Submitted:
31 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biofertilizers and Bioremediators
2.1. Agricultural Fertilization
2.2. Afforestation and Biostimulation
2.2.1. Temperature
2.2.2. Salinity
2.2.3. Flooding, Water Pooling, and Heavy Precipitation.
2.2.4. Imbalanced Nutrient Recycling
2.2.5. Soil Compaction by Cattle
2.3. Treatment of Polluted Soils (Bioremediation)
Genetically Modified Microorganisms for Bioremediation
3. Environmental Parameters Influencing Microbial Fertilization and Bioremediation in Soils
3.1. Temperature
3.2. Salinity
3.3. pH
3.4. Microbiological Diversity
3.5. Oxygen Levels
3.6. Soil Moisture
3.7. Nutrient Availability
3.8. Pollutant Complexity
4. Risks to Ecosystem Health: Impact on the Local Biota
5. Conclusions and Perspectives
Ethics Approval and Consent to Participate
Consent for Publication
Competing Interests
Author Contributions
Acknowledgments
References
- Benzerrouk, Z.; Abid, M.; Sekrafi, H. Pollution haven or halo effect? A comparative analysis of developing and developed countries. Energy Rep. 2021, 7, 4862–4871. [Google Scholar] [CrossRef]
- Ameen, R.F.M.; Mourshed, M. Urban environmental challenges in developing countries—A stakeholder perspective. Habitat Int. 2017, 64, 1–10. [Google Scholar] [CrossRef]
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Parkinson, D.; Coleman, D.C. Microbial communities, activity and biomass. Agriculture. Ecosystems & Environment 1991, 24, 3–33. [Google Scholar]
- Macrae, A., Coelho. Tropical Soil Microbial Communities. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Heidelberg, 2013. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, NX, Netherlands, 2015. [Google Scholar]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Sharma, A.K. Biofertilizer – A Key Player in Enhancing Soil Fertility and Agricultural Sustainability. Int. J. Agric. Environ. Biotechnol. 2023, 16. [Google Scholar] [CrossRef]
- Cui, K.; Xu, L.; Tao, T.; Huang, L.; Li, J.; Hong, J.; Li, H.; Chi, Y. Mechanical behavior of multiscale hybrid fiber reinforced recycled aggregate concrete subject to uniaxial compression. Journal of Building Engineering 2023, 71, 106504. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Homaee, M.; Darki, B.Z. Quality improvement of an erosion-prone soil through microbial enrichment. Soil Tillage Res. 2017, 165, 230–238. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, Z.; Yang, Z.; Nyumah, F.; Hu, C.; Lin, W.; Yuan, Z. Bio-fertilizer Affects Structural Dynamics, Function, and Network Patterns of the Sugarcane Rhizospheric Microbiota. Microb. Ecol. 2021, 84, 1195–1211. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.; Kumar, A. Plant Growth Promoting Rhizobacteria. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–66. [Google Scholar] [CrossRef]
- El-Ramady, H.; El-Ghamry, A.; Mosa, A.A.; Alshaal, T. Nanofertilizers vs. Biofertilizers: New Insights. Environ. Biodivers. Soil Secur. 2018, 2, 40–50. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Riaz, U.; Mehdi, S.M.; Iqbal, S.; Khalid, H.I.; Qadir, A.A.; Anum, W.; et al. Bio-fertilizers: eco-friendly approach for plant and soil environment,” in Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation, eds K. R. Hakeem, R. A. Bhat, and H. Qadri (Cham: Springer) 2020.
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-Producing Rhizobacteria as a Promising Tool for Empowering Plants to Cope with Iron Limitation in Saline Soils: A Review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.; Guan, Y.; Wu, L.; Zhang, L.; Pan, X.; Zhang, Z.; Zhang, Y.; et al. Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- Shirmohammadi, E.; Alikhani, H.A.; Pourbabaei, A.A.; Etesami, H. Improved Phosphorus (P) Uptake and Yield of Rainfed Wheat Fed with P Fertilizer by Drought-Tolerant Phosphate-Solubilizing Fluorescent Pseudomonads Strains: a Field Study in Drylands. J. Soil Sci. Plant Nutr. 2020, 20, 2195–2211. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Caracciolo, A.B.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground Microbiota and the Health of Tree Crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Tomar, O.; Akarca, G.; Çağlar, A.; Beykaya, M.; Gök, V. The effects of kefir grain and starter culture on kefir produced from cow and buffalo milk during storage periods. Food Sci. Technol. 2020, 40, 238–244. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; AlKahtani, M.D.F.; Khan, M.S. Heavy metal induced stress on wheat: phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Song, S.; Rao, P.; Wang, R.; Liu, S. Preparation and properties of soil conditioner microspheres based on self-assembled potassium alginate and chitosan. Int. J. Biol. Macromol. 2019, 147, 877–889. [Google Scholar] [CrossRef]
- Zahra, N.; Raza, Z.A.; Mahmood, S. Effect of Salinity Stress on Various Growth and Physiological Attributes of Two Contrasting Maize Genotypes. Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Narayanan, S. Effects of high temperature stress and traits associated with tolerance in wheat. Open Access J. Sci. 2018, 2, 1. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, J.O.; Abubakre, O.K.; Roos, W.D. Selected Heavy Metals Removal From Electroplating Wastewater by Purified and Polyhydroxylbutyrate Functionalized Carbon Nanotubes Adsorbents. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 1–11. [Google Scholar] [CrossRef]
- Luo, X.; Wu, C.; Lin, Y.; Li, W.; Deng, M.; Tan, J.; Xue, S. Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. J. Environ. Sci. 2022, 125, 662–677. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2021, 3, 100094. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhry, V.; Prakash, O. Editorial: Multi-omics profiling of unique niches to reveal the microbial and metabolite composition. Front. Microbiol. 2022, 13, 997191. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant Growth-Promoting Rhizobacteria Allow Reduced Application Rates of Chemical Fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Bertola, M.; Mattarozzi, M.; Sanangelantoni, A.M.; Careri, M.; Visioli, G. PGPB Colonizing Three-Year Biochar-Amended Soil: Towards Biochar-Mediated Biofertilization. J. Soil Sci. Plant Nutr. 2019, 19, 841–850. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Khalid, A.; Mehmood, S.; Rizwan, M.S.; Ashraf, M.; Mubeen, F.; Imtiaz, M.; Iqbal, M.M. Integrated Effect of Algal Biochar and Plant Growth Promoting Rhizobacteria on Physiology and Growth of Maize Under Deficit Irrigations. J. Soil Sci. Plant Nutr. 2019, 20, 346–356. [Google Scholar] [CrossRef]
- Murgese, P.; Santamaria, P.; Leoni, B.; Crecchio, C. Ameliorative Effects of PGPB on Yield, Physiological Parameters, and Nutrient Transporter Genes Expression in Barattiere (Cucumis melo L.). J. Soil Sci. Plant Nutr. 2020, 20, 784–793. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural Sustainability: Microbial Biofertilizers in Rhizosphere Management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, A.M.; Gastelum, L.A.S.; Pablos, C.M.F.; Parra-Cota, F.I.; Santoyo, G.; Puente, M.L.; Bhattacharya, D.; Mukherjee, J.; Santos-Villalobos, S.d.L. The Current and Future Role of Microbial Culture Collections in Food Security Worldwide. Front. Sustain. Food Syst. 2021, 4. [Google Scholar] [CrossRef]
- Panda, S.C. Organic Farming for Sustainable Agriculture, third ed.; Kalyani publisher: New Delhi, 2018; 9327216334. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Chakraborty, T.; Akhtar, N. Biofertilizers: Prospects and challenges for future. Biofertilizers 2021, 575–590. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Conway, G. One Billion Hungry: Can we Feed the World? Comstock Publishing Associates, 2012. [Google Scholar]
- Kumar, R.; Kumawat, N.; Sahu, Y.K. Role of biofertilizers in agriculture. Popular Kheti 2017, 5(4), 63–66. [Google Scholar]
- Odoh, C.K., Sam. Microbial consortium as biofertilizers for crops growing under extreme habitats. In Plant Microbiomes for Sustainable Agriculture; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Springer: Cham, 2020; pp. 381–424. [Google Scholar]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Berg, G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Brossi, M.J.d.L.; Kuramae, E.E.; Tsai, S.M. Land-use system shapes soil bacterial communities in Southeastern Amazon region. Appl. Soil Ecol. 2015, 95, 151–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Probiotic strain Stenotrophomonas acidaminiphila BJ1 degrades and reduces chlorothalonil toxicity to soil enzymes, microbial communities and plant roots. AMB Express 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.; Zhang, B.; Geng, M.; Cai, X.; Wang, J.; Wang, Y. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Appl. Soil Ecol. 2021, 172, 104369. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Y.; Yang, Y.; Zhang, M.; Mao, X.; Guo, Y.; Li, X.; Tao, B.; Qi, Y.; Ma, L.; et al. Co-application of biochar and microbial inoculants increases soil phosphorus and potassium fertility and improves soil health and tomato growth. J. Soils Sediments 2022, 23, 947–957. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2011, 14, 605–611. [Google Scholar] [CrossRef]
- Gangwar, R.K., Bhushan. Combined effects of plant growth promoting rhizobacteria and fungi on mung bean (Vigna radiata l.). International Journal of Pharmaceutical Sciences and Research 2013, 4, 4422–4426. [Google Scholar] [CrossRef]
- Niu, X.; Duiker, S.W. Carbon sequestration potential by afforestation of marginal agricultural land in the Midwestern U.S. For. Ecol. Manag. 2006, 223, 415–427. [Google Scholar] [CrossRef]
- Rudel, T.K.; Coomes, O.T.; Moran, E.; Achard, F.; Angelsen, A.; Xu, J.; Lambin, E. Forest transitions: towards a global understanding of land use change. Glob. Environ. Chang. 2005, 15, 23–31. [Google Scholar] [CrossRef]
- Benayas, J.R.; Martins, A.; Nicolau, J.M.; Schulz, J.J. Abandonment of agricultural land: An overview of drivers and consequences. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 1–14. [Google Scholar] [CrossRef]
- Asif, M.; Mughal, A.H.; Bisma, R.; Mehdi, Z.; Saima, S.; Ajaz, M.; Malik, M.A.; Masood, A.; Sidique, S. Application of Different Strains of Biofertilizers for Raising Quality Forest Nursery. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3680–3686. [Google Scholar] [CrossRef]
- Samec, P.; Kučera, A.; Tomášová, G. Soil Degradation Processes Linked to Long-Term Forest-Type Damage. Intech Open 2023. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant, Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Malhotra, S.K. Horticultural crops and climate change: A review. Indian J. Agric. Sci. 2017, 87, 12–22. [Google Scholar] [CrossRef]
- Gupta, R.; Kim, S.T. Depletion of RuBisCO protein using the protamine sulfate precipitation method. Methods in Molecular Biology 2017, 1295, 225–233. [Google Scholar] [CrossRef]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Ku, S.-B.; Edwards, G.E. Oxygen inhibition of photosynthesis. Planta 1978, 140, 1–6. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of High Temperature on Crop Productivity and Metabolism of Macro Molecules; Elsevier: Amsterdam, NX, Netherlands, 2019. [Google Scholar]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Qu, A.-L.; Ding, Y.-F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [PubMed]
- Byerlee, D.; Stevenson, J.; Villoria, N. Does intensification slow crop land expansion or encourage deforestation? Glob. Food Secur. 2014, 3, 92–98. [Google Scholar] [CrossRef]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal. Behav. 2020, 15. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The Potential Role of Microbial Biostimulants in the Amelioration of Climate Change-Associated Abiotic Stresses on Crops. Front. Microbiol. 2022, 12, 829099. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Esmaeel, Q.; Miotto, L.; Rondeau, M.; Leclère, V.; Clément, C.; Jacquard, C.; Sanchez, L.; Barka, E.A. Paraburkholderia phytofirmans PsJN-Plants Interaction: From Perception to the Induced Mechanisms. Front. Microbiol. 2018, 9, 2093. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Perinjelil, S.J.; Thakur, N.D. Chapter 11 - Soil salinization and bioremediation using halophiles and halotolerant microorganisms. Gustavo Santoyo, Ajay Kumar, Mohd Aamir, Ed.; Mitigation of Plant Abiotic Stress by Microorganisms, Academic Press, 2022. [Google Scholar] [CrossRef]
- Mitsuchi, M., Wichaidit, P., Jeungnijnirund, S. Soils of the Northeast Plateau, Thailand. Technical bulletin of the Tropical Agriculture Research Center. Japan, No. 25. 1989. https://www.jircas.go.jp/en/publication/techtarc/25/1.
- Sharifi, R.; Ryu, C.-M. Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 2018, 44, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Hameed, A.; Azooz, M.M.; Rehman, M.U.; Siddiqi, T.O.; Ahmad, P. Salt Stress: Causes, Types and Responses of Plants. In Ecophysiology and Responses of Plants under Salt Stress; Springer, 2012; pp. 1–24. [Google Scholar]
- Alonso-Español, A.; Bravo, E.; Ribeiro-Vidal, H.; Virto, L.; Herrera, D.; Alonso, B.; Sanz, M. The Antimicrobial Activity of Curcumin and Xanthohumol on Bacterial Biofilms Developed over Dental Implant Surfaces. Int. J. Mol. Sci. 2023, 24, 2335. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Zhang, Y.; Guan, X.; Wei, Y.; Guo, R. Global desertification vulnerability to climate change and human activities. Land Degrad. Dev. 2020, 31, 1380–1391. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2010, 27, 1231–1240. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Multifunctional Exopolysaccharides from Pseudomonas aeruginosa PF23 Involved in Plant Growth Stimulation, Biocontrol and Stress Amelioration in Sunflower Under Saline Conditions. Curr. Microbiol. 2014, 69, 484–494. [Google Scholar] [CrossRef]
- El-Ghany, M.F.A.; Attia, M. Effect of Exopolysaccharide-Producing Bacteria and Melatonin on Faba Bean Production in Saline and Non-Saline Soil. Agronomy 2020, 10, 316. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. A Halotolerant Bacterium Bacillus licheniformis HSW-16 Augments Induced Systemic Tolerance to Salt Stress in Wheat Plant (Triticum aestivum). Front. Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2013, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Gamalero, E.; Glick, B.R. Mycorrhizal-Bacterial Amelioration of Plant Abiotic and Biotic Stress. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2012, 44, 399–408. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Organic Farming Enhances the Diversity and Community Structure of Endophytic Archaea and Fungi in Maize Plant: a Shotgun Approach. J. Soil Sci. Plant Nutr. 2020, 20, 2587–2599. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozan, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Bray, E. A “Response to abiotic stress,” in Biochemistry and Molecular Biology of Plants. (2000). eds B. B. Buchanan, W. Gruissem, and R. L. Jones (Rockville, MD: ASPP), 1158–1203.
- Omomowo, I.O.; Fadiji, A.E.; Omomowo, O.I. Assessment of bio-efficacy of Glomus versiforme and Trichoderma harzianum in inhibiting powdery mildew disease and enhancing the growth of cowpea. Ann. Agric. Sci. 2018, 63, 9–17. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2015, 66, 35–42. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 2018, 44, 88–97. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Ettoumi, B.; Vigani, G.; Mapelli, F.; Borin, S.; Abou-Hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; et al. A Drought Resistance-Promoting Microbiome Is Selected by Root System under Desert Farming. PLOS ONE 2012, 7, e48479. [Google Scholar] [CrossRef]
- Jayakumar, A.; Padmakumar, P.; Nair, I.C.; Radhakrishnan, E.K. Drought tolerant bacterial endophytes with potential plant probiotic effects from Ananas comosus. Biologia 2020, 75, 1769–1778. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2003, 166, 525–530. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Samiyappan, R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 2006, 102, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Pei, H.; Xu, Z. Low nitrogen stress stimulating the indole-3-acetic acid biosynthesis of Serratia sp. ZM is vital for the survival of the bacterium and its plant growth-promoting characteristic. Arch. Microbiol. 2016, 199, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Survival of Pseudomonas extremorientalis TSAU20 and P. chlororaphis TSAU13 in the rhizosphere of common bean (Phaseolus vulgaris) under saline conditions. Plant, Soil Environ. 2011, 57, 122–127. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zhang, F.; Zhang, D.-J.; Srivastava, A.; Wu, Q.-S.; Zou, Y.-N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; You, Y.-H.; Khan, A.L.; Park, J.-M.; Lee, S.-M.; Lee, I.-J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B — Soil Plant Sci. 2014, 65, 36–44. [Google Scholar] [CrossRef]
- Shaffique, S.; Hussain, S.; Kang, S.-M.; Imran, M.; Hoque, I.U.; Khan, M.A.; Lee, I.-J. Phytohormonal modulation of the drought stress in soybean: outlook, research progress, and cross-talk. Front. Plant Sci. 2023, 14, 1237295. [Google Scholar] [CrossRef]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of Salinity on the In Vitro Development of Glomus intraradices and on the In Vivo Physiological and Molecular Responses of Mycorrhizal Lettuce Plants. Microb. Ecol. 2007, 55, 45–53. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2011, 11, 57–61. [Google Scholar] [CrossRef]
- Paul, S.; Bag, S.K.; Das, S.; Harvill, E.T.; Dutta, C. Molecular signature of hypersaline adaptation: insights from genome and proteome composition of halophilic prokaryotes. Genome Biol. 2008, 9, R70–R70. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.J.; Arndt, S.K. Osmotic Adjustment Under Drought Conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar] [CrossRef]
- Shintu, P. , Jayaram, K. Phosphate solubilising bacteria (Bacillus polymyxa)-An effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill.). Tropical Plant Research 2015, 2, 17–22. [Google Scholar]
- Dimkpa, C.O.; Merten, D.; Svatos, A.; Büchel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef]
- Bailly, A.; Groenhagen, U.; Schulz, S.; Geisler, M.; Eberl, L.; Weisskopf, L. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. 2014, 80, 758–771. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Garladinne, M.; Lee, Y.H. Volatile Indole Produced by Rhizobacterium Proteus vulgaris JBLS202 Stimulates Growth of Arabidopsis thaliana Through Auxin, Cytokinin, and Brassinosteroid Pathways. J. Plant Growth Regul. 2014, 34, 158–168. [Google Scholar] [CrossRef]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.-S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Paré, P.W. Choline and Osmotic-Stress Tolerance Induced in Arabidopsis by the Soil Microbe Bacillus subtilis (GB03). Mol. Plant-Microbe Interactions® 2010, 23, 1097–1104. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-Mediated Effects Predominate in Paraburkholderia phytofirmans Growth Promotion and Salt Stress Tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, F.M.; López-Bucio, J.; Altamirano-Hernández, J.; Valencia-Cantero, E.; de la Cruz, H.R.; Macías-Rodríguez, L. Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.-M.; Lee, I.-J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Else, M.A.; Jackson, M.B. Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Funct. Plant Biol. 1998, 25, 453–458. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.-C. Plant Growth Promotion Under Water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Grichko, V.P.; Glick, B.R. Amelioration of flooding stress by ACC deaminase-containingplant growth-promoting bacteria. Plant Physiol. Biochem. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.-J.; Hao, Z.-P.; Li, H.; Chen, B.-D. Aquaporin genesGintAQPF1andGintAQPF2fromGlomus intraradicescontribute to plant drought tolerance. Plant Signal. Behav. 2013, 8, e24030. [Google Scholar] [CrossRef]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean. Agric. Nat. Resour. 2018, 52, 330–334. [Google Scholar] [CrossRef]
- Fravel, D. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Manikandan, A., Anandham, R., Johnson, I., Krishnamoorthy, R., Senthilkumar, M., Raghu, R., et al. Bacillus and Streptomyces for Management of Biotic Stresses in Plants for Sustainable Agriculture. In S. Chhabra, R. Prasad, N. R. Maddela, & N. Tuteja (Eds.), Plant Microbiome for Plant Productivity and Sustainable Agriculture (pp. 263-288). Singapore: Springer Nature Singapore. 2023. [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Bernal, S.; Hedin, L.O.; Likens, G.E.; Gerber, S.; Buso, D.C. Complex response of the forest nitrogen cycle to climate change. Proc. Natl. Acad. Sci. 2012, 109, 3406–3411. [Google Scholar] [CrossRef]
- Vale, P.; Gibbs, H.; Vale, R.; Christie, M.; Florence, E.; Munger, J.; Sabaini, D. The Expansion of Intensive Beef Farming to the Brazilian Amazon. Glob. Environ. Chang. 2019, 57, 101922. [Google Scholar] [CrossRef]
- INPE, 2021. National Institute for Space Research. http://www.inpe.br/.
- Navarrete, A.A.; Tsai, S.M.; Mendes, L.W.; Faust, K.; de Hollander, M.; Cassman, N.A.; Raes, J.; van Veen, J.A.; Kuramae, E.E. Soil microbiome responses to the short-term effects of Amazonian deforestation. Mol. Ecol. 2015, 24, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Melo, V.F.; Orrutéa, A.G.; Motta, A.C.V.; Testoni, S.A. Land Use and Changes in Soil Morphology and Physical-Chemical Properties in Southern Amazon. Rev. Bras. De Cienc. Do Solo 2017, 41. [Google Scholar] [CrossRef]
- Braz, A.M.d.S.; Fernandes, A.R.; Alleoni, L.R.F. SOIL ATTRIBUTES AFTER THE CONVERSION FROM FOREST TO PASTURE IN AMAZON. Land Degrad. Dev. 2011, 24, 33–38. [Google Scholar] [CrossRef]
- Cenciani, K.; Lambais, M.R.; Cerri, C.C.; de Azevedo, L.C.B.; Feigl, B.J. Bacteria diversity and microbial biomass in forest, pasture and fallow soils in the southwestern Amazon basin. Rev. Bras. De Cienc. Do Solo 2009, 33, 907–916. [Google Scholar] [CrossRef]
- Carvalho, R.; Adami, M.; Amaral, S.; Bezerra, F.G.; de Aguiar, A.P.D. Changes in secondary vegetation dynamics in a context of decreasing deforestation rates in Pará, Brazilian Amazon. Appl. Geogr. 2019, 106, 40–49. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; Merloti, L.F.; Andreote, F.D.; Tsai, S.M. The natural recovery of soil microbial community and nitrogen functions after pasture abandonment in the Amazon region. FEMS Microbiol. Ecol. 2020, 96. [Google Scholar] [CrossRef]
- Porder, S.; Vitousek, P.M.; Chadwick, O.A.; Chamberlain, C.P.; Hilley, G.E. Uplift, Erosion, and Phosphorus Limitation in Terrestrial Ecosystems. Ecosystems 2007, 10, 159–171. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Sales, M.V.S.; Silva, P.S.D.; Comerford, N.B.; Cropper, W.P.; Gama-Rodrigues, E.F. An exploratory analysis of phosphorus transformations in tropical soils using structural equation modeling. Biogeochemistry 2014, 118, 453–469. [Google Scholar] [CrossRef]
- Alonso-Español, A.; Bravo, E.; Ribeiro-Vidal, H.; Virto, L.; Herrera, D.; Alonso, B.; Sanz, M. The Antimicrobial Activity of Curcumin and Xanthohumol on Bacterial Biofilms Developed over Dental Implant Surfaces. Int. J. Mol. Sci. 2023, 24, 2335. [Google Scholar] [CrossRef]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef]
- Shrivastava, M., Srivastava, P., D’Souza, S. Phosphate-Solubilizing Microbes: Diversity and Phosphates Solubilization Mechanism. In: Meena, V. (eds) Role of Rhizospheric Microbes in Soil. Springer, Singapore. 2018. [CrossRef]
- Chavarro-Bermeo, J.P.; Arruda, B.; Mora-Motta, D.A.; Bejarano-Herrera, W.; Ortiz-Morea, F.A.; Somenahally, A.; Silva-Olaya, A.M. Responses of Soil Phosphorus Fractions to Land-Use Change in Colombian Amazon. Sustainability 2022, 14, 2285. [Google Scholar] [CrossRef]
- Hamer, U.; Potthast, K.; Burneo, J.I.; Makeschin, F. Nutrient stocks and phosphorus fractions in mountain soils of Southern Ecuador after conversion of forest to pasture. Biogeochemistry 2012, 112, 495–510. [Google Scholar] [CrossRef]
- Garcia-Montiel, D.C.; Neill, C.; Melillo, J.; Thomas, S.; Steudler, P.A.; Cerri, C.C. Soil Phosphorus Transformations Following Forest Clearing for Pasture in the Brazilian Amazon. Soil Sci. Soc. Am. J. 2000, 64, 1792–1804. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry, fourth ed. Academic Press, London. 2014.
- Fu, D.; Wu, X.; Duan, C.; Chadwick, D.R.; Jones, D.L. Response of soil phosphorus fractions and fluxes to different vegetation restoration types in a subtropical mountain ecosystem. CATENA 2020, 193, 104663. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.J.; Silva, A.d.C.; Jamme, D.; Peña-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2019, 457, 117696. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Arenberg, M.R.; Arai, Y. Uncertainties in soil physicochemical factors controlling phosphorus mineralization and immobilization processes, first ed. Elsevier, London. 2019. [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, W.; Liu, G.; Liang, G.; He, P.; Liu, Z. Soil C/N and pH together as a comprehensive indicator for evaluating the effects of organic substitution management in subtropical paddy fields after application of high-quality amendments. Geoderma 2018, 337, 1116–1125. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Khan, S.M.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganism in sustainable agriculture: a review. Agronomy for Sustainable Development 2018, 27, 29–43. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ahmad, E.; Zaidi, A.; Oves, M. Functional aspect of phosphate solubilizing bacteria: importance in crop production. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D.K., Ed.; Springer: Berlin, 2013. [Google Scholar] [CrossRef]
- Bi, Q.-F.; Li, K.-J.; Zheng, B.-X.; Liu, X.-P.; Li, H.-Z.; Jin, B.-J.; Ding, K.; Yang, X.-R.; Lin, X.-Y.; Zhu, Y.-G. Partial replacement of inorganic phosphorus (P) by organic manure reshapes phosphate mobilizing bacterial community and promotes P bioavailability in a paddy soil. Sci. Total. Environ. 2019, 703, 134977. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Tsai, S.M.; Navarrete, A.A.; de Hollander, M.; van Veen, J.A.; Kuramae, E.E. Soil-Borne Microbiome: Linking Diversity to Function. Microb. Ecol. 2015, 70, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.-M.; Lee, I.-J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; Merloti, L.F.; Fonseca, M.d.C.d.; Cannavan, F.d.S.; Tsai, S.M. Forest-to-pasture conversion and recovery based on assessment of microbial communities in Eastern Amazon rainforest. FEMS Microbiol. Ecol. 2018, 95. [Google Scholar] [CrossRef]
- Batista, P.H.D.; de Almeida, G.L.P.; da Silva, J.L.B.; Pandorfi, H.; Da Silva, M.V.; da Silva, R.A.B.; de Melo, M.V.N.; Lins, F.A.C.; Junior, J.J.F.C. Short-term grazing and its impacts on soil and pasture degradation. DYNA 2020, 87, 123–128. [Google Scholar] [CrossRef]
- Sharrow, S.H. Soil compaction by grazing livestock in silvopastures as evidenced by changes in soil physical properties. Agrofor. Syst. 2007, 71, 215–223. [Google Scholar] [CrossRef]
- Pentoś, K.; Pieczarka, K.; Serwata, K. The Relationship between Soil Electrical Parameters and Compaction of Sandy Clay Loam Soil. Agriculture 2021, 11, 114. [Google Scholar] [CrossRef]
- de Moraes, M.T.; da Silva, V.R.; Zwirtes, A.L.; Carlesso, R. Use of penetrometers in agriculture: a review. Eng. Agricola 2014, 34, 179–193. [Google Scholar] [CrossRef]
- Krajco, J. Detection of soil compaction using soil electrical conductivity. Master’s Thesis, Cranfield University, Silsoe, UK, 2007.
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2012, 33, 291–309. [Google Scholar] [CrossRef]
- Mayerfeld, D.; Kruger, E.; Gildersleeve, R.; Rickenbach, M. Impacts of different grazing approaches on woodland ecosystem properties. Agrofor. Syst. 2021, 96, 527–540. [Google Scholar] [CrossRef]
- M., C.M. Effects of soil compaction by trampling of animals in soil productivity. Remediations. Rev. Colomb. de Cienc. Anim. - RECIA 2016, 8, 88–93. [Google Scholar] [CrossRef]
- Donkor, N.T.; Gedir, J.V.; Hudson, R.J.; Bork, E.W.; Chanasyk, D.S.; Naeth, M.A. Impacts of grazing systems on soil compaction and pasture production in Alberta. Can. J. Soil Sci. 2002, 82, 1–8. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Rangeland application of biochar and rotational grazing interact to influence soil and plant nutrient dynamics. Geoderma 2022, 408. [Google Scholar] [CrossRef]
- An, N.; Zhang, L.; Liu, Y.; Shen, S.; Li, N.; Wu, Z.; Yang, J.; Han, W.; Han, X. Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Chemosphere 2022, 298, 134304. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Song, S.; Rao, P.; Wang, R.; Liu, S. Preparation and properties of soil conditioner microspheres based on self-assembled potassium alginate and chitosan. Int. J. Biol. Macromol. 2019, 147, 877–889. [Google Scholar] [CrossRef]
- Cui, K.; Xu, L.; Tao, T.; Huang, L.; Li, J.; Hong, J.; Li, H.; Chi, Y. Mechanical behavior of multiscale hybrid fiber reinforced recycled aggregate concrete subject to uniaxial compression. Journal of Building Engineering 2023, 71, 106504. [Google Scholar] [CrossRef]
- Cui, K.; Xu, L.; Tao, T.; Huang, L.; Li, J.; Hong, J.; Li, H.; Chi, Y. Mechanical behavior of multiscale hybrid fiber reinforced recycled aggregate concrete subject to uniaxial compression. Journal of Building Engineering 2023, 71, 106504. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Cui, K.; Xu, L.; Tao, T.; Huang, L.; Li, J.; Hong, J.; Li, H.; Chi, Y. Mechanical behavior of multiscale hybrid fiber reinforced recycled aggregate concrete subject to uniaxial compression. Journal of Building Engineering 2023, 71, 106504. [Google Scholar] [CrossRef]
- Chander, M.P.; Kartick, C.; Gangadhar, J.; Vijayachari, P. Ethno medicine and healthcare practices among Nicobarese of Car Nicobar - an indigenous tribe of Andaman and Nicobar Islands. J. Ethnopharmacol. 2014, 158, 18–14. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L. M., Rezende, H.C., Coelho, L., M., de Sousa, P.A.R., Melo, D.F.O., Coelho, N.M.M. Bioremediation of Polluted Waters Using Microorganisms 2015. InTech. [CrossRef]
- Nicolaou, S.A.; Gaida, S.M.; Papoutsakis, E.T. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab. Eng. 2010, 12, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A., Bisht. Review on bioremediation of polluted environment: a management tool. International Journal of Environmental Sciences 2011, 1, 1079–1093. [Google Scholar]
- Wasilkowski, D., Swedziol. The applicability of genetically modified microorganisms in bioremediation of contaminated environments. Chemik 2012, 66, 822–826. [Google Scholar]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Mushtaq, S.; e Bareen, F.; Tayyeb, A. Equilibrium kinetics and thermodynamic studies on biosorption of heavy metals by metal-resistant strains of Trichoderma isolated from tannery solid waste. Environ. Sci. Pollut. Res. 2022, 30, 10925–10954. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Asyakina, L.K.; Serazetdinova, Y.R.; Frolova, A.S.; Velichkovich, N.S.; Prosekov, A.Y. Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals. Microorganisms 2023, 11, 864. [Google Scholar] [CrossRef]
- Effendi, A.J.; Mastroiani, L.J.; Suhardi, S.H.; Ramadan, B.S. Potential use of extracellular polymeric substances (EPS) of Bacillus subtilis for biosorption of mercury produced from soil-washing effluent. Bioresour. Technol. Rep. 2023, 22. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Srinivasan, R.; Charles, P.E.; Rajaram, R.; Zhang, R. Exopolymeric substances production by Bacillus cereus KMS3-1 enhanced its biosorption efficiency in removing Cd2+ and Pb2+ in single and binary metal mixtures. Environ. Res. 2023, 228, 115917. [Google Scholar] [CrossRef]
- Bioremediation of Heavy Metals Using Microorganisms. In Genetically Engineered Organisms in Bioremediation; Nagmote, M.S., Rai, A.R., Sharma, R., Desimone, M.F., Chaudhary, R.G., Singh, N.B., Eds.; CRC Press, 2024; pp. 168–190. [Google Scholar]
- Rahman, M.A.; Hassler, C. Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat. Toxicol. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- Mala, J.G.S.; Sujatha, D.; Rose, C. Inducible chromate reductase exhibiting extracellular activity in Bacillus methylotrophicus for chromium bioremediation. Microbiol. Res. 2015, 170, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Barkay, T.; Gu, B. Demethylation─The Other Side of the Mercury Methylation Coin: A Critical Review. ACS Environ. Au 2021, 2, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Thomas, L.; Chetri, S.P.K.; Bodhankar, S.; Kumar, V.; Naidu, R. A comprehensive review on chromium (Cr) contamination and Cr(VI)-resistant extremophiles in diverse extreme environments. Environ. Sci. Pollut. Res. 2023, 30, 59163–59193. [Google Scholar] [CrossRef] [PubMed]
- Elnabi, M.K.A.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Elaty, A.E.A.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Roane, T.M.; Josephson, K.L.; Pepper, I.L. Dual-Bioaugmentation Strategy To Enhance Remediation of Cocontaminated Soil. Appl. Environ. Microbiol. 2001, 67, 3208–3215. [Google Scholar] [CrossRef]
- Vullo, D.L.; Ceretti, H.M.; Daniel, M.A.; Ramírez, S.A.; Zalts, A. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Bioresour. Technol. 2007, 99, 5574–5581. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Chai, L.; Wang, Y.; Liu, Y.; Xiao, R. Bioleaching remediation of heavy metal-contaminated soils using Burkholderia sp. Z-90. J. Hazard. Mater. 2016, 301, 145–152. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2011, 201-202, 178–184. [Google Scholar] [CrossRef]
- Li, X.; Dong, S.; Yao, Y.; Shi, W.; Wu, M.; Xu, H. Inoculation of bacteria for the bioremediation of heavy metals contaminated soil by Agrocybe aegerita. RSC Adv. 2016, 6, 65816–65824. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Hossain, K.; Alam Saud, Z.; Saha, A.K.; Ghosh, S.; Olsson, B.; Mandal, A. Bioremediation of hexavalent chromium (VI) by a soil-borne bacterium,Enterobacter cloacaeB2-DHA. J. Environ. Sci. Heal. Part A 2015, 50, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Rafi, N.; Wani, U.; A, H.B.; Khan, M.S.A. Simultaneous bioremediation of heavy metals and biodegradation of hydrocarbons by metal resistantBrevibacillus parabrevisOZF5 improves plant growth promotion. Bioremediation J. 2021, 27, 20–31. [Google Scholar] [CrossRef]
- Ridene, S.; Werfelli, N.; Mansouri, A.; Landoulsi, A.; Abbes, C. Bioremediation potential of consortium Pseudomonas Stutzeri LBR and Cupriavidus Metallidurans LBJ in soil polluted by lead. PLOS ONE 2023, 18, e0284120. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Patil, A.; Pandey, A.; Arya, M. Biosorption of Heavy Metal (Mn2+) by Thermophilic Bacterial Strains Isolated from Surya Kund Hot Spring, Yamunotri, Uttarakhand. Appl. Biochem. Biotechnol. 2023, 196, 2518–2533. [Google Scholar] [CrossRef]
- Cam, N.; Benzerara, K.; Georgelin, T.; Jaber, M.; Lambert, J.-F.; Poinsot, M.; Skouri-Panet, F.; Cordier, L. Selective Uptake of Alkaline Earth Metals by Cyanobacteria Forming Intracellular Carbonates. Environ. Sci. Technol. 2016, 50, 11654–11662. [Google Scholar] [CrossRef]
- Al-Sherif, A.; Abd El-Hameed, M.S.; Mahmoud, M.A.; Ahmed, H.S. Use of cyanobacteria and organic fertilizer mixture as soil bioremediation. American-Eurasian Journal of Agricultural & Environmental Sciences 2015, 15, 794–799. [Google Scholar] [CrossRef]
- Jin, Z.; Deng, S.; Wen, Y.; Jin, Y.; Pan, L.; Zhang, Y.; Black, T.; Jones, K.C.; Zhang, H.; Zhang, D. Application of Simplicillium chinense for Cd and Pb biosorption and enhancing heavy metal phytoremediation of soils. Sci. Total. Environ. 2019, 697, 134148. [Google Scholar] [CrossRef]
- Chang, J.; Shi, Y.; Si, G.; Yang, Q.; Dong, J.; Chen, J. The bioremediation potentials and mercury(II)-resistant mechanisms of a novel fungus Penicillium spp. DC-F11 isolated from contaminated soil. J. Hazard. Mater. 2020, 396, 122638. [Google Scholar] [CrossRef]
- Din, G.; Hassan, A.; Dunlap, J.; Ripp, S.; Shah, A.A. Cadmium tolerance and bioremediation potential of filamentous fungus Penicillium chrysogenum FMS2 isolated from soil. Int. J. Environ. Sci. Technol. 2021, 19, 2761–2770. [Google Scholar] [CrossRef]
- Taştan, B.E.; Ertuğrul, S.; Dönmez, G. Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Bioresour. Technol. 2009, 101, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.K.; Congeevaram, S.; Thamaraiselvi, K. Evaluation of isolated fungal strain from e-waste recycling facility for effective sorption of toxic heavy metal Pb (II) ions and fungal protein molecular characterization-a Mycoremediation approach. Asian Journal of Experimental Biological Sciences 2011, 2, 342–347. [Google Scholar]
- Hasgul, E.; Malkoç, S.; Güven, A.; Dede, A.; Güven, K. Biosorption of cadmium and copper by Aspergillus spp. isolated from industrial ceramic waste sludge. Biol. Divers. Conserv. 2019, 12, 44–56. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ahmed, A.; Auta, H.S.; Hamid, F.S. Enhanced Bioremediation of Heavy Metal Contaminated Landfill Soil Using Filamentous Fungi Consortia: a Demonstration of Bioaugmentation Potential. Water, Air, Soil Pollut. 2019, 230, 215. [Google Scholar] [CrossRef]
- Bahafid, W.; Tahri Joutey, N.; Sayel, H.; Boularab, I.; EL Ghachtouli, N. Bioaugmentation of chromium-polluted soil microcosms with Candida tropicalis diminishes phytoavailable chromium. J. Appl. Microbiol. 2013, 115, 727–734. [Google Scholar] [CrossRef]
- Damodaran, D.; Suresh, G.; Mohan, R. Bioremediation of soil by removing heavy metals using Saccharomyces cerevisiae. In 2nd international conference on environmental science and technology; Singapore, 2011. [Google Scholar]
- Imam, S.S.A. Comparative Study of Heavy Metal Bioremediation in Soil by Bacillus Subtilis and Saccharomyces Cerevisiae. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Mao, Q.; Xie, X.; Pinzon-Nuñez, D.A.; Xie, Z.; Liu, T.; Irshad, S. Native microalgae and Bacillus XZM remediate arsenic-contaminated soil by forming biological soil crusts. J. Environ. Manag. 2023, 345, 118858. [Google Scholar] [CrossRef]
- Ri, C.; Tao, Y.; Tu, J.; Li, X.; She, S.; Hou, L.; Fu, Y.; Chen, L. Improvement of the Cd removal efficiency of a filamentous cyanobacterium Leptolyngbya sp. XZ1 through co-culture with Bacillus sp. S1. J. Appl. Phycol. 2023, 35, 2935–2944. [Google Scholar] [CrossRef]
- Gupta, R.; Khan, F.; Alqahtani, F.M.; Hashem, M.; Ahmad, F. Plant Growth–Promoting Rhizobacteria (PGPR) Assisted Bioremediation of Heavy Metal Toxicity. Appl. Biochem. Biotechnol. 2023, 196, 2928–2956. [Google Scholar] [CrossRef]
- Ledin, M. Accumulation of metals by microorganisms — processes and importance for soil systems. Earth-Science Rev. 2000, 51, 1–31. [Google Scholar] [CrossRef]
- Thapa, B.; Kc, A.K.; Ghimire, A. A Review On Bioremediation Of Petroleum Hydrocarbon Contaminants In Soil. 1970, 8, 164–170. [Google Scholar] [CrossRef]
- Gao, B.; Wang, X.; Ford, R.M. Chemotaxis along local chemical gradients enhanced bacteria dispersion and PAH bioavailability in a heterogenous porous medium. Sci. Total. Environ. 2022, 859, 160004. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, A.S.; Putra, S.R.; Putro, H.S.; Hamzah, A.; Rohma, N.A.; Rohmah, A.A.; Rizqi, H.D.; Asranudin; Tangahu, B.V.; Warmadewanthi, I.D.A.A. The application of biosurfactant-producing bacteria immobilized in PVA/SA/bentonite bio-composite for hydrocarbon-contaminated soil bioremediation. RSC Adv. 2023, 13, 21163–21170. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Hou, J.; Du, M.; Zhang, Y.; Liu, W.; Christie, P.; Luo, Y. Surfactant-enhanced bioremediation of petroleum-contaminated soil and microbial community response: A field study. Chemosphere 2023, 322, 138225. [Google Scholar] [CrossRef]
- Malik, G.; Arora, R.; Chaturvedi, R.; Paul, M.S. Implementation of Genetic Engineering and Novel Omics Approaches to Enhance Bioremediation: A Focused Review. Bull. Environ. Contam. Toxicol. 2021, 108, 443–450. [Google Scholar] [CrossRef]
- Shyam, K.; Kumar, N.; Chandel, H.; Dogra, A.S.; Sharma, G.; Saxena, G. Omics Technologies in Environmental Microbiology and Microbial Ecology: Insightful Applications in Bioremediation Research. Wiley Online Library, Genomics Approach to Bioremediation: Principles, Tools, and Emerging Technologies 2023, 433–454. [Google Scholar] [CrossRef]
- Wesseler, J.; Kleter, G.; Meulenbroek, M.; Purnhagen, K.P. EU regulation of genetically modified microorganisms in light of new policy developments: Possible implications for EU bioeconomy investments. Appl. Econ. Perspect. Policy 2022, 45, 839–859. [Google Scholar] [CrossRef]
- Directive 2009/41/EC, European Parliament 2009. Source: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC138068/.
- Perera, I.C.; Hemamali, E.H. Genetically Modified Organisms for Bioremediation: Current Research and Advancements. In Bioremediation of Environmental Pollutants; Suyal, D.C., Soni, R., Eds.; Springer: Cham, 2022. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, P. A comparative analysis of heavy metal bioaccumulation and functional gene annotation towards multiple metal resistant potential by Ochrobactrum intermedium BPS-20 and Ochrobactrum ciceri BPS-26. Bioresour. Technol. 2020, 320, 124330. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M. Genetically engineered microorganisms for environmental remediation. Chemosphere 2022, 310, 136751. [Google Scholar] [CrossRef]
- Tirkey, S.R.; Ram, S.; Chandrashekhar, P.; Mishra, S. Bioremediation of Soils Polluted with Hexavalent Chromium Using Bacteria. In Industrial Wastewater Reuse; Shah, M.P., Ed.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Han, F.; Liu, Z.; Zhao, C.; Lei, J.; Zhou, W. Enhanced degradation of petroleum in saline soil by nitrogen stimulation and halophilic emulsifying bacteria Bacillus sp. Z-13. J. Hazard. Mater. 2023, 459, 132102. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.R.; Dexter, G.N.; Baldino, H.; Huenemann, J.D.; Francis, R.; Peabody, G.L.; Martinez-Baird, J.; Riley, L.A.; Simmons, T.; Coleman-Derr, D.; et al. High-throughput genetic engineering of nonmodel and undomesticated bacteria via iterative site-specific genome integration. Sci. Adv. 2023, 9, eade1285. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P., Kandeler. Enzyme activities and microbiological and biochemical processes in soil. Enzymes in the Environment. CRC Press: Boca Raton, FL, USA, 2002; pp. 1–33. [Google Scholar]
- Khalid, F.; Hashmi, M.Z.; Jamil, N.; Qadir, A.; Ali, M.I. Microbial and enzymatic degradation of PCBs from e-waste-contaminated sites: a review. Environ. Sci. Pollut. Res. 2021, 28, 10474–10487. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Tripathi, M.; Srinath, T. Strategies for chromium bioremediation of tannery effluent. Reviews of Environmental Contamination and Toxicology 2012, 217, 75–140. [Google Scholar] [CrossRef] [PubMed]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The Role of Microorganisms in Bioremediation- A Review. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Haghollahi, A.; Fazaelipoor, M.H.; Schaffie, M. The effect of soil type on the bioremediation of petroleum contaminated soils. J. Environ. Manag. 2016, 180, 197–201. [Google Scholar] [CrossRef]
- da Silva, I.G.S.; de Almeida, F.C.G.; Silva, N.M.P.d.R.e.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Soil Bioremediation: Overview of Technologies and Trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Ng, Z.Y.; Ajeng, A.A.; Cheah, W.Y.; Ng, E.-P.; Abdullah, R.; Ling, T.C. Towards circular economy: Potential of microalgae – bacterial-based biofertilizer on plants. J. Environ. Manag. 2023, 349, 119445. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wang, J.; Deng, Y.; Liu, Y.; Peng, B. The potential impact on the biodegradation of organic pollutants from composting technology for soil remediation. Waste Manag. 2017, 72, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, P.; Vieira, G.A.; Otero, I.V.R.; Pellizzer, E.P.; Fontes, B.d.J.; Sette, L.D. Metal and organic pollutants bioremediation by extremophile microorganisms. J. Hazard. Mater. 2019, 382, 121024. [Google Scholar] [CrossRef]
- Hualpa-Cutipa E., Acosta R. A. S., Cariga O. J. M., Espinoza-Medina M. A., Hansen-Reyes M., Medina-Cerna D., et al. “Omics Insights into Cold Environments: Cold-Tolerant Microorganisms and their Potential Use in Bioremediation” in Omics Insights in Environmental Bioremediation. eds. Kumar V., Thakur I. S. (Singapore: Springer Nature Singapore;) 2022, 437–453.
- Chaturvedi, R. Extremophiles: Biofactories for Bioremediation. In Integrative Approaches to Biotechnology; CRC Press, 2024; pp. 171–185. ISBN 9781003324706. [Google Scholar]
- Abdelaziz, S.; Belal, E.E.; Al-Quwaie, D.A.; Ashkan, M.F.; Alqahtani, F.S.; El-Tarabily, K.A.; El-Mageed, T.A.A.; Shami, A.; Nader, M.M.; Hemeda, N.F. Extremophilic bacterial strains as plant growth promoters and biocontrol agents against Pythium ultimum and Rhizocotnia solani. J. Plant Pathol. 2023, 105, 1347–1369. [Google Scholar] [CrossRef]
- Santos, A.P.; Belfiore, C.; Úrbez, C.; Ferrando, A.; Blázquez, M.A.; Farías, M.E. Extremophiles as Plant Probiotics to Promote Germination and Alleviate Salt Stress in Soybean. J. Plant Growth Regul. 2022, 42, 946–959. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212. [Google Scholar] [CrossRef]
- Atai, E.; Jumbo, R.B.; Cowley, T.; Azuazu, I.; Coulon, F.; Pawlett, M. Efficacy of bioadmendments in reducing the influence of salinity on the bioremediation of oil-contaminated soil. Sci. Total. Environ. 2023, 892, 164720. [Google Scholar] [CrossRef]
- Rhykerd, R.L.; Weaver, R.W.; McInnes, K.J. Influence of salinity on bioremediation of oil in soil. Environ. Pollut. 1995, 90, 127–130. [Google Scholar] [CrossRef]
- Qin, X.; Tang, J.; Li, D.; Zhang, Q. Effect of salinity on the bioremediation of petroleum hydrocarbons in a saline-alkaline soil. Lett. Appl. Microbiol. 2012, 55, 210–217. [Google Scholar] [CrossRef]
- Kansour, M.K.; Al-Mailem, D.M. Bioremediation of two oil-contaminated Kuwaiti hyper-saline soils by cross bioaugmentation and the role of indigenous halophilic/halotolerant hydrocarbonoclastic bacteria. Environ. Technol. Innov. 2023, 32. [Google Scholar] [CrossRef]
- Quoc, K.N.; Le Vinh, T.; Le Thanh, Q.; Ngoc, H.T.; Thi, X.D.; Huu, D.H.; Thanh, X.L.N.; My, T.L.T. Effects of biofertilizer supplementation, Rhodopseudomonas spp., on nitrogen and phosphorus uptakes, growth, and yield of sesame ( Sesamum indicum L.) on salt-affected soil. J. Plant Nutr. 2023, 47, 1–17. [Google Scholar] [CrossRef]
- Hadibarata, T.; Kristanti, R.A.; Bilal, M.; Yilmaz, M.; Sathishkumar, P. Biodegradation mechanism of chlorpyrifos by halophilic bacterium Hortaea sp. B15. Chemosphere 2022, 312, 137260. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Sivaperumal, P. Marine halophilic archaeal isolate Halococcus sp. with emphasis on bioremediation of radionuclides through extracellular biomolecules. J. Chem. Technol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Iqbal, S., Hasni, M.S., Malik, Y., Baloch, S. and Aamir, R. Halophilic Fungi: Versatile Microorganisms for Biotechnological Advancements 2023. [CrossRef]
- Martínez-Espinosa, R.M.; Kumar, S.; Upadhyay, S.K.; Orhan, F. Editorial: Adaptation of halophilic/halotolerant microorganisms and their applications. Front. Microbiol. 2023, 14, 1252921. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Ma, Y.; Wang, X.; Zhang, B.; Zhang, G.; Bahadur, A.; Chen, T.; Liu, G.; Zhang, W.; et al. Research progress regarding the role of halophilic and halotolerant microorganisms in the eco-environmental sustainability and conservation. J. Clean. Prod. 2023, 418. [Google Scholar] [CrossRef]
- Hualpa-Cutipa, E., Acosta, R.A.S., Cariga, O.J.M., Espinoza-Medina, M.A., Hansen-Reyes, M., Medina-Cerna, D., Olanda, M.C., Cortez-Lázaro, A.A. Omics Insights into Cold Environments: Cold-Tolerant Microorganisms and their Potential Use in Bioremediation. In Omics Insights in Environmental Bioremediation 2022. (pp. 437-453). Singapore: Springer Nature Singapore. [CrossRef]
- Barghoth, M.G.; Desouky, S.E.; Radwan, A.A.; Shah, M.P.; Salem, S.S. Characterizations of highly efficient moderately halophilic toluene degrading exiguobacterium mexicanum M7 strain isolated from Egyptian saline sediments. Biotechnol. Genet. Eng. Rev. 2023, 1–19. [Google Scholar] [CrossRef]
- Kansour, M.K.; Al-Mailem, D.M. Bioremediation of two oil-contaminated Kuwaiti hyper-saline soils by cross bioaugmentation and the role of indigenous halophilic/halotolerant hydrocarbonoclastic bacteria. Environ. Technol. Innov. 2023, 32. [Google Scholar] [CrossRef]
- Rajkumar, R., Kurinjimalar, C. Microbiological Activity for Soil and Plant Health Management. Springer; Singapore. Microbes and Plant Mineral Nutrition 2021, 111–132. [CrossRef]
- Radhakrishnan, A.; Balaganesh, P.; Vasudevan, M.; Natarajan, N.; Chauhan, A.; Arora, J.; Ranjan, A.; Rajput, V.D.; Sushkova, S.; Minkina, T.; et al. Bioremediation of Hydrocarbon Pollutants: Recent Promising Sustainable Approaches, Scope, and Challenges. Sustainability 2023, 15, 5847. [Google Scholar] [CrossRef]
- Jalali, F.M.; Chahkandi, B.; Gheibi, M.; Eftekhari, M.; Behzadian, K.; Campos, L.C. Developing a smart and clean technology for bioremediation of antibiotic contamination in arable lands. Sustain. Chem. Pharm. 2023, 33. [Google Scholar] [CrossRef]
- Hwangbo, M.; Shao, Y.; Hatzinger, P.B.; Chu, K. Acidophilic methanotrophs: Occurrence, diversity, and possible bioremediation applications. Environ. Microbiol. Rep. 2023, 15, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Quoc, B.N.; Armenta, M.; Carter, J.A.; Bucher, R.; Sukapanpotharam, P.; Bryson, S.J.; Stahl, D.A.; Stensel, H.D.; Winkler, M.-K.H. An investigation into the optimal granular sludge size for simultaneous nitrogen and phosphate removal. Water Res. 2021, 198, 117119. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process. Eng. 2020, 39, 101858. [Google Scholar] [CrossRef]
- Abtahi, H.; Parhamfar, M.; Saeedi, R.; Villaseñor, J.; Sartaj, M.; Kumar, V.; Coulon, F.; Parhamfar, M.; Didehdar, M.; Seifi, H.; et al. Effect of competition between petroleum-degrading bacteria and indigenous compost microorganisms on the efficiency of petroleum sludge bioremediation: Field application of mineral-based culture in the composting process. J. Environ. Manag. 2020, 258, 110013. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Roane, T.M.; Josephson, K.L.; Pepper, I.L. Dual-Bioaugmentation Strategy To Enhance Remediation of Cocontaminated Soil. Appl. Environ. Microbiol. 2001, 67, 3208–3215. [Google Scholar] [CrossRef]
- Wani, P.A.; Rafi, N.; Wani, U.; A, H.B.; Khan, M.S.A. Simultaneous bioremediation of heavy metals and biodegradation of hydrocarbons by metal resistantBrevibacillus parabrevisOZF5 improves plant growth promotion. Bioremediation J. 2021, 27, 20–31. [Google Scholar] [CrossRef]
- Sar, P.; Kundu, S.; Ghosh, A.; Saha, B. Natural surfactant mediated bioremediation approaches for contaminated soil. RSC Adv. 2023, 13, 30586–30605. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Roller, B.R.K.; Schmidt, T.M. The physiology and ecological implications of efficient growth. ISME J. 2015, 9, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Neidhardt, F.C. Bacterial Growth: Constant Obsession with dN/dt. J. Bacteriol. 1999, 181, 7405–8. [Google Scholar] [CrossRef] [PubMed]
- Moqsud, M.; Yoshitake, J.; Bushra, Q.; Hyodo, M.; Omine, K.; Strik, D. Compost in plant microbial fuel cell for bioelectricity generation. Waste Manag. 2015, 36, 63–69. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.M.A.N.; Shafique, B.; Ullah, M.I.; Siddiqui, A.R.; Wang, L. Bioremediation of Soil: An Overview. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management; Malik, J.A., Ed.; Springer: Cham, 2022. [Google Scholar] [CrossRef]
- Narancic, T.; O'Connor, K.E. Microbial biotechnology addressing the plastic waste disaster. Microb. Biotechnol. 2017, 10, 1232–1235. [Google Scholar] [CrossRef]
- Ji, M.; Zhu, J.; Zhu, R.; Chen, J.; Wang, C. Experiments on the Effects of Dissolved Oxygen on the Free and Immobilization Effective Microorganisms (EM) in Treating Polluted River Water. International Conference on Material and Environmental Engineering (ICMAEE 2014). LOCATION OF CONFERENCE, ChinaDATE OF CONFERENCE;
- Wang, B.; Kuang, S.; Shao, H.; Wang, L.; Wang, H. Anaerobic-petroleum degrading bacteria: Diversity and biotechnological applications for improving coastal soil. Ecotoxicol. Environ. Saf. 2021, 224, 112646. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Wu, J.-H.; Wang, B.N. Intermittent Oxygen Supply Facilitates Codegradation of Trichloroethene and Toluene by Anaerobic Consortia. Environ. Sci. Technol. 2023, 57, 10252–10262. [Google Scholar] [CrossRef]
- Bhuyan, P.P.; Nayak, R.; Jena, M.; Pradhan, B. Convoluted role of cyanobacteria as biofertilizer: an insight of sustainable agriculture. Vegetos 2022, 36, 309–321. [Google Scholar] [CrossRef]
- Jangir, C.K.; Kumar, S.; Meena, R.S. Sustainable agriculture. Scientific Publisher; Jodhpur, India. Significance of soil organic matter to soil quality and evaluation of sustainability, 2019, 357–381.
- Bauder, D. L. Bioremediation of Contaminated Soil. Retrieved February 2006, 7, 2014 https://bioremediationofcontaminatedsoilwordpresscom/. [Google Scholar]
- Gao, H.; Wu, M.; Liu, H.; Ou, Y.; Zhang, T.; Duan, X. Unraveling the Positive Effect of Soil Moisture on the Bioaugmentation of Petroleum-Contaminated Soil Using Bioinformatics. Microb. Ecol. 2023, 1–11. [Google Scholar] [CrossRef]
- Naorem, A. Will Climate Change Alter the Efficiency of Bioremediation?. In: Malik, J.A. (eds) Advances in Bioremediation and Phytoremediation for Sustainable Soil Management. Springer, Cham. 2022. [CrossRef]
- Bahmani, F. , Ataei, S. A., Mikaili, M.A. The Effect of Moisture Content Variation on the Bioremediation of Hydrocarbon Contaminated Soils: Modeling and Experimental Investigation. Journal of Environmental Analytical Chemistry 2018, 5, 236. [Google Scholar] [CrossRef]
- Kumari, S.; Gautam, K.; Seth, M.; Anbumani, S.; Manickam, N. Bioremediation of polycyclic aromatic hydrocarbons in crude oil by bacterial consortium in soil amended with Eisenia fetida and rhamnolipid. Environ. Sci. Pollut. Res. 2023, 30, 82517–82531. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, R.; Peng, T.; Li, Q.; Wang, Q.; Wu, Y.; Song, X.; Lin, X. Low-temperature thermally enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil: Effects on fate, toxicity and bacterial communities. Environ. Pollut. 2023, 335, 122247. [Google Scholar] [CrossRef]
- Norozpour, M.; Sarikhani, M.R.; Aliasgharzad, N. Monitoring of soil respiration changes in a heavy naphtha-contaminated sandy loam soil under different bioremediation treatments. Water and Soil Science 2023, 33, 53–72. [Google Scholar] [CrossRef]
- Peekate, L.P.; Diepiriye, A.D.; Amadi, M.C. Effect of glucose on biodegradation of hydrocarbons in crude-oil polluted soil undergoing bioremediation. Asia J. Appl. Microbiol. 2023, 10, 61–69. [Google Scholar] [CrossRef]
- Mupambwa, H.A.; Mnkeni, P.N.S. Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers: a review. Environ. Sci. Pollut. Res. 2018, 25, 10577–10595. [Google Scholar] [CrossRef]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. J. Chem. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Cavazzoli, S.; Squartini, A.; Sinkkonen, A.; Romantschuk, M.; Rantalainen, A.-L.; Selonen, V.; Roslund, M.I. Nutritional additives dominance in driving the bacterial communities succession and bioremediation of hydrocarbon and heavy metal contaminated soil microcosms. Microbiol. Res. 2023, 270, 127343. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Microbial bioremediation as a robust process to mitigate pollutants of environmental concern. Case Stud. Chem. Environ. Eng. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, P. Futuristic avenues of metabolic engineering techniques in bioremediation. Biotechnol. Appl. Biochem. 2020, 69, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Dey, A. Bioremediation Approaches for Recalcitrant Pollutants: Potentiality, Successes and Limitation. 2015. [CrossRef]
- Li, Q.; Liu, J.; Gadd, G.M. Fungal bioremediation of soil co-contaminated with petroleum hydrocarbons and toxic metals. Appl. Microbiol. Biotechnol. 2020, 104, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Biswas, R.; Sarkar, A. Advancement of Omics: Prospects for Bioremediation of Contaminated Soils. In Microbial Bioremediation & Biodegradation; Shah, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Rathore, S.; Varshney, A.; Mohan, S.; Dahiya, P. An innovative approach of bioremediation in enzymatic degradation of xenobiotics. Biotechnol. Genet. Eng. Rev. 2022, 38, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, S.; Dong, Y.; Fan, H.; Bai, Z.; Zhuang, X. Engineering Microbial Consortia towards Bioremediation. Water 2021, 13, 2928. [Google Scholar] [CrossRef]
- Mali, H.; Shah, C.; Raghunandan, B.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2022, 127, 234–250. [Google Scholar] [CrossRef]
- Khatoon, N.; Jamal, A.; Ali, M.I. Polymeric pollutant biodegradation through microbial oxidoreductase: A better strategy to safe environment. Int. J. Biol. Macromol. 2017, 105, 9–16. [Google Scholar] [CrossRef]
- Peter, J.K.; Singh, R.; Yadav, A.K.; Kothari, R.; Mehta, P.K. Toxicity of nitriles/amides-based products in the environment and their enzymatic bioremediation. J. Hazard. Mater. Adv. 2023, 13. [Google Scholar] [CrossRef]
- Wang, L.; Du, X.; Li, Y.; Bai, Y.; Tang, T.; Wu, J.; Liang, H.; Gao, D. Enzyme immobilization as a sustainable approach toward ecological remediation of organic-contaminated soils: Advances, issues, and future perspectives. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1684–1708. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, F.; Guo, S. Highly efficient screening and optimal combination of functional isolates for bioremediation of hydrocarbon-polluted soil. Environ. Res. 2022, 219, 115064. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, J.R. Blight C Sci. Am. 1990, 263, 106–111. Available online: http://www.jstor.org/stable/24996872. [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- IPBES Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES secretariat 2019, 1–1148. [CrossRef]
- van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef]
- van Kleunen, M.; Pyšek, P.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Weigelt, P.; Stein, A.; Dullinger, S.; König, C.; et al. The Global Naturalized Alien Flora (GloNAF) database. Ecology 2018, 100, e02542. [Google Scholar] [CrossRef]
- Pyšek, P.; Pergl, J.; Essl, F.; Lenzner, B.; Dawson, W.; Kreft, H.; Weigelt, P.; Winter, M.; Kartesz, J.; Nishino, M.; et al. Naturalized alien flora of the world. Preslia 2017, 89, 203–274. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions. Springer Science and Business Media LLC, 2020; p. 383. [Google Scholar]
- Mawarda, P.C.; Le Roux, X.; van Elsas, J.D.; Salles, J.F. Deliberate introduction of invisible invaders: A critical appraisal of the impact of microbial inoculants on soil microbial communities. Soil Biol. Biochem. 2020, 148, 107874. [Google Scholar] [CrossRef]
- Arnold, B.J.; Huang, I.-T.; Hanage, W.P. Horizontal gene transfer and adaptive evolution in bacteria. Nat. Rev. Microbiol. 2021, 20, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, A.R. Horizontal Gene Transfer: Figure 1. Evol. Med. Public Heal. 2015, 2015, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Macedo, G.; Olesen, A.K.; Maccario, L.; Leal, L.H.; Maas, P.V.D.; Heederik, D.; Mevius, D.; Sørensen, S.J.; Schmitt, H. Horizontal Gene Transfer of an IncP1 Plasmid to Soil Bacterial Community Introduced by Escherichia coli through Manure Amendment in Soil Microcosms. Environ. Sci. Technol. 2022, 56, 11398–11408. [Google Scholar] [CrossRef]
- Ronchel, M.C.; Ramos-Díaz, M.A.; Ramos, J.L. Retrotransfer of DNA in the rhizosphere. Environ. Microbiol. 2000, 2, 319–323. [Google Scholar] [CrossRef]
- Martínez-Carranza, E.; García-Reyes, S.; González-Valdez, A.; Soberón-Chávez, G. Tracking the genome of four Pseudomonas aeruginosa isolates that have a defective Las quorum-sensing system, but are still virulent. Access Microbiol. 2020, 2, e000132. [Google Scholar] [CrossRef]
- European Regulation (EU) 2019/1009 soyce: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=PI_COM:Ares(2021)898281.
- Dastogeer, K.M.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Vacheron, J., Renoud, S., Muller, D., Babalola, O. O., Prigent-Combaret, C “Handbook for Azospirillum. Technical Issues and Protocols. Everything practical you always wanted to know about Azospirillum sp. but were afraid to ask,” in Alleviation of Abiotic and Biotic Stresses in Plants by Azospirillum, eds F. D. Cassan, Y. Okon, M. Cecilia, and M. C. Creus (Cham: Springer) 2015. 333–365.
- Jones, D.L. Organic acids in the rhizosphere – a critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef]
- Keswani, C.; Prakash, O.; Bharti, N.; Vílchez, J.I.; Sansinenea, E.; Lally, R.D.; Borriss, R.; Singh, S.P.; Gupta, V.K.; Fraceto, L.F.; et al. Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total. Environ. 2019, 690, 841–852. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant, Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, F.; Tebbe, C.C. Effect of Field Inoculation with Sinorhizobium meliloti L33 on the Composition of Bacterial Communities in Rhizospheres of a Target Plant ( Medicago sativa ) and a Non-Target Plant ( Chenopodium album )—Linking of 16S rRNA Gene-Based Single-Strand Conformation Polymorphism Community Profiles to the Diversity of Cultivated Bacteria. Appl. Environ. Microbiol. 2000, 66, 3556–3565. [Google Scholar] [CrossRef] [PubMed]
- Horácio, E.H.; Zucareli, C.; Gavilanes, F.Z.; Yunes, J.S.; dos Santos Sanzovo, A.W.; Andrade, D.S. Co-inoculation of rhizobia, azospirilla and cyanobacteria for increasing common bean production. Londrina 2020, 41, 2015–2028. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mhamdi, R. Microbial Inoculants and Their Impact on Soil Microbial Communities: A Review. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Harman, G.E.; Uphoff, N. Symbiotic Root-Endophytic Soil Microbes Improve Crop Productivity and Provide Environmental Benefits. Scientifica 2019, 2019, 1–25. [Google Scholar] [CrossRef]
- Baudoin, E.; Nazaret, S.; Mougel, C.; Ranjard, L.; Moënne-Loccoz, Y. Impact of inoculation with the phytostimulatory PGPR Azospirillum lipoferum CRT1 on the genetic structure of the rhizobacterial community of field-grown maize. Soil Biol. Biochem. 2009, 41, 409–413. [Google Scholar] [CrossRef]
- Herschkovitz, Y.; Lerner, A.; Davidov, Y.; Okon, Y.; Jurkevitch, E. Azospirillum brasilense does not affect population structure of specific rhizobacterial communities of inoculated maize (Zea mays). Environ. Microbiol. 2005, 7, 1847–1852. [Google Scholar] [CrossRef]
- Lerner, A.; Herschkovitz, Y.; Baudoin, E.; Nazaret, S.; Moenne-Loccoz, Y.; Okon, Y.; Jurkevitch, E. Effect of Azospirillum brasilense inoculation on rhizobacterial communities analyzed by denaturing gradient gel electrophoresis and automated ribosomal intergenic spacer analysis. Soil Biol. Biochem. 2006, 38, 1212–1218. [Google Scholar] [CrossRef]
- Correa, O.; Romero, A.; Montecchia, M.; Soria, M. Tomato genotype and Azospirillum inoculation modulate the changes in bacterial communities associated with roots and leaves. J. Appl. Microbiol. 2007, 102, 781–786. [Google Scholar] [CrossRef]
- Andreote, F.D.; de Araújo, W.L.; de Azevedo, J.L.; van Elsas, J.D.; da Rocha, U.N.; van Overbeek, L.S. Endophytic Colonization of Potato ( Solanum tuberosum L.) by a Novel Competent Bacterial Endophyte, Pseudomonas putida Strain P9, and Its Effect on Associated Bacterial Communities. Appl. Environ. Microbiol. 2009, 75, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yin, D.; Chen, S.; Xia, F.; Yang, J.; Li, Q.; Wang, W. Effect of Biocontrol Agent Pseudomonas fluorescens 2P24 on Soil Fungal Community in Cucumber Rhizosphere Using T-RFLP and DGGE. PLOS ONE 2012, 7, e31806. [Google Scholar] [CrossRef] [PubMed]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of Arbuscular Mycorrhizal Fungal Inoculant Benchmarks. Microorganisms 2020, 9, 81. [Google Scholar] [CrossRef]
- Koch, A.M.; Antunes, P.M.; Barto, E.K.; Cipollini, D.; Mummey, D.L.; Klironomos, J.N. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol. Invasions 2010, 13, 1627–1639. [Google Scholar] [CrossRef]
- Jin, H.; Germida, J.J.; Walley, F.L. Impact of arbuscular mycorrhizal fungal inoculants on subsequent arbuscular mycorrhizal fungi colonization in pot-cultured field pea (Pisum sativum L.). Mycorrhiza 2012, 23, 45–59. [Google Scholar] [CrossRef]
- Antunes, P.M.; Koch, A.M.; Dunfield, K.E.; Hart, M.M.; Downing, A.; Rillig, M.C.; Klironomos, J.N. Influence of commercial inoculation with Glomus intraradices on the structure and functioning of an AM fungal community from an agricultural site. Plant Soil 2008, 317, 257–266. [Google Scholar] [CrossRef]
- Jin, H.; Germida, J.J.; Walley, F.L. Impact of arbuscular mycorrhizal fungal inoculants on subsequent arbuscular mycorrhizal fungi colonization in pot-cultured field pea (Pisum sativum L.). Mycorrhiza 2012, 23, 45–59. [Google Scholar] [CrossRef]
- Marschner, P.; Timonen, S. Interactions between plant species and mycorrhizal colonization on the bacterial community composition in the rhizosphere. Appl. Soil Ecol. 2005, 28, 23–36. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Jackson, L.E.; Six, J.; Ferris, H.; Goyal, S.; Asami, D.; Scow, K.M. Arbuscular Mycorrhizas, Microbial Communities, Nutrient Availability, and Soil Aggregates in Organic Tomato Production. Plant Soil 2006, 282, 209–225. [Google Scholar] [CrossRef]
- Monokrousos, N.; Papatheodorou, E.M.; Orfanoudakis, M.; Jones, D.G.; Scullion, J.; Stamou, G.P. The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil Sci. 2019, 71, 265–278. [Google Scholar] [CrossRef]
- Salilh, F.Z., Tarekegn, M.M. Cleaning polluted environments by bioremediation: Review. IJESC 2020, 10(8),27112–21. Available at: https://ijesc.org/upload/36172711a56684478405d1ee977ff36a.Cleaning%20Polluted%20Environments%20by%20Bioremediation-Review.pdf.
- Agnolucci, M.; Avio, L.; Pepe, A.; Turrini, A.; Cristani, C.; Bonini, P.; Cirino, V.; Colosimo, F.; Ruzzi, M.; Giovannetti, M. Bacteria Associated With a Commercial Mycorrhizal Inoculum: Community Composition and Multifunctional Activity as Assessed by Illumina Sequencing and Culture-Dependent Tools. Front. Plant Sci. 2019, 9, 1956. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Omacini, M.; Montecchia, M.S.; Correa, O.S. Soil microbial community responses to the fungal endophyte Neotyphodium in Italian ryegrass. Plant Soil 2010, 340, 347–355. [Google Scholar] [CrossRef]
- Rojas, X.; Guo, J.; Leff, J.W.; McNear, D.H.; Fierer, N.; McCulley, R.L. Infection with a Shoot-Specific Fungal Endophyte (Epichloë) Alters Tall Fescue Soil Microbial Communities. Microb. Ecol. 2016, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M.P. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 2015, 400, 193–207. [Google Scholar] [CrossRef]
- Di Salvo, L.P.; Ferrando, L.; Fernández-Scavino, A.; de Salamone, I.E.G. Microorganisms reveal what plants do not: wheat growth and rhizosphere microbial communities after Azospirillum brasilense inoculation and nitrogen fertilization under field conditions. Plant Soil 2018, 424, 405–417. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Schulz, W.; Scheu, S.; Jousset, A. Niche dimensionality links biodiversity and invasibility of microbial communities. Funct. Ecol. 2012, 27, 282–288. [Google Scholar] [CrossRef]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Asiloglu, R.; Shiroishi, K.; Suzuki, K.; Turgay, O.C.; Murase, J.; Harada, N. Protist-enhanced survival of a plant growth promoting rhizobacteria, Azospirillum sp. B510, and the growth of rice (Oryza sativa L.) plants. Appl. Soil Ecol. 2020, 154, 103599. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Plant Growth and Soil Microbial Impacts of Enhancing Licorice With Inoculating Dark Septate Endophytes Under Drought Stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef]
- Hirsch, P.R. Release of transgenic bacterial inoculants - rhizobia as a case study. Plant Soil 2005, 266, 1–10. [Google Scholar] [CrossRef]
- Bellanger, X.; Guilloteau, H.; Bonot, S.; Merlin, C. Demonstrating plasmid-based horizontal gene transfer in complex environmental matrices: A practical approach for a critical review. Sci. Total. Environ. 2014, 493, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Mehendale, H.M. Oxidative Stress. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 735–737. ISBN 978-0-12-386455-0. [Google Scholar]
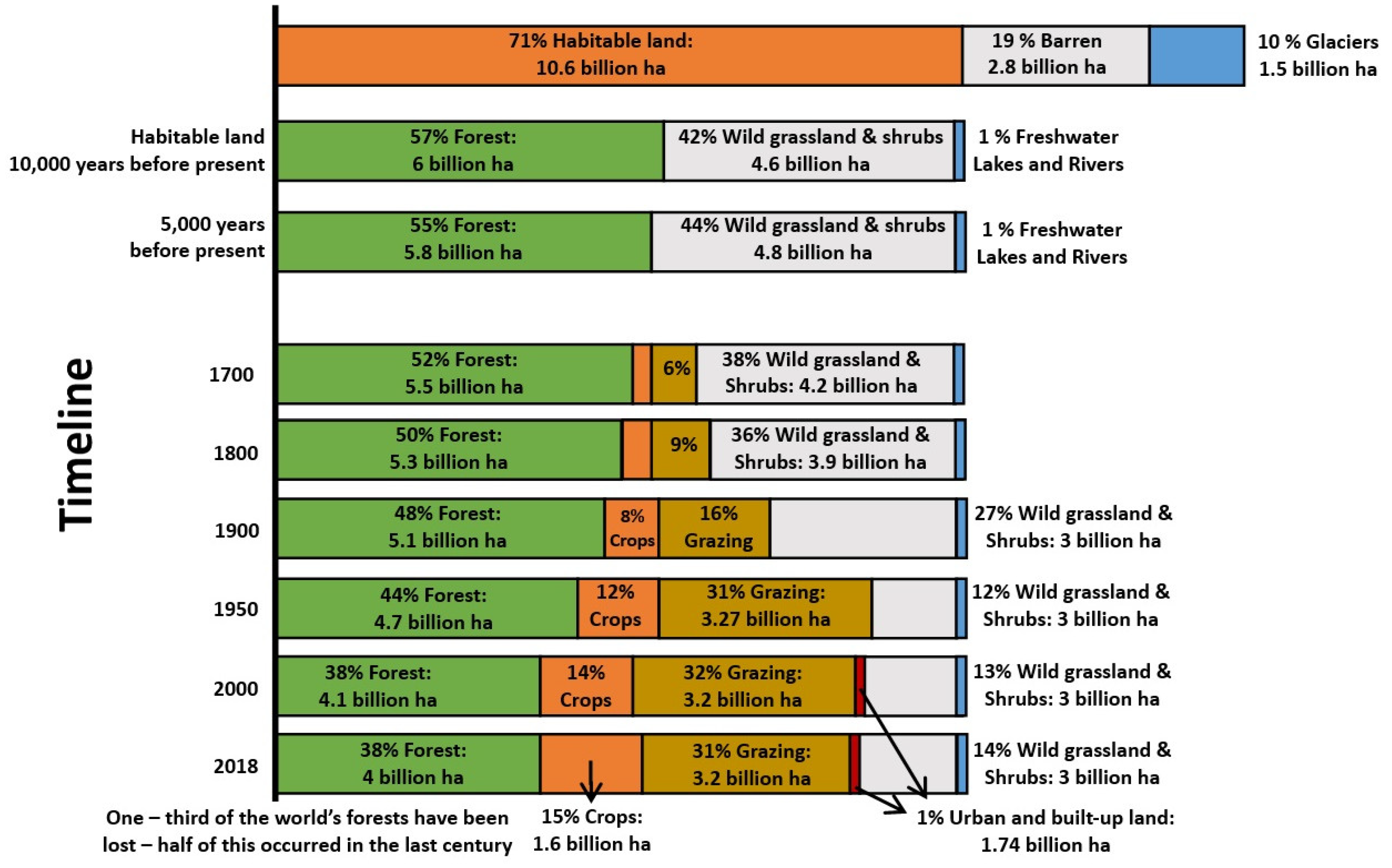
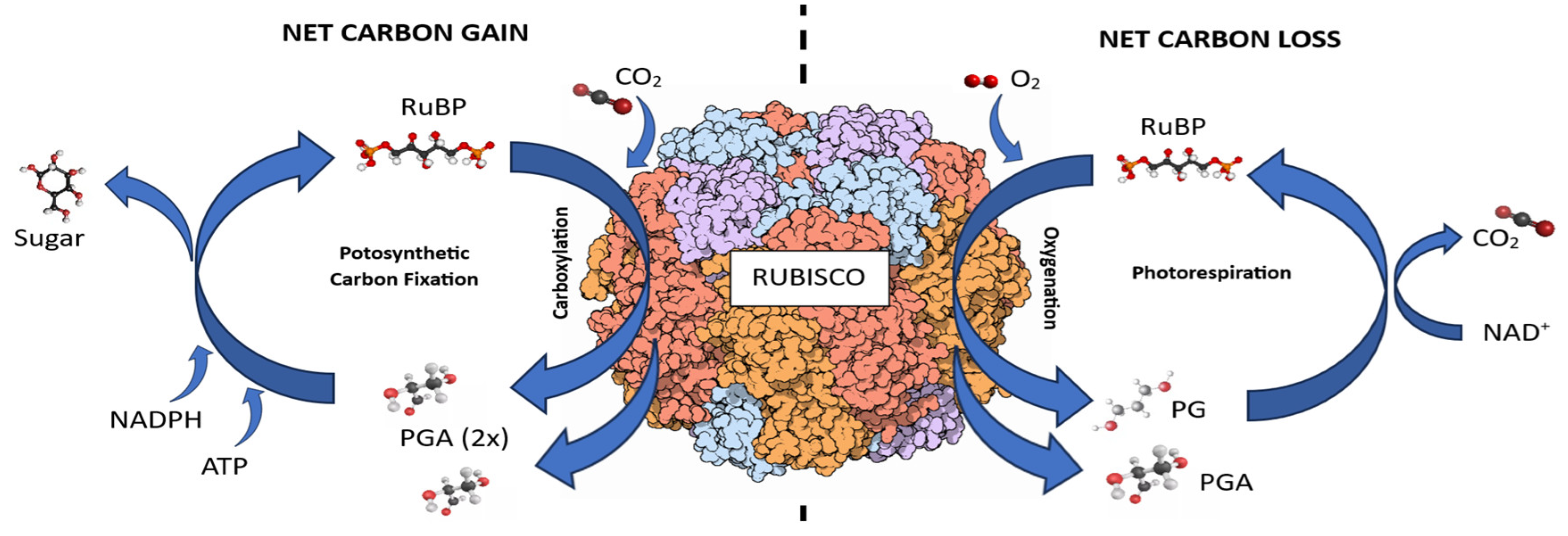
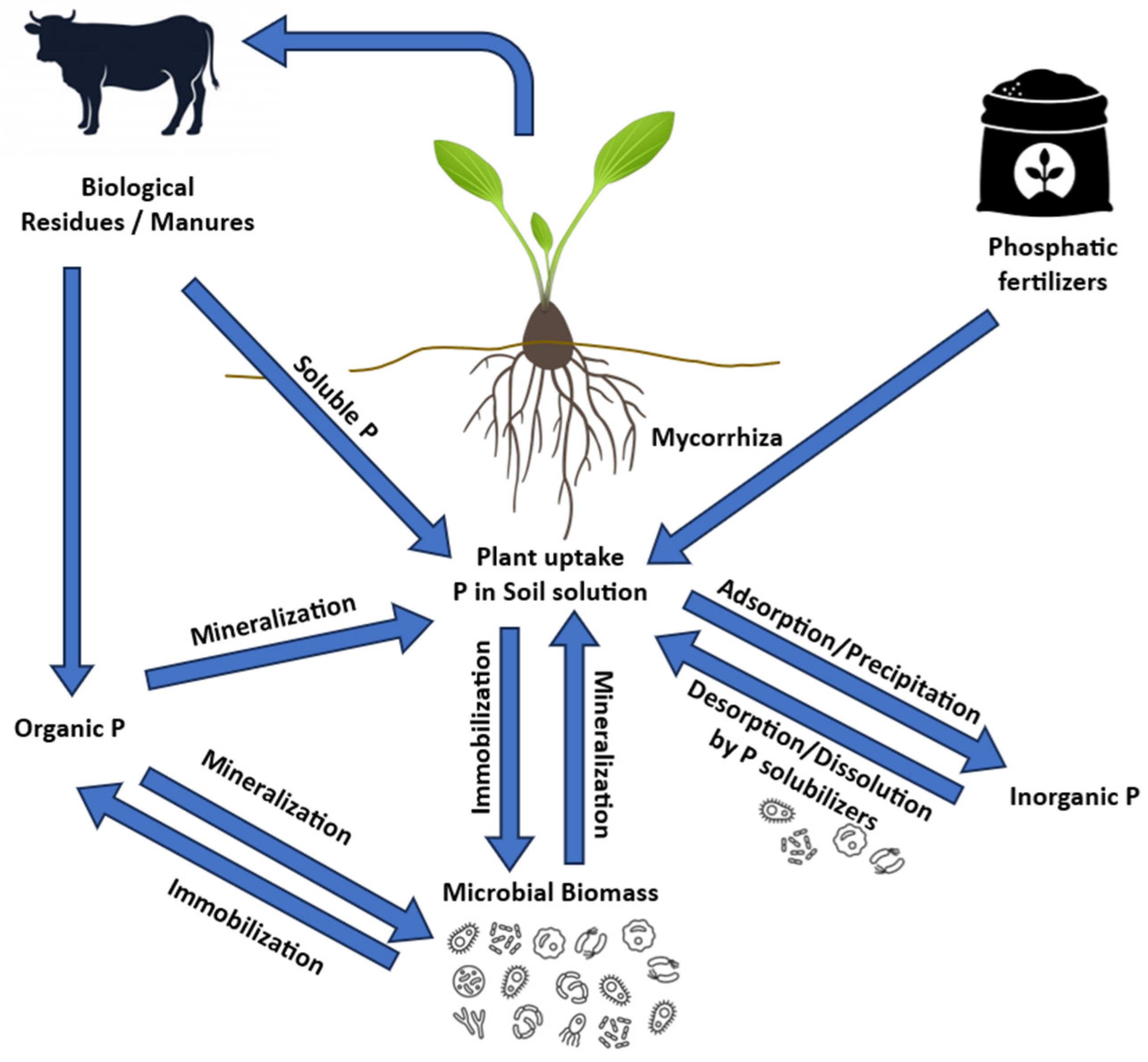
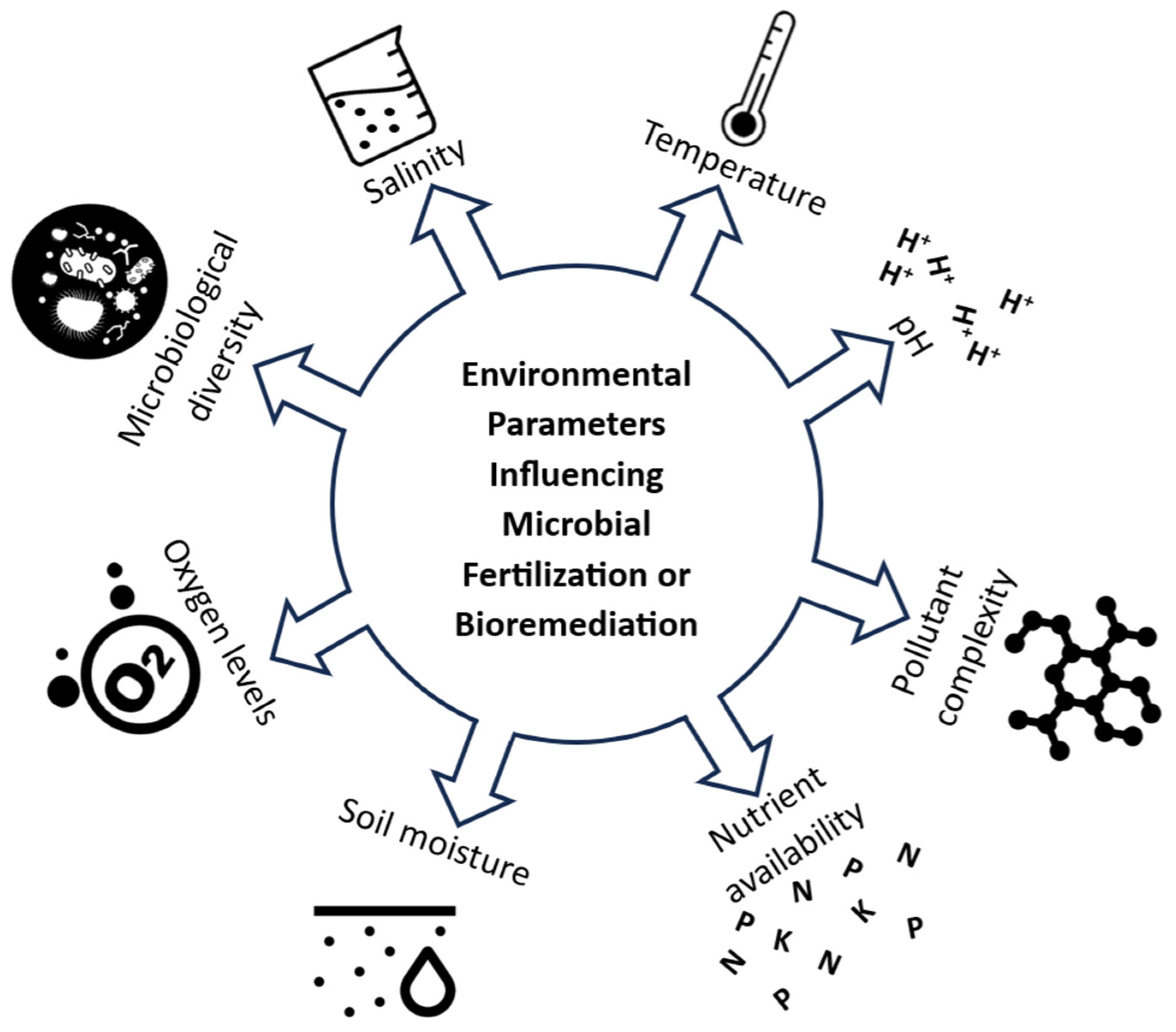
| Microbial inoculum | Heavy metal(s) | Reference |
|---|---|---|
| Bacteria: | ||
| Arthrobacter spp. | Cd | Roane et al., 2001 [197] |
| Pseudomonas veronii | Cd, Zn, Cu | Vullo et al., 2008 [198] |
| Burkholderia spp. | Zn, Pb, Mn, Cd, Cu, As | Yang et al., 2016 [199] |
| Kocuria flava | Cu | Achal et al., 2011 [200] |
| Bacillus cereus | Pb, Cd, Cr | Ayangbenro and Babalola, 2020 [201] |
| Sporosarcina ginsengisoli | As | Achal et al., 2012 [202] |
| Serratia sp. | Ni, Cd | Li et al., 2016 [203] |
| Enterobacter cloacae KJ-46, E. cloacae KJ-47, Sporosarcina soli B-22, and Viridibacillus arenosi B-21 | Cd, Pb, Cu | Kang et al., 2016 [204] |
| Enterobacter cloacae B2-DHA | Cr(VI) | Rahman et al., 2015 [205] |
| Brevibacillus parabrevis OZF 5 | Cr(VI), Zn | Wani et al., 2023 [206] |
| Cupriavidus metallidurans LBJ and Pseudomonas stutzeri LBR | Pb | Ridene et al., 2023 [207] |
| Acinetobacter sp. LSN-10 | Mn(II) | [208] |
| Achromobacter denitrificans, Klebsiella oxytoca, and Rhizobium radiobacter | Cd, Hg, As, Pb, Ni | [188] |
| Cyanobacteria | ||
| Gloeomargarita lithophora and Cyanothece sp | Sr | [209] |
| Nostoc minutum and Anabaena spiroides | Pb, Cd, Ni | [210] |
| Fungi | ||
| Simplicillium chinense | Cd, Pb | [211] |
| Penicillium sp. | Hg | [212] |
| Penicillium chrysogenum FMS2 | Cd | [213] |
| Aspergillus versicolor | Cr, Ni, Cu | [214] |
| Aspergillus fumigatus | Pb(II) | [215] |
| Aspergillus spp. | Cd, Cu | [216] |
| Gloeophyllum sepiarium | Cr | [200] |
| Perenniporia subtephropora, Daldinia starbaeckii, Phanerochaete concrescens, Cerrena aurantiopora, Fusarium equiseti, Polyporales sp., Aspergillus niger, Aspergillus fumigatus, and Trametes versicolor | As, Mn, Cu, Cr, and Fe | [217] |
| Yeasts | ||
| Candida tropicalis | Cr(VI) | [218] |
| Saccharomyces cerevisiae | Pb, Cd | [219] |
| Saccharomyces cerevisiae | Cd, Hg | [220] |
| Mixed consortia | ||
| Chlorella thermophila SM01, Leptolyngbya sp. XZMQ and Bacillus XZM | As | [221] |
| Leptolyngbya sp. XZ1 and Bacillus sp. S1 | Cd | [222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





