Background
According to COPE (Committee on Publication Ethics): «
Editors and publishers are dealing with increasing numbers of ethical concerns brought to their attention in a variety of ways, and increasingly concerning large numbers of papers. Journals may receive concerns directly or from different media and should investigate these regardless of the method of communication. » COPE has therefore published a number of guidelines giving recommendations to help publishers to deal with these different situations. COPE also reports that: «
The issues identified have become more complex. This includes situations where multiple concerns are raised simultaneously to multiple journals, from anonymous or named individuals, and which deal with a range of issues concerning the integrity of the published research. » [
2]
It is obviously very important that the rules governing the ethics of medical research are respected, and the contribution of researchers wishing to carry out independent checks can be a valuable asset for the scientific community but also, more generally, for maintaining a climate of confidence in medical research. However, a process designed to achieve constructive objectives can always have negative consequences if it is misused, and facilitating the process of communicating concerns to publishers can also present significant risks, including the risk generated by alerts issued by scientists unfamiliar with the research areas of the publications that they criticize and who may then be inclined to misinterpret the rules that apply to this type of research.
To illustrate these risks, we present the case of a team of independent researchers who, believing they had detected massive ethical fraud in the publications of a French institution, wrote to the publishers to communicate their concerns. But the laws on research ethics are complex and the first mistake made by Franck et al. is to confuse research involving human beings with research not involving human beings.
Through our work, we hope to enlighten publishers so that they are better equipped to carry out investigations into articles published by French researchers. This will maybe prevent other French teams from being suspected of fraud in the future on the basis of concerns generated by this same confusion.
Methods
To analyze the work of Franck et al., we have examined the texts of French legislation as well as the content of the training courses organized by various institutions for their researchers. We have compared French legislation with US legislation, with which publishers are more familiar, and examined in detail the legislation of the developing countries concerned by the publications singled out by the authors.
Franck et al. mention 456 publications that would be concerned by ethical problems, but the table they provide as a supplementary material only gives the references of 248 publications for which they discovered that they all mentioned the same ethical approval number. According to the authors, this reuse of the same approval number for a large number of publications does not comply with the French law on research on human beings (RIPH).
We therefore analyzed each of these 248 publications one by one in order to assess the type of research involved. We began by noting the titles of these publications, since they did not appear in the table, and this gave us an initial indication of the first major error. The vast majority of these titles showed that they were describing the genetic characteristics of a bacterium.
We assigned to each of these publications the terminology RIPH or non-RIPH according to the criteria defined by French legislation and checked whether the ethical mentions appearing in the texts corresponded to the legal requirements.
Detailed Examination of Research Ethics Laws
The terminology of « biomedical research » used in the Huriet-Serusclat law (applied since 1988 and revised in 2004) has been replaced in the Jardé law (applied since November 2016) by that of « research involving human person », which will be referred to in this work by the acronym RIPH. The Jardé law distinguish [
3]: interventional research (Category 1), interventional research with minimal risks and constraints (Category 2), non-interventional research (Category 3). For RIPH research, French legislation requires authorization from a Comité de Protection des Personnes (CPP). These committees correspond to the IRBs of Anglo-Saxon countries. In addition to RIPH research, there is also research that does not involve the human person: non-RIPH research. In the case of non-RIPH research, French law does not require authorization from a CPP.
1. The main difference between French law and the laws of Anglo-Saxon countries.
In Anglo-Saxon countries, there is only one type of ethics committee: the IRB, but in France, there are two: CPP (Comités de Protection de la Personne) and CEL (Comités d’Éthique Locaux). French CPPs are equivalent to the IRBs committees under US legislation, independent of research institutions or hospitals, and whose authorization is compulsory for research involving human beings. French CELs have no equivalent in Anglo-Saxon countries. Their authorizations are only advisory and are not legally binding. As there are only 39 CPPs to cover the entire French territory [
4], research outside the scope of the Jardé Law is no longer accepted for evaluation by CPPs, which can pose problems for publication. Scientists must then turn to their institution’s local ethics committee which will help them to determine whether the research they are planning is research involving human person (in which case, the CEL will direct them to a CPP) or research not involving the human person. For non-RIPH research, the authorizations of the CEL (even if they are not legally obligatory) are useful in providing the ethical endorsement publishers request (since the CPP refuse to evaluate them). [
5]
«
Retrospective research, involving biological samples or data already collected do not fall within the scope of the Jardé law on research involving human beings. » Such research involving the re-use of microbiological samples of those using sample collected during care are non-RIPH research and does not require CPP authorization. They are authorized by law, as stated in article L1211-2 of the French Public Health Code [
6]: «
The use of elements and products of the human body for a medical or scientific purpose other than that for which they were removed or collected is possible, unless opposition is expressed by the person from whom the removal or collection was carried out, duly informed in advance of this other purpose. » As long as the biological samples are not collected specifically for research purposes and are only pre-existing samples, French law does not require any ethical authorization. [
7]
2. Similarities between French law and US law.
Comparing American legislation will provide greater clarity for publishers of international scientific journals, as they are more familiar with it than with French legislation. But the same distinction is made between RIPH research (Research on Human Beings) and non-RIPH research (not on human beings).
In the USA, the HHS (Department of Health and Human Services) research privacy legislation [
8] in the Code of Federal Laws is referred to as 45 C.F.R 46. Certain types of research are excluded [
9,
10,
11,
12] from this regulation and are described in 45 CFR 46.104. Among the categories of research exempt from this regulation is secondary research on biological samples (biospecimens), provided that the data are anonymized.
The NIH website provides an infographic showing research exempt from IRB authorization. The fourth category of exemption describes research «
involving samples if they are recorded in such a way that the subjects cannot be identified. » [
13]
The concept of
« secondary research » is also defined in this presentation [
14] from the University of San Francisco:
« Not Human Subjects Research Description: Under some circumstances, research involving only unidentifiable/de-identified or coded private information or biological specimens is not human subjects research because investigators cannot readily ascertain the identities of the individuals to whom the data or samples belong. In such cases, IRB review is not required. »
Another presentation from the University of Virginia [
15] also outlines the conditions under which research on biological samples is not considered research involving human beings:
« Research with previously collected anonymous data and/or specimens does not meet the definition of “research involving human subjects” and may be performed without IRB approval only when the data/specimens to be studied were not collected specifically for the current research. »
These exemptions of IRBs in U.S. legislation correspond to which is defined as
« research involving existing data with a change of purpose and/or existing biological elements. » [
16,
17] The change of purpose Art L 1211-2 of the French Public Health Code simply requires the patient informed on the possible used of his sample for future research and the possibility he get to express an opposition to this [
18].
In their article: « French legislation on retrospective clinical research: what you need to know and what you need to do », Souche et al. summarize very well the fact that research on biological samples is not research involving the human person: «
Retrospective research is exterior to the framework of the Jardé law, the reason being that it involves not “natural” persons but rather, existing health-related data with a modified objective and/or existing biological collections. » [
19]
And, in the document: « A qualification guide for health research » published in 2021, the INSERM ethics committee (CEEI) states that: «
Three categories of research are not considered as RIPH and are listed in point II of article R 1121-2 of the CSP (...) The 3rd category corresponds to retrospective studies on data and biological samples. » [
20]
4. Confirmation by the French authorities.
The final report of the inspection carried out by the IGAS [
21] (Inspection Générale des Affaires Sociales) to which the authors refer states that, of the 140 IHU-MI studies inspected by them: « With the
exception of 19 of them, which were redirected to committees for the protection of individuals, these studies do not involve human beings within the meaning of the JARDÉ law » (page 106) and: «
In the case of research outside the scope of the RIPH, the regime is simpler: some research is carried out without any act on the person. They are not presented to the CPP. » (p 107) This IGAS report was published in August 2022. The authors therefore had official explanations for why most of the publications they listed should be classified as non-RIPH research. Moreover, in response to a question from Senator Alain Houpert, the Minister of Health, François Braun, replied on June 29, 2023: «
As human waste is not a person but a thing, research on it is not research involving human beings, but ‘scientific’ research. Research programs involving collections of biological samples fall within the remit of the ministry in charge of research, not the committees for the protection of individuals. » [
22]
5. Lack of ethics committees and specific legislation in some countries.
The particular case of Niger deserves some further explanation, firstly because it is representative of the situation in other developing countries and, secondly, because at the time of submitting this work, two publications involving the analysis of the microbiota of severely malnourished children had been retracted by the publisher. Indeed, in Niger, a 2013 decree issued by the Ministry of Education [
23] requires administrative authorization prior to research. Article 11 stipulates that for specific topics: «
it remains understood that the establishment of the administrative authorization for research is strictly subordinated to the opinion of the ethics committee created for this purpose. »
But Niger’s National Health Research Ethics Committee was only created in 2016 (decree 2016-644 /PRN/MSP) (see additional file n°4), and the stool samples sent to the IHU-MI for analysis by Dr Souleymane Brah, a physician at Niamey hospital, were taken in 2014, when Niger’s CNERS did not yet exist. Before the creation of the CNERS, there was a national consultative ethics committee (CCNE) but, as stated in the National Strategic Plan for Health Research [
24] issued by the Niger Ministry of Health in 2013, the legal framework governing its operation was very weak and its funding non-existent. In the absence of a functional ethics committee and legislation specifically framing human health research ethics, it was probably not possible in 2014 for Nigerien doctors collaborating with the IHU-MI to have a written document emanating from an official ethics committee. However, this does not mean that sampling did not respect the declaration of Helsinki. In the absence of national laws governing medical research ethics, researchers usually refer to the Declaration of Helsinki as an international standard reference. Anyway, as Chaudhry et al. [
25] point out in their conclusions, this problem of weak or non-existent specific legislation on research ethics relating to human health is quite common in developing countries: «
The findings show that most Research Ethics Committees (RECs) in Sub-Saharan Africa work under significant administrative and financial constraints, with few opportunities for capacity building for committee members. This impacts the quality of reviews and the overall performance of RECs. Although most countries have national governance systems for RECs, they lack regulations on accountability, transparency, and monitoring of RECs. The situation in Senegal is somewhat different, with ethical legislation in place since 2009. Nevertheless, according to the Senegalese CNERS report [
26] published in 2019: «
The law does not address the issue of the use, storage and transfer of biological material, nor that of the creation, use and transfer of databases ».
The specific context of certain developing countries must therefore always be taken into account when assessing whether local ethical legislation has been respected. In their article entitled « National ethics guidance in Sub-Saharan Africa on the collection and use of human biological specimens: a systematic review» [
27] published in 2016, Barchi et al. state in Tables 2 and 3 whether countries have ethical legislation and, if so, whether it includes clauses specific to the reuse or transfer of biological material. These tables confirm that, at the time the child malnutrition study was carried out, Niger had no legislation, regulations or instructions surrounding the ethics of human health research. While Senegal did have general legislation, it did not include any provisions concerning biological samples.
6. Analyzing the characteristics of bacteria kept in collections is not research involving human subjects.
Microbiology is a specific field of research, of which genomic sequencing is a sub-specialty. The rules that apply to it are therefore specific, since this research does not involve human beings, but bacteria, viruses or parasites. Biological samples collected during care or during an RIPH study are cultured and the growth of several different micro-organisms is regularly observed. These micro-organisms are isolated from the initial sample and stored in collections for later characterization. During subsequent research, it is not the initial samples that are reused, as they have been destroyed, but only the micro-organisms that were previously preserved. In France, this research on micro-organisms is not classified as biomedical research, but as scientific research in microbiology, since the researchers work exclusively on bacteria and the research does not involve humans.
And it is only fairly recently that publishers specializing in genome announcements have begun to establish more precise rules for the ethical mentions they require. In 2020, the Microbiology Resource Announcements published an article entitled: « Best Practices for Successfully Writing and Publishing a Genome Announcement in Microbiology Resource Announcements » [
28]. It stipulates that researchers must explain how the micro-organism was acquired, but a statement of the approval number and the name of their CPP is only required if the manuscripts describe research involving humans.
In Table S3 (“
Table S3 - Genome announcements.xlsx”) we listed nearly 20 publications of genome announcements resulting from research carried out by other teams than those at the IHU-MI. None of these publications describing the characteristics of a newly discovered bacterium mentions ethical approval. This demonstrates that it is common practice for the international community of researchers specializing in microorganism genome analysis not to mention IRB authorization when their research does not involve human subjects. French legislation is similar to that of other countries for this type of research.
Results
-
1.
A detailed analysis of their content shows that the publications concern secondary research on existing biological samples.
By consulting the full text of all 248 incriminating articles, it is possible to find the context of the samples taken and the references of the ethics committees that authorized the protocol of the studies in which the biological samples were collected.
In the
Table S1, we have grouped the publications according to the countries concerned and, for each country, we have highlighted the primary study(ies) in which the biological samples were taken, together with the references of the authorizations of the ethics committees consulted (see Additional file 2: “
Table S1 - Studies mentioning number 09-022.xlsx”).
The summary is presented in
Table 1.
From this summary of the international origin of biological samples, we can deduce that IHU-MI researchers collaborate with local doctors and scientists who take samples authorized by the ethics committees of the countries concerned. The IHU in this study only play a role of specialized laboratory for complement analysis of sample collected for care, collected from environment or being human waste that and have been subsequently the subject of single case report that do not need a specific authorization including in France. As for example article 1 and 2 from Niger (
Table S1) described each one a new bacterium identified in the stool of the same patient. No sample collection was done for research purpose only. Local doctors send the samples to the IHU-MI laboratory, which performs the analyses using a method they have developed (Culturomics and Taxogenomics).
The most important long-term international collaboration is with Senegal, where two Point-Of-Care (POC) laboratories were set up between 2010 and 2016 as part of the Dielmo Project. All the research carried out using the samples collected has been approved by the Senegalese authorities and the Comité National d’Ethique de la Recherche du Sénégal (CNERS), as indicated in the inspection report carried out at the request of the Minister of Health [
29]. CNERS authorizations are renewed annually [
30]. These biological analyses enable the IHU-MI to fulfil its mission of national and international surveillance of infectious diseases and epidemiological research, which is one of their objectives [
31,
32]. This mission includes introducing new infection detection tools, enabling IHU-MI researchers to detect previously unknown bacteria. The majority of the 248 publications bearing the number 09-022 describe the characteristics of the bacteria discovered by IHU-MI researchers. In addition, the IHU-MI has an international health monitoring structure and collaborates with numerous foreign partners in the international surveillance of emerging infectious diseases or those imported by travelers and migrants.The IHU-MI also houses the Centre National de Référence des Rickettsies, Coxiella et Bartonella (CNR), which, according to their website [
34]: «
receives more than 20,000 samples (serum, blood, various biopsies and arthropods) per year from over 300 public and private laboratories in France and many other countries, to diagnose infections caused by intracellular bacteria that are difficult to culture. » Secondary research on these biological samples taken for diagnostic purposes is authorized under French legislation, which does not consider it to be research involving human beings and, accordingly, does not require authorization from a CPP.
The stool samples listed as coming from Amazonia were collected in a village in the Amazon rainforest, part of French Guyana. The primary study in which these samples were collected was authorized by the ethics committee of the Institut Louis Malardé in French Polynesia, and the references to this authorization are given in the publication concerning this primary study: « Comparison of the gut microbiota of obese individuals from different geographic origins. » By reading the full text of the publications, it was possible to find the references of the ethics committee authorization and to understand that this authorization from the ethics committee of French Polynesia also applied to the part of the study taking place in French Guyana since they are both part of the French overseas territory (DOM-TOM) where French legislation applies. Any authorization from an ethics committee located on French territory is valid for studies taking place elsewhere on French territory.
For some publications, biological samples were collected as part of patient care, either at the IHU-MI (consultations and hospitalizations), or at other hospitals which sent their samples to the IHU-MI laboratory. Analyses carried out on samples taken as part of patient care (diagnostic samples) are also non-RIPH research and do not require authorization from a CPP.
-
2.
Document number 09-022 is an optional advice of the local ethics committee and not a compulsory CPP authorization.
By wrongly naming document 09-022 an IRB authorization and claiming that it is compulsory in France for all types of research, the authors are confusing the reader. This document is not in fact a legally binding authorization from an IRB for RIPH research, but an optional advice from the IHU-MI’s local ethics committee confirming that the legislation authorizes laboratory analyses (non-RIPH research) on biological samples, whether these samples have been obtained as part of an RIPH study authorized by an independent ethics committee or as part of patient care. The same document can be used for all research of this type, however numerous, and it remains valid as long as the law remains unchanged. It should be noted that many of the IHU-MI publications listed by the authors date from before 2016, i.e., before the Jardé law came into force. Nevertheless, the ethical rules were identical (Hurriet Act) and research on biological samples could already be carried out without the authorization of a CPP.
The primary RIPH studies from which the biological samples analyzed were obtained have all been approved by the CPP, whose references are given in the publications relating to these primary studies.
-
3.
All publications are part of a global project.
A complete analysis of the 248 articles leads to the conclusion that all these publications are part of a global, long-term research project that represents secondary research using laboratory analyses of biological samples already collected for other purposes.
Consequently, the document bearing the number 09-022 is only an optional opinion from the local ethics committee, confirming that French law authorizes this type of research. This justifies the fact that each sub-publication of the overall project mentions this same number. As the IHU-MI is a large infectious disease surveillance unit, it is normal that the number of publications is higher than for other institutions.
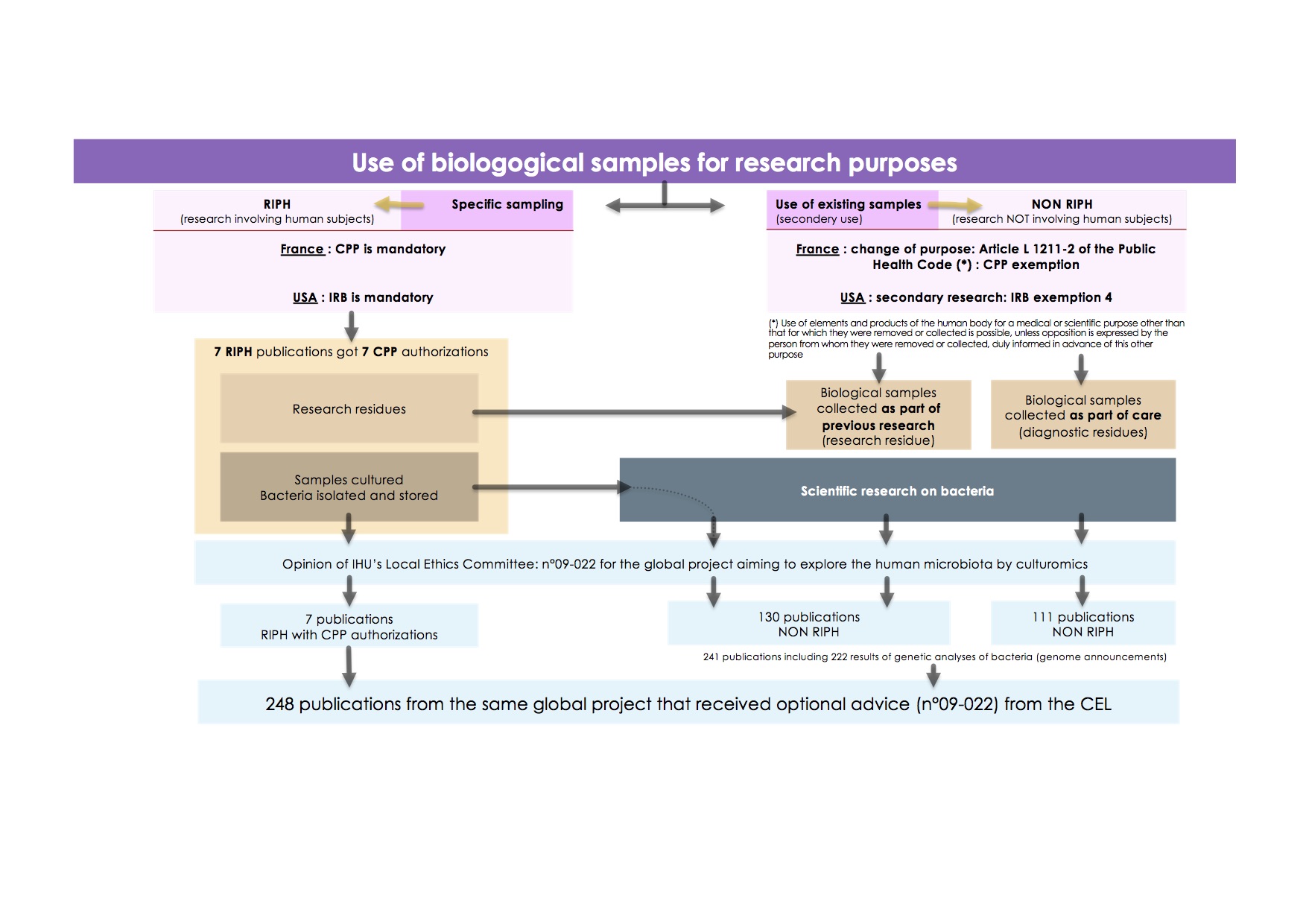
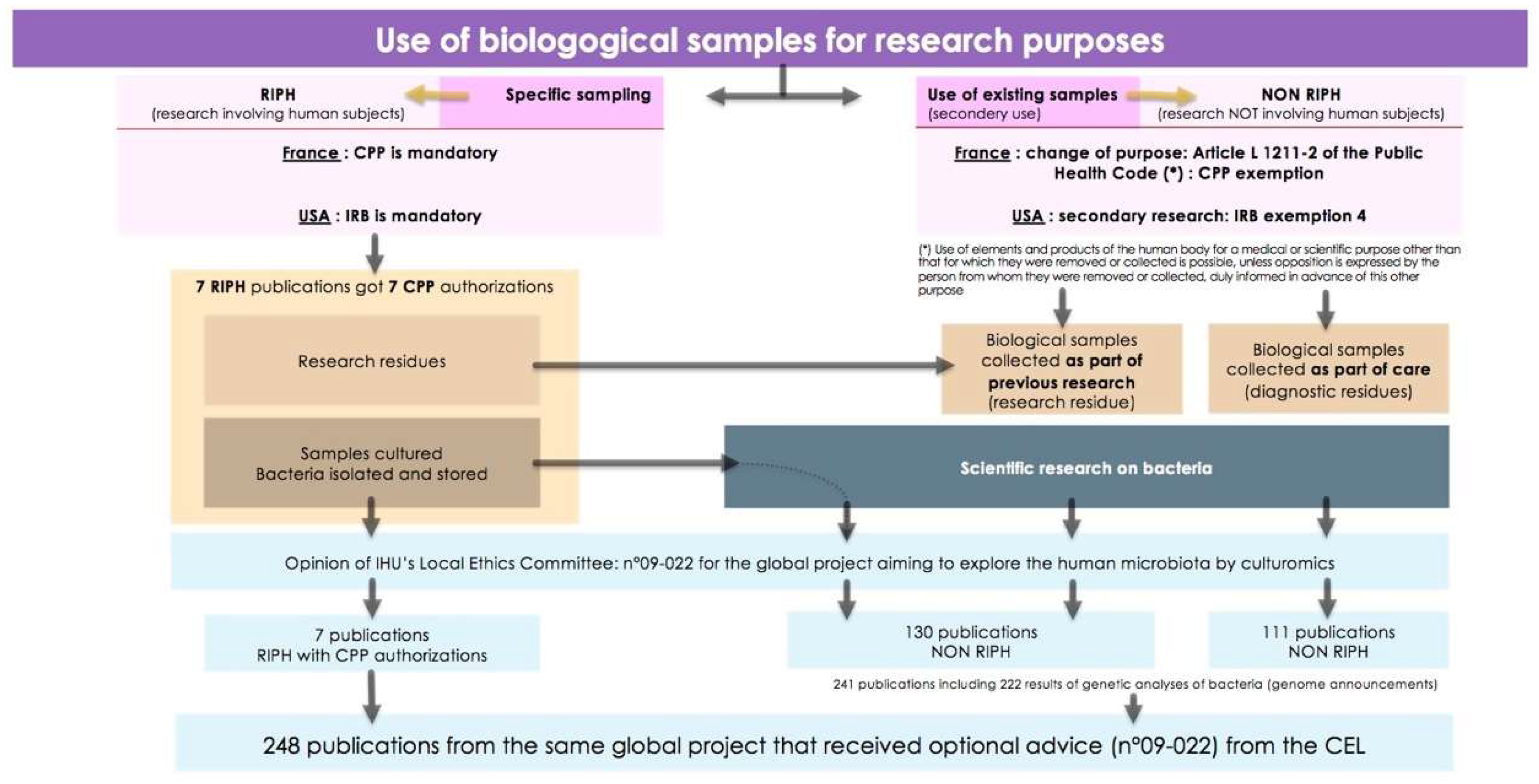
Figure 1.
Use of biological samples for research purposes.
Figure 1.
Use of biological samples for research purposes.
Conclusion
We share the authors’ concern for ethical principles in the protection of study participants. To assess the ethical compliance of scientific publications, we believe it is essential to rely on a precise knowledge of legislation and guidelines, as well as a rigorous analysis of each of the publications concerned. Our analysis of both French legislation and the 248 incriminated publications does not lead us to the same conclusion as Franck et al.
Indeed, of these 248 publications, only 7 concern research involving the human person (RIPH). All of them mention the references of the mandatory ethical authorization from a CPP for studies taking place in France, and references to authorizations received by local authorities in other countries, according to the legislation in force at the time the studies were carried out. These details appear either directly in the text, or in the references when there are several publications about the same RIPH study. The other 241 concern research not involving human subjects, since they are secondary research studies which re-use existing biological samples collected either during ancillary studies, during patient care or as part of an international health monitoring. They do not require any ethical authorization from a CPP. Of these 241 publications, 223 describe the characteristics of a new bacteria discovered by the IHU-MI. This is not biomedical research, but scientific research (research on bacteria). These 223 publications are genome announcements concerning bacteria discovered in biological samples already collected for other purposes. As secondary research on existing biological samples, they do not therefore require the authorization of a CPP ethics committee under French legislation.
The existence of local ethics committees is specific to France and has no equivalent in Anglo-Saxon countries. This may lead to confusion on the part of readers unfamiliar with this specific French feature. The opinion of a local ethics committee (CEL) may nevertheless be sought to check that the research does not involve human beings, and to allow publication by international publishers requiring ethical authorization from researchers. In France, the number of CPPs is very small compared with the large number of research institutions, so they refuse to examine research projects that do not involve human beings. Local ethics committees are therefore essential for such research, which falls outside the scope of the Jardé Law. In the USA, these local ethics committees would enable researchers to know whether their research meets the criteria for exemption from IRB authorization. This is the role played by the IHU-Mi’s local ethics committee, as indicated in the IGAS inspection report [
21] (p 112): « The IHU-MI’s local ethics committee is a body for the ethical evaluation of research projects that do not fall within the remit of a CPP. It intervenes upstream of clinical studies, before the research is carried out, and its aim is to enable research not covered by the CPP to obtain the opinion of an ethics committee with a view to publication or funding. » However, the opinions of local ethics committees are optional, so it is not possible for there to be any ethical or legal fraud regarding an authorization that is not legally required.
Publishers do not give instructions on the degree of precision required in ethical statements and it is therefore sometimes difficult for researchers to know how far to go in the information to be provided when submitting an article. Should they be content with a general statement to the effect that the research has received all the ethical approvals required by the legislation in force in the country where the research was carried out? Or should full details be given, including the reference number of the CPP or CEL and the reference number of the specific authorization, together with a copy of this document? In the case of RIPH research, it is probably preferable to give as much detail as possible and to provide a copy of the documents to the publisher, which could perhaps better guarantee compliance with legal requirements. But in the context of non-RIPH research, this case shows that in the end, by trying to give as much detail as possible to show that they had taken all the necessary information from their CEL and had therefore complied with all their ethical obligations, the IHU-MI researchers see their concern for precision backfire. If they had simply mentioned that the study had received approval from their CEL without mentioning a number, as most other French teams do, all these false alarms of potential fraud would not have occurred.
Nevertheless, we cannot exclude the possibility that some of the very large amount of research published by the IHU-MI may contain errors or inaccuracies relating to ethical authorizations. But this is certainly not a majority trend among their numerous works, and probably no more frequent than for other teams with such intense research activity.
Our work shows how confusion between research involving the human person and research not involving the human person, as well as superficial analysis of publications, can lead to scientists being wrongly accused of ethical fraud, and to publishers being misled by people who are not fully familiar with all the subtleties of ethical legislation. This risk of unfounded alerts could be considerably reduced by requiring authors of research not involving human beings to indicate this in their work, so that it is immediately clear to publishers and readers that the research is exempt from CPP authorization.
Supplementary Information
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Additional file 1, Table 1 - Summary - Classification of studies and IRB authorizations by country. Additional file 2, Figure 1: Use of biological samples for research purposes. Additional file 3, Table S1: Studies mentioning number 09-022. Additional file 4, République du Niger : Décret 2016-644 /PRN/MSP, Additional file 5, Table S3: Genome announcements.
Author Contributions
Conceptualization: VB, BN, MZ. Methodology: VB, BN, MZ. Investigation: VB. Writing – original draft: VB. Writing – review & editing: VB, BN, MZ.
Funding
The authors received no specific funding for this work.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets supporting this article are available. Not applicable.
Competing interests
No competing interest.
Abbreviations
|
AAR: Autorisation Administrative de Recherche.
ANSM: Agence Nationale de la Santé et du Médicament.
CCNE: Comité Consultatif National d’Ethique.
CEL: Comité Local d’Ethique.
CNERS: Comité National d’Ethique de la Recherche en Santé
CNR: Centre National de Référence des Rickettsies, Coxiella et Barnotella.
COPE: Committee on Publication Ethics.
CPP: Commission de Protection des Personnes i.e IRB.
IFR: Institut Fédératif de Recherche.
IGAS: Inspection Générale des Affaires Sociales.
IHU: Institut Hospitalo-Universitaire.
IHU-MI: IHU Méditerannée Infection.
IRB: Institutional Review Board.
INSERM: Institut National de la Santé et de la Recherche Médicale.
HHS: Department of Health and Human Services.
MESR: Ministère de l’Enseignement Supérieur et de la Recherche.
MST: Maladies Sexuellement Transmissibles.
RECs: Research Ethics Committees.
RHB: Research on Human Beings
RIPH: Recherche impliquant la personne humaine i.e., research involving human subjects.
Non-RIPH: Recherches n’impliquant pas la personne humaine i.e research non involving human subjects.
|
References
- Frank, F., Florens, N., Meyerowitz-katz, G. et al. Raising concerns on questionable ethics approvals – a case study of 456 trials from the Institut Hospitalo-Universitaire Méditerranée Infection. Res Integr Peer Rev 8, 9 (2023). [CrossRef]
- COPE Council – COPE Discussion Document: Dealing with concerns about the integrity of published research – English. Accessed Oct 6 2024. [CrossRef]
- Inserm – La science pour la santé – République Française. https://www.inserm.fr/nos-recherches/recherche-clinique/la-recherche-clinique/ (accessed on 7 October 2024).
- ARS île de France – Ministère des Solidarités et de la Santé – Comités de Protection des Personnes. https://www.iledefrance.ars.sante.fr/media/86137/download?inline (accessed on 3 October 2024).
- Infectiologie.com – Le site de l’infectiologie Française – La recherche clinique et la loi Jardé : https://www.infectiologie.com/UserFiles/File/formation/desc/2019/seminaire-avril-2019/jeudi-04-04-2019/recherche-6-jeudi-04-marion-noret.pdf. Accessed Oct 6 2024.
- Legifrance – Le service public de la diffusion du droit – République Française – Code de Santé Publique – Article L1211-2. https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000043895792. Accessed Oct 7 2024.
- GIRCI – La réutilisation d’échantillons biologiques humains à des fins de recherche – Webinaire 17 janvier 2023 – T. Roche, Avocat. https://girci-idf.fr/wp-content/uploads/2023/10/20230117_Webinaire-Juridique_Reutilisation-EBH-Recherche.pdf Accessed Oct 7 2024.
- U.S. Department of Health and Human Services – 45 CFR 46 FAQs. https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/45-cfr-46/index.html. Accessed Oct 7 2024.
- U.S. Department of Health and Human Services – 45 CFR 46 Exemptions. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/common-rule-subpart-a-46104/index.html. Accessed Oct 7 2024.
- U.S. Department of Health and Human Services – Exempt Research Determination – 45 CFR 46.101 (b). https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/exempt-research-determination/index.html. Accessed Oct 7 2024.
- U.S. Department of Health and Human Services - Human Subject Regulations Decision Charts: 2018 Requirements – Chart 01: Is an Activity Human Subjects Research Covered by 45 CFR Part 46? – And Chart 09: Does Exemption 45 CFR 46.104(d)(7), Storage for Secondary Research for Which Broad Consent Is Required, Apply? https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts-2018/index.html. Accessed Oct 7 2024.
- HHS.gov – Department of Health and Human Services – OHRP E-Learning Program – What is human subjects research ? : https://www.hhs.gov/ohrp/sites/default/files/OHRP-HHS-Learning-Module-Lesson2.pdf. Accessed Oct 7 2024.
- NIH – Training and Resources for Human Subjects – Exempt Human Subjects Research Infographic. https://grants.nih.gov/sites/default/files/exemption_infographic_v8_508c_1-15-2020.pdf Accessed Oct 7 2024.
- UCSF – University of California San Francisco – Not Human Subjects Research : https://irb.ucsf.edu/not-human-subjects-research. Accessed Oct 7 2024.
- University of Virginia – Research Involving Data and/or Biological Specimens : https://research.virginia.edu/sites/vpr/files/2019-08/25-03-Research-Involving-Data-and-or-Biological-Specimens.pdf. Accessed Oct 7 2024.
- Infectiologie.com – Le site de l’infectiologie Française – Outils de formation – Recherches « Hors Loi Jardé » M. NORET – Chef de projet Clinique des pathologies infectieuses – 6 Octobre 2021. https://www.infectiologie.com/UserFiles/File/formation/desc/2021/seminaire-octobre-2021/merc-610-t.27/conf-4-hors-loi-jarde-mnoret.pdf Accessed Oct 7 2024.
- Infectiologie.com – Le site de l’infectiologie Française – Outils de formation – Autorisations règlementaires : CPP, comité d’éthique, ANSM, CNIL. – Docteur Sébastien Gallien – Service d’immunologie et maladies infectieuses Université Paris Est Créteil. Equipe 16 INSERM U955 IMRB & VRI – 4 avril 2019. https://www.infectiologie.com/UserFiles/File/formation/desc/2019/seminaire-avril-2019/jeudi-04-04-2019/recherche-9-jeudi-04-dr-gallien.pdf. Accessed Oct 7 2024.
- Comité de Protection des Personnes (en recherche biomédicale) CPP Tours Ouest-1. – « Je veux démarrer une recherche sur des échantillons biologiques déjà prélevés à l’occasion du soin ou d’une recherche (changement de finalité) ». https://cppouest1.fr/mediawiki/index.php?title=CPP_Ouest-1:NOD0126. Accessed Oct 7 2024.
- R. Souche, S. Mas, O. Scatton, J.-M. Fabre, L. Gimeno, A. Herrero, S. Gaujoux, French legislation on retrospective clinical research: What to know and what to do, Journal of Visceral Surgery, Volume 159, Issue 3, 2022, Pages 222-228, ISSN 1878-7886. [CrossRef]
- Amiel, P., Dosquet C., Comité d’évaluation éthique de l’inserm (CEEI), Guide de qualification des recherches en santé [A qualification guide for health research], Inserm, 2021.
- Inspection Générale des Affaires Sociales – igas.gouv.fr – Contrôle de l’IHU Méditerranée infection – Rapport définitif tome 1 – Août 2023. https://www.igas.gouv.fr/sites/igas/files/2024-06/Tome%20I_Contr%C3%B4le%20de%20l%E2%80%99IHU%20M%C3%A9diterran%C3%A9e%20infection.pdf Accessed Oct 10 2024.
- Sénat Français – Question écrite n°05533 – 16e législature – Qualification des déchets résultant de la recherche médicale – Août 2023 https://www.senat.fr/questions/base/2023/qSEQ230305533.html. Accessed Oct 10 2024.
- Convention on Biological Diversity – Access and Benefit-sharing Clearing-House (ABS Clearing-House, ABSCH) – Arrêté du 17 mai 2013 du Ministère des Enseignements Moyen et Supérieur et de la Recherche Scientifique de la République du Niger fixant les conditions d’obtention d’Autorisation Administrative de Recherche (AAR) au Niger. https://absch.cbd.int/api/v2013/documents/F41B0FAB-46D1-5BF6-254E-83C88E73BE10/attachments/203719/Arr%C3%AAt%C3%A9%20106%20MEMS%20RS.PDF Accessed Oct 11 2024.
- Health Research Web – Plan stratégique national de la recherche en santé 2013-2020 – Ministère de la Santé du Niger. https://healthresearchwebafrica.org.za/files/PLAN_STRATEGIQUE_RECERCHE_EN_SANTE_2013_2020adoptjuin..pdf Accessed Oct 10 2024.
- Chaudhry I, Thurtle V, Foday E, et al. Strengthening ethics committees for health-related research in sub-Saharan Africa: a scoping review. BMJ Open 2022;12:e062847. https://bmjopen.bmj.com/content/12/11/e062847. [CrossRef]
- Centre National d’Ethique de la Recherche Scientifique du Sénégal – Ressources - Rapport consultant évaluation du système de revue éthique. https://cners.sn/public/docs/1587396889.pdf Accessed Oct 11 2024.
- Barchi, F. , Little, M.T. National ethics guidance in Sub-Saharan Africa on the collection and use of human biological specimens: a systematic review. BMC Med Ethics 17, 64 (2016). [CrossRef]
- Dunning Hotopp JC, Baltrus DA, Bruno VM, Dennehy JJ, Gill SR, Maresca JA, Matthijnssens J, Newton ILG, Putonti C, Rasko DA, Rokas A, Roux S, Stajich JE, Stedman KM, Stewart FJ, Thrash JC. Best Practices for Successfully Writing and Publishing a Genome Announcement in Microbiology Resource Announcements. Microbiol Resour Announc. 2020 Sep 3;9(36): e00763-20. PMCID: PMC7471381. [CrossRef] [PubMed]
- Health Research Web – Rapport du comité d’évaluation des sites de recherche de Dielmo et de Ndiop – Robin Bailey, Christophe Rogier, Samba Cor Sarr. – 1er Octobre 2009. https://healthresearchwebafrica.org.za/files/_RapportDielmoNdiop.pdf. Accessed Oct 11 2024.
- Abat Cédric, Colson P., Chaudet H., Rolain J. M., Bassene H., Diallo A., Mediannikov Oleg, Fenollar F., Raoult D., Sokhna Cheikh. (2016). Implementation of syndromic surveillance systems in two rural villages in Senegal. Plos Neglected Tropical Diseases, 10 (12), p. e0005212 [12 p.]. ISSN 1935-2735. [CrossRef]
- Institut Méditerranée Infection – Activité de surveillance épidémiologique. https://www.mediterranee-infection.com/veille-epidemiologique/lactivite-de-surveillance-epidemiologique-de-lihu/. Accessed Oct 11 2024.
- Méditerranée Infection – La veille sanitaire internationale de l’IHU. https://www.mediterranee-infection.com/veille-epidemiologique/lihu-et-la-surveillance-internationale-des-voyageurs/ Accessed Oct 11 2024.
- GéoSentinel – The Global Surveillance and Research Network – EuroTravNet. https://geosentinel.org/sites/eurotravnet Accessed Oct 11 2024.
- Méditerranée Infection – Présentation du CNR. https://www.mediterranee-infection.com/diagnostic/les-centres-nationaux-de-reference-cnr/cnr-rickettsioses/presentation-du-cnr/ Accessed Oct 11 2024.
Table 1.
Summary - Classification of studies and IRB authorizations by country.
Table 1.
Summary - Classification of studies and IRB authorizations by country.
| Countries |
Types of studies |
Ethical authorizations |
| Saoudi Arabia |
1 publication = primary RIPH study comparing the microbiota of people living in Saudi Arabia authorized by the ethics committee of King Abdul Aziz University to microbiota of people living in France (samples taken as part of care). 14 publications = non RIPH studies describing new bacteria isolated in cultures of samples collected as part of this RIPH study. Total: 15 publications |
These 14 publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| Congo |
Total: 16 publications |
All publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| France |
1 publication = primary RIPH study comparing the microbiota of HIV + patients to the microbiota of HIV patients - authorized by the ANRS ethics committee. 5 publications = non RIPH studies describing new bacteria isolated in cultures of samples collected as part of this RIPH study. 1 publications = non RIPH studies describing new bacteria isolated in cultures of samples collected as part of a RIPH study (microbiota of patients suffering from cancer). 12 publications = non RIPH studies describing new bacteria isolated in cultures of samples collected as part of a RIPH study (samples collected during a RIPH study on urinary tract infections). 88 publications = non RIPH studies each describing new bacteria isolated in cultures of samples collected as part of care. 8 publications = non RIPH studies presenting the results of microbiological analyses carried out on microorganisms isolated during culture of a biological samples collected as part of care. Total : 115 publications |
Authorization from the ethics committee of the ANRS / INSERM: (ANRS EP55 MICROGUT.) Authorization from B2M ethics committee under protocol number PP: 15-013 Ethical approval was obtained for the UTI project under the number 2015-A00884-45 Except for a single RIPH publication, all publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| Gabon |
|
All publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| Niger |
1 publication = primary RIPH study: Increased Gut Redox and Depletion of Anaerobic and Methanogenic Prokaryotes in Severe Acute Malnutrition: whose objective is to compare the microbiota of children suffering from severe malnutrition (Kwashiorkor and marasmus) with the microbiota of healthy children. The recruitment of healthy children was authorized by the CNERS and samples from sick children were samples taken as part of care at the local hospitals. 18 publications = non RIPH studies describing new bacteria isolated in cultures of samples collected as part of that primary RIPH study. Total : 19 publications |
For sick children: Recruitment of children <60 months attending the clinic ‘Notre Dame de L’Esperance’ for malnutrition in Thiaroye, Dakar, Senegal, occurred in April 2014. Children from Dielmo and Ndiop were recruited between September and December 2014 and recruitment of children from the National Hospital, Niamey, Niger ranged from February to November 2014. Professor DIALLO and Professor ADEHOSSI certified that this study was not in opposition to the declaration of Helsinki and in accordance with Senegalese and Nigerien laws respectively. Niger’s national ethics committee did not exist and was only created in 2016. For healthy children: authorization by the National Ethics Committee of Senegal (CNERS): Dielmo project. These 18 publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| Sénégal |
1 publication = primary RIPH study: Tropheryma whipplei: A Common Bacterium in Rural Senegal 1 publication = non RIPH study describing a new bacterium isolated in culture of a sample collected as part of this RIPH study. 1 publication = primary RIPH study: microbiota of children suffering of severe malnutrition: Increased Gut Redox and Depletion of Anaerobic and Methanogenic Prokaryotes in Severe Acute Malnutrition. 1 publication = primary RIPH study: Gut Microbiota Alteration is Characterized by a Proteobacteria and Fusobacteria Bloom in Kwashiorkor and a Bacteroidetes Paucity in Marasmus 20 publications: non RIPH studies describing new bacteria isolated in cultures of samples collected as part of these 2 primary RIPH studies. 1 publication = primary RIPH study Characterisation of the Vaginal Microbiota Using Culturomics and Metagenomics Suggests Transplantation of Gut Microbiota into the Vagina During Bacterial Vaginosis 28 publications = non RIPH studies describing new bacteria isolated in cultures of samples collected as part of the monitoring of emerging pathogens or as part of care. 4 publications = non RIPH studies present the results of microbiological analyses carried out on microorganisms isolated during culture of a biological samples collected as part of the monitoring of emerging pathogens. 3 publications = non RIPH studies describing new bacteria isolated in cultures of skin samples collected as part of a RIPH study (study on pathogenic bacteria in bedsores of acute febrile patients and comparison with healthy skin) authorized by the CNERS. Total : 60 publications |
by the National Ethics Committee of Senegal (CNERS): Dielmo project This publication concerns research on a bacterium isolated and stored for further analysis = scientific research in microbiology. Professor DIALLO and Professor ADEHOSSI certified that this study was not in opposition to the declaration of Helsinki and in accordance with Senegalese and Nigerien laws respectively These 20 publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used.
by the Senegalese CNERS, in accordance with the SEN protocol 16/04, approved this study under agreement number 00039 Dielmo project: authorization by the National Ethics Committee of Senegal, the Ministry of Health and Preventive Medicine, the Dakar Pasteur Institute and the IRD. Ethical approval is renewed on a yearly basis. by the National Ethics Committee of Senegal approved the project (N°0-00.87MSP/DS/CNERS and N°001380MSP/DS/CNERS) These 37 publications concern research on microorganisms isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| Various locations |
1 publication = primary RIPH study Treponema species enrich the gut microbiota of traditional rural populations but are absent from urban individuals 7 publications = non RIPH studies grouping by specific themes (related to the microbiota) the conclusions drawn on the basis of laboratory analyzes carried out on samples collected in different countries. These samples were authorized by ethics committees in the countries concerned or were collected as part of care by local doctors then sent to the IHU-MI. 1 publication = non RIPH secondary study describing the characteristics of a new bacteria discovered in a sample of breast milk taken as part of an RIPH study authorized by an ethics committee in Mali (link between infant microbiota and breastfeeding). 1 publication = non RIPH study describing the characteristics of a bacteria discovered in biological samples (blood and pleural fluid) collected as part of an RIPH study authorized by an ethics committee in Vietnam (link between microbiota and diabetes). 9 publications = non RIPH studies describing new bacteria isolated in cultures of stool samples collected during some RIPH study. Total : 18 publications |
Saudi Arabia: ethics committee of the King Abdul Aziz University under agreement Numbers 014-CEGMR-2-ETH-P French Polynesia + French Guiana – Amazonia: the agreement of the ethics committee of the Institute Louis Malardé (Comité d’éthique de Polynésie Française) was obtained under reference 67-CEPF. Congo: Agreement was also obtained from the Ministry for Health of the Republic of Congo (000208/MSP/CAB.15 du Ministère de la Santé et de la Population, 20 August 2015). For pilgrims returning from the Hajj: the protocol was approved by the Aix-Marseille University institutional review board (July 23rd, 2013; reference no. 2013-A00961-44) Senegal : CNERS (Dielmo Project) Mali: The study and the consent procedure were approved by the FMPOS institutional ethics committee under number 20l4/46/CE/FMPOS as of May 22, 2014. Vietnam: The study was approved by the Ministry of Science and Technology of Vietnam under the number NVQG-2018/08 These 11 publications concern research on bacteria isolated and stored for further analysis = scientific research in microbiology. The initial samples have been destroyed. Only the micro-organisms that were previously cultivated and stored are used. |
| Total : |
248 publications |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).