Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. The Major Challenge: Mechanism, Target, and Drug Discovery of Severe AP
2. What Makes XO a Drug Target
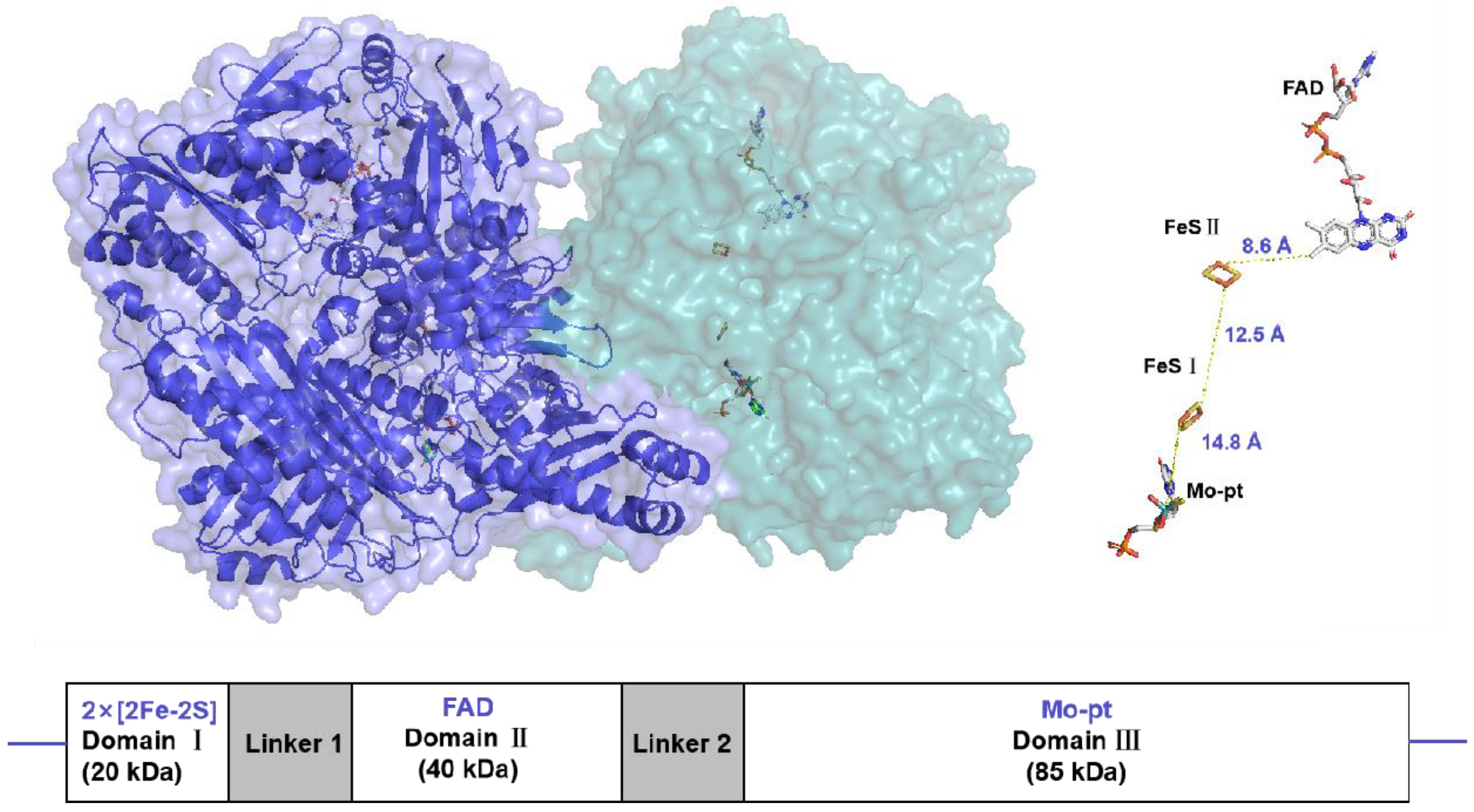
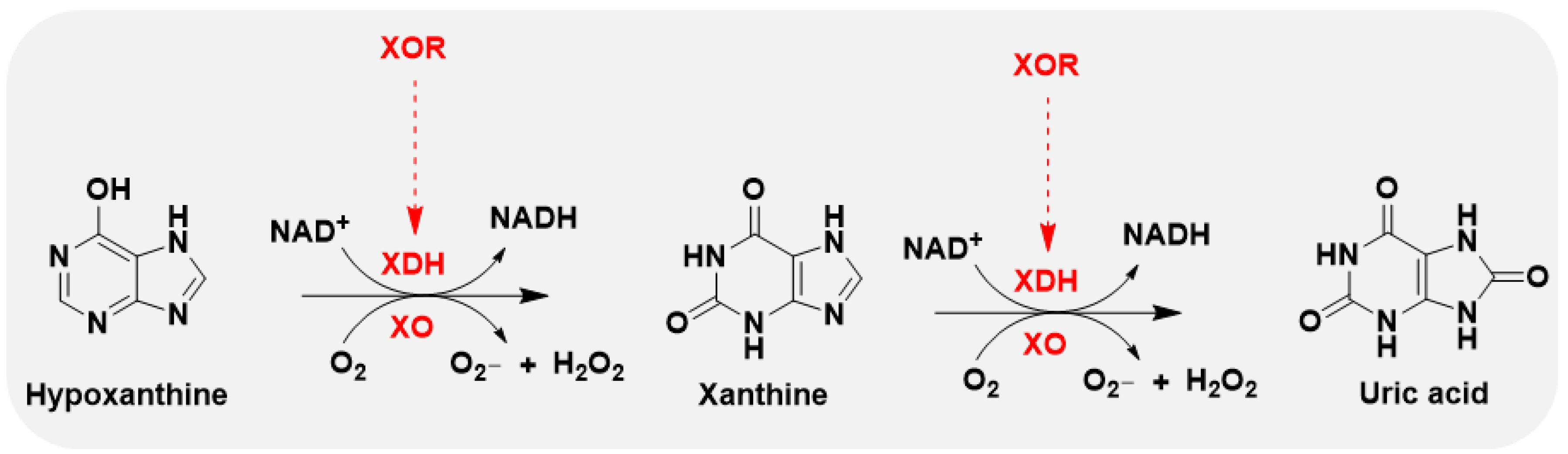
2.1. General Profile of XO and Target Characteristics
2.2. Inhibitors of XO
- (1)
- Purine analogs
- (2)
- Nonpurine analogs
Synthetic Nonpurine Analogs
Natural Nonpurine Analogs
3. Role of XO in AP Onset and Deterioration
3.1. Changes of XO Specifically Occur in Necrotic SAP Model Rather Than Edematous Model
3.2. Targeting XO in Experimental AP
3.3. Role of XO in Pancreatic Injury During AP
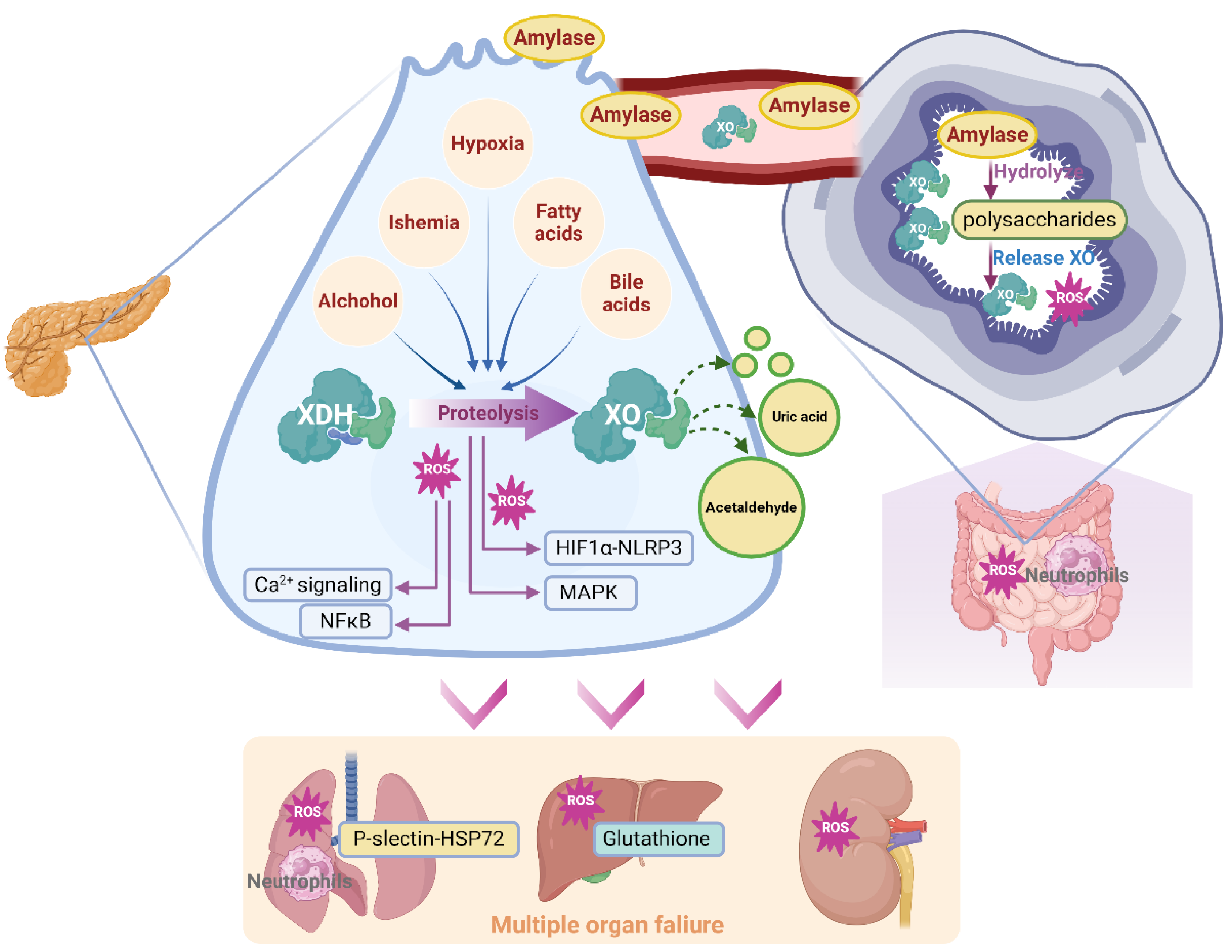
3.4. Effect of XO in AP-Associated Multiple Organ Failure
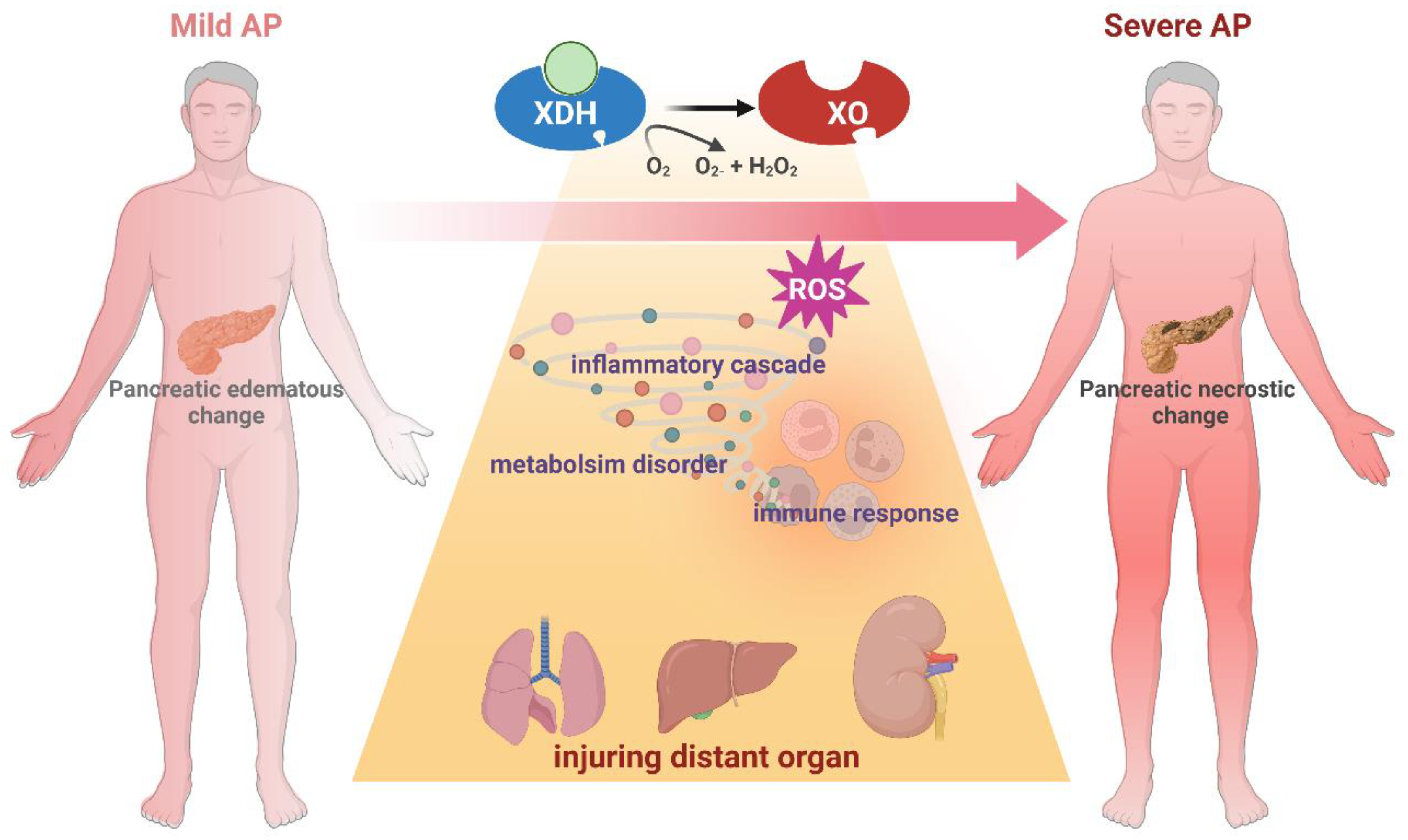
4. Clinical Trials of XO Inhibitors in AP Patients
5. Limitations and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012, Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Garg SK, Sarvepalli S, Campbell JP, Obaitan I, Singh D, Bazerbachi F, et al. Incidence, Admission Rates, and Predictors, and Economic Burden of Adult Emergency Visits for Acute Pancreatitis: Data From the National Emergency Department Sample, 2006 to 2012. J Clin Gastroenterol 2019, 53, 220–5.
- Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–90. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; Feuerstein, J.; Flamm, S.; Gellad, Z.; Gerson, L.; Gupta, S.; et al. American Gastroenterological Association Institute Clinical Guidelines Committee, American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef]
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet 2020, 396, 726–34. [Google Scholar] [CrossRef]
- Moggia, E.; Koti, R.; Belgaumkar, A.P.; Fazio, F.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst. Rev. 2017, 4, CD011384. [Google Scholar] [CrossRef]
- Barreto, S.G.; Habtezion, A.; Gukovskaya, A.; Lugea, A.; Jeon, C.; Yadav, D.; Hegyi, P.; Venglovecz, V.; Sutton, R.; Pandol, S.J. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut 2020, 70, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol 2019, 16, 479–96. [Google Scholar] [CrossRef]
- Shi, N.; Liu, T.; de la Iglesia-Garcia, D.; Deng, L.; Jin, T.; Lan, L.; Zhu, P.; Hu, W.; Zhou, Z.; Singh, V.; et al. Duration of organ failure impacts mortality in acute pancreatitis. Gut 2019, 69, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Matsumoto, K.; Hille, R.; Eger, B.T.; Pai, E.F.; Nishino, T. The crystal structure of xanthine oxidoreductase during catalysis: Implications for reaction mechanism and enzyme inhibition. Proc. Natl. Acad. Sci. USA 2004, 101, 7931–7936. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.V.; Bedi, P.M.S.; Singh, H.; Sharma, S. Xanthine oxidase inhibitors: patent landscape and clinical development (2015–2020). Expert Opin. Ther. Patents 2020, 30, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl 1986, 548, 87–99. [Google Scholar]
- Saksela, M.; Lapatto, R.; Raivio, K.O. Xanthine Oxidoreductase Gene Expression and Enzyme Activity in Developing Human Tissues. Neonatology 1998, 74, 274–280. [Google Scholar] [CrossRef] [PubMed]
- A Pritsos, C. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem. Interactions 2000, 129, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.M.; Vaitaitis, G.M.; Wilson, C.M.; Repine, T.B.; Terada, L.S.; E Repine, J. cDNA cloning, characterization, and tissue-specific expression of human xanthine dehydrogenase/xanthine oxidase. Proc. Natl. Acad. Sci. USA 1993, 90, 10690–10694. [Google Scholar] [CrossRef]
- Furuhashi, M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am. J. Physiol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef]
- Su A, Yuan X, Zhu G, Jiang X, Shu R, Yang W, et al. Association Between Baseline Uric Acid and the Risk of Acute Pancreatitis: A Prospective Cohort Study. Pancreas 2022, 51, 966–71. [Google Scholar] [CrossRef]
- Era, B.; Delogu, G.L.; Pintus, F.; Fais, A.; Gatto, G.; Uriarte, E.; Borges, F.; Kumar, A.; Matos, M.J. Looking for new xanthine oxidase inhibitors: 3-Phenylcoumarins versus 2-phenylbenzofurans. Int. J. Biol. Macromol. 2020, 162, 774–780. [Google Scholar] [CrossRef]
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half a Century after the Discovery of Allopurinol. Pharmacol. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef]
- Thankachen J, Agarwal V. Challenges in Diagnosis, Management, and Treatment of Allopurinol-Induced DRESS Syndrome: Case Report and Literature Review. Am J Ther 2015, 22, e77–e83. [Google Scholar] [CrossRef]
- Biagi, G.; Costantini, A.; Costantino, L.; Giorgi, I.; Livi, O.; Pecorari, P.; Rinaldi, M.; Scartoni, V. Synthesis and Biological Evaluation of New Imidazole, Pyrimidine, and Purine Derivatives and Analogs as Inhibitors of Xanthine Oxidase. J. Med. Chem. 1996, 39, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Tamta, H.; Thilagavathi, R.; Chakraborti, A.K.; Mukhopadhyay, A.K. 6-(N-benzoylamino)purine as a novel and potent inhibitor of xanthine oxidase: Inhibition mechanism and molecular modeling studies. J. Enzym. Inhib. Med. Chem. 2005, 20, 317–324. [Google Scholar] [CrossRef]
- Rodrigues MV, Barbosa AF, da Silva JF, dos Santos DA, Vanzolini KL, de Moraes MC, et al. 9-Benzoyl 9-deazaguanines as potent xanthine oxidase inhibitors. Bioorg Med Chem 2016, 24, 226–31. [Google Scholar] [CrossRef] [PubMed]
- 37. Šmelcerović, A.; Tomović, K.; Šmelcerović, Ž.; Petronijević, Ž.; Kocić, G.; Tomašič, T.; Jakopin, Ž.; Anderluh, M. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties, predicted pharmacokinetics and toxicity. Eur. J. Med. Chem. 2017, 135, 491–516. [Google Scholar] [CrossRef]
- Malik, U.Z.; Hundley, N.J.; Romero, G.; Radi, R.; Freeman, B.A.; Tarpey, M.M.; Kelley, E.E. Febuxostat inhibition of endothelial-bound XO: Implications for targeting vascular ROS production. Free. Radic. Biol. Med. 2011, 51, 179–184. [Google Scholar] [CrossRef]
- Sato T, Ashizawa N, Matsumoto K, Iwanaga T, Nakamura H, Inoue T, et al. Discovery of 3-(2-cyano-4-pyridyl)-5-(4-pyridyl)-1,2,4-triazole, FYX-051 - a xanthine oxidoreductase inhibitor for the treatment of hyperuricemia [corrected]. Bioorg Med Chem Lett 2009, 19, 6225–9.
- Jordan, A.; Gresser, U. Side Effects and Interactions of the Xanthine Oxidase Inhibitor Febuxostat. Pharmaceuticals 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Rullo, R.; Cerchia, C.; Nasso, R.; Romanelli, V.; De Vendittis, E.; Masullo, M.; Lavecchia, A. Novel Reversible Inhibitors of Xanthine Oxidase Targeting the Active Site of the Enzyme. Antioxidants 2023, 12, 825. [Google Scholar] [CrossRef]
- Xu, X.; Deng, L.; Nie, L.; Chen, Y.; Liu, Y.; Xie, R.; Li, Z. Discovery of 2-phenylthiazole-4-carboxylic acid, a novel and potent scaffold as xanthine oxidase inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 525–528. [Google Scholar] [CrossRef]
- Zhang, T.-J.; Zhang, Y.; Zhang, Z.-H.; Wang, Z.-R.; Zhang, X.; Hu, S.-S.; Lu, P.-F.; Guo, S.; Meng, F.-H. Discovery of 4-(phenoxymethyl)-1H-1,2,3-triazole derivatives as novel xanthine oxidase inhibitors. Bioorganic Med. Chem. Lett. 2022, 60, 128582. [Google Scholar] [CrossRef]
- Zhang, T.-J.; Tu, S.; Zhang, X.; Wang, Q.-Y.; Hu, S.-S.; Zhang, Y.; Zhang, Z.-H.; Wang, Z.-R.; Meng, F.-H. Amide-based xanthine oxidase inhibitors bearing an N-(1-alkyl-3-cyano-1H-indol-5-yl) moiety: Design, synthesis and structure-activity relationship investigation. Bioorganic Chem. 2021, 117, 105417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-J.; Zhang, Z.-H.; Zhang, X.; Wang, Z.-R.; Xu, E.-Y.; Tu, S.; Zhang, Y.; Meng, F.-H. Design, synthesis and biological evaluation of N-(4-alkoxy-3-(1H-tetrazol-1-yl)phenyl) heterocyclic aromatic amide derivatives as xanthine oxidase inhibitors. Bioorganic Chem. 2022, 127, 105938. [Google Scholar] [CrossRef]
- Tang H-J, Li W, Zhou M, Peng L-Y, Wang J-X, Li J-H, et al. Design, synthesis and biological evaluation of novel xanthine oxidase inhibitors bearing a 2-arylbenzo b furan scaffold. European Journal of Medicinal Chemistry 2018, 151, 849–60. [Google Scholar] [CrossRef]
- Zhao J, Mao Q, Lin F, Zhang B, Sun M, Zhang T, et al. Intramolecular hydrogen bond interruption and scaffold hopping of TMC-5 led to 2-(4-alkoxy-3-cyanophenyl)pyrimidine-4/5-carboxylic acids and 6-(4-alkoxy-3-cyanophenyl)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-ones as potent pyrimidine-based xanthine oxidase inhibitors. Eur J Med Chem 2022, 229, 114086. [Google Scholar]
- Fais A, Era B, Asthana S, Sogos V, Medda R, Santana L, et al. Coumarin derivatives as promising xanthine oxidase inhibitors. International Journal of Biological Macromolecules 2018, 120, 1286–93. [Google Scholar] [CrossRef] [PubMed]
- Mohos, V.; Fliszár-Nyúl, E.; Poór, M. Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates. Int. J. Mol. Sci. 2020, 21, 3256. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, L.; Sun, C.; Zhao, D.; Tang, H. Studies on the structure-activity relationship and interaction mechanism of flavonoids and xanthine oxidase through enzyme kinetics, spectroscopy methods and molecular simulations. Food Chem. 2020, 323, 126807. [Google Scholar] [CrossRef]
- Li J, Gong Y, Li J, Fan L. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: Identification, inhibitory activity, and action mechanism. Food Chem 2022, 379, 132100. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, D. Investigation of the interaction between salvianolic acid C and xanthine oxidase: Insights from experimental studies merging with molecular docking methods. Bioorganic Chem. 2019, 88, 102981. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Siguri, C.; Delogu, G.L.; Fais, A.; Era, B.; Floris, S.; Pintus, F.; Kumar, A.; Fantini, M.C.; Olla, S. Exploring Asphodelus microcarpus as a source of xanthine oxidase inhibitors: Insights from in silico and in vitro studies. Chem. Interactions 2024, 397, 111087. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Du, J.; He, P.; Wang, N.; Li, L.; Liu, Y.; Yang, C.; Xu, H.; Li, Y. Identification of natural xanthine oxidase inhibitors: Virtual screening, anti-xanthine oxidase activity, and interaction mechanism. Int. J. Biol. Macromol. 2024, 259, 129286. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Sun, S.; Lv, C.; Li, Z.; Guo, M.; Yin, Y.; Wang, H.; Wang, W. Discovery of mycotoxin alternariol as a potential lead compound targeting xanthine oxidase. Chem. Interactions 2022, 360, 109948. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, S.; Wang, X.; Fan, X.; Wilson, G.; Sa, Y.; Ma, X. A strategy for inhibitors screening of xanthine oxidase based on colorimetric sensor combined with affinity chromatography technology. Biosens. Bioelectron. 2024, 261, 116510. [Google Scholar] [CrossRef]
- Devenyi, Z.J.; Orchard, J.L.; Powers, R.E. Xanthine oxidase activity in mouse pancreas: Effects of caerulein-induced acute pancreatitis. Biochem. Biophys. Res. Commun. 1987, 149, 841–845. [Google Scholar] [CrossRef]
- Nordback, I.H.; Cameron, J.L. The mechanism of conversion of xanthine dehydrogenase to xanthine oxidase in acute pancreatitis in the canine isolated pancreas preparation. . 1993, 113, 90–7. [Google Scholar]
- Closa, D.; Bulbena, O.; Hotter, G.; Roseixó-Catafau, J.; Fernández-Cruz, L.; Gelpf, E. Xanthine oxidase activation in cerulein-and taurocholate-induced acute pancreatitis in rats. Arch. Int. de Physiol. de Biochim. et de Biophys. 1994, 102, 167–170. [Google Scholar] [CrossRef]
- Closa, D.; Bulbena, O.; Rosello-Catafau, J.; Fernandez-Cruz, L.; Gelpi, E. Effect of prostaglandins and superoxide dismutase administration on oxygen free radical production in experimental acute pancreatitis. Inflammation 1993, 17, 563–571. [Google Scholar] [CrossRef]
- Granell, S.; Gironella, M.; Bulbena, O.; Panés, J.; Mauri, M.; Sabater, L.; Aparisi, L.; Gelpí, E.; Closa, D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 2003, 31, 525–530. [Google Scholar] [CrossRef]
- Folch, E.; Gelpi, E.; Rosello-Catafau, J.; Closa, D. Free Radicals Generated by Xanthine Oxidase Mediate Pancreatitis-Associated Organ Failure. Dig. Dis. Sci. 1998, 43, 2405–2410. [Google Scholar] [CrossRef]
- Nordback, I.H.; Olson, J.L.; Chacko, V.P.; Cameron, J.L. Detailed characterization of experimental acute alcoholic pancreatitis. Surgery 1995, 117, 41–49. [Google Scholar] [CrossRef]
- Weber H, Merkord J, Jonas L, Wagner A, Schroder H, Kading U, et al. Oxygen radical generation and acute pancreatitis: effects of dibutyltin dichloride/ethanol and ethanol on rat pancreas. Pancreas 1995, 11, 382–8. [Google Scholar] [CrossRef]
- Rong, J.; Han, C.; Huang, Y.; Wang, Y.; Qiu, Q.; Wang, M.; Wang, S.; Wang, R.; Yang, J.; Li, X.; et al. Inhibition of xanthine oxidase alleviated pancreatic necrosis via HIF-1α-regulated LDHA and NLRP3 signaling pathway in acute pancreatitis. Acta Pharm. Sin. B 2024, 14, 3591–3604. [Google Scholar] [CrossRef]
- Shabanov VV, Sarbaeva NN, Milyakova MN. Generation of free oxygen radicals in the pathogenesis of experimental acute reflux pancreatitis. Bull Exp Biol Med 2002, 134, 26–7. [Google Scholar] [CrossRef] [PubMed]
- Czakó, L.; Takács, T.; Varga, I.S.; Tiszlavicz, L.; Hai, D.Q.; Hegyi, P.; Matkovics, B.; Lonovics, J. Oxidative Stress in Distant Organs and the Effects of Allopurinol During Experimental Acute Pancreatitis. J. Gastrointest. Cancer 2000, 27, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Czako, L.; Takacs, T.; Varga, I.S.; Tiszlavicz, L.; Hai, D.Q.; Hegyi, P.; Matkovics, B.; Lonovics, J. Involvement of Oxygen-Derived Free Radicals in L-Arginine-Induced Acute Pancreatitis. Dig. Dis. Sci. 1998, 43, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Czakó, L.; Takács, T.; Varga, I.S.; Hai, D.Q.; Tiszlavicz, L.; Hegyi, P.; Mándi, Y.; Matkovics, B.; Lonovics, J. The pathogenesis of L-arginine-induced acute necrotizing pancreatitis: Inflammatory mediators and endogenous cholecystokinin. J. Physiol. 2000, 94, 43–50. [Google Scholar] [CrossRef]
- Hirano, T.; Manabe, T.; Ohshio, G.; Nio, Y. Protective effects of combined therapy with a protease inhibitor, ONO 3307, and a xanthine oxidase inhibitor, allopurinol on temporary ischaemic model of pancreatitis in rats. . 1992, 61, 224–33. [Google Scholar]
- Degertekin H, Ertan A, Yater RD, Van Meter K, Akdamar K. Hyperbaric oxygen, allopurinol, and diet-induced acute pancreatitis. Ann Intern Med 1985, 103, 474–5. [Google Scholar] [CrossRef]
- Sanfey, H.; Bulkley, G.B.; Cameron, J.L. The Source and Role of Oxygen-derived Free Radicals in Three Different Experimental Models. Ann. Surg. 1985, 201, 633–639. [Google Scholar] [CrossRef]
- Nordback, I.H.; Macgowan, S.F.; Potter, J.J.B.; Cameron, J.L.M. The Role of Acetaldehyde in the Pathogenesis of Acute Alcoholic Pancreatitis. Ann. Surg. 1991, 214, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Sarr MG, Bulkley GB, Cameron JL. Temporal efficacy of allopurinol during the induction of pancreatitis in the ex vivo perfused canine pancreas. Surgery 1987, 101, 342–6. [Google Scholar]
- Marks, J.M.; Dunkin, B.J.; Shillingstad, B.L.; Youngelman, D.F.; Schweitzer, M.A.; Lash, R.H.; Singh, J.; Ponsky, L.; Ponsky, J.L. Pretreatment with allopurinol diminishes pancreatography-induced pancreatitis in a canine model. Gastrointest. Endosc. 1998, 48, 180–183. [Google Scholar] [CrossRef]
- Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, et al. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg 2004, 240, 108–16. [Google Scholar] [CrossRef]
- Comert B, Isik AT, Aydin S, Bozoglu E, Unal B, Deveci S, et al. Combination of allopurinol and hyperbaric oxygen therapy: a new treatment in experimental acute necrotizing pancreatitis? World J Gastroenterol 2007, 13, 6203–7.
- Escobar, J.; Pereda, J.; Arduini, A.; Sandoval, J.; Moreno, M.L.; Pérez, S.; Sabater, L.; Aparisi, L.; Cassinello, N.; Hidalgo, J.; et al. Oxidative and nitrosative stress in acute pancreatitis. Modulation by pentoxifylline and oxypurinol. Biochem. Pharmacol. 2012, 83, 122–130. [Google Scholar] [CrossRef]
- Isik, A.T.; Mas, M.R.; Yamanel, L.; Aydin, S.; Comert, B.; Akay, C.; Erdem, G.; Mas, N. The role of allopurinol in experimental acute necrotizing pancreatitis. Indian J Med Res 2006, 124, 709–14. [Google Scholar]
- Lankisch, P.G.; Pohl, U.; Otto, J.; Wereszczynska-Siemiatkowska, U.; Gröne, H.-J. *Xanthine Oxidase Inhibitor in Acute Experimental Pancreatitis in Rats and Mice. Pancreas 1989, 4, 436–440. [Google Scholar] [CrossRef]
- Wisner JR, Renner IG. Allopurinol attenuates caerulein induced acute pancreatitis in the rat. Gut 1988, 29, 926–9. [Google Scholar] [CrossRef]
- Suzuki H, Suematsu M, Miura S, Asako H, Kurose I, Ishii H, et al. Xanthine oxidase-mediated intracellular oxidative stress in response to cerulein in rat pancreatic acinar cells. Pancreas 1993, 8, 465–70. [Google Scholar] [CrossRef]
- Ito, T.; Nakao, A.; Kishimoto, W.; Nakano, M.; Takagi, H. The Involvement and Sources of Active Oxygen in Experimentally Induced Acute Pancreatitis. Pancreas 1996, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mantke, R.; Rocken, C.; Schubert, D.; Pross, M.; Sokolowski, A.; Halangk, W.; Lippert, H.; Schulz, H.-U. Enzymatic and histological alterations in the isolated perfused rat pancreas under conditions of oxidative stress. Langenbeck's Arch. Surg. 2002, 387, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Robles L, Vaziri ND, Ichii H. Role of Oxidative Stress in the Pathogenesis of Pancreatitis: Effect of Antioxidant Therapy. Pancreat Disord Ther 2013, 3, 112. [Google Scholar]
- Armstrong JA, Cash N, Soares PM, Souza MH, Sutton R, Criddle DN. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res 2013, 47, 917–33.
- Niederau, C.; Schulz, H.-U.; Klonowski, H. Lazaroids Protect Isolated Rat Pancreatic Acinar Cells Against Damage Induced by Free Radicals. Pancreas 1995, 11, 107–121. [Google Scholar] [CrossRef]
- Sanfey, H.; Bulkley, G.B.; Cameron, J.L. The Role of Oxygen-derived Free Radicals in the Pathogenesis of Acute Pancreatitis. Ann. Surg. 1984, 200, 405–413. [Google Scholar] [CrossRef]
- Rau B, Poch B, Gansauge F, Bauer A, Nussler AK, Nevalainen T, et al. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann Surg 2000, 231, 352–60.
- Schoenberg, M.; Büchler, M.; Beger, H. The role of oxygen radicals in experimental acute pancreatitis. Free. Radic. Biol. Med. 1992, 12, 515–522. [Google Scholar] [CrossRef]
- Pérez, S.; Pereda, J.; Sabater, L.; Sastre, J. Redox signaling in acute pancreatitis. Redox Biol. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Weber, H.; Roesner, J.; Nebe, B.; Rychly, J.; Werner, A.; Schröder, H.; Jonas, L.; Leitzmann, P.; Schneider, K.-P.; Dummler, W. Increased Cytosolic Ca2+ Amplifies Oxygen Radical-Induced Alterations of the Ultrastructure and the Energy Metabolism of Isolated Rat Pancreatic Acinar Cells. Digestion 1998, 59, 175–185. [Google Scholar] [CrossRef]
- Raghu, M.G.; Wig, J.D.; Kochhar, R.; Gupta, D.; Gupta, R.; Yadav, T.D.; Agarwal, R.; Kudari, A.K.; Doley, R.P.; Javed, A. Lung complications in acute pancreatitis. JOP 2007, 8, 177–85. [Google Scholar] [PubMed]
- Cuthbertson, C.M.M.; Su, K.H.B.; Muralidharan, V.M.; Millar, I.M.; Malcontenti-Wilson, C.B.; Christophi, C.M. Hyperbaric Oxygen Improves Capillary Morphology in Severe Acute Pancreatitis. Pancreas 2008, 36, 70–75. [Google Scholar] [CrossRef]
- Yu X, Li YG, He XW, Li XR, Din BN, Gan Y, et al. Hyperbaric oxygen reduces inflammatory response in acute pancreatitis by inhibiting NF-kappaB activation. Eur Surg Res 2009, 42, 130–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ge, B.; Yuan, Y.; Wang, G. Hyperbaric Oxygen Ameliorated Acute Pancreatitis in Rats via the Mitochondrial Pathway. Dig. Dis. Sci. 2020, 65, 3558–3569. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Klonowski, H.; Schulz, H.-U.; Sarbia, M.; Lüthen, R.; Häussinger, D. Oxidative injury to isolated rat pancreatic acinar cells vs. isolated zymogen granules. Free. Radic. Biol. Med. 1996, 20, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Jiang Q, Ding Y, Li F, Fayyaz AI, Duan H, Geng X. Modulation of NLRP3 inflammasome-related-inflammation via RIPK1/RIPK3-DRP1 or HIF-1alpha signaling by phenothiazine in hypothermic and normothermic neuroprotection after acute ischemic stroke. Redox Biol 2024, 73, 103169. [Google Scholar]
- de Groot H, Littauer A. Hypoxia, reactive oxygen, and cell injury. Free Radic Biol Med 1989, 6, 541–51. [Google Scholar] [CrossRef]
- Nonaka, A.; Manabe, T.; Tamura, K.; Asano, N.; Imanishi, K.; Tobe, T. Changes of Xanthine Oxidase, Lipid Peroxide and Superoxide Dismutase in Mouse Acute Pancreatitis. Digestion 1989, 43, 41–46. [Google Scholar] [CrossRef]
- Lopez Martin A, Carrillo Alcaraz A. Oxidative stress and acute pancreatitis. Rev Esp Enferm Dig 2011, 103, 559–62. [Google Scholar] [CrossRef]
- Granell, S.; Bulbena, O.; Genesca, M.; Sabater, L.; Sastre, J.; Gelpi, E.; Closa, D. Mobilization of xanthine oxidase from the gastrointestinal tract in acute pancreatitis. BMC Gastroenterol. 2004, 4, 1–1. [Google Scholar] [CrossRef]
- Shi, C.; Andersson, R.; Zhao, X.; Wang, X. Potential role of reactive oxygen species in pancreatitis-associated multiple organ dysfunction. Pancreatology 2005, 5, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Fink, MP. Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: potential benefits of resuscitation with Ringer's ethyl pyruvate solution. Curr Opin Clin Nutr Metab Care 2002, 5, 167–74. [Google Scholar] [CrossRef] [PubMed]
- Papathanassoglou, E.D.E.; Moynihan, J.A.; Ackerman, M.H. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Crit. Care Med. 2000, 28, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, R.; Sun, K.; Yan, C.; Jiang, J.; Kong, F.; Shi, J. The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3. Redox Biol. 2023, 62, 102707. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Zhang, L.; Zhao, J.; Li, P. Ferroptosis and its emerging roles in acute pancreatitis. Chin. Med J. 2022, 135, 2026–2034. [Google Scholar] [CrossRef]
- Gu, X.; Huang, Z.; Ying, X.; Liu, X.; Ruan, K.; Hua, S.; Zhang, X.; Jin, H.; Liu, Q.; Yang, J. Ferroptosis exacerbates hyperlipidemic acute pancreatitis by enhancing lipid peroxidation and modulating the immune microenvironment. Cell Death Discov. 2024, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Poch, B.; Gansauge, F.; Rau, B.; Wittel, U.; Gansauge, S.; Nüssler, A.K.; Schoenberg, M.; Beger, H.G. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett. 1999, 461, 268–272. [Google Scholar] [CrossRef]
- Folch E, Salas A, Panes J, Gelpi E, Rosello-Catafau J, Anderson DC, et al. Role of P-selectin and ICAM-1 in pancreatitis-induced lung inflammation in rats: significance of oxidative stress. Ann Surg 1999, 230, 792–8. [Google Scholar] [CrossRef]
- Folch, E.; Salas, A.; Prats, N.; Panés, J.; Piqué, J.M.; Gelpı́, E.; Roselló-Catafau, J.; Closa, D. H2O2 and PARS mediate lung P-selectin upregulation in acute pancreatitis. Free. Radic. Biol. Med. 2000, 28, 1286–1294. [Google Scholar] [CrossRef]
- Folch, E.; Closa, D.; Ñeco, P.; Solé, S.; Planas, A.; Gelpí, E.; Roselló-Catafau, J. Pancreatitis Induces HSP72 in the Lung: Role of Neutrophils and Xanthine Oxidase. Biochem. Biophys. Res. Commun. 2000, 273, 1078–1083. [Google Scholar] [CrossRef]
- Granell, S.; Serrano-Mollar, A.; Folch-Puy, E.; Navajas, D.; Farre, R.; Bulbena, O.; Closa, D. Oxygen in the alveolar air space mediates lung inflammation in acute pancreatitis. Free. Radic. Biol. Med. 2004, 37, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-L.; Yan, W.-S.; Xiang, X.-H.; Chen, K.; Xia, S.-H. Prevention Effect of Allopurinol on Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Meta-Analysis of Prospective Randomized Controlled Trials. PLOS ONE 2014, 9, e107350. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, Y.; Bai, J.; Xin, Y.; Pan, X.; Zhao, L. Meta-Analysis of Prophylactic Allopurinol Use in Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Pancreas 2008, 37, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Budzyńska, A.; Marek, T.; Nowak, A.; Kaczor, R.; Nowakowska-Dulawa, E. A Prospective, Randomized, Placebo-Controlled Trial of Prednisone and Allopurinol in the Prevention of ERCP-Induced Pancreatitis. Endoscopy 2001, 33, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Mosler, P.; Sherman, S.; Marks, J.; Watkins, J.L.; Geenen, J.E.; Jamidar, P.; Fogel, E.L.; Lazzell-Pannell, L.; Temkit, M.; Tarnasky, P.; et al. Oral allopurinol does not prevent the frequency or the severity of post-ERCP pancreatitis. Gastrointest. Endosc. 2005, 62, 245–250. [Google Scholar] [CrossRef]
- Romagnuolo, J.; Hilsden, R.; Sandha, G.S.; Cole, M.; Bass, S.; May, G.; Love, J.; Bain, V.G.; McKaigney, J.; Fedorak, R.N. Allopurinol to Prevent Pancreatitis After Endoscopic Retrograde Cholangiopancreatography: A Randomized Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2008, 6, 465–471. [Google Scholar] [CrossRef]
- Abbasinazari, M.; Alizadeh, A.H.M.; Moshiri, K.; Pourhoseingholi, M.A.; Zali, M.R. Does allopurinol prevent post endoscopic retrograde cholangio- pancreatography pancreatitis? A randomized double blind trial.. 2011, 49, 579–83. [Google Scholar]
- Romagnuolo, J.; Hilsden, R.; Sandha, G.S.; Cole, M.; Bass, S.; May, G.; Love, J.; Bain, V.G.; McKaigney, J.; Fedorak, R.N. Allopurinol to Prevent Pancreatitis After Endoscopic Retrograde Cholangiopancreatography: A Randomized Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2008, 6, 465–471. [Google Scholar] [CrossRef]
- Katsinelos, P.; Kountouras, J.; Chatzis, J.; Christodoulou, K.; Paroutoglou, G.; Mimidis, K.; Beltsis, A.; Zavos, C. High-dose allopurinol for prevention of post-ERCP pancreatitis: a prospective randomized double-blind controlled trial. Gastrointest. Endosc. 2005, 61, 407–415. [Google Scholar] [CrossRef]
- Martinez-Torres, H.; Rodriguez-Lomeli, X.; Davalos-Cobian, C.; Garcia-Correa, J.; Maldonado-Martinez, J.M.; Medrano-Muñoz, F.; Fuentes-Orozco, C.; Gonzalez-Ojeda, A. Oral allopurinol to prevent hyperamylasemia and acute pancreatitis after endoscopic retrograde cholangiopancreatography. World J. Gastroenterol. 2009, 15, 1600–1606. [Google Scholar] [CrossRef]
| Structure | Class | IC50 Value | Ref. |
|---|---|---|---|
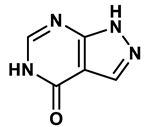 1 |
Purine derivatives | 0.2-50 μM | 19 |
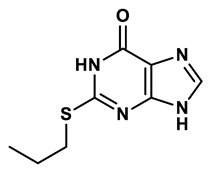 2 |
Purine derivatives | 0.115 μM | 21 |
 3 |
Purine derivatives | 0.45 μM | 22 |
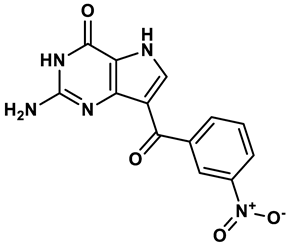 4 |
Purine derivatives | 0.065 μM | 23 |
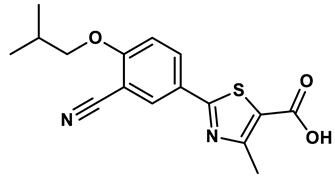 5 |
Thiazole derivative | 1.8 μM | 25 |
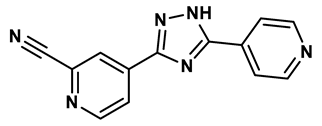 6 |
1,2,3-triazoles derivative | 0.0053 μM | 26 |
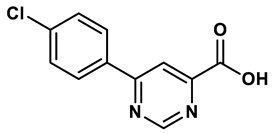 7 |
Pyrimidine derivative | 18 μM | 28 |
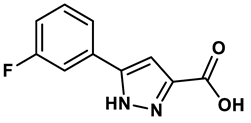 8 |
Pyrazole derivative | 30 μM | 28 |
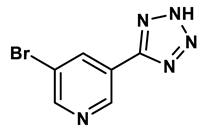 9 |
Isonicotinamide derivatives | 64 μM | 28 |
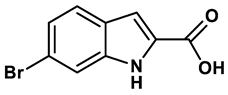 10 |
Imidazole derivatives | 82 μM | 28 |
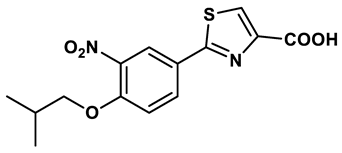 11 |
Thiazole derivative | 0.0486 μM | 29 |
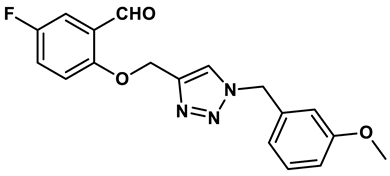 12 |
1,2,3-triazoles | 0.70 μM | 30 |
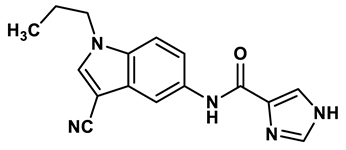 13 |
Imidazole derivatives | 0.018 μM | 31 |
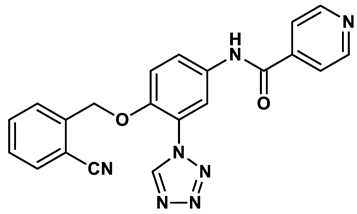 14 |
Isonicotinamide derivatives | 0.022 μM | 32 |
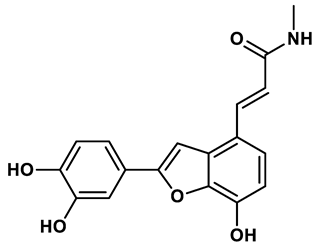 15 |
Benzo[b]furan derivatives | 4.45 μM | 33 |
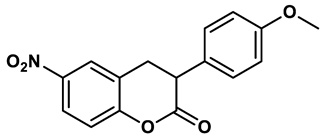 16 |
3-Phenylcoumarins derivatives | 8.4 μM | 18 |
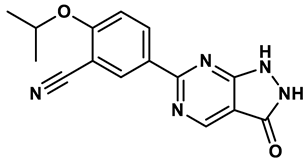 17 |
Pyrimidine derivatives | 0.039 μM | 34 |
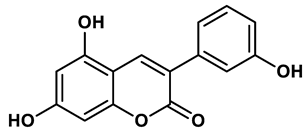 18 |
Coumarin analogues | 2.13 μM | 35 |
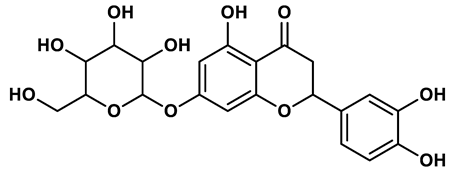 19 |
Flavonoid analogues | 10.6 μM | 41 |
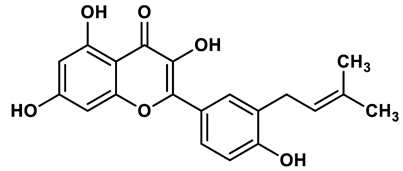 20 |
Flavonoid analogues | 8.45 μM | 42 |
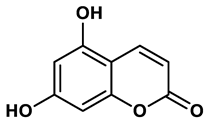 21 |
Coumarin analogues | 10.91 μM | 42 |
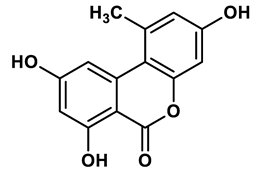 22 |
Ellagic acid analogues | 0.23 μM | 43 |
| Year | AP Model | Species | Drug | Dose | Administration | Effects | Drug effect | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1985 | CDE | Mice | Allopurinol | 24 mg/kg | Therapeutic | ↓Serum amylase, pancreatic histological damage; survival rate | Positive | 59 |
| 1985 | FFA POSS ISCH |
Dog | Allopurinol | 100 mg |
Prophylactic | ↓Serum amylase, pancreas edema; pancreatic weight | Positive | 60 |
| 1987 | Cerulein | Mice | Allopurinol | 0.7-7 mg/kg/h | Prophylactic | No effect on pancreas edema; no change in serum amylase level | Negative | 45 |
| 1988 | Cerulein | Rat | Allopurinol | 20 mg/kg/h | Therapeutic | ↓Serum amylase; pancreatic weight | Positive | 69 |
| 1989 | NaTC; CDE |
Rat; Mice | Allopurinol | 100 mg/kg for rats; 50 mg/kg for mice |
Prophylactic | No change in serum amylase/lipase; pancreatic trypsin levels; no effect on histological damage, inflammation and survival | Negative | 68 |
| 1991 | ISCH+AA | Dog | Allopurinol | 100 mg/kg | Therapeutic | ↓Serum amylase; pancreas edema; pancreatic weight; hemorrhage | Positive | 61 |
| 1992 | PBDO + ISCH | Rat | Allopurinol | 20 mg/kg | Prophylactic | ↓Serum amylase; histological changes | Positive | 58 |
| 1996 | Cerulein | Rat | Allopurinol | 50 mg/kg | Prophylactic | No change in serum amylase, lipase and trypsin level; no effect on pancreatic histological damage | Negative | 71 |
| 1998 | L-arginine | Rat | Allopurinol | 100 or 200 mg/kg | Prophylactic | ↓Serum amylase; pancreatic MDA;histological damage; catalase activity ↑SOD; GPx |
Positive | 56 |
| 1998 | NaTC | Rat | Oxypurinol | 10 mM; 0.066 ml/min | Therapeutic | ↓Pancreatic MPO, MOD ↑GSH |
Positive | 50 |
| 1998 | ERCP | Dog | Allopurinol | 5 mg/kg | Prophylactic | ↓Serum amylase and lipase; histologic changes | Positive | 63 |
| 1999 | NaTC | Rat | Oxypurinol | 10 mM; 0.066 ml/min | Therapeutic | ↓Plasma lipase, pancreatic MPO and MOD | Positive | 98 |
| 2000 | L-arginine | Rat | Allopurinol | 200 mg/kg | Prophylactic | ↓Serum amylase; pancreatic MDA; plasma CCK-like bioactivity; serum TNF-α and IL-6 levels; histological damage; ↑SOD; GPx; |
Positive | 57 |
| 2000 | L-arginine | Rat | Allopurinol | 200 mg/kg | Prophylactic | ↓Serum amylase; histologic changes; | Positive | 55 |
| 2000 | NaTC | Rat | Oxypurinol | 10 mM; 0.066 ml/min | Therapeutic | ↓Plasma lipase; pancreatic MPO | Positive | 100 |
| 2002 | Ethyl alchohol | Dog | Allopurinol | 10 mg/kg | Prophylactic | ↓Pancreatic MPO | Positive | 54 |
| 2004 | NaTC | Rat | Oxypurinol | 5 mM; 0.066 mL/min for 30 minutes | Therapeutic | ↓Serum TNF-α; lung MPO activity | Positive | 64 |
| 2006 | NaTC | Rat | Allopurinol | 200 mg/kg | Therapeutic | ↓Serum amylase; plasma MDA; histological damage ↑ Plasma SOD and GSH-Px |
Positive | 67 |
| 2007 | NaTC | Rat | Allopurinol | 200 mg/kg | Therapeutic | ↓Serum amylase; tissue MDA; histological damage ↑Tissue SOD and GSH-Px |
Positive | 65 |
| 2012 | NaTC | Rat | Oxypurinol | 5 mM, 0.066 ml/min for 30 min | Therapeutic | ↓GSSH/GSH ratio; serum lipase ↑GSSH |
Positive | 66 |
| Year | Sample size | Study design | Intervention | Clinical outcome | Ref |
|---|---|---|---|---|---|
| 2001 | 300 | RCT | Allopurinol, 40 mg, 15 h and 3 h before ERCP or prednisone, 200 mg, 15 h and 3 h before ERCP vs placebo, 15 h and 3 h before ERCP | Neither prednisone nor allopurinol showed a beneficial influence on the incidence and severity of post-ERCP pancreatitis | 104 |
| 2005 | 243 | RCT | Allopurinol, 600 mg, 15 h and 3 h before ERCP vs placebo, 15 h and 3 h before ERCP | Pretreatment with high-dose, orally administered allopurinol decreases the frequency of post-ERCP pancreatitis | 109 |
| 2005 | 701 | RCT | Allopurinol, 600 and 300 mg, 4 h and 1 h before ERCP vs placebo | Prophylactic oral allopurinol did not reduce the frequency or the severity of post-ERCP pancreatitis | 105 |
| 2008 | 586 | RCT | Allopurinol,300 mg, 1 h before ERCP vs placebo | Allopurinol does not appear to reduce the overall risk of post-ERCP pancreatitis | 108 |
| 2009 | 170 | RCT | Allopurinol, 300 mg, 15 h and 3 h before ERCP vs placebo | Oral allopurinol before ERCP decreased the incidences of hyperamylasemia and pancreatitis in patients submitted to high-risk procedures | 110 |
| 2011 | 74 | RCT | Allopurinol,300 mg, 3 h and just before ERCP vs placebo | Allopurinol does not reduce the occurrence and amylase rise of post ERCP pancreatitis | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





