Submitted:
05 November 2024
Posted:
06 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
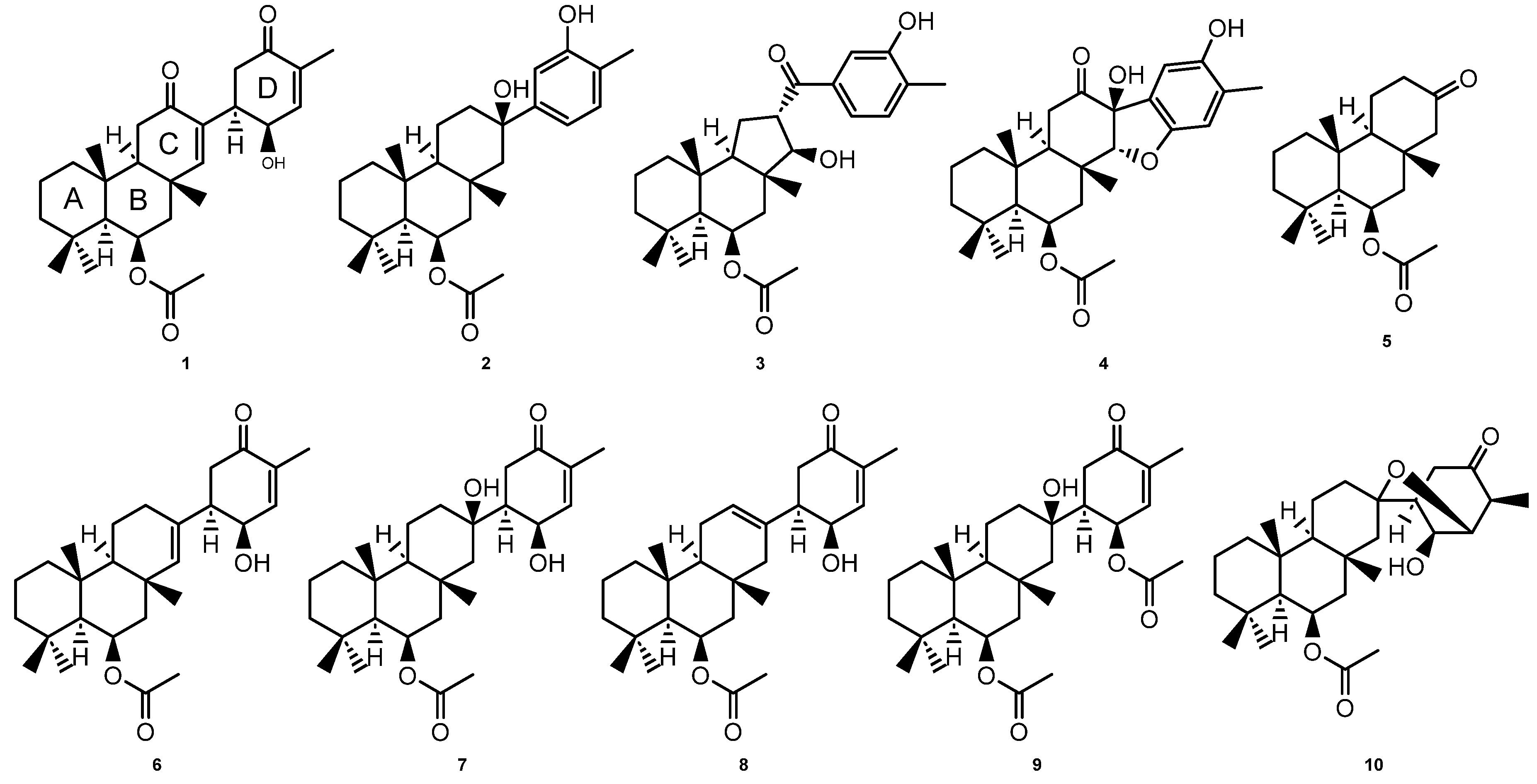
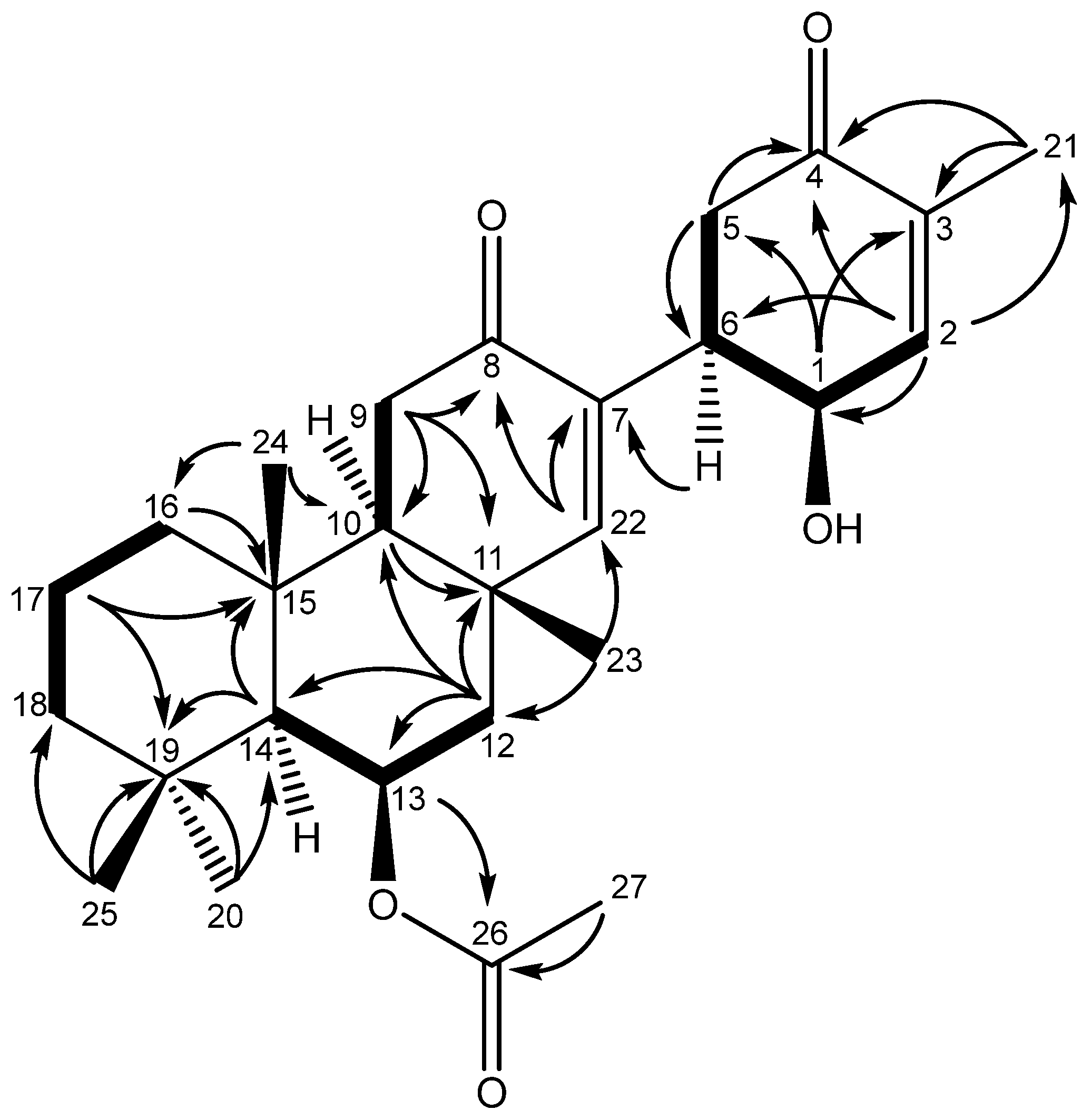
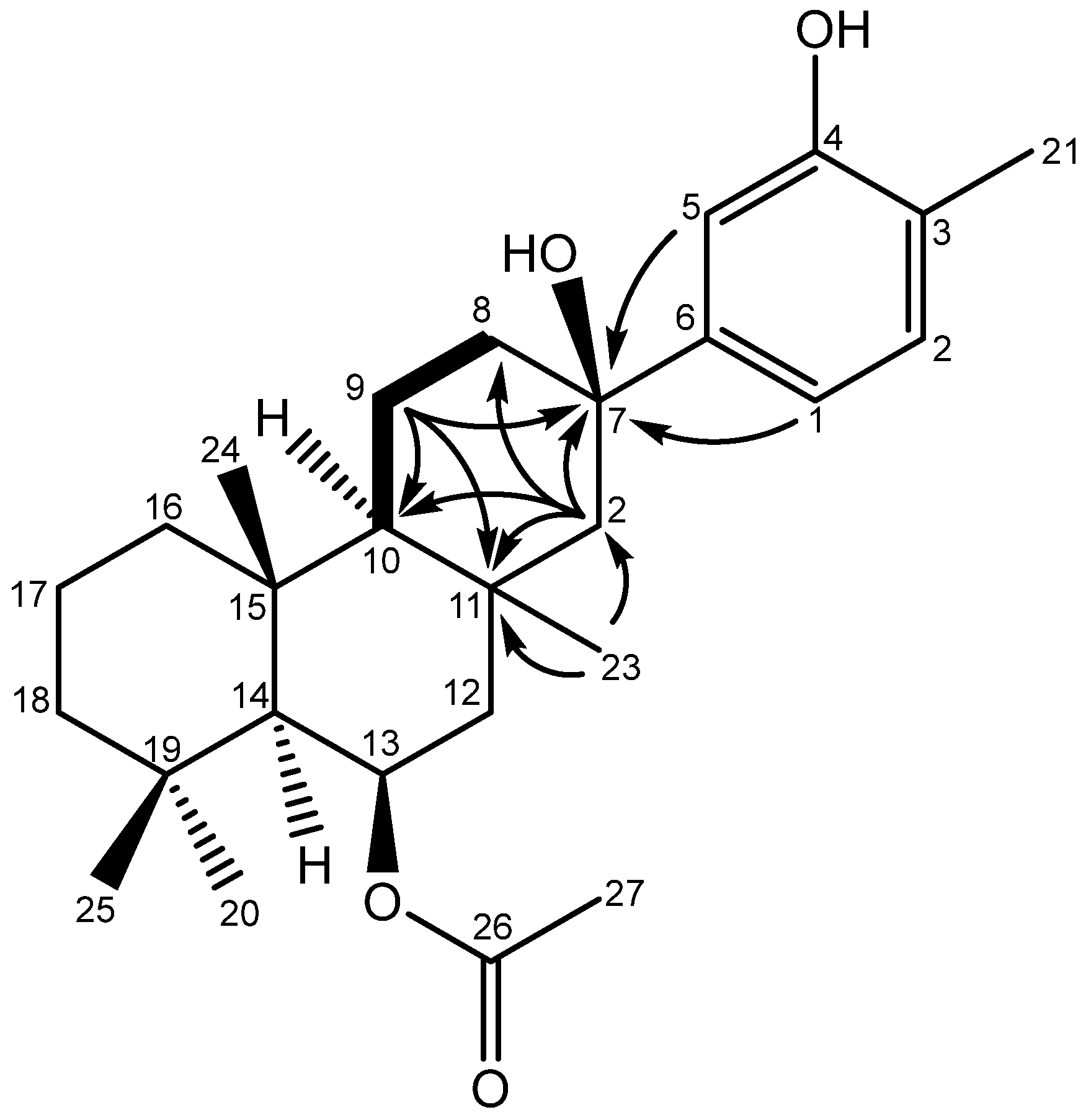
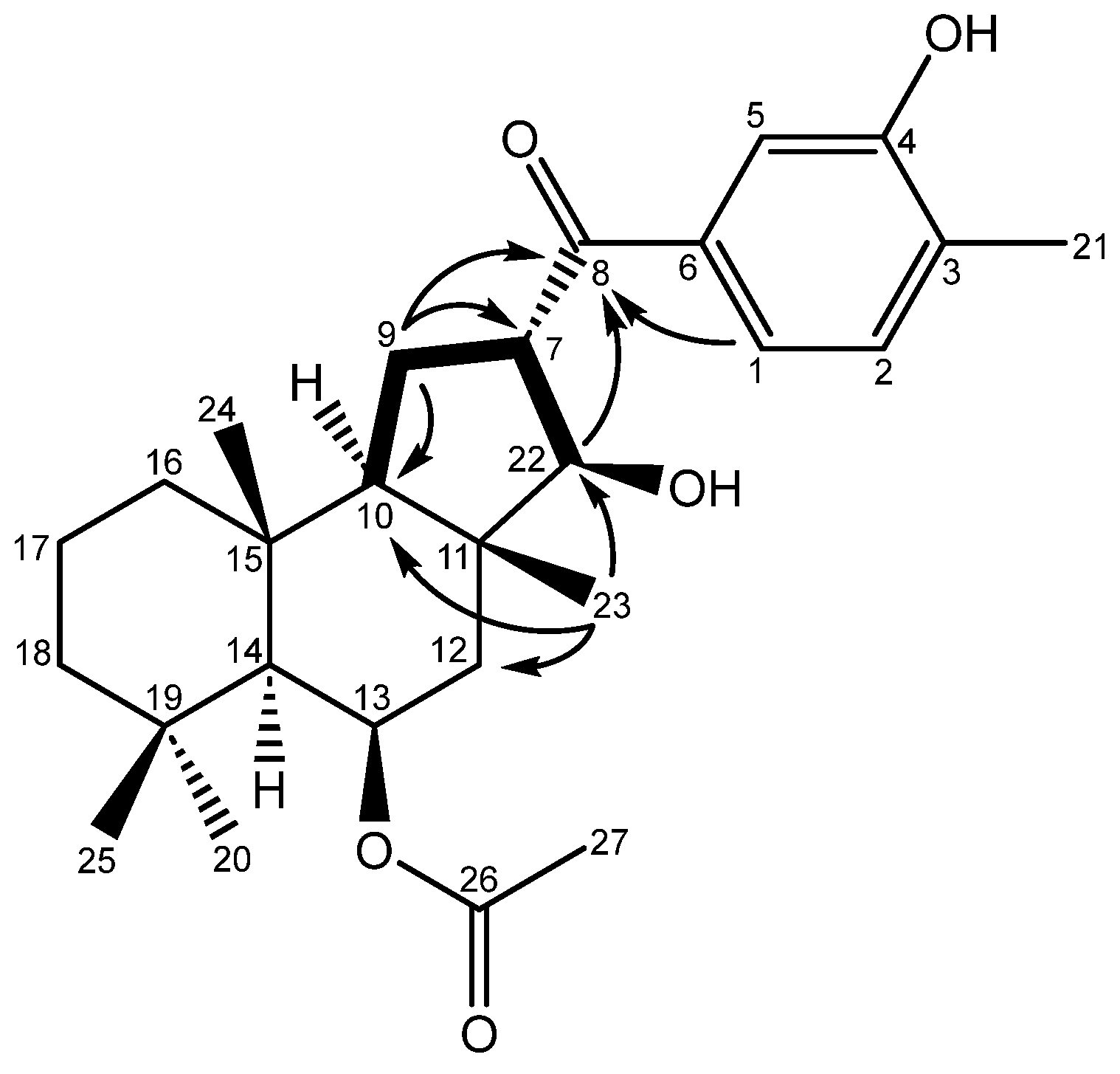
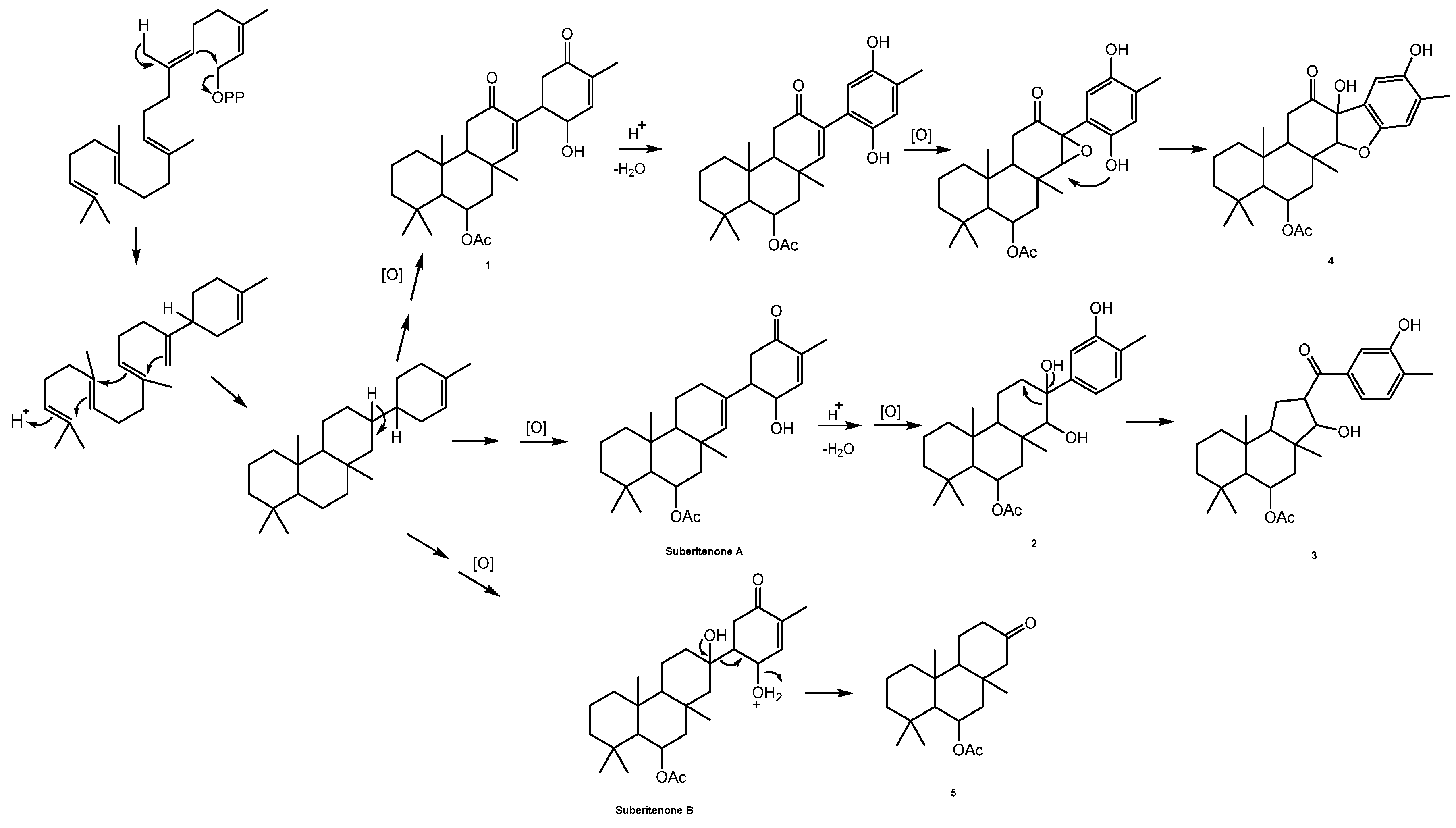
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Materials, Extraction, and Isolation
3.3. Spectroscopic Data for the Suberites (1-5)
3.4. RSV Antiviral Assay
3.5. X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amsler, C.D.; Moeller, C.B.; McClintock, J.B.; Iken, K.B.; Baker, B.J. Chemical defenses against diatom fouling in Antarctic marine sponges. Biofouling 2000, 16, 29–45. [Google Scholar] [CrossRef]
- McClintock, J.B.; Amsler, C.D.; Baker, B.J.; Van Soest, R.W. Ecology of Antarctic marine sponges: an overview. Integr. Comp. Biol. 2005, 45, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C. Marine natural products in medicinal chemistry. Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; de Rond, T.; Burkhardt, I.; Steele, T.S.; Schäfer, R.J.; Podell, S.; Allen, E.E.; Moore, B.S. Terpene biosynthesis in marine sponge animals. Proc. Natl. Acad. Sci. 2023, 120, e2220934120. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Pons, L.; Shilling, A.; Verde, C.; Baker, B.J.; Giordano, D. Marine terpenoids from polar latitudes and their potential applications in biotechnology. Mar. Drugs 2020, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Bracegirdle, J.; Olsen, S.S.; Teng, M.N.; Tran, K.C.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Neosuberitenone, a New Sesterterpenoid Carbon Skeleton; New Suberitenones; and Bioactivity against Respiratory Syncytial Virus, from the Antarctic Sponge Suberites sp. Mar. Drugs 2023, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Ahn, J.-W.; Lee, Y.-H.; Rho, J.-R.; Shin, J. New sesterterpenes from the antarctic sponge Suberites sp. J. Nat. Prod. 2004, 67, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, Y.; Rho, J.-R.; Baek, E.; Kwon, B.-M.; Jeong, T.-S.; Bok, S.-H. Suberitenones A and B: Sesterterpenoids of an unprecedented skeletal class from the Antarctic sponge Suberites sp. J. Org. Chem. 1995, 60, 7582–7588. [Google Scholar] [CrossRef]
- Dı́az-Marrero, A.R.; Brito, I.; Cueto, M.; San-Martı́n, A.; Darias, J. Suberitane network, a taxonomical marker for Antarctic sponges of the genus Suberites? Novel sesterterpenes from Suberites caminatus. Tetrahedron Lett. 2004, 45, 4707–4710. [Google Scholar] [CrossRef]
- Bruker APEX4, 2015.9; Bruker AXS Inc.: Madison, WI, USA, 2022.
- Bruker SAINT, 8.35A; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sec. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G., M. Crystal structure refinement with SHELXL. Acta Crystallographica Section C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
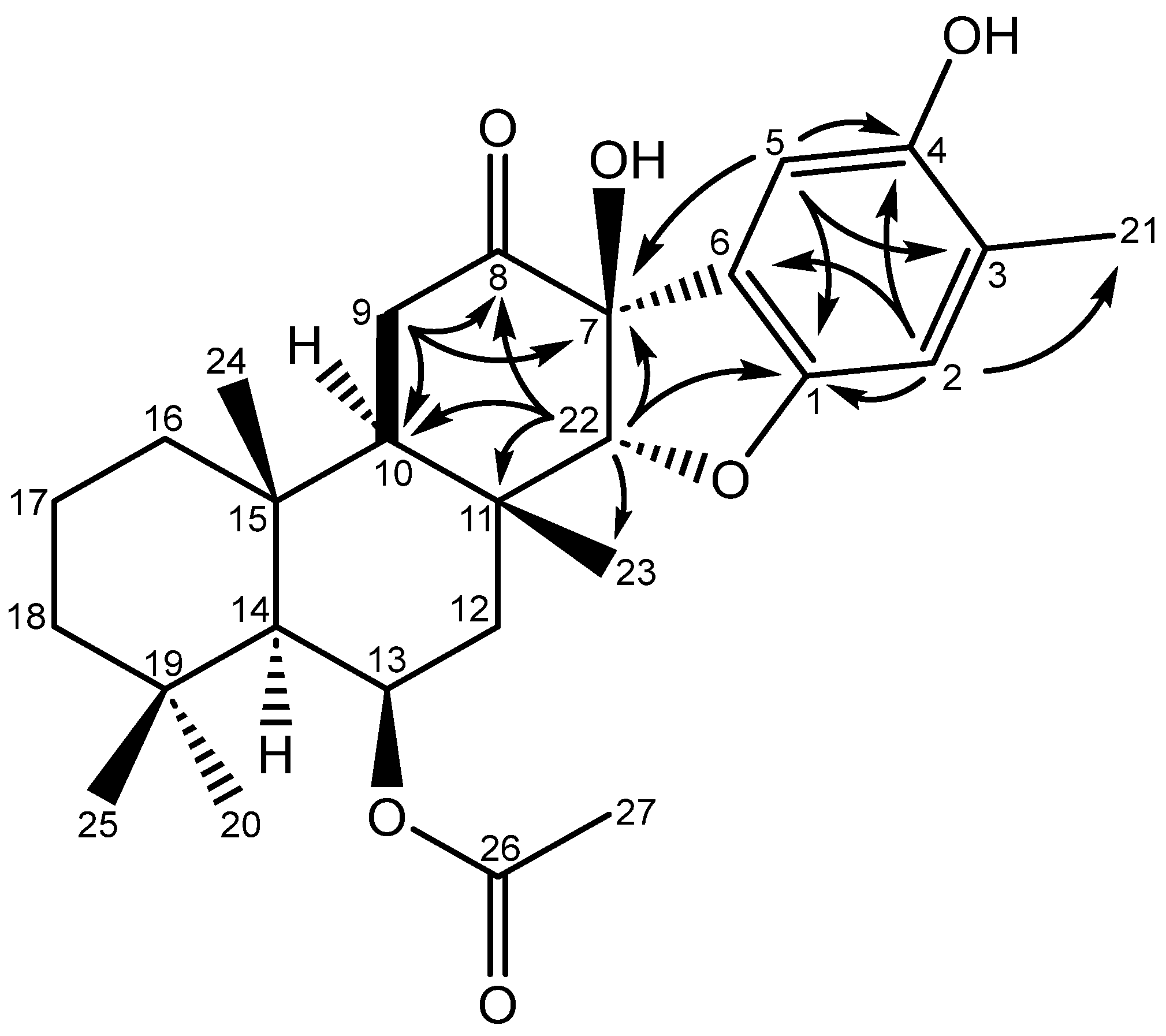
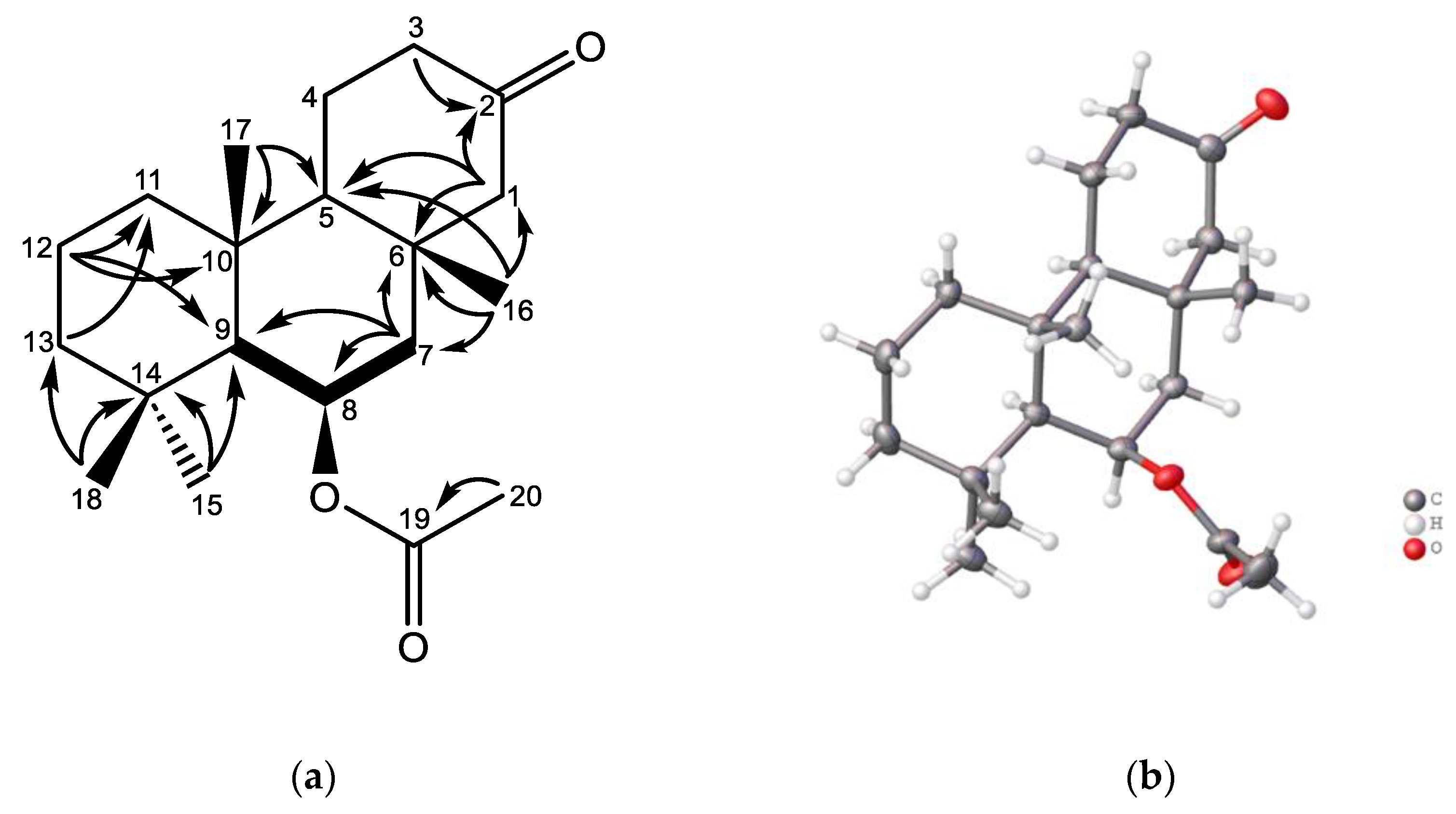
| Position | δC, type | δH (mult., (J)) | gCOSY | gHMBC |
|---|---|---|---|---|
| 1 | 65.2, CH | 4.25, dd (4.5, 5.0) | 2, 6 | 2, 3, 5 |
| 2 | 145.3, CH | 6.80, dq (1.3, 5.6) | 1, 21 | 1, 4, 6, 21 |
| 3 | 137.2, C | |||
| 4 | 202.2*, C | |||
| 5a | 37.6, C | 2.75, dd (13.5, 16.1) | 5b, 6 | 1, 4, 6, 7 |
| 5b | 2.15, dd (3.5, 16.1) | 5a, 6 | 1, 3, 4, 6, 7 | |
| 6 | 38.5, CH | 3.35, o/l* | 1, 5a, 5b | 1, 5, 7, 22 |
| 7 | 134.2, C | |||
| 8 | 202.1*, C | |||
| 9a | 35.6, CH2 | 2.52, d (12.6) | 9b, 10 | 8, 10, 11 |
| 9b | 2.50, d (4.9) | 9a, 10 | ||
| 10 | 54.9, CH | 1.96, dd (5.4, 12.5) | 9a, 9b | 8, 9, 11, 22, 23, 24 |
| 11 | 37.7, C | |||
| 12a | 43.9, CH2 | 2.06, o/l* | 12b, 13 | 10, 11, 13, 14, 22, 23 |
| 12b | 1.66, o/l* | 12a, 13 | 11, 22, 23 | |
| 13 | 72.0, CH | 5.59, ddd (2.6, 2.8, 2.9) | 12a, 12b, 14 | 12, 14, 26 |
| 14 | 57.3, CH | 1.23, o/l* | 13 | 15, 19, 20, 24 |
| 15 | 38.2, C | |||
| 16a | 42.2, CH2 | 1.69, o/l* | 16b, 17a, 17b | 15 |
| 16b | 0.98, o/l* | 16a, 17a, 17b | ||
| 17a | 19.5, CH2 | 1.81, o/l* | 16a, 16b, 17b, 18a, 18b | |
| 17b | 1.51, qd (3.1, 14.3) | 16a, 16b, 17a, 18a, 18b | 15, 19 | |
| 18a | 45.3, CH2 | 1.41, ddd (3.5, 4.0, 12.9) | 17a, 17b, 18b | 16 |
| 18b | 1.26, o/l* | 17a, 17b, 18a | ||
| 19 | 35.1, C | |||
| 20 | 33.3, CH3 | 0.94, s | 14, 18, 19, 25 | |
| 21 | 15.7, CH3 | 1.79, s | 2, 3, 4 | |
| 22 | 160.7, C | 6.40, s | 6, 7, 8, 10, 12, 23 | |
| 23 | 20.9, CH3 | 1.29, s | 10, 11, 12, 22 | |
| 24 | 17.4, CH3 | 1.34, s | 10, 14, 15, 16 | |
| 25 | 23.7, CH3 | 1.06, s | 14, 18, 19, 20 | |
| 26 | 172.1, C | |||
| 27 | 21.9, CH3 | 2.07, s | 26 |
| Position | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|
| δC, type | δH (mult., (J)) | δC, type | δH (mult., (J)) | δC, type | δH (mult., (J)) | |
| 1 | 116.6, CH | 6.90, d (7.8) | 121.4, CH | 7.43, dd (1.0, 7.9) | 155.3, C | |
| 2 | 130.7, CH | 7.07, d (7.9) | 131.0, CH | 7.19, d (7.8) | 112.1, CH | 6.70, s |
| 3 | 122.0, C | 130.6, C | 128.4, C | |||
| 4 | 153.6, C | 154.2, C | 148.4, C | |||
| 5 | 111.4, CH | 6.94, s | 114.5, CH | 7.41, d (1.0) | 109.7, CH | 6.42, s |
| 6 | 149.5, C | 136.0, C | 124.2, C | |||
| 7 | 74.4, C | 50.1, CH | 3.58, ddd (4.0, 7.9, 11.9) | 83.3, C | ||
| 8 | 41.1, CH2 | 1.90, o/l* | 202.1, C | 210.8, C | ||
| 9a | 17.5, CH2 | 1.86, o/l* | 26.2, CH2 | 2.09, o/l* | 33.9, CH2 | 2.48, dd (10.6, 19.0) |
| 9b | 1.69, o/l* | 1.66, ddd (3.0, 3.5, 12.8) | 2.33, dd (19.1, 9.0) | |||
| 10 | 58.9, CH | 1.06, o/l* | 56.4, CH | 1.30, dd (7.1, 12.9) | 45.9, CH | 1.88, t (9.6) |
| 11 | 34.8, C | 43.1, C | 38.3, C | |||
| 12a | 46.7, CH2 | 1.92, o/l* | 42.6, CH2 | 2.18, dd (2.1, 14.5) | 38.9, CH2 | 2.29, dd (4.2, 15.1) |
| 12b | 1.27, o/l* | 1.39, o/l* | 1.82, dd (2.4, 15.0) | |||
| 13 | 70.6, CH | 5.49, dt (2.5, 3.1) | 70.7, CH | 5.51, br dt (2.4, 2.5) | 69.8, CH | 5.64, td (2.1, 3.7) |
| 14 | 56.8, CH | 1.08, d (2.1) | 57.5, CH | 1.01, o/l* | 55.2, CH | 1.04, d (1.6) |
| 15 | 37.2, C | 36.9, C | 37.4, C | |||
| 16a | 41.9, CH2 | 1.82, o/l* | 41.5, CH2 | 1.40, o/l* | 41.8, CH2 | 1.37, o/l* |
| 16b | 0.92, o/l* | 0.94, o/l* | 0.76, dt (2.8, 12.2) | |||
| 17a | 18.6, CH2 | 1.75, o/l* | 18.1, CH2 | 1.70, o/l* | 18.1, CH2 | 1.62, m |
| 17b | 1.50, o/l* | 1.41, o/l* | 1.37, o/l* | |||
| 18a | 44.3, CH2 | 1.39, o/l* | 44.3, CH2 | 1.39, o/l* | 43.6, CH2 | 1.36, o/l* |
| 18b | 1.20, o/l* | 1.20, o/l* | 1.17, dd (2.5, 13.7) | |||
| 19 | 34.1, C | 33.7, C | 33.8, C | |||
| 20 | 32.9, CH3 | 0.94, s | 33.0, CH3 | 0.92, s | 33.1, CH3 | 0.98, s |
| 21 | 15.3, CH3 | 2.23, s | 16.1, CH3 | 2.30, s | 16.6, CH3 | 2.23, s |
| 22a | 57.2, CH2 | 1.59, d (14.3) | 83.5, CH | 4.02, d (7.8) | 97.4, CH | 4.19, s |
| 22b | 1.51, o/l* | |||||
| 23 | 22.7, CH3 | 1.43, s | 14.2, CH3 | 1.16, s | 18.9, CH3 | 1.00, s |
| 24 | 17.3, CH3 | 1.25, s | 16.7, CH3 | 1.26, s | 16.3, CH3 | 1.26, s |
| 25 | 23.0, CH3 | 1.03, s | 22.9, CH3 | 1.02, s | 23.2, CH3 | 1.00, s |
| 26 | 170.6, C | 170.4, C | 170.2, C | |||
| 27 | 21.9, CH3 | 2.07, s | 21.8, CH3 | 2.08, s | 21.8, CH3 | 2.06, s |
| Compound | IC50 (μM) |
|---|---|
| 1 | 41.8 |
| 2 | >50 |
| 3 | >50 |
| 4 | >50 |
| 5 | >50 |
| 9 | 15.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





