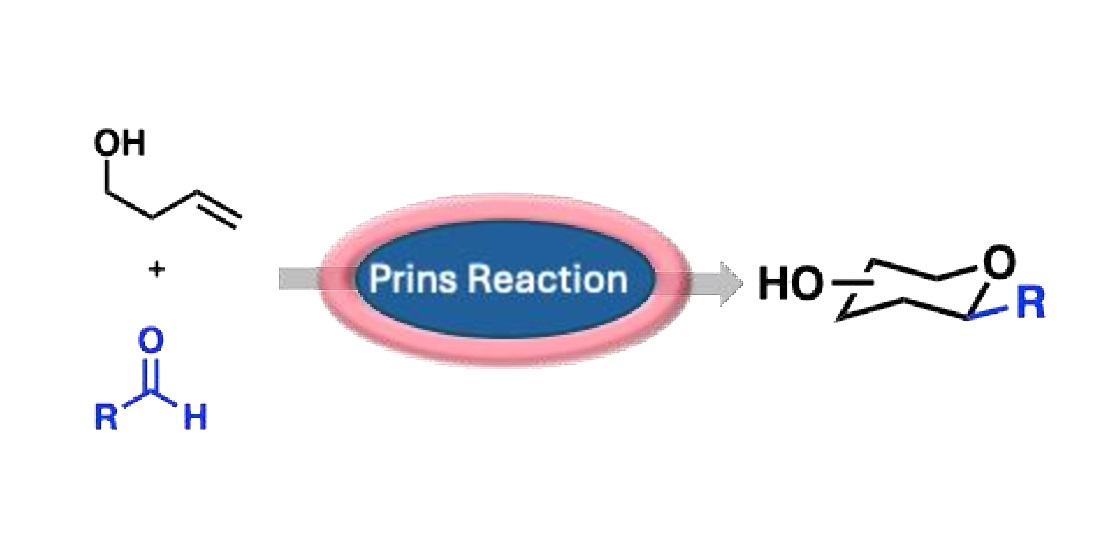
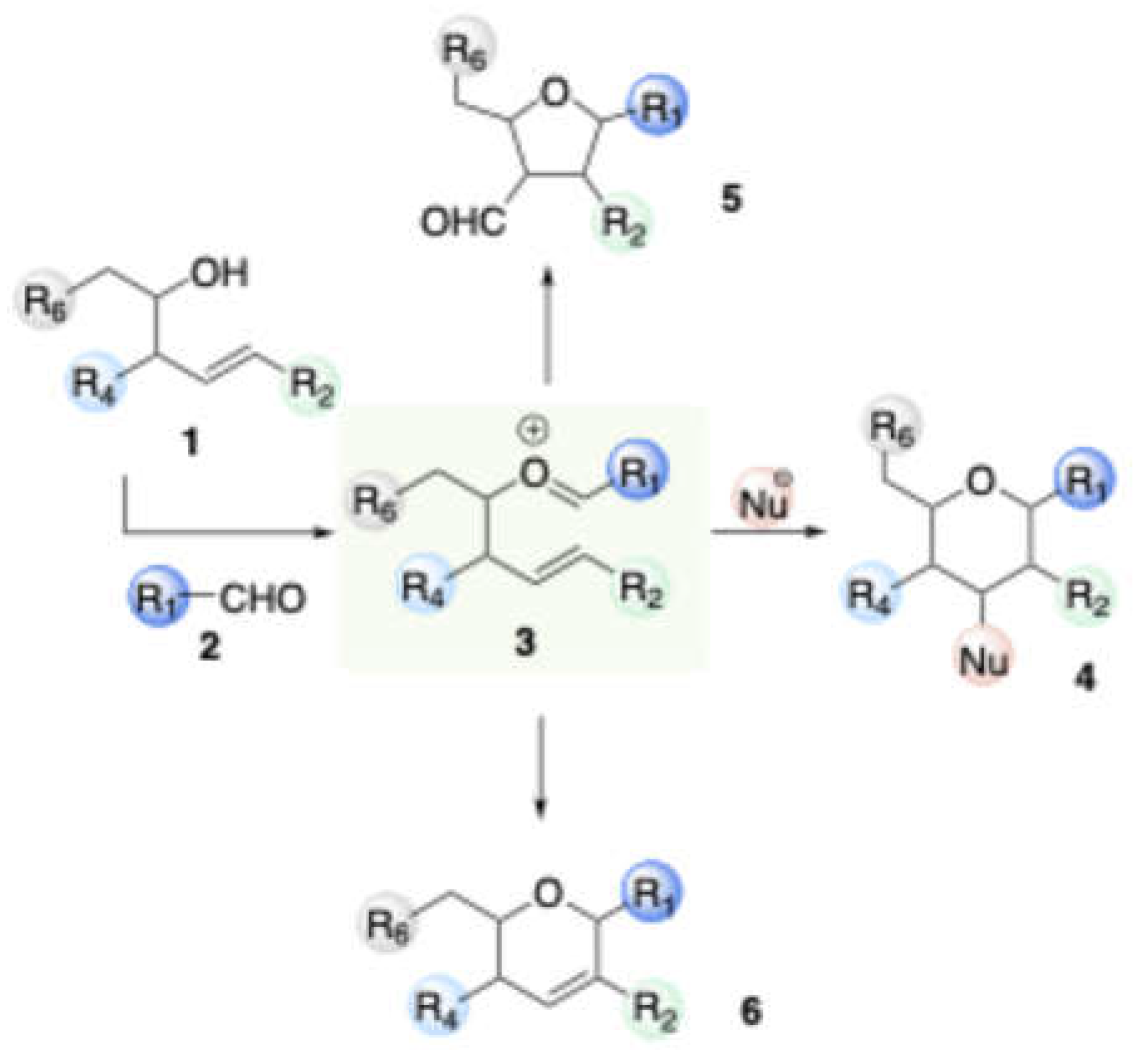
There is currently a wealth of inter and intra molecular Prins reactions are available within the modern synthetic chemist’s ‘toolbox’. However, among the most prevalent Prins reaction types is the addition of a nucleophiles to a secondary carbocation (typically alkenes or alkynes), Pinacol type rearrangement, Sakurai Prins reaction and
Ritter Prins reaction. Within the context of sugar moiety synthesis via Prins cyclization and based on the origin synthon of homoallylic alocohols, these reactions are divided into two distinct subclasses: those in which a synthesis of carbohydrate scaffolds from carbohydrate synthons (1.1) and those in which a carbohydrate scaffolds from non-carbohydrate synthons (2.2).
2.1. Carbohydrate Synthons to Carbohydrate Scaffolds
This section summarizes research that focuses on generating valuable carbohydrate scaffolds or structural units using carbohydrate-derived starting materials, particularly carbohydrate-derived homoallylic alcohols, through a process called Prins cyclization. In this specific study conducted by Yadav and colleagues, they reported [
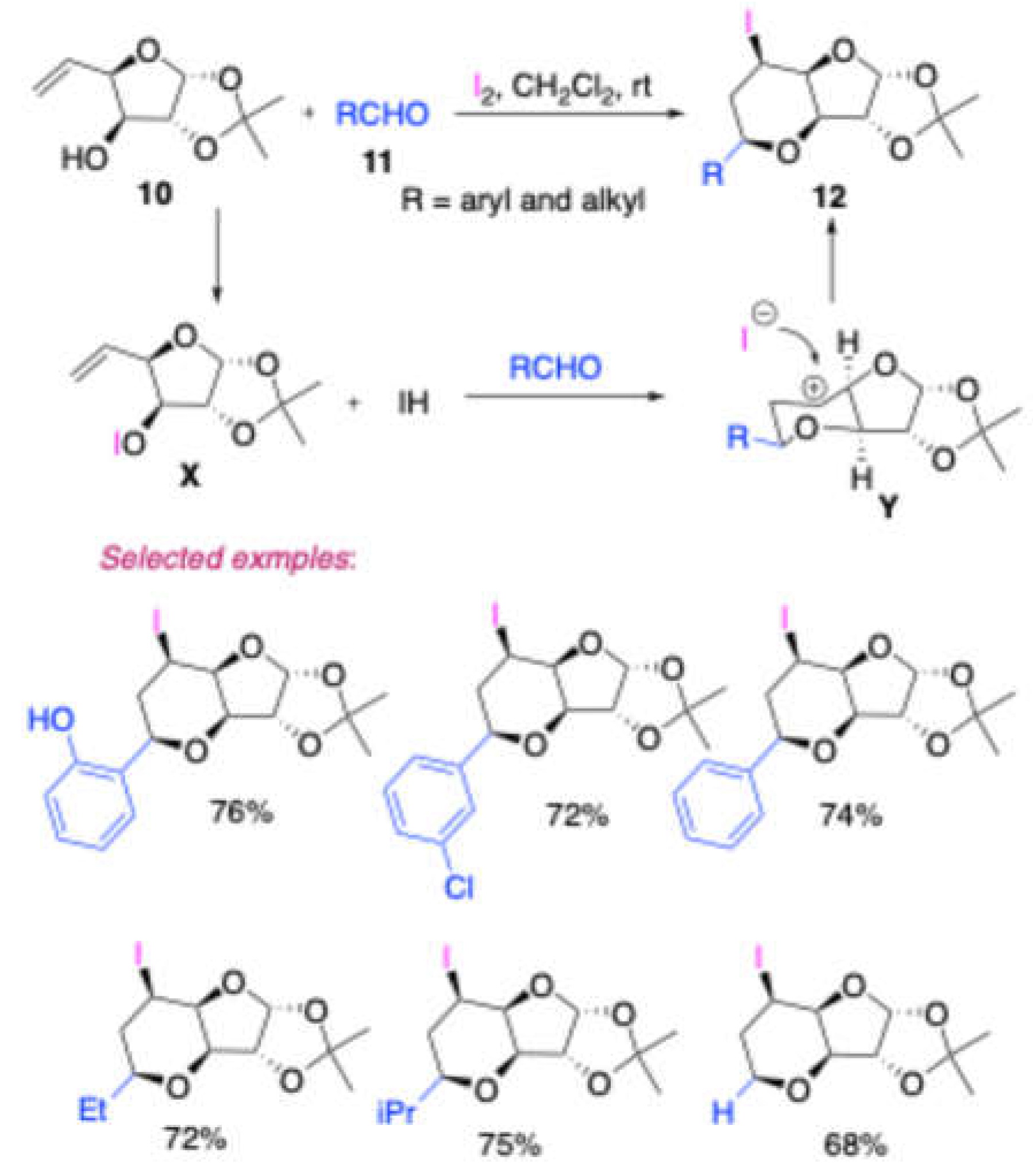
3] the synthesis of a sugar-annulated iodotetrahydropyran compound
12 by employing Prins cyclization. They combined a D-glucose-derived homoallylic alcohol
10 with an aldehyde
11 in their synthetic approach (
Scheme 2). The researchers explored the use of a variety of aromatic and aliphatic aldehydes as reactants in their experiments. Importantly, they successfully obtained the desired products in moderate to good yields, indicating the effectiveness of their synthesis method. The authors of this study proposed a reaction mechanism that involves an intermediate
X and a highly reactive carbocation
Y. This carbocation
Y is subsequently captured by an iodide ion, resulting in the formation of the sugar-annulated iodotetrahydropyran compound
12.
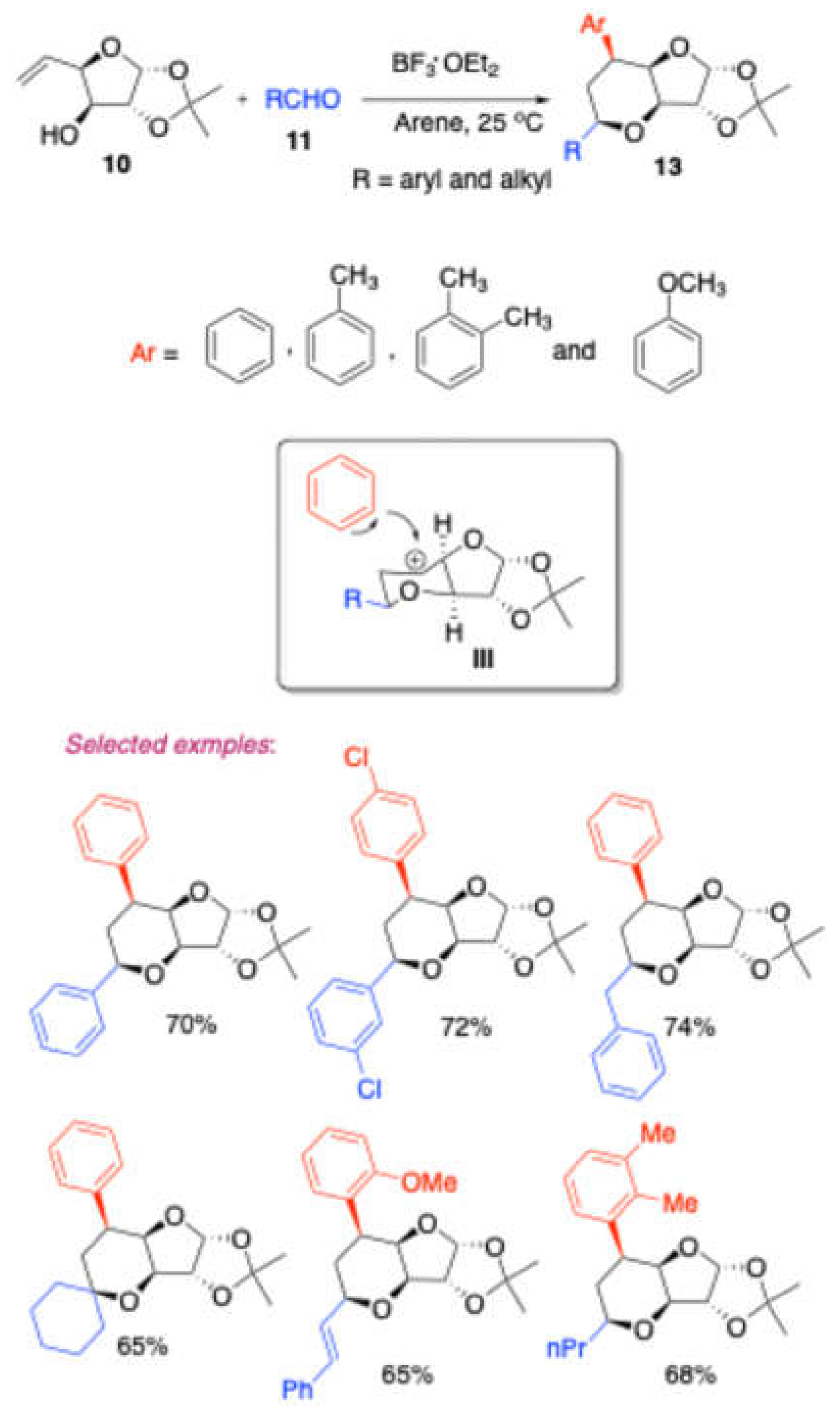
In a subsequent study, the same research group[
4] delved deeper into the Prins reaction of homoallylic alcohol
10, as shown in
Scheme 3. They investigated this reaction with various carbonyl compounds
11, employing BF
3·OEt
2 as a catalyst and various arene solvents. This reaction led to the formation of sugar-fused diaryl hexahydro-2
H-furo[3,2-b]pyran
13 via intermediate
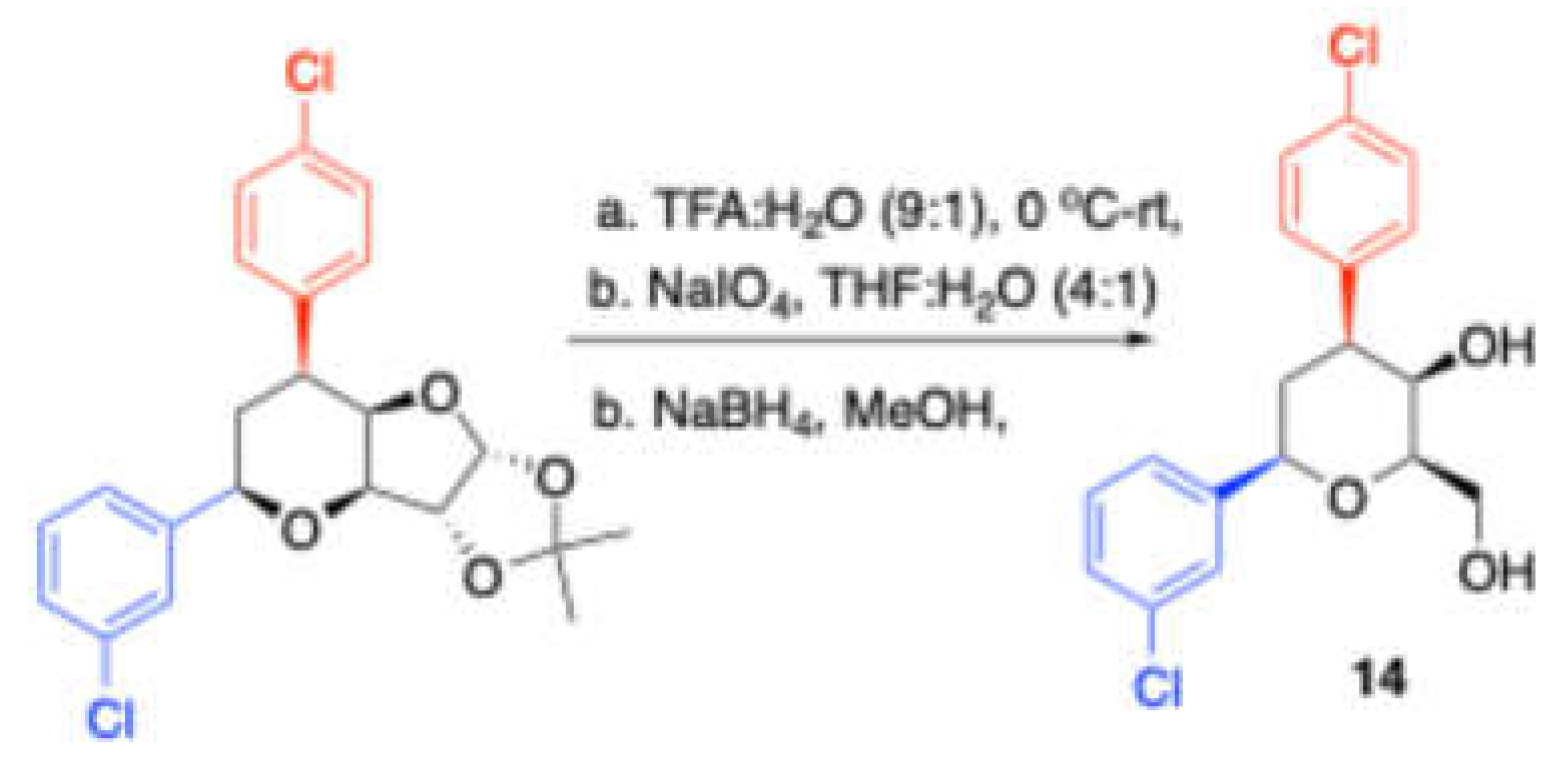
III. Notably, the researchers examined different arene solvents, including benzene, toluene, o-xylene, and anisole. The reaction proved compatible with a variety of aryl and alkyl aldehydes as well as cyclohexanone. Interestingly, one of the product further converted into diaryl dihydroxytetrahydropyara
14 (
Scheme 4).
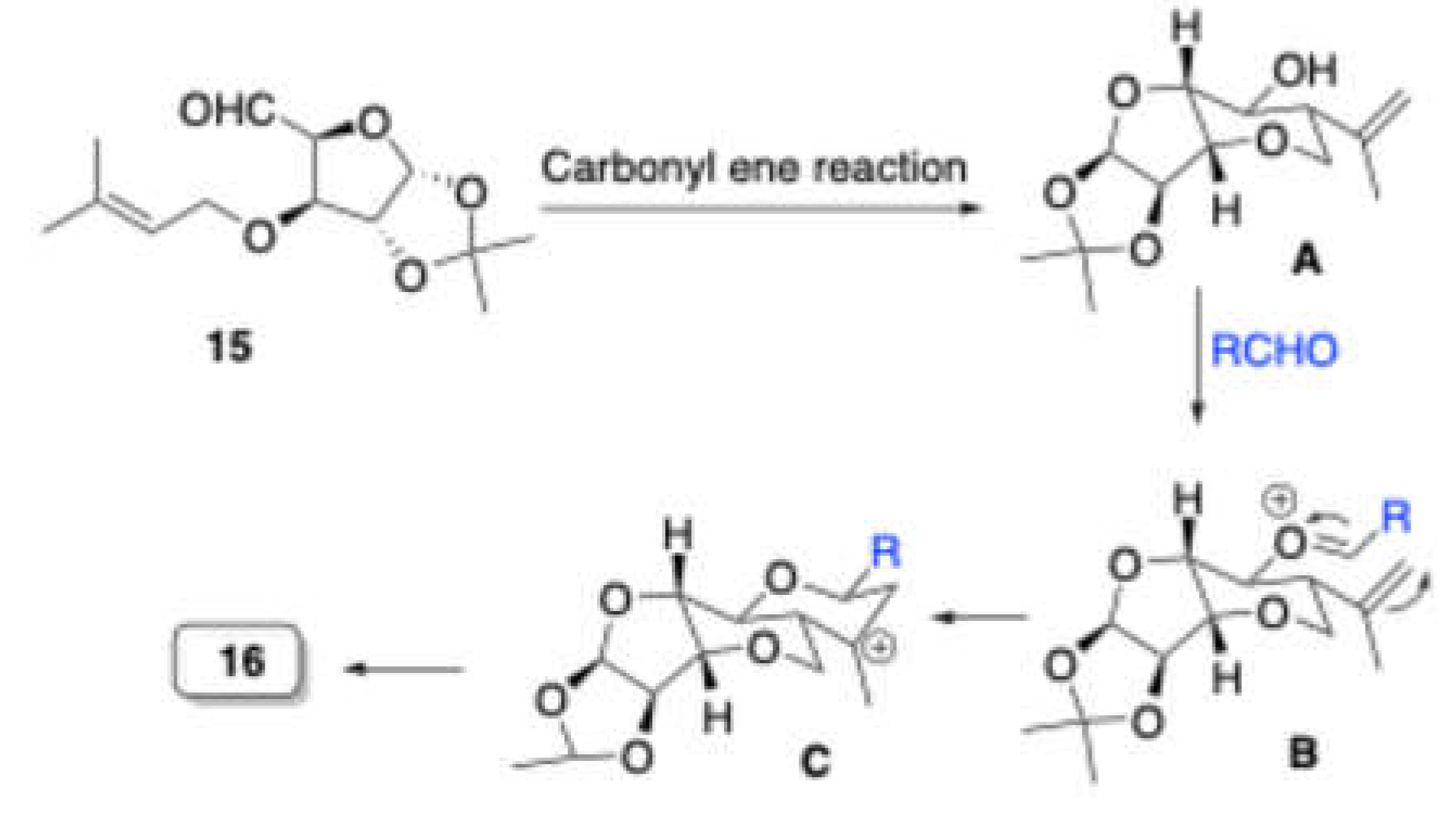
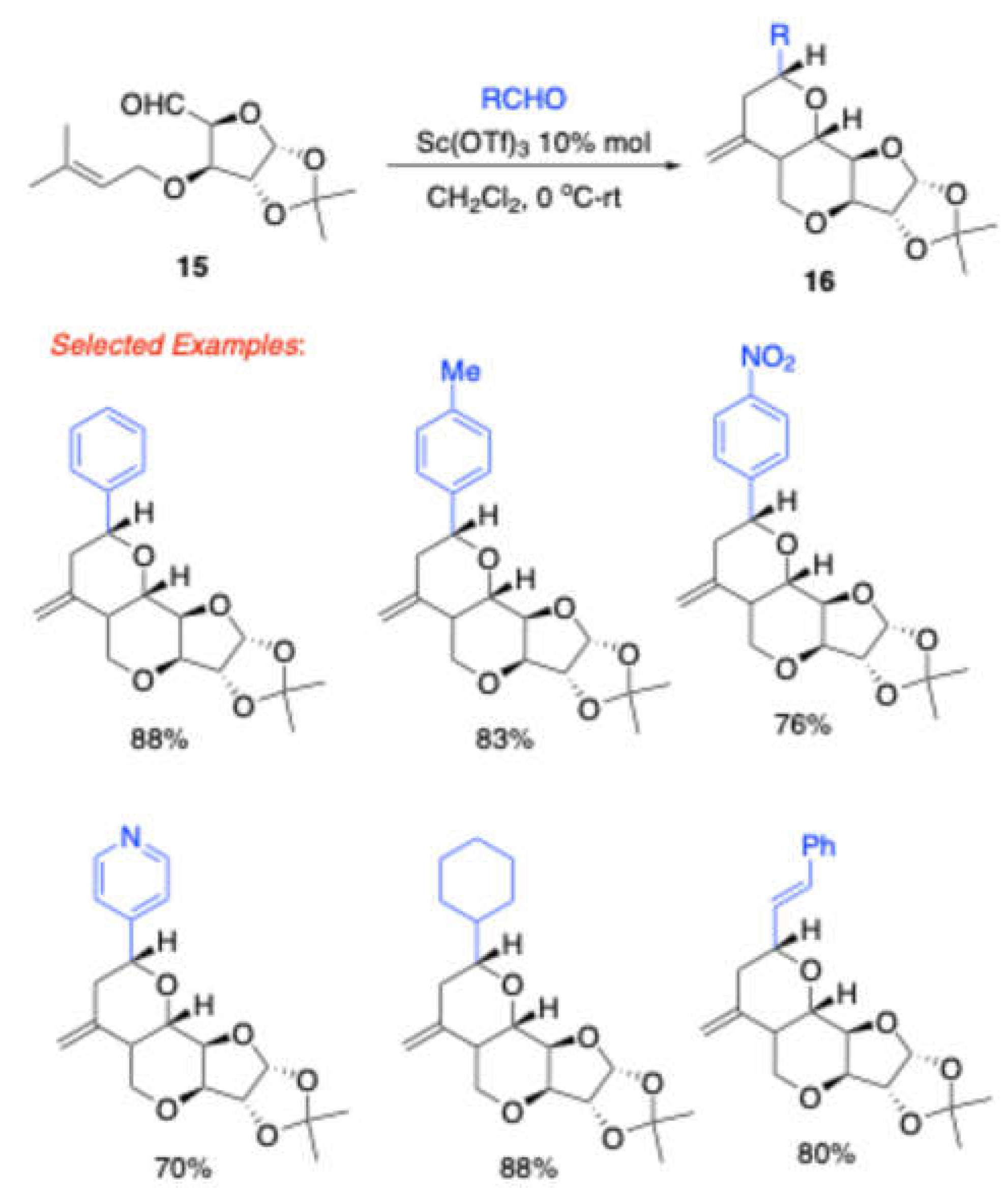
Reddy et al. reported[
5] the synthesis of the hexahydro-2
H-furo[3,2-
b]pyranopyran scaffold
16 from
O-prenyl tethered carbohydrate derived aldehyde
15 with various aldehydes in the presence of 10 mol% Sc(OTf)
3 in dichloromethane at 0
oC to room temperature (
Scheme 5). Further, this reaction was studied with a variety of aryl and alkyl aldehydes. Eventually, this reaction was quite successful with
p-bromobenzaldehyde and cyclohexylidene protected
O-prenyl tethered carbohydrate derived aldehyde to furnish the product tricyclic sugar derivative
16 (Scheme 7).
First, cyclization of O-prenyl tethered carbohydrate derived aldehyde 15 is proposed, facilitated by carbonyl ene reaction and led to homoallylic alcohol A, with this being the prins reaction defining step. Then A condes with aldehyde in presence of Sc(OTf)3 could give tertioary carbocation C via oxocarbonioum ion B. Further C could eliminate proton from methyl group and led to product 16.
Scheme 6.
Mechanism for the formation of hexahydro-2H-furo[3,2-b]pyranopyran.
Scheme 6.
Mechanism for the formation of hexahydro-2H-furo[3,2-b]pyranopyran.
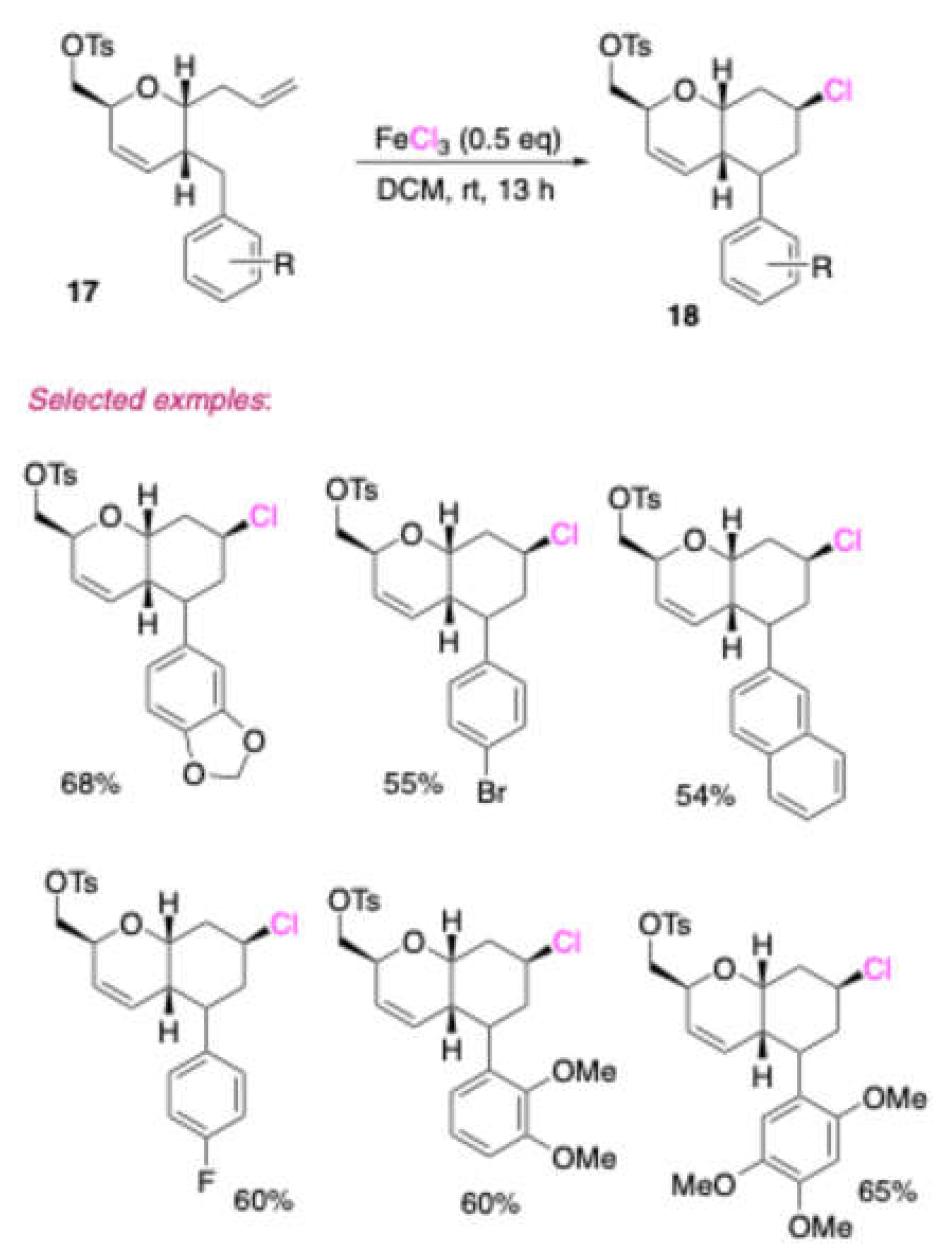
In 2014, Lumba and Mukherjee[
6] developed a practical protocol for the conversion of tri-
O-acetyl-D-glucal derived 2-C-branched sugars
17 to the corresponding
cis-1-oxadecalines
18. By using FeCl
3 as a catalyst system at room temperature, the target molecules could be afforded [
Scheme 8]. The advantages of this protocol are the use of a variety of 2-C-branched sugars.
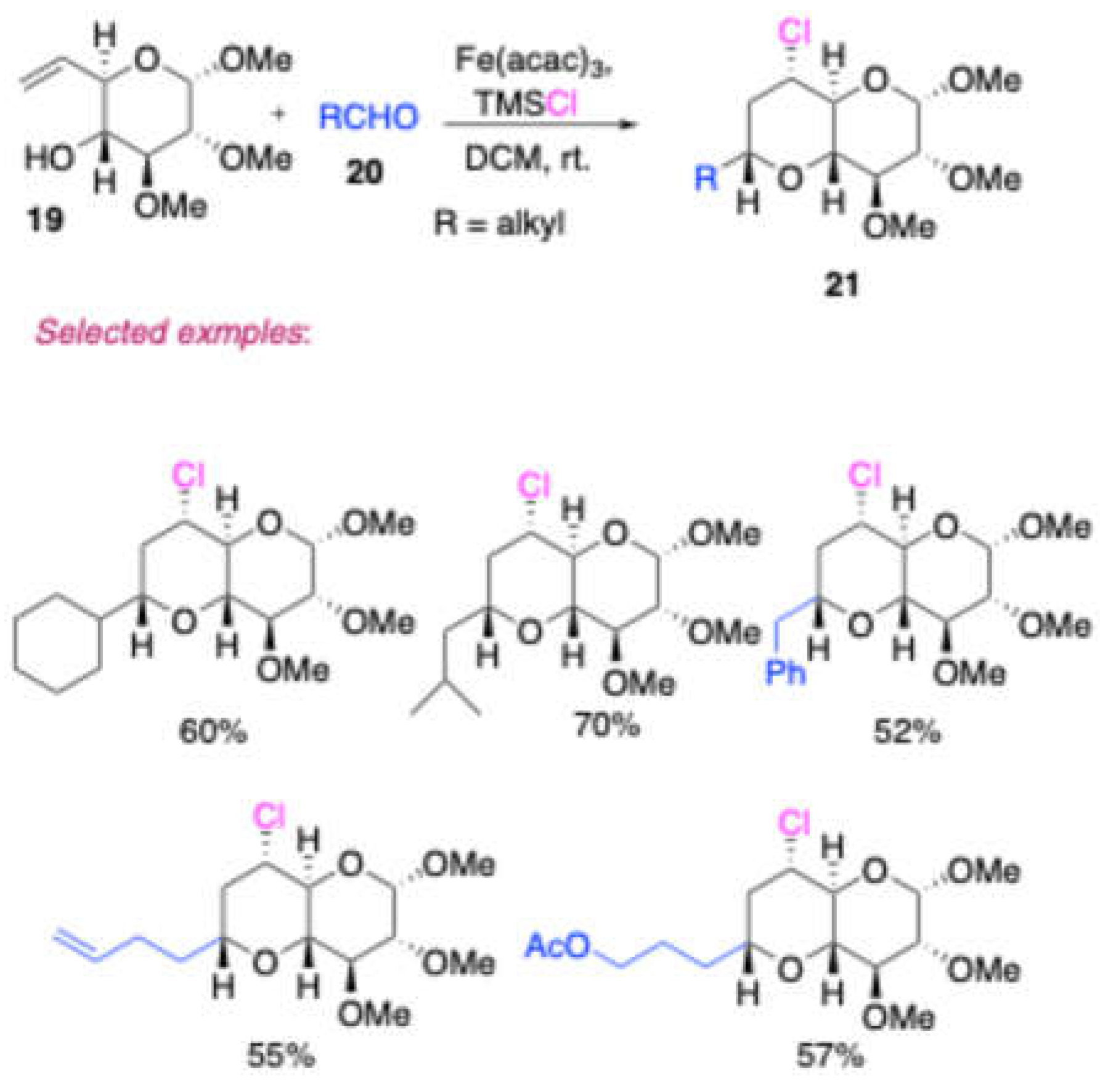
Padrón and co-workers[
7] reported a simple preparation method for
trans-fused bicyclic tetrahydropyran
21 by a iron(lll) catalyzed tandem reaction using tri-
O-acetyl-D-glucal derived homoallylic alcohol
16 and iso-valeraldehyde [
Scheme 9]. This reaction was further studied with more complex molecule
19, which is derived from α-methyl-D-glucopyranoside with aldehydes and led to the
trans-fused bicyclic tetrahydropyrans
20. Here, the features are the good substrate generality and the mild reaction conditions.
Cis-fused heterobicyclic systems are very important substrates in neuronally active agents such as marine-derived dysiherbaine and their analogues IKM-159 and MC-27. Oikawa and co-workers[
8] reported a BF
3·OEt
2 catalyzed condensation reaction between glucose derived enetiomerically pure homoallylic alcohol
22 with aldehydes 11 and nitrile solvent
23; the intended cis-fused 4-amidotetrahydropyrans
24 were obtained in a one-pot manner under relatively mild conditions (
Scheme 10). Based on these results and the scope was extended to substrate
24 with variety of aldehydes and nitrile solvent via Prins-Ritter reaction to obtained cis-fused heterobicyclics
24 which were further subjected to acid hydrolysis led to the formation for novel glutamates
25.
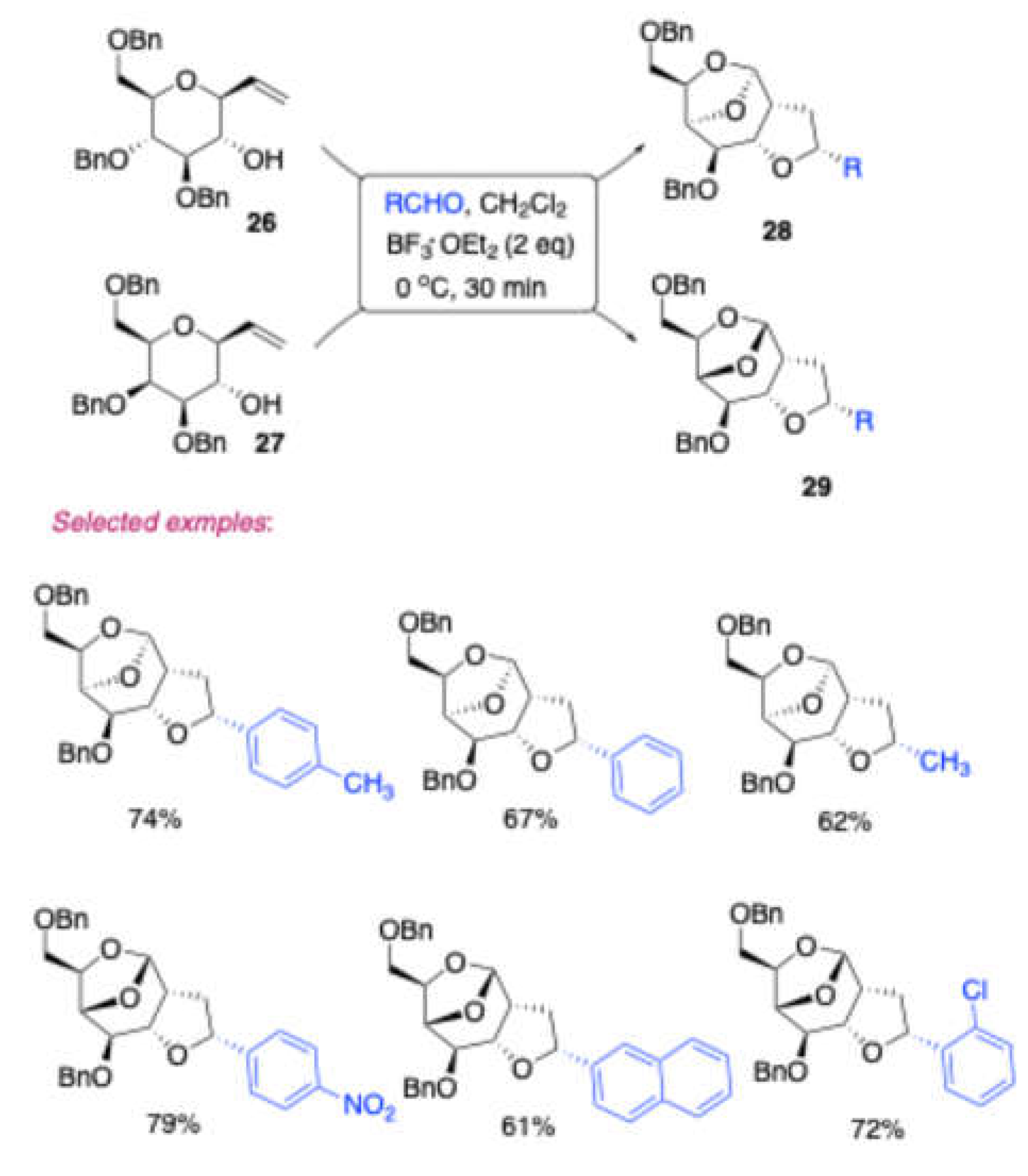
Vankar and co-workers[
9] developed an efficient synthesis of bridged tricyclic ketals
28/29 with good substrate generality starting from homoallylic alcohols
26/
27 derived from 1,2-anhydro and aldehydes; they used BF
3·OEt
2 as catalyst [
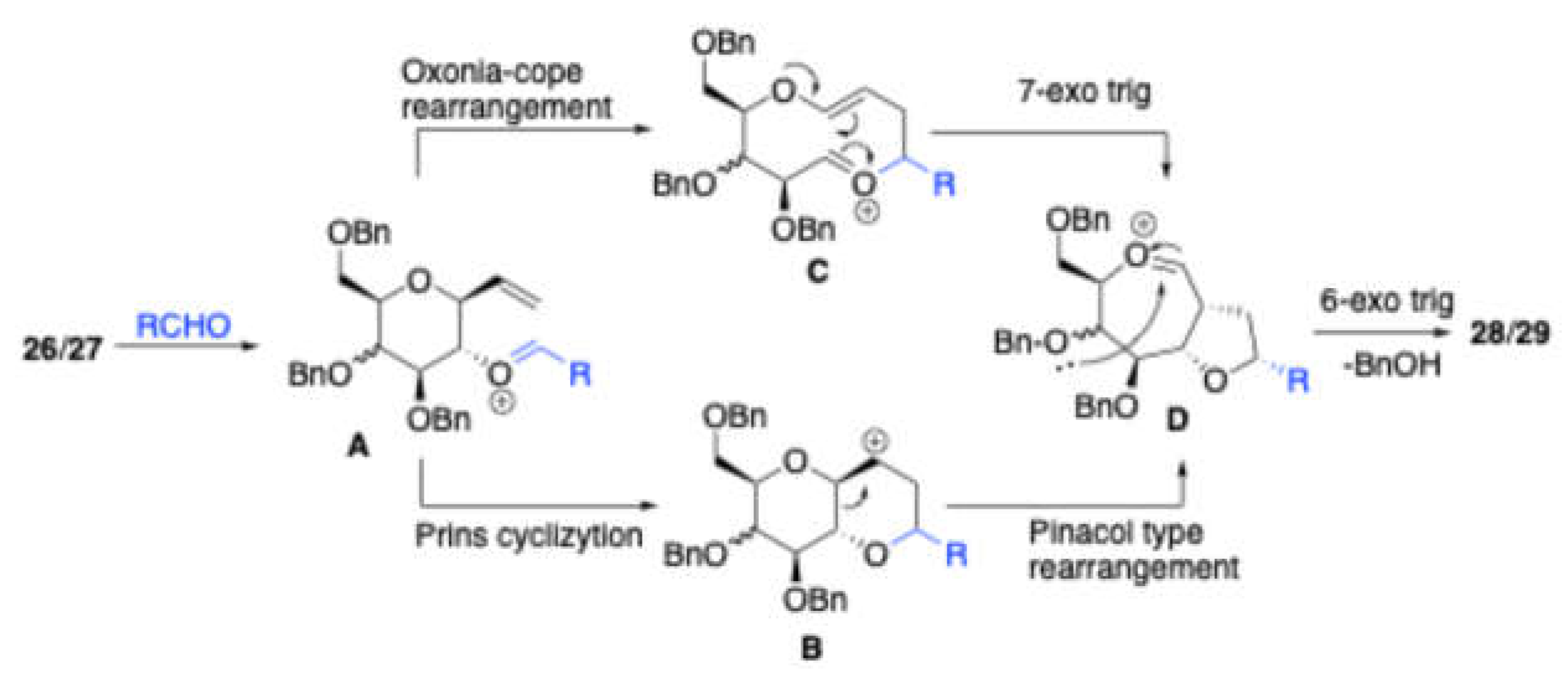
Scheme 11]. Here, the main advantages include the stereoselectivity and good yields. They proposed the plausible mechanism for the formation of bridged tricyclic ketals
28/
29 shown in the
Scheme 12. It is presumed that after initial formation of the oxocarbenium ion
A, it can either undergo Oxonia-Cope rearrangement to form
B, or simply a π-cation cyclization to form C. Both of these intermediates will then undergo 7-exo trig cyclization or pinacol-type rearrangement via the transition state
D, followed by cleavage of the C4-OBn participation resulting into to form bridged tricyclic ketals
28/
29. To get further insight into the proposed mechanism they tested homoallylic alcohol
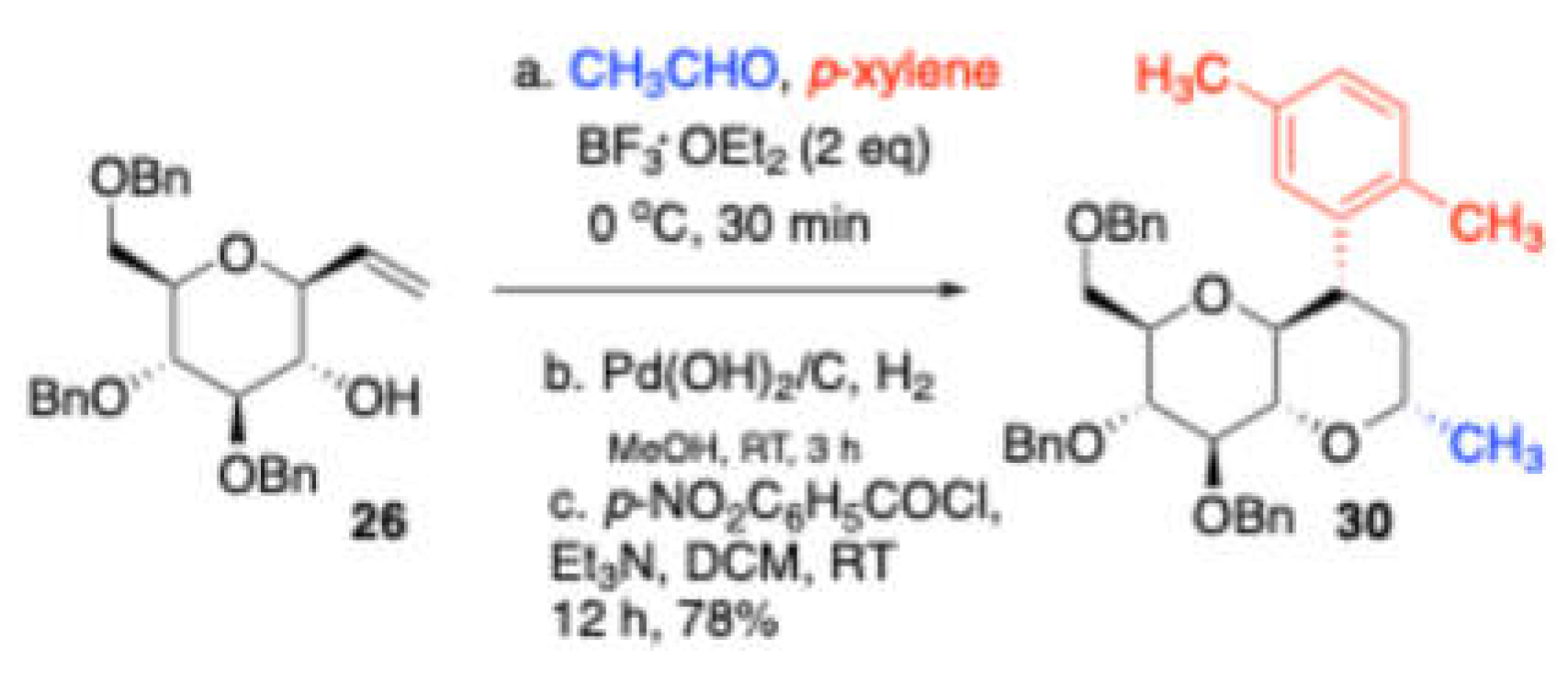
26 with acetaldehyde and
p-xylene as nucleophile observed the
p-xylene trapped prins product
30 (
Scheme 13).
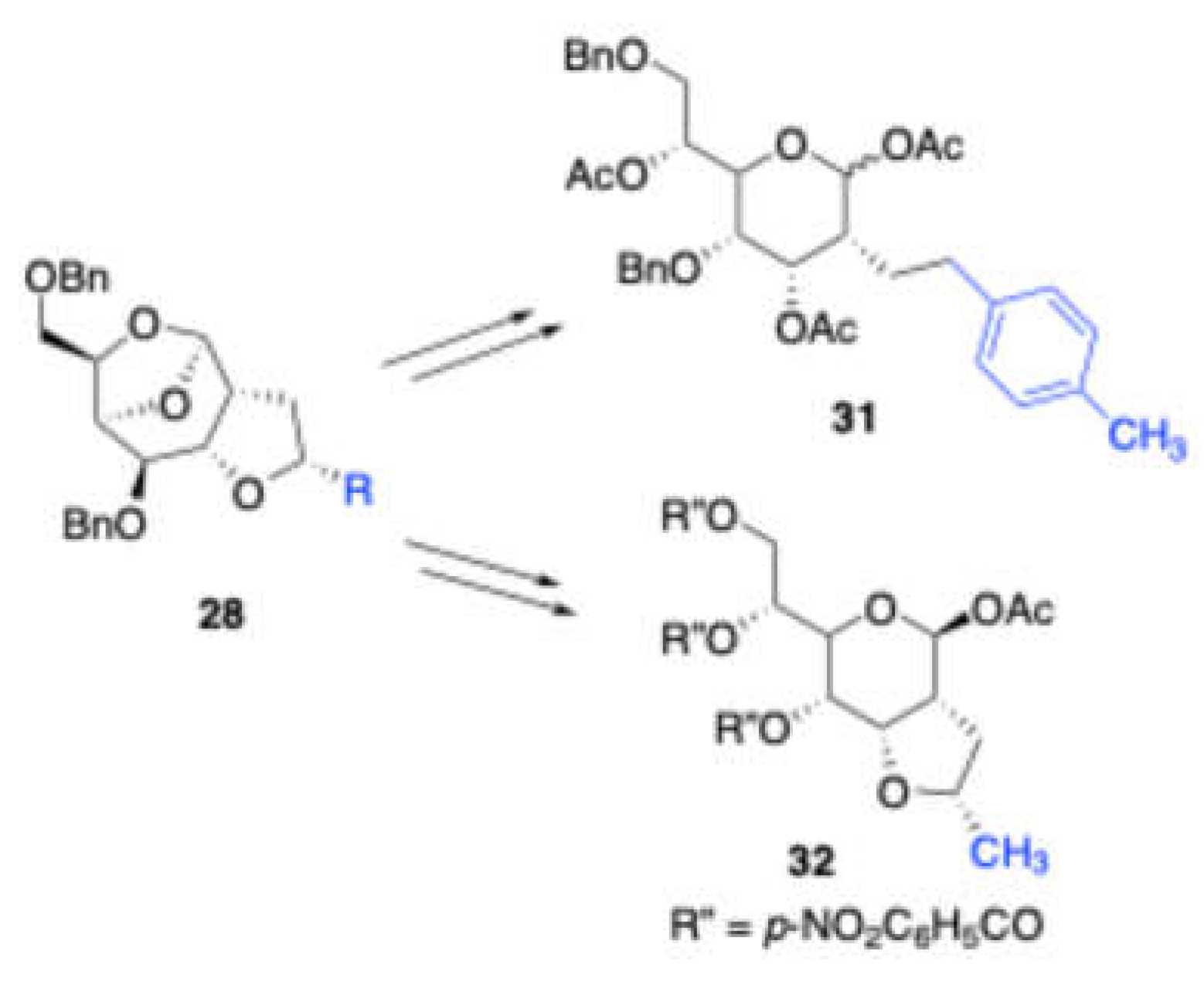
p-xylene trapped prins product was further hydrogenolysis with Pd(OH)
2/C followed by benzoylation of the resulting alcohol with p-nitrobenzoylchloride/Et
3N to give the corresponding annulated sugar
32. Later, these bridged tricyclic ketals were converted to (
Scheme 14) tetrahydrofuran ring fused heptose
31 and 2C-branched heptose
32.
Scheme 10.
Cis-fused 4-amidotetrahydropyrans towards a precursor for possible neuronal receptor ligands via Prins-Ritter reaction.
Scheme 10.
Cis-fused 4-amidotetrahydropyrans towards a precursor for possible neuronal receptor ligands via Prins-Ritter reaction.
Scheme 11.
Synthesis of bridged tricyclic ketals 28/29 thorugh Prins-Pinacol type rearrangement and C4-OBn participation.
Scheme 11.
Synthesis of bridged tricyclic ketals 28/29 thorugh Prins-Pinacol type rearrangement and C4-OBn participation.
Scheme 12.
Mechanism for the formation of bridged tricyclic ketals 30/31.
Scheme 12.
Mechanism for the formation of bridged tricyclic ketals 30/31.
Scheme 13.
Trapping with p-xylene.
Scheme 13.
Trapping with p-xylene.
Scheme 14.
Derivitization of bridged tricyclic ketal.
Scheme 14.
Derivitization of bridged tricyclic ketal.
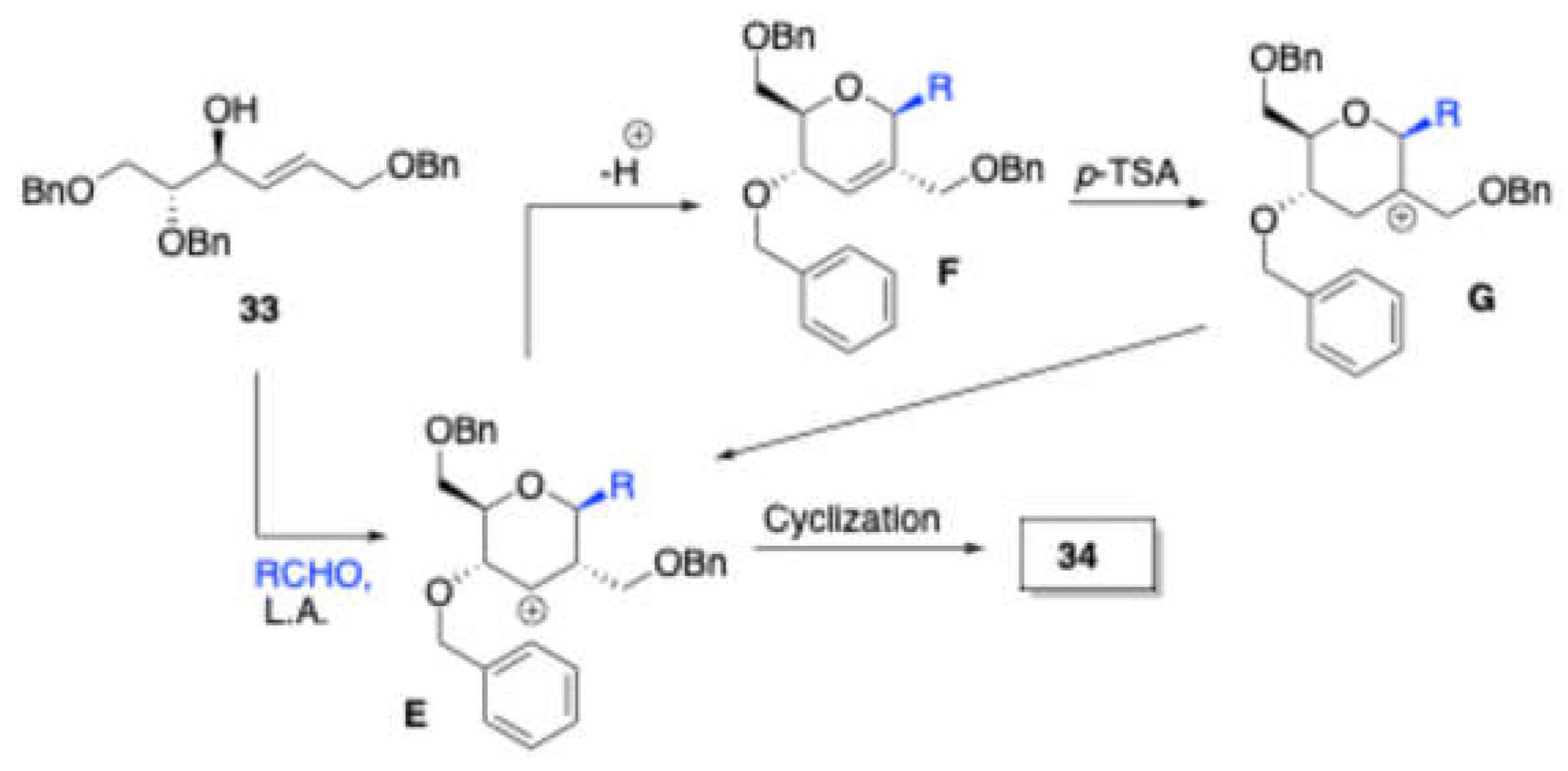
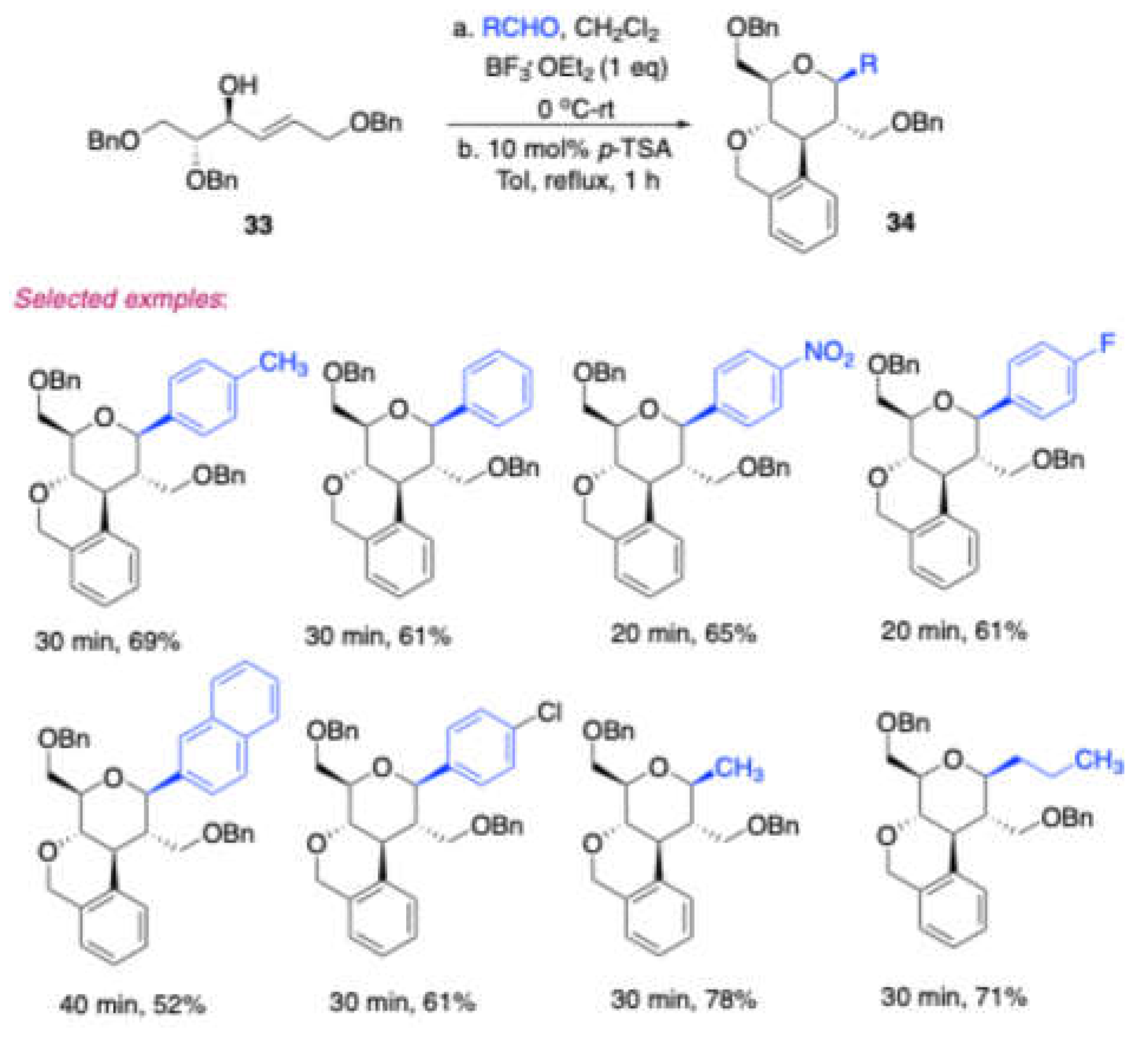
In 2017, Vankar et al. [
10] reported a very effective synthetic route by using BF
3·OEt
2 to synthesize 1C-aryl/alkyl 2C-branched sugar fused isochroman derivatives
34 from 2C-formyl glucal derived homoallylic alcohol
33 and aldehydes, in a moderate
Scheme 16.
Mechanism for the formation of 1C-aryl/alkyl 2C-branched sugar fused isochroman derivatives.
Scheme 16.
Mechanism for the formation of 1C-aryl/alkyl 2C-branched sugar fused isochroman derivatives.
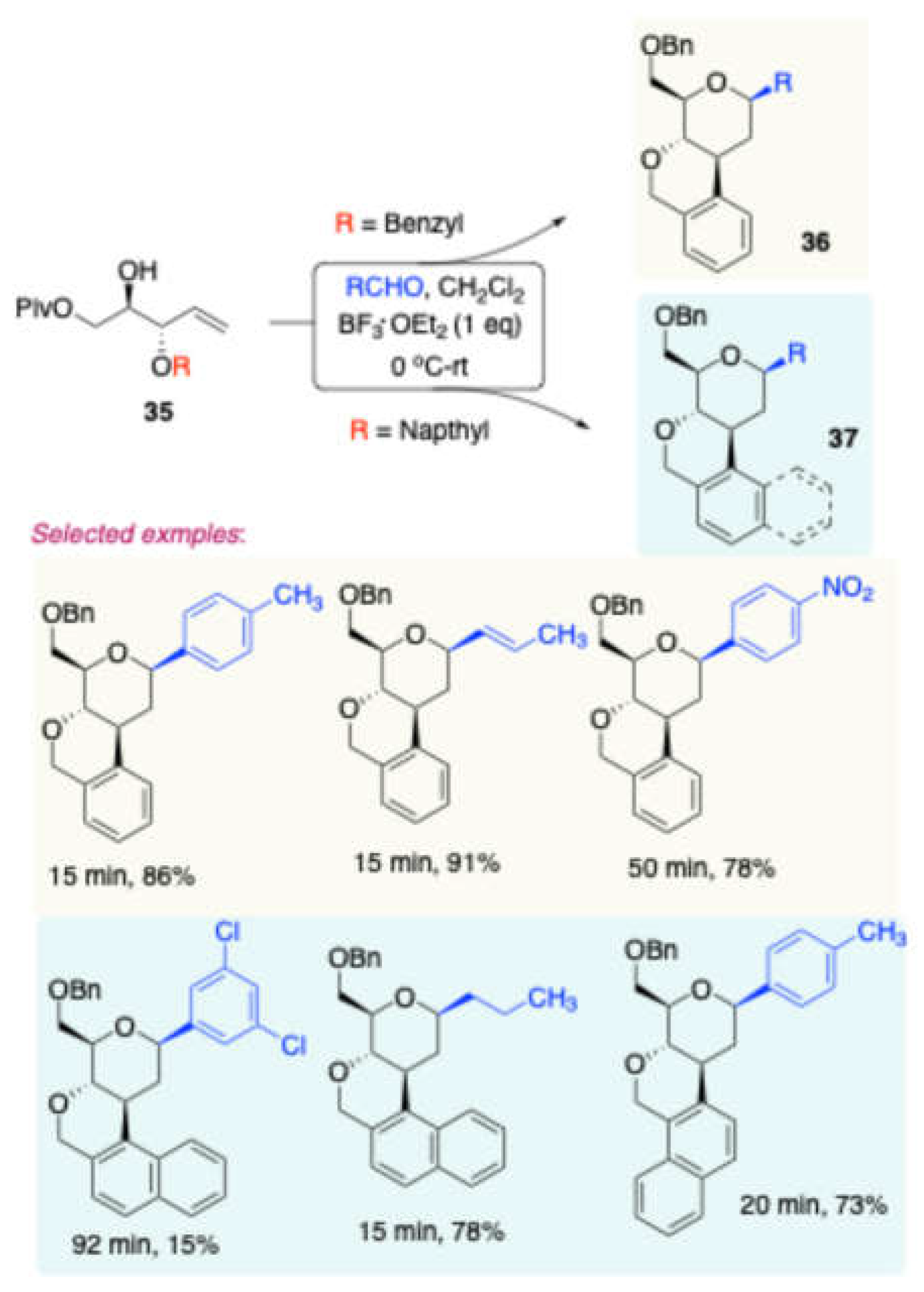
Later, Dubbu and Vankar strategically synthesized[
11] a series of 2-deoxy-3,4-fused-C-aryl/alkylglycosides through a cascade Prins cyclization of a D-mannitol-derived homoallylic alcohol, using BF₃·OEt₂ as a catalyst. Initially, (
Scheme 17) the D-mannitol-reaction time (1 h) in 60–70 % yields (
Scheme 15).
In the presence of the BF3·OEt2, substrate 33 and aldehyde were condensed to produce intermediate E, which would undergo elimination of adjacent proton and generate dihydropyran F. This dihydropyran F treated with PTSA gives tertiary carbocation G, which is equilibrium with intermediate E. then intermediate E may directly give the product 34.
derived homoallylic alcohol
35 was treated with various carbonyl compounds in the presence of BF₃·OEt₂ in DCM, yielding 2-deoxy-3,4-fused isochroman derivatives
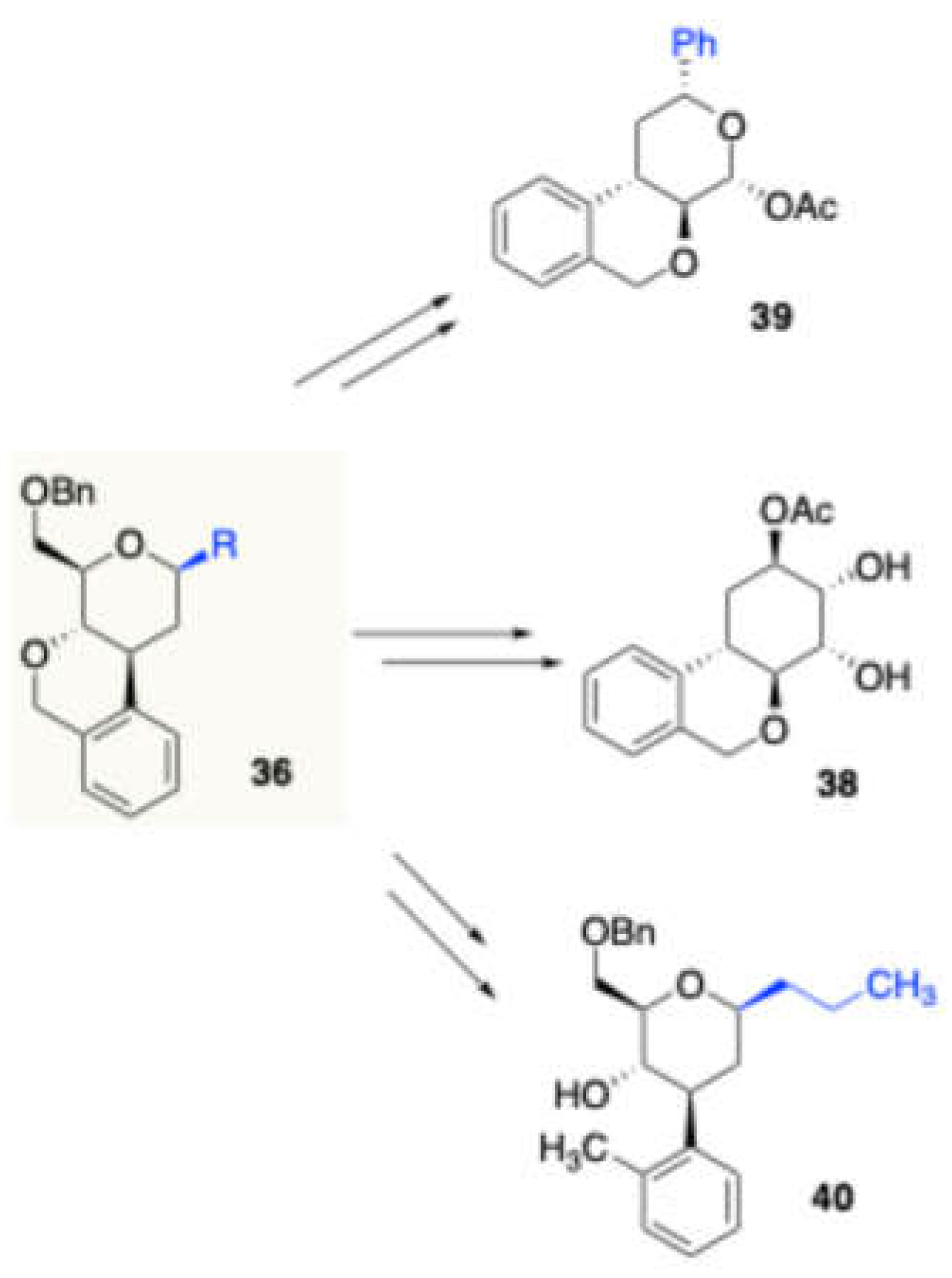
36/37 in good to excellent yields. The authors further modified these products to obtain potentially bioactive scaffolds 38, 39, and 40 (
Scheme 18).
Scheme 17.
Synthesis of 1C-aryl/alkyl fused isochroman derivatives.
Scheme 17.
Synthesis of 1C-aryl/alkyl fused isochroman derivatives.
Scheme 18.
Derivatization of sugar fused isochroman derivatives.
Scheme 18.
Derivatization of sugar fused isochroman derivatives.
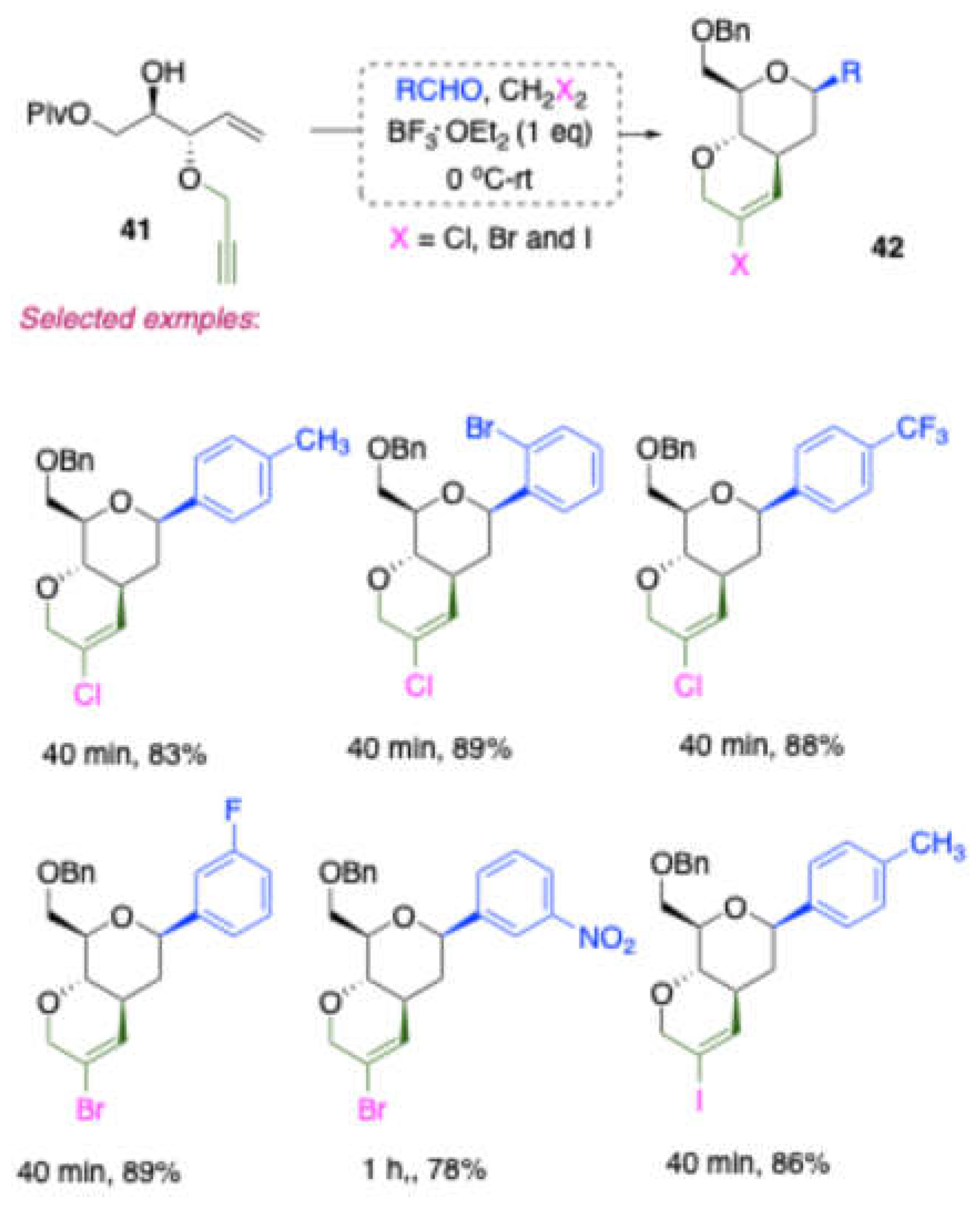
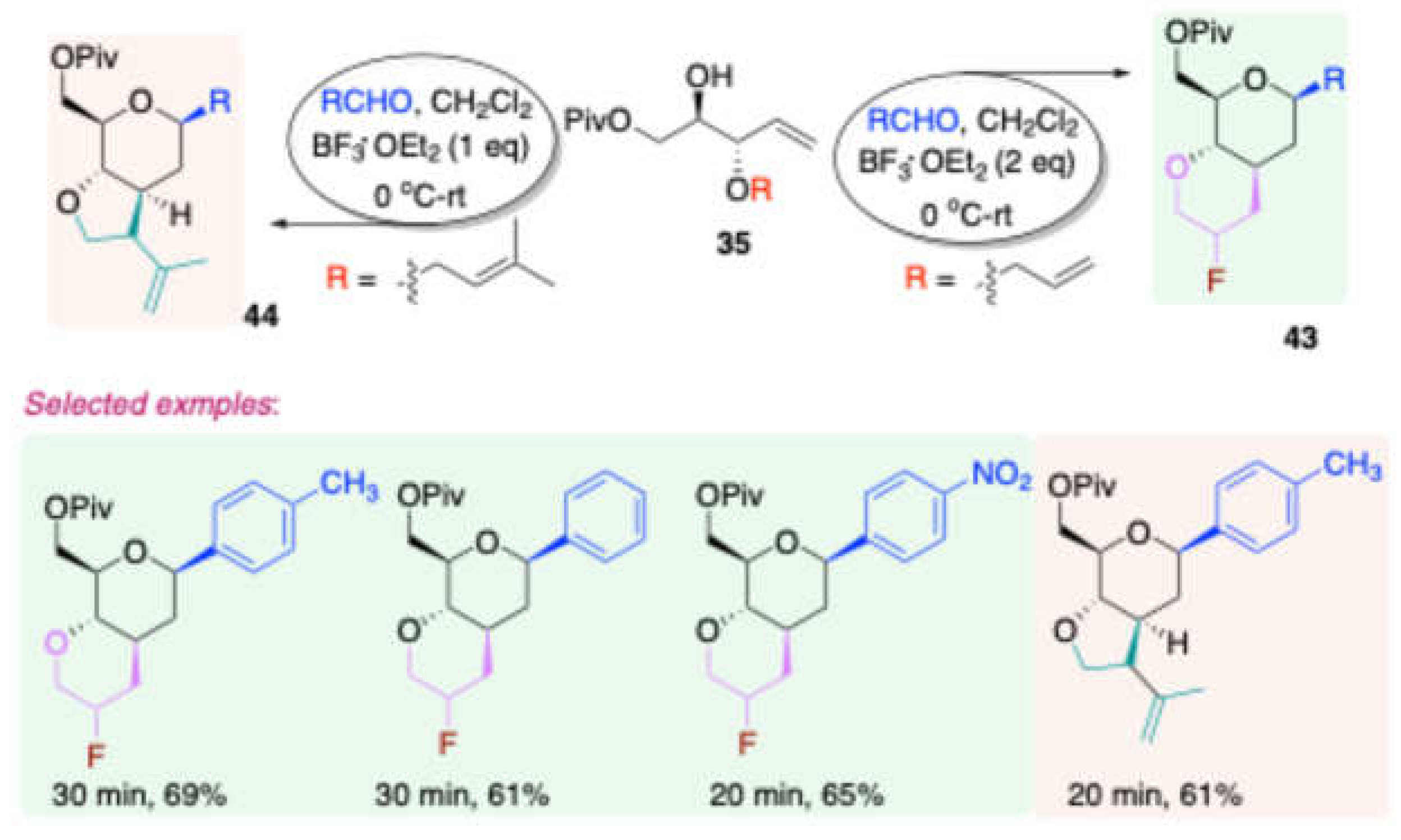
Using similar reaction conditions, but with a D-mannitol-derived homoallylic alcohol protected with a propargyl group, Dubbu and Vankar obtained [
11] 1C-aryl/alkyl-fused bicyclic vinyl halide derivatives
42 in good to excellent yields x. The halogen abstraction was achieved through the use of halogenated solvents. Depending on the solvent used—CH₂Cl₂, CH₂Br₂, CH₃I, etc.—the products obtained were 1C-aryl/alkyl-fused bicyclic vinyl chloride, vinyl bromide, or vinyl iodide derivatives, respectively.
Scheme 19.
Synthesis of 1C-aryl/alkyl fused bicyclic vinyl halide derivatives.
Scheme 19.
Synthesis of 1C-aryl/alkyl fused bicyclic vinyl halide derivatives.
By applying similar reaction conditions, Dubbu and Vankar further transformed[
11] the D-mannitol-derived homoallylic alcohol
35, protected with allyl and substituted allyl groups, to synthesize 1C-aryl/alkyl-fused bicyclic fluorine-substituted tetrahydropyran and furan derivatives 43/44, achieving good to excellent yields (
Scheme 20).
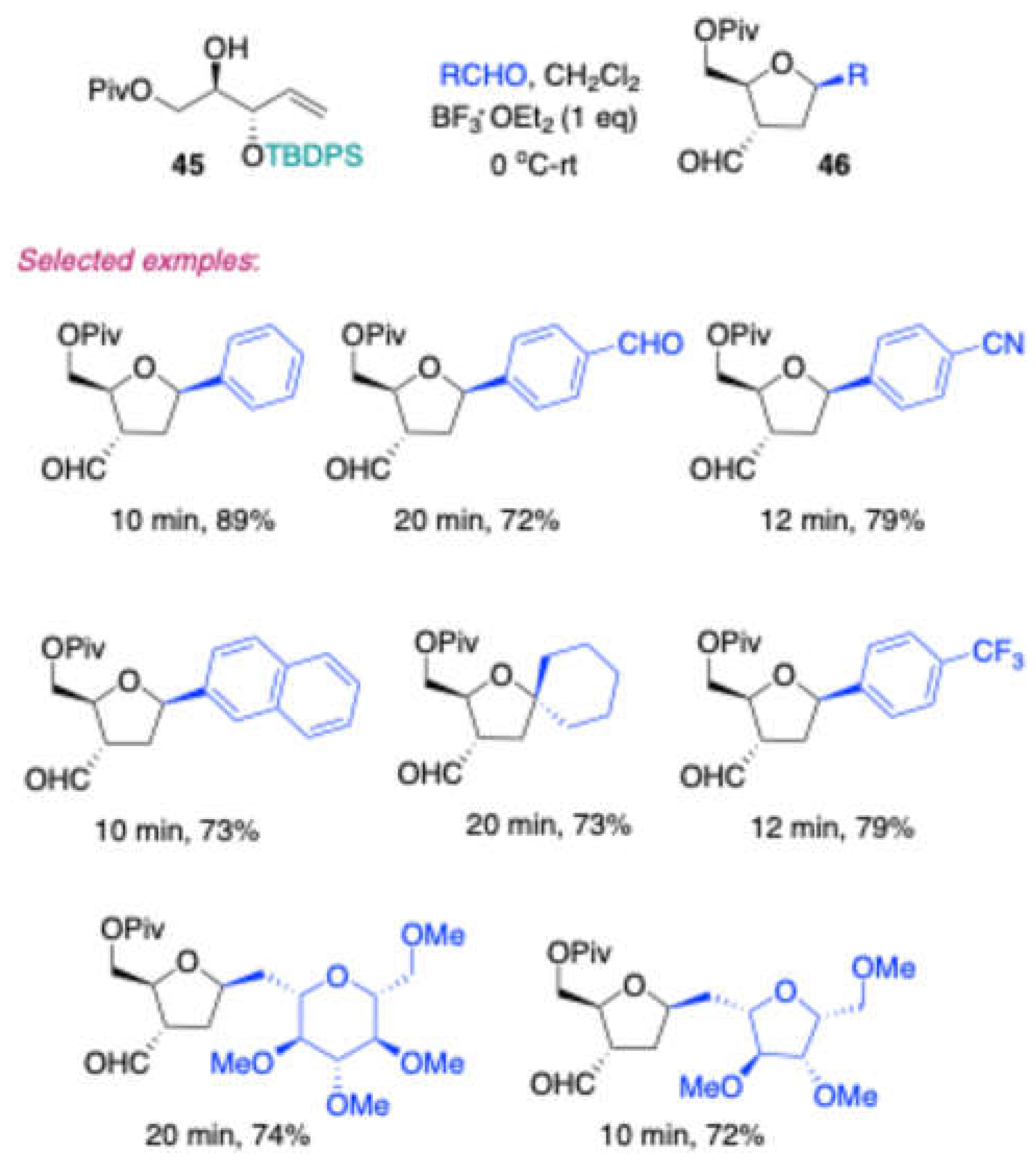
In 2019, Vankar reported the stereoselective synthesis of 3-deoxy-3C-formyl β-C-aryl/alkyl furanosides
46 (
Scheme 21) through a cascade Prins reaction followed by a pinacol-type rearrangement. [
12] This transformation involved an –OTBDPS-protected homoallylic alcohol
45, derived from D-mannitol, reacting with various carbonyl compounds in the presence of BF₃·OEt₂ in DCM. The reaction provided excellent yields and high selectivity.
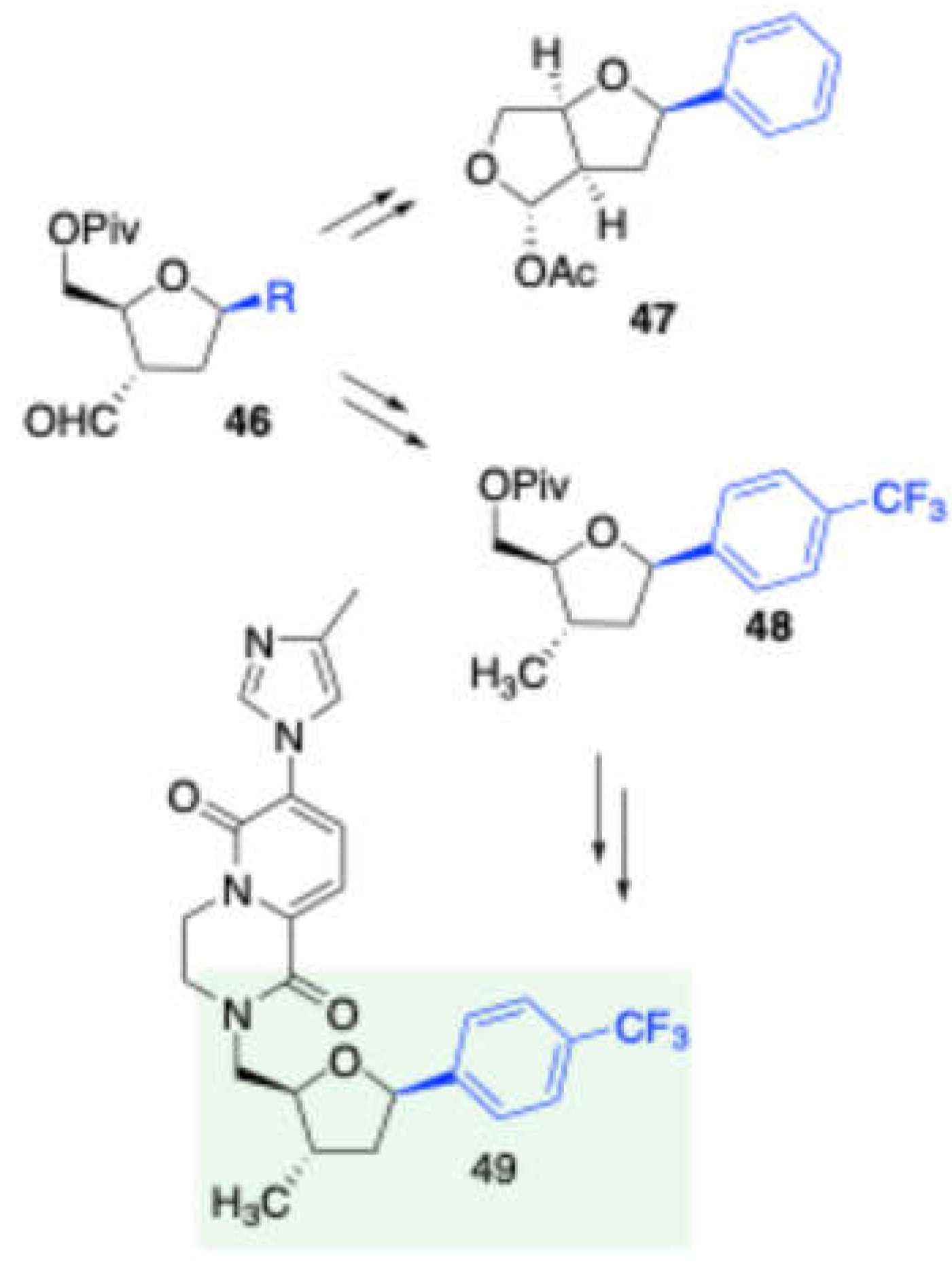
Furthermore, (
Scheme 22) this method was effectively applied to synthesize a fused-bicyclic β-C-aryl furanoside moiety
47 and a 2,3-dideoxy-3C-methyl β-C-aryl furanoside
48, both of which are found in the core structures of bioactive molecules.
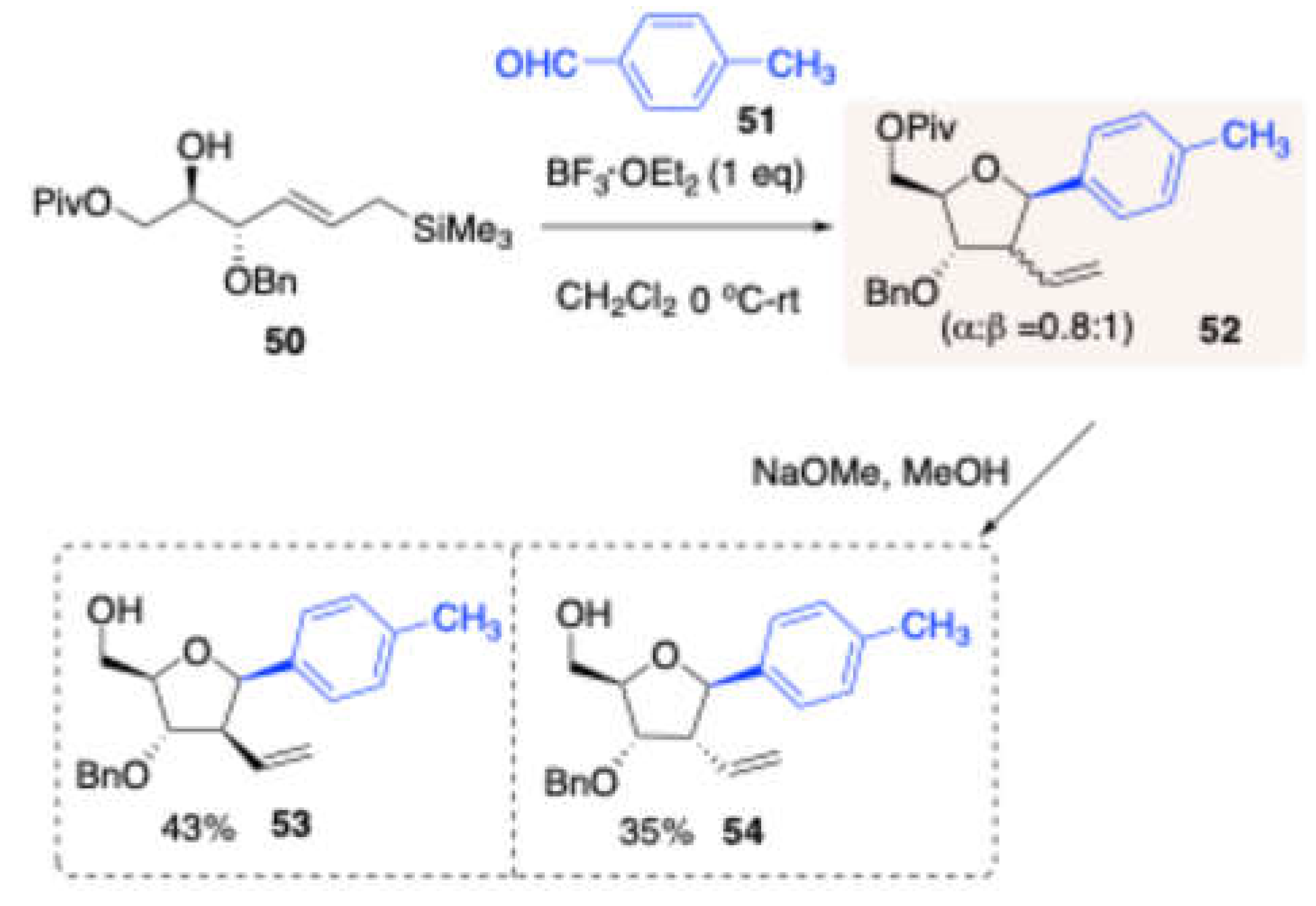
Later, Vankar and co-workers optimized (
Scheme 23) the Sakurai-Prins reaction of a D-mannitol-derived homologated allylsilane homoallylic alcohol
48 with
p-tolualdehyde
51 in the presence of BF₃·OEt₂.[
12] This reaction yielded 2-deoxy-2C-branched β-C-aryl furanosides in good yield (84%) as an inseparable diastereomeric mixture (α:β = 0.8:1 ratio) (
Scheme 9). To separate the stereoisomers, compound
48 was subsequently deprotected at the –OPiv group using NaOMe/MeOH, resulting in diastereomers
53 and
54, which were then separated by column chromatography with yields of 43% and 35%, respectively.
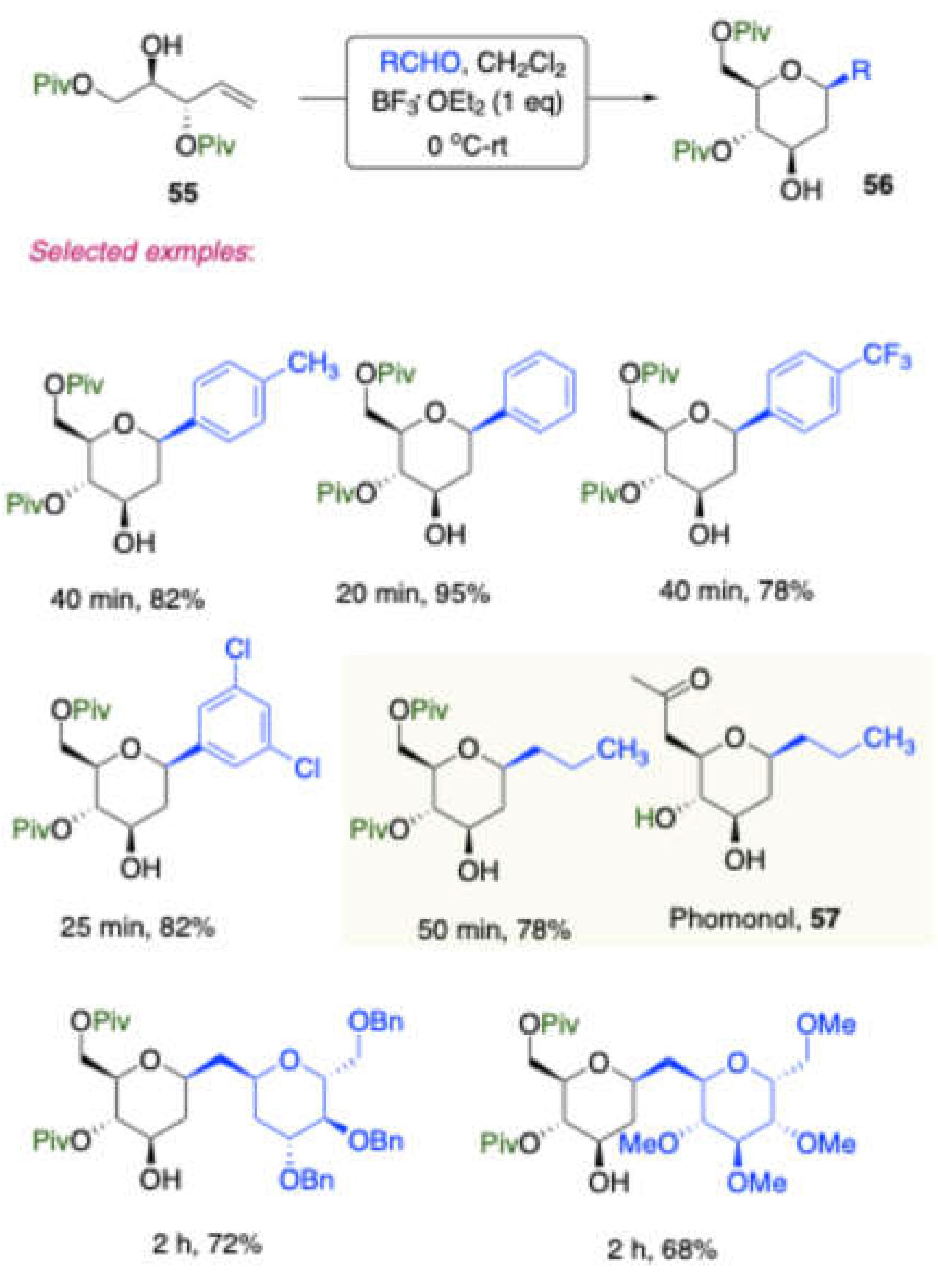
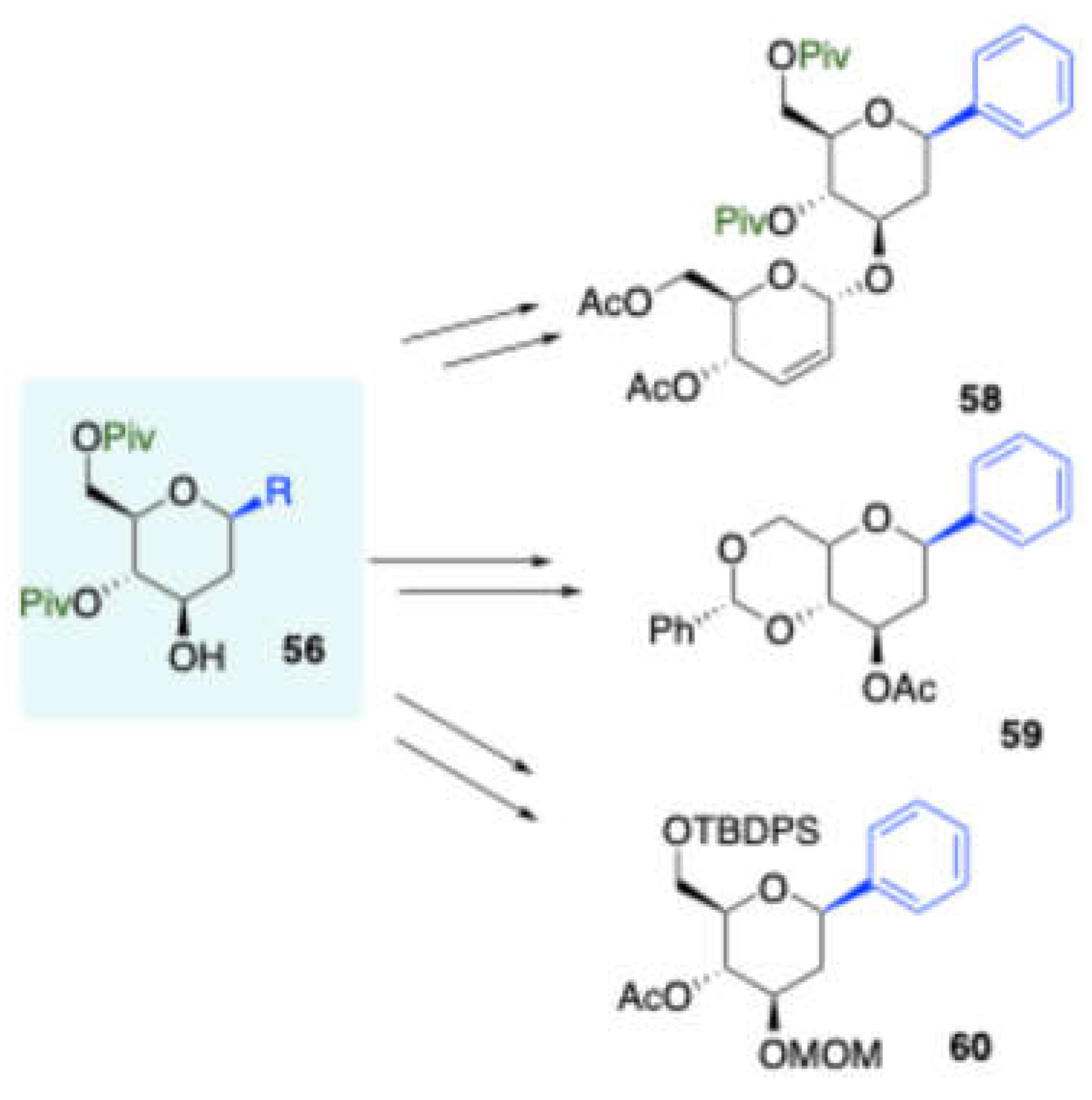
Vankar and co-workers further synthesized[
13] a non-participating protecting group at allylic position of D-mannitol derived homoallylic alcohol
55 and
which was subjected to the Prins reaction with a vairy of aldehydes in the presence of BF
3·OEt
2 as a catalyst and led to stereoselective 2-deoxy-C-aryl/alkyl glycosides
56 (
Scheme 24). The synthetic versatility of this approach has been demonstrated in the synthesis of C-disaccharide and O-linked disaccharides
58, and differently protected 2-deoxy-β-C-aryl glycosides
59,
60 (
Scheme 25).
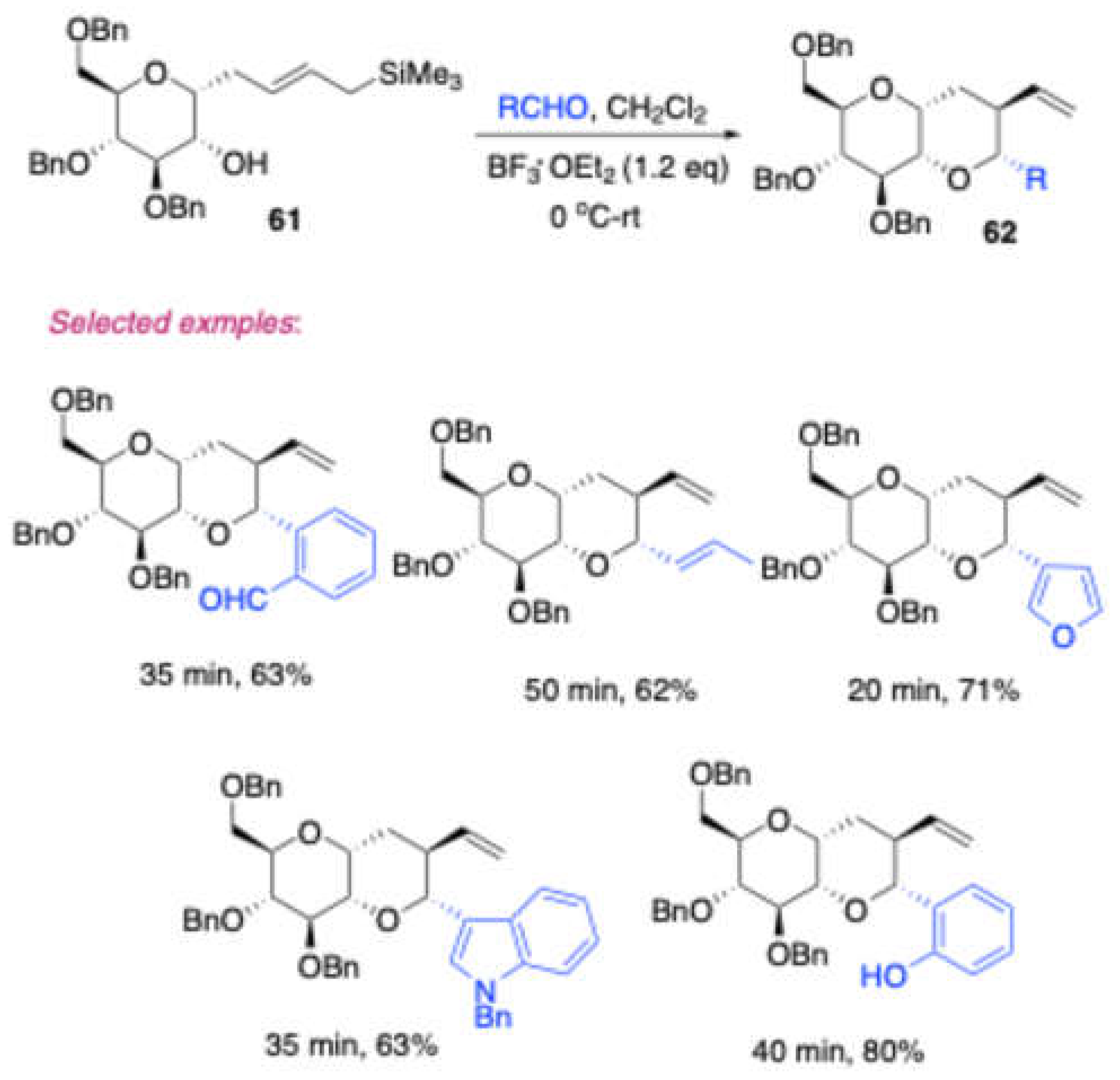
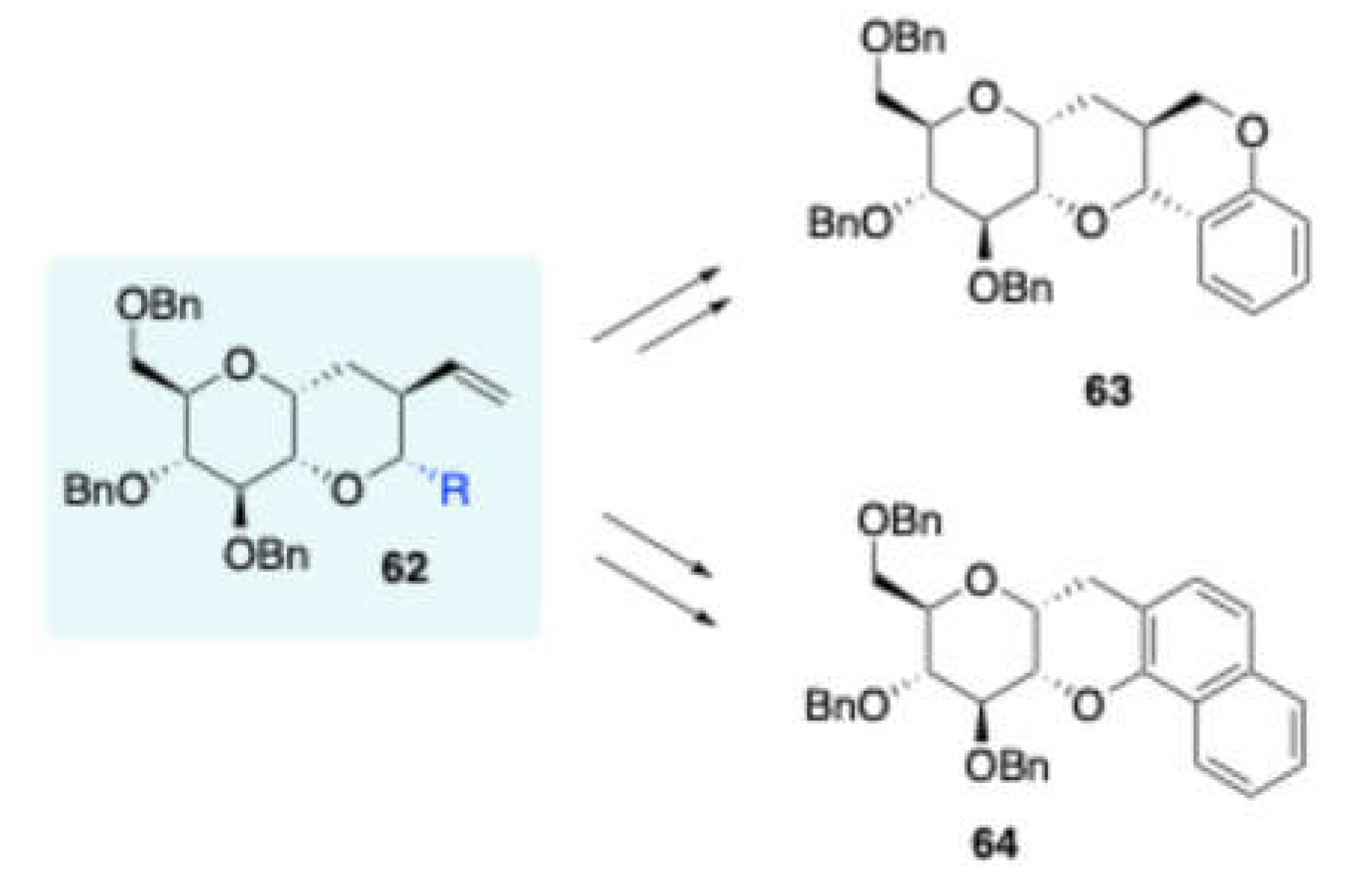
Furthermore, Vankar and co-workers reported[
14] (
Scheme 26) the synthesis of 1,2-annulated
tetrahydropyran fused sugar derivaties
62 by the reaction of a D-glucose derived alcohol
61 with various carbonyl compounds in the presence of BF
3 ∙ Et
2O, via Prins cyclization. The obtained products were converted to more useful scaffolds
cis-sugar fused pyrano[3,2-
c][
1]benzopyran
63 and
cis-sugar fused 4
H-naptho [1,2-
b] pyran
64 (
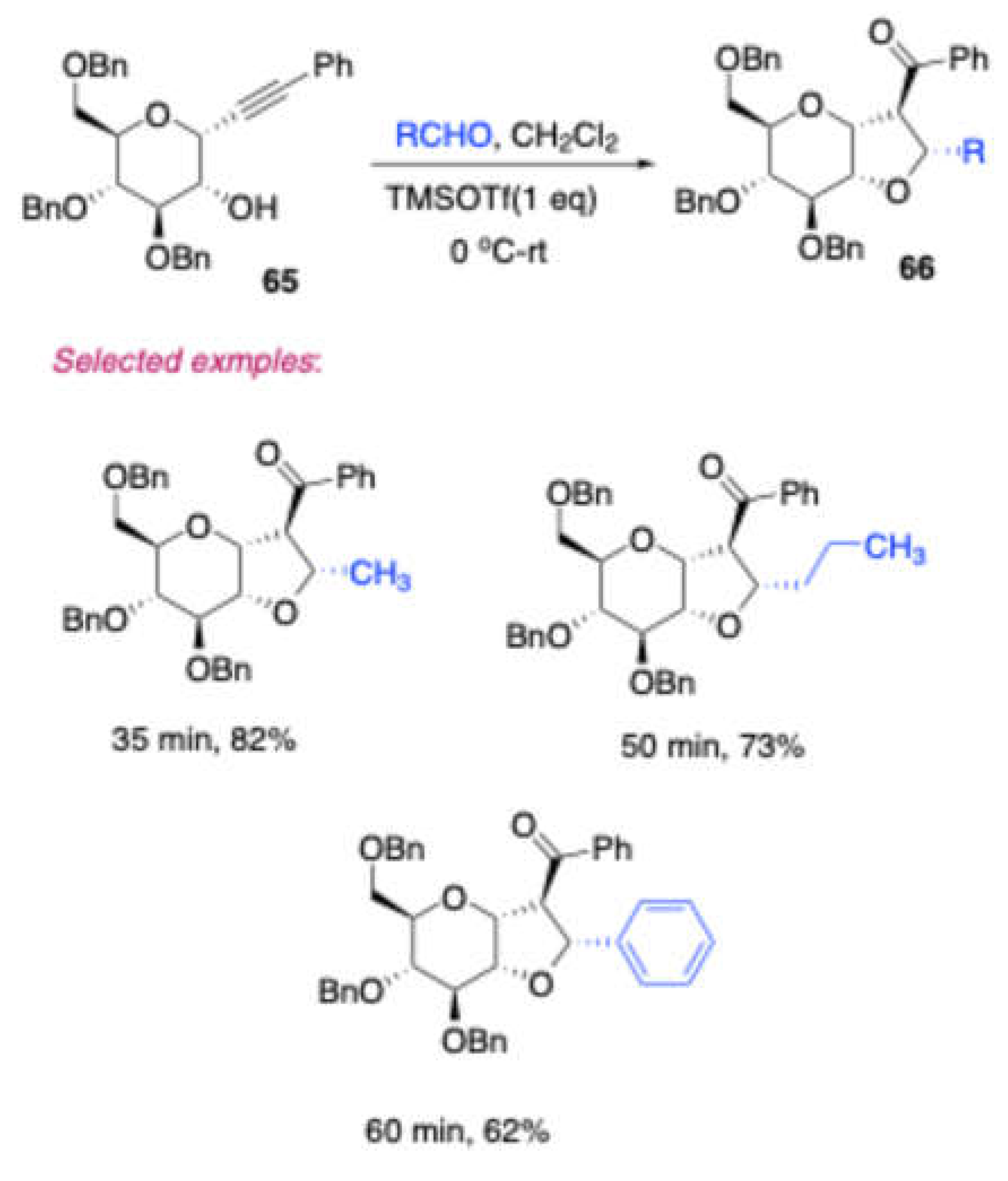
Scheme 27). Further studied that in the presence of TMSOTf, 1,2-annulated tetrahydrofuran fused sugar derivatives were obtained in moderate to excellent yields from D-glucose derived homopropargyl alcohol
65 and few aldehydes (
Scheme 28).
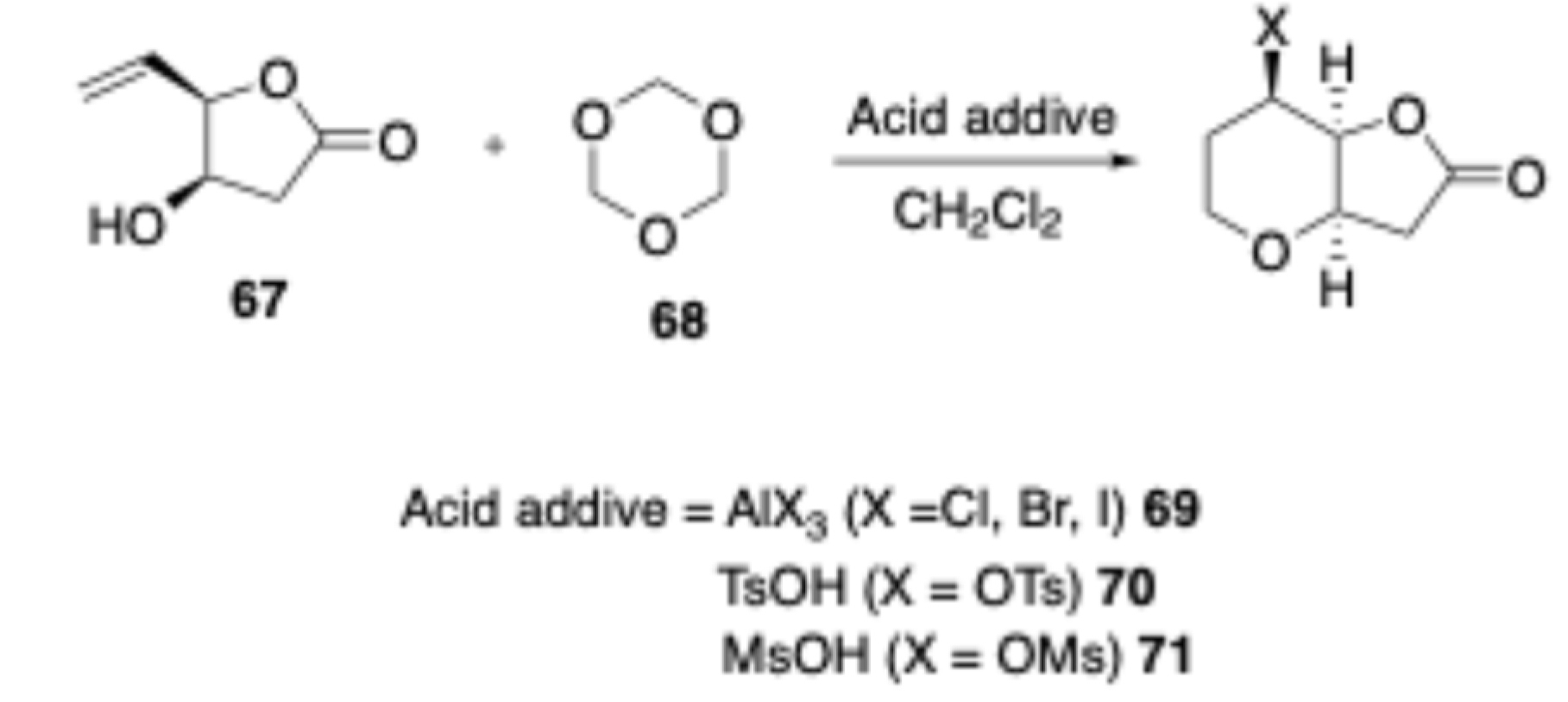
In 2008, Oikawa and co-workers reported[
15] the Prins reaction of glucose derived enetiomerically pure homoallylic alcohol
67 with unreactive formaldehyde equivalent
i.e., 1,3,5-trioxane
68 to trisubstituted
cis-fused hexahydro-2H-furo[3,2-b]pyran derivatives
69-71 (
Scheme 29).
2.2. Non-Carbohydrate Synthons to Carbohydrate Scaffolds
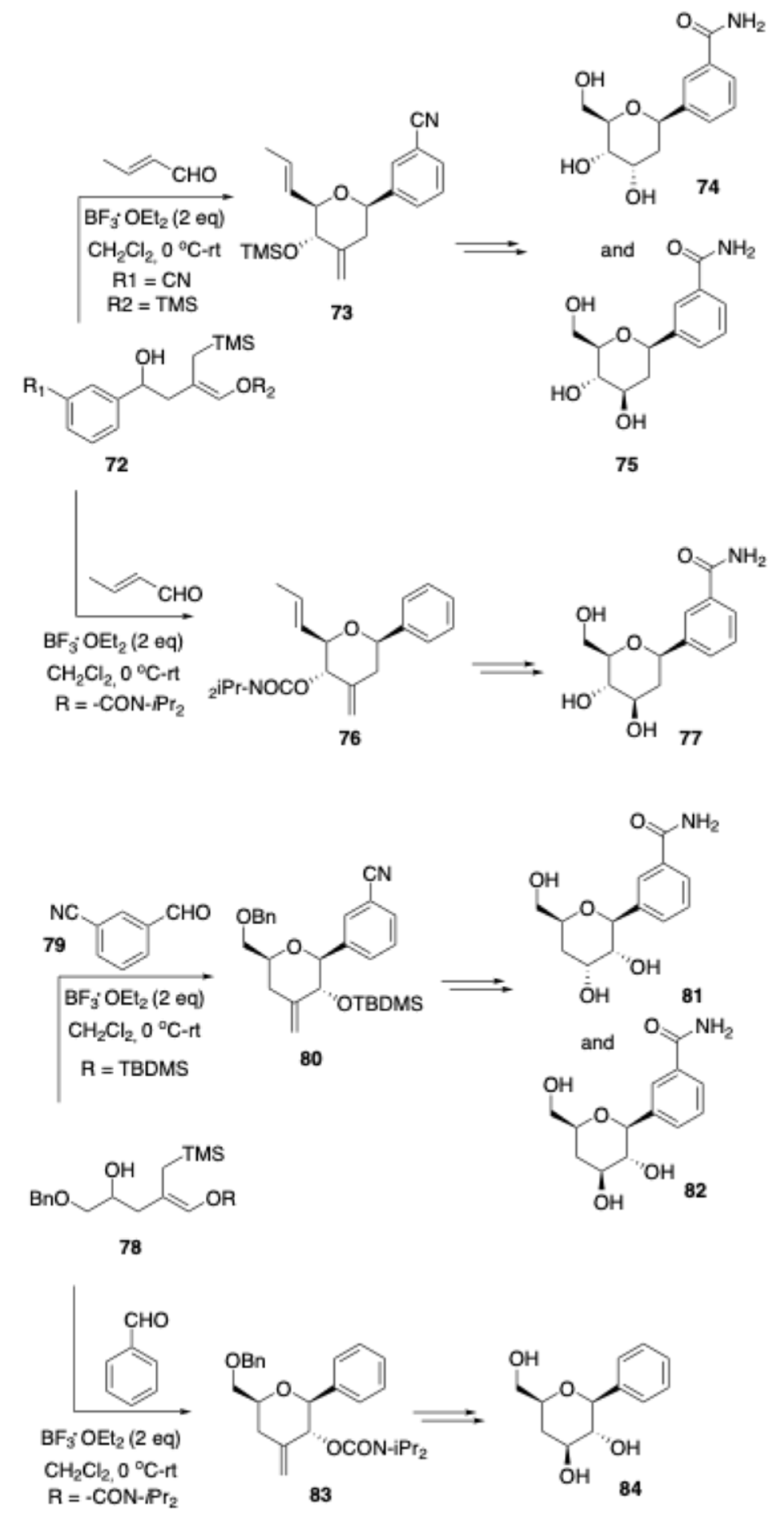
Besides exhibiting excellent biological activities, the carbohydrate-derived deoxy-C-aryl glycosides were found to be versatile substrates for the synthesis of various skeletal frameworks. Deoxy-C-aryl glycoside is a common structural motif found in a number of biologically relevant compounds, such as aquayamycin Adriamycin, pluramycin A, and kidamycin. An efficiently non-carbohydrate synthons source to sugar sketons by applying Prins reaction is a topic which has seen extensive study since the midtwentieth century.[32] Within this reaction manifold, Migaud and co-workers reported [
16] stereoselective synthesis of noncarbohydrate-based core sugar skeleton
73 via sakuri Prins cyclization of alcohol
72 with crotanaldehyde. The core sugar skeleton
73 further, the hydroxyl groups at C-6 and C-3 were introduced by oxidative cleavage of the alkenes in
73 followed by reduction of the dicarbonyl intermediate. Subsequent acetylation of these hydroxyls, purification, and acetyl removal then yielded the final C-nucleosides
74 and
75. Following the same reaction sequences and protocols, the synthesis of deoxy-C-aryl glycosides
77,
81,
82 and
84 were achieved using alcohol
72, 78 as a building block respectively.
Scheme 30.
Synthesis of deoxy-C-aryl glycosides from noncarbohydrate-based starting materials.
Scheme 30.
Synthesis of deoxy-C-aryl glycosides from noncarbohydrate-based starting materials.
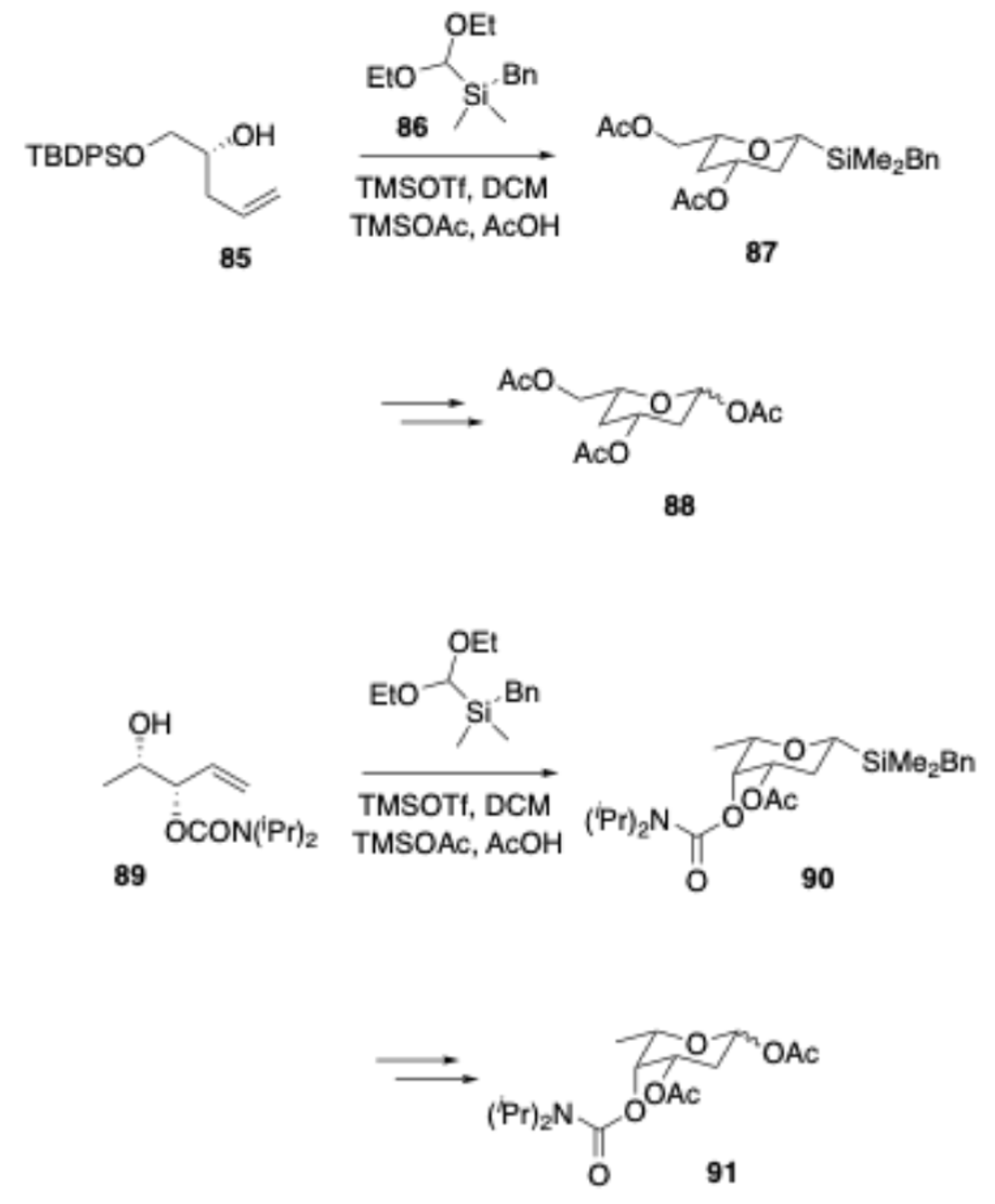
In 2016, Galan and coworkers reported[
17] a de novo approach for the rapid construction of orthogonally protected L- and D-deoxysugars and analogues via Prins cyclization. In this approach, homoallylic alcohol
85 was treated with aldehyde
86 in the presence of TMSOTf and TMSOAc/AcOH, resulting in the formation of silyltetrahydropyran
87 in good to excellent yield. This compound was subsequently subjected to Tamao–Fleming oxidation, leading to the formation of 2,4-dideoxysugar
88. By applying a similar reaction, compound
89 was converted into the 2,6-dideoxysugar
91.
Scheme 31.
Synthesis of dideoxysugars from silyltetrahydropyrans and noncarbohydrate-based starting materials via Prins reaction.
Scheme 31.
Synthesis of dideoxysugars from silyltetrahydropyrans and noncarbohydrate-based starting materials via Prins reaction.
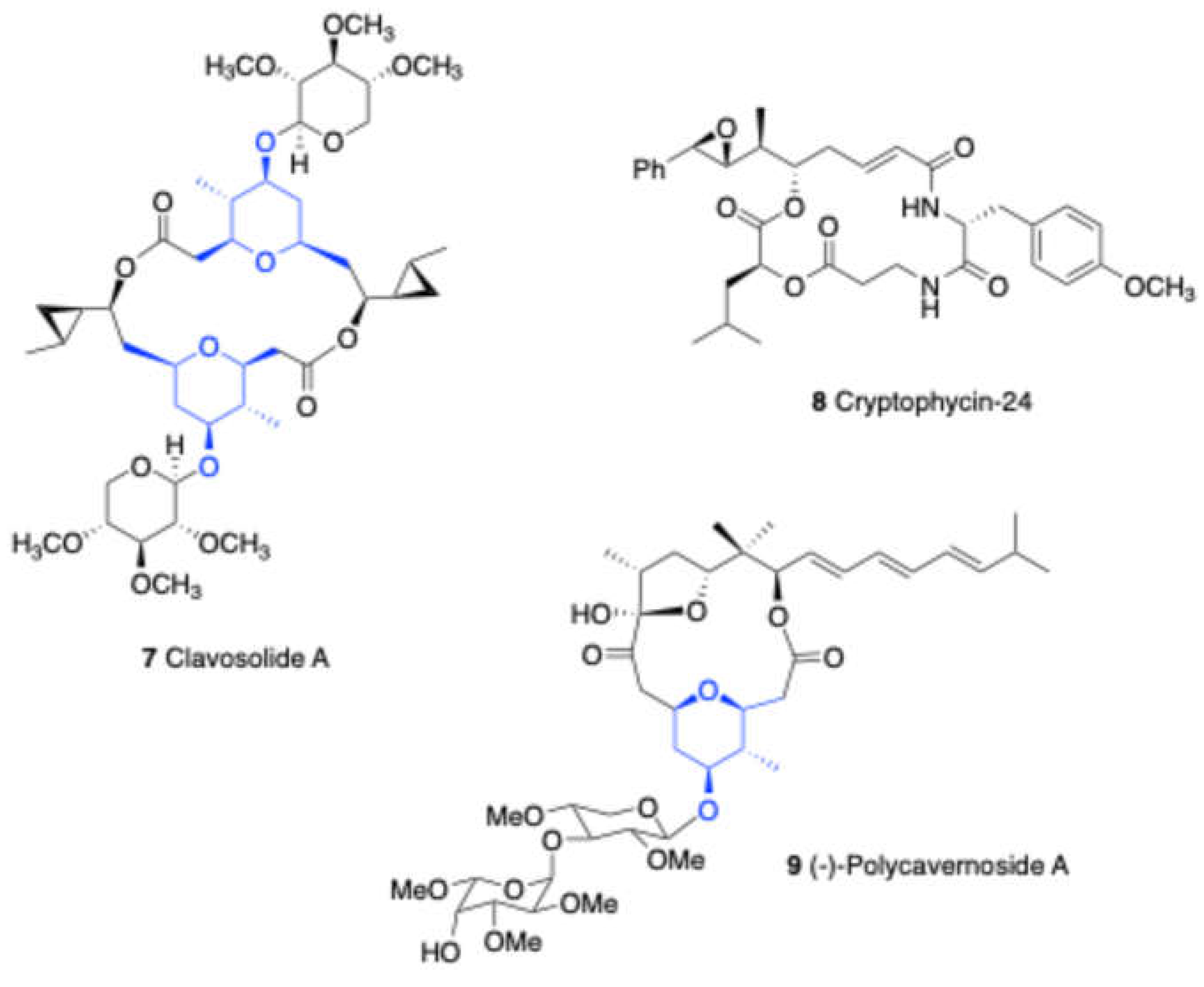
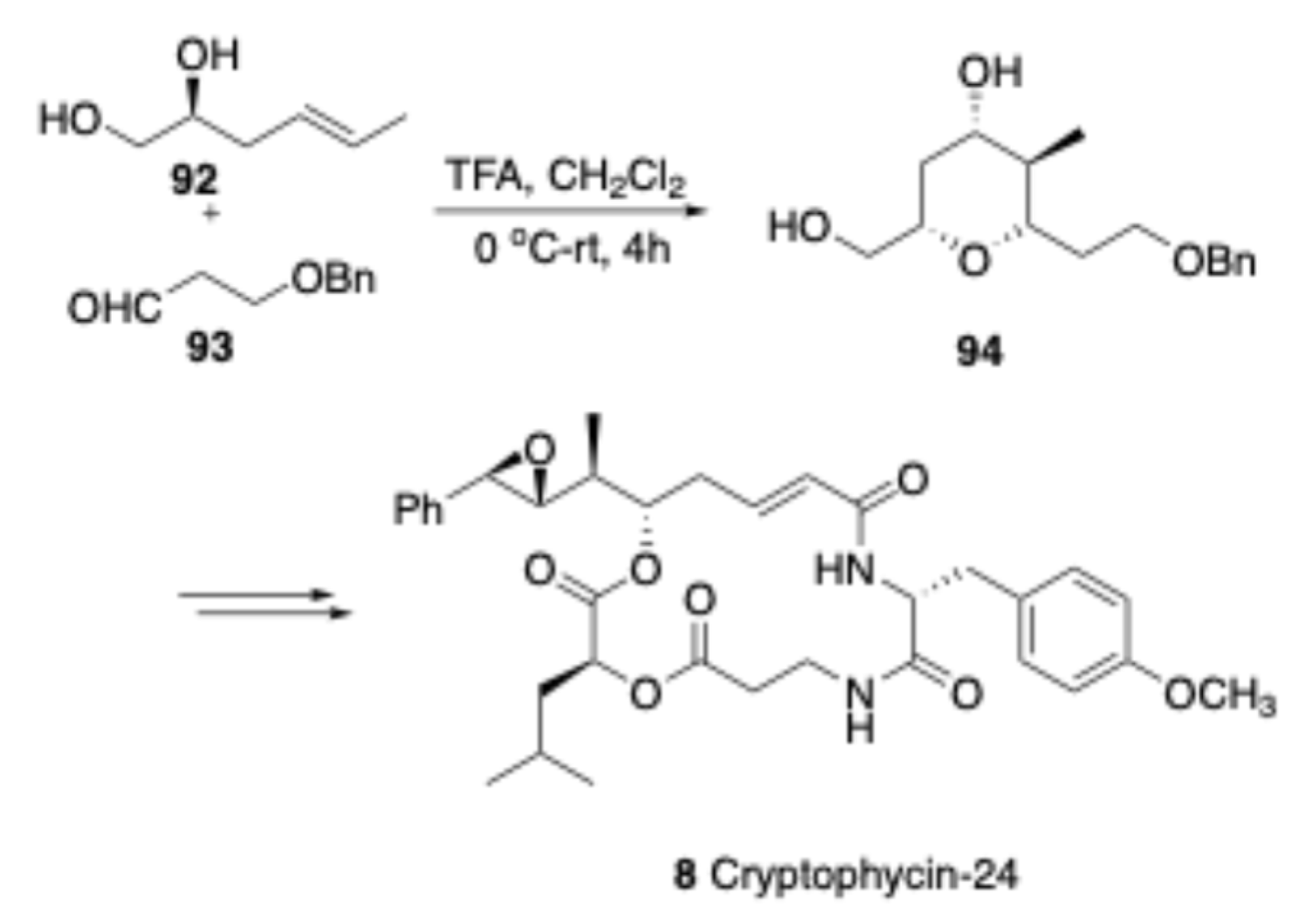
In 2016, Yadav and co-workers[
18] demonstrated a diastereoselective formal synthesis of cryptophycin-24. The key step for constructing the core center involved a Prins cyclization of homoallylic alcohol
92 with aldehyde
93 in the presence of TFA in DCM, yielding core sugar skeleton
94. Furthermore, this methodology was extended for the total synthesis of the natural product cryptophycin-24.
Scheme 32.
Synthesis of cryptophycin-24 from noncarbohydrate-based starting materials via Prins reaction.
Scheme 32.
Synthesis of cryptophycin-24 from noncarbohydrate-based starting materials via Prins reaction.
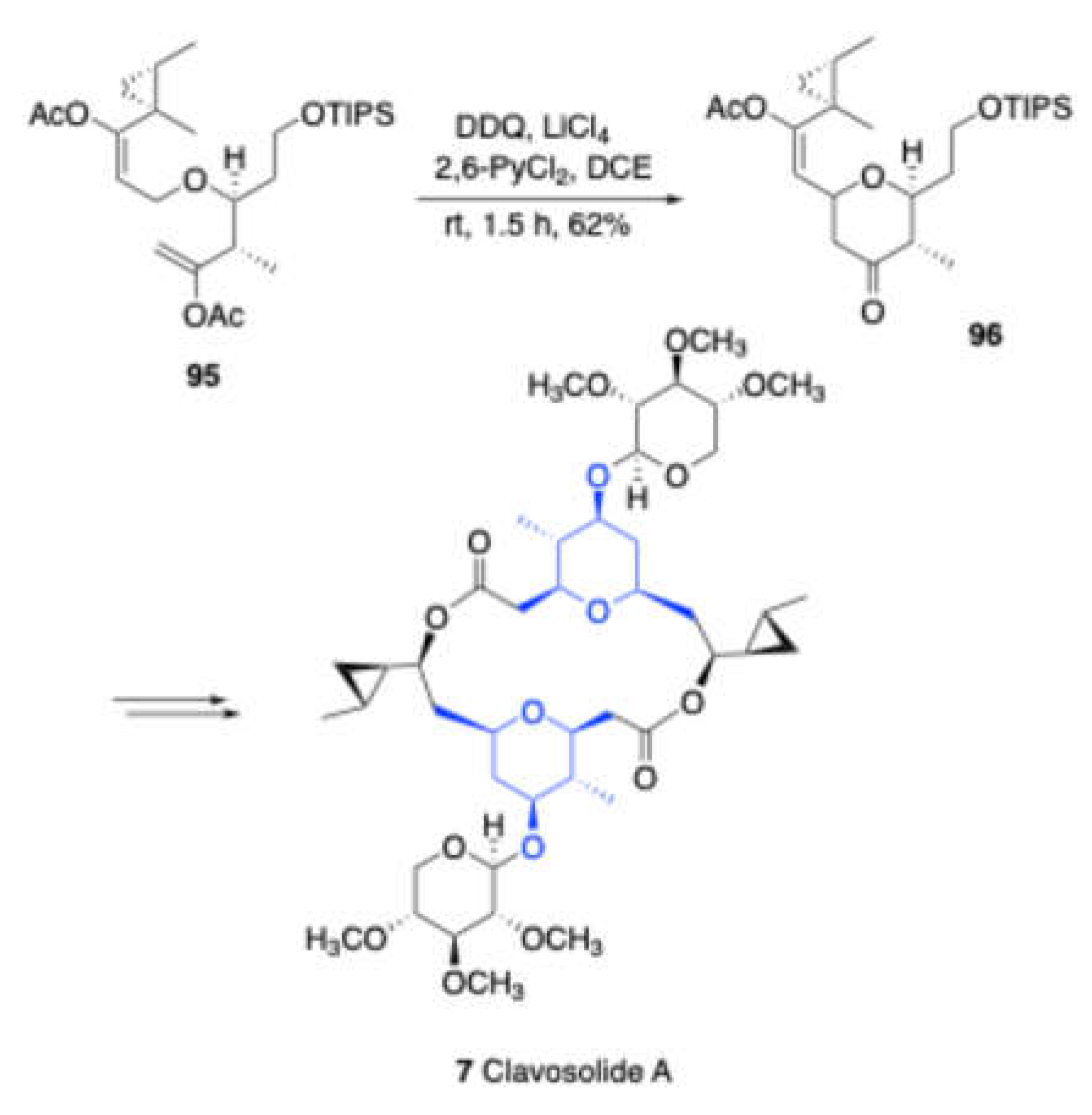
In 2012, Floreancig and Peh reported[
19] a DDQ-mediated oxidative intramolecular Prins cyclization of compound
95, yielding sugar core
96 in moderate yield (
Scheme 33). This reaction proceeds via a diene-type intermediate.
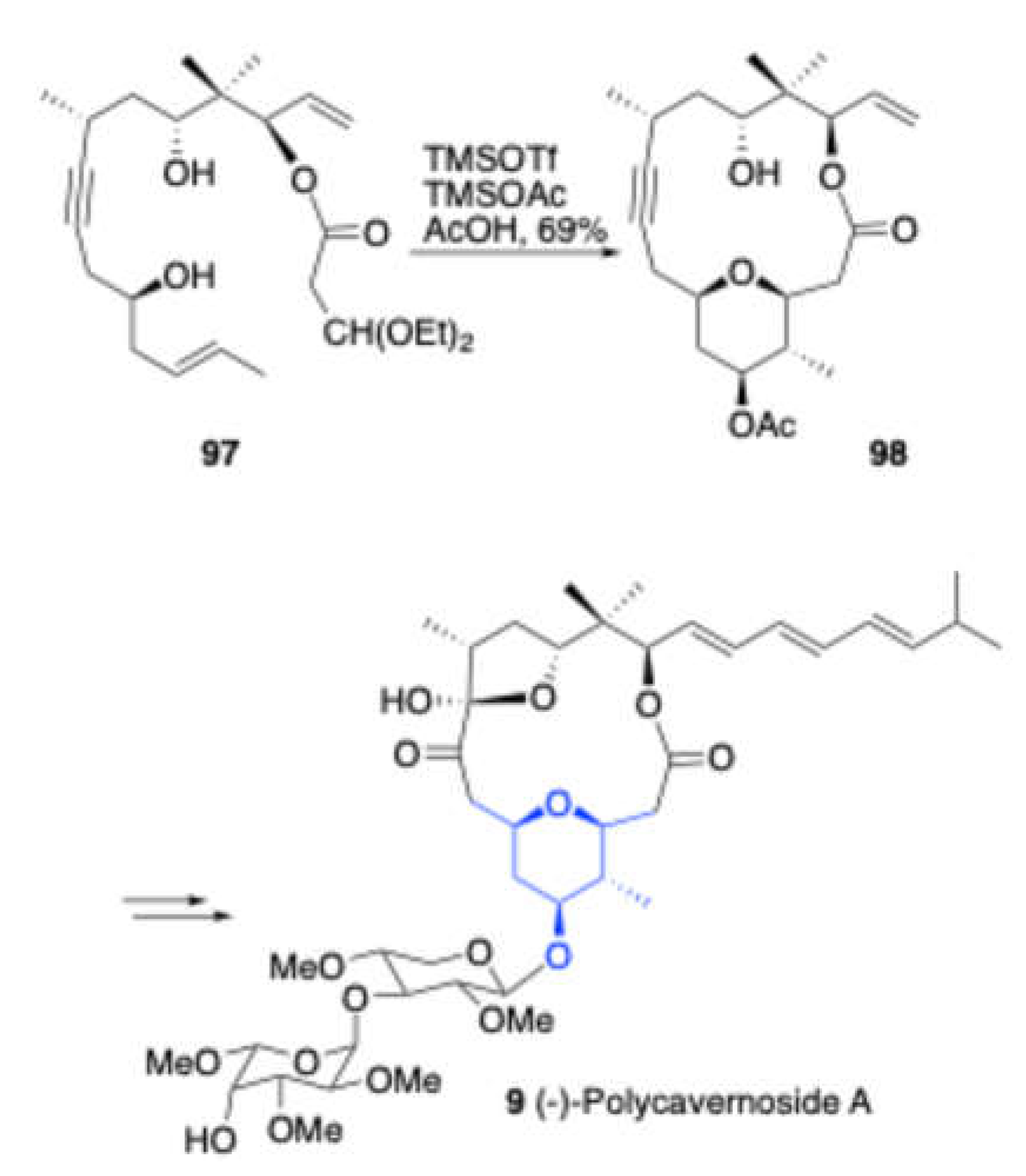
In 2010, Lee and Woo reported[
20]a intramolecular prins reaction of
97 in the presence of TMSOTf and
Scheme 34.
Total synthesis of (-)-Polycavernoside A 9 via Prins reaction.
Scheme 34.
Total synthesis of (-)-Polycavernoside A 9 via Prins reaction.
TMSOAc/AcOH, resulting in the formation of sugar core 98 in good yield. This compound was further subsequently used for the total synthesis of (-)-Polycavernoside A 7.