1. Introduction
The European sardine,
Sardina pilchardus (Walbaum, 1792), is one of the most abundant small pelagic fish in the northeast Atlantic Ocean, occurring from the North Sea to Senegal, as well as in the Mediterranean Sea [
1]. In European Atlantic waters, the commercial fishery targeting S. pilchardus is monitored by the International Council for the Exploration of the Sea (ICES) that surveys three major stocks: the southern Celtic Sea and English Channel stock, the Bay of Biscay stock, and the Cantabrian Sea and Atlantic Iberian waters stock (hereafter referred to as “the Iberian stock” for simplicity) [
2]. The Iberian stock distributes along the Atlantic coast of the Iberian Peninsula and is commercially explored by Portugal and Spain using purse-seines [
3]. According to the European Market Observatory for Fisheries and Aquaculture (EUMOFA),
S. pilchardus ranks among the top ten most landed and valued fishery products in Portugal [
4]. The Food and Agriculture Organization of the United Nations (FAO) further acknowledges that Portugal alone contributes an important part of S. pilchardus captures in European Union (EU) waters (as over 25,000 tons were landed in Portugal in 2022) [
5].
In 2017, the lowest level of recruitment ever recorded for
S. pilchardus in the Iberian stock prompted ICES to advise the closure of this fishery for 2018 [
6]. The social and economic impacts of such recommendation forced the governments of Portugal and Spain to adopt a multiannual medium-term management and recovery plan that could enable the Iberian stock of
S. pilchardus to bounce back under a maximum sustainable yield (MSY) approach [
7]. In Portugal, one of the measures implemented was a “reduction of the quantities allowed to be landed per day and trip including T4 category size (now 500 kg/day/vessel). Portugal can also reduce these limits for landings of all vessels in their representative ports to control catches, regulate the offer and promote a better valorization of catches” [
7]. This measure is prone to set the stage for several fraudulent practices, such as the illegal transfer of
S. pilchardus from one fishing vessel to another when daily quotas have already been achieved and the mislabeling of
S. pilchardus place of capture, so that fish can be landed on a more favorable fishing harbor to avoid landing limits and fetch better selling prices. The accurate tracing of
S. pilchardus place of origin is therefore of utmost importance to fight such practices of illegal, unreported, and unregulated (IUU) fishing. According to FAO, IUU fishing is a major threat to marine ecosystems due to its ability to undermine any efforts to manage fisheries sustainably and any endeavors to conserve marine biodiversity [
8]. Moreover, and from a social perspective, it has long been acknowledged that the threat posed by IUU fishing puts law-abiding fishers at an unfair disadvantage with those enrolled in such illegal practices [
9].
Seafood traceability has deserved increasing attention from the scientific community, particularly the verification of claims concerning geographic origin [
10,
11,
12,
13,
14]. The use of biochemical fingerprints, namely fatty acid (FA) and lipidomic profiles, have allowed achieving a remarkable level of confidence when verifying the accuracy of the geographic origin of less motile species of seafood, such as bivalves, namely Manila clams (
Ruditapes philippinarum) [
15] and common cockles (
Cerastoderma edule) [
16], as well as goose barnacles (
Pollicipes pollicipes) [
17] and Japanese sea cucumbers (
Apostichopus japonicus) [
18], among others. The FA profiles displayed by tissues with a high content of polar lipids are less prone to be shaped by the short-term turnover of FA related to dietary shifts or abrupt changes in abiotic conditions, as they rather reflect mid-to-long-term variations shaped by trophic history and more prevalent abiotic conditions [
10,
19]. Nonetheless, employing FA profiles to trace the geographic origin of highly motile species, such as
S. pilchardus, is certainly a more challenging task. Previous works on jumbo squid (
Dosidicus gigas) [
20], seabass (
Dicentrarchus labrax) [
13], and tunas (
Thunnus alalunga and
T. thynnus) [
21] have indicated that muscle FA profiles are also a promising tool for determining the geographic origin of highly motile species.
To the best of our knowledge, no study performed to date has investigated if the FA of small pelagic fish can be used as natural fingerprints to verify claims on their geographic origin. The present study employed a gas chromatography-mass spectrometry (GC-MS) approach to evaluate the potential of using the FA composition of S. pilchardus white muscle to confirm the geographic origin of individuals collected from several landing ports along the Iberian Atlantic coast. The validation of this traceability tool will be crucial for improving the management of fishing stocks and combat the increasingly reported IUU fishing practices for S. pilchardus.
2. Materials and Methods
2.1. Sampling
Landed
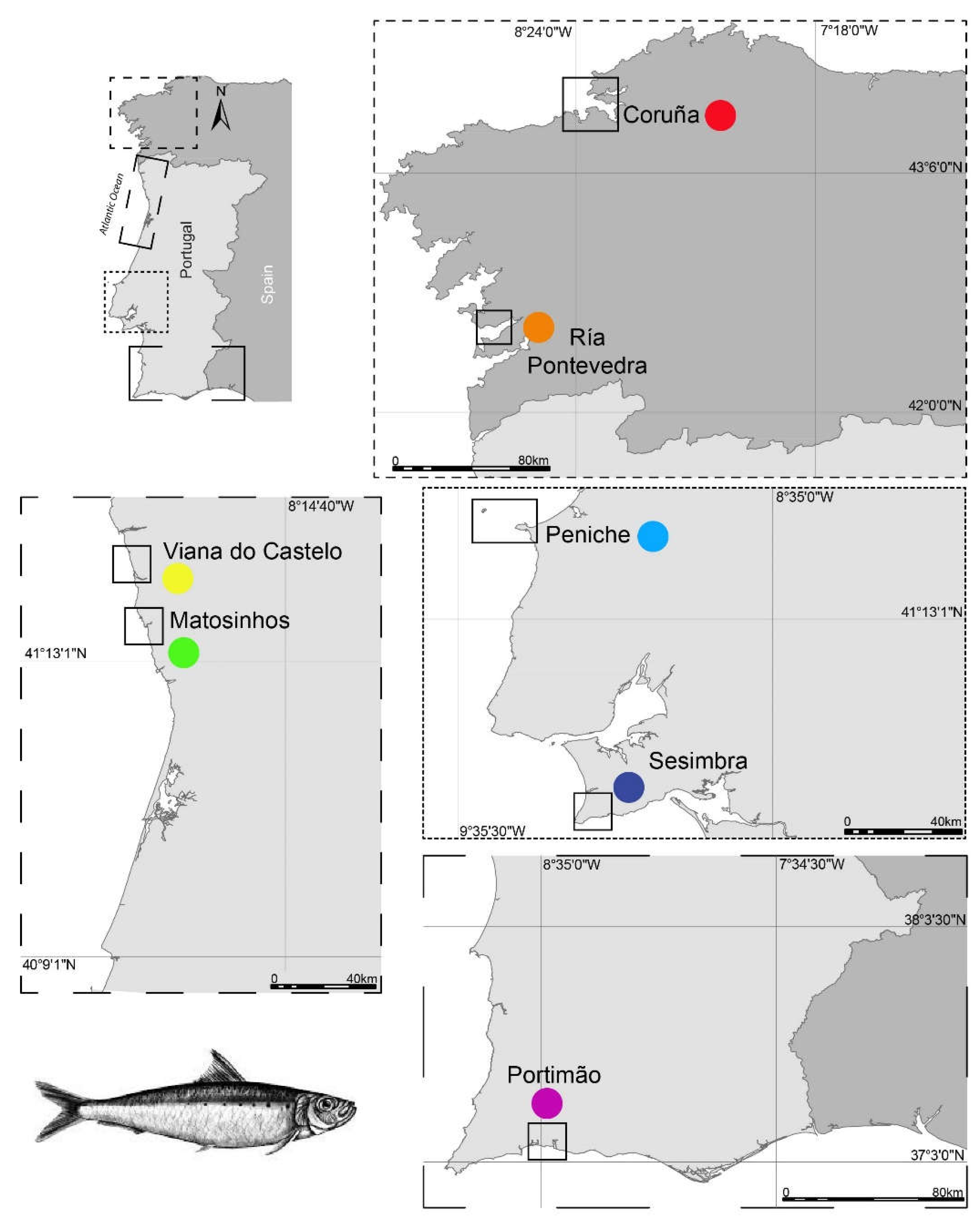
S. pilchardus of similar commercial size (T4 category) were obtained in seven relevant fishing harbors along the Iberian Atlantic coast during early summer (June and July) 2018: Malpica [Coruña (Cor), Spain; June 29, 2018], Bueu [Ria de Pontevedra (RP), Spain; June 27, 2018], Viana do Castelo [(VC), Portugal; July 16, 2018], Matosinhos [(Mat), Portugal; July 27, 2018], Peniche [(Pe), Portugal; July 13, 2018], Sesimbra [(Ses), Portugal; July 25, 2018], and Portimão [(Por), Portugal; July 27, 2018] (
Figure 1).
All specimens were acquired at the docking pier from trusted fishermen who ensured all specimens were captured in the coastal waters nearby the fishing harbor they were landed in. Sardines were fished and maintained refrigerated by dully licensed professional fishers onboard, being already dead when landed in the fishing harbor. As such, ethical issues concerning animal experimentation and welfare do not apply to the present study. Immediately after purchase, specimens were placed on aseptic bags and transported on cooler boxes to the University of Aveiro (Aveiro, Portugal) where their loins were removed and white and red muscles were separated before freeze-drying (CoolSafe 55-9L Pro; LaboGene, Lillerød, Denmark). The white muscle was subsequently macerated and freeze-dried again before preservation at -80 °C until biochemical analyses. Ten samples (n = 10) of white muscle from ten different specimens per fishing harbor were analyzed (n = 70 samples, i.e., 10 specimens × 7 fishing harbors, for FA analysis). The rationale for using white rather than red muscle of
S. pilchardus is that red muscle fibers are known for having a higher capacity to oxidize lipids than white muscle fibers (up to four-fold) [
22]; this feature could therefore prompt a faster shift of sardine red muscle FA profile post-harvesting and produce a blurring effect for the traceability of geographic origin.
2.2. Lipid Extraction and Fatty Acid Derivatization
All samples were first homogenized with a mortar grinder (RM200, Retsch, Hann, Germany). The biomass (20 mg per sample) was transferred to a glass tube containing 2500 μL of methanol (MeOH) and 1250 μL dichloromethane (CH2Cl2). Sample homogenization was performed by vortexing for 1 min followed by sonication for 1 min in cold water and incubation on ice in an orbital shaker for 30 min. Afterwards, the sample was centrifuged (Hettich universal 320, Tuttlingen, Germany) at 769 ×g and 4 ℃ for 10 min, and 3 mL of the organic phase was collected into a new tube. After the addition of 1250 μL CH2Cl2 and 1250 μL Milli Q water (MilliporeSigma, Burlington, MA, USA), centrifugation was performed at 492 ×g and 4 ℃ for 10 min to promote phase separation. An aliquot of the organic phase (75 μL final volume, fine-tuned after testing several aliquots of organic phase using GC-MS) was collected into a new tube previously rinsed with n-hexane. This aliquot was dried under a nitrogen gas stream and then used for derivation by transmethylation. Dry lipids were mixed with 1 mL n-hexane containing a C19:0 internal standard (4.4 μg mL−1, CAS number 1731-94-8; Merck, Darmstadt, Germany) and 200 μL KOH (2M) in MeOH, and vigorously homogenized by vortexing for 2 min. A saturated solution of NaCl (2 mL) was added, and the mixture was centrifuged at 492 ×g and 4 ℃ for 5 min. Subsequently, 600 μL were extracted from the organic phase to a microtube previously rinsed with n-hexane. This recovered volume, that holds the FA methyl esters (FAME), was then dried under a nitrogen gas stream, and kept at -20 °C until GC-MS analysis.
2.3. Gas Chromatography-Mass Spectrometry Analysis for Fatty Acid Profiling
Fatty acid methyl esters were dissolved in 150–200 μL n-hexane and 2 μL of this solution were then used for analysis on an 8860 GC system interfaced with a 5977B MS selective detector (both Agilent Technologies, Santa Clara, CA, USA) with electron impact ionization of 70 eV and scanning range of m/z 50–550 in a 1 s cycle in full scan acquisition mode. The GC-MS system was equipped with a DB-FFAP capillary column (J & W Scientific, Folsom, CA, USA; 30 m length, 0.32 mm internal diameter, and 0.25 μm film thickness). The oven temperature profile was as follows: initial temperature, 58 °C for 2 min; three consecutive linear increments to 160 °C at 25 °C min−1, 210 °C at 2 °C min−1, and 225 °C at 20 °C min−1; hold temperature, 225 °C for 20 min. Helium was used as the carrier gas at 1.4 mL min−1. The FA areas were integrated using the software GCMS5977B/Enhanced MassHunter, and qualitative data analysis was performed using the software MassHunter Qualitative Analysis 10.0 (both Agilent Technologies) to identify FAs. This identification was achieved considering the retention times and the MS spectra of FAME standards (Supelco 37 Component FAME Mix, ref. 47885-U; Sigma-Aldrich, St. Louis, MO, USA), and by MS spectrum comparison with chemical databases (Wiley 275 library, AOCS lipid library, and NIST 2014 Mass spectra library). The relative abundance (%) of each FA was calculated using the area of each peak obtained from integration with the Agilent MassHunter Qualitative10.0 software and considering the sum of all relative areas of the identified FAs.
2.4. Statistical Analyses
The relative FA composition per
S. pilchardus from each landing location was used to assemble a resemblance matrix among samples using Euclidean distances, following a log (x + 1) transformation to emphasize differences among locations [
23]. Only FAs with a relative abundance ≥ 1% of the total pool of FAs were considered for statistical analyses. The statistical differences (p < 0.05) in FA profiles among locations were tested using the vegan adonis() function for permutational multivariate analysis of variance (PERMANOVA) [
23]. A Boruta analysis, using the “TentativeRoughFix” function, was performed to select the most relevant FA to discriminate samples from different locations [
24]. Subsequently, a linear discriminant analysis (LDA) was employed to evaluate the possibility of successfully discriminating the geographic origin of sampled specimens using these FA present in the white muscle of
S. pilchardus. All statistical analyses were performed in R [
25].
2.5. Upwelling Index and Sea Surface Temperature
As upwelling plays a key role on the shaping and condition of S. pilchardus populations occurring in the Iberian Atlantic coast [
26], average upwelling indexes for each surveyed location were calculated using data from buoys operated by the Instituto Español de Oceanografía [
27]. Four measurements were retrieved from each location per day (one every 6 h), for every day of the month. Results presented are averages of 30 days prior to the day of capture, presented in m
3 s
-1 km
-1.
Sea surface temperature (SST) data were retrieved from The National Oceanic and Atmospheric Administration’s global temperature maps [
28] and calculated as monthly temperature percentiles. These allowed for relative temperature comparisons between the northern (Cor, RP, VC, and Mat) and the southern (Pe, Ses, and Por) locations surveyed in the present study.
3. Results
3.1. Fatty Acid Composition
A total of 35 FAs were identified from the white muscle of S. pilchardus, some of which (e.g., 12:0, 17:1n-9, 18:1n-5, 22:5n-6) were exclusive to one or two of the surveyed sampling sites (
Supplementary Table S1). Of these, the most abundant FA (relative abundance ≥ 1%) were selected for FA profiling and to investigate their potential use for the geographical discrimination of S. pilchardus samples collected at the seven locations surveyed along the Iberian Atlantic coast (
Table 1). Docosahexaenoic acid (22:6n-3, DHA), eicosapentaenoic acid (20:5n-3, EPA), and palmitic acid (16:0) were the most abundant FAs in all samples, representing 20.9%, 15.9%, and 17.9% of the total FA pool, respectively (
Supplementary Table S1). Polyunsaturated FAs (PUFAs) was the most abundant class across all locations (44.6% in RP to 52.4% in Ses, of the total FA pool), followed by saturated FAs (SFAs) in all samples, except Ses where monounsaturated FAs (MUFAs) were the second most abundant (
Table 1). Within PUFAs, DHA was the most abundant in all samples (from 20.9% in Por to 24.6% in Cor), except Pe where EPA was the most abundant (22.2%). Oleic acid (18:1 n-9) was the most abundant MUFA (from 7.1% in RP to 9.7% in Por) and 16:0 the most abundant SFA in all samples (from 14.2% in Ses to 23.3% in RP). In RP, 16:0 was the most abundant FA, surpassing the relative abundance of DHA. The EPA/DHA ratio varied largely among S. pilchardus samples, with a 2-fold increase from Cor (0.56) to Pe (1.22). The differences in FA profiles led to significant differences between all S. pilchardus landed in the fishing harbors surveyed, as revelead by PERMANOVA results (p < 0.05,
Table 2).
3.2. Fatty Acids as Markers of Geographic Origin
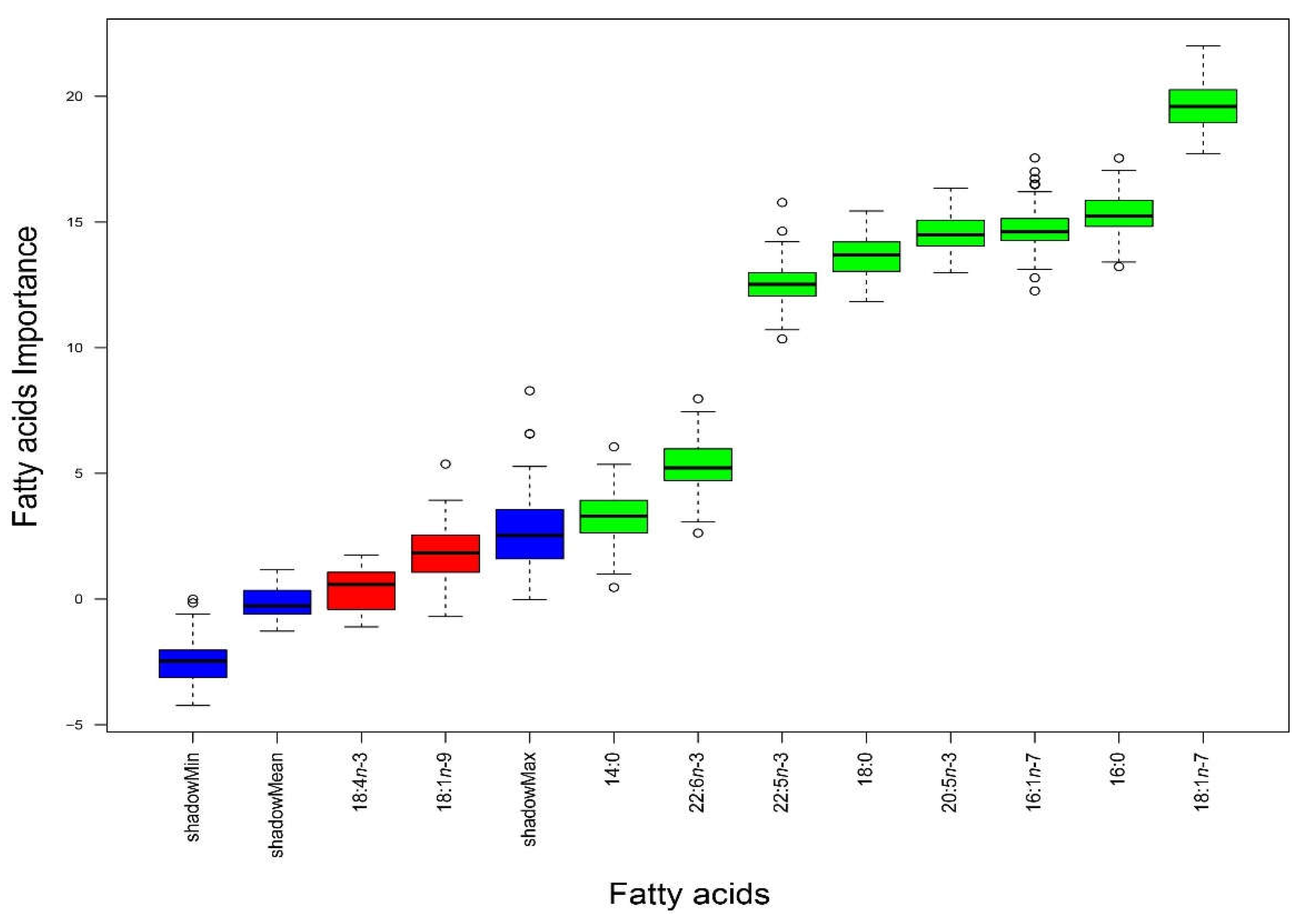
According to the Boruta analysis performed, the most important FAs to successfully discriminate between the seven landing locations were (in increasing order of relevance): myristic acid (14:0), DHA (22:6n-3), docosapentaenoic acid (DPA, 22:5n-3), stearic acid (18:0), EPA (20:5n-3), palmitoleic acid (16:1n-7), palmitic acid (16:0), and vaccenic acid (18:1n-7) (
Figure 2).
The relative abundances of these FAs were generally higher in samples from RP than in samples from Cor and Pe, except for 18:1n-7, 20:5n-3, and 22:5n-3, which were higher in Pe than in RP and Cor, and 22:6n-3, which was higher in Cor than in RP and Pe (
Table 1). These differences contributed to the contrasting levels of total SFAs, MUFAs, and PUFAs in S. pilchardus from Cor, RP, and Pe (
Table 1). Whereas Pe and RP displayed almost twice the relative abundance of total PUFAs than total MUFAs, differences between the relative abundances of the three FA classes were not that pronounced in Cor. Sardina pilchardus from Ses displayed the highest relative abundance of PUFAs and almost identical proportions of SFAs and MUFAs, in which they differed from S. pilchardus captured in Pe, RP, and Cor. These differences are reflected in the LDA plot (
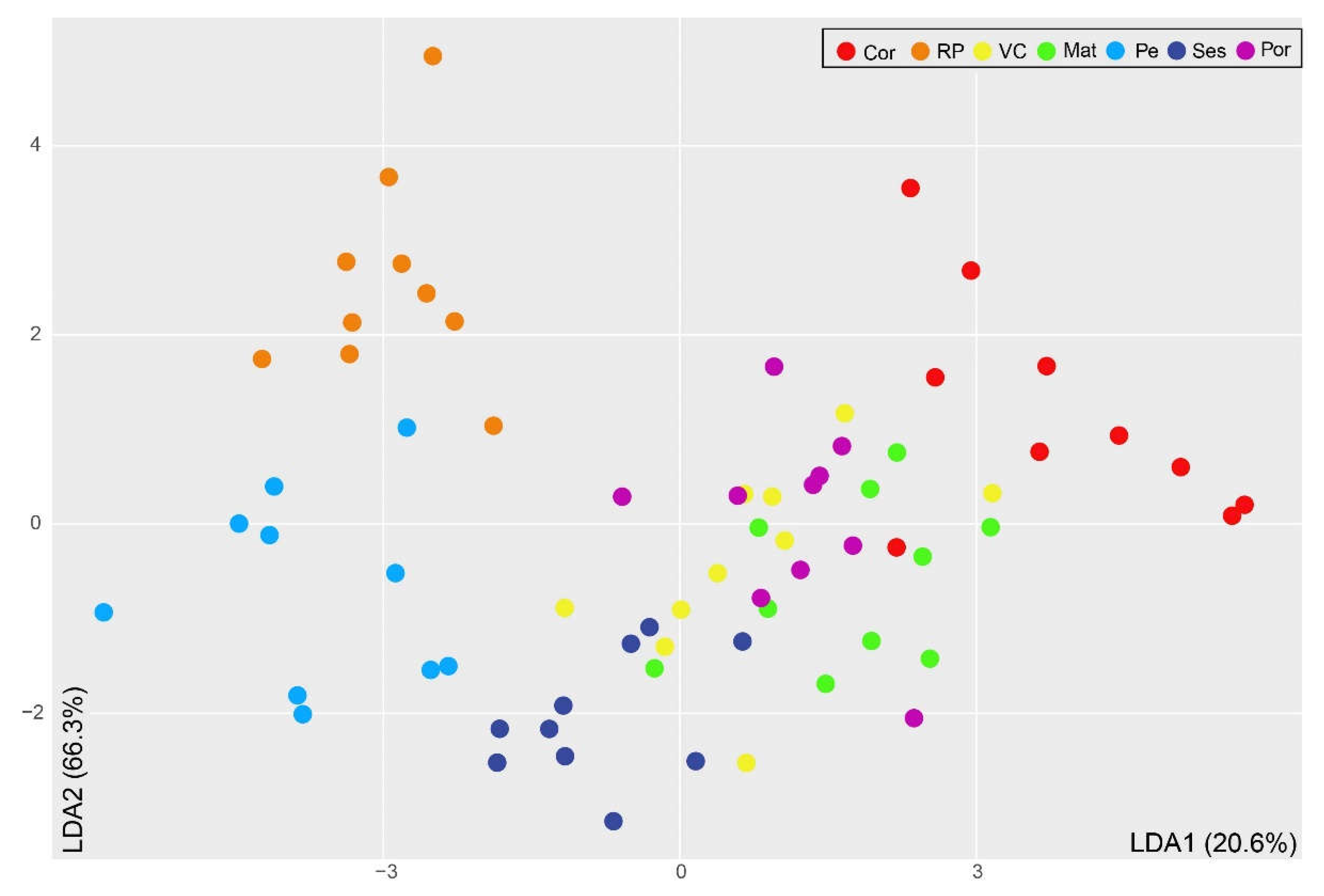
Figure 3), where samples from RP, Cor, and Pe appeared well separated from those collected in VC, Mat, Ses, and Por, which were overlapped. While the first discriminant function explained 20.6% of the variation recorded in the white muscle FA profiles, mostly associated with the relative abundance of 22:6n-3, the second discriminant function explained 66.3% of that variation, mostly associated with the relative abundances of 16:0 and 22:6n-3.
Results from the LDA were further supported by the percentages of correct classification (
Table 3). It is worth highlighting that RP and Pe samples, which appeared well separated in the LDA plot, showed the highest percentage of correct classification (100%), while VC and Mat, overlapping in the LDA plot, showed the highest misclassifications (40% and 20%, respectively, with a correct classification of 60% and 80%, respectively). Accordingly, four VC specimens were erroneously allocated to Cor, Mat, Ses, and Por, and two Mat specimens were erroneously allocated to VC. In S. pilchardus samples landed at Cor, Ses, and Por, a single specimen was misclassified (correct classification 90% in both locations).
3.3. Upwelling Index and Sea Surface Temperature
Upwelling index and SST percentile for each location are summarized in
Table 4. Cor and RP, the northernmost locations (
Figure 1), presented the highest upwelling values and ranked in the highest SST percentile. The lowest upwelling value was recorded in Ses, which ranked in the lower SST percentile.
4. Discussion
While, for the first time ever, the aquaculture volume of aquatic animals surpassed that of fisheries (accounting for 51 and 49%, respectively), marine finfish captures accounted for almost 70 million tons in 2022, representing 95% of the total volume of marine finfish production worldwide [
29]. Consequently, there is an increased concern with the sustainability of these fisheries, but also with the quality, safety, and origin of consumed fish. These concerns have intensified in latest decades due to globalization and disease outbreaks related to food consumption (e.g., mad cow disease, Avian flu, COVID-19) [
14,
30]), including bacterial or viral infections driven by the consumption of raw and/or undercooked fish [
31]. Traceability is therefore paramount for ensuring the safety of consumed fish, tracking sustainable fishing and to combat IUU fishing [
32]. As the FA composition of a marine organism reflects its diet and environment (FAs are linked to specific basal components of the food web), as well as its metabolism (certain essential FAs must be acquired from the diet and are selectively retained in the muscle), the profiling of FAs is particularly effective when one wants to confirm the geographical origin of seafood [
10,
13,
33]. In the present study, all sampled S. pilchardus belonged to the same metapopulation [
34]. Therefore, differences in metabolism produced from genetic differences were not expected (a priori), and differences in FA profiles are likely due to contrasting environmental conditions and/or dietary regimes that are experienced by these fish in different fishing areas. As previously referred, FA profiling was selected in the present study as the best approach to confirm the origin of
S. pilchardus landed in seven fishing ports along the Iberian Atlantic coast, from La Coruña in the north of Spain to Portimão in the South of Portugal.
Overall,
S. pilchardus from the seven locations had a higher proportion of total PUFAs, particularly n-3 PUFAs, than total SFAs or total MUFAs, in line with that which had already been reported for this species on the areas where it occurs, including the Iberian Atlantic coast [
35,
36]. This high proportion of PUFAs is likely promoted by the consumption of phytoplanktonic green algae and cryptophytes (single-cell biflagellate algae), as nearly 60% of their total FAs are PUFAs, while SFAs and MUFAs are the prevalent FAs in dinoflagellates and blue-green algae, respectively; diatoms and haptophytes have similar proportions of these three FA classes [
37].
The two most abundant FAs were, in general, DHA (22:6
n-3) and palmitic acid (16:0), in line with the FA profiles previously reported for S. pilchardus [
35,
36,
38]. However, EPA (20:5
n-3) was the most abundant FA in samples from Pe and the second most abundant in samples originating from Ses, with the proportions of all these three FAs varying among samples from different locations. The EPA/DHA ratios reflected the relative importance of these FAs in the seven locations, with these values suggesting that
S. pilchardus from Pe and Ses feed more on green algae and diatoms, while
S. pilchardus from Cor and Mat feed preferentially on dinoflagellates and haptophytes, as the former phytoplanktonic groups are richer in EPA and the latter in DHA [
37]. Regarding SFAs, those with 16 carbons are the signature of silica-rich diatoms [
37]. Therefore, the presence of 16:0 at a higher relative abundance in the white muscle of
S. pilchardus from all locations suggests that diatoms are an important part of this planktivorous fish diet along the Atlantic Iberian coast.
The contrasting FA compositions recorded suggest that, although geographically close,
S. pilchardus from RP appear to consume more diatoms and cryptophytes than those from Cor, thereby presenting higher levels of diatom-associated FA markers, namely palmitoleic acid (16:1
n-7) and EPA, whereas S
. pilchardus from Cor consume more phytoplanktonic green algae and dinoflagellates, thereby presenting higher levels of dinoflagellate-associated markers, namely stearidonic acid (18:4
n-3) and DHA [
39]. This is in agreement with diatoms being dominant throughout the year in Atlantic Galician Rias [
40] and cryptophytes preferentially inhabiting still and non-eutrophic waters [
41], as is the case of RP, whereas phytoplanktonic green algae and dinoflagellates are responsible for most of oceanic primary production[
37,
42].
Sardina pilchardus from Pe and Ses presented much higher relative abundances of 6,9,12,15-hexadecatetraenoic acid (16:4n-1) and EPA when compared to those from the other fishing harbors, supporting that they feed on diatoms. Specimens from VC, Mat, and Por appeared clustered in the center of the LDA plot, as their FA profiles were generally similar. Exceptions were the high relative abundance of oleic acid (18:1n-9) in S. pilchardus from Por (the highest among locations) and DHA in those from Mat (the second highest among locations).
On what concerns the environmental conditions, SST was generally warmer than average in all locations, but the upwelling indexes of Cor and RP, which were similar, were two to eight times higher than those in the other five locations. An obvious consequence of upwelling is an increased concentration of nutrients that originate from deeper water layers followed by phytoplankton blooms with high biomass (Favareto et al., 2023). Although presenting the lowest upwelling index, this does not seem to have affected the FA profile of S. pilchardus from Ses, as their FA profiles were similar to that of conspecifics landed in locations displaying higher upwelling intensity along the Portuguese coast (i.e., VC, Mat, and Por).
5. Conclusions
The present study highlights the discrimination potential of FA profiles in the white muscle of S. pilchardus. Indeed, individual FAs and FA classes present on this biological matrix allowed to confirm the geographic origin of specimens landed in different commercial fishing harbors, located at different spatial distances from each other, thus allowing to enforce traceability guidelines currently in place by national and EU authorities on fishery products. By using only eight of the 35 FAs identified in the white muscle of S. pilchardus, an average correct allocation of 80% on landing locations was achieved. Future studies are needed to refine this approach and investigate if and how seasonal and/or interannual variability in the FA profile of S. pilchardus can either fade or enhance the resolution already achieved with these natural biochemical fingerprints. The accurate identification of the geographic origin of landed S. pilchardus is paramount to successfully manage the Iberian stock of this commercially and ecologically important species, as overexploitation and IUU fishing practices (e.g., mislabeling of fishing area to dodge landing quotas) may continue to jeopardize the sustainability of this socially and economically important fishery.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Average fatty acid (FA) profiles (relative abundance of total pool of FA, %, ± SD) of Sardina pilchardus white muscle, sampled from individuals obtained in the seven landing locations along the Iberian Atlantic coast (n = 10 per location).
Author Contributions
Conceptualization, R.C. and F.Ri.; methodology, M.P., F.Ri and F.Re.; formal analysis, F.Ri. and F.Re.; investigation, M.P. and F.Ri; resources, R.C. and M.R.D.; data curation, F.Re.; writing—original draft preparation, M.P.; writing—review and editing, R.C., M.R.D., F.Ri and F.Re.; supervision, R.C., M.R.D. and F.Re.; project administration, R.C.; funding acquisition, R.C.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by project CITAQUA “Desenvolvimento do Projeto de Reforço do Polo de Aveiro (H4)”, framed within Measure 10 of Investment TC-C10-i01—Hub Azul—Rede de Infraestruturas para a Economia Azul, financed by the Recovery and Resilience Plan (PRR) and supported by Fundo Azul of the Portuguese Government. Thanks are also due to FCT/MCTES (Portugal) for the financial support to CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020) and LAQV-REQUIMTE (FCT UIDB/50006/2020), through national funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. Felisa Rey thanks FCT for her Junior Researcher contract in the scope of the Individual Call to Scientific Employment Stimulus 2017 (reference CEECIND/00580/2017, (https://doi.org/10.54499/CEECIND/00580/2017/CP1459/CT0005).
Data Availability Statement
All data supporting the present study is available as Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Froese, R.; & Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 11 November 2024).
- ICES. Working group on southern horse mackerel, anchovy and sardine (WGHANSA). In ICES Scientific Reports (Vol. 1, Issue 34, pp. 651–658). 2019.
- ICES. Report of the benchmark workshop on pelagic stocks (No. ICES CM 2017/ACOM:35). 2017.
- EUMOFA. European market observatory for fisheries and aquaculture. Country Profile—Portugal. Available online: https://www.eumofa.eu/portugal (accessed on 11 November 2024).
- FAO. Fisheries and Aquaculture. Global capture production Quantity (1950–2022). Available online: https://www.fao.org/fishery/statistics-query/en/capture/capture_quantity (accessed on 11 November 2024).
- ICES. Sardine (Sardina pilchardus) in divisions 8.C and 9.A (Cantabrian Sea and Atlantic Iberian waters). Bay of Biscay and Iberian coast ecoregion, (pp. 1–8). 2018.
- DGRM. Multiannual management and recovery plan for the Iberian sardine (2018-2023). 2022.
- FAO. Checklists and technical guidelines to combat illegal, unreported and unregulated (IUU) fishing. FAO, 2021.
- Bernal, M.; Stratoudakis, Y.; Coombs, S.; Angelico, M.M.; de Lanzós, A.L.; Porteiro, C.; Sagarminaga, Y.; Santos, M.; Uriarte, A.; Cunha, E.; et al. Sardine spawning off the European Atlantic coast:: Characterization of and spatio-temporal variability in spawning habitat. Prog. Oceanogr. 2007, 74, 210–227. [Google Scholar] [CrossRef]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood traceability: current needs, available tools, and biotechnological challenges for origin certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Cusa, M.; Glew, K.S.; Trueman, C.; Mariani, S.; Buckley, L.; Neat, F.; Longo, C. A future for seafood point-of-origin testing using DNA and stable isotope signatures. Rev. Fish Biol. Fish. 2022, 32, 597–621. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Montet, D. How to determine the geographical origin of seafood? Crit. Rev. Food Sci. Nutr. 2016, 56, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.F.; Duarte, I.A.; Matos, A.R.; Reis-Santos, P.; Duarte, B. Fatty acid profiles as natural tracers of provenance and lipid quality indicators in illegally sourced fish and bivalves. Food Control 2022, 134. [Google Scholar] [CrossRef]
- Gopi, K.; Mazumder, D.; Sammut, J.; Saintilan, N. Determining the provenance and authenticity of seafood: A review of current methodologies. Trends Food Sci. Technol. 2019, 91, 294–304. [Google Scholar] [CrossRef]
- Mamede, R.; Ricardo, F.; Santos, A.; Díaz, S.; Santos, S.A.O.; Bispo, R.; Domingues, M.R.M.; Calado, R. Revealing the illegal harvesting of Manila clams (Ruditapes philippinarum) using fatty acid profiles of the adductor muscle. Food Control 2020, 118. [Google Scholar] [CrossRef]
- Ricardo, F.; Pimentel, T.; Moreira, A.S.P.; Rey, F.; Coimbra, M.A.; Domingues, M.R.; Domingues, P.; Leal, M.C.; Calado, R. Potential use of fatty acid profiles of the adductor muscle of cockles (Cerastoderma edule) for traceability of collection site. Scientific Reports 2015, 5. [Google Scholar] [CrossRef]
- Mamede, R.; Santos, A.; Sousa, A.; Díaz, S.; Marques, S.C.; Leandro, S.M.; Domingues, M.R.; Calado, R.; Ricardo, F. Fatty acids profiling of goose barnacle (Pollicipes pollicipes) tissues to evaluate nutritional quality and confirm harvesting location. Journal of Food Composition and Analysis 2024, 127, 105930. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.F.; Li, Y.; Wang, H.X. The application of compound-specific isotope analysis of fatty acids for traceability of sea cucumber (Apostichopus japonicus) in the coastal areas of China. J. Sci. Food Agric. 2017, 97, 4912–4921. [Google Scholar] [CrossRef]
- Mateos, H.T.; Lewandowski, P.A.; Su, X.Q. Seasonal variations of total lipid and fatty acid contents in muscle, gonad and digestive glands of farmed Jade Tiger hybrid abalone in Australia. Food Chem. 2010, 123, 436–441. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.K.; Chen, X.J.; Chen, L. Potential use of stable isotope and fatty acid analyses for traceability of geographic origins of jumbo squid (Dosidicus gigas). Rapid Communications in Mass Spectrometry 2018, 32, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, F.; Tosi, F.; Tedeschi, P.; Gion, C.; Arcangeli, G.; Brandolini, V.; Giovanardi, O.; Maietti, A.; Berto, D. Preliminary multi analytical approach to address geographic traceability at the intraspecific level in Scombridae family. Isotopes Environ. Health Stud. 2020, 56, 260–279. [Google Scholar] [CrossRef] [PubMed]
- Teulier, L.; Thoral, E.; Queiros, Q.; McKenzie, D.J.; Roussel, D.; Dutto, G.; Gasset, E.; Bourjea, J.; Saraux, C. Muscle bioenergetics of two emblematic Mediterranean fish species: Sardina pilchardus and Sparus aurata. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology 2019, 235, 174–179. [Google Scholar] [CrossRef]
- Anderson, M.; Clarke, K.; Gorley, R. PERMANOVA+ for Primer. Guide to Software and Statistical Methods. 214 p; PRIMER-E Plymouth, 2008.
- Kursa, M.B.; Jankowski, A.; Rudnicki, W.R. Boruta—A system for feature selection. Fundamenta Informaticae 2010, 101, 271–286. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 11 November 2024).
- Santos, A.M.P.; Nieblas, A.E.; Verley, P.; Teles-Machado, A.; Bonhommeau, S.; Lett, C.; Garrido, S.; Peliz, A. Sardine (Sardina pilchardus) larval dispersal in the Iberian upwelling system, using coupled biophysical techniques. Prog. Oceanogr. 2018, 162, 83–97. [Google Scholar] [CrossRef]
- IEO. Instituto Español de Oceanografía. Available online: http://www.indicedeafloramiento.ieo.es (accessed on 11 November 2024).
- NOAA. The National Oceanic and Atmospheric Administration. Available online: www.ncdc.noaa.gov/temp-and-precip/global-maps/ (accessed on 11 October 2024).
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in action; Rome, 2024.
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Hopkins, C.R.; Roberts, S.I.; Caveen, A.J.; Graham, C.; Burns, N.M. Improved traceability in seafood supply chains is achievable by minimising vulnerable nodes in processing and distribution networks. Mar. Policy 2024, 159. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and condition in fish: the influence of lipids on membrane competency and immune response. In Lipids in Aquatic Ecosystems; Springer: 2009; pp. 237-256.
- Silva, A.; Garrido, S.; Ibaibarriaga, L.; Pawlowski, L.; Riveiro, I.; Marques, V.; Ramos, F.; Duhamel, E.; Iglesias, M.; Bryère, P.; et al. Adult-mediated connectivity and spatial population structure of sardine in the Bay of Biscay and Iberian coast. Deep-Sea Research Part Ii-Topical Studies in Oceanography 2019, 159, 62–74. [Google Scholar] [CrossRef]
- Bandarra, N.M.; Marçalo, A.; Cordeiro, A.R.; Pousao-Ferreira, P. Sardine (Sardina pilchardus) lipid composition: Does it change after one year in captivity? Food Chem. 2018, 244, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.; Brosset, P.; Soudant, P.; Lebigre, C. Spatial and ontogenetic variations in sardine feeding conditions in the Bay of Biscay through fatty acid composition. Mar. Environ. Res. 2022, 173. [Google Scholar] [CrossRef] [PubMed]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal changes in lipid composition of sardine (Sardina pilchardus). J. Food Sci. 1997, 62, 40–42. [Google Scholar] [CrossRef]
- Kohlbach, D.; Lange, B.A.; Schaafsma, F.L.; David, C.; Vortkamp, M.; Graeve, M.; van Franeker, J.A.; Krumpen, T.; Flores, H. Ice algae-produced carbon is critical for overwintering of Antarctic krill Euphausia superba. Frontiers in Marine Science 2017, 4. [Google Scholar] [CrossRef]
- Ospina-Alvarez, N.; Varela, M.; Doval, M.D.; Gómez-Gesteira, M.; Cervantes-Duarte, R.; Prego, R. Outside the paradigm of upwelling rias in NW Iberian Peninsula: Biogeochemical and phytoplankton patterns of a non-upwelling ria. Estuarine Coastal and Shelf Science 2014, 138, 1–13. [Google Scholar] [CrossRef]
- María Concepción Lora, V. Cryptophyte: Biology, Culture, and Biotechnological Applications. In Progress in Microalgae Research, Leila Queiroz, Z., Eduardo, J.-L., Mariany Costa, D., Eds.; IntechOpen: Rijeka, 2022; p. Ch. 3. [Google Scholar]
- García-Moreiras, I.; Oliveira, A.; Santos, A.; Oliveira, P.B.; Amorim, A. Environmental factors affecting spatial dinoflagellate cyst distribution in surface sediments off Aveiro-Figueira da Foz (Atlantic Iberian Margin). Frontiers in Marine Science 2021, 8. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).