1. Introduction
Bone resorption by osteoclasts plays a pivotal role in skeleton growth/modeling, homeostasis/remodeling, and fracture repair [
1]. The aberrant bone resorption due to either increased or demolished osteoclast number and/or activity is detrimental to skeleton health and causes anomalous bone mass and bone erosion in multiple bone diseases including osteoporosis, Paget’s disease of bone, periodontal disease, tumor bone metastasis, arthritis, and osteopetrosis. Thus, unveiling the molecules and pathways regulating osteoclast differentiation and function under physiological and pathological conditions will not only advance our knowledge in osteoclast biology but also identify therapeutic targets for treatment of developmental and degenerative bone diseases.
Osteoclasts are polykaryons formed by fusion of mononuclear precursors which are differentiated from the monocyte/macrophage lineage of hematopoietic system under the control of two cytokines, M-CSF (macrophage colony stimulating factor) and RANKL (receptor activator of nuclear factor-kB ligand) [
2]. Upon attachment of osteoclasts to bone matrix, the activating signals triggered by M-CSF and RANKL as well as those transduced through the integrins and the immunoreceptors act in concert to promote osteoclast actin cytoskeleton organization to form actin-rings at the sealing zone that tightly seals the resorptive microenvironment. Subsequently, the protons and hydrolases are released via lysosome secretion to dissolve bone minerals and degrade bone matrix, respectively [
3,
4]. Therefore, both cytoskeleton organization and lysosome secretory pathway are essential for osteoclast activation and bone resorption.
Of key molecules regulating osteoclastogenesis and function identified so far, PLEKHM1 is specifically indispensable for lysosome trafficking/secretion and bone resorption in osteoclasts [
5,
6]. Mutations in
PLEKHM1 gene cause an intermediate form of osteopetrosis in humans and in incisors absent (
ia/ia) rats [
7]. Moreover, both germline and osteoclast specific
Plekhm1 knockout mice display high bone mass phenotypes resulted from defective bone resorption [
8]. PLEKHM1 regulates osteoclast lysosomal trafficking and formation of the ruffled border, a highly convoluted plasma membrane domain circumscribed by the sealing zone, through its interaction with RAB7, a small GTPase specifically regulates lysosomal pathways in eukaryotic and mammalian cells [
9,
10,
11,
12]. RAB7 is localized at the ruffled border in bone-resorbing osteoclasts. Depletion of Rab7 expression or disruption of Plekhm1-Rab7 interaction attenuate lysosome secretion and bone resorption in cultured rat and murine osteoclasts
in vitro [
13,
14]. In addition to Rab7, we have previously identified several Plekhm1-binding proteins in murine osteoclasts including Fam98a (family with sequence similarity 98 member A) that may mediate PLEKHM1’s function in osteoclasts [
8,
15,
16].
The FAM98 family proteins have three members in human and mice (FAM98A, FAM98B, and FAM98C). The physiological functions of them remain largely unknown. FAM98A has been reported as a substrate of protein arginine methyltransferase 1 (PRMT1) and may be involved in tumor cell migration and invasion [
17]. It has been found to localize to stress granules upon various stress stimuli [
18]. We have unveiled that Fam98a interacts with Plekhm1 in murine osteoclasts and knockdown of Fam98a expression by lentiviral transduction of specific short hairpin RNAs (shRNAs) disrupts lysosome trafficking/secretion and bone resorption in osteoclasts
in vitro [
8]. FAM98B has been identified as a component of tRNA ligase and RNA capping/transporting complexes regulating RNA metabolism and translation [
19,
20,
21,
22]. FAM98C has been suggest as a candidate gene regulating ciliogenesis and mutation of which may cause ciliopathies [
23].
In this study, we investigated the roles of Fam98 family proteins in osteoclastogenesis and bone resorption by generation of Fam98a myeloid conditional knockout mice and depletion of Fam98b and Fam98c expression by lentiviral transduction of specific shRNAs in control and Fam98a-deficient bone marrow monocytes.
2. Materials and Methods
2.1. Reagents
The key reagents used in this study were listed in the following table.
2.2. Bone Marrow Monocyte and Osteoclast Cultures
The whole bone marrow cells were extracted from tibia and femurs of 6- to 8-week-old mice. Bone marrow monocytes (BMMs) and osteoclasts were cultured as described previously. In brief, red blood cells were lysed in 1× Red Blood Cell Lysis buffer for 5min at room temperature. A total of 5×10
6 bone marrow cells were plated onto a 100mm petri-dish and cultured in α-MEM containing 10% heat-inactivated FBS, 1× Penicillin–Streptomycin-L-Glutamine (PSG), and 1/10 volume of CMG 14–12 (conditioned medium supernatant containing recombinant M-CSF at 1 μg/mL) [
24] for 4 days. To generate osteoclasts from BMMs,1.5×10
4 or 3×10
4 BMMs were cultured in α-MEM culture medium with 1/100 volume of CMG 14–12 (equals to 10 ng/mL of M-CSF) and 100 ng/mL of recombinant RANKL in a well of 48-well or 24-well culture plates, respectively, for 4-5 days.
2.3. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
BMMs cultured in 48-well plate were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 min at room temperature. After washing with PBS for 5 min twice, TRAP was stained with NaK tartrate and Naphthol AS-BI phosphoric acid solution containing 0.12% of Pararosaniline as described previously . Images were taken with a Nikon Eclipse TE300 microscope equipped with a digital camera (Nikon DS-Fi1) and the Nikon Digit Sight system. The number of TRAP+ multinucleated osteoclasts with >3 nuclei/well in four wells were counted and analyzed by One-Way analysis of variance (ANOVA).
2.4. Real-Time Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNAs were purified using RNeasy mini kit and the first strand cDNAs were generated from 1μg of total RNA using the High-Capacity cDNA Reverse Transcription kit following the manufacturer’s instructions. The cDNA samples were 1:20 diluted with the molecular grade water. TaqMan real-time qPCR was performed using the specific primers and TaqMan Gene Expression Master Mix in the QuantStudio3 real-time PCR system (Thermo-Fisher Scientific) with an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The relative cDNA amount was calculated by normalizing to that of the mitochondrial gene Mrps2, which is steadily expressed during osteoclastogenesis, using the ΔCt method.
2.5. Lentiviral Transduction
The recombinant lentiviruses expressing shRNAs targeting the murine Fam98b, Fam98c, and the firefly luciferase (LUC) were generated as described in our previous publications. The 293-T cells were plated at a density of 0.75 × 106 cells/well in a 6-well tissue culture plate and cultured in DMEM medium containing 10% heat-inactivated FBS and 1 × PSG a day before transfection. The specific pLKO.1 gene transfer vectors (1.5µg each) were co-transfected respectively with the 1.5 µg of lentivirus packing vectors, pCMV-delta-R8.2 and pMD2.G (at 8:1 ratio), using the TransIT-LT1 transfection reagent (9µl in 100 µl of Opti-MEM/transfection). The medium was changed on the next day. The virus supernatants were collected after 24 hours and were filtrated through a 0.45µm sterile nylon syringe filter (CAT# 76479-028, VWR). For lentiviral transduction, 5 × 106 bone marrow cells were cultured in a 100mm petri-dish with α-MEM containing 10% heat-inactivated FBS, 1 × PSG, and 1/10 volume of CMG 14–12 for 3 days. The cells were then transduced with virus supernatant and 20µg/mL of Protamine for 24 hours. The positively transduced BMMs were selected in α-MEM culture medium containing 6µg/mL puromycin for 3 days before use.
2.6. Immunoblotting
Cells were washed twice with ice-cold PBS and were lysed in RIPA buffer containing protease inhibitor cocktail. After incubation on ice for 30 min, the cell lysates were centrifuged at 14, 000 rpm for 15 min at 4◦C. The supernatants of cell lysates were collected, and protein concentration was quantified using DC Protein Assay Kit II (Cat#5000112, Bio-Rad). A total of 20μg of proteins were separated by 8% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred electrophoretically onto polyvinylidene difluoride membrane (Cat# IPVH00010, MilliporeSigma) by a semi-dry blotting system (Bio-Rad). The membrane was blocked in 5% fat-free milk/Tris-buffered saline for 1 hour and incubated with primary antibodies at 4◦C overnight followed by incubation with HRP conjugated anti-mouse secondary antibody. After rinsing three times with Tris-buffered saline containing 0.1% Tween 20, the membrane was subjected to enhanced chemiluminescent detection reagents used at 1:10 dilution.
2.7. Immunofluorescence
The immunofluorescent staining was performed as described in our earlier work [
25]. Briefly, osteoclasts cultured on either glass coverslips in wells of 24-well plate or bovine cortical bone slices in wells of 48-well plate were fixed with 4% paraformaldehyde in PBS for 20 min. Non-specific binding was blocked by PBS/0.2% bovine serum albumin (BSA)/0.2% Saponin (PBSBS) for 30 min, followed by incubation with the rat monoclonal anti-mouse Lamp-2 antibody in PBSBS for 2 hours at room temperature. After three times 5-min-wash with PBSBS buffer, cells were incubated with the donkey anti-rat secondary antibody together with the Alexa Fluor-488 conjugated phalloidin for 45 min. After washing with PBSBS buffer for 5 min, the nuclei were stained with Hoechst 33342/PBS for 5 min. Samples were washed with PBS for 5 min and mounted with 80% glycerol/PBS. The triple fluorescence-labeled osteoclasts were examined and analyzed using a Zeiss LSM 900 laser confocal scanning microscope run by Zeiss ZenBlue software.
2.8. Resorption Pit Staining
The cells on bovine cortical bone slices were removed with a soft brush. The slices were then incubated with 20 μg/mL peroxidase-conjugated wheat germ agglutinin lectin in PBS for 60 min at room temperature. After washing in PBS for 5 min twice, bone chips were incubated with 0.52 mg/mL 3,3_-diaminobenzidine (DAB) and 0.1% H2O2 for 30 min. The stained bone slices were mounted and cleared in 80% glycerol/PBS at 4°C for 7 days before photographed with Zeiss AxioPlan2 microscope equipped with a digital camera (Nikon DS-Fi1) and the Nikon Digit Sight system. The pit area was quantified by NIH ImageJ software, and the percentage of pit area versus the total area of whole bone slice was calculated and analyzed by GraphPad Prism 9 software.
2.9. Generation of Fam98a-Flox and Fam98a-Flox;LysM-Cre Mice
The Fam98a-flox mice on C57BL6/J background were commercially generated by Cyagen Biosciences. The Lyz2-Cre (LysM-Cre) mice on C57BL6/J background (B6.129P2tm1(cre)Ifo, CAT# 004781) were obtained from The Jackson Laboratory (Bar Harbor, ME). The offsprings of Fam98a-flox/+;LysM-Cre/Cre breeding pairs were genotyped for Fam98a-flox and Lyz2-Cre using primers listed in the above table and from The Jackson Laboratory. The animal work follows the ARRIVE guidelines. All the in vivo and in vitro experiments were performed and analyzed in double-blinded manner.
2.10. Micro-CT
The femurs and L4 vertebrae were cleaned of soft tissues and fixed in 4% paraformaldehyde in PBS for 3 days at 4°C. After washing with PBS for three times, the bones were stored in PBS with 0.02% Sodium azide. The bones were loaded into a 12.3 mm diameter scanning tube and were imaged in a μCT (model vivaCT40, Scanco Medical AG, Switzerland). Trabecular bone parameters were measured at the epiphysis and the secondary spongiosa region of the distal femur with 55 to 70 kVp volts at a voxel size of 10.5 μm. Images were reconstructed using the 2D and 3D image software provided by the Scanco VIVA-CT 40 instrument. A threshold of 200 was applied to all scans, at medium resolution (E = 55 kVp, I = 145 μA, and integration time = 200 ms).
3. Results
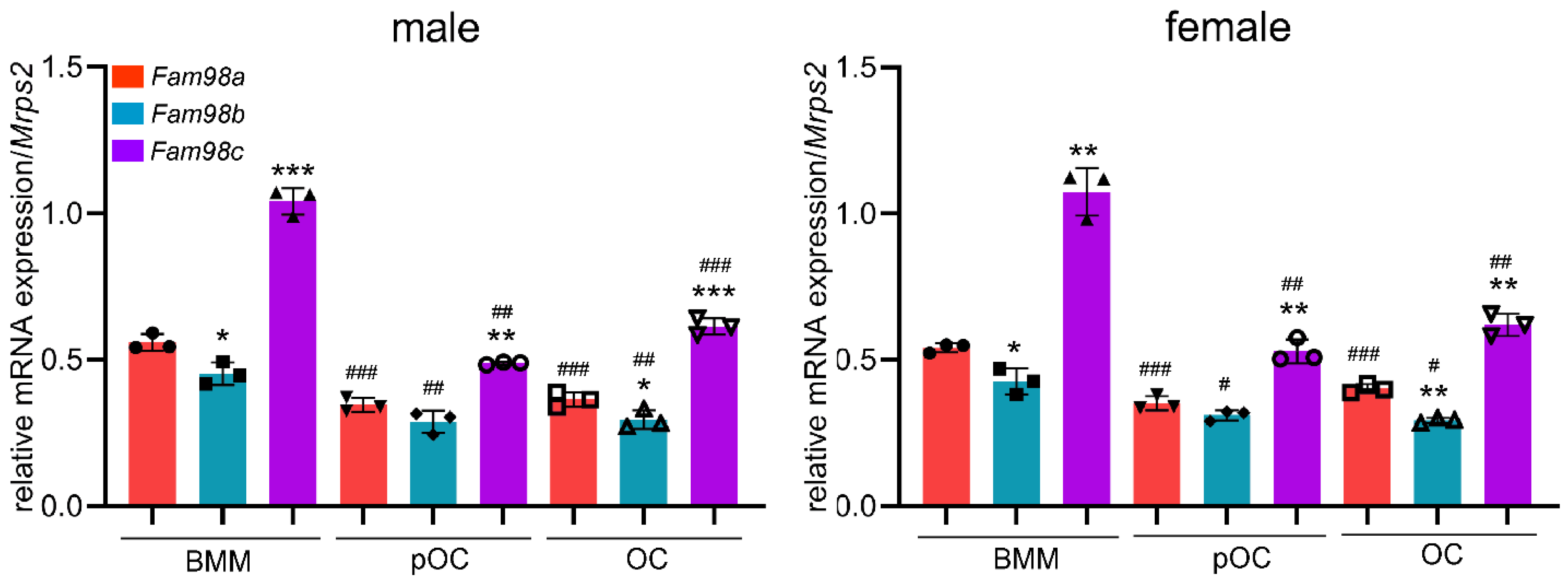
3.1. All Three Murine Fam98 Family Genes Are Expressed in Precursor and Mature Osteoclasts
In humans and mice, there are three FAM98 proteins (FAM98A, FAM98B, and FAM98C) which are encoded by the distinct genes located on different chromosomes. FAM98A is the longest and FAM98C is the shortest one among three FAM98 proteins. The murine Fam98a has 19.6% identity and 22.7% similarity with both Fam98b and Fam98c (
Figure 1). Fam98b is closer to Fam98a than Fam98c with 46.3% identity and 55.0% similarity while Fam98c has only 25% identity and 33.4% similarity with Fam98a. To dissect the roles of FAM98s in osteoclasts, we first examined their mRNA expression during
in vitro osteoclast differentiation from murine bone marrow monocytes (BMMs) by real-time quantitative RT-PCR. As shown in
Figure 2, the expression of
Fam98c was found to be the highest among three genes in male and female osteoclast precursor and mature cells. The expression of
Fam98b was slightly lower than that of
Fam98a in osteoclast lineage cells. The mRNA expression of all three
Fam98 genes decreased during differentiation of both male and female osteoclasts. The protein level of Fam98s in murine osteoclast lineage cells were not assessed in this study due to lack of reliable antibodies specifically recognizing each isoform of Fam98 family proteins.
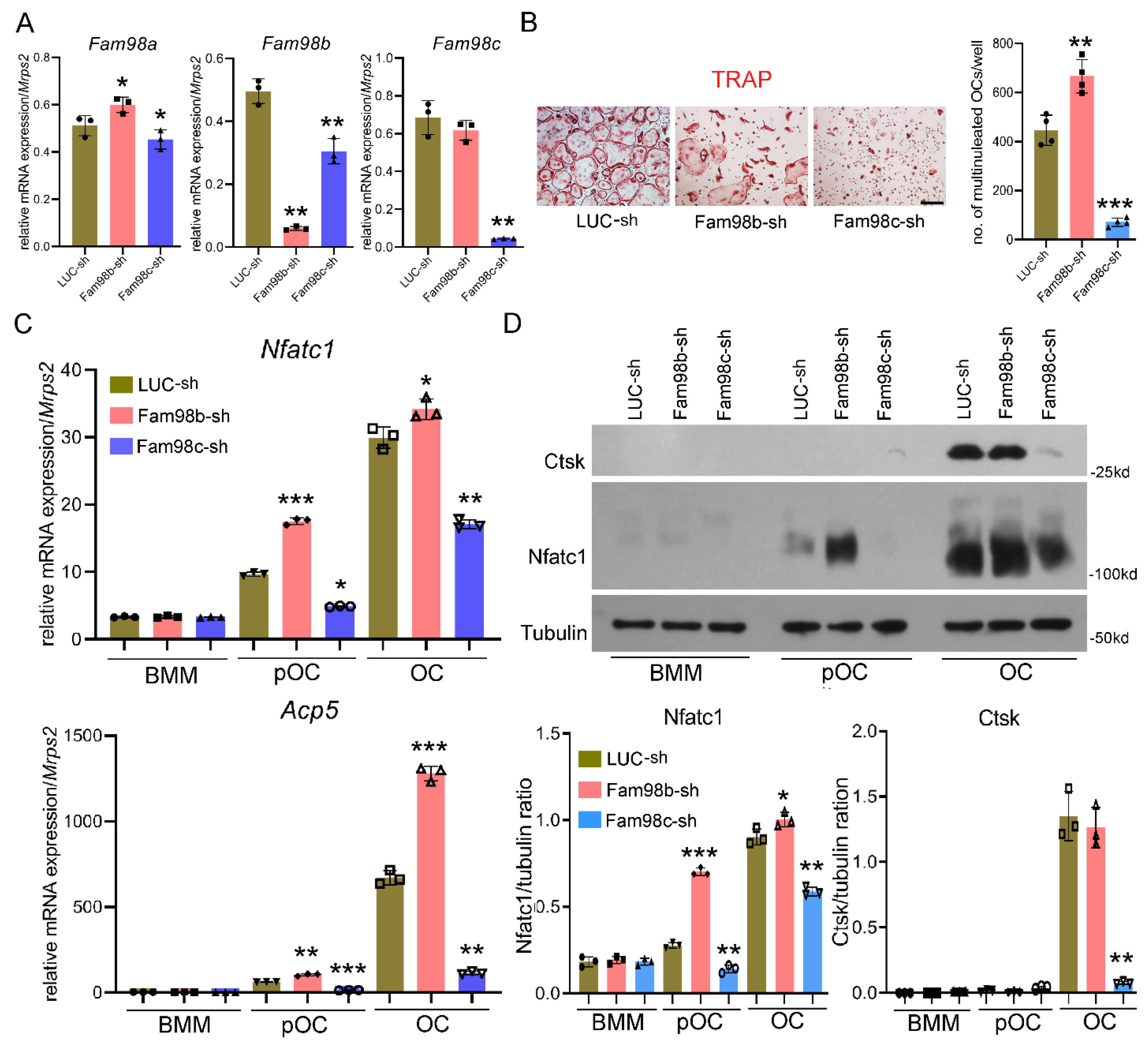
3.2. Knockdown of Fam98c but Not Fam98b Expression Attenuates Osteoclastogenesis In Vitro
We have previously reported that Fam98a binds to Plekhm1 and knockdown of Fam98a expression
in vitro attenuates lysosome trafficking and secretion in murine osteoclasts [
8]. However, whether and how Fam98b and Fam98c regulate osteoclasts remain unknown. To unveil the role of Fam98b and Fam98c in osteoclast differentiation and/or function, we set out to knockdown their expression in BMMs by lentiviral transduction of specific shRNA targeting Fam98b (Fam98b-sh) and Fam98c (Fam98c-sh), respectively. The expression of Fam98b-sh significantly reduced the mRNA level of
Fam98b and led to slightly increase in
Fam98a mRNA in BMMs relative to BMMs transduced with the control shRNA against firefly luciferase (LUC-sh) (
Figure 3A), whereas Fam98c-sh expression in BMMs robustly decreased the amount of
Fam98c mRNA but also resulted in a little decrease in
Fam98a and
Fam98b mRNAs compared to control BMMs (
Figure 3A).
We next cultured the different shRNA transduced BMMs with M-CSF and RANKL for 4 days to induce osteoclast differentiation. As shown by TRAP (tartrate-resistant acid phosphatase) staining and osteoclast number count (
Figure 3B), depletion of Fam98c greatly attenuated formation of multinucleated osteoclasts (> 3 nuclei/cell). Moreover, the mRNA and protein levels of Nfatc1, a master transcription factor of osteoclast differentiation, and the mRNA expression of osteoclast marker gene
Acp5 (encodes TRAP) as well as the protein level of Cathepsin K, a crucial bone matrix-digesting enzyme in mature osteoclasts, were decreased dramatically in Fam98c-suppressed osteoclast precursor and mature cells (
Figure 3C-
Figure 3D). This result indicates that Fam98c is critical for osteoclastogenesis probably by regulating Nfatc1 expression in osteoclast lineage cells. By contrast, repression of Fam98b expression in osteoclast precursors caused an increase in osteoclast number as affirmed by higher levels of Nfatc1 and Acp5 in mononuclear and mature osteoclasts compared to control cells (
Figure 3B-
Figure 3D). Of note, however, about 50% of Fam98b-knockdown osteoclasts (50.7 ± 8.4%, n=4) displayed a similar morphological cell shape to Plekhm1
-/- osteoclasts with aggregated TRAP
+ lysosomes surround nuclei (
Figure 5B, middle panel), suggesting that Fam98b like Fam98a might regulate osteoclast lysosome trafficking/secretion and bone resorption.
Figure 3.
Knockdown of Fam98c but not Fam98b expression in BMMs attenuates osteoclastogenesis in vitro. (A) The real time RT-qPCR detection of mRNA expression of Fam98 isoforms in BMMs transduced with the lentiviruses expressing control shRNA against firefly luciferase (LUC-sh) and the specific shRNAs targeting Fam98b and Fam98c, respectively. (B) The TRAP (tartrate-resistant acid phosphatase) staining and cell number count of osteoclasts with more than 3 nuclei/cell. Scale bar = 40µm. (C) The mRNA expression of osteoclast marker genes, Nfatc1 and Acp5, in lentiviral transduced BMMs, pre-OCs, and mature OCs detected by RT-qPCR. (D) The protein expression of osteoclast markers, Nfatc1 and cathepsin K (Ctsk), in lentiviral transduced BMMs, pre-OCs, and mature OCs probed by immunoblotting. The blot of tubulin served as a loading control. * p < 0.05, ** p < 0.01, *** p < 0.001 vs respective LUC-sh transduced cells analyzed by one-way ANOVA, n = 3-4.
Figure 3.
Knockdown of Fam98c but not Fam98b expression in BMMs attenuates osteoclastogenesis in vitro. (A) The real time RT-qPCR detection of mRNA expression of Fam98 isoforms in BMMs transduced with the lentiviruses expressing control shRNA against firefly luciferase (LUC-sh) and the specific shRNAs targeting Fam98b and Fam98c, respectively. (B) The TRAP (tartrate-resistant acid phosphatase) staining and cell number count of osteoclasts with more than 3 nuclei/cell. Scale bar = 40µm. (C) The mRNA expression of osteoclast marker genes, Nfatc1 and Acp5, in lentiviral transduced BMMs, pre-OCs, and mature OCs detected by RT-qPCR. (D) The protein expression of osteoclast markers, Nfatc1 and cathepsin K (Ctsk), in lentiviral transduced BMMs, pre-OCs, and mature OCs probed by immunoblotting. The blot of tubulin served as a loading control. * p < 0.05, ** p < 0.01, *** p < 0.001 vs respective LUC-sh transduced cells analyzed by one-way ANOVA, n = 3-4.
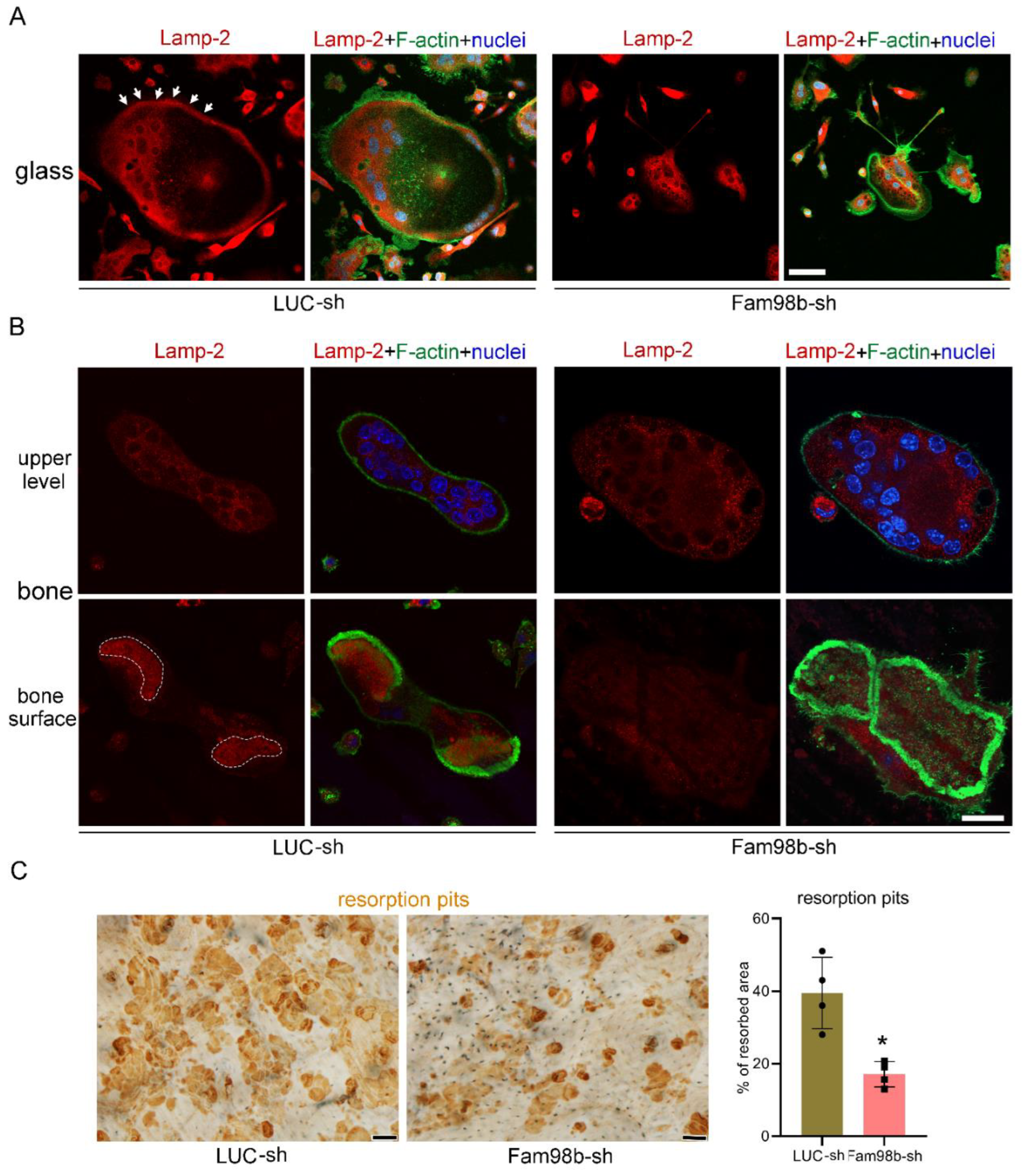
3.3. Decreased Expression of Fam98b in Osteoclasts Inhibits Lysosome Trafficking and Bone Resorption In Vitro
To further define the function of Fam98b in osteoclasts, we cultured the control (LUC-sh) and Fam98b-knockdown (Fam98b-sh) BMMs with M-CSF and RANKL for 4 days on glass coverslips and cortical bovine bone slices, respectively. Lysosomes were labeled with a rat monoclonal antibody against murine Lamp-2, an integral membrane protein and marker of lysosomes in mammalian cells [
26], co-stained with filament actin (F-actin) and nucleus. While a significant number of Lamp-2
+ lysosomes was observed to be transported to and localized at the peripheral area inside the podosome-ring in control osteoclasts cultured on glass coverslips, the lysosomes in Fam98b-deficient osteoclasts were aggregated around nuclei, void from the cell periphery (
Figure 4A). In control osteoclasts cultured on bone slices, the Lamp-2
+ lysosomes were targeted to the ruffled border membrane circumscribed by actin-rings (
Figure 4B, left panels). However, the Lamp-2 staining in Fam98b-deficient osteoclasts was mostly absent at the ruffled border (
Figure 4B, right panels). Consistent with an important role of lysosome secretion in osteoclast bone resorption, the defective lysosome transportation in Fam98b-depressed osteoclasts led to a 50% reduction in bone resorption compared to control osteoclasts (
Figure 4C).
Figure 4.
Decreased expression of Fam98b in osteoclasts inhibits lysosome trafficking and bone resorption in vitro. (A) The immunofluorescent staining of lysosome membrane protein Lamp-2 in control (LUC-sh) and Fam98b knockdown (Fam98b-sh) osteoclasts cultured on glass coverslips. The filament actin (F-actin) and nuclei were stained with the Alexa-488 conjugated phalloidin and Hoechst-33342, respectively. The white arrows point to the peripheral distributed Lamp-2 in control osteoclasts. Scale bar = 30µm. (B) The immunofluorescent staining of Lamp-2 in control and Fam98b knockdown osteoclasts cultured on bovine cortical bone chips. The white dash lines demarcate the Lamp-2 staining at the ruffled border circumscribed by actin-rings in control osteoclasts. Scale bar = 15 µm. (C) The resorption pits labeled by the horseradish peroxidase (HRP) conjugated wheat germ agglutinin (WGA) lectin were stained by 3,3’-diaminobenzidine (DAB). Scale bar = 10 µm. The percentage of resorbed area was quantified with the NIH ImageJ software and analyzed by Student’s t-test. * p < 0.05 vs control osteoclasts. n = 4.
Figure 4.
Decreased expression of Fam98b in osteoclasts inhibits lysosome trafficking and bone resorption in vitro. (A) The immunofluorescent staining of lysosome membrane protein Lamp-2 in control (LUC-sh) and Fam98b knockdown (Fam98b-sh) osteoclasts cultured on glass coverslips. The filament actin (F-actin) and nuclei were stained with the Alexa-488 conjugated phalloidin and Hoechst-33342, respectively. The white arrows point to the peripheral distributed Lamp-2 in control osteoclasts. Scale bar = 30µm. (B) The immunofluorescent staining of Lamp-2 in control and Fam98b knockdown osteoclasts cultured on bovine cortical bone chips. The white dash lines demarcate the Lamp-2 staining at the ruffled border circumscribed by actin-rings in control osteoclasts. Scale bar = 15 µm. (C) The resorption pits labeled by the horseradish peroxidase (HRP) conjugated wheat germ agglutinin (WGA) lectin were stained by 3,3’-diaminobenzidine (DAB). Scale bar = 10 µm. The percentage of resorbed area was quantified with the NIH ImageJ software and analyzed by Student’s t-test. * p < 0.05 vs control osteoclasts. n = 4.
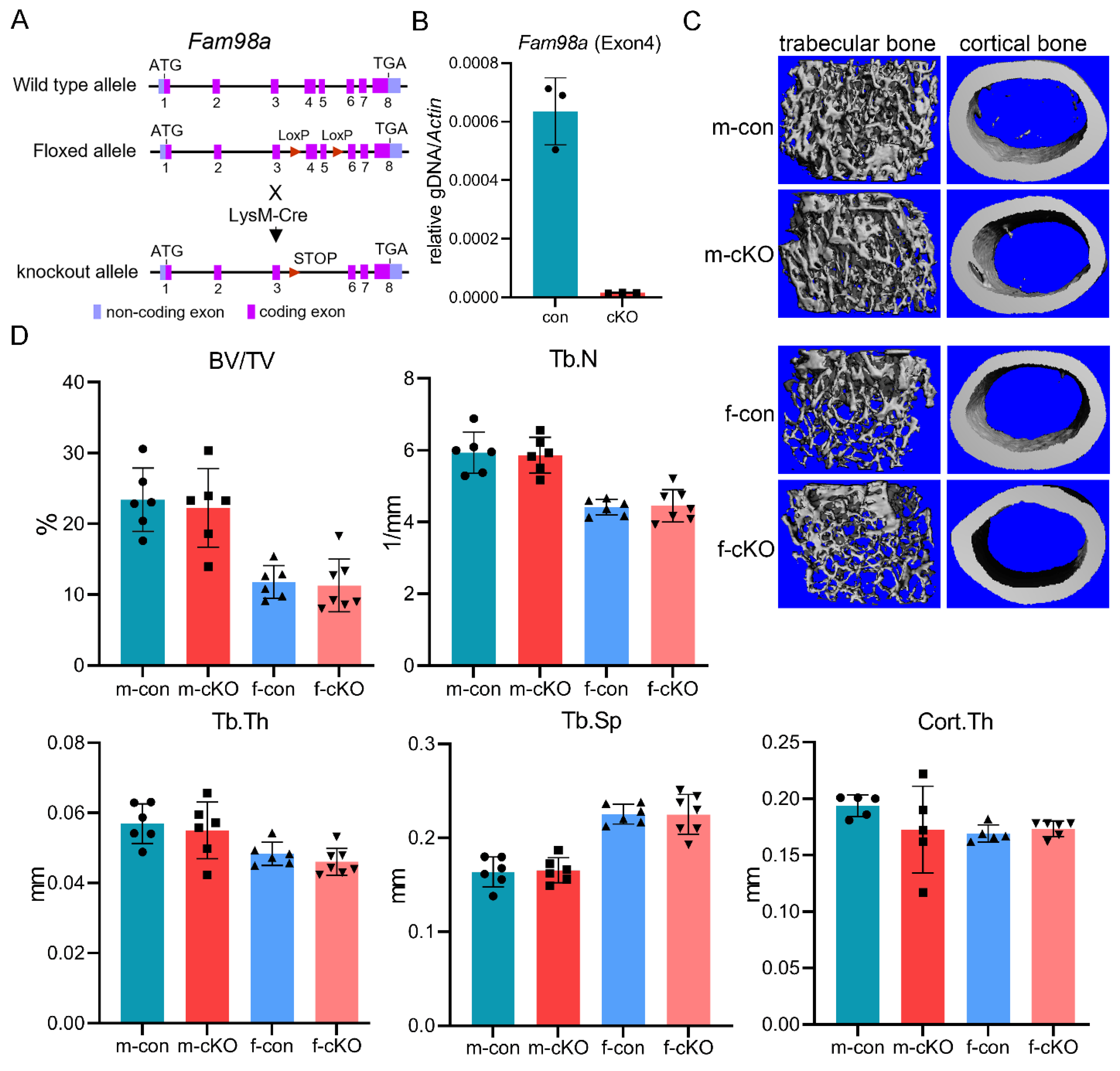
3.4. Fam98a and Fam98b Compensate in Regulation of Lysosome Secretion and Bone Resorption in Osteoclasts
To elucidate the role of FAM98A in regulation of osteoclasts and bone homeostasis
in vivo, we generated the
Fam98a-flox mice in which the exons 4 and 5 of murine
Fam98a gene were flanked by two loxP sites (
Figure 5A). By crossing
Fam98a-floxed mice with LysM-Cre mice, we created the Fam98a myeloid osteoclast precursor conditional knockout mice (cKO) (
Figure 5A). The real-time quantitative PCR confirmed deletion of exon 4 of the murine
Fam98a gene in BMMs cultured from Fam98a cKO mice (
Figure 5B). To maximize the deleting efficacy of Fam98a by LysM-Cre, we mated the
Fam98a-flox/+;LysM-Cre/Cre breeding pairs to generate the control (Fam98a-+/+;LysM-Cre/Cre) and the Fam98a cKO (Fam98a-flox/flox;LysM-Cre/Cre) mice, similar to what we did in our recent published work [
27]. The femurs and lumber vertebrae were harvested from 10-week-old male and female mice for micro-CT analysis of bone mass and structures. As presented in
Figure 5C and
Figure 5D of micro-CT images and analyses, the trabecular bone volume (BV/TV) and cortical bone thickness (Cort.Th) in distal femurs of Fam98a cKO male and female mice were similar to those of control mice. The trabecular number (Tb.N), thickness (Tb.Th), and trabecular spacing (Tb.Sp) in Fam98a cKO male and female mice were indistinguishable to those of control mice. Besides, there were no changes in the skeletal phenotypes of lumber vertebra in Fam98a cKO mice (data not shown). These results indicate that loss of Fam98a in osteoclasts is dispensable for bone homeostasis
in vivo.
Figure 5.
Loss of Fam98a in myeloid osteoclast precursor cells is dispensable for bone mass and structure in mice. (A) A cartoon illustration of the strategy to generate Fam98a conditional knockout (cKO) mice in LysM-Cre expressing myeloid osteoclast precursors. (B) Deletion of Fam98a exon 4 genomic DNA in BMMs isolated from Fam98a cKO mice detected by qPCR. n = 3. (C) and (D) The micro-CT images and analyses of trabecular and cortical bone compartments in distal femurs of 10-week-old male and female mice. n = 6-7.
Figure 5.
Loss of Fam98a in myeloid osteoclast precursor cells is dispensable for bone mass and structure in mice. (A) A cartoon illustration of the strategy to generate Fam98a conditional knockout (cKO) mice in LysM-Cre expressing myeloid osteoclast precursors. (B) Deletion of Fam98a exon 4 genomic DNA in BMMs isolated from Fam98a cKO mice detected by qPCR. n = 3. (C) and (D) The micro-CT images and analyses of trabecular and cortical bone compartments in distal femurs of 10-week-old male and female mice. n = 6-7.
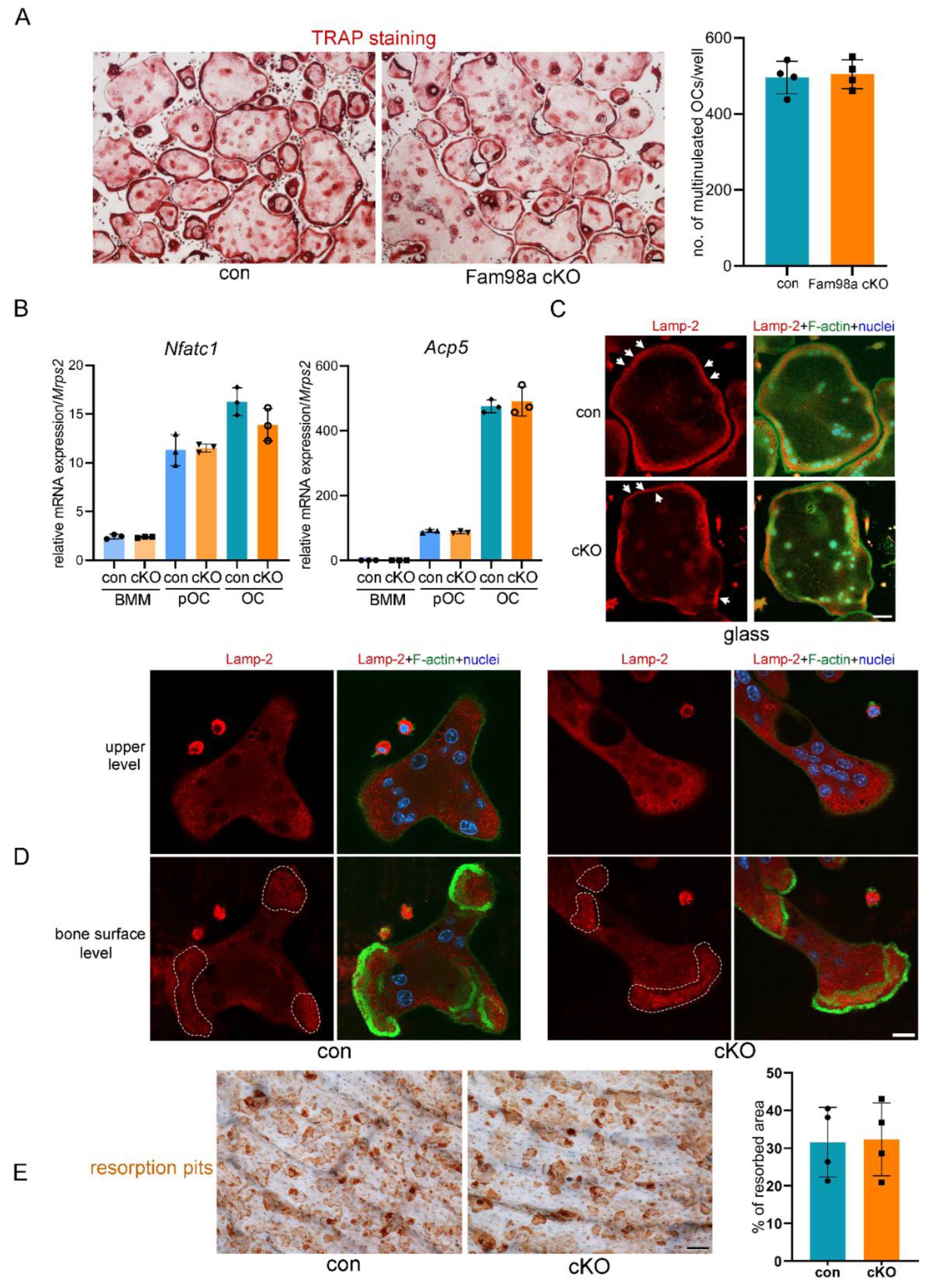
To unveil the impacts of loss of Fam98a in osteoclast precursors on osteoclastogenesis and bone resorption
in vitro, we cultured the BMMs isolated from control and Fam98a cKO mice, respectively, with M-CSF and RANKL for 4 days on plastic culture dishes, glass coverslips, and cortical bovine bone slices. The number of TRAP
+ multinucleated osteoclasts and the mRNA expression of
Nfatc1 and
Acp5 in Fam98a-deficient BMMs, pre-, and mature-osteoclasts were very close and insignificant to those in control cells (
Figure 6A-
Figure 6B). In contrast to our previous findings in Fam98a shRNA knockdown osteoclasts [
8], genetic ablation of Fam98a in osteoclasts had no effects on the peripheral distribution of Lamp-2
+ lysosomes in osteoclasts cultured on glass coverslips and lysosome trafficking and targeting to the ruffled border in osteoclasts cultured on bone slices (
Figure 6C-
Figure 6D). Thereafter, Fam98a
-/- osteoclasts had a similar bone-resorbing capacity as to control osteoclasts (
Figure 6E). Given that Fam98b is structurally and functionally related to Fam98a (
Figure 1 and
Figure 4), it is likely that Fam98b might compensate the loss of Fam98a in osteoclasts.
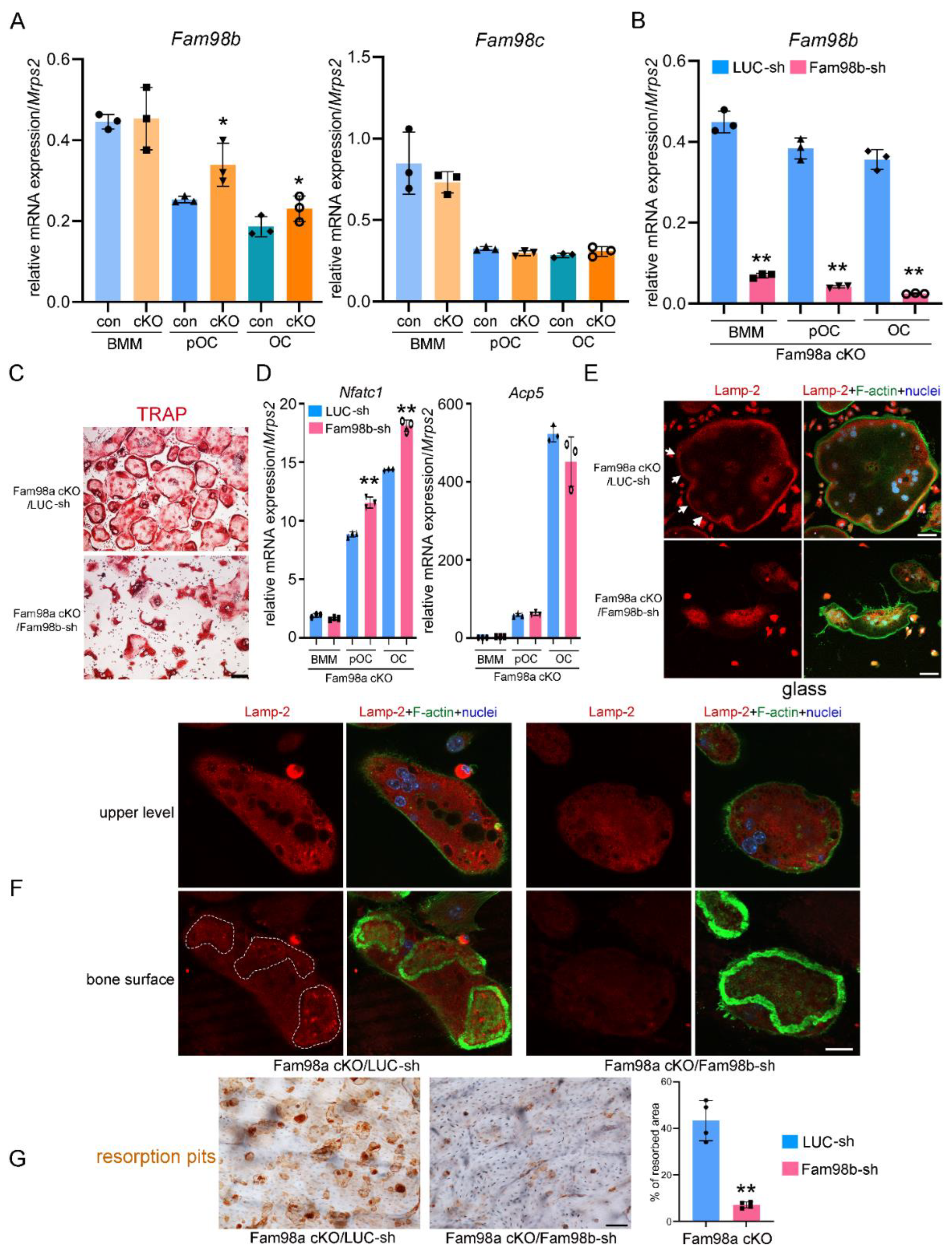
To test if lack of skeletal phenotype in Fam98a cKO mice can be explained by increased Fam98b expression , we fist examined the mRNA expression of
Fam98b and
Fam98c in control and Fam98a
-/- BMMs, pre- and mature-osteoclasts. The expression of
Fam98b but not
Fam98c was up regulated in Fam98a
-/- osteoclast precursor and mature cells compared to respective control osteoclasts (
Figure 7A). Next, we transduced the Fam98a
-/- BMMs with lentiviruses expressing the control (LUC-sh) and Fam98b-specific (Fam98b-sh) shRNAs, respectively. The mRNA level of
Fam98b was dramatically and significantly reduced in Fam98b-sh transduced osteoclast precursor and mature cells (
Figure 7B). The osteoclastogenesis from Fam98a
-/-/Fam98b
KD BMMs was comparable to control BMMs with slightly increase in
Nfatc1 but not
Acp5 mRNA expression (
Figure 7C-
Figure 7D) in Fam98a/b double-depleted osteoclast precursor and mature cells. However, the majority of Fam98a
-/-/Fam98b
KD osteoclasts (77 ± 12%, n = 4) exhibited irregular morphology with accumulation of TRAP
+ lysosomes around nuclei (
Figure 7C, lower panel) compared to Fam98b single knockdown osteoclasts (50.7 ± 8.4%, n=4, in
Figure 4B). The severe defects in lysosome trafficking and bone resorption in Fam98a
-/-/Fam98b
KD osteoclasts were observed by Lamp-2 immunofluorescent and bone resorption pit staining (
Figure 7E-
Figure 7G). These results postulate that Fam98a and Fam98b might compensate each other in regulation of lysosome secretion and bone resorption in osteoclasts
in vitro and
in vivo. This hypothesis warrants further investigation in the future.
4. Discussion
Three FAM98 family proteins (FAM98A/B/C) have been identified in human and mice based on sharing a unique structure domain with unknown function (DUF2465). By bioinformatic profiling and structural modeling, this domain is also found in several microtubule-associated proteins including the intraflagella transport (IFT) complex B subunits (IFT81, IFT57, and CLUAP1), suggesting a hypothetic role of FAM98 family proteins in microtubule dynamics and microtubule-based transportations [
28]. FAM98A and FAM98B have been shown working in an RNA-binding complex positively regulating RNA transcription, tRNA splicing, and RNA translation [
19,
20,
21,
22,
29]. FAM98C has been identified as a candidate gene linking to ciliogenesis [
23]. Nonetheless, the expression and physiological functions of FAM98A/B/C in bone cells and skeleton modeling/remodeling have not been fully elucidated and remain largely unknown.
We have previously reported that Fam98a interacts with Plekhm1 in murine osteoclasts and suppression of Fam98a expression by shRNA-mediated specific knockdown of
Fam98a mRNA in osteoclasts attenuates osteoclast lysosome trafficking and bone resorption, indicating that FAM98A might couple lysosomes to microtubules via its binding to lysosomal adaptor protein PLEKHM1 [
8]. Unexpectedly, however, we found in this study that loss of Fam98a by its genetical deletion in osteoclast myeloid precursor cells was dispensable for osteoclastogenesis and osteoclast function
in vitro as well as bone modeling/remodeling
in vivo. Since knockdown of Fam98b expression in control and Fam98a
-/- osteoclasts by shRNA exhibited defects in lysosome trafficking and bone resorption and that Fam98b expression was higher in Fam98a
-/- osteoclasts than control cells, it is likely that Fam98b compensates the loss-of-function of Fam98a in osteoclasts. Fam98b retains more structural similarity with Fam98a than Fam98c and FAM98A/B have been shown to function redundantly in colorectal cancer cells [
29]. As constituted in a protein complex regulating RNA metabolism and translation, FAM98A/B might activate PLEKHM1 mRNA maturation and/or translation. Thereby, suppression of FAM98A/B may cause decreased level of PLEKHM1 which is indispensable for lysosome trafficking and bone resorption in osteoclasts [
8]. Unfortunately, there is no reliable and high-quality antibody available to detect endogenous PLEKHM1 to test this hypothesis. Alternatively, FAM98A/B may anchor lysosomes onto microtubules via its interaction with PLEKHM1 and the N-terminal conserved DUF2465 domain. Nevertheless, the underlying mechanisms by which FAM98A/B regulate osteoclast lysosome trafficking and bone resorption warrant further investigation in the future.
The mRNA level of Fam98c was the highest among the three
Fam98 genes in cultured murine osteoclast precursor and mature cells as detected by RT-qPCR in present study. Moreover, repression of Fam98c but not loss of Fam98a/b greatly reduced the mRNA and protein levels of the master osteoclast differentiation transcription factor Nfatc1 and dramatically inhibited osteoclastogenesis
in vitro. These results indicate that FAM98C plays a critical role in osteoclast formation at least
in vitro. The exact mechanisms by which FAM98C specifically regulates osteoclast differentiation and/or survival are unknown. One possibility is that FAM98C may be involved in NFATC1 mRNA processing and translation. Since FAM98C has been linked to ciliogenesis [
23] and the primary cilium has been recently reported to regulate osteoclastogenesis [
30]. It is also likely that FAM98C regulates osteoclast differentiation through its action on the primary cilia dynamics. However, it should be noted that the important role of primary cilium in regulation of osteoclasts has not been well established yet as compared to osteoblasts and osteocytes [
31,
32] and requires more future research.
5. Conclusions
FAM98 family proteins play distinct roles in osteoclastogenesis and osteoclast bone resorption, respectively. FAM98C plays an important role in osteoclastogenesis, whereas FAM98A/B specifically regulate osteoclast lysosome trafficking and bone resorption.
Author Contributions
L.W. and T.M. (Methodology; Data curation; Investigation; Formal analysis; Writing-original draft), B.K.D., M.D.K., A.K. (Methodology; Data curation; Investigation; Formal analysis), L.G. (Validation; Supervision; Writing-review & editing), S.M., W.X., and K.I.V. (Conceptualization; Methodology; Investigation; Supervision; Writing-review & editing; H.Z. (Conceptualization; Methodology; Investigation; Validation; Formal analysis; Supervision; Writing-original draft; Writing-review & editing; Funding acquisition; Project administration). All authors have read and agreed to the published version of the manuscript.
Funding
L.W. was supported by a research grant AHWJ2023A30082 funded by Anhui Provincial Health Commission. The work was supported by grants from NIH/NIAMS R01AR073298, R21AR082842 to H.Z. and R01AR078843 to W.X. S.M. is a recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs (BX005381) and has grants support from NIH/NIAMS R01AR048139, R01AR070806.
Institutional Review Board Statement
All the animal protocols and procedures used in animal studies were approved by the Institutional Animal Care and Use Committees and the Subcommittees on Research Safety and Security in Long Beach VA Healthcare System and Loma Linda VA Healthcare System under the protocol number 1774.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated in this study are available from PI upon request.
Acknowledgments
The authors would like to thank Sheila Pourteymoor and Jillian Bray for help in micro-CT scan and analyses.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504-1508. [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337-342. [CrossRef]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J Cell Sci 2000, 113 ( Pt 3), 377-381. [CrossRef]
- Zhao, H. Membrane trafficking in osteoblasts and osteoclasts: new avenues for understanding and treating skeletal diseases. Traffic 2012, 13, 1307-1314. [CrossRef]
- Palagano, E.; Menale, C.; Sobacchi, C.; Villa, A. Genetics of Osteopetrosis. Curr Osteoporos Rep 2018, 16, 13-25. [CrossRef]
- Huybrechts, Y.; Van Hul, W. Osteopetrosis associated with PLEKHM1 and SNX10 genes, both involved in osteoclast vesicular trafficking. Bone 2022, 164, 116520. [CrossRef]
- Van Wesenbeeck, L.; Odgren, P.R.; Coxon, F.P.; Frattini, A.; Moens, P.; Perdu, B.; MacKay, C.A.; Van Hul, E.; Timmermans, J.P.; Vanhoenacker, F.; et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest 2007, 117, 919-930. [CrossRef]
- Fujiwara, T.; Ye, S.; Castro-Gomes, T.; Winchell, C.G.; Andrews, N.W.; Voth, D.E.; Varughese, K.I.; Mackintosh, S.G.; Feng, Y.; Pavlos, N.; et al. PLEKHM1/DEF8/RAB7 complex regulates lysosome positioning and bone homeostasis. JCI Insight 2016, 1, e86330. [CrossRef]
- Soldati, T.; Rancaño, C.; Geissler, H.; Pfeffer, S.R. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem 1995, 270, 25541-25548. [CrossRef]
- Méresse, S.; Gorvel, J.P.; Chavrier, P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci 1995, 108 ( Pt 11), 3349-3358. [CrossRef]
- Vitelli, R.; Santillo, M.; Lattero, D.; Chiariello, M.; Bifulco, M.; Bruni, C.B.; Bucci, C. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem 1997, 272, 4391-4397. [CrossRef]
- Mukhopadhyay, A.; Barbieri, A.M.; Funato, K.; Roberts, R.; Stahl, P.D. Sequential actions of Rab5 and Rab7 regulate endocytosis in the Xenopus oocyte. J Cell Biol 1997, 136, 1227-1237. [CrossRef]
- Zhao, H.; Laitala-Leinonen, T.; Parikka, V.; Väänänen, H.K. Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J Biol Chem 2001, 276, 39295-39302. [CrossRef]
- Das, B.K.; Minocha, T.; Kunika, M.D.; Kannan, A.; Gao, L.; Mohan, S.; Xing, W.; Varughese, K.I.; Zhao, H. Molecular and functional mapping of Plekhm1-Rab7 interaction in osteoclasts. JBMR Plus 2024, 8, ziae034. [CrossRef]
- Ye, S.; Fowler, T.W.; Pavlos, N.J.; Ng, P.Y.; Liang, K.; Feng, Y.; Zheng, M.; Kurten, R.; Manolagas, S.C.; Zhao, H. LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS One 2011, 6, e27285. [CrossRef]
- Das, B.K.; Gogoi, J.; Kannan, A.; Gao, L.; Xing, W.; Mohan, S.; Zhao, H. The Cytoplasmic Dynein Associated Protein NDE1 Regulates Osteoclastogenesis by Modulating M-CSF and RANKL Signaling Pathways. Cells 2021, 11. [CrossRef]
- Akter, K.A.; Mansour, M.A.; Hyodo, T.; Ito, S.; Hamaguchi, M.; Senga, T. FAM98A is a novel substrate of PRMT1 required for tumor cell migration, invasion, and colony formation. Tumour Biol 2016, 37, 4531-4539. [CrossRef]
- Ozeki, K.; Sugiyama, M.; Akter, K.A.; Nishiwaki, K.; Asano-Inami, E.; Senga, T. FAM98A is localized to stress granules and associates with multiple stress granule-localized proteins. Mol Cell Biochem 2019, 451, 107-115. [CrossRef]
- Pérez-González, A.; Pazo, A.; Navajas, R.; Ciordia, S.; Rodriguez-Frandsen, A.; Nieto, A. hCLE/C14orf166 associates with DDX1-HSPC117-FAM98B in a novel transcription-dependent shuttling RNA-transporting complex. PLoS One 2014, 9, e90957. [CrossRef]
- Popow, J.; Jurkin, J.; Schleiffer, A.; Martinez, J. Analysis of orthologous groups reveals archease and DDX1 as tRNA splicing factors. Nature 2014, 511, 104-107. [CrossRef]
- Pazo, A.; Pérez-González, A.; Oliveros, J.C.; Huarte, M.; Chavez, J.P.; Nieto, A. hCLE/RTRAF-HSPC117-DDX1-FAM98B: A New Cap-Binding Complex That Activates mRNA Translation. Front Physiol 2019, 10, 92. [CrossRef]
- Kroupova, A.; Ackle, F.; Asanović, I.; Weitzer, S.; Boneberg, F.M.; Faini, M.; Leitner, A.; Chui, A.; Aebersold, R.; Martinez, J.; et al. Molecular architecture of the human tRNA ligase complex. Elife 2021, 10. [CrossRef]
- Shaheen, R.; Szymanska, K.; Basu, B.; Patel, N.; Ewida, N.; Faqeih, E.; Al Hashem, A.; Derar, N.; Alsharif, H.; Aldahmesh, M.A.; et al. Characterizing the morbid genome of ciliopathies. Genome Biol 2016, 17, 242. [CrossRef]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 2000, 15, 1477-1488. [CrossRef]
- Zhao, H.; Väänänen, H.K. Pharmacological sequestration of intracellular cholesterol in late endosomes disrupts ruffled border formation in osteoclasts. J Bone Miner Res 2006, 21, 456-465. [CrossRef]
- Lewis, V.; Green, S.A.; Marsh, M.; Vihko, P.; Helenius, A.; Mellman, I. Glycoproteins of the lysosomal membrane. J Cell Biol 1985, 100, 1839-1847. [CrossRef]
- Das, B.K.; Wang, L.; Fujiwara, T.; Zhou, J.; Aykin-Burns, N.; Krager, K.J.; Lan, R.; Mackintosh, S.G.; Edmondson, R.; Jennings, M.L.; et al. Transferrin receptor 1-mediated iron uptake regulates bone mass in mice via osteoclast mitochondria and cytoskeleton. Elife 2022, 11. [CrossRef]
- Schou, K.B.; Andersen, J.S.; Pedersen, L.B. A divergent calponin homology (NN-CH) domain defines a novel family: implications for evolution of ciliary IFT complex B proteins. Bioinformatics 2014, 30, 899-902. [CrossRef]
- Akter, K.A.; Mansour, M.A.; Hyodo, T.; Senga, T. FAM98A associates with DDX1-C14orf166-FAM98B in a novel complex involved in colorectal cancer progression. Int J Biochem Cell Biol 2017, 84, 1-13. [CrossRef]
- Sutton, M.M.; Duffy, M.P.; Verbruggen, S.W.; Jacobs, C.R. Osteoclastogenesis Requires Primary Cilia Disassembly and Can Be Inhibited by Promoting Primary Cilia Formation Pharmacologically. Cells Tissues Organs 2024, 213, 235-244. [CrossRef]
- Yuan, X.; Cao, J.; He, X.; Serra, R.; Qu, J.; Cao, X.; Yang, S. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat Commun 2016, 7, 11024. [CrossRef]
- Moraes de Lima Perini, M.; Pugh, J.N.; Scott, E.M.; Bhula, K.; Chirgwin, A.; Reul, O.N.; Berbari, N.F.; Li, J. Primary cilia in osteoblasts and osteocytes are required for skeletal development and mechanotransduction. bioRxiv 2023. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).