1. Introduction
The gut-kidney axis reveals critical interactions between the gut microbiome and renal function that impact human health and disease. This bidirectional communication pathway plays a central role in kidney health by mediating inflammation, uremic toxicity, and metabolic processes [
1]. The gut microbiome, home to diverse microbes like short-chain fatty acid (SCFA)-producing bacteria from fiber fermentation, supports gut barrier integrity and reduces inflammation [
2]. However, dysbiosis—an imbalance in the gut microbiota commonly seen in chronic kidney disease (CKD)—can disrupt these beneficial functions, leading to systemic toxicity and increased kidney burden [
3].
In CKD, decreased kidney filtration restricts the excretion of uremic toxins, such as indoxyl sulfate and p-cresyl sulfate, leading to their accumulation in the bloodstream [
4]. These toxins cause oxidative stress and inflammation in kidney tissues, accelerating CKD progression. Furthermore, dysbiosis and constipation in CKD patients can result in excess harmful metabolites like ammonia and phenols, further stressing renal function and contributing to complications like cardiovascular disease [
5]. Increased gut permeability, often referred to as "leaky gut," is also associated with CKD, allowing endotoxins like lipopolysaccharides (LPS) to enter the bloodstream [
6]. These endotoxins induce systemic inflammation, worsening nephritis and further stressing the kidneys [
6]. Thus, the chronic inflammatory state seen in CKD patients is partly due to changes in the gut barrier and microbiota, emphasizing the role of the gut-kidney axis in disease progression.
Therapeutic strategies targeting the gut-kidney axis aim to restore microbial balance, reduce uremic toxins, and improve gut barrier function. Probiotics and prebiotics are increasingly studied for their potential to positively shift the gut microbiota, supporting beneficial bacteria and reducing harmful metabolites [
7,
8,
9]. Dietary interventions, particularly high-fiber diets, promote SCFA production, which can strengthen gut barrier integrity and reduce systemic inflammation [
10]. Treatments for constipation, fecal microbiota transplantation (FMT), and postbiotics (bioactive compounds produced by bacteria) are being investigated for their ability to correct dysbiosis and lower toxin loads in CKD patients [
3,
4,
5]. Although promising, further research is necessary to fully understand their long-term efficacy and safety. Additionally, studying the effects of specific microbial species and metabolites on kidney health could lead to precision therapies tailored to individual microbiomes.
In summary, the gut-kidney axis represents a promising frontier in understanding and managing CKD and other kidney diseases. Addressing gut microbiota imbalances, reducing uremic toxins, and strengthening the gut barrier may help slow disease progression and improve patient outcomes. As understanding of this axis deepens, innovative therapies targeting the gut microbiota may emerge to enhance kidney health. This review emphasizes the importance of an integrated approach to CKD management, focusing on the gut microbiota as a therapeutic target to reduce the disease burden and improve patients' quality of life.
2. Gut Microbiome Under Physiological Condition
2.1. Physiological Effects of Gut Microbiota
The gut microbiome consists of a variety of bacteria, fungi and viruses. Approximately 100 trillion bacteria inhabit the human intestines, forming the gut microbiota [
11]. Key members include
Firmicutes,
Bacteroides,
Ruminococcus and
Bifidobacterium [
12]. The gut microbiota exerts significant physiological effects on the human body, influencing metabolic, immune and neurobehavioral processes [
13]. This vast microbial community, mainly bacterial, exists symbiotically within the digestive tract, where its composition and diversity have profound implications for human health. These bacteria produce essential metabolites, such as SCFAs and vitamins, which regulate gut barrier integrity [
14]. Changes in microbiome composition, as seen in CKD, can substantially affect the kidneys, with pathogenic strains often outnumbering beneficial bacteria in CKD [
15].
2.2. Metabolic Effects and Integrity and Function of the Gut
The gut microbiota plays a crucial role in digestion and energy homeostasis [
16]. It can break down indigestible plant polysaccharides and resistant starch, thus facilitating the absorption of complex carbohydrates. In a healthy gut, probiotics continuously proliferate and smoothly synthesize vitamins [
17]. By breaking down dietary fiber and producing SCFAs, such as acetate, propionate and butyrate, the microbiota assists in nutrient absorption and energy extraction [
18]. Butyrate, in particular, serves as an energy source for colon cells, supporting gut health and maintaining the gut barrier [
19]. SCFAs also influence metabolic pathways like glucose regulation and lipid metabolism by acting on peripheral tissues through signaling pathways that affect insulin sensitivity and lipid processing [
20]. Dysbiosis (microbial imbalance) may contribute to metabolic disorders, including obesity, insulin resistance and type 2 diabetes [
21]. The gut microbiota also communicates bidirectionally with the brain, affecting neurobehavioral function via the gut-brain axis. Microbial metabolites such as SCFAs and neurotransmitter precursors (e.g., serotonin) influence mood, stress response and cognitive function by modulating the vagus nerve, immune pathways and hormonal signaling [
22]. For example, SCFAs can cross the blood-brain barrier, impacting brain function and behavior [
23]. Emerging research highlights correlations between gut microbiota imbalance and neuropsychiatric disorders like depression, anxiety and autism spectrum disorders, suggesting potential mental health benefits in restoring a healthy microbiome [
24]. Additionally, the gut microbiota aids in maintaining gut barrier integrity by promoting tight junction proteins that prevent pathogen invasion. Microbe-derived SCFAs, especially butyrate, strengthen the gut barrier and reduce inflammation, protecting against pathogens and lowering the risk of systemic infections [
25]. Dysbiosis weakens this barrier, increasing gut permeability and causing a condition known as “leaky gut,” where microbial endotoxins enter the bloodstream [
26], potentially leading to systemic inflammation associated with chronic diseases such as cardiovascular disease and type 2 diabetes.
2.3. Immunological Effects
The gut microbiota is central to immune system development, helping distinguish between beneficial and harmful microbes [
27]. Early exposure to microbes influences immune tolerance and shapes immune responses. Specific bacterial species, such as those in the
Bacteroides and
Lactobacillus genera, promote the generation of regulatory T cells (Tregs), which play a role in controlling inflammation and preventing autoimmune diseases [
28,
29]. Furthermore, the microbiota interacts with gut-associated lymphoid tissue (GALT), contributing to the secretion of immunoglobulin A (IgA), which protects against pathogens [
30]. Dysbiosis in the gut microbiota is associated with chronic inflammatory diseases, including inflammatory bowel disease (IBD), rheumatoid arthritis, and allergies [
31,
32].
3. Gut Microbiome in Kidney diseases: Dysbiosis in CKD, Microbial Metabolites and Toxins
3.1. Dysbiosis in CKD
In CKD, dysbiosis is characterized by a decrease in beneficial bacteria such as
Lactobacillus,
Prevotella and
Bifidobacteria, alongside an increase in pathogenic or opportunistic bacteria, including
Proteobacteria and
Enterococcus [
5,
33,
34,
35,
36,
37,
38]. The mechanisms underlying dysbiosis in CKD are not fully understood; however, several factors are implicated. These include the accumulation of uremic toxins due to renal failure progression, metabolic acidosis, the effects of chelating agents such as oral iron supplements, potassium and phosphorus used in CKD treatment, as well as impaired intestinal function, reduced dietary fiber intake, and prolonged intestinal transit time often due to constipation frequently seen in CKD patients [
5,
39]. Such changes in the gut microbiota are influenced by dietary factors (e.g., reduced fiber intake), medications and the uremic environment itself [
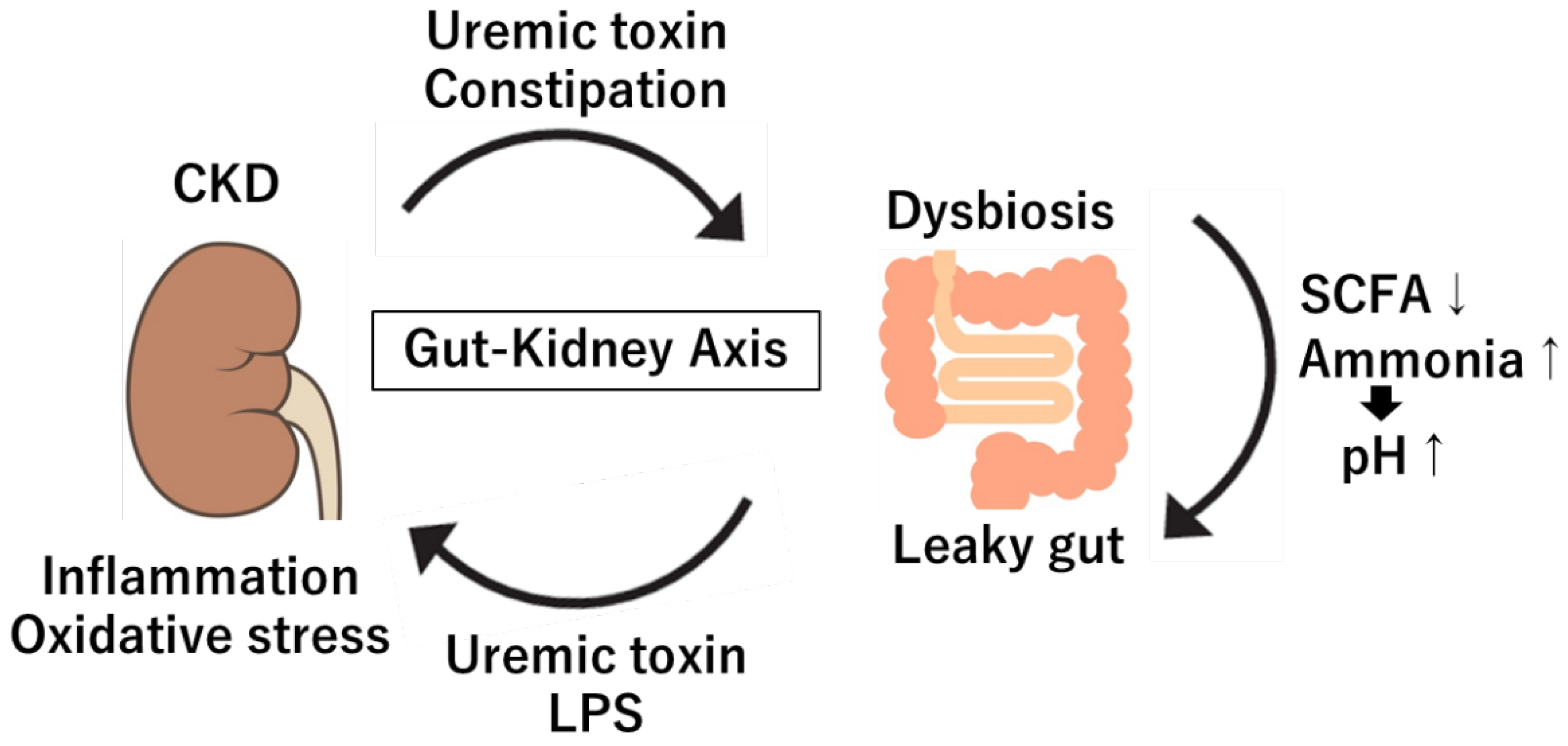
39]. As renal function declines, uremic toxins such as urea accumulate in the bloodstream, potentially infiltrating the gut and compromising the gut barrier [
40]. This disruption allows harmful metabolites to enter systemic circulation, triggering systemic inflammation and accelerating renal damage [
40] (
Figure 1). Additionally, CKD patients commonly exhibit "leaky gut" conditions due to increased intestinal permeability, which is exacerbated by uremia, intestinal edema and ischemic changes in the gut [
6]. This is associated with reduced expression of tight junction proteins in the intestinal epithelium (ZO-1, claudin, and occludin), allowing endotoxins like LPS to migrate into the bloodstream [
41,
42]. Endotoxemia subsequently provokes immune responses, stimulating pro-inflammatory cytokines (such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)) and perpetuating a chronic inflammatory state that may further degrade renal function [
41,
42]. Indeed, CKD patients show higher levels of circulating endotoxins than healthy individuals, suggesting compromised gut barrier function [
43]. The leaky gut phenomenon in CKD perpetuates a feedback loop where inflammation further disturbs the gut microbiome, creating a vicious cycle that exacerbates kidney disease progression.
3.2. Microbial Metabolites and Toxins
Gut-derived metabolites can have both beneficial and harmful effects on kidney health. SCFAs, for instance, help maintain the gut barrier function [
44]. On the other hand, toxins such as ammonia and phenols—produced during protein fermentation by urease-producing gut bacteria—promote systemic inflammation and damage to the kidneys [
45]. This metabolic burden creates a cycle of toxicity, as impaired kidneys reduce toxin clearance, intensifying renal and systemic injury. Harmful substances like ammonia, amines, thiols, phenols and indoles are produced by proteolytic gut bacteria such as
Bacteroides and
Clostridium, which proliferate in renal failure conditions [
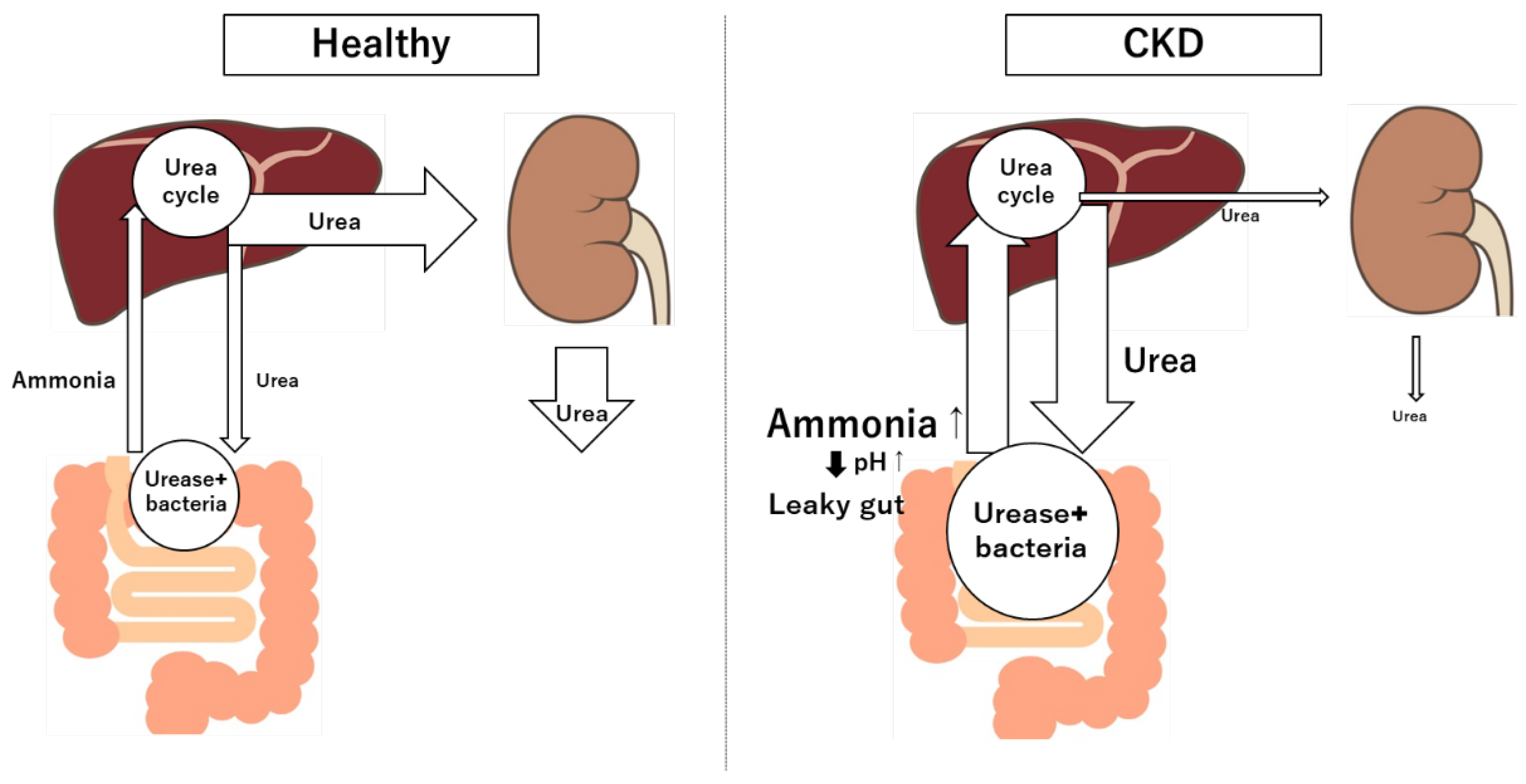
46]. Ammonia and urea are byproducts of protein catabolism, and the urea is converted back into ammonia by gut bacteria in the colon via urease [
47] (
Figure 2). In CKD, the buildup of urea in the blood allows it to pass into the gastrointestinal tract, where gut bacteria convert it to ammonia through urease activity [
48]. Elevated ammonia levels disrupt the local environment by raising pH, which can weaken the gut barrier and lead to epithelial cell damage [
48]. Ammonia has been found to affect the integrity of tight junction proteins like ZO-1 and occludin in the intestinal lining [
49]. This disruption in tight junctions compromises the gut barrier, potentially leading to a "leaky gut" state. Additionally, epithelial injury from high ammonia may trigger a cascade of immune responses, further increasing inflammatory cytokine production (e.g., IL-6, TNF-α) and aggravating chronic inflammation associated with CKD. Furthermore, altered gut environment due to ammonia may promote the growth of urease-producing, proteolytic bacteria, which produce additional toxins harmful to the kidneys and other tissues. This can create a vicious cycle where ammonia not only directly damages intestinal epithelial cells, but also promotes systemic inflammation that exacerbates kidney disease progression. Uremic toxins like indoxyl sulfate, p-cresyl sulfate and trimethylamine N-oxide (TMAO), which significantly contribute to CKD progression and vascular complications, are derived from dietary components and metabolized by gut bacteria [
50,
51,
52]. Protein fermentation in the gut generates various metabolites that greatly impact kidney health [
53]. A protein-rich diet and uremic conditions can lead to the overgrowth of protein-fermenting bacteria, producing toxic byproducts like indoxyl sulfate, p-cresyl sulfate,and ammonia [
54,
55]. These protein fermentation metabolites contribute to systemic toxicity, promoting the progression of CKD and associated cardiovascular complications.
3.2.1. Indoxyl Sulfate
Indoxyl sulfate originates from bacterial metabolism of tryptophan, an amino acid in protein-rich foods [
56]. After being absorbed, it undergoes hepatic modification and becomes indoxyl sulfate, which is excreted by the kidneys [
50]. In CKD, reduced kidney clearance allows indoxyl sulfate to accumulate in the bloodstream, acting as a uremic toxin. It promotes oxidative stress and inflammation in kidney tissues by stimulating reactive oxygen species (ROS) production and enhancing pro-fibrotic gene expression, accelerating tubular injury and interstitial fibrosis, both of which contribute to CKD progression [
50]. Research indicates that indoxyl sulfate also affects cardiovascular health, posing a greater risk to CKD patients who are already prone to cardiovascular complications [
57].
3.2.2. p-Cresyl Sulfate
Similar to indoxyl sulfate, p-cresyl sulfate is a gut bacteria-derived metabolite from amino acids, specifically tyrosine and phenylalanine [
56]. After undergoing sulfation in the liver, it circulates in the bloodstream, accumulating when renal function is impaired. p-Cresyl sulfate exerts pro-inflammatory effects on both the kidneys and vascular system, contributing to endothelial dysfunction and promoting atherosclerosis [
50,
58]. In kidney tissues, it induces oxidative stress and inflammation, impairing kidney tubular cells and accelerating disease progression [
58]. Elevated p-cresyl sulfate levels correlate with higher mortality rates in CKD patients [
59], underscoring its role as a significant uremic toxin.
3.2.3. Trimethylamine-N-oxide (TMAO)
TMAO is produced from dietary sources, especially from choline and carnitine intake found in red meat and eggs [
51,
60]. Gut bacteria convert these compounds into trimethylamine (TMA), which is then oxidized in the liver to form TMAO [
51,
60]. Elevated TMAO levels are associated with increased cardiovascular risk, a leading cause of death among CKD patients [
61]. TMAO has been shown to promote vascular inflammation, enhance platelet aggregation, and impair lipid metabolism, further exacerbating cardiovascular complications [
51]. In the context of CKD, TMAO’s cardiovascular impact adds an additional layer of risk, as CKD patients often face both kidney and heart issues. TMAO may also worsen renal damage by influencing inflammatory pathways [
61], though more research is needed to fully understand its role in CKD.
4. Mechanisms of Interaction Between Gut and Kidney
4.1. Systemic Inflammation and Immune Activation
CKD is associated with systemic inflammation, with gut dysbiosis playing a significant role in this process. A decrease in beneficial bacteria and an overgrowth of pathogenic bacteria increase exposure to endotoxins and other harmful molecules, which activate the immune system [
4,
5]. Specifically, harmful bacteria produce metabolites such as LPS that cross the intestinal barrier due to increased permeability [
6], the "leaky gut." When LPS enters the circulation, it stimulates immune cells to produce pro-inflammatory cytokines like IL-6 and TNF-α, which are known to contribute to CKD progression and vascular inflammation [
5,
6]. This immune activation not only impacts renal function, but also raises the risk of cardiovascular disease, as inflammatory mediators damage blood vessels and promote plaque formation [
5]. Cytokine-induced inflammation directly harms kidney tissue, exacerbating symptoms like glomerulosclerosis and fibrosis, which progressively deteriorate renal function over time.
4.2. Endotoxemia and Kidney Inflammation and Oxidative Stress:
The presence of endotoxins like LPS in the bloodstream is a key factor in kidney inflammation [
62,
63]. In CKD, the gut barrier is often compromised by both gut dysbiosis and uremic toxins, increasing intestinal permeability [
48]. As LPS and other microbial products leak into the bloodstream, they trigger an inflammatory response that has significant effects on renal function [
41,
42]. When endotoxins reach the kidneys, they activate Toll-like receptors (TLRs) on renal cells, initiating a cascade of pro-inflammatory and oxidative stress responses [
64]. These include upregulation of cytokines and chemokines, leading to increased immune cell infiltration in kidney tissue. This process is further aggravated by oxidative stress, a phenomenon where excess ROS damage cellular components and intensify inflammation [
64]. Oxidative stress, partially induced by gut-derived toxins like indoxyl sulfate, can directly harm renal tubular cells, promoting fibrosis and impairing renal function [
65]. The combined effects of endotoxemia and oxidative stress establish a harmful feedback loop, wherein chronic inflammation and oxidative stress gradually worsen renal function, exacerbating the clinical symptoms of CKD.
4.3. Dietary Carbohydrates Fermentation
The fermentation of dietary carbohydrates in the gut primarily produces SCFAs such as acetate, propionate and butyrate, which play a protective role in kidney health. SCFAs are known to have anti-inflammatory effects and help maintain intestinal barrier integrity [
44]. In CKD, however, gut dysbiosis reduces SCFA-producing bacteria (Bifidobacterium, Lactobacillus), weakening this protective effect [
66]. The decreased intake of dietary fiber often observed in CKD also reduces SCFA production, potentially allowing pathogenic bacteria to proliferate [
67]. Reduced SCFA levels weaken the gut barrier, increasing permeability and endotoxemia, which subsequently heighten inflammation and oxidative stress in kidney tissue [
68,
69]. This breakdown of tight junctions is associated with endotoxemia from gut-derived sources, elevated blood CRP levels, and increased mortality rates, with gut microbiome-derived DNA from species like Klebsiella spp., Proteus spp., Escherichia spp., Enterobacter spp. and Pseudomonas spp. detected in the bloodstream of approximately 20% of end stage renal disease (ESRD) patients [
70,
71]. Furthermore, low SCFA levels impair inflammation regulation, worsening immune activation [
68]. This pro-inflammatory state, exacerbated by endotoxemia and oxidative stress, contributes to CKD progression.
4.4. Advanced Glycation Products
Advanced glycation end products (AGEs) are compounds formed through the reaction of sugars with proteins or lipids, a process that can occur endogenously or through dietary intake, especially from high-temperature cooking methods like frying or grilling [
72]. AGEs accumulate in CKD due to reduced renal clearance, and they can also be absorbed from certain foods, especially processed foods [
72]. AGEs can contribute to renal damage through multiple pathways [
73]. When AGEs bind to their receptor (RAGE), they activate pro-inflammatory pathways and promote oxidative stress, exacerbating renal inflammation and fibrosis [
72,
73]. This process damages endothelial cells, leading to vascular stiffness and increased blood pressure, which are additional stressors on the kidneys [
73]. Additionally, AGEs contribute to glomerular sclerosis, one of the primary pathological features of CKD [
72,
74]. Dietary intake of AGEs further compounds this issue. High-AGE diets have been shown to elevate systemic levels of AGEs [
72,
75], leading to more significant kidney injury in CKD patients. Reducing dietary AGE intake, along with minimizing the production of endogenous AGEs, has potential therapeutic value in managing CKD progression.
4.5. Gut-Kidney Axis
The gut-kidney axis involves complex interactions mediated by systemic inflammation, endotoxemia, oxidative stress and metabolites derived from protein and carbohydrate fermentation, as well as dietary AGEs [
3,
4,
5,
6]. Each of these pathways contributes to a cycle of inflammation, immune activation and oxidative stress, creating a damaging feedback loop that accelerates CKD progression. Addressing these mechanisms, potentially through dietary interventions, prebiotics, and therapeutics targeting gut-derived toxins, may offer new pathways to slow kidney disease progression and improve patient outcomes. This comprehensive understanding of the gut-kidney axis highlights the importance of gut microbiota management in CKD and underscores the need for future research focused on targeted treatments to interrupt these pathological processes. The gut-kidney axis is part of a larger network, sometimes called the gut-kidney-cardiovascular (heart) axis, due to the overlapping effects that dysbiosis and gut-derived toxins have on both kidney and cardiovascular health [
5]. The gut-kidney axis significantly impacts cardiovascular disease associated with CKD. Dysbiosis and uremic toxins in the gut can contribute to increased rates of atherosclerosis, hypertension, and heart disease in CKD patients [
5]. The accumulation of indoxyl sulfate, p-cresyl sulfate and TMAO contributes to a pro-inflammatory and pro-oxidative state that aggravates kidney and cardiovascular diseases. This relationship highlights the importance of managing kidney health and cardiovascular risk factors through a gut-focused approach.
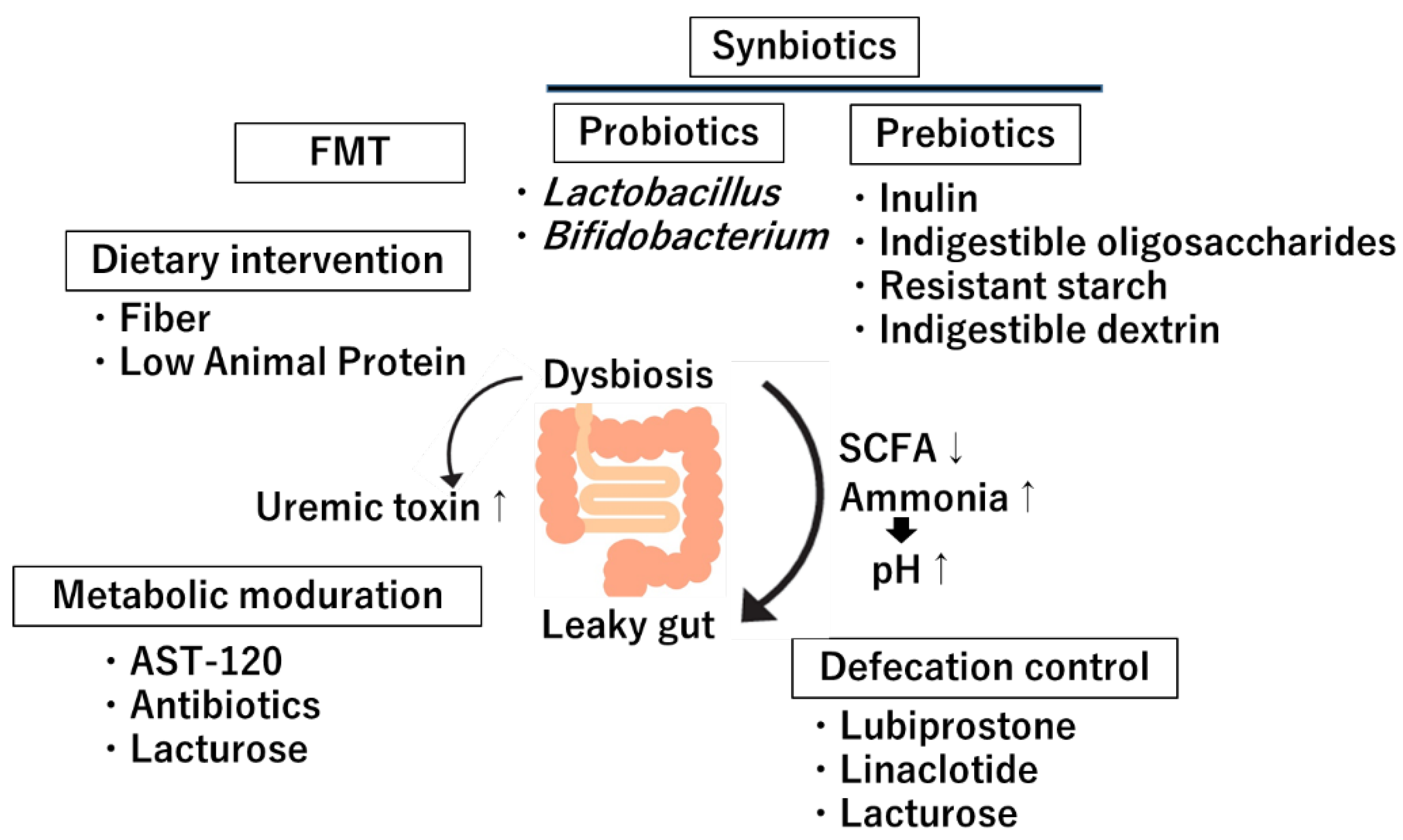
5. Clinical Implications and Therapeutic Approaches
The gut-kidney axis has emerged as a significant area of study due to the growing understanding of the microbiota's role in influencing kidney health. Interventions targeting this axis are increasingly considered for CKD management. These strategies encompass dietary interventions, probiotics and prebiotics, FMT, metabolite modulation and enhancing defecation (
Figure 3).
5.1. Dietary Intervention
Dietary interventions are foundational for CKD management, as diet directly influences the composition and metabolic activity of the gut microbiota [
76]. A diet high in fiber and low in animal protein promotes the growth of beneficial bacteria that produce SCFAs [
76,
77], which are associated with reduced production of uremic toxins. SCFAs like butyrate have anti-inflammatory properties that help strengthen the gut barrier and reduce gut-derived inflammation impacting the kidneys [
2]. Conversely, diets high in red or processed meats increase the production of indoxyl sulfate and p-cresyl sulfate, which are metabolites linked to renal damage [
78,
79]. Plant-based and Mediterranean diets are particularly beneficial as they supply prebiotic fibers that promote beneficial bacteria, lower uremic toxin levels, and enhance overall gut and kidney health [
80]. Regular inclusion of these dietary modifications can reduce systemic inflammation and oxidative stress, potentially slowing CKD progression. Dietary fiber alleviated the gut microbiota, elevating the level of Bacteroides acidifaciens [
81]. A high-fiber diet not only restores the gut microbiota and metabolome in plasma, cecum and urine but may also slow CKD progression [
82]. These effects may be mediated by restoration of tight junctions in the gut epithelium, attenuation of oxidative stress, and reduction in inflammation and fibrosis partly via increase in SCFA-producing bacteria [
83,
84].
5.2. Probiotics
Probiotics, often referred to as "good bacteria," are live microorganisms that offer health benefits by improving the balance of the gut microbiota [
85]. Common strains include
Lactobacillus and
Bifidobacterium, both known to support gut health and alleviate dysbiosis—a microbial imbalance frequently observed in CKD patients [
86]. Probiotics help by competing with harmful bacteria for resources, reducing toxin levels, and promoting gut barrier integrity [
87,
88]. Studies have demonstrated that probiotic supplementation in CKD patients can reduce levels of uremic toxins, potentially slowing renal function deterioration. [
89,
90,
91,
92,
93,
94] For instance, supplementation with
Lactobacillus acidophilus and
Bifidobacterium has been associated with decreased inflammatory markers in CKD, indicating benefits beyond the gastrointestinal tract [
95]. Probiotics may also modulate immune responses, influencing cytokine production (e.g., reducing pro-inflammatory cytokines like IL-6 and TNF-α), which lowers systemic inflammation and benefits overall kidney health [
87,
88]. Additionally, using probiotics in CKD may have cardiovascular benefits, a crucial consideration since CKD often coexists with cardiovascular issues [
85,
96]. However, the results are varied, and further research is needed to determine the optimal strains, dosages, and frequency of administration for renal protection in CKD.
5.3. Prebiotics
Prebiotics are indigestible food components that stimulate the growth and activity of beneficial bacteria in the gut [
97]. Examples of prebiotics include fibers like inulin, fructo-oligosaccharides, resistant starch, indigestible dextrin (such as resistant dextrin) and galacto-oligosaccharides, which ferment in the gut to produce SCFAs such as butyrate, acetate and propionate [
98,
99,
100]. As described, these SCFAs play a crucial role in maintaining gut barrier function by promoting immune tolerance and reducing systemic inflammation. In the context of CKD, where gut permeability is often compromised, prebiotics help strengthen the gut barrier and reduce the translocation of endotoxins into the bloodstream, which can exacerbate kidney inflammation [
89,
99,
100]. Prebiotic supplementation has shown effectiveness in CKD by reducing gut-derived uremic toxins [
101,
102]. By promoting the growth of SCFA-producing bacteria, prebiotics indirectly lower levels of indoxyl sulfate and p-cresyl sulfate. SCFAs also support kidney health by inhibiting pro-inflammatory pathways and improving glucose and lipid metabolism, both essential for managing the metabolic disorders associated with CKD.
Clinical studies evaluating prebiotics in CKD patients show promising results in improving gut microbiota composition and reducing inflammatory markers and uremic toxins [
98,
99,
100,
101,
102]. For example, inulin-type prebiotics are associated with increased levels of beneficial
Bifidobacterium and
Faecalibacterium prausnitzii [
103], known for their anti-inflammatory effects. Galacto-oligosaccharides have been found to increase beneficial gut bacteria while reducing pathogenic bacteria [
104,
105]. Combining probiotics and prebiotics as synbiotics (a strategy that utilizes both in parallel) may have even greater potential in modulating the gut microbiota and slowing kidney disease progression [
99,
100]. Synbiotic formulations are thought to enhance the survival and efficacy of probiotics by providing a nutritional source (prebiotics), more effectively promoting colonization in the gut. In summary, probiotics and prebiotics represent promising, non-invasive interventions for CKD management. They offer potential benefits through gut microbiota modulation, reduction of uremic toxin levels, and improved gut barrier integrity. However, further research is necessary to refine dosage guidelines, optimize strain selection, and understand the long-term impacts on CKD progression and patient outcomes.
5.4. Fecal Microbiota Transplantation (FMT)
FMT involves transferring fecal material from a healthy donor to a recipient, effectively replacing a dysbiotic gut microbiome with a balanced one [
106]. Although still in experimental stages for CKD, FMT has shown promising results in inflammatory and metabolic disorders [
107,
108], suggesting potential benefits in kidney disease. The rationale is that FMT can restore a healthy microbiota composition, reducing endotoxemia and systemic inflammation—key contributors to CKD [
109,
110]. In preclinical studies, FMT has been shown to reduce renal damage and inflammation by normalizing gut barrier integrity and reducing levels of pro-inflammatory metabolites [
111]. However, FMT carries risks, such as the transmission of infections or unforeseen immune reactions, so further research is required to assess safety, efficacy, and the best protocols for its application in CKD [
5]. Nonetheless, as a therapeutic tool, FMT represents an innovative approach with the potential to significantly modify the gut-kidney axis and alleviate CKD symptoms.
5.5. Metabolites Modulation
The modulation of gut-derived metabolites, particularly uremic toxins, is an important approach to addressing gut-kidney axis dysfunction. Targeting specific toxins like indoxyl sulfate and p-cresyl sulfate can mitigate adverse effects on kidney health. This can be achieved through dietary interventions, prebiotics and adsorbents that capture and reduce the absorption of harmful metabolites. Pharmaceutical interventions are also promising. For example, AST-120 is an oral adsorbent that binds indole and other indoxyl sulfate precursors in the gut, reducing systemic levels and renal impact [
112]. Although AST-120 is not widely accepted in all medical guidelines, randomized controlled trials in Japan involving pre-dialysis CKD patients (CAP-KD trial) demonstrated a significantly improved rate of renal function decline with AST-120 administration [
113,
114]. Its effectiveness in slowing CKD progression by reducing indoxyl sulfate levels has been validated. However, a large-scale study in Western countries (the EPPIC trial) found no significant CKD progression delay with AST-120 compared to a placebo [
115], potentially due to adherence issues and adverse impacts on the gut environment. Further research may identify other compounds or pharmacologic agents capable of modulating gut-derived toxins and providing additional therapeutic options for kidney disease patients. Recent studies suggest that administering the oral antibiotic vancomycin to remove gut microbiota significantly reduced plasma concentrations of indoxyl sulfate and p-cresyl sulfate, two gut-derived uremic solutes, in ESRD patients [
116]. On the other hand, germ-free mice with ischemia-reperfusion (I/R)-induced acute kidney injury (AKI) and CKD models demonstrated more severe renal damage than mice with a symbiotic microbiota [
117], possibly due to the beneficial effects of SCFAs. A sodium glucose co-transporter (SGLT)-2 inhibitor, Canagliflozin, in renal failure mice, changed the gut microbiota composition and reduced gut-derived uremic toxins by increasing glucose delivery to the distal intestine through SGLT-1 inhibition [
118].
5.6. Defecation Modulation
Regular bowel movements are crucial for gut-kidney health, as they affect the retention of gut-derived toxins [
119]. CKD patients, particularly those on maintenance dialysis, experience a high prevalence of constipation [
119,
120]. Mechanisms contributing to constipation include impaired blood flow to the intestines, neurological issues affecting intestinal peristalsis, side effects of certain medications (e.g., ion-exchange resins or oral iron supplements), dietary restrictions (e.g., potassium restrictions reducing fiber intake) and changes in the gut microbiota [
119,
121]. Constipation in CKD can prolong colonic transit time, increasing the retention of intestinal contents, promoting putrefaction reactions, and potentially worsening CKD. An epidemiological study in non-CKD patients (eGFR > 60 ml/min/1.73 m²) indicated that those with constipation had a higher rate of progression to CKD and increased risk of ESRD, compared to those without constipation [
39]. Therefore, bowel regulation interventions, such as fiber supplementation, laxatives and stool softeners, may serve as an effective, indirect approach to reducing gut-kidney axis dysfunction. Increased intake of dietary fiber and water encourages regular bowel movements, allowing uremic toxins to be excreted before entering systemic circulation [
122]. Among laxatives, lubiprostone activates chloride channels in intestinal epithelial cells, increasing intestinal fluid secretion and promoting content movement, providing a laxative effect [
123]. Studies in renal failure mice show that lubiprostone improves the altered gut environment associated with renal failure, reduces the accumulation of gut-derived blood uremic toxins, and slows CKD progression [
36]. It has also been shown to increase bacteria typically reduced in CKD, such as
Lactobacillus and
Prevotella species. Linaclotide has shown effects in renal failure mice, reducing TMAO levels and alleviating renal damage and myocardial fibrosis [
124].
Furthermore, drugs like lactulose and polyethylene glycol, designed to enhance motility and shorten intestinal transit time, may aid CKD patients in maintaining a regular bowel schedule, thus reducing the risk of endotoxemia. Lactulose also demonstrated a CKD progression-slowing effect in renal failure mice [
125]. Importantly, lactulose may act not only as defecation modulation, but also as prebiotic and metabolic modulation. As a prebiotic, lactulose resists digestion in the upper gastrointestinal tract, reaching the colon where it is fermented by beneficial bacteria, such as
Bifidobacteria and
Lactobacillus [
126,
127], which outcompete ammonia-producing bacteria and enhance SCFA production. Furthermore, as a modulation of ammonia metabolism, SCFAs acidify the gut lumen, leading to an increase in NH4+ ions and a decrease in freely absorbable NH3 [
128]. These modulation of ammonia metabolism may reduce serum ammonia levels and help limit the accumulation of uremic toxins that are toxic to the kidneys. Indeed, lactulose significantly decreased urea levels in the clinical prospective study [
129]. In addition, a randomized clinical trial revealed that lactulose increased
Bifidobacteria and
Lactobacillus, and decreased serum creatinine levels, suggesting the renoprotective effect presumably via modulation of microbiome [
130].
Each of these therapeutic approaches—dietary interventions, probiotics and prebiotics, FMT, metabolite modulation and bowel movement regulation—shows potential in addressing CKD via the gut-kidney axis. Together, they represent a multifaceted approach to managing CKD progression, reducing inflammation, systemic toxicity and oxidative stress. Further research is essential for specific interventions, especially FMT and metabolite-focused treatments, but the gut-kidney axis remains a promising therapeutic target in CKD management. These strategies collectively emphasize the potential of modulating gut health to improve kidney outcomes, a potential that continues to expand with advances in microbiome and nephrology research.
6. Conclusions
There remain gaps in understanding species-specific microbiota roles and causative mechanisms within the gut-kidney axis. Further research on microbial signaling pathways, the role of individual microbial species and longitudinal studies tracking microbiome changes are crucial to refining therapeutic strategies. Next-generation probiotics, designed to target specific bacterial strains and precision microbiome-modulation techniques, represent future treatment avenues. Individualized therapies based on a patient's unique microbiome profile may optimize gut-kidney health by reducing toxin levels and reinforcing the gut barrier.
In summary, the gut-kidney axis represents a promising target for managing CKD and related complications. By addressing gut dysbiosis, reducing uremic toxins, and improving gut barrier integrity, therapeutic interventions aimed at the microbiome hold potential to slow CKD progression and enhance patient outcomes. Continued research is essential for developing targeted, microbiome-based treatments that could transform kidney disease management.
Author Contributions
Conceptualization, K.T.; formal analysis, K.T., H.N. and K.F.; investigation, K.T., H.N. and K.F.; writing—original draft preparation, K.T.; writing—review and editing, K.T., H.N., K.F. S.K. and J.W.; supervision, J.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japanese Society for the Promotion of Science (JSPS)/Grant-in-Aid for Young Scientists (24K11411, to K.Ts.). The funder had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
There are no datasets generated during and/or analyzed during the current study.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut-Kidney Axis in Health and Disease. Front Med (Lausanne) 2020, 7, 620102. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhu, H.; Yao, Y.; Zeng, R. Gut Dysbiosis and Kidney Diseases. Front Med (Lausanne) 2022, 9, 829349. [Google Scholar] [CrossRef] [PubMed]
- Lehto, M.; Groop, P.H. The Gut-Kidney Axis: Putative Interconnections Between Gastrointestinal and Renal Disorders. Front Endocrinol (Lausanne) 2018, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Kovesdy, C.P. The gut-kidney-heart axis in chronic kidney disease. Physiol Int 2019, 106, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front Pharmacol 2022, 13, 837500. [Google Scholar] [CrossRef]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Barengolts, E. Gut Microbiota, Prebiotics, Probiotics, and Synbiotics in Management of Obesity and Prediabetes: Review of Randomized Controlled Trials. Endocr Pract 2016, 22, 1224–1234. [Google Scholar] [CrossRef]
- Lazaro, A.; Vila-Donat, P.; Manyes, L. Emerging mycotoxins and preventive strategies related to gut microbiota changes: Probiotics, prebiotics, and postbiotics—a systematic review. Food Funct 2024, 15, 8998–9023. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct Target Ther 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Mlynarska, E.; Budny, E.; Saar, M.; Wojtanowska, E.; Jankowska, J.; Marciszuk, S.; Mazur, M.; Rysz, J.; Franczyk, B. Does the Composition of Gut Microbiota Affect Chronic Kidney Disease? Molecular Mechanisms Contributed to Decreasing Glomerular Filtration Rate. Int J Mol Sci 2024, 25, 10429. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front Endocrinol (Lausanne) 2019, 10, 9. [Google Scholar] [CrossRef]
- Nysten, J.; Van Dijck, P. Can we microbe-manage our vitamin acquisition for better health? PLoS Pathog 2023, 19, e1011361. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate's role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin Nutr 2023, 42, 61–75. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol Sci 2020, 21, 6356. [Google Scholar] [CrossRef]
- Hamjane, N.; Mechita, M.B.; Nourouti, N.G.; Barakat, A. Gut microbiota dysbiosis -associated obesity and its involvement in cardiovascular diseases and type 2 diabetes. A systematic review. Microvasc Res 2024, 151, 104601. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int J Mol Sci 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- O'Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fulling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota-brain axis in neurological disorder. Front Neurosci 2023, 17, 1225875. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int J Mol Sci 2021, 22, 6729. [Google Scholar] [CrossRef]
- Liang, L.; Saunders, C.; Sanossian, N. Food, gut barrier dysfunction, and related diseases: A new target for future individualized disease prevention and management. Food Sci Nutr 2023, 11, 1671–1704. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed Pharmacother 2023, 164, 114985. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: The importance of being specific. Mucosal Immunol 2020, 13, 12–21. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, J.; Zhang, W.; Doherty, M.; Zhang, Y.; Xie, H.; Li, W.; Wang, N.; Lei, G.; Zeng, C. Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies. EBioMedicine 2022, 80, 104055. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, D.; Saadah, O.; Mosli, M.; Edris, S.; Alhindi, R.; Bahieldin, A. Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosn J Basic Med Sci 2021, 21, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Tajima, T.; Hasegawa, K.; Kanda, T.; Tokuyama, H.; Hayashi, K.; Itoh, H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol Dial Transplant 2016, 31, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Mishima, E.; Fukuda, S.; Shima, H.; Hirayama, A.; Akiyama, Y.; Takeuchi, Y.; Fukuda, N.N.; Suzuki, T.; Suzuki, C.; Yuri, A.; et al. Alteration of the Intestinal Environment by Lubiprostone Is Associated with Amelioration of Adenine-Induced CKD. J Am Soc Nephrol 2015, 26, 1787–1794. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, H.; Tang, Y.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; Xu, D.; et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis 2019, 284, 121–128. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Wang, S.; Xiong, J.; Chen, Y.; Liu, Y.; Xiao, T.; Li, Y.; He, T.; Li, Y.; et al. Indoxyl sulfate induces intestinal barrier injury through IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics 2020, 10, 7384–7400. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Incident CKD. J Am Soc Nephrol 2017, 28, 1248–1258. [Google Scholar] [CrossRef]
- Niwa, T. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci 2010, 72, 1–11. [Google Scholar]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat Rev Gastroenterol Hepatol 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Harrison, L.E.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 2011, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanave, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-derived short chain fatty acids in pediatric health and diseases: From gut development to neuroprotection. Front Microbiol 2024, 15, 1456793. [Google Scholar] [CrossRef]
- Li, F.; Wang, M.; Wang, J.; Li, R.; Zhang, Y. Alterations to the Gut Microbiota and Their Correlation With Inflammatory Factors in Chronic Kidney Disease. Front Cell Infect Microbiol 2019, 9, 206. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl Res 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Shen, T.C.; Albenberg, L.; Bittinger, K.; Chehoud, C.; Chen, Y.Y.; Judge, C.A.; Chau, L.; Ni, J.; Sheng, M.; Lin, A.; et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 2015, 125, 2841–2850. [Google Scholar] [CrossRef]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol 2019, 316, F1211–F1217. [Google Scholar] [CrossRef]
- Yokoo, K.; Yamamoto, Y.; Suzuki, T. Ammonia impairs tight junction barriers by inducing mitochondrial dysfunction in Caco-2 cells. FASEB J 2021, 35, e21854. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J Am Soc Nephrol 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, D.; Duan, S.; Chen, X. The role of gut-dependent molecule trimethylamine N-oxide as a novel target for the treatment of chronic kidney disease. Int Urol Nephrol 2023, 55, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.G.; Wang, C.H.; Lin, Y.L.; Lai, Y.H.; Tsai, J.P. Serum Trimethylamine N-Oxide Level Is Associated with Peripheral Arterial Stiffness in Advanced Non-Dialysis Chronic Kidney Disease Patients. Toxins (Basel) 2022, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; Farre, R.; Evenepoel, P.; Overbeek, S.A.; Meijers, B. Food-Derived Uremic Toxins in Chronic Kidney Disease. Toxins (Basel) 2023, 15, 116. [Google Scholar] [CrossRef]
- Jackson, R.; Yao, T.; Bulut, N.; Cantu-Jungles, T.M.; Hamaker, B.R. Protein combined with certain dietary fibers increases butyrate production in gut microbiota fermentation. Food Funct 2024, 15, 3186–3198. [Google Scholar] [CrossRef]
- Sun, C.Y.; Li, J.R.; Wang, Y.Y.; Lin, S.Y.; Ou, Y.C.; Lin, C.J.; Wang, J.D.; Liao, S.L.; Chen, C.J. Indoxyl sulfate caused behavioral abnormality and neurodegeneration in mice with unilateral nephrectomy. Aging (Albany NY) 2021, 13, 6681–6701. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins (Basel) 2016, 8, 358. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins (Basel) 2017, 9, 52. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J Immunol Res 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, J.; Liu, Y.; Dong, Z.; Liu, H.; Liu, Y.; Zhou, X.; Liu, F.; Chen, G. Lipopolysaccharide Induces Chronic Kidney Injury and Fibrosis through Activation of mTOR Signaling in Macrophages. Am J Nephrol 2015, 42, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Cheng, T.H.; Ma, M.C.; Liao, M.T.; Zheng, C.M.; Lu, K.C.; Liao, C.H.; Hou, Y.C.; Liu, W.C.; Lu, C.L. Indoxyl Sulfate, a Tubular Toxin, Contributes to the Development of Chronic Kidney Disease. Toxins (Basel) 2020, 12, 684. [Google Scholar] [CrossRef]
- Huang, H.W.; Chen, M.J. Exploring the Preventive and Therapeutic Mechanisms of Probiotics in Chronic Kidney Disease through the Gut-Kidney Axis. J Agric Food Chem 2024, 72, 8347–8364. [Google Scholar] [CrossRef]
- Cigarran Guldris, S.; Latorre Catala, J.A.; Sanjurjo Amado, A.; Menendez Granados, N.; Pineiro Varela, E. Fibre Intake in Chronic Kidney Disease: What Fibre Should We Recommend? Nutrients 2022, 14, 4419. [Google Scholar] [CrossRef]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int J Mol Sci 2022, 23, 5354. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, L.; Guo, H.; Xu, Y.; Xu, Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 2017, 68, 20–30. [Google Scholar] [CrossRef]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Du, J.; Zhao, X.; Ding, X.; Han, Q.; Duan, Y.; Ren, Q.; Wang, H.; Song, C.; Wang, X.; Zhang, D.; et al. The Role of the Gut Microbiota in Complications among Hemodialysis Patients. Microorganisms 2024, 12, 1878. [Google Scholar] [CrossRef] [PubMed]
- Fotheringham, A.K.; Gallo, L.A.; Borg, D.J.; Forbes, J.M. Advanced Glycation End Products (AGEs) and Chronic Kidney Disease: Does the Modern Diet AGE the Kidney? Nutrients 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Steenbeke, M.; Speeckaert, R.; Desmedt, S.; Glorieux, G.; Delanghe, J.R.; Speeckaert, M.M. The Role of Advanced Glycation End Products and Its Soluble Receptor in Kidney Diseases. Int J Mol Sci 2022, 23, 3439. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, A.; Yap, F.Y.T.; Borg, D.J.; McCarthy, D.; Fotheringham, A.; Leung, S.; Penfold, S.A.; Sourris, K.C.; Coughlan, M.T.; Schulz, B.L.; et al. The AGE receptor, OST48 drives podocyte foot process effacement and basement membrane expansion (alters structural composition). Endocrinol Diabetes Metab 2021, 4, e00278. [Google Scholar] [CrossRef] [PubMed]
- Vaaler, S.; Hanssen, K.F.; Aagenaes, O. The effect of cooking upon the blood glucose response to ingested carrots and potatoes. Diabetes Care 1984, 7, 221–223. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int J Mol Sci 2022, 23, 9588. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Liu, J.; Sun, J.; Wang, X.; Fan, G.; Meng, X.; Zhang, J.; Zhang, Y. The regulatory roles of dietary fibers on host health via gut microbiota-derived short chain fatty acids. Curr Opin Pharmacol 2022, 62, 36–42. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.A.; Cardozo, L.; Anjos, J.S.; Black, A.P.; Moraes, C.; Bergman, P.; Lindholm, B.; Stenvinkel, P. Red meat intake in chronic kidney disease patients: Two sides of the coin. Nutrition 2018, 46, 26–32. [Google Scholar] [CrossRef]
- Avesani, C.M.; Cardozo, L.; Yee-Moon Wang, A.; Shiels, P.G.; Lambert, K.; Lindholm, B.; Stenvinkel, P.; Mafra, D. Planetary Health, Nutrition, and Chronic Kidney Disease: Connecting the Dots for a Sustainable Future. J Ren Nutr 2023, 33, S40–S48. [Google Scholar] [CrossRef]
- D'Alessandro, C.; Giannese, D.; Panichi, V.; Cupisti, A. Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet. Nutrients 2023, 15, 1256. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, L.; Zhang, H.; Yang, Y.; Jiang, L.; Wu, D.; Shu, H.; Zhang, H.; Xie, L.; Zhou, K.; et al. Dietary fiber alleviates alcoholic liver injury via Bacteroides acidifaciens and subsequent ammonia detoxification. Cell Host Microbe 2024, 32, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2015, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, X.; Riserus, U.; Krishnamurthy, V.M.; Cederholm, T.; Arnlov, J.; Lindholm, B.; Sjogren, P.; Carrero, J.J. Dietary fiber, kidney function, inflammation, and mortality risk. Clin J Am Soc Nephrol 2014, 9, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, L.; Wei, W.; Fu, P. Efficacy of probiotics/synbiotics supplementation in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Front Nutr 2024, 11, 1434613. [Google Scholar] [CrossRef]
- Kalidindi, R.K.; Reddy, C.P.; Pv, K.; Kompella, P. The Efficacy and Safety of Probiotic Combinations Lobun Forte(R) Versus Renadyl(R) in Patients With Chronic Kidney Disease: A Comparative, Phase IV, Randomized, Open-Label, Active-Controlled, Parallel Study. Cureus 2024, 16, e67987. [Google Scholar] [CrossRef]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front Microbiol 2022, 13, 929346. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int J Mol Sci 2024, 25, 6022. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Wagner, S.; Merkling, T.; Metzger, M.; Koppe, L.; Laville, M.; Boutron-Ruault, M.C.; Frimat, L.; Combe, C.; Massy, Z.A.; Stengel, B.; et al. Probiotic Intake and Inflammation in Patients With Chronic Kidney Disease: An Analysis of the CKD-REIN Cohort. Front Nutr 2022, 9, 772596. [Google Scholar] [CrossRef]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Delcour, J.A.; Courtin, C.M.; Kuypers, D.; Augustijns, P.; Verbeke, K.; Meijers, B. The Influence of Prebiotic Arabinoxylan Oligosaccharides on Microbiota Derived Uremic Retention Solutes in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0153893. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B.; Vyas, U.; Ranganathan, P.; Weinberg, A.; Liang, P.; Mallappallil, M.C.; Norin, A.J.; Friedman, E.A.; Saggi, S.J. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int 2014, 2014, 568571. [Google Scholar] [CrossRef] [PubMed]
- De Mauri, A.; Carrera, D.; Bagnati, M.; Rolla, R.; Vidali, M.; Chiarinotti, D.; Pane, M.; Amoruso, A.; Del Piano, M. Probiotics-Supplemented Low-Protein Diet for Microbiota Modulation in Patients with Advanced Chronic Kidney Disease (ProLowCKD): Results from a Placebo-Controlled Randomized Trial. Nutrients 2022, 14, 1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.K.; Wu, Y.Y.; Yang, Y.F.; Ting, I.W.; Lin, C.C.; Yen, T.H.; Chen, J.H.; Wang, C.H.; Huang, C.C.; Lin, H.C. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: A randomised, double-blind, placebo-controlled trial. Benef Microbes 2015, 6, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Fasoulas, A.; Mantzorou, M.; Giaginis, C. Clinical Evidence on the Potential Beneficial Effects of Probiotics and Prebiotics in Cardiovascular Disease. Int J Mol Sci 2022, 23, 15898. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Wlodarczyk, M.; Slizewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef]
- Cooper, T.E.; Khalid, R.; Chan, S.; Craig, J.C.; Hawley, C.M.; Howell, M.; Johnson, D.W.; Jaure, A.; Teixeira-Pinto, A.; Wong, G. Synbiotics, prebiotics and probiotics for people with chronic kidney disease. Cochrane Database Syst Rev 2023, 10, CD013631. [Google Scholar]
- Bakhtiary, M.; Morvaridzadeh, M.; Agah, S.; Rahimlou, M.; Christopher, E.; Zadro, J.R.; Heshmati, J. Effect of Probiotic, Prebiotic, and Synbiotic Supplementation on Cardiometabolic and Oxidative Stress Parameters in Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Clin Ther 2021, 43, e71–e96. [Google Scholar] [CrossRef]
- Nguyen, T.T.U.; Kim, H.W.; Kim, W. Effects of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins, Inflammation, and Oxidative Stress in Hemodialysis Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med 2021, 10, 4456. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ss-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 2009, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Cohen Kadosh, K. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18-25 years) with corresponding changes in gut bacterial composition. Sci Rep 2021, 11, 8302. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J Clin Med 2022, 11, 4119. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yan, Y.; Tian, S.; Zheng, D.; Leng, D.; Wang, C.; Jiao, J.; Wang, Z.; Bai, Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front Cell Infect Microbiol 2019, 9, 455. [Google Scholar] [CrossRef]
- Boicean, A.; Bratu, D.; Fleaca, S.R.; Vasile, G.; Shelly, L.; Birsan, S.; Bacila, C.; Hasegan, A. Exploring the Potential of Fecal Microbiota Transplantation as a Therapy in Tuberculosis and Inflammatory Bowel Disease. Pathogens 2023, 12, 1149. [Google Scholar] [CrossRef]
- Lou, X.; Xue, J.; Shao, R.; Yang, Y.; Ning, D.; Mo, C.; Wang, F.; Chen, G. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front Immunol 2022, 13, 1063543. [Google Scholar] [CrossRef]
- Airola, C.; Severino, A.; Porcari, S.; Fusco, W.; Mullish, B.H.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. Future Modulation of Gut Microbiota: From Eubiotics to FMT, Engineered Bacteria, and Phage Therapy. Antibiotics (Basel) 2023, 12, 868. [Google Scholar] [CrossRef]
- Caggiano, G.; Cosola, C.; Di Leo, V.; Gesualdo, M.; Gesualdo, L. Microbiome modulation to correct uremic toxins and to preserve kidney functions. Curr Opin Nephrol Hypertens 2020, 29, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Hosomi, K.; Sekimoto, A.; Mishima, E.; Oe, Y.; Saigusa, D.; Ito, S.; Abe, T.; Sato, H.; Kunisawa, J.; et al. Effects of the oral adsorbent AST-120 on fecal p-cresol and indole levels and on the gut microbiota composition. Biochem Biophys Res Commun 2020, 525, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Fukuhara, S.; Akizawa, T.; Asano, Y.; Kurokawa, K. Study design and methods for a clinical trial of an oral carbonaceous adsorbent used to prevent the progression of chronic kidney disease (CAP-KD). Clin Exp Nephrol 2005, 9, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Akizawa, T.; Asano, Y.; Morita, S.; Wakita, T.; Onishi, Y.; Fukuhara, S.; Gejyo, F.; Matsuo, S.; Yorioka, N.; Kurokawa, K.; et al. Effect of a carbonaceous oral adsorbent on the progression of CKD: A multicenter, randomized, controlled trial. Am J Kidney Dis 2009, 54, 459–467. [Google Scholar] [CrossRef]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Arita, K.; Kato, A.; Shimizu, M. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. J Am Soc Nephrol 2015, 26, 1732–1746. [Google Scholar] [CrossRef]
- Nazzal, L.; Roberts, J.; Singh, P.; Jhawar, S.; Matalon, A.; Gao, Z.; Holzman, R.; Liebes, L.; Blaser, M.J.; Lowenstein, J. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol Dial Transplant 2017, 32, 1809–1817. [Google Scholar] [CrossRef]
- Jang, H.R.; Gandolfo, M.T.; Ko, G.J.; Satpute, S.; Racusen, L.; Rabb, H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol 2009, 297, F1457–F1465. [Google Scholar] [CrossRef]
- Mishima, E.; Fukuda, S.; Kanemitsu, Y.; Saigusa, D.; Mukawa, C.; Asaji, K.; Matsumoto, Y.; Tsukamoto, H.; Tachikawa, T.; Tsukimi, T.; et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Renal Physiol 2018, 315, F824–F833. [Google Scholar] [CrossRef]
- Cha, R.R.; Park, S.Y.; Camilleri, M.; The Constipation Research Group of Korean Society of Neurogastroenterology Motility. Constipation in Patients With Chronic Kidney Disease. J Neurogastroenterol Motil 2023, 29, 428–435. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.E.; Kim, J.H.; Ahn, S.H.; Jung, C.Y.; Hwang, S.D.; Lee, S.W.; Song, J.H. Real-world evidence of constipation and laxative use in the Korean population with chronic kidney disease from a common data model. Sci Rep 2024, 14, 6610. [Google Scholar] [CrossRef]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int Rep 2020, 5, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.; Pohl, D.; Azpiroz, F.; Chiarioni, G.; Ducrotte, P.; Gourcerol, G.; Hungin, A.P.S.; Layer, P.; Mendive, J.M.; Pfeifer, J.; et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol Motil 2020, 32, e13762. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Bharucha, A.E.; Ueno, R.; Burton, D.; Thomforde, G.M.; Baxter, K.; McKinzie, S.; Zinsmeister, A.R. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2006, 290, G942–G947. [Google Scholar] [CrossRef] [PubMed]
- Nanto-Hara, F.; Kanemitsu, Y.; Fukuda, S.; Kikuchi, K.; Asaji, K.; Saigusa, D.; Iwasaki, T.; Ho, H.J.; Mishima, E.; Suzuki, T.; et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant 2020, 35, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, M.; Fukunaga, M.; Mei, M.; Nakajima, A.; Tanaka, G.; Murase, T.; Narita, Y.; Hirata, S.; Kadowaki, D. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin Exp Nephrol 2019, 23, 908–919. [Google Scholar] [CrossRef]
- Vicic, V.; Pandel Mikus, R.; Ferjancic, B. Review of history and mechanisms of action of lactulose (4-O-beta-D-Galactopyranosyl-beta-D-fructofuranose): Present and future applications in food. J Food Sci Technol 2024, 61, 2036–2045. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front Nutr 2021, 8, 672925. [Google Scholar] [CrossRef]
- de Lorenzo-Pinto, A.; Garcia-Sanchez, R.; Lorenzo-Salinas, A. Lactulose enemas in the treatment of hepatic encephalopathy. Do we help or harm? Rev Esp Enferm Dig 2017, 109, 736–737. [Google Scholar] [CrossRef]
- Tayebi Khosroshahi, H.; Habibzadeh, A.; Khoshbaten, M.; Rahbari, B.; Chaichi, P.; Badiee, A.H. Lactulose for reduction of nitrogen products in patients with chronic kidney disease. Iran J Kidney Dis 2014, 8, 377–381. [Google Scholar]
- Tayebi-Khosroshahi, H.; Habibzadeh, A.; Niknafs, B.; Ghotaslou, R.; Yeganeh Sefidan, F.; Ghojazadeh, M.; Moghaddaszadeh, M.; Parkhide, S. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Renal Inj Prev 2016, 5, 162–167. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).