Submitted:
19 November 2024
Posted:
21 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Intervention
2.3. Data and Study Variables
2.4. Measurement of Neuropathic Pain Symptoms
2.5. Measurements of MLKL, ATG5, and EFABP5 Serum Levels by ELISA
2.6. Statistical Analysis
3. Results
3.1. Lipidomic Data Analysis
3.2. Assessment of Associated Markers of Painful Diabetic Neuropathy Levels by ELISA
3.2.1. Measurement of MLKL Serum levels by ELISA
3.2.2. Measurement of ATG5 Serum Levels by ELISA
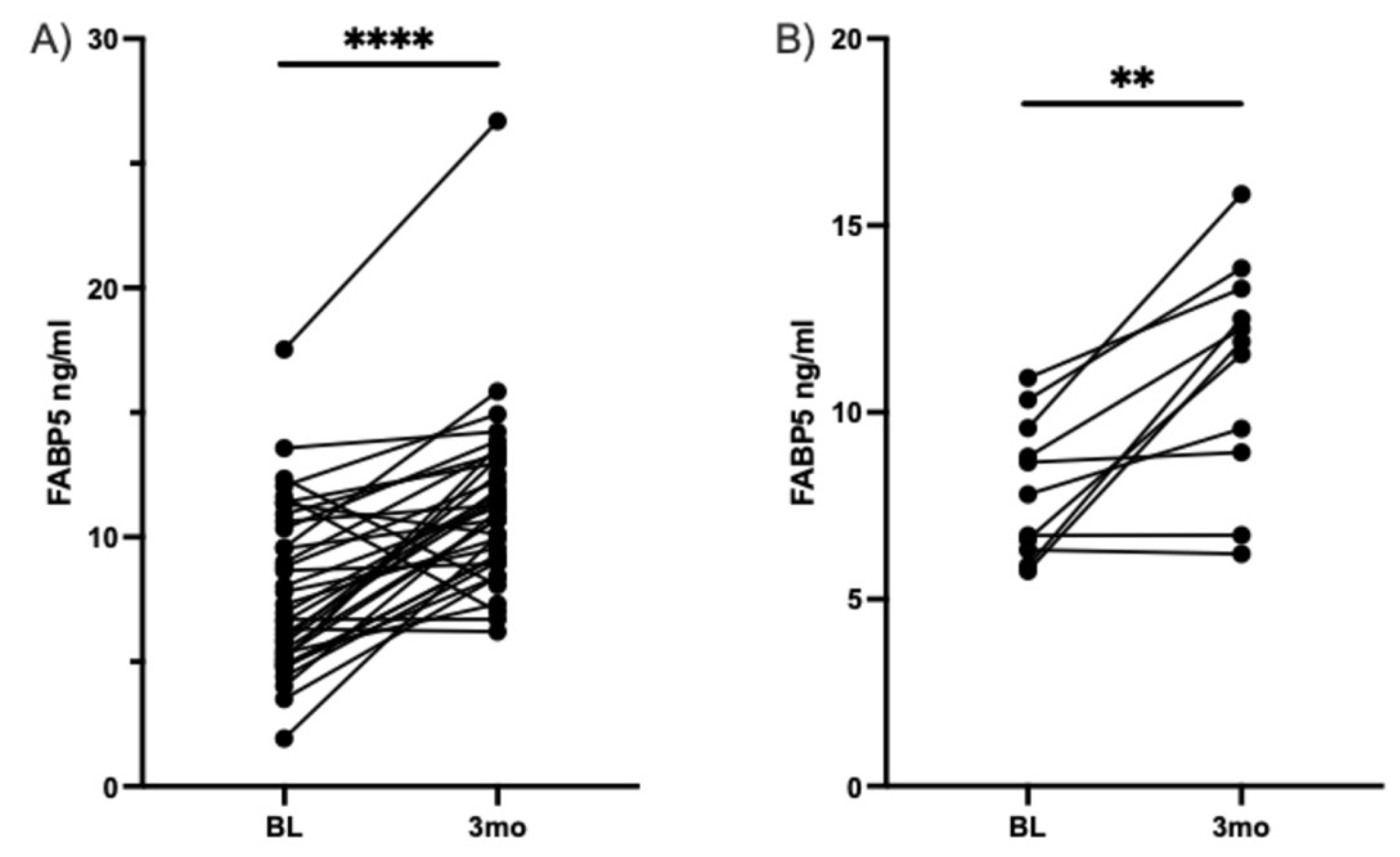
3.2.3. Measurement of FABP5 Serum Levels by ELISA
4. Discussion
4.1. Lipidomic implications of a DHA-rich supplementation in pDN
4.2. Implicaions of Associated Markers of Neuronal Protection after DHA-Rich Dietary Intervention
4.3. Study Limitations and Strengths
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callaghan, B. C.; Gallagher, G.; Fridman, V.; Feldman, E. L. , Diabetic neuropathy: what does the future hold? Diabetologia 2020, 63(5), 891–897. [Google Scholar] [CrossRef] [PubMed]
- Sloan, G.; Shillo, P.; Selvarajah, D.; Wu, J.; Wilkinson, I. D.; Tracey, I.; Anand, P.; Tesfaye, S. , A new look at painful diabetic neuropathy. Diabetes Res Clin Pract 2018, 144, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Tang, H. Y.; Jiang, A. J.; Ma, J. L.; Wang, F. J.; Shen, G. M. , Understanding the Signaling Pathways Related to the Mechanism and Treatment of Diabetic Peripheral Neuropathy. Endocrinology 2019, 160(9), 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T. S.; Karlsson, P.; Gylfadottir, S. S.; Andersen, S. T.; Bennett, D. L.; Tankisi, H.; Finnerup, N. B.; Terkelsen, A. J.; Khan, K.; Themistocleous, A. C.; Kristensen, A. G.; Itani, M.; Sindrup, S. H.; Andersen, H.; Charles, M.; Feldman, E. L.; Callaghan, B. C. , Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 2021, 144(6), 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M. A. , The Potential Role of Fatty Acids in Treating Diabetic Neuropathy. Curr Diab Rep 2018, 18(10), 86. [Google Scholar] [CrossRef]
- Feldman, E. L.; Callaghan, B. C.; Pop-Busui, R.; Zochodne, D. W.; Wright, D. E.; Bennett, D. L.; Bril, V.; Russell, J. W.; Viswanathan, V. , Diabetic neuropathy. Nat Rev Dis Primers 2019, 5(1), 41. [Google Scholar] [CrossRef]
- Callaghan, B. C.; Gao, L.; Li, Y.; Zhou, X.; Reynolds, E.; Banerjee, M.; Pop-Busui, R.; Feldman, E. L.; Ji, L. , Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 2018, 5(4), 397–405. [Google Scholar] [CrossRef]
- Callaghan, B. C.; Little, A. A.; Feldman, E. L.; Hughes, R. A. C. , Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews 2012, (6). [Google Scholar] [CrossRef]
- Rodriguez-Gutierrez, R.; Gonzalez-Gonzalez, J. G.; Zuniga-Hernandez, J. A.; McCoy, R. G. , Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ 2019, 367, l5887. [Google Scholar] [CrossRef]
- Afshinnia, F.; Reynolds, E. L.; Rajendiran, T. M.; Soni, T.; Byun, J.; Savelieff, M. G.; Looker, H. C.; Nelson, R. G.; Michailidis, G.; Callaghan, B. C.; Pennathur, S.; Feldman, E. L. , Serum lipidomic determinants of human diabetic neuropathy in type 2 diabetes. Ann Clin Transl Neurol 2022, 9(9), 1392–1404. [Google Scholar] [CrossRef]
- Handzlik, M. K.; Gengatharan, J. M.; Frizzi, K. E.; McGregor, G. H.; Martino, C.; Rahman, G.; Gonzalez, A.; Moreno, A. M.; Green, C. R.; Guernsey, L. S.; Lin, T.; Tseng, P.; Ideguchi, Y.; Fallon, R. J.; Chaix, A.; Panda, S.; Mali, P.; Wallace, M.; Knight, R.; Gantner, M. L.; Calcutt, N. A.; Metallo, C. M. , Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature 2023, 614(7946), 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S. M.; Baker, N. L.; El Abiad, J. M.; Spassieva, S. D.; Pierce, J. S.; Rembiesa, B.; Bielawski, J.; Lopes-Virella, M. F.; Klein, R. L. , Increased Plasma Levels of Select Deoxy-ceramide and Ceramide Species are Associated with Increased Odds of Diabetic Neuropathy in Type 1 Diabetes: A Pilot Study. Neuromolecular Med 2017, 19(1), 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X. T.; Huang, N.; Sheng, Z. H. , Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron 2022, 110(12), 1899–1923. [Google Scholar] [CrossRef] [PubMed]
- Rumora, A. E.; Lentz, S. I.; Hinder, L. M.; Jackson, S. W.; Valesano, A.; Levinson, G. E.; Feldman, E. L. , Dyslipidemia impairs mitochondrial trafficking and function in sensory neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2018, 32(1), 195–207. [Google Scholar] [CrossRef] [PubMed]
- Rumora, A. E.; LoGrasso, G.; Haidar, J. A.; Dolkowski, J. J.; Lentz, S. I.; Feldman, E. L. , Chain length of saturated fatty acids regulates mitochondrial trafficking and function in sensory neurons. J Lipid Res 2019, 60(1), 58–70. [Google Scholar] [CrossRef] [PubMed]
- Almaguel, F. G.; Liu, J. W.; Pacheco, F. J.; Casiano, C. A.; De Leon, M. , Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. Journal of neuroscience research 2009, 87(5), 1207–18. [Google Scholar] [CrossRef]
- Montero, M. L.; Liu, J. W.; Orozco, J.; Casiano, C. A.; De Leon, M. , Docosahexaenoic acid protection against palmitic acid-induced lipotoxicity in NGF-differentiated PC12 cells involves enhancement of autophagy and inhibition of apoptosis and necroptosis. J Neurochem 2020, 155(5), 559–576. [Google Scholar] [CrossRef]
- Guerbette, T.; Rioux, V.; Bostoen, M.; Ciesielski, V.; Coppens-Exandier, H.; Buraud, M.; Lan, A.; Boudry, G. , Saturated fatty acids differently affect mitochondrial function and the intestinal epithelial barrier depending on their chain length in the in vitro model of IPEC-J2 enterocytes. Front Cell Dev Biol 2024, 12, 1266842. [Google Scholar] [CrossRef]
- Feldman, E. L.; Nave, K. A.; Jensen, T. S.; Bennett, D. L. H. , New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93(6), 1296–1313. [Google Scholar] [CrossRef]
- Mignard, V.; Dubois, N.; Lanoe, D.; Joalland, M. P.; Oliver, L.; Pecqueur, C.; Heymann, D.; Paris, F.; Vallette, F. M.; Lalier, L. , Sphingolipid distribution at mitochondria-associated membranes (MAMs) upon induction of apoptosis. J Lipid Res 2020, 61(7), 1025–1037. [Google Scholar] [CrossRef]
- Descorbeth, M.; Figueroa, K.; Serrano-Illan, M.; De Leon, M. , Protective effect of docosahexaenoic acid on lipotoxicity-mediated cell death in Schwann cells: Implication of PI3K/AKT and mTORC2 pathways. Brain Behav 2018, 8(11), e01123. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J. D.; Cordero, K.; Serrano-Illan, M.; Almeyda, A.; Baldeosingh, K.; Almaguel, F. G.; De Leon, M. , Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience 2013, 255, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Coppey, L.; Davidson, E.; Shevalye, H.; Torres, M. E.; Yorek, M. A. , Effect of dietary oils on peripheral neuropathy-related endpoints in dietary obese rats. Diabetes Metab Syndr Obes 2018, 11, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Veigas, J. M.; Williams, P. J.; Halade, G.; Rahman, M. M.; Yoneda, T.; Fernandes, G. , Fish oil concentrate delays sensitivity to thermal nociception in mice. Pharmacol Res 2011, 63(5), 377–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, C. T.; Tsai, Y. J. , Docosahexaenoic acid confers analgesic effects after median nerve injury via inhibition of c-Jun N-terminal kinase activation in microglia. J Nutr Biochem 2016, 29, 97–106. [Google Scholar] [CrossRef]
- Pacheco, F. J.; Almaguel, F. G.; Evans, W.; Rios-Colon, L.; Filippov, V.; Leoh, L. S.; Rook-Arena, E.; Mediavilla-Varela, M.; De Leon, M.; Casiano, C. A. , Docosahexanoic acid antagonizes TNF-α-induced necroptosis by attenuating oxidative stress, ceramide production, lysosomal dysfunction, and autophagic features. Inflamm Res 2014, 63(10), 859–71. [Google Scholar] [CrossRef]
- Figueroa, J. D.; Serrano-Illan, M.; Licero, J.; Cordero, K.; Miranda, J. D.; De Leon, M. , Fatty Acid Binding Protein 5 Modulates Docosahexaenoic Acid-Induced Recovery in Rats Undergoing Spinal Cord Injury. J Neurotrauma 2016, 33(15), 1436–49. [Google Scholar] [CrossRef]
- Liu, J. W.; Almaguel, F. G.; Bu, L.; De Leon, D. D.; De Leon, M. , Expression of E-FABP in PC12 cells increases neurite extension during differentiation: involvement of n-3 and n-6 fatty acids. J Neurochem 2008, 106(5), 2015–29. [Google Scholar] [CrossRef]
- Liu, J. W.; Montero, M.; Bu, L.; De Leon, M. , Epidermal fatty acid-binding protein protects nerve growth factor-differentiated PC12 cells from lipotoxic injury. J Neurochem 2015, 132(1), 85–98. [Google Scholar] [CrossRef]
- Liu, Y.; Longo, L. D.; De León, M. , In situ and immunocytochemical localization of E-FABP mRNA and protein during neuronal migration and differentiation in the rat brain. Brain Res 2000, 852(1), 16–27. [Google Scholar] [CrossRef]
- Durán, A. M.; Beeson, W. L.; Firek, A.; Cordero-MacIntyre, Z.; De León, M. , Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy. Nutrients 2022, 14(4). [Google Scholar] [CrossRef] [PubMed]
- Durán, A. M.; Salto, L. M.; Câmara, J.; Basu, A.; Paquien, I.; Beeson, W. L.; Firek, A.; Cordero-MacIntyre, Z.; De León, M. , Effects of omega-3 polyunsaturated fatty-acid supplementation on neuropathic pain symptoms and sphingosine levels in Mexican-Americans with type 2 diabetes. Diabetes Metab Syndr Obes 2019, 12, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, L.; Ståhlman, M.; Forsberg, G. B.; Saarinen, S.; Nilsson, R.; Hansson, G. I. , The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res 2012, 53(8), 1690–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Y.; Tsai, Y. J.; Chen, S. H.; Lin, C. T.; Lue, J. H. , Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol Biochem Behav 2013, 106, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lin, J.; Yu, L.; Yan, M. , Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain. Int J Mol Sci 2022, 23(15). [Google Scholar] [CrossRef]
- Saberi Firouzi, S.; Namazi Sarvestani, N.; Bakhtiarian, A.; Ghazi Khansari, M.; Karimi, M. Y.; Ranjbar, A.; Safa, M.; Hosseini, A. , Sildenafil protective effects on high glucose-induced neurotoxicity in PC12 cells: the role of oxidative stress, apoptosis, and inflammation pathways in an in vitro cellular model for diabetic neuropathy. Neurol Res 2018, 40(8), 624–636. [Google Scholar] [CrossRef]
- Lelkes, E.; Unsworth, B. R.; Lelkes, P. I. , Reactive oxygen species, apoptosis and alte1red NGF-induced signaling in PC12 pheochromocytoma cells cultured in elevated glucose: AnIn Vitro cellular model for diabetic neuropathy. Neurotoxicity Research 2001, 3(2), 189–203. [Google Scholar] [CrossRef]
- Ulloth, J. E.; Almaguel, F. G.; Padilla, A.; Bu, L.; Liu, J. W.; De Leon, M. , Characterization of methyl-beta-cyclodextrin toxicity in NGF-differentiated PC12 cell death. Neurotoxicology 2007, 28(3), 613–21. [Google Scholar] [CrossRef]
- Huang, H. C.; Chen, L.; Zhang, H. X.; Li, S. F.; Liu, P.; Zhao, T. Y.; Li, C. X. , Autophagy Promotes Peripheral Nerve Regeneration and Motor Recovery Following Sciatic Nerve Crush Injury in Rats. J Mol Neurosci 2016, 58(4), 416–23. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, L.; Wang, P.; Xue, Y.; Li, X.; Qiao, X.; Zhang, X.; Xu, T.; Liu, G.; Li, P.; Chen, C. , Autophagy impairment mediated by S-nitrosation of ATG4B leads to neurotoxicity in response to hyperglycemia. Autophagy 2017, 13(7), 1145–1160. [Google Scholar] [CrossRef]
- Mohseni, S.; Badii, M.; Kylhammar, A.; Thomsen, N. O. B.; Eriksson, K. F.; Malik, R. A.; Rosen, I.; Dahlin, L. B. , Longitudinal study of neuropathy, microangiopathy, and autophagy in sural nerve: Implications for diabetic neuropathy. Brain Behav 2017, 7(8), e00763. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.; Kim, S. R.; Jang, Y.; Lee, S. H. , Neuronal Autophagy: Characteristic Features and Roles in Neuronal Pathophysiology. Biomol Ther (Seoul) 2021, 29(6), 605–614. [Google Scholar] [CrossRef] [PubMed]
- de Le�n, M.; Welcher, A. A.; Nahin, R. H.; Liu, Y.; Ruda, M. A.; Shooter, E. M.; Molina, C. A. , Fatty acid binding protein is induced in neurons of the dorsal root ganglia after peripheral nerve injury. Journal of Neuroscience Research 1996, 44(3), 283–292. [Google Scholar] [CrossRef]
- Liu, Y.; Molina, C. A.; Welcher, A. A.; Longo, L. D.; De Leon, M. , Expression of DA11, a neuronal-injury-induced fatty acid binding protein, coincides with axon growth and neuronal differentiation during central nervous system development. J Neurosci Res 1997, 48(6), 551–62. [Google Scholar] [CrossRef]
- Kagawa, Y.; Jin, L.; Sun, J.; Nicolazzo, J.; Owada, Y.; Pan, Y. , Microglia with fatty acid-binding protein 5 deficiency exhibit proinflammatory phenotype contributing to neuroinflammation. Alzheimer's & Dementia 2022, 18(S4). [Google Scholar]
- Pan, Y.; Short, J. L.; Choy, K. H.; Zeng, A. X.; Marriott, P. J.; Owada, Y.; Scanlon, M. J.; Porter, C. J.; Nicolazzo, J. A. , Fatty Acid-Binding Protein 5 at the Blood-Brain Barrier Regulates Endogenous Brain Docosahexaenoic Acid Levels and Cognitive Function. J Neurosci 2016, 36(46), 11755–11767. [Google Scholar] [CrossRef]
- Bertea, M.; Rütti, M. F.; Othman, A.; Marti-Jaun, J.; Hersberger, M.; von Eckardstein, A.; Hornemann, T. , Deoxysphingoid bases as plasma markers in Diabetes mellitus. Lipids in health and disease 2010, 9(1), 84. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K. M.; Abcouwer, S. F.; Feldman, E. L.; Gardner, T. W.; Pennathur, S.; Fort, P. E. , New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia 2019, 62(9), 1539–1549. [Google Scholar] [CrossRef]
- Padilla, A.; Descorbeth, M.; Almeyda, A. L.; Payne, K.; De Leon, M. , Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res 2011, 1370, 64–79. [Google Scholar] [CrossRef]
- Ren, C.; Liu, J.; Zhou, J.; Liang, H.; Wang, Y.; Sun, Y.; Ma, B.; Yin, Y. , Lipidomic analysis of serum samples from migraine patients. Lipids in health and disease 2018, 17(1), 22. [Google Scholar] [CrossRef]
- Arima, H.; Hanada, M.; Hayasaka, T.; Masaki, N.; Omura, T.; Xu, D.; Hasegawa, T.; Togawa, D.; Yamato, Y.; Kobayashi, S.; Yasuda, T.; Matsuyama, Y.; Setou, M. , Blockade of IL-6 signaling by MR16-1 inhibits reduction of docosahexaenoic acid-containing phosphatidylcholine levels in a mouse model of spinal cord injury. Neuroscience 2014, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Boulton, A. J.; Feldman, E. L.; Bril, V.; Freeman, R.; Malik, R. A.; Sosenko, J. M.; Ziegler, D. , Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40(1), 136–154. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; Mizushima, N. , Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441(7095), 885–9. [Google Scholar] [CrossRef] [PubMed]
- Yerra, V. G.; Kalvala, A. K.; Kumar, A. , Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J Nutr Biochem 2017, 47, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yang, Y.; Zhou, F.; Xiao, Y.; Shi, L. , Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy and relieves hyperalgesia in diabetic rats. Neuroreport 2020, 31(9), 644–649. [Google Scholar] [CrossRef]
- Li, J.; Tian, M.; Hua, T.; Wang, H.; Yang, M.; Li, W.; Zhang, X.; Yuan, H. , Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy 2021, 17(12), 4062–4082. [Google Scholar] [CrossRef]
- Hernández-Cáceres, M. P.; Cereceda, K.; Hernández, S.; Li, Y.; Narro, C.; Rivera, P.; Silva, P.; Ávalos, Y.; Jara, C.; Burgos, P.; Toledo-Valenzuela, L.; Lagos, P.; Cifuentes Araneda, F.; Perez-Leighton, C.; Bertocchi, C.; Clegg, D. J.; Criollo, A.; Tapia-Rojas, C.; Burgos, P. V.; Morselli, E. , Palmitic acid reduces the autophagic flux in hypothalamic neurons by impairing autophagosome-lysosome fusion and endolysosomal dynamics. Mol Cell Oncol 2020, 7(5), 1789418. [Google Scholar] [CrossRef]
- Ortiz-Rodriguez, A.; Acaz-Fonseca, E.; Boya, P.; Arevalo, M. A.; Garcia-Segura, L. M. , Lipotoxic Effects of Palmitic Acid on Astrocytes Are Associated with Autophagy Impairment. Mol Neurobiol 2019, 56(3), 1665–1680. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Lacombe, R. J. S.; Bazinet, R. P. , Mechanisms regulating brain docosahexaenoic acid uptake: what is the recent evidence? Curr Opin Clin Nutr Metab Care 2018, 21(2), 71–77. [Google Scholar] [CrossRef]
- Pan, Y.; Morris, E. R.; Scanlon, M. J.; Marriott, P. J.; Porter, C. J. H.; Nicolazzo, J. A. , Dietary docosahexaenoic acid supplementation enhances expression of fatty acid-binding protein 5 at the blood-brain barrier and brain docosahexaenoic acid levels. J Neurochem 2018, 146(2), 186–197. [Google Scholar] [CrossRef]
- Wagner, E. F.; Swenson, C. C.; Henggeler, S. W. , Practical and Methodological Challenges in Validating Community-Based Interventions. Children's Services 2000, 3(4), 211–231. [Google Scholar] [CrossRef]
- Stevens, J. R.; Herrick, J. S.; Wolff, R. K.; Slattery, M. L. , Power in pairs: assessing the statistical value of paired samples in tests for differential expression. BMC Genomics 2018, 19(1), 953. [Google Scholar] [CrossRef] [PubMed]
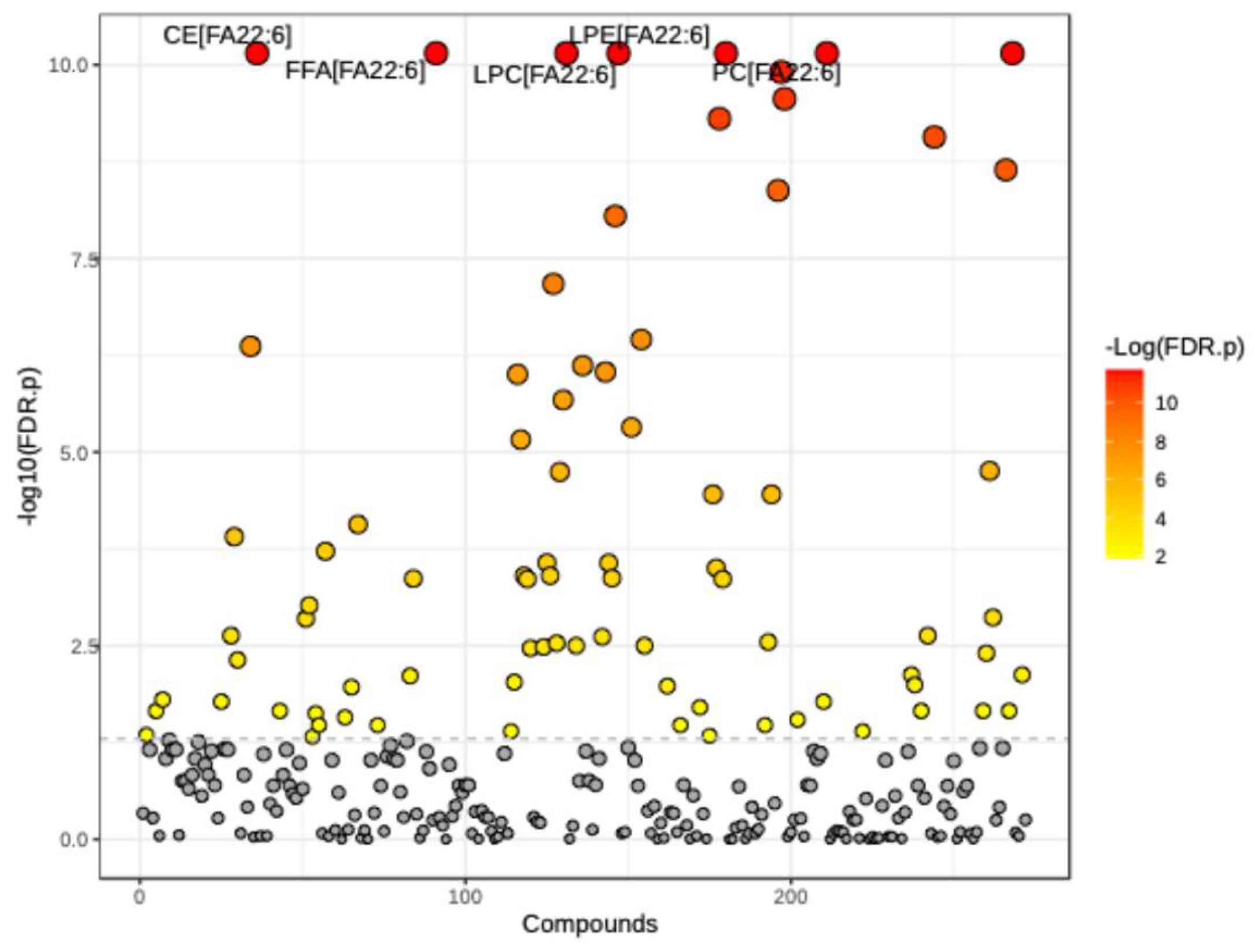
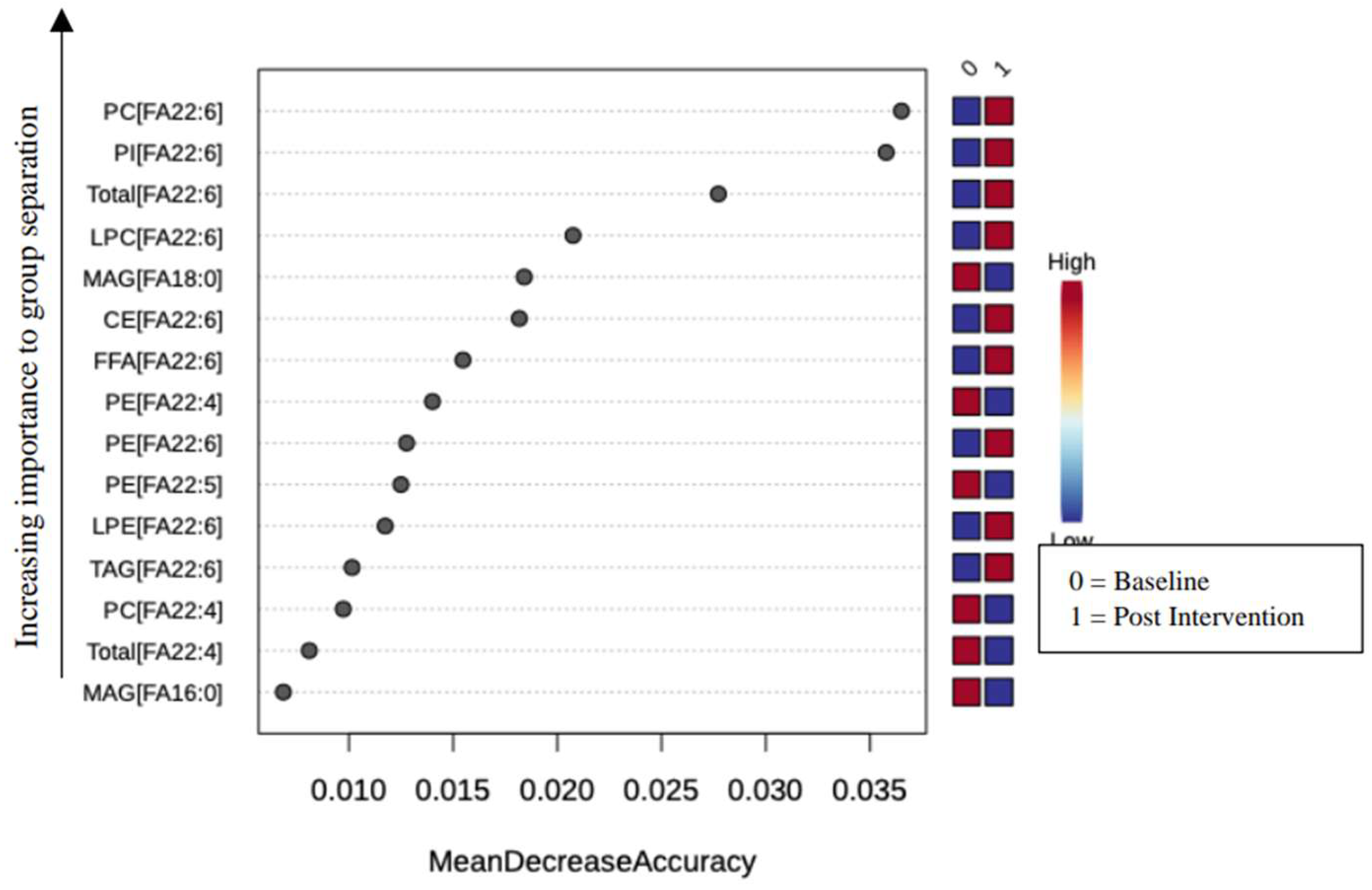
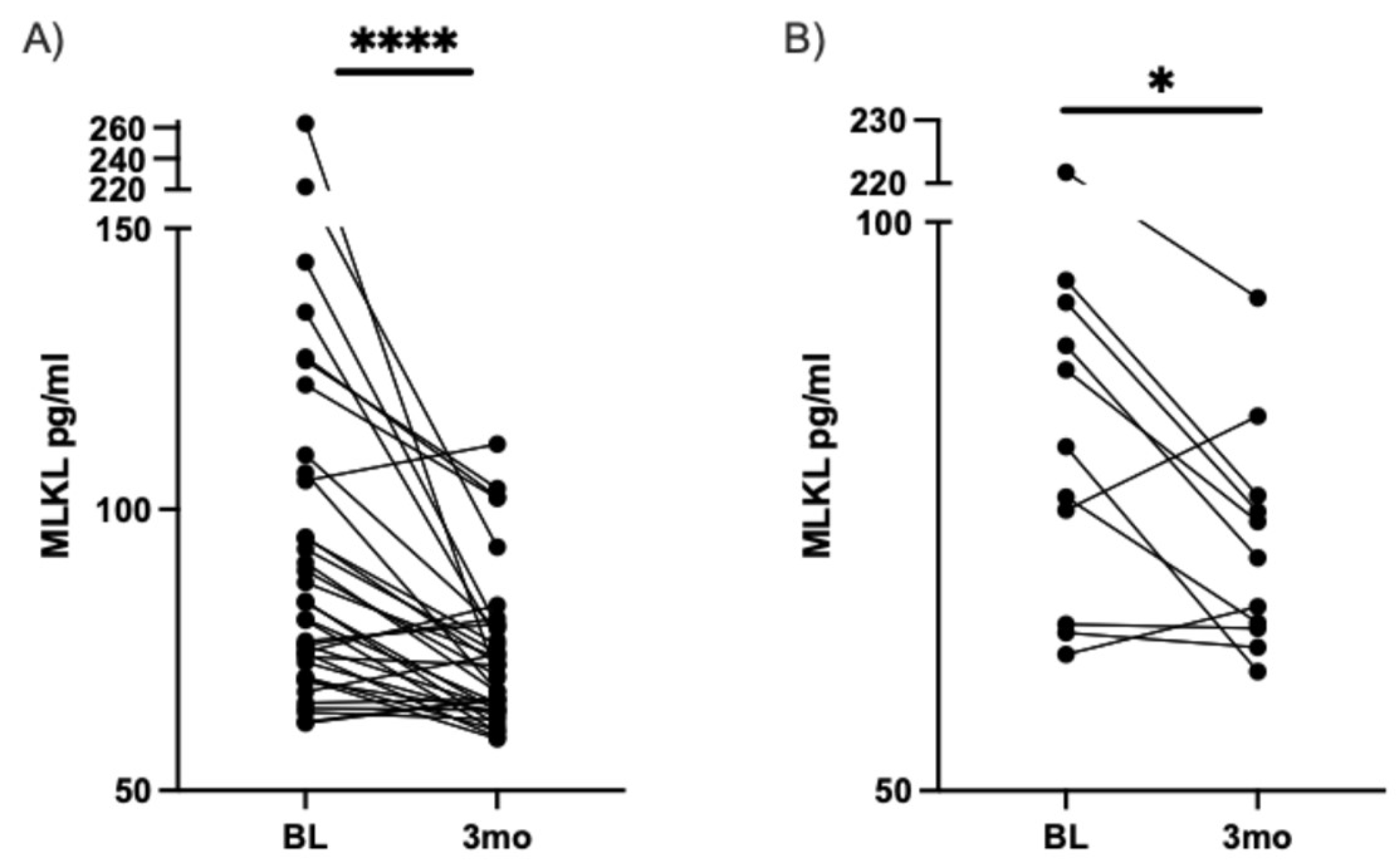
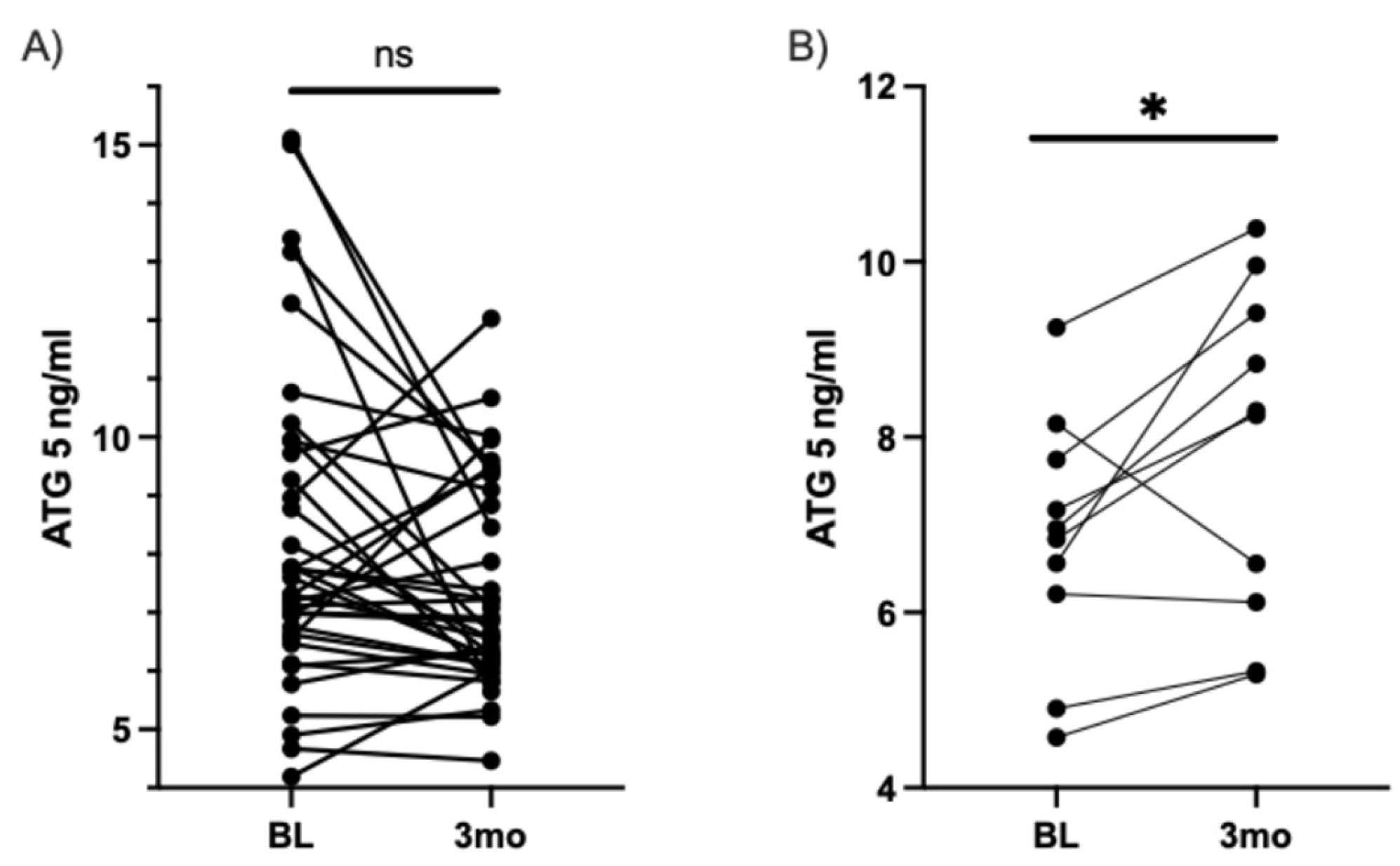
| Total Biochemicals Identified | 283 | |||
| Total Biochemicals p ≤ 0.05, adjusted P-value (FDR) cutoff, matched paired t-test | 81 | |||
| Biochemicals (↑|↓) | 22 |59 |
| Group | Lipid Class | Name | p-value | FDR |
| Neutral Lipids | Cholesteryl ester (CE) | CE[FA22:6] | 1.819E-12 | 7.0681E-11 |
| CE[FA22:4] | 2.6917E-08 | 4.3068E-07 | ||
| CE[FA20:4] | 1.3218E-05 | 0.00012397 | ||
| CE[FA20:3] | 0.0003791 | 0.0023435 | ||
| CE[FA20:5] | 0.00094117 | 0.0048302 | ||
| CE[FA20:0] | 0.0038675 | 0.016698 | ||
| Monoacylglycerol (MAG) | MAG[FA18:0] | 2.07E-08 | 3.519E-07 | |
| MAG[FA16:0] | 3.8752E-07 | 4.7911E-06 | ||
| MAG[FA18:1] | 0.00056988 | 0.0031634 | ||
| Triacylglycerol (TAG) | TAG[FA22:6] | 3.4561E-11 | 8.5459E-10 | |
| TAG[FA22:4] | 0.0003791 | 0.0023435 | ||
| TAG[FA20:2] | 0.0015176 | 0.0075053 | ||
| TAG[FA20:3] | 0.0021663 | 0.010159 | ||
| TAG[FA20:5] | 0.0053322 | 0.021975 | ||
| Diacylglycerol (DAG) | DAG[FA22:6] | 2.1048E-05 | 0.00019083 | |
| DAG[FA20:3] | 0.00014039 | 0.00095468 | ||
| DAG[FA20:2] | 0.0002192 | 0.0014196 | ||
| DAG[FA16:0] | 0.0053322 | 0.021975 | ||
| DAG[FA20:5] | 0.0060965 | 0.023689 | ||
| DAG[FA22:4] | 0.0093813 | 0.033575 | ||
| DAG[FA20:4] | 0.014108 | 0.047375 | ||
| Free fatty acid (FFA) | FFA[FA22:6] | 1.819E-12 | 7.0681E-11 | |
| FFA[FA20:4] | 5.8459E-05 | 0.00042975 | ||
| FFA[FA20:3] | 0.0015981 | 0.0077621 | ||
| FFA[FA16:0] | 0.0093813 | 0.033575 | ||
| Phospholipids | Phosphatidylcholine (PC) | PC[FA22:6] | 1.819E-12 | 7.0681E-11 |
| PC[FA22:4] | 1.819E-11 | 4.9477E-10 | ||
| PC[FA20:4] | 3.4888E-06 | 3.5146E-05 | ||
| PC[FA20:5] | 3.813E-05 | 0.00031428 | ||
| PC[FA22:5] | 6.2683E-05 | 0.00043717 | ||
| PC[FA14:1] | 0.0022767 | 0.010496 | ||
| PC[FA20:0] | 0.0046535 | 0.019777 | ||
| PC[FA17:0] | 0.0093813 | 0.033575 | ||
| PC[FA20:3] | 0.013558 | 0.046096 | ||
| Phosphatidylethanolamine (PE) | PE[FA22:5] | 3.638E-12 | 1.2369E-10 | |
| PE[FA22:6] | 9.0949E-12 | 2.7487E-10 | ||
| PE[FA22:4] | 2.0009E-10 | 4.1865E-09 | ||
| PE[FA20:5] | 3.4888E-06 | 3.5146E-05 | ||
| PE[FA20:4] | 0.00047945 | 0.002835 | ||
| PE[FA20:3] | 0.008995 | 0.033516 | ||
| Phosphatidylinositol (PI) | PI[FA22:6] | 1.819E-12 | 7.0681E-11 | |
| PI[FA22:5] | 0.0038675 | 0.016698 | ||
| PI[FA18:1] | 0.0075849 | 0.028654 | ||
| Lysophosphatidylcholine (LPC) | LPC[FA22:6] | 1.819E-12 | 7.0681E-11 | |
| LPC[FA20:4] | 3.7016E-09 | 6.7123E-08 | ||
| LPC[FA16:0] | 7.2841E-08 | 9.9064E-07 | ||
| LPC[FA22:5] | 1.6344E-07 | 2.1169E-06 | ||
| LPC[FA16:1] | 5.8416E-07 | 6.9084E-06 | ||
| LPC[FA22:4] | 1.6604E-06 | 1.8065E-05 | ||
| LPC[FA20:2] | 3.0617E-05 | 0.00026864 | ||
| LPC[FA17:0] | 5.0783E-05 | 0.00039466 | ||
| LPC[FA20:3] | 5.0783E-05 | 0.00039466 | ||
| LPC[FA18:0] | 6.2683E-05 | 0.00043717 | ||
| LPC[FA20:5] | 0.00050804 | 0.0029401 | ||
| LPC[FA20:1] | 0.00060329 | 0.0032819 | ||
| LPC[FA18:1] | 0.00063846 | 0.0034051 | ||
| LPC[FA15:0] | 0.0019595 | 0.0093506 | ||
| LPC[FA14:0] | 0.011537 | 0.040231 | ||
| Lysophosphatidylethanolamine (LPE) | LPE[FA22:6] | 1.819E-12 | 7.0681E-11 | |
| LPE[FA22:5] | 4.602E-10 | 8.9411E-09 | ||
| LPE[FA18:0] | 5.0639E-08 | 7.6521E-07 | ||
| LPE[FA20:4] | 6.4607E-08 | 9.249E-07 | ||
| LPE[FA20:5] | 3.1753E-05 | 0.0002699 | ||
| LPE[FA22:4] | 5.623E-05 | 0.00042485 | ||
| LPE[FA20:3] | 0.00040222 | 0.0024312 | ||
| LPE[FA16:1] | 0.00056988 | 0.0031634 | ||
| Sphingolipids | Sphingomyelin (SM) | SM[FA26:0] | 0.011537 | 0.040231 |
| Ceramide (CER) | CER[FA22:0] | 0.0035205 | 0.015698 | |
| CER[FA20:0] | 0.005577 | 0.021985 | ||
| CER[FA16:0] | 0.013026 | 0.044848 | ||
| Dihydroceramide (DCER) | DCER[FA26:0] | 8.848E-06 | 8.5952E-05 | |
| DCER[FA24:0] | 0.0023922 | 0.010845 | ||
| DCER[FA22:0] | 0.0069552 | 0.026645 | ||
| Total | N/A | Total [FA22:6] | 1.819E-12 | 7.0681E-11 |
| Total [FA22:4] | 1.0004E-10 | 2.2677E-09 | ||
| Total [FA20:4] | 1.5435E-06 | 1.7493E-05 | ||
| Total [FA20:5] | 0.0002059 | 0.001366 | ||
| Total [FA20:3] | 0.00075535 | 0.0039511 | ||
| Total [FA26:0] | 0.0015176 | 0.0075053 | ||
| Total [FA20:2] | 0.005577 | 0.021985 | ||
| Total [FA22:5] | 0.005577 | 0.021985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





