Submitted:
16 November 2024
Posted:
19 November 2024
You are already at the latest version
Abstract
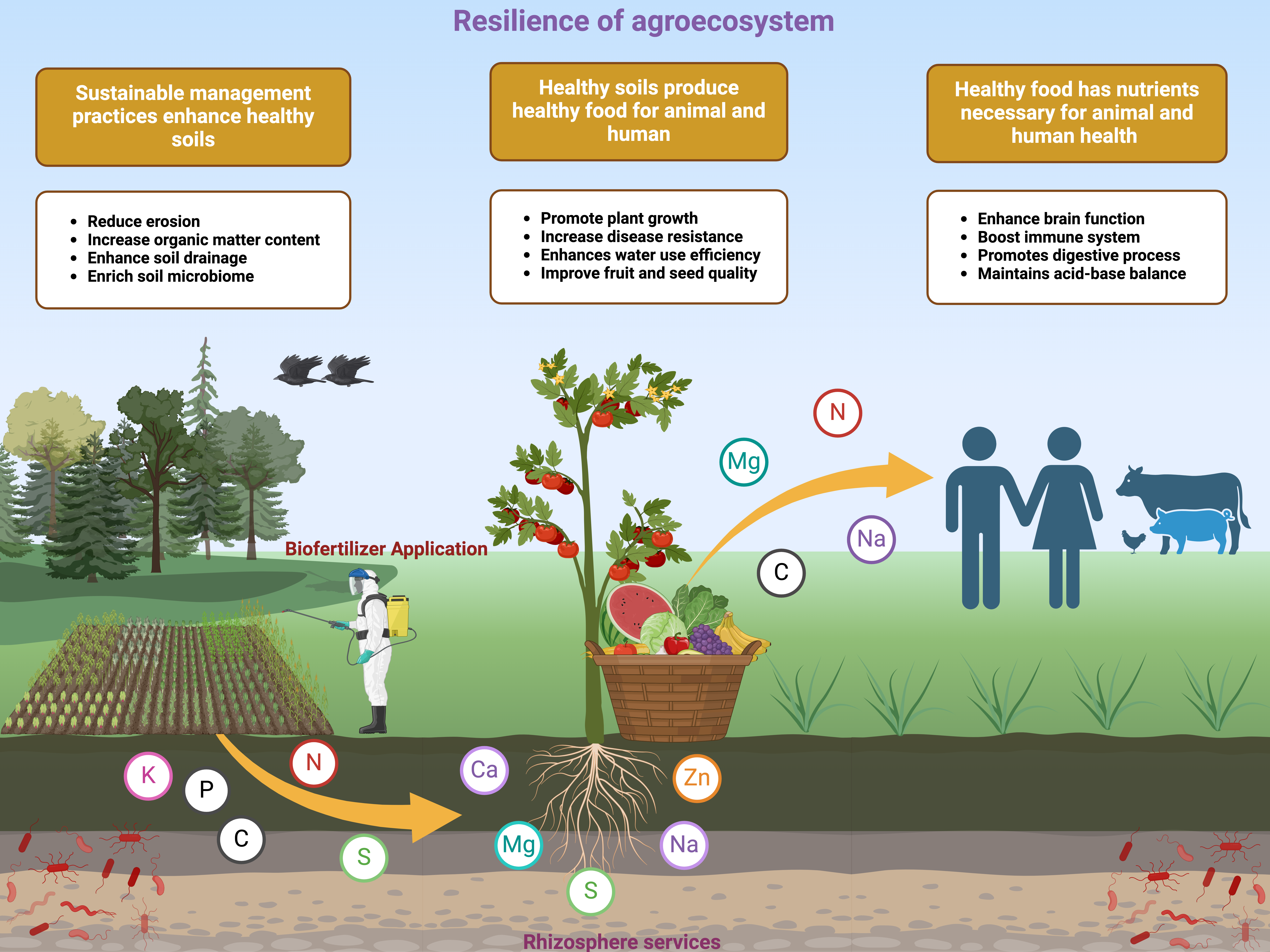
Keywords:
1. Introduction
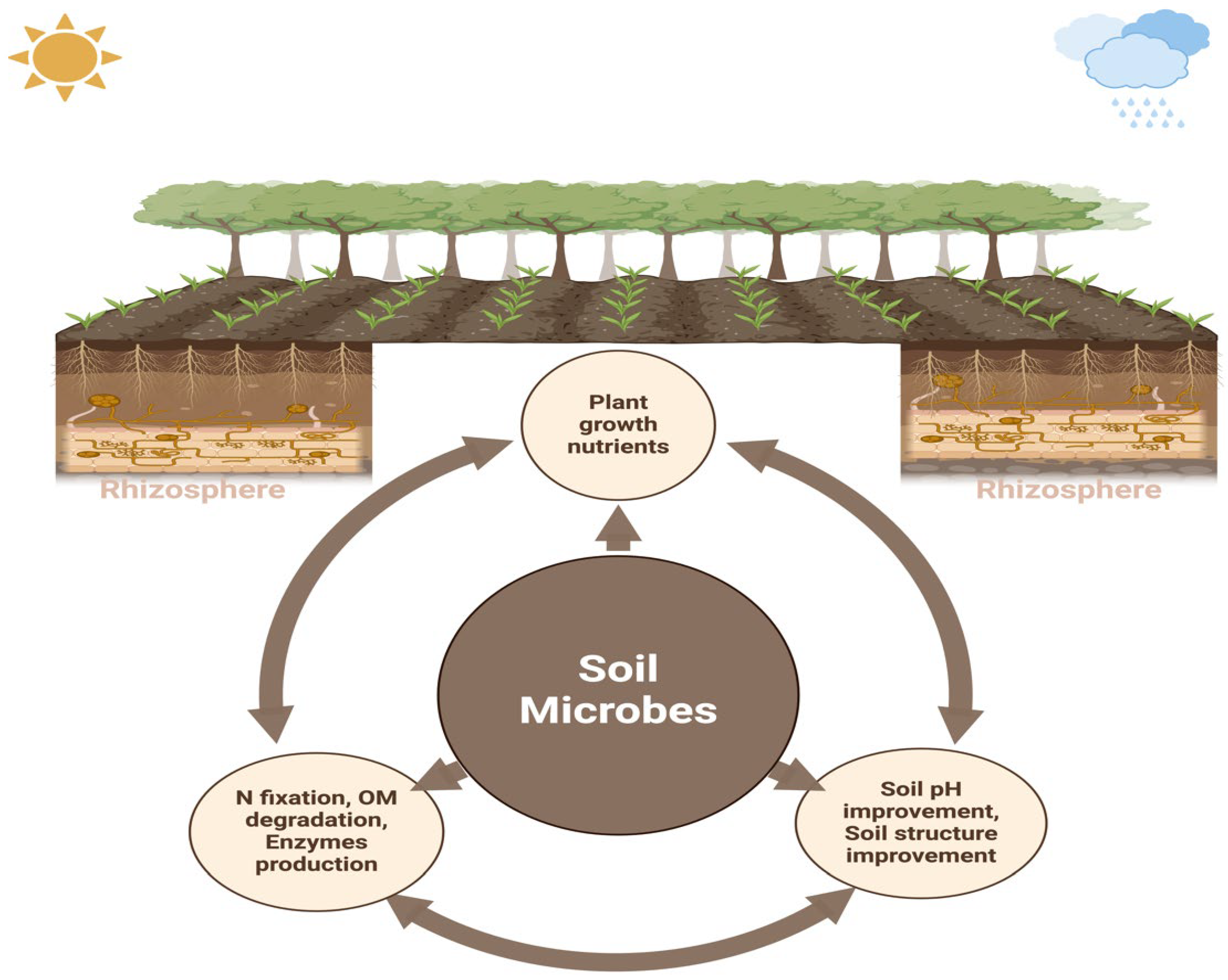
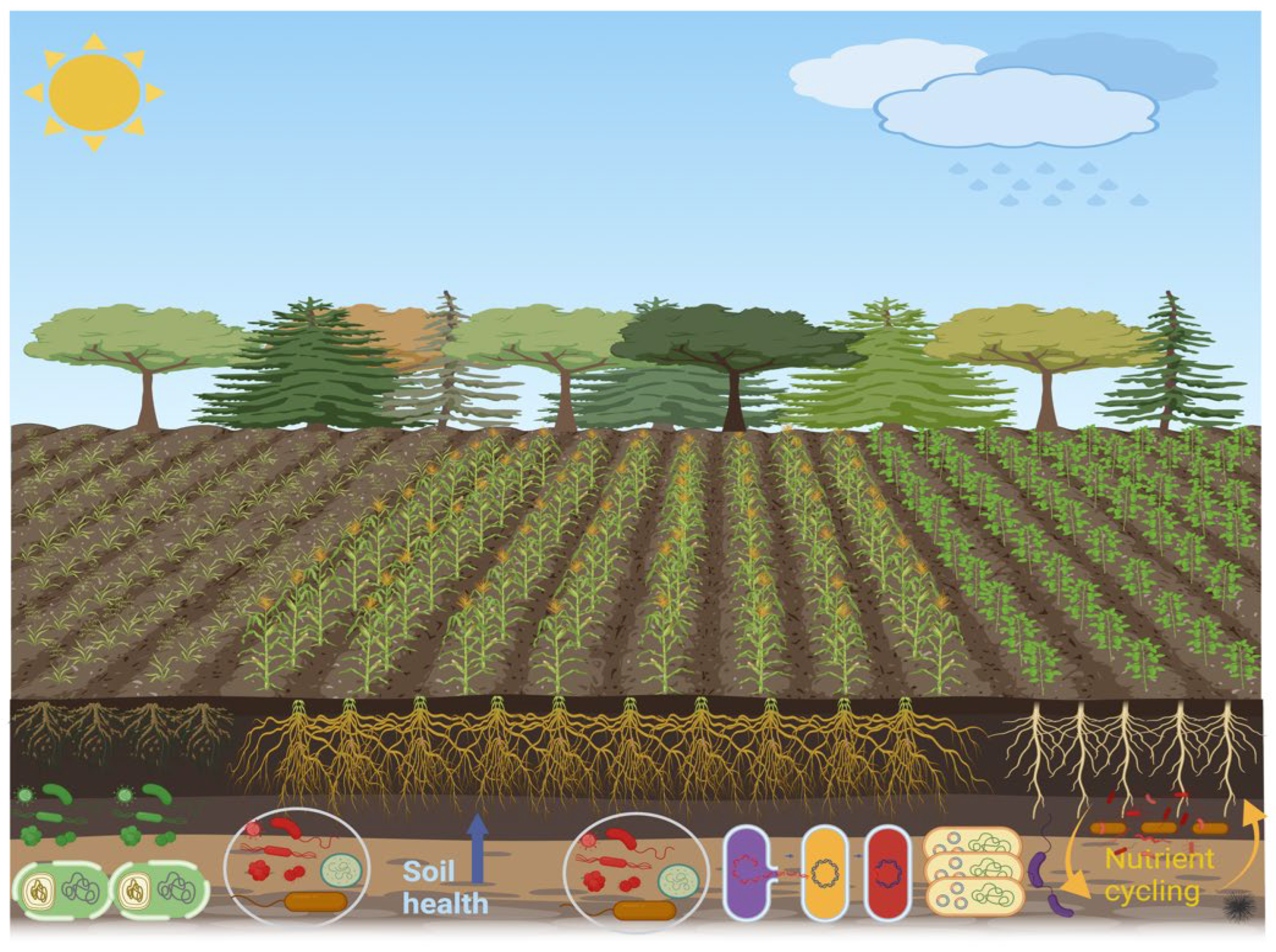
2. The Rhizosphere: Its Mechanism and Roles
2.1. Rhizosphere Processes: Nutrient Retention in Agroecosystems
2.2. Rhizosphere Engineering: Turn into Sustainably Increases the Agroecosystem and Its Productivity
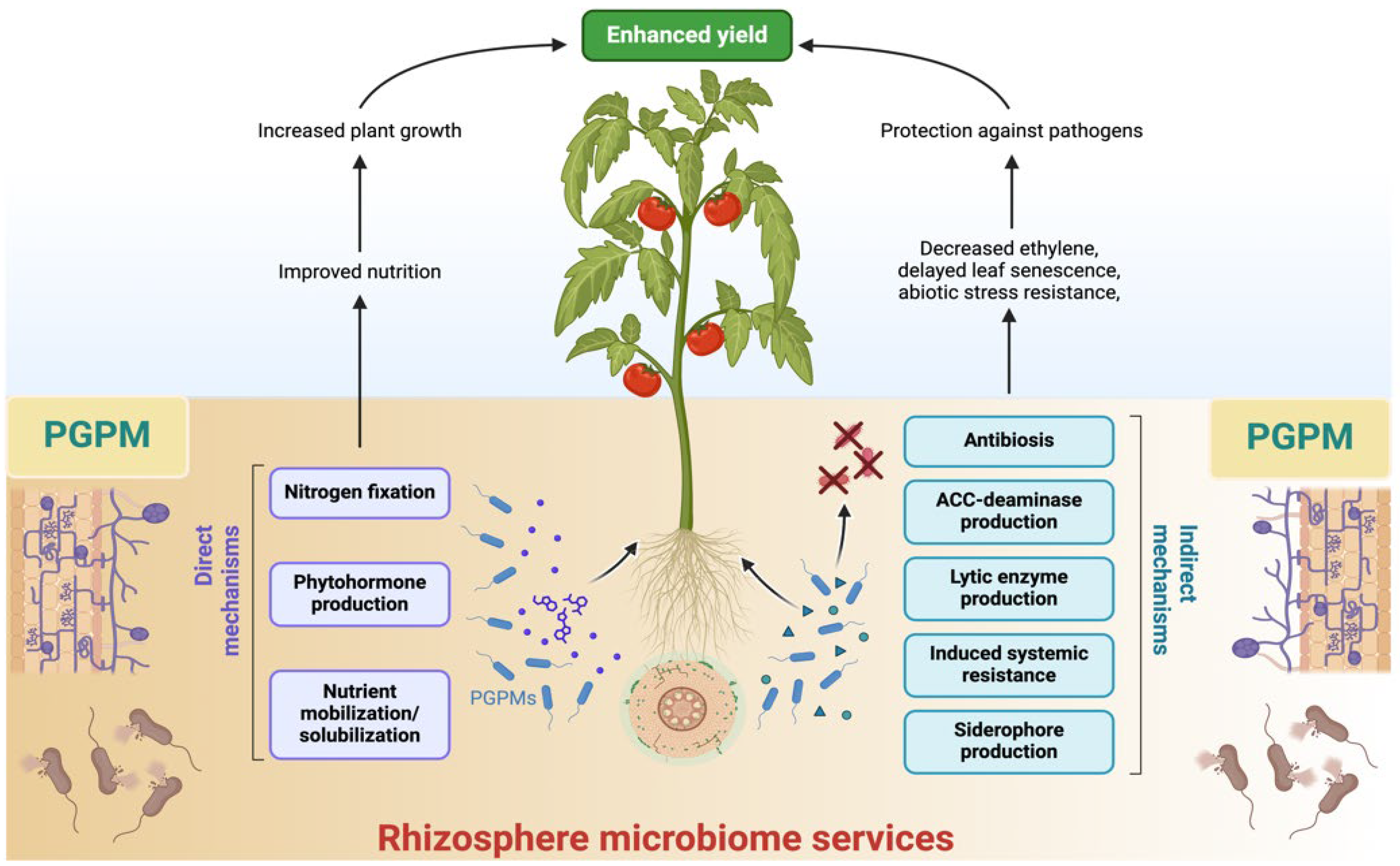
3. Role of Soil Microbes in the Rhizosphere Processes
3.1. The Role of Microbes in Agroecosystems
3.2. Plant Growth Promotion Via Microbes
3.3. Singling Between Plants and Microbes in the Rhizosphere
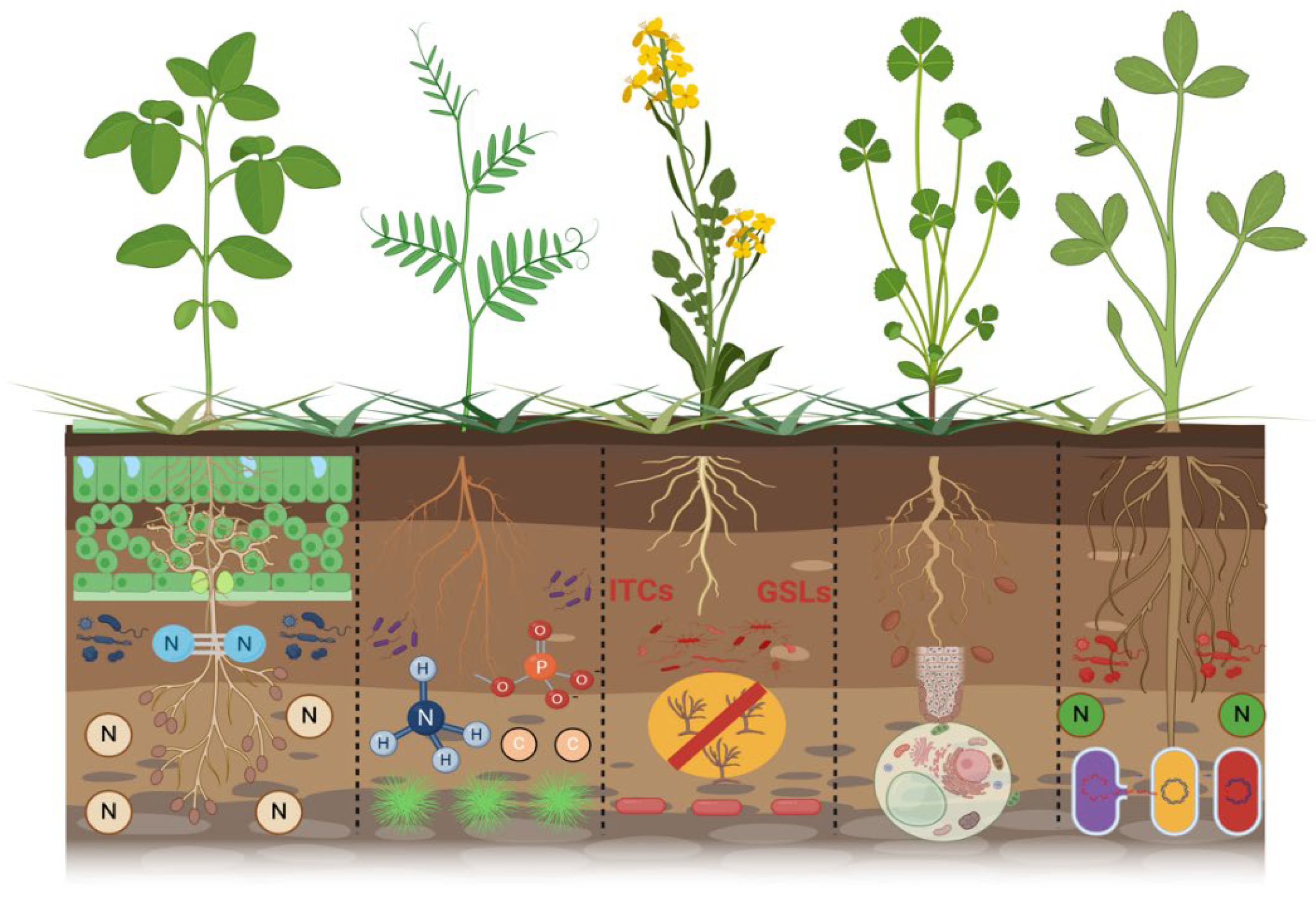
4. The Role of Green Manures in Agroecosystems
4.1. Green Manure and Their Beneficial Effects on the Rhizosphere Processes
4.2. Green Manure and Their Effects on Soil Health
5. Role of Cropping Systems in Agroecosystems
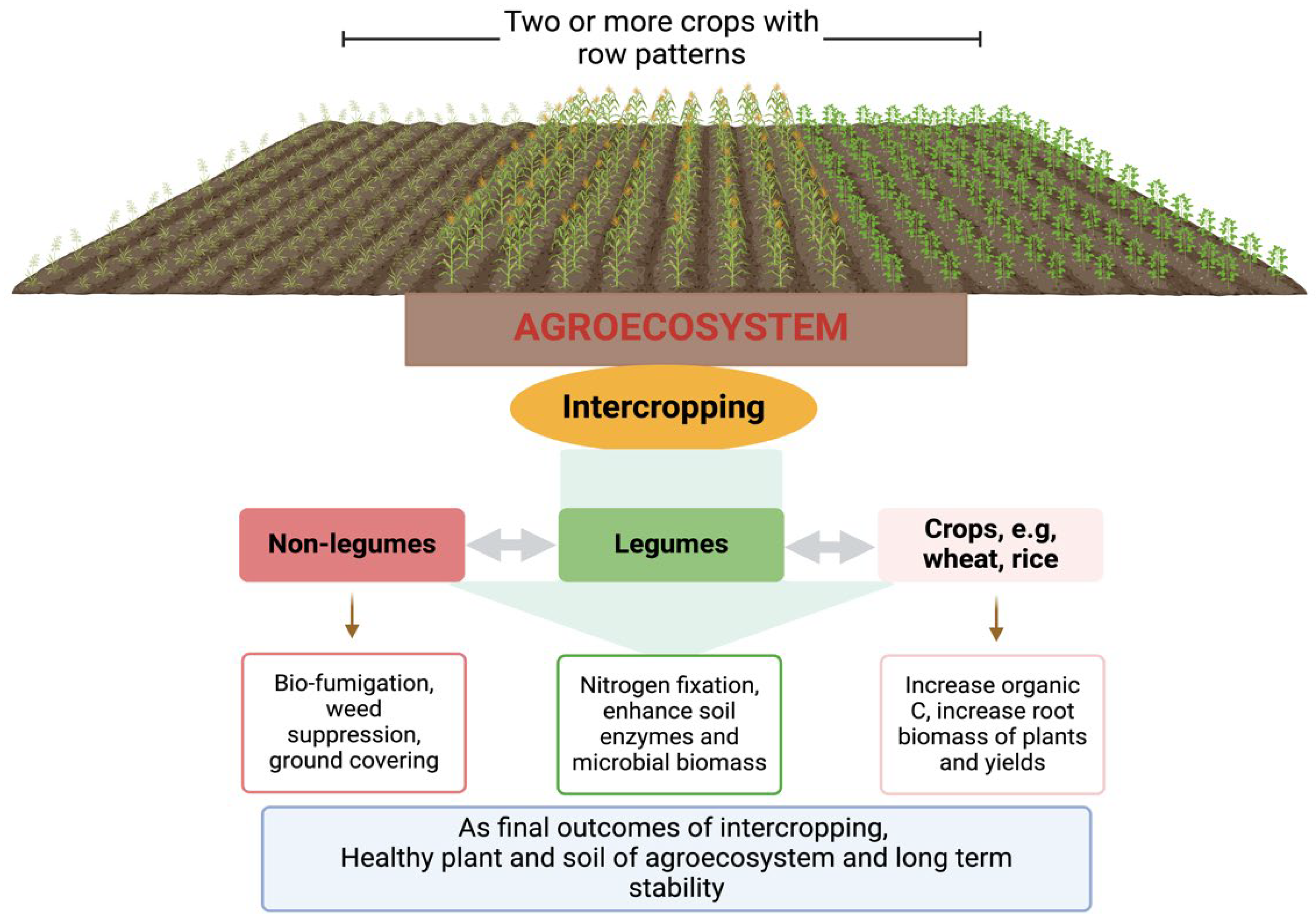
5.1. Intercropping and Their Effects on Rhizosphere Processes and Soil Health
5.2. Crop Rotation and Their Effects on Rhizosphere Processes and Soil
6. Indicators for Assessing Soil Health in Agroecosystems
7. Integrated Agricultural Practices for Improving Rhizospheric Processes
8. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omotayo, O.P.; Babalola, O.O. Resident rhizosphere microbiome’s ecological dynamics and conservation: Towards achieving the envisioned Sustainable Development Goals, a review. International Soil and Water Conservation Research 2020. [Google Scholar] [CrossRef]
- Liang, H.; Wang, X.; Yan, J.; Luo, L. Characterizing the intra-vineyard variation of soil bacterial and fungal communities. Front microbiol 2019, 10, 1239. [Google Scholar]
- Schmidt, J.E.; Vannette, R.L.; Igwe, A.; Blundell, R.; Casteel, C.L.; Gaudin, A.C. Effects of agricultural management on rhizosphere microbial structure and function in processing tomato plants. AEM 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Asghar, W.; Kataoka, R. Co-application of Green Manure and Trichoderma spp. Induced Plant Growth Promotion by Nutrient Improvement and Increased Fungal Biomass in Soil. Agric. Res 2024, 1–10. [Google Scholar]
- Asghar, W.; Craven, K.D.; Kataoka, R.; Mahmood, A.; Asghar, N.; Raza, T.; Iftikhar, F. The application of Trichoderma spp., an old but new useful fungus, in sustainable soil health intensification: A comprehensive strategy for addressing challenges. Plant Stress 2024, 100455. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Sturz, A.; Carter, M.; Johnston, H. A review of plant disease, pathogen interactions and microbial antagonism under conservation tillage in temperate humid agriculture. Soil Till Res 1997, 41, 169–189. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; Zhang, D.; Cheng, H.; Hao, B.; Cao, A.; Yan, D.; Wang, Q.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS One 2022, 17, e0266347. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Yu, Y.-N.; Gao, R.-W.; Wang, H.; Zhang, J.; Li, R.; Long, X.-H.; Shen, Q.-R.; Chen, W.; Cai, F. High-throughput absolute quantification sequencing reveals the effect of different fertilizer applications on bacterial community in a tomato cultivated coastal saline soil. Science of the total environment 2019, 687, 601–609. [Google Scholar] [CrossRef]
- Bajsa, N.; Morel, M.A.; Braña, V.; Castro-Sowinski, S. The effect of agricultural practices on resident soil microbial communities: focus on biocontrol and biofertilization. Mol Microb Ecol Rhizosphere 2013, 2, 687–700. [Google Scholar]
- Meena, H.; Meena, R.S.; Rajput, B.S.; Kumar, S. Response of bio-regulators to morphology and yield of clusterbean [Cyamopsis tetragonoloba (L.) Taub.] under different sowing environments. Journal of Applied and Natural Science 2016, 8, 715–718. [Google Scholar] [CrossRef]
- Dubey, R.K.; Dubey, P.K.; Chaurasia, R.; Singh, H.B.; Abhilash, P.C. Sustainable agronomic practices for enhancing the soil quality and yield of Cicer arietinum L. under diverse agroecosystems. Journal of environmental management 2020, 262, 110284. [Google Scholar] [PubMed]
- Bruinsma, M.; Kowalchuk, G.; Van Veen, J. Effects of genetically modified plants on microbial communities and processes in soil. Biol Fertil Soils 2003, 37, 329–337. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ramesh, A.; Sharma, M.P.; Joshi, O.P.; Govaerts, B.; Steenwerth, K.L.; Karlen, D.L. Microbial community structure and diversity as indicators for evaluating soil quality. In Biodiversity, biofuels, agroforestry and conservation agriculture; Springer, 2010; pp. 317–358. [Google Scholar]
- Asghar, W.; Kataoka, R. Green manure incorporation accelerates enzyme activity, plant growth, and changes in the fungal community of soil. Arch Microbiol 2022, 204, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, X.; Liao, Y.; Lu, Y.; Nie, J.; Cao, W. Co-incorporation of rice straw and green manure benefits rice yield and nutrient uptake. Crop Sci. 2019, 59, 749–759. [Google Scholar] [CrossRef]
- Khan, M.I.; Gwon, H.S.; Alam, M.A.; Song, H.J.; Das, S.; Kim, P.J. Short term effects of different green manure amendments on the composition of main microbial groups and microbial activity of a submerged rice cropping system. Appl Soil Ecol 2020, 147, 103400. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. Journal of experimental botany 2016, 67, 3629–3643. [Google Scholar] [CrossRef]
- Solomon, W.; Janda, T.; Molnár, Z. Unveiling the significance of rhizosphere: implications for plant growth, stress response, and sustainable agriculture. Plant Physiology and Biochemistry 2023, 108290. [Google Scholar] [CrossRef]
- De Luna, L.Z.; Stubbs, T.L.; Kennedy, A.C.; Kremer, R.J. Deleterious bacteria in the rhizosphere. roots and soil management: interactions between roots and the soil 2005, 48, 233-261.
- RUEss, L.; Ferris, H. Decomposition pathways and successional changes. Nematology Monographs and Perspectives 2004, 2, 547–556. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sc 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Asghar, W.; Kondo, S.; Iguchi, R.; Mahmood, A.; Kataoka, R. Agricultural utilization of unused resources: liquid food waste material as a new source of plant growth-promoting microbes. Agronomy 2020, 10, 954. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; Tuinen, D.v.; Berg, G. Plant-driven selection of microbes; Springer, 2009. [Google Scholar]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front Plant Sci 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Barea, J. Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbiome interactions. J. Soil Sci. Plant Nutr 2015, 15, 261–282. [Google Scholar] [CrossRef]
- He, Z.; Shang, X.; Zhang, T.; Yun, J. Ca and mg stimulate protein synthesis in maize kernel through the action of endogenous hormones and defense enzymes. Plant Physiology and Biochemistry 2024, 206, 108280. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, M.S.; Akter, M. Challenges faced by plant growth-promoting bacteria in field-level applications and suggestions to overcome the barriers. Physiological and Molecular Plant Pathology 2023, 126, 102029. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Frontiers in Sustainable Food Systems 2021, 5, 16. [Google Scholar] [CrossRef]
- de Graaff, M.-A. Interactions between plants and soil nutrient cycling under elevated CO2; 2007.
- Joseph, S.; Downie, A.; Munroe, P.; Crosky, A.; Lehmann, J. Biochar for carbon sequestration, reduction of greenhouse gas emissions and enhancement of soil fertility; a review of the materials science. In Proceedings of Proceedings of the Australian combustion symposium; pp. 130–133.
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci Plant Nutr 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: a win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agronomy journal 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; De Courcelles, V.d.R.; Singh, K. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric Ecosyst Environ 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Science of the Total Environment 2019, 659, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Neogi, S.; Dutta, T.; Powel, M.A.; Banik, P. The impact of biochar on soil carbon sequestration: meta-analytical approach to evaluating environmental and economic advantages. Journal of environmental management 2019, 250, 109466. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, N. CO 2 emission and structural characteristics of two calcareous soils amended with municipal solid waste and plant residue. Solid Earth 2016, 7, 105–114. [Google Scholar] [CrossRef]
- Kenney, I.; Blanco-Canqui, H.; Presley, D.R.; Rice, C.W.; Janssen, K.; Olson, B. Soil and crop response to stover removal from rainfed and irrigated corn. Gcb Bioenergy 2015, 7, 219–230. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Scheer, C.; Rowlings, D.W.; Grace, P.R. Rice husk biochar and crop residue amendment in subtropical cropping soils: effect on biomass production, nitrogen use efficiency and greenhouse gas emissions. Biol Fertil Soils 2016, 52, 261–270. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch Microbiol 2021, 10.1007/s00203-021-02413-4. [Google Scholar] [CrossRef]
- Kataoka, R.; Nagasaka, K.; Tanaka, Y.; Yamamura, H.; Shinohara, S.; Haramoto, E.; Hayakawa, M.; Sakamoto, Y. Hairy vetch (Vicia villosa), as a green manure, increases fungal biomass, fungal community composition, and phosphatase activity in soil. Appl Soil Ecol 2017, 117, 16–20. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar Role in the Sustainability of Agriculture and Environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: an introduction; Routledge, 2015. [Google Scholar]
- Lu, L.; Yu, W.; Wang, Y.; Zhang, K.; Zhu, X.; Zhang, Y.; Wu, Y.; Ullah, H.; Xiao, X.; Chen, B. Application of biochar-based materials in environmental remediation: from multi-level structures to specific devices. Biochar 2020, 2, 1–31. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass and bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Arif, M.; Ali, S.; Ilyas, M.; Riaz, M.; Akhtar, K.; Ali, K.; Adnan, M.; Fahad, S.; Khan, I.; Shah, S. Enhancing phosphorus availability, soil organic carbon, maize productivity and farm profitability through biochar and organic–inorganic fertilizers in an irrigated maize agroecosystem under semi-arid climate. Soil Use Manag 2021, 37, 104–119. [Google Scholar] [CrossRef]
- Uzoma, K.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Lu, H.; Yan, M.; Wong, M.H.; Mo, W.Y.; Wang, Y.; Chen, X.W.; Wang, J.-J. Effects of biochar on soil microbial community and functional genes of a landfill cover three years after ecological restoration. Science of The Total Environment 2020, 717, 137133. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Science of the Total Environment 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol Biochem 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Lagos, L.; Maruyama, F.; Nannipieri, P.; Mora, M.; Ogram, A.; Jorquera, M. Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: a mini‒review. J. Soil Sci. Plant Nutr 2015, 15, 504–523. [Google Scholar]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. PNAS 2013, 110, 6548–6553. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Mahmood, A.; Kataoka, R.; Takagi, K. Dichlorodiphenyltrichloroethane (DDT) degradation by Streptomyces sp. isolated from DDT contaminated soil. Bioremediation Journal 2020, 1–14. [Google Scholar]
- Asghar, W.; Kataoka, R. Fungal volatiles from green manure-incorporated soils promote the growth of lettuce (Lactuca sativa) and mediate antifungal activity against Fusarium oxysporum in vitro. Plant Soil 2023, 1–12. [Google Scholar] [CrossRef]
- Selvasekaran, P.; Chidambaram, R. Agriculturally Important Fungi for Crop Protection. In Agriculturally Important Fungi for Sustainable Agriculture; Springer, 2020; pp. 1–53. [Google Scholar]
- Ito, K.; Mahmood, A.; Kataoka, R.; Takagi, K. Dichlorodiphenyltrichloroethane (DDT) degradation by Streptomyces sp. isolated from DDT contaminated soil. Bioremediation Journal 2021, 25, 148–158. [Google Scholar]
- Mahmood, A.; Kataoka, R. Metabolite profiling reveals a complex response of plants to application of plant growth-promoting endophytic bacteria. Microbiol Res 2020, 234, 126421. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Iguchi, R.; Kataoka, R. Multifunctional food waste fertilizer having the capability of Fusarium-growth inhibition and phosphate solubility: A new horizon of food waste recycle using microorganisms. Waste Manage 2019, 94, 77–84. [Google Scholar] [CrossRef]
- Da Costa, P.B.; Beneduzi, A.; de Souza, R.; Schoenfeld, R.; Vargas, L.K.; Passaglia, L.M. The effects of different fertilization conditions on bacterial plant growth promoting traits: guidelines for directed bacterial prospection and testing. Plant Soil 2013, 368, 267–280. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatalysis and Agricultural Biotechnology 2020, 23, 101487. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A. Future of bio-fertilizers in Indian agriculture: an overview. International Journal of Agricultural and Food Research 2015, 3. [Google Scholar] [CrossRef]
- Murali, M.; Amruthesh, K.; Sudisha, J.; Niranjana, S.; Shetty, H. Screening for plant growth promoting fungi and their ability for growth promotion and induction of resistance in pearl millet against downy mildew disease. Journal of Phytology 2012, 4, 30–36. [Google Scholar]
- Murali, M.; Amruthesh, K.N. Plant Growth-promoting Fungus Penicillium oxalicum enhances plant growth and induces resistance in pearl millet against downy mildew disease. J Phytopathol 2015, 163, 743–754. [Google Scholar] [CrossRef]
- Sayyed, R.; Chincholkar, S.; Reddy, M.; Gangurde, N.; Patel, P. Siderophore producing PGPR for crop nutrition and phytopathogen suppression. In Bacteria in agrobiology: disease management; Springer, 2013; pp. 449–471. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. The plant growth-promoting fungus Penicillium simplicissimum GP17-2 induces resistance in Arabidopsis thaliana by activation of multiple defense signals. Plant and cell physiology 2007, 48, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Miyazawa, M.; Hyakumachi, M. The plant growth-promoting fungus Penicillium spp. GP15-1 enhances growth and confers protection against damping-off and anthracnose in the cucumber. Journal of oleo science 2014, 63, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Abdel-lateif, K.S. Trichoderma as biological control weapon against soil borne plant pathogens. Afr. J. Biotechnol 2017, 16, 2299–2306. [Google Scholar]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Cervantes-Badillo, M.G.; Rosales-Saavedra, M.T.; Islas-Osuna, M.A.; Casas-Flores, S. The plant growth-promoting fungus Aspergillus ustus promotes growth and induces resistance against different lifestyle pathogens in Arabidopsis thaliana. Journal of microbiology and biotechnology 2011, 21, 686–696. [Google Scholar] [CrossRef]
- Cavello, I.A.; Crespo, J.M.; García, S.S.; Zapiola, J.M.; Luna, M.F.; Cavalitto, S.F. Plant growth promotion activity of keratinolytic fungi growing on a recalcitrant waste known as “Hair Waste”. Biotechnology research international 2015. [Google Scholar] [CrossRef]
- Yamagiwa, Y.; Inagaki, Y.; Ichinose, Y.; Toyoda, K.; Hyakumachi, M.; Shiraishi, T. Talaromyces wortmannii FS2 emits β-caryphyllene, which promotes plant growth and induces resistance. J. Gen. Plant Pathol 2011, 77, 336–341. [Google Scholar] [CrossRef]
- Kumar, V.; Behl, R.K.; Narula, N. Establishment of phosphate-solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under green house conditions. Microbiol Res 2001, 156, 87–93. [Google Scholar] [CrossRef]
- Joseph, B.; Ranjan Patra, R.; Lawrence, R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). International Journal of Plant Production 2012, 1, 141–152. [Google Scholar]
- Ahemad, M.; Khan, M.S. Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek] using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi journal of biological sciences 2012, 19, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S. Plant growth promoting rhizobacteria. Resonance 2013, 18, 275–281. [Google Scholar] [CrossRef]
- Sunpapao, A.; Chairin, T.; Ito, S.-i. The biocontrol by Streptomyces and Trichoderma of leaf spot disease caused by Curvularia oryzae in oil palm seedlings. Biocontrol 2018, 123, 36–42. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere signaling: Insights into plant–rhizomicrobiome interactions for sustainable agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Koprivova, A.; Kopriva, S. Pinpointing secondary metabolites that shape the composition and function of the plant microbiome. Journal of Experimental Botany 2021, 72, 57–69. [Google Scholar] [CrossRef]
- O’Banion, B.S.; O’Neal, L.; Alexandre, G.; Lebeis, S.L. Bridging the gap between single-strain and community-level plant-microbe chemical interactions. MPMI 2020, 33, 124–134. [Google Scholar] [CrossRef]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The chemistry of stress: understanding the ‘cry for help’of plant roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Chen, L.; Schwier, M.; Koprivova, A.; Kopriva, S. Recent advances in the role of plant metabolites in shaping the root microbiome. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: from community assembly to plant health. Nature reviews microbiology 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Mönchgesang, S.; Strehmel, N.; Schmidt, S.; Westphal, L.; Taruttis, F.; Müller, E.; Herklotz, S.; Neumann, S.; Scheel, D. Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. Sci Rep 2016, 6, 29033. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: revisiting the synergy of plant-microbe interactions. Annals of Microbiology 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Science 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhou, Q.; Hao, Y.; Huang, A.C. Crafting the plant root metabolome for improved microbe-assisted stress resilience. New Phytologist 2022, 234, 1945–1950. [Google Scholar] [CrossRef]
- Toom, M.; Tamm, S.; Talgre, L.; Tamm, I.; Tamm, Ü.; Narits, L.; Hiiesalu, I.; Mäe, A.; Lauringson, E. The effect of cover crops on the yield of spring barley in Estonia. Agriculture 2019, 9, 172. [Google Scholar] [CrossRef]
- Florentín, M.A.; Peñalva, M.; Calegari, A.; Derpsch, R.; McDonald, M. Green manure/cover crops and crop rotation in conservation agriculture on small farms. Integrated Crop Management 2010, 12. [Google Scholar]
- Ntakirutimana, L.; Li, F.; Huang, X.; Wang, S.; Yin, C. Green manure planting incentive measures of local authorities and farmers’ perceptions of the utilization of rotation fallow for sustainable agriculture in Guangxi, China. Sustainability 2019, 11, 2723. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Global Ecology and Conservation 2020, e01118. [Google Scholar] [CrossRef]
- Gan, Y.; Miller, P.; McConkey, B.; Zentner, R.; Stevenson, F.; McDonald, C. Influence of diverse cropping sequences on durum wheat yield and protein in the semiarid northern Great Plains. Agronomy Journal 2003, 95, 245–252. [Google Scholar] [CrossRef]
- Chavarria, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. Eur J Soil Biol 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Balota, E.L.; Calegari, A.; Nakatani, A.S.; Coyne, M.S. Benefits of winter cover crops and no-tillage for microbial parameters in a Brazilian Oxisol: A long-term study. Agric Ecosyst Environ 2014, 197, 31–40. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Gao, J.; Wang, X.; Fan, F.; Ma, X.; Yin, H.; Zhang, C.; Feng, K.; Deng, Y. Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol Biochem 2017, 104, 208–217. [Google Scholar] [CrossRef]
- Khan, M.I.; Hwang, H.Y.; Kim, G.W.; Kim, P.J.; Das, S. Microbial responses to temperature sensitivity of soil respiration in a dry fallow cover cropping and submerged rice mono-cropping system. Appl Soil Ecol 2018, 128, 98–108. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover crops for sustainable cropping systems: a review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- Lazcano, C.; Gonzalez-Maldonado, N.; Yao, E.H.; Wong, C.T.; Merrilees, J.J.; Falcone, M.; Peterson, J.D.; Casassa, L.F.; Decock, C. Sheep grazing as a strategy to manage cover crops in Mediterranean vineyards: Short-term effects on soil C, N and greenhouse gas (N2O, CH4, CO2) emissions. Agric Ecosyst Environ 2022, 327, 107825. [Google Scholar] [CrossRef]
- Fiorini, A.; Maris, S.C.; Abalos, D.; Amaducci, S.; Tabaglio, V. Combining no-till with rye (Secale cereale L.) cover crop mitigates nitrous oxide emissions without decreasing yield. Soil Till Res 2020, 196, 104442. [Google Scholar] [CrossRef]
- Doltra, J.; Olesen, J.E. The role of catch crops in the ecological intensification of spring cereals in organic farming under Nordic climate. Eur J Agron 2013, 44, 98–108. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.; Kahlon, C.; Brar, A.; Grover, K.; Dia, M.; Steiner, R. The Role of Cover Crops towards Sustainable Soil Health and Agriculture—A Review Paper. American Journal of Plant Sciences 2018, 09, 1935–1951. [Google Scholar] [CrossRef]
- Brennan, R.J.B.; Glaze-Corcoran, S.; Robert, W.; HASHeMi, M. Biofumigation: An alternative strategy for the control of plant parasitic nematodes. Journal of Integrative Agriculture 2020, 19, 1680–1690. [Google Scholar] [CrossRef]
- Srivastava, J.; Ghatak, A. Biofumigation: a control method for the soil-borne diseases. International Journal of Plant Protection 2017, 10, 453–460. [Google Scholar] [CrossRef]
- Dubey, R.K.; Dubey, P.K.; Abhilash, P. Sustainable soil amendments for improving the soil quality, yield and nutrient content of Brassica juncea (L.) grown in different agroecological zones of eastern Uttar Pradesh, India. Soil Till Res 2019, 195, 104418. [Google Scholar] [CrossRef]
- Clark, A. Managing cover crops profitably; Diane Publishing, 2008. [Google Scholar]
- Thapa, V.R.; Ghimire, R.; Acosta-Martínez, V.; Marsalis, M.A.; Schipanski, M.E. Cover crop biomass and species composition affect soil microbial community structure and enzyme activities in semiarid cropping systems. Appl Soil Ecol 2021, 157, 103735. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management impact and benefit of cover crops on soil quality: A review. Soil Till Res 2020, 204, 104717. [Google Scholar] [CrossRef]
- Magdoff, F.; Van Es, H. Building soils for better crops; Sustainable Agriculture Network Beltsville, 2000; Volume 4. [Google Scholar]
- Boselli, R.; Fiorini, A.; Santelli, S.; Ardenti, F.; Capra, F.; Maris, S.C.; Tabaglio, V. Cover crops during transition to no-till maintain yield and enhance soil fertility in intensive agro-ecosystems. Field Crops Res 2020, 255, 107871. [Google Scholar] [CrossRef]
- Patra, D.; Anwar, M.; Chand, S. Integrated nutrient management and waste recycling for restoring soil fertility and productivity in Japanese mint and mustard sequence in Uttar Pradesh, India. Agric Ecosyst Environ 2000, 80, 267–275. [Google Scholar] [CrossRef]
- Annabi, M.; Houot, S.; Francou, C.; Poitrenaud, M.; Bissonnais, Y.L. Soil aggregate stability improvement with urban composts of different maturities. Soil Science Society of America Journal 2007, 71, 413–423. [Google Scholar] [CrossRef]
- Belachew, T.; Abera, Y. Effect of green manuring in combination with nitrogen on soil fertility and yield of bread wheat (Triticum aestivum) under double cropping system of Sinanadinsho, Southeast Ethiopia. Journal of Biodiversity and Environmental Sciences 2011, 1, 1–11. [Google Scholar]
- Baysal-Gurel, F.; Gardener, B.; Miller, S.A. Soil Borne Disease Management in Organic Vegetable Production. Organic Agri 2012. [Google Scholar]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Mokhtar, M.; El-Mougy, N. Bio-compost application for controlling soil-borne plant pathogens–a review. International Journal of Engineering and Innovative Technology 2014, 4, 2277–3754. [Google Scholar]
- Hao, J.; Subbarao, K.V.; Koike, S.T. Effects of broccoli rotation on lettuce drop caused by Sclerotinia minor and on the population density of sclerotia in soil. Plant Disease 2003, 87, 159–166. [Google Scholar] [CrossRef]
- Whalen, J. Crop profile for watermelon in Maryland. Online. Natl. Info. System for the Regional IPM Centers, North Carolina State Univ., Raleigh, NC 1999.
- Keinath, A.P.; Hassell, R.L.; Everts, K.L.; Zhou, X.-G. Cover crops of hybrid common vetch reduce Fusarium wilt of seedless watermelon in the eastern United States. Plant Health Progress 2010, 11, 8. [Google Scholar] [CrossRef]
- Papp, R.; Marinari, S.; Moscatelli, M.C.; van der Heijden, M.; Wittwer, R.; Campiglia, E.; Radicetti, E.; Mancinelli, R.; Fradgley, N.; Pearce, B. Short-term changes in soil biochemical properties as affected by subsidiary crop cultivation in four European pedo-climatic zones. Soil Till Res 2018, 180, 126–136. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochemistry Reviews 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Waisen, P.; Cheng, Z.; Sipes, B.S.; Koon-Hui, W. Biofumigation effects of brassicaceous cover crops on soil health in cucurbit agroecosystems in Hawaii, USA. Pedosphere 2022, 32, 521–531. [Google Scholar] [CrossRef]
- Aydınlı, G.; Mennan, S. Biofumigation studies by using Raphanus sativus and Eruca sativa as a winter cycle crops to control root-knot nematodes. Brazilian Archives of Biology and Technology 2018, 61, e18180249. [Google Scholar] [CrossRef]
- Gabriel, J.; Quemada, M. Replacing bare fallow with cover crops in a maize cropping system: yield, N uptake and fertiliser fate. Eur J Agron 2011, 34, 133–143. [Google Scholar] [CrossRef]
- Mukherjee, R.; Sen, S. Agricultural sustainability through nitrogen fixation: approaches and techniques. Harvest 2021, 6, 48–55. [Google Scholar]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Meena, R.S.; Vijayakumar, V.; Yadav, G.S.; Mitran, T. Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant Growth Regul 2018, 84, 207–223. [Google Scholar] [CrossRef]
- Lai, H.; Gao, F.; Su, H.; Zheng, P.; Li, Y.; Yao, H. Nitrogen distribution and soil microbial community characteristics in a legume–cereal intercropping system: A review. Agronomy 2022, 12, 1900. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Lombardo, S.; Fontanazza, S.; Abbate, C.; Pandino, G.; Anastasi, U.; Onofri, A.; Mauromicale, G. Improving soil health, weed management and nitrogen dynamics by Trifolium subterraneum cover cropping. Agron Sustain Dev 2020, 40, 1–12. [Google Scholar] [CrossRef]
- Campiglia, E.; Mancinelli, R.; Radicetti, E.; Baresel, J.P. Evaluating spatial arrangement for durum wheat (Triticum durum Desf.) and subclover (Trifolium subterraneum L.) intercropping systems. Field Crops Res 2014, 169, 49–57. [Google Scholar] [CrossRef]
- Guardia, G.; Aguilera, E.; Vallejo, A.; Sanz-Cobena, A.; Alonso-Ayuso, M.; Quemada, M. Effective climate change mitigation through cover cropping and integrated fertilization: A global warming potential assessment from a 10-year field experiment. Journal of Cleaner Production 2019, 241, 118307. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Sauer, T.J.; Cruse, R.M. Chapter one—soil: the forgotten piece of the water, food, energy nexus. Advances in agronomy 2017, 143, 1–46. [Google Scholar]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron Sustain Dev 2016, 36, 1–14. [Google Scholar] [CrossRef]
- Fargione, J.E.; Bassett, S.; Boucher, T.; Bridgham, S.D.; Conant, R.T.; Cook-Patton, S.C.; Ellis, P.W.; Falcucci, A.; Fourqurean, J.W.; Gopalakrishna, T. Natural climate solutions for the United States. Science Advances 2018, 4, eaat1869. [Google Scholar] [CrossRef]
- Lemaire, G.; Franzluebbers, A.; de Faccio Carvalho, P.C.; Dedieu, B. Integrated crop–livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric Ecosyst Environ 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Bukovsky-Reyes, S.; Isaac, M.E.; Blesh, J. Effects of intercropping and soil properties on root functional traits of cover crops. Agric Ecosyst Environ 2019, 285, 106614. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol 2011, 156, 1078–1086. [Google Scholar] [CrossRef]
- Sun, F.; Pan, K.; Olatunji, O.A.; Li, Z.; Chen, W.; Zhang, A.; Song, D.; Sun, X.; Huang, D.; Tan, X. Specific legumes allay drought effects on soil microbial food web activities of the focal species in agroecosystem. Plant Soil 2019, 437, 455–471. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Six, J.; Sun, J.H.; Bao, X.G.; Zhang, F.S.; Van Der Werf, W. Intercropping enhances soil carbon and nitrogen. Global change biology 2015, 21, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, E.V.; Notaro, K.d.A.; de Barros, J.A.; Duda, G.P.; Moraes, M.d.C.H.d.S.; Ambrósio, M.M.d.Q.; Negreiros, A.M.P.; Sales Júnior, R. Soils from intercropped fields have a higher capacity to suppress black root rot in cassava, caused by Scytalidium lignicola. J Phytopathol 2019, 167, 209–217. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Santos, G.A.; Moitinho, M.R.; de Oliveira Silva, B.; Xavier, C.V.; Teixeira, D.D.B.; Corá, J.E.; Júnior, N.L.S. Effects of long-term no-tillage systems with different succession cropping strategies on the variation of soil CO2 emission. Science of the total environment 2019, 686, 413–424. [Google Scholar] [CrossRef]
- Graf, D.R.; Saghaï, A.; Zhao, M.; Carlsson, G.; Jones, C.M.; Hallin, S. Lucerne (Medicago sativa) alters N2O-reducing communities associated with cocksfoot (Dactylis glomerata) roots and promotes N2O production in intercropping in a greenhouse experiment. Soil Biol Biochem 2019, 137, 107547. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.; Jones, H.G.; Karley, A.J. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytologist 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Chai, Q.; Nemecek, T.; Liang, C.; Zhao, C.; Yu, A.; Coulter, J.A.; Wang, Y.; Hu, F.; Wang, L.; Siddique, K.H. Integrated farming with intercropping increases food production while reducing environmental footprint. PNAS 2021, 118, e2106382118. [Google Scholar] [CrossRef]
- Xiao, J.; Yin, X.; Ren, J.; Zhang, M.; Tang, L.; Zheng, Y. Complementation drives higher growth rate and yield of wheat and saves nitrogen fertilizer in wheat and faba bean intercropping. Field Crops Res 2018, 221, 119–129. [Google Scholar] [CrossRef]
- Luo, S.; Yu, L.; Liu, Y.; Zhang, Y.; Yang, W.; Li, Z.; Wang, J. Effects of reduced nitrogen input on productivity and N2O emissions in a sugarcane/soybean intercropping system. Eur J Agron 2016, 81, 78–85. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.-Y.; Wu, H.-M.; Zhang, F.-F.; Li, C.-J.; Li, X.-X.; Lambers, H.; Li, L. Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. PNAS 2016, 113, 6496–6501. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Dang, K.; Lv, S.; Zhao, G.; Wang, H.; Feng, B. Interspecific competition and nitrogen application alter soil ecoenzymatic stoichiometry, microbial nutrient status, and improve grain yield in broomcorn millet/mung bean intercropping systems. Field Crops Res 2021, 270, 108227. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, H.; Zhang, W.; Bezemer, T.M.; Liang, W.; Li, Q.; Li, L. Interspecific interactions between crops influence soil functional groups and networks in a maize/soybean intercropping system. Agric Ecosyst Environ 2023, 355, 108595. [Google Scholar] [CrossRef]
- Teshita, A.; Feng, Y.; Qian, R.; Wang, X.; Khan, W.; Gao, Y. Alfalfa and maize intercropping enhances soil nematode structure and food web complexity in low-nitrogen soils. Appl Soil Ecol 2023, 186, 104809. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, J.; Ge, J.; Nie, J.; Zhao, J.; Xue, Z.; Hu, Y.; Yang, Y.; Peixoto, L.; Zang, H. Intercropping improves soil ecosystem multifunctionality through enhanced available nutrients but depends on regional factors. Plant Soil 2022, 480, 71–84. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C. Enhancing climate change resilience in agricultural crops. Current Biology 2023, 33, R1246–R1261. [Google Scholar] [CrossRef]
- Mugi-Ngenga, E.; Bastiaans, L.; Zingore, S.; Anten, N.; Giller, K. The role of nitrogen fixation and crop N dynamics on performance and legacy effects of maize-grain legumes intercrops on smallholder farms in Tanzania. Eur J Agron 2022, 141, 126617. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, L.; Chen, P.; Liu, X.; Song, C.; Yang, F.; Wang, X.; Liu, W.; Sun, X.; Du, J. Relay-intercropping soybean with maize maintains soil fertility and increases nitrogen recovery efficiency by reducing nitrogen input. The Crop Journal 2020, 8, 140–152. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of production in intercropping impact yield gains. Nature Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Chen, Y.; Wang, M.; Lu, Q.; Wang, K.; Dou, Z.; Chi, Z.; Qiu, W.; Dai, J. Microbiome convergence enables siderophore-secreting-rhizobacteria to improve iron nutrition and yield of peanut intercropped with maize. Nature Communications 2024, 15, 839. [Google Scholar] [CrossRef]
- Barbieri, P.; Pellerin, S.; Seufert, V.; Nesme, T. Changes in crop rotations would impact food production in an organically farmed world. Nature Sustainability 2019, 2, 378–385. [Google Scholar] [CrossRef]
- Tiemann, L.; Grandy, A.S.; Atkinson, E.; Marin-Spiotta, E.; McDaniel, M. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecology letters 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jousset, A.; de Boer, W.; Carrión, V.J.; Zhang, T.; Wang, X.; Kuramae, E.E. Legacy of land use history determines reprogramming of plant physiology by soil microbiome. The ISME journal 2019, 13, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Hilton, S.; Bennett, A.J.; Keane, G.; Bending, G.D.; Chandler, D.; Stobart, R.; Mills, P. Impact of shortened crop rotation of oilseed rape on soil and rhizosphere microbial diversity in relation to yield decline. PLOS one 2013, 8, e59859. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Global Ecology and Conservation 2020, 23, e01118. [Google Scholar] [CrossRef]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotations. Biological reviews 2012, 87, 52–71. [Google Scholar] [CrossRef]
- Crookston, R.; Kurle, J.; Copeland, P.; Ford, J.; Lueschen, W. Rotational cropping sequence affects yield of corn and soybean. Agronomy Journal 1991, 83, 108–113. [Google Scholar] [CrossRef]
- Benitez, M.-S.; Osborne, S.L.; Lehman, R.M. Previous crop and rotation history effects on maize seedling health and associated rhizosphere microbiome. Sci Rep 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS microbiology ecology 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: the microbial ecology of the rhizosphere. Nature Reviews Microbiology 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Gan, Y.; Vujanovic, V. Pyrosequencing reveals how pulses influence rhizobacterial communities with feedback on wheat growth in the semiarid Prairie. Plant Soil 2013, 367, 493–505. [Google Scholar] [CrossRef]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nature plants 2016, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vilich, V. Crop rotation with pure stands and mixtures of barley and wheat to control stem and root rot diseases. Crop Prot. 1993, 12, 373–379. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Larney, F.J.; Blackshaw, R.E.; Pearson, D.C.; Eastman, A.H. Soil microbial biomass and its relationship with yields of irrigated wheat under long-term conservation management. Soil Sci. 2018, 183, 179–187. [Google Scholar] [CrossRef]
- Hamel, C.; Gan, Y.; Sokolski, S.; Bainard, L.D. High frequency cropping of pulses modifies soil nitrogen level and the rhizosphere bacterial microbiome in 4-year rotation systems of the semiarid prairie. Appl Soil Ecol 2018, 126, 47–56. [Google Scholar] [CrossRef]
- Bainard, L.D.; Navarro-Borrell, A.; Hamel, C.; Braun, K.; Hanson, K.; Gan, Y. Increasing the frequency of pulses in crop rotations reduces soil fungal diversity and increases the proportion of fungal pathotrophs in a semiarid agroecosystem. Agric Ecosyst Environ 2017, 240, 206–214. [Google Scholar] [CrossRef]
- Merz, U.; Falloon, R.E. powdery scab of potato—increased knowledge of pathogen biology and disease epidemiology for effective disease management. Potato Research 2009, 52, 17–37. [Google Scholar] [CrossRef]
- Nayyar, A.; Hamel, C.; Lafond, G.; Gossen, B.D.; Hanson, K.; Germida, J. Soil microbial quality associated with yield reduction in continuous-pea. Appl Soil Ecol 2009, 43, 115–121. [Google Scholar] [CrossRef]
- Qaswar, M.; Ahmed, W.; Jing, H.; Hongzhu, F.; Xiaojun, S.; Xianjun, J.; Kailou, L.; Yongmei, X.; Zhongqun, H.; Asghar, W. Soil carbon (C), nitrogen (N) and phosphorus (P) stoichiometry drives phosphorus lability in paddy soil under long-term fertilization: A fractionation and path analysis study. PLoS One 2019, 14, e0218195. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P. Soil quality–A critical review. Soil Biol Biochem 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.d.; Alves, P.R.L.; Paula, A.M.d.; Nakatani, A.S.; Pereira, J.d.M.; Nogueira, M.A. Soil health: looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Scientia Agricola 2013, 70, 274–289. [Google Scholar]
- Marchiori Junior, M.; Melo, W. Carbon, microbial biomass carbon and enzyme activity of a soil under natural forest, grassland and cotton culture. Revista Brasileira de Ciencia do Solo (Brazil) 1999. [Google Scholar]
- Sicardi, M.; Garcı́a-Préchac, F.; Frioni, L. Soil microbial indicators sensitive to land use conversion from pastures to commercial Eucalyptus grandis (Hill ex Maiden) plantations in Uruguay. Appl Soil Ecol 2004, 27, 125–133. [Google Scholar] [CrossRef]
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Soil enzymes as indication of soil quality. In Soil enzymology, Springer: 2010; pp. 119-148.
- Nannipieri, P.; Kandeler, E.; Ruggiero, P. Enzyme activities and microbiological and biochemical processes in soil. Enzymes in the Environment 2002, 1–33. [Google Scholar]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol Biochem 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Dick, W.; Tabatabai, M.A. Significance and potential uses of soil enzymes. Soil microbial ecology: applications in agricultural and environmental management. 1992, 95-127.
- Barrutia, O.; Garbisu, C.; Epelde, L.; Sampedro, M.; Goicolea, M.; Becerril, J. Plant tolerance to diesel minimizes its impact on soil microbial characteristics during rhizoremediation of diesel-contaminated soils. Science of the total environment 2011, 409, 4087–4093. [Google Scholar] [CrossRef]
- Galende, M.A.; Becerril, J.M.; Barrutia, O.; Artetxe, U.; Garbisu, C.; Hernández, A. Field assessment of the effectiveness of organic amendments for aided phytostabilization of a Pb–Zn contaminated mine soil. Journal of Geochemical Exploration 2014, 145, 181–189. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Epelde, L.; Garbisu, C.; Bernal, M. Evaluation of the phytostabilisation efficiency in a trace elements contaminated soil using soil health indicators. Journal of hazardous materials 2014, 268, 68–76. [Google Scholar] [CrossRef]
- Dubey, R.K.; Dubey, P.K.; Chaurasia, R.; Rao, C.S.; Abhilash, P.C. Impact of Integrated Agronomic Practices on Soil Fertility and Respiration on the Indo-Gangetic Plain of North India. Agronomy 2021, 11, 402. [Google Scholar] [CrossRef]
- Viaux, P.; Rieu, C. Integrated farming systems and sustainable agriculture in France. MONOGRAPHS-BRITISH CROP PROTECTION COUNCIL 1995, 297-297.
- Sarkar, D.; Rakshit, A.; Al-Turki, A.I.; Sayyed, R.; Datta, R. Connecting bio-priming approach with integrated nutrient management for improved nutrient use efficiency in crop species. Agriculture 2021, 11, 372. [Google Scholar] [CrossRef]
- Duarah, I.; Deka, M.; Saikia, N.; Boruah, H.D. Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. 3 Biotech 2011, 1, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Entesari, M.; Sharifzadeh, F.; Ahmadzadeh, M.; Farhangfar, M. Seed biopriming with Trichoderma species and Pseudomonas fluorescent on growth parameters, enzymes activity and nutritional status of soybean. International Journal of Agronomy and Plant Production 2013, 4, 610–619. [Google Scholar]
- Kim, P.J.; Chung, D.Y.; Malo, D. Characteristics of phosphorus accumulation in soils under organic and conventional farming in plastic film houses in Korea. Soil Sci Plant Nutr 2001, 47, 281–289. [Google Scholar] [CrossRef]
| PGPM | Function and role | References |
|---|---|---|
| Penicillium spp. | Promotion of plant growth, increase systemic resistance, and confrontation to Pseudomonas syringae pv.Tomato | Hossain, et al. [70] Hossain, et al. [71] |
| Trichoderma spp. | Plant growth promotion, production of indole-3-acetic acid IAA, production of siderophores, resistance against soil-borne disease | Contreras-Cornejo, et al. [72] Abdel-lateif [73] Asghar and Kataoka [42] |
| Aspergillus spp. | Induces systemic resistance against necrotrophic fungus, plant growth promotion and phytohormones production | Salas-Marina, et al. [74] |
| Purpureocillium spp. | Plant growth promotion and siderophores and enzymes production | Cavello, et al. [75] |
| Talaromyces spp. | Promotion of plant growth, phosphate solubilization, siderophores production | Yamagiwa, et al. [76] |
| Bacillus spp. | Promotion of plant growth, Indole-3-acetic acid (IAA) and phosphate solubilization, siderophore production | Mahmood and Kataoka [62] |
| Azotobacter spp. | Ammonia production, Indole-3-acetic acid, growth enhancement of canola and lettuce plant, Phosphorus solubilization | Kumar, et al. [77] Joseph, et al. [78] |
| Pseudomonas spp. | Growth improvement of Canola and tomato plants, hormones production such as IAA, production of siderophore | Ahemad and Khan [79] Singh [80] |
| Azospirillum spp. | Indole-3-acetic acid (IAA) production, siderophore and exo-polysaccharides, and promote canola plant growth | Singh [80] Ahemad and Khan [79] |
| Streptomyces spp. | Streptomyces inhibit Curvularia oryzae and volatile organic compounds (VOCs) releasing, promote plant growth as well as activities of defense related enzymes | Sunpapao, et al. [81] |
| Green manure | Their beneficial effects | References |
|---|---|---|
| Oats, Hairy vetch | Nutrients availability, soil organic matter, enhancing of soil enzymes and fungal biomass and soil inorganic N and P, reducing soil erosion | Toom, Tamm, Talgre, Tamm, Tamm, Narits, Hiiesalu, Mäe and Lauringson [93] Kataoka, Nagasaka, Tanaka, Yamamura, Shinohara, Haramoto, Hayakawa and Sakamoto [43] Sharma, et al. [106] |
| Winter rye, Brassica | Biofumigation, reduction of soil-borne diseases, enhancement of soil enzymes and nitrogen fixation as well as improvement of soil biodiversity and structure | Sharma, Singh, Kahlon, Brar, Grover, Dia and Steiner [106] Brennan, et al. [107] Srivastava and Ghatak [108] Dubey, et al. [109] |
| Mustard, Barley | Soil and ground covering, nutrient cycling and increasing microbial biomass, as well as weed suppression, Increasing fungi and bacteria ration in soil | Khan, Gwon, Alam, Song, Das and Kim [17] Clark [110] |
| Red clover, Peas, Oat | Enhancement of biological activity in rhizosphere, nitrogen fixation and reduction of soil erosion, improve biological health, increase fungal abundance and soil enzymes activity | Clark [110] Thapa, et al. [111] |
| Cereal rye, Annual ryegrass | Enhancement of SOM, control weed growth, control N2O emissions, NO3- reduction, C sequestration and sustain cash crops yields | Adetunji, et al. [112] Magdoff and Van Es [113] Boselli, et al. [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




