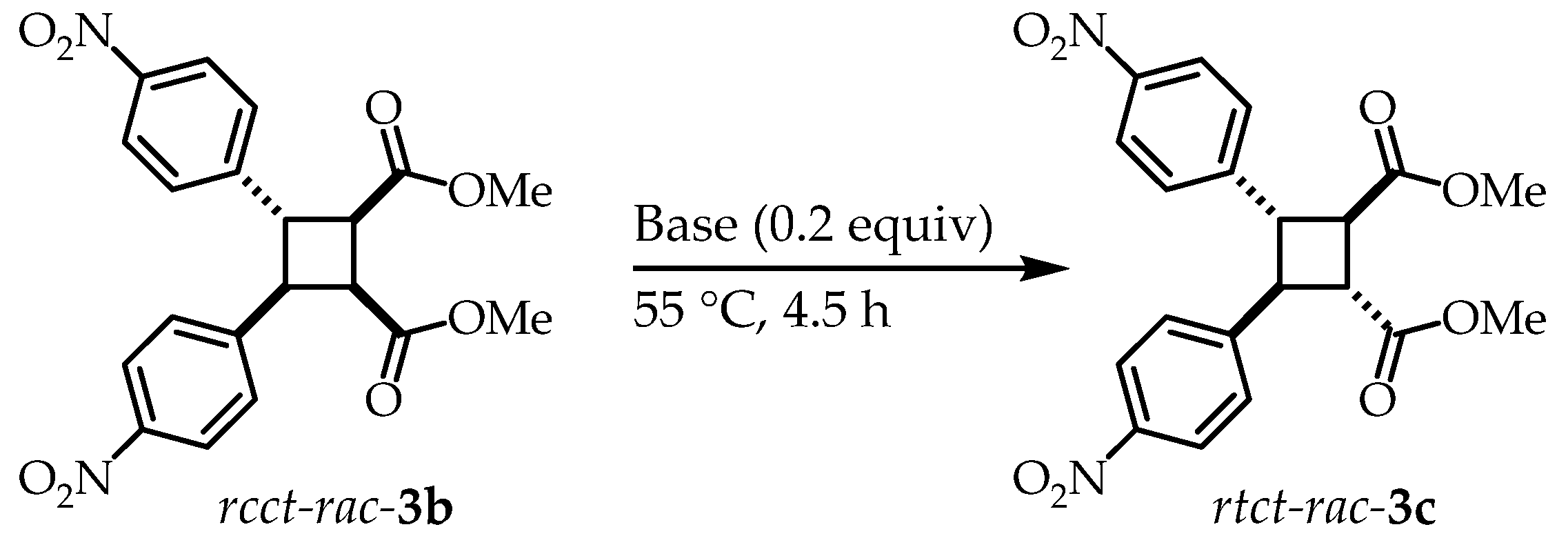1. Introduction
Green Chemistry, a field that emerged in the 1990s, is a way of designing processes and chemical products through methodologies that reduce or eliminate the production of substances that are hazardous to humans and the environment.[
1] This is supported by The Twelve Principles introduced by Paul Anastas and John Warren in 1998.[
2] Mechanochemistry, on the other hand, is a synthesis technique that is distinguished by the absence of solvent and by promoting chemical reactions through kinetic energy.[
3] This technique was developed from Principle five of Green Chemistry, which is oriented to avoid the use of solvents in chemical reactions. Mechanochemistry has become a powerful alternative to traditional solvent-based processes, which allows for a cleaner route for chemical transformations. The liquid-assisted grinding (LAG) technique is a method derived from mechanochemistry that consists in the use of catalytic quantities of solvent to facilitate the integration process of the reactants in a chemical reaction.[
4] The use of the LAG technique serves to improve the efficiency of solvent-free reactions by introducing small amounts of solvent to facilitate the reaction and proper integration of the starting material. LAG is quantified by ways of the parameter η (μL/mg), which is the ratio of the amount of solvent used (μL) to the total mass of the reactants (mg). A value η = 0 corresponds to a reaction in grinding, values of η ≈ 0-1 correspond to LAG reactions, η ≈ 1-10 correspond to slurry reactions, and when η > 10 it is considered a reaction in solution.[
5] LAG Technique has been widely used for the synthesis of Active Pharmaceutical Ingredients (APIs) and pharmaceutically relevant fragments, providing a cleaner, safer and more efficient synthesis route.[
6]
Truxinic and truxillic acids are structures derived from cyclobutanes with more than 100 derived structures found in nature.[
7] In addition, some derivatives have been reported to have biological activities, highlighting anticancer, anti-inflammatory, anti-neuroinflammatory, neuroprotective and antidiabetic activities.[
8] Other applications of truxinic/truxillic derivatives include their use in the synthesis of biobased functional materials,[
9] and the truxilline forms present in cocaine serves as its geographical, manufacture, and storage “fingerprint“ of cocaine samples.[
10]
The main method of formation of truxinic and truxillic acid derivatives is by [2+2] cycloaddition reactions. This way of synthesizing cyclobutane derivatives is limited by the poor regio- and diastereoselectivity of the intermediates, which are formed by irradiating the unsaturated structures with ultraviolet light. To redeem this, techniques have been developed that promote [2+2] dimerization in a regio- and diastereoselective manner.[
11] In addition, cycloadducts obtained by [2+2] cycloadditions can be isomerized to obtain structures with less accessible stereochemistry.[
12]
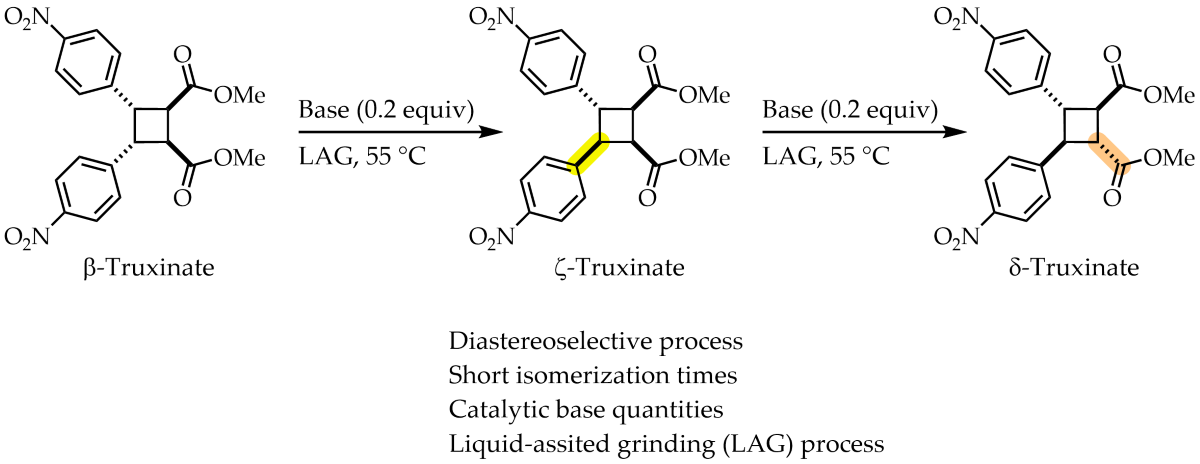
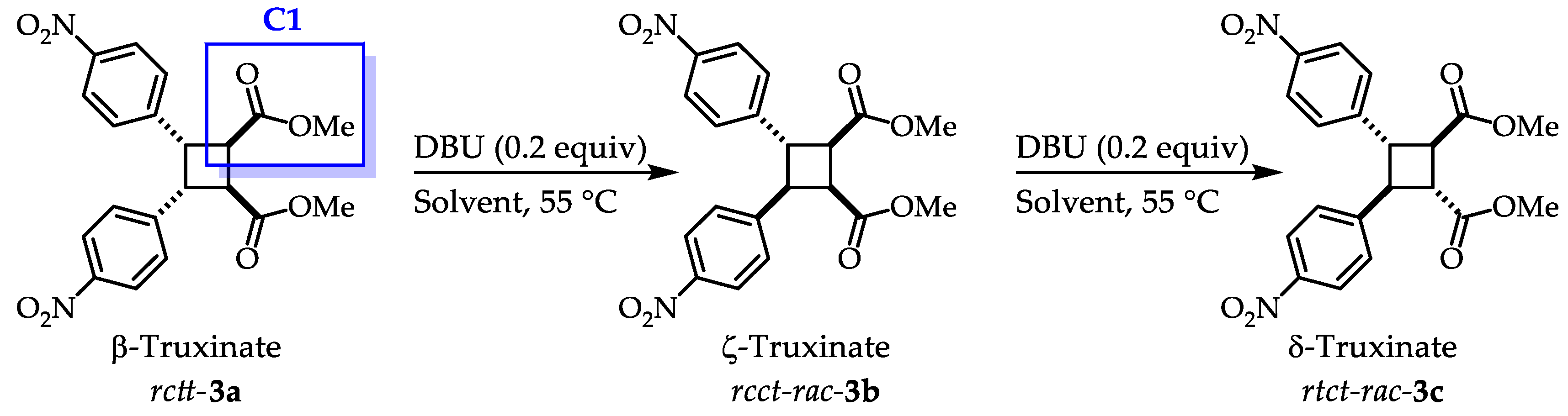
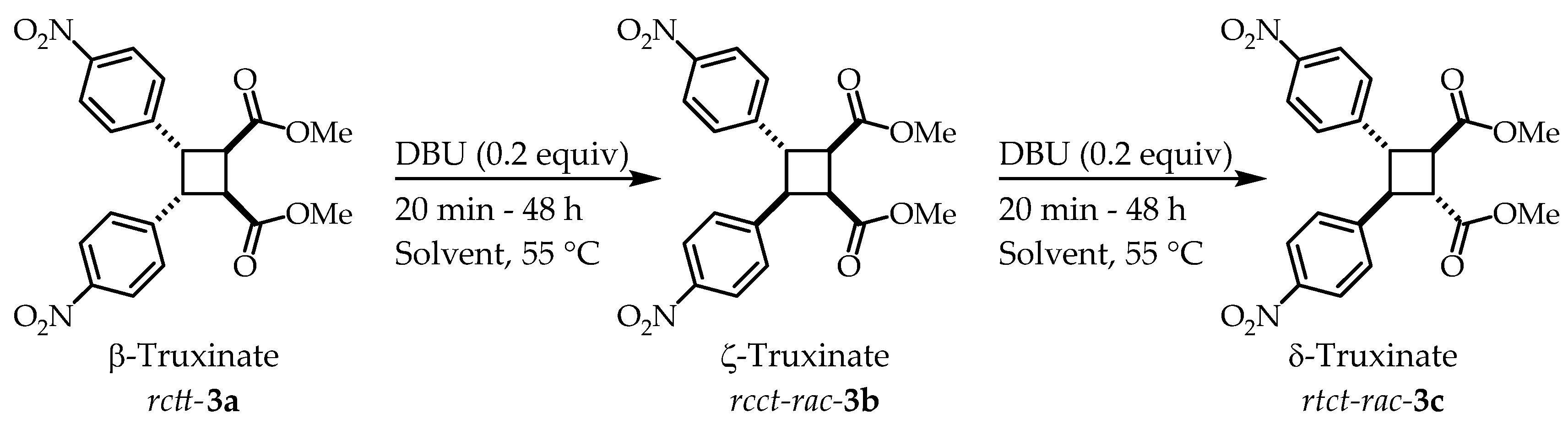
Recently, the synthesis of the derivative β-truxinate
rctt-
3a from methyl 4-nitrocinnamate and its isomerization with DBU forming the corresponding ζ- and δ-truxinate
in situ has been reported in our research group (Error! Reference source not found.).[
13] With this methodology it is possible to access three truxinate diastereoisomers out of the six possible ones. Also, in this work the diastereoisomers are named as
cis,
trans,
trans (
rctt-3a);
cis,
cis,
trans (
rcct-
3b) and
trans,
cis,
trans (
rtct-3 c) according to the relative orientation of the substituents with respect to the carbonyl group (
C1) marked in
Error! Reference source not found..[
14]
Scheme 1.
Previous published work β- → ζ- → δ-truxinate isomerization using DBU (0.2 equiv) in different deuterated solvents.
Scheme 1.
Previous published work β- → ζ- → δ-truxinate isomerization using DBU (0.2 equiv) in different deuterated solvents.
In this work we present the process of β- → ζ- → δ-truxinate isomerization by LAG technique using bases with different pKa values. This novel methodology provides a high proportion of the diastereoisomers was reached in short reaction times and with small amounts of solvents.
2. Materials and Methods
All chemicals were obtained commercially (Aldrich) and used without further purification. Reactions were monitored by TLC on Merck Al plates coated with silica gel of 0.25 mm with fluorescent indicator (60F-254) using ultraviolet light, iodine, and potassium permanganate as relay agents as appropriate. The [2+2] cycloaddition reactions were carried out in a 110 Volt RPR 100 Reactor Rayonet equipped with model RPR-2537A lamps with a wavelength of 254 nm.
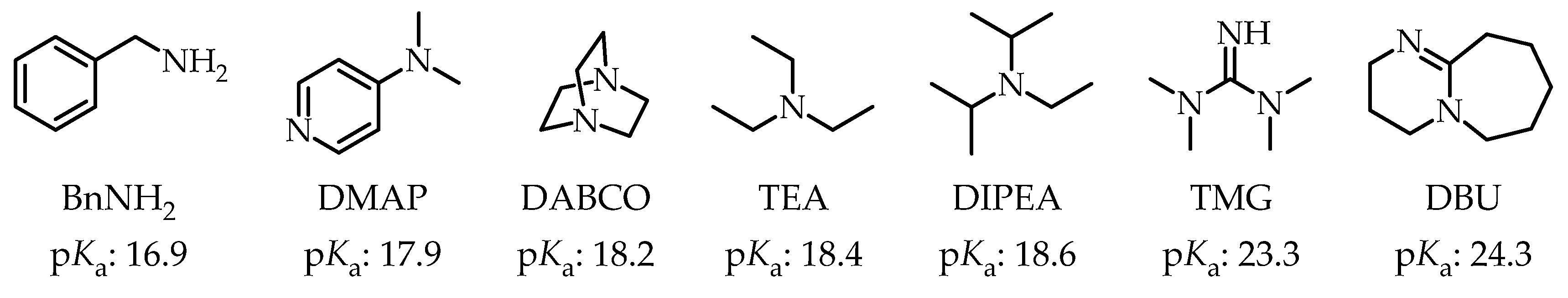
For the liquid-assisted grinding isomerization process β- → ζ- → δ-truxinate it was decided to explore the following bases:
N,N-diisopropylethylamine (DIPEA), benzylamine (BnNH
2), 4-dimethylamino-pyridine (DMAP), 1,4-diazabicyclo[2.2.2]octane (DABCO), triethylamine (TEA),
N,N,N′,N′-tetramethylguanidine (TMG) and 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU). The p
Ka values of the bases in acetonitrile are presented in Error! Reference source not found..[
16,
17,
18] Dissolutions of the bases in acetonitrile were prepared. Since the η values of the reactions are found to be between 0.8 - 1.4 these processes are classified as LAG.
Figure 1.
Bases used for the isomerization process β- → ζ- → δ-truxinate isomerization process and the pKa values of their conjugated acids (pKa value in MeCN).
Figure 1.
Bases used for the isomerization process β- → ζ- → δ-truxinate isomerization process and the pKa values of their conjugated acids (pKa value in MeCN).
3. Results and Discussion
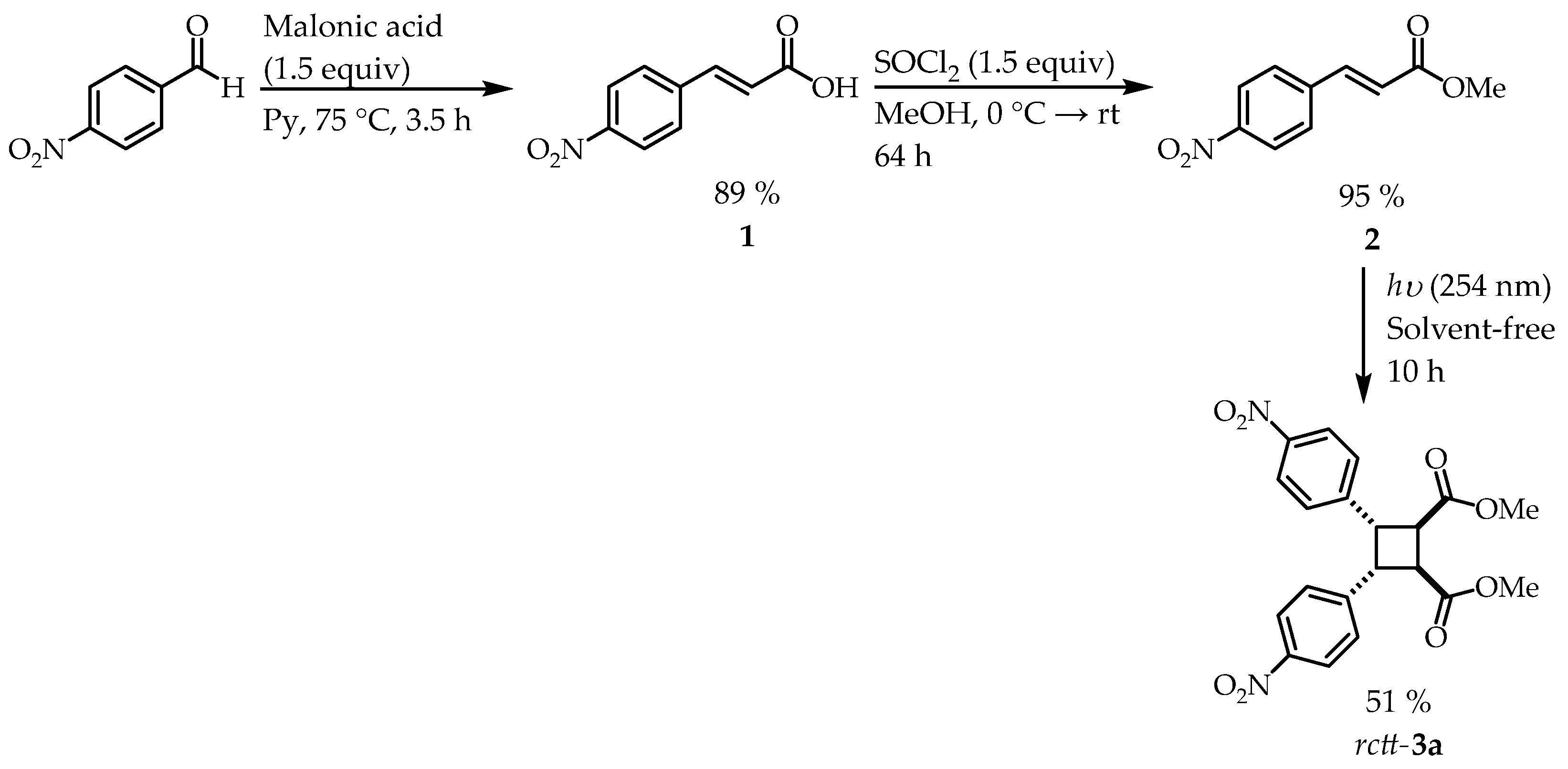
3.1. Synthesis of β-Truxinato rctt-3a
β-Truxinate rctt-3a was obtained by the synthesis route shown in Error! Reference source not found..
Scheme 2.
Synthesis route for the formation of β-truxinate rctt-3a.
Scheme 2.
Synthesis route for the formation of β-truxinate rctt-3a.
We started with a Knoevenagel condensation reaction between 4-nitrobenzaldehyde and malonic acid in pyridine.[
19] 4-Nitrocinnamic acid
1 was obtained as a white solid with a mp = 292-296 °C and 89 % yield, consistent with literature data.[
20]
The formation of methyl 4-nitrocinnamate ester
2 was achieved from the esterification of α,β-unsaturated acid
1 using thionyl chloride and methanol. Ester
2 was obtained as a slightly yellow solid with a mp = 161-164 °C and a yield of 95 %, coinciding with that reported in the literature.[
21]
β-Truxinate derivative
rctt-
3a was synthesized from ultraviolet light irradiation (λ = 254 nm) of the ester α,β-unsaturated
2 in an RPR-100 Photochemical Reactor via solvent-free. The product obtained was isolated as a slightly yellow solid with a mp = 128-130 °C and a yield of 51 %, consistent with literature data.[
22]
3.2. Truxinate Isomerization Process Using Different Bases
The isomerization reactions were carried out in a vial of 1 mL at 55 °C in an oil bath. For each reaction, 20 mg (4.8x10-2 mmol) of rctt-3a and 0.2 equiv (9.6x10-3 mmol) of base were used (Error! Reference source not found.).
Scheme 3.
General isomerization process β- → ζ- → δ-truxinate in situ using LAG technique.
Scheme 3.
General isomerization process β- → ζ- → δ-truxinate in situ using LAG technique.
The reactions were monitored by thin layer chromatography in a 7:3 hexane:EtOAc system eluted twice. The reactions were stopped by performing percolations in silica using a 1:1 hexane:EtOAc system, thus removing the bases and recovering the reaction crudes. The ratios of the diastereoisomers formed were determined by
1H NMR (
See Supplementary Material). The results of the ratios are presented in the Error! Reference source not found..
Table 1.
Ratios of the isomers β-, ζ- and δ-truxinate obtained by 1H NMR.
Table 1.
Ratios of the isomers β-, ζ- and δ-truxinate obtained by 1H NMR.
| Entry |
pKa
|
Base |
η (μL/mg) |
Time |
rctt-3a (%) |
rcct-rac-3b (%) |
rtct-rac-3c (%) |
| 1 |
16.9 |
BnNH2
|
0.8 |
48 h |
98 |
2 |
0 |
| 2 |
17.9 |
DMAP |
1.0 |
48 h |
74 |
26 |
0 |
| 3 |
18.2 |
DABCO |
0.9 |
48 h |
72 |
28 |
0 |
| 4a
|
18.4 |
TEA |
1.1 |
48 h |
100 |
0 |
0 |
| 5a
|
18.6 |
DIPEA |
1.4 |
48 h |
100 |
0 |
0 |
| 6 |
23.3 |
TMG |
1.0 |
90 min |
2 |
91 |
7 |
| 7 |
24.3 |
DBU |
1.2 |
20 min |
2 |
61 |
37 |
| 8 |
24.3 |
DBU |
1.2 |
45 min |
1 |
37 |
62 |
|
aNo reaction progress was observed by thin layer chromatography. |
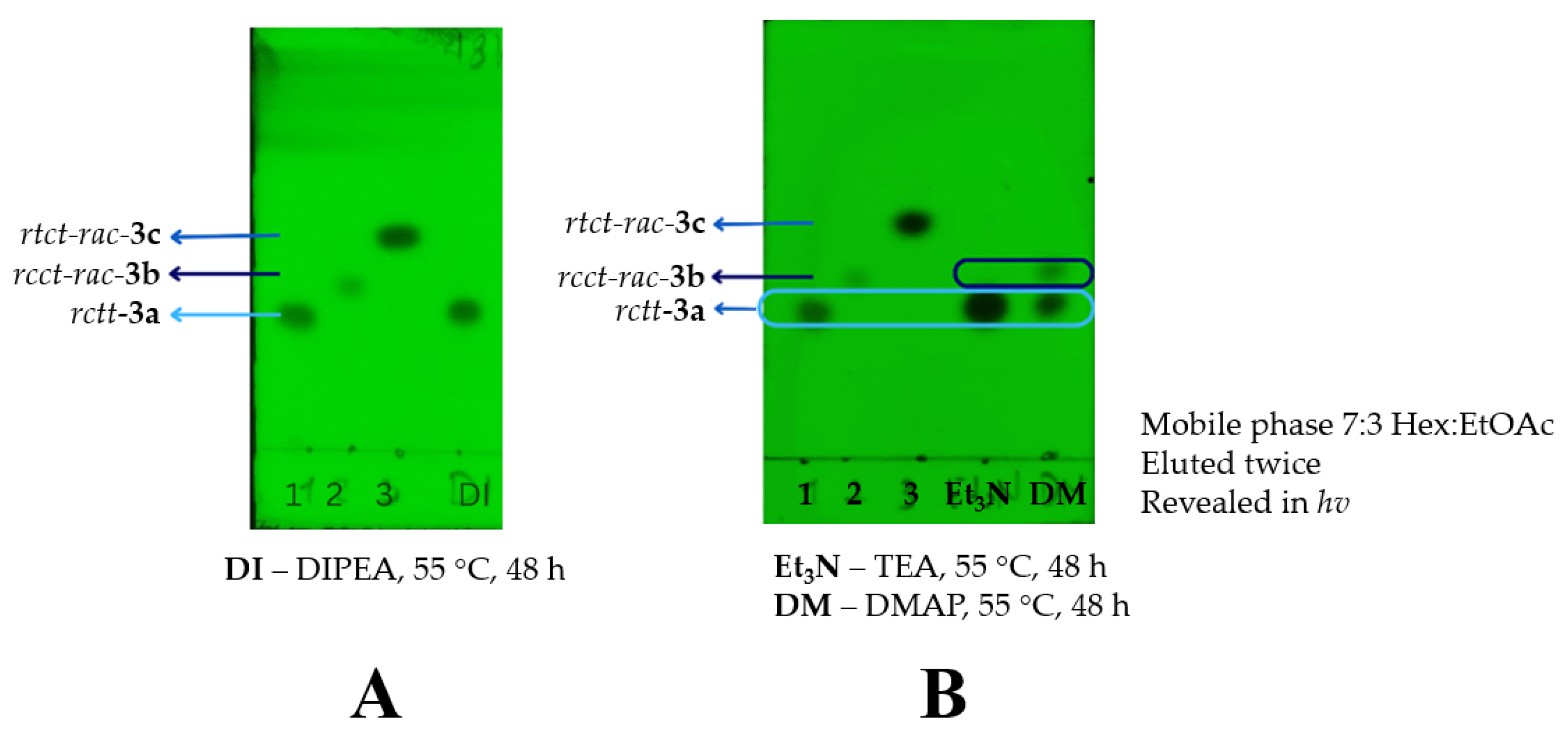
It was observed that bases with lower pKa values (Entry 1-5) resulted in longer reaction times (48 h) and lower diastereoisomer ratios. As can be seen by TLC (Error! Reference source not found.). On the other hand, no reaction advance is observed when the DIPEA and TEA are used.
Figure 2.
Thin layer chromatography (hexane:EtOAc, 7:3) of the reactions using DIPEA, TEA and DMAP.
Figure 2.
Thin layer chromatography (hexane:EtOAc, 7:3) of the reactions using DIPEA, TEA and DMAP.
When TMG and DBU bases were used, reaction times were considerably reduced (Entry 6-8). As shown in Error! Reference source not found., in 45 min of reaction using DBU a yield of 37 % for rcct-rac-3b and 62 % for rtct-rac-3c was obtained. With TMG, ζ-truxinate was obtained with 91 % yield in 90 min. This difference in reaction rates between β- → ζ- and ζ- → δ-truxinate processes employing TMG can be explained in steric hindrance terms of the nucleophilic nitrogen of the bases.
3.3. ζδ-Truxinate Isomerization Process Using TMG and DBU
To analyze the rate difference of the ζ- → δ-truxinate process using DBU and TMG, ζ-truxinate was isolated and the ζ- → δ-truxinate process was evaluated as shown in Error! Reference source not found..
Scheme 4.
ζ- → δ-Truxinate isomerization process using DBU and TMG.
Scheme 4.
ζ- → δ-Truxinate isomerization process using DBU and TMG.
First, isomerization reactions using DBU were carried out in a 1 mL vial at 55 °C in an oil bath. For this reaction, 20 mg (4.8x10
-2 mmol) of
rctt-
3a and 0.2 equiv (9.6x10
-3 mmol) of DBU were used. The reaction was monitored by thin layer chromatography and the reaction was terminated at 4.5 h due to no longer perceptible reaction progress observed. The proportions of the diastereoisomers were evaluated by
1H NMR (
See Supplementary Material). Subsequently, the same reaction was carried out but using TMG instead as a base. In the same manner the reaction was monitored by thin layer chromatography and stopped at 4.5 h. The proportions of the diastereoisomers were determined by
1H NMR and shown in Error! Reference source not found..
Table 2.
Ratios of the isomers ζ- and δ-truxinate obtained by 1H NMR.
Table 2.
Ratios of the isomers ζ- and δ-truxinate obtained by 1H NMR.
| Entry |
pKa
|
Base |
η (μL/mg) |
Time |
rcct-rac-3b (%) |
rtct-rac-3c (%) |
| 1 |
23.3 |
TMG |
1.0 |
4.5 h |
70 |
30 |
| 2 |
24.3 |
DBU |
1.2 |
4.5 h |
31 |
69 |
4. Conclusions
In summary, the formation of the cycloadducts ζ-truxinate and δ-truxinate from the isomerization of β-truxinate was achieved with high yields using the Liquid-Assisted Grinding technique. This novel technique provides shorter reaction times compared to the solution analogue. It was observed that bases with low pKa result in longer reaction times, while bases with higher pKa values resulted in short reaction times. By selection of the base the process can be directed to obtain preferentially the β- or ζ-truxinate.
Supplementary Material
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. The Supplementary Material contains experimental procedures, spectroscopic characterization of compounds and
1H NMR spectra of the reaction crudes.
Acknowledgments
We would like to thank CONAHCYT for financial support (Project No. CB2019/610262). José Daniel Bahena-Martínez thanks CONAHCYT for postgraduate scholarship with number 812421. Also, we would like to thank Laboratorio Nacional de Estructuras de Macromoléculas (LANEM, CIQ) for the acquisition of the NMR spectra.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Anastas:, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem Soc Rev 2010, 39(1), 301–312. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford [England]; New York, 1998. [Google Scholar]
- Tan, D.; García, F. Main Group Mechanochemistry: From Curiosity to Established Protocols. Chem. Soc. Rev. 2019, 48(8), 2274–2292. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3(1), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Childs, S.; Rizvi, S.; Jones, W. The Role of Solvent in Mechanochemical and Sonochemical Cocrystal Formation: A Solubility-Based Approach for Predicting Cocrystallisation Outcome. CrystEngComm 2009, 11(3), 418–426. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.; Su, W. Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals. Adv. Synth. Catal. 2021, 363(5), 1246–1271. [Google Scholar] [CrossRef]
- Yuan, X.; Men, L.; Liu, Y.; Qiu, Y.; He, C.; Huang, W. Truxillic and Truxinic Acid Derivatives: Configuration, Source, and Bioactivities of Natural Cyclobutane Dimers. J. Holist. Integr. Pharm. 2020, 1(1), 48–69. [Google Scholar] [CrossRef]
- Yang, P.; Jia, Q.; Song, S.; Huang, X. [2 + 2]-Cycloaddition-Derived Cyclobutane Natural Products: Structural Diversity, Sources, Bioactivities, and Biomimetic Syntheses. Nat. Prod. Rep. 2023, 40(6), 1094–1129. [Google Scholar] [CrossRef] [PubMed]
- Takada, K. Synthesis of Biobased Functional Materials Using Photoactive Cinnamate Derivatives. Polym. J. 2023, 55(10), 1023–1033. [Google Scholar] [CrossRef]
- Portada, T.; Brkljača, Z. Counting the Truxillines—11 or More, the Question Is Now. Chirality 2024, 36(6), e23680. [Google Scholar] [CrossRef] [PubMed]
- Plachinski, E. F.; Kim, H. J.; Genzink, M. J.; Sanders, K. M.; Kelch, R. M.; Guzei, I. A.; Yoon, T. P. A General Synthetic Strategy toward the Truxillate Natural Products via Solid-State Photocycloadditions. J. Am. Chem. Soc. 2024, 146(22), 14948–14953. [Google Scholar] [CrossRef] [PubMed]
- Kole, G. K.; Vittal, J. J. Isomerization of Cyclobutane Ligands in the Solid State and Solution. J. Indian Chem. Soc. 2022, 99(9), 100630. [Google Scholar] [CrossRef]
- Bahena-Martínez, J. D.; Flores, R.; Domínguez-Mendoza, B. E.; Hernández-Vázquez, L. G.; Juaristi, E.; Escalante, J. Synthesis of β-Truxinate via [2+2] Cycloaddition of Methyl 4-Nitrocinnamate: Kinetic Study of Its Isomerization with DBU. ChemistrySelect 2024, 9(22), e202400178. [Google Scholar] [CrossRef]
- Kole, G. K.; Vittal, J. J. Isomerization of Cyclobutane Ligands in the Solid State and Solution. J. Indian Chem. Soc. 2022, 99(9), 100630. [Google Scholar] [CrossRef]
- Blanchette, M. A.; Choy, W.; Davis, J. T.; Essenfeld, A. P.; Masamune, S.; Roush, W. R.; Sakai, T. Horner-Wadsworth-Emmons Reaction: Use of Lithium Chloride and an Amine for Base-Sensitive Compounds. Tetrahedron Lett. 1984, 25(21), 2183–2186. [Google Scholar] [CrossRef]
- Chen, Y.; Marri, G.; Lin, W. The Integral Role of Conjugate Acids in Brønsted Base-Catalyzed Regiodivergent Synthesis. ChemCatChem 2024, e202400405. [Google Scholar] [CrossRef]
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 p K a Units: Unification of Different Basicity Scales. J. Org. Chem. 2005, 70(3), 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Gibson, T. J.; Mashuta, M. S.; Buchanan, R. M.; Grapperhaus, C. A. Copper Catalysed Aerobic Oxidation of Benzylic Alcohols in an Imidazole Containing N 4 Ligand Framework. Dalton Trans. 2016, 45(45), 18356–18364. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.; Leadbeater, N. Synthesis of Trans-Cinnamic Acid: Knoevenagel Condensation. In Clean, Fast Organic Chemistry; CEM Publishing: Matthews, NC, 2006; p. 101. [Google Scholar]
- Szymanski, W.; Wu, B.; Weiner, B.; De Wildeman, S.; Feringa, B. L.; Janssen, D. B. Phenylalanine Aminomutase-Catalyzed Addition of Ammonia to Substituted Cinnamic Acids: A Route to Enantiopure α- and β-Amino Acids. J. Org. Chem. 2009, 74(23), 9152–9157. [Google Scholar] [CrossRef] [PubMed]
- Oger, N.; Le Callonnec, F.; Jacquemin, D.; Fouquet, E.; Le Grognec, E.; Felpin, F. Heck–Matsuda Arylation of Olefins Through a Bicatalytic Approach: Improved Procedures and Rationalization. Adv. Synth. Catal. 2014, 356(5), 1065–1071. [Google Scholar] [CrossRef]
- Golfmann, M.; Glagow, L.; Giakoumidakis, A.; Golz, C.; Walker, J. C. L. Organophotocatalytic [2+2] Cycloaddition of Electron-Deficient Styrenes. Chem. – Eur. J. 2023, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).