Submitted:
25 November 2024
Posted:
26 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Statistical Methods
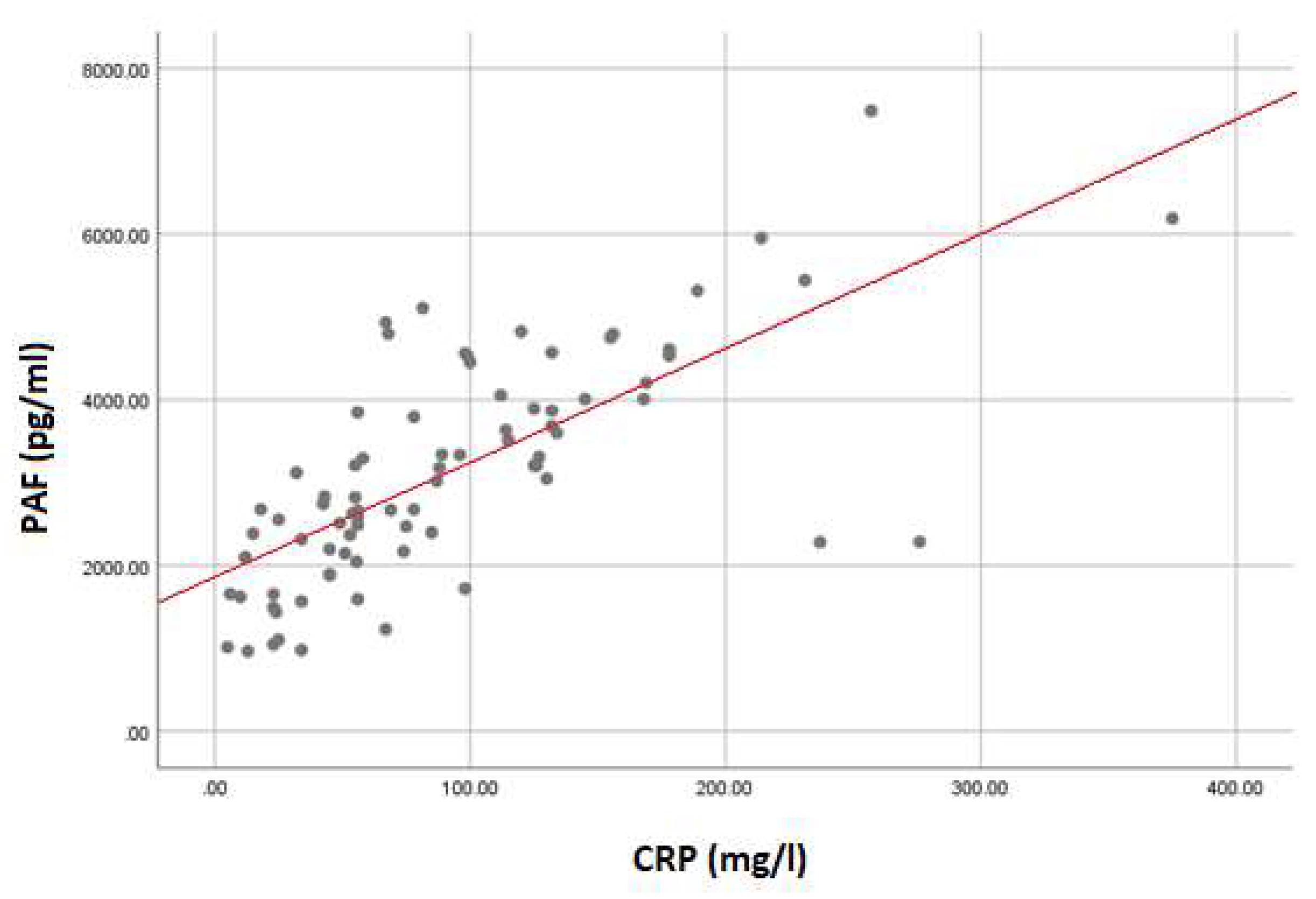
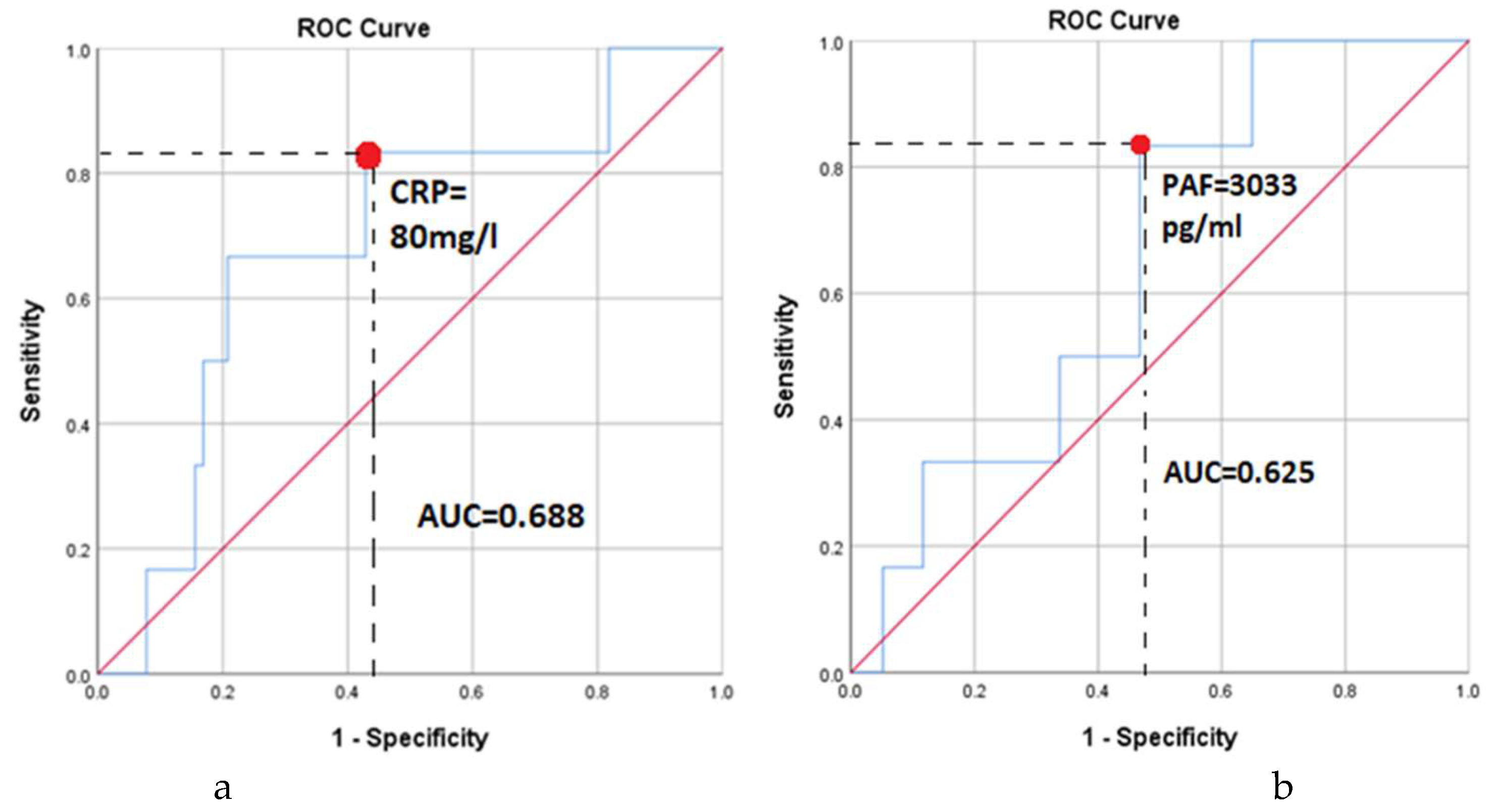
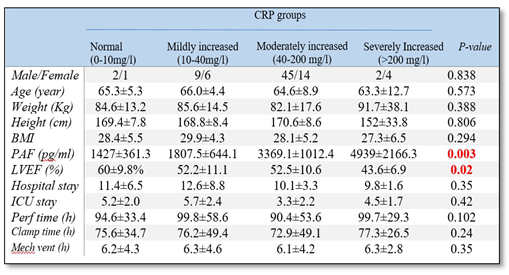
3. Results
| Variable | Odds ratio | 95% Confidence Intervals |
|---|---|---|
| Age | 1.10 | 1.02-1.19 |
| BMI | 1.75 | 0.33-9.3 |
| PAF | 2.62 | 0.37-20.0 |
| VIS | 0.89 | 0.78-0.99 |
| Clamp time >90 min | 0.34 | 0.38-3.1 |
| Perf time >180 min | 0.89 | 0.84-0.98 |
| Mech ventil | 1.85 | 0.32-10.7 |
| CRP | 2.45 | 0.48-18.3 |
| Variable | Odds ratio | 95% Confidence Intervals |
|---|---|---|
| Age | 0.25 | 0.03-2.56 |
| BMI | 0.48 | 0.08-2.82 |
| PAF | 0.36 | 0.01-8.9 |
| Mech ventil | 0.81 | 0.12-5.41 |
| CRP | 0.37 | 0.01-8.96 |
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernard, N.A., Williams, R.W. and Spencer, E.M. Preoperative Patient Assessment: A Review of Literature and Recommendations. Annals of the Royal College of Surgeons of England 1994, 76, 293–297. [Google Scholar]
- Van Klei, W.A., Grobbee, D.E., Rutten, C.L., Hennis, P.J., Knape, J.T., Kalkman, C.J., et al. Role of History and Physical Examination in Preoperative Evaluation. European Journal of Anaesthesiology 2003, 20, 612–618. [Google Scholar] [CrossRef]
- Johnson, R.K. and Mortimer, A.J. Routine Preoperative Blood Testing: Is It a Necessary? Anaesthesia 2002, 57, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Krishnammurthy, A. , Dutta, D., Phililps, J. and Methal, N. (2007) Are We Still Performing too Many Blood Tests? Quality and Safety in Health Care, 16, 400.
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998 Jun 01;187(11):1779-88.
- Won Song, S. , Yi, G.J., Lee, S., Nam Youn, Y., Young Sul, S. and Jong Yoo, K. (2008) Perioperative Indicators of Stress Response and Postoperative Inflammatory Complications in Patients Undergoing Off-Pump Coronary Artery Bypass Surgery.
- Benveniste J, Henson PM, Cochrane CJ: Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med 136:1365- 1377.1972 10.
- Chignard M, Le Couedic JP, Ten& M, et al: The role of platelet-activating factor in platelet aggregation. Nature 279:799- 800, 1979.
- Benveniste J: PAF-acether, an ether phospholipid with biological activity, in Karnovsky ML, Leaf A, Bolis LC (ed): Biological Membranes. New-York, NY, Liss, 1988, pp 75-85.
- Pinckard RN, Ludwig JC, MC Manus LM: Platelet-activating factors, in Gallin JI, Goldstein I, Snyderman R (ed): Inflammation. Basic Principles and Clinical Correlates. New York, NY, Raven, 1988, pp 139-167.
- Hedlund P: Clinical and experimental studies on C-reactive protein (acute phase protein). Acta Med Scand Suppl 1961, 361: 1-71.
- Balbay Y, Tikiz H, Baptiste RJ, Ayaz S, Sasmaz H, Korkmaz S: Circulating interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha, and soluble ICAM-1 in patients with chronic stable angina and myocardial infarction. Angiology 2001, 52: 109-114.
- Filep JG, Hermán F, Kelemen E, Földes-Filep E. C-reactive protein inhibits binding of platelet-activating factor to human platelets. Thromb Res. 1991 Feb 15;61(4):411-21.
- Sato A, Oe K, Yamanaka H, Yokoyama I, Ebina K. C-reactive protein specifically enhances platelet-activating factor-induced inflammatory activity in vivo. Eur J Pharmacol. 2014 Dec 15;745:46-51.
- Brunetti ND, Troccoli R, Correale M, Pellegrino PL, Di Biase M: C-reactive protein in patients with acute coronary syndrome: correlation with diagnosis, myocardial damage, ejection fraction and angiographic findings. Int J Cardiol 2006, 109: 248-256.
- Tian H, Jiang X, Duan G, Chen J, Liu Q, Zhang Y, Li S, Bao X, Huang H. Preoperative inflammatory markers predict postoperative clinical outcomes in patients undergoing heart valve surgery: A large-sample retrospective study. Front Immunol. 2023 Mar 31;14:1159089. [CrossRef] [PubMed]
- Tjörvi E. Perry, Jochen D. Muehlschlegel, Kuang-Yu Liu, Amanda A. Fox, Charles D. Collard, Simon C. Body, Stanton K. Shernan, for the CABG Genomics Investigators; Preoperative C-reactive Protein Predicts Long-term Mortality and Hospital Length of Stay after Primary, Nonemergent Coronary Artery Bypass Grafting. Anesthesiology 2010, 112, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Min JJ, Nam K, Kim TK, Kim HJ, Seo JH, Hwang HY, Kim KB, Murkin JM, Hong DM, Jeon Y. Relationship between early postoperative C-reactive protein elevation and long-term postoperative major adverse cardiovascular and cerebral events in patients undergoing off-pump coronary artery bypass graft surgery: a retrospective study. Br J Anaesth. 2014 Sep;113(3):391-401. Erratum in: Br J Anaesth. 2014 Nov;113(5):895. PMID: 24829443. [CrossRef] [PubMed]
- Benveniste J, Henson PM, Cochrane CG: Leukocyte-dependent histamine release from rabbit platelets: the role of IgE, basophils and a platelet-activating factor. J Exp Med 136: 1356, 1972.
- Benveniste J, Chignard M. A role for PAF-acether (platelet-activating factor) in platelet-dependent vascular diseases? Circulation. 1985 Oct;72(4):713-7. [CrossRef] [PubMed]
- Choi, B.Y.; Ye, Y.-M. Role of Platelet-Activating Factor in the Pathogenesis of Chronic Spontaneous Urticaria. Int. J. Mol. Sci. 2024, 25, 12143. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, J.F. , Zvaifler N.J. Antigen induced histamine release from platelets of rabbits producing homologous PGA antibody. Proc. Soc. Exp. Biol. Med. 1966;122:1245–1247.
- Goldstein RE, Feuerstein GZ, Bradley LM, Stambouly JJ, Laurindofrm, Davenport NJ. Cardiovascular effects of platelet activat-ing factor. Lipids 26: 1250 –1256, 1991.
- Tillett WS, Francis T, Jr. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med (1930) 52:561–71.
- Calu, V.; Piriianu, C.; Miron, A.; Grigorean, V.T. Utilizing C-Reactive Protein (CRP) and CRP Ratios for Early Detection of Postoperative Complications Following Rectal Cancer Surgery. Life 2024, 14, 1465. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.T.; Haessler, J.; Taylor, K.D.; Tuftin, B.; Briggs, M.; Parikh, M.A.; Peterson, S.J.; Gerszten, R.E.; Wilson, J.G.; Kelsey, K.; et al. The Relationship of Duffy Gene Polymorphism with High-Sensitivity C-Reactive Protein, Mortality, and Cardiovascular Outcomes in Black Individuals. Genes 2024, 15, 1382. [Google Scholar] [CrossRef] [PubMed]
- Engler, RL. Dahlgren MD, Peterson MA. et al: Accumulation of polymorphonuclear leukocytes during 3h experimental myocardial ischemia. Am J Physiol 1986:25I:H9.3-H]OO.
- Dreyer WJ. Michael LH, West MS, et al: Neutrophil accumulation in ischemic canine myocardium. Circulation 1991:84:400-411.
- Go, LO. Murry CE. Richard VJ. et al: Myocardial neutrophil accumulation during reperfusion after reversible or irreversible ischemic injury. Am J Physiol 1988;255:H188-HI9«.
- Corr PB, Witkowski FX: Potential electrophysiologic mechanisms responsible for dysrhythmias associated with reperfusion of ischaemic myocardium. Circulation t983:68(Suppl 0:1-16-1-24.
- Manning, AS. Hearse DJ: Reperftision-induced arrhythmias: Mechanisms and prevention. J Mol Cell Cardiol 1984:16:497-518.
- Annable CR, McManus LM, Carey KD, Pinckard RN. Isolationof platelet-activating factor (PAF) from ischemic baboon myocar-dium. Federation Proc 44: 1271, 1985.
- Montrucchio G, Camussi G, Tetta C, Emanuelli G, Orzan F, Libero L, Brusca A. Intravascular release of platelet-activating factorduring atrial pacing. Lancet 2: 293, 1986.
- Ko W, Hawes AS, Lazenby WD, Calvano SE, Shin YT, Zelano JA, Antonacci AC, Isom OW, Krieger KH. Myocardial reperfusion injury. Platelet-activating factor stimulates polymorphonuclear leukocyte hydrogen peroxide production during myocardial reperfusion. J Thorac Cardiovasc Surg. 1991 Aug;102(2):297-308. [PubMed]
- Ridker PM: C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007, 49: 2129-2138.
- Vigo, C. Effect of C-reactive protein on platelet-activating factor-induced platelet aggregation and membrane stabilization. J. Biol. Chem., 260, 3418-3422, 1985.
- Mortensen, R.F. , Osmond, A.P., Lint, T.F. and Gewurz, H.Interaction of C-reactive protein with lymphocytes and monocytes: complement-de endent adherence and phagocytosis. J Immunol., 117, 774-7[1, 1976.
- Fiedel, B.A. , Simpson, R.M. and Gewurz, H. Activation of latelets b modified C-reactive protein. Immunology, s, c39-447, 1982.
- Muller, H. and Fehr, J. Binding of C-reactive protein to human polymorphonuclear leukocytes: evidence for association of binding sites with Fc receptors. J. Immunol., I36, 2202-2207, 1986.
- Buchta, R. , Pontet, M. and Fridkin, M. Binding of C-reactive protein to human neutrophils. FEBS Lett.,a, 165-168, 1987.
- Miyazawa, K. , Kiyono, S. and Inoue,K. Modulation of stimulusdependent human platelet-activation by C-reactive protein modified with active oxygen species. J. Immunol., 141, 570-574, 1988.
- Fiedel, B.A. and Gewurz, H, Effects of C-reactive protein on platelet function I. Inhibition of platelet aggregation and release reactions. J. Immunol., 116, 1289-1294, 1976.
- Fiedel, B.A. and Gewurz, H. Effects of C-reactive protein on platelet function II. Inhibition by CRP of platelet reactivities stimulated by poly-L-lysine, ADP, epinephrine, and collagen. J. Immunol., 117, 1073-1078, 1976.
- Page, C.P. processes. CRP, PAF AND PLATELETS 421 The involvement of platelets in non-thrombotic Trends Pharmacol. Sci., 2, 66-71, 1988.
- Braquet, P., Touqui, L., Shen, T. Y. and Vargaftig, B. B. Perspectives in platelet-activating factor research. Pharmacol. Rev., 3, 98-145, 1987.
- Filep, J. and Foldes-Filep, E. Effects of C-reactive protein on human neutrophil granulocytes challenged with N-formylmethionyl-leucyl-phenylalanine and platelet-activating factor. Life Sci., 44, 517-524, 1989.
- Dalmaso, B.; Silva-Junior, I.A.d.; Jancar, S.; Del Debbio, C.B. Platelet-Activating Factor Receptor (PAFR) Regulates Retinal Progenitor/Stem Cells Profile in Ciliary Epithelium Cells. Int. J. Mol. Sci. 2024, 25, 3084. [Google Scholar] [CrossRef] [PubMed]
- Margariti, A.; Papakonstantinou, V.D.; Stamatakis, G.M.; Demopoulos, C.A.; Machalia, C.; Emmanouilidou, E.; Schnakenburg, G.; Nika, M.-C.; Thomaidis, N.S.; Philippopoulos, A.I. First-Row Transition Metal Complexes Incorporating the 2-(2′-pyridyl)quinoxaline Ligand (pqx), as Potent Inflammatory Mediators: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin. Molecules 2023, 28, 6899. [Google Scholar] [CrossRef] [PubMed]
- Maruyama M, Farber NE, Vercellotti GM, Jacob HS, Gross GJ. Evidence for a role of platelet activating factor in the pathogenesis of irreversible but not reversible myocardial injury after reperfusion in dogs. Am Heart J. 1990 Sep;120(3):510-20.
- Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343-348.
- Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444-2453.
- Swaminathan M, Hudson CCC, Phillips-Bute BG, et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg. 2010;89(4):1098-1104.
- Olivero JJ, Olivero JJ, Nguyen PT, Kagan A. Acute kidney injury after cardiovascular surgery: an overview. Methodist Debakey Cardiovasc J. 2012;8(3):31-36.
- Salazar JD, Wityk RJ, Grega MA, et al. Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg2001;72:1195–202.
- Stamou SC, Hill PC, Dangas G, et al. Stroke after coronary artery bypass: incidence, predictors, and clinical outcome.
- Stroke 2001;32:1508–13.
- Borger MA, Ivanov J, Weisel RD, Rao V, Peniston CM. Stroke during coronary bypass surgery: principal role of cerebral macroemboli. Eur J Cardiothorac Surg 2001;19:627–32.
- Newman MF, Wolman R, Kanchuger M, et al. Multicenter preoperative stroke risk index for patients undergoing coronary artery bypass graft surgery. Circulation 1996;94(Suppl 2):74–80.
- Head T, Daunert S, Goldschmidt-Clermont PJ. The Aging Risk and Atherosclerosis: A Fresh Look at Arterial Homeostasis. Front Genet. 2017 Dec 14;8:216.
- Kollef MH, Wragge T, Pasque C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest. 1995;107:1395–401.
- Harker LA, Malpass TW, Branson HE, Hessel EA, 2nd, Slichter SJ. Mechanism of abnormal bleeding in patients undergoing cardiopulmonary bypass: Acquired transient platelet dysfunction associated with selective alpha-granule release. Blood. 1980;56:824–34.
- Floyd RD, Sabiston DC, Jr, Lee KL, Jones RH. The effect of duration of hypothermic cardioplegia in ventricular function. J Thorac Cardiovasc Surg. 1983;85:606–11.
- Hugot P, Sicsic JC, Schaffuser A, Sellin M, Corbineau H, Chaperon J, et al. Base deficit in immediate postoperative period of coronary surgery with cardiopulmonary bypass and length of stay in intensive care unit. Intensive Care Med. 2003;29:257–61.
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





