1. Introduction
4-aminopyridine (4-AP; fampridine) is a non-selective blocker of voltage-dependent K
+ channels (K
v) widely used in basic research as a convulsant agent. It increases action potential firing and neurotransmission leading to acute epileptiform activity [
1,
2,
3,
4].
Interestingly, in the clinical scenario, the depolarizing action endows 4-AP with the therapeutic effectiveness to improve walking in patients with multiple sclerosis when used at low doses [
1,
5]. In addition, clinical trials and experimental studies suggest that 4-AP might be useful for the treatment of nystagmus, vertigo and various other cerebellar disorders [
6].
The depolarizing action of 4-AP stimulates neurotransmitter release and facilitates inward Ca
2+ currents therefore causing neuronal hyperactivity [
2,
7,
8,
9,
10,
11]. Increased excitatory glutamate-mediated neurotransmission in multiple brain areas such as hippocampus, entorhinal cortex and striatum is one of the main contributors to the epileptiform activity induced by 4-AP [
12,
13,
14]. This is supported by the protective effects of antagonists of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, but not by GABAergic drug, against 4-AP-induced epileptiform activity [
15,
16,
17,
18].
Both intrahippocampal and intraperitoneal 4-AP administration are known to chemically induce seizure activity in rodents and are used as epilepsy models to study neuroprotection [
19,
20,
21]. Nonetheless, in rodents, some behavioral, electrophysiological, excitotoxic and neuronal damage profiles differ between both routes of administration. Thus, i.p. 4-AP administration results in generalized tonic convulsions characterized by electrical discharges of relatively short duration [
2,
22] and, usually no extensive neuronal damage is found [
17,
18,
20,
23]. By contrast, intrahippocampal injection results in limbic seizures and frequent wet-dog shakes that correlate with hippocampal discharges that rapidly propagate to other structures [
22] and encompasses intense brain damage, including loss of pyramidal neurons [
17,
18,
21]. Such neuronal damage is thought to be linked to glutamate-induced excitotoxicity [
17,
18] through overactivation of NMDA receptors [
24]. And, at low doses, i.c.v. administration of 4-AP produces epileptiform activity without affecting glutamate levels [
25].
It has been shown that 4-AP has different affinity for the K
v subtypes. Thus, at low concentrations primarily blocks the K
v1 family whereas at high concentrations like those that induce convulsions, it blocks a wide range of K
v channels in neuronal and glial cells [
19,
26,
27,
28]. Therefore, 4-AP-induced effects seem to depend significantly on the route of administration that determines its concentration in the different brain areas as well as the cell types affected.
2-deoxy-2-[
18F]fluoro-D-glucose ([
18F]FDG) positron emission tomography (PET) neuroimaging is a minimally-invasive valuable tool for studying regional cerebral glucose metabolism, being a reflection of its functionality. Furthermore, many clinical and basic molecular neuroimaging studies have shown that the brain damage associated either to epilepsy or to seizures is characteristically associated with brain glucose metabolism alterations [
20,
29,
30,
31,
32]. In this context, we have previously reported that intrahippocampal 4-AP administration induces acute brain hypermetabolism that, reflecting increased local neuronal activity, leads to short-term detrimental consequences in hippocampal integrity and neuroinflammation [
21].
In the current study our aim was to evaluate the consequences of a single i.p. administration of 4-AP on brain glucose metabolism during the acute depolarizing phase by using in vivo [18F]FDG PET. Our second objective was to determine the short-term (3d after seizure) consequences on neuronal viability and signs of neuroinflammation by evaluating different markers of neuronal integrity, brain damage, astrocytic activation and microglia-mediated neuroinflammation.
2. Results
The administration of 4-AP was accompanied by intense seizure behavior. In fact, the dose of 5 mg/kg triggered generalized convulsions in all rats between 5 and 10 min after injection, which is in line with our previous work [
20]. Besides, no mortality was recorded at this dose. When the [
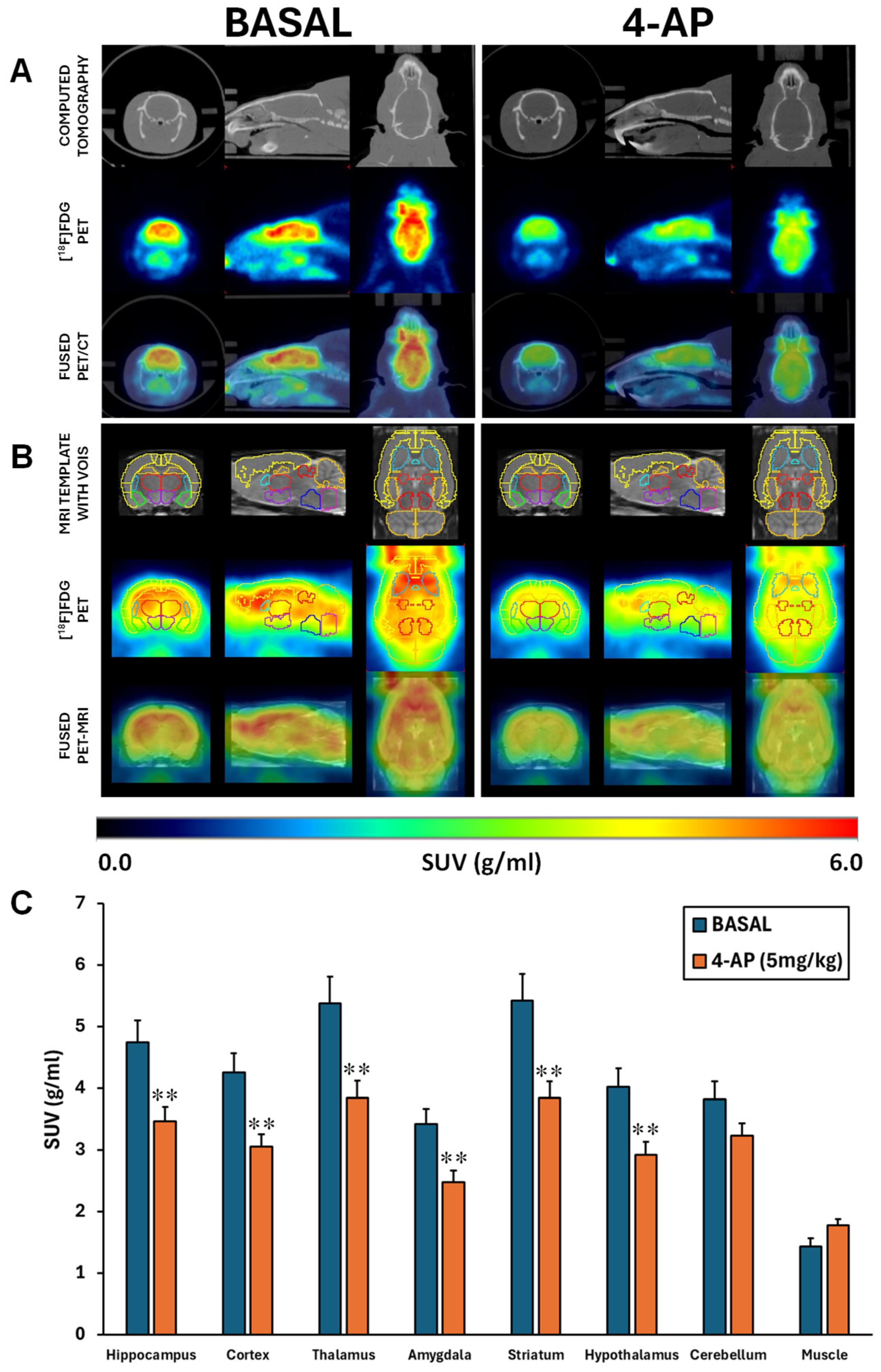
18F]FDG brain uptake was analyzed by SUV, 4-AP induced an acute hypometabolism of approximately 28% in all areas studied (p<0.01) except in the cerebellum (p=0.067) (
Figure 1A-B-C).
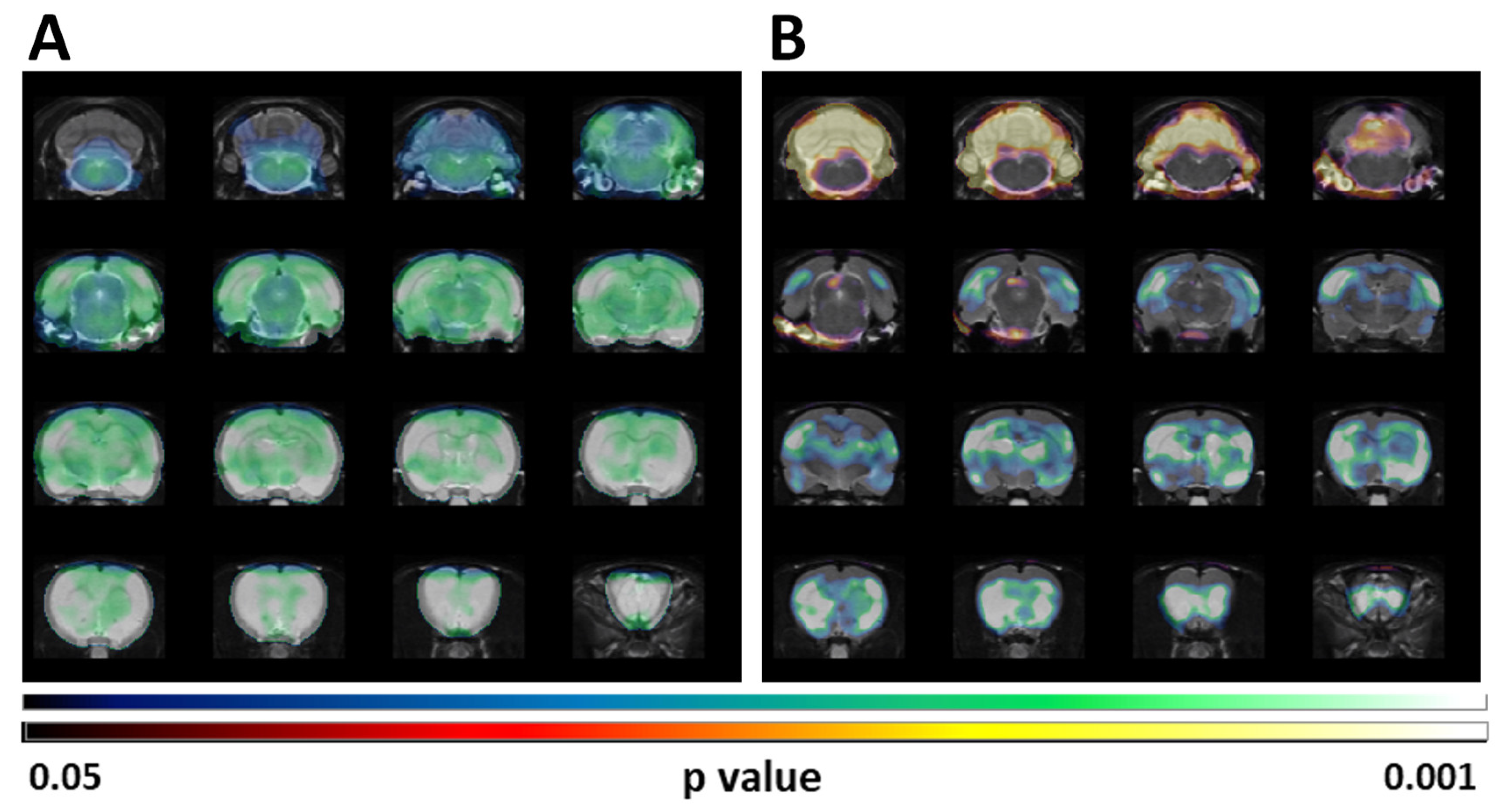
These results coincided with those obtained when the SUV differences were analyzed by SPM (
Figure 2A). However, when the SPM analysis was done after normalization of the [
18F]FDG uptake by the whole brain, the reduced uptake in clusters localized in telencephalic and diencephalic regions was maintained but a statistically significant increase of the glucose metabolism in the cerebellum is perceivable (
Figure 2B).
Considering the 4-AP was administered systemically, we also evaluated the SUV [
18F]FDG uptake in the forepaw muscle. Although not significant, our results suggest that 4-AP might acutely increase the muscle glucose metabolism (p=0.057;
Figure 1B).
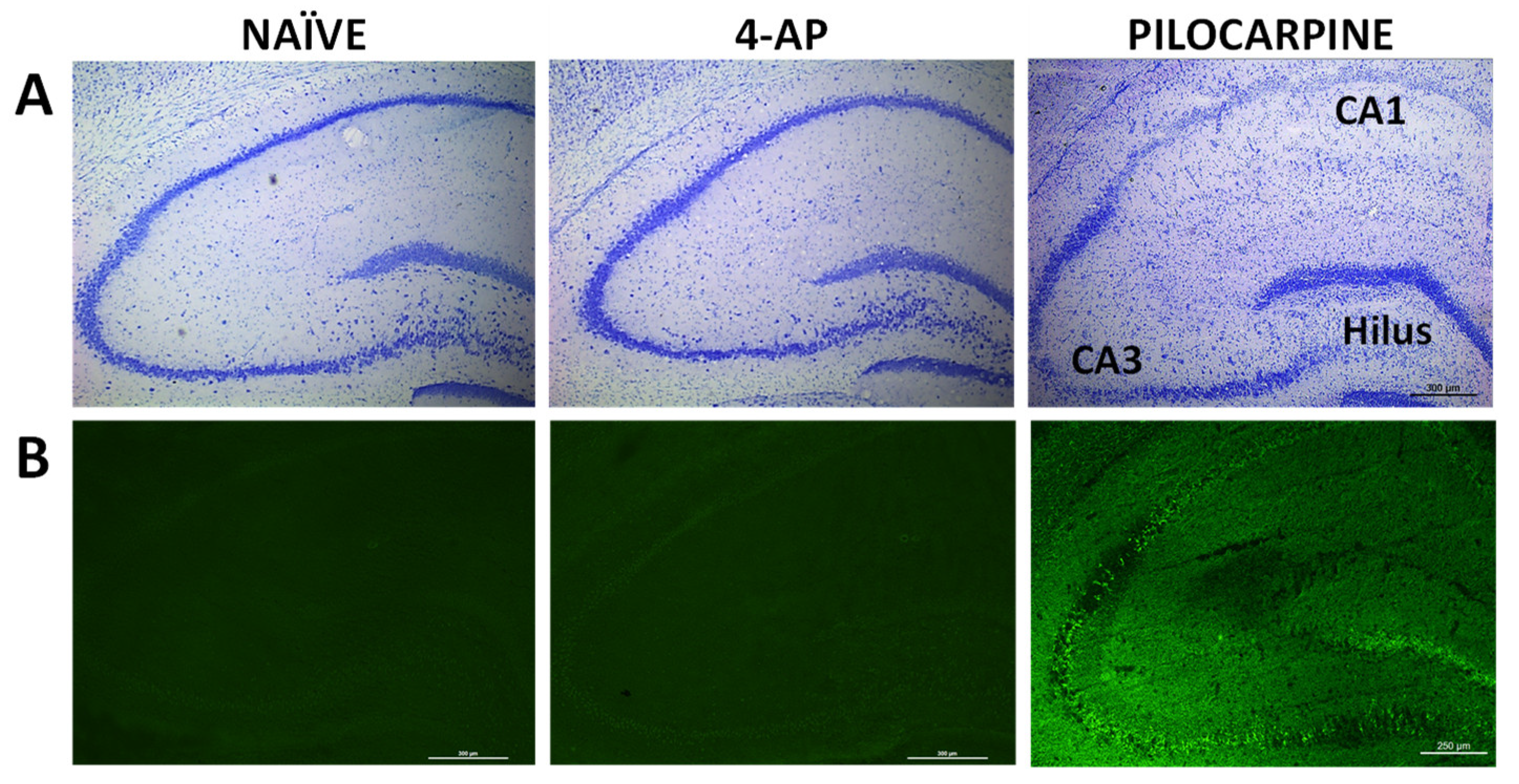
We also evaluated the short-term effects of 4-AP on neuron integrity, neurodegeneration, astrocyte reactivity and microglia-mediated neuroinflammation in hippocampus, 3 days after 4-AP induced seizures. Our results revealed that 4-AP had no detrimental effects either on hippocampal neurodegeneration (Nissl and Fluoro-Jade C stainings;
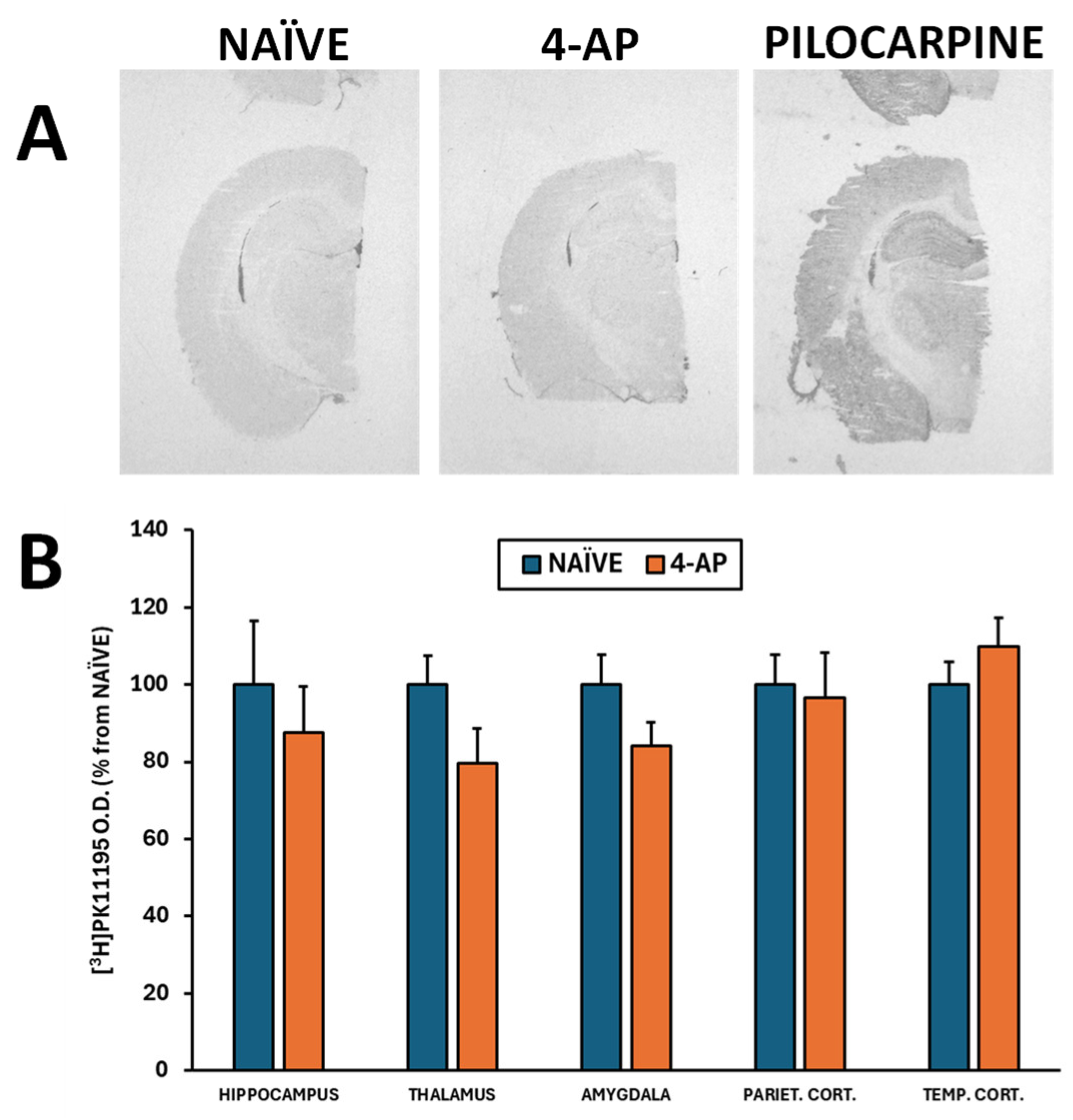
Figure 3) or on microglia-mediated neuroinflammation when evaluating the optical density of different brain areas (
Figure 4A-B; p>0.05 in all areas studied).
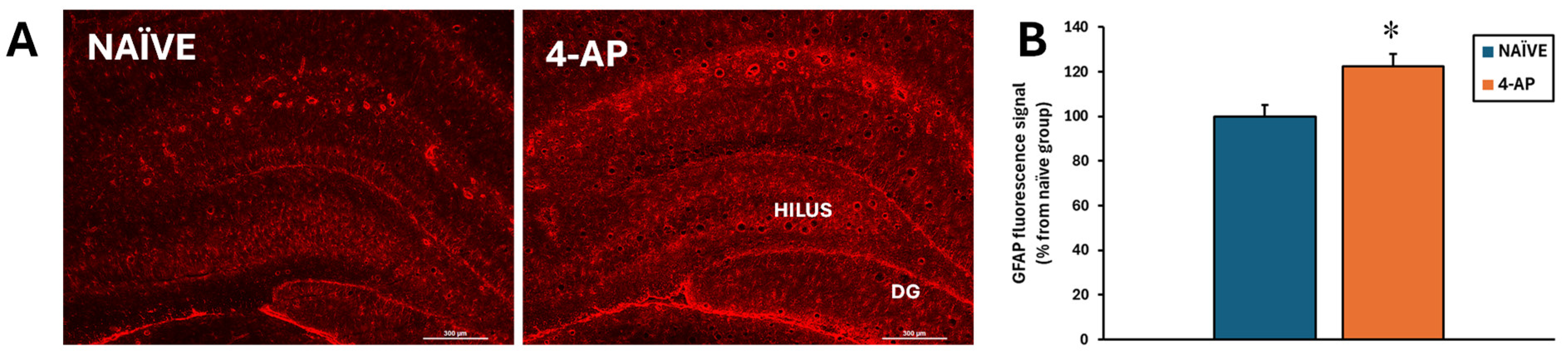
By contrast, astrocyte reactivity was detected based on a significant increase of approximately 22% (p<0.05) on GFAP fluorescence signal in the hippocampus (
Figure 5).
3. Discussion
In this study we have evaluated brain glucose metabolism by longitudinal [18F]FDG PET neuroimaging in rats under baseline conditions as well as, in the same rats, immediately after i.p. 4-AP administration (5 mg/kg). This dose and route of administration resulted in convulsive seizures and acute generalized brain glucose hypometabolism except in the cerebellum. Besides, 4-AP resulted in short-term astrocyte reactivity measured 3 days after the insult without apparent effects on microglia-mediated neuroinflammation.
3.1. VOIs Analysis Showed That 4-AP Administered i.p. Was Followed by Acute Generalized Brain Glucose Hypometabolism
Our results showing acute brain hypometabolism in response to 4-AP i.p. injection (
Figure 1), was at first surprising considering two-fold. On one hand, all rats responded with a marked convulsive activity, and on the other hand, we had previously reported that, when administered in the hippocampus, 4-AP (7 µg in 5 µl) induced acute local hypermetabolism around the injection site, reflecting increased local neuronal activity and leading to detrimental consequences in hippocampal integrity and neuroinflammation. Likewise, local hypermetabolism at the intracortical injection site of 4-AP in rats has also been found when measured by the uptake of 2-(
N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) a fluorescent deoxyglucose substitute [
33].
However, we have also previously shown that systemic 4-AP (3 mg/kg, i.p.) had no effects on short-term brain glucose metabolism, as measured 3 days after the 4-AP administration [
20]. Supporting the hypometabolic response describe herein, hypometabolism in response to 4-AP-induced seizures has been also reported in mouse hippocampal slices [
29]. Thus, it seems the metabolic responses to 4-AP go in the opposite direction depending on the dose and route of administration. Furthermore, when 4-AP is administered systemically it may induce a dose-dependently transient brain hypometabolism.
Despite these differences, 4-AP is used either by systemic or by intracerebral route as a model to induce seizure activity in rodents [
3,
20,
21]. However, the results highlight that the brain glucose metabolic profile clearly differs between both routes of administration. This route-dependent difference in metabolic profile is in line with the differences reported regarding the differential behavioral, electrophysiologic as well as neuronal damage profiles which characterize these routes [
2,
22].
3.2. Cerebellar Metabolism is Increased in Response to 4-AP as Evaluated by SPM Normalized to Whole Brain [18F]FDG Uptake
We also evaluated brain glucose metabolism by SPM after normalizing the values to the whole brain [
18F]FDG uptake. In our current study this analysis detected significantly hypometabolic clusters that overlapped with the regions detected by traditional VOIs analyses. These clusters included telencephalic as well as diencephalic structures (
Figure 2). Interestingly, this type of analysis highlighted a significant increase in cerebellar uptake (
Figure 2). This type of normalization of regional values
vs the whole brain reference value allows detecting the impact of localized changes, minimizes individual differences and avoids the impact of peripheral uptake.
In addition to the central convulsive activity, 4-AP is known to have noticeable peripheral effects increasing synaptic neuromuscular transmission and directly increasing skeletal muscle twitch tension [
34,
35,
36]. It has been suggested that the latter may contribute to their therapeutic effects in multiple sclerosis patients [
36]. Considering that in our study 4-AP was administered systemically, and to determine whether the actions of 4-AP on skeletal muscle might have interfered with brain [
18F]FDG uptake, we measured glucose metabolism in the forepaw muscle. Our results showed that there is a visually apparent but statistically non-significant increase in muscle glucose metabolism (
Figure 1B), a result that would be in concert with its effects increasing muscle contraction, but that also indicates that brain hypometabolism found in our study might not be attributed to [
18F]FDG uptake being deviated from brain towards muscle.
One limitation associated with the SPM analyses after normalizing the values to the whole brain [
18F]FDG uptake has been raised, in particular, when studying diseases characterized by whole brain hypometabolism. It has been noticed that this analysis may lead to underestimation of reduced metabolism in some regions as well as to overestimations in others, which might be inconsistent with the clinical or experimental conditions [
37,
38,
39].
However, in our case, and independently of the type of analysis (VOIs and SPM analyses), our data suggest that the cerebellum might be more resilient to 4-AP-induced hypometabolism. 4-AP has been shown to increase Purkinje cells activity in brain slices of the rat [
40,
41], and consequently to increase the GABAergic neurotransmission that restores cerebellar inhibition, effect that has been related to the therapeutic effects of 4-AP on cerebellar disorders [
42]. In a clinical case study evaluating brain glucose metabolism by [
18F]FDG PET, treatment with 4-AP lessened the cerebellar hypometabolism associated to downbeat nystagmus. The authors suggested that this effect might indicate an improvement of the cerebellar inhibition [
43]. Besides, 4-AP has been reported to exert a robust neuroprotective effect on apoptotic cerebellar granule cells [
44]. Furthermore, 4-AP (3 mg/kg, i.v.) has been shown to increase blood flow to various brain regions being particularly significant in the cerebellum [
45]. Thus, our results regarding the effects of 4-AP on cerebellar metabolism might be related to some of the above-mentioned actions of 4-AP.
3.3. Systemic 4-AP Administration Does Not Alter Hippocampal Integrity
4-AP (5 mg/kg, i.p.) administration had no detrimental short-term effects on hippocampal neuronal integrity and survival as measured 3 days after the insult (
Figure 3). This result agrees with a previous study where we showed that 4-AP i.p. at a dose of 3 mg/kg did not induce signs of neurodegeneration or neuronal death [
20].
Many studies support the neuroprotective effects of K
v channels blockade [
28,
46]. For example, 4-AP (5 mg/kg, i.p.) abolishes the kainate-induced hippocampal neuronal cell death, as measured 3 days after kainate injection [
47]. Both, in vivo as well as in vitro stroke models have shown that potassium channel blockade attenuates ischemia-induced neuronal death and apoptosis [
27]. Likewise, studies in cell cultures have reported that 4-AP prevents cerebellar granule neuronal cell death and apoptosis induced by low K
+ (5 mM) serum-free conditions [
44].
3.4. Effects of Systemic 4-AP Administration on Short-Term Neuroinflammation
Gliosis is a histopathological feature of epilepsy, including microglia and astrocyte activation [
48,
49,
50,
51]. Furthermore, complex and reciprocal regulatory interactions between microglia and astrocytes exist and, the temporal order of events is still unclear [
52,
53]. Nonetheless, it seems that the inflammatory effects of microglia may precede those of astrocytes [
54,
55,
56].
Herein we show that systemic 4-AP administration did not result in microglia-mediated neuroinflammation analyzed by [
3H]PK11195 autoradiography (
Figure 4). By contrast, intrahippocampal 4-AP injection induced microglia-mediated neuroinflammation measured by in vitro [
18F]GE180 autoradiography [
21]. In other models of microglia activation, 4-AP has been shown to inhibit K
+ outward current in rat [
57] and to lessens the microglial production of neurotoxins and the consequent neuronal damage [
58,
59]. Thus, again it seems that despite being able to induce seizures the effects of 4-AP, when administered systemically, clearly differ from those observed after intracerebral administration.
Astrocytes have been shown to be key players in the etiology and pathogenesis of epilepsy [
49,
51] and 4-AP has been also reported to non-selectively block K
+v in astrocytes [
26]. In our current study, astrocyte reactivity was observed 3 days after i.p. 4-AP-induced seizures. This result agrees with our previously reported data obtained in rats (4-AP, 3mg/kg, i.p.) [
20] as well as by data reported in mice (4-AP, 5.6 mg/kg, i.p.) [
60]. Likewise, pre-treatment with 4-AP in rodent C8D1A cultured astroglial cells increases LPS-stimulated astrocyte reactivity and production of pro-inflammatory cytokines [
61].
Even though astrocyte reactivity is commonly associated with neurodegeneration, in our study, as depicted in
Figure 5, we found astrocyte reactivity in the absence of hippocampal damage. Herein, it is interesting to notice that 4-AP directly acts on both neurons and astrocytes, the latter critically modulating the effects of 4-AP on neuronal integrity and survival [
62]. Our result suggests that the astrocyte reactivity could be consequence of the direct action of 4-AP on astrocytes and not as a response to neuronal damage. At this point, we cannot exclude the possibility that the astrocyte reactivity observed 3 days after the insult might in turn result in longer-term changes in hippocampal integrity.
4. Materials and Methods
4.1. Animals
Thirteen adult Sprague-Dawley male rats (Charles Rivers, Spain) were housed in pairs in standard cages on a ventilated rack (Tecniplast, Buguggiate, Italy). The animal room was under controlled conditions of temperature (22 ± 2ºC) and was under a light/dark cycle of 12 h (8 am – 8 pm). Rats were left undisturbed for a minimum of 5 d to allow adaptation to the new environment. Animals had free access to food chow and water. Access to food only was restricted for the 12h before the PET scan procedures to ensure low blood glucose plasma concentrations for minimizing any eventual interference with the radiotracer tissue uptake.
All procedures were carried out in accordance with animal welfare regulations of the European Union (2010/63/UE) and Spain (RD53/2013) and was approved by the Animal Research Ethical Committee of the Complutense University of Madrid and by the Autonomous Community of Madrid (PROEX 222.1/20). All efforts were made to minimize the suffering of the animals.
4.2. Experimental Design
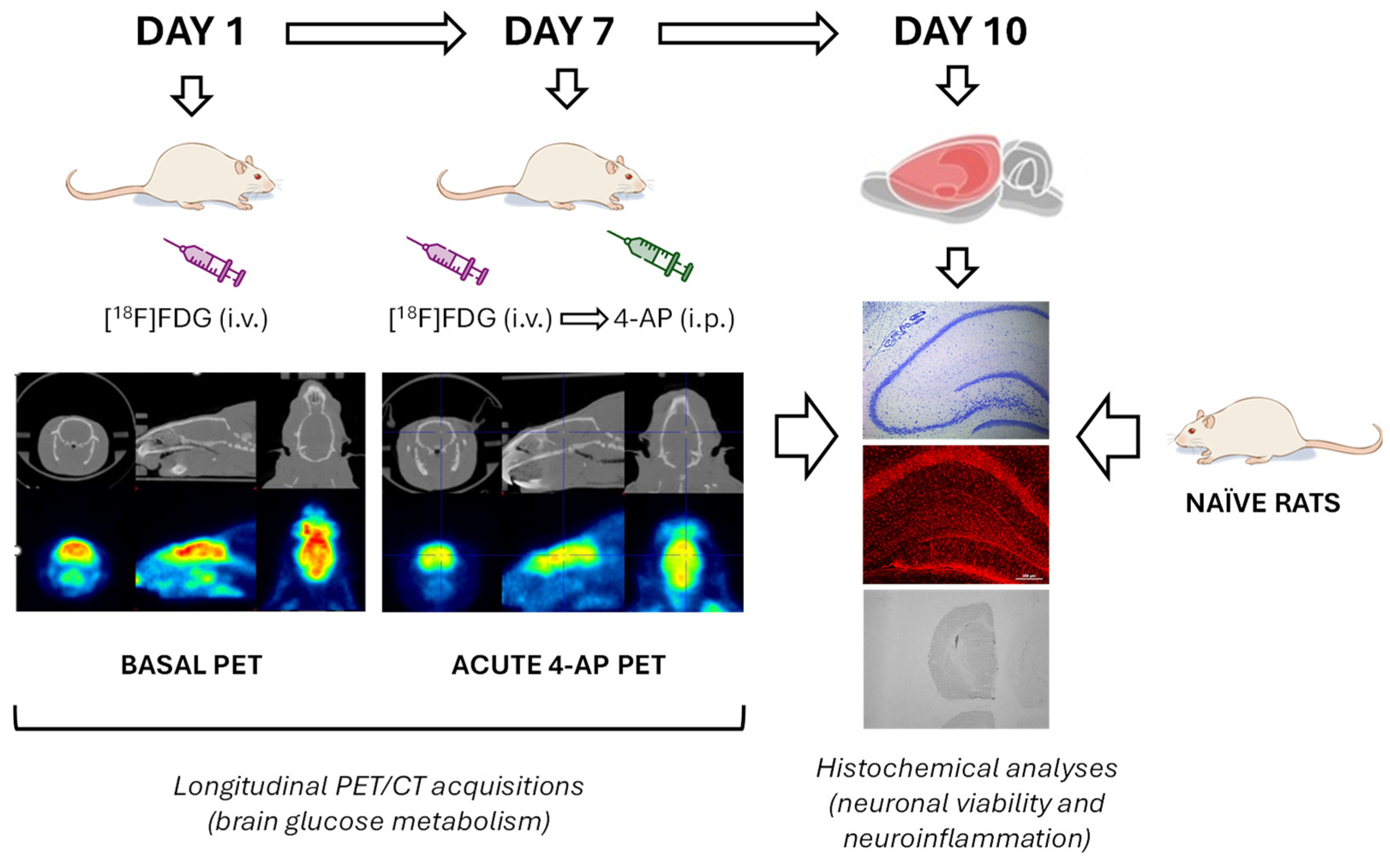
Longitudinal brain glucose metabolism activity was evaluated by [18F]FDG PET. By obtaining longitudinal measurements within the same animal, we could reduce the number of animals needed without put at risk statistical power. Thus, on experimental day 1, we evaluated the basal metabolic activity (BASAL group) and on experimental day 7, the acute metabolic effects of 4-AP (Merck; 5 mg/kg, i.p. in 0.9% NaCl at a volume of 1 ml/kg) in the same animals (4-AP group) were evaluated 1 min after [18F]FDG injection.
In parallel, a group of rats were kept naïve throughout the whole experiment to serve as a control group for histological studies (NAÏVE group; n = 6). All animals were sacrificed on experimental day 10. The experimental design is schematized in
Figure 6.
4.3. [18F]FDG PET/CT Imaging
Brain glucose metabolism was evaluated by in vivo [
18F]FDG PET imaging as previously reported [
21,
63]. Briefly, the rats were fasted overnight before the procedure to minimize the eventual competition between circulating glucose and [
18F]FDG. For basal metabolic activity evaluation (day 1), [
18F]FDG was injected into the tail vein (13.05 ± 0.05 MBq in approximately 0.2 ml of 0.9% NaCl; Curium Pharma, Madrid, Spain). On experimental day 7, the acute metabolic effects of 4-AP in the same animals (4-AP group) were evaluated by administering 4-AP (Merck; 5 mg/kg, i.p. in 0.9% NaCl at a volume of 1 ml/kg) 1 min after [
18F]FDG injection.
Immediately after the radiotracer was injected, the rats were returned to their cages for a 30 min period to ensure the brain uptake of the radiotracer. After this uptake period, rats were anesthetized by inhalation of isoflurane/oxygen and placed on the bed of the tomograph. Image tomographic acquisitions were done using a dedicated-small animal dual PET/CT scanner (Albira PET/CT dual scanner, Bruker NMI, Germany). The acquisitions consisted of a 20 min static PET scan followed by a high-resolution computed tomography (CT) scan.
In both cases, once the acquisitions were finished, PET images were reconstructed by using a maximum likelihood expectation maximization algorithm, applying decay, random and scatter corrections (Bruker, Germany).
4.4. PET Image Analysis by VOIs Analysis
The CT image of the skull of each rat was manually co-registered to a magnetic resonance imaging (MRI) rat brain template that containing the pre-defined brain regions allows for the obtention of a fitted spatial mathematical transformation. For each animal, the later CT transformation was applied to the corresponding PET image, allowing for the correct matching between the PET image and the MRI template as previously described (Jupp and O’Brien, 2007). Then, this co-registered PET image was overlayed onto the MRI template, allowing for the quantification of the tracer regional uptake values (kBq/ml; PMOD 4.1, PMOD Technologies Ltd., Zurich, Switzerland). Afterwards, the standard uptake value (SUV) was calculated applying the following equation: [Tracer uptake (kBq/ml) * BW (g)) / (Tracer dose administered (kBq))], that corrects by the differential individual body weights and [18F]FDG doses administered.
4.5. PET Evaluation by Statistical Parametric Mapping (SPM)
In experimental PET imaging studies, the regions that eventually show effects are often unknown in advance and they might not correspond to the predefined anatomical VOIs. Thus, different from to the traditional quantification using VOIs (see section 4.4), SPM includes the whole brain in the analysis, without any preconception about the structures involved in the experimental paradigm [
64]. This approach might unveil differences in subregions that are not evident in the VOI analysis. Besides, when the regional uptake is normalized to whole brain, it allows to compare relative regional changes without interferences of changes in blood brain flow and tracer peripheral uptake.
SPM comparisons were performed using MATLAB software (The MathWorks, Natick, Ma, USA) and SPM12 (Wellcome Trust Center for Neuroimaging, UCL, London, UK). To evaluate the effects of acute 4-AP injection, differences between BASAL and 4-AP-treated rats were calculated by paired t-test, setting a significance level threshold of 0.05 (uncorrected for multiple comparisons). The minimum cluster size of voxels was set to 100. The t-value threshold was set to show voxels with a correspondent p value lower than 0.05. Parametric t maps resulting from the comparison between the basal and the 4-AP groups were loaded in PMOD and fused to a rat brain T2-MRI template.
4.6. Brain Tissue Collection and Processing for Neurohistochemical Assessments
All rats were sacrificed by decapitation on experimental day 10 (3 days after 4-AP-induced seizures). Brains were collected, cut longitudinally into two halves and stored at -80ºC. Twenty-five µm thickness coronal slices at −20 °C were obtained using a cryostat (Leica CM1850, Leica Biosystems, Germany). Sections containing the dorsal hippocampus were thaw-mounted onto Superfrost Plus slides (Thermo Scientific, Germany), dried on a hot plate and stored into slide boxes at −80 °C until the day of the assays.
4.7. Nissl Staining
Qualitative evaluation of neuronal viability and hippocampal disruption was done with toluidine blue staining. Thus, slides were stained in 0.5% toluidine blue for 2 min, decolorized and dehydrated in graded ethanols: (i) 96% ethanol (10 s) and (ii) 100% ethanol (2x10 s). Then, the sections were cleared twice (2 min and 5 min) in Neo-ClearTM xylene substitute (Merck-Sigma). Finally, the slides were mounted with DPX mounting medium (Herter, Barcelona, Spain). The images were captured with a digital camera (Leica DFC425, Leica, Germany) coupled to a microscope (Leica DM 2000 LED, Leica, Germany).
4.8. Fluoro-Jade C Labeling
Fluoro-Jade C staining was performed following the protocol previously described [
65]. Thus, the brain sections were immersed for 2 min in 0.0001% Fluoro-Jade C (Millipore, Darmstadt, Germany) in PBS. Then, the slides were air-dried and cover-slipped with DPX mounting medium (Herter, Barcelona, Spain). The images were captured with a digital camera (Leica DFC3000G) coupled to a microscope (Leica DM 2000 LED) by using the FITC filter. At the hippocampal CA1, CA3 and hilus, the fluorescence signal was measured using ImageJ 1.46r software. The average value for each rat was calculated and the results were expressed as percentage
vs the naïve group.
Images from Fluoro-Jade C were acquired using a Leitz Laborlux S microscope (Leica, Germany). The filters used were as follows: FITC filter for Fluoro-Jade C The hippocampal subareas CA1, CA3 and hilus of each brain slice were manually delimited by a blind operator and the mean fluorescence signal value of those regions were obtained. The average value for each animal was obtained from 4 different brain slices containing dorsal hippocampus. Both delimitation, and quantification steps were performed with ImageJ software (NIH, available at the following website:
https://imagej.nih.gov/ij/).
4.9. Microglia-Mediated Neuroinflammation by [3H]PK11195 Autoradiography
As a marker of microglia-mediated neuroinflammation, we evaluated the translocator protein (TSPO; 18 kDa) by [
3H]PK11195 autoradiography following the protocol previously reported [
66] with minor modifications. The slides were dried on a hot plate at 37ºC (10 min), preincubated with 50 mM Tris-HCl pH 7.4 at RT (15 min) and then incubated with 1 nM [
3H]PK11195 (Perkin Elmer) in preincubation buffer. After 60 min incubating, the samples were washed in an ice cold preincubation buffer (2x5 min) and dipped in ice-cold distilled water. The slides were air-dried and exposed to Kodak BioMax MR autoradiography film (Carestream, U.S.A.) for approximately 2 months. After manual development, the film was placed onto a light box (Kaiser Prolite 5000, Kaiser Fototechnik, Germany) and the images were captured with a camera (Leica DFC425) coupled to a stereomicroscope (Leica MZ6). The optical densities of the selected brain regions were used as index of neuroinflammation degree.
4.10. Reactive Astrogliosis
Glial fibrillary acidic protein (GFAP) immunofluorescence was performed as previously reported [
21,
67]. Briefly, the slices were fixed in 4% formaldehyde, washed and then blocked and permeabilized with 3% BSA, 0.1% triton X-100 in TBS (60 min). The slides were incubated overnight with the anti-GFAP-Cy3 antibody (1:500, Sigma Aldrich) in 1% BSA in TBS at 4 °C. Afterwards, they were washed in 0.1% Tween 20 dissolved in TBS (3 x for 5 min each) and cover slipped with Mowiol mounting medium. The images were captured and evaluated using the same optical systems used for Fluoro-Jade C, but in this case using the TRITC filter. For each brain section containing the dorsal hippocampus, the fluorescence intensity was measured using ImageJ 1.46r software. As with the Fluoro-Jade C measurement. The results were expressed as percentage
vs the NAÏVE group.
4.11. Statistics
Data are shown as mean ± SEM. The longitudinal data corresponding to within-individual differences in brain glucose metabolism between BASAL and 4-AP-treated groups were evaluated using paired t-test. The data corresponding the neurohistochemical studies was compared between the NAÏVE and 4-AP groups by unpaired t-test. The analyses were carried out using the SigmaPlot 11.0 (Systat Software Inc., IL). The statistical significance level threshold was p < 0.05.
5. Conclusions
Summing up, even though 4-AP is relatively selective for A-type K+ channels at low concentrations, the selectivity is lost when used at higher doses. Thus, despite that 4-AP acts as a convulsant agent independently of the route of administration, acute generalized brain glucose hypometabolism and lack of neurodegeneration in response to 4-AP i.p. is clearly in opposition to the acute local hypermetabolism and extensive neuronal damage reported when 4-AP is administered intracerebrally. Therefore, it is evident that the route of administration and the dose have a significant impact on the 4-AP concentrations that reach the brain and, according to its potential beneficial or deleterious consequences. In addition, our study further points towards cerebellum glucose metabolism as a potential marker of systemic 4-AP-induced effects.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, L.G.-G. and M.A.P.; methodology, L.G.-G.; formal analysis, L.G.-G. and F.G-O.; investigation, L.G.-G., R.F.-R., P.B.; data curation, L.G.-G. and R.F.-R.; writing—original draft preparation, F.G.-O. and L.G.-G.; writing and editing, F.G.-O. and L.G.-G.; review, P.B., M.B., M.A.P. and R.F.-R.; funding acquisition, M.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministerio de Ciencia e Innovación, grant number Retos PID2019-106968RB-100.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Complutense University of Madrid, being ratified by the Autonomous Community of Madrid (PROEX 222.1/20).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hayes, K.C. The Use of 4-Aminopyridine (Fampridine) in Demyelinating Disorders. CNS Drug Rev 2004, 10, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Mihály, A.; Komáromi, Á.; Gyengési, E.; Szente, M.; Weiczner, R.; Krisztin-Péva, B.; Szabó, G.; Telegdy, G. Seizure, Neurotransmitter Release, and Gene Expression Are Closely Related in the Striatum of 4-Aminopyridine-Treated Rats. Epilepsy Res 2003, 55, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Pozo, M. Animal Models for the Development of New Neuropharmacological Therapeutics in the Status Epilepticus. Curr Neuropharmacol 2005, 4, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Ragavendran, J.; Thirumurugan, R.; Saxena, A.; Sriram, D. Ion Channels as Important Targets for Antiepileptic Drug Design. Curr Drug Targets 2005, 5, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Keune, P.M.; Cocks, A.J.; Young, W.R.; Burschka, J.M.; Hansen, S.; Hofstadt-van Oy, U.; Oschmann, P.; Muenssinger, J. Dynamic Walking Features and Improved Walking Performance in Multiple Sclerosis Patients Treated with Fampridine (4-Aminopyridine). BMC Neurol 2015, 15. [Google Scholar] [CrossRef]
- Strupp, M.; Teufel, J.; Zwergal, A.; Schniepp, R.; Khodakhah, K.; Feil, K. Aminopyridines for the Treatment of Neurologic Disorders. Neurol Clin Pract 2017, 7, 65–76. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Barker, J.L. Effects of 4-Aminopyridine on Calcium Action Potentials and Calcium Current under Voltage Clamp in Spinal Neurons. Brain Res 1983, 280, 180–185. [Google Scholar] [CrossRef]
- Rudy, B. Diversity and Ubiquity of K Channels. Neuroscience 1988, 25, 729–749. [Google Scholar] [CrossRef]
- Szente, M.; Baranyi, A. Mechanism of Aminopyridine-Induced Ictal Seizure Activity in the Cat Neocortex. Brain Res 1987, 413, 368–373. [Google Scholar] [CrossRef]
- Tapia, R.; Sitges, M. Effect of 4-Aminopyridine on Transmitter Release in Synaptosomes. Brain Res 1982, 250, 291–299. [Google Scholar] [CrossRef]
- Tapia, R.; Sitges, M.; Morales, E. Mechanism of the Calcium-Dependent Stimulation of Transmitter Release by 4-Aminopyridine in Synaptosomes. Brain Res 1985, 361, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ceja, L.; Morales-Villagrán, A.; Tapia, R. Action of 4-Aminopyridine on Extracellular Amino Acids in Hippocampus and Entorhinal Cortex: A Dual Microdialysis and Electroencehalographic Study in Awake Rats. Brain Res Bull 2000, 53, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mihály, A.; Szakács, R.; Bohata, C.; Dobó, E.; Krisztin-Péva, B. Time-Dependent Distribution and Neuronal Localization of c-Fos Protein in the Rat Hippocampus Following 4-Aminopyridine Seizures. Epilepsy Res 2001, 44, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Morales-Villagrán, A.; Tapia, R. Preferential Stimulation of Glutamate Release by 4-Aminopyridine in Rat Striatum in Vivo. Neurochem Int 1996, 28, 35–40. [Google Scholar] [CrossRef]
- Dóczi, J.; Banczerowski-Pelyhe, I.; Barna, B.; Világi, I. Effect of a Glutamate Receptor Antagonist (GYKI 52466) on 4-Aminopyridine-Induced Seizure Activity Developed in Rat Cortical Slices. Brain Res Bull 1999, 49, 435–440. [Google Scholar] [CrossRef]
- Fragoso-Veloz, J.; Tapia, R. NMDA Receptor Antagonists Protect against Seizures and Wet-Dog Shakes Induced by 4-Aminopyridine. Eur J Pharmacol 1992, 221, 275–280. [Google Scholar] [CrossRef]
- Pea, F.; Tapia, R. Seizures and Neurodegeneration Induced by 4-Aminopyridine in Rat Hippocampus in Vivo: Role of Glutamate- and GABA-Mediated Neurotransmission and of Ion Channels. Neuroscience 2000, 101, 547–561. [Google Scholar] [CrossRef]
- Peña, F.; Tapia, R. Relationships among Seizures, Extracellular Amino Acid Changes, and Neurodegeneration Induced by 4-Aminopyridine in Rat Hippocampus: A Microdialysis and Electroencephalographic Study. J Neurochem 1999, 72, 2006–2014. [Google Scholar] [CrossRef]
- Ventura-mejía, C.; Nuñez-ibarra, B.H.; Medina-ceja, L. An Update of 4-aminopyride as a Useful Model of Generalized Seizures for Testing Antiseizure Drugs: In Vitro and in Vivo Studies. Acta Neurobiol Exp (Wars) 2023, 83, 63–70. [Google Scholar] [CrossRef]
- Shiha, A.A.; de la Rosa, R.F.; Delgado, M.; Pozo, M.A.; García-García, L. Subacute Fluoxetine Reduces Signs of Hippocampal Damage Induced by a Single Convulsant Dose of 4-Aminopyridine in Rats. CNS Neurol Disord Drug Targets 2017, 16. [Google Scholar] [CrossRef]
- García-García, L.; Fernández de la Rosa, R.; Delgado, M.; Silván, Á.; Bascuñana, P.; Bankstahl, J.P.; Gomez, F.; Pozo, M.A. Metyrapone Prevents Acute Glucose Hypermetabolism and Short-Term Brain Damage Induced by Intrahippocampal Administration of 4-Aminopyridine in Rats. Neurochem Int 2018, 113, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Fragoso-Veloz, J.; Massieu, L.; Alvarado, R.; Tapia, R. Seizures and Wet-Dog Shakes Induced by 4-Aminopyridine, and Their Potentiation by Nifedipine. Eur J Pharmacol 1990, 178, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Takács, E.; Nyilas, R.; Szepesi, Z.; Baracskay, P.; Karlsen, B.; Røsvold, T.; Bjørkum, A.A.; Czurkó, A.; Kovács, Z.; Kékesi, A.K.; et al. Matrix Metalloproteinase-9 Activity Increased by Two Different Types of Epileptic Seizures That Do Not Induce Neuronal Death: A Possible Role in Homeostatic Synaptic Plasticity. Neurochem Int 2010, 56, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.X.; Tapia, R. Late N-Methyl-D-Aspartate Receptor Blockade Rescues Hippocampal Neurons from Excitotoxic Stress and Death after 4-Aminopyridine-Induced Epilepsy. Eur J Neurosci 2005, 22, 3067–3076. [Google Scholar] [CrossRef]
- Medina-Ceja, L.; Pardo-Peña, K.; Morales-Villagrán, A.; Ortega-Ibarra, J.; López-Pérez, S. Increase in the Extracellular Glutamate Level during Seizures and Electrical Stimulation Determined Using a High Temporal Resolution Technique. BMC Neurosci 2015, 16. [Google Scholar] [CrossRef]
- Bordey, A.; Sontheimer, H. Differential Inhibition of Glial K(+) Currents by 4-AP. J Neurophysiol 1999, 82, 3476–3487. [Google Scholar] [CrossRef]
- Wei, L.; Yu, S.P.; Gottron, F.; Snider, B.J.; Zipfel, G.J.; Choi, D.W. Potassium Channel Blockers Attenuate Hypoxia- and Ischemia-Induced Neuronal Death in Vitro and in Vivo. Stroke 2003, 34, 1281–1286. [Google Scholar] [CrossRef]
- Yu, S.P. Regulation and Critical Role of Potassium Homeostasis in Apoptosis. Prog Neurobiol 2003, 70, 363–386. [Google Scholar] [CrossRef]
- Malkov, A.; Ivanov, A.I.; Buldakova, S.; Waseem, T.; Popova, I.; Zilberter, M.; Zilberter, Y. Seizure-Induced Reduction in Glucose Utilization Promotes Brain Hypometabolism during Epileptogenesis. Neurobiol Dis 2018, 116, 28–38. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, F.; Wang, S.; Ding, Y.; Zhang, H.; Wang, J.; Ding, M.P. In Vivo Mapping of Temporospatial Changes in Glucose Utilization in Rat Brain during Epileptogenesis: An 18F-Fluorodeoxyglucose-Small Animal Positron Emission Tomography Study. Neuroscience 2009, 162, 972–979. [Google Scholar] [CrossRef]
- Jupp, B.; Williams, J.; Binns, D.; Hicks, R.J.; Cardamone, L.; Jones, N.; Rees, S.; O’Brien, T.J. Hypometabolism Precedes Limbic Atrophy and Spontaneous Recurrent Seizures in a Rat Model of TLE. Epilepsia 2012, 53, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, C.C.; Adelson, P.D.; Brenner, R.P.; Crumrine, P.K.; Van Cott, A.; Schiff, D.P.; Townsend, D.W.; Scheuer, M.L. Planned Ictal FDG PET Imaging for Localization of Extratemporal Epileptic Foci. Epilepsia 2000, 41, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tsytsarev, V.; Maslov, K.I.; Yao, J.; Parameswar, A.R.; Demchenko, A. V.; Wang, L. V. In Vivo Imaging of Epileptic Activity Using 2-NBDG, a Fluorescent Deoxyglucose Analog. J Neurosci Methods 2012, 203, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Agoston, S.; Bowman, W.C.; Houwertjes, M.C.; Rodger, I.W.; Savage, A.O. DIRECT ACTION OF 4-AMINOPYRIDINE ON THE CONTRACTILITY OF A FAST-CONTRACTING MUSCLE IN THE CAT. Clin Exp Pharmacol Physiol 1982, 9, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.O. A Comparison of the Effects of 4-Dimethylaminopyridine and 4-Aminopyridine on Isolated Cardiac and Skeletal Muscle Preparations. Arch Int Pharmacodyn Ther 1985, 273, 262–276. [Google Scholar]
- Smith, K.J.; Felts, P.A.; John, G.R. Effects of 4-Aminopyridine on Demyelinated Axons, Synapses and Muscle Tension. Brain 2000, 123, 171–184. [Google Scholar] [CrossRef]
- Borghammer, P. Perfusion and Metabolism Imaging Studies in Parkinson’s Disease. Dan Med J 2012, 59. [Google Scholar]
- Borghammer, P.; Jonsdottir, K.Y.; Cumming, P.; Ostergaard, K.; Vang, K.; Ashkanian, M.; Vafaee, M.; Iversen, P.; Gjedde, A. Normalization in PET Group Comparison Studies-The Importance of a Valid Reference Region. Neuroimage 2008, 40, 529–540. [Google Scholar] [CrossRef]
- Yakushev, I.; Hammers, A.; Fellgiebel, A.; Schmidtmann, I.; Scheurich, A.; Buchholz, H.G.; Peters, J.; Bartenstein, P.; Lieb, K.; Schreckenberger, M. SPM-Based Count Normalization Provides Excellent Discrimination of Mild Alzheimer’s Disease and Amnestic Mild Cognitive Impairment from Healthy Aging. Neuroimage 2009, 44, 43–50. [Google Scholar] [CrossRef]
- Yazdi, H.H.; Janahmadi, M.; Behzadi, G. The Role of Small-Conductance Ca2+-Activated K+ Channels in the Modulation of 4-Aminopyridine-Induced Burst Firing in Rat Cerebellar Purkinje Cells. Brain Res 2007, 1156, 59–66. [Google Scholar] [CrossRef]
- Haghdoost-Yazdi, H.; Janahmadi, M.; Behzadi, G. Iberiotoxin-Sensitive Large Conductance Ca2+-Dependent K+ (BK) Channels Regulate the Spike Configuration in the Burst Firing of Cerebellar Purkinje Neurons. Brain Res 2008, 1212, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Streng, M.L.; Krook-Magnuson, E. The Cerebellum and Epilepsy. Epilepsy and Behavior 2021, 121. [Google Scholar] [CrossRef] [PubMed]
- Bense, S.; Best, C.; Buchholz, H.G.; Wiener, V.; Schreckenberger, M.; Bartenstein, P.; Dieterich, M. 18F-Fluorodeoxyglucose Hypometabolism in Cerebellar Tonsil and Flocculus in Downbeat Nystagmus. Neuroreport 2006, 17, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.L.; Liu, Z.; Zeng, X.M.; Liu, Z.Q.; Chen, X.H.; Zhang, Z.H.; Mei, Y.A. 4-Aminopyridine, a Kv Channel Antagonist, Prevents Apoptosis of Rat Cerebellar Granule Neurons. Neuropharmacology 2006, 51, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Hardebo, J.E.; Lundh, H. Action of 4-Aminopyridine on the Cerebral Circulation. Acta Neurol Scand 1981, 63, 122–130. [Google Scholar] [CrossRef]
- Leung, Y.M. Voltage-Gated K+ Channel Modulators as Neuroprotective Agents. Life Sci 2010, 86, 775–780. [Google Scholar] [CrossRef]
- Ogita, K.; Okuda, H.; Watanabe, M.; Nagashima, R.; Sugiyama, C.; Yoneda, Y. In Vivo Treatment with the K+ Channel Blocker 4-Aminopyridine Protects against Kainate-Induced Neuronal Cell Death through Activation of NMDA Receptors in Murine Hippocampus. Neuropharmacology 2005, 48, 810–821. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and Epilepsy: Excitability and Inflammation. Trends Neurosci 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Purnell, B.S.; Alves, M.; Boison, D. Astrocyte-Neuron Circuits in Epilepsy. Neurobiol Dis 2023, 179. [Google Scholar] [CrossRef]
- Sanz, P.; Garcia-Gimeno, M.A. Reactive Glia Inflammatory Signaling Pathways and Epilepsy. Int J Mol Sci 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Verhoog, Q.P.; Holtman, L.; Aronica, E.; van Vliet, E.A. Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front Neurol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune Cell Regulation of Glia during CNS Injury and Disease. Nat Rev Neurosci 2020, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Bascuñana, P.; Gendron, T.; Sander, K.; Jahreis, I.; Polyak, A.; Ross, T.L.; Bankstahl, M.; Arstad, E.; Bankstahl, J.P. Ex Vivo Characterization of Neuroinflammatory and Neuroreceptor Changes during Epileptogenesis Using Candidate Positron Emission Tomography Biomarkers. Epilepsia 2019, 60, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Sano, F.; Shigetomi, E.; Shinozaki, Y.; Tsuzukiyama, H.; Saito, K.; Mikoshiba, K.; Horiuchi, H.; Cheung, D.L.; Nabekura, J.; Sugita, K.; et al. Reactive Astrocyte-Driven Epileptogenesis Is Induced by Microglia Initially Activated Following Status Epilepticus. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Wimberley, C.; Truillet, C.; Jego, B.; Caillé, F.; Pottier, G.; Boisgard, R.; Buvat, I.; Bouilleret, V. Longitudinal Positron Emission Tomography Imaging of Glial Cell Activation in a Mouse Model of Mesial Temporal Lobe Epilepsy: Toward Identification of Optimal Treatment Windows. Epilepsia 2018, 59, 1234–1244. [Google Scholar] [CrossRef]
- Nörenberg, W.; Gebicke-Haerter, P.J.; Illes, P. Voltage-Dependent Potassium Channels in Activated Rat Microglia. J Physiol 1994, 475, 15–32. [Google Scholar] [CrossRef]
- Liu, J.; Xu, E.; Tu, G.; Liu, H.; Luo, J.; Xiong, H. Methamphetamine Potentiates HIV-1gp120-Induced Microglial Neurotoxic Activity by Enhancing Microglial Outward K+ Current. Mol Cell Neurosci 2017, 82, 167–175. [Google Scholar] [CrossRef]
- Franciosi, S.; Ryu, J.K.; Choi, H.B.; Radov, L.; Kim, S.U.; McLarnon, J.G. Broad-Spectrum Effects of 4-Aminopyridine to Modulate Amyloid Beta1-42-Induced Cell Signaling and Functional Responses in Human Microglia. J Neurosci 2006, 26, 11652–11664. [Google Scholar] [CrossRef]
- Kong, S.; Chen, T. xiang; Jia, X. lei; Cheng, X. lei; Zeng, M. liu; Liang, J. yi; He, X. hua; Yin, J.; Han, S.; Liu, W. hong; et al. Cell-Specific NFIA Upregulation Promotes Epileptogenesis by TRPV4-Mediated Astrocyte Reactivity. J Neuroinflammation 2023, 20. [Google Scholar] [CrossRef]
- ELBini, I.; Neili, N. elhouda Potassium Channels at the Crossroads of Neuroinflammation and Myelination in Experimental Models of Multiple Sclerosis. Biochem Biophys Res Commun 2023, 653, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ahtiainen, A.; Genocchi, B.; Tanskanen, J.M.A.; Barros, M.T.; Hyttinen, J.A.K.; Lenk, K. Astrocytes Exhibit a Protective Role in Neuronal Firing Patterns under Chemically Induced Seizures in Neuron-Astrocyte Co-Cultures. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Slowing, K.; Gomez, F.; Delgado, M.; Fernández De La Rosa, R.; Hernández-Martín, N.; Pozo, M.Á.; García-García, L. PET Imaging and Neurohistochemistry Reveal That Curcumin Attenuates Brain Hypometabolism and Hippocampal Damage Induced by Status Epilepticus in Rats. Planta Med 2023, 89, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Prieto, E.; Collantes, M.; Delgado, M.; Juri, C.; García-García, L.; Molinet, F.; Fernández-Valle, M.E.; Pozo, M.A.; Gago, B.; Martí-Climent, J.M.; et al. Statistical Parametric Maps of 18F-FDG PET and 3-D Autoradiography in the Rat Brain: A Cross-Validation Study. Eur J Nucl Med Mol Imaging 2011, 38, 2228–2237. [Google Scholar] [CrossRef]
- Gu, Q.; Schmued, L.C.; Sarkar, S.; Paule, M.G.; Raymick, B. One-Step Labeling of Degenerative Neurons in Unfixed Brain Tissue Samples Using Fluoro-Jade C. J Neurosci Methods 2012, 208, 40–43. [Google Scholar] [CrossRef]
- Foucault-Fruchard, L.; Doméné, A.; Page, G.; Windsor, M.; Emond, P.; Rodrigues, N.; Dollé, F.; Damont, A.; Buron, F.; Routier, S.; et al. Neuroprotective Effect of the Alpha 7 Nicotinic Receptor Agonist PHA 543613 in an in Vivo Excitotoxic Adult Rat Model. Neuroscience 2017, 356, 52–63. [Google Scholar] [CrossRef]
- García-García, L.; Gomez, F.; Delgado, M.; Fernández de la Rosa, R.; Pozo, M.Á. The Vasodilator Naftidrofuryl Attenuates Short-Term Brain Glucose Hypometabolism in the Lithium-Pilocarpine Rat Model of Status Epilepticus without Providing Neuroprotection. Eur J Pharmacol 2023, 939. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).