Submitted:
06 August 2025
Posted:
06 August 2025
You are already at the latest version
Abstract
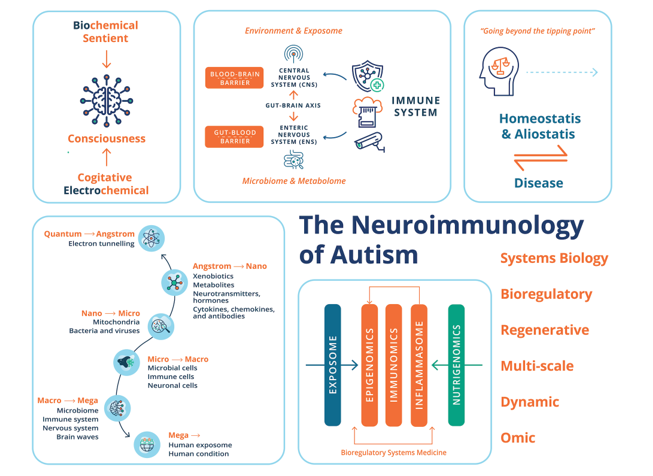
Keywords:
1. Introduction
2. Epidemiological Preambles of ASD
3. Underpinnings of ASD: From Genetic to Environmental and Gene/Environment Etiologies
4. The Immune & Nervous Systems: “Systems of Relations”
6. The Pathophysiology of ASD
6.1. ASD, Inflammation & Oxidative Stress
6.2. Innate Immune Deregulation, Encephalitis & ASD
6.2.1. The Pathophysiology of Glial Cells in ASD
6.2.2. The Pathophysiology of NK Cells in ASD
6.2.3. The Pathophysiology of Mast Cells & Dendritic Cells in ASD
6.2.4. The Pathophysiology of Platelets in ASD
6.3. Adaptive Immune Dysfunction, Autoimmune Conditions, & The Pathophysiology of ASD
6.4. Autoimmune Encephalitis, N-Methyl-D-Aspartate (NMDAR) Encephalitis & ASD
6.5. GI Pathology, the Immune System & Pathophysiology of ASD
6.6. Sex Hormones, the Pathogenesis of ASD & the Immune System
7. ASD: From Biochemical, Immune, & Electrophysiological Correlates of Cognition, Sentience & Consciousness to the Biology of the Self
7.1. ASD from Underpinnings of Homeostasis/Allostasis & Sentience
7.2. From Neuroanatomical to Neurofunctional Underpinnings of ASD
7.3. ASD at the Level of Brainwaves & Synchronized Neural Activity
7.4. Neurocognitive Theories of ASD
7.5. ASD the Biology of the Self
8. ASD and Biological Influences: A Two-Way Street
9. Future Directions & Therapeutic Modalities for ASD
9.1. ASD: Viewpoints from Environmental Exposomes, Omics Technologies & BrSYS Medicine
9.2. ASD Therapeutic Modalities: Standard of Care & State-Of-The-Art
9.3. ASD Therapeutics: Personalized & Precision Nutrition and Mind-Body Modalities
10. Concluding Remarks
References
- Abbaoui, A.; Fatoba, O.; Yamashita, T. Meningeal T cells function in the central nervous system homeostasis and neurodegenerative diseases. Front. Cell. Neurosci. 2023, 17, 1181071. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Abnave, P.; Ghigo, E. Role of the immune system in regeneration and its dynamic interplay with adult stem cells. Semin. Cell Dev. Biol. 2019, 87, 160–168. [Google Scholar] [CrossRef]
- Abo-Shaban, T.; Sharna, S.S.; Hosie, S.; Lee, C.Y.Q.; Balasuriya, G.K.; McKeown, S.J.; Franks, A.E.; Hill-Yardin, E.L. Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. J. Neural Transm. 2022, 130, 269–280. [Google Scholar] [CrossRef]
- Ackerman, L.S. Sex Hormones and the Genesis of Autoimmunity. Arch. Dermatol. 2006, 142, 371–376. [Google Scholar] [CrossRef]
- Adams, J.B.; Borody, T.J.; Kang, D.-W.; Khoruts, A.; Krajmalnik-Brown, R.; Sadowsky, M.J. Microbiota transplant therapy and autism: lessons for the clinic. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1033–1037. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Attia, S.M. Elevated IL-16 expression is associated with development of immune dysfunction in children with autism. Psychopharmacology 2018, 236, 831–838. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Attia, S.M.; Zoheir, K.M.; Al-Ayadhi, L.Y.; Alzahrani, M.Z.; Alsaad, A.M.; Alotaibi, M.R.; et al. Imbalance between the anti- and pro-inflammatory milieu in blood leukocytes of autistic children. Mol. Immunol. 2017, 82, 57–65. [Google Scholar] [CrossRef]
- Ahmad, S.F.; A Zoheir, K.M.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Alzahrani, M.Z.; Al-Shabanah, O.A.; Al-Harbi, M.M.; Attia, S.M. Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol. Neurobiol. 2016, 54, 4390–4400. [Google Scholar] [CrossRef]
- Aideyan, B.; Martin, G.C.; Beeson, E.T. A Practitioner’s Guide to Breathwork in Clinical Mental Health Counseling. J. Ment. Heal. Couns. 2020, 42, 78–94. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Akers, J.S.; Davis, T.N.; Gerow, S.; Avery, S. Decreasing motor stereotypy in individuals with autism spectrum disorder: A systematic review. Res. Autism Spectr. Disord. 2020, 77. [Google Scholar] [CrossRef]
- Albert, L.J.; Inman, R.D. Gram-negative pathogens and molecular mimicry: is there a case for mistaken identity? Trends Microbiol. 2000, 8, 444–445. [Google Scholar] [CrossRef]
- Albert, L.J.; Inman, R.D.; Epstein, F.H. Molecular Mimicry and Autoimmunity. New Engl. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef]
- Allen, J.S. “Theory of food” as a neurocognitive adaptation. Am. J. Hum. Biol. 2012, 24, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Huang, B.S.; Notaras, M.J.; Lodhi, A.; Barrio-Alonso, E.; Lituma, P.J.; Wolujewicz, P.; Witztum, J.; Longo, F.; Chen, M.; et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol. Psychiatry 2022, 27, 2470–2484. [Google Scholar] [CrossRef]
- Almahayni, O.; Hammond, L.; Hamasaki, H. Does the Wim Hof Method have a beneficial impact on physiological and psychological outcomes in healthy and non-healthy participants? A systematic review. PLOS ONE 2024, 19, e0286933. [Google Scholar] [CrossRef]
- Alsayouf, H.A. Growing evidence of pharmacotherapy effectiveness in managing attention-deficit/hyperactivity disorder in young children with or without autism spectrum disorder: a minireview. Front. Psychiatry 2024, 15, 1408876. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association, D. , & American Psychiatric Association, D. (2013). Diagnostic & statistical manual of mental disorders: DSM-5 (Vol. 5). American Psychiatric Association Washington, DC.
- American Psychiatric Association Staff, & Spitzer, R. L. (1980). Diagnostic & Statistical Manual of Mental Disorders (DSM-III). American Psychiatric Publishing.
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef]
- Anderson, W.F.; Dien, B.S.; Brandon, S.K.; Peterson, J.D. Assessment of Bermudagrass and Bunch Grasses as Feedstock for Conversion to Ethanol. Appl. Biochem. Biotechnol. 2007, 145, 13–21. [Google Scholar] [CrossRef]
- Angajala, A.; Lim, S.; Phillips, J.B.; Kim, J.-H.; Yates, C.; You, Z.; Tan, M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef]
- Angelidou, A.; Alysandratos, K.-D.; Asadi, S.; Zhang, B.; Francis, K.; Vasiadi, M.; Kalogeromitros, D.; Theoharides, T.C. Brief Report: “Allergic Symptoms” in Children with Autism Spectrum Disorders. More than Meets the Eye? J. Autism Dev. Disord. 2011, 41, 1579–1585. [Google Scholar] [CrossRef]
- Ansaldo, E.; Farley, T.K.; Belkaid, Y. Control of Immunity by the Microbiota. Annu. Rev. Immunol. 2021, 39, 449–479. [Google Scholar] [CrossRef]
- Ardalan, M.; Mallard, C. From hormones to behavior through microglial mitochondrial function. Brain, Behav. Immun. 2024, 117, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P. , & Van de Water, J. (2004). A review of autism and the immune response. Clinical & Developmental Immunology, 11(2), 165. [CrossRef]
- Asperger, H. Die „Autistischen Psychopathen” im Kindesalter. Eur. Arch. Psychiatry Clin. Neurosci. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Azzalini, D.; Rebollo, I.; Tallon-Baudry, C. Visceral Signals Shape Brain Dynamics and Cognition. Trends Cogn. Sci. 2019, 23, 488–509. [Google Scholar] [CrossRef]
- Bach, J.-F. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat. Rev. Immunol. 2017, 18, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Systems biology approaches to understand the effects of nutrition and promote health. Br. J. Clin. Pharmacol. 2016, 83, 38–45. [Google Scholar] [CrossRef]
- Bahi, C.; Irrmischer, M.; Franken, K.; Fejer, G.; Schlenker, A.; Deijen, J.B.; Engelbregt, H. Effects of conscious connected breathing on cortical brain activity, mood and state of consciousness in healthy adults. Curr. Psychol. 2023, 43, 10578–10589. [Google Scholar] [CrossRef]
- Balcells, C.; Xu, Y.; Gil-Solsona, R.; Maitre, L.; Gago-Ferrero, P.; Keun, H.C. Blurred lines: Crossing the boundaries between the chemical exposome and the metabolome. Curr. Opin. Chem. Biol. 2023, 78, 102407. [Google Scholar] [CrossRef] [PubMed]
- Baniel, A.; Almagor, E.; Sharp, N.; Kolumbus, O.; Herbert, M.R. From fixing to connecting—developing mutual empathy guided through movement as a novel path for the discovery of better outcomes in autism. Front. Integr. Neurosci. 2025, 18, 1489345. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Galán, J.C.; Martinez, J.L. The Origin of Niches and Species in the Bacterial World. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Barbaro, J.; Dissanayake, C. Autism Spectrum Disorders in Infancy and Toddlerhood: A Review of the Evidence on Early Signs, Early Identification Tools, and Early Diagnosis. J. Dev. Behav. Pediatr. 2009, 30, 447–459. [Google Scholar] [CrossRef]
- Barlattani, T. , D’Amelio, C., Cavatassi, A., De Luca, D., Di Stefano, R., Di Berardo, A., et al. (2023). Autism spectrum disorders and psychiatric comorbidities: a narrative review. Journal of Psychopathology. [CrossRef]
- Baron-Cohen, S. (2000). Theory of mind and autism: A review. In International Review of Research in Mental Retardation (Vol. 23, pp. 169–184). Academic Press. https://doi.org/10.1016/S0074-7750(00)80010-5.
- Baron-Cohen, S. The cognitive neuroscience of autism. J. Neurol. Neurosurg. Psychiatry 2004, 75, 945–948. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Ring, H.; Bullmore, E.; Wheelwright, S.; Ashwin, C.; Williams, S. The amygdala theory of autism. Neurosci. Biobehav. Rev. 2000, 24, 355–364. [Google Scholar] [CrossRef]
- Basheer, S.; Venkataswamy, M.M.; Christopher, R.; Van Amelsvoort, T.; Srinath, S.; Girimaji, S.C.; Ravi, V. Immune aberrations in children with Autism Spectrum Disorder: a case-control study from a tertiary care neuropsychiatric hospital in India. Psychoneuroendocrinology 2018, 94, 162–167. [Google Scholar] [CrossRef]
- Bauman, M.L. Medical Comorbidities in Autism: Challenges to Diagnosis and Treatment. Neurotherapeutics 2010, 7, 320–327. [Google Scholar] [CrossRef]
- Bauman, M.L.; Kemper, T.L. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 2005, 23, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Baumann, N.; Pham-Dinh, D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef] [PubMed]
- Becker, E. B. E., & Stoodley, C. J. (2013). Chapter One - Autism Spectrum Disorder and the Cerebellum. In G. Konopka (Ed.), International Review of Neurobiology (Vol. 113, pp. 1–34). Academic Press. https://doi.org/10.1016/B978-0-12-418700-9.00001-0.
- Bei, R.; Masuelli, L.; Palumbo, C.; Modesti, M.; Modesti, A. A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: Inflammation in their induction and impact on tumor growth. Cancer Lett. 2009, 281, 8–23. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Cohen, G.; van Schaik, A.; Ooi, L.; Morley, J.W.; Buskila, Y. Astrocytic modulation of cortical oscillations. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.-P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef]
- Beopoulos, A.; Gea, M.; Fasano, A.; Iris, F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome–Host Interaction Mechanistic Hypothesis. Nutrients 2021, 14, 65. [Google Scholar] [CrossRef]
- Bernard, S.; Enayati, A.; Redwood, L.; Roger, H.; Binstock, T. Autism: a novel form of mercury poisoning. Med Hypotheses 2001, 56, 462–471. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Bethell, C.D.; Kogan, M.D.; Strickland, B.B.; Schor, E.L.; Robertson, J.; Newacheck, P.W. A National and State Profile of Leading Health Problems and Health Care Quality for US Children: Key Insurance Disparities and Across-State Variations. Acad. Pediatr. 2011, 11, S22–S33. [Google Scholar] [CrossRef] [PubMed]
- Bhagavati, S. Autoimmune Disorders of the Nervous System: Pathophysiology, Clinical Features, and Therapy. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Parr, T.; Ramstead, M.; Friston, K. Immunoceptive inference: why are psychiatric disorders and immune responses intertwined? Biol. Philos. 2021, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bien, C.G.; Elger, C.E. Limbic encephalitis: A cause of temporal lobe epilepsy with onset in adult life. Epilepsy Behav. 2007, 10, 529–538. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Bjørklund, G.; Mkhitaryan, M.; Sahakyan, E.; Fereshetyan, K.; A Meguid, N.; Hemimi, M.; Nashaat, N.H.; Yenkoyan, K. Linking Environmental Chemicals to Neuroinflammation and Autism Spectrum Disorder: Mechanisms and Implications for Prevention. Mol. Neurobiol. 2024, 61, 6328–6340. [Google Scholar] [CrossRef]
- E Blalock, J. The immune system as a sensory organ. J. Immunol. 1984, 132, 1067–1070. [Google Scholar] [CrossRef]
- Blaxill, M.F.; Redwood, L.; Bernard, S. Thimerosal and autism? A plausible hypothesis that should not be dismissed. Med Hypotheses 2004, 62, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Bleuler, E. (1911). Dementia praecox: oder Gruppe der Schizophrenien. F. Deuticke.
- Bleuler, E. (1951). Autistic thinking. In Organization & pathology of thought: Selected sources (pp. 399–437). Columbia University Press.
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hermansen, G.H.; Åbjørsbråten, K.S.; Chambers, A.R.; Sprengel, R.; Vervaeke, K.; Tang, W.; et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R.; Waldor, M.K. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLOS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, D.; Ashwood, P.; Krakowiak, P.; Hertzpicciotto, I.; Hansen, R.; Croen, L.A.; Pessah, I.N.; Vandewater, J.; Van de Water, J. Autism: Maternally derived antibodies specific for fetal brain proteins. NeuroToxicology 2007, 29, 226–231. [Google Scholar] [CrossRef]
- Breece, E.; Paciotti, B.; Nordahl, C.W.; Ozonoff, S.; Van de Water, J.A.; Rogers, S.J.; Amaral, D.; Ashwood, P. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain, Behav. Immun. 2013, 31, 69–75. [Google Scholar] [CrossRef]
- Brincker, M.; Torres, E.B. Noise from the periphery in autism. Front. Integr. Neurosci. 2013, 7, 52681. [Google Scholar] [CrossRef]
- Brink, W.v.D.; van Bilsen, J.; Salic, K.; Hoevenaars, F.P.M.; Verschuren, L.; Kleemann, R.; Bouwman, J.; Ronnett, G.V.; van Ommen, B.; Wopereis, S. Current and Future Nutritional Strategies to Modulate Inflammatory Dynamics in Metabolic Disorders. Front. Nutr. 2019, 6, 129. [Google Scholar] [CrossRef]
- Brix, G.; Nekolla, E.A.; Nosske, D.; Griebel, J. Risks and safety aspects related to PET/MR examinations. Eur. J. Nucl. Med. 2008, 36, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Mehl-Madrona, L. Autoimmune and gastrointestinal dysfunctions: does a subset of children with autism reveal a broader connection? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., 3rd; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals With ASDs: A Consensus Report. Pediatrics 2010, 125 (Suppl. 1), S1–S18. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Walker, T.L. The multifaceted role of platelets in mediating brain function. Blood 2022, 140, 815–827. [Google Scholar] [CrossRef]
- Burns-Naas, L.A.; Dearman, R.J.; Germolec, D.R.; Kaminski, N.E.; Kimber, I.; Ladics, G.S.; Luebke, R.W.; Pfau, J.C.; Pruett, S.B. “Omics” Technologies and the Immune System. Toxicol. Mech. Methods 2006, 16, 101–119. [Google Scholar] [CrossRef]
- Buskila, Y.; Bellot-Saez, A.; Morley, J.W. Generating Brain Waves, the Power of Astrocytes. Front. Neurosci. 2019, 13, 1125. [Google Scholar] [CrossRef]
- Buzsáki, G.; Watson, B.O. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialog- Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef]
- Cagnin, A. In vivo imaging of neuroinflammation. Eur. Neuropsychopharmacol. 2002, 12, 581–586. [Google Scholar] [CrossRef]
- Cakir, J.; Frye, R.E.; Walker, S.J. The lifetime social cost of autism: 1990–2029. Res. Autism Spectr. Disord. 2020, 72. [Google Scholar] [CrossRef]
- Calçada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A.; et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136-137, 138–147. [Google Scholar] [CrossRef]
- Cantando, I.; Centofanti, C.; D’aLessandro, G.; Limatola, C.; Bezzi, P. Metabolic dynamics in astrocytes and microglia during post-natal development and their implications for autism spectrum disorders. Front. Cell. Neurosci. 2024, 18, 1354259. [Google Scholar] [CrossRef]
- Carré, A.; Chevallier, C.; Robel, L.; Barry, C.; Maria, A.-S.; Pouga, L.; Philippe, A.; Pinabel, F.; Berthoz, S. Tracking Social Motivation Systems Deficits: The Affective Neuroscience View of Autism. J. Autism Dev. Disord. 2015, 45, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- CDC. (2021). Managing Chronic Health Conditions. CDC Healthy Schools. https://www.cdc.gov/healthyschools/chronicconditions.htm. 1 June.
- Cekici, H.; Sanlier, N. Current nutritional approaches in managing autism spectrum disorder: A review. Nutr. Neurosci. 2017, 22, 145–155. [Google Scholar] [CrossRef]
- Cellot, G.; Cherubini, E. GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Front. Pediatr. 2014, 2, 70. [Google Scholar] [CrossRef]
- Chambers, C., & Schaefer, C. (2015). 2.10 - Epilepsy and antiepileptic medications. In C. Schaefer, P. Peters, & R. K. Miller (Eds.), Drugs During Pregnancy and Lactation (Third Edition) (pp. 251–291). San Diego: Academic Press. https://doi.org/10.1016/B978-0-12-408078-2.00011-1.
- Chavez, J.A.; Zappaterra, M. Can Wim Hof Method breathing induce conscious metabolic waste clearance of the brain? Med Hypotheses 2023, 177. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chiu, C.-H. Current and future applications of fecal microbiota transplantation for children. Biomed. J. 2021, 45, 11–18. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Tjia, M.; Thapliyal, S. Homeostatic plasticity and excitation-inhibition balance: The good, the bad, and the ugly. Curr. Opin. Neurobiol. 2022, 75, 102553–102553. [Google Scholar] [CrossRef]
- Chia, S.L.; Kapoor, S.; Carvalho, C.; Bajénoff, M.; Gentek, R. Mast cell ontogeny: From fetal development to life-long health and disease. Immunol. Rev. 2023, 315, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Bjørklund, G. PERM Hypothesis: The Fundamental Machinery Able to Elucidate the Role of Xenobiotics and Hormesis in Cell Survival and Homeostasis. Int. J. Mol. Sci. 2017, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, J.H.; Yang, H.S.; Kim, J.Y.; Cortese, S.; Smith, L.; Koyanagi, A.; Dragioti, E.; Radua, J.; Fusar-Poli, P.; et al. Pharmacological and non-pharmacological interventions for irritability in autism spectrum disorder: a systematic review and meta-analysis with the GRADE assessment. Mol. Autism 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Cieślińska, A.; Kostyra, E.; Savelkoul, H.F. ; Wageningen University & Research Treating Autism Spectrum Disorder with Gluten-Free and Casein-Free Diet: The Underlying Microbiota-Gut-Brain Axis Mechanisms. Clin. Immunolgy Immunother. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef]
- Copf, T. Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neurosci. Biobehav. Rev. 2016, 68, 946–978. [Google Scholar] [CrossRef]
- Costantini, M. Bodily self and immune self: is there a link? Front. Hum. Neurosci. 2014, 8, 138. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Coury, D.L.; Anagnostou, E.; Manning-Courtney, P.; Reynolds, A.; Cole, L.; McCoy, R.; Whitaker, A.; Perrin, J.M. Use of Psychotropic Medication in Children and Adolescents With Autism Spectrum Disorders. Pediatrics 2012, 130, S69–S76. [Google Scholar] [CrossRef]
- Crépeaux, G.; Authier, F.-J.; Exley, C.; Luján, L.; Gherardi, R.K. The role of aluminum adjuvants in vaccines raises issues that deserve independent, rigorous and honest science. J. Trace Elements Med. Biol. 2020, 62, 126632. [Google Scholar] [CrossRef]
- Cryan, J.F.; O'RIordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Csaba, G. Hormones in the immune system and their possible role. A critical review. Acta Microbiol. Et Immunol. Hung. 2014, 61, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Capellino, S.; Sulli, A.; Serioli, B.; Secchi, M.E.; Villaggio, B.; Straub, R.H. Estrogens and Autoimmune Diseases. Ann. New York Acad. Sci. 2006, 1089, 538–547. [Google Scholar] [CrossRef]
- Cwik, J. C. (2021). Spiritual needs of people with autism spectrum disorder. In Spiritual Needs in Research and Practice: The Spiritual Needs Questionnaire as a Global Resource for Health and Social Care (pp. 265–280). Springer.
- D’adamo, C.R.; Nelson, J.L.; Miller, S.N.; Hong, M.R.; Lambert, E.; Ruhm, H.T. Reversal of Autism Symptoms among Dizygotic Twins through a Personalized Lifestyle and Environmental Modification Approach: A Case Report and Review of the Literature. J. Pers. Med. 2024, 14, 641. [Google Scholar] [CrossRef] [PubMed]
- Daëron, M. The immune system as a system of relations. Front. Immunol. 2022, 13, 984678. [Google Scholar] [CrossRef] [PubMed]
- D’aGostino, P.M.; Gottfried-Blackmore, A.; Anandasabapathy, N.; Bulloch, K. Brain dendritic cells: biology and pathology. Acta Neuropathol. 2012, 124, 599–614. [Google Scholar] [CrossRef]
- Daly, E., D. Tricklebank, M., & Wichers, R. (2019). Chapter Two - Neurodevelopmental roles and the serotonin hypothesis of autism spectrum disorder. In M. D. Tricklebank & E. Daly (Eds.), The Serotonin System (pp. 23–44). Academic Press. https://doi.org/10.1016/B978-0-12-813323-1.00002-5.
- Damasio, A. Mental self: The person within. Nature 2003, 423, 227–227. [Google Scholar] [CrossRef]
- D’Angelo, E. (2018). Physiology of the cerebellum. Handbook of Clinical Neurology, 154, 85–108. https://doi.org/10.1016/B978-0-444-63956-1.00006-0.
- Davenport, P.; Sola-Visner, M. Platelets in the neonate: Not just a small adult. Res. Pr. Thromb. Haemost. 2022, 6, e12719. [Google Scholar] [CrossRef]
- Davies, S.; Bishop, D.; Manstead, A.S.R.; Tantam, D. Face Perception in Children with Autism and Asperger's Syndrome. J. Child Psychol. Psychiatry 1994, 35, 1033–1057. [Google Scholar] [CrossRef]
- De Giorgio, R.; Guerrini, S.; Barbara, G.; Stanghellini, V.; De Ponti, F.; Corinaldesi, R.; Moses, P.L.; A Sharkey, K.; Mawe, G.M. Inflammatory neuropathies of the enteric nervous system☆. Gastroenterology 2004, 126, 1872–1883. [Google Scholar] [CrossRef]
- Del Casale, A.; Ferracuti, S.; Alcibiade, A.; Simone, S.; Modesti, M.N.; Pompili, M. Neuroanatomical correlates of autism spectrum disorders: A meta-analysis of structural magnetic resonance imaging (MRI) studies. Psychiatry Res. Neuroimaging 2022, 325, 111516. [Google Scholar] [CrossRef]
- Delafield-Butt, J.; Ciaunica, A. Sensorimotor foundations of self-consciousness in utero. Curr. Opin. Behav. Sci. 2024, 59. [Google Scholar] [CrossRef]
- Dempsey, J.L.; Little, M.; Cui, J.Y. Gut microbiome: An intermediary to neurotoxicity. NeuroToxicology 2019, 75, 41–69. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yi, P.; Xiong, Y.; Ying, J.; Lin, Y.; Dong, Y.; Wei, G.; Wang, X.; Hua, F. Gut Metabolites Acting on the Gut-Brain Axis: Regulating the Functional State of Microglia. Aging Dis. 2024, 15, 480–502. [Google Scholar] [CrossRef]
- Denman, A.M. Sex hormones, autoimmune diseases, and immune responses. BMJ 1991, 303, 2–3. [Google Scholar] [CrossRef]
- Deth, R.C. Autism: A Redox/Methylation Disorder. Glob. Adv. Heal. Med. 2013, 2, 68–73. [Google Scholar] [CrossRef]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Di Liberto, D.; D’anneo, A.; Carlisi, D.; Emanuele, S.; De Blasio, A.; Calvaruso, G.; Giuliano, M.; Lauricella, M. Brain Opioid Activity and Oxidative Injury: Different Molecular Scenarios Connecting Celiac Disease and Autistic Spectrum Disorder. Brain Sci. 2020, 10, 437. [Google Scholar] [CrossRef]
- Dietz, P.M.; Rose, C.E.; McArthur, D.; Maenner, M. National and State Estimates of Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 4258–4266. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Ding, S.; A Rando, T.; Trounson, A. Translational strategies and challenges in regenerative medicine. Nat. Med. 2014, 20, 814–821. [Google Scholar] [CrossRef]
- D'MEllo, A.M.; Stoodley, C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. 2015, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Foley, K.-R.; Schloss, J. Complementary and Alternative Medicine for Autism – A Systematic Review. J. Autism Dev. Disord. 2024, 1–11. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.P.A.; Basson, M.A. The neuroanatomy of autism – a developmental perspective. Am. J. Anat. 2016, 230, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Dukhinova, M.; Kuznetsova, I.; Kopeikina, E.; Veniaminova, E.; Yung, A.W.; Veremeyko, T.; Levchuk, K.; Barteneva, N.S.; Wing-Ho, K.K.; Yung, W.-H.; et al. Platelets mediate protective neuroinflammation and promote neuronal plasticity at the site of neuronal injury. Brain, Behav. Immun. 2018, 74, 7–27. [Google Scholar] [CrossRef]
- Meimand, S.E.; Rostam-Abadi, Y.; Rezaei, N. Autism spectrum disorders and natural killer cells: a review on pathogenesis and treatment. Expert Rev. Clin. Immunol. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Ecker, C. The neuroanatomy of autism spectrum disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2016, 21, 18–28. [Google Scholar] [CrossRef]
- Edmiston, E.; Ashwood, P.; Van de Water, J. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 383–390. [Google Scholar] [CrossRef]
- El-Ansary, A.; Bhat, R.S.; Zayed, N. Gut Microbiome and Sex Bias in Autism Spectrum Disorders. Curr. Behav. Neurosci. Rep. 2020, 7, 22–31. [Google Scholar] [CrossRef]
- Enstrom, A.M.; Lit, L.; Onore, C.E.; Gregg, J.P.; Hansen, R.L.; Pessah, I.N.; Hertz-Picciotto, I.; Van de Water, J.A.; Sharp, F.R.; Ashwood, P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain, Behav. Immun. 2008, 23, 124–133. [Google Scholar] [CrossRef]
- Epel, E.S.; McEwen, B.S.; Ickovics, J.R. Embodying Psychological Thriving: Physical Thriving in Response to Stress. J. Soc. Issues 1998, 54, 301–322. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Exley, C. Human exposure to aluminium. Environ. Sci. Process. Impacts 2013, 15, 1807–1816. [Google Scholar] [CrossRef]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Famitafreshi, H.; Karimian, M. Overview of the Recent Advances in Pathophysiology and Treatment for Autism. CNS Neurol. Disord. - Drug Targets 2018, 17, 590–594. [Google Scholar] [CrossRef]
- Fan, G.; Ma, J.; Ma, R.; Suo, M.; Chen, Y.; Zhang, S.; Zeng, Y.; Chen, Y. Microglia Modulate Neurodevelopment in Autism Spectrum Disorder and Schizophrenia. Int. J. Mol. Sci. 2023, 24, 17297. [Google Scholar] [CrossRef]
- Farmer, C.A.; Thurm, A.E.; Honnekeri, B.; Kim, P.; Swedo, S.E.; Han, J.C. The contribution of platelets to peripheral BDNF elevation in children with autism spectrum disorder. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Fasano, A. Leaky Gut and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2011, 42, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Halt, A.R.; Realmuto, G.; Earle, J.; Kist, D.A.; Thuras, P.; Merz, A. Purkinje Cell Size Is Reduced in Cerebellum of Patients with Autism. Cell. Mol. Neurobiol. 2002, 22, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex Differences in Autism Spectrum Disorder: a Review. Curr. Psychiatry Rep. 2018, 20, 1–17. [Google Scholar] [CrossRef]
- Filiano, A.J.; Xu, Y.; Tustison, N.J.; Marsh, R.L.; Baker, W.; Smirnov, I.; Overall, C.C.; Gadani, S.P.; Turner, S.D.; Weng, Z.; et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 2016, 535, 425–429. [Google Scholar] [CrossRef]
- Filipek, P.A.; Juranek, J.; Nguyen, M.T.; Cummings, C.; Gargus, J.J. Relative Carnitine Deficiency in Autism. J. Autism Dev. Disord. 2004, 34, 615–623. [Google Scholar] [CrossRef]
- Fincham, G.W.; Strauss, C.; Montero-Marin, J.; Cavanagh, K. Effect of breathwork on stress and mental health: A meta-analysis of randomised-controlled trials. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Fleming, D. Walter B. Cannon and homeostasis.. 1984, 51, 609–40. [Google Scholar]
- Folstein, S.; Rutter, M. Genetic influences and infantile autism. Nature 1977, 265, 726–728. [Google Scholar] [CrossRef]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; Díaz-Caneja, C.M.; Pina-Camacho, L.; Moreno, C.; Durán-Cutilla, M.; Ayora, M.; González-Vioque, E.; de Matteis, M.; Hendren, R.L.; Arango, C.; et al. Dietary Interventions for Autism Spectrum Disorder: A Meta-analysis. Pediatrics 2019, 144, e20183218. [Google Scholar] [CrossRef] [PubMed]
- Frith, U. Autism and theory of mind in everyday life. Soc. Dev. 1994, 3, 108–124. [Google Scholar] [CrossRef]
- Frith, U.; Happé, F. Autism: beyond “theory of mind”. Cognition 1994, 50, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Frith, U.; Happé, F. Theory of Mind and Self-Consciousness: What Is It Like to Be Autistic? Mind Lang. 1999, 14, 82–89. [Google Scholar] [CrossRef]
- Frye, R.E.; Rincon, N.; McCarty, P.J.; Brister, D.; Scheck, A.C.; Rossignol, D.A. Biomarkers of mitochondrial dysfunction in autism spectrum disorder: A systematic review and meta-analysis. Neurobiol. Dis. 2024, 197, 106520. [Google Scholar] [CrossRef]
- Fu, J.; Schroder, K.; Wu, H. Mechanistic insights from inflammasome structures. Nat. Rev. Immunol. 2024, 24, 518–535. [Google Scholar] [CrossRef]
- Fuller, R.; Rahona, E.; Fisher, S.; Caravanos, J.; Webb, D.; Kass, D.; Matte, T.; Landrigan, P.J. Pollution and non-communicable disease: time to end the neglect. Lancet Planet. Heal. 2018, 2, e96–e98. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: Berlin, Germany, 2014; Volume 817, pp. 39–71. [Google Scholar] [CrossRef]
- Gable, M.S.; Sheriff, H.; Dalmau, J.; Tilley, D.H.; Glaser, C.A. The Frequency of Autoimmune N-Methyl-D-Aspartate Receptor Encephalitis Surpasses That of Individual Viral Etiologies in Young Individuals Enrolled in the California Encephalitis Project. Clin. Infect. Dis. 2012, 54, 899–904. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Persico, A.M. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2014, 24, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Gallotto, S.; Sack, A.T.; Schuhmann, T.; de Graaf, T.A. Oscillatory Correlates of Visual Consciousness. Front. Psychol. 2017, 8, 1147–1147. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Zarate-Lopez, D.; Torres-Chavez, A.L.; Gonzalez-Perez, O. Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder. Brain Sci. 2020, 10, 951. [Google Scholar] [CrossRef]
- Garg, D.; Mohammad, S.S.; Sharma, S. Autoimmune Encephalitis in Children: An Update. Indian Pediatr. 2020, 57, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, T.; Klei, L.; Sanders, S.J.; A Bodea, C.; Goldberg, A.P.; Lee, A.B.; Mahajan, M.; Manaa, D.; Pawitan, Y.; Reichert, J.; et al. Most genetic risk for autism resides with common variation. Nat. Genet. 2014, 46, 881–885. [Google Scholar] [CrossRef]
- D. A., G.; J.K., K.; M.R., G. The biological basis of autism spectrum disorders: Understanding causation and treatment by clinical geneticists. Acta Neurobiol. Exp. 2010, 70, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; King, P.G.; Hooker, B.S.; Dórea, J.G.; Kern, J.K.; Sykes, L.K.; Geier, M.R. Thimerosal: Clinical, epidemiologic and biochemical studies. Clin. Chim. Acta 2015, 444, 212–220. [Google Scholar] [CrossRef]
- Geng, Z.-H.; Zhu, Y.; Li, Q.-L.; Zhao, C.; Zhou, P.-H. Enteric Nervous System: The Bridge Between the Gut Microbiota and Neurological Disorders. Front. Aging Neurosci. 2022, 14, 810483. [Google Scholar] [CrossRef]
- Genuis, S.J. What's Out There Making Us Sick? J. Environ. Public Heal. 2011, 2012, 1–10. [Google Scholar] [CrossRef]
- Gershon, M.D. The Enteric Nervous System: A Second Brain. Hosp. Pr. 1999, 34, 31–52. [Google Scholar] [CrossRef]
- Gershon, M. D. (2022). The Shaggy Dog Story of Enteric Signaling: Serotonin, a Molecular Megillah. In N. J. Spencer, M. Costa, & S. M. Brierley (Eds.), The Enteric Nervous System II (pp. 307–318). Cham: Springer International Publishing.
- Ghanizadeh, A.; Akhondzadeh, S.; Hormozi, M.; Makarem, A.; Abotorabi-Zarchi, M.; Firoozabadi, A. Glutathione-Related Factors and Oxidative Stress in Autism, A Review. Curr. Med. Chem. 2012, 19, 4000–4005. [Google Scholar] [CrossRef] [PubMed]
- Ghaziuddin, M.; Al-Owain, M. Autism Spectrum Disorders and Inborn Errors of Metabolism: An Update. Pediatr. Neurol. 2013, 49, 232–236. [Google Scholar] [CrossRef]
- Ghosh, A.; Michalon, A.; Lindemann, L.; Fontoura, P.; Santarelli, L. Drug discovery for autism spectrum disorder: challenges and opportunities. Nat. Rev. Drug Discov. 2013, 12, 777–790. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK cells in autoimmune diseases: Linking innate and adaptive immune responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef]
- Giron, M.C.; Mazzi, U. Molecular imaging of microbiota-gut-brain axis: searching for the right targeted probe for the right target and disease. Nucl. Med. Biol. 2021, 92, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gładysz, D.; Krzywdzińska, A.; Hozyasz, K.K. Immune Abnormalities in Autism Spectrum Disorder—Could They Hold Promise for Causative Treatment? Mol. Neurobiol. 2018, 55, 6387–6435. [Google Scholar] [CrossRef] [PubMed]
- Gobin, V.; Van Steendam, K.; Denys, D.; Deforce, D. Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. Int. Immunopharmacol. 2014, 20, 148–156. [Google Scholar] [CrossRef]
- Goldberg, W.A.; Osann, K.; Filipek, P.A.; Laulhere, T.; Jarvis, K.; Modahl, C.; Flodman, P.; Spence, M.A. Language and Other Regression: Assessment and Timing. J. Autism Dev. Disord. 2003, 33, 607–616. [Google Scholar] [CrossRef]
- Goldman, A.W.; Burmeister, Y.; Cesnulevicius, K.; Herbert, M.; Kane, M.; Lescheid, D.; McCaffrey, T.; Schultz, M.; Seilheimer, B.; Smit, A.; et al. Bioregulatory systems medicine: an innovative approach to integrating the science of molecular networks, inflammation, and systems biology with the patient's autoregulatory capacity? Front. Physiol. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Goubau, C.; Buyse, G.M.; Van Geet, C.; Freson, K. The contribution of platelet studies to the understanding of disease mechanisms in complex and monogenetic neurological disorders. Dev. Med. Child Neurol. 2014, 56, 724–731. [Google Scholar] [CrossRef]
- Graeber, M.B.; Streit, W.J. Microglia: biology and pathology. Acta Neuropathol. 2009, 119, 89–105. [Google Scholar] [CrossRef]
- Graeber, M.B.; Stre'RT, W.J. Microglia: Immune Network in the CNS. Brain Pathol. 1990, 1, 2–5. [Google Scholar] [CrossRef]
- Green, D.J.; Park, K.; Bhatt-Mehta, V.; Snyder, D.; Burckart, G.J. Regulatory Considerations for the Mother, Fetus and Neonate in Fetal Pharmacology Modeling. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef]
- Greenberg, G. , & Tobach, E. (2014). Behavioral Evolution & Integrative Levels: The T.C. Schneirla Conferences Series, Volume 1. Taylor & Francis.
- Gropman, A., & Sadle, C. J. (2024). Epigenetics of autism spectrum disorder. In Neuropsychiatric Disorders & Epigenetics (pp. 81–102). Elsevier. https://doi.org/10.1016/B978-0-443-18516-8.00017-X.
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The role of gamma oscillations in central nervous system diseases: Mechanism and treatment. Front. Cell. Neurosci. 2022, 16, 962957. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, M. Off-label psychopharmacological interventions for autism spectrum disorders: strategic pathways for clinicians. CNS Spectrums 2023, 29, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Gzielo, K.; Nikiforuk, A. Astroglia in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 11544. [Google Scholar] [CrossRef]
- Hacohen, Y.; Wright, S.; Gadian, J.; Vincent, A.; Lim, M.; Wassmer, E.; Lin, J. N-methyl-d-aspartate (NMDA) receptor antibodies encephalitis mimicking an autistic regression. Dev. Med. Child Neurol. 2016, 58, 1092–1094. [Google Scholar] [CrossRef]
- Hafizi, S.; Tabatabaei, D.; Lai, M.-C. Review of Clinical Studies Targeting Inflammatory Pathways for Individuals With Autism. Front. Psychiatry 2019, 10, 849. [Google Scholar] [CrossRef]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Hameroff, S. The “conscious pilot”—dendritic synchrony moves through the brain to mediate consciousness. J. Biol. Phys. 2009, 36, 71–93. [Google Scholar] [CrossRef]
- Hampe, C.S.; Mitoma, H. A Breakdown of Immune Tolerance in the Cerebellum. Brain Sci. 2022, 12, 328. [Google Scholar] [CrossRef]
- Han, A.; Peng, T.; Xie, Y.; Zhang, W.; Sun, W.; Xie, Y.; Ma, Y.; Wang, C.; Xie, N. Mitochondrial-regulated Tregs: potential therapeutic targets for autoimmune diseases of the central nervous system. Front. Immunol. 2023, 14, 1301074. [Google Scholar] [CrossRef]
- Hanaford, A.; Johnson, S.C. The immune system as a driver of mitochondrial disease pathogenesis: a review of evidence. Orphanet J. Rare Dis. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.G.E. Wechsler IQ Profile and Theory of Mind in Autism: A Research Note. J. Child Psychol. Psychiatry 1994, 35, 1461–1471. [Google Scholar] [CrossRef]
- Hardy, D. Autoimmune Encephalitis in Children. Pediatr. Neurol. 2022, 132, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Harkey, J. The Epidemiology of Selected Chronic Childhood Health Conditions. Child. Heal. Care 1983, 12, 62–71. [Google Scholar] [CrossRef]
- Harris, J. Leo Kanner and autism: a 75-year perspective. Int. Rev. Psychiatry 2018, 30, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Al Hasib, R.; Ali, C.; Rahman, H.; Ahmed, S.; Sultana, S.; Summa, S.Z.; Shimu, M.S.S.; Afrin, Z.; Jamal, M.A.H.M. Integrated gene expression profiling and functional enrichment analyses to discover biomarkers and pathways associated with Guillain-Barré syndrome and autism spectrum disorder to identify new therapeutic targets. J. Biomol. Struct. Dyn. 2023, 42, 11299–11321. [Google Scholar] [CrossRef] [PubMed]
- Hawgood, S.; Hook-Barnard, I.G.; O’bRien, T.C.; Yamamoto, K.R. Precision medicine: Beyond the inflection point. Sci. Transl. Med. 2015, 7, 300ps17–300ps17. [Google Scholar] [CrossRef]
- Hazan, S.; Spradling-Reeves, K.D.; Papoutsis, A.; Walker, S.J. Shotgun Metagenomic Sequencing Identifies Dysbiosis in Triplet Sibling with Gastrointestinal Symptoms and ASD. Children 2020, 7, 255. [Google Scholar] [CrossRef]
- Heberling, C.A.; Dhurjati, P.S.; Sasser, M. Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: Links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses 2013, 80, 264–270. [Google Scholar] [CrossRef]
- Heemskerk, V.H.; Daemen, M.A.; A Buurman, W. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999, 10, 5–14. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocr. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Helt, M.; Kelley, E.; Kinsbourne, M.; Pandey, J.; Boorstein, H.; Herbert, M.; Fein, D. Can Children with Autism Recover? If So, How? Neuropsychol. Rev. 2008, 18, 339–366. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Herberman, R.B.; Ortaldo, J.R. Natural killer cells: their roles in defenses against disease. Science 1981, 214, 24–30. [Google Scholar] [CrossRef]
- Herbert, M. , & Weintraub, K. (2013). The Autism Revolution: Whole-Body Strategies for Making Life All It Can Be. Random House Publishing Group.
- Herbert, M.R. Large Brains in Autism: The Challenge of Pervasive Abnormality. Neurosci. 2005, 11, 417–440. [Google Scholar] [CrossRef]
- Herbert, M. S. 01.03 Autism: from static genetic brain defect to dynamic gene-environment modulated pathophysiology. Eur. Neuropsychopharmacol. 2013, 23. [Google Scholar] [CrossRef]
- Herbert, M. R. (2014). Translational implications of a whole-body approach to brain health in autism: how transduction between metabolism and electrophysiology points to mechanisms for neuroplasticity. In Frontiers in Autism Research: New Horizons for Diagnosis & Treatment, 515–556. http://dx.doi.org/10.1142/9789814602167_0021.
- Herbert, M.R.; Sage, C. Autism and EMF? Plausibility of a pathophysiological link – Part I. Pathophysiology 2013, 20, 191–209. [Google Scholar] [CrossRef]
- Herbert, M.R.; Sage, C. Autism and EMF? Plausibility of a pathophysiological link part II. Pathophysiology 2013, 20, 211–234. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.K.; O'Brien, L.M.; Kennedy, D.N.; Filipek, P.A.; Bakardjiev, A.I.; Hodgson, J.; Takeoka, M.; Makris, N.; et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 2004, 128, 213–226. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.K.; O'BRien, L.M.; Lange, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Steele, S.; Makris, N.; et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 2003, 126, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Picciotto, I.; Schmidt, R.J.; Krakowiak, P. Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Res. 2018, 11, 554–586. [Google Scholar] [CrossRef]
- Hickey, A.; Crabtree, J.; Stott, J. ‘Suddenly the first fifty years of my life made sense’: Experiences of older people with autism. Autism 2017, 22, 357–367. [Google Scholar] [CrossRef]
- Higdon, R.; Earl, R.K.; Stanberry, L.; Hudac, C.M.; Montague, E.; Stewart, E.; Janko, I.; Choiniere, J.; Broomall, W.; Kolker, N.; et al. The Promise of Multi-Omics and Clinical Data Integration to Identify and Target Personalized Healthcare Approaches in Autism Spectrum Disorders. OMICS: A J. Integr. Biol. 2015, 19, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L. Executive dysfunction in autism☆. Trends Cogn. Sci. 2004, 8, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L. Evaluating the theory of executive dysfunction in autism. Dev. Rev. 2004, 24, 189–233. [Google Scholar] [CrossRef]
- Hill, K. (1990). The decline of childhood mortality.
- Hiller-Sturmhöfel, S. , & Bartke, A. (1998). The endocrine system - An overview. Alcohol health & Research World, 22, 153–64. https://pubmed.ncbi.nlm.nih. 1570. [Google Scholar]
- Hirahara, K.; Nakayama, T. CD4 + T-cell subsets in inflammatory diseases: beyond the T h 1/T h 2 paradigm. Int. Immunol. 2016, 28, 163–171. [Google Scholar] [CrossRef]
- Ho, P.; Ross, D.A. More Than a Gut Feeling: The Implications of the Gut Microbiota in Psychiatry. Biol. Psychiatry 2017, 81, e35–e37. [Google Scholar] [CrossRef]
- Hooker, B.; Kern, J.; Geier, D.; Haley, B.; Sykes, L.; King, P.; Geier, M. Methodological Issues and Evidence of Malfeasance in Research Purporting to Show Thimerosal in Vaccines Is Safe. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Horlin, C.; Falkmer, M.; Parsons, R.; Albrecht, M.A.; Falkmer, T.; Mulle, J.G. The Cost of Autism Spectrum Disorders. PLOS ONE 2014, 9, e106552. [Google Scholar] [CrossRef] [PubMed]
- Hridi, S.U.; Barbour, M.; Wilson, C.; Franssen, A.J.; Harte, T.; Bushell, T.J.; Jiang, H.-R. Increased Levels of IL-16 in the Central Nervous System during Neuroinflammation Are Associated with Infiltrating Immune Cells and Resident Glial Cells. Biology 2021, 10, 472. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Chow, J.; Mazmanian, S.K.; Patterson, P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. 2012, 109, 12776–12781. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Chen, Z.; Zou, L.; Healy, S.; Tse, C.Y.A.; Li, C. Effects of Mind-Body Exercises on Health-related Outcomes in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2023, 1–15. [Google Scholar] [CrossRef]
- Hughes, H.K.; Ko, E.M.; Rose, D.; Ashwood, P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell. Neurosci. 2018, 12, 405. [Google Scholar] [CrossRef]
- Hughes, H.K.; Rose, D.; Ashwood, P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.R.; Jones, O.D.; Raymond, C.R.; Sah, P.; Abraham, W.C. Mechanisms of heterosynaptic metaplasticity. Philos. Trans. R. Soc. B: Biol. Sci. 2014, 369, 20130148. [Google Scholar] [CrossRef]
- Humphries S, Bystrianyk R (2013) Dissolving illusions: Disease, vaccines and the forgotten history. CreateSpace Independent Publishing Platform.
- Hurley-Hanson, A. E., Giannantonio, C. M., Griffiths, A. J., Hurley-Hanson, A. E., Giannantonio, C. M., & Griffiths, A. J. (2020). The costs of autism. Springer. https://doi.org/10.1007/978-3-030-29049-8_3.
- Hussaini, S.M.Q.; Jang, M.H. New Roles for Old Glue: Astrocyte Function in Synaptic Plasticity and Neurological Disorders. Int. Neurourol. J. 2018, 22, S106–114. [Google Scholar] [CrossRef] [PubMed]
- E Ichim, T.; Solano, F.; Glenn, E.; Morales, F.; Smith, L.; Zabrecky, G.; Riordan, N.H. Stem Cell Therapy for Autism. J. Transl. Med. 2007, 5, 30–30. [Google Scholar] [CrossRef]
- Irwin, J. K. , MacSween, J., & Kerns, K. A. (2011). History and evolution of the autism spectrum disorders. In International Handbook of Autism & Pervasive Developmental disorders (pp. 3–16). Springer.
- Jaga, K.; Dharmani, C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Rev. Environ. Heal. 2007, 22, 57–74. [Google Scholar] [CrossRef]
- Jarrold, C.; Russell, J. Counting Abilities in Autism: Possible Implications for Central Coherence Theory. J. Autism Dev. Disord. 1997, 27, 25–37. [Google Scholar] [CrossRef]
- Jaswal, V.K.; Wayne, A.; Golino, H. Eye-tracking reveals agency in assisted autistic communication. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Jaswal, V.K.; Lampi, A.J.; Stockwell, K.M. Literacy in nonspeaking autistic people. Autism 2024, 28, 2503–2514. [Google Scholar] [CrossRef]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.M.; Cowan, M.; Moonah, S.N.; Petri, W.A. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol. Med. 2018, 24, 794–804. [Google Scholar] [CrossRef]
- John, E. The neurophysics of consciousness. Brain Res. Rev. 2002, 39, 1–28. [Google Scholar] [CrossRef]
- Jones, J.P.; Williamson, L.; Konsoula, Z.; Anderson, R.; Reissner, K.J.; Parker, W. Evaluating the Role of Susceptibility Inducing Cofactors and of Acetaminophen in the Etiology of Autism Spectrum Disorder. Life 2024, 14, 918. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.-P.; Barreau, F. Peyer's Patches: The Immune Sensors of the Intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H. Food allergy and autism spectrum disorders: Is there a link? Curr. Allergy Asthma Rep. 2009, 9, 194–201. [Google Scholar] [CrossRef]
- Jyonouchi, H. Autism spectrum disorder and a possible role of anti-inflammatory treatments: experience in the pediatric allergy/immunology clinic. Front. Psychiatry 2024, 15, 1333717. [Google Scholar] [CrossRef]
- Kaminska, A.; Cheliout-Heraut, F.; Eisermann, M.; de Villepin, A.T.; Lamblin, M.D. EEG in children, in the laboratory or at the patient's bedside. Neurophysiol. Clin. 2015, 45, 65–74. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Karhu, E.; Zukerman, R.; Eshraghi, R.S.; Mittal, J.; Deth, R.C.; Castejon, A.M.; Trivedi, M.; Mittal, R.; A Eshraghi, A. Nutritional interventions for autism spectrum disorder. Nutr. Rev. 2019, 78, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef]
- Karlsson, O. Chemical safety and the exposome. Emerg. Contam. 2023, 9. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, H.; Kumar, A.; Arora, S.; Akter, R. Role of metallic pollutants in neurodegeneration: effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ. Sci. Pollut. Res. 2021, 28, 8989–9001. [Google Scholar] [CrossRef]
- Kayser, M.S.; Dalmau, J. Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Schizophr. Res. 2016, 176, 36–40. [Google Scholar] [CrossRef]
- Keating, B. A., Lees, J. G., & Moalem-Taylor, G. (2019). The Roles of Regulatory T Cells in Central Nervous System Autoimmunity. In H. Mitoma & M. Manto (Eds.), Neuroimmune Diseases: From Cells to the Living Brain (pp. 167–193). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-030-19515-1_6.
- Keller, A.; Rimestad, M.L.; Rohde, J.F.; Petersen, B.H.; Korfitsen, C.B.; Tarp, S.; Lauritsen, M.B.; Händel, M.N. The Effect of a Combined Gluten- and Casein-Free Diet on Children and Adolescents with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 470. [Google Scholar] [CrossRef]
- Kelly, L.S.; Apple, C.G.; Gharaibeh, R.; Pons, E.E.B.; Thompson, C.W.B.; Kannan, K.B.; Darden, D.B.; Efron, P.A.; Thomas, R.M.; Mohr, A.M. Stress-related changes in the gut microbiome after trauma. J. Trauma Acute Care Surg. 2021, 91, 192–199. [Google Scholar] [CrossRef]
- Kemper, T.L.; Bauman, M. Neuropathology of Infantile Autism. J. Neuropathol. Exp. Neurol. 1998, 57, 645–652. [Google Scholar] [CrossRef]
- Kepser, L.-J.; Homberg, J.R. The neurodevelopmental effects of serotonin: A behavioural perspective. Behav. Brain Res. 2015, 277, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Geier, D.; Audhya, T.; King, P.; Sykes, L.; Geier, M. Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiol. Exp. 2012, 72, 113–153. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Deth, R.C.; Sykes, L.K.; Hooker, B.S.; Love, J.M.; Bjørklund, G.; Chaigneau, C.G.; Haley, B.E.; Geier, M.R. Systematic Assessment of Research on Autism Spectrum Disorder (ASD) and Mercury Reveals Conflicts of Interest and the Need for Transparency in Autism Research. Sci. Eng. Ethic- 2017, 23, 1691–1718. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Geier, M.R. Relevance of Neuroinflammation and Encephalitis in Autism. Front. Cell. Neurosci. 2016, 9, 519. [Google Scholar] [CrossRef]
- Khachadourian, V.; Mahjani, B.; Sandin, S.; Kolevzon, A.; Buxbaum, J.D.; Reichenberg, A.; Janecka, M. Comorbidities in autism spectrum disorder and their etiologies. Transl. Psychiatry 2023, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Wang, G. Environmental agents, oxidative stress and autoimmunity. Curr. Opin. Toxicol. 2018, 7, 22–27. [Google Scholar] [CrossRef]
- Khan, S.; Gramfort, A.; Shetty, N.R.; Kitzbichler, M.G.; Ganesan, S.; Moran, J.M.; Lee, S.M.; Gabrieli, J.D.E.; Tager-Flusberg, H.B.; Joseph, R.M.; et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. 2013, 110, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Michmizos, K.; Tommerdahl, M.; Ganesan, S.; Kitzbichler, M.G.; Zetino, M.; Garel, K.-L.A.; Herbert, M.R.; Hämäläinen, M.S.; Kenet, T. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 2015, 138, 1394–1409. [Google Scholar] [CrossRef]
- Kharrazian, D.; Herbert, M.; Lambert, J. The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 16352. [Google Scholar] [CrossRef] [PubMed]
- Khetrapal, N. The framework for disturbed affective consciousness in autism. Neuropsychiatr. Dis. Treat. 2008, 4, 531–533. [Google Scholar] [CrossRef]
- Khundakji, Y.; Masri, A.; Khuri-Bulos, N. Anti-NMDA receptor encephalitis in a toddler. Int. J. Pediatr. Adolesc. Med. 2018, 5, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Camilleri, M. Serotonin: A Mediator of The Brain–Gut Connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, M.-H.; Shim, W.H.; Kim, J.K.; Jeon, E.-Y.; Kim, D.-H.; Yoon, S.-Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 2016, 22, 1576–1584. [Google Scholar] [CrossRef]
- King, B.H.; Hollander, E.; Sikich, L.; McCracken, J.T.; Scahill, L.; Bregman, J.D.; Donnelly, C.L.; Anagnostou, E.; Dukes, K.; Sullivan, L.; et al. Lack of Efficacy of Citalopram in Children With Autism Spectrum Disorders and High Levels of Repetitive Behavior. Arch. Gen. Psychiatry 2009, 66, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kissane, D.W. The Relief of Existential Suffering. Arch. Intern. Med. 2012, 172, 1501–1505. [Google Scholar] [CrossRef]
- Kleine, B.; Rossmanith, W.G. Hormones and the Endocrine System; Springer Nature: Dordrecht, GX, Netherlands, 2016. [Google Scholar]
- Knapp, M.; Romeo, R.; Beecham, J. Economic cost of autism in the UK. Autism 2009, 13, 317–336. [Google Scholar] [CrossRef]
- Knudsen, G.P. Gender bias in autoimmune diseases: X chromosome inactivation in women with multiple sclerosis. J. Neurol. Sci. 2009, 286, 43–46. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef]
- Ko, C.-L.; Lin, C.-K.; Lin, C.-L. Relationship between executive function and autism symptoms in preschoolers with autism spectrum disorder. Res. Dev. Disabil. 2024, 147, 104692. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The Roles of Peyer's Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front. Immunol. 2019, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef]
- Konteh, F.H. Urban sanitation and health in the developing world: Reminiscing the nineteenth century industrial nations. Heal. Place 2009, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, E.; Gevezova, M.; Maes, M.; Sarafian, V. Mast Cells in Autism Spectrum Disorder—The Enigma to Be Solved? Int. J. Mol. Sci. 2024, 25, 2651. [Google Scholar] [CrossRef]
- Koyama, R.; Ikegaya, Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 2015, 100, 1–5. [Google Scholar] [CrossRef]
- Krausová, M.; Braun, D.; Buerki-Thurnherr, T.; Gundacker, C.; Schernhammer, E.; Wisgrill, L.; Warth, B. Understanding the Chemical Exposome During Fetal Development and Early Childhood: A Review. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 517–540. [Google Scholar] [CrossRef]
- Kushak, R.I.; Winter, H.S. Gut Microbiota and Gender in Autism Spectrum Disorders. Curr. Pediatr. Rev. 2020, 16, 249–254. [Google Scholar] [CrossRef]
- Lacivita, E.; Perrone, R.; Margari, L.; Leopoldo, M. Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. J. Med. Chem. 2017, 60, 9114–9141. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Maloney, B.; Wang, R.; Sokol, D.K.; Rogers, J.T.; Westmark, C.J. How autism and Alzheimer’s disease are TrAPPed. Mol. Psychiatry 2020, 26, 26–29. [Google Scholar] [CrossRef]
- Lai, Y.; Dhingra, R.; Zhang, Z.; Ball, L.M.; Zylka, M.J.; Lu, K. Toward Elucidating the Human Gut Microbiota–Brain Axis: Molecules, Biochemistry, and Implications for Health and Diseases. Biochemistry 2021, 61, 2806–2821. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Aman, M.G.; Arnold, L.E. Neurochemical correlates of autistic disorder: A review of the literature. Res. Dev. Disabil. 2006, 27, 254–289. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Reissmann, A. Gluten-free and casein-free diets in the therapy of autism. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 572–575. [Google Scholar] [CrossRef]
- Lebow, M., Kuperman, Y., & Chen, A. (2024). Prenatal-induced psychopathologies: All roads lead to microglia. In Stress: Immunology & Inflammation (pp. 199–214). Elsevier.
- Lederberg, J. Infectious History. Science 2000, 288, 287–293. [Google Scholar] [CrossRef]
- Lee, J.-S.; Sato, W.; Son, C.-G. Brain-regional characteristics and neuroinflammation in ME/CFS patients from neuroimaging: A systematic review and meta-analysis. Autoimmun. Rev. 2023, 23, 103484. [Google Scholar] [CrossRef] [PubMed]
- Leiter, O.; Walker, T.L. Platelets: The missing link between the blood and brain? Prog. Neurobiol. 2019, 183, 101695. [Google Scholar] [CrossRef]
- Leo, E.E.M.; Campos, M.R.S. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrients 2020, 71, 110609. [Google Scholar] [CrossRef]
- Lerer, L.; Varia, J. A long trip into the universe: Psychedelics and space travel. Front. Space Technol. 2022, 3. [Google Scholar] [CrossRef]
- Lesch, K.-P.; Waider, J. Serotonin in the Modulation of Neural Plasticity and Networks: Implications for Neurodevelopmental Disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef]
- Leventhal, J.; Miller, J.; Abecassis, M.; Tollerud, D.J.; Ildstad, S.T. Evolving Approaches of Hematopoietic Stem Cell–Based Therapies to Induce Tolerance to Organ Transplants: The Long Road to Tolerance. Clin. Pharmacol. Ther. 2012, 93, 36–45. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; He, X.; Zhou, J.; Jin, C.; Shen, L.; Gao, Y.; Tian, M.; Zhang, H. Structural, Functional, and Molecular Imaging of Autism Spectrum Disorder. Neurosci. Bull. 2021, 37, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, Y.; Fu, X.; Li, Y. Postmortem Studies of Neuroinflammation in Autism Spectrum Disorder: a Systematic Review. Mol. Neurobiol. 2020, 57, 3424–3438. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, S.O.; Marshall, J.; Todd, J.T.; Shane, H.C. The persistence of fad interventions in the face of negative scientific evidence: Facilitated communication for autism as a case example. Evidence-Based Commun. Assess. Interv. 2014, 8, 62–101. [Google Scholar] [CrossRef]
- Liu, G.; Pearl, A.M.; Kong, L.; Leslie, D.L.; Murray, M.J. A Profile on Emergency Department Utilization in Adolescents and Young Adults with Autism Spectrum Disorders. J. Autism Dev. Disord. 2016, 47, 347–358. [Google Scholar] [CrossRef]
- Liu, M.; Liang, S.; Zhang, C. NK Cells in Autoimmune Diseases: Protective or Pathogenic? Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shi, X.-J.; Fan, F.-C.; Cheng, Y. Peripheral blood neurotrophic factor levels in children with autism spectrum disorder: a meta-analysis. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Lleo, A. (2014). Chapter 2 - What Is an Autoantibody? In Y. Shoenfeld, P. L. Meroni, & M. E. Gershwin (Eds.), Autoantibodies (Third Edition) (pp. 13–20). San Diego: Elsevier. https://doi.org/10.1016/B978-0-444-56378-1.00002-2.
- Lleo, A.; Invernizzi, P.; Gao, B.; Podda, M.; Gershwin, M.E. Definition of human autoimmunity — autoantibodies versus autoimmune disease. Autoimmun. Rev. 2010, 9, A259–A266. [Google Scholar] [CrossRef] [PubMed]
- López-Cacho, J.M.; Gallardo, S.; Posada, M.; Aguerri, M.; Calzada, D.; Mayayo, T.; Lahoz, C.; Cárdaba, B. Characterization of Immune Cell Phenotypes in Adults with Autism Spectrum Disorders. J. Investig. Med. 2016, 64, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional foods and the immune system: a review. Eur. J. Clin. Nutr. 2002, 56, S29–S33. [Google Scholar] [CrossRef]
- Lord, C.; Shulman, C.; DiLavore, P. Regression and word loss in autistic spectrum disorders. J. Child Psychol. Psychiatry 2004, 45, 936–955. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Z. The Impact of Microglia on Neurodevelopment and Brain Function in Autism. Biomedicines 2024, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Luvián-Morales, J.; Varela-Castillo, F.O.; Flores-Cisneros, L.; Cetina-Pérez, L.; Castro-Eguiluz, D. Functional foods modulating inflammation and metabolism in chronic diseases: a systematic review. Crit. Rev. Food Sci. Nutr. 2021, 62, 4371–4392. [Google Scholar] [CrossRef]
- Luyster, R.; Richler, J.; Risi, S.; Hsu, W.-L.; Dawson, G.; Bernier, R.; Dunn, M.; Hepburn, S.; Hyman, S.L.; McMahon, W.M.; et al. Early Regression in Social Communication in Autism Spectrum Disorders: A CPEA Study. Dev. Neuropsychol. 2005, 27, 311–336. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Machado, A.P.; Ratliff, H.; Abdelwahab, A.; Vohra, M.H.; Kuang, A.; Shatila, M.; Khan, M.A.; Shafi, M.A.; Thomas, A.S.; Philpott, J.; et al. The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: a Systematic Review. J. Cancer 2023, 14, 2956–2963. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Pachnis, V.; Prinz, M. Boundaries and integration between microbiota, the nervous system, and immunity. Immunity 2023, 56, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR. Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Maier, S.F.; Watkins, L.R. Bidirectional communication between the brain and the immune system: implications for behaviour. Anim. Behav. 1999, 57, 741–751. [Google Scholar] [CrossRef]
- Maitre, L.; Bustamante, M.; Hernández-Ferrer, C.; Thiel, D.; Lau, C.-H.E.; Siskos, A.P.; Vives-Usano, M.; Ruiz-Arenas, C.; Pelegrí-Sisó, D.; Robinson, O.; et al. Multi-omics signatures of the human early life exposome. Nat. Commun. 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. Part C: Embryo Today: Rev. 2016, 108, 224–242. [Google Scholar] [CrossRef]
- Mamuladze, T.; Kipnis, J. Type 2 immunity in the brain and brain borders. Cell. Mol. Immunol. 2023, 20, 1290–1299. [Google Scholar] [CrossRef]
- Manji, H.; Kato, T.; Di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef]
- Margulis, L. , & Fester, R. (1991). Symbiosis as a Source of Evolutionary Innovation: Speciation & Morphogenesis. MIT Press.
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Marques, P.E.; Guabiraba, R.; Teixeira, M.M. Exploring the Homeostatic and Sensory Roles of the Immune System. Front. Immunol. 2016, 7, 125–125. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L., & Born, J. (2002). Brain-Immune interactions in sleep. In International Review of Neurobiology (Vol. 52, pp. 93–131). Academic Press. https://doi.org/10.1016/S0074-7742(02)52007-9.
- Martin, A.; Scahill, L.; Anderson, G.M.; Aman, M.; Arnold, L.E.; McCracken, J.; McDougle, C.J.; Tierney, E.; Chuang, S.; Vitiello, B.; et al. Weight and Leptin Changes Among Risperidone-Treated Youths With Autism: 6-Month Prospective Data. Am. J. Psychiatry 2004, 161, 1125–1127. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev. Neurobiol. 2016, 77, 393–404. [Google Scholar] [CrossRef]
- Marwaha, A.K.; Leung, N.J.; McMurchy, A.N.; Levings, M.K. TH17 Cells in Autoimmunity and Immunodeficiency: Protective or Pathogenic? Front. Immunol. 2012, 3, 129. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Aguilar, S.V.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; González, F.Z.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune Regulatory Function of B Cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef]
- McCormick, C.M.; Furey, B.F.; Child, M.; Sawyer, M.J.; Donohue, S.M. Neonatal sex hormones have `organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Dev. Brain Res. 1998, 105, 295–307. [Google Scholar] [CrossRef]
- McCracken, J.T.; Anagnostou, E.; Arango, C.; Dawson, G.; Farchione, T.; Mantua, V.; McPartland, J.; Murphy, D.; Pandina, G.; Veenstra-VanderWeele, J. Drug development for Autism Spectrum Disorder (ASD): Progress, challenges, and future directions. Eur. Neuropsychopharmacol. 2021, 48, 3–31. [Google Scholar] [CrossRef]
- McCruden, A. B., & Stimson, W. H. (1991). Sex Hormones and Immune Function. In R. ADER, D. L. FELTEN, & N. COHEN (Eds.), Psychoneuroimmunology (pp. 475–493). Academic Press. https://doi.org/10.1016/B978-0-12-043780-1.50021-X.
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Megremi, A.S. Is fever a predictive factor in the autism spectrum disorders? Med Hypotheses 2013, 80, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mezzacappa, A.; Lasica, P.-A.; Gianfagna, F.; Cazas, O.; Hardy, P.; Falissard, B.; Sutter-Dallay, A.-L.; Gressier, F. Risk for Autism Spectrum Disorders According to Period of Prenatal Antidepressant Exposure. JAMA Pediatr. 2017, 171, 555–563. [Google Scholar] [CrossRef]
- Miclotte, L.; Van de Wiele, T. Food processing, gut microbiota and the globesity problem. Crit. Rev. Food Sci. Nutr. 2019, 60, 1769–1782. [Google Scholar] [CrossRef]
- Miller, H. , Zhang, J., Kuolee, R., Patel, G., & Chen, W. (2007). Intestinal M cells: The fallible sentinels? World Journal of Gastroenterology : WJG, 13, 1477–86. [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef]
- Misquita, A.G.; da Silva, C.N.F.; Souza, D.R.O.; dos Santos, D.N.B.; Brito, I.D.S.M.; Ribeiro, J.D.O.S.; Almeida, J.F.L.; Silva, J.A.G.; Barreto, N.P.M.; Rodrigues, S.C.P.; et al. Cognitive behavioral therapy: A valuable intervention in the autistic universe. Int. Seven- J. Heal. Res. 2024, 3, 754–760. [Google Scholar] [CrossRef]
- Mitsea, E.; Drigas, A.; Skianis, C. Cutting-Edge Technologies in Breathwork for Learning Disabilities in Special Education. Tech. Soc. Sci. J. 2022, 34, 136–157. [Google Scholar] [CrossRef]
- Modafferi, S.; Lupo, G.; Tomasello, M.; Rampulla, F.; Ontario, M.; Scuto, M.; Salinaro, A.T.; Arcidiacono, A.; Anfuso, C.D.; Legmouz, M.; et al. Antioxidants, Hormetic Nutrition, and Autism. Curr. Neuropharmacol. 2024, 22, 1156–1168. [Google Scholar] [CrossRef]
- Nordin, A.M.; Ismail, J.; Nor, N.K. Motor Development in Children With Autism Spectrum Disorder. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef]
- Molloy, C.; Morrow, A.; Meinzenderr, J.; Schleifer, K.; Dienger, K.; Manningcourtney, P.; Altaye, M.; Willskarp, M. Elevated cytokine levels in children with autism spectrum disorder. J. Neuroimmunol. 2006, 172, 198–205. [Google Scholar] [CrossRef]
- Moncrieff, J. Why is it so difficult to stop psychiatric drug treatment? Med Hypotheses 2006, 67, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cohen, D.; Porter, S. The Psychoactive Effects of Psychiatric Medication: The Elephant in the Room. J. Psychoact. Drugs 2013, 45, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Money, J.; Bobrow, N.A.; Clarke, F.C. Autism and autoimmune disease: A family study. J. Autism Dev. Disord. 1971, 1, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.A.; dos Santos, A.A.A.; Gomes, L.M.M.; Rito, R.V.V.F. AUTISM SPECTRUM DISORDER: A SYSTEMATIC REVIEW ABOUT NUTRITIONAL INTERVENTIONS. Rev. Paul. de Pediatr. 2020, 38, e2018262. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Bowers, W.J. Tumor Necrosis Factor-alpha and the Roles it Plays in Homeostatic and Degenerative Processes Within the Central Nervous System. J. Neuroimmune Pharmacol. 2011, 7, 42–59. [Google Scholar] [CrossRef]
- Moore, J.B.; Weeks, M.E. Proteomics and Systems Biology: Current and Future Applications in the Nutritional Sciences. Adv. Nutr. Int. Rev. J. 2011, 2, 355–364. [Google Scholar] [CrossRef]
- Mordelt, A.; de Witte, L.D. Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: Hype or hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Moroncini, G.; Calogera, G.; Benfaremo, D.; Gabrielli, A. Biologics in Inflammatory Immune-mediated Systemic Diseases. Curr. Pharm. Biotechnol. 2018, 18, 1008–1016. [Google Scholar] [CrossRef]
- Moroni, L.; Bianchi, I.; Lleo, A. Geoepidemiology, gender and autoimmune disease. Autoimmun. Rev. 2011, 11, A386–A392. [Google Scholar] [CrossRef]
- Mosconi, M.W.; Wang, Z.; Schmitt, L.M.; Tsai, P.; Sweeney, J.A. The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front. Neurosci. 2015, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Mulder, S.J.; Mulder-Bos, G.C. Most probable origin of coeliac disease is low immune globulin A in the intestine caused by malfunction of Peyer’s patches. Med Hypotheses 2006, 66, 757–762. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Sarawi, W.; Attia, S.M.; Bakheet, S.A.; Alqarni, S.A.; Ali, N.; AsSobeai, H.M. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol. Immunol. 2022, 141, 297–304. [Google Scholar] [CrossRef]
- Naviaux, R.K. Perspective: Cell danger response Biology—The new science that connects environmental health with mitochondria and the rising tide of chronic illness. Mitochondrion 2020, 51, 40–45. [Google Scholar] [CrossRef]
- Nelissen, S.; Lemmens, E.; Geurts, N.; Kramer, P.; Maurer, M.; Hendriks, J.; Hendrix, S. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013, 125, 637–650. [Google Scholar] [CrossRef]
- Nelson, A.D.; Bender, K.J. Dendritic Integration Dysfunction in Neurodevelopmental Disorders. Dev. Neurosci. 2021, 43, 201–221. [Google Scholar] [CrossRef]
- Nelson, I. Exploring the influence of Schumann resonance and electromagnetic fields on bioelectricity and human health. Electromagn. Biol. Med. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’nEill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098–aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Nevison, C.; Blaxill, M.; Zahorodny, W. California Autism Prevalence Trends from 1931 to 2014 and Comparison to National ASD Data from IDEA and ADDM. J. Autism Dev. Disord. 2018, 48, 4103–4117. [Google Scholar] [CrossRef]
- Nevison, C.D.; Blaxill, M. Diagnostic Substitution for Intellectual Disability: A Flawed Explanation for the Rise in Autism. J. Autism Dev. Disord. 2017, 47, 2733–2742. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012, 100, 656–664. [Google Scholar] [CrossRef]
- Nie, Z.-Q.; Han, D.; Zhang, K.; Li, M.; Kwon, H.-K.; Im, S.-H.; Xu, L.; Yang, J.-C.; Li, Z.-W.; Huang, X.-W.; et al. TH1/Treg ratio may be a marker of autism in children with immune dysfunction. Res. Autism Spectr. Disord. 2022, 101. [Google Scholar] [CrossRef]
- NIEHS. (2024). Autoimmune Diseases. https://www.niehs.nih.gov/health/topics/conditions/autoimmune. 1 May.
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.-H. Disorders of the enteric nervous system — a holistic view. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- NIMH. (2024). Autism Spectrum Disorder. https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd. 16 July.
- Complication of encephalitis in children, an innovative approach for the treatment. Int. J. Pharm. Res. 2020, 12. [CrossRef]
- Njotto, L.L.; Simin, J.; Fornes, R.; Odsbu, I.; Mussche, I.; Callens, S.; Engstrand, L.; Bruyndonckx, R.; Brusselaers, N. Maternal and Early-Life Exposure to Antibiotics and the Risk of Autism and Attention-Deficit Hyperactivity Disorder in Childhood: a Swedish Population-Based Cohort Study. Drug Saf. 2023, 46, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Nyati, K.K.; Prasad, K.N. Role of Cytokines and Toll-Like Receptors in the Immunopathogenesis of Guillain-Barré Syndrome. Mediat. Inflamm. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nelson, I. Exploring the influence of Schumann resonance and electromagnetic fields on bioelectricity and human health. Electromagn. Biol. Med. 2025, 1–11. [Google Scholar] [CrossRef]
- van Ommen, B.; Stierum, R. Nutrigenomics: exploiting systems biology in the nutrition and health arena. Curr. Opin. Biotechnol. 2002, 13, 517–521. [Google Scholar] [CrossRef]
- Onore, C.; Careaga, M.; Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain, Behav. Immun. 2012, 26, 383–392. [Google Scholar] [CrossRef]
- Orekhova, E.V.; Stroganova, T.A.; Nygren, G.; Tsetlin, M.M.; Posikera, I.N.; Gillberg, C.; Elam, M. Excess of High Frequency Electroencephalogram Oscillations in Boys with Autism. Biol. Psychiatry 2007, 62, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Iosif, A.-M.; Baguio, F.; Cook, I.C.; Hill, M.M.; Hutman, T.; Rogers, S.J.; Rozga, A.; Sangha, S.; Sigman, M.; et al. A Prospective Study of the Emergence of Early Behavioral Signs of Autism. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 256–266.e2. [Google Scholar] [CrossRef]
- Padmakumar, M.; Van Raes, E.; Van Geet, C.; Freson, K. Blood platelet research in autism spectrum disorders: In search of biomarkers. Res. Pr. Thromb. Haemost. 2019, 3, 566–577. [Google Scholar] [CrossRef]
- Palmieri, L.; Persico, A.M. Mitochondrial dysfunction in autism spectrum disorders: Cause or effect? Biochim. et Biophys. Acta (BBA) - Bioenerg. 2010, 1797, 1130–1137. [Google Scholar] [CrossRef]
- Panksepp, J. A neurochemical theory of autism. Trends Neurosci. 1979, 2, 174–177. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Pardo, C.A.; Eberhart, C.G. The Neurobiology of Autism. Brain Pathol. 2007, 17, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Pardo, C.A.; Vargas, D.L.; Zimmerman, A.W. Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 2005, 17, 485–495. [Google Scholar] [CrossRef]
- Park, K.M.; Bowers, W.J. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell. Signal. 2010, 22, 977–983. [Google Scholar] [CrossRef]
- Pathak, D.; Sriram, K. Neuron-astrocyte omnidirectional signaling in neurological health and disease. Front. Mol. Neurosci. 2023, 16, 1169320. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Paveenakiattikhun, S.; Likhitweerawong, N.; Sanguansermsri, C. EEG findings and clinical severity and quality of life in non-epileptic patients with autism spectrum disorders. Child Neuropsychol. 2024, 31, 255–265. [Google Scholar] [CrossRef]
- Peper, E.; Shufor, J. Reflections on the Increase in Autism, ADHD, Anxiety, and Depression: Part 2 – Exposure to Neurotoxins and Ultraprocessed Foods. NeuroRegulation 2024, 11, 219–228. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci. Rep. 2024, 14, 1–11. [Google Scholar] [CrossRef]
- Pereira, A. (2020). Chapter Seven - Classical-quantum interfaces in living neural tissue supporting conscious functions. In R. R. Poznański & E. J. Brändas (Eds.), Advances in Quantum Chemistry (Vol. 82, pp. 213–252). Academic Press. https://doi.org/10.1016/bs.aiq.2020.08.002.
- Pereira, A.; Furlan, F.A. On the role of synchrony for neuron–astrocyte interactions and perceptual conscious processing. J. Biol. Phys. 2009, 35, 465–480. [Google Scholar] [CrossRef]
- Pereira, A.; Furlan, F.A. Astrocytes and human cognition: Modeling information integration and modulation of neuronal activity. Prog. Neurobiol. 2010, 92, 405–420. [Google Scholar] [CrossRef]
- Pereira, J. A. (2020). The Role of Sentience in the Theory of Consciousness and Medical Practice. [CrossRef]
- Pereira, A.; Wang, F. Neuromodulation, Emotional Feelings and Affective Disorders. Mens Sana Monogr. 2016, 14, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Perrin, J.M.; Anderson, L.E.; Van Cleave, J. The Rise In Chronic Conditions Among Infants, Children, And Youth Can Be Met With Continued Health System Innovations. Heal. Aff. 2014, 33, 2099–2105. [Google Scholar] [CrossRef]
- Petrelli, F.; Pucci, L.; Bezzi, P. Astrocytes and Microglia and Their Potential Link with Autism Spectrum Disorders. Front. Cell. Neurosci. 2016, 10, 21. [Google Scholar] [CrossRef]
- Pierce, S.; Kadlaskar, G.; Edmondson, D.A.; Keehn, R.M.; Dydak, U.; Keehn, B. Associations between sensory processing and electrophysiological and neurochemical measures in children with ASD: an EEG-MRS study. J. Neurodev. Disord. 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ploog, B. O. (2023). Theory of Mind in Autism. In A. El Idrissi & D. McCloskey (Eds.), Neurobiology of Autism Spectrum Disorders (pp. 23–35). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-031-42383-3_2.
- Gerber, J.S.; Offit, P.A. Vaccines and Autism: A Tale of Shifting Hypotheses. Clin. Infect. Dis. 2009, 48, 456–461. [Google Scholar] [CrossRef]
- Pociūtė, A.; Pivoriūnas, A.; Verkhratsky, A. Astrocytes dynamically regulate the blood-brain barrier in the healthy brain. Neural Regen. Res. 2023, 19, 709–710. [Google Scholar] [CrossRef]
- Poling, J.S.; Frye, R.E.; Shoffner, J.; Zimmerman, A.W. Developmental Regression and Mitochondrial Dysfunction in a Child With Autism. J. Child Neurol. 2006, 21, 170–172. [Google Scholar] [CrossRef]
- Politte, L.C.; Howe, Y.; Nowinski, L.; Palumbo, M.; McDougle, C.J. Evidence-Based Treatments for Autism Spectrum Disorder. Curr. Treat. Options Psychiatry 2015, 2, 38–56. [Google Scholar] [CrossRef]
- Pourgholaminejad, A., & Tahmasebinia, F. (2019). The Role of Th17 Cells in Immunopathogenesis of Neuroinflammatory Disorders. In H. Mitoma & M. Manto (Eds.), Neuroimmune Diseases: From Cells to the Living Brain (pp. 83–107). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-030-19515-1_3.
- Pretzsch, C.M.; Schäfer, T.; Lombardo, M.V.; Warrier, V.; Mann, C.; Bletsch, A.; Chatham, C.H.; Floris, D.L.; Tillmann, J.; Yousaf, A.; et al. Neurobiological Correlates of Change in Adaptive Behavior in Autism. Am. J. Psychiatry 2022, 179, 336–349. [Google Scholar] [CrossRef]
- Prüss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021, 21, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Yang, J.; Chang, L.; Qu, Y.; Wang, S.; Zhang, K.; Xiong, Z.; Zhang, J.; Tan, Y.; Wang, X.; et al. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. 2020, 117, 11753–11759. [Google Scholar] [CrossRef]
- Pulikkan, J., Mazumder, A., & Grace, T. (2019). Role of the gut microbiome in autism spectrum disorders. Reviews on Biomarker Studies in Psychiatric & Neurodegenerative disorders, 253–269. https://doi.org/10.1007/978-3-030-05542-4_13.
- Puricelli, C.; Rolla, R.; Gigliotti, L.; Boggio, E.; Beltrami, E.; Dianzani, U.; Keller, R. The Gut-Brain-Immune Axis in Autism Spectrum Disorders: A State-of-Art Report. Front. Psychiatry 2022, 12, 755171. [Google Scholar] [CrossRef]
- Qi, H. Neuroimmunology: reviews and perspectives on recent advances. Cell. Mol. Immunol. 2023, 20, 1257–1258. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Stephens, M.L.; Possin, K.L. Executive Dysfunction. Contin. Lifelong Learn. Neurol. 2015, 21, 646–659. [Google Scholar] [CrossRef]
- Ramautar, R.; Berger, R.; van der Greef, J.; Hankemeier, T. Human metabolomics: strategies to understand biology. Curr. Opin. Chem. Biol. 2013, 17, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.S.; Woods, S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 2014, 121, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Randolph-Gips, M.; Srinivasan, P. Modeling autism: a systems biology approach. J. Clin. Bioinform. 2012, 2, 17–17. [Google Scholar] [CrossRef]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef]
- Reid, G.; Younes, J.A.; Van Der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat. Rev. Genet. 2011, 9, 27–38. [Google Scholar] [CrossRef]
- Renz, H.; Holt, P.G.; Inouye, M.; Logan, A.C.; Prescott, S.L.; Sly, P.D. An exposome perspective: Early-life events and immune development in a changing world. J. Allergy Clin. Immunol. 2017, 140, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Réthelyi, J.M.; Vincze, K.; Schall, D.; Glennon, J.; Berkel, S. The role of insulin/IGF1 signalling in neurodevelopmental and neuropsychiatric disorders – Evidence from human neuronal cell models. Neurosci. Biobehav. Rev. 2023, 153, 105330. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The crucial role of mast cells in blood–brain barrier alterations. Exp. Cell Res. 2015, 338, 119–125. [Google Scholar] [CrossRef]
- Ribeiro, S. Whole Organisms or Pure Compounds? Entourage Effect Versus Drug Specificity. In Plant Medicines, Healing and Psychedelic Science; Labate, B., Cavnar, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 133–149. [Google Scholar] [CrossRef]
- Riikonen, R. Insulin-Like Growth Factors in the Pathogenesis of Neurological Diseases in Children. Int. J. Mol. Sci. 2017, 18, 2056. [Google Scholar] [CrossRef]
- Robertson, J.M. The Astrocentric Hypothesis: proposed role of astrocytes in consciousness and memory formation. J. Physiol. 2002, 96, 251–255. [Google Scholar] [CrossRef]
- Rogers, S. J. , Cook, I., & Meryl, A. (2005). Imitation and play in autism. In Handbook of Autism a& Pervasive Developmental Disorders (Vol. 1, pp. 382–405). Wiley Online Library.
- Romagnani, S. Th1/Th2 Cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef]
- Rook, G.A.W. Hygiene Hypothesis and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2011, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Rosin, J.M.; Kurrasch, D.M. Emerging roles for hypothalamic microglia as regulators of physiological homeostasis. Front. Neuroendocr. 2019, 54, 100748. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Johnstone, J.M.; Kaplan, B.J. Nutrition Provides the Essential Foundation for Optimizing Mental Health. Evidence-Based Pr. Child Adolesc. Ment. Heal. 2021, 6, 131–154. [Google Scholar] [CrossRef]
- Rueda-Ruzafa, L.; Cruz, F.; Roman, P.; Cardona, D. Gut microbiota and neurological effects of glyphosate. NeuroToxicology 2019, 75, 1–8. [Google Scholar] [CrossRef]
- Saad, K.; Zahran, A.M.; Elsayh, K.I.; Abdel-Rahman, A.A.; Al-Atram, A.A.; Hussein, A.; El-Gendy, Y.G. Frequency of Dendritic Cells and Their Expression of Costimulatory Molecules in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2017, 47, 2671–2678. [Google Scholar] [CrossRef]
- Safari-Alighiarloo, N. , Taghizadeh, M., Rezaei tavirani, M., Goliaei, B., & Peyvandi, A. (2014). Protein-Protein Interaction Networks (PPI) and complex diseases. Gastroenterology & Hepatology From Bed to Bench, 7, 17–31. https://pubmed.ncbi.nlm.nih. 2543. [Google Scholar]
- Salmond, C.H.; de Haan, M.; Friston, K.J.; Gadian, D.G.; Vargha-Khadem, F.; Frith, U.; Hill, E.L. Investigating individual differences in brain abnormalities in autism. Philos. Trans. R. Soc. B: Biol. Sci. 2003, 358, 405–413. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Samanipour, S.; Barron, L.P.; van Herwerden, D.; Praetorius, A.; Thomas, K.V.; O’bRien, J.W. Exploring the Chemical Space of the Exposome: How Far Have We Gone? JACS Au 2024, 4, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Samsam, M.; Ahangari, R.; Naser, S.A. Pathophysiology of autism spectrum disorders: Revisiting gastrointestinal involvement and immune imbalance. World J. Gastroenterol. 2014, 20, 9942–51. [Google Scholar] [CrossRef] [PubMed]
- Samsel, A.; Seneff, S. Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases. Entropy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Faure, F. Self and the Brain. The Immune Metaphor. Front. Psychiatry 2020, 11, 540676. [Google Scholar] [CrossRef]
- Santarone, M.E.; Zambrano, S.; Zanotta, N.; Mani, E.; Minghetti, S.; Pozzi, M.; Villa, L.; Molteni, M.; Zucca, C. EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children. Brain Sci. 2023, 13, 345. [Google Scholar] [CrossRef]
- Saresella, M.; Piancone, F.; Marventano, I.; Zoppis, M.; Hernis, A.; Zanette, M.; Trabattoni, D.; Chiappedi, M.; Ghezzo, A.; Canevini, M.P.; et al. Multiple inflammasome complexes are activated in autistic spectrum disorders. Brain, Behav. Immun. 2016, 57, 125–133. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Schwartz, M.; Cahalon, L. The vicious cycle governing the brain–immune system relationship in neurodegenerative diseases. Curr. Opin. Immunol. 2022, 76, 102182. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Kyriakopoulos, A.M.; Nigh, G. Is autism a PIN1 deficiency syndrome? A proposed etiological role for glyphosate. J. Neurochem. 2024, 168, 2124–2146. [Google Scholar] [CrossRef]
- Seneff, S. , & Orlando, L. (2018). ( 8(1), 541. [CrossRef]
- Serrano-Pozo, A. (2018). Encephalopathy. In The Wiley Handbook on the Aging Mind and Brain (pp. 553–590). https://doi.org/10.1002/9781118772034.ch25.
- Shah, P.A.; Park, C.J.; Shaughnessy, M.P.; Cowles, R.A. Serotonin as a Mitogen in the Gastrointestinal Tract: Revisiting a Familiar Molecule in a New Role. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1093–1104. [Google Scholar] [CrossRef]
- Shahar, O.; Botvinnik, A.; Shwartz, A.; Lerer, E.; Golding, P.; Buko, A.; Hamid, E.; Kahn, D.; Guralnick, M.; Blakolmer, K.; et al. Effect of chemically synthesized psilocybin and psychedelic mushroom extract on molecular and metabolic profiles in mouse brain. Mol. Psychiatry 2024, 29, 2059–2073. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S.; Baranov, A.; Khan, W.I. Diverse Effects of Gut-Derived Serotonin in Intestinal Inflammation. ACS Chem. Neurosci. 2017, 8, 920–931. [Google Scholar] [CrossRef]
- Shaw, C.A.; Seneff, S.; Kette, S.D.; Tomljenovic, L.; Oller, J.W.; Davidson, R.M. Aluminum-Induced Entropy in Biological Systems: Implications for Neurological Disease. J. Toxicol. 2014, 2014, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.C.; Crombie, G.K.; Palliser, H.K.; Hirst, J.J. Impaired Oligodendrocyte Development Following Preterm Birth: Promoting GABAergic Action to Improve Outcomes. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef]
- Shaw, K.A. Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years — Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. MMWR. Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef]
- Shin, Y.-W.; Lee, S.-T.; Park, K.-I.; Jung, K.-H.; Jung, K.-Y.; Lee, S.K.; Chu, K. Treatment strategies for autoimmune encephalitis. Ther. Adv. Neurol. Disord. 2017, 11. [Google Scholar] [CrossRef]
- Siegel, M.; Milligan, B.; Chemelski, B.; Payne, D.; Ellsworth, B.; Harmon, J.; Teer, O.; Smith, K.A. Specialized Inpatient Psychiatry for Serious Behavioral Disturbance in Autism and Intellectual Disability. J. Autism Dev. Disord. 2014, 44, 3026–3032. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Cruz-Machado, S.d.S.; Campos, L.M.G.; Fadini, C.C.; Anderson, G.; Markus, R.P.; Pinato, L. Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. J. Pineal Res. 2021, 70, e12715. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.S.; Ni, G.; Lam, V.; Demopoulos, C. Beyond words: an investigation of fine motor skills and the verbal communication spectrum in autism. Front. Psychiatry 2024, 15, 1379307. [Google Scholar] [CrossRef]
- Simons, M.; Levin, J.; Dichgans, M. Tipping points in neurodegeneration. Neuron 2023, 111, 2954–2968. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Siniscalco, D.; Bradstreet, J.J.; Antonucci, N. Therapeutic Role of Hematopoietic Stem Cells in Autism Spectrum Disorder-Related Inflammation. Front. Immunol. 2013, 4, 140. [Google Scholar] [CrossRef]
- Sloan, S.A.; Barres, B.A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 2014, 27, 75–81. [Google Scholar] [CrossRef]
- Smith, A.; Storti, S.; Lukose, R.; Jr, R.J.K. Structural and Functional Aberrations of the Auditory Brainstem in Autism Spectrum Disorder. J. Am. Osteopat. Assoc. 2019, 119, 41–50. [Google Scholar] [CrossRef]
- Smyth, M.J.; Godfrey, D.I.; Trapani, J.A. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001, 2, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ssucharewa, G. Die schizoiden Psychopathien im Kindesalter. (Part 1 of 2). Eur. Neurol. 1926, 60, 235–247. [Google Scholar] [CrossRef]
- Starr, A.; Amlie, R.N.; Martin, W.H.; Sanders, S. Development of Auditory Function in Newborn Infants Revealed by Auditory Brainstem Potentials. Pediatrics 1977, 60, 831–839. [Google Scholar] [CrossRef]
- Stein, Y., Hänninen, O., Huttunen, P., Ahonen, M., & Ekman, R. (2015). Electromagnetic Radiation and Health: Human Indicators. In R. H. Armon & O. Hänninen (Eds.), Environmental Indicators (pp. 1025–1046). Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-9499-2_57.
- Steinman, G. Plausible etiology of brain dysconnectivity in autism – Review and prospectus. Med Hypotheses 2015, 85, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Steinman, G.; Mankuta, D. Insulin-like growth factor and the etiology of autism. Med Hypotheses 2013, 80, 475–480. [Google Scholar] [CrossRef]
- Stilling, R.M.; Bordenstein, S.R.; Dinan, T.G.; Cryan, J.F. Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Front. Cell. Infect. Microbiol. 2014, 4, 147–147. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S.; Keelan, J.A. Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit. Rev. Microbiol. 2016, 43, 352–369. [Google Scholar] [CrossRef]
- Storch, E.A.; Arnold, E.B.; Lewin, A.B.; Nadeau, J.M.; Jones, A.M.; De Nadai, A.S.; Mutch, P.J.; Selles, R.R.; Ung, D.; Murphy, T.K. The Effect of Cognitive-Behavioral Therapy Versus Treatment as Usual for Anxiety in Children With Autism Spectrum Disorders: A Randomized, Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 132–142.e2. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Streng, M.L.; Krook-Magnuson, E. The cerebellum and epilepsy. Epilepsy Behav. 2021, 121, 106909–106909. [Google Scholar] [CrossRef]
- Stubbs, E.G.; Crawford, M.L.; Burger, D.R.; Vandenbark, A.A. Depressed lymphocyte responsiveness in autistic children. J. Autism Child. Schizophr. 1977, 7, 49–55. [Google Scholar] [CrossRef]
- Su, L.-D.; Xu, F.-X.; Wang, X.-T.; Cai, X.-Y.; Shen, Y. Cerebellar Dysfunction, Cerebro-cerebellar Connectivity and Autism Spectrum Disorders. Neuroscience 2021, 462, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sugihara, G.; Ouchi, Y.; Nakamura, K.; Futatsubashi, M.; Takebayashi, K.; Yoshihara, Y.; Omata, K.; Matsumoto, K.; Tsuchiya, K.J.; et al. Microglial Activation in Young Adults With Autism Spectrum Disorder. JAMA Psychiatry 2013, 70, 49–58. [Google Scholar] [CrossRef]
- Swatzyna, R.J.; Boutros, N.N.; Genovese, A.C.; MacInerney, E.K.; Roark, A.J.; Kozlowski, G.P. Electroencephalogram (EEG) for children with autism spectrum disorder: evidential considerations for routine screening. Eur. Child Adolesc. Psychiatry 2018, 28, 615–624. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Sweeten, T.L.; Posey, D.J.; Shekhar, A.; McDougle, C.J. The amygdala and related structures in the pathophysiology of autism. Pharmacol. Biochem. Behav. 2002, 71, 449–455. [Google Scholar] [CrossRef]
- Tahmasebinia, F.; Pourgholaminejad, A. The role of Th17 cells in auto-inflammatory neurological disorders. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2017, 79, 408–416. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, A.; Emon, N.U.; Islam, M.; Hong, S.-T.S.; Podder, B.R.; Mimi, A.A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef]
- Tarabeux, J.; Kebir, O.; Gauthier, J.; Hamdan, F.F.; Xiong, L.; Piton, A.; Spiegelman, D.; Henrion, É.; Millet, B.; Fathalli, F.; et al. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl. Psychiatry 2011, 1, e55–e55. [Google Scholar] [CrossRef]
- Tarnowska, K.; Gruczyńska–Sękowska, E.; Kowalska, D.; Majewska, E.; Kozłowska, M.; Winkler, R. The opioid excess theory in autism spectrum disorders - is it worth investigating further? Crit. Rev. Food Sci. Nutr. 2021, 63, 3980–3993. [Google Scholar] [CrossRef]
- Tartaglione, A.; Camoni, L.; Calamandrei, G.; Chiarotti, F.; Venerosi, A. The contribution of environmental pollutants to the risk of autism and other neurodevelopmental disorders: A systematic review of case-control studies. Neurosci. Biobehav. Rev. 2024, 164, 105815. [Google Scholar] [CrossRef]
- Terrabuio, E.; Zenaro, E.; Constantin, G. The role of the CD8+ T cell compartment in ageing and neurodegenerative disorders. Front. Immunol. 2023, 14, 1233870. [Google Scholar] [CrossRef]
- Tetreault, N.A.; Hakeem, A.Y.; Jiang, S.; Williams, B.A.; Allman, E.; Wold, B.J.; Allman, J.M. Microglia in the Cerebral Cortex in Autism. J. Autism Dev. Disord. 2012, 42, 2569–2584. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 2017, 18, 716–724. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844–e844. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Angelidou, A.; Alysandratos, K.-D.; Zhang, B.; Asadi, S.; Francis, K.; Toniato, E.; Kalogeromitros, D. Mast cell activation and autism. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2012, 1822, 34–41. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Doyle, R. Autism, Gut-Blood-Brain Barrier, and Mast Cells. J. Clin. Psychopharmacol. 2008, 28, 479–483. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kavalioti, M.; Tsilioni, I. Mast Cells, Stress, Fear and Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 3611. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M.; Panagiotidou, S.; Melamed, I. Mast cells, brain inflammation and autism. Eur. J. Pharmacol. 2016, 778, 96–102. [Google Scholar] [CrossRef]
- Thomson, A.R.; Pasanta, D.; Arichi, T.; Puts, N.A. Neurometabolite differences in Autism as assessed with Magnetic Resonance Spectroscopy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2024, 162, 105728. [Google Scholar] [CrossRef]
- Tillisch, K.; Wang, Z.; Kilpatrick, L.; Holschneider, D.P.; Mayer, E.A. Studying the Brain–Gut Axis with Pharmacological Imaging. Ann. New York Acad. Sci. 2008, 1144, 256–264. [Google Scholar] [CrossRef]
- Tillmann, T.; Gibson, A.R.; Scott, G.; Harrison, O.; Dominiczak, A.; Hanlon, P. Systems Medicine 2.0: Potential Benefits of Combining Electronic Health Care Records With Systems Science Models. J. Med Internet Res. 2015, 17, e64–e64. [Google Scholar] [CrossRef]
- Toledano, M.; Pittock, S.J. Autoimmune Epilepsy. Semin. Neurol. 2015, 35, 245–258. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Toomey, R.; Kang, H.K.; Karlinsky, J.; Baker, D.G.; Vasterling, J.J.; Alpern, R.; Reda, D.J.; Henderson, W.G.; Murphy, F.M.; Eisen, S.A. Mental health of US Gulf War veterans 10 years after the war. Br. J. Psychiatry 2007, 190, 385–393. [Google Scholar] [CrossRef]
- Tordjman, S.; Celume, M.; Denis, L.; Motillon, T.; Keromnes, G. Reframing schizophrenia and autism as bodily self-consciousness disorders leading to a deficit of theory of mind and empathy with social communication impairments. Neurosci. Biobehav. Rev. 2019, 103, 401–413. [Google Scholar] [CrossRef]
- Tordjman, S.; Somogyi, E.; Coulon, N.; Kermarrec, S.; Cohen, D.; Bronsard, G.; Bonnot, O.; Weismann-Arcache, C.; Botbol, M.; Lauth, B.; et al. Gene-Environment Interactions in Autism Spectrum Disorders: Role of Epigenetic Mechanisms. Front. Psychiatry 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.B.; Denisova, K. Motor noise is rich signal in autism research and pharmacological treatments. Sci. Rep. 2016, 6, 37422. [Google Scholar] [CrossRef]
- Torres, E.B.; Varkey, H.; Vero, J.; London, E.; Phan, H.; Kittler, P.; Gordon, A.; E Delgado, R.; Delgado, C.F.; A Simpson, E.; et al. Sensing echoes: temporal misalignment in auditory brainstem responses as the earliest marker of neurodevelopmental derailment. PNAS Nexus 2023, 2, pgac315. [Google Scholar] [CrossRef]
- Tuchman, R.; Moshé, S.L.; Rapin, I. Convulsing toward the pathophysiology of autism. Brain Dev. 2008, 31, 95–103. [Google Scholar] [CrossRef]
- Tzang, R.-F.; Chang, C.-H.; Chang, Y.-C.; Lane, H.-Y. Autism Associated With Anti-NMDAR Encephalitis: Glutamate-Related Therapy. Front. Psychiatry 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Üçok, A.; Gaebel, W. Side effects of atypical antipsychotics: a brief overview. World Psychiatry 2008, 7, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P. Neural synchrony in cortical networks: history, concept and current status. Front. Integr. Neurosci. 2009, 3, 17. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron 2006, 52, 155–168. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113. [Google Scholar] [CrossRef]
- Ullah, F.; Kaelber, D.C. Using Large Aggregated De-Identified Electronic Health Record Data to Determine the Prevalence of Common Chronic Diseases in Pediatric Patients Who Visited Primary Care Clinics. Acad. Pediatr. 2021, 21, 1084–1093. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Vakilzadeh, G.; Martinez-Cerdeño, V. Pathology and Astrocytes in Autism. Neuropsychiatr. Dis. Treat. 2023, ume 19, 841–850. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, G. Understanding Schizophrenia as a Disorder of Consciousness: Biological Correlates and Translational Implications from Quantum Theory Perspectives. Clin. Psychopharmacol. Neurosci. 2015, 13, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef]
- Vicedo, M. Moving beyond the search for the first discoverer of autism. Front. Psychiatry 2024, 15, 1266486. [Google Scholar] [CrossRef]
- Victoria, H.K.; Caldwell, C. Breathwork in body psychotherapy: Clinical applications. Body, Mov. Dance Psychother. 2013, 8, 216–228. [Google Scholar] [CrossRef]
- Vineis, P.; Robinson, O.; Chadeau-Hyam, M.; Dehghan, A.; Mudway, I.; Dagnino, S. What is new in the exposome? Environ. Int. 2020, 143, 105887. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Vojdani, A.; Mumper, E.; Granpeesheh, D.; Mielke, L.; Traver, D.; Bock, K.; Hirani, K.; Neubrander, J.; Woeller, K.N.; O'HAra, N.; et al. Low natural killer cell cytotoxic activity in autism: The role of glutathione, IL-2 and IL-15. J. Neuroimmunol. 2008, 205, 148–154. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Nelson, D.S. Seizure Disorders in Autism. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef]
- Wakefield, A.J. The Gut–Brain Axis in Childhood Developmental Disorders. J. Pediatr. Gastroenterol. Nutr. 2002, 34, S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.J.; Puleston, J.M.; Montgomery, S.M.; Anthony, A.; O'LEary, J.J.; Murch, S.H. The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Aliment. Pharmacol. Ther. 2002, 16, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Hill, C.; Ross, R.P. Impact of glyphosate (Roundup TM ) on the composition and functionality of the gut microbiome. Gut Microbes 2023, 15, 2263935. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Wang, T.; Sternes, P.R.; Guo, X.-K.; Zhao, H.; Xu, C.; Xu, H. Autoimmune diseases exhibit shared alterations in the gut microbiota. Rheumatology 2023, 63, 856–865. [Google Scholar] [CrossRef]
- Ward, J.H.; Weir, E.; Allison, C.; Baron-Cohen, S. Increased rates of chronic physical health conditions across all organ systems in autistic adolescents and adults. Mol. Autism 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Ward, L.M. The thalamic dynamic core theory of conscious experience. Conscious. Cogn. 2011, 20, 464–486. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.P.; Foster, A.; Margaretten, N.C. Reduced Natural Killer Cell Activity in Autism. J. Am. Acad. Child Adolesc. Psychiatry 1987, 26, 333–335. [Google Scholar] [CrossRef]
- Wegiel, J.; Chadman, K.; London, E.; Wisniewski, T.; Wegiel, J. Contribution of the serotonergic system to developmental brain abnormalities in autism spectrum disorder. Autism Res. 2024, 17, 1300–1321. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the Regulation of Innate and Adaptive Immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Weitlauf, A.S.; Gotham, K.O.; Vehorn, A.C.; Warren, Z.E. Brief Report: DSM-5 “Levels of Support:” A Comment on Discrepant Conceptualizations of Severity in ASD. J. Autism Dev. Disord. 2013, 44, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Alshikho, M.J.; Herbert, M.R.; Hu, V.W. Pathway Network Analyses for Autism Reveal Multisystem Involvement, Major Overlaps with Other Diseases and Convergence upon MAPK and Calcium Signaling. PLOS ONE 2016, 11, e0153329–e0153329. [Google Scholar] [CrossRef]
- Wen, Y.; Herbert, M.R. Connecting the dots: Overlaps between autism and cancer suggest possible common mechanisms regarding signaling pathways related to metabolic alterations. Med Hypotheses 2017, 103, 118–123. [Google Scholar] [CrossRef]
- Werling, D.M. The role of sex-differential biology in risk for autism spectrum disorder. Biol. Sex Differ. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.; Dawson, G. Validation of the Phenomenon of Autistic Regression Using Home Videotapes. Arch. Gen. Psychiatry 2005, 62, 889–895. [Google Scholar] [CrossRef]
- West, L.; Brunssen, S.H.; Waldrop, J. Review of the Evidence for Treatment of Children with Autism with Selective Serotonin Reuptake Inhibitors. J. Spéc. Pediatr. Nurs. 2009, 14, 183–191. [Google Scholar] [CrossRef]
- West, L.; Waldrop, J.; Brunssen, S. Pharmacologic Treatment for the Core Deficits and Associated Symptoms of Autism in Children. J. Pediatr. Heal. Care 2009, 23, 75–89. [Google Scholar] [CrossRef]
- White, J.F. Intestinal Pathophysiology in Autism. Exp. Biol. Med. 2003, 228, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.; Carr, K.; Shattock, P. Is Autism Inborn And Lifelong For Everyone? Neuropsychiatr. Dis. Treat. 2019, ume 15, 2885–2891. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiology Biomarkers Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Willison, H.J.; Jacobs, B.C.; A van Doorn, P. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Wills, S.; Cabanlit, M.; Bennett, J.; Ashwood, P.; Amaral, D.G.; Van de Water, J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain, Behav. Immun. 2008, 23, 64–74. [Google Scholar] [CrossRef]
- Witkamp, R.F.; van Norren, K. Let thy food be thy medicine….when possible. Eur. J. Pharmacol. 2018, 836, 102–114. [Google Scholar] [CrossRef]
- Wood, J.D.; Alpers, D.H.; Andrews, P.L.R. Fundamentals of neurogastroenterology. Gut 1999, 45, ii6–ii16. [Google Scholar] [CrossRef]
- Wood, J.J.; Kendall, P.C.; Wood, K.S.; Kerns, C.M.; Seltzer, M.; Small, B.J.; Lewin, A.B.; Storch, E.A. Cognitive Behavioral Treatments for Anxiety in Children With Autism Spectrum Disorder. JAMA Psychiatry 2020, 77, 474–483. [Google Scholar] [CrossRef]
- Wu, M.; Zheng, W.; Song, X.; Bao, B.; Wang, Y.; Ramanan, D.; Yang, D.; Liu, R.; Macbeth, J.C.; Do, E.A.; et al. Gut complement induced by the microbiota combats pathogens and spares commensals. Cell 2024, 187, 897–913.e18. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Li, Y. Microglia and astrocytes underlie neuroinflammation and synaptic susceptibility in autism spectrum disorder. Front. Neurosci. 2023, 17, 1125428. [Google Scholar] [CrossRef]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of Food Allergy and Other Allergic Conditions With Autism Spectrum Disorder in Children. JAMA Netw. Open 2018, 1, e180279–e180279. [Google Scholar] [CrossRef]
- Xu, S.-C.; Zhong, Y.; Jiang, H.-Y.; Tang, J. Exposure to anti-seizure medication during pregnancy and the risk of autism and ADHD in offspring: a systematic review and meta-analysis. Front. Neurol. 2024, 15, 1440145. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.R.; Arici, M.K.; Demirel, H.C.; Tsai, C.-J.; Jang, H.; Nussinov, R.; Tuncbag, N. Neurodevelopmental disorders and cancer networks share pathways, but differ in mechanisms, signaling strength, and outcome. npj Genom. Med. 2023, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Heinrich, M.; Kijjoa, A.; Tzvetkov, N.T.; Atanasov, A.G. The ethnopharmacological literature: An analysis of the scientific landscape. J. Ethnopharmacol. 2020, 250, 112414. [Google Scholar] [CrossRef]
- Yngve, M.; Lidström, H. Implementation of information and communication technology to facilitate participation in high school occupations for students with neurodevelopmental disorders. Disabil. Rehabilitation: Assist. Technol. 2023, 19, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Rivest, S. Taking Advantage of the Systemic Immune System to Cure Brain Diseases. Neuron 2009, 64, 55–60. [Google Scholar] [CrossRef]
- Zanchi, M.M.; Marins, K.; Zamoner, A. Could pesticide exposure be implicated in the high incidence rates of depression, anxiety and suicide in farmers? A systematic review. Environ. Pollut. 2023, 331, 121888. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Peeva, E.; Shoenfeld, Y. Gender and autoimmunity. Autoimmun. Rev. 2007, 6, 366–372. [Google Scholar] [CrossRef]
- Zarate-Lopez, D.; Torres-Chávez, A.L.; Gálvez-Contreras, A.Y.; Gonzalez-Perez, O. Three Decades of Valproate: A Current Model for Studying Autism Spectrum Disorder. Curr. Neuropharmacol. 2024, 22, 260–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M. , & An, J. ( 45(2), 27–37. [CrossRef]
- Zhang, S.; Han, F.; Wang, Q.; Fan, F. Probiotics and Prebiotics in the Treatment of Autism Spectrum Disorder: A Narrative Review. J. Integr. Neurosci. 2024, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Ziv, Y.; Schwartz, M. Immune-based regulation of adult neurogenesis: Implications for learning and memory. Brain, Behav. Immun. 2008, 22, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Riederer, A.M.; Ettinger, A.S.; A Schaider, L.; Shine, J.P.; Amarasiriwardena, C.J.; O Wright, R.; Spengler, J.D. Associations between metals in residential environmental media and exposure biomarkers over time in infants living near a mining-impacted site. J. Expo. Sci. Environ. Epidemiology 2015, 26, 510–519. [Google Scholar] [CrossRef]
- Zürcher, N.R.; Bhanot, A.; McDougle, C.J.; Hooker, J.M. A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: Current state and future research opportunities. Neurosci. Biobehav. Rev. 2015, 52, 56–73. [Google Scholar] [CrossRef]
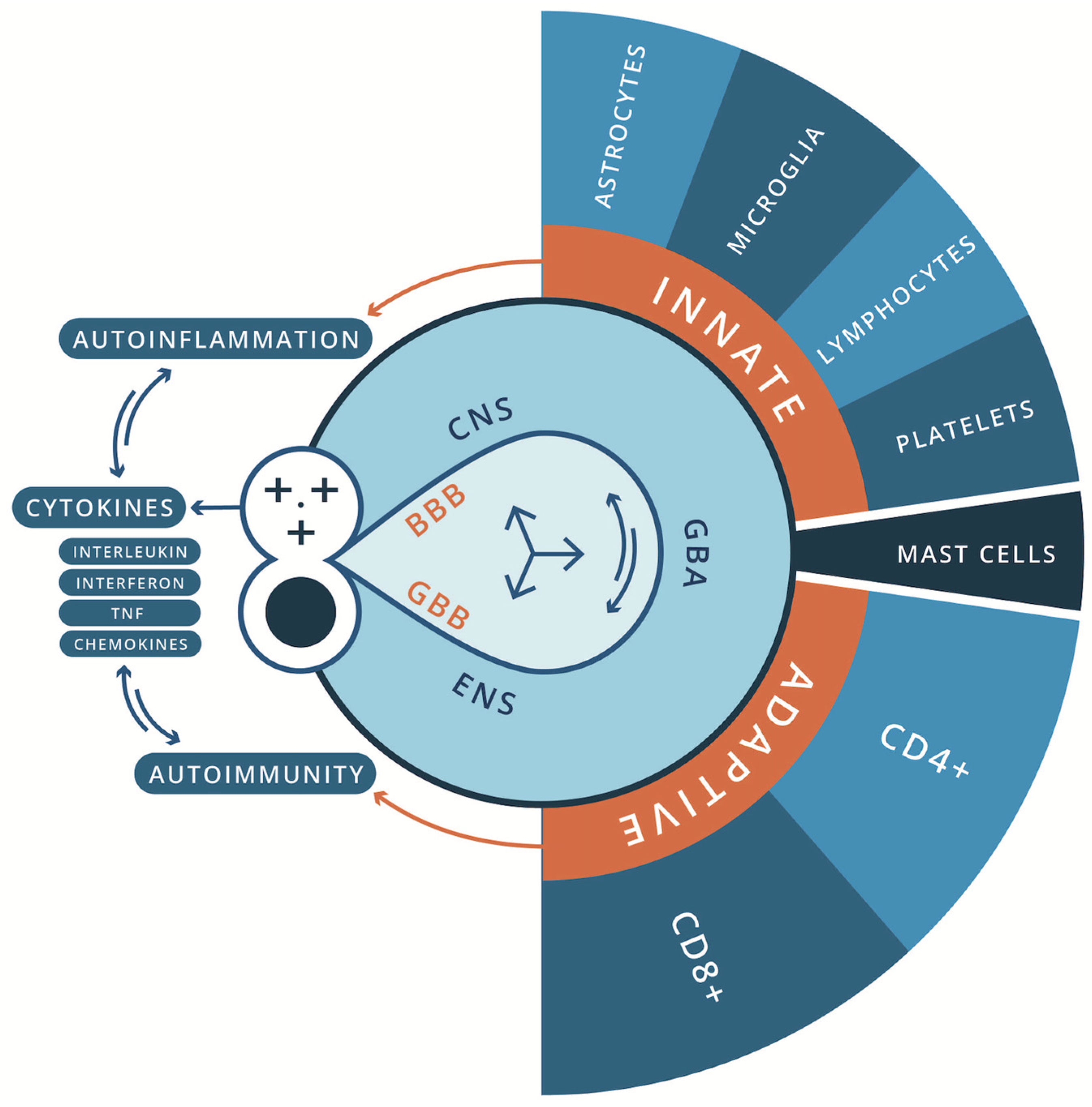
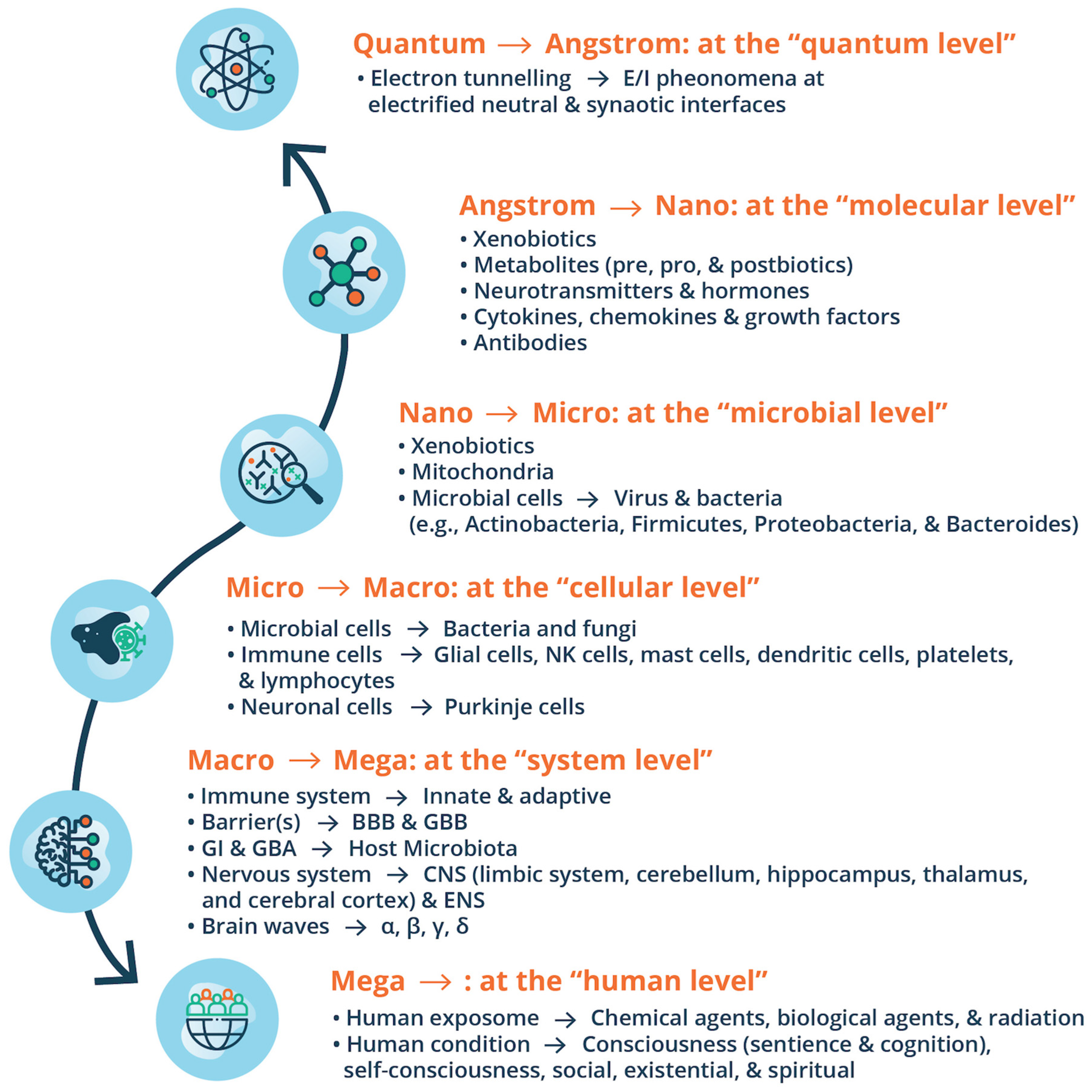
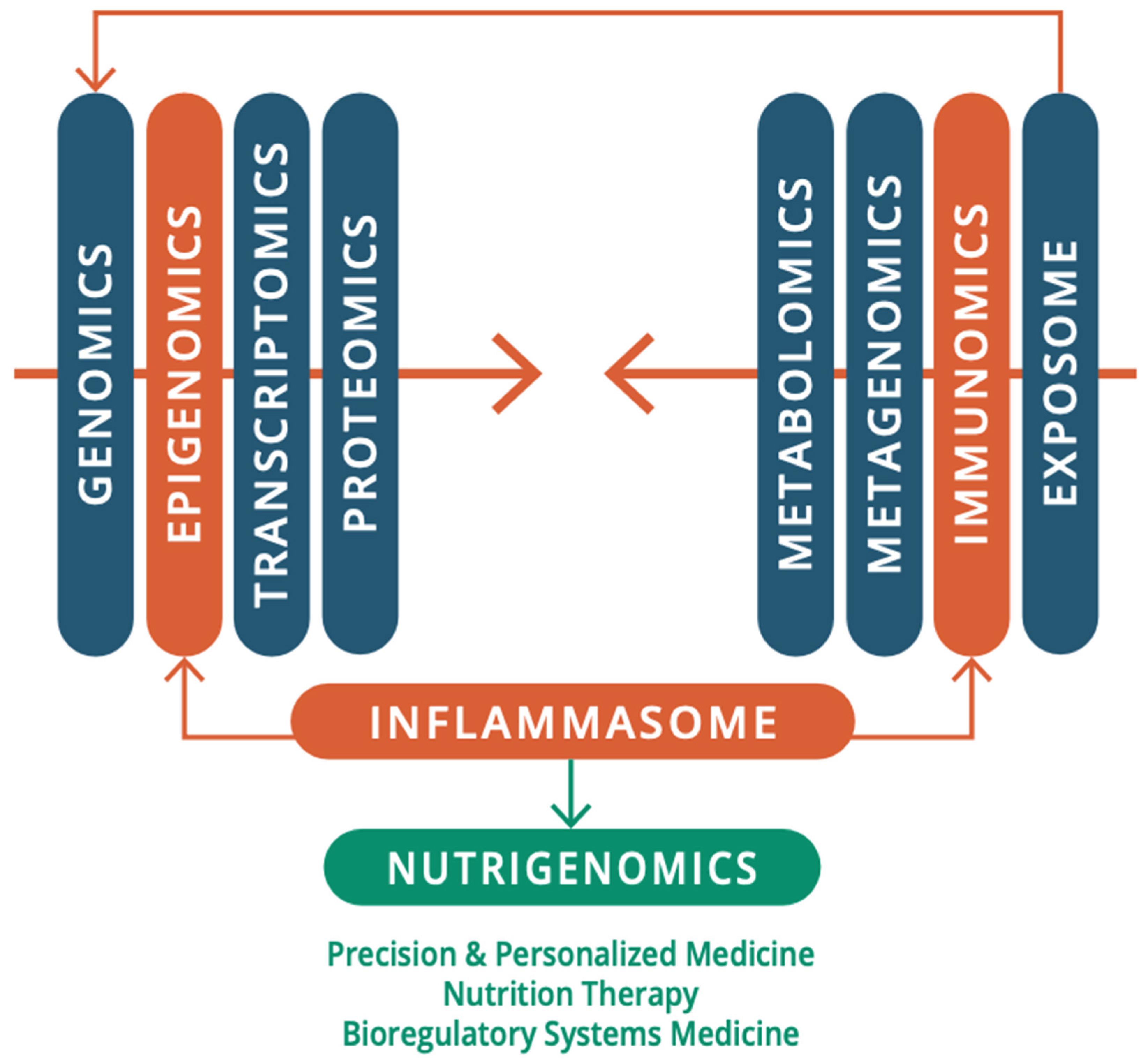
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




