Submitted:
05 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
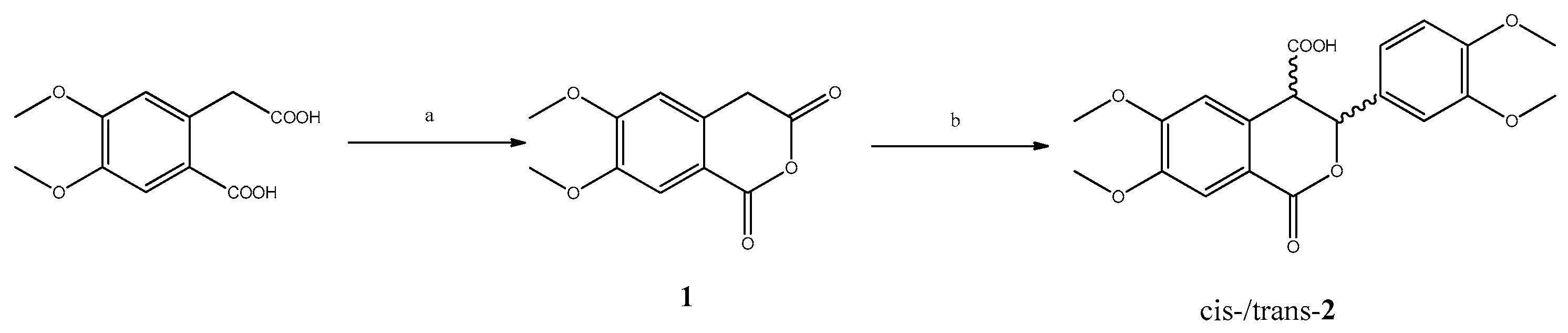
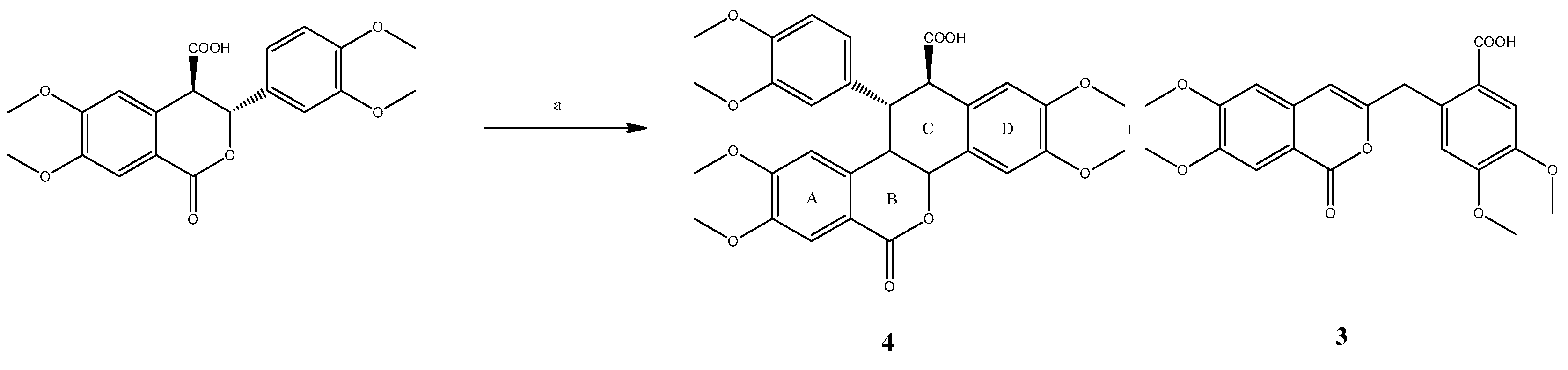
2. Results and Discussion
3. Materials and Methods
3.1. trans-11-(3,4-dimethoxyphenyl)-2,3,8,9-tetramethoxy-6-oxo-11,12-dihydro-6H-dibenzo[c,h]chromene-12-carboxylic acid (4):
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González-López, M., & Shaw, J. T. (2009). Cyclic Anhydrides in Formal Cycloadditions and Multicomponent Reactions. In Chemical Reviews (Vol. 109, Issue 1, pp. 164–189). American Chemical Society (ACS). [CrossRef]
- Moshnenko, N., Kazantsev, A., Bakulina, O., Dar’in, D., & Krasavin, M. (2022). The Use of Aryl-Substituted Homophthalic Anhydrides in the Castagnoli–Cushman Reaction Provides Access to Novel Tetrahydroisoquinolone Carboxylic Acid Bearing an All-Carbon Quaternary Stereogenic Center. In Molecules (Vol. 27, Issue 23, p. 8462). MDPI AG. [CrossRef]
- Liu, J., Wang, Z., Levin, A., Emge, T. J., Rablen, P. R., Floyd, D. M., & Knapp, S. (2014). N-Methylimidazole Promotes the Reaction of Homophthalic Anhydride with Imines. In The Journal of Organic Chemistry (Vol. 79, Issue 16, pp. 7593–7599). American Chemical Society (ACS). [CrossRef]
- Tietze, L. F., Brasche, G., & Gericke, K. M. (2006). Domino Reactions in Organic Synthesis. Wiley. [CrossRef]
- Beck, D. E., Agama, K., Marchand, C., Chergui, A., Pommier, Y., & Cushman, M. (2014). Synthesis and Biological Evaluation of New Carbohydrate-Substituted Indenoisoquinoline Topoisomerase I Inhibitors and Improved Syntheses of the Experimental Anticancer Agents Indotecan (LMP400) and Indimitecan (LMP776). In Journal of Medicinal Chemistry (Vol. 57, Issue 4, pp. 1495–1512). American Chemical Society (ACS). [CrossRef]
- Bogdanov, M. G., Mitrev, Y., & Tiritiris, I. (2010). New Highly Diastereoselective Perkin/Michael Addition Domino Reaction between Homophthalic Anhydride and Aromatic Aldehydes: A Facile Approach to Blue-Fluorescent Diben-zo[c,h]chromenones. In European Journal of Organic Chemistry (Vol. 2011, Issue 2, pp. 377–384). Wiley. [CrossRef]
- Pramanik, S., Jash, M., Mondal, D., & Chowdhury, C. (2019). Palladium-Catalyzed Synthesis of 6H-Dibenzo[c,h]chromenes and 5,6-Dihydrobenzo[c]phenanthridines: Application to the Synthesis of Dibenzo[c,h]chromene-6-ones, Benzo[c]phenanthridines, and Arnottin I. In Advanced Synthesis & Catalysis (Vol. 361, Issue 22, pp. 5223–5238). and studies cited within. [CrossRef]
- Misra, R., Tritch, H. R., & Pandey, R. C. (1985). Defucogilvocarcin V, a new antibiotic from Streptomyces arenae 2064: Isolation, characterization, partial synthesis and biological activity. In The Journal of Antibiotics (Vol. 38, Issue 9, pp. 1280–1283). Japan Antibiotics Research Association. [CrossRef]
- Oyola, R., Arce, R., Alegria, A. E., & Garcia, C. (1997). Photophysical Properties of Gilvocarcins V and M and Their Binding Constant to Calf Thymus DNA. In Photochemistry and Photobiology (Vol. 65, Issue 5, pp. 802–810). Wiley. [CrossRef]
- Sehgal, S. N., Czerkawski, H., Kudelski, A., Pandev, K., Saucier, R., & Vézina, C. (1983). Ravidomycin (AY-25,545),a new antitumor antibiotic. In The Journal of Antibiotics (Vol. 36, Issue 4, pp. 355–361). Japan Antibiotics Research Association. [CrossRef]
- Albert, L. M., Mewshaw, R. E., Edsall, R. J., Cohn, S. T., Harris, H. A., & Keith, J. C. (2002). Substituted 6h-dibenzo[c,h]chromenes as estrogenic agents EP1453820B1.
- Chiodi, D., & Ishihara, Y. (2024). The role of the methoxy group in approved drugs. In European Journal of Medicinal Chemistry (Vol. 273, p. 116364). Elsevier BV. [CrossRef]
- Bogdanov, M. G., & Palamareva, M. D. (2004). cis/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. In Tetrahedron (Vol. 60, Issue 11, pp. 2525–2530). Elsevier BV. [CrossRef]
- Karnik, M.; Usgaonkar, R.N. (1974). Indian Journal of Chemistry (Vol. 12, p.573).
- Schmid, C. R., Beck, C. A., Cronin, J. S., & Staszak, M. A. (2004). Demethylation of 4-Methoxyphenylbutyric Acid Using Molten Pyridinium Hydrochloride on Multikilogram Scale. In Organic Process Research & Development (Vol. 8, Issue 4, pp. 670–673). American Chemical Society (ACS). [CrossRef]
- Karplus, M. (1959). Contact Electron-Spin Coupling of Nuclear Magnetic Moments. In The Journal of Chemical Physics (Vol. 30, Issue 1, pp. 11–15). AIP Publishing. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



