Submitted:
27 October 2024
Posted:
29 October 2024
Read the latest preprint version here
Abstract
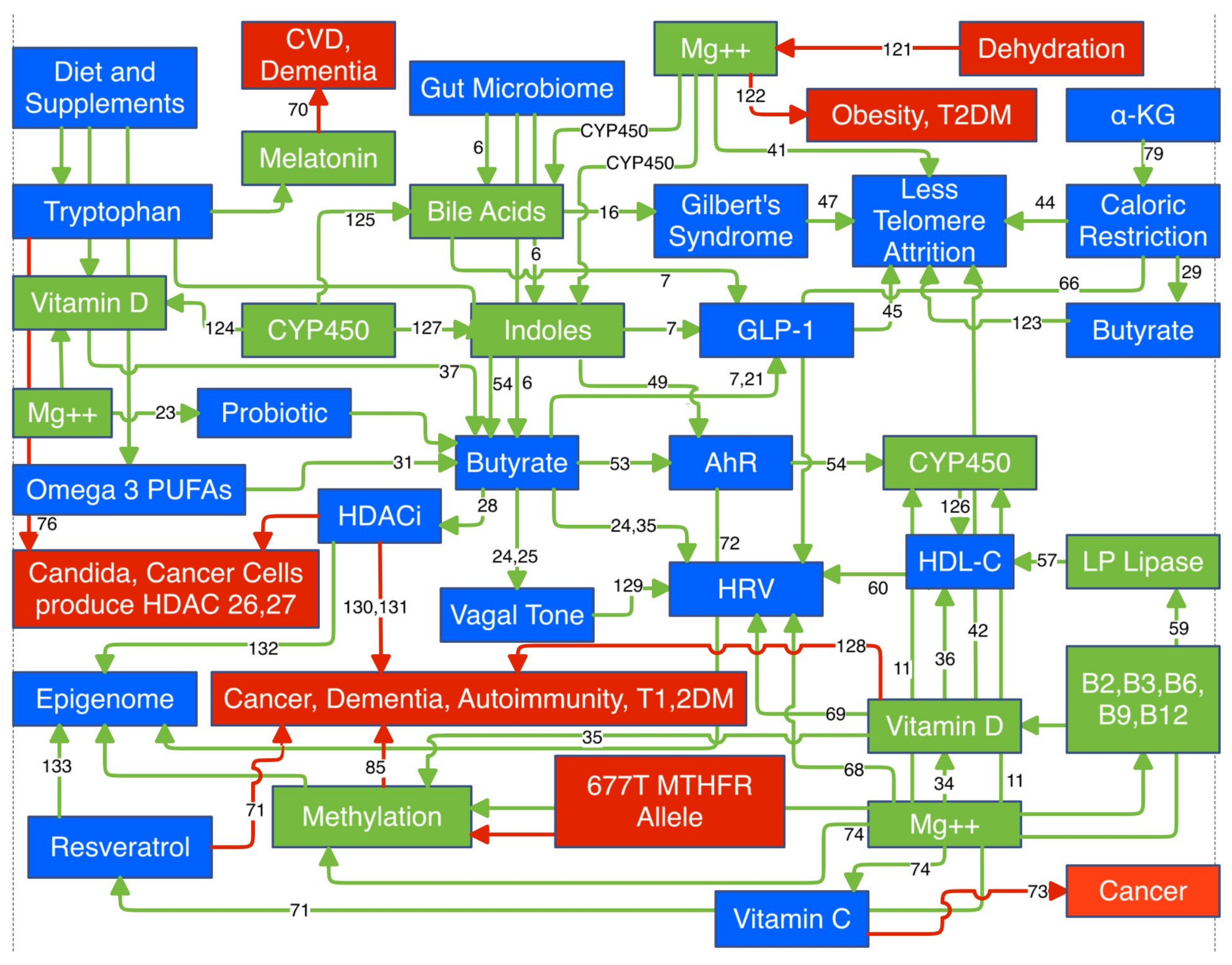
Magnesium (Mg) is not prominent among the list of well known anti-aging agents. Yet the signs and symptoms of aging mimic those of Mg deficiency. Mg is a required cofactor for over 800 enzymatic reactions (as of 2022). This review does not correlate Mg status with clinical data on agents linked to longevity. The approach is physiologic and highlights specific Mg dependent reactions required by these longevity linked biomarkers. Many of these share common pathways to extend healthspan. Mg is a required cofactor in the synthesis of vitamin D and melatonin and activation of five of the eight B vitamins. It is a required cofactor for all CYP450 enzymes. It is directly responsible for the appropriate methylation of proteins and DNA, which control the epigenome. The MTHFR (methylenetetrahydrofolate reductase) 677T allele that compromises methylation is present in a majority of Americans. Aberrant methylation predicts the severity of Covid-19 and its persistence into long Covid. Mg is a silent benefactor that may indirectly link these longevity agents, but only if viewed in context with calcium (Ca), i.e., Ca:Mg. Both compete for the same receptor. To fully exploit these longevity agents sufficient Mg is required. The pertinent physiology is presented.
Keywords:
Introduction
DISCUSSION
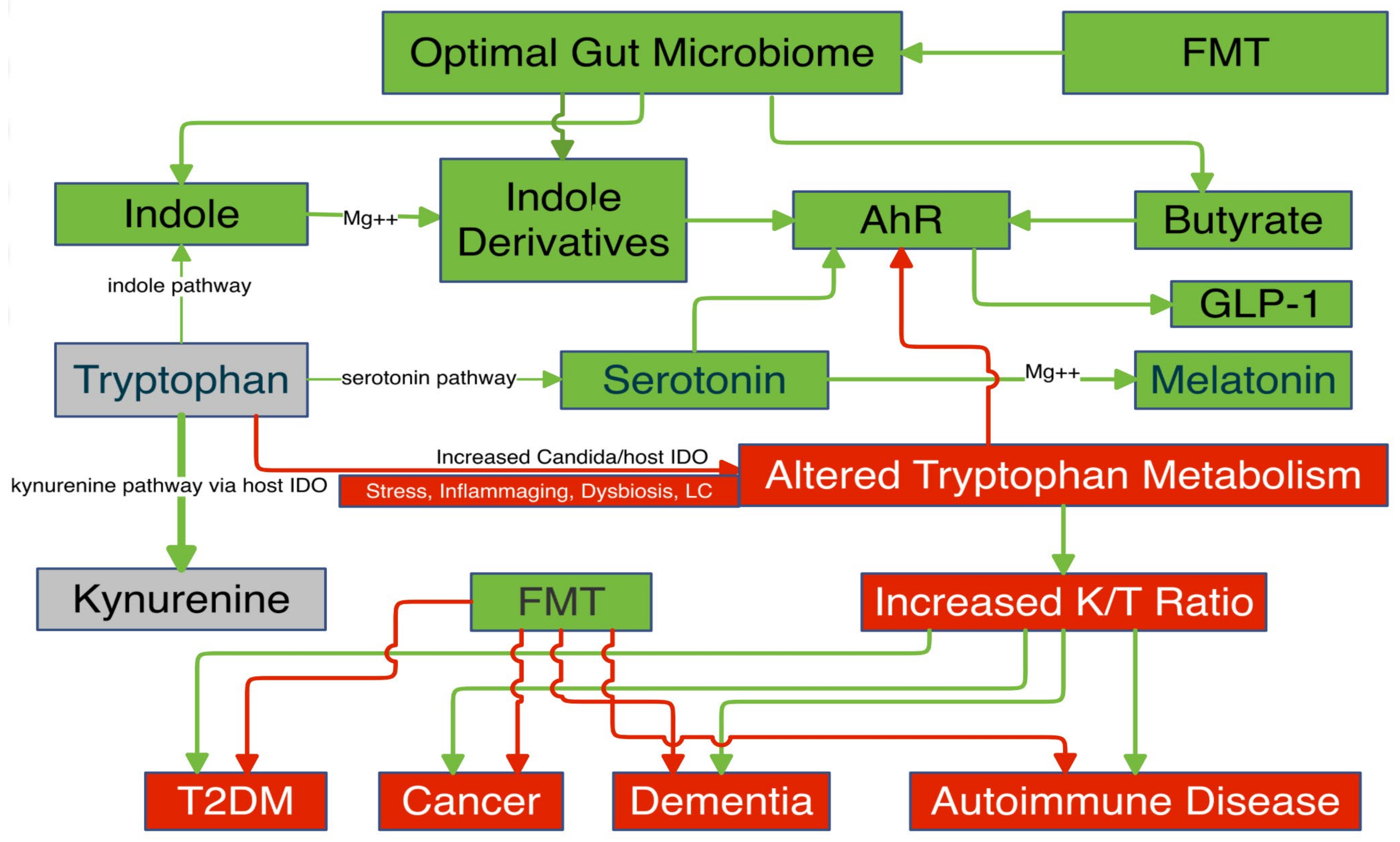
I. Gut Microbiome
A. Secondary Bile Acids
A. Indoles
A. Butyrate
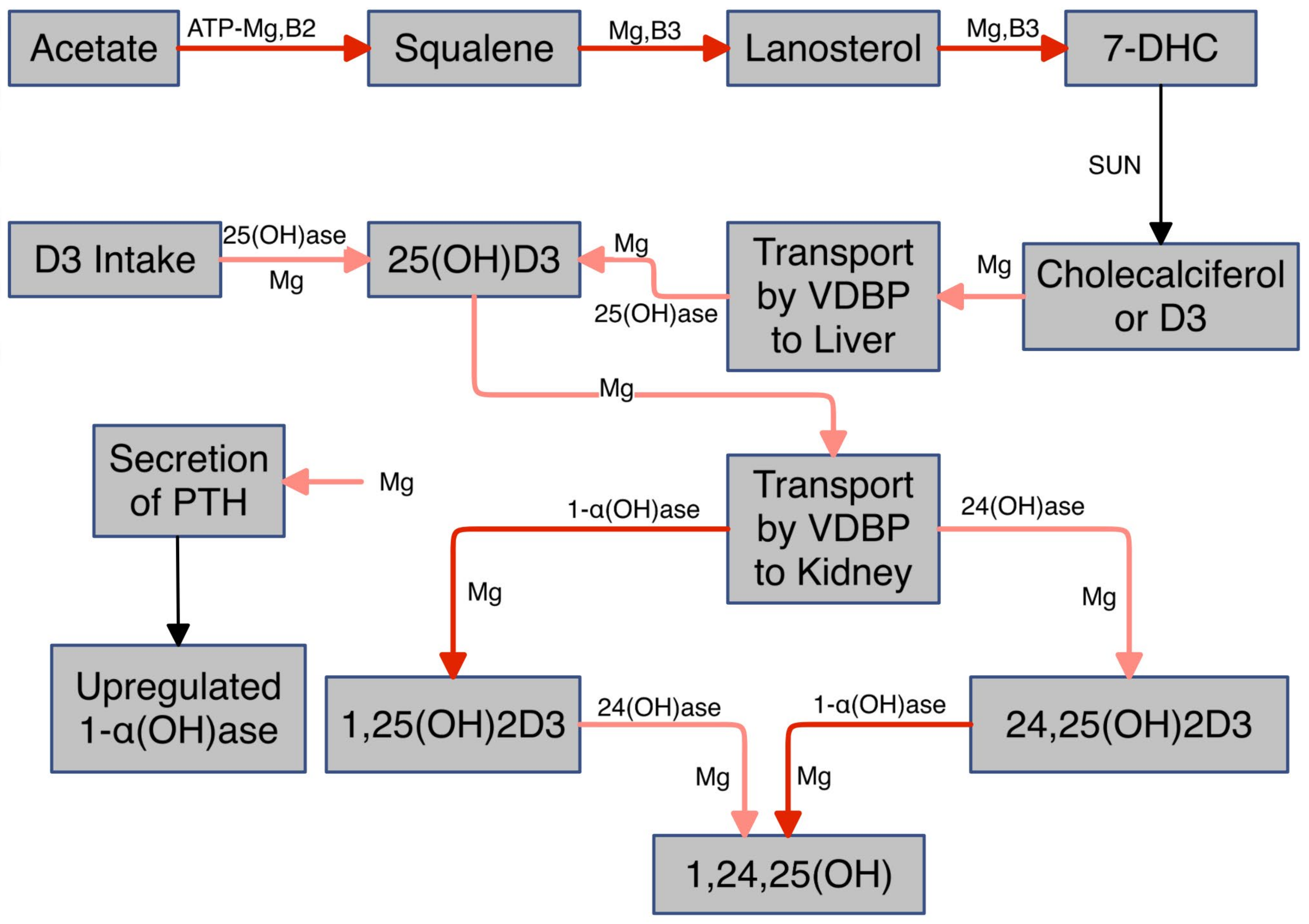
I. Vitamin D
I. Telomeres
I. AhR
I. HDL-C
I. HRV
Melatonin, Resveratrol, Vitamin C, Tryptophan, and α-Ketoglutarate
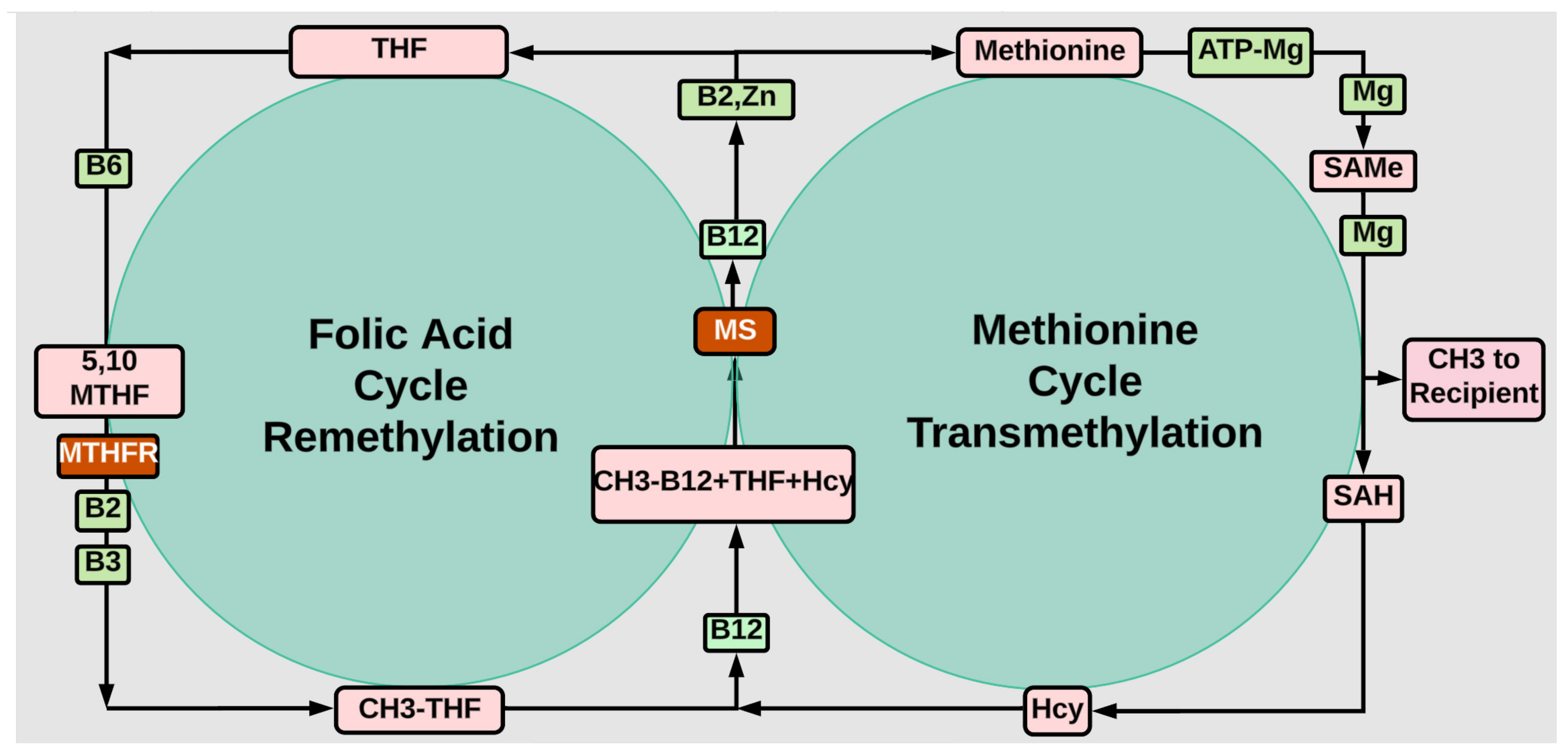
Methylation
I. Magnesium
A. Ca:Mg
A. Magnesium and Covid-19
A. Therapeutic Interventions
Conclusions
References
- Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients. 2021 Mar 30;13(4):1136. [CrossRef]
- Workinger JL, Doyle RP, Bortz J. Challenges in the Diagnosis of Magnesium Status. Nutrients. 2018 Sep 1;10(9):1202. [CrossRef]
- Dominguez LJ, Veronese N, Barbagallo M. Magnesium and the Hallmarks of Aging. Nutrients. 2024; 16(4):496. [CrossRef]
- Killilea DW, Maier JA. A connection between magnesium deficiency and aging: new insights from cellular studies. Magnes Res. 2008 Jun;21(2):77-82. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc2790427/.
- Mansmann, H.C. (1993) Consider Magnesium Homeostasis: II: Staging of Magnesium Deficiencies. Pediatric Allergy, Immunology and Pulmonology, 7, 211-215. [CrossRef]
- Jin L, Shi L, Huang W. The role of bile acids in human aging. Med Rev (2021). 2024 Mar 7;4(2):154-157. [CrossRef]
- Masse KE, Lu VB. Short-chain fatty acids, secondary bile acids and indoles: gut microbial metabolites with effects on enteroendocrine cell function and their potential as therapies for metabolic disease. Front Endocrinol (Lausanne). 2023 Jul 25;14:1169624. [CrossRef]
- Chavda VP, Balar PC, Vaghela DA, Dodiya P. Unlocking longevity with GLP-1: A key to turn back the clock? Maturitas. 2024 Aug;186:108028. [CrossRef]
- Wei Peng , Rui Zhou , Ze-Fang Sun , Jia-Wei Long , Yong-Qiang Gong. Novel Insights into the Roles and Mechanisms of GLP-1 Receptor Agonists against Aging-Related Diseases. Aging and disease. 2022, 13(2): 468-490. [CrossRef]
- Huang LY, Liu CH, Chen FY, Kuo CH, Pitrone P, Liu JS. Aging Affects Insulin Resistance, Insulin Secretion, and Glucose Effectiveness in Subjects with Normal Blood Glucose and Body Weight. Diagnostics (Basel). 2023 Jun 24;13(13):2158. [CrossRef]
- Mansmann, H.C. (1994). Consider magnesium homeostasis: III: cytochrome P450 enzymes and drug toxicity. Applied Immunohistochemistry & Molecular Morphology, 8, 7-28. [CrossRef]
- Fuchs, C.D., Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol 19, 432–450 (2022). [CrossRef]
- Wise JL, Cummings BP. The 7-α-dehydroxylation pathway: An integral component of gut bacterial bile acid metabolism and potential therapeutic target. Front Microbiol. 2023 Jan 9;13:1093420. [CrossRef]
- Vico-Oton, E., Volet, C., Jacquemin, N. et al. Strain-dependent induction of primary bile acid 7-dehydroxylation by cholic acid. BMC Microbiol 24, 286 (2024). [CrossRef]
- Ji S, Pan Y, Zhu L, Tan J, Tang S, Yang Q, Zhang Z, Lou D, Wang B. A novel 7α-hydroxysteroid dehydrogenase: Magnesium ion significantly enhances its activity and thermostability. Int J Biol Macromol. 2021 Apr 30;177:111-118. [CrossRef]
- Horsfall LJ, Nazareth I, Pereira SP, Petersen I. Gilbert’s syndrome and the risk of death: a population-based cohort study. J Gastroenterol Hepatol. 2013 Oct;28(10):1643-7. [CrossRef]
- Wagner KH, Khoei NS, Hana CA, Doberer D, Marculescu R, Bulmer AC, Hörmann-Wallner M, Mölzer C. Oxidative Stress and Related Biomarkers in Gilbert’s Syndrome: A Secondary Analysis of Two Case-Control Studies. Antioxidants (Basel). 2021 Sep 15;10(9):1474. [CrossRef]
- Matoba N, Une M, Hoshita T. Identification of unconjugated bile acids in human bile. J Lipid Res. 1986 Nov;27(11):1154-62. [CrossRef]
- Li XJ, Fang C, Zhao RH, Zou L, Miao H, Zhao YY. Bile acid metabolism in health and ageing-related diseases. Biochem Pharmacol. 2024 Jul;225:116313. [CrossRef]
- Oxenkrug G, Navrotska V. Extension of life span by down-regulation of enzymes catalyzing tryptophan conversion into kynurenine: Possible implications for mechanisms of aging. Exp Biol Med (Maywood). 2023 Apr;248(7):573-577. [CrossRef]
- Gribble, F.M., Reimann, F. Metabolic Messengers: glucagon-like peptide 1. Nat Metab 3, 142–148 (2021). [CrossRef]
- Chaudhary, P., Kathuria, D., Suri, S., Bahndral, A., & Kanthi Naveen, A. (2023). Probiotics- its functions and influence on the ageing process: A comprehensive review. Food Bioscience. [CrossRef]
- Mahboobi S, Ghasvarian M, Ghaem H, Alipour H, Alipour S, Eftekhari MH. Effects of probiotic and magnesium co-supplementation on mood, cognition, intestinal barrier function and inflammation in individuals with obesity and depressed mood: A randomized, double-blind placebo-controlled clinical trial. Front Nutr. 2022 Sep 28;9:1018357. [CrossRef]
- Seefeldt, J.M., Homilius, C., Hansen, J., Lassen, T.R., Jespersen, N.R., Jensen, R.V., et al. (2024). Short-Chain Fatty Acid Butyrate Is an Inotropic Agent With Vasorelaxant and Cardioprotective Properties. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 13. [CrossRef]
- Yu Z, Han J, Chen H, Wang Y, Zhou L, Wang M, et al. Oral Supplementation With Butyrate Improves Myocardial Ischemia/Reperfusion Injury via a Gut-Brain Neural Circuit. Front Cardiovasc Med. 2021 Sep 23;8:718674. [CrossRef]
- Su S, Li X, Yang X, Li Y, Chen X, Sun S, Jia S. Histone acetylation/deacetylation in Candida albicans and their potential as antifungal targets. Future Microbiol. 2020 Jul;15:1075-1090. [CrossRef]
- Alseksek RK, Ramadan WS, Saleh E, El-Awady R. The Role of HDACs in the Response of Cancer Cells to Cellular Stress and the Potential for Therapeutic Intervention. Int J Mol Sci. 2022 Jul 24;23(15):8141. [CrossRef]
- Yu, R., Cao, X., Sun, L. et al. Inactivating histone deacetylase HDA promotes longevity by mobilizing trehalose metabolism. Nat Commun12, 1981 (2021). [CrossRef]
- Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020 Jan 31;11:25. [CrossRef]
- Sasaki H, Hayashi K, Imamura M, Hirota Y, Hosoki H, Nitta L, et al. Combined resistant dextrin and low-dose Mg oxide administration increases short-chain fatty acid and lactic acid production by gut microbiota. J Nutr Biochem. 2023 Oct;120:109420. [CrossRef]
- Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021 Jan-Dec;13(1):1-24. [CrossRef]
- Menni, C., Zierer, J., Pallister, T. et al. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep 7, 11079 (2017). [CrossRef]
- Fantini C, Corinaldesi C, Lenzi A, Migliaccio S, Crescioli C. Vitamin D as a Shield against Aging. Int J Mol Sci. 2023 Feb 25;24(5):4546. [CrossRef]
- Rude RK, Adams JS, Ryzen E, Endres DB, Niimi H, Horst RL, Haddad JG Jr, Singer FR. Low serum concentrations of 1,25-dihydroxyvitamin D in human magnesium deficiency. J Clin Endocrinol Metab. 1985 Nov;61(5):933-40. [CrossRef]
- Ong LTC, Booth DR, Parnell GP. Vitamin D and its Effects on DNA Methylation in Development, Aging, and Disease. Mol Nutr Food Res. 2020 Dec;64(23):e2000437. [CrossRef]
- Daniel T Dibaba, Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis, Nutrition Reviews, Volume 77, Issue 12, December 2019, Pages 890–902. [CrossRef]
- Thomas, R.L., Jiang, L., Adams, J.S. et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun 11, 5997 (2020). [CrossRef]
- Kherad Z, Yazdanpanah S, Saadat F, Pakshir K, Zomorodian K. Vitamin D3: A promising antifungal and antibiofilm agent against Candida species. Curr Med Mycol. 2023 Jun;9(2):17-22. Available online: https://pubmed.ncbi.nlm.nih.gov/38375518/.
- DeBoy EA, Tassia MG, Schratz KE, Yan SM, Cosner ZL, McNally EJ, Gable DL, Xiang Z, Lombard DB, Antonarakis ES, Gocke CD, McCoy RC, Armanios M. Familial Clonal Hematopoiesis in a Long Telomere Syndrome. N Engl J Med. 2023 Jun 29;388(26):2422-2433. [CrossRef]
- Ye Q, Apsley AT, Etzel L, Hastings WJ, Kozlosky JT, Walker C, et al. Telomere length and chronological age across the human lifespan: A systematic review and meta-analysis of 414 study samples including 743,019 individuals. Ageing Res Rev. 2023 Sep;90:102031. [CrossRef]
- Maguire D, Neytchev O, Talwar D, McMillan D, Shiels PG. Telomere Homeostasis: Interplay with Magnesium. Int J Mol Sci. 2018 Jan 5;19(1):157. [CrossRef]
- Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, et al., Swaminathan R, Spector TD, Aviv A. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007 Nov;86(5):1420-5. [CrossRef]
- Hu L, Bai Y, Hu G, Zhang Y, Han X, Li J. Association of Dietary Magnesium Intake With Leukocyte Telomere Length in United States Middle-Aged and Elderly Adults. Front Nutr. 2022 May 19;9:840804. [CrossRef]
- Hastings WJ, Ye Q, Wolf SE, Ryan CP, Das SK, Huffman KM, Kobor MS, Kraus WE, MacIsaac JL, Martin CK, Racette SB, Redman LM, Belsky DW, Shalev I. Effect of long-term caloric restriction on telomere length in healthy adults: CALERIE™ 2 trial analysis. Aging Cell. 2024 Jun;23(6):e14149. [CrossRef]
- Ridout KK, Syed SA, Kao HT, Porton B, Rozenboym AV, Tang J, et al. Relationships Between Telomere Length, Plasma Glucagon-like Peptide 1, and Insulin in Early-Life Stress-Exposed Nonhuman Primates. Biol Psychiatry Glob Open Sci. 2021 Aug 3;2(1):54-60. [CrossRef]
- Wang J, Dong X, Cao L, Sun Y, Qiu Y, Zhang Y, Cao R, Covasa M, Zhong L. Association between telomere length and diabetes mellitus: A meta-analysis. J Int Med Res. 2016 Dec;44(6):1156-1173. [CrossRef]
- Tosevska A, Moelzer C, Wallner M, Janosec M, Schwarz U, Kern C, et al. Longer telomeres in chronic, moderate, unconjugated hyperbilirubinaemia: insights from a human study on Gilbert’s Syndrome. Sci Rep. 2016 Mar 1;6:22300. [CrossRef]
- Dogan F, Forsyth NR. Telomerase Regulation: A Role for Epigenetics. Cancers (Basel). 2021 Mar 10;13(6):1213. [CrossRef]
- Ojo ES, Tischkau SA. The Role of AhR in the Hallmarks of Brain Aging: Friend and Foe. Cells. 2021 Oct 13;10(10):2729. [CrossRef]
- Wang Z, Snyder M, Kenison JE, Yang K, Lara B, Lydell E, et al. How the AhR Became Important in Cancer: The Role of Chronically Active AhR in Cancer Aggression. Int J Mol Sci. 2020 Dec 31;22(1):387. [CrossRef]
- Zhu K, Meng Q, Zhang Z, Yi T, He Y, Zheng J, Lei W. Aryl hydrocarbon receptor pathway: Role, regulation and intervention in atherosclerosis therapy (Review). Mol Med Rep. 2019 Dec;20(6):4763-4773. [CrossRef]
- Seo, SK., Kwon, B. Immune regulation through tryptophan metabolism.Exp Mol Med 55, 1371–1379 (2023). [CrossRef]
- Marinelli, L., Martin-Gallausiaux, C., Bourhis, JM. et al. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep 9, 643 (2019). [CrossRef]
- Li X, Zhang B, Hu Y, Zhao Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front Pharmacol. 2021 Dec 13;12:769501. [CrossRef]
- Rahilly-Tierney C, Sesso HD, Michael Gaziano J, Djoussé L. High-density lipoprotein and mortality before age 90 in male physicians. Circ Cardiovasc Qual Outcomes. 2012 May;5(3):381-6. [CrossRef]
- Franczyk B, Rysz J, Ławiński J, Rysz-Górzyńska M, Gluba-Brzózka A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines. 2021; 9(9):1083. [CrossRef]
- Feng M, Darabi M, Tubeuf E, Canicio A, Lhomme M, Frisdal E, et al. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol. 2020 Oct;27(15):1606-1616. [CrossRef]
- Basu D, Goldberg IJ. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr Opin Lipidol. 2020 Jun;31(3):154-160. [CrossRef]
- Mathew AA, Panonnummal R. ‘Magnesium’-the master cation-as a drug-possibilities and evidences. Biometals. 2021 Oct;34(5):955-986. [CrossRef]
- Balikai FA, Javali SB, Shindhe VM, Deshpande N, Benni JM, Shetty DP, et al. Correlation of serum HDL level with HRV indices using multiple linear regression analysis in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2022 Aug;190:109988. [CrossRef]
- Lin, S., Lee, I.H., Tsai, H.C., Chi, M.H., Chang, W.H., Chen, P.S., Chen, K., & Yang, Y. (2019). The association between plasma cholesterol and the effect of tryptophan depletion on heart rate variability. The Kaohsiung Journal of Medical Sciences, 35, 440—445. [CrossRef]
- Hernández-Vicente A, Hernando D, Santos-Lozano A, Rodríguez-Romo G, Vicente-Rodríguez G, Pueyo E, et al. Heart Rate Variability and Exceptional Longevity. Front Physiol. 2020 Sep 17;11:566399. [CrossRef]
- Jarczok MN, Weimer K, Braun C, Williams DP, Thayer JF, Gündel HO, Balint EM. Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations. Neurosci Biobehav Rev. 2022 Dec;143:104907. [CrossRef]
- Gidron Y, Deschepper R, De Couck M, Thayer JF, Velkeniers B. The Vagus Nerve Can Predict and Possibly Modulate Non-Communicable Chronic Diseases: Introducing a Neuroimmunological Paradigm to Public Health. J Clin Med. 2018 Oct 19;7(10):371. [CrossRef]
- Goswami C, Iwasaki Y, Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem. 2018 Jul;57:130-135. [CrossRef]
- 66 Nicoll R, Henein MY. Caloric Restriction and Its Effect on Blood Pressure, Heart Rate Variability and Arterial Stiffness and Dilatation: A Review of the Evidence. Int J Mol Sci. 2018 Mar 7;19(3):751. [CrossRef]
- Tsubokawa M, Nishimura M, Mikami T, Ishida M, Hisada T, Tamada Y. Association of Gut Microbial Genera with Heart Rate Variability in the General Japanese Population: The Iwaki Cross-Sectional Research Study. Metabolites. 2022 Aug 7;12(8):730. [CrossRef]
- Kim YH, Jung KI, Song CH. Effects of serum calcium and magnesium on heart rate variability in adult women. Biol Trace Elem Res. 2012 Dec;150(1-3):116-22. [CrossRef]
- Lopresti AL. Association between Micronutrients and Heart Rate Variability: A Review of Human Studies. Adv Nutr. 2020 May 1;11(3):559-575. [CrossRef]
- Martín Giménez VM, de Las Heras N, Lahera V, Tresguerres JAF, Reiter RJ, Manucha W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front Aging Neurosci. 2022 Jun 3;14:888292. [CrossRef]
- Meng X, Zhou J, Zhao CN, Gan RY, Li HB. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods. 2020 Mar 14;9(3):340. [CrossRef]
- Abudahab S, Price ET, Dozmorov MG, Deshpande LS, McClay JL. The Aryl Hydrocarbon Receptor, Epigenetics and the Aging Process. J Nutr Health Aging. 2023;27(4):291-300. [CrossRef]
- Roa FJ, Peña E, Gatica M, Escobar-Acuña K, Saavedra P, Maldonado M, Cuevas ME, Moraga-Cid G, Rivas CI, Muñoz-Montesino C. Therapeutic Use of Vitamin C in Cancer: Physiological Considerations. Front Pharmacol. 2020 Mar 3;11:211. [CrossRef]
- Cho S, Chae JS, Shin H, Shin Y, Kim Y, Kil EJ, Byun HS, Cho SH, Park S, Lee S, Yeom CH. Enhanced Anticancer Effect of Adding Magnesium to Vitamin C Therapy: Inhibition of Hormetic Response by SVCT-2 Activation. Transl Oncol. 2020 Feb;13(2):401-409. [CrossRef]
- Dang, H., Castro-Portuguez, R., Espejo, L. et al. On the benefits of the tryptophan metabolite 3-hydroxyanthranilic acid in Caenorhabditis elegans and mouse aging. Nat Commun 14, 8338 (2023). [CrossRef]
- Bozza, S, Fallarino, F, Pitzurra, L, Zelante, T, Montagnoli, C, Bellocchio, S, et al.; A Crucial Role for Tryptophan Catabolism at the Host/Candida albicans Interface. J Immunol 1 March 2005; 174 (5): 2910–2918. [CrossRef]
- Chambers, PW, Hyphae and Healthspan: Hypothesis. ResearchGate June (2024). [CrossRef]
- Xue C, Li G, Zheng Q, Gu X, Shi Q, Su Y, et al. Tryptophan metabolism in health and disease. Cell Metab. 2023 Aug 8;35(8):1304-1326. [CrossRef]
- Naeini SH, Mavaddatiyan L, Kalkhoran ZR, Taherkhani S, Talkhabi M. Alpha-ketoglutarate as a potent regulator for lifespan and healthspan: Evidences and perspectives. Exp Gerontol. 2023 May;175:112154. [CrossRef]
- Abraham KJ, Chan JN, Salvi JS, Ho B, Hall A, Vidya E, Guo R, Killackey SA, Liu N, Lee JE, Brown GW, Mekhail K. Intersection of calorie restriction and magnesium in the suppression of genome-destabilizing RNA-DNA hybrids. Nucleic Acids Res. 2016 Oct 14;44(18):8870-8884. [CrossRef]
- Cox MF, Hascup ER, Bartke A, Hascup KN. Friend or Foe? Defining the Role of Glutamate in Aging and Alzheimer’s Disease. Front Aging. 2022 Jun 16;3:929474. [CrossRef]
- Seale, K., Horvath, S., Teschendorff, A. et al. Making sense of the ageing methylome. Nat Rev Genet 23, 585–605 (2022)2). [CrossRef]
- Salameh Y, Bejaoui Y, El Hajj N. DNA Methylation Biomarkers in Aging and Age-Related Diseases. Front Genet. 2020 Mar 10;11:171. [CrossRef]
- Horvath, S., Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet 19, 371–384 (2018). [CrossRef]
- Milicic L, Porter T, Vacher M, Laws SM. Utility of DNA Methylation as a Biomarker in Aging and Alzheimer’s Disease. J Alzheimers Dis Rep. 2023 May 31;7(1):475-503. [CrossRef]
- MTHFR Gene Variant and Folic Acid Facts. Available online: https://www.cdc.gov/folic-acid/data-research/mthfr/index.
- Moll S and Varga E, Homocysteine and MTHFRMutations, Circulation 132(1). [CrossRef]
- Barbagallo M, Veronese N, Dominguez LJ. Magnesium in Aging, Health and Diseases. Nutrients. 2021; 13(2):463. [CrossRef]
- Zhang C, Zhang T, Zou J, Miller CL, Gorkhali R, Yang JY, et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci Adv. 2016 May 27;2(5):e1600241. [CrossRef]
- Ferrè S, Hoenderop JG, Bindels RJ. Sensing mechanisms involved in Ca2+ and Mg2+ homeostasis. Kidney Int. 2012 Dec;82(11):1157-66. [CrossRef]
- Hardwick LL, Jones MR, Brautbar N, Lee DB. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991 Jan;121(1):13-23. [CrossRef]
- Rosanoff, A., Dai, Q. and Shapses, S.A. (2016) Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Advances in Nutrition, 7, 25-43. [CrossRef]
- Ashique S, Kumar S, Hussain A, Mishra N, Garg A, Gowda BHJ, et al. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J Health Popul Nutr. 2023 Jul 27;42(1):74. [CrossRef]
- Yang Z, Zhang Y, Gao J, Yang Q, Qu H, Shi J. Association between dietary magnesium and 10-year risk of a first hard atherosclerotic cardiovascular disease event. Am J Med Sci. 2024 May 26:S0002-9629(24)01261-8. [CrossRef]
- Du K, Zheng X, Ma ZT, Lv JY, Jiang WJ, Liu MY. Association of Circulating Magnesium Levels in Patients With Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front Aging Neurosci. 2022 Jan 10;13:799824. [CrossRef]
- Deng, X., Song, Y., Manson, J.E. et al. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med 11, 187 (2013). [CrossRef]
- Costello R.B., Elin R.J., Rosanoff A., Wallace T.C., Guerrero-Romero F., Hruby A., Lutsey P.L., Nielsen F.H., Rodriguez-Moran M., Song Y., et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016;7:977–993. [CrossRef]
- Razzaque MS. Magnesium: Are We Consuming Enough? Nutrients. 2018; 10(12):1863. [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010 Dec;23(4):S194-8. [CrossRef]
- Micke, O., Vormann, J., Kraus, A. and Kisters, K. (2021) Serum Magnesium: Time for a Standardized and Evidence-Based Reference Range. Magnetic Resonance, 34, 84-89. Available online: https://www.magnesium-ges.de/Micke_et_al._2021.pdf.
- Rosanoff A, West C, Elin RJ, Micke O, Baniasadi S, Barbagallo M, et al. MaGNet Global Magnesium Project (MaGNet). Recommendation on an updated standardization of serum magnesium reference ranges. Eur J Nutr. 2022 Oct;61(7):3697-3706. [CrossRef]
- Weiss D, Brunk DK, Goodman DA. Scottsdale Magnesium Study: Absorption, Cellular Uptake, and Clinical Effectiveness of a Timed-Release Magnesium Supplement in a Standard Adult Clinical Population. J Am Coll Nutr. 2018 May-Jun;37(4):316-327. [CrossRef]
- Hoy MK, Goldman JD. Calcium intake of the U.S. population: What We Eat in America, NHANES 2009-2010. 2014 Sep. Available online: https://www.ncbi.nlm.nih.gov/books/NBK589560/.
- Shlisky J, Mandlik R, Askari S, Abrams S, Belizan JM, Bourassa MW, Cormick G, Driller-Colangelo A, Gomes F, Khadilkar A, Owino V, Pettifor JM, Rana ZH, Roth DE, Weaver C. Calcium deficiency worldwide: prevalence of inadequate intakes and associated health outcomes. Ann N Y Acad Sci. 2022 Jun;1512(1):10-28. [CrossRef]
- Guerrero-Romero F, Mercado M, Rodriguez-Moran M, et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients. 2022 Apr;14(9):1686. [CrossRef]
- La Carrubba A, Veronese N, Di Bella G, Cusumano C, Di Prazza A, Ciriminna S, et al. Prognostic Value of Magnesium in COVID-19: Findings from the COMEPA Study. Nutrients. 2023 Feb 6;15(4):830. [CrossRef]
- Fan L, Zhu X, Zheng Y, Zhang W, Seidner DL, Ness R, Murff HJ, Yu C, Huang X, Shrubsole MJ, Hou L, Dai Q. Magnesium treatment on methylation changes of transmembrane serine protease 2 (TMPRSS2). Nutrition. 2021 Sep;89:111340. [CrossRef]
- Balnis J, Madrid A, Drake LA, Vancavage R, Tiwari A, Patel VJ, et al. Blood DNA methylation in post-acute sequelae of COVID-19 (PASC): a prospective cohort study. EBioMedicine. 2024 Aug;106:105251. [CrossRef]
- Ponti G, Pastorino L, Manfredini M, Ozben T, Oliva G, Kaleci S, Iannella R, Tomasi A. COVID-19 spreading across world correlates with C677T allele of the smethylenetetrahydrofolate reductase (MTHFR) gene prevalence. J Clin Lab Anal. 2021 Jul;35(7):e23798. [CrossRef]
- Abraham, G.E.; Schwartz, U.D.; Lubran, M.M. Effect of vitamin B-6 on plasma and red blood cell magnesium levels in premenopausal women. Ann. Clin. Lab. Sci. 1981, 11, 333–336. Available online: https://pubmed.ncbi.nlm.nih.gov/7271227/.
- Boylan, L.M.; Spallholz, J.E. In vitro evidence for a relationship between magnesium and vitamin B-6. Magnes. Res. 1990, 3, 79–85. Available online: https://pubmed.ncbi.nlm.nih.gov/2133627/.
- Noah, L., Pickering, G., Dubray, C., Mazur, A., Hitier, S., & Pouteau, E. (2020). Effect of vitamin B6 supplementation, in combination with magnesium, on severe stress and magnesium status: Secondary analysis from an RCT. Proceedings of the Nutrition Society, 79(OCE2), E491. [CrossRef]
- Pouteau E, Kabir-Ahmadi M, Noah L, Mazur A, Dye L, Hellhammer J, Pickering G, Dubray C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018 Dec 18;13(12):e0208454. [CrossRef]
- Eisinger J, Dagorn J. Vitamin B6 and magnesium. Magnesium. 1986;5(1):27-32. Available online: https://pubmed.ncbi.nlm.nih.gov/3959594/.
- Planells E, Lerma A, Sánchez-Morito N, Aranda P, LLopis J. Effect of magnesium deficiency on vitamin B2 and B6 status in the rat. J Am Coll Nutr. 1997 Aug;16(4):352-6. [CrossRef]
- Vrolijk MF, Opperhuizen A, Jansen EHJM, Hageman GJ, Bast A, Haenen GRMM. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol In Vitro. 2017 Oct;44:206-212. [CrossRef]
- Mikkelsen K, Apostolopoulos V. B Vitamins and Ageing. Subcell Biochem. 2018;90:451-470. [CrossRef]
- Stoff R, Wolf Y, Boursi B. Fecal Microbiota Transplantation as a Cancer Therapeutic. Cancer J. 2023 Mar-Apr 01;29(2):102-108. [CrossRef]
- Liu X, Liu M, Zhao M, Li P, Gao C, Fan X, Cai G, Lu Q, Chen X. Fecal microbiota transplantation for the management of autoimmune diseases: Potential mechanisms and challenges. J Autoimmun. 2023 Dec;141:103109. [CrossRef]
- Wang, H., Yang, F., Zhang, S. et al. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. npj Parkinsons Dis. 7, 70 (2021). [CrossRef]
- Matsuoka, H. (2005) Aldosterone and Magnesium. Clinical Calcium, 15, 187-191. Available online: https://pubmed.ncbi.nlm.nih.gov/15692156/.
- Piuri G, Zocchi M, Della Porta M, Ficara V, Manoni M, Zuccotti GV, Pinotti L, Maier JA, Cazzola R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients. 2021 Jan 22;13(2):320. [CrossRef]
- Dan J, Yang J, Liu Y, Xiao A, Liu L. Roles for Histone Acetylation in Regulation of Telomere Elongation and Two-cell State in Mouse ES Cells. J Cell Physiol. 2015 Oct;230(10):2337-44. [CrossRef]
- Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014 Jan;55(1):13-31. [CrossRef]
- Chen J, Zhao KN, Chen C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann Transl Med. 2014 Jan;2(1):7. [CrossRef]
- Pikuleva, I.A. Cholesterol-metabolizing cytochromes P450: implications for cholesterol lowering. Expert Opin Drug Metab Toxicol. 2008 Nov;4(11):1403-14. [CrossRef]
- Li X, Zhang B, Hu Y, Zhao Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front Pharmacol. 2021 Dec 13;12:769501. [CrossRef]
- Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmunity Reviews. 2013 Aug;12(10):976-989. [CrossRef]
- Laborde S, Mosley E, Thayer JF. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front Psychol. 2017 Feb 20;8:213. [CrossRef]
- Shanmukha KD, Paluvai H, Lomada SK, Gokara M, Kalangi SK. Histone deacetylase (HDACs) inhibitors: Clinical applications. Prog Mol Biol Transl Sci. 2023;198:119-152. [CrossRef]
- Banik D, Moufarrij S, Villagra A. Immunoepigenetics Combination Therapies: An Overview of the Role of HDACs in Cancer Immunotherapy. International Journal of Molecular Sciences. 2019; 20(9):2241. [CrossRef]
- Guha, S., Jagadeesan, Y., Pandey, M.M., Mittal, A., & Chitkara, D. (2024). Targeting the epigenome with advanced delivery strategies for epigenetic modulators. Bioengineering & Translational Medicine. [CrossRef]
- Zhang S, Kiarasi F. Therapeutic effects of resveratrol on epigenetic mechanisms in age-related diseases: A comprehensive review. Phytother Res. 2024 May;38(5):2347-2360. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




