Submitted:
24 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biological functions of Inositols in female reproductive system
3. Clinical Applications of Inositols in fertility treatments and healthy pregnancy
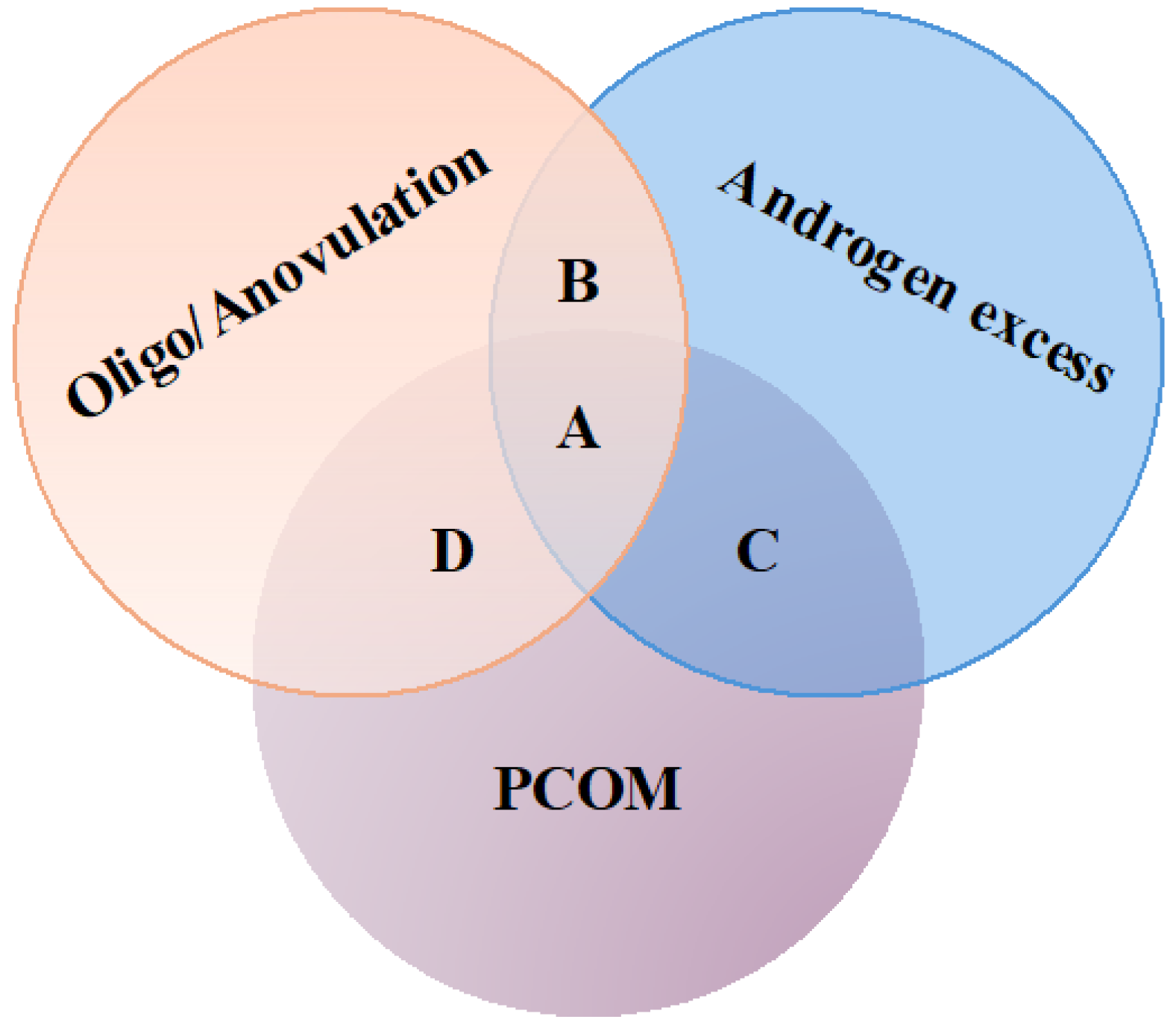
3.1. Inositols and Polycystic Ovary Syndrome (PCOS)
3.2. Inositols in ovarian response to hormonal stimulation by exogenous gonadotropins during Assisted Reproductive Technologies (ART)
3.3. Impact of inositols administration on gestational diabetes mellitus
4. Dietary intake, safety and tolerability of Inositols
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ombelet, W. WHO Fact Sheet on Infertility Gives Hope to Millions of Infertile Couples Worldwide. Facts Views Vis Obgyn 12, 249–251.
- Joardar, S.; Duarah, P.; Purkait, M.K. Recent Advances in Myo-Inositol Recovery and Purification from Agricultural Sources as Potential Dietary Supplements: A Review. Sustainable Chemistry and Pharmacy 2023, 36, 101331. [Google Scholar] [CrossRef]
- Brusco, G.F.; Mariani, M. Inositol: Effects on Oocyte Quality in Patients Undergoing ICSI. An Open Study. Eur Rev Med Pharmacol Sci 2013, 17, 3095–3102. [Google Scholar] [PubMed]
- Zacchè, M.M.; Caputo, L.; Filippis, S.; Zacchè, G.; Dindelli, M.; Ferrari, A. Efficacy of Myo-Inositol in the Treatment of Cutaneous Disorders in Young Women with Polycystic Ovary Syndrome. Gynecological Endocrinology 2009, 25, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Carlomagno, G.; Rizzo, P.; Raffone, E.; Roseff, S. Myo-Inositol Rather than D-Chiro-Inositol Is Able to Improve Oocyte Quality in Intracytoplasmic Sperm Injection Cycles. A Prospective, Controlled, Randomized Trial. Eur Rev Med Pharmacol Sci 2011, 15, 452–457. [Google Scholar] [PubMed]
- Granata, R.; Settanni, F.; Biancone, L.; Trovato, L.; Nano, R.; Bertuzzi, F.; Destefanis, S.; Annunziata, M.; Martinetti, M.; Catapano, F.; et al. Acylated and Unacylated Ghrelin Promote Proliferation and Inhibit Apoptosis of Pancreatic Beta-Cells and Human Islets: Involvement of 3’,5’-Cyclic Adenosine Monophosphate/Protein Kinase A, Extracellular Signal-Regulated Kinase 1/2, and Phosphatidyl Inositol 3-Kinase/Akt Signaling. Endocrinology 2007, 148, 512–529. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Carlomagno, G.; Gerli, S.; Montanino Oliva, M.; Devroey, P.; Lanzone, A.; Soulange, C.; Facchinetti, F.; Carlo Di Renzo, G.; Bizzarri, M.; et al. Results from the International Consensus Conference on Myo-Inositol and D-Chiro-Inositol in Obstetrics and Gynecology--Assisted Reproduction Technology. Gynecol Endocrinol 2015, 31, 441–446. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol Metab 2018, 29, 768–780. [Google Scholar] [CrossRef]
- Cheang, K.I.; Baillargeon, J.-P.; Essah, P.A.; Ostlund, R.E.; Apridonize, T.; Islam, L.; Nestler, J.E. Insulin-Stimulated Release of D-Chiro-Inositol-Containing Inositolphosphoglycan Mediator Correlates with Insulin Sensitivity in Women with Polycystic Ovary Syndrome. Metabolism 2008, 57, 1390–1397. [Google Scholar] [CrossRef]
- Chiu, T.T.Y.; Rogers, M.S.; Briton-Jones, C.; Haines, C. Effects of Myo-Inositol on the in-Vitro Maturation and Subsequent Development of Mouse Oocytes. Hum Reprod 2003, 18, 408–416. [Google Scholar] [CrossRef]
- Chiu, T.T.Y.; Rogers, M.S.; Law, E.L.K.; Briton-Jones, C.M.; Cheung, L.P.; Haines, C.J. Follicular Fluid and Serum Concentrations of Myo-Inositol in Patients Undergoing IVF: Relationship with Oocyte Quality. Hum Reprod 2002, 17, 1591–1596. [Google Scholar] [CrossRef]
- Zuluaga, A.M.; Mena-García, A.; Soria Monzón, A.C.; Rada-Mendoza, M.; Chito, D.M.; Ruiz-Matute, A.I.; Sanz, M.L. Microwave Assisted Extraction of Inositols for the Valorization of Legume By-Products. LWT 2020, 133, 109971. [Google Scholar] [CrossRef]
- Gambioli, R.; Forte, G.; Aragona, C.; Bevilacqua, A.; Bizzarri, M.; Unfer, V. The Use of D-Chiro-Inositol in Clinical Practice. Eur Rev Med Pharmacol Sci 2021, 25, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Beemster, P.; Groenen, P.; Steegers-Theunissen, R. Involvement of Inositol in Reproduction. Nutr Rev 2002, 60, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Larner, J. D-Chiro-Inositol--Its Functional Role in Insulin Action and Its Deficit in Insulin Resistance. Int J Exp Diabetes Res 2002, 3, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Uarquin, F.; Kenéz, Á.; Rodehutscord, M.; Huber, K. Dietary Phytase and Myo-Inositol Supplementation Are Associated with Distinct Plasma Metabolome Profile in Broiler Chickens. Animal 2020, 14, 549–559. [Google Scholar] [CrossRef]
- Clements, R.; Darnell, B. Myo-Inositol Content of Common Foods: Development of a High-Myo-Inositol Diet. The American journal of clinical nutrition 1980, 33, 1954–1967. [Google Scholar] [CrossRef]
- Dikeman, C.; Bauer, L.; Fahey, G. Carbohydrate Composition of Selected Plum/Prune Preparations. Journal of agricultural and food chemistry 2004, 52, 853–859. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Krakowska, A.; Górecki, R.; Buszewski, B. Simultaneous Determination of Cyclitols and Sugars Following a Comprehensive Investigation of 40 Plants. Food Anal. Methods 2019, 12, 1466–1478. [Google Scholar] [CrossRef]
- Soria, A.C.; Sanz, M.L.; Villamiel, M. Determination of Minor Carbohydrates in Carrot (Daucus Carota L.) by GC–MS. Food Chemistry 2009, 114, 758–762. [Google Scholar] [CrossRef]
- Indyk, H.E.; Saldo, S.C.; White, P.M.; Dole, M.N.; Gill, B.D.; Woollard, D.C. The Free and Total Myo-Inositol Contents of Early Lactation and Seasonal Bovine Milk. International Dairy Journal 2016, 56, 33–37. [Google Scholar] [CrossRef]
- Byun, S.M.; Jenness, R. Estimation of Free Myo-Inositol in Milks of Various Species and Its Source in Milk of Rats (Rattus Norvegicus). J Dairy Sci 1982, 65, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aceituno, L.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Ramos, L.; Soria, A.C.; Sanz, M.L. Optimisation of a Biotechnological Procedure for Selective Fractionation of Bioactive Inositols in Edible Legume Extracts. Journal of the Science of Food and Agriculture 2013, 93, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Chiu, T.T.Y.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The Rationale of the Myo-Inositol and D-Chiro-Inositol Combined Treatment for Polycystic Ovary Syndrome. J Clin Pharmacol 2014, 54, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, I.; Michikawa, T.; Mikoshiba, K.; Ikura, M. Structural Insights into the Regulatory Mechanism of IP3 Receptor. Biochim Biophys Acta 2004, 1742, 89–102. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Reiter, E.; Crépieux, P. FSH Receptor Signaling: Complexity of Interactions and Signal Diversity. Endocrinology 2018, 159, 3020–3035. [Google Scholar] [CrossRef]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr Rev 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Cogram, P.; Hynes, A.; Dunlevy, L.P.E.; Greene, N.D.E.; Copp, A.J. Specific Isoforms of Protein Kinase C Are Essential for Prevention of Folate-Resistant Neural Tube Defects by Inositol. Hum Mol Genet 2004, 13, 7–14. [Google Scholar] [CrossRef]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.E.; Copp, A.J. Neural Tube Closure: Cellular, Molecular and Biomechanical Mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef]
- Unfer, V.; Dinicola, S.; Laganà, A.S.; Bizzarri, M. Altered Ovarian Inositol Ratios May Account for Pathological Steroidogenesis in PCOS. Int J Mol Sci 2020, 21, 7157. [Google Scholar] [CrossRef]
- Merviel, P.; James, P.; Bouée, S.; Le Guillou, M.; Rince, C.; Nachtergaele, C.; Kerlan, V. Impact of Myo-Inositol Treatment in Women with Polycystic Ovary Syndrome in Assisted Reproductive Technologies. Reprod Health 2021, 18, 13. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S.; Camajani, E. The 40:1 Myo-Inositol/D-Chiro-Inositol Plasma Ratio Is Able to Restore Ovulation in PCOS Patients: Comparison with Other Ratios. Eur Rev Med Pharmacol Sci 2019, 23, 5512–5521. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.H.; Russo, M.; Dinicola, S.; Forte, G.; Unfer, V. Questioning PCOS Phenotypes for Reclassification and Tailored Therapy. Trends in Endocrinology & Metabolism 2023, 34, 694–703. [Google Scholar] [CrossRef]
- Papaleo, E.; Unfer, V.; Baillargeon, J.-P.; De Santis, L.; Fusi, F.; Brigante, C.; Marelli, G.; Cino, I.; Redaelli, A.; Ferrari, A. Myo-Inositol in Patients with Polycystic Ovary Syndrome: A Novel Method for Ovulation Induction. Gynecol Endocrinol 2007, 23, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Benelli, E.; Del Ghianda, S.; Di Cosmo, C.; Tonacchera, M. A Combined Therapy with Myo-Inositol and D-Chiro-Inositol Improves Endocrine Parameters and Insulin Resistance in PCOS Young Overweight Women. Int J Endocrinol 2016, 2016, 3204083. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Bizzarri, M. Physiological Role and Clinical Utility of Inositols in Polycystic Ovary Syndrome. Best Pract Res Clin Obstet Gynaecol 2016, 37, 129–139. [Google Scholar] [CrossRef]
- Minozzi, M.; D’Andrea, G.; Unfer, V. Treatment of Hirsutism with Myo-Inositol: A Prospective Clinical Study. Reprod Biomed Online 2008, 17, 579–582. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, D.; Zhang, Y.; Lin, Y.; Song, J.; Li, S.; Sun, Y. Inositol Supplement Improves Clinical Pregnancy Rate in Infertile Women Undergoing Ovulation Induction for ICSI or IVF-ET. Medicine (Baltimore) 2017, 96, e8842. [Google Scholar] [CrossRef]
- Colazingari, S.; Treglia, M.; Najjar, R.; Bevilacqua, A. The Combined Therapy Myo-Inositol plus D-Chiro-Inositol, Rather than D-Chiro-Inositol, Is Able to Improve IVF Outcomes: Results from a Randomized Controlled Trial. Arch Gynecol Obstet 2013, 288, 1405–1411. [Google Scholar] [CrossRef]
- Akbari Sene, A.; Tabatabaie, A.; Nikniaz, H.; Alizadeh, A.; Sheibani, K.; Mortezapour Alisaraie, M.; Tabatabaie, M.; Ashrafi, M.; Amjadi, F. The Myo-Inositol Effect on the Oocyte Quality and Fertilization Rate among Women with Polycystic Ovary Syndrome Undergoing Assisted Reproductive Technology Cycles: A Randomized Clinical Trial. Arch Gynecol Obstet 2019, 299, 1701–1707. [Google Scholar] [CrossRef]
- Artini, P.G.; Di Berardino, O.M.; Papini, F.; Genazzani, A.D.; Simi, G.; Ruggiero, M.; Cela, V. Endocrine and Clinical Effects of Myo-Inositol Administration in Polycystic Ovary Syndrome. A Randomized Study. Gynecol Endocrinol 2013, 29, 375–379. [Google Scholar] [CrossRef]
- Mendoza, N.; Pérez, L.; Simoncini, T.; Genazzani, A. Inositol Supplementation in Women with Polycystic Ovary Syndrome Undergoing Intracytoplasmic Sperm Injection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Reprod Biomed Online 2017, 35, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bhide, P.; Pundir, J.; Gudi, A.; Shah, A.; Homburg, R.; Acharya, G. The Effect of Myo-Inositol/Di-Chiro-Inositol on Markers of Ovarian Reserve in Women with PCOS Undergoing IVF/ICSI: A Systematic Review and Meta-Analysis. Acta Obstet Gynecol Scand 2019, 98, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, E.; Unfer, V.; Baillargeon, J.-P.; Fusi, F.; Occhi, F.; De Santis, L. Myo-Inositol May Improve Oocyte Quality in Intracytoplasmic Sperm Injection Cycles. A Prospective, Controlled, Randomized Trial. Fertil Steril 2009, 91, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of Myo-Inositol and Melatonin versus Myo-Inositol, in a Randomized Controlled Trial, for Improving in Vitro Fertilization of Patients with Polycystic Ovarian Syndrome. Gynecol Endocrinol 2016, 32, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Dragotto, J.; Giuliani, A.; Bizzarri, M. Myo-Inositol and D-Chiro-Inositol (40:1) Reverse Histological and Functional Features of Polycystic Ovary Syndrome in a Mouse Model. J Cell Physiol 2019, 234, 9387–9398. [Google Scholar] [CrossRef]
- Carlomagno, G.; Unfer, V.; Roseff, S. The D-Chiro-Inositol Paradox in the Ovary. Fertil Steril 2011, 95, 2515–2516. [Google Scholar] [CrossRef]
- Tarlatzis, B.C.; Zepiridis, L.; Grimbizis, G.; Bontis, J. Clinical Management of Low Ovarian Response to Stimulation for IVF: A Systematic Review. Hum Reprod Update 2003, 9, 61–76. [Google Scholar] [CrossRef]
- Caprio, F.; D’Eufemia, M.D.; Trotta, C.; Campitiello, M.R.; Ianniello, R.; Mele, D.; Colacurci, N. Myo-Inositol Therapy for Poor-Responders during IVF: A Prospective Controlled Observational Trial. J Ovarian Res 2015, 8, 37. [Google Scholar] [CrossRef]
- Humaidan, P.; Alviggi, C.; Fischer, R.; Esteves, S.C. The Novel POSEIDON Stratification of “Low Prognosis Patients in Assisted Reproductive Technology” and Its Proposed Marker of Successful Outcome. F1000Res 2016, 5, 2911. [Google Scholar] [CrossRef]
- Kailasam, C.; Keay, S.D.; Wilson, P.; Ford, W.C.L.; Jenkins, J.M. Defining Poor Ovarian Response during IVF Cycles, in Women Aged <40 Years, and Its Relationship with Treatment Outcome. Hum Reprod 2004, 19, 1544–1547. [Google Scholar] [CrossRef]
- Ata, B.; Yakin, K.; Balaban, B.; Urman, B. Embryo Implantation Rates in Natural and Stimulated Assisted Reproduction Treatment Cycles in Poor Responders. Reprod Biomed Online 2008, 17, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Balaban, B.; Ebner, T.; Mandelbaum, J. The Oocyte. Hum Reprod 2012, 27 Suppl 1, i2-21. [Google Scholar] [CrossRef]
- Pandian, Z.; McTavish, A.R.; Aucott, L.; Hamilton, M.P.; Bhattacharya, S. Interventions for “poor Responders” to Controlled Ovarian Hyper Stimulation (COH) in in-Vitro Fertilisation (IVF). Cochrane Database Syst Rev 2010, CD004379. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, T.; Kozinoglu, H.; Kaba, A. Growth Hormone Co-Treatment within a GnRH Agonist Long Protocol in Patients with Poor Ovarian Response: A Prospective, Randomized, Clinical Trial. J Assist Reprod Genet 2008, 25, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ciotta, L.; Stracquadanio, M.; Pagano, I.; Carbonaro, A.; Palumbo, M.; Gulino, F. Effects of Myo-Inositol Supplementation on Oocyte’s Quality in PCOS Patients: A Double Blind Trial. Eur Rev Med Pharmacol Sci 2011, 15, 509–514. [Google Scholar] [PubMed]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Saharkhiz, N.; Azizi, E.; Hashemi, T.; Ghodssi-Ghassemabadi, R. Effect of Myo-Inositol Supplementation on ICSI Outcomes among Poor Ovarian Responder Patients: A Randomized Controlled Trial. J Gynecol Obstet Hum Reprod 2020, 49, 101698. [Google Scholar] [CrossRef]
- Lisi, F.; Carfagna, P.; Oliva, M.M.; Rago, R.; Lisi, R.; Poverini, R.; Manna, C.; Vaquero, E.; Caserta, D.; Raparelli, V.; et al. Pretreatment with Myo-Inositol in Non Polycystic Ovary Syndrome Patients Undergoing Multiple Follicular Stimulation for IVF: A Pilot Study. Reprod Biol Endocrinol 2012, 10, 52. [Google Scholar] [CrossRef]
- Simmons, D. Diabetes and Obesity in Pregnancy. Best Pract Res Clin Obstet Gynaecol 2011, 25, 25–36. [Google Scholar] [CrossRef]
- Murphy, A.; Shamshirsaz, A.; Markovic, D.; Ostlund, R.; Koos, B. Urinary Excretion of Myo-Inositol and D-Chiro-Inositol in Early Pregnancy Is Enhanced in Gravidas With Gestational Diabetes Mellitus. Reprod Sci 2016, 23, 365–371. [Google Scholar] [CrossRef]
- Corrado, F.; D’Anna, R.; Di Vieste, G.; Giordano, D.; Pintaudi, B.; Santamaria, A.; Di Benedetto, A. The Effect of Myoinositol Supplementation on Insulin Resistance in Patients with Gestational Diabetes. Diabet Med 2011, 28, 972–975. [Google Scholar] [CrossRef]
- Fraticelli, F.; Celentano, C.; Zecca, I.A.; Di Vieste, G.; Pintaudi, B.; Liberati, M.; Franzago, M.; Di Nicola, M.; Vitacolonna, E. Effect of Inositol Stereoisomers at Different Dosages in Gestational Diabetes: An Open-Label, Parallel, Randomized Controlled Trial. Acta Diabetol 2018, 55, 805–812. [Google Scholar] [CrossRef] [PubMed]
- De Grazia, S.; Carlomagno, G.; Unfer, V.; Cavalli, P. Myo-Inositol Soft Gel Capsules May Prevent the Risk of Coffee-Induced Neural Tube Defects. Expert Opin Drug Deliv 2012, 9, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does It Improve Sperm Mitochondrial Function and Sperm Motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and Pharmacokinetics of Inositol(s) in Health and Disease. Expert Opin Drug Metab Toxicol 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Jany, K.D.; Berk, A.; Schulz, E.; Rechkemmer, G. Degradation of Phytate in the Gut of Pigs--Pathway of Gastro-Intestinal Inositol Phosphate Hydrolysis and Enzymes Involved. Arch Tierernahr 2001, 55, 255–280. [Google Scholar] [CrossRef]
- Caputo, M.; Bona, E.; Leone, I.; Samà, M.T.; Nuzzo, A.; Ferrero, A.; Aimaretti, G.; Marzullo, P.; Prodam, F. Inositols and Metabolic Disorders: From Farm to Bedside. J Tradit Complement Med 2020, 10, 252–259. [Google Scholar] [CrossRef]
- Mukai, T.; Kishi, T.; Matsuda, Y.; Iwata, N. A Meta-Analysis of Inositol for Depression and Anxiety Disorders. Hum Psychopharmacol 2014, 29, 55–63. [Google Scholar] [CrossRef]
- Lepore, E.; Lauretta, R.; Bianchini, M.; Mormando, M.; Di Lorenzo, C.; Unfer, V. Inositols Depletion and Resistance: Principal Mechanisms and Therapeutic Strategies. Int J Mol Sci 2021, 22, 6796. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Bizzarri, M. Inositols in Insulin Signaling and Glucose Metabolism. Int J Endocrinol 2018, 2018, 1968450. [Google Scholar] [CrossRef]
- Montanino Oliva, M.; Buonomo, G.; Calcagno, M.; Unfer, V. Effects of Myo-Inositol plus Alpha-Lactalbumin in Myo-Inositol-Resistant PCOS Women. J Ovarian Res 2018, 11, 38. [Google Scholar] [CrossRef]
| Source | Quantity | References |
|---|---|---|
| Cereals | ||
| Rice bran | 7.85-8.52 mg/g | [16] |
| Fruits | ||
| Grapefruit juice | 120g of grapefruit juice provides about 468.8 mg of the compound | [17] |
| Fresh mandarin orange | 3.07 mg/g | [17] |
| Dates, palms, prunes | 46 mg/g | [18] |
| Vegetables | ||
| Lettuce | 1,07 mg/g | [19] |
| White onion | 0.6642 mg/g | [19] |
| Carrots | 2.2–9.8 mg/g | [20] |
| Milk derived products | ||
| Bovine milk | 5.3-8.7 mg/100 ml | [21] |
| Sweetened condensed milk | 0.26 mg/g | [22] |
| Meat | ||
| Beef liver | 0.64 mg/g | [17] |
| Legumes | ||
| Soybean | 1.22 mg/g in pod, 8 mg/g in seed | [12] |
| Dried fruit | ||
| Pine nuts | 0.7 mg/g | [23] |
| Peanut butter | 3.04 mg/g | [17] |
| Almond | 2.78 mg/g | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





