Submitted:
23 October 2024
Posted:
25 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Body Composition Changes
2.1. Skeletal Muscle

2.2. Bone

2.3. Adipose Tissue
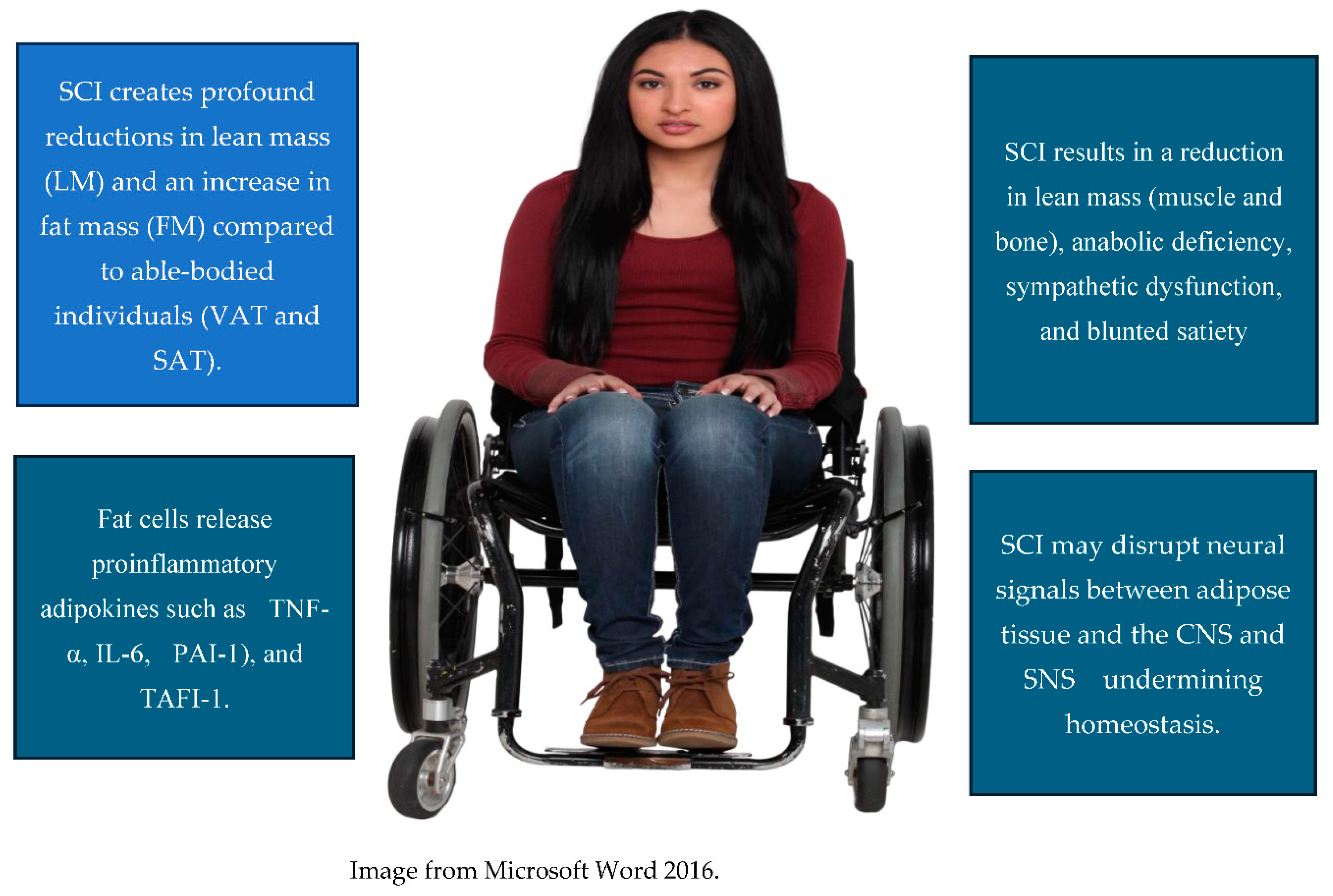
3. Endometabolic

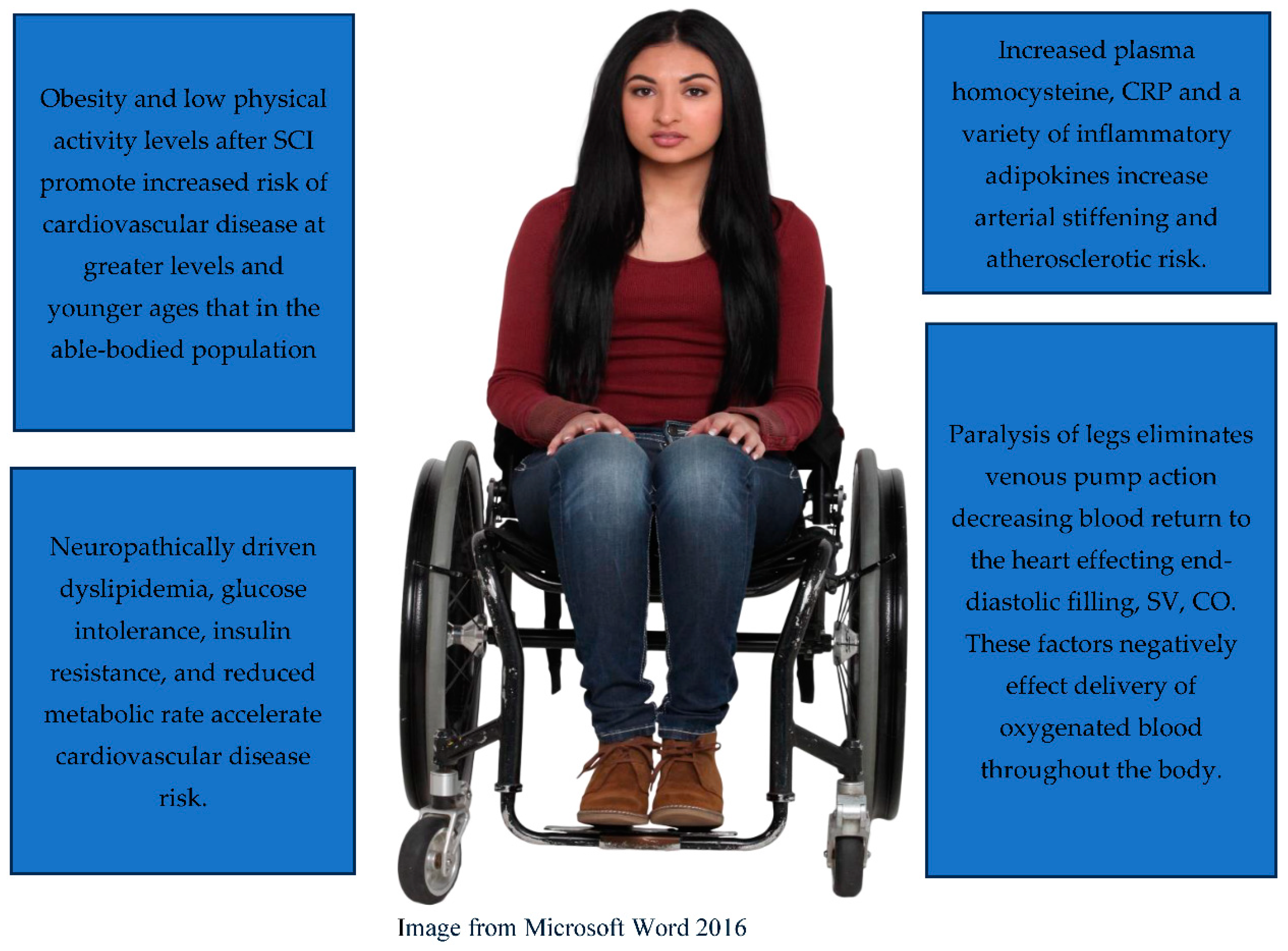
4. Cardiovascular
5. Conclusions
References
- Bennett J, M Das J, Emmady PD. Spinal Cord Injuries. [Updated 2022 May 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from:.
- Traumatic Spinal Cord Injury Facts and Figures at a Glance. National Spinal Cord Injury Statistical Center. 2024. Accessed April 18, 2024. https://www.nscisc.uab.edu/Public/Facts and Figures 2020.pdf.
- Middleton, J.W.; Dayton, A.; Walch, J.; Rutkowski, S.B.; Leong, G.; Duong, S. Life expectancy after spinal cord injury: a 50-year study. Spinal Cord 2012, 50, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.; Yakura, J. Aging related functional changes in persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2006, 6, 69–82. [Google Scholar] [CrossRef]
- Hitzig, S.; Eng, J.; Miller, W.; et al. An evidence-based review of aging of the body systems following spinal cord injury. Spinal Cord 2011, 49, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.; Thompson, L. Aging and spinal cord injury: medical, functional, and psychosocial changes. SCI Nursing. 2002, 19, 51–60. [Google Scholar]
- Groah, S.L.; Charlifue, S.; Tate, D.; Jensen, M.P.; Molton, I.R.; Forchheimer, M.; Krause, J.S.; Lammertse, D.P.; Campbell, M. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012, 91, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.E.; Mollett, P. Aging with Spinal Cord Injury: An Update. Phys Med Rehabil Clin N Am. 2017, 28, 821–828. [Google Scholar] [CrossRef]
- Charlifue, S.; Jha, A.; Lammertse, D. Aging with spinal cord injury. Phys Med Rehabil Clin N Am. 2010, 21, 383–402. [Google Scholar] [CrossRef]
- Miljkovic, N.; Lim, J.-Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Annals of Rehabilitation Medicine. 2015, 39, 155. [Google Scholar] [CrossRef]
- Xu, X.; Talifu, Z.; Zhang, C.J.; et al. Mechanism of skeletal muscle atrophy after spinal cord injury: A narrative review. Front Nutr. 2023, 10, 1099143. [Google Scholar] [CrossRef]
- Alazzam, A.M.; Goldsmith, J.A.; Khalil, R.E.; Khan, M.R.; Gorgey, A.S. Denervation impacts muscle quality and knee bone mineral density after spinal cord injury. Spinal Cord. 2023, 61, 276–284. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Davis, J.; Bersch, I.; Goldberg, G.; Gorgey, A.S. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen Res. 2020, 15, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, B.; Kristensen, I.B.; Kjær, M.; Biering-Sørensen, F. Muscle after Spinal Cord Injury. Muscle & Nerve. 2009, 40, 499–519. [Google Scholar] [CrossRef]
- Grimby, G.; Bruberg, G.; Krotkiewski, I.; Krotkiewiski, M. Muscle fiber composition in patients with traumatic cord liesion. Scand J Rehabil. Med. 1976, 8, 37–42. [Google Scholar] [PubMed]
- Crameri, R.M.; Weston, A.R.; Rutkowski, S.; Middleton, J.W.; Davis, G.M.; Sutton, J.R. Effects of electrical stimulation leg training during the acute phase of Spinal Cord Injury: A pilot study. European Journal of Applied Physiology. 2002, 83, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, R.; Marchetti, C.; Poggi, P.; Lotta, S.; Lommi, G. Muscle fiber type morphology and distribution in paraplegic patients with traumatic cord lesion. Acta Neuropathologica. 1982, 57, 243–248. [Google Scholar] [CrossRef]
- Lotta, S.; Scelsi, R.; Alfonsi, E.; et al. Morphometric and neurophysiological analysis of skeletal muscle in paraplegic patients with traumatic cord lesion. Spinal Cord. 1991, 29, 247–252. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.C.; Wade, R.C.; Segal, L.; Chen, Q.; Savas, J.; Lesnefsky, E.J.; Gorgey, A.S. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol Rep. 2017, 5, e13080. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Chen, Q.; Savas, J.; Lesnefsky, E.J.; Gorgey, A.S. Skeletal muscle mitochondrial mass is linked to lipid and metabolic profile in individuals with spinal cord injury. Eur J Appl Physiol. 2017, 117, 2137–2147. [Google Scholar] [CrossRef]
- Burnham, R.; Martin, T.; Stein, R.; Bell, G.; MacLean, I.; Steadward, R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord. 1997, 35, 86–91. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Dirks, M.L.; Snijders, T.; et al. Reduced satellite cell numbers with spinal cord injury and aging in humans. Medicine & Science in Sports & Exercise. 2012, 44, 2322–2330. [Google Scholar] [CrossRef]
- Bazgir, B.; Fathi, R.; Rezazadeh Valojerdi, M.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J. 2017, 18, 473–484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holman, M.E.; Gorgey, A.S. Testosterone and Resistance Training Improve Muscle Quality in Spinal Cord Injury. Med Sci Sports Exerc. 2019, 51, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Graham, Z.A.; Chen, Q.; Rivers, J.; Adler, R.A.; Lesnefsky, E.J.; Cardozo, C.P. Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J Appl Physiol (1985). 2020, 128, 1487–1496. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury on Cardio-Metabolic Risk Factors: An Open-Label Randomized Clinical Trial. J Neurotrauma. 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Bersch, I.; Gorgey, A.S.; Davis, G.M. The Clinical Management of Electrical Stimulation Therapies in the Rehabilitation of Individuals with Spinal Cord Injuries. J Clin Med 2024, 13, 2995. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Sutor, T.W.; Musselman, K.; Bochkezanian, V.; Davis, G.M. Electrical Stimulation Exercise Recommendations for Individuals With Spinal Cord Injury. Arch Phys Med Rehabil Epub ahead of print. 2023. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Coleman, R. Aging and Bone. Journal of Dental Research. 2010, 89, 1333–1348. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. European Journal of Rheumatology. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Battaglino, R.A.; Lazzari, A.A.; Garshick, E.; Morse, L.R. Spinal cord injury-induced osteoporosis: pathogenesis and emerging therapies. Curr Osteoporos Rep. 2012, 10, 278–285. [Google Scholar] [CrossRef]
- Antoniou, G.; Benetos, I.S.; Vlamis, J.; Pneumaticos, S.G. Bone Mineral Density Post a Spinal Cord Injury: A Review of the Current Literature Guidelines. Cureus. 2022, 14, e23434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sezer, N.; Akkuş, S.; Gülçin Uğurlu, F. Chronic complications of Spinal Cord Injury. World Journal of Orthopedics. 2015, 6, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Hangartner, T.N.; Rodgers, M.M.; Glasser, R.M.; Barre, P.S. Tibial Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev. 1994, 31, 50–61. [Google Scholar] [PubMed]
- Dolbow, D.R.; Gorgey, A.S.; Daniels, J.A.; Adler, R.A.; Moore, J.R.; Gater, D.R. The effects of spinal cord injury and exercise on Bone Mass: A literature review. NeuroRehabilitation. 2011, 29, 261–269. [Google Scholar] [CrossRef]
- Modlesky, C.M.; Slade, J.M.; Bickel, C.S.; Meyer, R.A.; Dudley, G.A. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005, 36, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Cardozo, C.P. Osteoporosis in individuals with spinal cord injury. PM R. 2015, 7, 188–201. [Google Scholar] [CrossRef]
- Haider, I.T.; Lobos, S.M.; Simonian, N.; Schnitzer, T.J.; Edwards, W.B. Bone fragility after spinal cord injury: reductions in stiffness and bone mineral at the distal femur and proximal tibia as a function of time. Osteoporos Int. 2018, 29, 2703–2715. [Google Scholar] [CrossRef]
- Edwards, W.B.; Simonian, N.; Troy, K.L.; Schnitzer, T.J. Reduction in torsional stiffness and strength at the proximal tibia as a function of time since spinal cord injury. J Bone Miner Res. 2015, 30, 1422–1430. [Google Scholar] [CrossRef]
- Abderhalden, L.; Weaver, F.M.; Bethel, M.; et al. Dual-energy X-ray absorptiometry and fracture prediction in patients with spinal cord injuries and disorders. Osteoporos Int. 2017, 28, 925–934. [Google Scholar] [CrossRef]
- Berger, C.; Langsetmo, L.; Joseph, L.; et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Canadian Medical Association Journal. 2008, 178, 1660–1668. [Google Scholar] [CrossRef]
- Wilmet, E.; Ismail, A.; Heilporn, A.; et al. ; Longitudinal study of bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995, 33, 674–677. [Google Scholar] [CrossRef]
- Garland, D.E.; Adkins, R.H. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil 2001, 6, 37–46. [Google Scholar] [CrossRef]
- Modlesky, C.M.; Majumdar, S.; Narasimhan, A.; Dudley, G.A. Trabecular Bone microarchitecture is deteriorated in men with Spinal Cord Injury. Journal of Bone and Mineral Research. 2004, 19, 48–55. [Google Scholar] [CrossRef]
- Slade, J.M.; Bickel, C.S.; Modlesky, C.M.; Majumdar, S.; Dudley, G.A. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos Int. 2005, 16, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Dudley-Javoroski, S.; Boaldin, K.M.; Corey, T.A.; Fog, D.B.; Ruen, J.M. Peripheral quantitative computed tomography: Measurement sensitivity in persons with and without spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2006, 87, 1376–1381. [Google Scholar] [CrossRef]
- Dauty, M.; Perrouin Verbe, B.; Maugars, Y.; Dubois, C.; Mathe, J.F. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 2000, 27, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Grassner, L.; Klein, B.; Maier, D.; Bühren, V.; Vogel, M. Lower extremity fractures in patients with spinal cord injury characteristics, outcome and risk factors for non-unions. J Spinal Cord Med. 2018, 41, 676–683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maynard, F.M.; Karunas, R.S.; Adkins, R.H. Maynard, F.M.; Karunas, R.S.; Adkins, R.H. Management of the neuromuscular system. In: Stover SL, Delisa JA, Whiteneck GG, editors. Spinal cord injury: clinical outcomes of the model systems. Aspen; Gaitersburg, MD: 1995. pp. 163–169.
- Kostovski, E.; Hjeltnes, N.; Eriksen, E.F.; Kolset, S.O.; Iversen, P.O. Differences in bone mineral density, markers of bone turnover and extracellular matrix and daily life muscular activity among patients with recent motor incomplete versus motor-complete spinal cord injury. Calcif Tissue Int. 2015, 96, 145-014-154. [Google Scholar] [CrossRef]
- Mai'moun, L.; Ben Bouallegue, F.; Gelis, A.; et al. Periostin and sclerostin levels in individuals with spinal cord injury and their relationship with bone mass, bone turnover, fracture and osteoporosis status. Bone. 2019, 127, 612–619. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Poarch, H.J.; Adler, R.A.; Khalil, R.E.; Gater, D.R. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM R. 2013, 5, 939–948. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Johnston, T.E.; Bersch, I. Electrical Stimulation Exercise for People with Spinal Cord Injury: A Healthcare Provider Perspective. J Clin Med. 2023, 12, 3150. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Sutor, T.W.; Musselman, K.; Bochkezanian, V.; Davis, G.M. Electrical Stimulation Exercise Recommendations for Individuals With Spinal Cord Injury. Arch Phys Med Rehabil Epub ahead of print. 2023. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.E.; Shields, D.C.; Haque, A.; Banik, N.L. Rehabilitation: Neurogenic Bone Loss after Spinal Cord Injury. Biomedicines. 2023, 11, 2581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- JafariNasabian, P.; Inglis, J.E.; Reilly, W.; Kelly, O.J.; Ilich, J.Z. Aging human body: Changes in bone, muscle, and body fat with consequent changes in nutrient intake. Journal of Endocrinology 2017, 234. [Google Scholar] [CrossRef]
- Gater, D.R.; Farkas, G.J.; Tiozzo, E.T. Pathophysiology of neurogenic obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2021, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spungen, A.M.; Adkins, R.H.; Stewart, C.A.; Wang, J.; Pierson, R.N., Jr.; Waters, R.L.; et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003, 95, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Mather, K.J.; Poarch, H.J.; Gater, D.R. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J Spinal Cord Med. 2011, 34, 99–109. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Wells, K.M.; Austin, T.L. Adiposity and spinal cord injury. World J Orthop. 2015, 6, 567–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rankin, K.C.; O'Brien, L.C.; Segal, L.; Khan, M.R.; Gorgey, A.S. Liver Adiposity and Metabolic Profile in Individuals with Chronic Spinal Cord Injury. Biomed Res Int. 2017, 2017, 1364818. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. The influence of level of spinal cord injury on adipose tissue and its relationship to inflammatory adipokines and cardiometabolic profiles. The Journal of Spinal Cord Medicine. 2017, 41, 407–415. [Google Scholar] [CrossRef]
- Spungen, A.M.; Wang, J.; Pierson, R.N.; Bauman, W.A. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. Journal of Applied Physiology. 2000, 88, 1310–1315. [Google Scholar] [CrossRef]
- Ogawa, M.; Lester, R.; Akima, H.; Gorgey, A.S. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen Res. 2017, 12, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.B.; Tataranni, P.A.; Pratley, R.; Manore, M.M.; Skinner, J.S.; Ravussin, E. Lower Daily Energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. The American Journal of Clinical Nutrition. 1998, 68, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Gater, D.R. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with Spinal Cord Injury. Applied Physiology, Nutrition, and Metabolism. 2011, 36, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gater, D.R.; Farkas, G.J.; Dolbow, D.R.; Berg, A.; Gorgey, A.S. Body composition assessment after motor complete spinal cord injury: Development of a clinically relevant equation to estimate body fat. Topics in Spinal Cord Injury Rehabilitation. 2021, 27, 11–22. [Google Scholar] [CrossRef]
- McMillan, D.W.; Bigford, G.E.; Farkas, G.J. The Physiology of Neurogenic Obesity: Lessons from Spinal Cord Injury Research. Obes Facts. 2023, 16, 313–325. [Google Scholar] [CrossRef]
- McMillan, D.W.; Maher, J.L.; Jacobs, K.A.; Nash, M.S.; Gater, D.R., Jr. Exercise Interventions Targeting Obesity in Persons With Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2021, 27, 109–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shojaei, M.H.; Alavinia, S.M.; Craven, B.C. Management of obesity after spinal cord injury: a systematic review. J Spinal Cord Med. 2017, 40, 783–794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes. 2012, 61, 1315–1322. [Google Scholar] [CrossRef]
- Nelson, M.D.; Widman, L.M.; Abresch, R.T.; et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007, 30 (Suppl 1), S127–S139. [Google Scholar] [CrossRef]
- Manns, P.J.; McCubbin, J.A.; Williams, D.P. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Archives of Physical Medicine and Rehabilitation. 2005, 86, 1176–1181. [Google Scholar] [CrossRef]
- LaVela, S.L.; Weaver, F.M.; Goldstein, B.; et al. Diabetes mellitus in individuals with spinal cord injury or disorder. The Journal of Spinal Cord Medicine. 2006, 29, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, T.E.; Moore, P.; Harman, J.; et al. Body composition changes with testosterone replacement therapy following spinal cord injury and aging: A mini review. The Journal of Spinal Cord Medicine. 2017, 41, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.J.; Sneij, A.; Gater, D.R. Energy expenditure following spinal cord injury: A delicate balance. Topics in Spinal Cord Injury Rehabilitation. 2021, 27, 92–99. [Google Scholar] [CrossRef]
- Bauman, W.A.; Spungen, A.M.; Wang, J.; Pierson, R.N. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. The Journal of Rehabilitation Research and Development. 2004, 41, 1. [Google Scholar] [CrossRef]
- Barbonetti, A.; Caterina Vassallo, M.R.; Cotugno, M.; Felzani, G.; Francavilla, S.; Francavilla, F. Low testosterone and non-alcoholic fatty liver disease: Evidence for their independent association in men with Chronic Spinal Cord Injury. The Journal of Spinal Cord Medicine. 2016, 39, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Muzumdar, R.; Atzmon, G.; Barzilai, N. The paradox of the insulin/IGF-1 signaling pathway in longevity. Mechanisms of Ageing and Development. 2004, 125, 397–403. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.J.; Lu, Z.; Warneryd, A.C.; Swanson, R.L., 2nd. A Systematic Review of Testosterone Therapy in Men With Spinal Cord Injury or Traumatic Brain Injury. Cureus. 2023, 15, e34264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macvanin, M.; Gluvic, Z.; Radovanovic, J.; Essack, M.; Gao, X.; Isenovic, E.R. New insights on the cardiovascular effects of IGF-1. Front Endocrinol (Lausanne). 2023, 14, 1142644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peterson, M.D.; Berri, M.; Lin, P.; et al. Cardiovascular and metabolic morbidity following spinal cord injury. The Spine Journal. 2021, 21, 1520–1527. [Google Scholar] [CrossRef]
- Nash, M.S.; Bilzon, J.L.J. Guideline Approaches for Cardioendocrine Disease Surveillance and Treatment Following Spinal Cord Injury. Curr Phys Med Rehabil Rep 2018, 6, 264–276. [Google Scholar] [CrossRef]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac changes associated with vascular aging. Clin Cardiol. 2020, 43, 92–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panemi, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J Am Coll Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Lavis, T.D.; Scelza, W.M.; Bockenek, W.L. Cardiovascular health and fitness in persons with spinal cord injury. Phys Med Rehabil Clin N Am. 2007, 18, 317–vii. [Google Scholar] [CrossRef] [PubMed]
- Capoor, J.; Stein, A.B. Aging with spinal cord injury. Physical Medicine and Rehabilitation Clinics of North America. 2005, 16, 129–161. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Spungen, A.M.; Zhong, Y.-G.; Rothstein, J.L.; Petry, C.; Gordon, S.K. Depressed serum high density lipoprotein cholesterol levels in veterans with Spinal Cord Injury. Spinal Cord. 1992, 30, 697–703. [Google Scholar] [CrossRef]
- Pradhan, A.D. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001, 286, 327. [Google Scholar] [CrossRef]
- Figoni, S.F.; Dolbow, D.R.; Crawford, E.C.; White, M.L.; Pattanaik, S. Does aerobic exercise benefit persons with tetraplegia from spinal cord injury? A systematic review. J Spinal Cord Med. 2021, 44, 690–703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hodgkiss, D.D.; Bhangu, G.S.; Lunny, C.; et al. Exercise and aerobic capacity in individuals with spinal cord injury: A systematic review with meta-analysis and meta-regression. PLoS Med. 2023, 20, e1004082. [Google Scholar] [CrossRef]
- Hopman, M.T.E.; Monroe, M.; Dueck, C.; et al. Blood redistribution and Circulatory Responses to Sub-maximal Arm exercises in persons with spinal cord injury. Scand J Rehabil Med. 1998, 30, 167–175. [Google Scholar]
- Gater, D.R.; Dolbow, D.; Tsui, B.; Gorgey, A.S. Functional Electrical Stimulation Therapies after Spinal Cord Injury. NeuroRehabilitation. 2011, 28, 231–48. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dolbow, D.R.; Dolbow, J.D.; Gater, D.R. The Effects of Electrical Stimulation on Body Composition and Metabolic Profile after Spinal Cord Injury- Part II. Journal of Spinal Cord Medicine. 2015, 38, 23–37. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Syndrome (Adapted from Gater et al., 2021)67 | n (%) |
| Metabolic syndrome = 3 abnormal measures out of the 5 from the following list. | 3/5 |
| SCI-Specific BMI ≥ 22 kg/m2 (n=72) | 59 (82%) |
| 4 Compartment Model % body fat | |
| All (n=72) | 70 (97%) |
| ≥ 22 for men (n=59) | 59 (100%) |
| ≥ 35 for women (n=13) | 11 (85%) |
| Triglycerides ≥ 150 mg/dl or under Treatment (n=70) | 23 (33%) |
| High density lipoprotein cholesterol < 40 (men) or < 50 (women) mg/dl or under Treatment | |
| All (n=72) | 60 (83%) |
| < 40 for men (n=59) | 50 (85%) |
| < 50 for women (n=13) | 10 (77%) |
| Systolic blood pressure ≥ 130 or Diastolic blood pressure ≥ 85 mmHg, or under Treatment (n=72) | 31 (43%) |
| Fasting Glucose ≥ 100 mg/dl or under Treatment (n=71) | 23 (32%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





