Submitted:
05 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Keywords:
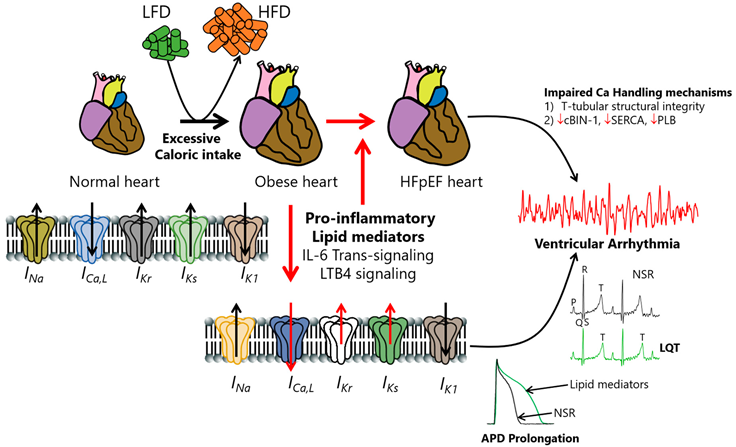
Introduction
Pathophysiology and Promotion of Molecular Changes in Cardiac Ionic Currents By Va
Voltage-Gated Sodium Channels and Sodium Currents
Voltage-Gated l-Type Calcium Channels, Calcium Currents, and Calcium-Handling Proteins
Voltage-Gated Potassium Channels and Potassium Currents
Inflammation and Structural Changes in Hfpef
Interleukin 6 Signaling in Heart Failure
Leukotriene B4 Signaling in Heart Failure
Cardiac Bridging Integrator 1 Signaling in Heart Failure
Mechanical Circulatory Support Devices in Hfpef
Conclusions and Future Directions
| Current | Gene | mRNA | Protein | Current Density | Animal Model | HFpEF/Obese | Cardiac Tissue | QTC | Drug Tx (effect) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| late INa | SCN5A | NR | NR | ↑ | Mouse (C57BL/6J) |
+/+ | Ventricle | NR | Pre-incubation with Empagliflozin (↓ current density) |
[57] |
| NR | NR | ↔ | Rat (SD) | -/+ | Ventricle | ↔ (↑QRS) | NR | [79] | ||
| NR | NR | ↑ (male˃ female) |
Mouse (db/db+Aldo) | +/+ | Ventricle | NR | Empagliflozin (↓ APD prolongation, male + female) AIP (↓ APD prolongation, male + female) Vericiguat (↓ APD prolongation, only female) |
[116] | ||
| Peak INa | SCN5A | NR | NR | ↔ | Rat (SD) | -/+ | Ventricle | ↔ (↑QRS) | NR | [79] |
| ↔ | NR | ↑* | Rat (WR) | -/+ | Ventricle | NR | NR | [106] | ||
| ICa,L | CACNA1c | ↔ | ↑ | ↑ | Rat (Dahl/SS) | +/- | Ventricle | NR | NR | [86] |
| ↑ | NR | ↑* | Rat (WR) | -/+ | Ventricle | NR | NR | [106] | ||
| NR | ↔ | ↑ | Rat (WR) | +/- | Ventricle | NR | [88] | |||
| NR | NR | ↑ | Rat (HHR) | +/- | Ventricle | ↔ (↑QRS) | NR | [98] | ||
| NR | ↔ | NR | Rat (WR) | -/+ | Ventricle | NR | NR | [99] | ||
| ↓ | NR | NR | Rat (WR, 15 weeks) | -/+ | Ventricle | NR | NR | [102] | ||
| ↑ | NR | NR | Rat (WR, 30 weeks) | -/+ | Ventricle | NR | NR | [102] | ||
| ↔ | NR | NR | Rat (WR, 45 weeks) | -/+ | Ventricle | NR | NR | [102] | ||
| NR | ↔ | NR | Rat (WR) | -/+ | Ventricle | NR | NR | [105] | ||
| NR | ↔ | NR | Mouse (db/db) | +/- | Ventricle | NR | cBIN1 | [213] | ||
| ↔ | NR | ↑ | Rat (Dahl/SS) | +/- | Ventricle | ↑ | NR | [34] | ||
| NR | NR | ↓ (only male) | Mouse (db/db+Aldo) | +/+ | Ventricle | NR | Empagliflozin (↓ APD prolongation, male + female) AIP (↓ APD prolongation, male + female) Vericiguat (↓ APD prolongation, only female) |
[116] | ||
| Ito | KCND2/3, KCNA4 | NR | NR | ↔ | Rat (WR) | +/- | Ventricle | NR | NR | [87] |
| ↔ | NR | ↑* | Rat (WR) | -/+ | Ventricle | NR | NR | [106] | ||
| NR | NR | ↓ (male + female) | Mouse (db/db+Aldo) | +/+ | Ventricle | NR | Empagliflozin (↓ APD prolongation, male + female) AIP (↓ APD prolongation, male + female) Vericiguat (↓ APD prolongation, only female) |
[116] | ||
| NR | NR | ↑ | Mouse (C57BL6) | -/+ | Ventricle | NR | NR | [126] | ||
| KCND2 | ↔ | ↔ | ↓ | Rat (Dahl/SS) | +/- | Ventricle | ↑ | NR | [34] | |
| KCND3 | ↓ | ↓ | ↓ | Rat (Dahl/SS) | +/- | Ventricle | ↑ | NR | [34] | |
| KCNA4 | ↔ | ↔ | ↓ | Rat (Dahl/SS) | +/- | Ventricle | ↑ | NR | [34] | |
| IKr | hERG | ↔ | ↔ | ↓ | Rat (Dahl/SS) | +/- | Ventricle | ↑ | NR | [34] |
| ↓ | NR | NR | Rat (WR) | -/+ | Ventricle | NR | NR | [106] | ||
| NR | ↓ | ↓ | Guinea pig (HFD) | -/+ | Ventricle | ↑ | NR | [39] | ||
| IKs | KCNQ1, KCNE1 |
↓ | NR | ↓ | Guinea pig (HFD) | -/+ | Ventricle | ↑ | NR | [39] |
| IK1 | KCNJ12/14/4 | ↔ | NR | ↓ | Rat (WR) | +/- | Ventricle | NR | NR | [87] |
| ↔ | ↔ | NR | Rat (Dahl/SS) | +/- | Ventricle | ↑ | NR | [34] | ||
| ↑ | NR | ↑* | Rat (WR) | -/+ | Ventricle | NR | NR | [106] | ||
| NR | NR | ↓ (male + female) | Mouse (db/db+Aldo) | +/+ | Ventricle | NR | Empagliflozin (↓ APD prolongation, male + female) AIP (↓ APD prolongation, male + female) Vericiguat (↓ APD prolongation, only female) |
[116] |
| Ca handling protein | mRNA | Protein | Animal Model | HFpEF/Obese | Cardiac Tissue | Drug Tx (effect) | Ref. |
|---|---|---|---|---|---|---|---|
| SERCA2a | ↑ | NR | Rat (WR) | -/+ | Ventricle | NR | [106] |
| ↓ | ↓ | Rat (Dahl/SS) | +/- | Ventricle | Intraperitoneal Ranolazine (↑ expression) | [59] | |
| NR | ↔ | Rat (WR) | +/- | Ventricle | NR | [87] | |
| NR | ↓ | Rat (WR) | +/- | Ventricle | NR | [88] | |
| NR | ↓ | Rat (HHR) | +/- | Ventricle | NR | [98] | |
| NR | ↔ | Rat (WR) | -/+ | Ventricle | NR | [99] | |
| ↓ | NR | Rat (WR, 15 weeks) | -/+ | Ventricle | NR | [102] | |
| ↑ | NR | Rat (WR, 30 weeks) | -/+ | Ventricle | NR | [102] | |
| ↓ | NR | Rat (WR, 45 weeks) | -/+ | Ventricle | NR | [102] | |
| NR | ↔ | Rat (WR) | -/+ | Ventricle | NR | [105] | |
| ↑ | NR | Rat (WR) | -/+ | Ventricle | NR | [101] | |
| NR | ↔ | Rat (ZSF1) | +/+ | Ventricle | NR | [100] | |
| NR | ↔ | Mouse (C57BL/6J) | *+/+ | NR | NR | [104] | |
| NR | ↓ | Mouse (db/db) | +/- | Ventricle | cBIN1 (↑expression) | [213] | |
| NR | ↓ | Rat (Dahl/SS) | +/- | Ventricle | NR | [90] | |
| NR | ↓ | Rat (ZSF1) | +/+ | Ventricle | NR | [90] | |
| RyR2 | ↑ | NR | Rat (WR) | -/+ | Ventricle | NR | [106] |
| NR | ↔ | Rat (WR) | +/- | Ventricle | NR | [87] | |
| NR | NR | Rat (SD) | +/- | Ventricle | Dantrolene (↑RyR2 inhibition) | [89] | |
| ↔ | NR | Rat (WR, 15 weeks) | -/+ | Ventricle | NR | [102] | |
| ↑ | NR | Rat (WR, 30 weeks) | -/+ | Ventricle | NR | [102] | |
| ↔ | NR | Rat (WR, 45 weeks) | -/+ | Ventricle | NR | [102] | |
| ↑ | NR | Rat (WR) | -/+ | Ventricle | NR | [101] | |
| NR | ↔ | Mouse (db/db) | +/- | Ventricle | cBIN1 | [213] | |
| NCX | ↑ | NR | Rat (WR) | -/+ | Ventricle | NR | [106] |
| ↑ | ↑ | Rat (Dahl/SS) | +/- | Ventricle | Intraperitoneal Ranolazine (↓ expression) | [59] | |
| NR | ↔ | Rat (WR) | +/- | Ventricle | NR | [87] | |
| NR | ↔ | Rat (WR) | +/- | Ventricle | NR | [88] | |
| ↔ | Rat (WR) | +/- | [98] | ||||
| ↓ | Rat (WR, 15 weeks) | -/+ | Ventricle | NR | [102] | ||
| ↑ | NR | Rat (WR, 30 weeks) | -/+ | Ventricle | NR | [102] | |
| ↓ | NR | Rat (WR, 45 weeks) | -/+ | Ventricle | NR | [102] | |
| NR | ↔ | Rat (Dahl/SS) | +/- | Ventricle | NR | [90] | |
| NR | ↓ | Rat (ZSF1) | +/+ | Ventricle | NR | [90] | |
| PLB | NR | ↔ | Rat (WR) | +/- | Ventricle | NR | [87] |
| NR | ↔ | Rat (WR) | +/- | Ventricle | NR | [88] | |
| NR | ↓ | Rat (HHR) | +/- | Ventricle | NR | [98] | |
| NR | ↔ | Rat (WR) | -/+ | Ventricle | NR | [99] | |
| ↓ | NR | Rat (WR, 15 weeks) | -/+ | Ventricle | NR | [102] | |
| ↑ | NR | Rat (WR, 30 weeks) | -/+ | Ventricle | NR | [102] | |
| ↔ | NR | Rat (WR, 45 weeks) | -/+ | Ventricle | NR | [102] | |
| NR | ↓ | Rat (WR) | -/+ | Ventricle | NR | [105] | |
| ↑ | NR | Rat (WR) | -/+ | Ventricle | NR | [101] | |
| NR | ↑ | Rat (ZSF1) | +/+ | Ventricle | NR | [100] | |
| NR | ↔ | Rat (Dahl/SS) | +/- | Ventricle | NR | [90] | |
| NR | ↔ | Rat (ZSF1) | +/+ | Ventricle | NR | [90] |
Author Contributions
Funding
Data Availability
Conflicts of Interest
| Nonstandard Abbreviations and Acronyms | |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| SCD | Sudden cardiac death |
| EAT | Epicardial adipose tissue |
| INa | Sodium current |
| ICa, L | L-type calcium current |
| Ito | Transient outward potassium current |
| IKr | Rapid delayed rectifier potassium current |
| IKs | Slow delayed rectifier potassium current |
| IK1 | Inward rectifier potassium current |
| QTc | Corrected QT interval |
| NCX | Sodium/calcium exchanger |
| EAD | Early after depolarization |
| DAD | Delayed after depolarization |
| SGLT2 | Sodium–glucose cotransporter-2 |
| CamK | Calcium–calmodulin kinase |
| RyR | Ryanodine receptor |
| eNOS | Endothelial nitric oxide synthesis |
| NO | Nitric oxide |
| TNF | Tumor necrosis factor |
| TGF- β | Transforming growth factor-β |
| SERCA | Sarcoplasmic reticulum Ca+2-ATPase |
| PLB | Phospholamban |
| hERG | Human Ether-à-go-go-related gene |
| VT | Ventricular tachycardia |
| VF | Ventricular fibrillation |
| IL-6R | Interleukin 6 receptor |
| gp130 | Glycoprotein 130 |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| TLR | Toll-like receptor |
| NLRP3 | Leucine-rich repeat (LRR) and pyrin domain (PYD)-containing protein 3 |
| NF-κB | Nuclear factor kappa B |
| MyD88 | Myeloid differentiation primary response 88 |
| FFA | Free fatty acid |
| LTB4 | Leukotriene B4 |
| BLT1/2 | Leukotriene B4 receptor 1/2 |
| cBIN1 | Cardiac bridging integrator 1 |
| LVAD | Left ventricular assistance device |
| LAAD | Left atrial assistance device |
References
- Toth PP, Gauthier D: Heart failure with preserved ejection fraction: disease burden for patients, caregivers, and the health-care system. Postgraduate Medicine 2021, 133(2):140-145.
- Roh J, Houstis N, Rosenzweig A: Why Don’t We Have Proven Treatments for HFpEF? Circ Res 2017, 120(8):1243-1245.
- Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J: Mode of Death in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2017, 69(5):556-569.
- Yuyun MF, Kinlay S, Singh JP, Joseph J: Are arrhythmias the drivers of sudden cardiac death in heart failure with preserved ejection fraction? A review. ESC Heart Fail 2023, 10(3):1555-1569.
- Hooks M, Downey MC, Joppa S, Beard A, Gravely A, Tholakanahalli V, Adabag S: Arrhythmic causes of in-hospital cardiac arrest among patients with heart failure with preserved ejection fraction. Heart Rhythm O2 2021, 2(6Part A):665-667.
- Gutierrez A, Ash J, Akdemir B, Alexy T, Cogswell R, Chen J, Adabag S: Nonsustained ventricular tachycardia in heart failure with preserved ejection fraction. Pacing Clin Electrophysiol 2020, 43(10):1126-1131.
- Cho JH, Leong D, Cuk N, Ebinger JE, Bresee C, Yoon SH, Ehdaie A, Shehata M, Wang X, Chugh SS et al: Delayed repolarization and ventricular tachycardia in patients with heart failure and preserved ejection fraction. PLoS One 2021, 16(7):e0254641.
- Curtain JP, Adamson C, Kondo T, Butt JH, Desai AS, Zannad F, Rouleau JL, Rohde LE, Kober L, Anand IS et al: Investigator-reported ventricular arrhythmias and mortality in heart failure with mildly reduced or preserved ejection fraction. Eur Heart J 2023, 44(8):668-677.
- Cho JH: Sudden Death and Ventricular Arrhythmias in Heart Failure With Preserved Ejection Fraction. Korean Circ J 2022, 52(4):251-264.
- Crespo-García T, Cámara-Checa A, Dago M, Rubio-Alarcón M, Rapún J, Tamargo J, Delpón E, Caballero R: Regulation of cardiac ion channels by transcription factors: Looking for new opportunities of druggable targets for the treatment of arrhythmias. Biochemical Pharmacology 2022, 204:115206.
- Bers DM, Despa S: Na+ transport in cardiac myocytes; Implications for excitation-contraction coupling. IUBMB Life 2009, 61(3):215-221.
- Varró A, Nánási PP, Lathrop DA: Potassium currents in isolated human atrial and ventricular cardiocytes. Acta Physiol Scand 1993, 149(2):133-142.
- Aromolaran AS, Subramanyam P, Chang DD, Kobertz WR, Colecraft HM: LQT1 mutations in KCNQ1 C-terminus assembly domain suppress IKs using different mechanisms. Cardiovasc Res 2014, 104(3):501-511.
- Puckerin A, Aromolaran KA, Chang DD, Zukin RS, Colecraft HM, Boutjdir M, Aromolaran AS: hERG 1a LQT2 C-terminus truncation mutants display hERG 1b-dependent dominant negative mechanisms. Heart Rhythm 2016, 13(5):1121-1130.
- Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintrón M, Scott JD, Santana LF: Restoration of Normal L-Type Ca2+ Channel Function During Timothy Syndrome by Ablation of an Anchoring Protein. Circulation Research 2011, 109(3):255-261.
- Wit AL: Afterdepolarizations and triggered activity as a mechanism for clinical arrhythmias. Pacing and Clinical Electrophysiology 2018, 41(8):883-896.
- Anderson A, Kulkarni K, Marron Fernandez de Velasco E, Carlblom N, Xia Z, Nakano A, Martemyanov KA, Tolkacheva EG, Wickman K: Expression and relevance of the G protein-gated K(+) channel in the mouse ventricle. Sci Rep 2018, 8(1):1192.
- Liu X, Shi J, Xiao P: Associations between common ion channel single nucleotide polymorphisms and sudden cardiac death in adults: A MOOSE-compliant meta-analysis. Medicine 2018, 97(38):e12428.
- Hancox JC, McPate MJ, El Harchi A, Zhang YH: The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther 2008, 119(2):118-132.
- Garrido A, Lepailleur A, Mignani SM, Dallemagne P, Rochais C: hERG toxicity assessment: Useful guidelines for drug design. European Journal of Medicinal Chemistry 2020, 195:112290.
- Aromolaran AS, Srivastava U, Alí A, Chahine M, Lazaro D, El-Sherif N, Capecchi PL, Laghi-Pasini F, Lazzerini PE, Boutjdir M: Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One 2018, 13(12):e0208321.
- Sale H, Wang J, O’Hara TJ, Tester DJ, Phartiyal P, He JQ, Rudy Y, Ackerman MJ, Robertson GA: Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with Long-QT syndrome. Circ Res 2008, 103(7):e81-95.
- Jones DK, Liu F, Vaidyanathan R, Eckhardt LL, Trudeau MC, Robertson GA: hERG 1b is critical for human cardiac repolarization. Proc Natl Acad Sci U S A 2014, 111(50):18073-18077.
- Zünkler BJ: Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol Ther 2006, 112(1):12-37.
- Wu L, Ma J, Li H, Wang C, Grandi E, Zhang P, Luo A, Bers DM, Shryock JC, Belardinelli L: Late sodium current contributes to the reverse rate-dependent effect of IKr inhibition on ventricular repolarization. Circulation 2011, 123(16):1713-1720.
- Weiss JN, Garfinkel A, Karagueuzian HS, Chen P-S, Qu Z: Early afterdepolarizations and cardiac arrhythmias. Heart rhythm 2010, 7(12):1891-1899.
- Remme CA, Bezzina CR: REVIEW: Sodium Channel (Dys)Function and Cardiac Arrhythmias. Cardiovascular Therapeutics 2010, 28(5):287-294.
- Ravens U, Cerbai E: Role of potassium currents in cardiac arrhythmias. EP Europace 2008, 10(10):1133-1137.
- Choi BR, Burton F, Salama G: Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol 2002, 543(Pt 2):615-631.
- Paar V, Jirak P, Larbig R, Zagidullin NS, Brandt MC, Lichtenauer M, Hoppe UC, Motloch LJ: Pathophysiology of Calcium Mediated Ventricular Arrhythmias and Novel Therapeutic Options with Focus on Gene Therapy. Int J Mol Sci 2019, 20(21).
- Stienen S, Ferreira JP, Kobayashi M, Preud’homme G, Dobre D, Machu J-L, Duarte K, Bresso E, Devignes M-D, López N et al: Enhanced clinical phenotyping by mechanistic bioprofiling in heart failure with preserved ejection fraction: insights from the MEDIA-DHF study (The Metabolic Road to Diastolic Heart Failure). Biomarkers 2020, 25(2):201-211.
- Carella MJ, Mantz SL, Rovner DR, Willis 3rd PW, Gossain VV, Bouknight RR, Ferenchick GS: Obesity, adiposity, and lengthening of the QT interval: improvement after weight loss. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 1996, 20(10):938-942.
- Milovancev A, Stokic E: Corrected QT Interval and Corrected QT Dispersion in Obesity. CJournal of Clinical Cardiology and Diagnostics 2019, 2(1):1-4.
- Cho JH, Zhang R, Kilfoil PJ, Gallet R, de Couto G, Bresee C, Goldhaber JI, Marbán E, Cingolani E: Delayed Repolarization Underlies Ventricular Arrhythmias in Rats With Heart Failure and Preserved Ejection Fraction. Circulation 2017, 136(21):2037-2050.
- Cho JH, Zhang R, Aynaszyan S, Holm K, Goldhaber JI, Marbán E, Cingolani E: Ventricular Arrhythmias Underlie Sudden Death in Rats With Heart Failure and Preserved Ejection Fraction. Circ Arrhythm Electrophysiol 2018, 11(8):e006452.
- Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A: Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. European Journal of Preventive Cardiology 2019, 27(5):494-510.
- Paulus WJ, Tschöpe C: A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013, 62(4):263-271.
- Omran J, Bostick BP, Chan AK, Alpert MA: Obesity and Ventricular Repolarization: a Comprehensive Review. Progress in Cardiovascular Diseases 2018, 61(2):124-135.
- Corbin A, Aromolaran KA, Aromolaran AS: STAT4 Mediates IL-6 Trans-Signaling Arrhythmias in High Fat Diet Guinea Pig Heart. Int J Mol Sci 2024, 25(14).
- Biet M, Morin N, Benrezzak O, Naimi F, Bellanger S, Baillargeon JP, Chouinard L, Gallo-Payet N, Carpentier AC, Dumaine R: Lasting alterations of the sodium current by short-term hyperlipidemia as a mechanism for initiation of cardiac remodeling. American Journal of Physiology-Heart and Circulatory Physiology 2013, 306(2):H291-H297.
- Sánchez G, Araneda F, Peña JP, Finkelstein JP, Riquelme JA, Montecinos L, Barrientos G, Llanos P, Pedrozo Z, Said M et al: High-Fat-Diet-Induced Obesity Produces Spontaneous Ventricular Arrhythmias and Increases the Activity of Ryanodine Receptors in Mice. Int J Mol Sci 2018, 19(2).
- Załęska-Kocięcka M, Wojdyńska Z, Kalisz M, Litwiniuk A, Mączewski M, Leszek P, Paterek A: Epicardial fat and ventricular arrhythmias. Heart Rhythm 2024, 21(2):206-212.
- Qu Z, Weiss JN: Mechanisms of ventricular arrhythmias: from molecular fluctuations to electrical turbulence. Annu Rev Physiol 2015, 77:29-55.
- Kornreich BG: The patch clamp technique: principles and technical considerations. J Vet Cardiol 2007, 9(1):25-37.
- O’Shea C, Winter J, Kabir SN, O’Reilly M, Wells SP, Baines O, Sommerfeld LC, Correia J, Lei M, Kirchhof P et al: High resolution optical mapping of cardiac electrophysiology in pre-clinical models. Scientific Data 2022, 9(1):135.
- Zhang R, Mesquita T, Cho Jae H, Li C, Sanchez L, Holm K, Akhmerov A, Liu W, Li Y, Ibrahim Ahmed G, Cingolani E: Systemic Delivery of Extracellular Vesicles Attenuates Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction. JACC: Clinical Electrophysiology 2023, 9(2):147-158.
- Monitillo F, Leone M, Rizzo C, Passantino A, Iacoviello M: Ventricular repolarization measures for arrhythmic risk stratification. World J Cardiol 2016, 8(1):57-73.
- Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z: Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 2010, 7(12):1891-1899.
- Tse G: Mechanisms of cardiac arrhythmias. J Arrhythm 2016, 32(2):75-81.
- Liu MB, de Lange E, Garfinkel A, Weiss JN, Qu Z: Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm 2015, 12(10):2115-2124.
- Clayton RH, Holden AV: Dispersion of cardiac action potential duration and the initiation of re-entry: A computational study. BioMedical Engineering OnLine 2005, 4(1):11.
- Bouza AA, Isom LL: Voltage-Gated Sodium Channel β Subunits and Their Related Diseases. Handb Exp Pharmacol 2018, 246:423-450.
- Dong C, Wang Y, Ma A, Wang T: Life Cycle of the Cardiac Voltage-Gated Sodium Channel NaV1.5. Frontiers in Physiology 2020, 11.
- András V, Tomek J, Nagy N, Virág L, Passini E, Rodriguez B, Baczkó I: Cardiac transmembrane ion channels and action potentials: cellular physiology and arrhythmogenic behavior. Physiological Reviews 2021, 101(3):1083-1176.
- Gintant GA, Datyner NB, Cohen IS: Slow inactivation of a tetrodotoxin-sensitive current in canine cardiac Purkinje fibers. Biophys J 1984, 45(3):509-512.
- Patlak JB, Ortiz M: Slow currents through single sodium channels of the adult rat heart. J Gen Physiol 1985, 86(1):89-104.
- Hegyi B, Mira Hernandez J, Shen EY, Habibi NR, Bossuyt J, Bers DM: Empagliflozin Reverses Late Na+ Current Enhancement and Cardiomyocyte Proarrhythmia in a Translational Murine Model of Heart Failure With Preserved Ejection Fraction. Circulation 2022, 145(13):1029-1031.
- Maier LS: A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: inhibition of late I(Na) using ranolazine. J Cardiovasc Pharmacol 2009, 54(4):279-286.
- De Angelis A, Cappetta D, Piegari E, Rinaldi B, Ciuffreda LP, Esposito G, Ferraiolo FA, Rivellino A, Russo R, Donniacuo M et al: Long-term administration of ranolazine attenuates diastolic dysfunction and adverse myocardial remodeling in a model of heart failure with preserved ejection fraction. Int J Cardiol 2016, 217:69-79.
- Fink M, Noble PJ, Noble D: Ca²⁺-induced delayed afterdepolarizations are triggered by dyadic subspace Ca2²⁺ affirming that increasing SERCA reduces aftercontractions. Am J Physiol Heart Circ Physiol 2011, 301(3):H921-935.
- Iqbal SM, Lemmens-Gruber R: Phosphorylation of cardiac voltage-gated sodium channel: Potential players with multiple dimensions. Acta Physiol (Oxf) 2019, 225(3):e13210.
- Hale SL, Kloner RA: Ranolazine, an inhibitor of the late sodium channel current, reduces postischemic myocardial dysfunction in the rabbit. J Cardiovasc Pharmacol Ther 2006, 11(4):249-255.
- Belardinelli L, Shryock JC, Fraser H: Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 2006, 92(suppl 4):iv6-iv14.
- Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN: Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. Journal of cardiovascular electrophysiology 2006, 17:S169-S177.
- Cempaka Putri DKS, Andrianto A, Al-Farabi MJ, Saputra PBT, Nugraha RA: Efficacy of Ranolazine to Improve Diastolic Performance in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-analysis. Eur Cardiol 2023, 18:e02.
- Anker Stefan D, Butler J, Filippatos G, Ferreira João P, Bocchi E, Böhm M, Brunner–La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E et al: Empagliflozin in Heart Failure with a Preserved Ejection Fraction. New England Journal of Medicine 2021, 385(16):1451-1461.
- Solomon Scott D, McMurray John JV, Claggett B, de Boer Rudolf A, DeMets D, Hernandez Adrian F, Inzucchi Silvio E, Kosiborod Mikhail N, Lam Carolyn SP, Martinez F et al: Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. New England Journal of Medicine 2022, 387(12):1089-1098.
- Ozturk F, Tuner H, Atici A, Ali Barman H: Effect of Empagliflozin Treatment on Ventricular Repolarization Parameters. RCM 2024, 25(2):64-null.
- Fujiki S, Iijima K, Nakagawa Y, Takahashi K, Okabe M, Kusano K, Owada S, Kondo Y, Tsujita K, Shimizu W et al: Effect of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes treated with an implantable cardioverter-defibrillator: the EMPA-ICD trial. Cardiovascular Diabetology 2024, 23(1):224.
- Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, Barr A, Singh J, Wong J, Palechuk T et al: Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143(22):2188-2204.
- Withaar C, Lam CSP, Schiattarella GG, de Boer RA, Meems LMG: Heart failure with preserved ejection fraction in humans and mice: embracing clinical complexity in mouse models. European Heart Journal 2021, 42(43):4420-4430.
- Mohammed SF, Ohtani T, Korinek J, Lam CS, Larsen K, Simari RD, Valencik ML, Burnett JC, Jr., Redfield MM: Mineralocorticoid accelerates transition to heart failure with preserved ejection fraction via "nongenomic effects". Circulation 2010, 122(4):370-378.
- Beghi S, Furmanik M, Jaminon A, Veltrop R, Rapp N, Wichapong K, Bidar E, Buschini A, Schurgers LJ: Calcium Signalling in Heart and Vessels: Role of Calmodulin and Downstream Calmodulin-Dependent Protein Kinases. In: International Journal of Molecular Sciences. vol. 23; 2022.
- Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH et al: Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 2006, 116(12):3127-3138.
- Hegyi B, Pölönen R-P, Hellgren KT, Ko CY, Ginsburg KS, Bossuyt J, Mercola M, Bers DM: Cardiomyocyte Na+ and Ca2+ mishandling drives vicious cycle involving CaMKII, ROS, and ryanodine receptors. Basic Research in Cardiology 2021, 116(1):58.
- Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA et al: Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2016, 4(4):312-324.
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB: The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 1995, 95(5):2111-2119.
- Trayhurn P, Wood IS: Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004, 92(3):347-355.
- Axelsen LN, Calloe K, Braunstein TH, Riemann M, Hofgaard JP, Liang B, Jensen CF, Olsen KB, Bartels ED, Baandrup U et al: Diet-induced pre-diabetes slows cardiac conductance and promotes arrhythmogenesis. Cardiovascular Diabetology 2015, 14(1):87.
- Jafri MS: Models of excitation-contraction coupling in cardiac ventricular myocytes. Methods Mol Biol 2012, 910:309-335.
- Buonarati OR, Henderson PB, Murphy GG, Horne MC, Hell JW: Proteolytic processing of the L-type Ca (2+) channel alpha (1)1.2 subunit in neurons. F1000Res 2017, 6:1166.
- Locatelli J, de Assis LV, Isoldi MC: Calcium handling proteins: structure, function, and modulation by exercise. Heart Fail Rev 2014, 19(2):207-225.
- Bers DM: Cardiac excitation-contraction coupling. Nature 2002, 415(6868):198-205.
- Valentim MA, Brahmbhatt AN, Tupling AR: Skeletal and cardiac muscle calcium transport regulation in health and disease. Biosci Rep 2022, 42(12).
- Saad NS, Mashali MA, Repas SJ, Janssen PML: Altering Calcium Sensitivity in Heart Failure: A Crossroads of Disease Etiology and Therapeutic Innovation. Int J Mol Sci 2023, 24(24).
- Kilfoil PJ, Lotteau S, Zhang R, Yue X, Aynaszyan S, Solymani RE, Cingolani E, Marbán E, Goldhaber JI: Distinct features of calcium handling and β-adrenergic sensitivity in heart failure with preserved versus reduced ejection fraction. J Physiol 2020, 598(22):5091-5108.
- Rouhana S, Farah C, Roy J, Finan A, Rodrigues de Araujo G, Bideaux P, Scheuermann V, Saliba Y, Reboul C, Cazorla O et al: Early calcium handling imbalance in pressure overload-induced heart failure with nearly normal left ventricular ejection fraction. Biochim Biophys Acta Mol Basis Dis 2019, 1865(1):230-242.
- Røe Å T, Aronsen JM, Skårdal K, Hamdani N, Linke WA, Danielsen HE, Sejersted OM, Sjaastad I, Louch WE: Increased passive stiffness promotes diastolic dysfunction despite improved Ca2+ handling during left ventricular concentric hypertrophy. Cardiovasc Res 2017, 113(10):1161-1172.
- Nawata J, Yamamoto T, Tanaka S, Yano Y, Uchida T, Fujii S, Nakamura Y, Suetomi T, Uchinoumi H, Oda T et al: Dantrolene improves left ventricular diastolic property in mineralcorticoid-salt-induced hypertensive rats. Biochem Biophys Rep 2023, 34:101449.
- Frisk M, Le C, Shen X, Røe ÅT, Hou Y, Manfra O, Silva GJJ, van Hout I, Norden ES, Aronsen JM et al: Etiology-Dependent Impairment of Diastolic Cardiomyocyte Calcium Homeostasis in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology 2021, 77(4):405-419.
- Shou J, Huo Y: Changes of calcium cycling in HFrEF and HFpEF. Mechanobiology in Medicine 2023, 1(1):100001.
- Brette F, Orchard C: T-Tubule Function in Mammalian Cardiac Myocytes. Circulation Research 2003, 92(11):1182-1192.
- Benitah JP, Perrier R, Mercadier JJ, Pereira L, Gómez AM: RyR2 and Calcium Release in Heart Failure. Front Physiol 2021, 12:734210.
- Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S et al: Dantrolene, a Therapeutic Agent for Malignant Hyperthermia, Inhibits Catecholaminergic Polymorphic Ventricular Tachycardia in a RyR2R2474S/+ Knock-In Mouse Model. Circulation Journal 2010, 74(12):2579-2584.
- Koss KL, Kranias EG: Phospholamban: a prominent regulator of myocardial contractility. Circ Res 1996, 79(6):1059-1063.
- Mattiazzi A, Kranias E: The role of CaMKII regulation of phospholamban activity in heart disease. Frontiers in Pharmacology 2014, 5.
- Loffredo FS, Nikolova AP, Pancoast JR, Lee RT: Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 2014, 115(1):97-107.
- Curl CL, Danes VR, Bell JR, Raaijmakers AJA, Ip WTK, Chandramouli C, Harding TW, Porrello ER, Erickson JR, Charchar FJ et al: Cardiomyocyte Functional Etiology in Heart Failure With Preserved Ejection Fraction Is Distinctive-A New Preclinical Model. J Am Heart Assoc 2018, 7(11).
- Lima-Leopoldo AP, Leopoldo AS, da Silva DC, do Nascimento AF, de Campos DH, Luvizotto RA, de Deus AF, Freire PP, Medeiros A, Okoshi K, Cicogna AC: Long-term obesity promotes alterations in diastolic function induced by reduction of phospholamban phosphorylation at serine-16 without affecting calcium handling. J Appl Physiol (1985) 2014, 117(6):669-678.
- Miranda-Silva D, Wüst RCI, Conceição G, Gonçalves-Rodrigues P, Gonçalves N, Gonçalves A, Kuster DWD, Leite-Moreira AF, van der Velden J, de Sousa Beleza JM et al: Disturbed cardiac mitochondrial and cytosolic calcium handling in a metabolic risk-related rat model of heart failure with preserved ejection fraction. Acta Physiol (Oxf) 2020, 228(3):e13378.
- Lima-Leopoldo AP, Sugizaki MM, Leopoldo AS, Carvalho RF, Nogueira CR, Nascimento AF, Martinez PF, Luvizotto RA, Padovani CR, Cicogna AC: Obesity induces upregulation of genes involved in myocardial Ca2+ handling. Braz J Med Biol Res 2008, 41(7):615-620.
- Lima-Leopoldo AP, Leopoldo AS, Silva DC, Nascimento AF, Campos DH, Luvizotto Rde A, Oliveira Júnior SA, Padovani CR, Nogueira CR, Cicogna AC: Influence of long-term obesity on myocardial gene expression. Arq Bras Cardiol 2013, 100(3):229-237.
- Dia M, Gomez L, Thibault H, Tessier N, Leon C, Chouabe C, Ducreux S, Gallo-Bona N, Tubbs E, Bendridi N et al: Reduced reticulum–mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Research in Cardiology 2020, 115(6):74.
- Abdurrachim D, Ciapaite J, Wessels B, Nabben M, Luiken JJ, Nicolay K, Prompers JJ: Cardiac diastolic dysfunction in high-fat diet fed mice is associated with lipotoxicity without impairment of cardiac energetics in vivo. Biochim Biophys Acta 2014, 1842(10):1525-1537.
- Leopoldo AS, Lima-Leopoldo AP, Sugizaki MM, Nascimento AFd, de Campos DHS, Luvizotto RdAM, Castardeli E, Alves CAB, Brum PC, Cicogna AC: Involvement of L-type calcium channel and serca2a in myocardial dysfunction induced by obesity. Journal of Cellular Physiology 2011, 226(11):2934-2942.
- Ashrafi R, Yon M, Pickavance L, Yanni Gerges J, Davis G, Wilding J, Jian K, Zhang H, Hart G, Boyett M: Altered Left Ventricular Ion Channel Transcriptome in a High-Fat-Fed Rat Model of Obesity: Insight into Obesity-Induced Arrhythmogenesis. J Obes 2016, 2016:7127898.
- Zhao Z, Gordan R, Wen H, Fefelova N, Zang W-J, Xie L-H: Modulation of intracellular calcium waves and triggered activities by mitochondrial ca flux in mouse cardiomyocytes. PloS one 2013, 8(11):e80574.
- Landstrom AP, Dobrev D, Wehrens XHT: Calcium Signaling and Cardiac Arrhythmias. Circulation Research 2017, 120(12):1969-1993.
- Gordan R, Fefelova N, Gwathmey JK, Xie LH: Involvement of mitochondrial permeability transition pore (mPTP) in cardiac arrhythmias: Evidence from cyclophilin D knockout mice. Cell Calcium 2016, 60(6):363-372.
- Littlejohns B, Pasdois P, Duggan S, Bond AR, Heesom K, Jackson CL, Angelini GD, Halestrap AP, Suleiman MS: Hearts from mice fed a non-obesogenic high-fat diet exhibit changes in their oxidative state, calcium and mitochondria in parallel with increased susceptibility to reperfusion injury. PLoS One 2014, 9(6):e100579.
- Huo R, Sheng Y, Guo WT, Dong DL: The potential role of Kv4.3 K+ channel in heart hypertrophy. Channels (Austin) 2014, 8(3):203-209.
- Wulff H, Castle NA, Pardo LA: Voltage-gated potassium channels as therapeutic targets. Nature Reviews Drug Discovery 2009, 8(12):982-1001.
- Organ-Darling LE, Vernon AN, Giovanniello JR, Lu Y, Moshal K, Roder K, Li W, Koren G: Interactions between hERG and KCNQ1 α-subunits are mediated by their COOH termini and modulated by cAMP. Am J Physiol Heart Circ Physiol 2013, 304(4):H589-599.
- Aromolaran AS, Boutjdir M: Cardiac Ion Channel Regulation in Obesity and the Metabolic Syndrome: Relevance to Long QT Syndrome and Atrial Fibrillation. Front Physiol 2017, 8:431.
- Reisqs JB, Qu YS, Boutjdir M: Ion channel trafficking implications in heart failure. Front Cardiovasc Med 2024, 11:1351496.
- Mira Hernandez J, Shen EY, Ko CY, Hourani Z, Spencer ER, Smoliarchuk D, Bossuyt J, Granzier H, Bers DM, Hegyi B: Differential sex-dependent susceptibility to diastolic dysfunction and arrhythmia in cardiomyocytes from obese diabetic HFpEF model. Cardiovasc Res 2024.
- Armstrong Paul W, Pieske B, Anstrom Kevin J, Ezekowitz J, Hernandez Adrian F, Butler J, Lam Carolyn SP, Ponikowski P, Voors Adriaan A, Jia G et al: Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. New England Journal of Medicine 2020, 382(20):1883-1893.
- Chen L, Sampson KJ, Kass RS: Cardiac Delayed Rectifier Potassium Channels in Health and Disease. Card Electrophysiol Clin 2016, 8(2):307-322.
- Lu H, Ding W, Xiao H, Dai M, Xue Y, Jia Z, Guo J, Wu M, Shen B, Zhao R: Association of the P441L KCNQ1 variant with severity of long QT syndrome and risk of cardiac events. Frontiers in Cardiovascular Medicine 2022, 9.
- Wilson AJ, Quinn KV, Graves FM, Bitner-Glindzicz M, Tinker A: Abnormal KCNQ1 trafficking influences disease pathogenesis in hereditary long QT syndromes (LQT1). Cardiovascular Research 2005, 67(3):476-486.
- Aromolaran AS: Is there an emerging role for I(Ks) in aging-related ventricular arrhythmias? J Cell Physiol 2022, 237(12):4337-4338.
- Jost N, Virág L, Bitay M, Takács J, Lengyel C, Biliczki P, Nagy Z, Bogáts G, Lathrop DA, Papp JG, Varró A: Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation 2005, 112(10):1392-1399.
- Tomaselli GF, Marbán E: Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 1999, 42(2):270-283.
- He Q, Feng Y, Wang Y: Transient outward potassium channel: a heart failure mediator. Heart Fail Rev 2015, 20(3):349-362.
- Chowdhury MKH, Martinez-Mateu L, Do J, Aromolaran KA, Saiz J, Aromolaran AS: Macrophage-Dependent Interleukin-6-Production and Inhibition of I(K) Contributes to Acquired QT Prolongation in Lipotoxic Guinea Pig Heart. Int J Mol Sci 2021, 22(20).
- Haim TE, Wang W, Flagg TP, Tones MA, Bahinski A, Numann RE, Nichols CG, Nerbonne JM: Palmitate attenuates myocardial contractility through augmentation of repolarizing Kv currents. J Mol Cell Cardiol 2010, 48(2):395-405.
- Killeen MJ, Thomas G, Sabir IN, Grace AA, Huang CLH: Mouse models of human arrhythmia syndromes. Acta Physiologica 2008, 192(4):455-469.
- Abraham DM, Lee TE, Watson LJ, Mao L, Chandok G, Wang HG, Frangakis S, Pitt GS, Shah SH, Wolf MJ, Rockman HA: The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J Clin Invest 2018, 128(11):4843-4855.
- Frangogiannis NG: Cardiac fibrosis. Cardiovasc Res 2021, 117(6):1450-1488.
- Sweeney M, Corden B, Cook SA: Targeting cardiac fibrosis in heart failure with preserved ejection fraction: mirage or miracle? EMBO Mol Med 2020, 12(10):e10865.
- Nguyen TP, Qu Z, Weiss JN: Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol 2014, 70:83-91.
- Pitt B, Pfeffer Marc A, Assmann Susan F, Boineau R, Anand Inder S, Claggett B, Clausell N, Desai Akshay S, Diaz R, Fleg Jerome L et al: Spironolactone for Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine, 370(15):1383-1392.
- Solomon Scott D, McMurray John JV, Anand Inder S, Ge J, Lam Carolyn SP, Maggioni Aldo P, Martinez F, Packer M, Pfeffer Marc A, Pieske B et al: Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine 2019, 381(17):1609-1620.
- Peh ZH, Dihoum A, Hutton D, Arthur JSC, Rena G, Khan F, Lang CC, Mordi IR: Inflammation as a therapeutic target in heart failure with preserved ejection fraction. Front Cardiovasc Med 2023, 10:1125687.
- Correale M, Tricarico L, Fortunato M, Mazzeo P, Nodari S, Di Biase M, Brunetti ND: New Targets in Heart Failure Drug Therapy. Frontiers in Cardiovascular Medicine 2021, 8.
- Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ: Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). European heart journal 2018, 39(38):3499-3507.
- Libby P, Rocha VZ: All roads lead to IL-6: A central hub of cardiometabolic signaling. Int J Cardiol 2018, 259:213-215.
- Lazzerini PE, Laghi-Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, Selvi E, Bacarelli MR, Finizola F, Vanni F et al: Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart 2017, 103(22):1821.
- Ridker PM, Rane M: Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circulation Research 2021, 128(11):1728-1746.
- Deokar SA, Dandekar SP, Shinde GA, Prabhu SS, Patawardhan M: Role of serum interleukin-6 in heart failure. International Journal of Advances in Medicine 2018, 5(4):936-940.
- Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G: Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 2004, 94(4):534-541.
- Neri Serneri GG, Boddi M, Modesti PA, Coppo M, Cecioni I, Toscano T, Papa ML, Bandinelli M, Lisi GF, Chiavarelli M: Cardiac Angiotensin II Participates in Coronary Microvessel Inflammation of Unstable Angina and Strengthens the Immunomediated Component. Circulation Research 2004, 94(12):1630-1637.
- Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H: Expression of Angiotensin II and Interleukin 6 in Human Coronary Atherosclerotic Plaques. Circulation 2000, 101(12):1372-1378.
- Groot HE, Al Ali L, van der Horst ICC, Schurer RAJ, van der Werf HW, Lipsic E, van Veldhuisen DJ, Karper JC, van der Harst P: Plasma interleukin 6 levels are associated with cardiac function after ST-elevation myocardial infarction. Clin Res Cardiol 2019, 108(6):612-621.
- Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J et al: Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. Journal of the American Heart Association, 6(10):e005077.
- Zhao Z, Qi D, Zhang Z, Du X, Zhang F, Ma R, Liang Y, Zhao Y, Gao Y, Yang Y: Prognostic Value of Inflammatory Cytokines in Predicting Hospital Readmissions in Heart Failure with Preserved Ejection Fraction. J Inflamm Res 2024, 17:3003-3012.
- Berger M, März W, Niessner A, Delgado G, Kleber M, Scharnagl H, Marx N, Schuett K: IL-6 and hsCRP predict cardiovascular mortality in patients with heart failure with preserved ejection fraction. ESC Heart Fail 2024.
- Alogna A, Koepp KE, Sabbah M, Espindola Netto JM, Jensen MD, Kirkland JL, Lam CSP, Obokata M, Petrie MC, Ridker PM et al: Interleukin-6 in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail 2023, 11(11):1549-1561.
- Ratchford SM, Lee JF, Bunsawat K, Alpenglow JK, Zhao J, Ma CL, Ryan JJ, Khor LL, Wray DW: The impact of obesity on the regulation of muscle blood flow during exercise in patients with heart failure with a preserved ejection fraction. J Appl Physiol (1985) 2022, 132(5):1240-1249.
- Gui XY, Rabkin SW: C-Reactive Protein, Interleukin-6, Trimethylamine-N-Oxide, Syndecan-1, Nitric Oxide, and Tumor Necrosis Factor Receptor-1 in Heart Failure with Preserved Versus Reduced Ejection Fraction: a Meta-Analysis. Curr Heart Fail Rep 2023, 20(1):1-11.
- Ertunc ME, Hotamisligil GS: Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. Journal of Lipid Research 2016, 57(12):2099-2114.
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S: The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011, 1813(5):878-888.
- Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ: Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine 1992, 4(2):96-100.
- Taga T, Kishimoto T: Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 1997, 15:797-819.
- Fontes JA, Rose NR, Čiháková D: The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74(1):62-68.
- Collaboration IRGCERF: Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. The Lancet 2012, 379(9822):1205-1213.
- Garbers C, Heink S, Korn T, Rose-John S: Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 2018, 17(6):395-412.
- Hagiwara Y, Miyoshi S, Fukuda K, Nishiyama N, Ikegami Y, Tanimoto K, Murata M, Takahashi E, Shimoda K, Hirano T et al: SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent ICaL, [Ca2+]i transient, and APD increase in cardiomyocytes. Journal of Molecular and Cellular Cardiology 2007, 43(6):710-716.
- Alí A, Boutjdir M, Aromolaran AS: Cardiolipotoxicity, Inflammation, and Arrhythmias: Role for Interleukin-6 Molecular Mechanisms. Front Physiol 2018, 9:1866.
- Toldo S, Mezzaroma E, Buckley LF, Potere N, Di Nisio M, Biondi-Zoccai G, Van Tassell BW, Abbate A: Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol Ther 2022, 236:108053.
- Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR: Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-κB dependent inflammatory response. Cardiovascular Research 2006, 72(3):384-393.
- Qian C, Xu D, Wang J, Luo Y, Jin T, Huang L, Zhou Y, Cai Z, Jin B, Bao H, Wang Y: Toll-like receptor 2 deficiency ameliorates obesity-induced cardiomyopathy via inhibiting NF-κB signaling pathway. Int Immunopharmacol 2024, 128:111551.
- Higashikuni Y, Tanaka K, Kato M, Nureki O, Hirata Y, Nagai R, Komuro I, Sata M: Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1β upregulation via nuclear factor κB activation. J Am Heart Assoc 2013, 2(6):e000267.
- Zheng X-y, Sun C-c, Liu Q, Lu X-y, Fu L-l, Liang G, Zhang X-h, Chen G-z: Compound LM9, a novel MyD88 inhibitor, efficiently mitigates inflammatory responses and fibrosis in obesity-induced cardiomyopathy. Acta Pharmacologica Sinica 2020, 41(8):1093-1101.
- Hu N, Zhang Y: TLR4 knockout attenuated high fat diet-induced cardiac dysfunction via NF-κB/JNK-dependent activation of autophagy. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2017, 1863(8):2001-2011.
- Wang S, Han Y, Liu R, Hou M, Neumann D, Zhang J, Wang F, Li Y, Zhao X, Schianchi F et al: Glycolysis-Mediated Activation of v-ATPase by Nicotinamide Mononucleotide Ameliorates Lipid-Induced Cardiomyopathy by Repressing the CD36-TLR4 Axis. Circ Res 2024, 134(5):505-525.
- Liu H, Jia W, Tang Y, Zhang W, Qi J, Yan J, Ding W, Cao H, Liang G, Zhu Z et al: Inhibition of MyD88 by LM8 Attenuates Obesity-Induced Cardiac Injury. J Cardiovasc Pharmacol 2020, 76(1):63-70.
- Xu Z, Kong X-Q: Bixin ameliorates high fat diet-induced cardiac injury in mice through inflammation and oxidative stress suppression. Biomedicine & Pharmacotherapy 2017, 89:991-1004.
- Zhang Y, Wu J, Dong E, Wang Z, Xiao H: Toll-like receptors in cardiac hypertrophy. Frontiers in Cardiovascular Medicine 2023, 10.
- Gao J, Xie Q, Wei T, Huang C, Zhou W, Shen W: Nebivolol Improves Obesity-Induced Vascular Remodeling by Suppressing NLRP3 Activation. J Cardiovasc Pharmacol 2019, 73(5):326-333.
- Ralston JC, Lyons CL, Kennedy EB, Kirwan AM, Roche HM: Fatty Acids and NLRP3 Inflammasome–Mediated Inflammation in Metabolic Tissues. Annual Review of Nutrition 2017, 37(Volume 37, 2017):77-102.
- Yaron J, Gangaraju S, Rao M, Kong X, Zhang L, Su F, Tian Y, Glenn H, Meldrum D: K+ regulates Ca2+ to drive inflammasome signaling: Dynamic visualization of ion flux in live cells. Cell Death & Disease 2015, 6.
- Saeki K, Yokomizo T: Identification, signaling, and functions of LTB4 receptors. Seminars in Immunology 2017, 33:30-36.
- Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Mayoral R, Maris M: LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nature medicine 2015, 21(3):239-247.
- Corbin A, Aromolaran KA, Aromolaran AS: Leukotriene B4 is elevated in diabetes and promotes ventricular arrhythmogenesis in guinea pig. Journal of Cellular Physiology 2024, n/a(n/a):e31467.
- Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B: Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol 2004, 24(2):369-375.
- Lefebvre B, Pépin JL, Baguet JP, Tamisier R, Roustit M, Riedweg K, Bessard G, Lévy P, Stanke-Labesque F: Leukotriene B4: early mediator of atherosclerosis in obstructive sleep apnoea? Eur Respir J 2008, 32(1):113-120.
- Molaie M, Lotfi R, Heidari Moghadam R, Rezaiemanesh A, Karaji AG, Salari F: Imbalanced serum levels of resolvin E1 (RvE1) and leukotriene B4 (LTB4) may contribute to the pathogenesis of atherosclerosis. Prostaglandins & Other Lipid Mediators 2023, 169:106781.
- Horii Y, Nakaya M, Ohara H, Nishihara H, Watari K, Nagasaka A, Nakaya T, Sugiura Y, Okuno T, Koga T et al: Leukotriene B4 receptor 1 exacerbates inflammation following myocardial infarction. The FASEB Journal 2020, 34(6):8749-8763.
- Xu S, Tang L, Mi Y, Jiang J, Zhu M, Chen B, Wang B, Li T, Xu C, Wang J et al: Clinical significance of leukotriene b4 and extracellular matrix metalloproteinase inducer in acute coronary syndrome. Clin Invest Med 2013, 36(6):E282-289.
- Sasaki K, Ueno A, Katori M, Kikawada R: Detection of leukotriene B4 in cardiac tissue and its role in infarct extension through leucocyte migration. Cardiovascular Research 1988, 22(2):142-148.
- Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B: Deficiency of the Leukotriene B4 Receptor, BLT-1, Protects against Systemic Insulin Resistance in Diet-Induced Obesity. The Journal of Immunology 2011, 187(4):1942-1949.
- Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Mayoral R, Maris M et al: LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nature Medicine 2015, 21(3):239-247.
- Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N: The effects of doxorubicin on cardiac calcium homeostasis and contractile function. Journal of Cardiology 2022, 80(2):125-132.
- Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, Paccone A, Altucci L, Conte M, Canale ML et al: The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovascular Diabetology 2021, 20(1):150.
- Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ: Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res 2023, 118(18):3434-3450.
- Aitken-Buck HM, Moharram M, Babakr AA, Reijers R, Van Hout I, Fomison-Nurse IC, Sugunesegran R, Bhagwat K, Davis PJ, Bunton RW et al: Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte 2019, 8(1):412-420.
- Tager AM, Luster AD: BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids 2003, 69(2-3):123-134.
- Sánchez-Galán E, Gómez-Hernández A, Vidal C, Martín-Ventura JL, Blanco-Colio LM, Muñoz-García B, Ortega L, Egido J, Tuñón J: Leukotriene B4 enhances the activity of nuclear factor-κB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovascular Research 2009, 81(1):216-225.
- Yokomizo T, Shimizu T: The leukotriene B receptors BLT1 and BLT2 as potential therapeutic targets. Immunological Reviews 2023, 317(1):30-41.
- Huang L, Zhao A, Wong F, Ayala JM, Struthers M, Ujjainwalla F, Wright SD, Springer MS, Evans J, Cui J: Leukotriene B4 Strongly Increases Monocyte Chemoattractant Protein-1 in Human Monocytes. Arteriosclerosis, Thrombosis, and Vascular Biology 2004, 24(10):1783-1788.
- Wenzl FA, Ambrosini S, Mohammed SA, Kraler S, Lüscher TF, Costantino S, Paneni F: Inflammation in Metabolic Cardiomyopathy. Frontiers in Cardiovascular Medicine 2021, 8.
- Liu Y, Lu X, Li X, Du P, Qin G: High-fat diet triggers obesity-related early infiltration of macrophages into adipose tissue and transient reduction of blood monocyte count. Molecular Immunology 2020, 117:139-146.
- Gaudreault É, Paquet-Bouchard C, Fiola S, Le Bel M, Lacerte P, Shio MT, Olivier M, Gosselin J: TAK1 contributes to the enhanced responsiveness of LTB4-treated neutrophils to Toll-like receptor ligands. International Immunology 2012, 24(11):693-704.
- Wang Z, Filgueiras LR, Wang S, Serezani AP, Peters-Golden M, Jancar S, Serezani CH: Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. J Immunol 2014, 192(5):2349-2356.
- Santos-Bezerra DP, Filgueiras LR, Monteiro MB, Admoni SN, Perez RV, Cavaleiro AM, Machado CG, Machado UF, Passarelli M, Jancar S, Correa-Giannella ML: Leukotriene Pathway Activation Associates with Poor Glycemic Control and with Cardiovascular Autonomic Neuropathy in Type 1 Diabetes. Mediators Inflamm 2020, 2020:5704713.
- Duque A, Mediano MFF, De Lorenzo A, Rodrigues LF, Jr.: Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications. World J Diabetes 2021, 12(6):855-867.
- Bakkar N-MZ, Dwaib HS, Fares S, Eid AH, Al-Dhaheri Y, El-Yazbi AF: Cardiac Autonomic Neuropathy: A Progressive Consequence of Chronic Low-Grade Inflammation in Type 2 Diabetes and Related Metabolic Disorders. In: International Journal of Molecular Sciences. vol. 21; 2020.
- Bhati P, Alam R, Moiz JA, Hussain ME: Subclinical inflammation and endothelial dysfunction are linked to cardiac autonomic neuropathy in type 2 diabetes. Journal of Diabetes & Metabolic Disorders 2019, 18(2):419-428.
- Gonca E: The Effects of Zileuton and Montelukast in Reperfusion-Induced Arrhythmias in Anesthetized Rats. Current Therapeutic Research 2013, 75:27-32.
- Aromolaran AS: Leukotriene B4 signaling in diabetic ventricular arrhythmias. J Cell Physiol 2024.
- Jiang X-X, Zhu Y-R, Liu H-M, Chen S-L, Zhang D-M: Effect of BIN1 on cardiac dysfunction and malignant arrhythmias. Acta Physiologica 2020, 228(3):e13429.
- Zhou K, Hong T: Cardiac BIN1 (cBIN1) is a regulator of cardiac contractile function and an emerging biomarker of heart muscle health. Sci China Life Sci 2017, 60(3):257-263.
- Hong T, Yang H, Zhang S-S, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J et al: Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nature Medicine 2014, 20(6):624-632.
- Mackrill JJ: Evolution of the cardiac dyad. Philosophical Transactions of the Royal Society B: Biological Sciences 2022, 377(1864):20210329.
- Lawless M, Caldwell JL, Radcliffe EJ, Smith CER, Madders GWP, Hutchings DC, Woods LS, Church SJ, Unwin RD, Kirkwood GJ et al: Phosphodiesterase 5 inhibition improves contractile function and restores transverse tubule loss and catecholamine responsiveness in heart failure. Sci Rep 2019, 9(1):6801.
- Hong T-T, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM: BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS biology 2010, 8(2):e1000312.
- Hong T-T, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, Fang K, Halushka MK, Russell SD, Colecraft H: BIN1 is reduced and Cav1. 2 trafficking is impaired in human failing cardiomyocytes. Heart rhythm 2012, 9(5):812-820.
- Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T: Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)–Organized Dyads. Circulation 2016, 133(4):388-397.
- Liu Y, Zhou K, Li J, Agvanian S, Caldaruse A-M, Shaw S, Hitzeman Tara C, Shaw Robin M, Hong T: In Mice Subjected to Chronic Stress, Exogenous cBIN1 Preserves Calcium-Handling Machinery and Cardiac Function. JACC: Basic to Translational Science 2020, 5(6):561-578.
- Hong T-T, Cogswell R, James CA, Kang G, Pullinger CR, Malloy MJ, Kane JP, Wojciak J, Calkins H, Scheinman MM et al: Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2012, 9(6):961-967.
- on AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS et al: Plasticity of surface structures and β(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail 2012, 5(3):357-365.
- Li J, Richmond B, Cluntun AA, Bia R, Walsh MA, Shaw K, Symons JD, Franklin S, Rutter J, Funai K et al: Cardiac gene therapy treats diabetic cardiomyopathy and lowers blood glucose. JCI Insight 2023, 8(18).
- Harvey SE, Cheng C: Methods for Characterization of Alternative RNA Splicing. Methods Mol Biol 2016, 1402:229-241.
- Xu B, Fu Y, Liu Y, Agvanian S, Wirka RC, Baum R, Zhou K, Shaw RM, Hong T: The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol 2017, 15(8):e2002354.
- Nikolova AP, Hitzeman TC, Baum R, Caldaruse A-M, Agvanian S, Xie Y, Geft DR, Chang DH, Moriguchi JD, Hage A et al: Association of a Novel Diagnostic Biomarker, the Plasma Cardiac Bridging Integrator 1 Score, With Heart Failure With Preserved Ejection Fraction and Cardiovascular Hospitalization. JAMA Cardiology 2018, 3(12):1206-1210.
- Marcel R, Meyer DM: An overview of approved and investigational left ventricular assist devices. Proc (Bayl Univ Med Cent) 2004, 17(4):407-410.
- Rosenblum H, Brener M, Burkhoff D: Theoretical considerations for a left atrial pump in heart failure with preserved ejection fraction. Heart Fail Rev 2023, 28(2):273-280.
- Rose Eric A, Gelijns Annetine C, Moskowitz Alan J, Heitjan Daniel F, Stevenson Lynne W, Dembitsky W, Long James W, Ascheim Deborah D, Tierney Anita R, Levitan Ronald G et al: Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. New England Journal of Medicine, 345(20):1435-1443.
- Galand V, Flécher E, Auffret V, Pichard C, Boulé S, Vincentelli A, Rollin A, Mondoly P, Barandon L, Pernot M et al: Early Ventricular Arrhythmias After LVAD Implantation Is the Strongest Predictor of 30-Day Post-Operative Mortality. JACC: Clinical Electrophysiology 2019, 5(8):944-954.
- Ito K, Nakayama M, Hasan F, Yan X, Schneider MD, Lorell BH: Contractile reserve and calcium regulation are depressed in myocytes from chronically unloaded hearts. Circulation 2003, 107(8):1176-1182.
- Fischer TH, Kleinwächter A, Herting J, Eiringhaus J, Hartmann N, Renner A, Gummert J, Haverich A, Schmitto JD, Sossalla S: Inhibition of CaMKII Attenuates Progressing Disruption of Ca2+ Homeostasis Upon Left Ventricular Assist Device Implantation in Human Heart Failure. Artificial Organs 2016, 40(8):719-726.
- Refaat M, Chemaly E, Lebeche D, Gwathmey JK, Hajjar RJ: Ventricular arrhythmias after left ventricular assist device implantation. Pacing Clin Electrophysiol 2008, 31(10):1246-1252.
- Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR, Burkhoff D: Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol 2005, 45(5):668-676.
- Terracciano CM, Harding SE, Adamson D, Koban M, Tansley P, Birks EJ, Barton PJ, Yacoub MH: Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur Heart J 2003, 24(14):1329-1339.
- Chaudhary KW, Rossman EI, Piacentino V, 3rd, Kenessey A, Weber C, Gaughan JP, Ojamaa K, Klein I, Bers DM, Houser SR, Margulies KB: Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol 2004, 44(4):837-845.
- Terracciano CM, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH: Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation 2004, 109(19):2263-2265.
- Yacoub MH: A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J 2001, 22(7):534-540.
- Khan MS, Kyriakopoulos CP, Taleb I, Dranow E, Scott M, Ranjan R, Yin M, Tseliou E, Alharethi R, Caine W et al: Baseline QRS duration associates with cardiac recovery in patients with continuous-flow left ventricular assist device implantation. Am Heart J Plus 2022, 22:100211.
- Gude E, Fiane AE: Can mechanical circulatory support be an effective treatment for HFpEF patients? Heart Fail Rev 2023, 28(2):297-305.
- Topilsky Y, Pereira NL, Shah DK, Boilson B, Schirger JA, Kushwaha SS, Joyce LD, Park SJ: Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail 2011, 4(3):266-275.
- Klaeske K, Messer EK, Klein S, Sieg F, Eifert S, Haunschild J, Jawad K, Saeed D, Dashkevich A, Borger MA, Dieterlen MT: Body mass index-dependent immunological profile changes after left ventricular assist device implantation. Front Immunol 2023, 14:1256725.
- Messer EK, Meyer AL, Klaeske K, Sieg F, Eifert S, Schmiedel D, Haunschild J, Jawad K, Saeed D, Hildebrandt L et al: The Impact of Obesity on T and NK Cells after LVAD Implantation. Obes Facts 2023, 16(4):364-373.
- Just IA, Schoenrath F, Roehrich L, Heil E, Stein J, Auer TA, Fehrenbach U, Potapov E, Solowjowa N, Balzer F et al: Artificial intelligence-based analysis of body composition predicts outcome in patients receiving long-term mechanical circulatory support. J Cachexia Sarcopenia Muscle 2024, 15(1):270-280.
- Kyriakopoulos CP, Taleb I, Tseliou E, Sideris K, Hamouche R, Maneta E, Nelson M, Krauspe E, Selko S, Visker JR et al: Impact of Diabetes and Glycemia on Cardiac Improvement and Adverse Events Following Mechanical Circulatory Support. J Am Heart Assoc 2024, 13(14):e032936.
- Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V et al: Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014, 16(10):1096-1103.
- Fukamachi K, Horvath DJ, Karimov JH, Kado Y, Miyamoto T, Kuban BD, Starling RC: Left atrial assist device to treat patients with heart failure with preserved ejection fraction: Initial in vitro study. The Journal of Thoracic and Cardiovascular Surgery 2021, 162(1):120-126.
- Miyagi C, Kuban BD, Flick CR, Polakowski AR, Miyamoto T, Karimov JH, Starling RC, Fukamachi K: Left atrial assist device for heart failure with preserved ejection fraction: initial results with torque control mode in diastolic heart failure model. Heart Failure Reviews 2023, 28(2):287-296.
- Lester RM: Update on ICH E14/S7B Cardiac Safety Regulations: The Expanded Role of Preclinical Assays and the "Double-Negative" Scenario. Clin Pharmacol Drug Dev 2021, 10(9):964-973.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





