Submitted:
21 November 2024
Posted:
22 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
| Symptoms | Overall patients with NDD |
Father’s patients | Mother’s patients | Statistical analysis | ||||||
| Total n. (ASD+ADHD + TS) | % | n. | % | . | % | O.R. | p-value | p-overall | ||
| Totals (n.) | 120 | |||||||||
| Striae roubre / Skin irregularities / Reddened skin | absent | 109 | 90.8 | 15 | 13.8 | 7 | 6.4 | 2.44 | 0.206 | 0.330 |
| present | 11 | 9.2 | 5 | 45.5 | 0 | 0.0 | ||||
| Excessive sweating of hands and feet | absent | 97 | 80.8 | 11 | 11.3 | 10 | 10.3 | 1.49 | 0.480 | 0.299 |
| present | 23 | 19.2 | 4 | 17.4 | 2 | 8.7 | ||||
| Mal di schiena / dolori muscolari transitori agli arti / stanchezza cronica | absent | 104 | 86.7 | 28 | 26.9 | 21 | 20.2 | 1.7 | 0.341 | 0.353 |
| present | 16 | 13.3 | 6 | 100.0 | 5 | 31.6 | ||||
| Back pain / transient muscle pain in the limbs / chronic fatigue | absent | 110 | 91.7 | 21 | 19.1 | 19 | 17.3 | 2.08 | 0.283 | 0.862 |
| present | 10 | 8.3 | 3 | 30.0 | 3 | 30.0 | ||||
| Flat foot | absent | 62 | 51.7 | 5 | 8.1 | 4 | 4.5 | 8.3 | <0.001 | <0.001 |
| present | 58 | 48.3 | 18 | 31.0 | 18 | 31.0 | ||||
| Constipation/diarrhoea / abdominal discomfort | absent | 103 | 85.8 | 23 | 22.3 | 8 | 7.8 | 3.01 | 0.041 | 0.302 |
| present | 17 | 14.2 | 8 | 47.1 | 4 | 23.5 | ||||
| Heartburn / gastroesophageal reflux / hiatal hernia | absent | 101 | 84.2 | 33 | 32.7 | 25 | 24.8 | 1.68 | 0.344 | 0.389 |
| present | 19 | 15.8 | 9 | 47.4 | 6 | 31.6 | ||||
| Heartburn / gastroesophageal reflux / hiatal hernia | absent | 84 | 70.0 | 23 | 27.4 | 10 | 11.9 | 1.48 | 0.372 | 0.109 |
| present | 36 | 30.0 | 12 | 33.3 | 10 | 27.8 | ||||
| Tactile, visual, auditory, olfactory or gustatory hyper sensoriality | absent | 54 | 45.0 | 8 | 14.8 | 3 | 5.6 | 3.31 | 0.006 | <0.001 |
| present | 66 | 55.0 | 24 | 36.4 | 10 | 15.2 | ||||
| Myopia or drooping eyelids / unilateral or bilateral eyelid ptosis | absent | 100 | 83.3 | 28 | 28.0 | 26 | 26.0 | 1.27 | 0.635 | 0.059 |
| present | 20 | 16,7 | 9 | 45,0 | 6 | 30,0 | ||||
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Male
- Female
- Autism,
- ADHD,
- Tourette's syndrome,
- Control
- Maschio
- Femmina
- Autismo
- ADHD
- Sindrome di Tourette
- Controllo
References
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin Neurosci 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Huisman-van Dijk, H.M.; Schoot, R.; Rijkeboer, M.M.; Mathews, C.A.; Cath, D.C. The relationship between tics, OC, ADHD and autism symptoms: A cross- disorder symptom analysis in Gilles de la Tourette syndrome patients and family-members. Psychiatry Res 2016, 237, 138–146. [Google Scholar] [CrossRef]
- Xie, S.; Karlsson, H.; Dalman, C.; Widman, L.; Rai, D.; Gardner, R.M.; Magnusson, C.; Sandin, S.; Tabb, L.P.; Newschaffer, C.J.; et al. The Familial Risk of Autism Spectrum Disorder with and without Intellectual Disability. Autism Res 2020, 13, 2242–2250. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, H.; Lee, P.H.; Tsetsos, F.; Davis, L.K.; Yu, D.; Lee, S.H.; Dalsgaard, S.; Haavik, J.; Barta, C.; et al. Investigating Shared Genetic Basis Across Tourette Syndrome and Comorbid Neurodevelopmental Disorders Along the Impulsivity-Compulsivity Spectrum. Biol Psychiatry 2021, 90, 317–327. [Google Scholar] [CrossRef]
- Hollander, E. Obsessive-compulsive disorder and spectrum across the life span. Int J Psychiatry Clin Pract 2005, 9, 79–86. [Google Scholar] [CrossRef]
- Darrow, S.M.; Grados, M.; Sandor, P.; Hirschtritt, M.E.; Illmann, C.; Osiecki, L.; Dion, Y.; King, R.; Pauls, D.; Budman, C.L.; et al. Autism Spectrum Symptoms in a Tourette's Disorder Sample. J Am Acad Child Adolesc Psychiatry 2017, 56, 610–617.e611. [Google Scholar] [CrossRef]
- Petti, T.; Gupta, M.; Fradkin, Y.; Gupta, N. Management of sleep disorders in autism spectrum disorder with co-occurring attention-deficit hyperactivity disorder: update for clinicians. BJPsych Open 2023, 10, e11. [Google Scholar] [CrossRef]
- Lau-Zhu, A.; Fritz, A.; McLoughlin, G. Overlaps and distinctions between attention deficit/hyperactivity disorder and autism spectrum disorder in young adulthood: Systematic review and guiding framework for EEG-imaging research. Neurosci Biobehav Rev 2019, 96, 93–115. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; King, P.G.; Sykes, L.K.; Mehta, J.A.; Geier, M.R. Shared Brain Connectivity Issues, Symptoms, and Comorbidities in Autism Spectrum Disorder, Attention Deficit/Hyperactivity Disorder, and Tourette Syndrome. Brain Connect 2015, 5, 321–335. [Google Scholar] [CrossRef]
- Zoccante, L.; Ciceri, M.L.; Gozzi, L.A.; Gennaro, G.D.; Zerman, N. The "Connectivome Theory": A New Model to Understand Autism Spectrum Disorders. Front Psychiatry 2021, 12, 794516. [Google Scholar] [CrossRef]
- Heinze, G.; Puhr, R. Bias-reduced and separation-proof conditional logistic regression with small or sparse data sets. Stat Med 2010, 29, 770–777. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat Med 2002, 21, 2409–2419. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.B.; Jeong, J.O.; Kwon, N.Y.; Jeong, S.M. Correlation of foot posture index with plantar pressure and radiographic measurements in pediatric flatfoot. Ann Rehabil Med 2015, 39, 10–17. [Google Scholar] [CrossRef]
- Pezaro, S.; Brock, I.; Buckley, M.; Callaway, S.; Demirdas, S.; Hakim, A.; Harris, C.; High Gross, C.; Karanfil, M.; Le Ray, I.; et al. Management of childbearing with hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders: A scoping review and expert co-creation of evidence-based clinical guidelines. PLoS One 2024, 19, e0302401. [Google Scholar] [CrossRef]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J Cell Sci 2002, 115, 2817–2828. [Google Scholar] [CrossRef]
- Hitoglou, M.; Ververi, A.; Antoniadis, A.; Zafeiriou, D.I. Childhood autism and auditory system abnormalities. Pediatr Neurol 2010, 42, 309–314. [Google Scholar] [CrossRef]
- Gage, N.M.; Siegel, B.; Callen, M.; Roberts, T.P. Cortical sound processing in children with autism disorder: an MEG investigation. Neuroreport 2003, 14, 2047–2051. [Google Scholar] [CrossRef]
- Lindly, O.J.; Chan, J.; Fenning, R.M.; Farmer, J.G.; Neumeyer, A.M.; Wang, P.; Swanson, M.; Parker, R.A.; Kuhlthau, K.A. Vision care among school-aged children with autism spectrum disorder in North America: Findings from the Autism Treatment Network Registry Call-Back Study. Autism 2021, 25, 840–853. [Google Scholar] [CrossRef]
- Guimarães-Souza, E.M.; Joselevitch, C.; Britto, L.R.G.; Chiavegatto, S. Retinal alterations in a pre-clinical model of an autism spectrum disorder. Mol Autism 2019, 10, 19. [Google Scholar] [CrossRef]
- Longo, R.; Allegrini, F.; Gusson, E.; Morbio, R.; Di Gennaro, G.; Gozzi, L.A.; Marchini, G.; Zoccante, L. Visual-motor involvement in autism spectrum disorder: could the stereopsis deficit affect motor coordination? Front Psychiatry 2023, 14, 1130185. [Google Scholar] [CrossRef]
- Jussila, K.; Junttila, M.; Kielinen, M.; Ebeling, H.; Joskitt, L.; Moilanen, I.; Mattila, M.L. Sensory Abnormality and Quantitative Autism Traits in Children With and Without Autism Spectrum Disorder in an Epidemiological Population. J Autism Dev Disord 2020, 50, 180–188. [Google Scholar] [CrossRef]
- Chou, W.P.; Chen, Y.L.; Hsiao, R.C.; Lai, Y.H.; Yen, C.F. Bidirectional associations between hyperopia, myopia, astigmatism, and strabismus, and attention-deficit/hyperactivity disorder in children: a national population-based cohort study. Braz J Psychiatry 2023, 45, 397–404. [Google Scholar] [CrossRef]
- Johnson, C.R.; DeMand, A.; Lecavalier, L.; Smith, T.; Aman, M.; Foldes, E.; Scahill, L. Psychometric properties of the children's sleep habits questionnaire in children with autism spectrum disorder. Sleep Med 2016, 20, 5–11. [Google Scholar] [CrossRef]
- Ikeda, J.; Davitt, B.V.; Ultmann, M.; Maxim, R.; Cruz, O.A. Brief report: incidence of ophthalmologic disorders in children with autism. J Autism Dev Disord 2013, 43, 1447–1451. [Google Scholar] [CrossRef]
- Asif, M.I.; Kalra, N.; Sharma, N.; Jain, N.; Sharma, M.; Sinha, R. Connective tissue disorders and eye: A review and recent updates. Indian J Ophthalmol 2023, 71, 2385–2398. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J Ophthalmol 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Bennett, S.M.; Hindin, J.S.; Mohatt, J.; Bauer, C.; Schild, J.; Falk, A.; Specht, M.; Woods, D.; Walkup, J. Proof of Concept Study of an Oral Orthotic in Reducing Tic Severity in Tourette Syndrome. Child Psychiatry Hum Dev 2022, 53, 953–963. [Google Scholar] [CrossRef]
- Murakami, J.; Tachibana, Y.; Akiyama, S.; Kato, T.; Taniguchi, A.; Nakajima, Y.; Shimoda, M.; Wake, H.; Kano, Y.; Takada, M.; et al. Oral splint ameliorates tic symptoms in patients with tourette syndrome. In Mov Disord; United States, 2019; Volume 34, pp. 1577–1578.
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. Jama 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Csecs, J.L.L.; Iodice, V.; Rae, C.L.; Brooke, A.; Simmons, R.; Quadt, L.; Savage, G.K.; Dowell, N.G.; Prowse, F.; Themelis, K.; et al. Joint Hypermobility Links Neurodivergence to Dysautonomia and Pain. Front Psychiatry 2021, 12, 786916. [Google Scholar] [CrossRef]
- Zoccante, L.; Zaffanello, M.; Di Gennaro, G. Editorial: The "Connectivome Theory": psyche, soma and the systemic involvement of connective tissue in neurodivergence. Front Psychiatry 2024, 15, 1436796. [Google Scholar] [CrossRef]
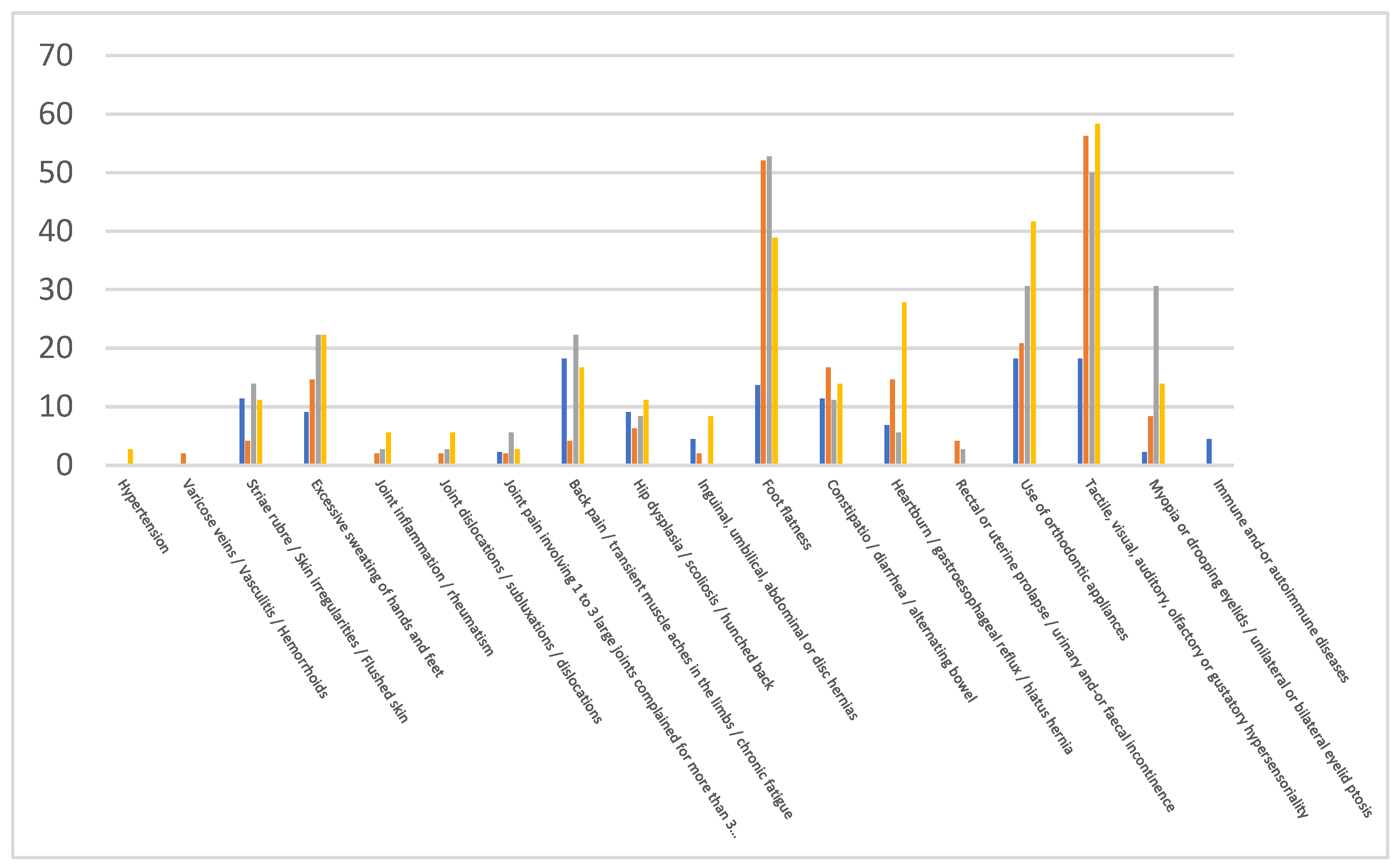
| NDD | ASD (%) | Δ | ADHD (%) | Δ | TS (%) | Δ | Controls (%) |
|---|---|---|---|---|---|---|---|
| Hypertension | 0.0 | 0 | 0.0 | 0 | 2.8 | 2.8 | 0.0 |
| Varicose veins / Vasculitis / Hemorrhoids | 2.1 | 2.1 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Striae rubrae / Skin irregularities / Reddened skin | 4.2 | -7.2 | 13.9 | 2.5 | 11.1 | -0.3 | 11.4 |
| Excessive sweating of hands and feet | 14.6 | 5.5 | 22.2 | 13.1 | 22.2 | 13.1 | 9.1 |
| Joint inflammation/rheumatism | 2.1 | 2.1 | 2.8 | 2.8 | 5.6 | 5.6 | 0.0 |
| Joint dislocations/subluxations/dislocations | 2.1 | 2.1 | 2.8 | 2.8 | 5.6 | 5.6 | 0.0 |
| Joint pain involving 1 to 3 large joints Complained for more than 3 months | 2.1 | -0.2 | 5.6 | 3.3 | 2.8 | 0.5 | 2.3 |
| Back pain / transient muscle pain in the limbs / chronic fatigue | 4.2 | -14 | 22.2 | 4 | 16.7 | -1.5 | 18.2 |
| Hip dysplasia/scoliosis / curved back | 6.3 | -2.8 | 8.3 | -0.8 | 11.1 | 2 | 9.1 |
| Inguinal / Umbilical abdominals or discs hernias | 2.1 | -2.4 | 0.0 | -4.5 | 8.3 | 3.8 | 4.5 |
| Flat plate | 52.1 | 38.5 | 52.8 | 39.2 | 38.9 | 25.3 | 13.6 |
| Constipation/diarrhoea/alternating bowel | 16.7 | 5.3 | 11.1 | -0.3 | 13.9 | 2.5 | 11.4 |
| Heartburn / gastroesophageal reflux / hiatal hernia | 14.6 | 7.8 | 5.6 | -1.2 | 27.8 | 21 | 6.8 |
| Rectal or uterine prolapse / urinary and/or faecal incontinence | 4.2 | 4.2 | 2.8 | 2.8 | 0.0 | 0 | 0.0 |
| Use of orthodontic appliances | 20.8 | 2.6 | 30.6 | 12.4 | 41.7 | 23.5 | 18.2 |
| Tactile / Visual / Auditory/olfactory or gustatory hyper sensoriality | 56.3 | 38.1 | 50.0 | 31.8 | 58.3 | 34.8 | 18.2 |
| Myopia or drooping eyelids / unilateral or bilateral eyelid ptosis | 8.3 | 6 | 30.6 | 28.3 | 13.9 | 11.6 | 2.3 |
| Immune and/or autoimmune diseases | 0.0 | -4.5 | 0.0 | 0 | 0.0 | 0 | 4.5 |
| Symptoms | O.R. | 95% C.I. | P value | ||
|---|---|---|---|---|---|
| Hypertension | ASD | 2.19 | 0.021 | 228.08 | 0.740 |
| ADHD | 1.86 | 0.011 | 306.46 | 0.812 | |
| TS | 4.90 | 0.033 | 734.81 | 0.534 | |
| Varicose veins / Vasculitis / Hemorrhoids | ASD | 3.58 | 0.069 | 185.13 | 0.526 |
| ADHD | 1.26 | 0.011 | 143.97 | 0.924 | |
| TS | 1.09 | 0.009 | 135.69 | 0.973 | |
| Striae rubrae / Skin irregularities / Reddened skin | ASD | 0.34 | 0.062 | 1.91 | 0.222 |
| ADHD | 0.81 | 0.188 | 3.45 | 0.771 | |
| TS | 0.52 | 0.107 | 2.54 | 0.419 | |
| Excessive sweating of hands and feet | ASD | 1.78 | 0.451 | 7.01 | 0.411 |
| ADHD | 2.12 | 0.523 | 8.56 | 0.293 | |
| TS | 1.79 | 0.422 | 7.62 | 0.429 | |
| Joint inflammation/rheumatism | ASD | 2.61 | 0.065 | 105.07 | 0.610 |
| ADHD | 2.43 | 0.059 | 99.60 | 0.639 | |
| TS | 3.40 | 0.082 | 141.57 | 0.520 | |
| Joint dislocations/subluxations/dislocations | ASD | 2.79 | 0.075 | 104.34 | 0.578 |
| ADHD | 3.10 | 0.071 | 134.14 | 0.557 | |
| TS | 4.82 | 0.116 | 201.07 | 0.409 | |
| Joint pain involving 1 to 3 large joints complained of for more than 3 months | ASD | 1.14 | 0.083 | 15.65 | 0.922 |
| ADHD | 1.79 | 0.151 | 21.20 | 0.645 | |
| TS | 0.90 | 0.053 | 15.23 | 0.942 | |
| Back pain / transient muscle pain in the limbs / chronic fatigue | ASD | 0.25 | 0.053 | 1.16 | 0.076 |
| ADHD | 1.21 | 0.353 | 4.12 | 0.765 | |
| TS | 0.82 | 0.211 | 3.15 | 0.769 | |
| Hip dysplasia/scoliosis / curved back | ASD | 0.91 | 0.170 | 4.92 | 0.915 |
| ADHD | 0.84 | 0.151 | 4.69 | 0.846 | |
| TS | 0.99 | 0.176 | 5.55 | 0.988 | |
| Inguinal, umbilical, abdominal or disc hernias | ASD | 0.55 | 0.060 | 5.03 | 0.596 |
| ADHD | 0.18 | 0.007 | 4.70 | 0.306 | |
| TS | 1.21 | 0.150 | 9.71 | 0.860 | |
| Flat foot | ASD | 7.20 | 2.438 | 21.23 | 0.000 |
| ADHD | 6.73 | 2.097 | 21.63 | 0.001 | |
| TS | 3.70 | 1.107 | 12.34 | 0.034 | |
| Constipation/diarrhoea/alternating bowel | ASD | 2.07 | 0.579 | 7.44 | 0.263 |
| ADHD | 1.45 | 0.325 | 6.49 | 0.625 | |
| TS | 1.85 | 0.414 | 8.28 | 0.421 | |
| Heartburn / gastroesophageal reflux / hiatal hernia | ASD | 1.92 | 0.460 | 7.98 | 0.372 |
| ADHD | 0.63 | 0.105 | 3.75 | 0.609 | |
| TS | 3.05 | 0.697 | 13.37 | 0.138 | |
| Rectal or uterine prolapse / urinary and/or faecal incontinence | ASD | 4.34 | 0.144 | 131.51 | 0.399 |
| ADHD | 2.58 | 0.068 | 97.84 | 0.609 | |
| TS | 0.71 | 0.009 | 56.36 | 0.880 | |
| Use of orthodontic appliances | ASD | 2.02 | 0.602 | 6.77 | 0.255 |
| ADHD | 2.36 | 0.650 | 8.57 | 0.192 | |
| TS | 3.20 | 0.887 | 11.51 | 0.076 | |
| Tactile, visual, auditory, olfactory or gustatory hyper sensoriality | ASD | 5.90 | 2.160 | 16.12 | 0.001 |
| ADHD | 4.11 | 1.385 | 12.19 | 0.011 | |
| TS | 5.35 | 1.738 | 16.47 | 0.003 | |
| Myopia or drooping eyelids / unilateral or bilateral eyelid ptosis | ASD | 3.18 | 0.433 | 23.37 | 0.255 |
| ADHD | 13.12 | 1.859 | 92.56 | 0.010 | |
| TS | 5.01 | 0.615 | 40.81 | 0.132 | |
| Immune and/or autoimmune diseases | ASD | 0.38 | 0.018 | 8.18 | 0.537 |
| ADHD | 0.30 | 0.010 | 9.11 | 0.488 | |
| TS | 0.38 | 0.011 | 13.29 | 0.594 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





