for CA125 Detection. Sensors and Actuators B: Chemical 2022, 367, 132063. [Google Scholar] [CrossRef]
- Lo, X.-C.; Li, J.-Y.; Lee, M.-T.; Yao, D.-J. Frequency Shift of a SH-SAW Biosensor with Glutaraldehyde and 3-Aminopropyltriethoxysilane Functionalized Films for Detection of Epidermal Growth Factor. Biosensors 2020, 10, 92. [Google Scholar] [CrossRef]
- Onen, O.; Sisman, A.; Gallant, N.D.; Kruk, P.; Guldiken, R. A Urinary Bcl-2 Surface Acoustic Wave Biosensor for Early Ovarian Cancer Detection. Sensors 2012, 12, 7423–7437. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yatsuda, H.; Goto, M.; Kondoh, J.; Liu, S.-H.; Wang, R. Application of Shear Horizontal Surface Acoustic Wave (SH-SAW) Immunosensor in Point-of-Care Diagnosis. Biosensors 2023, 13, 605. [Google Scholar] [CrossRef]
- Rauf, S.; Qazi, H.I.A.; Luo, J.; Fu, C.; Tao, R.; Rauf, S.; Yang, L.; Li, H.; Fu, Y. Ultrasensitive Leaky Surface Acoustic Wave Immunosensor for Real-Time Detection of Alpha-Fetoprotein in Biological Fluids. Chemosensors 2021, 9, 311. [Google Scholar] [CrossRef]
- Hadj-Larbi, F.; Serhane, R. Sezawa SAW Devices: Review of Numerical-Experimental Studies and Recent Applications. Sensors and Actuators A: Physical 2019, 292, 169–197. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly Stable, Love-Mode Surface Acoustic Wave Biosensor Using Au Nanoparticle-MoS2-rGO Nano-Cluster Doped Polyimide Nanocomposite for the Selective Detection of Carcinoembryonic Antigen. Materials Chemistry and Physics 2020, 246, 122800. [Google Scholar] [CrossRef]
- Tang, P.; Wang, Y.; Huo, J.; Lin, X. Love Wave Sensor for Prostate-Specific Membrane Antigen Detection Based on Hydrophilic Molecularly-Imprinted Polymer. Polymers 2018, 10, 563. [Google Scholar] [CrossRef]
- Tigli, O.; Bivona, L.; Berg, P.; Zaghloul, M.E. Fabrication and Characterization of a Surface-Acoustic-Wave Biosensor in CMOS Technology for Cancer Biomarker Detection. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Greco, G.; Cecchini, M. Full-SAW Microfluidics-Based Lab-on-a-Chip for Biosensing. IEEE Access 2019, 7, 70901–70909. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Quan, A.; Qiu, C.; Cao, W.; Fu, C.; Fu, Y.Q. Highly Selective and Label-Free Love-Mode Surface Acoustic Wave Biosensor for Carcinoembryonic Antigen Detection Using a Self-Assembled Monolayer Bioreceptor. Applied Surface Science 2020, 518, 146061. [Google Scholar] [CrossRef]
- Jandas, P.J.; Prabakaran, K.; Luo, J.; Fu, C.; Fu, Y.Q.; Holaday M G, D. Ti3C2Tx MXene-Au Nanoparticles Doped Polyimide Thin Film as a Transducing Bioreceptor for Real-Time Acoustic Detection of Carcinoembryonic Antigen. Sensors and Actuators A: Physical 2021, 331, 112998. [Google Scholar] [CrossRef]
- Wang, X.; Ji, J.; Yang, P.; Li, X.; Pang, Y.; Lu, P. A Love-Mode Surface Acoustic Wave Aptasensor with Dummy Fingers Based on Monolayer MoS2/Au NPs Nanocomposites for Alpha-Fetoprotein Detection. Talanta 2022, 243, 123328. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xie, H.; Luo, J.; Fu, C.; Tao, R.; Li, H.; Fu, Y. Highly Sensitive Love Mode Acoustic Wave Platform with SiO2 Wave-Guiding Layer and Gold Nanoparticles for Detection of Carcinoembryonic Antigens. Biosensors 2022, 12, 536. [Google Scholar] [CrossRef]
- Zou, Y. Love Wave Based Portable Sensing System for On-Line Detection of Carcinoembryonic Antigen in Exhaled Breath Condensate. Biomed Microdevices 2020. [Google Scholar] [CrossRef]
- Sisman, A.; Gur, E.; Ozturk, S.; Enez, B.; Okur, B.; Toker, O. A Low-Cost Biomarker-Based SAW-Biosensor Design for Early Detection of Prostate Cancer. Procedia Technology 2017, 27, 248–249. [Google Scholar] [CrossRef]
- Wang, T.; Green, R.; Nair, R.R.; Howell, M.; Mohapatra, S.; Guldiken, R.; Mohapatra, S.S. Surface Acoustic Waves (SAW)-Based Biosensing for Quantification of Cell Growth in 2D and 3D Cultures. Sensors (Basel) 2015, 15, 32045–32055. [Google Scholar] [CrossRef]
- Lee, D.S.; Fu, Y.Q.; Maeng, S.; Du, X.Y.; Tan, S.C.; Luo, J.K.; Flewitt, A.J.; Kim, S.H.; Park, N.M.; Choi, Y.J.; et al. Integrated ZnO Surface Acoustic Wave Microfluidic and Biosensor System. In Proceedings of the 2007 IEEE International Electron Devices Meeting; IEEE: Washington, DC, USA, 2007; pp. 851–854. [Google Scholar]
- Murillo, A.E.; Melo-Máximo, L.; García-Farrera, B.; Salas Martínez, O.; Melo-Máximo, D.V.; Oliva-Ramírez, J.; García, K.; Huerta, L.; Oseguera, J. Development of AlN Thin Films for Breast Cancer Acoustic Biosensors. Journal of Materials Research and Technology 2019, 8, 350–358. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. A Rayleigh Surface Acoustic Wave (R-SAW) Resonator Biosensor Based on Positive and Negative Reflectors with Sub-Nanomolar Limit of Detection. Sensors and Actuators B: Chemical 2018, 254, 1–7. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, X.; An, C.; Ran, C.; Ying, K.; Wang, P. A Point-of-Care Testing System with Love-Wave Sensor and Immunogold Staining Enhancement for Early Detection of Lung Cancer. Biomed Microdevices 2014, 16, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, Y.; An, C.; Ying, K.; Chen, X.; Wang, P. Sensitive Detection of Carcinoembryonic Antigen in Exhaled Breath Condensate Using Surface Acoustic Wave Immunosensor. Sensors and Actuators B: Chemical 2015, 217, 100–106. [Google Scholar] [CrossRef]
- Hao, H.-C.; Chang, H.-Y.; Wang, T.-P.; Yao, D.-J. Detection of Cells Captured with Antigens on Shear Horizontal Surface-Acoustic-Wave Sensors. SLAS Technology 2013, 18, 69–76. [Google Scholar] [CrossRef]
- Cai, H.-L.; Yang, Y.; Chen, X.; Mohammad, M.A.; Ye, T.-X.; Guo, C.-R.; Yi, L.-T.; Zhou, C.-J.; Liu, J.; Ren, T.-L. A Third-Order Mode High Frequency Biosensor with Atomic Resolution. Biosensors and Bioelectronics 2015, 71, 261–268. [Google Scholar] [CrossRef]
- Dejous, C.; Hallil, H.; Raimbault, V.; Lachaud, J.-L.; Plano, B.; Delépée, R.; Favetta, P.; Agrofoglio, L.; Rebière, D. Love Acoustic Wave-Based Devices and Molecularly-Imprinted Polymers as Versatile Sensors for Electronic Nose or Tongue for Cancer Monitoring. Sensors 2016, 16, 915. [Google Scholar] [CrossRef]
- Lebal, N.; Raimbault, V.; Hallil, H.; Plano, B.; Lachaud, J.L.; Dejous, C.; Rebière, D.; Krstulja, A.; Delepée, R.; Agrofoglio, L. Love Wave-Based Acoustic Components as Versatile Sensors for Electronic Nose or Tongue. In Application to Cancer Monitoring. In Proceedings of the IEEE SENSORS 2014 Proceedings; IEEE: Valencia, November, 2014; pp. 1380–1383. [Google Scholar]
- Aouled, N.O.; Hallil, H.; Plano, B.; Rebiere, D.; Dejous, C.; Delepee, R.; Agrofoglio, L. Love Wave Sensor Based on Thin Film Molecularly Imprinted Polymer : MIP Layer Morphology and Nucleosides Analogs Detection. In Proceedings of the 2013 IEEE SENSORS; IEEE: Baltimore, MD, USA, November, 2013; pp. 1–4. [Google Scholar]
- Zhang, X.-F.; Zhang, Z.-W.; He, Y.-L.; Liu, Y.-X.; Li, S.; Fang, J.-Y.; Zhang, X.-A.; Peng, G. Sniffing Lung Cancer Related Biomarkers Using an Oxidized Graphene SAW Sensor. Front. Phys. 2016, 11, 116801. [Google Scholar] [CrossRef]
- Wang, D.; Yu, K.; Wang, Y.; Hu, Y.; Zhao, C.; Wang, L.; Ying, K.; Wang, P. A Hybrid Electronic Noses’ System Based on MOS-SAW Detection Units Intended for Lung Cancer Diagnosis. J. Innov. Opt. Health Sci. 2012, 05, 1150006. [Google Scholar] [CrossRef]
- He, S.; Gao, Y.; Shao, J.; Lu, Y. Application of SAW Gas Chromatography in the Early Screening of Lung Cancer. In Proceedings of the 2015 Symposium on Piezoelectricity, Acoustic Waves, and Device Applications (SPAWDA); IEEE: Jinan, October, 2015; pp. 22–25. [Google Scholar]
- Tsou, P.-H.; Lin, Z.-L.; Pan, Y.-C.; Yang, H.-C.; Chang, C.-J.; Liang, S.-K.; Wen, Y.-F.; Chang, C.-H.; Chang, L.-Y.; Yu, K.-L.; et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers 2021, 13, 1431. [Google Scholar] [CrossRef]
- Saalberg, Y.; Wolff, M. VOC Breath Biomarkers in Lung Cancer. Clinica Chimica Acta 2016, 459, 5–9. [Google Scholar] [CrossRef]
- Vassilenko, V.; Moura, P.C.; Raposo, M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines 2023, 11, 3029. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Meta-Analysis. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, X.; Liu, J.; Li, S.; Liu, X. Metal Oxide Semiconductor Gas Sensors for Lung Cancer Diagnosis. Chemosensors 2023, 11, 251. [Google Scholar] [CrossRef]
- Šetka, M.; Bahos, F.A.; Matatagui, D.; Potoček, M.; Kral, Z.; Drbohlavová, J.; Gràcia, I.; Vallejos, S. Love Wave Sensors Based on Gold Nanoparticle-Modified Polypyrrole and Their Properties to Ammonia and Ethylene. Sensors and Actuators B: Chemical 2020, 304, 127337. [Google Scholar] [CrossRef]
- Alves Martins, B.A.; de Bulhões, G.F.; Cavalcanti, I.N.; Martins, M.M.; de Oliveira, P.G.; Martins, A.M.A. Biomarkers in Colorectal Cancer: The Role of Translational Proteomics Research. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Y.; An, C.; Ying, K.; Chen, X.; Wang, P. A Miniaturized Immunosensor Platform for Automatic Detection of Carcinoembryonic Antigen in EBC. Sensors and Actuators B: Chemical 2014, 205, 94–101. [Google Scholar] [CrossRef]
- Głowska-Ciemny, J.; Szymański, M.; Kuszerska, A.; Malewski, Z.; von Kaisenberg, C.; Kocyłowski, R. The Role of Alpha-Fetoprotein (AFP) in Contemporary Oncology: The Path from a Diagnostic Biomarker to an Anticancer Drug. International Journal of Molecular Sciences 2023, 24, 2539. [Google Scholar] [CrossRef]
- Holjencin, C.; Jakymiw, A. MicroRNAs and Their Big Therapeutic Impacts: Delivery Strategies for Cancer Intervention. Cells 2022, 11, 2332. [Google Scholar] [CrossRef]
- Hernández, J.; Thompson, I.M. Prostate-Specific Antigen: A Review of the Validation of the Most Commonly Used Cancer Biomarker. Cancer 2004, 101, 894–904. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Dahmardeh, M.; Sheybanifar, S.; Gharooni, M.; Janmaleki, M.; Abdolahad, M. Acoustic Wave Based Biosensor to Study Electroacoustic Based Detection of Progressive (SW-48) Colon Cancer Cells from Primary (HT-29) Cells. Sensors and Actuators A: Physical 2015, 233, 169–175. [Google Scholar] [CrossRef]
- Hsu-Chao Hao; Da-Jeng Yao A Sensitive, Rapid and Specific Technique for the Detection of Antigen-Specific Cells on Shear Horizontal Surface Acoustic Wave (SH-SAW) Sensors. In Proceedings of the 2010 IEEE Sensors; IEEE: Kona, HI, November 2010; pp. 2117–2121.
- Yu, Y.; Pan, H.-Y.; Zheng, X.; Yuan, F.; Zhou, Y.-L.; Zhang, X.-X. Ultrasensitive Simultaneous Detection of Multiple Rare Modified Nucleosides as Promising Biomarkers in Low-Put Breast Cancer DNA Samples for Clinical Multi-Dimensional Diagnosis. Molecules 2022, 27, 7041. [Google Scholar] [CrossRef] [PubMed]
- Rapanotti, M.C.; Cenci, T.; Scioli, M.G.; Cugini, E.; Anzillotti, S.; Savino, L.; Coletta, D.; Di Raimondo, C.; Campione, E.; Roselli, M.; et al. Circulating Tumor Cells: Origin, Role, Current Applications, and Future Perspectives for Personalized Medicine. Biomedicines 2024, 12, 2137. [Google Scholar] [CrossRef] [PubMed]
- Gruhl, F.J.; Rapp, M.; Länge, K. Label-Free Detection of Breast Cancer Marker HER-2/Neu with an Acoustic Biosensor. Procedia Engineering 2010, 5, 914–917. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. ZnO/ST-Quartz SAW Resonator: An Efficient NO2 Gas Sensor. Sensors and Actuators B: Chemical 2017, 252, 840–845. [Google Scholar] [CrossRef]
- Abraham, M.H.; Poole, C.F.; Poole, S.K. Classification of Stationary Phases and Other Materials by Gas Chromatography. Journal of Chromatography A 1999, 842, 79–114. [Google Scholar] [CrossRef]
- Aleixandre, M.; Nakamoto, T. Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances. Sensors 2020, 20, 4026. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Fu, W.; Lv, H.; Zu, X.; Guo, Y.; Gibson, D.; Fu, Y.-Q. Advances in Sensing Mechanisms and Micro/Nanostructured Sensing Layers for Surface Acoustic Wave-Based Gas Sensors. J. Mater. Chem. A 2023, 11, 9216–9238. [Google Scholar] [CrossRef]
- Qureshi, S.; Hanif, M.; Jeoti, V.; Stojanović, G.M.; Khan, M.T. Review of Fabrication of SAW Sensors on Flexible Substrates: Challenges and Future. Results in Engineering 2024, 22, 102323. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, Y.; Wang, Y.; Ji, Z.; Zhang, Q.; Zhuo, F.; Luo, J.; Tao, R.; Xie, J.; Reboud, J.; et al. Flexible and Wearable Acoustic Wave Technologies. Applied Physics Reviews 2023, 10, 021311. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Yang, Z.; Xia, H.; Zhang, C.; Wei, X. Surface Acoustic Wave Based Microfluidic Devices for Biological Applications. Sens. Diagn. 2023, 2, 507–528. [Google Scholar] [CrossRef]
- Jeng, M.-J.; Li, Y.-C.; Sharma, M.; Chen, C.-W.; Tsai, C.-L.; Lin, Y.-H.; Huang, S.-F.; Chang, L.-B.; Lai, C.-S. A Surface Acoustic Wave Sensor with a Microfluidic Channel for Detecting C-Reactive Protein. Chemosensors 2021, 9, 106. [Google Scholar] [CrossRef]
- Matatagui, D.; Bastida, A.; Horrillo, M.C. Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors. Biosensors 2024, 12(1), 17. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).