Introduction
Medium-chain fatty acid triglycerides (MCTs) such as hexanoic, octanoic, decanoic and dodecanoic acid esters of glycerol are well established as nontoxic compounds with important medicinal and nutritional properties. For example, a patient with an unresponsive cancer of the lymphatic channels and nodes was free of disease for two years upon treatment with MCTs [
1]. More recently, the anticancer activity of MCTs was reported in a murine pancreatic cancer model [
2]. The anti-inflammatory and antifibrotic properties of MCTs were reported to reverse alcoholic liver injury despite continued administration of alcohol [
3]. Subsequently, it was reported that the anti inflarnrnatory and antifibrotic activity of MCTs was sufficient to induce nephroprotection against renal fibrosis, sclerosis and end-stage renal disease [
4]. Other therapeutic benefits attributed to MCTs include stimulation of hematopoiesis and erythropoiesis [
5]. Indeed, MCTs, unlike their longer chain fatty acid triglyceride homologue LCTs, exhibit properties that endow health benefits which result in an improved metabolic profile that includes protection against metabolic syndrome. The latter includes diabetes, kidney and cardiovascular disease, hypertension, hyperlipidemia and obesity. In fact, the above health benefits combined with the excellent safety of human consumption of MCTs, up to 1 g/kg body weight, supports the GRAS (Generally Regarded As Safe) status granted by the US FDA to MCTs composed of octanoic and decanoic acid for use as a food ingredient. Additionally, in 2009 the US FDA granted medical food status to the MCT composed of octanoic acid; Axona Ltd, UK.
This triglyceride is used for the dietary management of metabolic processes associated with Alzheimer’s disease. In view of the above health benefits, the market for MCTs as a nutritional supplement is increasing. MCTs are also used in the pharmaceuticals industry as excipients that include emulsifiers, delivery vehicles and lubricants. Similar use of MCTs occurs in the cosmetics and perfume industries. Indeed, the commercial importance of MCTs is reflected by the multiple available reviews [
6].
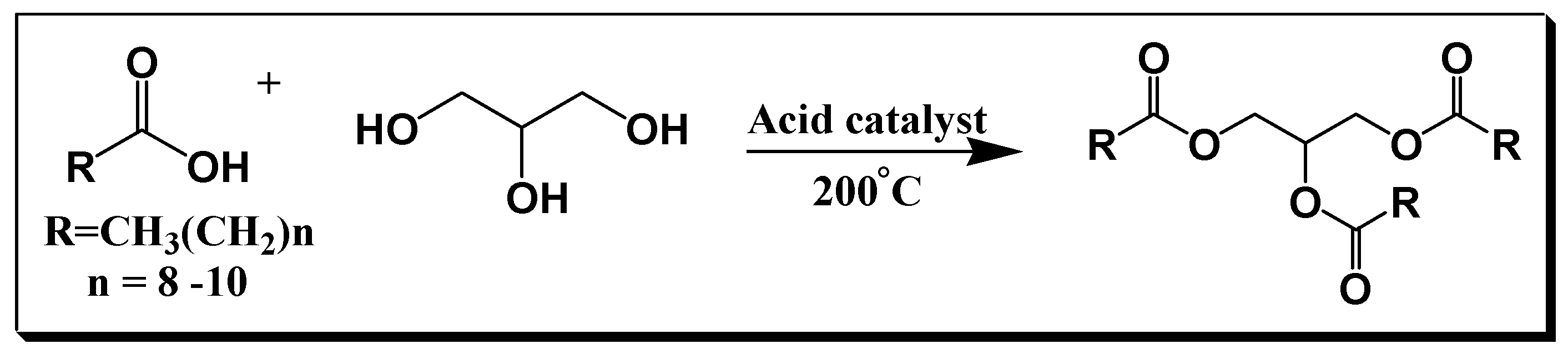
The synthesis of MCTs is typically accomplished by the reaction of glycerol with medium-chain fatty acids at high temperature (140-260°C) in the presence of an acid catalyst (
Scheme 1). Use of an enzyme catalyst (lipase)[
7] or ultrasonic reactors[
8] permits reaction at a lower temperature (70-90°C). However, the low purity of the triglyceride obtained by these techniques necessitates decolorization and chromatography purification or distillation which adds to the difficulty of large-scale synthesis. In general, the yield and purity obtained by known techniques does not exceed 75% due to multiple factors including incomplete esterification and product loss during workup and purification. Variations of the above are possible. For example, Lao
et al [
9]. claims the synthesis of MCTs enriched in lauric acid. As such, the MCTs are restricted to octanoic, decanoic and lauric acid obtained from coconut or palm kernel via fractionated methyl esters. In this case, synthesis takes place via transesterification between the appropriate fatty acid methyl ester and glycerol in the presence of acid, base or metal catalysts at 140-260°C. In spite of the multitude of existing methods, no satisfactory procedure has been developed for the preparation of glycerides of even (natural; 6, 8, 10 or 12) or odd-number (7, 9 or 11) of carbon atoms medium-chain fatty acids.
The general methods for esterification described above must lead to an almost complete elimination of mono- and diglycerides because of their bitter taste. The unreacted free fatty acid or methyl ester content of the end-product has to be very low. The necessity of using a large excess of fatty acid or ester in the esterification step to eliminate the mono- and diglycerides, results in considerable refining losses, which increase the cost of MCTs manufacture [
10]. Also, the high reaction temperature (>200°C) is likely to cause the polymerization or degradation of fatty acids, glycerol dehydration and oxidation, along with other side reactions, resulting in poor product color. As a result, product purification must be scrupulous. For example, to remove residual methanol from products or separate product from catalyst. These characteristics must be taken into consideration when scaling up the synthesis of MCTs. Here we report a general, simple, environmentally friendly, cost-effective new catalysts, high yield and purity one-step procedure for the synthesis of triglycerides of medium-chain length fatty acids.
Results and Discussion
Large amounts of high purity MCTs (>99.5%) of different chain length from six to twelve carbon atoms were required for our therapeutic program. Therefore, there was a need to develop a robust method to supply a kilogram of these compounds. Our first attempt was to treat glycerol with three and a half molar equivalents of capric acid in the presence of methanesulfonic acid [
11] (0.03 equivalent). The reaction was heated at 150°Cunder vacuum of 10 Torr. The residue was dissolved in isooctane and washed with sodium bicarbonate, followed by acetonitrile and water. The reaction tended to be problematic with regards to the purity of triglycerides, presence of side products and product yield (<55%). Also, it was reported in the literature that the catalysts used for the preparation of triglycerides are p-toluenesulfonic acid or hydrochloric acid [
12]. The activity and stability of these acid catalysts are affected by reaction conditions such as time and temperature. The above limits the generality of this method and scale-up of the triglycerides.
To overcome this problem, we examined a variety of metal salt catalysts for this reaction. The advantage of these catalysts might be recycling to permit multiple synthesis cycles. Overall, the potential low-cost heterogeneous catalysts have been demonstrated for transesterification applications. For example, it was reported that metal catalysts such as tungsten [
13], chromium [
14]
, calcium [
15,
16] and magnesium oxide [
16] were used for the esterification of used vegetable oils to produce biodiesel. These catalysts were investigated starting with the inexpensive metal salt tungsten trioxide.
Large Scale Production, 10 to 500 Grams
Decanoic acid was chosen as a representative medium chain fatty acid. Our process strategy was the treatment of a slight excess of decanoic acid (25%) with glycerol in the presence of tungsten trioxide (1-1.5%). lt is necessary to target a temperature range of 150 -180°C under partial vacuum (water pump vacuum). The ideal work-up is to dissolve the residue in a warm solvent that upon cooling would precipitate the corresponding triglyceride in high purity. A variety of solvents were investigated including methanol, isopropanol, acetonitrile, ethyl acetate and acetone, but ultimately the best choice is ethanol.
Thus, the use of 95% ethanol produced high solubility of the triglyceride in ethanol, is not toxic and facilitates crystallization of the product ester from the reaction medium. The reaction temperature was also optimized. The mixture was heated at a temperature that ranged from 150-230°C under partial vacuum for 22 h and then cooled to 25°C. Best results were obtained at a temperature of 175°C and partial vacuum of 10 Torr. With a vacuum of 1.0-0.7 Torr, the yield was lower because of the loss of decanoic acid under this pressure. The reaction was then cooled to 0-5°C for 2 h at a rate of 1°C/min to crystallize the triglyceride in high yield and purity. Encouraged by these results, we investigated other metal catalysts. In fact, a good result was obtained by use of an oxide or a chloride of one of the following metals: tungsten, calcium, magnesium, zinc, molybdenum or chromium. The preferred catalysts that gave high yield are the oxides of tungsten, calcium or zinc and the amount used in the reaction is 1 - 2%.
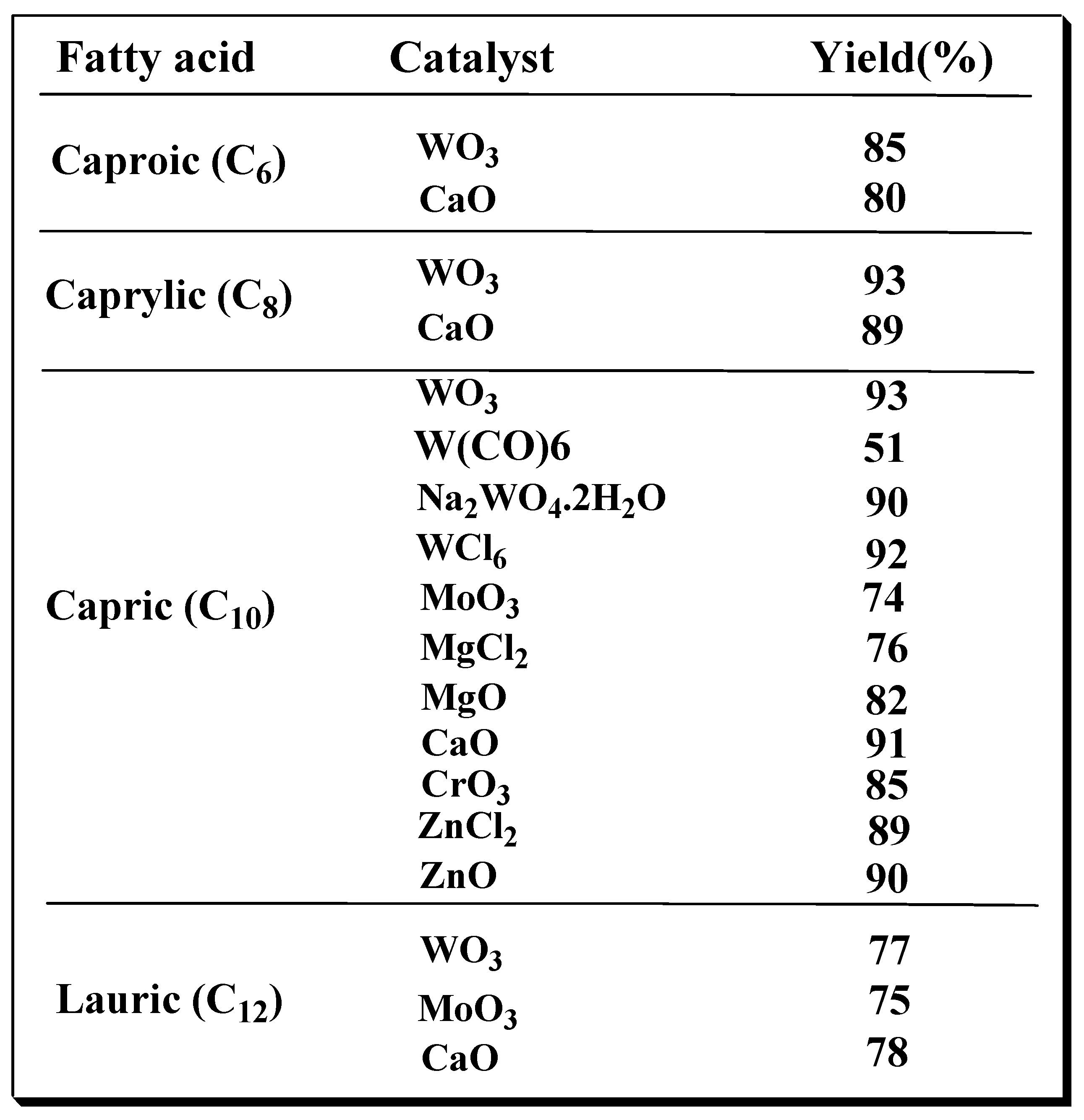
Table 1 summarizes the yield of triglycerides of medium-chain fatty acids with the use of different catalysts.
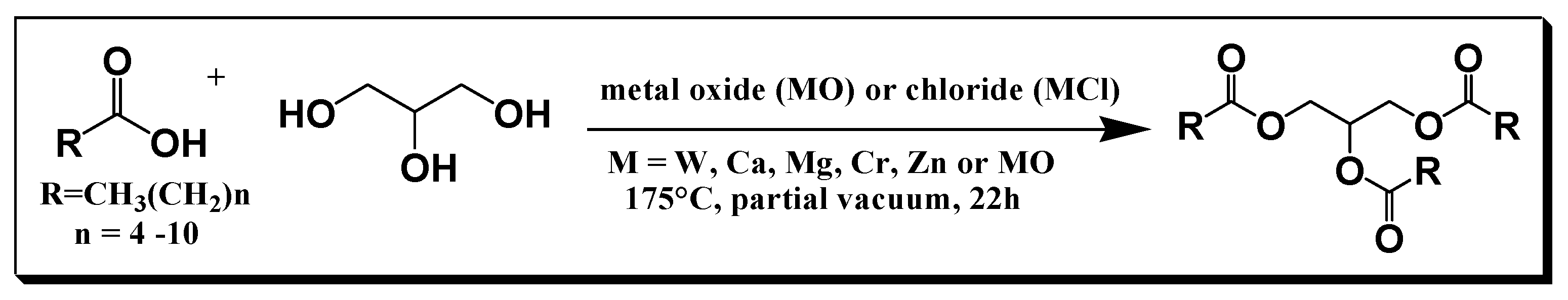
The above efforts resulted in the simplified general synthesis of the triglyceride of medium- chain length fatty acids which is described in
Scheme 2.
This is a general approach for the preparation of triglycerides of medium-chain length fatty acids. Glycerol is reacted with four molar equivalents of monocarboxylic fatty acids including C6 (caproic acid, hexanoic acid), C8 (caprylic acid, octanoic acid), C10 (capric acid, decanoic acid) and C12 (lauric acid, dodecanoic acid); in the presence of metal oxide or chloride catalyst such as calcium oxide or tungsten oxide and in absence of a solvent. The reaction is undertaken under partial vacuum (higher than 1 mm Hg) and at a temperature of 175°C for 22 h to produce the triglyceride product. The reaction was then cooled to room temperature and the residue dissolved in hot 95% ethanol. This solution was treated with charcoal, filtered over fiberglass and cooled in an ice bath at 0-5°C for 2 h. MCT product crystallized as a white solid which was filtered and washed with cold 95% ethanol. The crude cold product in ethanol facilitated the precipitation of the pure triglyceride product. Also, one of the best solvents used to wash the solid and remove impurities and the excess fatty acids was ethanol. All MCTs such as caproic, caprylic, lauric and capric triglycerides were isolated in high yield (80-95%) and high purity (>99.8%). Similarly, odd numbered carbon atom chain length carboxylic acid triglycerides were also conveniently prepared in high yield and purity using this triesterification protocol. Odd numbered carbon atom chains include 7 (heptanoic acid), 9 (nonanoic acid) and 11 (undecanoic acid) carbon fatty acids. This procedure served to produce 33 batches of 1- 50 grams of tricaprin using different metal salt catalysts; W(C0)6, Na2W04, WCl6, Mo03, MgCl2, MgO and Cr03. However, three catalysts, W(C0) 6, Mo03 and MgCl2, did not give high yield (50-76%) compared to the other metal catalysts (80-93%). The purity was almost the same for all catalysts (>99.2%). The best results were obtained using W03 and CaO. This allowed tricaprin to be isolated in 91% yield and 99.8% purity. This optimized procedure was successfully repeated at 5x25 g, 5x50 g and 5x100 g scale for W03 and 2x50 g and 2x100 g for CaO, then scaled up for both catalysts to 2x200 g. Our acceptance criteria for the final product included visual appearance, melting point, purity, solubility, NMR and mass spectra. In the case of tungsten as a catalyst, analysis of the metal in the final product was determined by neutron activation. The result was in the range of the detection limit (0.056µg/g or 0.056 ppm).
Large-Scale Production, One Kilogram
The optimized procedure was used for synthesis on a one- kilogram scale. However, the quantity of ethanol was reduced by 60% from the optimized small-scale procedure to solve the problem of low throughput. The rate of cooling for precipitation (1°C/min) was kept constant. The tricaprin obtained from this batch was a white dense uniform solid.
Conclusions
A simple, practical, one-step general procedure was developed for the preparation of triglycerides of medium-chain length fatty acids without solvent. The combination of the catalyst, partial vacuum and temperature offers ideal conditions for esterification of glycerol with medium chain length fatty acids. The starting materials are inexpensive, available and easy to handle. This approach overcomes many of the limitations previously reported for the preparation of triglycerides. The potential utility of this method is exemplified by the production on one-kilogram scale of high yield (91%) and purity (99.8%) tricaprin.
Experimental Section
All HPLC chromatograms and mass spectra were recorded on an HP 1100 LC-MS Agilent instrument using an analytical Zorbax SB-phenyl column with a gradient over 8 min of 15- 99% acetonitrile-water with 0.01% trifluoroacetic acid as the eluent and a flow of 2 mL/minute. An ELSD detector was used to analyze the triglycerides. NMR spectra were recorded on a 400 MHz Varian spectrometer.
Example 1: Preparation of Tricaprin (General Procedure)
To a flask containing glycerol (180 g, 1.95 mol) and equipped with a condenser, was added capric acid (1.346 kg, 7.813 mol) and calcium oxide (1.64 g, 29.31 mmol). The mixture was heated at 175°C under partial vacuum (10 Torr, water pump vacuum) for 22 h. The temperature of the water in the condenser was approximately 35°C in order to maintain a gentle reflux of the capric acid and to accelerate removal of water under vacuum. The reaction was cooled to room temperature and the residue dissolved in hot ethanol (95%, 6 L). This solution was treated with Charcoal, filtered over fiberglass and cooled in an ice bath at 0-5°C for 2 h. Tricaprin was crystallized as a white solid which was filtered, washed with cold ethanol (95%, 40 mL), with cold water (2 L) and dried under vacuum for 48 h. Yield of product: 979.7 g (91%); mp: 29-31°C; 1H NMR (CDCl3): δ 5.22-5.29 (m, lH), 4.29 (dd, J = 11.9, J = 4.3, 2H), 4.14 (dd, J = 11.9, J = 6.1, 2H), 2.26-2.34 (m, 6H), 1.54-1.65 (m, 6H), 1.18-1.36 (m, 36H), 0.87 (t, J = 7.0, 9H). 13C NMR (101 MHz, CDCl3): 8 73.54, 173.13, 69.07, 62.32, 34.44, 34.27, 32.09, 29.67, 29.65, 29.51, 29.50, 29.34, 29.30, 25.13, 25.08, 22.90, 14.33. UPLC: 99.8% purity.
Example 2: Trilaurin
Triglyceride of lauric acid was prepared as described in the general procedure above by use of 15 g of lauric acid (74.9 mmol), 1.7 g glycerol (18.7 mmol) and 15.7 mg calcium oxide (0.28 mmol). Yield of product: 9 g (78%); mp = 45-47°C; 1H NMR (CDCl3): δ 5.25-5.28 (m, lH), 4.29 (dd, J = 11.7, J = 4.3, 2H), 4.14 (dd, J = 11.9, J = 6.1, 2H), 2.28-2.34 (m, 6H), 1.55-1.66 (m, 6H), 1.20-1.36 (m, 48H), 0.87 (t, J= 7.0, 9H). 13C (101 MHz, CDCl3): ô 173.55, 173.14, 69.07, 62.33, 34.45, 34.29, 32.15, 29.86, 29.73, 29.71, 29.58, 29.53, 29.50, 29.35, 29.31, 25.10, 22.92, 14.36; HPLC: 7 min.
Example 3: Tricaprylin
Triglyceride of caprylic acid was prepared as described in the general procedure above by use of 11 g of caprylic acid (74.9 mmol), 1.7 g glycerol (18.7 mmol) and 15.7 mg calcium oxide (0.28 mmol). Since tricaprylin is a liquid, the crude product was filtered on silica gel, instead of fiberglass, using ethyl acetate/hexanes (5-10%). This gave the pure product as a colorless oil. Yield: 8 g (89%); 1H NMR (CDCl3): δ 5.25-5.28 (m, lH), 4. 29 (dd, J = 11.9, J = 4.3, 2H), 4.14 (dd, J = 11.9, J = 6.1, 2H), 2.28-2.34 (m, 6H), 1.56-1.66 (m, 6H), 1.20-1.36 (m, 24H), 0.87 (t, J = 7.0, 9H). 13C (101 MHz, CDCl3): () 173.56, 173.14, 69.07, 62.33, 34.45, 34.28, 31.89, 31.88, 29.28, 29.24, 29.16, 29.14, 25.13, 25.08, 22.83, 14.30; HPLC: 5 min.
Example 4: Tricaproin
Since tricaproin is a volatile compound, the general procedure was slightly modified. The procedure detailed here is applicable to the method of preparing the triglycerides of volatile medium-chain fatty acids such as those of chain length of 6 to 7 carbons. In a flask containing glycerol (1.73 g, 18.7 mmol), equipped with a condenser and a Dean-Stark trap filled with caproic acid, was added caproic acid (8.7 g, 74.9 mmol) and calcium oxide (15.7 mg, 0.3 mmol). The mixture was heated at 175°C under vacuum overnight (22 h, 10 mm Hg). The mixture was cooled and dissolved in ethyl acetate. This solution was washed with 10% sodium hydroxide, brine (NaCl), treated with magnesium sulfate- charcoal for water removal and filtered on fiberglass. The filtrate was concentrated to give a yellow oil which was dissolved in hexanes and poured on a 10x10 cm2 silica gel pad. The compound was eluted with 10% ethyl acetate/hexanes. The pure fractions were combined and concentrated to give a colorless oil. Yield: 5.8 g, 80%; 1H NMR (CDCl3): δ 5.23-5.29 (m, l H), 4.29 (dd, J = 11.9, J = 4.3, 2H), 4.14 (dd, J = 11.9, J = 6.1,2H), 2.27-2.34 (m, 6H), 1.56-1.66 (m, 6H), 1.22-1.37 (m, 12H), 0.89 (t, J = 7.0, 9H). 13C (101MHz, CDCl3): S 173.56, 173.14, 69.07, 62.32, 34.39, 34.23, 31.45, 31.41, 24.78, 24.75, 22.51, 14.12; HPLC: 4 min.
Author Contributions
Christopher Penney: Review and editing, Literature collection and analysis, Project administration. Boulos Zacharie: Project conceptualization, Drafting of original manuscript, Review and editing, Literature collection and analysis, Project administration, Methodology. Jean-Simon Duceppe: Project conceptualization Review and editing, Methodology, Experimentation, Literature collection and analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no grants were received in support of this work.
Institutional Review Board Statement
No animal studies or human trials were undertaken for this project by the authors thereby negating the need for animal (ethics) committee, IRB and human ethics committee approvals
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Abbreviations
MCT: medium-chain fatty acid triglyceride; LCT: longer-chain fatty acid triglyceride homologue; GRAS: generally regarded as safe.
References
- (a) Calabrese, P. R.; Frank, H. D.; Taubin, H. L. Cancer 1977, 40, 895-897. (b) Nebeling, L. C.; Lemer, E. J Am Diet Assac., 1995, 95(6 ), 693-697. (c) Cohen, L. A.; Thompson, D. O.; Maeura, Y.; Weisburger, J. H. Cancer Res. 1984, 44, 5023-5028.
- Gagnon, L.; Geerts, L.; Penney, C. US Patent 8,946,190, 2015.
- Paulson, S. K.; Vaughn, M. B.; Jessen, S. M.; Lawal, Y.; Gresk, C. J.; Yan, B.; Maziasz, T. J.; Cook, C. S.; Karim, A. J. Pharmacol. Exp. Ther. 2001, 299, 638-644.
- Pichette, V.; Leblond, F.; Lagraoui, M.; Gagnon, L. US Patent 9,532,962, 2017. (a) Gagnon, L.; Barabé, J.; Laurin, P.; Penney, C.; Zacharie, B. US Patent 7,745,488, 2010.
- (b) Gagnon, L.; Barabé, J.; Laurin, P.; Penney, C.; Zacharie, B. US Patent 8,487,001, 2013. (c) Gagnon, L.; Barabé, J.; Laurin, P.; Penney, C.; Zacharie, B. US Patent 9,682,054, 2017.
- (a) Greenberger, N. J.; Skillman, T. G. N Engl. J Med. 1969, 280, 1045-1058. (b) Bach, A.C.; Lutz, F. O. Clinical Nutrition 1989, 8, 223-235. (c) Traul, K. A.; Driedger, A.; Ingle, D. L.; Nakhasi, D. Food Chem. Toxicol. 2000, 38, 79-98. (d) Wanten, G.J.; Naber, A. H. Mini Rev. in Med. Chem. 2004, 4, 847-857.
- Langone, M. A.; Sant'Anna, G. L. Jr. Appl. Biochem Biotechnol. 2002, 98-100, 997-1008.
- Mohod, A. V.; Gogate, P. R.; Ultrasonics Sonochemistry 2018, 42, 347-355.
- Lao, D. A. Jr; Savador, S. D.; Apostol, G. C. US Patent W02016/007026, 2017.
- Hartman, L.; Reimann, D. Eur. J Lipid Sei. Technol. 1989, 91, 324-327.
- See the esterification reaction of glycerol and palm oil oleic acid using methyl ester sulfonate acid as catalyst. IOP conf. series: materials science and engineering 172, 2017, 012062.
- Tran, H.-L.; Ryu, Y.-J.; Seong, D. H.; Lim, S.-M.; Lee, C.-G. Biotechnol . Bioprocess Eng. 2013, 18, 242-247 and references cited in.
- (a) Park, Y. M.; Lee, J. Y.; Chung, S. H.; Park, I. S.; Lee, S. Y.; Kim, D. K.; Lee, J. S.; Lee, K. Y. Bioresour. Technol. 2010, 101 Suppl. 1 S59-61(b) Immobilized phosphotungstic acid (H3PW12040/C) was used to prepare medium-chain triglycerides. The drawbacks of this method are the preparation and activation of the catalyst and the many distillations at different temperatures 130-170°C under vacuum to purify and isolate the products. See CN1266107C.
- Wan, Z; Lim, J. K.; Hameed, B. H. Energy 2017, 141, 1989-1997.
- Kawashima A.; Matsubara K.; Honda K. Bioresour. Technol. 2009, 100, 696-700.
- Buasri, A.; Rochanakit, K.; Wong Vitvichot, W.; Masa-ard, U.; Loryuenyong,V. Energy Procedia 2015, 79, 562-566.
Scheme 1.
General synthesis for the preparation of medium-chain triglycerides.
Scheme 1.
General synthesis for the preparation of medium-chain triglycerides.
Scheme 2.
A large-scale synthesis for the preparation of medium-chain triglycerides.
Scheme 2.
A large-scale synthesis for the preparation of medium-chain triglycerides.
Table 1.
Yield of triglycerides of medium-chain length fatty acids with the use of different catalysts.
Table 1.
Yield of triglycerides of medium-chain length fatty acids with the use of different catalysts.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).