Submitted:
07 November 2024
Posted:
07 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fruits Harvesting and Post-Harvesting
2.2. Sorting and Grading
2.3. Juice Extraction
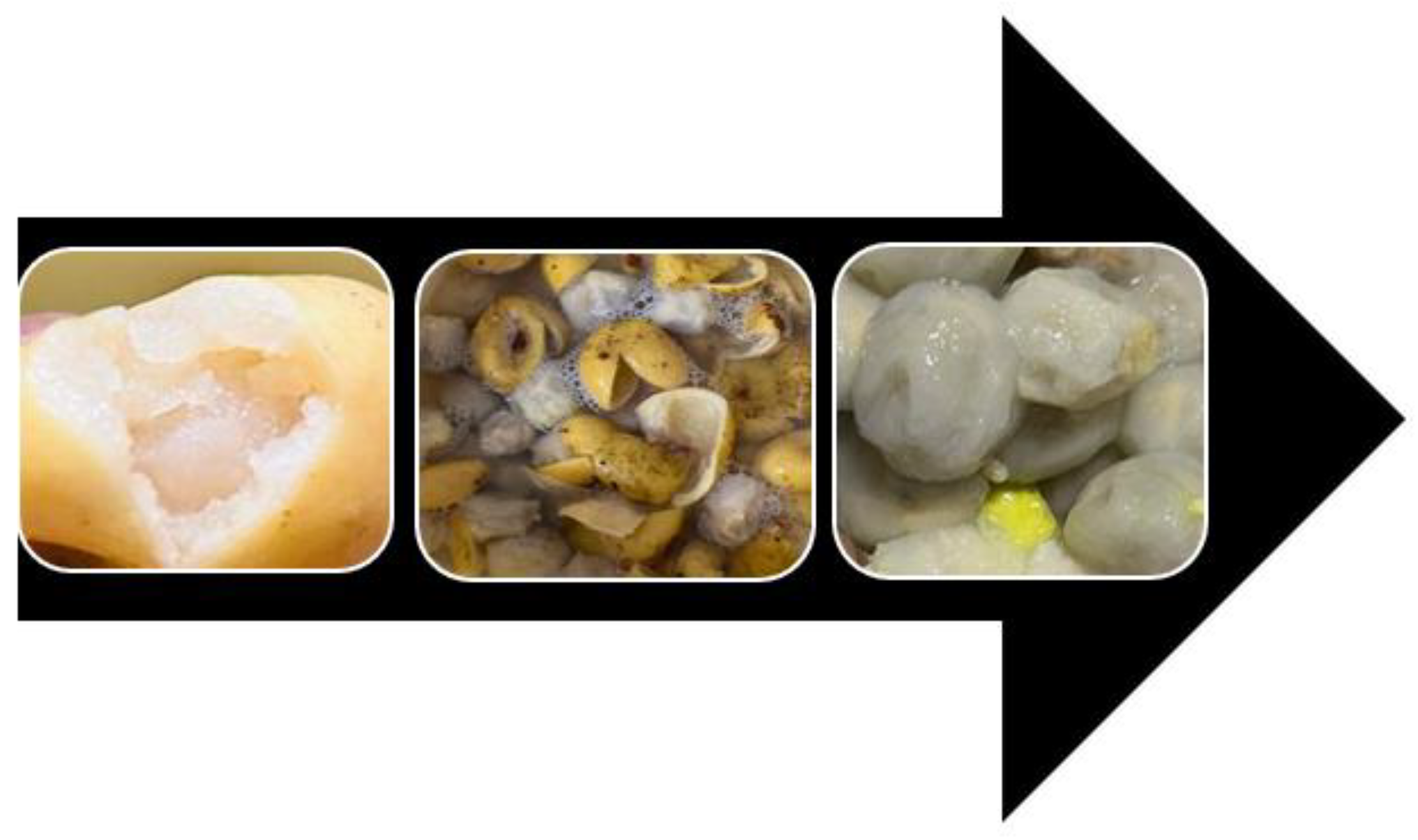
2.4. Sample Preparation Methods
| Beverage 1 (Sample 1) | Beverage 2 (Sample 2) | Beverage 3 (Sample 3) |
|---|---|---|
|
Ingredients: • Provitamin A Biofortified Maize (PVABM) • Marula juice (for fermentation and blending) • Clean water (for washing the grains) Method • In a clean container, combine PVABM with extracted Marula juice. Cover and allow the mixture to ferment at room temperature for 3 to 4 days. • After fermentation, the grains should be swollen and ready for further processing. Rinse the fermented grains thoroughly with clean water. • Heat a pan over medium heat. Add the washed grains and roast them until they turn golden brown. • Transfer the roasted grains to an electric blender. Add Marula juice and blend until smooth. • Sift the blended mixture to remove any coarse particles. Cover and let it ferment for an additional 3 days at room temperature. • After the second fermentation, refrigerate the mixture into clean glass bottles. |
Ingredients: Provitamin A Biofortified Maize grains Marula juice (For fermentation, cooking and cooling) Method • Using an electric miller or blender, grind Provitamin A Biofortified Maize grains into a fine powder. • In a clean container, mix the ground maize powder with 1 cup of extracted Marula juice. Cover and let the mixture ferment for 3 to 4 days at room temperature. This traditional fermentation process is known as "ukuncwancwisa," according to Indigenous Knowledge (IK) experts. • After 4 days of fermentation, transfer the wet maize mixture to a pot. Add Marula juice and cook over medium heat, stirring occasionally, until a soft porridge forms. • Once the porridge is cooked, gradually add cold Marula juice to cool it down and achieve a smooth, pouring consistency. • Pour the cooled porridge into a clean glass bottle. Seal and refrigerate for further use. |
• Ingredients: • Provitamin A Biofortified Maize grains • Marula juice (For fermentation and blending) • Clean water • Method • In a large container, add Marula juice to yellow maize and allow the mixture to ferment for 3 to 4 days at room temperature. • Wash the Fermented Maize**: • After fermentation, wash the maize thoroughly with clean running water to prepare for blending. • Place the fermented maize in a blender and add some Marula juice. Blend until a very smooth consistency is achieved. • Sift the blended maize to remove any unwanted chaff. Set aside the smooth mixture. • Let the mixture stand for 2 hours. During this time, it will separate into water and settle solids. • Carefully decant the water, leaving behind the settled part of the mixture. • Place the settled mixture into a muslin cloth and allow it to drain for 3 hours to release any remaining water. • Spread the drained mixture in an oven at 70°C with a fan for 3 hours to dehydrate. Alternatively, you can use a dehydrator or sun drying to achieve the same result. • Once dried, process the mixture using a dry miller or blender. Sift the resulting powder to ensure a fine consistency. • Store the powder in an airtight container or freezer for future use. • To make the final product, cook the powder in Marula juice over medium heat, stirring until a thick, drinkable consistency is achieved. • Allow the mixture to cool. Transfer to a glass bottle and refrigerate for further use. |
2.5. Nutritional Analysis
Vitamin A Content Profile Determination of Fermented Beverages
2.6. Minerals Profile Determination of Fermented Beverage
2.7. Amino Acid Profile Determination of Fermented Beverage
2.8. Sensory and Consumer Acceptability
2.9. Alcohol Content Determination in the Samples
2.10. Data Analysis
3. Results
3.1. Vitamin A Analysis of the Developed Beverages
| Test (Vitamin A (Retinol) |
Results [±Uncertainty] |
Units | Limits | LOQ |
|---|---|---|---|---|
| Sample 1 | < 50 | μg/100g | - | 50 |
| Sample 2 | < 50 | μg/100g | - | 50 |
| Sample 3 | < 50 | μg/100g | - | 50 |
3.2. Consumer Acceptability of the Fermented Non-Alcoholic Beverages Developed
| Sample ID: | Colour | Taste | Aroma | Thickness | No Lumps | Overall |
|---|---|---|---|---|---|---|
| Ave/STDV | Ave/STDV | Ave/STDV | Ave/STDV | Ave/STDV | Ave/STDV | |
| 1 | 4.05±1.14a | 3.65±1.16a | 3.45±1.26a | 3.83±1.01a | 3.80±1.33a | 3.95±1.01a |
| 2 | 3.49±1.52a | 3.26±1.16a | 3.18±1.39a | 3.78±1.37a | 3.71±1.45a | 3.40±1.31a |
| 3 | 4.00±1.15a | 3.29±1.16a | 3.58±1.17a | 3.74±1.25a | 3.88±1.19a | 3.77±1.00a |
| His |
Arg | Ser | Gly | Asp | Glu | Thr | Ala | Pro | Lys | Tyr | Met | Val | ILe | Leu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID |
mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg |
| 1 |
162,608 | 293,441 | 323,941 | 235,167 | 408,739 | 1446,506 | 219,815 | 589,229 | 777,83 | 203,747 | 361,566 | 92,373 | 291,309 | 242,616 | 1008,326 |
| 2 |
521,246 | 967,454 | 1213,703 | 736,675 | 1380,063 | 5312,344 | 835,091 | 2147,56 | 2462,819 | 504,686 | nd | 82,319 | 1093,02 | 901,447 | 3722,275 |
| 3 |
181,859 | 429,448 | 436,109 | 355,074 | 600,909 | 1934,364 | 331,016 | 817,788 | 1062,586 | 268,46 | 164,671 | nd | 398,461 | 332,799 | 1298,06 |
3.3. Minerals Profile Determination of Fermented Beverage
| Sample ID |
LIMS No |
ADF | amm | Ash | Ca | Cu | Fat | Fe | K | K/Ca+Mg | lac |
| % | % | % | % | ppm | % | ppm | % | % | % | ||
| 1 | 31466 | 1.84±0.01a | 0.07±0.005a | 2.64±0.01a | 0.07±0.005a | 4±1.00b | 1.47±0.01a | 30±1.00b | 0.92±0.01a | 2.16±0.01a | 2.12±0.01a |
| 2 | 31467 | 2.08±0.01a | 0.07±0.005a | 2.18±0.01a | 0.05±0.005a | 3±1.00b | 2.93±0.01a | 53±1.00b | 0.62±0.01a | 2.13±0.01a | 1.82±0.01a |
| 3 | 31468 | 3.33±0.01a | 0.06±0.005a | 3.15±0.01a | 0.05±0.005a | 31±1.00b | 1.68±0.01a | 26±1.00b | 0.55±0.01a | 2.13±0.01a | 2.15±0.01a |
| Sample ID |
LIMS No |
Mg | Mn | Moisture | Na | NDF | NPN | P | Protein | Starch | Zn |
| % | ppm | % | % | % | % | % | % | % | ppm | ||
| 1 | 31466 | 0.09±0.006a | 2±0.50b | 6.77±0.01a | 0.05±0.006a | 9.80±0.01a | 0.11±0.01a | 0.17±0.006a | 8.78±0.01a | 46.84±0.01a | 12±0.60b |
| 2 | 31467 | 0.06±0.006a | 1±0.006a | 5.50±0.01a | 0.04±0.006a | 10.66±0.01a | 0.10±0.01a | 0.13±0.006a | 9.52±0.01a | 44.79±0.01a | 9±0.58b |
| 3 | 31468 | 0.05±0.006a | 1±0.006a | 1.14±0.01a | 0.03±0.006a | 11.53±0.01a | 1.02±0.01a | 0.12±0.006a | 9.55±0.01a | 33.76± | 21±1.00b |
4. Discussion
5. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical clearance
References
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition quality of food purchases varies by household income: the SHoPPER study. BMC public health 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Bader-Ul-Ain, H.; Abbas, M.; Saeed, F.; Khalid, S.; Suleria, H.A.R. Functional nonalcoholic beverages: A global trend toward a healthy life. In Non-Alcoholic Beverages, Elsevier: 2019; pp. 73-105.
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A. Functional foods and nondairy probiotic food development: trends, concepts, and products. Comprehensive reviews in food science and food safety 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends in Food Science & Technology 2019, 88, 513–526. [Google Scholar]
- Takaidza, S. Indigenous South African Food: Nutrition and Health Benefits. 2023.
- Sileshi, G.W.; Dagar, J.C.; Akinnifesi, F.K.; Mng’omba, S.A. Potentials of Indigenous Fruit Trees in Enhancing Nutrition, Income and Biodiversity Conservation in African Agroforestry. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa, Springer: 2023; pp. 321-361.
- Msangi, J.P.; Msangi, J.P. Indigenous plant resources and food security among small-scale agricultural producers: Southern Africa. Food Security Among Small-Scale Agricultural Producers in Southern Africa 2014, 75-104.
- Dorothy, M.Z.; Suinyuy, T.N.; Lubaale, J.; Peter, B.O. Physicochemical properties and antioxidant activities of marula fruit (Sclerocarya birrea subsp. Caffra) steamed and boiled before juice extraction. Food Science & Nutrition 2023, 11, 4607–4615. [Google Scholar]
- Mokoena, D.Z.; Suinyuy, T.N.; Lubaale, J.; Peter, B.O. Physicochemical properties and antioxidant activities of marula fruit (Sclerocarya birrea subsp. Caffra) steamed and boiled before juice extraction. Food Science & Nutrition 2023, 11, 4607–4615. [Google Scholar] [CrossRef]
- Nwonwu, F.O. The socio-economic and economic relevance of the Marula tree and its sustainable use in South Africa. Africa Insight 2006, 36, 249–265. [Google Scholar]
- Murye, A.F.; Pelser, A.J. Commercial harvesting of marula (Sclerocarya birrea) in Swaziland: A quest for sustainability. In Selected Studies in Biodiversity, IntechOpen: 2018.
- Maluleke, E. CHARACTERISATION OF THE MICROORGANISMS AND DETERMINATION OF THE CHEMICAL CONSTITUENTS OF MARULA BREWS DURING FERMENTATION. University of Limpopo, Limpopo, 2019.
- Ndlovu, P.F. The development of indigenous marula (sclerocarya birrea) fruit leather: effect of drying temperature and sugar concentration on the drying characteristics, physico-chemical and consumer sensory properties of marula fruit leathers. 2016.
- Rampedi, I.T. Indigenous plants in the Limpopo Province: Potential for their commercial beverage production. University of South Africa Pretoria, 2010.
- Awobusuyi, T.D. Quality and storage stability of provitamin A biofortified amahewu, a non-alcoholic cereal beverage. 2015.
- Khush, G.S.; Lee, S.; Cho, J.-I.; Jeon, J.-S. Biofortification of crops for reducing malnutrition. Plant biotechnology reports 2012, 6, 195–202. [Google Scholar] [CrossRef]
- Zuma, M.K.; Kolanisi, U.; Modi, A.T. The potential of integrating provitamin A-biofortified maize in smallholder farming systems to reduce malnourishment in South Africa. International journal of environmental research and public health 2018, 15, 805. [Google Scholar] [CrossRef]
- Razzaq, A.; Tang, Y.; Qing, P. Towards sustainable diets: Understanding the cognitive mechanism of consumer acceptance of biofortified foods and the role of nutrition information. International Journal of Environmental Research and Public Health 2021, 18, 1175. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.; Winter-Nelson, A. Consumer acceptance of provitamin A-biofortified maize in Maputo, Mozambique. Food Policy 2008, 33, 341–351. [Google Scholar] [CrossRef]
- Ndwandwe, N.K. Sensory quality of provitamin A biofortified maize-based foods and the effect of a provitamin A biofortified maize awareness campaign on their acceptance in KwaZulu-Natal, South Africa. 2018.
- Awobusuyi, T.D.; Siwela, M.; Kolanisi, U.; Amonsou, E.O. Provitamin A retention and sensory acceptability of amahewu, a non-alcoholic cereal-based beverage made with provitamin A-biofortified maize. Journal of the Science of Food and Agriculture 2016, 96, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- De Groote, H.; Kimenju, S.C. Comparing consumer preferences for color and nutritional quality in maize: Application of a semi-double-bound logistic model on urban consumers in Kenya. Food policy 2008, 33, 362–370. [Google Scholar] [CrossRef]
- Pillay, K.; Siwela, M.; Derera, J.; Veldman, F.J. Provitamin A carotenoids in biofortified maize and their retention during processing and preparation of South African maize foods. Journal of food science and technology 2014, 51, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Applied microbiology and biotechnology 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Ravyts, F.; Vuyst, L.D.; Leroy, F. Bacterial diversity and functionalities in food fermentations. Engineering in Life Sciences 2012, 12, 356–367. [Google Scholar] [CrossRef]
- Khaleghi, M.K.; Savizi, I.S.P.; Lewis, N.E.; Shojaosadati, S.A. Synergisms of machine learning and constraint-based modeling of metabolism for analysis and optimization of fermentation parameters. Biotechnology Journal 2021, 16, 2100212. [Google Scholar] [CrossRef]
- Fandos, C.; Flavian, C. Intrinsic and extrinsic quality attributes, loyalty and buying intention: an analysis for a PDO product. British food journal 2006. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.d.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Comprehensive reviews in food science and food safety 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Pillay, K.; Derera, J.; Siwela, M.; Veldman, F. Consumer acceptance of yellow, provitamin A-biofortified maize in KwaZulu-Natal. South African Journal of Clinical Nutrition 2011, 24, 186–191. [Google Scholar] [CrossRef]
- Oyeyinka, A.T.; Pillay, K.; Siwela, M. Full title- In vitro digestibility, amino acid profile and antioxidant activity of cooked Bambara groundnut grain. Food Bioscience 2019, 31, 100428. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. AOAC International: Gaithersburg. MD, USA 2005, 18. [Google Scholar]
- Thiex, N.J.; Anderson, S.; Gildemeister, B.; F, C.A.W.B.J.B.E.C.R.C.K.D.A.F.E.G.M.H.P.K.T.M.J.l.J.R.R.R.M.S. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): collaborative study. Journal of AOAC International 2003, 86, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Tsomele, G.F.; Meiring, B.; Anyasi, T.A.; Mlambo, V.; Amonsou, E.; Lepule, S.P.; Siwela, M.; Wokadala, O.C. Influence of amino acid profile and secondary structure on nutritional and functional properties of Trichilia emetica and Trichilia dregeana protein concentrates. International Journal of Food Science & Technology 2023, 58, 5489–5500. [Google Scholar]
- Curtis, P.C. Chapter 12: Untrained sensory panels. Kerth, C., Ed. The science of meat quality, Wiley online library: 2013. [CrossRef]
- Qahtan, S.; Alsattar, H.A.; Zaidan, A.A.; Deveci, M.; Pamucar, D.; Martinez, L. A comparative study of evaluating and benchmarking sign language recognition system-based wearable sensory devices using a single fuzzy set. Knowledge-Based Systems 2023, 269, 110519. [Google Scholar] [CrossRef]
- Vargas, H.C.; Aggabao, C.M. Organoleptic Evaluation of Mixed Powdered Cotton Fruit (Sandoricum Koetjape) and Rattan Fruit (Calamus Manillensis) as Souring Agent. 2023.
- Ngwenya, M.; Nkambule, T.; Kidane, S. Physicochemical Attributes and Acceptability of Marula Wine Fermented with Natural Lactobacillus Plantarum and Saccharomyces Cerevisiae. Available at SSRN 4408467 2023. [Google Scholar]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: a paramount staple crop in the context of global nutrition. Comprehensive reviews in food science and food safety 2010, 9, 417–436. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of vitamin A in the immune system. Journal of clinical medicine 2018, 7, 258. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Carotenoids and food preparation: the retention of provitamin A carotenoids in prepared, processed and stored foods; Citeseer: 1997.
- Bechoff, A. Investigating carotenoid loss after drying and storage of orange-fleshed sweet potato. University of Greenwich, 2010.
- De Moura, F.F.; Miloff, A.; Boy, E. Retention of provitamin A carotenoids in staple crops targeted for biofortification in Africa: cassava, maize and sweet potato. Critical reviews in food science and nutrition 2015, 55, 1246–1269. [Google Scholar] [CrossRef]
- Allwood, M. The influence of light on vitamin A degradation during administration. Clinical Nutrition 1982, 1, 63–70. [Google Scholar] [CrossRef]
- Ejigui, J.; Savoie, L.; Marin, J.; Desrosiers, T. Beneficial changes and drawbacks of a traditional fermentation process on chemical composition and antinutritional factors of yellow maize (Zea mays). Journal of Biological Sciences 2005, 5, 590–596. [Google Scholar]
- Ortiz, D.; Nkhata, S.G.; Rocheford, T.; Ferruzzi, M.G. Steeping of biofortified orange maize genotypes for Ogi production modifies pasting properties and carotenoid stability. Agronomy 2019, 9, 771. [Google Scholar] [CrossRef]
- Huey, S.L.; Konieczynski, E.M.; Mehta, N.H.; Krisher, J.T.; Bhargava, A.; Friesen, V.M.; Mbuya, M.N.; Monterrosa, E.C.; Nyangaresi, A.M.; Mehta, S. A systematic review of the impacts of post-harvest handling on provitamin A, iron and zinc retention in seven biofortified crops. Nature Food 2023, 4, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food science and biotechnology 2018, 27, 333–342. [Google Scholar] [PubMed]
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Nitray, J. Dietary requirements for proteins and amino acids in human nutrition. Current Nutrition & Food Science 2019, 15, 638–645. [Google Scholar]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. The Journal of nutrition 2006, 136, 533S–537S. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E.; Bertolo, R.F.; Brunton, J.A. Methionine: a metabolically unique amino acid. Livestock Science 2007, 112, 2–7. [Google Scholar] [CrossRef]
- Ozols, J. [44] Amino acid analysis. In Methods in enzymology, Elsevier: 1990; Vol. 182, pp. 587-601.
- Garlick, P.J. The role of leucine in the regulation of protein metabolism. The Journal of nutrition 2005, 135, 1553S–1556S. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; McClung, H.L.; McClung, J.P.; Margolis, L.M.; Andersen, N.E.; Cloutier, G.J.; Pikosky, M.A.; Rood, J.C.; Fielding, R.A.; Young, A.J. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. The American journal of clinical nutrition 2011, 94, 809–818. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Mishra, A.; Clark, J.; Farmer, T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. Comptes Rendus. Chimie 2018, 21, 112–130. [Google Scholar] [CrossRef]
- Kendall, C.W.; Esfahani, A.; Jenkins, D.J. The link between dietary fibre and human health. Food hydrocolloids 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Bmj 2013, 347. [Google Scholar] [CrossRef]
- Liu, S.; Buring, J.E.; Sesso, H.D.; Rimm, E.B.; Willett, W.C.; Manson, J.E. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. Journal of the American College of Cardiology 2002, 39, 49–56. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Ötles, S.; Ozgoz, S. Health effects of dietary fiber. Acta scientiarum polonorum Technologia alimentaria 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed]
- McRorie Jr, J.W. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy. Nutrition today 2015, 50, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Nabrzyski, M. Functional role of some minerals in foods. In Mineral components in foods, CRC Press: 2006; pp. 123-161.
- Marshall, M.R. Ash analysis. Food analysis 2010, 4, 105–116. [Google Scholar]
- Heaney, R.P. Calcium, dairy products and osteoporosis. Journal of the American college of nutrition 2000, 19, 83S–99S. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Overview of calcium. In Dietary reference intakes for calcium and vitamin D, National Academies Press (US): 2011.
- Tapiero, H.; Townsend, D.á.; Tew, K. Trace elements in human physiology and pathology. Copper. Biomedicine & pharmacotherapy 2003, 57, 386–398. [Google Scholar]
- NIFH. Copper-Fact Sheet for Health Professionals. NIFH [elektroninis išteklius] 2021.
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L. Modern nutrition in health and disease; Jones & Bartlett Learning: 2020.
- Fairweather-Tait, S.J.; Harvey, L.J.; Collings, R. Risk–benefit analysis of mineral intakes: Case studies on copper and iron. Proceedings of the Nutrition Society 2011, 70, 1–9. [Google Scholar] [CrossRef]
- Prohaska, J.R. Impact of copper deficiency in humans. Annals of the New York Academy of Sciences 2014, 1314, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of utilizing healthy fats in foods. Advances in Nutrition 2015, 6, 309S–317S. [Google Scholar] [CrossRef]
- Ravisankar, P.; Reddy, A.A.; Nagalakshmi, B.; Koushik, O.S.; Kumar, B.V.; Anvith, P.S. The comprehensive review on fat soluble vitamins. IOSR Journal of Pharmacy 2015, 5, 12–28. [Google Scholar]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. The Journal of nutrition 2001, 131, 568S–580S. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and cognitive functions. Neuropsychiatric disease and treatment 2014, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Pivina, L.; Semenova, Y.; Doşa, M.D.; Dauletyarova, M.; Bjørklund, G. Iron deficiency, cognitive functions, and neurobehavioral disorders in children. Journal of Molecular Neuroscience 2019, 68, 1–10. [Google Scholar] [CrossRef]
- Kinabo, J.; SALAAM, D. Role of potassium in human and animal nutrition. In Proceedings of First National Potash Symposium Dares Salaam.
- Castro, H.; Raij, L. Potassium in hypertension and cardiovascular disease. In Proceedings of Seminars in nephrology; pp. 277-289.
- Bhor, R.J.; Damdhar, H.; Kokate, G.; Salve, M.; Andhale, S. An Overview on Cause of Muscles Cramps or Leg spasms; Types of Muscle Cramps and its Pharmacological Treatment by New Drugs. Research Journal of Pharmacology and Pharmacodynamics 2016, 8, 134–140. [Google Scholar] [CrossRef]
- Allahdadi, I. Impact de niveaux élevés de résidus de désencrage sur le rendement, la fixation symbiotique de l'azote et la nutrition minérale de quatre légumineuses fourragères; National Library of Canada= Bibliothèque nationale du Canada, Ottawa: 2001.
- Fouhy, L.E.; Mangano, K.M.; Zhang, X.; Hughes, B.D.; Tucker, K.L.; Noel, S.E. Association between a calcium-to-magnesium ratio and osteoporosis among Puerto Rican adults. The Journal of Nutrition 2023, 153, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Lactose and lactose derivatives as bioactive ingredients in human nutrition. International Dairy Journal 2008, 18, 458–465. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut microbes 2020, 12, 1799533. [Google Scholar] [CrossRef]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: implications for health and disease. Physiological reviews 2015. [Google Scholar] [CrossRef]
- Calsou, P.; Salles, B. Properties of damage-dependent DNA incision by nucleotide excision repair in human cell-free extracts. Nucleic acids research 1994, 22, 4937–4942. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Abo Nahas, H.H.; Elshaer, A.M.; El-Waseef, D.A.E.-D.A.; El-Kharashi, O.A.; Mohamed, S.M.; Sabry, Y.G.; Almaimani, R.A.; Almasmoum, H.A.; Altamimi, A.S. Unveiling the interplay between NSAID-induced dysbiosis and autoimmune liver disease in children: insights into the hidden gateway to autism spectrum disorders. Evidence from ex vivo, in vivo, and clinical studies. Frontiers in Cellular Neuroscience 2023, 17, 1268126. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Manganese: its role in disease and health. Met. Ions Life Sci 2019, 19, 253–266. [Google Scholar]
- Tuormaa, T.E. The adverse effects of manganese deficiency on reproduction and health: A literature review. cell 1996, 2, 7. [Google Scholar]
- Bowman, A.B.; Kwakye, G.F.; Hernández, E.H.; Aschner, M. Role of manganese in neurodegenerative diseases. Journal of trace elements in medicine and biology 2011, 25, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Antao, V.; Kaye, W.; Sanchez, M.; Williamson, D.; Bryan, L.; Muravov, O.; Horton, K. Division of Toxicology and Human Health Sciences, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia; Centers for Disease Control and Prevention (CDC). Prevalence of amyotrophic lateral sclerosis-United States, 2010-2011. MMWR suppl 2014, 63, 1–14. [Google Scholar]
- Nielsen, S.S. Determination of moisture content. Food analysis laboratory manual 2010, 17–27. [Google Scholar]
- Zhang, M.; Xing, S.; Fu, C.; Fang, F.; Liu, J.; Kan, J.; Qian, C.; Chai, Q.; Jin, C. Effects of Drying Methods on Taste Components and Flavor Characterization of Cordyceps militaris. Foods 2022, 11, 3933. [Google Scholar] [CrossRef]
- Doyle, M.E.; Glass, K.A. Sodium reduction and its effect on food safety, food quality, and human health. Comprehensive reviews in food science and food safety 2010, 9, 44–56. [Google Scholar] [CrossRef]
- Arce-Cordero, J.A.; Paula, E.M.; Daniel, J.L.; Silva, L.G.; Broderick, G.A.; Faciola, A.P. Effects of neutral detergent fiber digestibility estimation method on calculated energy concentration of canola meals from 12 Canadian processing plants. Journal of Animal Science 2021, 99, skab309. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional ecology of the ruminant; Cornell university press: 1994.
- Márton, M.; Mándoki, Z.; Csapo-Kiss, Z.; Csapó, J. The role of sprouts in human nutrition. A review. 2010. [Google Scholar]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Dietary phosphorus in bone health and quality of life. Nutrition reviews 2012, 70, 311–321. [Google Scholar] [CrossRef]
- Bird, R.P.; Eskin, N.M. The emerging role of phosphorus in human health. In Advances in food and Nutrition Research, Elsevier: 2021; Vol. 96, pp. 27-88.
- Wu, G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. Journal of animal science and biotechnology 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Cynober, L.; DeLegge, M.; Kreymann, G.; Wernerman, J.; Wolfe, R.R. Proteins and amino acids are fundamental to optimal nutrition support in critically ill patients. Critical care 2014, 18, 1–13. [Google Scholar] [CrossRef]
- NHS. Starchy foods and carbohydrates 15 March 2023 ed.; NHS: 2023.
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: the biological basis of altered resistance to infection. The American journal of clinical nutrition 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. The immune system and the impact of zinc during aging. Immunity & Ageing 2009, 6, 1–17. [Google Scholar]
- Prasad, A.S. Discovery of zinc for human health and biomarkers of zinc deficiency. In Molecular, genetic, and nutritional aspects of major and trace minerals, Elsevier: 2017; pp. 241-260.
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Muzhingi, T.; Langyintuo, A.S.; Malaba, L.C.; Banziger, M. Consumer acceptability of yellow maize products in Zimbabwe. Food Policy 2008, 33, 352–361. [Google Scholar] [CrossRef]
- Agume Ntso, A.S.; Njintang, Y.N.; Mbofung, C.M.F. Physicochemical and pasting properties of maize flour as a function of the interactive effect of natural-fermentation and roasting. Journal of Food Measurement and Characterization 2017, 11, 451–459. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Reviews International 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Das, P.P.; Duarah, P.; Purkait, M.K. Fundamentals of food roasting process. In High-Temperature Processing of Food Products, Elsevier: 2023; pp. 103-130.
- Rothschild, J.; Rosentrater, K.A.; Onwulata, C.; Singh, M.; Menutti, L.; Jambazian, P.; Omary, M.B. Influence of quinoa roasting on sensory and physicochemical properties of allergen-free, gluten-free cakes. International journal of food science & technology 2015, 50, 1873–1881. [Google Scholar]
- Okaru, A.O.; Lachenmeier, D.W. Defining no and low (NoLo) alcohol products. Nutrients 2022, 14, 3873. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




