Submitted:
02 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
Keywords:
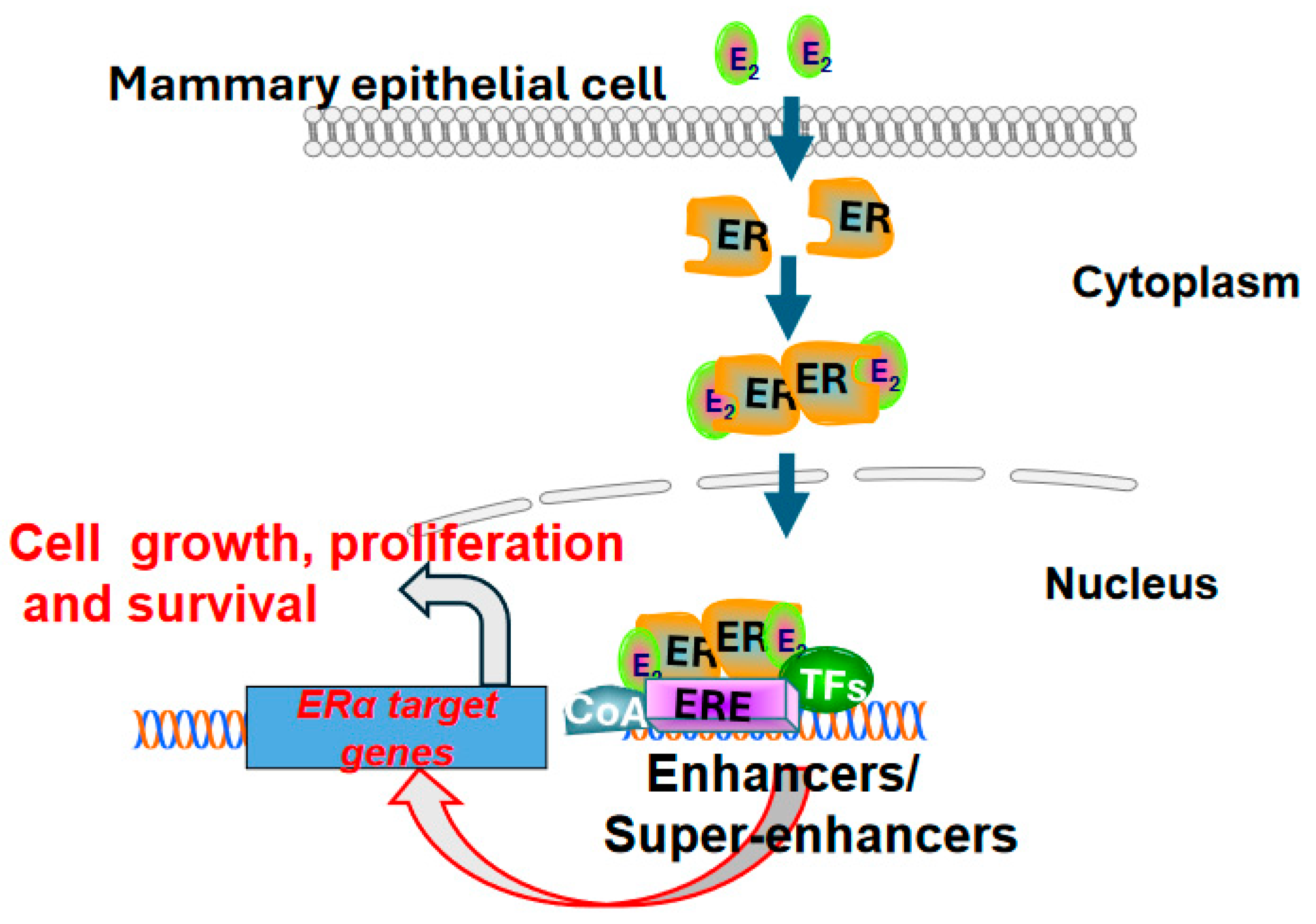
1. Introduction
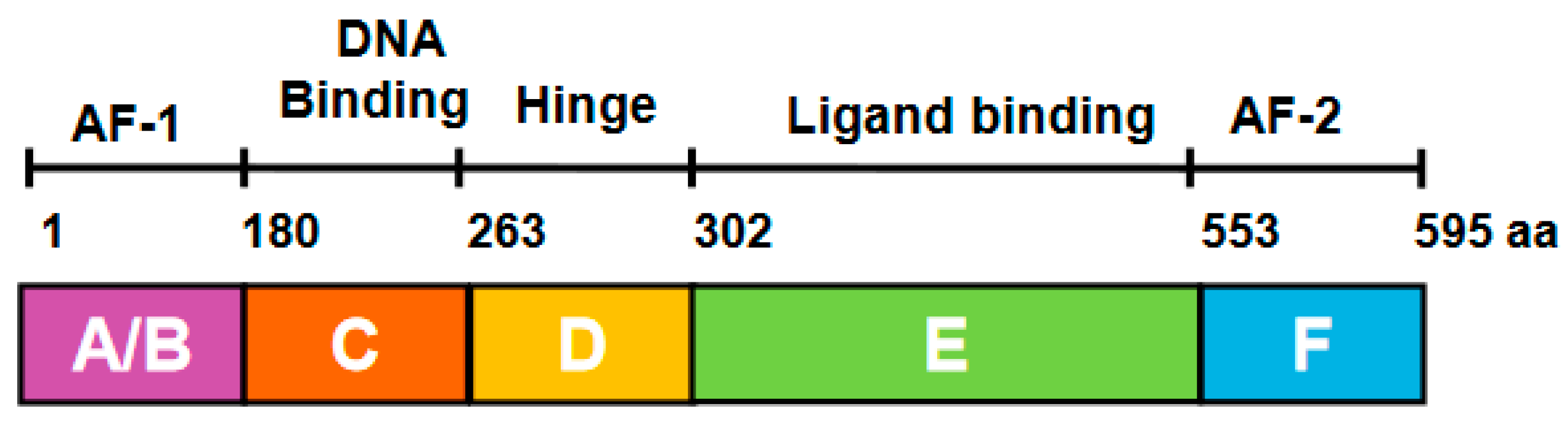
2. ERα Domain Structure
3. ERα in Human Breast Cancer
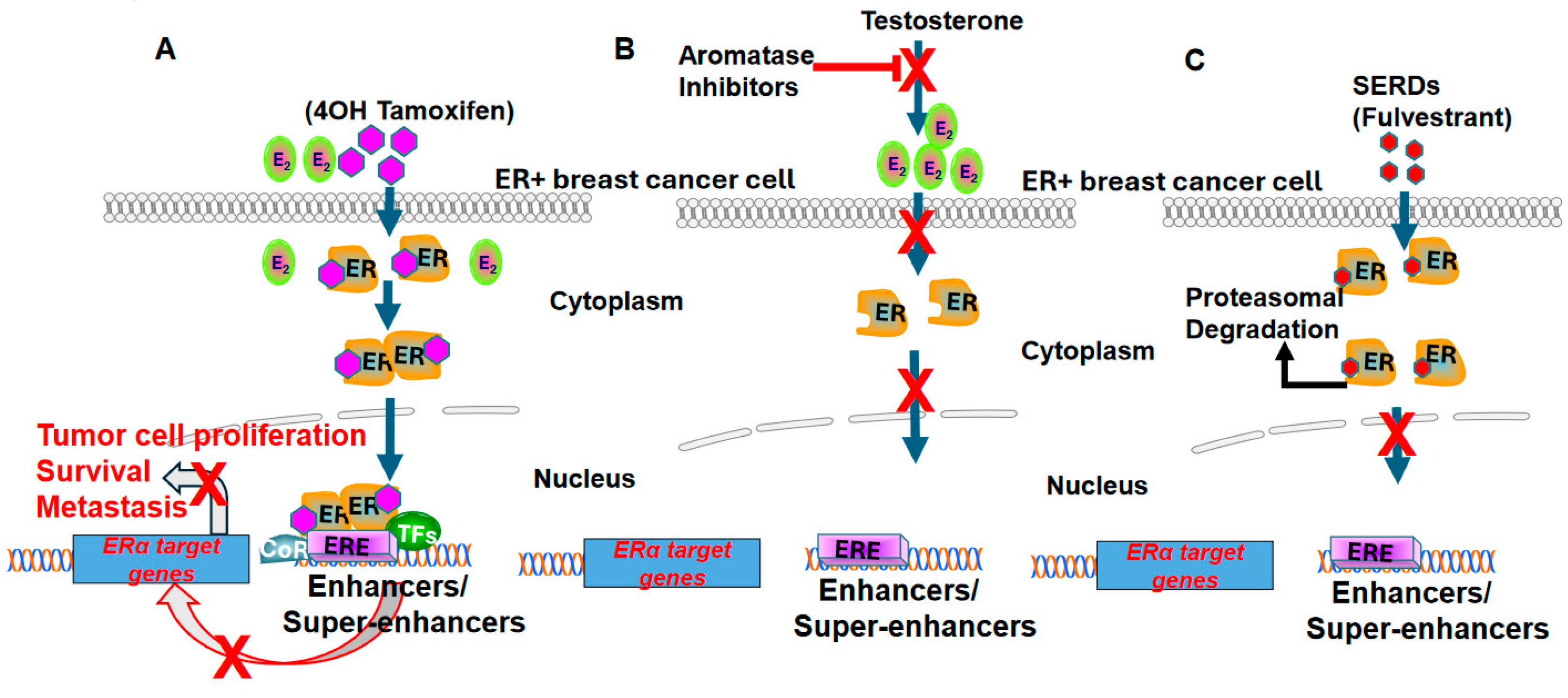
3.1. Endocrine Therapy in ER+ Breast Cancer
3.2. Endocrine-Resistant Breast Cancer
3.3. ESR1 Mutation
3.4. Therapeutic Strategies for ESR1 Mutant Breast Cancer.
4. SERDs in Clinical Trials
4.1. Giredestrant (GDC-9545)
4.2. Imlunestrant (LY3484356)
4.3. Camizestrant (AZD-9833)
4.4. Vepdegestrant (ARV-471)
4.5. Palazestrant (OP-1250)
5. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Toft, D.; Gorski, J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proceedings of the National Academy of Sciences of the United States of America 1966, 55, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Walter, P.; Kumar, V.; Krust, A.; Bornert, J.M.; Argos, P.; Chambon, P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 1986, 320, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.L.; Gilna, P.; Waterfield, M.; Baker, A.; Hort, Y.; Shine, J. Sequence and expression of human estrogen receptor complementary DNA. Science 1986, 231, 1150–1154. [Google Scholar] [CrossRef]
- Nilsson, S.; Makela, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol Rev 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Bjornstrom, L.; Sjoberg, M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005, 19, 833–842. [Google Scholar] [CrossRef]
- Gosden, J.R.; Middleton, P.G.; Rout, D. Localization of the human oestrogen receptor gene to chromosome 6q24----q27 by in situ hybridization. Cytogenet Cell Genet 1986, 43, 218–220. [Google Scholar] [CrossRef]
- Green, S.; Kumar, V.; Krust, A.; Walter, P.; Chambon, P. Structural and functional domains of the estrogen receptor. Cold Spring Harb Symp Quant Biol 1986, 51 Pt 2, 751–758. [Google Scholar] [CrossRef]
- Sarwar, N.; Kim, J.S.; Jiang, J.; Peston, D.; Sinnett, H.D.; Madden, P.; Gee, J.M.; Nicholson, R.I.; Lykkesfeldt, A.E.; Shousha, S.; et al. Phosphorylation of ERalpha at serine 118 in primary breast cancer and in tamoxifen-resistant tumours is indicative of a complex role for ERalpha phosphorylation in breast cancer progression. Endocr Relat Cancer 2006, 13, 851–861. [Google Scholar] [CrossRef]
- Rajbhandari, P.; Finn, G.; Solodin, N.M.; Singarapu, K.K.; Sahu, S.C.; Markley, J.L.; Kadunc, K.J.; Ellison-Zelski, S.J.; Kariagina, A.; Haslam, S.Z.; et al. Regulation of estrogen receptor alpha N-terminus conformation and function by peptidyl prolyl isomerase Pin1. Mol Cell Biol 2012, 32, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Klein-Hitpass, L.; Ryffel, G.U.; Heitlinger, E.; Cato, A.C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res 1988, 16, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Sentis, S.; Le Romancer, M.; Bianchin, C.; Rostan, M.C.; Corbo, L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol Endocrinol 2005, 19, 2671–2684. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Pestell, R.; Curran, E.M.; Welshons, W.V.; Fuqua, S.A. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer research 2004, 64, 9199–9208. [Google Scholar] [CrossRef]
- Berry, N.B.; Fan, M.; Nephew, K.P. Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol 2008, 22, 1535–1551. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engstrom, O.; Ohman, L.; Greene, G.L.; Gustafsson, J.A.; Carlquist, M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef]
- Arao, Y.; Korach, K.S. The physiological role of estrogen receptor functional domains. Essays Biochem 2021, 65, 867–875. [Google Scholar] [CrossRef]
- Bourguet, W.; Germain, P.; Gronemeyer, H. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol Sci 2000, 21, 381–388. [Google Scholar] [CrossRef]
- Lonard, D.M.; Nawaz, Z.; Smith, C.L.; O'Malley, B.W. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Molecular cell 2000, 5, 939–948. [Google Scholar] [CrossRef]
- Lonard, D.M.; O'Malley, B.W. Molecular Pathways: Targeting Steroid Receptor Coactivators in Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2016, 22, 5403–5407. [Google Scholar] [CrossRef]
- Dauvois, S.; Danielian, P.S.; White, R.; Parker, M.G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proceedings of the National Academy of Sciences of the United States of America 1992, 89, 4037–4041. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Rientjes, J.M.; Stewart, A.F. Different positioning of the ligand-binding domain helix 12 and the F domain of the estrogen receptor accounts for functional differences between agonists and antagonists. EMBO J 1998, 17, 765–773. [Google Scholar] [CrossRef]
- Schwartz, J.A.; Zhong, L.; Deighton-Collins, S.; Zhao, C.; Skafar, D.F. Mutations targeted to a predicted helix in the extreme carboxyl-terminal region of the human estrogen receptor-alpha alter its response to estradiol and 4-hydroxytamoxifen. The Journal of biological chemistry 2002, 277, 13202–13209. [Google Scholar] [CrossRef]
- Koide, A.; Zhao, C.; Naganuma, M.; Abrams, J.; Deighton-Collins, S.; Skafar, D.F.; Koide, S. Identification of regions within the F domain of the human estrogen receptor alpha that are important for modulating transactivation and protein-protein interactions. Mol Endocrinol 2007, 21, 829–842. [Google Scholar] [CrossRef]
- Arao, Y.; Korach, K.S. The F domain of estrogen receptor alpha is involved in species-specific, tamoxifen-mediated transactivation. The Journal of biological chemistry 2018, 293, 8495–8507. [Google Scholar] [CrossRef]
- Huang, B.; Omoto, Y.; Iwase, H.; Yamashita, H.; Toyama, T.; Coombes, R.C.; Filipovic, A.; Warner, M.; Gustafsson, J.A. Differential expression of estrogen receptor alpha, beta1, and beta2 in lobular and ductal breast cancer. Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, V.; Robinson-Rechavi, M. Meta-analysis of estrogen response in MCF-7 distinguishes early target genes involved in signaling and cell proliferation from later target genes involved in cell cycle and DNA repair. BMC Syst Biol 2011, 5, 138. [Google Scholar] [CrossRef]
- Welboren, W.J.; Sweep, F.C.; Span, P.N.; Stunnenberg, H.G. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer 2009, 16, 1073–1089. [Google Scholar] [CrossRef]
- Palaniappan, M.; Edwards, D.; Creighton, C.J.; Medina, D.; Conneely, O.M. Reprogramming of the estrogen responsive transcriptome contributes to tamoxifen-dependent protection against tumorigenesis in the p53 null mammary epithelial cells. PloS one 2018, 13, e0194913. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.S.; Meyer, C.A.; Song, J.; Li, W.; Geistlinger, T.R.; Eeckhoute, J.; Brodsky, A.S.; Keeton, E.K.; Fertuck, K.C.; Hall, G.F.; et al. Genome-wide analysis of estrogen receptor binding sites. Nature genetics 2006, 38, 1289–1297. [Google Scholar] [CrossRef]
- Hurtado, A.; Holmes, K.A.; Ross-Innes, C.S.; Schmidt, D.; Carroll, J.S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nature genetics 2011, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.S.; Liu, X.S.; Brodsky, A.S.; Li, W.; Meyer, C.A.; Szary, A.J.; Eeckhoute, J.; Shao, W.; Hestermann, E.V.; Geistlinger, T.R.; et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 2005, 122, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ross-Innes, C.S.; Stark, R.; Teschendorff, A.E.; Holmes, K.A.; Ali, H.R.; Dunning, M.J.; Brown, G.D.; Gojis, O.; Ellis, I.O.; Green, A.R.; et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 2012, 481, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen: a most unlikely pioneering medicine. Nature reviews. Drug discovery 2003, 2, 205–213. [Google Scholar] [CrossRef]
- Jordan, V.C. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer 2014, 21, R235–246. [Google Scholar] [CrossRef]
- Cole, M.P.; Jones, C.T.; Todd, I.D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. British journal of cancer 1971, 25, 270–275. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Buschmann, M.; Wiegand, A.; Schnellbacher, K.; Bonn, R.; Rehe, A.; Trenk, D.; Jahnchen, E.; Roskamm, H. Comparison of the effects of two different galenical preparations of glyceryl trinitrate on pulmonary artery pressure and on the finger pulse curve. Eur J Clin Pharmacol 1993, 44, 451–456. [Google Scholar] [CrossRef]
- Nabholtz, J.M. Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther Clin Risk Manag 2008, 4, 189–204. [Google Scholar] [CrossRef]
- Osborne, C.K.; Wakeling, A.; Nicholson, R.I. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. British journal of cancer 2004, 90 Suppl 1, S2–6. [Google Scholar] [CrossRef]
- Carlson, R.W. The history and mechanism of action of fulvestrant. Clin Breast Cancer 2005, 6 Suppl 1, S5–8. [Google Scholar] [CrossRef]
- Wardley, A.M. Fulvestrant: a review of its development, pre-clinical and clinical data. Int J Clin Pract 2002, 56, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, S.; Simigdala, N.; Ribas, R.; Schuster, E.; Leal, M.F.; Nikitorowicz-Buniak, J.; Rega, C.; Bihani, T.; Patel, H.; Johnston, S.R.; et al. Elacestrant demonstrates strong anti-estrogenic activity in PDX models of estrogen-receptor positive endocrine-resistant and fulvestrant-resistant breast cancer. NPJ Breast Cancer 2022, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.; Nicholson, R.I.; Bundred, N.J.; Anderson, E.; Rayter, Z.; Dowsett, M.; Fox, J.N.; Gee, J.M.; Webster, A.; Wakeling, A.E.; et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer research 2001, 61, 6739–6746. [Google Scholar]
- Bihani, T.; Patel, H.K.; Arlt, H.; Tao, N.; Jiang, H.; Brown, J.L.; Purandare, D.M.; Hattersley, G.; Garner, F. Elacestrant (RAD1901), a Selective Estrogen Receptor Degrader (SERD), Has Antitumor Activity in Multiple ER(+) Breast Cancer Patient-derived Xenograft Models. Clinical cancer research : an official journal of the American Association for Cancer Research 2017, 23, 4793–4804. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Jager, A.; de Vries, E.G.E.; der Houven van Oordt, C.W.M.; Neven, P.; Venema, C.M.; Glaudemans, A.; Wang, Y.; Bagley, R.G.; Conlan, M.G.; Aftimos, P. A phase 1b study evaluating the effect of elacestrant treatment on estrogen receptor availability and estradiol binding to the estrogen receptor in metastatic breast cancer lesions using (18)F-FES PET/CT imaging. Breast cancer research : BCR 2020, 22, 97. [Google Scholar] [CrossRef]
- Garner, F.; Shomali, M.; Paquin, D.; Lyttle, C.R.; Hattersley, G. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs 2015, 26, 948–956. [Google Scholar] [CrossRef]
- Patel, H.K.; Tao, N.; Lee, K.M.; Huerta, M.; Arlt, H.; Mullarkey, T.; Troy, S.; Arteaga, C.L.; Bihani, T. Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors. Breast cancer research : BCR 2019, 21, 146. [Google Scholar] [CrossRef]
- Gombos, A. Selective oestrogen receptor degraders in breast cancer: a review and perspectives. Current opinion in oncology 2019, 31, 424–429. [Google Scholar] [CrossRef]
- Fanning, S.W.; Greene, G.L. Next-Generation ERalpha Inhibitors for Endocrine-Resistant ER+ Breast Cancer. Endocrinology 2019, 160, 759–769. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Wardell, S.E. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Current opinion in pharmacology 2010, 10, 620–628. [Google Scholar] [CrossRef]
- McDonnell, D.P. The molecular pharmacology of estrogen receptor modulators: implications for the treatment of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2005, 11, 871s–877s. [Google Scholar] [CrossRef]
- Puhalla, S.; Bhattacharya, S.; Davidson, N.E. Hormonal therapy in breast cancer: a model disease for the personalization of cancer care. Molecular oncology 2012, 6, 222–236. [Google Scholar] [CrossRef]
- Nardone, A.; De Angelis, C.; Trivedi, M.V.; Osborne, C.K.; Schiff, R. The changing role of ER in endocrine resistance. Breast 2015, 24 Suppl 2, S60–66. [Google Scholar] [CrossRef]
- Will, M.; Liang, J.; Metcalfe, C.; Chandarlapaty, S. Therapeutic resistance to anti-oestrogen therapy in breast cancer. Nature reviews. Cancer 2023, 23, 673–685. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annual review of medicine 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.F.; Schiff, R.; Osborne, C.K. Targeting HER2 for the treatment of breast cancer. Annual review of medicine 2015, 66, 111–128. [Google Scholar] [CrossRef]
- Morrison, G.; Fu, X.; Shea, M.; Nanda, S.; Giuliano, M.; Wang, T.; Klinowska, T.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast cancer research and treatment 2014, 144, 263–272. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Sliwkowski, M.X.; Osborne, C.K.; Perez, E.A.; Puglisi, F.; Gianni, L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature reviews. Clinical oncology 2011, 9, 16–32. [Google Scholar] [CrossRef]
- Choi, H.J.; Joo, H.S.; Won, H.Y.; Min, K.W.; Kim, H.Y.; Son, T.; Oh, Y.H.; Lee, J.Y.; Kong, G. Role of RBP2-Induced ER and IGF1R-ErbB Signaling in Tamoxifen Resistance in Breast Cancer. Journal of the National Cancer Institute 2018, 110. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Agboke, F.A.; Cunliffe, H.E.; Ramos, P.; Jordan, V.C. A molecular model for the mechanism of acquired tamoxifen resistance in breast cancer. European journal of cancer 2014, 50, 2866–2876. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, S.A.; Powter, B.; Balakrishnar, B.; Mok, K.; Soon, P.; Franken, A.; Neubauer, H.; de Souza, P.; Becker, T.M. Endocrine Resistance in Breast Cancer: The Role of Estrogen Receptor Stability. Cells 2020, 9. [Google Scholar] [CrossRef]
- Gururaj, A.E.; Rayala, S.K.; Vadlamudi, R.K.; Kumar, R. Novel mechanisms of resistance to endocrine therapy: genomic and nongenomic considerations. Clinical cancer research : an official journal of the American Association for Cancer Research 2006, 12, 1001s–1007s. [Google Scholar] [CrossRef]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nature genetics 2013, 45, 1439–1445. [Google Scholar] [CrossRef]
- Merenbakh-Lamin, K.; Ben-Baruch, N.; Yeheskel, A.; Dvir, A.; Soussan-Gutman, L.; Jeselsohn, R.; Yelensky, R.; Brown, M.; Miller, V.A.; Sarid, D.; et al. D538G mutation in estrogen receptor-alpha: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer research 2013, 73, 6856–6864. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nature genetics 2013, 45, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Yelensky, R.; Buchwalter, G.; Frampton, G.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; Ferrer-Lozano, J.; Perez-Fidalgo, J.A.; Cristofanilli, M.; Gomez, H.; et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2014, 20, 1757–1767. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Borg, A.; Wolf, D.M.; Oesterreich, S.; Fuqua, S.A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer research 1997, 57, 1244–1249. [Google Scholar]
- Oesterreich, S.; Davidson, N.E. The search for ESR1 mutations in breast cancer. Nature genetics 2013, 45, 1415–1416. [Google Scholar] [CrossRef]
- Thomas, C.; Gustafsson, J.A. Estrogen receptor mutations and functional consequences for breast cancer. Trends in endocrinology and metabolism: TEM 2015, 26, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.T.; Gou, X.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. Journal of cancer metastasis and treatment 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Dustin, D.; Gu, G.; Fuqua, S.A.W. ESR1 mutations in breast cancer. Cancer 2019. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A.; Mayne, C.G.; Katzenellenbogen, B.S.; Greene, G.L.; Chandarlapaty, S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance. Nature reviews. Cancer 2018, 18, 377–388. [Google Scholar] [CrossRef]
- Carlson, K.E.; Choi, I.; Gee, A.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Altered ligand binding properties and enhanced stability of a constitutively active estrogen receptor: evidence that an open pocket conformation is required for ligand interaction. Biochemistry 1997, 36, 14897–14905. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, S.; Gustafsson, J.A. Nuclear Receptors: Recent Drug Discovery for Cancer Therapies. Endocrine reviews 2019, 40, 1207–1249. [Google Scholar] [CrossRef] [PubMed]
- Zundelevich, A.; Dadiani, M.; Kahana-Edwin, S.; Itay, A.; Sella, T.; Gadot, M.; Cesarkas, K.; Farage-Barhom, S.; Saar, E.G.; Eyal, E.; et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast cancer research : BCR 2020, 22, 16. [Google Scholar] [CrossRef]
- Hancock, G.R.; Gertz, J.; Jeselsohn, R.; Fanning, S.W. Estrogen Receptor Alpha Mutations, Truncations, Heterodimers, and Therapies. Endocrinology 2024, 165. [Google Scholar] [CrossRef] [PubMed]
- Dustin, D.; Gu, G.; Beyer, A.R.; Herzog, S.K.; Edwards, D.G.; Lin, H.; Gonzalez, T.L.; Grimm, S.L.; Coarfa, C.; Chan, D.W.; et al. RON signalling promotes therapeutic resistance in ESR1 mutant breast cancer. British journal of cancer 2021, 124, 191–206. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Yates, M.E.; Tasdemir, N.; Bahreini, A.; Chen, J.; Levine, K.M.; Priedigkeit, N.M.; Nasrazadani, A.; Ali, S.; et al. Hotspot ESR1 Mutations Are Multimodal and Contextual Modulators of Breast Cancer Metastasis. Cancer research 2022, 82, 1321–1339. [Google Scholar] [CrossRef]
- Grinshpun, A.; Sandusky, Z.M.; Jeselsohn, R. The Clinical Utility of ESR1 Mutations in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer. Hematology/oncology clinics of North America 2023, 37, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Grinshpun, A.; Chen, V.; Sandusky, Z.M.; Fanning, S.W.; Jeselsohn, R. ESR1 activating mutations: From structure to clinical application. Biochimica et biophysica acta. Reviews on cancer 2023, 1878, 188830. [Google Scholar] [CrossRef] [PubMed]
- Jeselsohn, R.; Bergholz, J.S.; Pun, M.; Cornwell, M.; Liu, W.; Nardone, A.; Xiao, T.; Li, W.; Qiu, X.; Buchwalter, G.; et al. Allele-Specific Chromatin Recruitment and Therapeutic Vulnerabilities of ESR1 Activating Mutations. Cancer cell 2018, 33, 173–186. [Google Scholar] [CrossRef]
- Arnesen, S.; Blanchard, Z.; Williams, M.M.; Berrett, K.C.; Li, Z.; Oesterreich, S.; Richer, J.K.; Gertz, J. Estrogen Receptor Alpha Mutations in Breast Cancer Cells Cause Gene Expression Changes through Constant Activity and Secondary Effects. Cancer research 2021, 81, 539–551. [Google Scholar] [CrossRef]
- Nettles, K.W.; Bruning, J.B.; Gil, G.; Nowak, J.; Sharma, S.K.; Hahm, J.B.; Kulp, K.; Hochberg, R.B.; Zhou, H.; Katzenellenbogen, J.A.; et al. NFkappaB selectivity of estrogen receptor ligands revealed by comparative crystallographic analyses. Nature chemical biology 2008, 4, 241–247. [Google Scholar] [CrossRef]
- Fanning, S.W.; Mayne, C.G.; Dharmarajan, V.; Carlson, K.E.; Martin, T.A.; Novick, S.J.; Toy, W.; Green, B.; Panchamukhi, S.; Katzenellenbogen, B.S.; et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife 2016, 5. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Norris, J.D.; Chang, C.Y. Neomorphic ERalpha Mutations Drive Progression in Breast Cancer and Present a Challenge for New Drug Discovery. Cancer cell 2018, 33, 153–155. [Google Scholar] [CrossRef]
- Li, Z.; Levine, K.M.; Bahreini, A.; Wang, P.; Chu, D.; Park, B.H.; Oesterreich, S.; Lee, A.V. Upregulation of IRS1 Enhances IGF1 Response in Y537S and D538G ESR1 Mutant Breast Cancer Cells. Endocrinology 2018, 159, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol 2016, 2, 1310–1315. [Google Scholar] [CrossRef]
- Henry, N.L.; Somerfield, M.R.; Dayao, Z.; Elias, A.; Kalinsky, K.; McShane, L.M.; Moy, B.; Park, B.H.; Shanahan, K.M.; Sharma, P.; et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J Clin Oncol 2022, 40, 3205–3221. [Google Scholar] [CrossRef]
- Bellward, G.D.; Norstrom, R.J.; Whitehead, P.E.; Elliott, J.E.; Bandiera, S.M.; Dworschak, C.; Chang, T.; Forbes, S.; Cadario, B.; Hart, L.E.; et al. Comparison of polychlorinated dibenzodioxin levels with hepatic mixed-function oxidase induction in great blue herons. J Toxicol Environ Health 1990, 30, 33–52. [Google Scholar] [CrossRef]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- Wang, P.; Bahreini, A.; Gyanchandani, R.; Lucas, P.C.; Hartmaier, R.J.; Watters, R.J.; Jonnalagadda, A.R.; Trejo Bittar, H.E.; Berg, A.; Hamilton, R.L.; et al. Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2016, 22, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidants and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson's disease, Alzheimer's disease, traumatic injury or stroke? Acta Neurol Scand Suppl 1989, 126, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.R.; Jhaveri, K.; Kalinsky, K.; Bardia, A.; Wander, S.A. Precision therapeutics and emerging strategies for HR-positive metastatic breast cancer. Nature reviews. Clinical oncology 2024, 21, 743–761. [Google Scholar] [CrossRef]
- Bardia, A.; Cortes, J.; Bidard, F.C.; Neven, P.; Garcia-Saenz, J.; Aftimos, P.; O'Shaughnessy, J.; Lu, J.; Tonini, G.; Scartoni, S.; et al. Elacestrant in ER+, HER2- Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups. Clinical cancer research : an official journal of the American Association for Cancer Research 2024, 30, 4299–4309. [Google Scholar] [CrossRef]
- Bhatia, N.; Thareja, S. Elacestrant: a new FDA-approved SERD for the treatment of breast cancer. Med Oncol 2023, 40, 180. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Bellet, M.; Turner, N.C.; Loi, S.; Bardia, A.; Boni, V.; Sohn, J.; Neilan, T.G.; Villanueva-Vazquez, R.; Kabos, P.; et al. Phase Ia/b Study of Giredestrant +/- Palbociclib and +/- Luteinizing Hormone-Releasing Hormone Agonists in Estrogen Receptor-Positive, HER2-Negative, Locally Advanced/Metastatic Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2024, 30, 754–766. [Google Scholar] [CrossRef]
- Liang, J.; Zbieg, J.R.; Blake, R.A.; Chang, J.H.; Daly, S.; DiPasquale, A.G.; Friedman, L.S.; Gelzleichter, T.; Gill, M.; Giltnane, J.M.; et al. GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J Med Chem 2021, 64, 11841–11856. [Google Scholar] [CrossRef]
- Martin, M.; Lim, E.; Chavez-MacGregor, M.; Bardia, A.; Wu, J.; Zhang, Q.; Nowecki, Z.; Cruz, F.M.; Safin, R.; Kim, S.B.; et al. Giredestrant for Estrogen Receptor-Positive, HER2-Negative, Previously Treated Advanced Breast Cancer: Results From the Randomized, Phase II acelERA Breast Cancer Study. J Clin Oncol 2024, 42, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Lim, E.; Jeselsohn, R.; Ma, C.X.; Hamilton, E.P.; Osborne, C.; Bhave, M.; Kaufman, P.A.; Beck, J.T.; Manso Sanchez, L.; et al. Imlunestrant, an Oral Selective Estrogen Receptor Degrader, as Monotherapy and in Combination With Targeted Therapy in Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Phase Ia/Ib EMBER Study. J Clin Oncol 2024, JCO2302733. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.V.Z.B.; Shen, W; Mur, C; Barr, R; Kindler, L.J; Rubio, A; Bastian, J.A; Cohen, J.D; Mattioni, B.E; Yuen, E; Baker, T.K; Castanares, M.A; Fei, D; Manro, J.R; Lallena, M.J; Peng, S.B; de Dios, A. Preclinical characterization of LY3484356, a novel, potent and orally bioavailable selective estrogen receptor degrader (SERD) In Proceedings of the American Association for Cancer Research Annual Meeting, 2021; p. abstr. 1236.
- Hamilton, E.; Oliveira, M.; Turner, N.; Garcia-Corbacho, J.; Hernando, C.; Ciruelos, E.M.; Kabos, P.; Ruiz-Borrego, M.; Armstrong, A.; Patel, M.R.; et al. A phase I dose escalation and expansion trial of the next-generation oral SERD camizestrant in women with ER-positive, HER2-negative advanced breast cancer: SERENA-1 monotherapy results. Ann Oncol 2024, 35, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Huang-Bartlett, C.; Kalinsky, K.; Cristofanilli, M.; Bianchini, G.; Chia, S.; Iwata, H.; Janni, W.; Ma, C.X.; Mayer, E.L.; et al. Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment. Future Oncol 2023, 19, 559–573. [Google Scholar] [CrossRef]
- Lawson, M.; Cureton, N.; Ros, S.; Cheraghchi-Bashi, A.; Urosevic, J.; D'Arcy, S.; Delpuech, O.; DuPont, M.; Fisher, D.I.; Gangl, E.T.; et al. The Next-Generation Oral Selective Estrogen Receptor Degrader Camizestrant (AZD9833) Suppresses ER+ Breast Cancer Growth and Overcomes Endocrine and CDK4/6 Inhibitor Resistance. Cancer research 2023, 83, 3989–4004. [Google Scholar] [CrossRef]
- Scott, J.S.; Moss, T.A.; Balazs, A.; Barlaam, B.; Breed, J.; Carbajo, R.J.; Chiarparin, E.; Davey, P.R.J.; Delpuech, O.; Fawell, S.; et al. Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist. J Med Chem 2020, 63, 14530–14559. [Google Scholar] [CrossRef]
- Oliveira, M.e.a. Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose Phase 2 SERENA-2 trial. 2023; pp. abstr. GS3-02.
- Gough, S.M.; Flanagan, J.J.; Teh, J.; Andreoli, M.; Rousseau, E.; Pannone, M.; Bookbinder, M.; Willard, R.; Davenport, K.; Bortolon, E.; et al. Oral Estrogen Receptor PROTAC Vepdegestrant (ARV-471) Is Highly Efficacious as Monotherapy and in Combination with CDK4/6 or PI3K/mTOR Pathway Inhibitors in Preclinical ER+ Breast Cancer Models. Clinical cancer research : an official journal of the American Association for Cancer Research 2024, 30, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.V. , V; Han,H.S; Ranciato,J. et al. First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2- locally advanced or metastatic breast cancer. 2022; pp. abstr. PD13-08.
- Schott, A.F.e.a. ARV-471, a PROTAC estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: phase 2 expansion (VERITAC) of a phase 1/2 study. 2023; pp. abstr. GS3-03.
- Hamilton, E.P.; Ma, C.; De Laurentiis, M.; Iwata, H.; Hurvitz, S.A.; Wander, S.A.; Danso, M.; Lu, D.R.; Perkins Smith, J.; Liu, Y.; et al. VERITAC-2: a Phase III study of vepdegestrant, a PROTAC ER degrader, versus fulvestrant in ER+/HER2- advanced breast cancer. Future Oncol 2024, 20, 2447–2455. [Google Scholar] [CrossRef]
- Parisian, A.D.; Barratt, S.A.; Hodges-Gallagher, L.; Ortega, F.E.; Pena, G.; Sapugay, J.; Robello, B.; Sun, R.; Kulp, D.; Palanisamy, G.S.; et al. Palazestrant (OP-1250), A Complete Estrogen Receptor Antagonist, Inhibits Wild-type and Mutant ER-positive Breast Cancer Models as Monotherapy and in Combination. Mol Cancer Ther 2024, 23, 285–300. [Google Scholar] [CrossRef]
| Study Drug | Mode of Action | Clinical Stage of Development | Status | Clinical Trial Identifier | Sponsor/Source |
|---|---|---|---|---|---|
| G1T48 | SERD | Phase 1 | Completed | NCT03455270 | G1 Therapeutics, Inc. |
| D-0502 | SERD | Phase 1 | Completed | NCT03471663 | InventisBio Co., Ltd |
| SIM0270 | SERD | Phase 1 | Recruiting | NCT05293964 | Jiangsu Simcere Pharmaceutical Co., Ltd. |
| H3B-654 | Covalent ERα antagonist |
Phase 1 | Completed | NCT03250676 | Eisai Inc. |
| ZN-c5 | SERD | Phase1/2 | Completed | NCT03560531 | Zeno Alpha Inc |
| GDC-9545 | SERD | Phase 3 | Recruiting | NCT06065748 | Hoffmann-La Roche |
| LY3484356 | SERD | Phase 3 | Recruiting | NCT05514054 | Eli Lilly and Company |
| AZD9833 | SERD | Phase 3 | Active, not recruiting | NCT04964934 | AstraZeneca |
| OP-1250 | SERD | Phase 3 | Recruiting | NCT06016738 | Olema Pharmaceuticals, Inc. |
| ARV-471 (PF- 07850327) |
ER PROTAC degrader |
Phase 3 | Recruiting | NCT05514054 | Pfizer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





