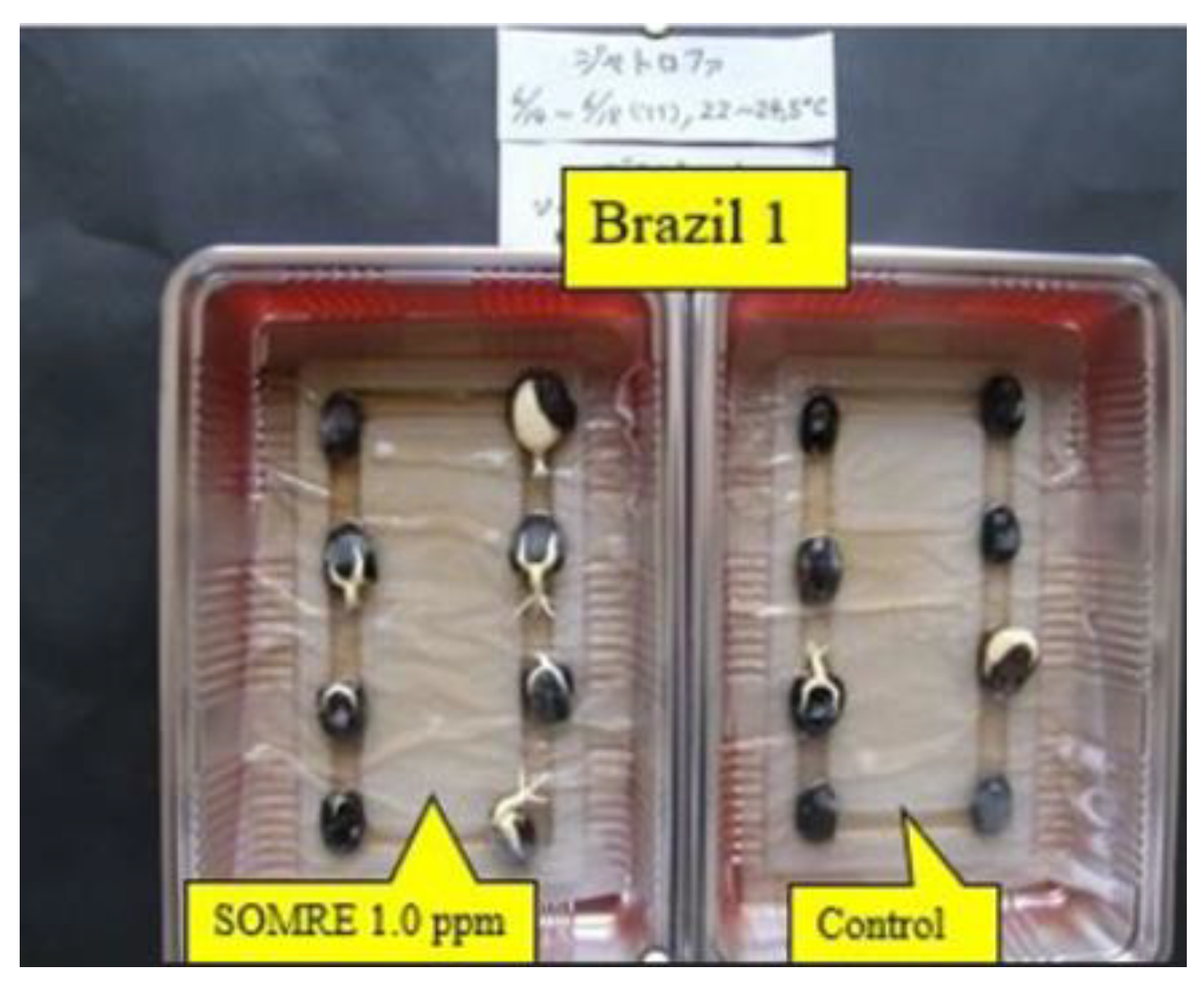
Melting points were determined on a Yanagimoto micro melting point apparatus and are uncorrected. Infrared (IR) spectra with a Shimadzu IR-420, a Shimadzu IR-460, and a Horiba FT-720 spectrophotometers, UV spectra with a Shimadzu UV 2400 PC spectrophotometer, and 1H-NMR spectra with JEOL FX-100, JEOL EX 270, and JEOL GSX 500 spectrometers, with tetramethyl silane as an internal standard. Mass spectra (MS) were recorded on a Hitachi M-80 or JEOL SX-102A spectrometer. Preparative thin-layer chromatography (p-TLC) was performed on Merck Kiesel-gel GF254 (Type 60) (SiO2) or Merck Aluminum Oxide GF254 (Type 60/E) (Al2O3). Column chromatography was performed on silica gel (SiO2, 100—200 mesh, from Kanto Chemical Co. Inc.) or activated alumina (Al2O3, 300 meshes, from Wako Pure Chemical Industries, Ltd.) throughout the present study.
23.1. Synthesis of SOMRE and Related Derivatives Based on the 1-Hydroxyindole Chemistry
Table 30.
Preparation of 1-methxyindole (6) from 2,3-dihydroindole (3)[54]..
Table 30.
Preparation of 1-methxyindole (6) from 2,3-dihydroindole (3)[54]..
1-Methoxyindole (6) from 2,3-dihydroindole (3)[
54] —
General method A (Table 30, Entry 1) :
A solution of Na
2WO
4·2H
2O[
70] (2.834 g, 8.42 mmol) in H
2O (40.0 mL) was added to a solution of
3 (5.015 g, 42.1 mmol) in MeOH (375 mL). 30% H
2O
2 (47.657 g, 421 mmol) was added to the resultant solution at 0 °C with stirring. After stirring for 15 min at rt (16 °C), K
2CO
3 (20.456 g, 147 mmol) and a solution of Me
2SO
4 (7.972 g, 631 mmol) in MeOH (25.0 mL) were added to the reaction mixture. After stirring for 90 min at rt (16 °C), brine (330 mL) was added and the whole was extracted with CHCl
3 (200 mL x 3). The extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave a black oil, which was column-chromatographed on SiO
2 with CHCl
3–hexane (1:4, v/v) to give
6 (3.361 g, 15%). [
12,
61]
6: colorless oil. Mass and all spectral data are identical with those reported by Acheson et al.[
62]
General method B (Table 1, Entry 3): A solution of Na2WO4·2H2O (13.2 mg, 0.04 mmol) in H2O (0.5 mL) was added to a solution of 3 (47.5 mg, 0.39 mmol) in MeOH (4.0 mL). 30% H2O2 (452.5 mg, 4.0 mmol) was added to the resultant solution at 0 °C with stirring. After stirring for 30 min at rt (17 °C), ethereal CH2N2 (excess) was added to the reaction mixture with stirring at rt until the starting material was not detected on tlc monitoring. Brine was added and the whole was extracted with CH2Cl2. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave oil, which was purified by p-TLC on SiO2 with CH2Cl2–hexane (7:3, v/v) as a developing solvent. Extraction of a band having an Rf value of 0.92–0.79 with CH2Cl2 afforded 6 (29.6 mg, 50%).
Entry 6: In the same procedure for Entry 3, Na2WO4·2H2O (27.0 mg, 0.08 mmol), 3 (48.8 mg, 0.41 mmol), 30% H2O2 (464.9 mg, 4.10 mmol) were used. And the same work-up as Entry 3 afforded 6 (31.1 mg, 52%).
Entry 12: In the same procedure for Entry 3, 2Na2O·P2O5·12WO4·18H2O (23.8 mg, 0.007 mmol), 3 (50.3 mg, 0.42 mmol), 30% H2O2 (479.2 mg, 4.22 mmol) were used. And the same work-up as Entry 3 afforded 6 (35.9 mg, 58%).
General method C (Table 1, Entry 13): A solution of Na2WO4·2H2O (591.4 mg, 1.79 mmol) in H2O (10.0 mL) and urea·H2O2 compound (8.437 g, 89.64 mmol) were added to a solution of 3 (1.068 g, 8.96 mmol) in MeOH (100.0 mL) at 0 °C with stirring. After stirring at rt for 15 min, K2CO3 (22.300 g, 161.3 mmol) and then a solution of Me2SO4 (3.391 g, 26.9 mmol) in MeOH (10.0 mL) were added to the reaction mixture. After the same work-up as Entry 3, 6 (717.5 mg, 54%) was obtained.
Entry 14: m-Chloroperbenzoic acid (231.6 mg, 0.94 mmol), (n-Bu)4NHSO4 (7.1 mg, 0.02 mmol) and sat. aq. NaHCO3 (5.0 mL) were added to a solution of 3 (111.4 mg, 0.94 mmol) in acetone–CH2Cl2 (1:1, v/v, 5.0 mL) at 0 °C with stirring. After stirring for 5 min, brine was added. The whole was extracted with CH2Cl2 and ethereal CH2N2 (excess) was added to the extract. After stirring for 3 min, the solvent was evaporated under reduced pressure to leave oil, which was purified by column-chromatography on SiO2 to afford 6 (47.5 mg, 35%).
Entry 15: In the same procedure as Entry 14, solvent was changed to CH2Cl2 (5.0 mL) only, where m-chloroperbenzoic acid (223.2 mg, 0.90 mmol), (n-Bu)4NHSO4 (7.2 mg, 0.02 mmol), 3 (107.0 mg, 0.90 mmol), and sat. aq. NaHCO3 (5.0 mL) were used. After usual work-up, 6 (52.9 mg, 40%) was obtained.
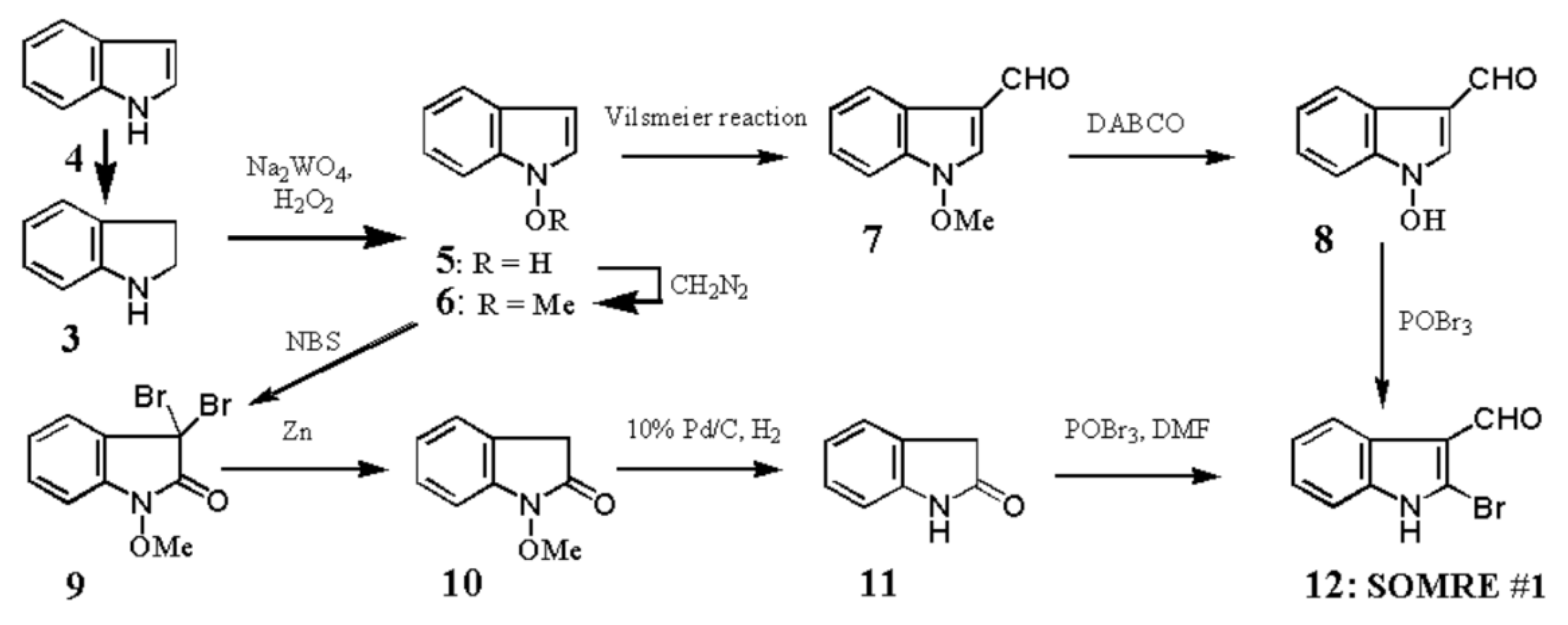
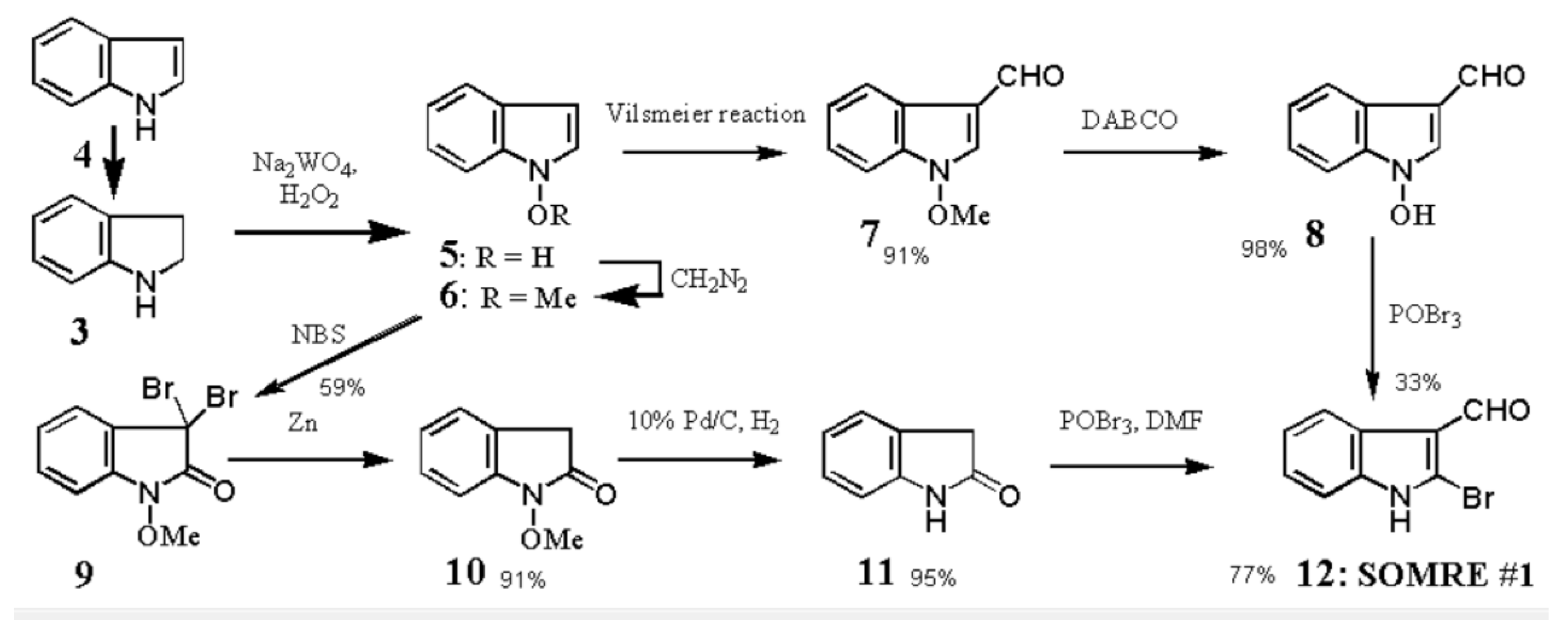
Scheme 4.
Synthesis of SOMRE #1 (12).
Scheme 4.
Synthesis of SOMRE #1 (12).
1-Methoxyindole-3-carbaldehyde (7)[63] from 1-methoxyindole (6) — POCl
3 (8.4mL) was added to an ice cooled anhydrous DMF (31.0 mL) with stirring. A solution of
6 (12.341 g) in anhydrous DMF (10.0 mL) was added to the resultant viscous solution and stirring was continued at rt for 2 h. Then, crushed ice and 16% aq. NaOH (100 mL) were added to the reaction mixture and the whole was extracted with ether. The extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to give a crystalline solid. Recrystallization from ether-hexane afforded
7 (13.411 g, 91.3%) as colorless prisms.
7: mp 50.0–51.0 °C. IR (KBr): 2810,1660–1650, 1375, 1240 cm
-1.
1H-NMR (CCl
4) δ: 3.98 (3H, s), 6.81–7.31(3H, m, 7.52 (1H, s), 7.80–8.16 (1H,m), 9.57 (1H, s). MS
m/z: 175 (M
+):
Anal. Calcd. for C
10H
9NO
2·1/2H
2O: C, 66.84; H, 5.33; N, 7.80. Found: C, 67.04; H, 5.11; N, 7.89.
3,3-Dibromo-1-methoxy-2-oxindole (9) from 1-methoxyindole (6) — NBS (3.637 g, 20.43 mmol) was added to a solution of 6 (1.001g, 6.81 mmol) in t-BuOH (70 mL) and the mixture was stirred at rt for 30 min. After evaporation of the solvent, H2O was added to the residue. The whole was extracted with benzene. The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a yellow solid, which was column-chromatographed on SiO2 with CH2Cl2–hexane (2:1, v/v) to give 9 (1.281 g, 59%). 9: mp 73—75°C (pale yellow prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 1745 cm–1. 1H-NMR (CDCl3) δ: 4.06 (3H, s), 6.95 (1H, dd, J=7.7, 1.5 Hz), 7.15 (1H, br dt, J=1.5, 7.7 Hz), 7.34 (1H, br dt, J=1.5, 7.7 Hz), 7.56 (1H, dd, J=7.8, 1.5 Hz). MS m/z: 319, 321, and 323 (M+, 79Br and 81Br). Anal. Calcd for C9H7Br2NO2: C, 33.64; H, 2.18; N, 4.36. Found: C, 33.51; H, 2.09; N, 4.51.
1-Methoxy-2-oxindole (
10) from 9 — Zink powder (103.2 mg, 1.6 mmol) was added to a solution of
9 (50.5 mg, 0.16 mmol) in AcOH (5 mL) and the mixture was stirred at rt for 1.5 h. Unreacted Zn was filtered off and washed with CH
2Cl
2–MeOH (95:5, v/v). H
2O was added to the combined washing and the filtrate. The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO
2 with CH
2Cl
2 to give
10 (16.6 mg, 65%). Spectral data are identical with the authentic sample prepared according to our previous procedures.[
73]
2-Oxindole (11) from 10 — A solution of 10 (155.3 mg, 0.95 mmol) in MeOH (10 mL) was hydrogenated in the presence of 10% Pd/C (50 mg) at rt and 1 atm for 1 h. Catalyst was filtered off and the filtrate was evaporated under reduced pressure to leave a crystalline solid, which was column-chromatographed on SiO2 with CH2Cl2 to give 11 (120.4 mg, 95%), whose physical data were identical with the commercially available sample.
1-Hydroxyindole-3-carbaldehyde (8) from 1-Methoxyindole-3-carbaldehyde (7) — i) Method A:
DABCO (386.3 mg, 3.45 mmol) was added to a solution of
7[3,17] (61.2 mg, 0.35 mmol) in DMF–H
2O (3:1, v/v, 2 mL) and the mixture was heated at 100°C for 21 h with stirring. After addition of H
2O, the whole was made acidic (pH 4) with 6% HCl and extracted with AcOEt. The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave a brown solid, which was column-chromatographed on SiO
2 with CHCl
3–MeOH (97:3, v/v) to give
8 (55.0 mg, 98%).
8: mp 154—156 °C (decomp, pale yellow prisms, recrystallized from AcOEt-hexane). IR (KBr): 3107, 1616 (br), 1558 (br), 1516, 1309, 1238 cm
-1.
1H-NMR (DMSO-
d6) δ: 7.26 (1H, dt,
J=1.3, 7.3 Hz), 7.34 (1H, dt,
J=1.3, 7.3 Hz), 7.52 (1H, d,
J=7.3 Hz), 8.11 (1H, d,
J=7.3 Hz), 8.43 (1H, s), 9.84 (1H, s), 12.20 (1H, br s, disappeared on addition of D
2O).
Anal. Calcd for C
9H
7NO
2: C, 67.07; H, 4.38; N, 8.69. Found: C, 66.86; H, 4.37; N, 8.66.
ii) Method B: KI (2.753 g, 16.6 mmol) was added to a solution of 7 (32.3 mg, 0.19 mmol) in DMF–H2O (3:1, v/v, 4 mL) and the mixture was heated at 160°C for 24 h with stirring. After the same work-up as described in the method B, unreacted 7 (10.5 mg, 33%), 8 (0.7 mg, 3%), and 8 (16.3 mg, 55%) were obtained in the order of elution.
SOMRE #1 (2-Bromoindole-3-carbaldehyde (12) from 2-Oxindole (11) — A solution of phosphorus tribromide (8.0 ml, 78.7 mmol) in anhydrous CHCl
3 (20 mL) was added dropwise to anhydrous DMF (30 mL) at 0 °C within 10 min with stirring. The mixture became viscous and then turned to yellow white solid. Although magnetic stirring bar stopped stirring, a solution of 2-oxindole (
11, 4.056 g, 30.5 mmol) in anhydrous CHCl
3 (60 mL) was added to the yellow white solid and the whole was immersed in an ultra sound bath for 1 h at rt and allowed to stand for 10 h. Again, the whole was immersed in an ultra sound bath for 1 h at rt. With ice cooling, H
2O (100 mL) was added to the reaction mixture and the yellow solid solved. The whole was then made slightly alkaline (pH 9) with 40% aqueous NaOH. After separation of organic layer, the water layer was extracted with CH
2Cl
2–MeOH (9:1, v/v, 4 times, total 400 mL). The combined organic layer and the extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave crystals. Repeated recrystallization from MeOH afforded
12. Total yield : 5.281 g (77%).
12: mp 209.0–210.5°C[1b] (colorless needles, , recrystallized from MeOH, lit.[
67] mp 196—198°C). IR (KBr): 3100, 1645 cm
-1.
1H-NMR (DMSO-
d6) δ: 6.96–7.54 (3H, m), 7.88–-8.14 (1H, m), 9.82 (1H, s). MS
m/z: 223 and 225 (M
+,
79Br and
81Br).
Anal. Calcd for C
9H
6BrNO: C, 48.25; H,2.70; N, 6.25. Found: C, 48.26; H, 2.70; N, 6.35.
SOMRE #1 (2-Bromoindole-3-carbaldehyde (12) from 1-Hydroxyindole-3-carbaldehyde (8) — A solution of 8 (27.6 mg, 0.17 mmol) in anhydrous THF (3 mL) was added to POBr3 (310.0 mg, 1.08 mmol) and the mixture was stirred at rt for 15 h. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give 12 (12.6 mg, 33%) and 24 (5.3 mg, 21%) in the order of elution.
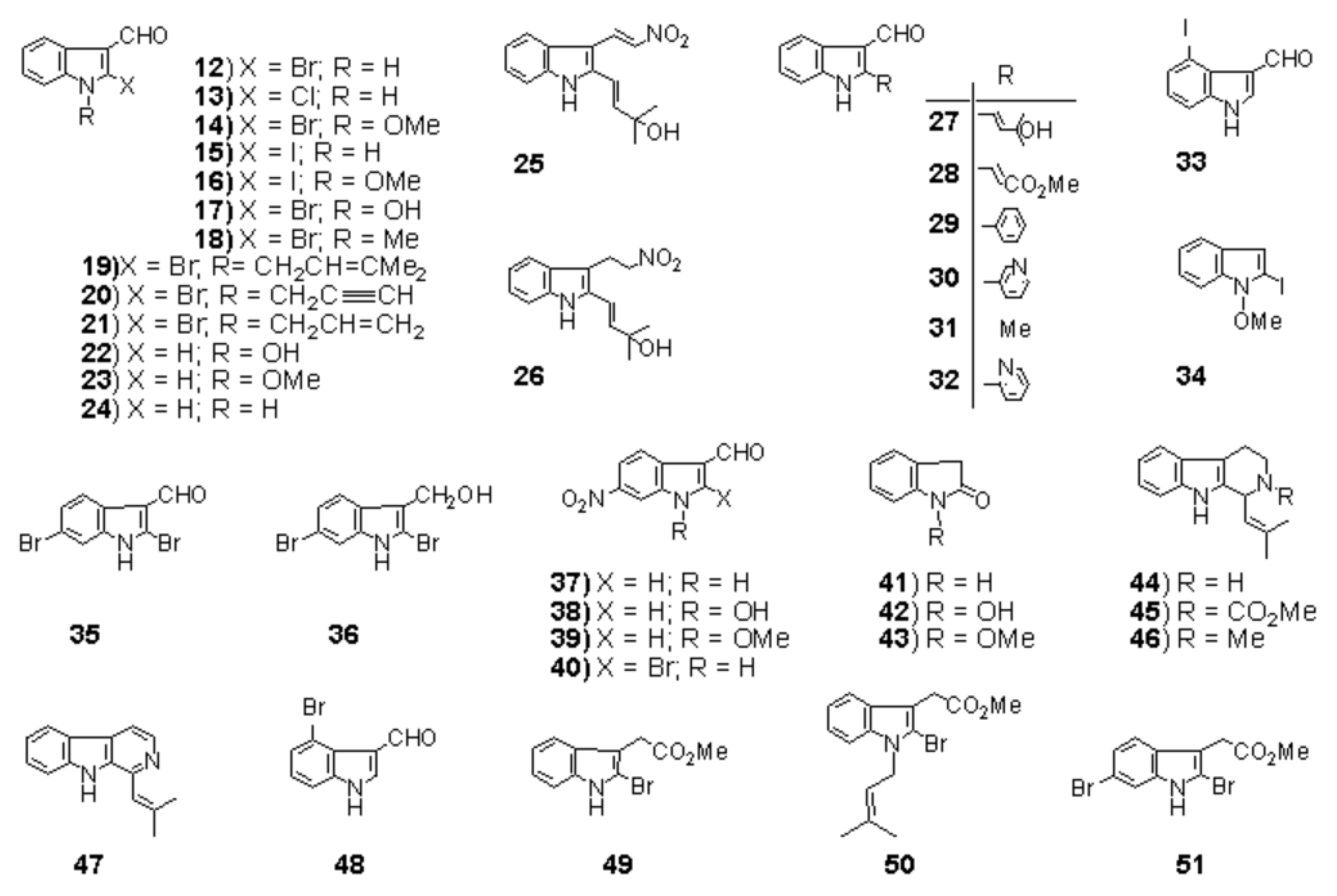
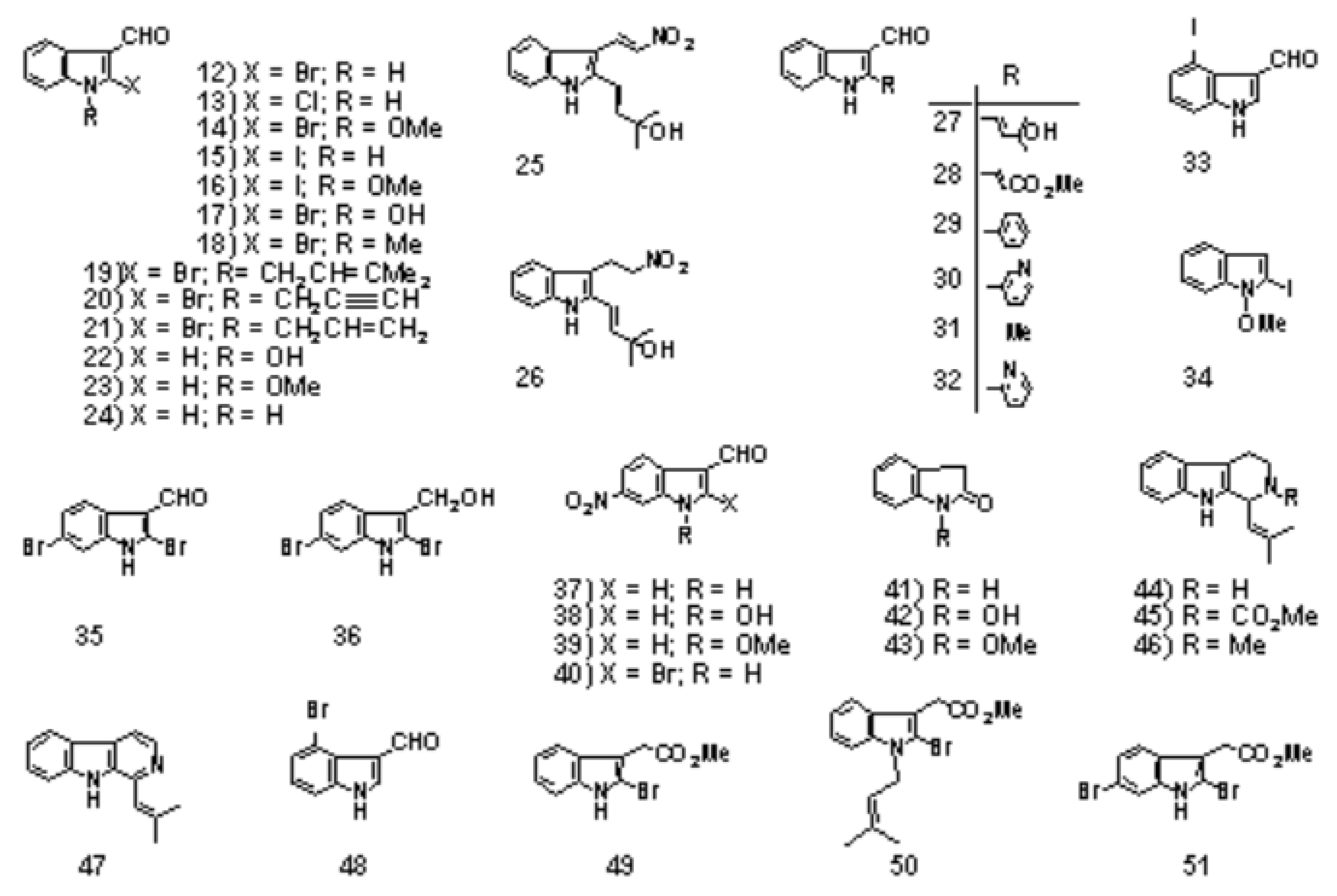
Figure 3.
SOMRE family compounds 12-51.
Figure 3.
SOMRE family compounds 12-51.
2-Chloroindole-3-carbaldehyde (13) from (8) — A solution of
8 (27.6 mg, 0.17 mmol) in anhydrous THF (3 mL) was added to POCl
3 (45.9 mg, 0.3 mmol) and DMF (22.0 mg, 0.3 mmol), and the mixture was stirred at rt for 5.5 h. The whole was made basic with 8% aqueous NaOH and extracted with AcOEt. The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave a solid, which was column-chromatographed on SiO
2 with CH
2Cl
2 to give
13 (20.6 mg, 67%). Physical data were identical with those of the authentic sample.[
68]
2-Bromo-1-methoxyindole-3-carbaldehyde (14) from 1-Methoxy-2-oxindole (10)[
69] — Anhydrous DMF (9 mL) was added to a solution of POBr
3 (0.2 mL) in anhydrous CHCl
3 (6 mL) at 0°C and stirring was continued at rt for 15 min. To the resulting solution was added a solution of
10[70] (236.3 mg, 1.45 mmol) in DMF (5 mL) at 0°C and the mixture was stirred at rt for 12 h. The whole was made basic with 8% aqueous NaOH and extracted with AcOEt. The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave a solid, which was column-chromatographed on SiO
2 with AcOEt–hexane (1:3, v/v) to give
14 (307.5 mg, 84%).
14: mp 97—98°C (colorless needles, recrystallized from MeOH). IR (KBr): 1653 cm
-1.
1H NMR (CDCl
3) δ: 4.19 (3H, s), 7.31 (1H, ddd,
J=7.6, 7.3, 1.2 Hz), 7.36 (1H, ddd,
J=7.8, 7.3, 1.2 Hz), 7.45 (1H, dd,
J=7.8, 1.2 Hz), 8.32 (1H, dd,
J=7.6, 1.2 Hz), 9.98 (1H, s). MS m/z: 253 and 255 (M
+,
79Br and
81Br).
Anal. Calcd for C
10H
8BrNO: C, 47.27; H, 3.17; N, 5.51. Found: C, 47.02; H, 3.22; N, 5.33.
2-Iodoindole-3-carbaldehyde (15) from 12 — KI (1.144 g, 6.89 mmol) and CuI (659.5 mg, 3.46 mmol) were added to a solution of 12 (152.8 mg, 0.68 mmol) in DMF (15 mL) and the mixture was heated at 120°C for 48 h. After evaporation of the solvent under reduced pressure, H2O was added to the residue. The whole was extracted with AcOEt. The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give an inseparable mixture (151.9 mg) of 12 and 15 in a ratio of 1:3.1 (1H-NMR analysis). The yields of 12 and 15 were calculated to be 29.8 mg (20%) and 122.1 mg (62%), respectively. To obtain 2 mg of pure 15, repeated HPLC and column-chromatography were required. 15: mp 224—226°C (colorless needles, recrystallized from MeOH). IR (KBr): 3138, 1639 cm–1. 1H-NMR (DMSO-d6) δ: 7.19 (1H, td, J=7.5, 1.2 Hz), 7.22 (1H, td, J=7.5, 1.2 Hz), 7.42 (1H, dd, J=7.5, 1.2 Hz), 8.09 (1H, dd, J=7.5, 1.2 Hz), 9.72 (1H, s), 12.81 (1H, br s). High-resolution MS m/z: Calcd for C9H6INO: 270.9494. Found: 270.9478.
2-Iodo-1-methoxyindole-3-carbaldehyde (16) from 2-Iodo-1-methoxyindole (34) — POCl3 (0.2 mL, 2.15 mmol) was added to DMF (2 mL, 25.8 mmol) at 0°C and the stirring was continued at rt for 15 min. To the solution was added a solution of 34 (137.1 mg, 0.50 mmol) in DMF (2 mL) at 0°C and the mixture was stirred at rt for 2 h. The whole was made basic with 8% aqueous NaOH and extracted with AcOEt. The organic layer was washed with brine, dried over Na2SO4, and evaporated under the reduced pressure to leave a solid, which was column-chromatographed on SiO2 with AcOEt–hexane (1:5, v/v) to give 16 (105.6 mg, 70%). 16: mp 132—134°C (colorless needles, recrystallized from MeOH). IR (KBr): 1645 cm–1. 1H-NMR (CDCl3) δ: 4.18 (3H, s), 7.29 (1H, t, J=7.3 Hz), 7.33 (1H, t, J=7.3 Hz), 7.46 (1H, d, J=7.3 Hz), 8.33 (1H, d, J=7.3 Hz), 9.79 (1H, s). MS m/z: 301 (M+). Anal. Calcd for C10H8INO2: C, 39.89; H, 2.68; N, 4.65. Found: C, 40.33; H, 2.87; 4.47.
2,6-Dibromoindole-3-carbaldehyde (35) with/without 2-Bromo-1-hydroxyindole-3-carbaldehyde (17) from 2-Bromo-1-methoxyindole-3-carbaldehyde (14) — i) Method A: BBr3 (2 mL, 21.2 mmol) was added to a solution of 14 (50.0 mg, 0.20 mmol) in anhydrous CH2Cl2 (5 mL) at 0°C and the mixture was refluxed for 21 h with stirring. The mixture was poured into an ice water and the whole was extracted with AcOEt. The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (95:5, v/v) to give 35 (36.3 mg, 61%). 35: mp 269—270°C (decomp., colorless prisms, recrystallized from MeOH). IR (KBr): 3082, 1631 cm-1. 1H NMR (CD3OD) δ: 7.35 (1H, dd, J=8.5, 1.8 Hz), 7.56 (1H, d, J=1.8 Hz), 8.04 (1H, d, J=8.5 Hz), 9.91 (1H, s). 1H NMR (CDCl3) δ: 7.41 (1H, dd, J=8.5, 1.2 Hz), 7.51 (1H, d, J=1.2 Hz), 8.17 (1H, d, J=8.5 Hz), 8.76 (1H, br s), 10.01 (1H, s). MS m/z: 301, 303, and 305 (M+ 79Br2, 79Br81Br, and 81Br2). Anal. Calcd for C9H5Br2NO: C, 35.68; H, 1.66; N, 4.62. Found: C, 35.64; H, 1.72; N, 4.57.
ii) Method B: BBr3 (4.0 mL, 42.3 mmol) was added to a solution of 14 (97.0 mg, 0.33 mmol) in anhydrous CH2Cl2 (10 mL) at 0°C and the mixture was stirred at rt for 24 h. After the same work-up as described in the Method A, the crude product was column-chromatographed on SiO2 with CHCl3 to give unreacted 14 (30.8 mg, 32%), 35 (18.2 mg, 16%), and 17 (12.7 mg, 14%) in the order of elution. 17: mp 192—194°C (decomp., colorless needles, recrystallized from AcOEt–hexane). IR (KBr): 1630 cm–1. 1H-NMR (CD3OD) δ: 7.27 (1H, dd, J=7.8, 7.3 Hz), 7.34 (1H, J=7.8, 7.3 Hz), 7.50 (1H, d, J=7.8 Hz), 8.16 (1H, d, J=7.8 Hz), 9.86 (1H, s). High-resolution MS m/z: Calcd for C9H679BrNO2: 238.9582. Found: 238.9579. Calcd for C9H681BrNO2: 240.9561. Found: 240.9556.
2-Bromo-1-methylindole-3-carbaldehyde (18) from 12 — A solution of MeI (969.2 mg, 6.83 mmol) in THF (10 mL) was added to a mixture of 12 (1.07 g, 4.75 mmol), Bu4NBr (306.9 mg, 0.952 mmol), and K2CO3 (3.55 g, 25.7 mmol) in THF (60 mL), and the mixture was stirred at rt for 5 h. After addition of brine, the whole was extracted with CH2Cl2–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CH2Cl2 to give 18 (1.11 g, 98%). 18: mp 118—118.5°C (colorless prisms, recrystallized from hexane). IR (KBr): 1638 cm–1. 1H-NMR (CDCl3) δ: 3.84 (3H, s), 7.14—7.36 (3H, m), 8.14—8.36 (1H, m), 9.98 (1H, s). MS m/z: 237 and 239 (M+, 79Br and 81Br). Anal. Calcd for C10H8BrNO: C, 50.45; H, 3.39; N, 5.88. Found: C, 50.44; H, 3.35; N, 6.09.
2-Bromo-1-(3-methyl-2-buten-1-yl)indole-3-carbaldehyde (19) from 12 — A solution of prenyl bromide (1.06 g, 7.17 mmol) in THF (10 mL) was added to a mixture of 12 (1.02 g, 4.54 mmol), Bu4NBr (300.8 mg, 0.933 mmol), and K2CO3 (3.57 g, 25.8 mmol) in THF (60 mL), and the mixture was stirred at rt for 6 h. After the same work-up as described in the preparation of 18, 1.22 g (92%) of 19 was obtained. 19: 78.5—79°C (colorless needles, recrystallized from hexane). IR (KBr): 1650 cm–1. 1H-NMR (CDCl3) δ: 1.75 (3H, d, J=1.2 Hz), 1.92 (3H, d, J=1.2 Hz), 4.83 (2H, d, J=7.0 Hz), 5.18 (1H, th, J=7.0, 1.2 Hz), 7.11—7.35 (3H, m), 8.15—8.39 (1H, m). 10.00 (1H, s). MS m/z: 291 and 293 (M+, 79Br and 81Br). Anal. Calcd for C14H14BrNO: C, 57.55; H, 4.83; N, 4.79. Found: C, 57.58; H, 4.84; N, 4.80.
2-Bromo-1-propargylindole-3-carbaldehyde (20) from 12 — A solution of propargyl bromide (826.6 mg, 6.95 mmol) in THF (10 mL) was added to a mixture of 12 (1.00 g, 4.50 mmol), Bu4NBr (280.1 mg, 0.88 mmol), and K2CO3 (3.19 g, 23.1 mmol) in THF (40 mL), and the mixture was stirred at rt for 22 h. After addition of brine, the whole was extracted with CH2Cl2–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a crystalline solid, which was recrystallized from MeOH to give 20 (1.01 g) as colorless flakes. The mother liquor was subjected to p-TLC on SiO2 with CH2Cl2–hexane (3:2, v/v) as a developing solvent. Extraction of the band having an Rf value of 0.35—0.43 with CH2Cl2–MeOH (95:5, v/v) gave 20 (60.9 mg). Total yield of 20 was 1.07 g (91%). 20: mp 151.5—152.5°C. IR (KBr): 1636 cm–1. 1H-NMR (CDCl3) δ: 2.37 (1H, t, J=2.5 Hz), 5.00 (2H, d, J=2.5 Hz), 7.14—7.54 (3H, m), 8.14—8.39 (1H, m), 10.00 (1H, s). MS m/z: 261 and 263 (M+, 79Br and 81Br). Anal. Calcd for C12H8BrNO: C, 54.98; H, 3.08; N, 5.34. Found: C, 54.82; H, 2.98; N, 5.47.
1-Allyl-2-bromoindole-3-carbaldehyde (21) from 12 — A solution of allyl bromide (108.0 mg, 0.89 mmol) in THF (2 mL) was added to a mixture of 12 (100.3 mg, 0.45 mmol), Bu4NBr (29.3 mg, 0.09 mmol), and K2CO3 (307.8 mg, 2.23 mmol) in THF (6 mL), and the mixture was stirred at rt for 1 h. After the same work-up as described in the preparation of 18, 115.3 mg (98%) of 21 was obtained. 21: mp 87—88°C (colorless prisms, recrystallized from CHCl3). IR (KBr): 1652 cm–1. 1H-NMR (CDCl3) δ: 4.90 (2H, ddd, J=5.0, 1.8, 1.2 Hz), 5.23 (1H, dt, J=17.0, 1.8 Hz), 5.26 (1H, dt, J=10.5, 1.2 Hz), 5.94 (1H, ddt, J=17.0, 10.5, 5.0 Hz), 7.26—7.33 (3H, m), 8.31—8.34 (1H, m), 10.06 (1H, s). MS m/z: 263 and 265 (M+, 79Br and 81Br). Anal. Calcd for C12H10BrNO·1/4H2O: C, 53.65; H, 3.94; N, 5.21. Found: C, 53.84; H, 3.73; N, 5.21.
(E,E)-2-Methyl-4-[3-(2-nitrovinyl)indol-2-yl]-3-buten-2-ol (25) from 27 — NH4OAc (736.1 mg, 9.50 mmol) was added to a solution of 27 (435.7 mg, 1.90 mmol) in MeNO2 (26 mL) and the mixture was heated at 90°C for 4 h with stirring. After cooling to rt, the resulting precipitates (16, 406.1 mg) were collected by filtration and washed with MeOH. The filtrate and washings were combined and H2O was added. The whole was extracted with CH2Cl2–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a crystalline solid, which was column-chromatographed on SiO2 with CH2Cl2–MeOH (95:5, v/v) to give 25 (75.7 mg). Total yield of 25 was 481.8mg (93%). 25: mp 246.5—247°C (decomp., red needles, recrystallized from MeOH). IR (KBr): 3250, 1578, 1360 cm–1. 1H NMR (pyridine-d5) δ: 1.55 (6H, s), 7.02 (1H, d, J=16.0 Hz), 7.26—7.59 (4H, m), 7.85—8.05 (1H, m), 8.52 (1H, d, J=13.2 Hz), 8.77 (1H, d, J=13.2 Hz). MS m/z: 272 (M+). Anal. Calcd for C15H16N2O3: C, 66.16; H, 5.92; N, 10.29. Found: C, 65.97; H, 5.89; N, 10.46.
(E)-2-Methyl-4-[3-(2-nitroethyl)indol-2-yl]-3-buten-2-ol (26) from 25 — NaBH4 (173.5 mg, 4.59 mmol) was added to a solution of 25 (206.0 mg, 0.76 mmol) in MeOH (30 mL) and the mixture was stirred at rt for 1 h. After addition of AcOEt, the whole was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give 26 (199.5 mg, 96%). 26: mp 122.5—123°C (colorless prisms, recrystallized from benzene). IR (KBr): 3520, 3310, 1554, 1380 cm–1. 1H-NMR (CD3OD) δ: 1.23 (6H, s), 3.48 (2H, t, J=7.5 Hz), 4.62 (2H, t, J=7.5 Hz), 6.31 (1H, d, J=16.0 Hz), 6.71 (1H, d, J=16.0 Hz), 6.83—7.52 (4H, m). MS m/z: 274 (M+). Anal. Calcd for C15H18N2O3: C, 65.67; H, 6.61; N, 10.21. Found: C, 65.89; H, 6.58; N, 10.23.
E-2-(3-Hydroxy-3-methyl-1-butenyl)indole-3-carbaldehyde (27) from 12 — General Procedure: A solution of 12 (405.4 mg, 1.81 mmol), (3-hydroxy-3-methyl-1-butenyl)tributyltin (1.00 g, 2.81 mmol), Pd(OAc)2 (42.3 mg, 0.19 mmol), and Bu4NCl (1.00 g, 3.61 mmol) in DMF (6 mL) was heated at 115—120°C for 3 h with stirring. After evaporation of the solvent under reduced pressure, brine was added, and the whole was extracted with AcOEt–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (2:1, v/v) to give 27 (362.1 mg, 87%). 27: mp 194—195°C (colorless prisms, recrystallized from MeOH). IR (KBr): 3165, 2975, 1614 cm–1. 1H-NMR (CD3OD) δ: 1.47 (6H, s), 6.75 (1H, d, J=16.0 Hz), 7.02—7.48 (3H, m), 7.20 (1H, d, J=16.0 Hz), 7.99—8.23 (1H, m), 10.18 (1H, s). MS m/z: 229 (M+). Anal. Calcd for C14H15NO2: C, 73.34; H, 6.59; N, 6.11. Found: C, 73.17; H, 6.60; N, 6.32.
Methyl (E)-3-(3-Formylindol-2-yl)acrylate (28) from 12 — In the general procedure for 27, 12 (102.2 mg, 0.46 mmol), 2-(methoxycarbonyl)vinyl tributyltin (254.2 mg, 0.68 mmol), Pd(OAc)2 (12.1 mg, 0.05 mmol), and Bu4NCl (248.6 mg, 0.90 mmol) were used. After the same work-up as described in the preparation of 27, 70.2 mg (67%) of 28 was obtained. 28: mp 261—262°C. IR (KBr): 3050, 1703, 1628 cm–1. 1H-NMR (pyridine-d5) δ: 3.72 (3H, s), 6.95 (1H, d, J=16.0 Hz), 7.25—7.49 (3H, m), 8.50 (1H, d, J=16.0 Hz), 8.59—8.77 (1H, m), 10.71 (1H, s). The NH proton signal was not observed. MS m/z: 229 (M+). Anal. Calcd for C13H11NO3: C, 68.11; H, 4.84; N, 6.11. Found: C, 67.89; H, 4.76; N, 6.04.
2-Phenylindole-3-carbaldehyde (29) from 12 — In the general procedure for 27, 12 (41.1 mg, 0.18 mmol), tetraphenyl tin (117.3 mg, 0.27 mmol), Pd(OAc)2 (4.3 mg, 0.02 mmol), and Bu4NCl (97.7 mg, 0.35 mmol) were used. After the same work-up and column-chromatography as described in the preparation of 27, 29 (27.7 mg, 68%) and 12 (3.9 mg, 10%) were obtained in the order of elution. 29: mp 260.5—263°C (colorless prisms, recrystallized from MeOH). IR (KBr): 3120, 1628 cm–1. 1H-NMR (pyridine-d5) δ: 7.18—7.90 (8H, m), 8.53—8.91 (1H, m), 10.25 (1H, s). MS m/z: 221 (M+). Anal. Calcd for C15H11NO: C, 81.43; H, 5.01; N, 6.33. Found: C, 81.51; H, 4.92; N, 6.35.
2-(3-Pyridyl)indole-3-carbaldehyde (30) from 12 — In the general procedure for 27, 12 (103.1 mg, 0.46 mmol), (3-pyridyl)trimethyl tin (221.0 mg, 0.91 mmol), Pd(OAc)2 (9.7 mg, 0.04 mmol), and Bu4NCl (261.3 mg, 0.94 mmol) were used. After the same work-up and column-chromatography as described in the preparation of 27, 30 (39.1 mg, 38%), 31 (10.9 mg, 15%), and indole-3-carbaldehyde (5.5 mg, 8%) were obtained in the order of elution. 30: mp 246—246.5°C (colorless needles, recrystallized from MeOH). IR (KBr): 3430, 3130, 1628 cm–1. 1H-NMR (pyridine-d5) δ: 7.28—7.70 (4H, m), 8.14 (1H, ddd, J=8.0, 2.2, 1.8 Hz), 8.75—8.96 (2H, m), 9.27 (1H, dd, J=2.2, 0.8 Hz), 10.41 (1H, s). MS m/z: 222 (M+). Anal. Calcd for C14H10N2O: C, 75.65; H, 4.54; N, 12.61. Found: C, 75.48; H, 4.77; N, 12.50.
2-Methylindole-3-carbaldehyde (31) from 12 — In the general procedure for 27, 12 (467.8 mg, 2.09 mmol), tetramethyl tin (562.2 mg, 3.12 mmol), Pd(OAc)2 (42.7 mg, 0.19 mmol), and Bu4NCl (1.34 g, 4.84 mmol) were used. After the same work-up and column-chromatography as described in the preparation of 27, 31 (130.0 mg, 39%) and indole-3-carbaldehyde (18.1 mg, 6%) were obtained in the order of elution. 31: mp 204—205°C (colorless needles, recrystallized from MeOH). IR (KBr): 3250, 1635 cm–1. 1H-NMR (CD3OD) δ: 2.63 (3H, s), 6.89—7.39 (3H, m), 7.79—8.12 (1H, m), 9.82 (1H, s). MS m/z: 159 (M+). Anal. Calcd for C10H9NO: C, 75.45; H, 5.70; N, 8.80. Found: C, 75.50; H, 5.62; N, 8.82.
2-(2-Pyridyl)indole-3-carbaldehyde (32) from 12 — In the general procedure for 27, 12 (100.5 mg, 0.45 mmol), (2-pyridyl)trimethyl tin (1.07 g, 4.43 mmol), Pd(OAc)2 (10.9 mg, 0.05 mmol), and Bu4NCl (246.1 mg, 0.89 mmol) were used. After the same work-up and column-chromatography as described in the preparation of 27, unreacted 12 (21.4 mg, 21%), 32 (5.0 mg, 5%), 31 (0.5 mg, 1%), and indole-3-carbaldehyde (2.4 mg, 4%) were obtained in the order of elution. 32: mp 225.5—226.5°C (colorless needles, recrystallized from MeOH). IR (KBr): 3060, 1619 cm–1. 1H-NMR (pyridine-d5) δ: 7.12—7.70 (4H, m), 7.75 (1H, dd, J=7.5, 2.0 Hz), 8.70 (1H, dt, J=8.0, 1.0 Hz), 8.65—8.78 (1H, m), 8.78—8.98 (1H, m), 11.04 (1H, s). MS m/z: 222 (M+). Anal. Calcd for C14H10N2O: C, 75.65; H, 4.54; N, 12.61. Found: C, 75.42; H, 4.38; N, 12.83.
Hydroxy-6-nitroindole-3-carbaldehyde (38) from 1-Methoxy-6-nitroindole-3-carbaldehyde (39) — i) Method A: The formation of 38 in 90% yield by the reaction of 39 with DABCO was reported in the preceding paper.
ii) Method B: A solution of KI (960.0 mg, 5.78 mmol) in H2O (1 mL) was added to a solution of 39 (32.8 mg, 0.15 mmol) in DMF (3 mL), and the mixture was heated at 120°C for 36 h with stirring. After evaporation of the solvent under reduced pressure, AcOEt was added to the residue. The same work-up of the organic layer as described in the Method A for 38, gave unreacted 39 (7.3 mg, 22%), 37 (1.1 mg, 4%), and 38 (17.9 mg, 58%) in the order of elution. 38 is identical with the authentic sample.72
2-Bromo-6-nitroindole-3-carbaldehyde (40) from 38 — POBr3 (529.4 mg, 1.85 mmol) was added to a solution of 38 (60.9 mg, 0.30 mmol) in anhydrous THF (4 mL) and the mixture was stirred at rt for 5 h. After evaporation of the solvent, H2O was added to the residue, and the whole was extracted with CHCl3–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give 40 (65.3 mg, 82%) and 37 (6.2 mg, 11%) in the order of elution. 40: mp 296—298°C (decomp., colorless prisms, recrystallized from AcOEt). IR (KBr): 1631 cm–1. 1H-NMR (DMSO-d6) δ: 8.12 (1H, dd, J=8.8, 1.6 Hz), 8.24 (1H, d, J=8.8 Hz), 8.27 (1H, d, J=1.6 Hz), 9.95 (1H, s). The proton at the 1-position did not appear. MS m/z: 268 and 270 (M+, 79Br and 81Br). Anal. Calcd for C9H5BrN2O3: C, 40.18; H, 1.87; N, 10.41. Found: C, 40.16; H, 1.93; N, 10.47.
2,6-Dibromoindol-3-ylmethanol (36) from 35 — NaBH4 (150.0 mg, 3.95 mmol) was added to a solution of 35 (65.2 mg, 0.22 mmol) in MeOH (10 mL), and the mixture was stirred at rt for 1 h. After evaporation of the solvent under reduced pressure, AcOEt was added to the residue. The whole was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (95:5, v/v) to give 36 (44.2 mg, 67%). 36: colorless oil. IR (film): 3398, 3213, 1614 cm–1. 1H-NMR (CDCl3) δ: 1.48 (1H, br s), 4.80 (2H, s), 7.26 (1H, dd, J=8.5, 1.7 Hz), 7.43 (1H, d, J=1.7 Hz), 7.55 (1H, d, J=8.5 Hz), 8.18 (1H, br s). High-resolution MS m/z: Calcd for C9H779Br2NO: 302.8895. Found: 302.8915. Calcd for C9H779Br81BrNO: 304.8874. Found: 304.8852. Calcd for C9H781Br2NO: 306.8854. Found: 306.8829.
1-(2-Methyl-1-propenyl)-1,2,3,4-tetrahydro-β-carboline (44) from 26 — A solution of
26 (174.9 mg, 0.64 mmol) in THF (19.5 mL) was added to a mixture of Zn powder (897.2 mg, 13.7 mmol) [washed with 6% HCl (4 mL)] in 6% HCl (6.5 mL) at 0°C and the mixture was refluxed for 5 min with stirring. Unreacted Zn was filtered off and the filtrate was evaporated under reduced pressure. The residue was made basic by adding 8% aqueous NaOH and the whole was extracted with CH
2Cl
2–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO
2 with CHCl
3–MeOH–28% aq. NH
3 (46:2:0.2, v/v) to give
44 (22.5 mg, 50%).
44: mp 160—161°C (colorless prisms, recrystallized from CH
2Cl
2, lit.,[
10] mp 158—159°C). IR (KBr): 3400, 1092 cm
–1.
1H-NMR (CDCl
3) δ: 1.82 (3H, d,
J=1.2 Hz), 1.88 (3H, d,
J=1.2 Hz), 2.65—2.89 (2H, m), 2.89—3.55 (2H, m), 4.83 (1H, br d,
J=9.5 Hz), 5.26 (1H, dt,
J=9.5, 1.2 Hz), 6.92—7.33 (3H, m), 7.33—7.53 (1H, m), 7.64 (1H, br s). MS
m/z: 226 (M
+).
Anal. Calcd for C
15H
18N
2·1/4H
2O: C, 78.05; H, 8.07; N, 12.13. Found: C, 78.47; H, 8.09; N, 11.84.
2-Methoxycarbonyl-1-(2-methyl-1-propenyl)-1,2,3,4-tetrahydro-β-carboline (45) from 44 — A solution of ClCO
2Me (38.6 mg, 0.41 mmol) in CH
2Cl
2 (1 mL) was added to a solution of
44 (54.0 mg, 0.24 mmol) and Et
3N (80.0 mg, 0.79 mmol) in CH
2Cl
2 (3 mL) and the mixture was stirred at rt for 2 h. Saturated NaHCO
3 was added and the whole was extracted with CH
2Cl
2–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was recrystallized from MeOH to give
45 (47.5 mg) as colorless prisms. The mother liquor was subjected to p-TLC on SiO
2 with CH
2Cl
2–MeOH (95:5, v/v) as a developing solvent. Extraction from the band having an
Rf value of 0.93—1.00 with CH
2Cl
2–MeOH (95:5, v/v) gave
45 (10.3 mg). Total yield of
45 was 58.7 mg (85%).
45: mp 186—189°C (lit.,[
74] mp 180—181°C). IR (KBr): 3400, 3040, 1092 cm
–1.
1H NMR (CDCl
3) δ: 1.77 (3H, d,
J=1.4 Hz), 1.98 (3H, d,
J=1.4 Hz), 2.64—2.88 (2H, m), 2.88—3.37 (1H, m), 3.73 (3H, s), 4.39 (1H, d,
J=12.5 Hz), 5.30 (1H, d,
J=10.0 Hz), 5.88 (1H, d,
J=10.0 Hz), 6.91—7.32 (3H, m), 7.36—7.52 (1H, m), 7.59 (1H, br s). High-resolution MS
m/z: Calcd for C
17H
20N
2O
2: 284.1524. Found: 284.1526.
Borrerine (46) from 45 — LiAlH
4 (98.6 mg, 2.60 mmol) was added to a solution of
45 (46.3 mg, 0.16 mmol) in THF (8 mL) at 0°C and the mixture was refluxed for 5 h with stirring. After addition of MeOH and 10% aqueous Rochelle salt under ice cooling, the whole was extracted with CH
2Cl
2–MeOH (95:5, v/v). The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was subjected to p-TLC on SiO
2 with CHCl
3–MeOH–28% aq. NH
3 (46:5:0.5, v/v) as a developing solvent. Extraction from the band having an
Rf value of 0.74—0.90 with CHCl
3–MeOH–28% aq. NH
3 (46:5:0.5, v/v) gave
46 (28.2 mg, 70%).
46: mp 105—106°C (colorless prisms, recrystallized from hexane, lit.[
10] mp 102—103°C). IR (KBr): 3200 cm
–1.
1H-NMR (CDCl
3) δ: 1.86 (3H, d,
J=1.2 Hz), 1.88 (3H, d,
J=1.2 Hz), 2.48—3.29 (4H, m), 4.05 (1H, dt,
J=9.5, 1.2 Hz), 5.18 (1H, br d,
J=9.5 Hz), 6.92—7.34 (3H, m), 7.34—7.63 (2H, m). High-resolution MS
m/z: Calcd for C
16H
20N
2: 240.1625. Found: 240.1630.
Vulcanine (47) from 44 — A solution of
t-BuOCl (42.4 mg, 0.39 mmol) in THF (1 mL) was added to a suspension of
44 (37.0 mg, 0.16 mmol) and powdered NaOH (32.1 mg, 0.80 mmol) in THF (4 mL) and the mixture was stirred at rt for 64 h. H
2O was added and the whole was extracted with AcOEt. The organic layer was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was subjected to p-TLC on SiO
2 with AcOEt–hexane (1:1, v/v) as a developing solvent. Extraction from the band having an
Rf value of 0.47—0.65 with AcOEt gave
47 (18.5 mg, 51%).
47: pale yellow oil. IR (CHCl
3): 1645, 1623, 1567, 1492, 1452, 1420, 1380, 1316, 1235 cm
–1.
1H-NMR (CDCl
3) δ: 2.00 (3H, d,
J=1.2 Hz), 2.01 (3H, d,
J=1.2 Hz), 6.60 (1H, t,
J=1.2 Hz), 7.28 (1H, ddd,
J=8.1, 6.4, 1.7 Hz), 7.51 (1H, ddd,
J=8.1, 1.7, 1.0 Hz), 7.53 (1H, ddd,
J=8.1, 6.4, 1.0 Hz), 7.82 (1H, d,
J=5.4 Hz), 8.11 (1H, d,
J=8.1 Hz), 8.46 (1H, d,
J=5.4 Hz), 8.57 (1H, br s, disappeared on addition of D
2O).
13C-NMR (CDCl
3) δ: 20.5, 26.7, 111.9, 113.0, 119.0, 120.3, 121.6, 121.8, 128.8, 129.5, 134.0, 136.8, 140.7, 140.8, 143.8. UV
λmax (MeOH) nm (log
ε): 214 (4.36), 239 (4.49), 260 (sh, 4.23), 292 (4.14), 356 (3.79). MS
m/z: 222 (M
+), 207, 182, 103. High-resolution MS
m/z: Calcd for C
15H
14N
2: 222.1157. Found: 222.1154.
47·HCl: mp 189—192°C (yellow needles, recrystallized from Et
2O–MeOH, lit.,[
75] mp 103°C). IR (KBr): 3340, 1640, 1599, 1442, 761 cm
–1.
1H-NMR (CD
3OD) δ: 1.93 (3H, d,
J=1.2 Hz), 2.22 (3H, d,
J=1.2 Hz), 6.74 (1H, t,
J=1.2 Hz), 7.47 (1H, ddd,
J=8.1, 6.8, 1.0 Hz), 7.76 (1H, dt,
J=8.3, 1.0 Hz), 7.80 (1H, ddd,
J=8.3, 6.8, 1.0 Hz), 8.35 (1H, d,
J=6.4 Hz), 8.41 (1H, dt,
J=8.1, 1.0 Hz), 8.55 (1H, d,
J=6.4 Hz).
13C-NMR (CD
3OD) δ: 20.9, 26.6, 113.9, 114.2, 116.6, 121.5, 123.0, 124.1, 129.5, 133.1, 134.8, 135.2, 137.4, 145.4, 152.5.
Anal. Calcd for C
15H
14N
2·HCl·1/4H
2O: C, 68.44; H, 5.93; N, 10.64. Found: C, 68.51; H, 5.83; N, 10.68.
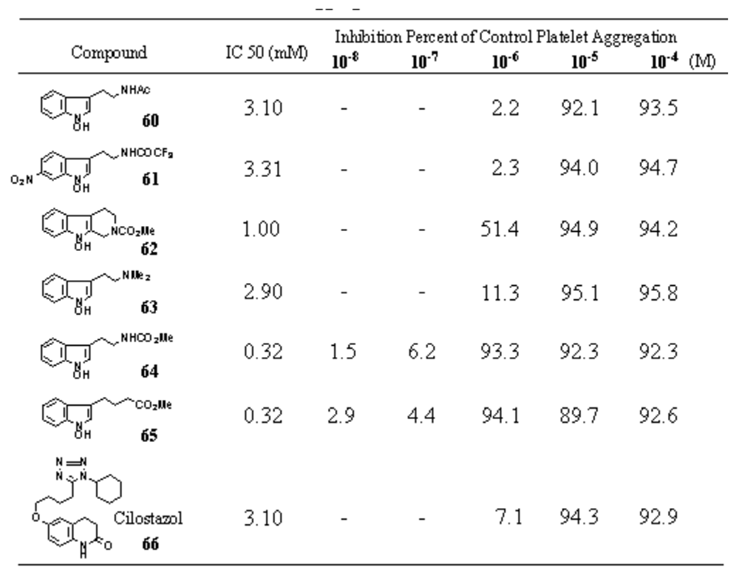
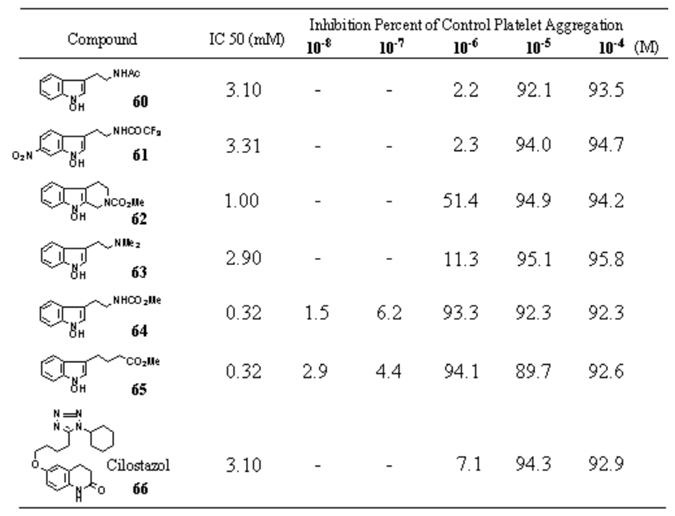
Table 28.
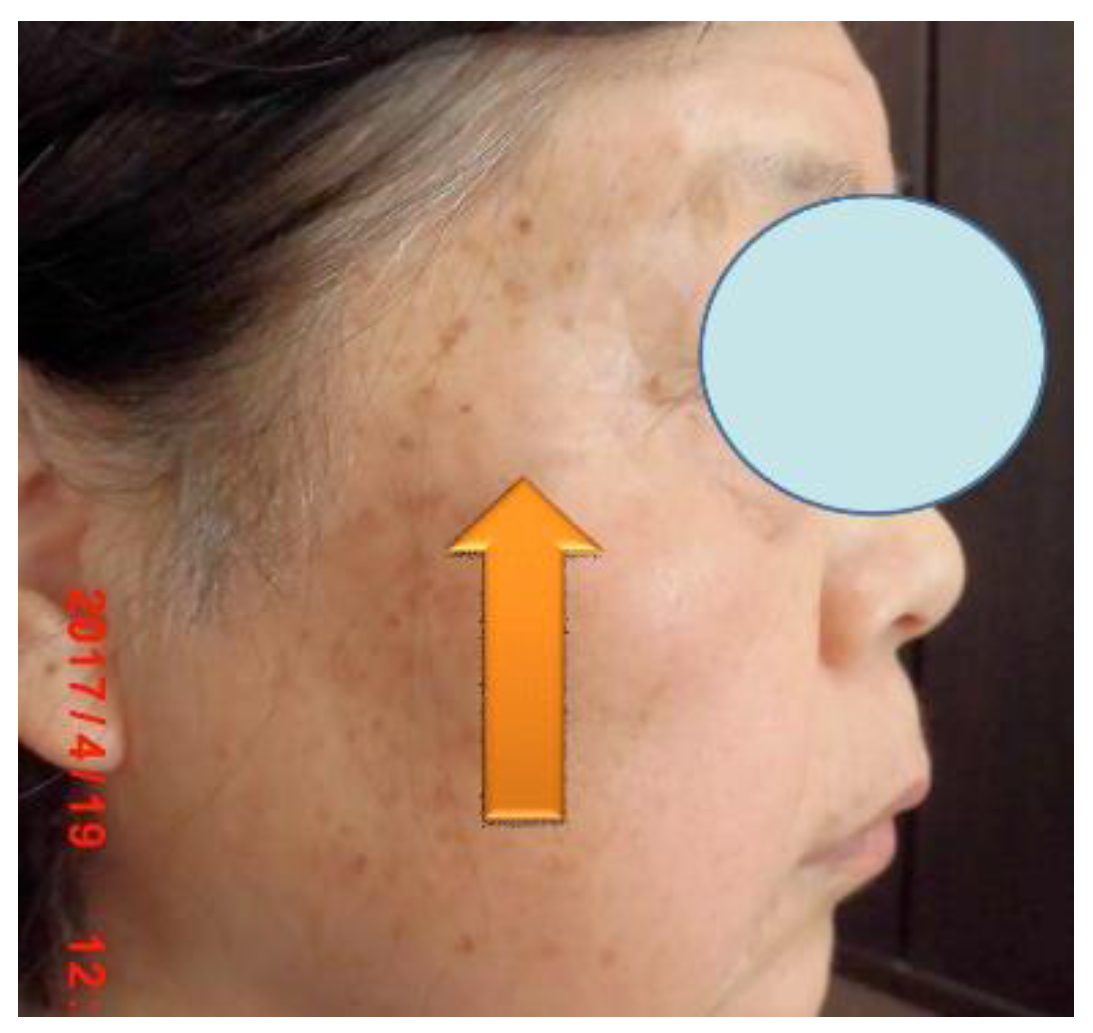
Effects of 1-Hydroxyindoles on Arachidonic Acid Induced Platelet Aggregation in Rabbit PRP 60-66.
Table 28.
Effects of 1-Hydroxyindoles on Arachidonic Acid Induced Platelet Aggregation in Rabbit PRP 60-66.
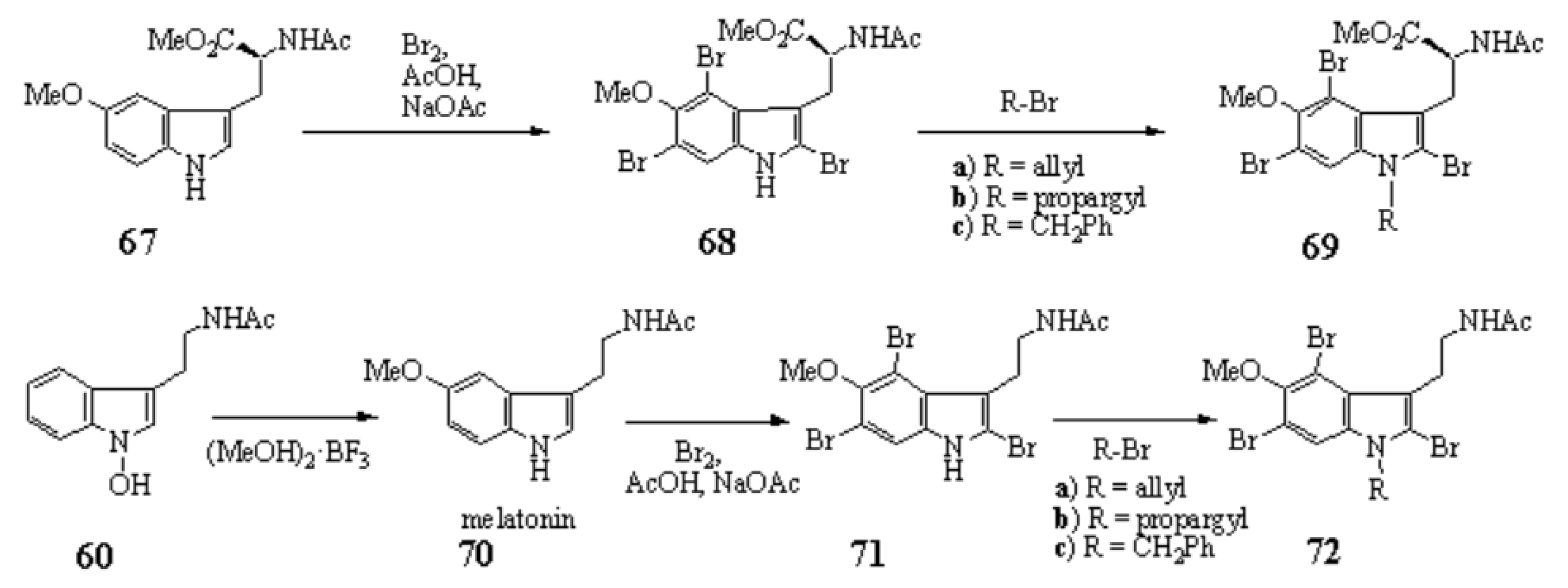
Scheme 6.
Synthesis of candidates of osteoporosis.
Scheme 6.
Synthesis of candidates of osteoporosis.
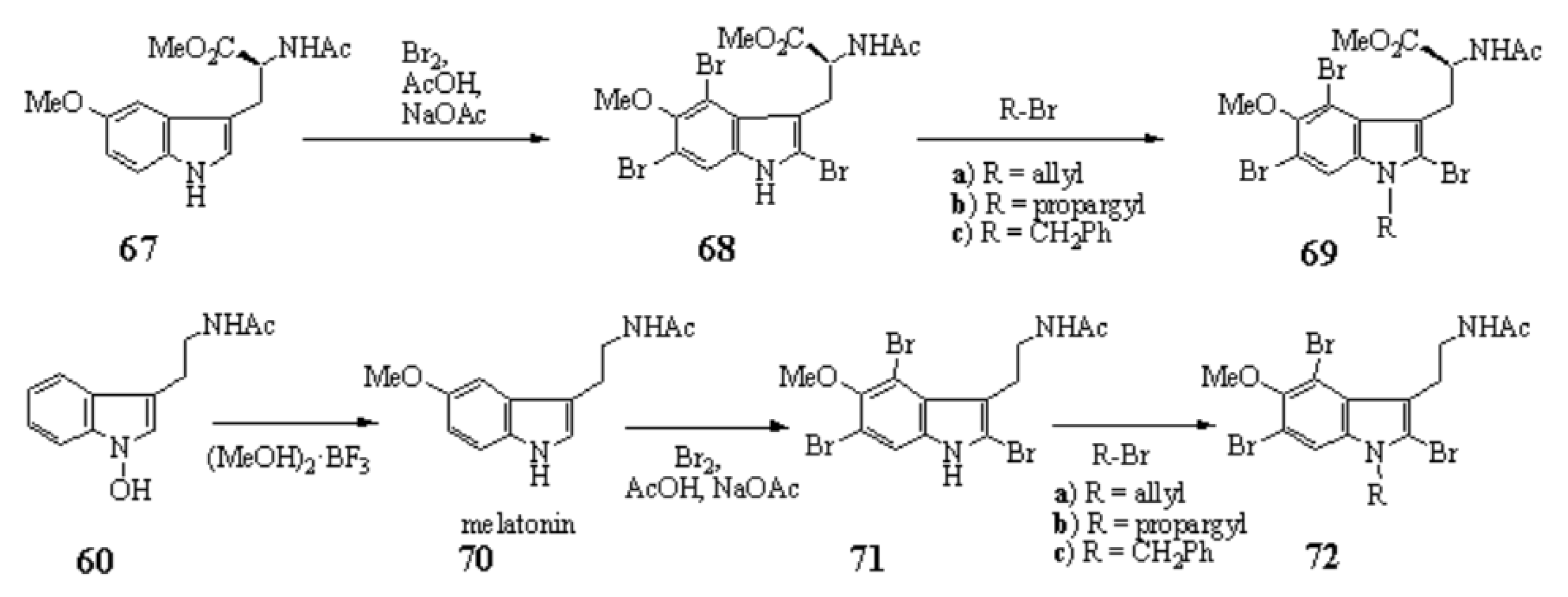
23.2. Tryptophan Derivatives:
Synthesis of (
S)-(+)-
N-acetyl-2,4,6-tribromo-5-methoxytryptophan methyl ester (68) from (
S)-(+)-
N-acetyl-5-methoxy tryptophan methyl ester (67) — To a solution of (
S)-(+)-
N-acetyl-5-methoxytryptophan methyl ester (67)[
84] (56.6 mg, 0.20 mmol) in 4.5 mL of AcOH was added a solution of Br
2 (1.0 mL, 0.59 mmol), separately prepared by dissolving 458.0 mg of Br
2 and 41.8 mg of NaOAc in 5.0 mL of AcOH, and the mixture was stirred at room temperature for 30 min. After the addition of 10% aqueous Na
2S
2O
2, the mixture was made alkaline by adding 40% aqueous NaOH under ice cooling and extracted with CHCl
3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave a yellow solid, which was column-chromatographed on SiO
2 with CHCl
3–MeOH (99.5:0.5, v/v) to give an inseparable 1:2 mixture (28.4 mg) of (S)-(+)-N-acetyl-2,6-dibromo- and (S)-(+)-N-acetyl-2,4,7-tribromo--5-methoxytryptophan methyl ester, and 68 (53.7 mg, 52%) in the order of elution. 68: mp 198–199°C (colorless granules, recrystallized from AcOEt). IR (KBr): 3307, 1730, 1647, 1556, 1300, 1232, 1028 cm
-1.
1H-NMR (CDCl
3) δ: 1.88 (3H, s), 3.29 (1H, dd,
J = 9.8, 14.6 Hz), 3.58 (1H, dd,
J = 5.2, 14.6 Hz), 3.77 (3H, s), 3.88 (3H, s), 5.01 (1H, ddd,
J = 5.2, 8.6, 9.8 Hz, changed to dd,
J = 5.2, 9.8 Hz on addition of D
2O), 6.17 (1H, br d,
J = 8.6 Hz, disappeared on addition of D
2O), 7.37 (1H, s), 8.70 (1H, br s, disappeared on addition of D
2O). MS
m/z: 530 (M
+), 528 (M
+), 526 (M
+), 524 (M
+).
Anal. Calcd for C
15H
15Br
3N
2O
4: C, 34.19; H, 2.87; N, 5.32. Found: C, 34.24; H, 2.89; N, 5.18. Optical Rotation [α]
D26 +14.8° (DMSO, c 0.200). [α]
D27 +1.47° (MeOH, c 0.204). [α]
D28 +4.4° (CHCl
3, c 0.203).
Synthesis of (S)-(+)-N-acetyl-1-allyl-2,4,6-tribromo-5-methoxytryptophan methyl ester (69a) from 68 — To a solution of 68 (39.8 mg, 0.08 mmol) in N,N-dimethylformamide (DMF, 2.5 mL) was added K2CO3 (36.5 mg, 0.26 mmol) and allyl bromide (0.13 mL, d = 1.398, 1.51 mmol). After stirring at room temperature for 30 min, water was added to the reaction mixture. The whole was extracted with AcOEt. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a yellow oil. Purification by column-chromatography on SiO2 with CHCl3 to give 69a (42.4 mg, 99%). 69a: mp 191–192°C (colorless needles, recrystallized from AcOEt). IR (KBr): 3303, 1732, 1645, 1547, 1228, 1016 cm-1. 1H-NMR (CDCl3) δ: 1.86 (3H, s), 3.34 (1H, dd, J = 9.8, 14.7 Hz), 3.62 (1H, dd, J = 5.4, 14.7 Hz), 3.74 (3H, s), 3.89 (3H, s), 4.75 (2H, m), 4.83 (1H, d, J = 17.1 Hz), 5.01 (1H, ddd, J = 5.4, 8.7, 9.8 Hz, changed to dd, J = 5.4, 9.8 Hz on addition of D2O), 5.19 (1H, d, J = 10.3 Hz), 5.86 (1H, tdd, J = 4.8, 10.3, 17.1 Hz), 6.12 (1H, br d, J = 8.7 Hz, disappeared on addition of D2O), 7.41 (1H, s). MS m/z: 570 (M+), 568 (M+), 566 (M+), 564 (M+). Anal. Calcd for C18H19Br3N2O4: C, 38.12; H, 3.38; N, 4.94. Found: C, 37.97; H, 3.43; N, 4.86. Optical Rotation [α]D26 +13.8° (CHCl3, c 0.203).
Synthesis of (S)-(+)-N-acetyl-2,4,6-tribromo-5-methoxy-1-propargyltryptophan methyl ester (69b) from 68 — To a solution of 68 (23.6 mg, 0.04 mmol) in DMF (2.0 mL) was added K2CO3 (21.6 mg, 0.16 mmol) and propargyl bromide (0.08 mL, d = 1.335, 0.9 mmol). After stirring at room temperature for 30 min, water was added to the reaction mixture. The whole was extracted with AcOEt. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a pink oil. Purification by column-chromatography on SiO2 with CHCl3 to give 69b (23.9 mg, 94%). 69b: mp 284–285°C (decomp., measured with a sealed tube, colorless needles, recrystallized from CHCl3–MeOH). IR (KBr): 3284, 3224, 2114, 1724, 1647, 1552, 1230, 1016 cm-1. 1H-NMR (DMSO-d6) δ: 1.82 (3H, s), 3.28 (1H, dd, J = 7.1, 14.9 Hz), 3.33 (1H, dd, J = 8.7, 14.9 Hz), 3.33 (1H, t, J = 2.4 Hz), 3.48 (3H, s), 3.80 (3H, s), 4.47 (1H, ddd, J = 7.1, 7.1, 8.7 Hz, changed to dd, J = 7.1, 8.7 Hz on addition of D2O), 5.13 (2H, dt, J = 2.4, 4.2 Hz), 8.02 (1H, s), 8.44 (1H, br d, J = 7.1 Hz, disappeared on addition of D2O). MS m/z: 568 (M+), 566 (M+), 564 (M+), 562 (M+). Anal. Calcd for C18H17Br3N2O4: C, 38.26; H, 3.03; N, 4.96. Found: C, 38.11; H, 3.12; N, 4.83. Optical Rotation [α]D24 +7.7° (DMSO, c 0.202).
Synthesis of (S)-(+)-N-acetyl-1-benzyl-2,4,6-tribromo-5-methoxytryptophan methyl ester (69c) from 68 — To a solution of 68 (19.6 mg, 0.04 mmol) in DMF (1.5 mL) was added K2CO3 (18.0 mg, 0.13 mmol) and benzyl bromide (0.09 mL, d = 1.44, 0.7 mmol). After stirring at room temperature for 30 min, water was added to the reaction mixture. The whole was extracted with AcOEt–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a yellow oil. Preparative thin-layer chromatography was performed on SiO2 with CHCl3–MeOH (99:1, v/v) as a developing solvent. Extraction of the band having an Rf value of 0.18 to 0.29 with CHCl3–MeOH (95:5, v/v) afforded 69c (22.0 mg, 96%). 69c: mp 226–227°C (colorless needles, recrystallized from MeOH). IR (KBr): 3298, 1732, 1643, 1550, 1414, 1230, 1018 cm-1. 1H-NMR (DMSO-d6) δ: 1.80 (3H, s), 3.28 (1H, dd, J = 7.1, 14.4 Hz), 3.40 (1H, dd, J = 8.5, 14.7 Hz, appeared on addition of D2O), 3.47 (3H, s), 3.79 (3H, s), 4.55 (1H, ddd, J = 7.1, 7.1, 8.5 Hz, changed to dd, J = 7.1, 8.5 Hz on addition of D2O), 5.53 (2H, s), 6.96 (2H, d, J = 7.1 Hz), 7.25 (1H, t, J = 7.1 Hz), 7.31 (2H, t, J = 7.1 Hz), 7.90 (1H, s) 8.45 (1H, d, J = 7.1 Hz, disappeared on addition of D2O). MS m/z: 620 (M+), 618 (M+), 616 (M+), 614 (M+). Anal. Calcd for C22H21Br3N2O4: C, 42.82; H, 3.43; N, 4.54. Found: C, 42.69; H, 3.47; N, 4.56. Optical Rotation [α]D24 +8.3° (CHCl3, c 0.204)
-
1)
Tryptamine derivatives
Melatonin (70) from Nb-acetyl-1-hydroxytryptamine (60) — 50% (MeOH)2·BF3 (15.0 mL) was added to a solution of 60 (150.0 mg, 0.688 mmol) in MeOH (10.0 mL) under ice cooling, and the mixture was refluxed for 30 min with stirring. After evaporation of the solvent, the whole was made neutral by adding 8% NaOH under ice cooling and extracted with CH2Cl2–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CH2Cl2–MeOH (98:2, v/v) to give 70 (121.6 mg, 72%). 70 was identical with commercially available authentic sample in every spectral data.
2,4,6-Tribromomelatonin (71) from melatonin (70) — A 0.56 M solution of Br2 in AcOH (containing 1 mmol of NaOAc, 3.30 mL, 1.85 mmol) was added to a solution of 70 (144.5 mg, 0.62 mmol) in AcOH (12 mL), and the mixture was stirred at rt for 1.5 h. After the addition of Na2S2O3 (1.0 mL) and H2O, the mixture was made basic with 40% NaOH under ice cooling and extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–MeOH (99:1, v/v) to give 71 (272.2 mg, 94%). 71: mp >300 °C (decomp., colorless powder, recrystallized from MeOH). IR (KBr): 3371, 3370, 1653, 1543, 1446, 1406, 1306, 1022 cm-1. 1H-NMR (CDCl3) δ: 1.78 (3H, s), 2.97 (2H, t, J=7.3 Hz), 3.25 (2H, q, J=7.3 Hz), 3.77 (3H, s), 7.52 (1H, s), 7.89 (1H, br t, J=5.6 Hz, disappeared on addition of D2O), 12.15 (1H, br t, J=7.3 Hz, disappeared on addition of D2O). Anal. Calcd for C13H13Br3N2O2: C, 33.29; H, 2.79; N, 5.97. Found: C, 33.27; H, 2.82; N, 5.85.
1-Allyl-2,4,6-tribromomelatonin (72a) from 2,4,6-tribromomelatonin (71) — General procedure. K2CO3 (31.1mg, 0.22 mmol) was added to a solution of 71 (30.2 mg, 0.064 mmol) in DMF (2.0 mL), and the mixture was stirred at rt for 1.5 h. To the resultant mixture, allyl bromide (0.11 mL, 1.28 mmol) was added and stirred at rt for 1.5 h. After the addition of H2O, the mixture was extracted with AcOEt–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt to give 72a (31.0 mg, 95%). 72a: mp 142–143 °C (colorless fine needles, recrystallized from AcOEt–hexane). IR (KBr): 3284, 1633, 1562, 1456, 1412, 1298, 1018 cm-1. 1H-NMR (CDCl3) δ: 1.93 (3H, s), 3.24 (2H, t, J=6.6 Hz), 3.58 (2H, q, J=6.6 Hz), 3.89 (3H, s), 4.76 (2H, dt, J=4.9, 1.7 Hz), 4.89 (1H, d, J=16.6 Hz), 5.20 (1H, d, J=10.3 Hz), 5.55 (1H, br t, disappeared on addition of D2O), 5.87 (1H, ddt, J=16.6, 10.3, 4.9 Hz), 7.4 (1H, s). Anal. Calcd for C16H17Br3N2O2: C, 37.75; H, 3.37; N, 5.50. Found: C, 37.75; H, 3.37; N, 5.42.
1-Propargyl-2,4,6-tribromomelatonin (72b) from 2,4,6-tribromomelatonin (71) — In the general procedure for the preparation of 72a, K2CO3 (31.9mg, 0.22 mmol), 71 (30.1 mg, 0.064mmol), and propargyl chloride (0.09 mL, 1.28 mmol) were used. After work–up, 31.6 mg (97%) of 72b was obtained. 72b: mp 199–200 °C (colorless fine needles, recrystallized from AcOEt–hexane). IR (KBr): 3286, 2117, 1628, 1558, 1456, 1435, 1410, 1294, 1018 cm-1. 1H-NMR (CDCl3) δ: 1.93 (3H, s), 2.34 (1H, t, J=2.4 Hz), 3.23 (2H, t, J=6.6 Hz), 3.58 (2H, q, J=6.6 Hz), 3.89 (3H, s), 4.91 (2H, d, J=2.4 Hz), 5.54 (1H, br t, J=6.6 Hz, disappeared on addition of D2O), 7.58 (1H, s). Anal. Calcd for C13H15Br3N2O2: C, 37.90; H, 2.98; N, 5.53. Found: C, 37.78; H, 3.00; N, 5.44.
1-Benzyl-2,4,6-tribromomelatonin (72c) from 2,4,6-tribromomelatonin (71) — In the general procedure for the preparation of 74a, K2CO3 (31.8mg, 0.30 mmol), 71 (40.1 mg, 0.086 mmol), and benzyl bromide (0.20 mL, 1.72 mmol) were used. After work–up, 40.3 mg (83%) of 72c was obtained. 72c: mp 218–219 °C (colorless fine needles, recrystallized from MeOH). IR (KBr): 3280, 1630, 1547, 1454, 1414, 1360, 1298, 1014 cm-1. 1H-NMR (CDCl3) δ: 1.91 (3H, s), 3.26 (2H, t, J=6.6 Hz), 3.61 (2H, td, J=12.7, 6.6 Hz), 3.88 (3H, s), 5.36 (2H, s), 5.54 (1H, br t, J=6.6 Hz, disappeared on addition of D2O), 7.01 (2H, d, J=6.6 Hz), 7.27-7.33 (3H, m), 7.39 (1H, s). Anal. Calcd for C20H19Br3N2O2: C, 42.97; H, 3.43; N, 5.01. Found: C, 42.76; H, 3.40; N, 4.86.
23-2. Synthesis of VED and related derivatives through 1-hydroxy tryptamines
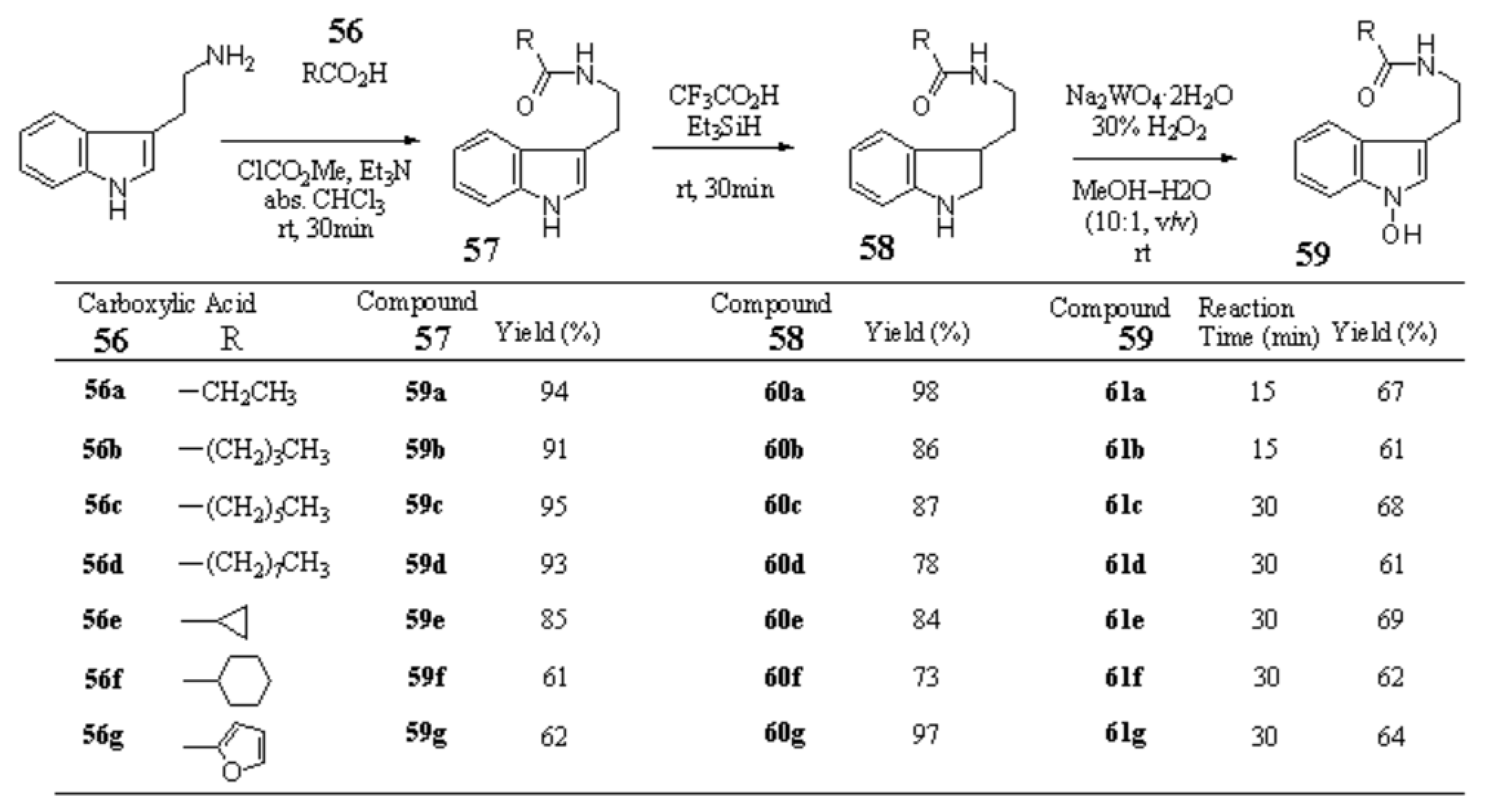
Scheme 5 Synthesis of 1-hydroxy-Nb-acyltryptamines
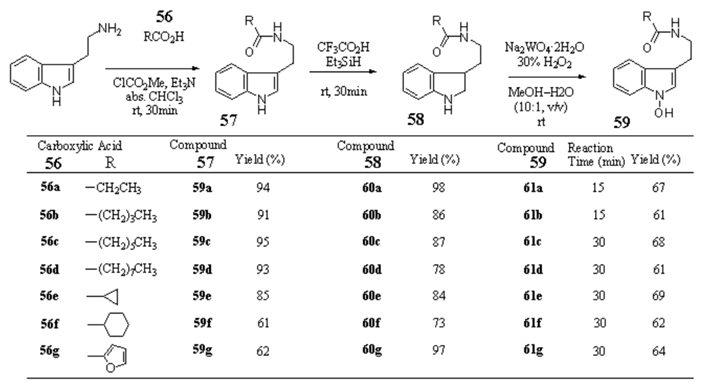
Nb-Propionyl tryptamine (57a) from tryptamine — Et3N (1.89 mL, 13.6 mmol) and ClCO2Me (1.05 mL, 1.36 mmol) were added to a solution of propionic acid (913 mg, 12.3 mmol) in anhydrous CHCl3 (30 mL) and the mixture was stirred at 0 °C for 30 min. To the resulting mixture, tryptamine (2.17 g, 13.6 mmol) was added and the mixture was stirred at rt for 30 min. After addition of H2O the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a residue, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give 57a (2.51g, 94%). 57a: mp 88–89 °C (colorless fine needles, recrystallized from Et2O). IR (KBr): 3377, 1635, 1563, 1453, 1368, 1250 cm-1. 1H-NMR (CDCl3) δ: 1.11 (3H, t, J=7.0 Hz), 2.14 (2H, q, J=7.6 Hz), 2.98 (2H, dt, J=6.6, 0.7 Hz), 3.61 (2H, q, J=6.6 Hz), 5.50 (1H, br s), 7.04 (1H, d, J=2.2 Hz), 7.13 (1H, ddd, J=7.8, 7.1, 1.0 Hz), 7.21 (1H, ddd, J=7.8, 7.1, 1.2 Hz) 7.37 (1H, dt, J=7.8, 1.0 Hz), 7.61 (1H, ddd, J=7.8, 1.2, 0.7 Hz), 8.09 (1H, br s). MS m/z: 216 (M+). Anal. Calcd. For C13H16N2O·1/8H2O: C, 71.45; H, 7.50; N, 12.82. Found: C, 71.77; H, 7.34; N, 12.52.
Nb-Valeryl tryptamine (57b) from tryptamine — Et3N (1.57 mL, 11.3 mmol) and ClCO2Me (0.87 mL, 11.3 mmol) were added to a solution of valeric acid (1.05 g, 10.3 mmol) in anhydrous CHCl3 (30 mL) and the mixture was stirred at 0 °C for 30 min. To the resulting mixture, tryptamine (1.81 g, 11.3 mmol) was added and the mixture was stirred at rt for 30 min. After addition of H2O the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a residue, which was column-chromatographed on SiO2 with AcOEt–hexane (2:3, v/v) to give 57b (2.29g, 91%). 57b: mp 93–94 °C (colorless powder, recrystallized from AcOEt–hexane). IR (KBr): 3377, 3237, 2927, 1630, 1561, 1450 cm-1. 1H-NMR (CDCl3) δ: 0.88 (3H, t, J=7.3 Hz), 1.26–1.34 (2H, m), 1.53–1.60 (2H, m), 2.11 (2H, t, J=7.5 Hz), 2.98 (2H, t, J=6.6 Hz), 3.61 (2H, q, J=6.6 Hz), 5.56 (1H, br s), 7.04 (1H, s), 7.13 (1H, ddd, J=7.9, 7.0, 0.9 Hz), 7.22 (1H, ddd, J=7.9, 7.0, 0.9 Hz), 7.38 (1H, dt, J=7.9 Hz), 7.61 (1H, d, J=7.9 Hz), 8.09 (1H, br s). Anal. Calcd. For C15H20N2O: C, 73.73; H, 8.25; N, 11.47. Found: C, 73.48; H, 8.23; N, 11.42.
Nb-Heptanoyl tryptamine (57c) from tryptamine — Et3N (1.19 mL, 8.58 mmol) and ClCO2Me (0.66 mL, 8.58 mmol) were added to a solution of heptanoic acid (1.01 g, 7.80 mmol) in anhydrous CHCl3 (30 mL) and the mixture was stirred at 0 °C for 30 min. To the resulting mixture, tryptamine (1.37 g, 8.58 mmol) was added and the mixture was stirred at rt for 30 min. After addition of H2O the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a residue, which was column-chromatographed on SiO2 with CHCl3 to give 57c (2.02 g, 95%). 57c: mp 97–98 °C (colorless powder, recrystallized from AcOEt–hexane). IR (KBr): 3410, 1632, 1565, 1457, 1425 cm-1. 1H-NMR (CDCl3) δ: 0.87 (3H, t, J=7.3 Hz), 1.21–1.31 (6H, m), 1.57 (2H, quint, J=7.5 Hz), 2.10 (2H, t, J=7.5 Hz), 2.98 (2H, t, J=6.6 Hz), 3.61 (2H, q, J =6.6 Hz), 5.52 (1H, br s), 7.04 (1H, d, J=2.2 Hz), 7.13 (1H, ddd, J= 8.1, 7.0, 1.0 Hz), 7.22 (1H, ddd, J= 8.1, 7.0, 1.0 Hz, 7.38 (1H, dt, J=8.1, 1.0 Hz) 7.61 (1H, d, J=8.1 Hz), 8.08 (1H, br s). Anal. Calcd. For C17H24N2O: C, 74.96; H, 8.88; N, 10.29. Found: C, 74.80; H, 8.92; N, 10.26.
VED #1 [Nb-Nonanoyl tryptamine (57d)] from tryptamine — Et3N (0.99 mL, 7.09 mmol) and ClCO2Me (0.55 mL, 7.09 mmol) were added to a solution of nonanoic acid (1.02g, 6.45 mmol) in anhydrous CHCl3 (30 mL) and the mixture was stirred at 0 °C for 30 min. To the resulting mixture, tryptamine (1.14 g, 7.09 mmol) was added and the mixture was stirred at rt for 30 min. After addition of H2O the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a residue, which was column-chromatographed on SiO2 with AcOEt–hexane (1:2, v/v) to give 57d (1.78g, 93%). 57d: mp 101–102 °C (colorless fine needles, recrystallized from CHCl3–hexane). IR (CHCl3): 2950, 1652, 1506, 1165 cm-1. 1H-NMR (CDCl3) δ: 0.87 (3H, t, J=7.0 Hz), 1.22–1.31 (10H, m), 1.57 (2H, br quint, J=7.0 Hz), 2.10 (2H, t, J=7.6 Hz), 2.98 (2H, t, J=6.7 Hz), 3.61 (2H, q, J=6.7 Hz, collapsed to t , J=6.7 Hz on addition of D2O), 5.52 (1H, br s, disappeared on addition of D2O), 7.04 (1H, s), 7.13 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.21 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.38 (1H, d, J=8.1 Hz), 7.61 (1H, d, J=8.1 Hz), 8.09 (1H, br s, disappeared on addition of D2O). Anal. Calcd for C19H28N2O: C, 75.96; H, 9.39; N, 9.33. Found: C, 75.66; H, 9.49; N, 9.24.
2,3-Dihydro-Nb-propionyltryptamine (58a) from 57a — A mixture of 57a (1.02 g, 4.74 mmol) and Et3SiH (1.89 mL, 11.9 mmol) in TFA (20 mL) was stirred at rt for 30 min. After evaporation of the solvent, the residue was made alkaline with 8% NaOH and extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH–28%NH4H (46:1:0.1, v/v) to give 58a (1.01 g, 98%). 58a: yellow viscous oil. IR (film): 3315, 2970, 1635, 1606, 1547, 1486, 1461 cm-1. 1H-NMR (CDCl3) δ: 1.13 (3H, t, J=7.5 Hz), 1.78 (1H, dtd, J=13.6, 7.9, 6.0 Hz), 2.00 (1H, dddd, J=13.6, 7.9, 7.0, 5.0 Hz), 2.16 (2H, q, J=7.5 Hz), 2.82(1H, br s, disappeared on addition of D2O), 3.26–3.42 (4H, m), 3.72 (1H, t, J=8.8 Hz), 5.62 (1H, br s, disappeared on addition of D2O), 6.67 (1H, d, J=7.3 Hz), 6.75 (1H, td, J=7.3, 0.9 Hz), 7.05 (1H, br t, J=7.3 Hz), 7.10 (1H, d, J=7.3 Hz). HR–MS m/z: Calcd for C13H18N2O: 218.1419. Found: 218.1431.
2,3-Dihydro-Nb-valeryltryptamine (58b) from 57b — A mixture of 57b (102.1 mg, 0.42 mmol) and Et3SiH (0.17 mL, 1.05 mmol) in TFA (3.0 mL) was stirred at rt for 30 min. After evaporation of the solvent, the residue was made alkaline with 8% NaOH and extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH–28%NH4H (46:1:0.1, v/v) to give 58b (88.1 mg, 86%). 58b: yellow viscous oil. IR (film): 3290, 2930, 1640, 1605, 1552, 1484, 1461 cm-1. 1H-NMR (CDCl3) δ: 0.91 (3H, t, J= 7.5 Hz), 1.33 (2H, sext, J=7.5 Hz), 1.59 (2H, quint, J=7.5 Hz), 1.73 (1H, dtd, J=13.6, 8.0, 6.1 Hz), 1.99 (1H, dddd, J=13.6, 8.0, 7.0, 5.0 Hz), 2.13 (2H, t, J=7.5 Hz), 2.75 (1H, br s, disappeared on addition of D2O), 3.27–3.42 (4H, m), 3.72 (1H, t, J=8.6 Hz), 5.60 (1H, br s, disappeared on addition of D2O), 6.68 (1H, d, J=7.3 Hz), 6.75 (1H, td, J=7.3, 0.9 Hz), 7.05 (1H, br t, J=7.3 Hz), 7.11 (1H, d, J=7.3 Hz). HR–MS m/z: Calcd for C15H22N2O: 246.1732. Found: 246.1743.
Nb-Heptanoyl-2,3-dihydrotryptamine (58c) from 57c — A mixture of 57c (1.04 g, 3.82 mmol) and Et3SiH (1.52 mL, 9.54 mmol) in TFA (20 mL) was stirred at rt for 30 min. After evaporation of the solvent, the residue was made alkaline with 8% NaOH and extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (2:1, v/v) to give 58c (912.7 mg, 87%). 58c: pale yellow viscous oil. IR (film): 3310, 2960, 1634, 1606, 1544, 1484, 1461 cm-1. 1H-NMR (CDCl3) δ: 0.88 (3H, t, J= 7.5 Hz), 1.25–1.34 (6H, m), 1.60 (2H, quint, J=7.5 Hz), 1.78 (1H, dtd, J=13.6, 7.9, 5.9 Hz), 1.99 (1H, dddd, J=13.6, 7.9, 7.1, 5.1 Hz), 2.12 (2H, t, J=7.7 Hz), 2.68 (1H, br s, disappeared on addition of D2O), 3.27–3.42 (4H, m), 3.72 (1H, t, J=8.6 Hz), 5.60 (1H, br s, disappeared on addition of D2O), 6.68 (1H, d, J=7.5 Hz), 6.75 (1H, td, J=7.5, 1.1 Hz), 7.05 (1H, br t, J=7.5 Hz), 7.10 (1H, d, J=7.5 Hz). HR–MS m/z: Calcd for C17H26N2O: 274.2045. Found: 274.2057.
2,3-Dihydro-Nb-nonanoyltryptamine (58d) from 57d — A mixture of 57d (1.10 g, 3.65 mmol) and Et3SiH (1.45 mL, 9.10 mmol) in TFA (20 mL) was stirred at rt for 30 min. After evaporation of the solvent, the residue was made alkaline with 8% NaOH and extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give 58d (862.4 mg, 78%). 58d: mp 41–42.5 °C (colorless powder, recrystallized from AcOEt–hexane). IR (KBr): 3300, 2935, 2870, 1638, 1546, 1486, 1465 cm-1. 1H-NMR (DMSO-d6) δ: 0.84 (3H, t, J=7.0 Hz), 1.20–1.27 (10H, m), 1.45–1.57 (3H, m). 1.83 (1H, dtd, J=13.2, 7.6, 5.6 Hz), 2.04 (2H, t, J=7.5 Hz), 3.05 (1H, ddd, J=9.3, 8.1, 2.2 Hz), 3.09–3.16 (3H, m), 3.54 (1H, td, J=8.6, 1.7 Hz), 5.40 (1H, br s, disappeared on addition of D2O), 6.47 (1H, d, J=7.5 Hz), 6.52 (1H, td, J= 7.5, 0.7 Hz), 6.90 (1H, br t, J=7.5 Hz), 7.00 (1H, d, J=7.5 Hz), 7.80 (1H, br t, J= 6.1 Hz, disappeared on addition of D2O). Anal. Calcd for C19H30N2O: C, 75.45; H, 10.00; N, 9.26. Found: C, 75.25; H,10.16; N, 9.24.
1-Hydroxy-Nb-propionyltryptamine (59a) from 58a — A solution of 30% H2O2 (1.11 g, 9.80 mmol) in MeOH (3.0 mL) was added to a solution of 58a (211.8 mg, 0.97 mmol) and Na2WO4·2H2O (64.1 mg, 0.19 mmol) in MeOH (7.0 mL) and H2O (1.0 mL) under ice cooling with stirring. Stirring was continued at rt for 15 min. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (99:1, v/v) to give 59a (150.2 mg, 67%). 59a: mp 132–133 °C (colorless fine prisms, recrystallized from CHCl3). IR (KBr): 3290, 3100, 2935, 1598, 1566, 1352 cm-1. 1H-NMR (DMSO-d6) δ: 0.99 (3H, t, J=7.6 Hz), 2.06 (2H, q, J=7.6 Hz), 2.79 (2H, t, J=7.3 Hz), 3.30 (2H, td, J=7.3, 6.1 Hz, collapsed to t, J=7.3 Hz, on addition of D2O), 6.98 (1H, dd, J=8.0, 7.3 Hz), 7.13 (1H,dd, J=8.0, 7.3 Hz), 7.24 (1H, s), 7.32 (1H, d, J=8.0 Hz), 7.53 (1H, d, J=8.0 Hz), 7.84 (1H, br t, J=6.1 Hz, disappeared on addition of D2O), 11.01 (1H, s, disappeared on addition of D2O). Anal. Calcd for C13H16N2O2: C, 67.22; H, 6.94; N, 12.06. Found: C, 66.94; H, 6.95; N, 12.02.
1-Hydroxy-Nb-valeryltryptamine (59b) from 58b — A solution of 30% H2O2 (2.37 g, 20.9 mmol) in MeOH (5.0 mL) was added to a solution of 58b (513.3 mg, 2.09 mmol) and Na2WO4·2H2O (138.0 mg, 0.42 mmol) in MeOH (20 mL) and H2O (2.5 mL) under ice cooling with stirring. Stirring was continued at rt for 15 min. After addition of H2O, the whole was extracted with CHCl3. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (1:1, v/v) to give 59b (331.2 mg, 61%). 59b: mp 114.5–115 °C (colorless powder, recrystallized from CHCl3). IR (CHCl3): 3125, 2922, 1649, 1513 cm-1. 1H-NMR (DMSO-d6) δ: 0.86 (3H, t, J=7.4 Hz), 1.25 (2H, sext, J=7.4 Hz), 1.47 (2H, quint, J=7.4 Hz), 2.05 (2H, t, J=7.4 Hz), 2.78 (2H, t, J=7.4 Hz), 3,30 (2H, td, J=7.4, 6.1 Hz, collapsed to t, J=7.4 Hz, on addition of D2O), 6.98 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.12 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.23(1H, s), 7.32 (1H, d, J=8.1 Hz), 7.53 (1H, d, J=8.1 Hz), 7.86 (1H, br t, J=6.1 Hz, disappeared on addition of D2O), 11.00 (1H, s, disappeared on addition of D2O). Anal. Calcd for C15H20N2O2: C, 69.20; H, 7.74; N, 10.76. Found: C, 69.17; H, 7.70; N, 10.68.
1-Hydroxy-Nb-heptanoyltryptamine (59c) from 58c — A solution of 30% H2O2 (461 4 mg, 4.07 mmol) in MeOH (1.0 mL) was added to a solution of 58c (111.3 mg, 0.41 mmol) and Na2WO4·2H2O (27.3 mg, 0.08 mmol) in MeOH (4.0 mL) and H2O (0.5 mL) under ice cooling with stirring. Stirring was continued at rt for 30 min. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–hexane (2:1, v/v) to give 59c (79.8 mg, 68%). 59c: mp 83–83.5 °C (colorless prisms, recrystallized from CHCl3–hexane). IR (KBr): 3280, 2930, 1601, 1555, 1435, 1358, 1241 cm-1. 1H-NMR (DMSO-d6) δ: 0.86 (3H, t, J=7.5 Hz), 1.21–1.29 (6H, m), 1.47 (2H, quint., J=7.5 Hz), 2.04 (2H, t, J=7.5 Hz), 2.78 (2H, t, J=7.5 Hz), 3.29 (2H, td, J=7.5, 6.1 Hz, collapsed to t, J=7.5 Hz, on addition of D2O), 6.98 (1H, ddd, J= 8.1, 7.1, 1.0 Hz), 7.12(1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.24 (1H, s), 7.32 (1H, dt, J=8.1, 1.0 Hz), 7.52 (1H, dt, J=8.1, 1.0 Hz), 7.86 (1H, br t, J=6.1 Hz, disappeared on addition of D2O), 11.00 (1H, s, disappeared on addition of D2O). Anal. Calcd for C17H24N2O2: C, 70.80; H, 8.39; N, 9.71. Found: C, 70.73; H, 8.40; N, 9.64.
1-Hydroxy-Nb-nonanyltryptamine (59d) from 58d — A solution of 30% H2O2 (451.3 mg, 3.98 mmol) in MeOH (1.0 mL) was added to a solution of 58d (119.3 mg, 0.40 mmol) and Na2WO4·2H2O (26.7 mg, 0.08 mmol) in MeOH (4.0 mL) and H2O (0.5 mL) under ice cooling with stirring. Stirring was continued at rt for 30 min. After addition of H2O, the whole was extracted with AcOEt. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (99:1, v/v) to give 59d (75.8 mg, 61%). 59d: mp 82.5–83 °C (colorless powder, recrystallized from CHCl3–hexane). IR (CHCl3): 3155, 2915, 1648, 1510, 1457 cm-1. 1H-NMR (DMSO-d6) δ: 0.86 (3H, t, J=7.4 Hz), 1.15–1.30 (10H, m), 1.47 (2H, quint., J=7.4 Hz), 2.03 (2H, t, J=7.4 Hz), 2.78 (2H, t, J=7.4 Hz), 3.30 (2H, td, J=7.4, 6.1 Hz, collapsed to t, J=7.4 Hz, on addition of D2O), 6.98 (1H, ddd, J= 8.1, 7.1, 1.0 Hz),7.12(1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.24 (1H, s), 7.32 (1H, d, J=8.1 Hz), 7.52 (1H, d, J=8.1 Hz), 7.86 (1H, br t, J=6.1 Hz, disappeared on addition of D2O), 11.01 (1H, s, disappeared on addition of D2O). Anal. Calcd for C19H28N2O2: C, 72.11; H, 8.92; N, 8.85. Found: C, 72.09; H, 8.96; N, 8.85.
1-Allyloxyindole (73a) from (3) — Prepared according to the general method B for 6, where Na2WO4·2H2O (611.6 mg, 1.85 mmol) in H2O (10.0 mL) was added to a solution of 3 (1.108 g, 9.29 mmol) in MeOH (20.0 mL). 30% H2O2 (10.561 g, 101.2 mmol) in MeOH (20.0 mL) was added to the resultant solution at 0 °C with stirring. After stirring for 15 min at rt (20 °C), K2CO3 (3.86 g, 27.8 mmol) and allyl bromide (3.313 g, 27.4 mmol) were added and stirred at rt for 1.5 h. Brine was added and the whole was extracted with CH2Cl2. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave oil, which was purified by column-chromatography on SiO2 with CH2Cl2–hexane (1:9, v/v) to give 73a (711.3 mg, 44%). 73a: colorless oil. IR (film): 3050, 1449, 1220, 740 cm-1. 1H-NMR (CDCl3) δ: 4.67 (2H, dt, J=6.6, 1.2 Hz), 5.21 (1H, m), 5.35 (1H, d, J=3.4 Hz), 5.86–6.25 (1H, m), 6.30 (1H, dd, J=3.5, 1.0 Hz), 6.94–7.61 (5H, m). High resolution MS m/z: Calcd for C11H11NO: 173.0782. Found: 173.0811.
Scheme 7 Synthesis of 1-hydroxyindole derivatives (1)
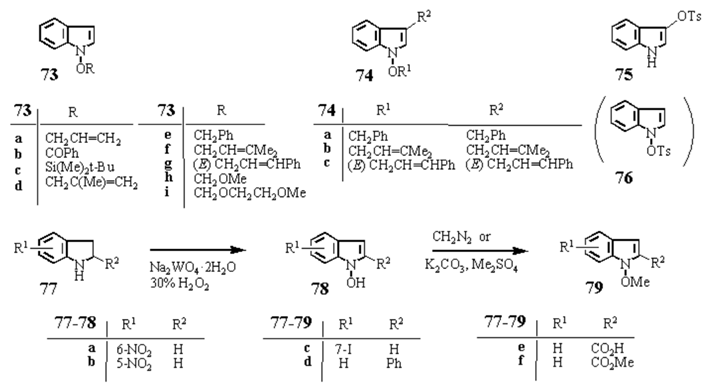
1-Benzoyloxyindole (73b) from 3 — Prepared according to the general method B for
6, where Na
2WO
4·2H
2O (57.1 mg, 0.17 mmol) in H
2O (1.0 mL),
3 (103.0 mg, 0.86 mmol) in MeOH (8.0 mL), and 30% H
2O
2 (981.2 mg, 8.66 mmol) in MeOH (2.0 mL) were used. The reaction mixture was extracted with benzene and benzene layer was dried over Na
2SO
4. After filtering off Na
2SO
4, K
2CO
3 (583.3 mg, 3.89 mmol) and benzoyl chloride (483.2 mg, 2.59 mmol) were added to the benzene solution and stirred at rt for 1.5 h. H
2O was added and the whole was extracted with CH
2Cl
2. The extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave oil, which was purified by column-chromatography on SiO
2 with CH
2Cl
2–hexane (3:7, v/v) to give
73b (100.8 mg, 49%).
73b: mp 55.5–56.0 °C (lit.[
64] mp 49–50 °C, pale brown needles, recrystallized from MeOH). IR (KBr): 1767, 1600, 1446, 1323, 1236, 1184, 1075, 1039, 1012, 1002, 754, 729, 700 cm
-1. UV λ
maxMeOH nm (log ε): 217 (4.50), 265 (3.94), 293 (3.60).
1H-NMR (CDCl
3) δ: 6.53 (1H, d,
J=3.7 Hz), 6.96–7.35 (4H, m), 7.35–7.82 (4H, m), 8.21 (2H, dd,
J=8.2, 1.7 Hz). MS
m/z: 237 (M
+).
Anal. Calcd for C
15H
11NO
2: C, 75.94; H, 4.67; N, 5.90. Found: C, 75.85; H, 4.62; N, 5.84.
1-t-Butyldimethylsilyloxyindole (73c) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (75.9 mg, 0.23 mmol) in H2O (1.3 mL), 3 (136.9 mg, 1.15 mmol) in MeOH (10.0 mL), and 30% H2O2 (1.304 g, 11.5 mmol) in MeOH (3.0 mL) were used. Silylation was carried out according to the method for 73b with K2CO3 (715.5 mg, 5.18 mmol) and t-butyldimethylsilyl chloride (520.2 mg, 3.45 mmol). After usual work-up and purification, 73c (133.1 mg, 47%) was obtained. 73c: colorless oil. IR (film): 1472, 1436, 1266, 1074, 1035, 836, 787, 739 cm-1. 1H-NMR (CDCl3) δ: 0.23 (6H, s), 1.10 (9H, s), 6.31 (1H, d, J=3.4 Hz), 7.01 (1H, t, J=6.6 Hz), 7.07 (1H, d, J=3.4 Hz), 7.17 (1H, t, J=6.6 Hz), 7.31 (1H, d, J=6.6 Hz), 7.53 (1H, d, J=6.6 Hz). High resolution MS m/z: Calcd for C14H21NOSi: 247.1390. Found: 247.1376.
1-Methallyloxyindole (73d) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (26.7 mg, 0.08 mmol) in H2O (0.5 mL), 3 (48.0 mg, 0.40 mmol) in MeOH (4.0 mL), and 30% H2O2 (461.7 mg, 4.0 mmol) in MeOH (1.0 mL) were used. Methallylation was carried out with K2CO3 (253.3 mg, 1.80 mmol) and methallyl chloride (110.5 mg, 1.20 mmol). After usual work-up and purification, 73d (4.2 mg, 6%) was obtained. 73d: colorless oil. IR (film): 1653, 1450, 1323, 1222, 740 cm-1. 1H-NMR (CDCl3) δ: 1.97 (3H, t, J=1.2 Hz), 4.60 (2H, s), 5.03 (2H, m), 6.32 (1H, dd, J=3.4, 0.7 Hz), 7.00–7.63 (5H, m). High resolution MS m/z: Calcd for C12H13NO: 187.0972. Found: 187.0984.
1-Benzyloxyindole (73e) and 3-benzyl-1-benzyloxyindole (74a) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (60.3 mg, 0.18 mmol) in H2O (1.0 mL), 3 (108.7 mg, 0.91 mmol) in MeOH (8.0 mL), and 30% H2O2 (1.036 g, 9.13 mmol) in MeOH (2.0 mL) were used. Benzylation was carried out with K2CO3 (568.1 mg, 4.11 mmol) and benzyl bromide (483.2 mg, 2.74 mmol). After usual work-up and purification, 73e (96.0 mg, 47%) and 74a (14.7 mg, 5%) were obtained. 73e: colorless oil. IR (film): 1455, 1323, 1221, 1074, 1032, 756, 740, 697 cm-1. 1H-NMR (CDCl3) δ: 5.15 (2H, s), 6.23 (1H, d, J=3.4 Hz), 6.98 (1H, d, J=3.4 Hz), 6.98–7.22 (2H, m), 7.22–7.44 (6H, m), 7.44–7.60 (1H, m). High resolution MS m/z: Calcd for C15H13NO: 223.0996. Found: 223.0992. 74a: colorless oil. IR (KBr, film): 1494, 1450, 734, 695 cm–1. 1H-NMR (CDCl3) δ: 4.00 (2H, s), 5.12 (2H, s), 6.11 (1H, s), 6.85–7.11 (14H, m). High resolution MS m/z: Calcd for C22H15NO: 313.1465. Found: 313.1468.
1-Prenyloxyindole (73f) and 3-prenyl-1-prenyloxyindole (74b) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (61.9 mg, 0.19 mmol) in H2O (1.0 mL), 3 (111.2 mg, 0.93 mmol) in MeOH (8.0 mL), and 30% H2O2 (1.087 g, 10.1 mmol) in MeOH (2.0 mL) were used. Prenylation was carried out with K2CO3 (470.6 mg, 6.84 mmol) and prenyl bromide (380.2 mg, 0.51 mmol). After usual work-up and purification 73f (13.5 mg, 7%) and 74b (9.3 mg, 4%) were obtained. 73f: pale yellow oil. IR (film): 3050, 2980, 2930, 1670, 1450, 1324, 1222, 1075, 1030, 740 cm-1. 1H-NMR (CDCl3) δ: 1.48 (3H, s), 1.68 (3H, s), 4.54 (2H, d, J=7.9 Hz), 5.44 (1H, t, J=7.2 Hz), 6.21 (1H, dd, 3.6, 1.0 Hz), 6.88–7.50 (5H, m). High resolution MS m/z: Calcd for C13H15NO: 201.1153. Found: 201.1155. 74b: pale red oil. IR (film): 2980, 2920, 1666, 1612, 1447, 1375, 1088, 1008, 735 cm–1. 1H-NMR (CDCl3) δ: 1.58 (3H, s), 1.74 (3H, s), 1.76 (6H, s), 3.38 (2H, dd, J=7.0, 1.0 Hz), 4.60 (2H, d, J=7.5 Hz), 5.28–5.61 (2H, m), 6.94–7.56 (5H, m). High resolution MS m/z: Calcd for C18H23NO: 269.1753. Found: 269.1765.
1-Prenyloxyindole (73f) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (65.2 mg, 0.19 mmol) in H2O (1.0 mL), 3 (116.4 mg, 0.98 mmol) in MeOH (9.0 mL), and 30% H2O2 (1.143 g, 10.0 mmol) in MeOH (1.0 mL) were used. Prenylation was carried out with NEt3 (1.4 mL, 9.8 mmol), (n-Bu)4NBr (32.3 mg, 0.10 mmol), and prenyl bromide (1.339 g, 8.71 mmol). After usual work-up and purification,73f (35.7 mg, 18%) was obtained.
1-Prenyloxyindole (73f) from 73c — A solution of prenyl bromide (112.2 mg, 0.72 mmol) in anhydrous THF (1.0 mL), KOt-Bu (91.7 mg, 0.82 mmol), and a solution of (n-Bu)4NF·3H2O (120.6 mg, 0.34 mmol) in anhydrous THF (1.0 mL) were added to a solution of 73c (75.4 mg, 0.37 mmol) in anhydrous THF (1.0 mL) at rt. After usual work-up and purification, 73f (75.4 mg, 100%) was obtained.
1-Cinnamyloxyindole (73g) and 3-cinnamyl-1-cinnanyloxyindole (74c) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (57.9 mg, 0.17 mmol) in H2O (1.0 mL). 3 (104.1 mg, 0.87 mmol) in MeOH (8.0 mL), and 30% H2O2 (991.9 mg, 8.7 mmol) in MeOH (2.0 mL) were used. Cinnamylation was carried out with K2CO3 (544.6 mg, 3.91 mmol) and cinnamyl bromide (520.8 mg, 2.61 mmol). After usual work-up and purification, 73g (110.5 mg, 51%) and 74c (80.2 mg, 22%) were obtained. 73g: pale brown oil. IR (film): 3050, 3017, 2920, 1495, 1449, 1323, 1221, 1074, 1030, 965, 757, 738, 691 cm-1. 1H-NMR (CDCl3) δ: 4.81 (2H, d, J=6.0 Hz), 6.31 (1H, dd, J=3.5, 1.0 Hz), 6.25–6.72 (2H, m), 6.96–7.60 (10H, m). High resolution MS m/z: Calcd for C17H15NO: 249.1148. Found: 249.1150. 74c: pale yellow oil. IR (film): 3050, 3017, 2920, 1496, 1449, 964, 738, 692 cm–1. 1H-NMR (CDCl3) δ: 3.58 (2H, dd, J=5.3, 1.0 Hz), 4.77 (2H, d, J=5.5 Hz), 6.13–6.72 (4H, m), 6.93–7.61 (15H, m). High resolution MS m/z: Calcd for C26H23NO: 365.1800. Found: 365.1789.
1-Methoxymethoxyindole (73h) from 3— Prepared according to the general method C for 6, where Na2WO4·2H2O (63.7 mg, 0.19 mmol) in H2O (1.0 mL), 3 (115.1 mg, 0.96 mmol) in MeOH (10.0 mL), and urea·H2O2 compound (927.2 mg, 9.66 mmol) were used. Methoxymethylation was carried out with K2CO3 (2.403 g, 17.38 mmol), (n-Bu)4NBr (31.1 mg, 0.09 mmol), and methoxymethyl chloride (233.3 mg, 2.89 mmol) in benzene (1.0 mL). After usual work-up and purification, 73h (66.0 mg, 39%) was obtained. 73h: mp 27.0–27.5 °C (colorless prisms, recrystallized from hexane). IR (KBr): 2970, 1445, 1330, 1230, 1182, 1100, 1089, 1030, 920, 740 cm-1. 1H-NMR (CDCl3) δ: 3.66 (3H, s), 5.17 (2H, s), 6.37 (1H, d, J=3.5 Hz), 7.11 (1H, t, J=7.4 Hz), 7.20–7.26 (3H, m), 7.42 (1H, d, J=8.1 Hz), 7.58 (1H, d, J=7.9 Hz). MS m/z: 177 (M+). Anal. Calcd for C10H11NO2: C, 67.78; H, 6.26; N, 7.90. Found: C, 67.64; H, 6.33; N, 7.86.
1-Methoxymethoxyindole (73h) from 73c — A solution of methoxymethyl chloride (56.2 mg, 0.69 mmol) in anhydrous THF (1.0 mL), KOt-Bu (91.2 mg, 0.81 mmol), and a solution of (n-Bu)4NF·3H2O (111.1 mg, 0.35 mmol) in anhydrous THF (1.0 mL) were added to a solution of 73c (85.4 mg, 0.34 mmol) in anhydrous THF (1.0 mL) at rt. After usual work-up and purification, 73h (59.7 mg, 98%) was obtained.
1-(2-Methoxyethoxymethoxy)indole (73i) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (61.4 mg, 0.19 mmol) in H2O (1.0 mL), 3 (111.1 mg, 0.93 mmol) in MeOH (9.0 mL), and 30% H2O2 (1.073 g, 9.46 mmol) in MeOH (1.0 mL) were used. Methoxyethoxymethylation was carried out with NEt3 (2.6 mL, 18.6 mmol), (n-Bu)4NBr (29.9 mg, 0.09 mmol), and 2-methoxyethoxymethyl chloride (1.038 g, 8.35 mmol). After usual work-up and purification, 73i (23.2 mg, 11%) and indole (2.2 mg, 2%) were obtained. 73i: colorless oil. IR (KBr): 2920, 2890, 1450, 1320, 1220, 1105, 1030, 920, 875, 845, 760, 740 cm-1. 1H-NMR (CDCl3) δ: 3.41 (3H, s), 3.59–3.65 (2H, m), 3.93–3.99 (2H, m), 5.27 (2H, s), 6.36 (1H, dd, J=3.5, 1.0 Hz), 7.10 (1H, ddd, J=7.9, 6.9, 1.0 Hz), 7.22 (1H, ddd, J=8.3, 6.9, 1.0 Hz), 7.33 (1H, d, J=3.5 Hz), 7.42 (1H, dd, J=8.3, 1.0 Hz), 7.58 (1H, ddd, J=7.9, 1.0, 1.01 Hz). High resolution MS m/z: Calcd for C12H15NO3: 221.1050. Found: 221.1049.
1-(2-Methoxyethoxymethoxy)indole (73i) from 73c — A solution of 2-methoxyethoxymethyl chloride (98.8 mg, 0.79 mmol) in anhydrous THF (1.0 mL), KOt-Bu (97.2 mg, 0.86 mmol), and a solution of (n-Bu)4NF·3H2O (125.8 mg, 0.39 mmol) in anhydrous THF (1.0 mL) were added to a solution of 73c (94.9 mg, 0.38 mmol) in anhydrous THF (1.0 mL) at rt. After usual work-up and purification, 73i (83.3 mg, 98%) was obtained.
3-Tosyloxyindole (75) from 3 — Prepared according to the general method B for 6, where Na2WO4·2H2O (559.6 mg, 1.69 mmol) in H2O (10.0 mL), 3 (1.009 g, 8.48 mmol) in MeOH (80.0 mL), and 30% H2O2 (9.610 g, 84.8 mmol) in MeOH (20.0 mL) were used. Tosylation was carried out with K2CO3 (5.270 g, 38.2 mmol) and tosyl chloride (4.848 g, 25.5 mmol). After usual work-up and purification, 75 (252.2 mg, 10%) was obtained. 75: mp 112–114°C (colorless prisms, recrystallized from MeOH). IR (KBr): 3390, 3120, 1595, 1453, 1370, 1190, 1175, 1090, 1063, 843, 812, 742, 723, 657, 555, 545, 503 cm-1. 1H-NMR (CDCl3) δ: 2.41 (3H, s), 6.95–7.30 (8H, m), 7.75 (2H, d, J=8.3 Hz). MS m/z: 287 (M+). Anal. Calcd for C15H13NO3S: C, 62.70; H, 4.56; N, 4.87. Found: C, 62.70; H, 4.53; N, 4.72. 76 was not isolated because of its instant instability to change to 75.
1-Hydroxy-6-nitroindole (78a) from 77a — Prepared according to the general method B for 6, where Na2WO4·2H2O (18.8 mg, 0.06 mmol), 77a (101.3 mg, 0.57 mmol) in MeOH (10.0 mL), and 30% H2O2 (0.58 mL, 5.7 mmol) were used. After usual work-up and purification,78a (80.1 mg, 79%) was obtained. 78a: mp 153–155 °C (decomp., pale orange needles, recrystallized from CHCl3). IR (KBr): 3240, 1617, 1586, 1514, 1481, 1357, 1332, 1280, 1095, 1056, 863, 811, 751, 731 cm-1. UV λmaxMeOH nm (log ε): 264 (4.01), 321 (3.94), 361 (3.73). 1H-NMR (CDCl3–CD3OD. 95:5, v/v) δ: 6.43 (1H, d, J=3.3 Hz), 7.51 (1H, d, J=3.3 Hz), 7.61 (H, d, J=8.8 Hz), 7.95 (1H, dd, J=8.8, 2.1 Hz), 8.42 (1H, dd, J=2.1 Hz). MS m/z: 178 (M+). Anal. Calcd for C8H6N2O3: C, 53.94; H, 3.39; N, 15.72. Found: C, 54.03; H, 3.45; N, 15.73.
1-Hydroxy-5-nitroindole (78b) from 77b — Prepared according to the general method B, where Na2WO4·2H2O (81.6 mg, 0.25 mmol) in H2O (2.0 mL), 77b (202.9 mg, 1.23 mmol) in MeOH (20.0 mL), and 30% H2O2 (1.26 mL, 12.3 mmol) were used. After usual work-up and purification, 77b (52.7 mg, recovery, 26%), 5-nitroindole (9.4 mg, 5%), and 78b (91.7 mg, 42%) were obtained. The mixture of 78b and 5-nitroindoe was successfully separated by column chromatography on Al2O3 with benzene–EtOAc (10:1, v/v). 78b: mp 175–176 °C (decomp., brown needles, recrystallized from CHCl3). IR (KBr, film): 1613, 1584, 1508, 1358, 1339, 756, 720 cm-1. UV λmaxMeOH nm (log ε): 254 (4.07), 275 (4.20), 331 (3.81). 1H–NMR (CDCl3–CD3OD, 95:5, v/v) δ: 6.45 (H, d, J=3.4 Hz), 7.52 (1H, d, J=3.4 Hz), 7.60 (1H, d, J=8.9 Hz), 7.98 (1H, dd, J=8.8, 2.2 Hz), 8.38 (1H, br s). MS m/z: 178 (M+). Anal. Calcd for C8H6N2O3: C, 53.94; H, 3.39; N, 15.72. Found: C, 53.66; H, 3.37; N, 15.71.
1-Hydroxy-2-phenylindole (78d) from 2,3-dihydro-2-phenylindole (77d) — Prepared according to the general method B for
6, where Na
2WO
4·2H
2O (17.4 mg, 0.053 mmol),
77d (51.5 mg, 0.26 mmol) in 4.0 mL of MeOH, and 30% H
2O
2 (299.4 mg, 2.64 mmol) in MeOH (1.0 mL) were used. The crude product was purified by p-TLC on SiO
2 with CH
2Cl
2–MeOH (98:2, v/v) as a developing solvent to afford
78d (30.9 mg, 56%).
78d: mp 174.0–175.0 °C (decomp., pale yellow needles, recrystallized from CHCl
3, lit.,[
65] mp 175 °C). IR (KBr): 2400, 1625, 1370, 756, 740, 683 cm
-1.
1H-NMR (10% CD
3OD in CDCl
3) δ: 6.52 (1H, s, C3–H, deuterated during measuring), 6.88–7.62 (7H, m), 7.68–7.92 (2H, m). MS
m/z: 209 (M
+). Identical with the authentic sample prepared from benzoin oxime.[
66]
1-Methoxy-6-nitroindole (79a) from 2,3-dihydro-6-nitroindole (77a) — Prepared according to the general method B for 6, where Na2WO4·2H2O (9.0 mg, 0.027 mmol), 77a (44.8 mg, 0.27 mmol) in MeOH (3.0 mL), and 30% H2O2 (309.7 mg, 2.73 mmol) were used. After methylation, usual work-up, and purification, 79a (31.3 mg, 60%) and 6-nitroindole (3.8 mg, 9%) were obtained. 79a: mp 90.0–91.0 °C (yellow needles, recrystallized from MeOH). IR (KBr): 1613, 1584, 1508, 1358, 1339, 756, 720 cm-1. 1H-NMR (CDCl3) δ: 4.17 (3H, s), 6.45 (1H, dd, J=3.4 and 1.0 Hz), 7.52 (1H, d, J=3.4 Hz), 7.60 (1H, d, J=8.8 Hz), 7.98 (1H, dd, J=8.8, 2.2 Hz), 8.38 (1H, br d, J=2.2 Hz). MS m/z: 192 (M+). Anal. Calcd for C9H8N2O3: C, 56.25; H, 4.20; N, 14.58. Found: C, 56.21; H, 4.17; N, 14.73.
1-Methoxy-5-nitroindole (79b) from 2,3-dihydro-5-nitroindole (77b) — Prepared according to the general method B for 6, where Na2WO4·2H2O (16.4 mg, 0.05 mmol), 77b (40.8 mg, 0.29 mmol) in MeOH (3.0 mL), and 30% H2O2 (282.0 mg, 2.48 mmol) in MeOH (2.0 mL) were used. After methylation, usual work-up, and purification, 79b (23.2 mg, 49%), unreacted starting material (17.1 mg, 42%), and 5-nitroindole (1.1 mg, 3%) were obtained. 79b: mp 89.5–90.5 °C (yellow plates, recrystallized from MeOH). IR (KBr): 1615, 1580, 1512, 1324, 1066, 737 cm-1. 1H-NMR (CDCl3) δ: 4.14 (3H, s), 6.54 (1H, dd, J=3.6, 0.8 Hz), 7.38 (1H, d, J=3.6 Hz), 7.44 (1H, d, J=9.0 Hz), 8.12 (1H, dd, J=9.0, 2.1 Hz), 8.54 (1H, d, J=2.1 Hz). MS m/z: 192 (M+). Anal. Calcd for C9H8N2O3: C, 56.25; H, 4.20; N, 14.58. Found: C, 56.25; H, 4.17; N, 14.50.
7-Iodo-1-methoxyindole (79c) from 2,3-dihydro-7-iodoindole (77c) — Prepared according to the general method B for 6, where Na2WO4·2H2O (14.7 mg, 0.045 mmol), 79c (218.7 mg, 0.89 mmol) in MeOH (5.0 mL), and 30% H2O2 (303.6 mg, 2.68 mmol) in MeOH (4.0 mL) were used. After usual work-up and purification, 79c (62.6 mg, 26%), 7-iodoindole (9.9 mg, 5%), and unreacted starting material (117.5 mg, 54%) were obtained. 79c: mp 35.0–35.5 °C (colorless plates, recrystallized from hexane). IR (KBr): 1544, 1333, 1276, 1033, 948, 775 cm-1. 1H-NMR (CDCl3) δ: 4.08 (3H, s), 6.31 (1H, d, J=3.4 Hz), 6.82 (1H, t, J=7.6 Hz), 7.29 (1H, d, J=3.4 Hz), 7.54 (1H, dd, J=7.6, 1.0 Hz), 7.68 (1H, dd, J=7.6, 1.0 Hz). MS m/z: 273 (M+). Anal. Calcd for C9H8INO: C, 39.59; H, 2.95; N, 5.12. Found: C, 39.53; H, 2.99; N, 5.13.
1-Methoxy-2-phenylindole (79d) —
a) From 2,3-dihydro-2-phenylindole (77d); prepared according to the general method B for
6, where Na
2WO
4·2H
2O (17.7 mg, 0.054 mmol),
77d (52.3 mg, 0.27 mmol) in MeOH (4.0 mL), and 30% H
2O
2 (304.1 mg, 2.68 mmol) in MeOH (1.0 mL) were used. After usual work-up and purification,
79d (40.0 mg, 67%) and 2-phenylindole (3.9 mg, 8%) were obtained.
79d: mp 47.0–48.0 °C (lit.[
15] mp 49–51 °C, pale yellow plates, recrystallized from MeOH). IR (KBr): 1597, 956, 760, 741 cm
-1.
1H-NMR (CDCl
3) δ: 3.73 (3H, s), 6.56 (1H, s), 7.00–7.66 (7H, m), 7.73–7.91 (2H, m).
Anal. Calcd for C
15H
13NO: C, 80.69; H, 5.87; N, 6.27. Found: C, 80.77; H, 5.91; N, 6.04.
b) From 1-Hydroxy-2-phenylindole (78d): ethereal CH2N2 (excess) was added to a solution of 78d (30.9 mg, 0.15 mmol) in MeOH (3.0 mL) with stirring at rt until the starting material was not detected on tlc monitoring. The crude product was purified by p-TLC on SiO2 with EtOAc–hexane (1:4, v/v) as a developing solvent to afford 79d (28.3 mg, 86%).
Methyl 1-methoxyindole-2-carboxylate (79f) from 2,3-dihydroindole-2-carboxylic acid (77e) — Prepared according to the general method B, where Na2WO4·2H2O (20.8 mg, 0.063 mmol), 77e (51.5 mg, 0.31 mmol) in MeOH (4.0 mL), and 30% H2O2 (358.2 mg, 3.16 mmol) in MeOH (1.0 mL) were used. After methylation and usual work-up and purification, 4 (10.4 mg, 22%) and 79f (11.4 mg, 18%) were obtained. 79f: mp 40.5–41.5 °C (colorless plates, recrystallized from MeOH). IR (KBr): 1723, 1239, 1209, 1085, 738 cm-1. 1H-NMR (CDCl3) δ: 3.93 (3H, s), 4.19 (3H, s), 7.08 (1H, d, J=1.2 Hz), 7.02–7.54 (3H, m), 7.61 (1H, dt, J=7.9, 1.0 Hz). MS m/z: 205 (M+). Anal. Calcd for C11H11NO3: C, 64.38; H, 5.40; N, 6.83. Found: C, 64.19; H, 5.45; N, 7.00.
Scheme 8.
Synthesis of 1-hydroxyindole derivative (2)
Scheme 8.
Synthesis of 1-hydroxyindole derivative (2)
3-Acetylaminomethyl-1-hydroxyindole (82b) from 3-acetylaminomethyl-2,3-dihydroindole (80) — Prepared according to the method for 6, where Na2WO4·2H2O (149.8 mg, 0.45 mmol) in H2O (4.5 mL), 80 (688.0 mg, 3.16 mmol) in MeOH (55.0 mL), and 30% H2O2 (2.574 g, 22.7 mmol) in MeOH (5.0 mL) were used. After usual work-up and purification, 82b (302.5 mg, 66%) was obtained. 82b: mp 132.5–133.0 °C (pale yellow prisms, recrystallized from CH2Cl2). IR (KBr): 3330, 2810, 1603, 1538, 1406, 1366, 1320, 1244, 1103, 1028, 1006, 743, 667, 570 cm-1. 1H-NMR (5% CD3OD–CDCl3) δ: 1.96 (3H, s), 4.49 (2H, s), 7.08 (1H, dd, J=7.7, 7.3 Hz), 7.18 (1H, s), 7.22 (1H, dd, J=8.1, 7.3 Hz), 7.45 (1H, d, J=8.1 Hz), 7.54 (1H, d, J=7.7 Hz). MS m/z: 204 (M+). Anal. Calcd for C11H12N2O2·1/8H2O: C, 63.99; H, 5.98; N, 13.57. Found: C, 64.15; H, 5.79; N, 13.60.
1-Hydroxy-Nb-trifluoroacetylindole-3-methanamine (82a) from 81 — Prepared according to the method for 82b, where Na2WO4·2H2O (148.7 mg, 0.45 mmol) in H2O (4.5 mL), 81 (551.2 mg, 2.26 mmol) in MeOH (50.0 mL), and 30% H2O2 (2.575 g, 22.6 mmol) in MeOH (5.0 mL) were used. After usual work-up, the crude product was column-chromatographed on SiO2 with EtOAc–hexane (3:1, v/v) to give 82a (414.5 mg, 71%). 82a: mp 123.5–124.5 °C (colorless needles, recrystallized from CH2Cl2). IR (KBr): 3390, 3305, 1694, 1566, 1541, 1353, 1252, 1206, 1178, 1169, 1151, 1103, 755, 747, 682 cm-1. 1H-NMR (CD3OD) δ: 4.59 (2H, s), 7.03 (1H, dd, J=8.0, 0.9 Hz), 7.17 (1H, dd, J=8.0, 0.9 Hz), 7.28 (1H, s), 7.38 (1H, ddd, J=8.0, 0.9, 0.7 Hz), 7.57 (1H, ddd, J=8.0, 0.9, 0.7 Hz). High resolution MS m/z: Calcd for C11H9F3N2O2: 258.0615. Found: 258.0617.
1-Methoxy-Nb-trifluoroacetylindole-3-methanamine (83a) from 2,3-dihydro-3-trifluoroacetyl- indole-3-methanamine (81) — Prepared according to the method for 80b, where Na2WO4·2H2O (14.8 mg, 0.04 mmol) in H2O (0.5 mL), 81 (54.1 mg, 0.22 mmol) in MeOH (6.0 mL), and 30% H2O2 (266.6 mg, 2.35 mmol) in MeOH (1.0 mL) were used. After methylation and work-up, the product was purified by column-chromatography on SiO2 with CHCl3–hexane (3:1, v/v) to give 83a (46.5 mg, 77%). 83a: mp 70.5–71.0 °C (colorless prisms, recrystallized from benzene–hexane). IR (KBr): 3290, 1715, 1695, 1561, 1208, 1180, 1159, 738, 722 cm-1. UV λmaxMeOH nm (log ε): 219 (4.57), 272 (3.73), 288 (3.72). 1H-NMR (CDCl3) δ: 4.10 (3H, s), 4.68 (2H, d, J=5.3 Hz), 6.43 (1H, br s), 7.18 (1H, t, J=7.8 Hz), 7.31 (1H, s), 7.31 (1H, t, J=7.8 Hz), 7.46 (1H, d, J=7.8 Hz), 7.57 (1H, d, J=7.8 Hz). High resolution MS m/z: Calcd for C12H11F3N2O2: 272.0772. Found: 272.0756.
1-Methoxy-Nb-acetylindole-3-methanamine (83b) from 3-acetylaminomethyl-2,3-dihydroindole (80) — Prepared according to the method for 82b, where Na2WO4·2H2O (22.8 mg, 0.07 mmol) in H2O (0.5 mL), 80 (64.9 mg, 0.34 mmol) in MeOH (6.0 mL), and 30% H2O2 (388.0 mg, 3.42 mmol) in MeOH (1.0 mL) were used. After methylation and work-up, the product was purified by column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (100:1:0.1, v/v) to give 83b (43.6 mg, 59%). 83b: mp 132.5–133.0 °C (colorless prisms, recrystallized from benzene). IR (KBr): 3200, 3040, 1623, 1533, 1450, 1245, 735 cm-1. UV λmaxMeOH nm (log ε): 222 (4.51), 273 (3.72), 288 (3.72). 1H-NMR (CDCl3) δ: 1.98 (3H, s), 4.07 (3H, s), 4.56 (2H, d, J=5.1 Hz), 5.65 (1H, br s), 7.14 (1H, t, J=8.2 Hz), 7.24 (1H, s), 7.28 (1H, t, J=8.2 Hz), 7.43 (1H, d, J=8.2 Hz), 7.60 (1H, d, J=8.2 Hz). MS m/z: 218 (M+). Anal. Calcd for C12H14N2O2: C, 66.04; H, 6.47; N, 12.84. Found: C, 65.91; H, 6.47; N, 12.71.
3-Acetylaminomethyl-1-methoxyindole (83b) from 82b — Ethereal CH2N2 (excess) was added to a solution of 82b (11.8 mg, 0.058 mmol) in MeOH (1.0 mL) and stirring was continued at rt for 30 min. After evaporation of the solvent under reduced pressure, the residue was column-chromatographed on SiO2 with CH2Cl2–MeOH (97:3, v/v) to give 81b (10.7 mg, 85%).
2,3-Dihydro-3-tosylaminomethylindole (85) from 3-tosylaminomethylindole (84) — 95% NaBH3CN (247.7 mg, 3.74 mmol) was added to a solution of 84 (201.8 mg, 0.67 mmol) in AcOH (10.0 mL) at rt and stirring was continued for 5 h. After adding H2O under ice cooling, the solvent was evaporated under reduced pressure. The residue was made alkaline by adding H2O and 8% NaOH. The whole was extracted with CH2Cl2–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a residue, which was column-chromatographed on SiO2 with CH2Cl2–MeOH (99:1, v/v) to give 84 (16.5 mg, recovery, 8%) and 85 (144.3 mg, 71%) in the order of elution. 85: colorless hard oil. IR (KBr): 3320, 3050, 2910, 1592, 1481, 1460, 1423, 1323, 1155, 1041, 755 cm-1. 1H-NMR (CD3OD) δ: 2.42 (3H, s), 2.92 (1H, dd, J=12.8, 5.3 Hz), 3.06 (1H, dd, J=12.8, 5.3 Hz), 3.27 (1H, dd, J=9.3, 5.7 Hz), 3.30–3.38 (1H, m), 3.51 (1H, dd, J=9.3, 8.6 Hz), 6.63 (1H, d, J=7.9 Hz), 6.65 (1H, td, J=7.3, 0.9 Hz), 6.98 (1H, t, J=7.9 Hz), 7.03 (1H, d, J=7.3 Hz), 7.37 (2H, m), 7.72 (2H, m). MS m/z: 302 (M+). Anal. Calcd for C16H18N2O2S: C, 63.55; H, 6.00; N, 9.26. Found: C, 63.45; H, 6.04; N, 9.16.
1-Hydroxy-3-tosylaminomethylindole (86) from 85 — Prepared according to the method for 82b, where Na2WO4·2H2O (60.6 mg, 0.18 mmol) in H2O (1.8 mL), 85 (277.4 mg, 0.92 mmol) in MeOH (13.0 mL), and 30% H2O2 (1.049 g, 9.26 mmol) in MeOH (5.0 mL) were used. After usual work-up, 86 (197.5 mg, 68%) was obtained. 86: mp 134.0–135.5 °C (colorless needles, recrystallized from CHCl3). IR (KBr): 3390, 3320, 1396, 1318, 1303, 1230, 1157, 1097, 1020, 817, 732 cm-1. 1H-NMR (CD3OD) δ: 2.39 (3H, s), 4.18 (2H, s), 6.94 (1H, ddd, J=8.0, 7.0, 1.0 Hz), 7.08 (1H, s), 7.11 (1H, ddd, J=8.0, 7.0, 1.0 Hz), 7.28 (2H, m), 7.29 (1H, dt, J=8.0, 1.0 Hz), 7.39 (1H, dt, J=8.0, 1.0 Hz), 7.68 (2H, m). MS m/z: 316 (M+). Anal. Calcd for C16H16N2O3S·1/4H2O: C, 59.89; H, 5.18; N, 8.73. Found: C, 59.91; H, 5.00; N, 8.71.
1-Methoxy-3-tosylaminomethylindole (87) from 86 — Ethereal CH2N2 (excess) was added to a solution of 86 (32.3 mg, 0.10 mmol) in MeOH (1.0 mL) and stirring was continued at rt for 30 min. After evaporation of the solvent under reduced pressure, the residue was column-chromatographed on SiO2 with CH2Cl2 to give 87 (32.2 mg, 96%). 87: mp 176.0–178.5 °C (colorless prisms, recrystallized from MeOH). IR (KBr): 3300, 1600, 1423, 1315, 1243, 1145, 1089, 1024, 952, 866, 812, 746, 681, 541 cm-1. 1H-NMR (5% CD3OD–CDCl3) δ: 2.43 (3H, s), 4.02 (3H, s), 4.25 (2H, s), 7.07 (1H, ddd, J=8.0, 7.1, 1.0 Hz), 7.10 (1H, s), 7.23 (1H, ddd, J=8.2, 7.1, 1.0 Hz), 7.28 (2H, m), 7.37 (1H, dt, J=8.2, 1.0 Hz), 7.41 (1H, dt, J=8.0, 1.0 Hz), 7.74 (2H, m). MS m/z: 330 (M+). Anal. Calcd for C17H18N2O3S: C, 61.80; H, 5.49; N, 8.48. Found: C, 61.71; H, 5.50; N, 8.41.
Methyl N-(2,3-dihydroindol-3-yl)methyl succinamate (90) and N-(2,3-Dihydroindol-3-yl)methyl- succinimide (89) from methyl N-(indol-3-yl)methyl succinamate (88) — Prepared according to the method for 85, where 95% NaBH3CN (66.0 mg, 1.00 mmol) and 88 (49.8 mg, 0.19 mmol) in AcOH (2.0 mL) were used. After usual work-up and purification, 89 (6.5 mg, 15%) and 90 (41.0 mg, 82%) were obtained. 90: mp 73.0–74.0 °C (colorless prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 3290, 1726, 1638, 1609, 1542, 1483, 1433, 1338, 1197, 1174, 745 cm-1. 1H-NMR (CD3OD) δ: 2.50 (2H, t, J=6.8 Hz), 2.61 (2H, t, J=6.8 Hz), 3.25 (1H, dd, J=9.3, 5.5 Hz), 3.28–3.32 (1H, m), 3.41–3.47 (2H, m), 3.56 (1H, t, J=9.3 Hz), 3.66 (3H, s), 6.68 (1H, d, J=7.6 Hz), 6.69 (1H, td, J=7.6, 1.0 Hz), 7.00 (1H, t, J=7.6 Hz), 7.14 (1H, d, J=7.6 Hz). MS m/z: 262 (M+). Anal. Calcd for C14H18N2O3: C, 64.10; H, 6.92; N, 10.68. Found: C, 64.11; H, 6.90; N, 10.69. 89: mp 94.5–96.0 °C (colorless prisms, recrystallized from MeOH). IR (KBr): 3370, 2990, 2820, 1778, 1689, 1608, 1405, 1351, 1247, 1124, 1151, 755 cm-1. 1H-NMR (CD3OD) δ: 2.71 (4H, s), 3.29 (1H, dd, J=9.5, 5.1 Hz), 3.48 (1H, dd, J=9.5, 8.4 Hz), 3.57 (1H, m), 3.64 (1H, dd, J=13.1, 8.7 Hz), 3.71 (1H, dd, J=13.1, 5.4 Hz), 6.66 (1H, t, J=7.8 Hz), 6.68 (1H, td, J=7.3, 1.0 Hz), 7.01 (1H, m), 7.08 (1H, d, J=7.3 Hz). MS m/z: 230 (M+). Anal. Calcd for C13H14N2O2: C, 67.81; H, 6.13; N, 12.17. Found: C, 67.77; H, 6.11; N, 12.08.
Methyl N-(1-hydroxyindol-3-yl)methyl succinamate (91a) from 90 — Prepared according to the method for 82b, where Na2WO4·2H2O (14.6 mg, 0.04 mmol) in H2O (0.4 mL), 90 (56.8 mg, 0.21 mmol) in MeOH (3.5 mL), and 30% H2O2 (249.9 mg, 2.21 mmol) in MeOH (1.0 mL) were used. After usual work-up and purification, 91a (37.5 mg, 63%) was obtained. 91a: mp 115.5–116.0 °C (colorless needles, recrystallized from EtOAc). IR (KBr): 3350, 3120, 2930, 1710, 1637, 1535, 1443, 1387, 1358, 1243, 1218, 1174, 735 cm-1. 1H-NMR (CD3OD) δ: 2.48 (2H, t, J=6.7 Hz), 2.62 (2H, t, J=6.7 Hz), 3.61 (3H, s), 4.48 (2H, s), 7.01 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.15 (1H, ddd, J=8.3, 7.1, 1.0 Hz), 7.24 (1H, s), 7.36 (1H, dt, J=8.3, 1.0 Hz), 7.54 (1H, dt, J=8.1, 1.0 Hz). MS m/z: 276 (M+). Anal. Calcd for C14H16N2O4: C, 60.86; H, 5.84; N, 10.14. Found: C, 60.72; H, 5.85; N, 10.12.
Methyl N-(1-methoxyindol-3-yl)methyl succinamate (91b) from 91a — Ethereal CH2N2 (excess) was added to a solution of 91a (25.5 mg, 0.09 mmol) in MeOH (1.0 mL) and stirring was continued at rt for 30 min. After evaporation of the solvent under reduced pressure, the residue was column-chromato- graphed on SiO2 with CH2Cl2–MeOH (99:1, v/v) to give 91b (25.5 mg, 95%). 91b: mp 75.0–76.5 °C (colorless prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 3280, 1730, 1634, 1537, 1445, 1349, 1319, 1201, 1136, 736 cm-1. 1H-NMR (CD3OD) δ: 2.49 (2H, t, J=7.1 Hz), 2.62 (2H, t, J=7.1 Hz), 3.61 (3H, s), 4.05 (3H, s), 4.48 (2H, s), 7.06 (1H, ddd, J=7.8, 7.1, 1.0 Hz), 7.20 (1H, ddd, J=8.3, 7.1, 1.0 Hz), 7.36 (1H, s), 7.39 (1H, dt, J=8.3, 1.0 Hz), 7.58 (1H, dt, J=7.8, 1.0 Hz). MS m/z: 290 (M+). Anal. Calcd for C15H18N2O4: C, 62.05; H, 6.25; N, 9.65. Found: C, 62.05; H, 6.33; N, 9.60.
2-Methylthiomethylindole (93) from gramine (92) — MeI (1.45 mL, 23.3 mmol) was added to a solution of 92 (399.3 mg, 2.30 mmol) in THF (23.0 mL) and stirred at rt for 1 h. The solvent was evaporated under reduced pressure to leave a residue, which was dissolved in MeOH (20.0 mL). To the resultant solution, 15% aqueous NaSMe (10.7 mL, 23.3 mmol) was added and stirred at rt for 15 h. After addition of H2O, the whole was extracted with CH2Cl2–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a residue, which was column-chromatographed on SiO2 with CHCl3–MeOH–28% aq. NH3 (100:20:2, v/v) to give 93 (325.7 mg, 80%) and 92 (65.7 mg, recovery, 17%) in the order of elution. 93: mp 91.0–92.5 °C (colorless prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 3310, 1645, 1555, 1456, 1421, 1354, 1253, 1097, 745, 639 cm-1. 1H-NMR (CD3OD) δ: 1.98 (3H, s), 3.89 (2H, s), 7.00 (1H, ddd, J=8.1, 7.2, 0.9 Hz), 7.09 (1H, ddd, J=8.2, 7.2, 1.1 Hz), 7.14 (1H, s), 7.32 (1H, dd, J=8.2, 0.9 Hz), 7.62 (1H, dd, J=8.1, 1.1 Hz). Anal. Calcd for C10H11NS: C, 67.79; H, 6.25; N, 7.88. Found: C, 67.76; H, 6.25; N, 7.90.
Scheme 9.
Synthesis of 1-hydroxyinole derivative (3)
Scheme 9.
Synthesis of 1-hydroxyinole derivative (3)
2,3-Dihydro-3-methylthiomethylindole (94) from 93 — Prepared according to the method for 85, where 95% NaBH3CN (355.5 mg, 5.37 mmol) and 93 (100.2 mg, 0.56 mmol) in AcOH (6.0 mL) were used. After usual work-up and purification, 93 (21.8 mg, recovery, 22%) and 94 (55.5 mg, 55%) were obtained. 94: colorless oil. IR (film): 3370, 2920, 1607, 1488, 1465, 1249, 747 cm-1. 1H-NMR (CDCl3) δ: 2.15 (3H, s), 2.68 (1H, dd, J=12.8, 9.4 Hz), 2.91 (1H, dd, J=12.8, 5.1 Hz), 3.44 (1H, dd, J=9.2, 6.2 Hz), 3.49–3.55 (1H, m), 3.75 (1H, t, J=9.2 Hz), 6.68 (1H, d, J=7.6 Hz), 6.75 (1H, t, J=7.6 Hz), 7.06 (1H, t, J=7.6 Hz), 7.17 (1H, d, J=7.6 Hz). MS m/z: 179 (M+). Anal. Calcd for C10H13NS: C, 66.99; H, 7.31; N, 7.81. Found: C, 67.10; H, 7.36; N, 7.93.
1-Hydroxy-3-methylsulfinylmethylindole (95) from 94 — Prepared according to the method for 82b, where Na2WO4·2H2O (47.5 mg, 0.14 mmol) in H2O (0.7 mL), 94 (128.9 mg, 0.72 mmol) in MeOH (6.0 mL), and 30% H2O2 (803.7 mg, 7.09 mmol) in MeOH (1.0 mL) were used. Then, a solution of Me2S (0.42 mL, 5.76 mmol) in MeOH (1.0 mL) was added to the reaction mixture. After usual work-up and purification, 95 (41.2 mg, 27%) was obtained. 95: mp 114.0–115.0 °C (pale orange prisms, recrystallized from EtOAc). IR (KBr): 2580, 1349, 1322, 1240, 1093, 1007, 947, 735 cm-1. 1H-NMR (CD3OD) δ: 2.53 (3H, s), 4.23 (1H, d, J=13.7 Hz), 4.30 (1H, d, J=13.7 Hz), 7.08 (1H, ddd, J=8.1, 7.0, 1.0 Hz), 7.20 (1H, ddd, J=8.1, 7.0, 1.0 Hz), 7.39 (1H, s), 7.42 (1H, d, J=8.1 Hz), 7.62 (1H, d, J=8.1 Hz). High resolution MS m/z: Calcd for C10H11NO2S: 209.0510. Found: 209.0508.
1-Methoxy-3-methylsulfinylmethylindole (96) and 1-methoxy-3-methylsulfonylmethylindole (97) from 94 — [Entry 1]: Prepared according to the method for 82b, where Na2WO4·2H2O (35.5 mg, 0.11 mmol) in H2O (0.5 mL), 94 (94.7 mg, 0.53 mmol) in MeOH (4.0 mL), and 30% H2O2 (600.3 mg, 5.30 mmol) in MeOH (1.0 mL) were used. After stirring at rt for 5 min, a solution of Me2S (0.31 mL, 4.23 mmol) in MeOH (1.0 mL) was added and stirred for 30 min. Ethereal CH2N2 (excess) was then added and stirred for 30 min. After usual work-up and purification by column-chromatography on SiO2 with CH2Cl2–MeOH (99:1, v/v), 97 (20.1 mg, 16%) and 96 (31.8 mg, 27%) were obtained. 96: mp 67.0–69.0 °C (colorless prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 3420, 1453, 1435, 1349, 1321, 1093, 1063, 1025, 965, 946, 747 cm-1. 1H-NMR (CD3OD) δ: 2.54 (3H, s), 4.11 (3H, s), 4.21 (1H, dd, J=13.8, 0.6 Hz), 4.30 (1H, dd, J=13.8, 0.6 Hz), 7.13 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.25 (1H, ddd, J=8.3, 7.1, 1.0 Hz), 7.45 (1H, dt, J=8.3, 1.0 Hz), 7.53 (1H, s), 7.66 (1H, dt, J=8.1, 1.0 Hz). MS m/z: 223 (M+). Anal. Calcd for C11H13NO2S·1/8H2O: C, 58.58; H, 5.81; N, 6.21. Found: C, 58.49; H, 5.84; N, 6.14. 97: mp 101.5–102.5 °C (colorless plates, recrystallized from CH2Cl2–hexane). IR (KBr): 3100, 2930, 1455, 1320, 1263, 1244, 1147, 1120, 968, 945, 747, 736 cm-1. 1H-NMR (CDCl3) δ: 2.75 (3H, s), 4.13 (3H, s), 4.41 (2H, s), 7.21 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.31 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.48 (1H, dt, J=8.1, 1.0 Hz), 7.48 (1H, s), 7.62 (1H, dt, J=8.1, 1.0 Hz). MS m/z: 239 (M+). Anal. Calcd for C11H13NO3S: C, 55.21; H, 5.48; N, 5.85. Found: C, 55.19; H, 5.47; N, 5.81.
3-Acetylthiomethylindole (99) from 92 — MeI (0.12 mL, 1.85 mmol) was added to a solution of 92 (32.2 mg, 0.19 mmol) in THF (2.0 mL) at rt and stirring was continued for 1 h. The solvent was evaporated under reduced pressure to leave a residue, which was dissolved in DMF–H2O (3:1, v/v, 2.0 mL). To the resultant solution, KSCOMe (31.7 mg, 0.28 mmol) was added and stirred at rt for 2 h. After usual work-up and purification by column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (100:20:2, v/v), 99 (29.8 mg, 79%) and 92 (6.9 mg, recovery, 21%) were obtained. 99: colorless oil. IR (film): 3350, 1676, 1454, 1419, 1352, 1339, 1136, 1116, 1095, 959, 740 cm-1. 1H-NMR (CDCl3) δ: 2.34 (3H, s), 4.35 (2H, d, J=0.7 Hz), 7.14 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.18 (1H, d, J=2.4 Hz), 7.21 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.35 (1H, dt, J=8.1, 1.0 Hz), 7.60 (1H, d, J=8.1 Hz), 8.03 (1H, br s). High resolution MS m/z: Calcd for C11H11NOS: 205.0561. Found: 205.0541.
3-Acetylthiomethyl-2,3-dihydroindole (100) from 99 — Prepared according to the method for 94, where 95% NaBH3CN (42.6 mg, 0.64 mmol) and 99 (26.2 mg, 0.13 mmol) in AcOH–CF3CO2H (3:1, v/v, 1.5 mL) were used. After usual work-up, 100 (17.8 mg, 67%) was obtained. 100: colorless oil. IR (film): 3370, 1692, 1611, 1487, 1465, 1252, 1138, 957, 748 cm-1. 1H-NMR (CDCl3) δ: 2.36 (3H, s), 3.10 (1H, dd, J=13.6, 8.3 Hz), 3.28 (1H, dd, J=13.6, 5.4 Hz), 3.30 (1H, dd, J=9.1, 6.1 Hz), 3.48–3.53 (1H, m), 3.68 (1H, t, J=9.1 Hz), 6.65 (1H, d, J=7.5 Hz), 6.73 (1H, td, J=7.5, 1.0 Hz), 7.06 (1H, t, J=7.5 Hz), 7.19 (1H, d, J=7.5 Hz). High resolution MS m/z: Calcd for C11H13NOS: 207.0718. Found: 207.0763.
3-Acetylthiomethyl-1-methoxyindole (101) from 99 — Crude 100, prepared with 95% NaBH3CN (60.6 mg, 0.92 mmol) and 99 (37.9 mg, 0.18 mmol) in AcOH–CF3CO2H (3:1, v/v, 2.0 mL), was dissolved in MeOH (1.5 mL). To the resultant solution, a solution of Na2WO4·2H2O (12.3 mg, 0.04 mmol) in H2O (0.2 mL) and then a solution of 30% H2O2 (207.8 mg, 1.83 mmol) in MeOH (0.5 mL) were added under ice cooling and stirred at rt for 20 min. Ethereal CH2N2 (excess) was added to the mixture and stirring was continued at rt for 1 h. After usual work-up and purification, 101 (6.6 mg, 15%) was obtained. 101: colorless oil. IR (film): 2940, 1689, 1452, 1354, 1232, 1133, 1097, 1031, 954, 758, 737 cm-1. 1H-NMR (CDCl3) δ: 2.34 (3H, s), 4.06 (3H, s), 4.29 (2H, d, J=0.7 Hz), 7.13 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.24–7.27 (2H, m), 7.43 (1H, dt, J=8.1, 1.0 Hz), 7.57 (1H, dt, J=8.1, 1.0 Hz). High resolution MS m/z: Calcd for C12H13NO2S: 235.0667. Found: 235.0685.
Scheme 10.
Synthesis of 1-hydroxyindoles (4).
Scheme 10.
Synthesis of 1-hydroxyindoles (4).
2,3-Dihydro-N,N-dimethylindole-3-acetamide (103a) from N,N-dimethylindole-3-acetamide (102a) — Prepared according to the method for 94, where 95% NaBH3CN (394.5 mg, 5.96 mmol) and 102a (241.1 mg, 1.20 mmol) in AcOH (10.0 mL) were used. After work-up, 103a (236.6 mg, 97%) was obtained.103a: colorless oil. IR (film): 3310, 2930, 1630, 1488, 1465, 1407, 1322, 1254, 1143, 748 cm-1. 1H-NMR (CDCl3) δ: 2.58 (1H, dd, J=16.0, 8.9 Hz), 2.73 (1H, dd, J=16.0, 4.8 Hz), 2.94 (3H, s), 2.97 (3H, s), 3.23–3.26 (1H, m), 3.78–3.86 (2H, m), 6.65 (1H, d, J=7,7 Hz), 6.71 (1H, td, J=7.3, 1.0 Hz), 7.04 (1H, d, J=7.7 Hz), 7.10 (1H, d, J=7.3 Hz). High resolution MS m/z: Calcd for C12H16N2O: 204.1263. Found: 204.1255.
2,3-Dihydro-N,N-dimethylindole-3-propionamide (103b) from N,N-dimethylindole-3-propionamide (102b) — Prepared according to the method for 94, where 95% NaBH3CN (303.5 mg, 4.59 mmol) and 102b (201.4 mg, 0.93 mmol) in AcOH (10.0 mL) were used. After usual work-up and purification, 103b (199.8 mg, 98%) was obtained. 103b: colorless oil. IR (film): 3290, 2920, 1628, 1486, 1459, 1399, 1247, 1143, 747 cm-1. 1H-NMR (CDCl3) δ: 1.88–1.96 (1H, m), 2.11–2.18 (1H, m), 2.33–2.45 (2H, m), 2.95 (3H, s), 2.99 (3H, s), 3.24 (1H, dd, J=8.8, 6.4 Hz), 3.33–3.39 (1H, m), 3.70 (1H, t, J=8.8 Hz), 6.64 (1H, d, J=7.6 Hz), 6.72 (1H, td, J=7.6, 1.0 Hz), 7.03 (1H, d, J=7.6 Hz), 7.11 (1H, d, J=7.6 Hz). High resolution MS m/z: Calcd for C13H18N2O: 218.1419. Found: 218.1427.
N,N-Dimethyl-1-hydroxyindole-3-acetamide (104a) from 103a — Prepared according to the method for 82b where Na2WO4·2H2O (131.6 mg, 0.40 mmol) in H2O (4.0 mL), 103a (407.4 mg, 2.00 mmol) in MeOH (3.0 mL), and 30% H2O2 (2.214 g, 19.5 mmol) in MeOH (5.0 mL) were used. After usual work-up, 104a (321.4 mg, 74%) was obtained. 104a: mp 146.0–147.0 °C (colorless prisms, recrystallized from CHCl3–hexane). IR (KBr): 2600, 1590, 1405, 1316, 1216, 1086, 758, 741 cm-1. 1H-NMR (CDCl3) δ: 2.97 (3H, s), 2.97 (3H, s), 3.61 (2H, s), 6.52 (1H, s), 6.98 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.16 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.23 (1H, d, J=8.1 Hz), 7.43 (1H, d, J=8.1 Hz), 10.72 (1H, s, D2O exchange). Anal. Calcd for C12H14N2O2: C, 66.04; H, 6.47; N, 12.84. Found: C, 65.74; H, 6.36; N, 12.69.
N,N-Dimethyl-1-hydroxyindole-3-propionamide (104b) from 103b — Prepared according to the method for 82b, where Na2WO4·2H2O (208.4 mg, 0.63 mmol) in H2O (6.0 mL), 103b (688.0 mg, 3.16 mmol) in MeOH (55.0 mL), and 30% H2O2 (3.543 g, 31.3 mmol) in MeOH (5.0 mL) were used. After usual work-up and purification, 104b (483.9 mg, 66%) was obtained. 104b: mp 144.0–145.0 °C (colorless prisms, recrystallized from CHCl3–hexane). IR (KBr): 2760, 1598, 1402, 1310, 1140, 1026, 736 cm-1. 1H-NMR (DMSO-d6) δ: 2.63 (2H, dd, J=8.2, 7.2 Hz), 2.82 (3H, s), 2.88 (2H, dd, J=8.2, 7.2 Hz), 2.93 (3H, s), 6.97 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.12 (1H, ddd, J=8.1, 7.1, 1.0 Hz), 7.23 (1H, s), 7.31 (1H, d, J=8.1 Hz), 7.51 (1H, d, J=8.1 Hz), 10.98 (1H, s, D2O exchange). Anal. Calcd for C13H16N2O2·1/4H2O: C, 65.94; H, 7.02; N, 11.83. Found: C, 66.14; H, 6.85; N, 11.80.
9-Hydroxy-1,2,3,4-tetrahydrocarbazole (106) from 1,2,3,4,4a,9a-hexahydrocarbazole (105) — Prepared according to the method for 82b, where Na2WO4·2H2O (28.7 mg, 0.087 mmol) in H2O (1.0 mL), 105 (71.8 mg, 0.41 mmol) in MeOH (10.0 mL), and 30% H2O2 (0.45 mL, 3.92 mmol) were used. After usual work-up and purification, 105 (13.3 mg, 18%) and 106 (50.1 mg, 65%) were obtained. 106: yellow oil. IR (film): 3061, 2931, 2857, 1458, 1238, 1178, 740 cm-1. 1H-NMR (CD3OD) δ: 1.73–1.84 (4H, m), 2.55–2.57 (2H, m), 2.64–2.66 (2H, m), 6.83 (1H, t, J=7.9 Hz), 6.94 (1H, t, J=7.9 Hz), 7.18 (1H, d, J=7.9 Hz), 7.23 (1H, d, J=7.9 Hz). High resolution MS m/z: Calcd for C12H13NO: 187.0997. Found: 187.1001.
9-Methoxy-1,2,3,4-tetrahydrocarbazole (107) from 1,2,3,4,4a,9a-hexahydrocarbazole (105) — Prepared according to the method for 82b, where Na2WO4·2H2O (19.3 mg, 0.028 mmol), 105 (50.6 mg, 0.29 mmol) in MeOH (4.0 mL), and 30% H2O2 (331.6 mg, 2.92 mmol) in MeOH (1.0 mL) were used. After methylation with CH2N2 and work-up, product purification was carried out by p-TLC on SiO2 with CH2Cl2–hexane (7:3, v/v) as a developing solvent to afford 107 (32.2 mg, 55%). 107: colorless oil. IR (KBr): 2942, 2842, 1459, 1443, 1230, 1046, 735 cm-1. 1H-NMR (CDCl3) δ: 1.68–2.08 (4H, m), 2.52–2.92 (4H, br m), 3.99 (3H, s), 6.88–7.48 (4H, m). High resolution MS m/z: Calcd for C13H15NO: 201.1152. Found: 201.1134.
9-Methoxy-1,2,3,4-tetrahydrocarbazole (107) from 105 — K2CO3 (173.3 mg, 1.25 mmol) and Me2SO4 (0.053 mL, 0.56 mmol) were added to a solution of 105 (67.0 mg, 0.33 mmol) in acetone (10.0 mL) and the mixture was stirred at rt for 2 h. After usual work-up and purification, 107 (54.8 mg, 70%) was obtained.
9-Methoxycarbazole (108) from 107 — Dichlorodicyanoquinone (469.4 mg, 2.38 mmol) was added to a solution of 107 (188. 9 mg, 0.94 mmol) in benzene (30.0 mL) and stirred at rt (14 °C) for 3 h. Precipitates were filtered off through silica gel and washed with CH2Cl2. Washings and filtrates were combined and evaporated under reduced pressure to leave a crystalline solid, which was column-chromatographed on SiO2 with hexane–EtOAc (9:1, v/v) as an eluent to afford 108 (121.1 mg, 65%). 108: mp 40.0–41.0 °C (colorless needles, recrystallized from MeOH). IR (KBr): 1601, 1450, 1320, 1233, 1052, 946 cm-1. 1H-NMR (CDCl3) δ: 4.12 (3H, s), 7.19 (1H, dd, J=7.3, 2.7 Hz), 7.25 (1H, dd, J=7.3, 2.7 Hz), 7.34–7.58 (4H, m), 8.02 (2H, dt, J=7.3, 1.0 Hz). MS m/z: 197 (M+). Anal. Calcd for C13H11NO: C, 79.17; H, 5.62; N, 7.10. Found: C, 79.36; H, 5.55; N, 7.21.
A mixture of diastereoisomers, 4-nitro-1,2,2a,3,4,5-hexahydrobenz[cd]indole (110) from 4-nitro- 1,3,4,5-tetrahydrobenz[cd]indole (109) — Prepared according to the method for 94, where 95% NaBH3CN (60.8 mg, 0.97 mmol) and 109 (35.9 mg, 0.18 mmol) in AcOH–CF3CO2H (3:2, v/v, 2.0 mL) were used. After usual work-up, crude 110 was subjected to p-TLC on SiO2 with CH2Cl2–hexane (3:1, v/v) as a developing solvent. Extraction of the band having an Rf value of 0.39–0.14 with CH2Cl2–MeOH (95:5, v/v) afforded pure 110 (34.4 mg, 95%). Although 1H-NMR analysis of 110 showed 2:1 mixture of diastereoisomers, further separation was not examined.
1-Hydroxy-4-nitro-1,3,4,5-tetrahydrobenz[cd]indole (111) from a diastereoisomer’s mixture (110) — Prepared according to general method C for 82b, where Na2WO4·2H2O (10.5 mg, 0.03 mmol) in H2O (0.2 mL), urea·H2O2 (138.6 mg, 1.47 mmol), and diastereoisomer’s mixture, 110 (30.2 mg, 0.15 mmol), in MeOH (2.0 mL) were used. The reaction mixture was adjusted to pH 4 by adding 0.6% HCl and extracted with CH2Cl2–MeOH (95:5, v/v). After usual work-up and purification, 111 (16.9 mg, 52%) was obtained. 111: mp 134–134.5 ℃ (colorless prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 3427, 3112, 2971, 1604, 1530, 1442, 1419, 1349, 1149, 1001, 848, 769, 751 cm-1. 1H-NMR (CDCl3) δ: 3.50 (2H, br s), 3.52 (1H, dd, J=15.6, 4.4 Hz), 3.61 (1H, dd, J=15.6, 9.3 Hz), 4.98 (1H, ddd, J=13.7, 9.3, 4.4 Hz), 6.79 (1H, br s, D2O exchange), 6.89 (1H, br s), 7.01 (1H, br s), 7.21 (1H, dd, J=7.8, 7.3 Hz). High resolution MS m/z: Calcd for C11H10N2O3: 218.0690. Found: 218.0692.
Scheme 11.
Synthesis of 1-hydroxyinoles (5).
Scheme 11.
Synthesis of 1-hydroxyinoles (5).
1-Methoxy-4-nitro-1,3,4,5-tetrahydrobenz[cd]indole (112) from 111 — Diazomethane-ether solution (excess) was added to a solution of 111 (7.5 mg, 0.04 mmol) in MeOH (1.0 mL) at rt with stirring for 0.5 h. After evaporation of solvent under reduced pressure, the residue was purified by p-TLC on SiO2 with CH2Cl2–hexane (1:1, v/v) as a developing solvent. Extraction of the band having an Rf value of 0.50–0.31 with CH2Cl2–MeOH (95:5, v/v) afforded 112 (5.1 mg, 64%). 112: pale brown oil. IR (KBr): 3450, 2941, 1605, 1521, 1440, 1561, 1540, 983, 761, 747 cm-1. 1H-NMR (CDCl3) δ: 3.48 (2H, dd, J=17.3, 1.0 Hz), 3.54 (1H, dd, J=15.6, 4.6 Hz), 3.61 (1H, dd, J=15.6, 9.3 Hz), 4.07 (3H, s), 4.99 (1H, ddt, J=9.3, 7.3, 4.6 Hz), 6.90 (1H, d, J J=7.3 Hz), 7.02 (1H, s), 7.20 (1H, dd, J=7.8, 7.3 Hz), 7.25 (1H, d, J=7.8 Hz). High resolution MS m/z: Calcd for C12H12N2O3: 232.0847. Found: 232.0893.
A mixture of diastereoisomers, 4-(N-phenylacetylamino)-1,2,2aβ,3,4β,5-hexahydrobenz[cd]indole (114a) and 4-(N-phenylacetylamino)-1,2,2aα,3,4β,5-hexahydrobenz[cd]indole (114b) from 4-(N-phenylacetylamino)-1,3,4,5-tetrahydrobenz[cd]indole (113) — Prepared according to the method for 94, where 95%NaBH3CN (46.0 mg, 0.73 mmol) and 113 (40.1 mg, 0.14 mmol) in AcOH–CF3CO2H (4:1, v/v, 2.0 mL) were used. After work-up and purification, 114a (17.0 mg, 47%) and 114b (16.7 mg, 41%) were obtained. 114a: colorless oil. IR (KBr) : 3261, 3037, 2910, 2843, 1637, 1603, 1532, 1491, 1452, 1333, 1247, 1233, 761, 718, 692 cm-1. 1H-NMR (CD3OD) δ: 1.45 (1H, dt, J=12.8, 3.2 Hz), 2.29 (1H, dt, J=12.8, 4.6 Hz), 2.70 (1H, d, J=18.3 Hz), 2.96 (1H, dd, J=18.3, 6.4 Hz), 3.00 (1H, dd, J=11.9, 8.3 Hz), 3.10–3.19 (1H, m), 3.46 (1H, d, J=14.2 Hz), 3.50 (1H, d, J=14.2 Hz), 3.57 (1H, dd, J=8.3, 7.3 Hz), 4.39–4.46 (1H, m), 6.51 (2H, t, J=7.3 Hz), 6.94 (1H, dd, J=8.3, 7.3 Hz), 7.19–7.24 (1H, m), 7.27 (2H, s), 7.28 (2H, s). High resolution MS m/z: Calcd for C19H20N2O: 292.1575. Found: 292.1578. 114b: mp 161–162℃ (colorless prisms, recrystallized from EtOAc–hexane). IR (KBr): 3233, 3054, 2928, 2883, 1637, 1551, 1452, 1280, 1243, 762, 727, 691 cm-1. 1H-NMR (CD3OD) δ: 1.39 (1H, q, J=11.9 Hz), 2.24 (1H, dt, J=11.9, 3.7 Hz), 2.48 (1H, dd, J=16.5, 11.9 Hz), 2.99 (1H, dd, J=11.9, 8.3 Hz), 3.06 (1H, dd, J=16.5, 6.4 Hz), 3.12–3.22 (1H, m), 3.51 (2H, s), 3.58 (1H, t, J=8.3 Hz), 4.20 (1H, ddt, J=11.9, 6.4, 3.7 Hz), 6.49 (2H, d, J=7.3 Hz), 6.91 (1H, dd, J=8.3, 7.3 Hz), 7.20– 7.26 (1H, m), 7.30 (2H, s), 7.31 (2H,s). MS m/z: 292 (M+). Anal. Calcd for C19H20N2O: C, 78.05; H, 6.90; N, 9.58. Found: C, 77.91; H, 6.92; N, 9.42.
1-Methoxy-4-(N-phenylacetylamino)-1,3,4,5-tetrahydrobenz[cd]indole (115) from a mixture of diastereoisomers, 4-(N-phenylacetylamino)-1,2,2a,3,4,5-hexahydrobenz[cd]indoles (114a, 114b) — Prepared according to general method C for 6, where Na2WO4·2H2O (6.6 mg, 0.02 mmol) in H2O (0.2 mL), urea·H2O2 (95.2 mg, 1.01 mmol), and diastereoisomer’s mixture, 114a and 114b (29.1 mg, 0.10 mmol) in MeOH (2.0 mL) were used. To the reaction mixture, K2CO3 (247.1 mg, 1.79 mmol) and Me2SO4 (80.0 mg, 0.64 mmol) were added and stirred at rt for 1.5 h. After usual work-up and purification, 115 (12.8 mg, 40%) was obtained. 115: mp 138–139 ℃ (colorless prisms, recrystallized from CH2Cl2–hexane). IR (KBr): 3301, 3053, 2943, 1635, 1543, 1493, 1442, 1342, 985, 752, 731 cm-1. 1H-NMR (CDCl3) δ: 2.74 (1H, dd, J=15.6, 5.9 Hz), 2.90 (1H, dd, J=15.6, 5.9 Hz), 3.01 (1H, dd, J=15.6, 3.7 Hz), 3.11 (1H, dd, J=15.6, 3.7 Hz), 3.43 (2H, s), 4.05 (3H, s), 4.61–4.69 (1H, m), 5.44 (1H, br d, J=8.3 Hz), 6.79 (1H, d, J=7.3 Hz), 6.89 (1H, s), 7.03 (1H, dd, J=7.3, 1.8 Hz), 7.13–7.22 (2H, m). MS m/z: 320 (M+). Anal. Calcd for C20H20N2O2: C, 74.98; H, 6.29; N, 8.74. Found: C, 74.70; H, 6.20; N, 8.31.
A mixture of diastereoisomers, 4-N,N-di(n-propylamino)-1,2,2a,3,4,5-hexahydrobenz[cd]indole (117), from 4-N,N-di(n-propylamino)-1,3,4,5-tetrahydrobenz[cd]indole (116) — Prepared according to the method for 94, where 95% NaBH3CN (27.2 mg, 0.43 mmol) and 116 (22.0 mg, 0.09 mmol) in AcOH–CF3CO2H (2:1, v/v, 1.5 mL) were used. After usual work-up, the reaction residue was subjected to p-TLC on SiO2 with CHCl3–MeOH–aq. 30% NH3–hexane (92:10:1:1, v/v) as a developing solvent. Extraction of the band having an Rf value of 0.53–0.24 with CHCl3–MeOH–aq. 30% NH3 (46:5:0.5, v/v) afforded 117 (19.0 mg, 86%). Although 1H-NMR analysis of 117 showed 6:1 mixture of diastereoisomers, further separation was not examined.
4-N,N-Di(n-propylamino)-1-methoxy-1,3,4,5-tetrahydrobenz[cd]indole (118) from a diastereoiso- mer’s mixture (117) — Prepared according to the method for 6, where Na2WO4·2H2O (8.1 mg, 0.02 mmol) in H2O (0.2 mL), urea·H2O2 (116.8 mg, 1.24 mmol), and diastereoisomer’s mixture, 117 (30.7 mg, 0.12 mmol) in MeOH (2.0 mL) were used. Then, the reaction mixture was treated with ethereal CH2N2 (excess), followed by addition of PPh3 (319.5 mg, 1.26 mmol) under ice cooling, and the whole was stirred at rt for 20 min. After usual work-up, products were separated by p-TLC on Al2O3 with EtOAc–hexane (1:14, v/v) to give 118 (15.8 mg, 46%) and 117 (4.2 mg, 14%). 118: pale brown oil. IR (KBr): 2950, 1606, 1460, 1441, 1375, 1152, 1071, 984, 747 cm-1. 1H-NMR (pyridine-d5) δ: 0.90 (6H, t, J=7.3 Hz), 1.42 (4H, sex, J=7.3 Hz), 2.47 (4H, t, J J=7.3 Hz), 2.73 (1H, ddd, J=15.1, 12.0, 1.5 Hz), 2.92 (1H, dd, J=15.1, 4.1 Hz), 2.95 (1H, d, J=12.0 Hz), 3.02 (1H, dd, J=15.1, 4.1 Hz), 3.20–3.27 (1H, m), 3.97 (3H, s), 6.98 (1H, d, J=7.8 Hz), 7.16 (1H, d, J=1.5 Hz), 7.29 (1H, dd, J=7.8, 6.8 Hz), 7.36 (1H, d, J=7.8 Hz). High resolution MS m/z: Calcd for C18H26N2O: 286.2043. Found: 286.2044.
Scheme 12.
Synthesis of 1-hydroxyinoles (6).
Scheme 12.
Synthesis of 1-hydroxyinoles (6).
1-Hydroxy-Nb-acetyltryptamine (120a) from Nb-acetyl-2,3-dihydrotryptamine (119a)— Prepared according to the general method for 6, where Na2WO4·2H2O (66.4 mg, 0.20 mmol), 119a (205.2 mg, 1.00 mmol) in MeOH (20.0 mL), and 30% H2O2 (1.0 mL, 10.0 mmol) were used. After usual work-up and purification, 120a (121.5 mg, 55%) was obtained. 120a: mp 138.0–139.0 °C (colorless prisms, recrystallized from EtOAc). IR (KBr): 3250, 3105, 1619, 1602, 1580, 743 cm-1. UV λmaxMeOH nm (log ε): 225 (4.52), 281 (3.62), 295 (3.66). 1H-NMR (CD3OD) δ: 1.89 (3H, s), 2.89 (2H, t, J=7.3 Hz), 3.43 (2H, t, J=7.3 Hz), 6.99 (1H, dd, J=8.3, 8.3 Hz), 7.10 (1H, s), 7.12 (1H, dd, J=8.3, 8.3 Hz), 7.34 (1H, d, J=8.3 Hz), 7.52 (1H, d, J=8.3 Hz), 7.46 (1H, d, J=8.3 Hz). MS m/z: 218 (M+). Anal. Calcd for C12H14N2O2: C, 66.04; H, 6.47; N, 12.84. Found: C, 66.02; H, 6.53; N, 12.77.
1-Hydroxy-Nb-methoxycarbonyltryptamine (120b) from Nb-methoxycarbonyl-2,3-dihydro- tryptamine (119b) — Prepared according to the general method for 6, where Na2WO4·2H2O (56.1 mg, 0.17 mmol) in H2O (1.8 mL), 119b (185.9 mg, 0.85 mmol) in MeOH (18.0 mL), and 30% H2O2 (0.86 mL, 8.42 mmol) were used. After usual work-up and purification, 120b (131.5 mg, 67%) was obtained. 120b: mp 114.0–115.0 °C (colorless needles, recrystallized from CH2Cl2–hexane). IR (KBr): 3380, 3190, 1698, 1533, 1267, 983, 751 cm-1. UV λmaxMeOH nm (log ε): 225 (4.53), 295 (3.66). 1H-NMR (CD3OD) δ: 2.89 (2H, t, J=7.5 Hz), 3.36 (2H, t, J=7.9 Hz), 3.61 (3H, s), 6.99 (1H, t, J=7.9 Hz), 7.09 (1H, s), 7.13 (1H, t, J=7.9 Hz), 7.34 (1H, d, J=7.9 Hz), 7.53 (1H, d, J=7.9 Hz). MS m/z: 234 (M+). Anal. Calcd for C12H14N2O3: C, 61.53; H, 6.02; N, 11.96. Found: C, 61.40; H, 6.02; N, 11.90.
1-Hydroxy-Nb-trifluoroacetyltryptamine (120c) from Nb-trifluoroacetyl-2,3-dihydrotryptamine (119c) — Prepared according to the general method for 6, where Na2WO4·2H2O (57.8 mg, 0.18 mmol) in H2O (2.2 mL), 119c (218.6 mg, 0.85 mmol) in MeOH (20.0 mL), and 30% H2O2 (984.0 mg, 8.68 mmol) in MeOH (2.0 mL) were used. After usual work-up, the crude product was purified by column-chromatography on SiO2 with CH2Cl2–MeOH (99:1, v/v) to give 120c (165.3 mg, 72%). 120c: colorless oil. IR (film): 3310, 2935, 1721, 1698, 1566, 1553, 1451, 1354, 1205, 1098, 1008, 741 cm-1. 1H-NMR (5% CD3OD in CDCl3) δ: 2.99 (2H, t, J=6.6 Hz), 3.62 (2H, q, J=6.6 Hz), 7.07 (1H, s), 7.08 (1H, t, J=8.0 Hz), 7.22 (1H, t, J=8.0 Hz), 7.38 (1H, br s), 7.44 (1H, d, J=8.0 Hz), 7.53 (1H, d, J=8.0 Hz). High resolution MS m/z: Calcd for C12H11F3N2O2: 272.0772. Found: 272.0779.
Nb,Nb-Dimethyl-1-hydroxytryptamine (120d) from Nb,Nb-dimethyl-2,3-dihydrotryptamine (119d)— Prepared according to the general method for 6, where Na2WO4·2H2O (132.5 mg, 0.40 mmol) in H2O (4.0 mL), 119d (378.9 mg, 1.99 mmol) in MeOH (40.0 mL), and 30% H2O2 (2.0 mL, 19.6 mmol) in MeOH (40.0 mL) were used. After usual work-up, the crude product was purified by column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:5:0.5, v/v) to give 120d (70.5 mg, 55%). 120d: mp 179.5–180.0 °C (colorless needles, recrystallized from MeOH–H2O). IR (KBr): 2415, 1470, 1447, 1320, 1226, 838, 737 cm-1. UV λmaxMeOH nm (log ε): 223 (4.48), 292 (3.62). 1H-NMR (CD3OD) δ: 2.35 (6H, s), 2.64–2.68 (2H, m), 2.89–2.93 (2H, m), 6.99 (1H, dt, J=0.9 and 8.1 Hz), 7.09 (1H, s), 7.13 (1H, dt, J=0.9, 8.1 Hz), 7.34 (1H, dt, J=8.1, 0.9 Hz), 7.50 (1H, dt, J=8.1, 0.9 Hz). MS m/z: 204 (M+). Anal. Calcd for C12H16N2O: C, 70.56; H, 7.90; N, 13.71. Found: C, 70.35; H, 8.04; N, 13.66.
1-Hydroxy-Nb-n-propyltryptamine (120e) from Nb-n-propyl-2,3-dihydrotryptamine (119e) — Prepared according to the general method for 6, where Na2WO4·2H2O (56.5 mg, 0.17 mmol) in H2O (1.8 mL), 119e (173.4 mg, 0.85 mmol) in MeOH (18.0 mL), and 30% H2O2 (0.85 mL, 8.42 mmol) were used. After usual work-up, the crude product was purified by column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:5:0.5, v/v) to give 120e (96.3 mg, 52%). 120e: mp 147.0–148.0 °C (colorless needles, recrystallized from MeOH). IR (KBr): 2960, 2840, 1970, 1515, 1447, 1343, 1322, 1221, 1089, 785, 734 cm-1. 1H-NMR (CD3OD) δ: 0.90 (3H, t, J=7.3 Hz), 1.52 (2H, sext, J=7.3 Hz), 2.63 (2H, m), 2.94 (4H, m), 6.96 (1H, dd, J=8.0, 1.1 Hz), 7.09 (1H, s), 7.11 (1H, dd, J=8.0, 1.1 Hz), 7.37 (1H, ddd, J=8.0, 1.1, 0.7 Hz), 7.50 (1H, ddd, J=8.0, 1.1, 0.7 Hz). MS m/z: 218 (M+). Anal. Calcd for C13H18N2O·1/8H2O: C, 70.80; H, 8.34; N, 12.70. Found: C, 70.91; H, 8.29; N, 12.70.
1-Methoxy-Nb-acetyltryptamine (121a) from 120a — Ethereal CH2N2 (excess) was added to a solution of 120a (51.6 mg, 0.23 mmol) and stirred at rt for 1 h. After evaporation of the solvent under reduced pressure, the residue was column-chromatographed on SiO2 with CH2Cl2–MeOH (99:1, v/v) to give 121a (46.7 mg, 85%). 121a: colorless oil. IR (film): 3280, 3075, 1650, 1551, 1451, 740 cm-1. 1H-NMR (CDCl3) δ: 1.93 (3H, s), 2.93 (2H, t, J=6.6 Hz), 3.56 (2H, dt, J=5.9, 6.6 Hz), 4.06 (3H, s), 5.66 (1H, br s, D2O exchange), 7.11 (1H, s), 7.12 (1H, br d, J=8.3 Hz), 7.26 (1H, br d, J=8.3 Hz), 7.42 (1H, d, J=8.2 Hz), 7.56 (1H, d, J=8.3 Hz). High resolution MS m/z: Calcd for C13H16N2O2: 232.1210. Found: 232.1214.
1-Methoxy-Nb-methoxycarbonyltryptamine (121b) from 120b — Ethereal CH2N2 (excess) was added to a solution of 120b (39.1 mg, 0.18 mmol) and stirred at rt for 1 h. After usual work-up and purification, 121b (34.3 mg, 83%) was obtained. 121b: colorless oil. IR (film): 3320, 2930, 1705, 1525, 1452, 1254, 737 cm-1. 1H-NMR (CDCl3) δ: 2.93 (2H, t, J=6.6 Hz), 3.49 (2H, q, J=6.6 Hz), 3.66 (3H, s), 4.06 (3H, s), 4.76 (1H, br s), 7.10 (1H, s), 7.12 (1H, dt, J=1.1, 8.0 Hz), 7.25 (1H, dt, J=1.1, 8.0 Hz), 7.42 (1H, d, J=8.0 Hz), 7.57 (1H, d, J=8.0 Hz). High resolution MS m/z: Calcd for C13H16N2O3: 248.1160. Found: 248.1163.
1-Methoxy-Nb-trifluoroacetyltryptamine (121c) from 120c — Ethereal CH2N2 (excess) was added to a solution of 120c (32.7 mg, 0.12 mmol) and stirred at rt for 1 h. After usual work-up, 121c (26.8 mg, 78%) was obtained. 121c: mp 70.5–71.0 °C (colorless prisms, recrystallized from benzene–hexane). IR (KBr): 3270, 1732, 1702, 1567, 1454, 1215, 1186, 1148, 735 cm-1. UV λmaxMeOH nm (log ε): 223 (4.53), 276 (3.68), 290 (3.69). 1H-NMR (CDCl3) δ: 3.02 (2H, t, J=6.7 Hz), 3.67 (2H, q, J=6.7 Hz), 4.07 (3H, s), 6.35 (1H, br s), 7.12 (1H, s), 7.14 (1H, t, J=8.0 Hz), 7.28 (1H, t, J=8.0 Hz), 7.44 (1H, d, J=8.0 Hz), 7.56 (1H, d, J=8.0 Hz). High resolution MS m/z: Calcd for C13H13F3N2O3: 286.0928. Found: 286.0877.
Lespedamine (Nb,Nb-Dimethyl-1-methoxytryptamine, 121d) from 120d — Ethereal CH
2N
2 (excess) was added to a solution of
120d (13.1 mg, 0.064 mmol) in MeOH (5.0 mL) with stirring at rt until the starting material was not detected on tlc monitoring. After usual work-up and purification,
121d (8.0 mg, 57%) was obtained.
121d: colorless oil. IR (film): 2930, 2855, 2820, 2770, 1460, 1093, 1051, 1034, 1007, 953 cm
-1 (lit.[
18] 1459 cm
-1).
1H-NMR (CDCl
3) δ: 2.37 (6H, s), 2.65 (2H, t,
J=8.0 Hz), 2.93 (2H, t,
J=8.0 Hz), 4.05 (3H, s), 7.10 (1H, dt,
J=0.9, 7.8 Hz), 7.10 (1H, s), 7.23 (1H, dt,
J=0.9, 7.8 Hz), 7.40 (1H, dd,
J=7.8, 0.9 Hz), 7.57 (1H, dd,
J=7.8, 0.9 Hz) (lit.
7b,18 1H-NMR (CCl
4) δ: 2.19 (6H, s), 2.32–2.96 (4H, m), 3.92 (3H, s), 6.62–7.45 (5H, m)).
Lespedamine (121d) and lespedamine-Nb-oxide (122) from 2,3-dihydro-Nb,Nb-dimethyltryptamine (119d) — Prepared according to the general method for 6, where Na2WO4·2H2O (15.1mg, 0.04 mmol) in H2O (0.5 mL), 119d (43.9 mg, 0.23 mmol) in MeOH (5 mL), and 30% H2O2 (0.24 mL, 2.35 mmol) were used. After methylation and work-up, the product was purified by column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:2:0.2, v/v) to give 121d (13.1 mg, 26%) and 122 (17.0 mg, 31%) in the order of elution. 122: colorless oil. IR (film): 3420, 1644, 1453, 954, 742 cm-1. 1H-NMR (CDCl3) δ: 3.31 (6H, s), 3.37–3.40 (2H, m), 3.57–3.60 (2H, m), 4.06 (3H, s), 7.13 (1H, dt, J=1.1, 7.9 Hz), 7.18 (1H, s), 7.25 (1H, dt, J=1.1, 7.9 Hz), 7.42 (1H, ddd, J=7.9, 1.1, 0.9 Hz), 7.59 (1H, ddd, J=7.9, 1.1, 0.9 Hz). MS m/z: 234 (M+). Anal. Calcd for C13H18N2O2·MeOH: C, 63.13; H, 8.33; N, 10.52. Found: C, 63.24; H, 8.14; N, 10.74. High resolution MS m/z: Calcd for C13H18N2O2: 234.1368. Found: 234.1370.
1-Methoxy-Nb-n-propyltryptamine (121e) and 1-methoxy-Nb-methyl-Nb-n-propyltryptamine (121) from Nb-n-propyl-2,3-dihydrotryptamine (119e)— Prepared according to the general method for 6, where Na2WO4·2H2O (13.9 mg, 0.04 mmol) in H2O (0.4 mL), 119e (41.9 mg, 0.21 mmol) in MeOH (4.0 mL), and 30% H2O2 (0.21 mL, 2.06 mmol) were used. After methylation and work-up, the products were purified by column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:2:0.2, v/v) to give 123 (4.6 mg, 9%) and 121e (23.3 mg, 49%) in the order of elution. 121e: pale yellow oil. IR (film): 2960, 2930, 2875, 2820, 1451, 1094, 738 cm-1. 1H-NMR (CDCl3) δ: 0.90 (3H, t, J=7.5 Hz), 1.53 (2H, sext, J=7.5 Hz), 2.63 (2H, t, J=7.5 Hz), 2.97 (4H, m), 4.05 (3H, s), 7.10 (1H, dt, J=1.1 and 8.0 Hz), 7.11 (1H, s), 7.24 (1H, dt, J=1.1, 8.0 Hz), 7.41 (1H, dt, J=8.0, 1.1 Hz), 7.59 (1H, dt, J=8.0, 1.1 Hz). High resolution MS m/z: Calcd for C14H20N2O: 232.1575. Found: 232.1575. 123: colorless oil. IR (film): 2955, 2940, 2870, 2780, 1450, 1098, 1010, 956, 736 cm–1. 1H-NMR (CDCl3) δ: 0.93 (3H, t, J=7.3 Hz), 1.58 (2H, br sext, J=7.3 Hz), 2.40 (3H, s), 2.47 (2H, br t, J=7.3 Hz), 2.75 (2H, br t, J=7.5 Hz), 2.95 (2H, br t, J=7.5 Hz), 4.05 (3H, s), 7.11 (1H, dt, J=0.9, 8.1 Hz), 7.11 (1H, s), 7.24 (1H, dt, J=0.9, 8.1 Hz), 7.40 (1H, d, J=8.1 Hz), 7.57 (1H, d, J=8.1 Hz). High resolution MS m/z: Calcd for C15H22N2O: 246.1731. Found: 246.1734.
2,3-Dihydromelatonin (124) from melatonin (70) — A solution of 70 (1.01 g, 4.35 mmol) in CF3CO2H (20.0 mL) was added to Et3SiH (0.85 mL, 5.32 mmol) and stirred at 58 °C for 1 h. After usual work-up and purification, by column-chromatography on SiO2 with CHCl3–MeOH (97:3, v/v), 124 (847.7 mg, 83%) was obtained. 124: mp 83–84 °C (colorless prisms, recrystallized from EtOAc–hexane). IR (KBr): 3550, 1645, 1550, 1495, 1360, 1220, 1110, 1025, 890, 795, 740, 690, 585, 535 cm-1. 1H-NMR (CDCl3) δ: 1.73–1.79 (1H, m), 1.94–2.03 (1H, m), 1.95 (3H, s), 3.22–3.43 (4H, m), 3.70 (1H, br t, J=8.8 Hz), 3.75 (3H, s), 5.71 (1H, br s), 6.60 (1H, d, J=8.5 Hz), 6.62 (1H, dd, J=8.5, 2.20 Hz), 6.73 (1H, d, J=2.20 Hz). Anal. Calcd for C13H18N2O2: C, 66.64; H, 7.74; N, 11.96. Found: C, 66.47; H, 7.80; N, 11.91.
1-Hydroxymelatonin (125a) from 2,3-dihydromelatonin (124) — Prepared according to the general method B for 6, where Na2WO4·2H2O, (107.1 mg, 0.325 mmol) in H2O (3.8 mL), 124 (379.5 mg, 1.62 mmol) in MeOH (38.0 mL), and 30% H2O2 (1.8 mL, 15.9 mmol) were used. After usual work-up and purification by column-chromatographed on SiO2 with EtOAc, 125a (234.9 mg, 58%) was obtained. 125a: mp 113–114 °C (colorless prisms recrystallized from CHCl3–hexane. IR (KBr): 3600, 3200, 2900, 2850, 1610, 1560, 1480, 1360, 1280, 1260, 1215, 1170, 1095, 1035, 995, 950, 900, 823, 795, 760, 600 cm-1. 1H-NMR (CD3OD) δ: 1.91 (3H, s), 2.86 (2H, t, J=7.3 Hz), 3.42 (2H, t, J=7.3 Hz), 3.82 (3H, s), 6.80 (1H, dd, J=8.8, 2.4 Hz), 7.03 (1H, d, J=2.4 Hz), 7.07 (1H, s), 7.23 (1H, d, J=8.8 Hz). MS m/z: 248 (M+). Anal. Calcd for C13H16N2O3·1/8H2O: C, 62.32; H, 6.54; N, 11.18. Found: C, 62.20; H, 6.40; N, 11.01.
1-Methoxymelatonin (125b) from 1-hydroxymelatonin (125a) — Excess CH2N2 in Et2O was added to a solution of 125a (40.2 mg, 0.16 mmol) in MeOH (5.0 mL) at rt and stirred for 15 min. Evaporation of the solvent under reduced pressure afforded an oil, which was column-chromatographed on SiO2 with AcOEt to give 125b (39.1 mg,75.1%). 125b: pale yellow oil. UV λmaxMeOH nm: 306, 277. IR (film): 3280, 2930, 1643, 1553, 1480, 1440, 1220, 1090 cm-1. 1H-NMR (5% CD3OD in CDCl3) δ: 1.93 (3H, s), 2.87 (2H, t, J=6.8 Hz), 3.53 (2H, t, J=6.8 Hz), 3.85 (3H, s), 4.04 (3H, s), 6.91 (1H, dd, J=8.9, 2.3 Hz), 7.00 (1H, d, J=2.3 Hz), 7.08 (1H, s), 7.30 (1H, dd, J=8.9, 2.3 Hz). MS m/z: 262 (M+). High resolution MS m/z: Calcd for C14H18N2O3: 262.1317. Found: 262.1331.
(dl)-2-Acetoamino-3-(1-hydroxyindol-3-yl)propanol ((dl)-127) from (dl)-2-acetoamino-3-(2,3- dihydroindol-3-yl)propanol ((dl)-126) — Prepared according to the general method B for 6, where Na2WO4·2H2O (44.6 mg, 0.14 mmol), (dl)-126 (158.2 mg, 0.68 mmol) in MeOH (16 mL), and 30% H2O2 (0.69 mL, 6.76 mmol) were used. After usual work-up and purification, (dl)-127 (40.8 mg, 30%) was obtained. (dl)-127: colorless unstable oil. IR (film): 3265, 3110, 1629, 1547, 738 cm-1. 1H-NMR (CD3OD) δ: 1.88 (3H, s), 2.80 (1H, dd, J=14.5, 7.5 Hz), 3.00 (1H, dd, J=14.5, 6.5 Hz), 3.53 (2H, d, J=5.1 Hz), 4.14 (1H, m), 6.96 (1H, dd, J=7.6, 7.1 Hz), 7.10 (1H, s), 7.12 (1H, dd, J=7.6, 6.8 Hz), 7.32 (1H, d, J=7.1 Hz), 7.57 (1H, d, J=6.8 Hz). High resolution MS m/z: Calcd for C13H16N2O3: 248.1159. Found: 248.1146.
Scheme 13.
Synthesis of 1-hydroxyinole derivative (7).
Scheme 13.
Synthesis of 1-hydroxyinole derivative (7).
(dl)-2-Acetoamino-3-(1-methoxyindol-3-yl)propanol ((dl)-128) from (dl)-127) — Ethereal CH2N2 (excess) was added to a solution of (dl)-127 (32.8 mg, 0.13 mmol) in MeOH (3.0 mL) and stirring was continued at rt for 10 min. After usual work-up and purification by column-chromatography on SiO2 with CH2Cl2–MeOH (95:5, v/v), (dl)-128 (26.7 mg, 77%) was obtained. (dl)-128: mp 117–118°C (colorless prisms, recrystallized from EtOAc). IR (KBr): 3275, 3180, 3090, 1630, 1584, 1440, 1081, 1052, 757, 737 cm-1. UV λmaxMeOH nm (log ε): 223 (4.43), 278 (3.63), 291 (3.65). 1H-NMR (CDCl3) δ: 1.96 (3H, s), 2.54 (1H, br s, D2O exchange), 2.97 (2H, d, J=6.3 Hz), 3.63 (2H, dd, J=5.6, 3.9 Hz), 4.04 (3H, s), 4.08–4.38 (1H, m), 5.88 (1H, d, J=7 Hz), 7.08 (1H, dd, J=7.1, 6.8 Hz), 7.12 (1H, s), 7.23 (1H, dd, J=7.3, 6.8 Hz), 7.40 (1H, d, J=7.1 Hz), 7.60 (1H, d, J=7.3 Hz). MS m/z: 262 (M+). Anal. Calcd for C14H18N2O2: C, 64.11; H, 6.92; N, 10.68. Found: C, 63.89; H, 7.24; N, 10.54.
(S)-(+)-Nb-Acetyl-1-hydroxytryptophan methyl ester ((S)-(+)-131) from (S)-(+)-Nb-acetyl-2,3-di- hydrotryptophan methyl ester ((S)-(+)-130) — Prepared according to the general method B for 6, where Na2WO4·2H2O (40.2 mg, 0.12 mmol), (S)-(+)-130 (159.5 mg, 0.61 mmol) in MeOH (15.0 mL), and 30% H2O2 (0.62 mL, 6.09 mmol) were used. After usual work-up, the product was purified by p-TLC on SiO2 with CH2Cl2–MeOH (98:2, v/v) to give (S)-(+)-131 (89.7 mg, 53%). (S)-(+)-131: mp 116.0–117.0 °C (colorless prisms, recrystallized from MeOH–H2O). [α]D24 +11.8° (c=0.102, MeOH). IR (KBr): 3370, 3240, 1733, 1655, 1534, 745 cm-1. UV λmaxMeOH nm (log ε): 224 (4.53), 282 (3.64), 293 (3.66). 1H-NMR (5% CD3OD in CDCl3) δ: 1.90 (3H, s), 3.19 (1H, dd, J=15.0, 5.8 Hz), 3.27 (1H, dd, J=15.0, 5.2 Hz), 3.71 (3H, s), 4.86 (1H, dd, J=5.8, 5.2 Hz), 7.01 (1H, s), 7.06 (1H, t, J=8.3 Hz), 7.19 (1H, t, J=8.3 Hz), 7.42 (1H, d, J=8.3 Hz), 7.45 (1H, d, J=8.3 Hz). MS m/z: 276 (M+). Anal. Calcd for C14H16N2O4: C, 60.86; H, 5.84; N, 10.14. Found: C, 60.85; H, 5.88; N, 10.14.
(dl)-Nb-Acetyl-1-hydroxytryptophan methyl ester ((dl)-131) from (dl)-130 — Prepared under the same reaction conditions as described in the procedure for (S)-(+)-131. Yield was 73%. (dl)-131: mp 153.0–154.0 °C (decomp., colorless prisms, recrystallized from MeOH). IR (KBr): 3259, 3125, 1739, 1640, 1547, 727 cm-1. UV λmaxMeOH nm (log ε): 224 (4.55), 282 (3.65), 294 (3.68). 1H-NMR (CD3OD) δ: 1.92 (3H, s), 3.06 (1H, dd, J=13.9, 7.6 Hz), 3.28 (1H, dd, J=13.9, 5.9 Hz), 3.65 (3H, s), 4.66 (1H, dd, J=7.6, 5.9 Hz), 6.97 (1H, ddd, J=7.1, 6.8, 1.5 Hz), 7.09 (1H, s), 7.12 (1H, ddd, J=7.6, 6.8, 1.5 Hz), 7.32 (1H, dm, J=7.1 Hz), 7.47 (1H, dm, J=7.6 Hz). MS m/z: 276 (M+). Anal. Calcd for C14H16N2O4: C, 60.86; H, 5.84; N, 10.14. Found: C, 60.78; H, 5.92; N, 10.09.
(S)-(+)-Nb-Acetyl-1-methoxytryptophan methyl ester ((S)-(+)-132) from (S)-(+)-131 — Ethereal CH2N2 (excess) was added to a solution of (S)-(+)-131 (46.8 mg, 0.17 mmol) in MeOH (2.0 mL) and stirring was continued at rt for 15 min. After work-up and purification by column-chromatography on SiO2 with CH2Cl2–MeOH (98:2, v/v), (S)-(+)-132 (38.3 mg, 78%) was obtained. (S)-(+)-132: colorless oil. [α]D20 +16.8° (c=0.107, MeOH). IR (film): 3270, 1741, 1658, 1540, 736 cm-1. UV λmaxMeOH nm (log ε): 223 (4.47), 276 (3.66), 289 (3.68). 1H-NMR (CDCl3) δ: 1.97 (3H, s), 3.25 (1H, dd, J=14.6, 4.9 Hz), 3.31 (1H, dd, J=14.6, 5.4 Hz), 3.70 (3H, s), 4.05 (3H, s), 4.93 (1H, ddd, J=7.8, 5.4, 4.9 Hz), 6.03 (1H, d, J=7.8 Hz), 7.04 (1H, s), 7.11 (1H, dd, J=8.3, 7.8 Hz), 7.24 (1H, t, J=8.3 Hz), 7.40 (1H, d, J=8.3 Hz), 7.49 (1H, d, J=7.8 Hz). High resolution MS m/z: Calcd for C15H18N2O4: 290.1266. Found: 290.1296.
(dl)-Nb-Acetyl-1-methoxytryptophan methyl ester ((dl)-132) from (dl)-131 — Ethereal CH2N2 (excess) was added to a solution of (dl)-131 (40.1 mg, 0.15 mmol) in MeOH (2.0 mL) and stirring was continued at rt for 30 min. After usual work-up, (dl)-132 (35.1 mg, 83%) was obtained. (dl)-132: mp 95–96°C (colorless plates, recrystallized from MeOH-H2O). IR (KBr): 3235, 1737, 1657, 1545, 1443, 1376, 1313, 1241, 1210, 1173, 747, 741 cm-1. UV λmaxMeOH nm (log ε): 224 (4.50), 277 (3.70), 290 (3.17). 1H-NMR (CDCl3) δ: 1.97 (3H, s), 3.26 (1H, dd, J=15.1, 4.9 Hz), 3.31 (1H, dd, J=15.1, 5.4 Hz), 3.71 (3H, s), 4.05 (3H, s), 4.93 (1H, ddd, J=7.8, 5.4, 4.9 Hz), 5.88 (1H, d, J=7.8 Hz, D2O exchange), 7.04 (1H, s), 7.11 (1H, br d, J=8.3 Hz), 7.24 (1H, br d, J=8.3 Hz), 7.41 (1H, d, J=8.3 Hz), 7.49 (1H, d, J=8.3 Hz). MS m/z: 290 (M+). Anal. Calcd for C15H18N2O4: C, 62.06; H, 6.25; N, 9.65. Found: C, 62.04; H, 6.37; N, 9.52.
23.3. 1-Hydroxyyohimbine Derivatives, a Group of Potential New Medicine Candidates
23.3.1. Synthesis of 1-Hydroxyyohimbine Derivatives
2β,7β- (133) and 2α,7α-Dihydroyohimbine (134) from Yohimbine (52·Free) — General Procedure: NaBH3CN (610.7 mg, 9.72 mmol) was added to a solution of 52·Free (1056.4 mg, 2.98 mmol) in CF3COOH (20.0 mL) at 0°C. The mixture was stirred at rt for 3 h. After evaporation of the solvent, the whole was made alkaline with initially aq. 8% and then 0.8% NaOH under ice cooling, and extracted with CHCl3. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with AcOEt–CHCl3–MeOH–28% aq. NH3 (51.5:46:5:0.5, v/v) to give 134 (843.3 mg, 79%) and 133 (189.1 mg, 18%) in the order of elution. 133: mp 191—193.5°C (pale yellow needles, recrystallized from AcOEt–hexane). IR (KBr): 3502, 1722, 1608, 754 cm-1. 1H-NMR (CDCl3) δ: 1.05 (1H, q, J=11.5 Hz), 1.30—1.53 (5H, m), 1.60—1.78 (4H, m), 1.90—1.96 (1H, m), 2.06 (1H, dt, J=3.9, 11.5 Hz), 2.15—2.27 (2H, m), 2.25 (1H, dd, J=11.5, 2.0 Hz), 2.60 (1H, dt, J=11.5, 3.4 Hz), 2.71 (1H, dd, J=11.5, 2.7 Hz), 3.00 (1H, br s, disappeared on addition of D2O), 3.31 (1H, t, J=8.1 Hz), 3.42 (1H, br s), 3.78 (3H, s), 4.16 (1H, br s), 6.65 (1H, d, J=7.8 Hz), 6.77 (1H, dd, J=7.6, 7.3 Hz), 7.02—7.06 (2H, m). High-resolution MS m/z: calcd for C21H28N2O3: 356.2100, found 356.2096. [α]29D –69.9° (c=0.33, MeOH). 134: mp 190—193°C (colorless fine needles, recrystallized from AcOEt–hexane). IR (KBr): 3471, 2906, 1707, 1020 cm-1. 1H-NMR (CDCl3) δ: 1.36—1.61 (7H, m), 1.68 (1H, br s, disappeared on addition of D2O), 1.71—1.77 (1H, m), 1.83—2.06 (4H, m), 2.18 (1H, dt, J=11.5, 2.7 Hz), 2.30 (1H, dd, J=11.5, 2.2 Hz), 2.77 (1H, ddd, J=11.5, 3.4, 3.2 Hz), 2.83 (1H, dd, J=11.5, 2.2 Hz), 2.93 (1H, dt, J=6.6, 2.7 Hz), 3.10 (1H, s, disappeared on addition of D2O), 3.57 (1H, dd, J=6.6, 2.7 Hz), 3.76 (3H, s), 4.19 (1H, br s), 6.68 (1H, dd, J=7.8, 1.0 Hz), 6.72 (1H, ddd, J=7.6, 7.3, 1.0 Hz), 7.01 (1H, ddd, J=7.8, 7.6, 1.0 Hz), 7.08 (1H, dd, J=7.3, 1.0 Hz). High-resolution MS m/z: calcd for C21H28N2O3: 356.2100, found: 356.2111. Anal. Calcd for C21H28N2O3·1/8H2O: C, 70.31; H, 7.94; N, 7.81. Found: C, 70.30; H, 7.93; N, 7.78. [α]25D +90.64° (c=0.20, CHCl3).
Scheme 14.
Synthesis of new yohimbine derivatives (1).
Scheme 14.
Synthesis of new yohimbine derivatives (1).
2α,7α-Dihydroyohimbine (134) from Yohimbine hydrochloride (52·HCl) — According to the general procedure, NaBH3CN (36.4 mg, 0.55 mmol), 52·HCl (107.6mg, 0.28 mmol), and CF3COOH (2.0 mL) were used. After column-chromatography on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:3:0.3, v/v), 134 (98.0 mg, 100%) was obtained.
Table 29.
Effect of yohimbine derivative on the contraction induced by clonidine. in rat thoracic aort.
Table 29.
Effect of yohimbine derivative on the contraction induced by clonidine. in rat thoracic aort.
1-Hydroxyyohimbine (53b) from Yohimbine hydrochloride (52·HCl) — According to the general procedure, NaBH3CN (85.5 mg, 1.3 mmol), 52·HCl (101.0mg, 0.26 mmol), and CF3COOH (2.0 mL) were used. The resultant oil, obtained after general procedure, was dissolved in MeOH (9.0 mL). A solution of Na2WO4·2H2O (17.0 mg, 0.05 mmol) in H2O (1.0 mL) and 30% H2O2 (0.59 mL, 5.2 mmol) were added to the solution. The mixture was stirred at 0°C for 1 h. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a solid, which was column-chromatographed on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:5:0.5, v/v) to give 53b (82.1 mg, 86%). 53b: mp 224—226°C (decomp., colorless fine needles, recrystallized from MeOH). IR (KBr): 3505, 2945, 1711, 751 cm-1. 1H-NMR (CD3OD) δ: 1.19 (1H, q, J=11.5 Hz), 1.33—1.39 (1H, m), 1.43—1.57 (2H, m), 1.65 (1H, br t, J=13.4 Hz), 1.91 (1H, dq, J=13.4, 2.7 Hz), 1.99 (1H, dq, J=2.7, 11.5 Hz), 2.31 (1H, br d, J=11.5 Hz), 2.40 (1H, t, J=11.5 Hz), 2.63—2.76 (2H, m), 2.88—2.98 (3H, m), 3.10—3.15 (1H, m), 3.62 (1H, d, J=11.5 Hz), 3.73 (3H, s), 4.22 (1H, q, J=2.7 Hz), 6.98 (1H, t, J=7.6 Hz), 7.09 (1H, t, J=7.6 Hz), 7.29 (1H, d, J=7.6 Hz), 7.37 (1H, d, J=7.6 Hz). MS m/z: 370 (M+), 354 (M+–O), 353 (M+–OH). Anal. Calcd for C21H26N2O4: C, 68.09; H, 7.07; N, 7.56. Found: C, 67.97; H, 7.13; N, 7.60. [α]30D +7.75° (c=0.20, DMF).
1-Hydroxyyohimbine (53b) from 2β,7β-Dihydroyohimbine (133) — A solution of Na2WO4·2H2O (6.8 mg, 0.03 mmol) in H2O (0.3 mL) and 30% H2O2 (0.10 mL, 0.80 mmol) were added to a solution of 133 (28.2 mg, 0.80 mmol) in MeOH (3.0 mL). The mixture was stirred at rt for 2 h. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave a solid, which was column-chromatographed on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:5:0.5, v/v) to give 53b (12.7mg, 43%).
1-Methoxyyohimbine (135a) from 53b — An excess amount of ethereal CH
2N
2 was added to a solution of
53b (52.6 mg, 0.14 mmol) in MeOH (20.0 mL) and the whole was stirred at 0°C for 1 h. The solution was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO
2 with CHCl
3–MeOH–28% aq. NH
3 (46:3:0.3, v/v) to give
135a (42.2 mg, 77%).
135a: mp 201—203°C (decomp., colorless prisms, recrystallized from acetone. Lit.[
76] mp 198—201°C). IR (KBr): 3145, 1737, 743 cm
-1.
1H-NMR (CDCl
3) δ: 1.36—1.45 (2H, m), 1.49—1.62 (3H, m), 1.97—2.09 (2H, m), 2.32—2.39 (2H, m), 2.46 (1H, ddd,
J=12.7, 3.2, 2.9 Hz), 2.63 (1H, dt,
J=4.2, 11.2 Hz), 2.66—2.71 (1H, m), 2.89—2.98 (2H, m), 3.03—3.08 (1H, m), 3.36 (1H, br s, disappeared on addition of D
2O), 3.49 (1H, br d,
J=11.2 Hz), 3.77 (3H, s), 3.89 (3H, s), 4.21 (1H, br s), 7.09 (1H, ddd,
J=7.8, 7.1, 1.0 Hz), 7.19 (1H, ddd,
J=8.1, 7.1, 1.0 Hz), 7.34 (1H, dd,
J=8.1, 1.0 Hz), 7.44 (1H, dd,
J=7.8, 1.0 Hz). MS
m/z: 384 (M
+), 353 (M
+–OMe).
Anal. Calcd for C
22H
28N
2O
4: C, 68.72; H, 7.34; N, 7.29. Found: C, 68.65; H, 7.35; N, 7.23. [α]
29D +20.54° (
c=0.20, CHCl
3).
1-n-Butyloxyyohimbine (135b) from 53b — According to the general procedure for 135c, K2CO3 (56.7 mg, 0.41 mmol), n-butyl iodide (30.8 mg, 0.17 mmol), and 53b (50.5 mg, 0.14 mmol) were used. After column-chromatography, 135b (57.8 mg, 99%) was obtained. 135b: mp 126—128.5°C (decomp., colorless fine needles, recrystallized from hexane). IR (KBr): 3458, 2920, 1739, 1151, 737 cm-1. 1H-NMR (CDCl3) δ: 1.34—1.43 (2H, m), 1.48—1.64 (3H, m), 1.97—2.06 (2H, m), 2.34 (1H, dd, J=11.5, 2.2 Hz), 2.35 (1H, t, J=11.5 Hz), 2.55 (1H, dt, J=12.9, 2.9 Hz), 2.62 (1H, dt, J=4.2, 10.7 Hz), 2.66—2.72 (1H, m), 2.90—2.98 (1H, m), 2.96 (1H, dd, J=11.5, 2.9 Hz), 3.05 (1H, ddd, J=11.5, 5.6, 2.2 Hz), 3.30 (1H, s, disappeared on addition of D2O), 3.51 (1H, dd, J=11.5, 2.2 Hz), 3.75 (3H, s), 4.20 (1H, d, J=1.2 Hz), 4.49 (1H, dddd, J=11.0, 6.6, 1.2, 1.0 Hz), 4.55 (1H, dddd, J=11.0, 6.1, 1.2, 1.0 Hz), 5.39 (1H, ddd, J=10.7, 1.2, 1.0 Hz), 5.44 (1H, dq, J=17.1, 1.2 Hz), 6.05 (1H, dddd, J=17.1, 10.7, 6.6, 6.1 Hz), 7.08 (1H, ddd, J=8.1, 7.8, 1.0 Hz), 7.18 (1H, dt, J=1.0, 8.1 Hz), 7.34 (1H, ddd, J=8.1, 1.0, 0.7 Hz), 7.43 (1H, br d, J=7.8 Hz). MS m/z: 410 (M+), 353 (M+–OCH2CH=CH2). Anal. Calcd for C24H30N2O4: C, 70.22; H, 7.37; N, 6.82. Found: C, 70.13; H, 7.50; N, 6.57. [α]30D +18.4° (c=0.21, CHCl3).
1-Allyloxyyohimbine (135c) from 53b — General procedure: K2CO3 (59.2 mg, 0.43 mmol) and a solution of allyl bromide (24.7 mL, 0.3 mmol) in DMF (1.0 mL) were successively added to a solution of 53b (52.8 mg, 0.14 mmol) in DMF (4.0 mL) and the whole was stirred at rt for 30 min. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (95:5, v/v) to give 135c (54.6 mg, 93%). 135c: mp 150—152°C (decomp., colorless fine needles, recrystallized from hexane). IR (KBr): 3464, 2935, 1738, 1151, 737 cm-1. 1H-NMR (CDCl3) δ: 1.01 (3H, t, J=7.3 Hz), 1.34—1.43 (2H, m), 1.48—1.63 (5H, m), 1.65—1.79 (2H, m), 1.97—2.07 (2H, m), 2.34 (1H, dd, J=11.2, 2.0 Hz), 2.36 (1H, t, J=11.2 Hz), 2.53 (1H, dt, J=12.9, 2.9 Hz), 2.63 (1H, dt, J=4.2, 11.2 Hz), 2.66—2.72 (1H, m), 2.90—2.98 (1H, m), 2.96 (1H, dd, J=11.2, 2.9 Hz), 3.05 (1H, ddd, J=11.2, 5.6, 2.0 Hz), 3.29 (1H, s, disappeared on addition of D2O), 3.48 (1H, d, J=11.2 Hz), 3.76 (3H, s), 3.98 (1H, dt, J=6.6, 8.5 Hz), 4.06 (1H, dt, J=6.6, 8.5 Hz), 4.20 (1H, br s), 7.07 (1H, ddd, J=8.1, 7.8, 1.0 Hz), 7.17 (1H, dt, J=1.0, 8.1 Hz), 7.31 (1H, dd, J=8.1, 1.0 Hz), 7.43 (1H, dd, J=7.8, 1.0 Hz). MS m/z: 426 (M+), 353 (M+–On-Bu). Anal. Calcd for C25H34N2O4: C, 70.39; H, 8.03; N, 6.57. Found: C, 70.26; H, 8.12; N, 6.48. [α]32D +21.46° (c=0.21, CHCl3).
1-p-Nitrobenzyloxyyohimbine (135d) from 1-Hydroxyyohimbine (53b) — According to the general procedure for 135c, K2CO3 (56.8 mg, 0.41 mmol), p-nitrobenzyl bromide (35.4 mg, 0.16 mmol), and 53b (50.3 mg, 0.14 mmol) were used. After column-chromatography, 135d (61.7 mg, 90%) was obtained. 135d: mp 148—149°C (decomp., yellow fine needles, recrystallized from AcOEt–hexane). IR (KBr): 3430, 2924, 1734 and 1703 (collapsed to 1710 in CHCl3), 1523, 1348, 739 cm-1. 1H-NMR (CDCl3) δ: 1.33—1.43 (2H, m), 1.50—1.61 (3H, m), 1.94—2.03 (2H, m), 2.28 (1H, br t, J=11.0 Hz), 2.33 (1H, dd, J=11.7, 2.2 Hz), 2.54 (1H, dt, J=12.7, 2.9 Hz), 2.57 (1H, dt, J=4.2, 11.0 Hz), 2.66—2.72 (1H, m), 2.89—2.98 (2H, m), 3.01—3.07 (1H, m), 3.04 (1H, s, disappeared on addition of D2O), 3.23 (1H, br d, J=11.0 Hz), 3.59 (3H, s), 4.21 (1H, br s), 5.02 (1H, d, J=10.6 Hz), 5.07 (1H, d, J=10.6 Hz), 7.12 (1H, ddd, J=8.1, 7.8, 1.0 Hz), 7.21 (1H, dt, J=1.0, 8.1 Hz), 7.32 (1H, dd, J=8.1, 1.0 Hz), 7.46 (1H, dd, J=7.8, 1.0 Hz), 7.59—7.62 (2H, A2 part of A2B2), 8.29—8.33 (2H, B2 part of A2B2). Anal. Calcd for C28H31N3O6: C, 66.52; H, 6.18; N, 8.31. Found: C, 66.40; H, 6.22; N, 8.18. [α]31D +48.77° (c=0.20, CHCl3).
1-Propargyloxyyohimbine (135e) from 53b — According to the general procedure for 135c, K2CO3 (223.6 mg, 1.62 mmol), propargyl bromide (70.8 mg, 0.60 mmol), and 53b (200.1 mg, 0.54 mmol) were used. After column-chromatography with AcOEt–hexane (1:1, v/v), 135e (57.8 mg, 99%) was obtained. 135e: mp 158—161°C (decomp., colorless fine needles, recrystallized from AcOEt–hexane). IR (KBr): 3565, 1720, 1265, 742 cm-1. 1H-NMR (CDCl3) δ: 1.35—1.43 (2H, m), 1.51—1.63 (3H, m), 1.97—2.07 (2H, m), 2.34 (1H, dd, J=11.5, 2.2 Hz), 2.36 (1H, d, J=11.2 Hz), 2.55 (1H, dt, J=12.7, 3.4 Hz), 2.60-2.71 (3H, m), 2.89—2.96 (1H, m), 2.96 (1H, dd, J=11.2, 3.4 Hz), 3.05 (1H, ddd, J=11.2, 6.1, 1.7 Hz), 3.46 (1H, br s, disappeared on addition of D2O), 3.58 (1H, dd, J=11.2, 1.7 Hz), 3.79 (3H, s), 4.21 (1H, d, J=1.2 Hz), 4.63 (1H, dd, J=15.1, 2.4 Hz), 4.71 (1H, dd, J=15.1, 2.4 Hz), 7.09 (1H, dt, J=1.0, 7.8 Hz), 7.19 (1H, dt, J=1.0, 7.8 Hz), 7.38 (1H, d, J=7.8 Hz), 7.43 (1H, d, J=7.8 Hz). MS m/z: 408 (M+). Anal. Calcd for C24H28N2O4·1/2H2O: C, 69.04; H, 7.00; N, 6.71. Found: C, 68.99; H, 6.82; N, 6.56. [α]24D +96.19° (c=0.21, MeOH).
1-Isopropyloxyyohimbine (135f) from 53b — a) Cs2CO3 Method: According to the general procedure for 135c, 53b (29.4 mg, 0.08 mmol), Cs2CO3 (28.6 mg, 0.09 mmol), and isopropyl bromide (29.3 mg, 0.24 mmol) were used. After column-chromatography with CHCl3–MeOH–28% aq. NH3 (46:1:0.1, v/v), 135f (32.3 mg, 99%) was obtained. 135f: pale brown viscous oil. IR (film): 3456, 2922, 1734 (br), 750 cm-1. 1H-NMR (CDCl3) δ: 1.29 (3H, d, J=6.1 Hz), 1.31 (3H, d, J=6.1 Hz), 1.41—1.64 (4H, m), 1.95—2.07 (2H, m), 2.38 (1H, dd, J=11.6, 2.0 Hz), 2.64—2.77 (3H, m), 2.97—3.07 (2H, br s), 3.07—3.17 (2H, br s), 3.55—3.65 (1H, br), 3.76 (3H, s), 4.23 (1H, br s), 4.50 (1H, sep, J=6.1 Hz), 4.72 (2H, br s, disappeared on addition of D2O), 7.02 (1H, t, J=7.3 Hz), 7.19 (1H, t, J= 7.3 Hz), 7.30 (1H, d, J=7.9 Hz), 7.43 (1H, d, J=7.3 Hz). MS m/z: 412 (M+), 353 (M+–OCHMe2). High-resolution MS m/z: calcd for C24H32N2O4: 412.2362, found: 412.2366. [α]25D +16.45° (c=0.15, CHCl3).
b) K2CO3 Method: According to the general procedure for 135c, K2CO3 (18.9 mg, 0.14 mmol), isopropyl bromide (19.8 mg, 0.16 mmol), and 53b (16.8 mg, 0.05 mmol) were used. After work-up, 135f (13.3 mg, 71%) was obtained.
(dl)-1-(1-Methoxycarbonyl)ethoxyyohimbine (135g) from 53b — According to the general procedure for 135c, 53b (30.3 mg, 0.08 mmol), Cs2CO3 (29.4 mg, 0.09 mmol), and (dl)-methyl-2-bromopropionate (41.0 mg, 0.25 mmol) were used. After column-chromatography with AcOEt, 135g (37.0 mg, 99%) was obtained. 135g: pale brown viscous oil. IR (film): 3509, 2923, 1739, 1710, 752 cm-1. 1H-NMR (CDCl3) δ: 1.30—1.45 (2H, m), 1.52—1.61 (3H, m), 1.55 (3H, d, J=6.8 Hz), 1.95—2.07 (2H, m), 2.34 (1H, dd, J=11.6, 2.0 Hz), 2.37—2.44 (1H, m), 2.52 (1H, dt, J=13.2, 2.7 Hz), 2.61—2.71 (2H, m), 2.86—2.95 (1H, m), 2.98 (1H, dd, J=11.6, 2.7 Hz), 3.01—3.08 (1H, m), 3.16 (1H, br s, disappeared on addition of D2O), 3.53 (1H, d, J=10.0 Hz), 3.66 (3H, s), 3.77 (3H, s), 4.20 (1H, br s), 4.66 (1H, q, J=6.8 Hz), 7.06 (1H, t, J=7.8 Hz), 7.16 (1H, t, J=7.8 Hz), 7.39 (1H, d, J=7.8 Hz), 7.44 (1H, d, J=7.8 Hz). High-resolution MS m/z: calcd for C25H32N2O6: 456.2260, found: 456.2263. [α]26D –22.11° (c=0.015, CHCl3).
1-Cyclohexyloxyyohimbine (135h) from 53b — According to the general procedure for 135c, 53b, (30.0 mg, 0.08 mmol), and Cs2CO3 (29.1 mg, 0.09 mmol) were used. The reaction time with cyclohexyl bromide (40.7 mg, 0.25 mmol) was prolonged to 130 min. After column-chromatography with CHCl3–MeOH–28% aq. NH3 (46:0.5:0.05, v/v), 135h (4.0 mg, 11%) and unreacted 53b (20 mg, 67%) were obtained. 135h: pale brown viscous oil. IR (film): 3446, 2927, 1738 (br), 739 cm-1. 1H-NMR (CD3OD) δ: 1.25—1.41 (8H, m), 1.46—1.71 (5H, m), 1.77—2.08 (6H, m), 2.35 (1H, dd, J=11.7, 2.7 Hz), 2.45 (1H, br t, J=11.0 Hz), 2.68—2.79 (2H, m), 2.90—3.02 (3H, m), 3.09—3.18 (1H, m), 3.73 (3H, s), 4.12—4.19 (1H, m), 4.20—4.25 (1H, m), 7.01 (1H, t, J=7.8 Hz), 7.13 (1H, t, J=8.3 Hz), 7.30 (1H, d, J=8.3 Hz), 7.39 (1H, d, J=7.8 Hz). High-resolution MS (EI) m/z: calcd for C27H34N2O4: 452.2675, found: 452.2677. [α]25D +8.46° (c=0.14, CHCl3).
N-Methoxycarbonyl-1-isopropyloxytryptamine (136a) from 1-Hydroxy-N-methoxy- carbonyltryptamine (64) — General procedure: 1-Hydroxy-N-methoxycarbonyltryptamine (64, 50.9 mg, 0.22 mmol) was added to a solution of Cs2CO3 (83.9 mg, 0.23 mmol) in MeOH (2.0 mL) and the whole was stirred at rt for 20 min. To the resultant residue obtained after evaporation of the solvent under reduced pressure, a solution of isopropyl bromide (160.0 mg, 1.32 mmol) in DMF (3.0 mL) was added and the whole was stirred at rt for 1 h. After addition of H2O, the whole was extracted with AcOEt. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 136a (49.7 mg, 83%). 136a: colorless oil. IR (film): 1710 (br), 740 cm-1. 1H-NMR (CDCl3) δ: 1.36 (3H, d, J=6.2 Hz), 1.37 (3H, d, J=6.2 Hz), 2.93 (2H, t, J=6.6 Hz), 3.49 (2H, br q, J=6.6 Hz, collapsed to t on addition of D2O), 3.66 (3H, s), 4.52 (1H, sep, J=6.2 Hz), 4.74 (1H, br s, disappeared on addition of D2O), 7.06 (1H, s), 7.09 (1H, dt, J=0.8, 7.7 Hz), 7.22 (1H, dt, J=0.8, 7.7 Hz), 7.38 (1H, dd, J=7.7, 0.8 Hz), 7.55 (1H, dd, J=7.7, 0.8 Hz). High-resolution MS m/z: calcd for C15H20N2O3: 276.1482, found: 276.1474.
(dl)-N-Methoxycarbonyl-1-(1-methoxycarbonyl)ethoxy tryptamine (136b) from 64 — According to the general procedure for 136a, 64 (49.1 mg, 0.21 mmol), Cs2CO3 (76.0 mg, 0.23 mmol), and (dl)-methyl 2-bromopropionate (212.8 mg, 1.3 mmol) were used. After column-chromatography with AcOEt–hexane (1:2, v/v), 136b (64.9 mg, 97%) was obtained. 136b: colorless oil. IR (film): 3405, 1749, 1716 (br), 742 cm-1. 1H-NMR (CDCl3) δ: 1.66 (3H, d, J=7.0 Hz), 2.89 (2H, t, J=6.6 Hz), 3.47 (2H, br q, J=6.6 Hz, collapsed to t on addition of D2O), 3.66 (3H, s), 3.77 (3H, s), 4.73 (1H, br s, disappeared on addition of D2O), 4.85 (1H, q, J=7.0 Hz), 7.12 (1H, dt, J=0.7, 7.6 Hz), 7.21 (1H, s), 7.24 (1H, dt, J=0.7, 7.6 Hz), 7.40 (1H, d, J=7.6 Hz), 7.54 (1H, d, J=7.6 Hz). High-resolution MS m/z: calcd for C16H20N2O5: 320.1372, found: 320.1371.
Scheme 15.
New yohimbine derivatives (2).
Scheme 15.
New yohimbine derivatives (2).
7α-Acetoxy- (137a) and 7α,17α-dacetoxy-7H-yohimbine (137b) from 1-hydroxyyohimbine (53b) — 98% NaOAc (93.6 mg, 1.1 mmol) was added to a solution of
53b (207.1 mg, 0.56 mmol) in Ac
2O (10.0 mL) at rt and stirred at 65 °C for 1 h. After evaporation of the solvent and adding H
2O, the whole was made alkaline with 0.8% NaOH under ice cooling. The whole was extracted with CHCl
3 and the extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO
2 with CHCl
3–MeOH (97:3, v/v) to give
137a (163.4 mg, 66%) and
137b (21.4 mg, 8%).
137a:Lit. [
81] mp 120—122 °C (yellow powder, recrystallized from Et
2O–hexane). IR (KBr): 1747, 1726, 1597, 1203, 1146, 1111, 1078, 1018, 775, 758 cm
-1.
1H-NMR (CDCl
3) δ: 1.36—1.63 (5H, m), 1.78—2.09 (4H, m), 2.06 (3H, s), 2.18 (1H, t,
J=11.0 Hz), 2.37 (1H, dd,
J=11.0, 2.0 Hz), 2.61—2.77 (3H, m), 2.91 (1H, dd,
J=11.0, 3.4 Hz), 2.94 (1H, dd,
J=11.0, 2.7 Hz), 3.15 (1H, s, disappeared on addition of D
2O), 3.76 (3H, s), 4.18 (1H, s), 7.20 (1H, dd,
J=7.6, 7.3 Hz), 7.37 (1H, dd,
J=7.6, 7.3 Hz), 7.38 (1H, d,
J=7.6 Hz), 7.61 (1H, d,
J=7.6 Hz). MS
m/z: 412 (M
+).
Anal. Calcd for C
23H
28N
2O
5·3/2H
2O: C, 62.85; H, 7.11; N, 6.37. Found: C, 62.86; H, 7.28; N, 6.18. [α]
23D +189° (c=0.221, CHCl
3).
137b: mp 190—191 °C (pale yellow prisms, recrystallized from Et
2O–hexane). IR (KBr): 1736, 1597, 1369, 1254, 1147, 1024, 754 cm
-1.
1H-NMR (CDCl
3) δ: 1.38—1.52 (4H, m), 1.59—1.68 (1H, m), 1.69 (1H. q,
J=12.0 Hz), 1.90—2.03 (2H, m), 2.05 (3H, s), 2.06 (3H, s), 2.22 (1H, t,
J=10.5 Hz), 2.30 (1H, dt,
J=12.9, 3.0 Hz), 2.42 (1H, dd,
J=11.5, 2.4 Hz), 2.64—2.78 (3H, m), 2.93 (1H, dd,
J=11.0, 3.0 Hz), 3.06 (1H, dd,
J=11.0, 2.4 Hz), 3.64 (3H, s), 5.41 (1H, dd,
J=5.4, 2.4 Hz), 7.19 (1H, ddd,
J=7.6, 7.3, 1.0 Hz), 7.36 (1H, ddd,
J=7.8, 7.6. 1.0 Hz), 7.39 (1H, dd,
J=7.3, 1.0 Hz), 7.59 (1H, dd,
J=7.8, 1.0 Hz).
Anal. Calcd for C
25H
30N
2O
6: C, 66.06; H, 6.65; N, 6.16. Found: C, 65.99; H, 6.78; N, 6.06. [α]
30D +188° (c=0.201, CHCl
3).
(2α,7α)- (141) and (2β,7β)-26-Nitro-benzofurano[2,3-n]yohimbine (142) from 53b — A solution of K2CO3 (374.2 mg, 2.71 mmol) and p-bromonitrobenzene (329.2 mg, 1.63 mmol) in DMF (3.0 mL) was added to a solution of 53b (500.5 mg, 1.35 mmol) in DMF (12.0 mL) and the whole was stirred at rt for 22 h. After addition of H2O, the whole was extracted with EtOAc. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with EtOAc–hexane (1:1, v/v) to give 141 (358.0 mg, 53%) and 142 (89.6 mg, 13%). 141: mp 275—276 °C (decomp., yellow powder, recrystallized from EtOAc–hexane). IR (film): 3545, 3392, 2360, 1596, 1506, 1387, 1327 cm-1. 1H-NMR (CDCl3) δ: 1.30—1.77 (5H, m), 1.84—2.17 (6H, m), 2.20—2.32 (1H, m), 2.35 (1H, dd, J=12.5, 2.5 Hz), 2.60—2.97 (3H, m), 3.07 (1H, brs, disappeared on addition of D2O), 3.83 (3H, s), 4.20 (1H, brs), 5.21 (1H, brs, disappeared on addition of D2O), 6.77 (1H, d, J=9.2 Hz), 6.82 (1H, dd, J=8.3, 1.7 Hz), 6.82 (1H, t, J=7.5 Hz), 7.01 (1H, dd, J=8.3, 1.7 Hz), 7.10 (1H, ddd, J=8.3, 8.3, 1.2 Hz), 8.12 (1H, dd, J=8.8, 2.5 Hz), 8.30 (1H, d, J=2.4 Hz). 13C-NMR (CDCl3) δ: 175.62 (C), 164.64 (C), 145.02 (C), 142.25 (C), 132.98 (C), 132.43 (C), 128.47 (CH), 126.02 (CH), 122.47 (CH), 121.04 (CH), 118.91 (CH), 111.93 (C), 110.68 (CH), 109.94 (CH), 66.52 (CH), 64.73 (CH), 61.42 (CH2), 55.11 (C), 52.25 (CH3), 52.03 (CH), 51.44 (CH3), 40.09 (CH), 36.14 (CH), 31.73 (CH2), 31.06 (CH2), 29.74 (CH2), 23.03 (CH2). MS m/z: 491 (M+). Anal. Calcd for C27H29N3O6·1/4H2O: C, 65.38; H, 5.99; N, 8.47. Found: C, 65.41; H, 5.89; N, 8.54. [α]23D +310° (c=0.221, MeOH). 142: yellow viscous oil. IR (film): 3508, 3352, 1728, 1518, 1338 cm-1. 1H-NMR (CDCl3) δ: 1.23—1.62 (4H, m), 1.74—1.92 (4H, m), 1.92—2.00 (2H, m), 2.06 (1H, ddd, J=11.7, 11.7, 2.9 Hz), 2.33 (1H, dd, J=10.7, 2.4 Hz), 2.38 (1H, dd, J=10.9, 2.1 Hz), 2.59 (1H, dd, J=14.2, 3.4 Hz), 2.72 (1H, ddd, J=11.7, 4.2, 4.2 Hz), 2.81 (1H, brs, disappeared on addition of D2O), 2.84 (1H, dd, J=11.2, 2.7 Hz), 3.83 (3H, s), 4.23 (1H, brs), 4.71 (1H, brs, disappeared on addition of D2O), 6.63 (1H, d, J=7.8 Hz), 6.90 (1H, t, J=7.6 Hz), 6.95 (1H, d, J=8.8 Hz), 7.13 (1H, td, J=7.7, 1.2 Hz), 7.39 (1H, d, J=7.1 Hz), 7.94 (1H, d, J=2.4 Hz), 8.09 (1H, d, J=8.8, 2.4 Hz). 13C-NMR (CDCl3) δ: 175.62 (C), 162.15 (C), 147.60 (C), 142.87 (C), 136.19 (C), 129.03 (CH), 128.39 (C), 125.57 (CH), 122.57 (CH), 122.55 (CH), 120.44 (CH), 119.41 (CH), 110.92 (CH), 110.21 (C), 109.68 (CH), 66.82 (CH), 66.27 (CH), 61.63 (CH2), 54.82 (C), 52.30 (CH), 52.04 (CH3), 51.20 (CH3), 39.95 (CH), 36.24 (CH), 31.78 (CH2), 31.29 (CH2), 29.40 (CH2), 23.04 (CH2). HR–MS m/z: Calcd for C27H29N3O6: 491.2057. Found: 491.2058. [α]23D +59.0° (c=0.370, MeOH).
Scheme 16 New yohimbine derivatives (3)
7α-(2-Methoxy-5-nitrophenyl)-7H-yohimbine (143) from 141 — Excess CH2N2 in Et2O was added to a solution of 141 (165.0 mg, 0.34 mmol) in MeOH (5 mL) and the whole was stirred at rt for 30 min. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 143 (142.8 mg, 84%) and unreacted 141 (26.7 mg, 16%). 143: mp 171.5—172 °C (yellow prisms, recrystallized from CHCl3–hexane). IR (KBr): 3392, 1734, 1589, 1344, 1269 cm-1. 1H-NMR (CDCl3) δ: 1.33—1.85 (5H, m), 1.87—2.04 (2H, m), 2.05—2.32 (2H, m), 2.41—2.71 (3H, m, 1H disappeared on addition of D2O), 2.87—3.24 (4H, m), 3.55 (3H, brs), 3.77 (3H, s), 4.67 (1H,, brs), 6.86 (1H, d, J=10.0 Hz), 7.05 (1H, d, J=8.3 Hz), 7.15 (1H, t, J=8.3 Hz), 7.35 (1H, ddd, J=8.3, 8.3, 1.7 Hz), 7.73 (1H, d, J=8.3 Hz), 8.22 (1H, dd, J=10.0, 3.3 Hz), 8.51 (1H, brs). 13C-NMR (CDCl3) δ: 184.96 (C), 175.18 (C), 162.15 (C), 155.02 (C), 142.95 (C), 141.78 (C), 128.12 (CH), 125.54 (CH), 125.09 (CH), 123.85 (CH), 121.76 (CH), 120.95 (CH), 111.37 (CH), 67.06 (CH), 61.40 (C), 61.26 (CH2), 59.09 (C), 55.85 (CH3), 52.26 (CH3), 51.80 (CH), 51.13 (CH2), 50.66 (CH), 40.11 (CH), 36.59 (CH2), 35.99 (CH), 31.45 (CH2), 31.41 (CH2), 23.06 (CH2). HR–MS m/z: Calcd for C28H31N3O6: 505.2213. Found: 505.2206. [α]29D +289° (c=0.141, DMF).
7α-(5-Amino-2-methoxyphenyl)-7H-yohimbine (144) from 143 — Tin powder (75.6 mg, 0.64 mmol) was added to a solution of 143 (30.9 mg, 0.06 mmol) in MeOH–8% HCl (3:1, v/v, 6.0 mL) and the whole was stirred at rt for 40 min. The resulting solution was made alkaline with 6% NaOH under ice cooling, and the whole was extracted with CHCl3–MeOH–28% aq. NH3 (46:3:0.3, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH–28% aq. NH3 (46:2:0.2, v/v) to give 144 (26.7 mg, 90%). 144: mp 262—264 °C (decomp., yellow prisms, recrystallized from EtOAc–MeOH). IR (KBr): 3452, 3336, 3234, 2933, 1745, 1500, 1236, 1144, 1109, 1018 cm-1. 1H-NMR (DMSO-d6) δ: 1.04 (1H, t, J=13.1 Hz), 1.61—1.43 (3H, m), 1.52—1.65 (2H, m), 1.70—1.79 (2H, m), 1.88 (1H, t, J=10.4 Hz), 2.15 (1H, brd, J=12.7 Hz), 2.25—2.33 (2H, m), 2.38—2.54 (1H, m), 2.65 (1H brd, J=11.5 Hz), 2.76 (1H, dd, J=10.9, 3.1 Hz), 2.83—3.11 [1H, m, on addition of D2O, it changed to 2.89 (1H, brd, J=10.3 Hz)], 3.27 (3H, s), 3.64 (3H, s), 4.12 (1H, s), 4.20 (1H, brd, J=4.4 Hz, disappeared on addition of D2O), 4.55 (2H, brs, disappeared on addition of D2O), 6.48 (1H, dd, J=8.5, 2.7 Hz), 6.63 (1H, d, J=8.5 Hz), 6.84 (1H, brs), 7.06 (1H, t, J=7.4 Hz), 7.14 (1H, d, J=7.4 Hz), 7.23 (1H, t, J=7.4 Hz), 7.49 (1H, d, J=7.4 Hz). MS m/z: 475 (M+). Anal. Calcd for C28H33N3O4·1/2H2O: C, 69.40; H, 7.07; N, 8.67. Found: C, 69.60; H, 7.10; N, 8.60. [α]30D +255° (c=0.210, MeOH).
17α-Acetoxy- (145b) and 7α-(5-acetylamino-2-methoxyphenyl)-7H-yohimbine (145a) from 144 — [Entry 1] Ac2O (1.0 mL) was added to a solution of 144 (33.5 mg, 0.07 mmol) in pyridine (2.0 mL) and the whole was stirred at rt for 40 min. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH–30% aq. NH3 (46:3:0.3, v/v) to give 145a (30.3 mg, 80%) and 145b (2.6 mg, 7%).
[Entry 2] Ac2O (1.0 mL) was added to a solution of 144 (28.4 mg, 0.06 mmol) in pyridine (2.0 mL) and the whole was stirred at rt for 2 days. The same work-up and purification as Entry 1 afforded 145b (31.8 mg, 94%). 145a: mp 180—182 °C (colorless powder, recrystallized from EtOAc–hexane). IR (KBr): 3434, 2927, 1731, 1668, 1608, 1548, 1500, 1244, 1146, 1024 cm-1. 1H-NMR (DMSO-d6) δ: 1.09 (1H, td, J=3.3, 13.2 Hz), 1.17—1.41 (3H, m), 1.50—1.58 (1H, m), 1.62 (1H, q, J=11.9 Hz), 1.70—1.78 (2H, m), 1.84 (1H, t, J=10.8 Hz), 2.03 (3H, s), 2.14—2.19 (1H, m), 2.26—2.30 (2H, m), 2.43—2.53 (1H, m), 2.69—2.64 (1H, m), 2.76 (1H, dd, J=10.8, 3.5 Hz), 2.83 (1H, brd, J=10.8 Hz), 3.42 (3H, s), 3.64 (3H, s), 4.10—4.14 (1H, m), 4.21 (1H, d, J=4.6 Hz, disappeared on addition of D2O), 6.84 (1H, d, J=8.8 Hz), 7.07 (1H, td, J=1.2, 7.5 Hz), 7.13 (1H, brd, J=7.5 Hz), 7.25 (1H, td, J=1.2, 7.5 Hz), 7.46 (1H, dd, J=7.8, 2.3 Hz), 7.51 (1H, brd, J=7.5 Hz), 7.72 (1H, brs), 9.56 (1H, s, disappeared on addition of D2O). MS m/z: 517 (M+). Anal. Calcd for C30H35N3O5·H2O: C, 67.27; H, 6.96; N, 7.84. Found: C, 67.29; H, 6.92; N, 7.54. [α]28D +249° (c=0.411, MeOH). 145b: mp 218—220 °C (orange prisms, recrystallized from EtOAc–hexane). IR (KBr): 3430, 2927, 1739, 1664, 1610, 1500, 1244, 1025 cm-1. 1H-NMR (DMSO-d6) δ: 1.05—1.14 (1H, m), 1.19—1.45 (3H, m), 1.63—1.75 (3H, m), 1.79—1.86 (1H, m), 1.87—1.93 (1H, m), 1.94 (3H, s), 2.03 (3H, s), 2.06—2.12 (1H, m), 2.30 (1H, t, J=12.9 Hz), 2.38—2.55 (2H, m), 2.69 (1H, d, J=11.7 Hz), 2.80 (1H, d, J=8.3 Hz), 2.82—3.15 (1H, m), 3.40 (3H, s), 3.63 (3H, s), 5.28 (1H, brd, J=2.7 Hz), 6.84 (1H, d, J=8.8 Hz), 7.08 (1H, t, J=7.7 Hz), 7.13 (1H, brd, J=7.7 Hz), 7.25 (1H, t, J=7.7 Hz), 7.47 (1H, dd, J=8.8, 2.1 Hz), 7.52 (1H, d, J=7.7 Hz), 7.72 (1H, brs), 9.58 (1H, brs, disappeared on addition of D2O). MS m/z: 559 (M+). Anal. Calcd for C32H37N3O6·1/2H2O: C, 67.59; H, 6.74; N, 7.39. Found: C, 67.38; H, 6.57; N, 7.42. [α]24D +215° (c=0.204, CHCl3).
7α-(5-Benzoylamino-2-methoxyphenyl)-7H-yohimbine (146) from 144 — Benzoyl chloride (0.02 mL, 0.16 mmol) was added to a solution of 144 (37.0 mg, 0.08 mmol) in pyridine (5.0 mL) and the mixture was stirred at rt for 1 h. After adding H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (95:5, v/v) to give 146 (38.8 mg, 83%). 146: mp 191—193 °C (decomp., colorless prisms, recrystallized from CHCl3–hexane). IR (KBr): 3435, 2927, 1732, 1649, 1500, 1111, 1026 cm-1. 1H-NMR (DMSO-d6) δ: 1.13 (1H, td, J=13.2, 3.4 Hz), 1.19—1.59 (4H, m), 1.66 (1H, q, J=11.8 Hz), 1.73—1.92 (2H, m), 2.19 (1H, d, J=12.7 Hz), 2.25—2.37 (2H, m), 2.39—2.55 (1H, m), 2.70 (1H, brd, J=11.8 Hz), 2.78 (1H, dd, J=10.9, 3.1 Hz), 2.84—3.00 (1H, m), 3.14 (1H, brd, J=13.7 Hz), 3.44 (3H, brs), 3.65 (3H, s), 4.09 (1H, brs, disappeared on addition of D2O), 4.13 (1H, brs), 6.90 (1H, d, J=8.8 Hz), 7.08 (1H, td, J=7.5, 1.1 Hz), 7.17 (1H, brd, J=7.5 Hz), 7.25 (1H, td, J=7.5, 1.1 Hz), 7.48—7.58 (4H, m), 7.68 (1H, dd, J=8.8, 2.4 Hz), 7.90—8.00 (3H, m), 9.86 (1H, brs, disappeared on addition of D2O). MS m/z: 579 (M+). Anal. Calcd for C35H37N3O5·H2O: C, 70.33; H, 6.58; N, 7.03. Found: C, 70.30; H, 6.36; N, 7.16. [α]28D +239° (c=0.232, MeOH).
7α-(5-Benzenesulfonylamino-2-methoxyphenyl)-7H-yohimbine (147) from 144 — Benzene sulfonyl chloride (0.03 mL, 0.24 mmol) was added to a solution of 144 (54.8 mg, 0.12 mmol) in pyridine (6.0 mL) and the mixture was stirred at rt for 20 min. After adding H2O, the whole was extracted with CHCl3–MeOH (97:3, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (95:5, v/v) to give 147 (68.0 mg, 95%). 147: mp 277—279 °C (decomp., colorless prisms, recrystallized from MeOH). IR (KBr): 3388, 2924, 1738, 1500, 1460, 1441, 1163 cm-1. 1H-NMR (DMSO-d6) δ: 1.03 (1H, td, J=13.4, 3.2 Hz), 1.18—1.32 (2H, m), 1.40 (1H, qd, J=12.5, 3.2 Hz), 1.50—1.64 (2H, m), 1.70—1.82 (2H, m), 2.01 (1H, t, J=11.8 Hz), 2.13 (1H, td, J=12.5, 3.2 Hz), 2.26 (1H, dd, J=11.8, 2.4 Hz), 2.45—2.52 (1H, m), 2.56 (1H, d, J=11.8 Hz), 2.67 (1H, d, J=11.0 Hz), 2.73 (1H, dd, J=11.0, 3.2 Hz), 2.80—3.00 (1H, m), 3.41 (3H, s), 3.65 (3H, s), 4.13 (2H, brs, disappeared 1H, on addition of D2O), 6.80 (1H, d, J=8.5 Hz), 6.92 (1H, brd, J=7.6 Hz), 7.00 (1H, dd, J=8.5, 2.4 Hz), 7.04 (1H, t, J=7.6 Hz), 7.13 (1H, brs), 7.23 (1H, td, J=7.6, 1.2 Hz), 7.48 (1H, t, J=7.6 Hz), 7.53 (2H, t, J=7.6 Hz), 7.60 (1H, tt, J=7.6, 1.7 Hz), 7.73 (2H, dd, J=7.6, 1.7 Hz), 9.55 (1H, brs, disappeared on addition of D2O). MS m/z: 615 (M+). Anal. Calcd for C34H37N3O6S·1/2H2O: C, 65.84; H, 6.09; N, 6.77. Found: C, 65.91; H, 6.12; N, 6.75. [α]25D +223° (c=0.200, MeOH).
7α-(5-Mesylamino-2-methoxyphenyl)-7H-yohimbine (148) from 144 — MsCl (0.014 mL, 0.18 mmol) was added to a solution of 144 (42.3 mg, 0.09 mmol) in pyridine (5.0 mL) and the whole was stirred at rt for 40 min. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with EtOAc–MeOH (93:7, v/v) to give 148 (48.1 mg, 95%). 148: mp 183.0—185.0 °C (colorless fine prisms, recrystallized from MeOH). IR (KBr): 3438, 1732, 1498, 1327, 1146 cm-1. 1H-NMR (DMSO-d6) δ: 1.12 (1H, td, J=13.3, 3.4 Hz), 1.18—1.34 (2H, m), 1.37 (1H, qd, J=12.3, 3.4 Hz), 1.50—1.58 (1H, m), 1.65 (1H, q, J=11. 5 Hz), 1.71—1.80 (2H, m), 1.83—1.91 (1H, m), 2.16 (1H, d, J=12.3Hz), 2.28 (1H, dd, J=11.5, 2.0 Hz), 2.43—2.52 (1H, m), 2.65—2.71 (1H, m), 2.77 (1H, brd, J=11.5 Hz), 2.80—3.00 [1H, m, on addition of D2O, it changed to 2.80—2.90 (1H, m)], 2.80—3.00 [3H, m, changed to 2.94 (3H, s) on addition of D2O], 3.11 (1H, brd, J=13.3 Hz), 3.44 (3H, s), 3.65 (3H, s), 4.08 (1H, brs, disappeared on addition of D2O), 4.13 (1H, brs), 6.89 (1H, d, J=8.9 Hz), 7.07 (1H, t, J=7.2 Hz), 7.13 (1H, d, J=7.2 Hz), 7.14 (1H, dd, J=8.9, 2.7 Hz), 7.25 (1H, td, J=7.2, 1.2 Hz), 7.42 (1H, brs), 7.52 (1H, d, J=7.2 Hz), 9.05 (1H, brs, disappeared on addition of D2O). 13C-NMR (CDCl3) δ: 186.12 (C), 175.25 (C), 155.61 (C), 154.80 (C), 143.70 (C), 129.97 (C), 128.19 (C), 127.80 (CH), 125.36 (CH), 123.68 (CH), 123.10 (CH), 121.98 (CH), 120.74 (CH), 112.92 (CH), 67.15 (CH), 61.52 (CH), 61.35 (CH2), 59.22 (C), 55.59 (CH3), 52.27 (CH), 51.86 (CH3), 51.34 (CH2), 40.08 (CH), 39.00 (CH3), 36.89 (CH2), 36.04 (CH), 31.50 (CH2), 31.40 (CH2), 23.08 (CH2). Anal. Calcd for C29H35N3O6S·3/4H2O: C, 61.41; H, 6.49; N, 7.41. Found: C, 61.48; H, 6.39; N, 7.49. [α]25D +204° (c=0.250, CHCl3).
(2α,7α)- (150) and (2β,7β)-24,26-Dinitrobenzofurano[2,3-n]yohimbine (151) from 53b — A solution of K2CO3 (76.7 mg, 0.55 mmol) and 2,4-dinitrofluorobenzene (62.8 mg, 0.34 mmol) in DMF (1.0 mL) was added to a solution of 53b (102.8 mg, 0.28 mmol) in DMF (4.0 mL) and the whole was stirred at –8 °C for 70 min. After addition of H2O, the whole was extracted with EtOAc. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (99:1, v/v) to give 150 (106.0 mg, 71%) and 151 (35.2 mg, 24%). 150: yellow viscous oil. IR (film): 3481, 3286, 2934, 1701, 1622, 1606, 1535, 1523, 1473, 1437, 1375, 910 cm-1. 1H-NMR (CDCl3) δ: 1.34—1.39 (1H, m), 1.44—1.54 (4H, m), 1.88—1.91 (5H, m), 2.15 (1H, dt, J=12.4, 2.4 Hz), 2.22 (1H, dd, J=11.5, 2.4 Hz), 2.33 (1H, dd, J=11.5, 1.7 Hz), 2.67—2.70 (1H, m), 2.75—2.78 (1H, m), 2.87 (1H, dd, J=11.5, 1.7 Hz), 3.11 (1H, disappeared on addition of D2O), 3.84 (3H, s), 4.22 (1H, d, J=0.7 Hz), 5.41 (1H, s, disappeared on addition of D2O), 6.84 (1H, dd, J=8.0, 0.7 Hz), 6.85 (1H, td, J=8.0, 0.7 Hz), 6.99 (1H, dd, J=8.0, 0.7 Hz), 7.15 (1H, dd, J=8.0, 0.7 Hz), 8.48 (1H, d, J=2.2 Hz), 8.89 (1H, d, J=2.2 Hz). 13C-NMR (CDCl3) δ: 175.17 (C), 158.84 (C), 144.70 (C), 141.06 (C), 138.25 (C), 131.69 (C), 131.63 (C), 129.17 (CH), 122.59 (CH), 122.35 (CH), 121.76 (CH), 121.58 (CH), 116.78 (C), 115.15 (CH), 66.50 (CH), 64.76 (CH), 61.39 (CH2), 54.86 (C), 52.34 (CH3), 52.17 (CH), 51.44 (CH2), 40.08 (CH), 36.07 (CH), 32.01 (CH2), 31.06 (CH2), 29.73 (CH2), 22.97 (CH2). HR–MS m/z: Calcd for C27H28N4O8: 536.1907. Found: 536.1903. [α]28D +258° (c=0.106, MeOH). 151: yellow viscous oil. IR (film): 3556, 3381, 2935, 1716, 1619, 1608, 1535, 1336, 738 cm-1. 1H-NMR (CDCl3) δ: 1.25—1.60 (5H, m), 1.81—2.00 (5H, m), 2.16—2.22 (2H, m), 2.39—2.50 (2H, m), 2.64—2.67 (1H, m), 2.69 (1H, brs, disappeared on addition of D2O), 2.77—2.81 (2H, m), 3.82 (3H, s), 5.14 (1H, s, disappeared on addition of D2O), 6.67 (1H, d, J=7.8 Hz), 6.89 (1H, td, J=7.8, 1.0 Hz), 7.14 (1H, td, J=7.8, 1.0 Hz), 7.31 (1H, d, J=7.8 Hz), 8.21 (1H, dd, J=2.4, 1.5 Hz), 8.85 (1H, dd, J=2.4, 1.5 Hz). 13C-NMR (CDCl3) δ: 175.44 (C), 157.82 (C), 147.04 (C), 141.32 (C), 141.03 (C), 131.82 (C), 129.58 (CH), 128.37 (C), 122.78 (CH), 122.59 (CH), 121.30 (CH), 120.77 (CH), 114.19 (C), 109.92 (CH), 66.94 (CH), 64.13 (CH), 61.40 (CH2), 54.80 (C), 52.06 (CH3), 52.04 (CH), 50.00 (CH2), 39.38 (CH), 35.70 (CH), 31.25 (CH2), 30.22 (CH2), 29.07 (CH2), 22.89 (CH2). HR–MS m/z: Calcd for C27H28N4O8: 536.1907. Found: 536.1902. [α]28D +121° (c=0.101, MeOH).
(2α,7α)-1-Acetyl- (152) and -17-Acetoxy-24,26-dinitrobenzofurano[2,3-n]yohimbine (153) from 150 — Ac2O (1.0 mL) was added to a solution of 150 (40.4 mg, 0.08 mmol) in pyridine (2.0 mL) and the whole was stirred at 60 °C for 4 h. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with EtOAc–hexane (2:1, v/v) to give 153 (26.1 mg, 59%) and 152 (2 mg, 4%). 153: mp 200—201 °C (decomp., yellow powder, recrystallized from EtOAc–hexane). IR (KBr): 3423, 1749, 1728, 1599, 1572, 1543, 1373, 1355 cm-1. 1H-NMR (CDCl3) δ: 1.23—1.58 (5H, m), 1.63 (1H, m), 1.85—2.07 (4H, m), 2.02 (3H, s), 2.26—2.33 (1H, m), 2.36—2.45 (2H, m), 2.65—2.81 (2H, m), 2.90 (1H, dd, J=11.1, 3.1 Hz), 3.71 (3H, s), 5.42 (1H, brs, disappeared on addition of D2O), 5.43 (1H, d, J=2.4 Hz), 6.83 (1H, d, J=7.6 Hz), 6.85 (1H, t, J=7.6 Hz), 6.99 (1H, d, J=7.6 Hz), 7.15 (1H, t, J=7.6 Hz), 8.49 (1H, d, J=2.3 Hz), 8.90 (1H, d, J=2.3 Hz). 13C-NMR (CDCl3) δ: 171.65 (C), 169.99 (C), 158.87 (C), 144.76 (C), 141.02 (C), 138.31 (C), 131.71 (C), 131.52 (C), 129.22 (CH), 122.51 (CH), 122.33 (CH), 121.68 (CH), 121.53 (CH), 116.77 (C), 111.14 (CH), 69.26 (CH), 64.79 (CH), 61.25 (CH2), 54.83 (C), 52.01 (CH3), 51.42 (CH2), 51.36 (CH), 39.45 (CH), 36.16 (CH), 31.96 (CH2), 29.70 (CH2), 29.60 (CH2), 23.68 (CH2), 20.94 (CH3). MS m/z: 578 (M+). Anal. Calcd for C29H30N4O9·1/2H2O: C, 59.28; H, 5.32; N, 9.54. Found: C, 59.19; H, 5.33; N, 9.23. [α]24D +148° (c=0.220, CHCl3). 153: yellow viscous oil. IR (film): 1743, 1673, 1622, 1533, 1373, 1338 cm-1. 1H-NMR (CDCl3) δ: 1.02 (1H, dd, J=24.7, 12.7 Hz), 1.20—1.36 (2H, m), 1.37—1.47 (1H, m), 1.49—1.66 (2H, m), 1.94—2.03 (2H, m), 2.06 (3H, s), 2.21—2.27 (2H, m), 2.56—2.69 (2H, m), 2.67 (3H, s), 2.74—2.89 (1H, m), 2.83—2.91 (2H, m), 3.63 (3H, s), 4.25 (1H, dd, J=12.8, 2.1 Hz), 5.37 (1H, d, J=2.7 Hz), 7.19 (1H, t, J=7.6 Hz), 7.29 (1H, t, J=7.6 Hz), 7.42 (1H, d, J=7.8 Hz), 8.14 (1H, d, J=7.8 Hz), 8.18 (1H, d, J=2.3 Hz), 8.91 (1H, d, J=2.3 Hz). 13C-NMR (CDCl3) δ: 171.30 (C), 170.36 (C), 169.96 (C), 155.86 (C), 143.10 (C), 142.45 (C), 140.51 (C), 132.36 (C), 130.05 (CH), 129.00 (C), 125.00 (CH), 123.03 (CH), 121.57 (CH), 121.56 (CH), 117.45 (CH), 111.74 (C), 69.12 (CH), 62.77 (CH), 60.35 (CH2), 54.33 (C), 51.90 (CH3), 50.70 (CH), 41.10 (CH2), 38.71 (CH), 33.30 (CH), 32.34 (CH2), 29.74 (CH2), 25.51 (CH2), 24.52 (CH3), 23.11 (CH2), 21.02 (CH3). HR–MS m/z: Calcd for C31H32N4O10: 620.2118. Found: 620.2124. [α]31D –38.2° (c=0.192, CHCl3).
Scheme 17.
Synthesis of new yohimbine derivatives (4).
Scheme 17.
Synthesis of new yohimbine derivatives (4).
7α-(2-Methoxy-3,5-dinitrophenyl)-7H-yohimbine (154) from 150 — Excess CH2N2 in Et2O was added to a solution of 150 (5.0 mg, 0.008 mmol) in MeOH (0.5 mL) and the whole was stirred at rt for 30 min. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (95:5, v/v) to give 154 (5.0 mg, 98%). 154: yellow oil. IR (KBr): 2927, 1736, 1595, 1541, 1456, 1342, 1267, 1207 cm-1. 1H-NMR (CDCl3) δ: 1.13—1.36 (5H, m), 1.51—1.63 (2H, m), 1.72—1.76 (2H, m), 1.91 (1H, d, J=10.5 Hz), 2.12 (1H, d, J=10.8 Hz), 2.30 (1H, dd, J=10.5, 2.4 Hz), 2.33 (1H, t, J=10.0 Hz), 2.76 (2H, dd, J=10.5, 2.4 Hz), 2.84 (1H, brd, J=10.0 Hz), 3.02 (3H, brs), 3.62 (3H, s), 4.10 (1H, brt, J=2.4 Hz), 4.55 (1H, d, J=2.8 Hz, disappeared on addition of D2O), 7.18 (1H, t, J=7.6 Hz), 7.24 (1H, d, J=7.6 Hz), 7.36 (1H, t, J=7.6 Hz), 7.64 (1H, d, J=7.6 Hz), 8.75 (1H, d, J=2.4 Hz), 8.92 (1H, brs). 13C-NMR (CD3OD) δ: 186.15 C), 175.24 (C), 157.33 (C), 155.44 (C), 144.48 (C), 144.38 (C), 144.33 (C), 137.81 (C), 130.01 (CH), 128.51 (CH), 127.69 (CH), 123.72 (CH), 122.37 (CH), 121.82 (CH), 68.52 (CH), 62.92 (CH), 62.65 (CH3), 62.04 (CH2), 60.94 (C), 53.94 (CH), 52.09 (CH3), 51.85 (CH2), 41.22 (CH), 37.71 (CH2), 36.54 (CH), 33.40 (CH2), 32.56 (CH2), 24.18 (CH2). HR–MS m/z: Calcd for C28H30N4O8: 550.2063. Found: 550.2059. [α]26D +258° (c=0.101, MeOH).
(
S)-(4aα,9aα)- (159) and (
S)-(4aβ,9aβ)-3β-Methoxycarbonyl-11,13-dinitro-1,2,3,4-tetrahydro-9
H- benzofurano[2,3-
m]-β-carboline (160) from 155[
77] — A solution of K
2CO
3 (168.5 mg, 1.2 mmol) and 2,4-dinitrofluorobenzene (84.6 mg, 0.45 mmol) in DMF (3.0 mL) was added to a solution of 155 (100.0 mg, 0.41 mmol) in DMF (7.0 mL) and the whole was stirred at 0 °C for 30 min. After addition of H
2O, the whole was extracted with EtOAc. The extract was washed with brine, dried over Na
2SO
4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO
2 with EtOAc–hexane–Et
2O (1:1:1, v/v) to give 159 (77.5 mg, 46%), 160 (58.0 mg, 35%), and unreacted 155 (7.1 mg, 7%). 159: mp 158.0—160.0 °C (yellow needles, recrystallized from Et
2O–hexane). IR (KBr): 3392, 1736, 1620, 1608, 1533, 1473, 1437, 1336 cm
-1.
1H-NMR (CDCl
3) δ: 2.02 (1H, dd,
J=14.4, 11.4 Hz), 2.04 (1H, brs, disappeared on addition of D
2O), 3.02 (1H, dd,
J=14.4, 4.7 Hz), 3.23 (1H, d,
J=14.2 Hz), 3.38 (1H, dd,
J=11.4, 4.4 Hz), 3.69 (1H, d,
J=14.2 Hz), 3.71 (3H, s), 5.41 (1H, brs, disappeared on addition of D
2O), 6.80 (1H, d,
J=7.8 Hz), 6.84 (1H, t,
J=7.8 Hz), 7.12 (1H, d,
J=7.8 Hz), 7.16 (1H, t,
J=7.8 Hz), 8.51 (1H, dd,
J=2.0, 1.0 Hz), 8.90 (1H, dd,
J=2.0, 1.0 Hz).
13C-NMR (CDCl
3) δ: 172.66 (C), 159.04 (C), 145.78 (C), 141.04 (C), 138.00 (C), 131.25 (C), 130.33 (C), 129.61 (CH), 122.78 (CH), 122.71 (CH), 121.88 (CH), 121.24 (CH), 113.33 (C), 110.54 (CH), 54.69 (C), 52.93 (CH), 52.47 (CH
3), 48.65 (CH
2), 34.50 (CH
2).
Anal. Calcd for C
19H
16N
4O
7·1/8H
2O: C, 55.03; H, 3.95; N, 13.41. Found: C, 55.13; H, 3.94; N, 13.19. [α]
28D +206° (c=0.111, MeOH). 160: yellow viscose oil. IR (film): 3402, 1732, 1618, 1608, 1533, 1473, 1433, 1333, 1255, 744 cm
-1.
1H-NMR (CDCl
3) δ: 2.21 (1H, brs, disappeared on addition of D
2O), 2.55 (1H, dd,
J=14.6, 6.6 Hz), 2.83 (1H, dd,
J=14.6, 6.1 Hz), 3.39 (1H, d,
J=14.4 Hz), 3.57 (1H, d,
J=14.4 Hz), 3.60 (3H, s), 3.69 (1H, t,
J=6.4 Hz), 5.17 (1H, s, disappeared on addition of D
2O), 6.75 (1H, d,
J=8.1 Hz), 6.89 (1H, td,
J=8.1, 0.7 Hz), 7.17 (1H, td,
J=8.1, 0.7 Hz), 7.24 (1H, d,
J=8.1 Hz), 8.36 (1H, d,
J=2.2 Hz), 8.87 (1H, d,
J=2.2 Hz).
13C-NMR (CDCl
3) δ: 173.22 (C), 158.70 (C), 146.70 (C), 141.03 (C), 138.80 (C), 131.45 (C), 129.82 (CH), 129.24 (C), 123.57 (CH), 122.78 (CH), 121.82 (CH), 121.07 (CH), 113.09 (C), 110.14 (CH), 54.49 (C), 52.39 (CH
3), 51.51 (CH), 46.57 (CH
2), 32.30 (CH
2). HR–MS
m/z: Calcd for C
19H
16N
4O
7: 412.1019. Found: 412.1024. [α]
27D –171° (c=0.100, MeOH).
(S)-(4aα,9aα)-3β-Methoxycarbonyl-Nb-Methyl- (162), -Na-methyl- (161), and -Na,Nb-dimethyl- 11,13-dinitro-1,2,3,4-tetrahydro-9H-benzofurano[2,3-m]-β-carboline (163) from 159 — Excess CH2N2 in Et2O was added to a solution of 159 (33.0 mg, 0.08 mmol) in MeOH (1.5 mL) and the whole was stirred at rt for 145 min. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 162 (5.4 mg, 16%), 161 (9.2 mg, 27%), 163 (6.4 mg, 18%), and unreacted 159 (5.4 mg, 16%). 162: pale viscose oil. IR (film): 3380, 1743, 1621, 1610, 1373, 1336 cm-1. 1H-NMR (DMSO-d6:D2O=5:1, v/v) δ: 2.26 (1H, dd, J=15.0, 11.7 Hz), 2.37 (3H, s), 2.86 (1H, dd, J=15.0, 5.0 Hz), 3.02 (1H, d, J=14.2 Hz), 3.11 (1H, dd, J=11.7, 5.0 Hz), 3.70 (3H, s), 3.70 (1H, m), 4.52—4.81 (1H, brs, disappeared on addition of D2O), 6.77 (1H, d, J=8.3 Hz), 6.85 (1H, t, J=8.3 Hz), 7.13 (1H, d, J=8.3 Hz), 7,16 (1H, t, J=8.3 Hz), 8.44 (1H, d, J=2.5 Hz), 8.89 (1H, d, J=2.5 Hz). 13C-NMR (CDCl3) δ: 172.30 (C), 159.35 (C), 146.16 (C), 140.95 (C), 138.36 (C), 130.91 (C), 129.68 (CH), 129.63 (C), 122.86 (CH), 122.36 (CH), 121.71 (CH), 121.07 (CH), 114.78 (C), 110.50 (CH), 59.85 (CH), 55.79 (CH2), 54.19 (C), 52.04 (CH3), 42.21 (CH3), 33.77 (CH2). HR–MS (FAB+) m/z: Calcd for C20H18N4O7: 426.1175. Found: 427.1257. [α]31D +154° (c=0.234, CHCl3). 161: yellow oil. IR (film): 2952, 1736, 1620, 1606, 1489, 1435, 1338, 1246, 756 cm-1. 1H-NMR (CDCl3) δ: 2.01 (1H, brs, disappeared on addition of D2O), 2.02 (1H, dd, J=14.4, 11.2 Hz), 2.99 (1H, dd, J=14.4, 5.4 Hz), 3.14 (3H, s), 3.24 (1H, d, J=14.4 Hz), 3.42 (1H, dd, J=11.2, 5.4 Hz), 3.71 (3H, s), 3.75 (1H, d, J=14.4 Hz), 6.67 (1H, d, J=7.8 Hz), 6.83 (1H, d, J=7.8 Hz), 7.10 (1H, d, J=7.8 Hz), 7.21 (1H, t, J=7.8 Hz), 8.47 (1H, d, J=2.4 Hz), 8.87 (1H, d, J=2.4 Hz). 13C-NMR (CDCl3) δ: 172.98 (C), 159.40 (C), 148.41 (C), 140.81 (C), 138.45 (C), 131.17 (C), 129.85 (C), 129.71 (CH), 122.36 (CH), 122.35 (CH), 121.71 (CH), 120.35 (CH), 116.24 (C), 108.17 (CH), 54.26 (C), 52.60 (CH), 52.41 (CH3), 46.12 (CH2), 33.57 (CH2), 28.55 (CH3). HR–MS m/z: Calcd for C20H18N4O7: 426.1175. Found: 426.1165. [α]26D +42.9° (c=0.107, MeOH). 163: yellow oil. IR (film): 2952, 1736, 1620, 1608, 1533, 1489, 1435, 1335, 1255, 1007, 744, 594 cm-1. 1H-NMR (CDCl3) δ: 2.14 (1H, dd, J=14.7, 11.0 Hz), 2.36 (3H, s), 2.88 (1H, dd, J=14.7, 6.1 Hz), 3.10 (1H, d, J=14.0 Hz), 3.12 (3H, s), 3.21 (1H, dd, J=11.0, 6.1 Hz), 3.70 (3H, s), 3.77 (1H, d, J=14.0 Hz), 6.61 (1H, d, J=7.8 Hz), 6.79 (1H, t, J=7.8 Hz), 7.10 (1H, d, J=7.8 Hz), 7.19 (1H, t, J=7.8 Hz), 8.39 (1H, dd, J=2.2, 0.7 Hz), 8.85 (1H, dd, J=2.2, 0.7 Hz). 13C-NMR (CDCl3) δ: 172.50 (C), 159.95 (C), 148.56 (C), 140.60 (C), 138.88(C), 130.61(C), 129.68 (CH), 129.36 (C), 122.54 (CH), 121.75 (CH), 121.43 (CH), 119.95 (CH), 117.92 (C), 107.61 (CH), 58.98 (CH), 53.64 (C), 53.56 (CH2), 51.83 (CH3), 41.71 (CH3), 33.04 (CH2), 28.53 (CH3). HR–MS m/z: Calcd for C21H20N4O7: 440.1332. Found: 440.1334. [α]27D +18.8° (c=0.110, MeOH).
Scheme 18.
Synthesis of new β-carboline derivatives (1).
Scheme 18.
Synthesis of new β-carboline derivatives (1).
162 from 159 — Me2SO4 (12.3 mg, 0.09 mmol) in MeOH (0.5 mL) was added to a solution of 159 (11.1 mg, 0.027 mmol) in MeOH (0.5 mL) and K2CO3 (13.4 mg, 13.0 mmol) at 0°C and the mixture was stirred at rt for 19 h. After addition of H2O, the whole was extracted with EtOAc. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (97:3, v/v) to give 162 (6.3 mg, 55%) and unreacted 159 (1.9 mg, 17%).
(S)-(4aα,9aα)-Nb-Acetyl- (164a) and -Na,Nb-diacetyl-3β-methoxycarbonyl-11,13-dinitro-1,2,3,4- tetrahydro-9H-benzofurano[2,3-m]-β-carboline (164b) from 159 — Ac2O (1.0 mL) was added to a solution of 159 (40.0 mg, 0.09 mmol) in pyridine (2.0 mL) and the whole was stirred at rt for 3 h. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 164a (30.8 mg, 59%) and 164b (14.6 mg, 30%). 164a: mp 198—200 °C (yellow prisms, recrystallized from EtOAc). IR (KBr): 3292, 1647, 1620, 1610, 1533, 1336 cm-1. 1H-NMR (CDCl3) δ: 2.19 (3H, s), 2.24 (1H, dd, J=14.8, 12.7 Hz), 3.14 (1H, dd, J=12.6, 6.7 Hz), 3.72 (3H, s), 3.88 (1H, d, J=15.1 Hz), 4.42 (1H, dd, J=12.6, 6.7 Hz), 4.53 (1H, d, J=15.1 Hz), 5.27 (1H, s, disappeared on addition of D2O), 6.74 (1H, d, J=8.1 Hz), 6.89 (1H, t, J=7.6 Hz), 7.18 (1H, t, J=7.8 Hz), 7.25 (1H, d, J=8.1 Hz), 8.46 (1H, d, J=2.2 Hz), 8.86 (1H, d, J=2.2 Hz). 13C-NMR (CDCl3) δ: 170.97 (C), 170.84 (C), 158.50 (C), 146.28 (C), 141.53 (C), 136.52 (C), 130.89 (C), 130.18 (CH), 128.16 (C), 123.36 (CH), 122.85 (CH), 122.17 (CH), 121.25 (CH), 113.31 (C), 110.26 (CH), 55.88 (C), 52.63 (CH3), 50.98 (CH), 48.31 (CH2), 31.74 (CH2), 20.98 (CH3). Anal. Calcd for C21H18N4O8·1/2EtOAc: C, 55.42; H, 4.45; N, 11.24. Found: C,55.22; H, 4.45; N, 11.03. [α]31D +63.1° (c=0.194, MeOH). 164b: yellow viscous oil. IR (film): 1747, 1685, 1655, 1614, 1541, 1340 cm-1. 1H-NMR (CDCl3) δ: 2.27 (3H, s), 2.28 (1H, dd, J=14.9, 12.9 Hz), 2.68 (3H, s), 3.16 (1H, dd, J=15.0, 6.7 Hz), 3.72 (3H, s), 3.95 (1H, d, J=15.4 Hz), 4.40 (1H, dd, J=12.9, 6.6 Hz), 5.26 (1H, brs), 7.19 (1H, t, J=7.6 Hz), 7.26 (1H, d, J=7.6 Hz), 7.35 (1H, t, J=7.9 Hz), 7.39 (1H, d, J=7.6 Hz), 8.49 (1H, d, J=2.2 Hz), 8.90(1H, d, J=2.2 Hz). 13C-NMR (CDCl3) δ: 170.89 (C), 170.66 (C), 169.04 (C), 157.24 (C), 142.52 (C), 141.49 (C), 135.21 (C), 131.63 (C), 130.43 (CH), 130.31 (C), 125.60 (CH), 123.17 (CH), 122.78 (CH), 122.56 (CH), 116.76 (CH), 110.67 (C), 56.16 (C), 52.59(CH3), 50.69 (CH), 47.74 (CH2), 32.66 (CH2), 25.44 (CH3), 20.83 (CH3). HR–MS m/z: Calcd for C23H20N4O9: 496.1231. Found: 496.1232. [α]31D +89.3° (c=0.273, CHCl3).
Scheme 19.
Synthesis of new β-carboline derivatives (2).
Scheme 19.
Synthesis of new β-carboline derivatives (2).
164b from 164a — Ac2O (0.5 mL) was added to a solution of 164a (10.0 mg, 0.02 mmol) in pyridine (1.0 mL) and the whole was refluxed for 3 h. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 164b (7.7 mg, 71%).
164a from 164b — Sat. NaHCO3 (1.0 mL) was added to a solution of 164b (16.6 mg, 0.03 mmol) in MeOH (4.0 mL) and the whole was stirred at rt for 100 min. After addition of H2O, the whole was extracted with CHCl3. The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 164a (14.7 mg, 97%).
(S)-(4aα,9aα)-Nb-Chloroacetyl- (165a) and -Na,Nb-bis(chloroacetyl)-3β-methoxycarbonyl-11,13-di- nitro-1,2,3,4-tetrahydro-9H-benzofurano[2,3-m]-β-carboline (165b) from 159 — Chloroacetyl chloride (0.012 mL, 0.18 mmol) and Et3N (0.15 mL) was added to a solution of 159 (60.8 mg, 0.15 mmol) in CHCl3 (1.5 mL) and the whole was stirred at rt for 20 min. The solvent was evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3 to give 163a (17.2 mg, 24%), 165b (32.8 mg, 39%), and unreacted 159 (4.3 mg, 7%). 165a: mp 198—200 °C (yellow prisms, recrystallized from EtOAc). IR (KBr): 3382, 1749, 1729, 1668, 1654, 1619, 1610, 1373, 1336 cm-1. 1H-NMR (CDCl3) δ: 2.31 (1H, dd, J=14.9, 12.6 Hz), 3.17 (1H, dd, J=14.9, 6.5 Hz), 3.73 (3H, s), 3.95 (1H, d, J=15.1 Hz), 4.11 (1H, d, J=12.7 Hz), 4.29 (1H, d, J=12.7 Hz), 4.42 (1H, dd, J=12.6, 6.5 Hz), 4.61 (1H, d, J=15.1 Hz), 5.40 (1H, brs, disappeared on addition of D2O), 6.75 (1H, d, J=7.8 Hz), 6.89 (1H, t, J=7.8 Hz), 7.18 (1H, t, J=7.8 Hz), 7.24—7.28 (1H, m), 8.48 (1H, d, J=2.3 Hz), 8.85 (1H, d, J=2.3 Hz). 13C-NMR (CDCl3) δ: 170.21 (C), 166.82 (C), 158.16 (C), 146.09 (C), 141.75 (C), 136.20 (C), 131.17 (C), 130.33 (CH), 127.97 (C), 123.30 (CH), 122.85 (CH), 122.26 (CH), 121.50 (CH), 112.59 (C), 110.40 (CH), 55.88 (C), 52.81 (CH), 51.61 (CH3), 48.13 (CH2), 40.17 (CH2), 31.60 (CH2). HR–MS m/z: Calcd for C21H17N4O8Cl: 488.0734, 490.0705. Found: 488.0756, 490.0707. [α]28D +135° (c=0.141, DMF). 165b: mp 223—224 °C (pale yellow prisms, recrystallized from EtOAc). IR (KBr): 1747, 1735, 1662, 1541, 1373, 1340 cm-1. 1H-NMR (CDCl3) δ: 2.30 (1H, dd, J=15.3, 12.8 Hz), 3.13 (1H, dd, J=15.3, 6.1 Hz), 3.63 (3H, s), 3.98 (1H, d, J=15.9 Hz), 4.14 (1H, d, J=12.8 Hz), 4.23 (1H, d, J=13.1 Hz), 4.27 (1H, dd, J=12.8, 6.1 Hz), 4.42 (1H, d, J=12.8 Hz), 4.64 (1H, d, J=13.1 Hz), 5.26 (1H, d, J=15.9 Hz), 7.14—7.22 (1H, m), 7.31 (1H, t, J=7.8 Hz), 7.39 (1H, t, J=7.8 Hz), 7.76 1H, brd, J=7.8 Hz), 8.46 (1H, d, J=1.8 Hz), 8.81 (1H, d, J=1.8 Hz). 13C-NMR (CDCl3) δ: 170.90 (C), 167.15 (C), 165.56 (C), 156.52 (C), 142.97 (C), 140.39 (C), 134.65 (C), 132.04 (C), 130.77 (CH), 130.47 (C), 126.51 (CH), 123.47 (CH), 122.94 (CH), 122.74 (CH), 117.07 (CH), 109.90 (C), 56.20 (C), 52.79 (CH3), 51.59 (CH), 47.01 (CH2), 42.55 (CH2), 40.23 (CH2), 32.73 (CH3). Anal. Calcd for C23H18N4O9Cl2: C, 48.87; H, 3.21; N, 9.91. Found: C, 48.87; H, 3.29; N, 9.87. [α]30D +122° (c=0.200, DMF).
165b from 165a — Chloroacetyl chloride (54.8 mg, 0.49 mmol) and Et3N (0.1 mL) was added to a solution of 165a (11.7 mg, 0.02 mmol) in CHCl3 (2.0 mL) and the whole was stirred at rt for 20 min. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (99:1, v/v) to give 165b (17.9 mg, 88%).
165a from 165b — Sat. NaHCO3 (0.5 mL) was added to a solution of 165b (10.4 mg, 0.02 mmol) in MeOH (2.0 mL) and the whole was stirred at rt for 30 min. After addition of H2O, the whole was extracted with CHCl3–MeOH (95:5, v/v). The extract was washed with brine, dried over Na2SO4, and evaporated under reduced pressure to leave an oil, which was column-chromatographed on SiO2 with CHCl3–MeOH (99:1, v/v) to give 165a (7.5 mg, 83%).
In summary, we demonstrated further examples of rearrangement reactions based on 1-hydroxyindole chemistry and succeeded in the production of thus far unknown 7β- and 7α-heteroaryl yohimbines, and 4aα- and 4aβ-heteroaryl-1,2,3,4-tetrahydro-β-carboline derivatives. We hope these novel compounds reported in this text would become a new member of our group of biologically active compounds that we have created so far.[
10]
23.3.2. Yohimbine α2-Blocker Test Method
Animals: Male Wistar rats were used in the present study. Animals were housed under controlled conditions (21–22ºC, relative humidity 50±5%). Food and water were freely available to all animals. This study was performed according to the Guideline for the Care and Use of Laboratory Animals of Toho University School of Pharmaceutical Sciences (which is accredited by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan), and the protocol of this study was approved by the Institutional Animal Care and Use Committee.
Preparation of rat thoracic aortic rings: Rats were killed by cervical dislocation and exsanguinated from the common carotid arteries. A section of the thoracic aorta between aortic arch and diaphragm was carefully removed and immersed in oxygenated Krebs-HEPES solution of the following composition (in mM): NaCl, 126.9; KCl, 5.9; CaCl2, 2.36; MgCl2, 1.18; HEPES, 10.03 and glucose, 11.8 (pH=7.4). The aorta was cleaned of loosely adhering fat and connective tissues and cut into ring segments about 2 mm in length. In this series of experiments, the endothelium was not removed.
Measurement of tension changes: The aortic tissue was then mounted using stainless steel hooks (outer diameter, 200 µm) under the resting tension of 2.0 g in a 5 mL organ bath (UC-5, UFER Medical Instrument, Kyoto, Japan) containing normal Tyrode’s solution (mM): NaCl, 158.3; KCl, 4.0; NaHCO3, 10.0; NaH2PO4:, 0.42; CaCl2, 2.0; MgCl2, 1.05 mM, glucose, 5.6), which was continuously gassed with 95% O2–5% CO2 being kept at 37±1ºC (pH=7.4). Tension changes of the muscle preparation were isometrically recorded with a force-displacement transducer (T7-8-240; Orientec, Tokyo, Japan; TB-612T, Nihon Kohden, Tokyo, Japan) connected to a carrier amplifier (AP-600G/AP-621G, Nihon Kohden, Tokyo, Japan; Signal Conditioner: Model MSC-2, Labo Support, Suita-City, Japan). Vascular preparations were equilibrated for 90 min in normal Tyrode’s solution, which was exchanged every 20–30 min. Before starting assessment of yohimbine derivatives, aortic preparations were contracted with isotonic high-KCl (80 mM) Tyrode’s solution (mM: NaCl, 82.3; KCl, 80.0; CaCl2, 2.0; MgCl2, 1.05; NaH2PO4, 0.42; NaHCO3, 10.0 and glucose, 5.6), in order to confirm the muscle normal contractility. After washing out, experiments were started after a subsequent 30 min equilibration period.
Assessment of relaxant potencies of yohimbine derivatives: Aortic ring preparations were contracted with an α2-adrenoceptor (α2-AR) agonist clonidine (10-7–10-6 M) in the presence of an NO synthase inhibitor nitro-l-arginine methyl ester (l-NAME, 10-4 M). When the sustained contraction induced by clonidine reached a steady-state level, yohimbine derivatives (10-5 M) were applied to the bath solution. When the relaxant effects of yohimbine derivatives reached their maximum level, yohimbine at 10-5 M was applied. The steady-state tension level before application of each yohimbine derivative and the tension level corresponding to yohimbine-induced maximum relaxation were defined as 0% and 100% relaxation, respectively. Relaxant potency of tested yohimbine derivatives was expressed as percentage relaxation to the maximum response to 10-5 M yohimbine.
Drugs: The following drugs were used in the present study: clonidine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA); yohimbine hydrochloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan); NG-nitro-l-arginine methyl ester hydrochloride (l-NAME) (Dojindo Laboratories, Kumamoto, Japan). Yohimbine derivatives tested in this study were dissolved in pure dimethyl sulfoxide (DMSO) at 10-2 M. Final DMSO concentrations in the bath medium did not exceed 0.1%, which did not affect the vascular responses. Other drugs were dissolved/diluted in/with distilled water. All drugs are expressed in molar concentrations (mol/L, M) in bathing solution.
Statistics: Data are presented as means±S.E.M. and n refers to the number of experiments.
23.3.3. Candidates for Curing Osteoporosis
We subjected 1-substituted 2,4,6-tribromotryptophan derivatives (
69a-c) and -melatonin derivatives
(72a-c) to the gills assay test (synthesis of chemicals are reported in
Scheme 6). Happily, we discovered that
69c as a strong activator for osteoblast and suppressor for osteoclast. The compound
72c was also discovered to be strong promotor of osteoblasts and suppressor for osteoclasts.
The test compounds were synthesized as shown in
Scheme 6. In the acute toxicity test of 1-benzyl- 2,4,6-tribromomelatonin (
69c), according to the OECD (Europe) guidelines, 2 g per 1 kg of rat body weight was administered, and after 2 weeks of breeding, toxicity was not observed. Furthermore, no toxicity was observed in a mutagenicity test in which rats were administered 2 g per kg of body weight and evaluated after feeding for 2 weeks according to the OECD guidelines. Addition of S-9 mix (rat liver homogenate) did not change.[
57,
58]
Therefore, we were able to discover compounds
69c and
72c as potential therapeutic candidate compounds.[
42,
43,
57,
58] Even now, no effective therapeutic agent for osteoporosis that has an osteoblast-activating action is known. Combined use of
69c and
72c with VED #1 can not only treat osteoporosis, fractures, arthralgia, and dental treatment in humans, but also improve the bone metabolism of shrimp, shellfish, goldfish, ‘Nishikigoi’, farming, poultry, birds, and animals. It opens up the possibility of treating osteoporosis caused by aging in humans and guaranteeing a healthy life.
As noted previously in the safety test of VED #1, ‘medaka’ a kind of fish grew for 2 years and 6 months without any problems, and the body length became more than doubled.[
40] From these results, we believe that mixing VED with fish feed and breeding them will contribute to the improvement of production in the fishery and aquaculture industries.
It is predicted that by the 2030, the aging of the population will progress worldwide and become a social problem in countries around the world. In an aging society, the number of osteoporosis patients is rapidly increasing. We can provide society with these compounds that have the potential to cure osteoporosis.
23-4. Structure elucidation by X ray analysis – A single crystal (0.10 x 0.20 x 0.30 mm) of (
dl)-
131was obtained by recrystallization from MeOH. Measurements were made on a Rigaku AFC5R diffractometer with graphite monochromated Cu-
Kα radiation (
λ=1.54178 Å). Crystal data for (
dl)-
131: C
14H
16N
2O
4,
M=276.29; triclinic, space group
P1(_) (
#2),
a=8.163 (1)Å,
b=12.086 (1)Å,
c=8.0126 (9)Å,
α=107.940 (8)°,
β=109.560 (9)°,
γ=73.161 (8)°,
V=693.2 (1)Å
3,
Z=2,
Dcalc=1.324 g/cm
3,
F(000)=292, and
μ(Cu
Kα)=7.77 cm
–1. The structure was solved by direct methods using MITHRIL[
78] The non-hydrogen atoms were refined anisotropically. The final cycle of full-matrix least-squares refinement was based on 2685 observed reflections (
I>3.00
σ (
I), 2
θ <120.1°) and 245 variable parameters. The final refinement converged with
R=0.039 and
Rw=0.047.
Contrary to our expectation, (
dl)-
131 and (
S)-(+)-
131 are stable crystalline compounds enough to X-ray crystallographic analysis.
Figure 116 (
H,
I) is an ORTEP drawing of (
dl)-
131. Numbering of (
dl)-
131 is shown in
J. It should be stressed that 1-hydroxy group is deviated from the indole plane by 15.2°. This is the reason why 1-hydroxytryptophan derivatives undergo nucleophilic substitution.
The following measurements were made on a Rigaku/MSC Mercury diffractometer with graphite monochromated Mo-Kα radiation (
λ=0.71069 Å). All calculations were performed using the teXsan package.[
79] The structure was solved by a direct method (SIR).[
80] The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were included but not refined.
Crystal data for 53b: C
23H
36N
2O
9S,
M=516.61; orthorhombic; space group,
P2
12
12
1 (#19);
a=8.738(3) Å,
b=14.732(4) Å,
c=19.428 (6) Å,
V=2501 (1) Å
3,
Z=4,
Dcalc= 1.372 g/cm
3. The final
R- and
Rw-factors after full-matrix least-squares refinements were 0.038 and 0.049 for 3499 observed reflections [
I>3.00
σ (
I)], respectively. Positional parameters and
B(eq) for
53b are shown in
Table 32. Crystallization solvent is CH
3SO
3H.
Figure 118.
ORTEP Drawing and Numbering of 135a.
Figure 118.
ORTEP Drawing and Numbering of 135a.
Crystal data for 135a: C22H28N2O4, M=384.47; monoclinic; space group, P21 (#4); a=8.200(3) Å, b=12.873(4) Å, c=9.296 (4) Å, β=97.769(4)°, V=972.2(6) Å3, Z=2, Dcalc= 1.313 g/cm3. The final R- and Rw-factors after full-matrix least-squares refinements were 0.035 and 0.050 for 4169 observed reflections [I>3.00σ (I)], respectively. Positional parameters and B(eq) for 135a are shown in Table33. The N-O bond is deviated from the indole plane by12.7°.
Crystal data for 137b: C
25H
30N
2O
6,
M=454.52; monoclinic; space group,
P2
1 (#4);
a=8.1888(6) Å,
b=10.7944(9) Å,
c=13.8942(9) Å,
β=97.262(1)°,
V=1218.3(2) Å
3,
Z=2,
Dcalc= 1.239 g/cm
3, F(000)=484 and μ(Cu–Kα)=6.92 cm
-1. The final
R- and
Rw-factors after full-matrix least-squares refinements were 0.030 and 0.036 for 1632 observed reflections [
I>3.00
σ (
I)], respectively. Positional parameters and
B(eq) for
137b are shown in
Table 34.
Figure 119.
ORTEP Drawing and Numbering of 137b.
Figure 119.
ORTEP Drawing and Numbering of 137b.
Figure 120.
ORTEP Drawing and Numbering of 148.
Figure 120.
ORTEP Drawing and Numbering of 148.
Crystal data for 148: C
23H
36N
2O
9S,
M=516.61; orthorhombic; space group,
P2
12
12
1 (#19);
a=8.738(3) Å,
b=14.732(4) Å,
c=19.428(6) Å,
V=2501(1) Å
3,
Z=4,
Dcalc= 1.372 g/cm
3. The final
R- and
Rw-factors after full-matrix least-squares refinements were 0.038 and 0.049 for 3499 observed reflections [
I>3.00
σ (
I)], respectively. Positional parameters and
B(eq) for
148 are shown in
Table 35. Crystallization solvents are H
2O, CH
3OH, and CH
3SO
3H.
Crystal data for 164a: C
21H
18N
4O
8,
M=542.50; tetragonal; space group,
P4
3 (#78);
a=14.441(2) Å,
b=14.449(1) Å,
c=12.626(2) Å,
V=2633.2(7) Å
3,
Z=4,
Dcalc=1.368 g/cm
3. The final
R- and
Rw-factors after full-matrix least-squares refinements were 0.060 and 0.091 for 5781 observed reflections [
I>3.00
σ (
I)], respectively. Positional parameters and
B(eq) for
164a are shown in
Table 36. Crystallization solvent is CH
3CO
2Et.
Figure 121.
ORTEP Drawing and Numbering of 164a.
Figure 121.
ORTEP Drawing and Numbering of 164a.