Submitted:
22 October 2024
Posted:
23 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
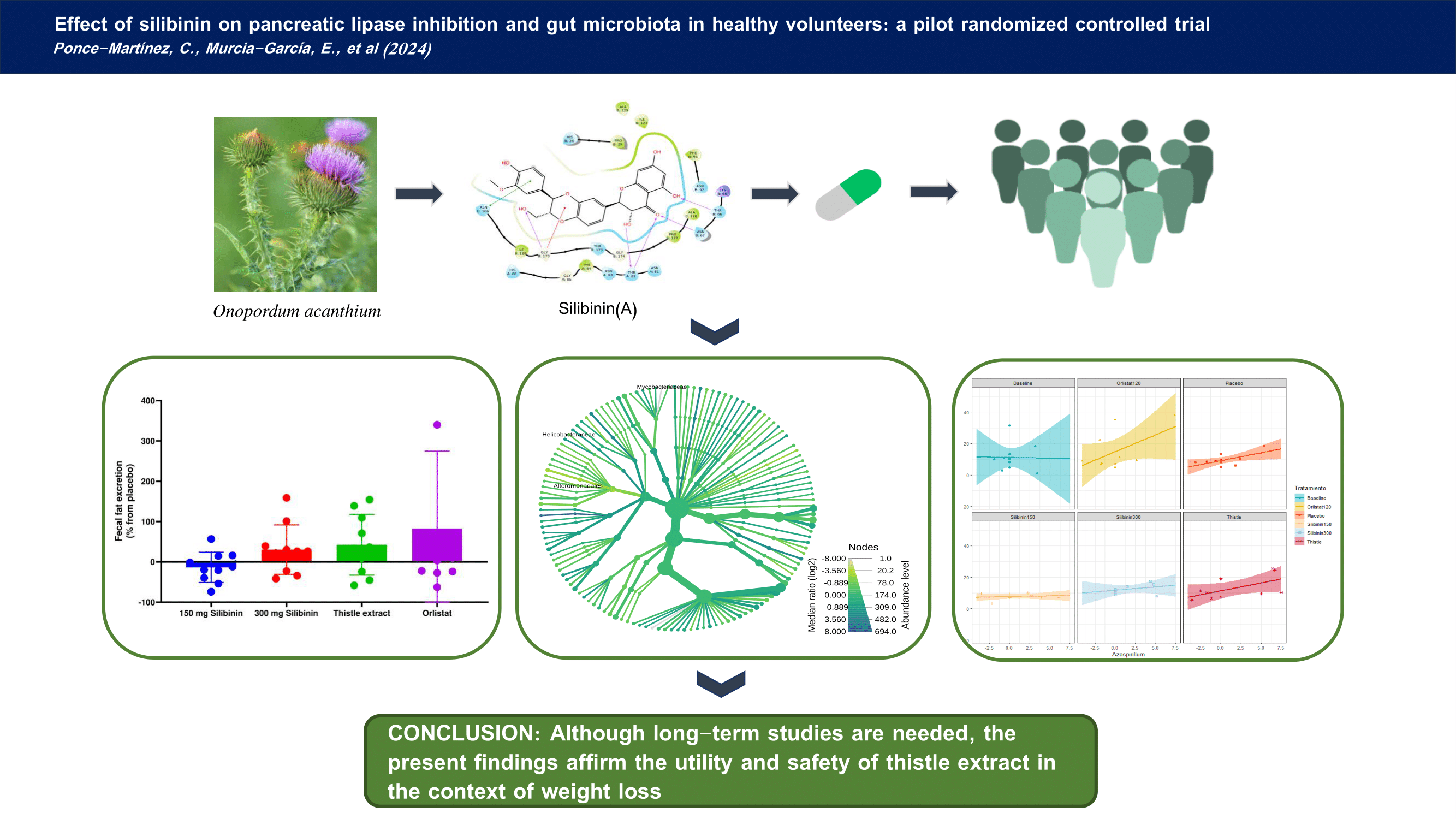
2. Results
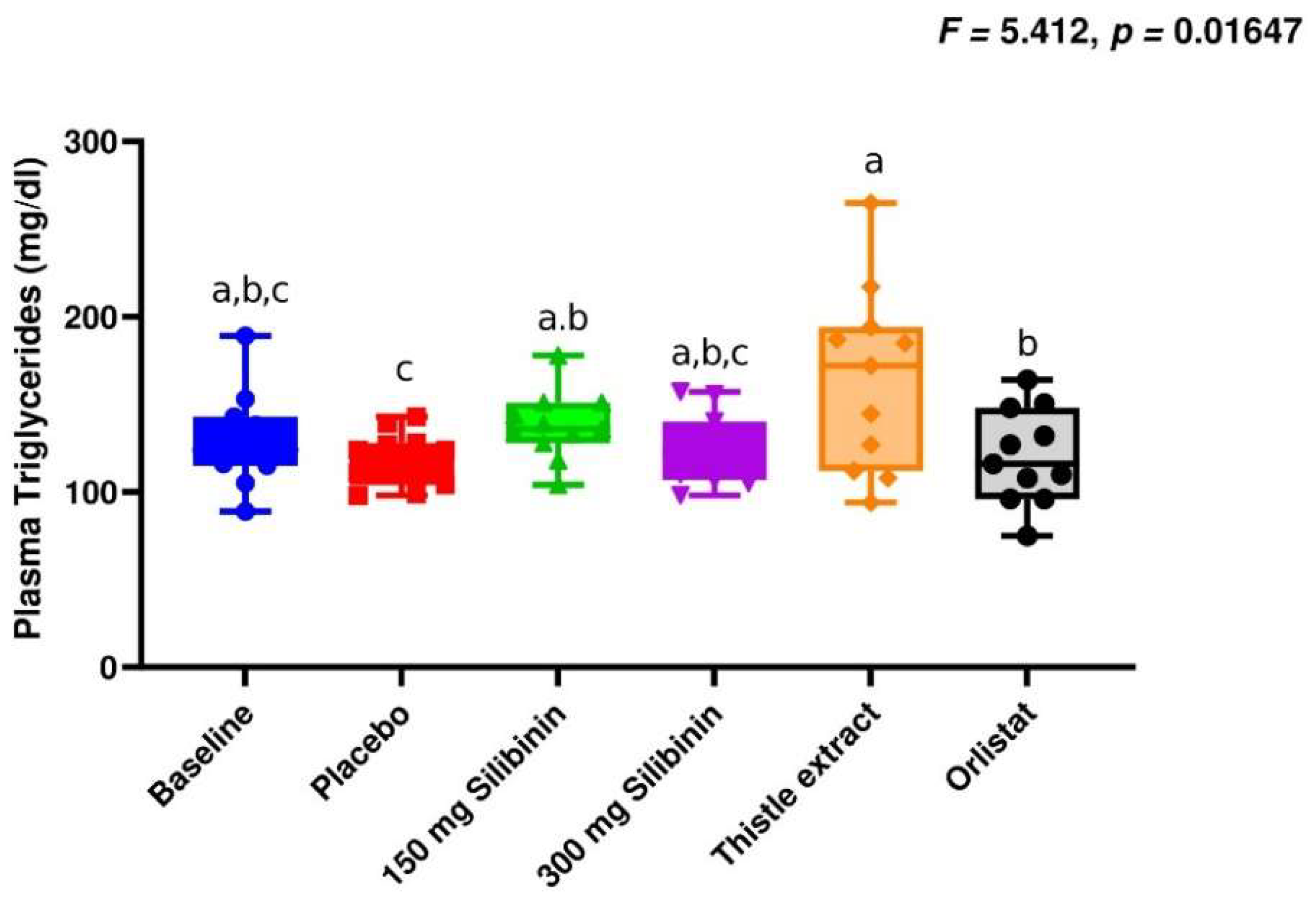
2.1. Effect of Silibinin on Clinical Parameters
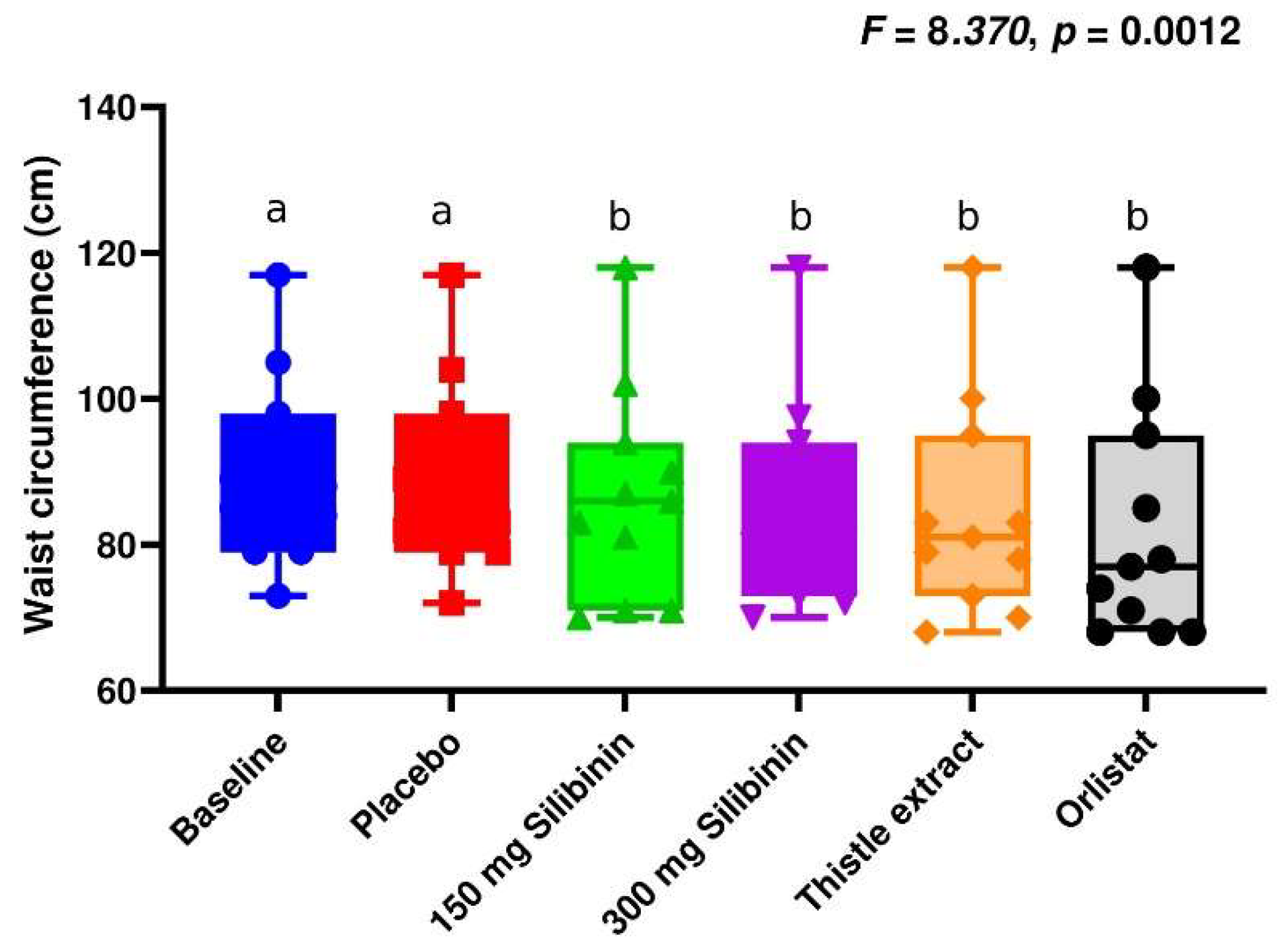
2.2. Effect of Silibinin on Anthropometric Parameters
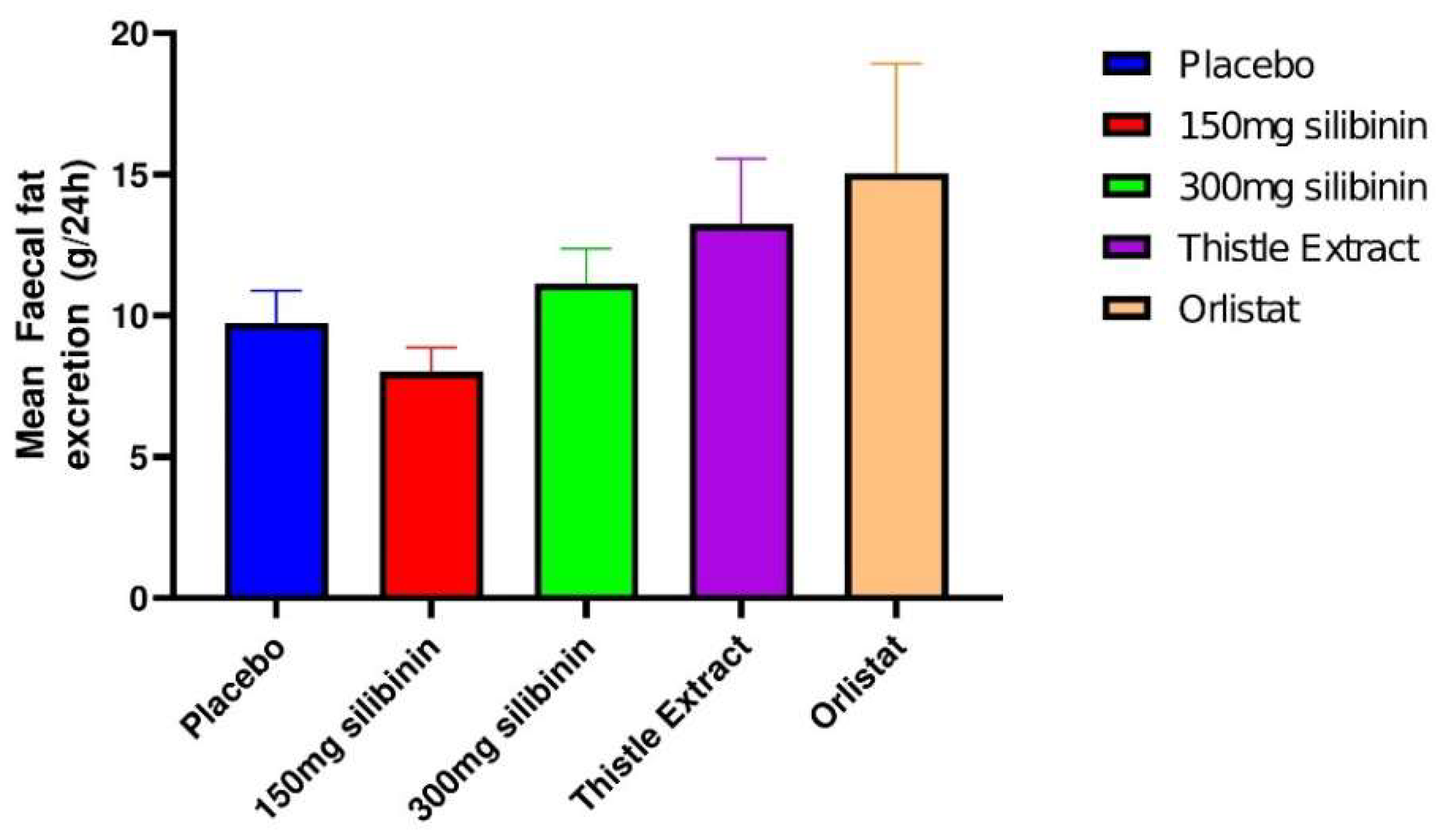
2.3. Effect of Silibinin on Faecal Fat Excretion
2.4. Effect of Silibinin on Gut Microbiota
3. Discussion
4. Materials and Methods
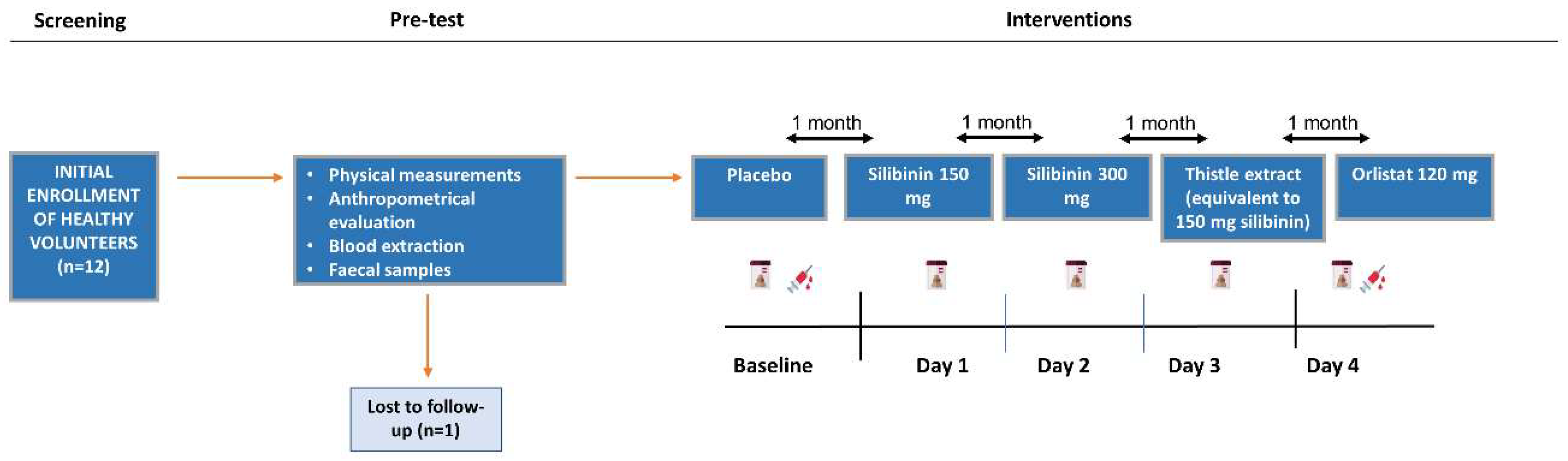
4.1. Study Design
4.2. Outcome Measurement
4.3. Participants
4.4. Anthropometric and Clinical Measurements
4.5. Biochemical Parameters
4.6. Faecal Sample Collection and Metagenomic Data
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 May 2022).
- Overweight and Obesity - What Are Overweight and Obesity? | NHLBI, NIH Available online: https://www.nhlbi.nih.gov/health/overweight-and-obesity (accessed on 12 December 2023).
- Bryson, A.; De La Motte, S.; Dunk, C. Reduction of dietary fat absorption by the novel gastrointestinal lipase inhibitor cetilistat in healthy volunteers. Br. J. Clin. Pharmacol. 2009, 67, 309–315. [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [CrossRef]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obes. Metab. 2017, 19, 1242–1251. [CrossRef]
- Chao, A.M.; Tronieri, J.S.; Amaro, A.; A Wadden, T. Clinical Insight on Semaglutide for Chronic Weight Management in Adults: Patient Selection and Special Considerations. Drug Des. Dev. Ther. 2022, ume 16, 4449–4461. [CrossRef]
- Uehira, Y.; Ueno, H.; Miyamoto, J.; Kimura, I.; Ishizawa, Y.; Iijima, H.; Muroga, S.; Fujita, T.; Sakai, S.; Samukawa, Y.; et al. Impact of the lipase inhibitor orlistat on the human gut microbiota. Obes. Res. Clin. Pr. 2023, 17, 411–420. [CrossRef]
- Sierra, A.C.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [CrossRef]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [CrossRef]
- Del Castillo-Santaella, T.; Hernández-Morante, J.J.; Suárez-Olmos, J.; Maldonado-Valderrama, J.; Peña-García, J.; Martínez-Cortés, C.; Pérez-Sánchez, H. Identification of the thistle milk component Silibinin(A) and Glutathione-disulphide as potential inhibitors of the pancreatic lipase: Potential implications on weight loss. J. Funct. Foods 2021, 83, 104479. [CrossRef]
- Loguercio, C. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-Associated Adverse Effects and Drug Interactions. Drug Saf. 2008, 31, 53–65. [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study. Diabetes Care 2004, 27, 155–161. [CrossRef]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity – An update. Biomed. Pharmacother. 2021, 140, 111789. [CrossRef]
- McDuffie, J.R.; Calis, K.A.; Booth, S.L.; Uwaifo, G.I.; Yanovski, J.A. Effects of Orlistat on Fat-Soluble Vitamins in Obese Adolescents. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 814–822. [CrossRef]
- Wilde, P.; Chu, B. Interfacial & colloidal aspects of lipid digestion. Adv. Colloid Interface Sci. 2011, 165, 14–22. [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020, 11, 1199–1216. [CrossRef]
- Orlistat (Marketed as Alli and Xenical) Information | FDA Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/orlistat-marketed-alli-and-xenical-information (accessed on 12 December 2023).
- Kheong, C.W.; Mustapha, N.R.N.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940–1949.e8. [CrossRef]
- MacDonald-Ramos, K.; Michán, L.; Martínez-Ibarra, A.; Cerbón, M. Silymarin is an ally against insulin resistance: A review. Ann. Hepatol. 2020, 23, 100255. [CrossRef]
- Liu, X.; Hu, M.; Ye, C.; Liao, L.; Ding, C.; Sun, L.; Liang, J.; Chen, Y. Isosilybin regulates lipogenesis and fatty acid oxidation via the AMPK/SREBP-1c/PPARα pathway. Chem. Interactions 2022, 368, 110250. [CrossRef]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martínez, J.A.; Riezu-Boj, J.I. Gut Microbiota Differences According to Ultra-Processed Food Consumption in a Spanish Population. Nutrients 2021, 13, 2710. [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of Linoleic Acid by Human Gut Bacteria: Different Routes for Biosynthesis of Conjugated Linoleic Acid. J. Bacteriol. 2007, 189, 2566–2570. [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of Linoleic Acid by Human Gut Bacteria: Different Routes for Biosynthesis of Conjugated Linoleic Acid. J. Bacteriol. 2007, 189, 2566–2570. [CrossRef]
- Albornoz, S.P.P.; Fraga-Silva, T.F.d.C.; Gembre, A.F.; de Oliveira, R.S.; de Souza, F.M.; Rodrigues, T.S.; Kettelhut, I.D.C.; Manca, C.S.; Jordao, A.A.; Ramalho, L.N.Z.; et al. Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection. Cells 2021, 10, 1732. [CrossRef]
- Amoroso, M.; Böttcher, A.; Lowry, C.A.; Langgartner, D.; Reber, S.O. Subcutaneous Mycobacterium vaccae promotes resilience in a mouse model of chronic psychosocial stress when administered prior to or during psychosocial stress. Brain, Behav. Immun. 2020, 87, 309–317. [CrossRef]
- Nesmiyanov, P.P.; Petrov, V.I.; Tolkachev, B.E.; Morkovin, E.I.; Gutov, M.V.; Strygina, A.O. Mycobacterium vaccae Lysate Induces Anti-Allergic Immune Response In Vitro. Bull. Exp. Biol. Med. 2020, 170, 226–229. [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2019, 78, 108324. [CrossRef]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [CrossRef]
- Peirotén, .; Bravo, D.; Landete, J.M. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit. Rev. Food Sci. Nutr. 2019, 60, 1922–1937. [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 1–14. [CrossRef]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [CrossRef]
- Franson, K.; Rossner, S. Fat intake and food choices during weight reduction with diet, behavioural modification and a lipase inhibitor. J. Intern. Med. 2000, 247, 607–614. [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 270–279. [CrossRef]
- Horowitz, M.; Flint, A.; Jones, K.L.; Hindsberger, C.; Rasmussen, M.F.; Kapitza, C.; Doran, S.; Jax, T.; Zdravkovic, M.; Chapman, I.M. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res. Clin. Pr. 2012, 97, 258–266. [CrossRef]
- Muñoz, J.S.G.; Morillas-Ruiz, J.M.; Gallego, M.G.; Soler, I.D.; Ortega, M.d.C.B.; Martínez, C.M.; Morante, J.J.H. Cognitive Training Therapy Improves the Effect of Hypocaloric Treatment on Subjects with Overweight/Obesity: A Randomised Clinical Trial. Nutrients 2019, 11, 925. [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. WITHDRAWN: CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2010, 152, 726–732.
- Fernández, M.G.; Marset, J.B.; Lesmes, I.B.; Izquierdo, J.Q.; Sala, X.F.; Salas-Salvadó, J. Resumen del consenso FESNAD-SEEDO: recomendaciones nutricionales basadas en la evidencia para la prevención y el tratamiento del sobrepeso y la obesidad en adultos. 2012, 59, 429–437. [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [CrossRef]
| Placebo | 150 mg silibinin | 300 mg silibinin | Thistle extract | Orlistat | Total | |
|---|---|---|---|---|---|---|
| Abdominal pain | 1 | 1 | 1 | 3 | 2 | 8 |
| Change in bowel habits | 1 | 4 | 0 | 2 | 4 | 11 |
| Constipation | 4 | 3 | 4 | 1 | 3 | 15 |
| Diarrhoea | 0 | 0 | 0 | 1 | 3 | 4 |
| Dysphagia | 0 | 0 | 0 | 0 | 0 | 0 |
| Dry mouth | 1 | 4 | 2 | 2 | 2 | 11 |
| Flatulence | 2 | 0 | 1 | 1 | 4 | 8 |
| Melaena | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting/nausea | 0 | 1 | 0 | 0 | 2 | 3 |
| Headache | 0 | 1 | 1 | 1 | 0 | 3 |
| Fatty stools | 0 | 0 | 0 | 0 | 3 | 3 |
| Other | 1 | 1 | 0 | 0 | 0 | 2 |
| Total | 10 | 15 | 9 | 11 | 23 | 68 |
| Bacteria Name | Log2CF | P-value | FDR |
|---|---|---|---|
| Placebo vs silibinin (150mg) | |||
| ● Genus | |||
| Lachnospiracea incertae sedis | -0.897 | 0.00237 | 0.0138 |
| Alkalibacter | -1.54 | 0.00862 | 0.0415 |
| Acholeplasma | -1.51 | 0.00902 | 0.0430 |
| ● Family | |||
| Acholeplasmataceae | -1.51 | 0.00902 | 0.0431 |
| Placebo vs silibinin (300 mg) | |||
| ● Genus | |||
| Lachnospiracea incertae sedis | -0.788 | 0.00907 | 0.0434 |
| Placebo vs thistle extract | |||
| ● Genus | |||
| Dorea | 1.45 | 0.00254 | 0.0146 |
| Mycobacterium | 1.41 | 0.00489 | 0.0257 |
| Nevskia | 0.961 | 0.00540 | 0.0277 |
| Pectinatus | 0.699 | 0.00922 | 0.0435 |
| Acidaminococcus | 0.736 | 0.01040 | 0.0480 |
| Rikettsia | 1.15 | 0.01090 | 0.0499 |
| ● Family | |||
| Mycobacteriaceae | 1.41 | 0.00489 | 0.0254 |
| Placebo vs orlistat (120 mg) | |||
| ● Genus | |||
| Propionispora | -1.36 | 0.00367 | 0.0203 |
| Haemophilus | -3 | 0.00382 | 0.0210 |
| Desulfosporomusa | -1.33 | 0.00409 | 0.0222 |
| Ethanoligenens | 1.22 | 0.00592 | 0.0300 |
| Dysgonomonas | -0.565 | 0.00682 | 0.0337 |
| Oxobacter | 0.887 | 0.00750 | 0.0367 |
| Slackia | 1.31 | 0.00850 | 0.0408 |
| Veillonella | -1.95 | 0.00990 | 0.0464 |
| ● Family | |||
| Pasteurellaceae | -2.66 | 0.00759 | 0.0373 |
| Bacteria Name | Coefficient | Sig |
|---|---|---|
| ● Family | ||
| Veillonellaceae | 0.259* | 0.0435 |
| Flavobacteriaceae | 0.259* | 0.0439 |
| ● Genus | ||
| Azospirillum | 0.359** | 0.0046 |
| Lachnoanaerobaculum | -0.318* | 0.0124 |
| Butyricimonas | -0.307* | 0.0161 |
| Lishizhenia | -0.300* | 0.0187 |
| Peptoniphilus | 0.296* | 0.0207 |
| Citrobacter | -0.280* | 0.0290 |
| Selenomonas | 0.264* | 0.0397 |
| Salinivibrio | -0.260* | 0.0428 |
| ● Species | ||
| Massiliomicrobiota timonensis | -0.332** | 0.0090 |
| Halothermothrix orenii | -0.295* | 0.0210 |
| Clostridium XVIII | -0.286* | 0.0254 |
| Clostridium ramosum | -0.279* | 0.0292 |
| Clostridium populeti | 0.274* | 0.0326 |
| Clostridium scindens | 0.265* | 0.0387 |
| Marvinbryantia formatexigens | -0.263* | 0.0402 |
| Dysgonomona salginatilytica | -0.262* | 0.0413 |
| Alistipes sp. | -0.255* | 0.0473 |
| Peptoniphilus grossensis | 0.252* | 0.0498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





