1. Introduction
Mpox is an emerging zoonotic disease caused by the Mpox virus (MPXV), which belongs to the Poxviridae family and the Orthopoxvirus genus. Mpox was first detected in 1958 in Denmark when two cases similar to smallpox appeared in monkeys under study, hence named ‘Monkeypox’ or Mpox. It was then first identified in humans in the Democratic Republic of Congo (Zaire/DRC) in 1970, marking the initial discovery of the disease in a human population. The disease shows symptoms closely resembled to smallpox, a globally eradicated disease in 1980. Despite the success in eliminating smallpox, Mpox continues to spread sporadically and has become endemic in certain regions of Africa, particularly in Central and West Africa, where it persists as a public health challenge. However, in 2022, Mpox cases began to emerge in Europe, marking the start of a wider geographical spread of the disease. By July 2022, Mpox had slowly extended to parts of Asia, with South-East Asia experiencing fluctuating case numbers between May and September 2023. By September 2024, Indonesia reported 88 confirmed cases, underscoring the growing presence of this reemerging disease in the region, and Jakarta has the highest number of cases. In response to the first reported case in 2022, Indonesia implemented a specialized epidemiological investigation form as part of its efforts to monitor and address the ongoing threat posed by Mpox.[
1,
2,
3,
4]
Mpox virus could be transmitted from human to human by droplets, respiratory secretions, intimate contact, skin lesions, and contaminated objects. MSM (men who have sex with men) and bisexuals are commonly found in Mpox cases, this indicates that Mpox could spread among people within the close-contact community. Usually, the incubation period would take 2-3 weeks, and then there will be two phases of clinical manifestations, the first one is the invasion period which consists of fever, myalgia, lymphadenopathy, arthralgia, and severe asthenia. Then it was followed by the skin eruption phase. Normally they will resolve in 3-4 weeks, depending on the immunology status.[
5,
6]
According to the Indonesian guideline for the prevention and control of Mpox by the Indonesian Health Ministry, the severity index could correlate to the prognosis of the case. The criteria of the severity index are whether the patient is in the high-risk groups (children under eight years old, pregnant women, immunocompromised patients, and patients with chronic skin conditions); one sign of complication symptoms; three abnormal laboratory examination results, whether it is AST or ALT increase, leukocytosis, decreasing BUN, thrombocyte, or albumin; and severe or very severe skin lesion amount. The case is considered a mild degree case if they didn't meet the criteria mentioned above. It would be regarded as a severe degree case if they fulfilled at least one of the criteria.[
1]
Most Mpox cases experience mild to moderate symptoms and recover well by using therapy such as antivirus.[
6] Although it could recover well, a study found that 30.18% of the confirmed cases were HIV positive.[
7] Which possess a higher risk of mortality when the patient is immunocompromised.[
8] Therefore, more attention would be needed for Mpox patients with HIV.
One of the recommended prevention of Mpox is vaccination. Modified vaccinia Ankara (MVA) vaccine, Bavarian Nordic (JYNNEOS), and ACAM2000 are currently two available vaccines that are already approved by the Food and Drug Administration (FDA) to prevent Mpox and smallpox disease. In 2007, FDA approved the ACAM2000 immunization for individuals who are at high risk of smallpox. ACAM2000 is also believed to have some cross-protective immunity against Mpox. [
9,
10] JYENNEOS vaccine was approved by the FDA in 2019, administered subcutaneously in two-dose series, 0.5 ml per dose, 4 weeks apart, later in 2022 emergency use authorization (EUA) was issued for intradermal administration in a two-dose series, 0.1 ml per dose, 4 weeks apart to increase the supply of vaccine. [
11]
Data in this study were collected from August 2022 to December 2023. This study aims to describe the epidemiological data, clinical features, and mortality of Mpox patients. In addition, this study aims to provide a comprehensive analysis by identifying and examining the differences both epidemiologically and clinically between Mpox patients who are living with HIV (PLHIV) and those who are not (non-PLHIV). Through this approach, this study seeks to find any variations in the disease's progression, symptoms, and transmission patterns between these two groups, thereby contributing to a deeper understanding of how Mpox affects individuals with different immunological status.
2. Material and Methods
This study used a cross-sectional study design to determine the epidemiology, clinical features, and outcomes of Mpox infection and the differences between PLHIV and non-PLHIV. The target population in this study is all the Mpox patients in Indonesia. The accessible population is Mpox patients who are treated in health facilities in Jakarta. The inclusion criteria in this study are patients who resided in Jakarta and nearby cities between August 2022 and December 2023 who were diagnosed with Mpox and confirmed by PCR. The samples were taken by whole sampling methods. Data were obtained from the epidemiological and clinical investigation form of Mpox cases provided by the Indonesian Ministry of Health (Figure S1) that is integrated into the reporting system in DKI Jakarta Health District and we also collected our sample HIV data from the Indonesian HIV/AIDS Information System (SIHA). We collected character demography data such as assigned sex at birth, age, occupation, HIV status, Anti-retroviral Therapy (ART), total CD4, and viral load; we also collected clinical manifestations; complications; outcome; treatment; isolation period; and laboratory examination data. The data collection involved primary health care facilities in Jakarta and also 17 national and private hospitals with reported Mpox cases. This data was coded to be subsequently analyzed. All the categorical variables were analyzed for the frequencies and the percentages, while the continuous variables were analyzed for mean and SD.
3. Results
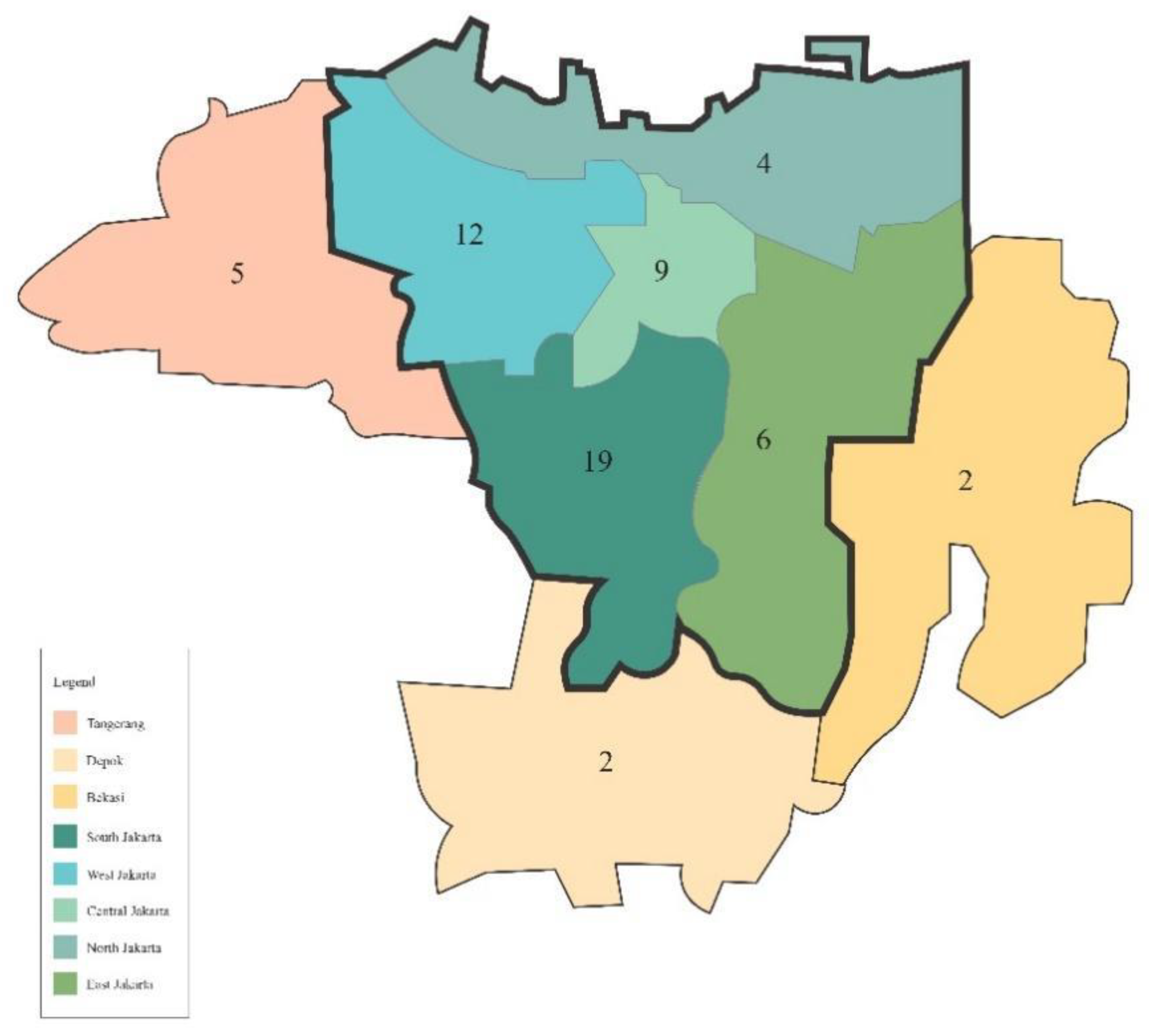
Out of 59 cases, 50 patients reportedly resided in Jakarta, while 9 patients resided in cities nearby Jakarta (
Figure 1). Most of the cases are reported from South Jakarta, with a total of 19 cases (
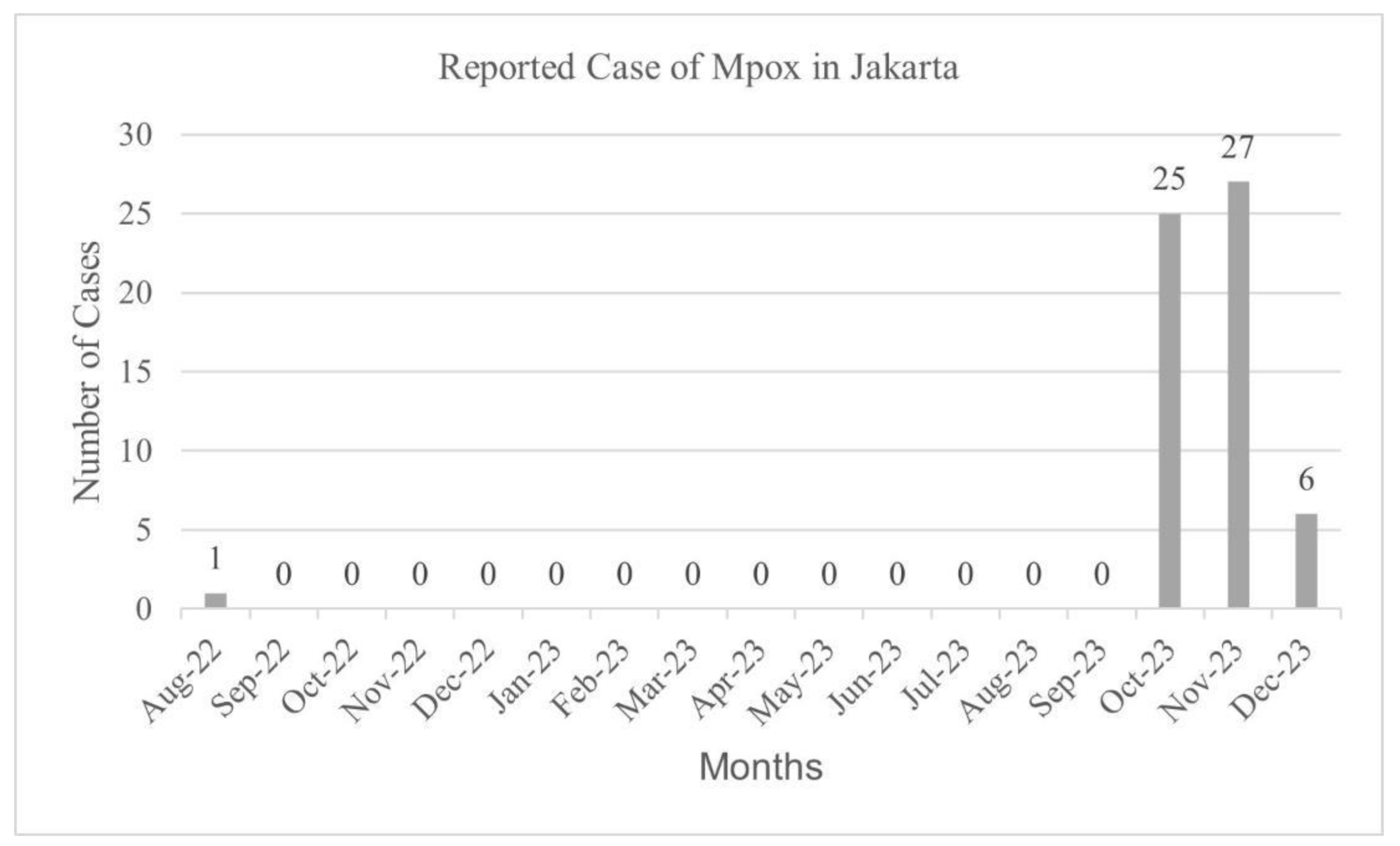
Table 1). According to the report by DKI Jakarta Health District, the first case was found in August 2022, then the case started to appear again in October 2023 and the number peaked in November 2023 (
Figure 2). Majority of the cases (74.58%) were reported by primary health care, 15.25% were reported by general hospitals, and 10.17% were reported by private hospitals.
In this study, all the cases are male (59 cases) and most of them are identified as MSM, comprising 76.3% of the population. The population’s mean age is 30.34 years old (21-49, SD 5.310). Majority of the cases (46/59, 78%) are PLHIV with 39/46 patients (84.78%) already consumed ART before their diagnosis of Mpox. Two (3.4%) patients just find out that they’re infected with HIV after being diagnosed with Mpox. There were three patients (6.5%) whose ART status were unknown. We can only obtain eighteen data for total CD4 and twenty-one data for viral load. Most of the identified total CD4 from PLHIV with ART are 200-500 cells/mm3, while PLHIV without ART total CD4 is mostly <200 cells/mm3.
About 40 (67.8%) cases are reported to have sexual intercourse history for the last twenty-one days, and 29 (72.5%) of them are confirmed to be PLHIV while 11 (27.5%) cases are non-PLHIV. None of the non-PLHIV patients had a history of consuming Pre-Exposure Prophylaxis (PreP). Marital status was not recorded in this study. Thus, there is no data on spouse tracing.
There are five confirmed cases reported with a history of traveling abroad. Two reportedly visited countries in Europe, two patients visited Malaysia, and one patient visited China. Further demographic data can be seen in
Table 1.
Clinical manifestations reported in Mpox cases may vary. Signs and symptoms were separated in this study. Out of 59 Mpox cases, the most frequently reported symptoms are skin lesions (93.2%), followed by fever (84.3%) and rash (66.1%). Lymphadenopathy is also a common finding in Mpox patients and in this study, mostly manifested in the inguinal area (39.0%).
In general, most skin lesions are located on the face (64.4%), lower extremities (44.1%), and palm (42.4%). Out of seven (11.9%) patients who manifest perioral lesions, 3/7 (42.85%) patients reportedly have mucosal manifestations inside the mouth.
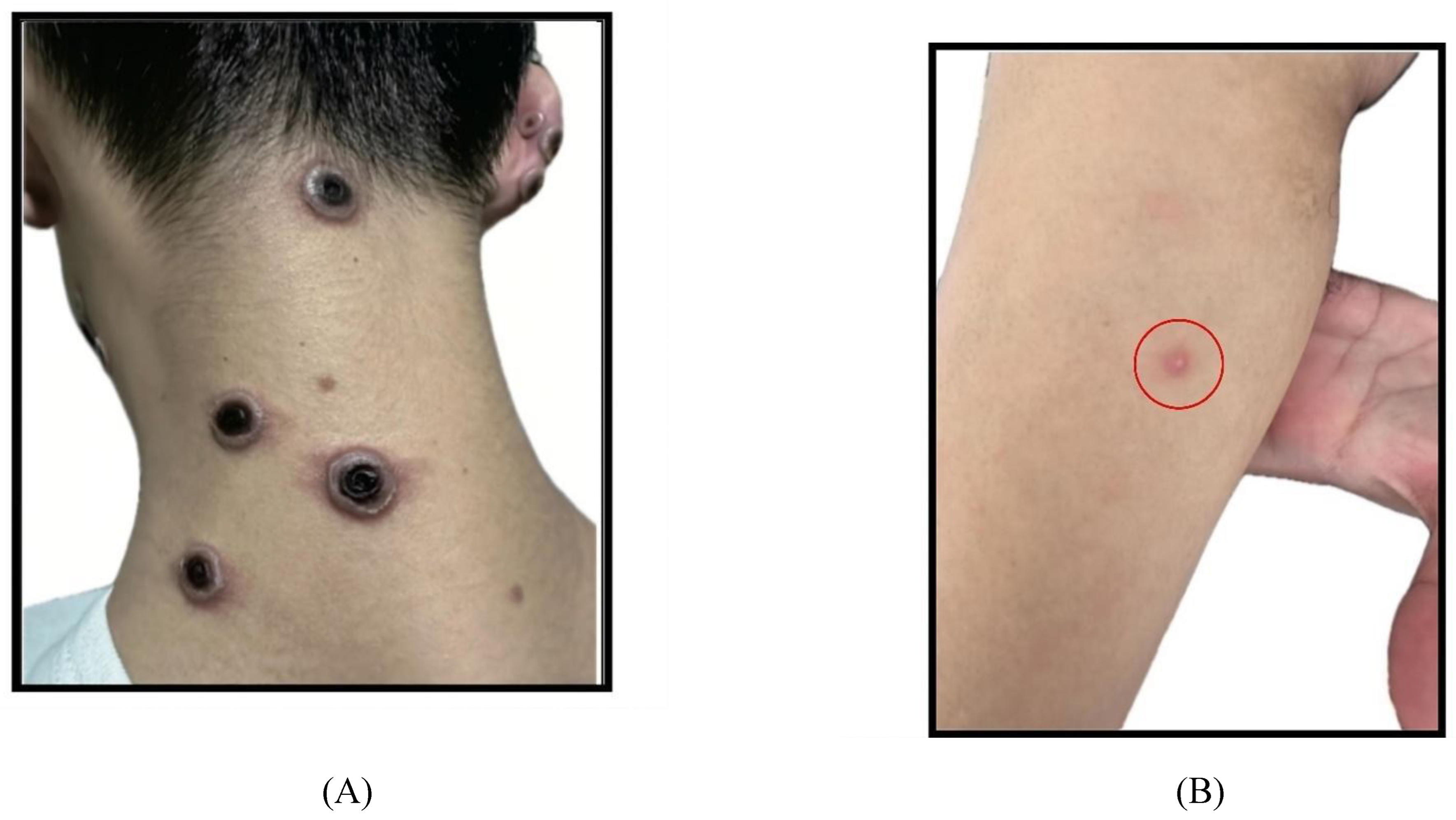
Most lesion morphology are vesicles (52.5%) and pustules (49.2%) (
Figure 3-B). There is a difference in the lesion morphology tendencies in PLHIV who received ART and those who haven’t received ART. In PLHIV, more than half of PLHIV with ART manifested with vesicle (56%) and pustule (59%) lesions, while PLHIV without ART are all manifested with necrotic lesions (
Figure 3-A). Half of the PLHIV without ART shows vesicles, umbilicated pustules, and ulcers as their most common skin lesion manifestations.
The majority of the patients (62.7%) have <25 lesions. There is also a difference in the number of lesions between PLHIV with ART and without ART, the majority of PLHIV with ART (25/39, 64%) have lesions <25, while PLHIV without ART (3/4, 75%) mostly had 25 to 99 lesions. Another important characteristic of a lesion is its pain and pruritus. Most cases reportedly have pain on the lesions, which comprised 54.2% of the cases. In contrast, only 5.1% of patients complained about pruritus on the lesion. Other clinical manifestations can be seen in
Table 2.
Based on the clinical manifestations, only nine patients (15.3%) are classified as severe Mpox according to the Indonesian guideline for the prevention and control of Mpox, five patients are reported to have cervical lymphadenopathy and complained dysphagia, two patients have more than 250 number of lesions, one patient complained of vomiting and nausea, and one patient are classified as severe due to eye pain and laboratory findings. Most of these patients are PLHIV (77.78%). There are 2 (3.4%) cases that have experienced organ involvement such as gastrointestinal involvement, vision involvement, and cellulitis around the perianal area due to Mpox.
Our study showed that 23/59 cases (38.98%) are concurrently diagnosed with sexually transmitted infection (STI), 22 patients are diagnosed with syphilis and 17/22 (77.3%) of them are PLHIV. Only 1 (1.7%) patient is diagnosed with HSV, and they’re also PLHIV. Other than STIs, TB is also found among the patients. There are three patients (5.1%) with TB history and two patients presented with active TB (3.4%). Other comorbidities that are also reported are hypertension (3.4%), liver disease (3.4%), malignancy (1.7%), diabetes (1.7%), and other comorbidities (5.1%) such as pneumonia, CMV, and IRIS.
Most of the cases are examined for PCR for their specimens. None of the patients are tested using serology testing. Based on the result, PLHIV showed a higher percentage of positive results when using necrotic lesions as the specimen (100%). In comparison, non-PLHIV showed a higher percentage of positive results when using lesion fluid as the specimen (100%). (
Table 3)
From 51 data we successfully collected, the median interval for isolation is 20 days, with the shortest isolation period is six days and the longest is 46 days. Our patients are predominantly isolated in the hospital (55.9%), while the rest (44.1%) are self-isolated at home.
Two (3.4%) severe Mpox patients reportedly died due to HIV-related opportunistic infection, not Mpox. Neither patient had initiated antiretroviral therapy (ART) and both patient's CD4 are below 200 cells/mm3. Their viral load data were unavailable due to the Indonesian policy that viral load testing would only proceed after consuming ART for six months. One of the patients already received tecovirimat for 14 days and previously was given cidofovir.
4. Discussion
Since the first case emerged in 2022. The government had taken a measure regarding the disease by raising Mpox awareness among healthcare and health workers on how to diagnose and manage Mpox. Thus, the case finding and management of Mpox became better and we could find 59 confirmed cases in Jakarta and analyzed them. Our analysis finds the majority of the Mpox patient characteristics are male in productive age (mean age 30.34 years old, SD 5.310). A large portion of the cases are reported to have a history of sexual contact with men 21 days before the diagnosis. Mpox is mostly transmitted through direct skin-to-skin and mucosal contact during sexual activities. However, transmission via sperm should be studied further. This aligns with a previous study in 16 countries in 2022, most patients diagnosed with Mpox were identified among men who have sex with men (MSM) (98% of patients in a report of 528 cases from 16 countries) and many reported high-risk sexual behaviors as a potential risk factor.[
13] Another study conducted in the UK also stated that 98% of their population were male with a mean age of 35 years old.[
14]
Our population also presented a large number of PLHIV, out of 59 cases, 78% of them are PLHIV, and 84.78% of them already received ART. The CD4 was also checked among PLHIV. However, out of 59 patients, only 18 patients had available CD4 data to be displayed and most of the PLHIV total CD4 are 200-500 cells/mm3. Additionally, viral load data were collected for 21 patients, and most of these individuals had undetectable viral loads, indicating the patients underwent an effective treatment. None of the non-PLHIV patients are taking PreP. Compared to other studies, our population shows a higher prevalence of PLHIV. A prospective cohort study in France shows that only 29% of them are PLHIV,[
15] and a retrospective study in Germany also identified only 46.9% of their population are PLHIV.[
16] The difference in this result might be caused by the usage of PreP in their population is much higher compared to our study. The French study described that 71% of their non-PLHIV patients are taking PreP, and at least 42.5% of patients from the German study were using PreP[
15,
16]
Between PLHIV and non-PLHIV, both mostly manifested skin lesions (93.2%), fever (81.4%), rashes (66.1%), and lymphadenopathy (59.3%). A rash is a skin discoloration that has not yet developed into primary lesions. The confirmed Mpox patients without showing skin lesions were those found from contact tracing and those who came by themselves for screening. Compared to other studies in another country, we find that fever and rashes were the most common manifestation of Mpox. A study from Nigeria in 2023 stated that their most common symptoms were skin rash (100%), fever (92%), and headache (77%).[
17] While another study from Chinese population, also described that skin lesions or rash (97%) and fever (78%) are the most common complaints in Mpox patients.[
18]
Most skin lesions manifest on the face (64.4%), lower extremities (44.1%), and palm (42.4%). The characteristics are more similar to the study from Nigeria in 2023 where the most common regions are the face (93%), lower extremities (81%), trunk (79%), and palms (61%).[
17] We also noticed that lesion manifestation in the genital area from our study (35.6%) is lower than other studies in Nigeria and China, where more than half of their population were showing genital lesions.[
18]
Both PLHIV and non-PLHIV manifested vesicles (52.5%) and pustules (49.2%) as the most common skin lesion morphology. However, our study finds that there’s a different trend in lesion morphology between PLHIV who received ART and those who haven’t received ART. PLHIV with ART mostly manifested vesicle and pustule lesions, while PLHIV without ART lesions are mostly manifested with necrotic lesions (4/4, 100%), the percentage is higher if we compare to PLHIV with ART who only 26% (10/39) of them manifested necrotic lesions. This shows an intriguing result since the lesion progressions from rash to pustules took approximately a week for each stage to develop and it took 2-4 weeks to develop necrotic lesion shedding from the onset,[
19] while a lot of the PLHIV without ART in our population presented with necrotic lesion, we don't have the data on how long the PLHIV without ART would develop necrotic lesion, therefore the progression of the lesion in PLHIV without ART needs to be studied in the future.
A study stated that PLHIV with CD4 counts <200 cells/mm3 usually shows a severe necrotizing lesion.[
2] This data suits our findings since all PLHIV without ARV cases CD4 count are <200 cells/mm3 and most of them are showing necrotic lesions. Therefore, we should be giving more attention to patients who are presented with a more advanced lesion, because the patients could be HIV patients with low CD4 count and need more attention to the treatment.
Concurrent STIs were also common findings in Mpox infections, about 39% of our cases are diagnosed with syphilis. This number is higher than other studies in the UK, where syphilis was found in 11% of the population.[
14] Most of our patients are also examined by PCR and the highest positive rate is using necrotic lesions (100%) and lesion fluid (94%) as the specimens. In our study, we noticed that a positive rate of lesion fluid is higher in non-PLHIV compared to PLHIV. We found a similar result with another study in China, their symptomatic cases have a 100% positive rate when using lesion fluid, skin lesion swabs, and necrotic lesions as the specimens.[
18] Considering the high positive rate in the lesion sample, the amount of HIV population in this study, and also the findings of syphilis in the population, it is very possible that the spread of Mpox in Jakarta were most likely due to close contact, especially when they do sexual activity.
In the beginning, Indonesia regulated that all Mpox patients should be isolated in hospitals, but the recent policy stated that it is allowed to do self-isolation at home if the case doesn’t require hospital aid. Therefore, our hospitalization rate is higher (55.9%) than another study such as the study from Germany that has a low hospitalization number (4.0%),[
16] the study stated their patients will be hospitalized due to severe clinical manifestations only. Another study from Spain in 2022 also have a lower hospitalization number (2.0%) because they only admit patients that have complications and social reasons.[
20]
Most of the cases are fully recovered, with only two death cases due to other causes than Mpox and it occurs in the PLHIVs without ART with CD4 <200 cells per mm3. Both patients are severe Mpox patients and the only patients who suffered systemic complications. According to a study by Mitja (2023), PLHIV with CD4 below 200 cells/mm3 are more at risk for mortality. Uncontrolled HIV is also one of the risk factors for developing long or complicated Mpox. One of the patients from our study is also suffering IRIS from the ART initiation, and according to the same study, 57% of the Mpox patients who suffered IRIS died. Although these patients didn’t die due to Mpox, AIDS could burden the Mpox condition. Therefore, more attention is needed to treat people with severe cases of Mpox, especially people with uncontrolled HIV.[
2,
21]
Prevention is needed to reduce the number of Mpox cases and vaccines are one of the ways to prevent Mpox. According to the World Health Organization (WHO), there are two strategies for Mpox vaccination. The first is Primary Preventive (Pre-exposure) Vaccination (PPV), which is administered to individuals at high risk of Mpox virus exposure and the second strategy is Post-exposure Preventive Vaccination (PEPV), given to those who are in close contact within 4 days of exposure, or up to 14 days if there are no symptoms have appeared.[
1] In Indonesia, the vaccine is recommended for those who are at high risk of Mpox, such as healthcare workers who handle Mpox cases and MSM who had a history of sexual contact in two weeks. The vaccine that is used in Indonesia is MVA-BN (JYENNEOS), and as of September 2024, the vaccines were still available for high-risk patients. Despite the readiness of Indonesia in providing vaccines, some of the high-risk patients were not complying due to the patients’ worrying about the vaccines and lack of awareness. In 2023, a total of 495 (100%) of the high-risk population were vaccinated for the first dose of Mpox vaccine, however, only 430 (86.9%) patients were vaccinated for the second dose. This result suggests that a prevalent and adequate education regarding Mpox, especially vaccination, in high-risk populations and healthcare workers was important to improve the vaccination rate.[
22]
Mpox is a reemerging viral infection that could manifest with various clinical appearances. In particular, our findings suggest that Mpox may present with distinct symptoms in PLHIV who are not on ART, potentially leading to more severe disease progression. This underscores the need for more attention to PLHIV who have not yet initiated or maintained ART, as they may be at a greater risk of experiencing more severe clinical appearance or may have complications due to Mpox infections. Given the vulnerability of this group, targeted interventions and careful clinical monitoring are essential to mitigate the potential for severe outcomes in these patients.
Our study still has several limitations, such as the limited availability of our laboratory data can be attributed to the fact that not all patients underwent the same set of examinations. Laboratory examinations are not uniformly standardized across all receiving laboratories. Consequently, the amount of data varies across different specimens and the results were not reported thoroughly. Additionally, since the epidemiological forms were completed by various healthcare professionals across different facilities, there is a possibility that some of the information provided may be subjective. Whereas Mpox is an emerging disease, we have conducted several Mpox education and socialization for healthcare professionals, however, it has not reached all healthcare professionals effectively, leading to inconsistent reports due to differences in interpretation and reporting practices.
5. Conclusion
Mpox is an infectious reemerging disease that affects Indonesia. In this study, the majority of the identified Mpox cases were found among male PLHIV in productive age and MSM community. Syphilis was found as a concomitant infection in this group. Notably, severe manifestations of Mpox were predominantly seen in PLHIV who were not receiving ART, indicating that the absence of treatment could exacerbate the disease's severity. Given these findings, it is crucial to establish a strong surveillance system and conduct thorough clinical examinations to ensure early detection, effective management, and prevention of Mpox, particularly in high-risk populations such as PLHIV. Considering vaccines also play a part in the prevention of Mpox, it is required to educate the high-risk population and healthcare workers regarding vaccination. These approaches will be essential to reduce the impact of Mpox and prevent further transmission.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: Epidemiological and clinical investigation form of Mpox cases.
Author Contributions
Conceptualization, Hanny Nilasari and Eliza Miranda; Data curation, Supriadi Supriadi and Sekar Hasanah; Formal analysis, Hanny Nilasari, Eliza Miranda, Melani Marissa, Ani Ruspitawati, Dwi Handayani, Budi Setiawan, Inggrita Wisnuwardani, Erni Nelwan, Robert Sinto, Adityo Susilo, Yulia Saharman, Suratno Ratnoglik, Sekar Hasanah, Megandhita Sharasti and Evy Yunihastuti; Investigation, Ani Ruspitawati, Dwi Handayani, Ngabila Salama, Budi Setiawan, Supriadi Supriadi, Tiranti Aisyah, Inggariwati Inggariwati, Arif Haq, Siti Zuhroh, Eka Safitri, Rahmat Pramono, Inggrita Wisnuwardani, Ni Pitawati and Muhamad Fauzan; Methodology, Hanny Nilasari, Eliza Miranda, Erni Nelwan, Robert Sinto, Yulia Saharman, Suratno Ratnoglik and Evy Yunihastuti; Project administration, Budi Setiawan, Supriadi Supriadi and Sekar Hasanah; Resources, Hanny Nilasari, Ani Ruspitawati, Dwi Handayani, Ngabila Salama, Tiranti Aisyah, Inggariwati Inggariwati, Arif Haq, Siti Zuhroh, Eka Safitri and Rahmat Pramono; Software, Megandhita Sharasti; Supervision, Hanny Nilasari, Eliza Miranda, Melani Marissa, Yulia Saharman and Evy Yunihastuti; Validation, Hanny Nilasari, Melani Marissa, Dwi Handayani, Ngabila Salama, Budi Setiawan, Arif Haq, Ni Pitawati, Muhamad Fauzan and Evy Yunihastuti; Visualization, Megandhita Sharasti; Writing – original draft, Sekar Hasanah and Megandhita Sharasti; Writing – review & editing, Hanny Nilasari, Eliza Miranda, Melani Marissa, Erni Nelwan, Robert Sinto, Adityo Susilo, Yulia Saharman, Suratno Ratnoglik and Evy Yunihastuti. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, governmental, or institutional sources.
Institutional Review Board Statement
The ethical authorization for this research was requested to the Health Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia – Cipto Mangunkusumo Hospital. The approval was granted on 28th November 2023 with the letter number KET-1815/UN2.F1/ETIK/PPM.00.02/2023.
Data Availability Statement
The details of the data in this study couldn't be accessed publicly due to the medical records policy in Indonesia.
Acknowledgments
We are thankful for all the supports from physicians, staffs, and nurses at Cipto Mangunkusumo Hospital, National Infectious Disease Center Sulianti Saroso Hospital, Fatmawati Hospital, Persahabatan Hospital and participating hospitals such as Mampang Prapatan General Hospital, Kembangan General Hospital, Kalideres General Hospital, Matraman General Hospital, Kebayoran Baru General Hospital, Kebayoran Lama General Hospital, Pasar Minggu General Hospital, Ciracas General Hospital, Brawijaya Saharjo Hospital, Juwita Bekasi Hospital, Siloam TB Simatupang Hospital, Grha Kedoya Hospital, Cengkareng General Hospital, also 25 primary health care that are participating.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Direktorat Jenderal Pencegahan dan Pengendalian Penyakit. Pedoman Pencegahan Dan Pengendalian Mpox (Monkeypox); Kementerian Kesehatan RI: Jakarta, 2023. [Google Scholar]
- Mitjà, O.; Alemany, A.; Marks, M.; Lezama Mora, J. I.; Rodríguez-Aldama, J. C.; Torres Silva, M. S.; Corral Herrera, E. A.; Crabtree-Ramirez, B.; Blanco, J. L.; Girometti, N.; et al. Mpox in People with Advanced HIV Infection: A Global Case Series. The Lancet, 2023, 401 (10380), 939–949. [CrossRef]
- World Health Organization. Mpox (monkeypox) outbreak https://www.who.int/europe/emergencies/situations/monkeypox (accessed , 2024). 16 May.
- World Health Organizations. Multi-Country Outbreak of Mpox: External Situation Report 32; 2024.
- Kannan, S.; Ali, P. S. S.; Sheeza, A. Monkeypox: Epidemiology, Mode of Transmission, Clinical Features, Genetic Clades and Molecular Properties. Eur Rev Med Pharmacol Sci, 2022, 26 (16), 5983–5990.
- Minhaj, F. S.; Ogale, Y. P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C. M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Monkeypox Outbreak - Nine States, 22. MMWR Morb Mortal Wkly Rep, 2022, 71 (23), 764–769. 20 May. [CrossRef]
- Sharma, A.; Prasad, H.; Kaeley, N.; Bondalapati, A.; Edara, L.; Kumar, Y. A. Monkeypox Epidemiology, Clinical Presentation, and Transmission: A Systematic Review. Int J Emerg Med, 2023, 16 (1), 20. [CrossRef]
- Yinka-Ogunleye, A.; Dalhat, M.; Akinpelu, A.; Aruna, O.; Garba, F.; Ahmad, A.; Adeleye, A.; Botson, I.; Oluwafemi, B.; Ogunbode, O.; et al. Mpox (Monkeypox) Risk and Mortality Associated with HIV Infection: A National Case-Control Study in Nigeria. BMJ Glob Health, 2023, 8 (11). [CrossRef]
- Ghazy, R. M.; Elrewany, E.; Gebreal, A.; ElMakhzangy, R.; Fadl, N.; Elbanna, E. H.; Tolba, M. M.; Hammad, E. M.; Youssef, N.; Abosheaishaa, H.; et al. Systematic Review on the Efficacy, Effectiveness, Safety, and Immunogenicity of Monkeypox Vaccine. Vaccines, 11(11), 1708. [CrossRef]
- Pischel, L.; Martini, B. A.; Yu, N.; Cacesse, D.; Tracy, M.; Kharbanda, K.; Ahmed, N.; Patel, K. M.; Grimshaw, A. A.; Malik, A. A.; et al. Vaccine Effectiveness of 3rd Generation Mpox Vaccines Against Mpox and Disease Severity: A Systematic Review and Meta-analysis. Vaccine, 2024, 42(25), 126053. [CrossRef]
- Deputy, N. P.; Deckert, J.; Chard, A. N.; Sandberg, N.; Moulia, D. L.; Barkley, E.; Dalton, A. F.; Sweet, C.; Cohn, A. C.; Little, D. R.; et al. Vaccine Effectiveness of JYNNEOS Against Mpox Disease in the United States. New England Journal of Medicine, 2023, 388(26), 2434–2443. [CrossRef]
- Sinto, R.; Johan, A.; Nilasari, H.; Yunihastuti, E.; Nelwan, E. J. Mpox Skin Lesion. Acta Med Indones-Indones J Intern Med •, 2024, 56.
- Thornhill, J. P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L. B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M. S.; et al. Monkeypox Virus Infection in Humans across 16 Countries April-22. N Engl J Med, 2022, 387 (8), 679–691. 20 June. [CrossRef]
- Fink, D. L.; Callaby, H.; Luintel, A.; Beynon, W.; Bond, H.; Lim, E. Y.; Gkrania-Klotsas, E.; Heskin, J.; Bracchi, M.; Rathish, B.; et al. Clinical Features and Management of Individuals Admitted to Hospital with Monkeypox and Associated Complications across the UK: A Retrospective Cohort Study. Lancet Infect Dis, 2023, 23 (5), 589–597. [CrossRef]
- Mailhe, M.; Beaumont, A. L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V. M.; Cortier, M.; et al. Clinical Characteristics of Ambulatory and Hospitalized Patients with Monkeypox Virus Infection: An Observational Cohort Study. Clinical Microbiology and Infection, 2023, 29 (2), 233–239. [CrossRef]
- Hoffmann, C.; Jessen, H.; Wyen, C.; Grunwald, S.; Noe, S.; Teichmann, J.; Krauss, A. S.; Kolarikal, H.; Scholten, S.; Schuler, C.; et al. Clinical Characteristics of Monkeypox Virus Infections among Men with and without HIV: A Large Outbreak Cohort in Germany. HIV Med, 2023, 24 (4), 389–397. [CrossRef]
- Ogoina, D.; Dalhat, M. M.; Denue, B. A.; Okowa, M.; Chika-Igwenyi, N. M.; Yusuff, H. A.; Christian, U. C.; Adekanmbi, O.; Ojimba, A. O.; Aremu, J. T.; et al. Clinical Characteristics and Predictors of Human Mpox Outcome during the 2022 Outbreak in Nigeria: A Cohort Study. Lancet Infect Dis, 2023, 23 (12), 1418–1428. [CrossRef]
- Dou, X.; Li, F.; Ren, Z.; Zhang, D.; Li, J.; Li, D.; Sun, Y.; Jin, H.; Li, R.; Li, W.; et al. Clinical, Epidemiological, and Virological Features of Mpox in Beijing, China - -June 21, 2023. Emerg Microbes Infect, 2023, 12 (2), 2254407. 31 May. [CrossRef]
- Wang, X.; Lun, W. Skin Manifestation of Human Monkeypox. J Clin Med, 2023, 12 (3). [CrossRef]
- Tarín-Vicente, E. J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. The Lancet, 2022, 400 (10353), 661–669. [CrossRef]
- Corma-Gómez, A.; Cabello, A.; Orviz, E.; Morante-Ruiz, M.; Ayerdi, O.; Al-Hayani, A.; Muñoz-Gómez, A.; Santos, I. D. L.; Gómez-Ayerbe, C.; Rodrigo, D.; et al. Long or Complicated Mpox in Patients with Uncontrolled HIV Infection. J Med Virol, 2024, 96 (3). [CrossRef]
-
Laporan Penilaian Risiko Cepat Mpox di Indonesia Tahun 2024; Kementerian Kesehatan RI: Jakarta, 2024.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).