1. Introduction
Cisplatin (cis-diamminedichloroplatinum II) (CDDP) is a commonly used anticancer therapy with FDA approval, despite its advanced toxicity, for hematologic and solid tumor malignancies like bladder, testicular, and ovarian cancers. It can be applied as a neoadjuvant or induction therapy alone or in combination [
1]. Cisplatin is occasionally used in conjunction with other chemotherapeutic agents such as doxorubicin, taxane derivatives, and 5-FU, to cure cancers that are commonly seen in women, such as breast, cervical, endometrial, and gestational trophoblastic cancers [
2].
Before the age of forty, primary or secondary amenorrhea with consistently low estrogen or elevated gonadotropin, is known as premature ovarian insufficiency/failure (POI/POF) [
3]. The rising POI prevalence observed in the recent years could be explained by the increased rate of cancer survivors among young women who often receive chemotherapy [
4].
The granulosa cells (GCs), which surround the oocyte in the ovarian follicles, are an essential cell type that promotes oocyte growth and play a crucial role in the ovarian reproductive and endocrine functions [
5]. During the physiological process of follicular atresia, the apoptosis of GCs precedes that of oocytes and theca cells. The proper growth and maturation of follicles may therefore be affected by excessive GCs apoptosis [
6]. Most chemotherapeutic agents, such as cisplatin, could induce GCs apoptosis, which could result in disruption of follicular growth with subsequent ovarian dysfunction and failure. It is therefore possible to hypothesize that ovarian function could be protected from the cytotoxic effects of the chemotherapeutic agents by preventing GCs apoptosis [
7].
In addition, Oxidative stress is an essential mechanism underlying the CDDP cytotoxicity. According to Saad et al. [
8], the main molecular mechanism of CDDP-induced cytotoxicity is mitochondrial oxidative stress, which leads to mitochondrial protein-SH loss, impairment of calcium uptake, and mitochondrial membrane potential reduction. This is further supported by the protective effects of the antioxidant silymarin, which was shown to counteract the cytotoxic effect of cisplatin on the liver [
9].
Chinese traditional patent remedies primarily contain hyperoside (HYP), which is a flavonoid that was identified from Rhododendron ponticum L. Occasionally, it is referred to as quercetin 3-O-β-d-galactoside. HYP is known for its wide range of pharmacological beneficial effects, such as anti-cancer, anti-fibrosis, anti-inflammatory, and antiapoptotic effects [
10]. In addition, HYP treatment displayed prevention in maladaptive remodeling of the cardiac muscles, which was depending partially on Akt signaling pathway regulation. Hence the current work investigated the cytoprotective effect of HYP against CDDP-induced apoptosis and other cytotoxic effects in human ovarian GCs.
2. Results
The study was conducted to evaluate the protective effect of HYP on CDDP induced toxicity in the ovarian granulosa cells using
invitro cell line model. MTT assay showed that CDDP significantly decreased the viability of the treated granulosa cells 48 hrs postexposure at concentrations 1,5, 10, 15, and 20 µM to 90.2±4.5, 74.3±5.2, 49.2±4,7, 42±3.9, and 37±4.8% of the control nontreated cells viability (
Figure 1A). While treatment with HYP was shown to significantly increase the viability of GCs 48hrs post-exposure at concentrations 40 and 80 µM (figure 1B). However, cells that were co-treated by CDDP at concentrations 5 and 10 µM in presence of HYP (40 µM), HYP was shown to significantly alleviate CDDP induced negative effect on cell’s viability (
Figure 1C)
We first aimed to identify which of the three common molecular pathways namely mitochondrial inhibition, oxidative stress, and apoptosis could be the main underlying molecular mechanism of CDDP-induced cytotoxicity. To achieve this, we investigated the effects of mitochondrial enhancer Co-Q10, the antioxidant reduced glutathione, or the anticaspase-3 z-vAD-fmk on CDDP-induced cytotoxicity. GCs were co-treated with Co-Q10 (1 µM), reduced glutathione (10 µM), or z-vAD-fmk (0.2 µM) in addition to CDDP (5 or 10 µM). Data revealed that both reduced glutathione (10 µM), and z-vAD-fmk (0.2 µM) were found to alleviate CDDP cytotoxic effects to a variable extent with more beneficial effects of the anticaspase-3 z-vAD-fmk (
Figure 1D). These findings directed the project to more deeply investigate the oxidative damage and apoptosis as main molecular pathways in CDDP cytoxicity and as targets for HYP protective action.
The effect of CDDP and HYP on ATP production and MMP was investigated. Cells were treated by CDDP at concentrations 5 and 10 µM in presence and absence of HYP (40 µM). CDDP administration significantly decreased ATP production and MMP levels in the treated GCs in a concentration dependent manner. While HYP was shown to significantly alleviated CDDP induced mitochondrial disruption (
Figure 1E,F)
The effect of CDDP and HYP on the cells secretory function was also evaluated. CDDP (5 or 10 µM) exposure for 48 hrs was found to significantly decrease the treated GCs’ secretion of Progesterone, while estradiol secretion was only significantly inhibited by CDDP (10 µM) (
Figure 1E,F). CDDP was found to decrease Progesterone and estradiol secretion to 50.3±6.7 and 58.2±5.6 %, respectively, of the non-treated cells secretory functions. Moreover, HYP was found to significantly counteract the effect of CDDP on progesterone secretion, but no significant effect on estradiol.
Regarding the effect on oxidative stress, CDDP (5 and 10 µM) significantly increased reactive oxygen species (ROS) and lipid peroxidation levels. At 10 µM treatment with CDDP, ROS and thiobarbituric acid derivatives (TBARS) were induced around 2.5 and 2.1 folds of the nontreated cells levels. HYP significantly ameliorated CDDP effects on ROS levels and lipid peroxidation (
Figure 2A,B). Regarding the impact of CDDP on antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD) and their coding genes expression, CDDP was found to reduce both enzymes’ activities and their gene expression in a concentration dependent manner. HYP cotreatment with CDDP was found to significantly lessen the CDDP effect on CAT and SOD activities and their coding genes expression (
Figure 2C–F).
Regarding Nrf2 and Ho-1 gene expression assays in GCs, CDDP caused significant inhibition in the expression of both genes to variable extents, with more effect on HO-1 genes. This inhibitory action was counteracted by HYP (40µM) cotreatment (
Figure 2G,H).
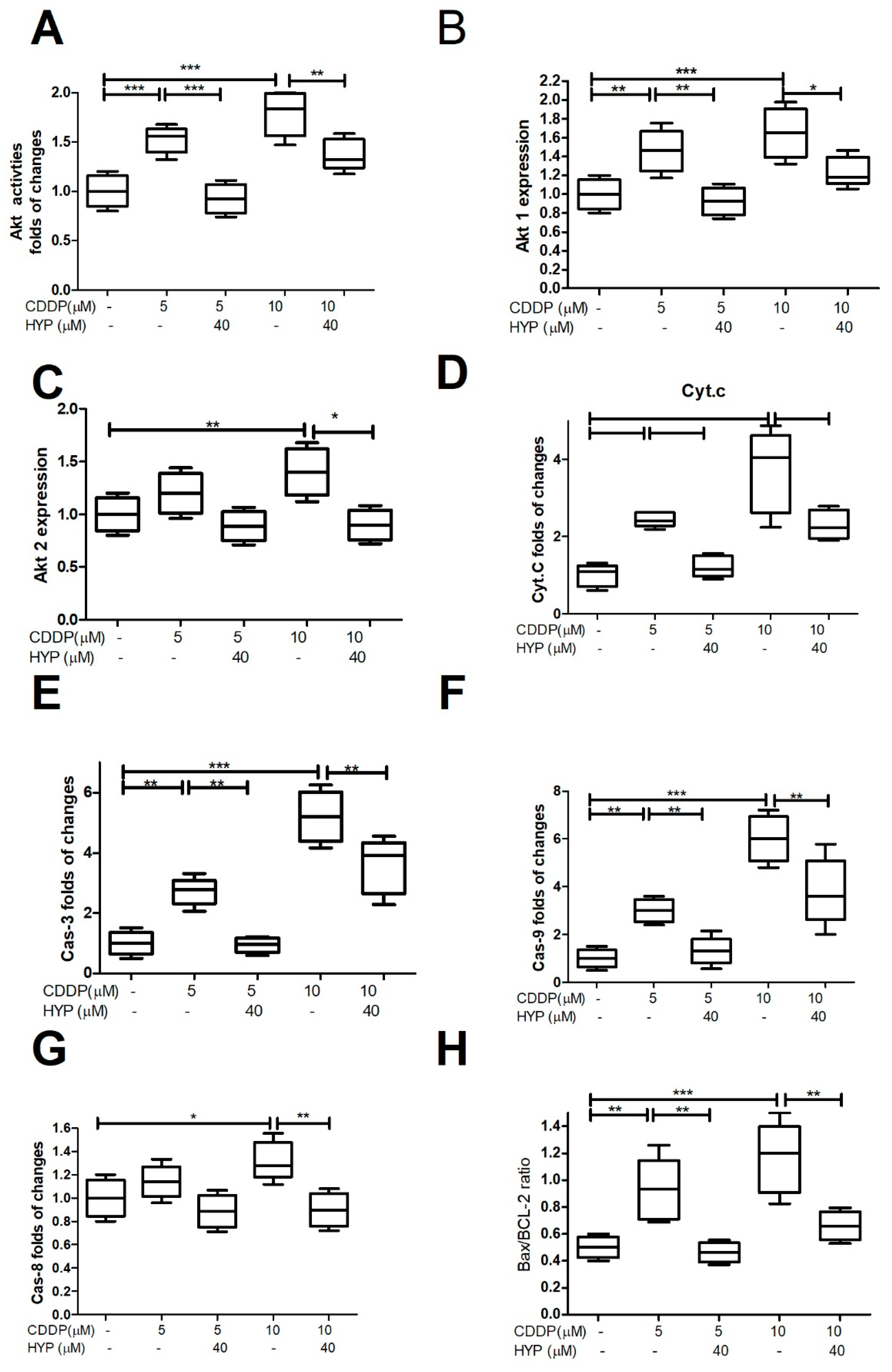
CDDP with or without cotreatment with HYP effect on Akt kinase activity was assessed by ELISA and Q-PCR assays. Data displayed that CDDP increased the Akt kinase activity and its coding genes expression (Akt-1, and Akt-2) significantly in a concentration dependent manner (
Figure 3A–E). HYP was found to significantly attenuate the effect of CDDP on Akt signaling pathways. The CDDP effect on Akt-2 expression was less significant than its effect on Akt-1(
Figure 3A–
3C).
Moreover, Cytochrome c assay showed that CDDP (5 and 10µM) significantly increased cytochrome c levels in the treated cells to about 2.1 and 3.7 folds of the non-treated cells, respectively. CDDP effect on cytochrome c was significantly reversed by cotreatment with HYP (
Figure 3D). Caspases assays also revealed that CDDP (10 µM) significantly increased caspases -3, -8, and -9 levels in comparison to the non-treated cells by about 5.7, 1.3, and 6.2 folds of their activities in the control caspases activity levels, respectively, with concomitant increase in Bax/Bcl2 ratio up to 193.3±5.9% of the control levels (
Figure 3E–H). The effect of PQ on cytochrome c, caspases and apoptosis-related gene expression were significantly antagonized by HYP cotreatment (40 µM).
3. Discussion
The current work evaluated the cytoprotective effect of HYP against CDDP-induced cytotoxicity in the human ovarian GCs. The human cell line was selected to avoid the effect of the interspecies variation in their response to chemicals, while the secondary cell line was chosen as it is difficult to get the primary human cells with the considered ethical issues. Also, the secondary cell line gives a continuous source of cells for experiments with a high degree of data reproducibility. CDDP cytotoxicity was assessed in concentrations range 1-20 µM, which was used in previous studies to establish CDDP toxicity model [
11,
12]. HYP protective effect was evaluated within the concentration range utilized in previously published studies [
13,
14].
The data revealed that CDDP was cytotoxic 48 hrs postexposure at a concentration range of 1-20µM to the human GCs in a concentration dependent pattern. While HYP was shown to enhance the viability of the GCs cells. Furthermore, HYP cotreatment was found to attenuate the CDDP cytotoxic effects on the treated GCs. Our findings are consistent with a previous studies, which showed HYP to induce the viability of hydrogen peroxide treated rats’ ovarian granulosa cells [
15], Chinese hamster lung fibroblast (V79-4) cells [
16], and human umbilical vein endothelial cells (HUVECs) [
17], via inhibition of hydrogen peroxide induced oxidative damage and apoptosis.
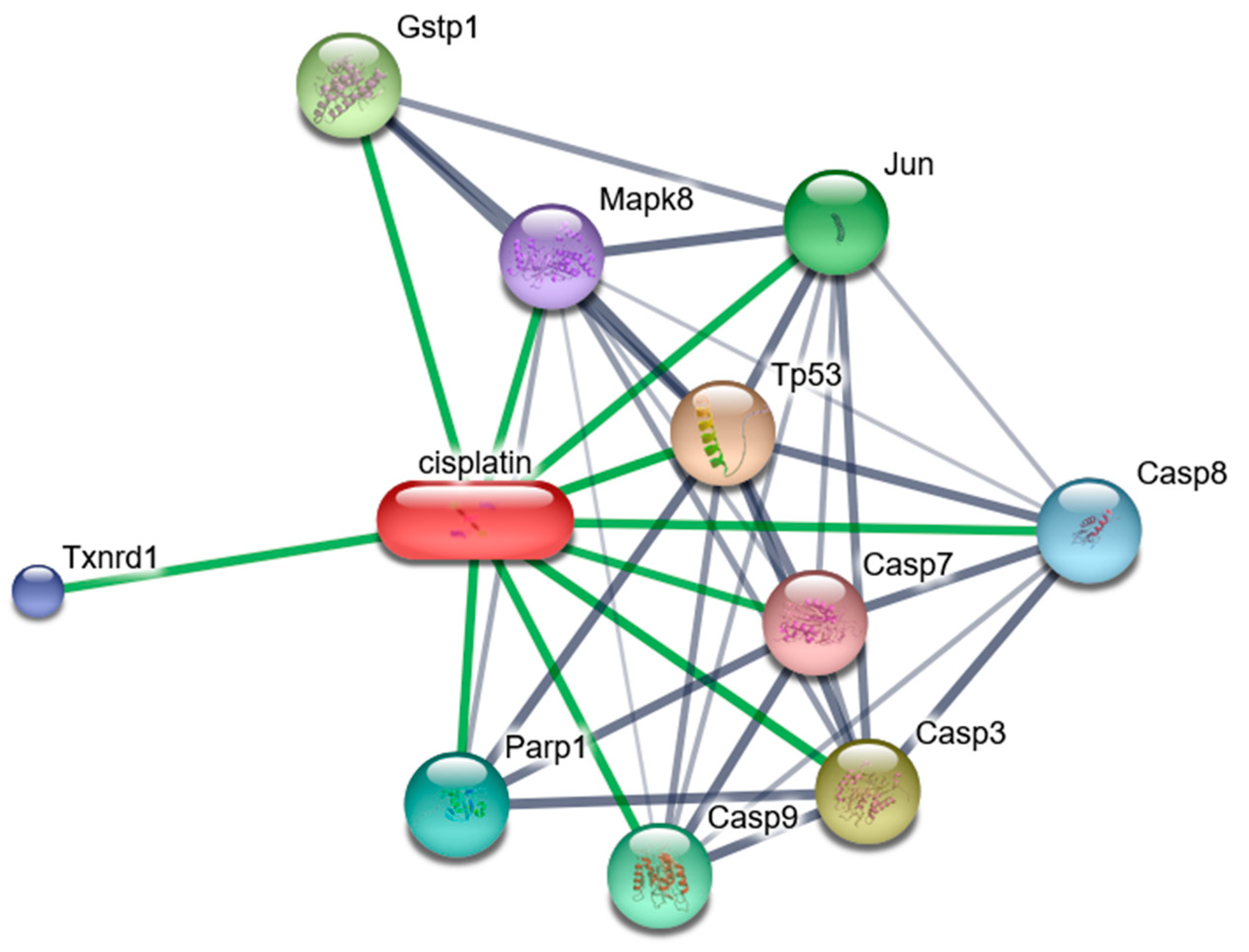
We have investigated the role of the three common cytotoxicity mechanisms as the possible underlying mechanisms of CDDP-induced cytotoxicity in the human GCs via testing the protective effect of Co-Q10 (common mitochondrial enhancer), reduced glutathione (common antioxidant), and z-vAD-fmk (common anti-caspase 3). Data revealed major roles of oxidative stress and apoptosis in CDDP induced GC cytotoxicity with more effect of apoptosis. Hence the study was focused on both mechanisms-related assays. These findings are supported by stitch database Using the "STITCH platform (http://stitch.embl.de/)" (last accessed 18, Oct 2024) [
18], the main molecules and interacting proteins related to CDDP effect were identified (
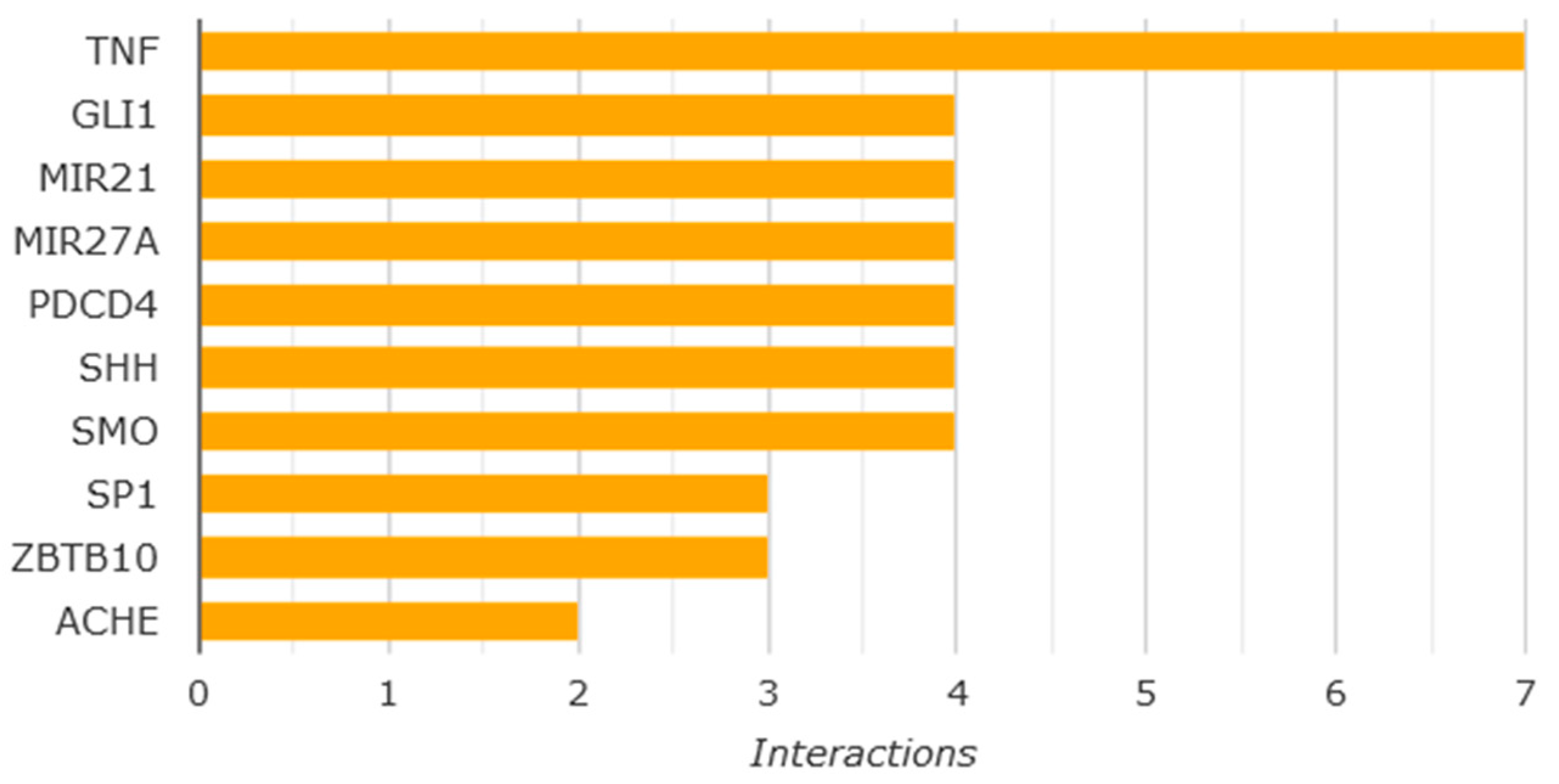
Figure 4). It has been shown that CDDP interacted with members of caspases (-3, -7, -8, and -9) pathway which is crucial to induce apoptosis. Also, CDDP interacts with tumor suppressor gene (TP53), mitogen-activated protein kinase 8 (Mapk8), and transcription factor AP-1 (Jun) which control cell growth and apoptosis. In addition, CDDP interacts with Thioredoxin reductase 1(Txnrd1) which has a role to protect the cells from oxidative damage, Poly [ADP-ribose] polymerase 1 (Parp-1) required for DNA repair as well as Glutathione S-transferase P (Gstp1) which plays an essential role to prevent neurodegeneration. While the top genes interacting with the protective effect HYP was revised using” Comparative toxicogenomics database (CTD) (
https://ctdbase.org/)” (last accessed 25, Oct, 2024) [
19]. Data revealed that the top interacting genes were related to cell growth, differentiation, apoptosis and necrosis, genes expression and signal transuction in pathways as Akt pathway(
Figure 5).
ATP and MMP assays showed that CDDP significantly attenuated ATP production and MMP of the treated GCs to a lesser extent than its total effect of the cell’s viability at the same tested concentration. Viability, ATP production and MMP were decreased to 49.2±4.7, 58.3±5.5, and 62.9±4.9 while using CDDP at concentration 10µM. This can be explained by the fact that mitochondrial dysfunction may be secondary event to its oxidative damage and other CDDP induced cytotoxic events rather than an initial event in CDDP cytotoxicity pathways. HYP was shown to alleviate the damage in mitochondria induced by CDDP in the treated cells. This is consistent with the data that was previously presented, where HYP was shown to reduce mitochondrial dysfunction caused by amyloid beta in HT22 brain cells. Furthermore, Li et al. [
20] demonstrated that HYP reduced the cell mortality and mitochondrial dysfunction brought on by oxidative stress in 661W photoreceptor cells. Zeng et al. [
21] reported that HYP also inhibited the cytotoxicity and apoptosis caused by Amyloid β-protein (Aβ) 25–35 in the primary cortical neuronal cell line by reversing Aβ-induced mitochondrial dysfunction, which counteracted the effect on MMP decrease, ROS production, and mitochondrial release of cytochrome c.
Oxidative stress assays showed that CDDP induced oxidative stress in the treated GCs with inhibition of the antioxidant enzymes; CAT, SOD, and its coding genes with parallel inhibition of the antioxidants pathways genes Nrf2 and HO-1. HYP was shown to alleviate CDDP oxidative damage in the treated GCs. This fining supports the previously published data showing that HYP mitigated oxidative stress and apoptosis induced by ischemia/reperfusion injury in the kidney in both in vitro and in vivo (HK-2 cell and Male C57BL/6 mice) models [
22].
The current data showed that CDDP induced apoptosis in the treated GCs with increased levels of cytochrome C, caspases activation and increased Bax/Bcl2 gene family’s expression. The Bcl-2 protein family, which involves both pro-apoptotic (like Bax) and anti-apoptotic (like Bcl-2) proteins, that play critical roles in follicular development and atresia [
23]. Cytochrome C release and the induction of the downstream protease caspase-3 can be regulated by the Bcl-2/Bax ratio, which is a critical determinant of cell fate [
24]. The follicular destiny is partially determined by molecules and apoptotic signals released by GCs. Follicle atresia may begin because of GCs apoptosis. Hence granulosa cells apoptosis may hasten the onset of POI/POF in young women receiving chemotherapy [
25]. However, HYP was shown to significantly counteract CDDP induced apoptosis via decreasing cytochrome C and the associated caspases activation and lowering Bax/Bcl2 ratio. This agrees with what was reported by Wu et al. [
26], who revealed that HYP decreased cytochrome C, caspases -3, -8, and -9 activities, and Bax/Bcl2 ratio in the primary rats’ retinal vascular endothelial cells. Also, HYP was shown to decrease apoptosis in the HUVECs treated with hydrogen peroxide with decreasing the cleaved caspase-3 levels and reducing Bax/Bcl2 expression ratio [
27].
In various cell types, protein kinase B or Akt, mediated the cell survival effect of cytokines and growth factors and prevents apoptosis caused by various apoptotic triggers. Cheng et al. [
28], Franke et al. [
29], and Vara et al. [
30] all included it as one of the primary downstream targets of phosphatidylinositol 3-OH-kinase (PI3K). It has been demonstrated that after exposure to CDDP, Akt activation regulates the levels of p53 and X-linked apoptotic protein inhibitor [
31,
32]. Additionally, it was shown that p53 activated the caspase-dependent pathway that results in apoptosis through the release of mitochondrial Smac into the cytosol in a manner that is sensitive to Akt [
33]. The current study showed that CDDP significantly increased Akt kinase activity and expression. This effect was significantly mitigated by HYP. Earlier study showed that HYP inactivated Akt pathway signaling in Neonatal rat cardiac myocytes and prevented hypertrophy with protection of the myocytes function [
34].
Cytotoxic events are overlapping and complex, the apoptotic pathways were demonstrated to be activated by oxidative stress, and this was used as an underlying mechanism in cancer oxidation therapy [
35]. Furthermore, cytochrome c leaking to the cytoplasm and apoptosis induction are caused by mitochondrial rupture, which also activates the apoptotic pathways [
36]. While impaired mitochondrial electron transport chain is known to be the principal source for leaking electrons which interact with nitric oxide to form the highly reactive peroxynitrites, which have been demonstrated to be a significant contributor to oxidative damage [
37]. Furthermore, during apoptosis, the mitochondria undergo several further alterations. These include mitochondrial fragmentation and cristae remodeling, as well as the loss of critical mitochondrial processes like the production of ATP and the maintenance of calcium homeostasis. Moreover, significant changes in the distribution and composition of the lipidic component of mitochondrial membranes have been found to be pertinent regulatory events for the proteins implicated in apoptotic mitochondrial damage.
4. Materials & Methods
4.1. Reagents and Cell Culture Conditions
All kits and reagents used, unless defined, were acquired from Sigma (St. Louis, MO, USA). The human ovarian granulosa cell line KGN (CVCL_0375, Cellcook Biotech Co., Ltd., Guangzhou, China) was cultivated in 5% CO2, at 37°C in DMEM/F-12 medium supplemented with 10% (vol:vol) charcoal-stripped fetal bovine serum (VivaCell), 1% penicillin/streptomycin (Beyotime, Shanghai, China), and no phenol red. Following cell adhesion, a fresh culture medium was added every other day.
4.2. Cytotoxicity Assessment Using MTT Assay
GCs were grown in 96-well plates till 80-90% confluency and treated with CDDP for 48hrs at concentrations 1, 5, 10, 15, and 20 µM or HYP at concentrations of 5, 10, 20, 40, and 80 µM. The essay was conducted using a commercial kit following the manufacturer's recommendations (Sigma). Finally, the solution absorbance was measured at 590 nm using the "TopCount" plate reader (Perkin Elmer, Ueberlingen, Germany). The assay was repeated with further cotreatment, GCs were subjected to CDDP (5 or 10µM) with or without HYP (40 µM) cotreatment. Then the assay was performed again, and the cells were exposed to CDDP (5 or 10µM) with or without reduced glutathione, Co-Q10 or z-VAD-fmk for 48hrs. All experiments were conducted in triplicates.
4.3. Measurement of Progesterone and Estradiol Levels
Cells were grown and treated with CDDP (5 or 10 µM) for 48 hrs and cotreated or not with HYP (40 µM). At the time point, progesterone and estradiol secretory levels in serum-media free of the treated groups were assessed using gonadal hormone ELISA kits (Cusabio, China) and absorbance was taken at 450 nm. Triplicates were conducted for each experiment.
4.4. Effect of CDDP and HYP on Intracellular ATP Level
GCs were seeded in 96-well plate and subjected to CDDP (5 or 10 µM) for 48 hrs in presence of absence of HYP (40 µM). Following the instructions provided by the manufacturer, intracellular ATP levels were assessed after 48 hrs using a commercial kit (Abcam, Cambridge, UK). A Perkin Elmer "TopCount" luminometer was used to measure the amount of signal produced. For every treatment concentration, a minimum of three duplicate trials were conducted.
4.5. Effect of CDDP and HYP on Mitochondrial Membrane Potential (MMP)
The CDDP and HYP impact on the MMP of the treated cells was evaluated using a MitoTracker Green (MG) probe. Plates of cells were prepared for the ATP test. After removing the medium, the plates were incubated with a 50 nM MG staining solution for 30 minutes at 37ºC. A TopCount Perkin Elmer microplate reader with excitation/emission filters set at 490/515 nm was then used to monitor the cells' fluorescence after they had been cleaned and submerged in new phosphate-buffered saline.
4.6. Measurements of Oxidative Stress Markers
The cells were administered CDDP (5 or 10 µM) for 48 hrs in presence of absence of HYP (40 µM). At the time point, the following oxidative stress related assays were conducted.
4.6.1. Reactive Oxygen Species (ROS) Production
The ROS assay was based on dichlorofluorescein diacetate (DCFDA) solution (25 µM in Hank's solution) following the method described by Elmorsy et al. [
37]. Finally, the excitation and emission wavelengths used to measure fluorescence were 485 and 535 nm. For every time point, the experiments were run in triplicate.
4.6.2. Lipid Peroxidation Assay
The amounts of thiobarbituric acid reactive compounds (TBARS), more especially malondialdehyde (MDA), in treated homogenized cells were measured using a commercial Abcam kit (Catalog Number: ab118970). The manufacturer's (Abcam, Cambridge, UK) instructions were carefully followed. At 532 nm, the absorbance was measured. Every experiment was carried out three times.
4.6.3. Antioxidant Enzymes Activity
The impact of CDDP and HYP exposure on the catalase (CAT) and superoxide dismutase (SOD) activities in GCs was evaluated. Following the given guideline, CAT and SOD activities were colorimetrically evaluated using a colorimetric commercial assay kit (Abcam, Cambridge, UK). For CAT and SOD, the absorbance was assessed at 570 nm and 440 nm, respectively. Every experiment was carried out three times.
4.7. Apoptosis Assays
Cells were subjected to CDDP (5 or 10 µM) for 48 hrs, either cotreated or not with HYP (40 µM) for apoptosis tests’ investigation. At the time point, caspase -3, -8, and -9 activities were evaluated via a Flourometric kits following the recommended directions (Clontech Laboratories Inc., Mountain View, CA, USA). For caspases -3, -8, and -9, the fluorescence was measured at excitation/emission wavelengths of 400/505, 400/505, and 380/460 nm, respectively.
4.8. Akt Kinase Assay
Akt kinase was assessed utilizing a merchant kit (Abcam) following the recommended protocol. Briefly, At the time point, the medium was removed, and the cells were washed using ice cold PBS, then the cells were scraped in the relevant kit supplied lysis buffer on ice. Cell lysate was collected and centrifuged 13000 rpm for 15 minutes and the supernatant was collected for the assay. By the end of the assay, the stop solution was added, and the absorbance was read at 450 nm for both assays. The assay was repeated trice for robust data.
4.9. Determination of Gene Expression by Quantitative Polymerase Chain Reaction
GCs were subjected to CDDP (5 or 10 µM) for 48 hrs in presence of absence of HYP (40 µM). Following cell harvesting, a commercial kit from Qiagen, Germany, was used to extract RNA. The Bio-Rad Laboratories, Inc. CFX96 real-time System was used to perform quantitative PCR experiments. As directed by the manufacturer, the RT2 SYBR® Green qPCR Mastermixes kit (Qiagen) was utilized.
Table S1 lists all the primer sequences that were employed. In accordance with Martínez et al. [
32], the thermocycling parameters were modified. Transcript levels were measured and normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the reference housekeeping gene. Every experiment was carried out three times.
4.10. Statistical Analysis
Tukey's post-test was used in conjunction with a one-way ANOVA to assess data from three or more groups. In the statistical studies performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA), and P<0.05 taken as statistical significance.
Figure 1.
The effect of CDDP and HYP on the GCs’ viability and secretory activities. (A-D) Histograms demonstrating the results of MTT assay for cell viability after using a wide range of CDDP concentrations 1-20µM and 48hrs exposure to assess its cytotoxic effect and HYP in a concentration ranging between 5-80µM to assess its effect on the cells and following co-administration of HYP in 40µM concentration. (A) The histogram shows significant cytotoxic effect of CDDP on the GCs, starting at 5µM concentration, while at 10µM, viability was reduced much significantly. However, no significant differences noticed at the concentrations 10-20µM. (B) The histogram reveals that, HYP mitigated the GCs viability in a concentration dependent pattern ranging between 5-10µM. (C&D) Histogram demonstrating the effect of cotreatment of HYP (40µM), where it counteracts CDDP induced cytotoxicity depending on its concentration. (E&F) Histograms show progesterone and estradiol secretion after exposure to variable concentrations of CDDP alone or cotreated with HYP. CDDP reduced both progesterone and estradiol secretion starting at 5µM concentration, however, HYP reverse CDDP effect at 40µM. (G) CDDP alleviates ATP gene’s expression, and HYP cotreatment at concentrations 40 µM shows significant downregulation on CDDP induced inhibition ATP gene’s expression to variable extents in a dose dependent. (H) CDDP reduces MMP activities in a dose dependent, while HYP reverses the effect at 40µM. The data were displayed as mean ± standard deviation and P ≤ 0.05 was considered as statistically significant. The data demonstrated the significant differences among groups (P*** = P < 0.001, P** = P < 0.01, P* = P < 0.05).
Figure 1.
The effect of CDDP and HYP on the GCs’ viability and secretory activities. (A-D) Histograms demonstrating the results of MTT assay for cell viability after using a wide range of CDDP concentrations 1-20µM and 48hrs exposure to assess its cytotoxic effect and HYP in a concentration ranging between 5-80µM to assess its effect on the cells and following co-administration of HYP in 40µM concentration. (A) The histogram shows significant cytotoxic effect of CDDP on the GCs, starting at 5µM concentration, while at 10µM, viability was reduced much significantly. However, no significant differences noticed at the concentrations 10-20µM. (B) The histogram reveals that, HYP mitigated the GCs viability in a concentration dependent pattern ranging between 5-10µM. (C&D) Histogram demonstrating the effect of cotreatment of HYP (40µM), where it counteracts CDDP induced cytotoxicity depending on its concentration. (E&F) Histograms show progesterone and estradiol secretion after exposure to variable concentrations of CDDP alone or cotreated with HYP. CDDP reduced both progesterone and estradiol secretion starting at 5µM concentration, however, HYP reverse CDDP effect at 40µM. (G) CDDP alleviates ATP gene’s expression, and HYP cotreatment at concentrations 40 µM shows significant downregulation on CDDP induced inhibition ATP gene’s expression to variable extents in a dose dependent. (H) CDDP reduces MMP activities in a dose dependent, while HYP reverses the effect at 40µM. The data were displayed as mean ± standard deviation and P ≤ 0.05 was considered as statistically significant. The data demonstrated the significant differences among groups (P*** = P < 0.001, P** = P < 0.01, P* = P < 0.05).
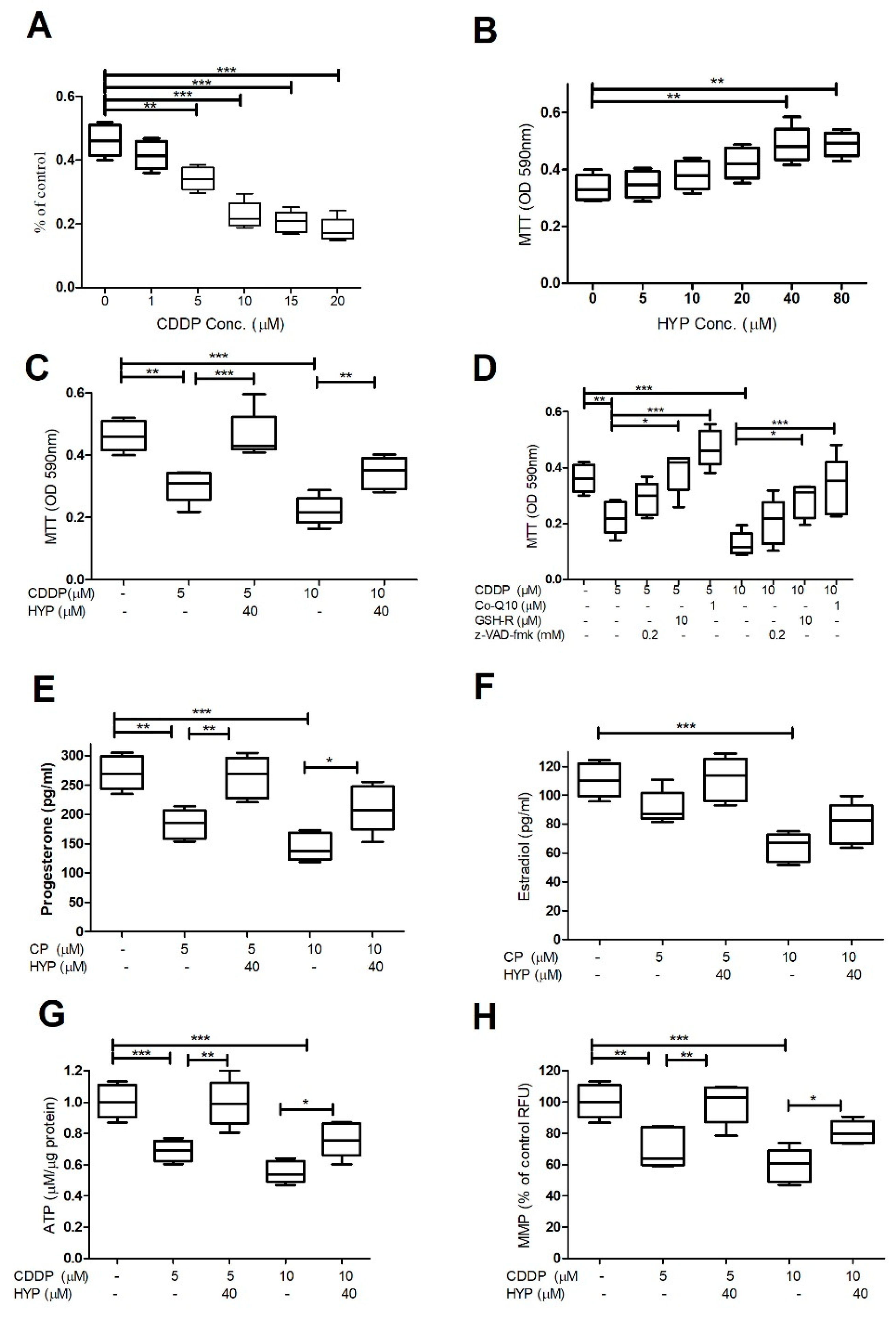
Figure 2.
The effect of CDDP and HYP on oxidative stress tests in GCs. (A-G) Histograms demonstrating the results of oxidative stress tests; ROS, TBARS, CAT, SOD activities. (H&I) Histograms demonstrating Nrf2 and HO-1 gene expression in GCs treated with 5-10µM CDDP for 48hrs and cotreatment with HYP (40 µM concentrations). (A) Shows that CDDP (5&10µM) significantly induced ROS and TBARS (B) levels, however, it reduced the antioxidant enzymes CAT (C&E) and SOD (D&F) activities. The significant dose dependent induction in oxidative stress by CDDP, was significantly attenuated by HYP cotreatment at 40 µM concentrations (A-G). (H&I) CDDP downregulated Nrf2 (E), and HO-1 (F) gene expression in a dose-dependent. While cotreatment + HYP reverses the CDDP inhibitory effects on both genes. The data were displayed as mean ± standard deviation and P ≤ 0.05 was considered as statistically significant. The data demonstrated a significant difference among groups (P*** = P < 0.001, P** = P < 0.01, P* = P < 0.05).
Figure 2.
The effect of CDDP and HYP on oxidative stress tests in GCs. (A-G) Histograms demonstrating the results of oxidative stress tests; ROS, TBARS, CAT, SOD activities. (H&I) Histograms demonstrating Nrf2 and HO-1 gene expression in GCs treated with 5-10µM CDDP for 48hrs and cotreatment with HYP (40 µM concentrations). (A) Shows that CDDP (5&10µM) significantly induced ROS and TBARS (B) levels, however, it reduced the antioxidant enzymes CAT (C&E) and SOD (D&F) activities. The significant dose dependent induction in oxidative stress by CDDP, was significantly attenuated by HYP cotreatment at 40 µM concentrations (A-G). (H&I) CDDP downregulated Nrf2 (E), and HO-1 (F) gene expression in a dose-dependent. While cotreatment + HYP reverses the CDDP inhibitory effects on both genes. The data were displayed as mean ± standard deviation and P ≤ 0.05 was considered as statistically significant. The data demonstrated a significant difference among groups (P*** = P < 0.001, P** = P < 0.01, P* = P < 0.05).
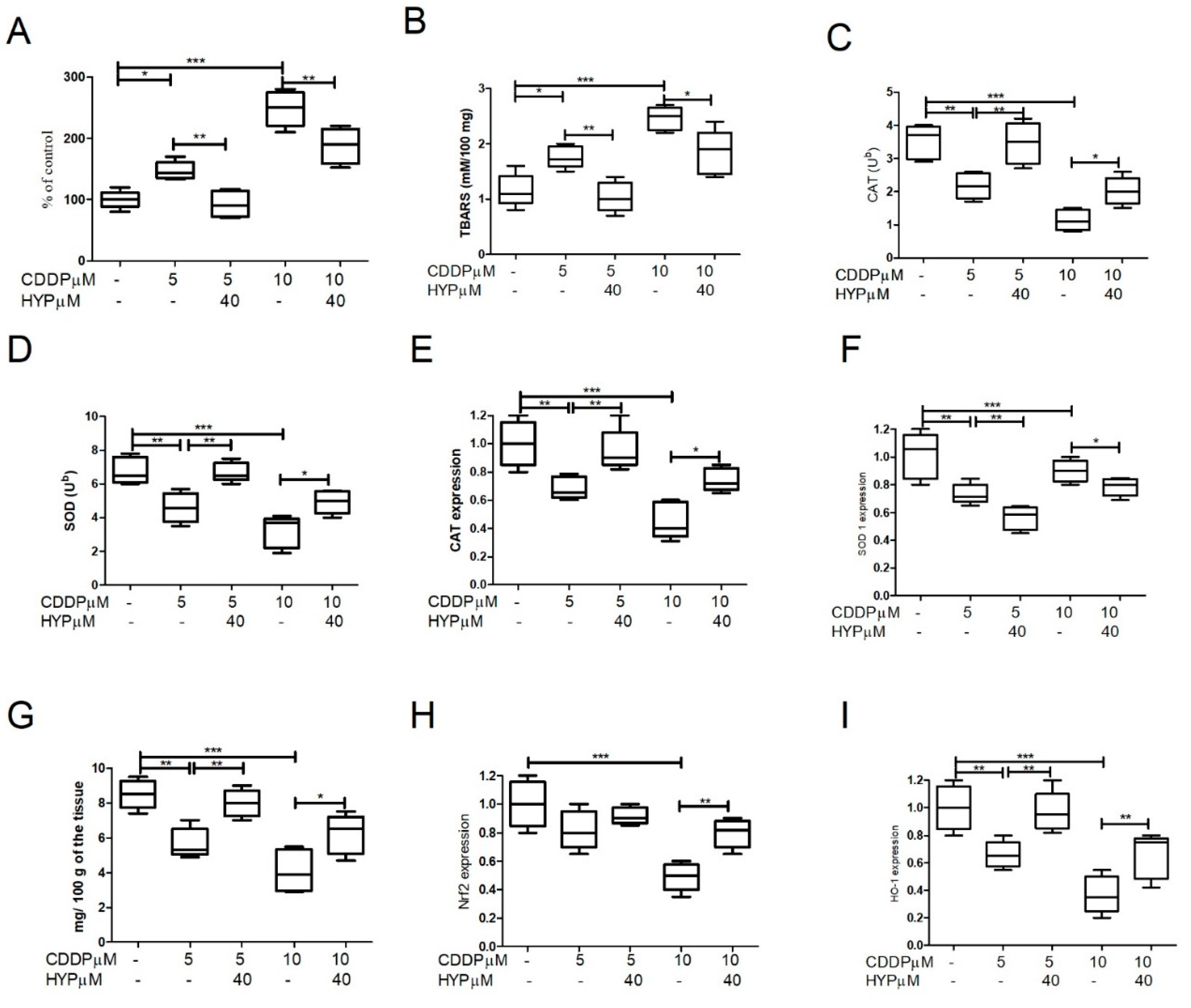
Figure 3.
The CDDP and HYP impact on the apoptosis pathways in granulosa cells. (A-C) Histograms demonstrating the activities and the expression of Akt 1&2. (D) showing cytochrome c activities, and (E-H) Histograms demonstrating the expression of Caspases -3, -8/-9 and Bax/Bcl2 ratio. CDDP induces Akt 1&2 activities and expression(A-C) and Cytochrome c (D) depending on the used dose (5-10 µM). (E-G) CDDP also aggravates caspases -3 (A) caspases -8 (B), and caspase -9 (C)expression depending on the dose. CDDP also markedly upregulates the Bax/Bcl2 ratio in the treated cells compared to the untreated cells (H). However, cotreatment with HYP at 40 µM concentrations, mitigates the CDDP effects on Akt signaling, apoptotic pathways and the Bax/Bcl2 ratio. The data were displayed as mean ± standard deviation and P ≤ 0.05 was used as significant. The data demonstrated significant differences among groups (P*** = P < 0.001, P** = P < 0.01, P* = P < 0.05).
Figure 3.
The CDDP and HYP impact on the apoptosis pathways in granulosa cells. (A-C) Histograms demonstrating the activities and the expression of Akt 1&2. (D) showing cytochrome c activities, and (E-H) Histograms demonstrating the expression of Caspases -3, -8/-9 and Bax/Bcl2 ratio. CDDP induces Akt 1&2 activities and expression(A-C) and Cytochrome c (D) depending on the used dose (5-10 µM). (E-G) CDDP also aggravates caspases -3 (A) caspases -8 (B), and caspase -9 (C)expression depending on the dose. CDDP also markedly upregulates the Bax/Bcl2 ratio in the treated cells compared to the untreated cells (H). However, cotreatment with HYP at 40 µM concentrations, mitigates the CDDP effects on Akt signaling, apoptotic pathways and the Bax/Bcl2 ratio. The data were displayed as mean ± standard deviation and P ≤ 0.05 was used as significant. The data demonstrated significant differences among groups (P*** = P < 0.001, P** = P < 0.01, P* = P < 0.05).
Figure 4.
Predicted Protein Interaction (PPI) Network of CDDP: It has been shown that CDDP interacted with members of caspases (Casp) -3, -7, -8, and -9 pathway which is crucial to induce apoptosis. Also, CDDP interacts with tumor suppressor gene (TP53), mitogen-activated protein kinase 8 (Mapk8), and transcription factor AP-1 (Jun), that control cell growth and apoptosis. In addition, CDDP interacts with Thioredoxin reductase 1(Txnrd1) which has a role to protect the cells from oxidative damage, Poly [ADP-ribose] polymerase 1 (Parp-1) required for DNA repair as well as Glutathione S-transferase P (Gstp1) which plays an essential role to prevent cellular oxidative damage by catalyzing the conjugation of oxidative stress related compounds with reduced glutathione.
Figure 4.
Predicted Protein Interaction (PPI) Network of CDDP: It has been shown that CDDP interacted with members of caspases (Casp) -3, -7, -8, and -9 pathway which is crucial to induce apoptosis. Also, CDDP interacts with tumor suppressor gene (TP53), mitogen-activated protein kinase 8 (Mapk8), and transcription factor AP-1 (Jun), that control cell growth and apoptosis. In addition, CDDP interacts with Thioredoxin reductase 1(Txnrd1) which has a role to protect the cells from oxidative damage, Poly [ADP-ribose] polymerase 1 (Parp-1) required for DNA repair as well as Glutathione S-transferase P (Gstp1) which plays an essential role to prevent cellular oxidative damage by catalyzing the conjugation of oxidative stress related compounds with reduced glutathione.
Figure 5.
Top genes that have shown to be affected by protective effect of HYP. HYP is expected to interact with TNF; tumor necrosis, factor genes, GLI1; GLI family zinc finger 1 which is negatively affect TP53 activities,MIR21 and MIR27A which are related to genes expression activities, PDCD4; Programmed Cell Death 4 which is related to apoptosis, SHH; Coding for Sonic Hedgehog which is essential for cell growth, specialization, and shaping, SMO; coding for G protein-coupled receptor which controls Akt signaling, SP1; SP1 transcription factor involved in various cellular processes including apoptosis, post-translation modification, and DNA repair, ZBTB10; involved in transcription regulation, ACHE; Acetyl cholinesterase gene.
Figure 5.
Top genes that have shown to be affected by protective effect of HYP. HYP is expected to interact with TNF; tumor necrosis, factor genes, GLI1; GLI family zinc finger 1 which is negatively affect TP53 activities,MIR21 and MIR27A which are related to genes expression activities, PDCD4; Programmed Cell Death 4 which is related to apoptosis, SHH; Coding for Sonic Hedgehog which is essential for cell growth, specialization, and shaping, SMO; coding for G protein-coupled receptor which controls Akt signaling, SP1; SP1 transcription factor involved in various cellular processes including apoptosis, post-translation modification, and DNA repair, ZBTB10; involved in transcription regulation, ACHE; Acetyl cholinesterase gene.