1. Introduction
Ventilator-associated pneumonia (VAP) is the most frequent infection acquired at the intensive care unit (ICU) that occurs>48 h after endotracheal intubation necessary for mechanical ventilation [
1]. VAP incidences vary widely from 5% to 40%, depending on ICU hygienic regimes, pathogen prevalence, and diagnostic criteria in local settings [
1]. Patients suffering from VAP are at high risk of life-threatening complications, resulting in mortality exceeding 30% [
2]. They also had significantly longer ICU and hospital length of stay, required longer time under invasive mechanical ventilation (MV), and were more often subjected to tracheostomy [
3]. Considering the wide range of possible causative pathogens and severe complications if treatment is not induced timely, patients with suspected VAP commonly receive broad-spectrum antibiotics still before the diagnosis is confirmed, accounting for nearly half of all antibiotic use in the ICUs, which contributes to the escalation of antimicrobial resistance [
4]. Moreover, despite available guidelines, different diagnosis criteria and management protocols exist across ICUs [
5].
It was proven that applying different diagnostic criteria to the same patient population resulted in delayed diagnosis (from 4 to 8 days) with increasingly stringent criteria, thus altering treatment and increasing mortality from 50% to 80% [
6]. Also, the diagnostic methods lack specificity and sensitivity, whereby clinical examination, although essential, has a low sensitivity of 66% and specificity of 54% [
6] and is nonspecific to VAP but often related to systemic inflammatory response. In turn, the presence of new or progressive infiltrates on chest X-Ray (CXR) is a valuable adjunct for diagnosing VAP, offering as high sensitivity as 89%, but by the cost of specificity of only 26% (lung infiltrates on CXR imaging often overlap of with other confounding pathologies, e.g., ARDS). The microbiological examination is considered a gold standard in VAP diagnosis, but the variety of sampling techniques determines the limitations of this approach. For instance, although simple and safe, endotracheal aspirates cannot differentiate lower respiratory tract infection (LRTI) from colonization of upper airways [
4]. Techniques that bypass upper airway colonization when collecting specimens from distal airways are bronchoalveolar lavage (BAL) and protected specimen brush (PSB) utilizing a fiberoptic bronchoscope. Both BAL and PSB techniques require an invasive procedure but yield good specificity (80% and 76%, respectively) and sensitivity (71% and 61%, respectively) [
6]. To overcome the pitfalls of individual diagnostic methods mentioned above, a scoring system was proposed, where the clinical pulmonary infection score (CPIPS) is considered the most relevant for VAP diagnosis as it combines clinical, radiological and microbiological criteria. The scores >6 (for a scale ranging from 0-12) allow VAP diagnosis with a sensitivity of 74% and specificity of 66% [
4,
7].
Given the limitations of currently used methods, the biomarkers for VAP diagnosis are gaining increasing interest. Serum biomarkers like C-reactive protein (CRP) and procalcitonin have shown similar to previous diagnostic accuracies of around 60-80% in distinguishing bacterial infections from uninfected controls, but these values vary between studies, making them inconsistent in diagnosing VAP [
4]. The other type of biomarkers are volatile organic compounds (VOCs) related to the pathological processes in infected patients. Volatile biomarkers can be readily analyzed in the exhaled breath from critically ill patients in a non-invasive way, using mass spectrometry, either offline in the laboratory [
8,
9,
10] or in real-time directly at the bedside of mechanically ventilated patients [
11]. It was recently demonstrated that breath gas analysis is a valuable tool in diagnosing VAP with area under the curve (AUC) for receiver operating characteristic (ROC) analysis ranging from 0.67 to 0.77 [
12] in the case of individual VOCs, alternatively reaching AUC=0.86 for a ROC model composed of 10 VOCs in another study [
13]. However, in the later study, a model differentiating VAP from control patients included some compounds of unknown identity and other compounds of unknown or exogenous origin (enflurane).
We propose another approach to arrive at a breath test for VAP diagnosis focusing on volatile metabolites with unambiguously proven bacterial origin. Detection of such microbial metabolites in breath gas would testify to the presence of metabolically active bacteria, reflecting the ongoing infection. The increasing prevalence of antibiotic-resistant pathogens is observed for decades [
14], with a particular emphasis on the so-called ESKAPE group of microorganisms dominating the variety of hospital-acquired infections (HAI) [
15]. Amongst them, the gram-negative
Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and
Escherichia coli are the most prevalent pathogens causing VAP [
16,
17].
Therefore, these four species have been chosen to in vitro experiments investigating the production of volatile organic compounds. This study aims to identify volatile compounds metabolized by these four bacteria, verify whether they can be detected in the breath gas of ventilated patients infected with those pathogens, and to evaluate their diagnostic potential for the VAP detection. To clarify the putative bias of incorporating exogenous compounds into a diagnostic model, the emission of volatiles from medical respiratory devices (endotracheal tube and disposable catheter mount) was additionally examined.
2. Materials and Methods
2.1. Chemicals
Gaseous and liquid chemicals (GC-MS standards) manufactured by Tokyo Chemical Industry (Tokyo, Japan), Sigma Aldrich (Merck KGaA, Darmstadt, Germany) or Acros Organics, Alfa Aesar, Honeywell (all three belong to the Thermo Scientific Chemicals, Waltham, MA, USA) were purchased from AlChem (Torun, Poland).
2.2. Setup for Bacteria Cultivation and Headspace Sampling
Acinetobacter baumannii (strain DSM 30008), Klebsiella pneumoniae (strain DSM 681), and Pseudomonas aeruginosa (strain DSM 10273) were purchased from the German Collection of Microorganisms and Cell Cultures GmbH (Leibniz Institute DSMZ, Leibniz, Germany) whereas Escherichia coli (strain ATCC 25922) was purchased from the American Type Culture Collection (ATCC, Manassas, Virginia, USA). Before experiments, strains were stored in Tryptic Soy Broth (TSB, Becton Dickinson, Franklin Lakes, NJ, USA) with 15% glycerol at -80°C.
An in-house system previously described in detail was used for bacteria cultivation and headspace gas sampling [
18]. Briefly, glass bottles containing 100 ml of bacteria suspension in TSB (stirred with a rate of 80 rpm) were kept at 37°C within a water bath, which was additionally placed inside an incubator at 45°C to prevent water condensation in transfer lines that could lead to loss of volatiles. Synthetic air of purity 6.0 enriched with 5% CO2 (Air Products, Belgium) was additionally purified with a Supelcarb filter (Merck KGaA, Darmstadt, Germany) and served as a carrier gas to transfer bacterial volatiles from cultures to sorption tubes filled with 140 mg of Carbotrap B (20/40 mesh) and 330 mg of Carbotrap X (60/80 mesh). The flow of carrier gas was split into two lines: (A) 5 ml/min passing the bacteria culture and (B) 40 ml/min used to dilute (hence decrease the humidity) the bacterial headspace. All flows were precisely controlled with Mass Flow Controllers with an option of automatic flow compensation (Vögtlin Red-Y smart series, NewTech, Gliwice, Poland). As a quality control for the tightness of the entire headspace sampling system, the terminal flows at the outlet from sorption tubes during the sample extraction were also checked using a Mass Flow Meter (Vögtlin Red-Y Compact, NewTech, Gliwice, Poland). The volumes of 200 ml of headspace gas for GC-MS analysis were taken at 0 h (T0), 2h 40min (T1), 4h 10min (T2), 5h 40min (T3), 7h 10min (T4), 8h 40min (T5), 24h (T6) and 26h (T7) after inoculation of bacteria to a pure TSB medium (except Acinetobacter baumannii which were discontinued after T5). Altogether, five biological replicates of each strain were measured in this study. After each headspace sampling, 300 µl of bacterial suspension was collected through a gas-tight septum port to assess bacterial growth and its quantification (CFU/ml).
2.3. VOC Emission from Medical Respiratory Devices
The emission of volatiles from medical respiratory devices (MRD) was studied to verify whether the breath samples collected from mechanically ventilated patients were not contaminated with exogenous VOCs. For this purpose, the endotracheal tube cuffed model RIM-80 8.0mm I.D. (ZARYS International Group, Zabrze, Poland), and the disposable catheter mount of the ventilator circuit model RB08-18-15 (Yangzhou Beswin Medical Equipment Co., Ltd, Yizheng Yangzhou, China), which were used at the ICU ward participating in this study, were examined. The experimental setup was as follows: Nitrogen 6.0 (Air Products, Warsaw, Poland), additionally purified on Carboxen 1000 filter (Merck KGaA, Darmstadt, Germany), was used as a carrier gas passing (at the constant flow of 50 ml/min) through the examined respiratory devices, which were placed in incubator kept at 37°C (to mimic the temperature of a human body). The flow of nitrogen was initiated immediately after removal of both parts (endotracheal tubes and catheter mount) from their original sterile housing and their connection via short FEP capillary (5cm length, 1/16" O.D., BOLA Bohlender GmbH, Grünsfeld, Germany) to the sorption tube of the same composition as used for breath sampling and in vitro experiments, i.e., 140 mg of Carbotrap B (20/40 mesh) and 330 mg of Carbotrap X (60/80 mesh). The total volume of 750ml of nitrogen with the stripped-off contaminants released from medical respiratory devices was collected at 0, 1, 2, 3, 24, 25, 26, 27, 48, and 72 hours counted since assembling the system and switching on the nitrogen flow. The same setup but without investigated medical devices was used to collect blank samples to estimate a systemic background for this test. The samples were measured on TD-GC-MS immediately after extraction on a sorption tube, using the same method as in vitro and in vivo experiments (bacteria cultures and breath gas, respectively).
2.4. Selection of Patients and Diagnosis of Pneumonia
All mechanically ventilated patients were recruited from the Anesthesiology and Intensive Care Unit of the 10th Military Research Hospital and Polyclinic in Bydgoszcz, Poland. Ethical approval for this study was obtained from the local Bioethics Committee (KB-218/2018). Patients who received mechanical ventilation for at least three days and developed VAP symptoms (i.e., with already confirmed VAP or at high risk) were eligible for breath sampling. The exclusion criteria were (1) age under 18, (2) pregnancy, (3) positive end-expiratory pressure (PEEP) >10, (4) increased intracranial pressure (ICP), (5) confirmed pre-existing structural lung disease (traumatic lung injury or pulmonary cancer), (6) chest or lung injury, (7) extracorporal heart and lung assistance devices, and (8) strict isolation at the ICU. Ventilator settings such as ventilation mode, oxygen concentration in inspired air, and positive end-expiratory pressure (PEEP) were adjusted individually to prevent hypoxia or ventilation-associated lung injury. Antimicrobial therapy was introduced presumptively upon suspicion of pneumonia, considering pathogens' local prevalence, resistance patterns, and patient factors (underlying disease, age, comorbidities, and immune status).
VAP was suspected based on radiology criteria: new and persisting infiltrates on chest X-ray or CT, assisted with the systemic signs: fever >38°C (with no other recognized cause), purulent secretion in the bronchial tree, auscultatory changes, and concomitantly elevated laboratory parameters: CRP >50 mg/dL and PCT >1 ng/mL. To confirm the clinical diagnosis of VAP, the positive result of microbiological culture from the BAL specimen was required at the quantity of >10^5 CFU/ml with the coexistence of leucocytes. Patients with continuous VAP progression or resolution, as reflected by inflammatory parameters, oxygenation, and ventilator settings (i.e., PaO2/FiO2), were eligible for additional collection of breath samples in subsequent days to cover the broadest possible range of clinical conditions that could be observed in real-life ICU settings. In this manner, 77 breath samples were collected from thirty-two patients suffering from VAP, and 17 breath samples were collected from six uninfected patients receiving mechanical ventilation (i.e., from whom collected BAL samples occurred to be sterile).
2.5. Collection of Breath Samples from Mechanically Ventilated Patients
The end-tidal air was collected directly from the respiratory circuit under continuous capnography control (i.e., visual monitoring of an exhaled CO2 profile) to maximize endogenous VOCs' content and minimize exogenous VOCs' contribution. Breath samples were collected at least 30 minutes after the last activity related to the patient (e.g., hygienic care). Each portion of exhaled gas (typically 20-40 ml, depending on the ventilation mode applied to the patient) was collected from the T-connector of the catheter mount of the ventilator circuit (Ref: RB08-18-15, Yangzhou Beswin Medical Equipment Co., Ltd, Yizheng Yangzhou, China), through a sterile bacterial filter (Ref: 7699822, pore size 0.20 μm, LabSolute, TH. Geyer GmbH, Renningen, Germany) to the glass syringe (250ml total volume, Socorex Isba S.A., Ecublens, Switzerland). The glass syringes filled with 250ml of breath gas samples were tightly closed and immediately placed into an incubator held at 45°C for five to ten minutes to prevent water condensation and loss of polar analytes. Subsequently, a total volume of 750ml of breath gas (i.e., three syringes) was extracted on a sorption tube (also thermostated at 45°C) with a stable flow of 70 ml/min generated by a vacuum pump (Vacuubrand, Wertheim, Germany) and governed by a Mass Flow Controllers (Vögtlin Red-Y smart series, NewTech, Gliwice, Poland). Immediately after extraction, sorption tubes were tightly sealed with Swagelok brass caps and transported from the Anesthesiology and Intensive Care Unit of the 10th Military Research Hospital to the Analytical Laboratory at the Department of Pharmacodynamics and Molecular Pharmacology of the Collegium Medicum NCU (both in Bydgoszcz, Poland) so the TD-GC-MS analyses were accomplished within several hours according to the protocol described below.
Notably, the respiratory conditions, such as oxygen content in the inspired air, value of PEEP, and ventilation mode, were not changed for the duration of breath sampling to not affect the composition of the collected sample (and results of GC-MS analysis). Similarly, bronchoalveolar lavage was performed only for microbiological testing upon suspicion of pneumonia (described above) and tailoring the antimicrobial therapy, and it was not related to the collection of breath samples from MV patients.
2.6. TD-GC-MS Analysis
One analytical protocol was used for TD-GC-MS analysis of all samples, including bacterial cultures, emission from medical respiratory devices, and breath samples. Sorption tubes with extracted analytes were thermally desorbed at 320°C over 15 min in a TD-30R autosampler (Shimadzu, ShimPol, Warsaw, Poland), and released VOCs were cryofocused at -20°C on a cold trap filled with Carboxen. Rapid heating of the cold trap to 350°C triggered splitless injection over 2 min into a Nexis 2030 Gas Chromatograph (Shimadzu, Shim-Pol, Warsaw, Poland). Sample constituents were separated on an Rt-Q-Bond capillary column 30 m x 0.25 mm x 8 µm (Restek, Bellefonte, PA, USA) using the following temperature program: initial 60°C held for 2 min, ramp 8°C/min to 110°C (hold 1 min), ramp 3°C/min to 120°C (7 min), ramp 3°C/min to 155°C (7 min), ramp 3°C/min to 225°C (4 min), ramp 10°C/min to 300°C (7 min). Data were acquired with a QP-2020-NX Mass Spectrometer (Shimadzu, Shim-Pol, Warsaw, Poland) operating in a SCAN mode range of 33–235 m/z.
2.7. Data Processing and Statistical Analysis
Chromatographic data was processed according to the previously described protocol [
18]. Shimadzu GCMS PostRun Analysis software (combined with the NIST 2018 library) was used to identify and integrate peaks based on the predefined database of analytes. Notably, the experienced GC-MS analyst processing the data was blinded to the breath sampling procedure and any information related to the patient's conditions, including clinical, microbiological, and laboratory results. To prevent errors in the integration of peak areas, the “Target Ion” was assigned to each analyte as the most selective ion (unique for only one compound in the case of coeluted peaks) or the most abundant ion (for peaks resolved to the baseline). Characteristic reference ions were also assigned to each analyte to verify peak identity during integration. Analytes were identified using the two-stage approach: by NIST 2018 spectra library match and by additional confirmation of retention times with injected standards. The integrated areas of all chromatographic peaks were pre-processed using Log10-transformation and Pareto scaling before performing the statistical tests using the Metaboanalyst 6.0 online software.
In the in vitro experiments, the significance between VOC levels in bacterial suspensions at different growth times and reference TSB medium was calculated using the Kruskal-Wallis test, which is a non-parametric test to compare samples from two or more groups of independent observations, whereby p-values <0.05 were considered to be significant. This test was selected because of its stability to outliers and no requirement for the groups to be normally distributed.
The bacterial metabolites elucidated in the in vitro experiments were then monitored in the breath gas collected from VAP and uninfected MV patients (sterile BAL). The significant p-values of the non-parametric Wilcoxon rank-sum test with correction to false discovery rate (FDR), as well as the values of Area Under the Curve (AUC) from Receiver Operating Characteristic (ROC) calculated with Linear Supported Vector Machine algorithm (robust for features with low occurrence), and the high values of frequency in the Least Absolute Shrinkage and Selection Operator (LASSO) test were all considered to select individual compounds. Then, the AUC (calculated with the Random Forest algorithm, potentially capturing more complex non-linear relationships between the metabolites) and the True Positive Rate were used to evaluate the ROC model's performance for chosen metabolite sets.
For the secondary aim of differentiating VAP patients based on the causative pathogen, a separate set of metabolites that potentially may be more pathogen-specific needed to be elucidated. For this reason, only patients with a single-pathogen infection were included in the model utilizing the Partial Least Squares Discriminant Analysis (PLS-DA). The metabolites with discrimination power between pathogen strains (based firstly on variable importance in projection, i.e. VIP scores, and secondarily on the statistical significance of the non-parametric one-way ANOVA test) were prioritized.
All statistical calculations and plotting were performed with Statistica 13.3 PL software (TIBCO Software Inc., Tulsa, OK, United States) and the Metaboanalyst 6.0 online software using Log10-transformation of chromatographic peak areas and Paretto-scaling.
3. Results and Discussion
3.1. Bacteria Cultures
3.1.1. Microbial Growth
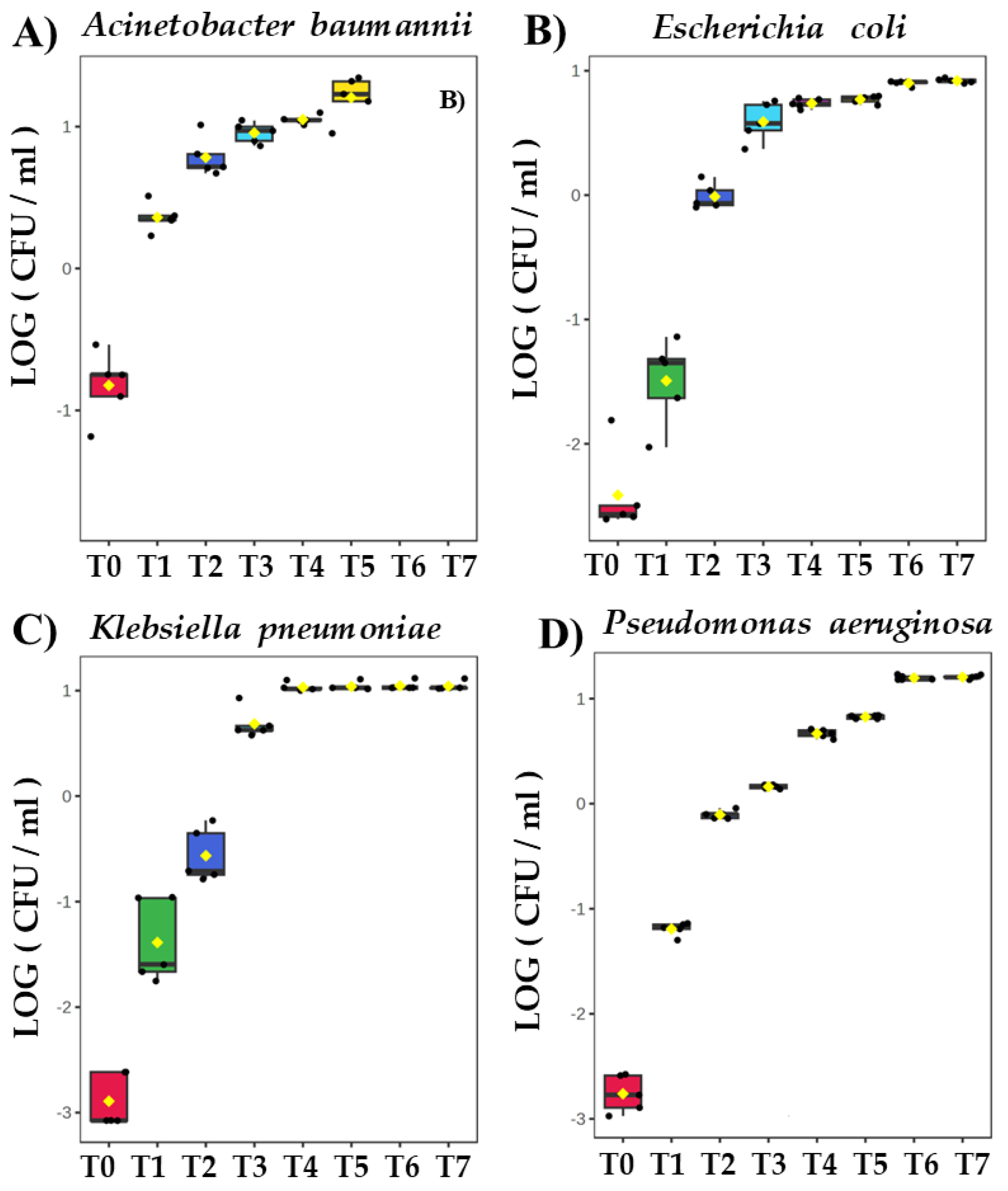
All bacteria strains were studied in five biological replicates. The initial bacteria density immediately after inoculation to TSB medium ranged from 4.0x10
4 CFU/ml for
Klebsiella pneumoniae to 1.7x10
6 CFU/ml for
Acinetobacter baumannii. The latter exhibits the slowest proliferation rate and the lowest absolute cell density amongst all bacteria tested in this study, reaching only 3x10
8 CFU/ml at T5 (8.6 h after inoculation), being two orders of magnitude less than for other species at the same tie point (Supplementary Table ST1).
Escherichia coli,
Klebsiella pneumoniae, and
Pseudomonas aeruginosa exhibit nearly identical kinetics, whereby the cell density in experimental cultures ranged from approximately 1x10
5 CFU/ml just after inoculation (T0) to approximately 3x10
10 CFU/ml after 26 h (T7). The control measurements performed overnight (24h for T6 and 26h for T7) confirmed that these three bacteria species reached a steady state of growth, after which the experiment was discontinued (
Figure 1).
3.1.2. VOC of Microbial Origin
The primary aim of the performed in vitro experiments was to identify the volatile metabolites released by particular pathogenic bacteria. The secondary aim was to characterize the relation of their production kinetic to cultivation time or bacteria load.
The primary aim was approached by comparing the peak areas for respective metabolites analyzed at the same time points in the headspace of bacteria cultures and sterile reference medium. Using the non-parametric Kruskal-Wallis test, 39 VOCs were observed at significantly different levels in the headspace of bacteria cultures compared to the control medium. Amongst them, Acinetobacter baumannii released fifteen VOCs and consumed another three substances from the broth medium. The relatively low number of compounds significant for this specie was probably related to the slow metabolism of this bacteria, reflected also by its low proliferation rate. In turn, Escherichia coli produced eighteen and utilized another seven metabolites. The most active metabolism of volatiles was observed for Klebsiella pneumoniae, whereby twenty-one VOCs were significantly released, and four were taken up by this specie. On the contrary, only sparse production of VOCs was reported for Pseudomonas aeruginosa strain DSM10273, whereby fourteen compounds were secreted and eight taken up from the medium.
The adequately chosen time points for sampling bacterial headspace gas allowed to cover the most dynamic phase of bacterial metabolism, namely the logarithmic phase of microbes proliferation. Consequently, three time-dependent profiles of VOC metabolism could be observed, including the release proportional to the bacteria load (RPB), release with a temporary maximum (RTM), after which VOC’s level decreases and the uptake (UPT) of analyte from surrounding medium (see
Table 1 for examples).
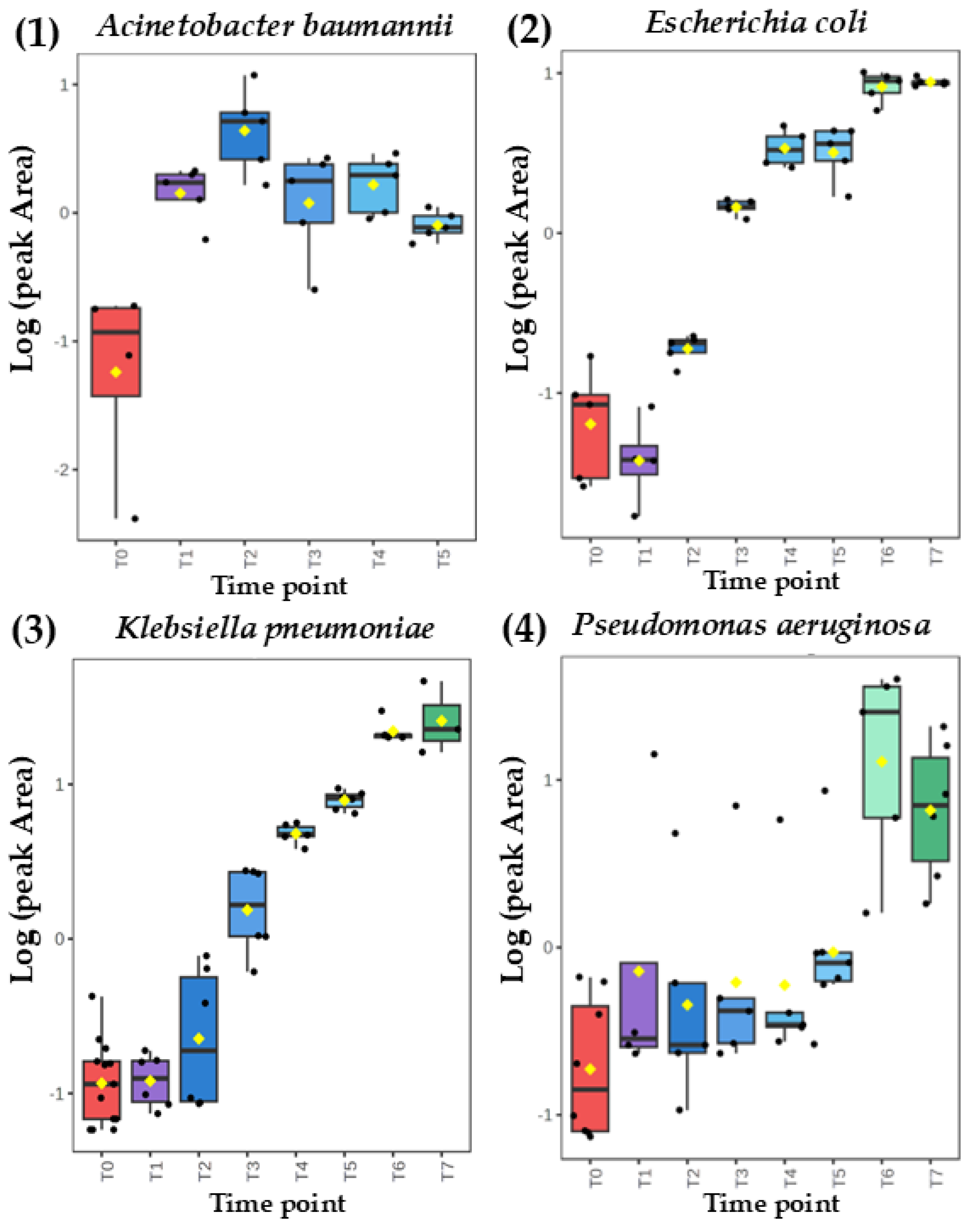
Intriguingly, the same metabolite can exhibit a distinct secretion profile for different microorganisms. This can be well exemplified with ethyl acetate released with a temporary maximum by
Acinetobacter baumannii but in the quantities proportional to the absolute bacteria load for the remaining three species (
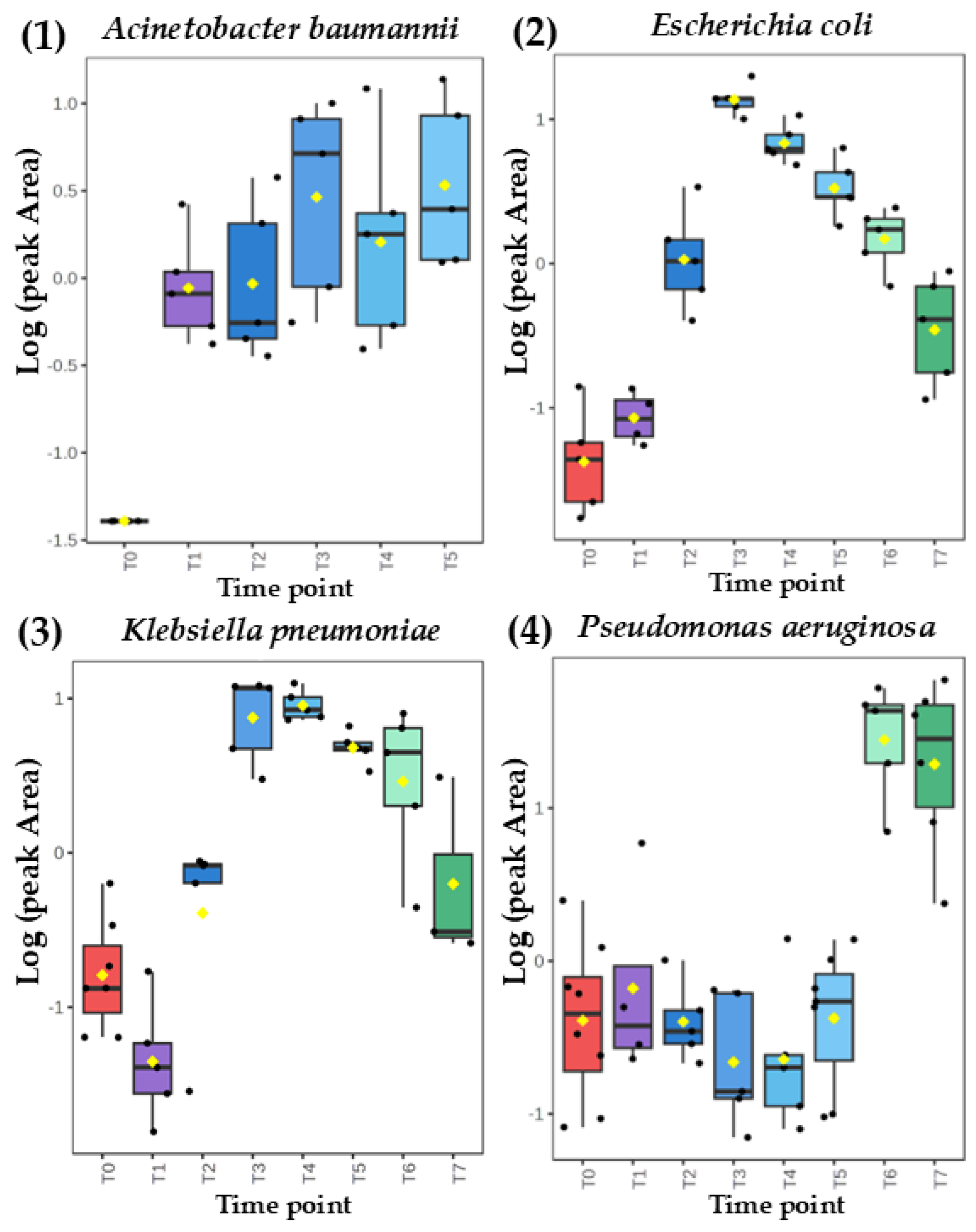
Figure 2). On the other hand, the abundance of dimethyl sulfide was dependent on the bacterial cell density for
Acinetobacter baumannii and
Pseudomonas aeruginosa but exhibits a clear and abundant temporary maximum for
Klebsiella pneumoniae and
Escherichia coli. (
Figure 3).
A closer look at the dynamically changing time profile allows to find a few VOCs that exhibit even opposite profiles amongst bacteria, such as the case of 2-butene, which was produced by
Acinetobacter baumannii and
Pseudomonas aeruginosa but consumed by
Escherichia coli. A similar situation concerns aldehydes, where methacrolein and 2-methylpropanal were secreted by
Acinetobacter baumannii but taken up by all remaining microorganisms studied, as well as 3-methyl butanal released by
Klebsiella pneumoniae but degraded by
Escherichia coli and
Pseudomonas aeruginosa (
Table 1).
3.2. Breath Analysis
3.2.1. VOC Emission from Medical Respiratory Devices
The most popular approach to elucidate biomarkers for certain diseases, including Ventilation-Associated Pneumonia, relies on untargeted analysis of collected samples to cover as broad a range of metabolites as possible with their subsequent reduction through adequately chosen statistical models. Apart from the problem with the correct identification of unknown analytes, there is also a problem with the appropriate processing of results obtained. Despite sound statistical models, incorrect substances, including exogenous contaminants or anesthetics, are often proposed as biomarkers. For this reason, the emission of volatiles from plastic materials constituting the respiratory circuit in mechanically ventilated patients placed near the infection site (endotracheal tube) or the breath sampling site (disposable catheter mount) was examined.
The results confirm that numerous substances of diverse chemical classes are released from the tested parts of a respiratory circuit (Supplementary Table ST2). Amongst them, aromatic compounds (e.g., ethylbenzene, styrene, xylene -
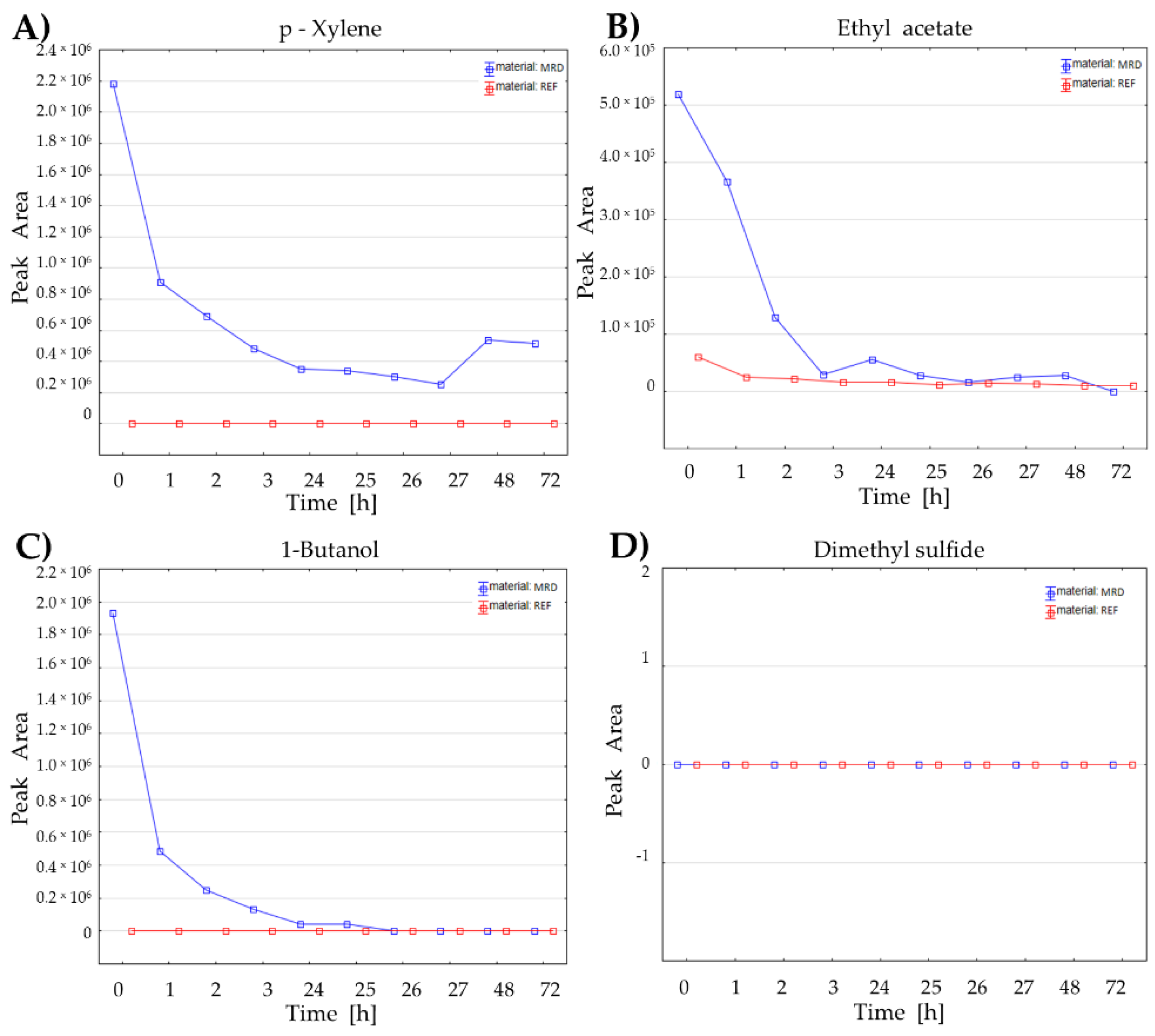
Figure 4A) and heavier esters (containing more than six carbon atoms, >C6) do not reach the low level of reference blank samples within the tested time frame, suggesting a continuous emission of these impurities at a fixed level (
Figure 4A). Importantly, also the low-molecular, very volatile compounds typically considered endogenous, including those with confirmed bacterial origin, e.g., ethyl acetate (
Figure 4B), 2-methyl-1-propanol, and 1-butanol (
Figure 4C), occurred to be secreted from MRD tested, but their levels decrease rapidly and reach nearly zero level within 24 hours of a continuous rinse with nitrogen. Therefore, from a practical point of view, there is no risk of spoiling the results of breath analysis due to the emission of these endogenous VOCs from plastic materials if patients underwent mechanical ventilation for at least two or three days preceding collection of breath gas. Notably, the volatile sulfur-containing compounds (VSCs), such as dimethyl sulfide (
Figure 4D) or dimethyl disulfide, which were proved to be of bacterial origin in the here presented and previous in vitro studies (thoroughly discussed elsewhere [
19]), are not secreted from the tested MRD parts at all.
3.2.2. Differentiation of VAP from Uninfected Patients
The most frequent reasons for admission to the intensive care unit in the studied population were cardiac (mostly cardiac arrest) and neurological (mainly stroke and intracerebral bleeding), less frequent organ failure (mostly pancreatitis and hepatic failure), or post-surgical complications. Amongst injury cases, those concerning chest and lung structure were considered exclusion criteria.
Table 2.
Demographic characteristics of patients with confirmed Ventilation-Associated Pneumoniae (VAP) and Control individuals in whom VAP was excluded based on sterile microbiological culture results from BAL specimens. APACHE II – Acute Physiology and Chronic Health Evaluation score; BAL culture results may concern multiple pathogens detected.
Table 2.
Demographic characteristics of patients with confirmed Ventilation-Associated Pneumoniae (VAP) and Control individuals in whom VAP was excluded based on sterile microbiological culture results from BAL specimens. APACHE II – Acute Physiology and Chronic Health Evaluation score; BAL culture results may concern multiple pathogens detected.
| |
|
Control (n=6) |
VAP (n=32) |
| Age, years |
median (IQR) |
80 (64-86) |
68 (56-75) |
| Male |
n (%) |
5 (83) |
19 (57) |
| Admission Type |
cardiac |
2 |
10 |
| |
neurological |
2 |
5 |
| |
organ failure |
0 |
4 |
| |
post-surgical |
0 |
4 |
| |
injury |
1 |
2 |
| |
other |
1 |
7 |
| APACHE II |
median (IQR) |
37 (27-40) |
30 (27-33) |
| Days from intubation to VAP diagnosis |
median (IQR) |
- |
7 (4-18) |
| Ventilation days at the 1st breath sampling |
median (IQR) |
6 (3-14) |
9 (6-19) |
| ICU days |
median (IQR) |
15 (8-32) |
25 (14-33) |
| ICU mortality |
n (%) |
5 (83%) |
16 (48%) |
| * BAL culture results |
n (single) |
|
|
| Acinetobacter baumannii |
|
- |
16 (11) |
| Pseudomonas aeruginosa |
|
- |
11 (6) |
| Escherichia coli |
|
- |
5 (1) |
| Klebsiella pneumoniae |
|
- |
4 (2) |
| Staphylococcus aureus |
|
- |
6 (3) |
| Enterococcus aerogenes |
|
- |
5 (1) |
| Proteus mirabilis |
|
- |
3 (0) |
| Other |
|
- |
8 (0) |
The onset of VAP occurred on average on the 9th day of the patient's mechanical ventilation, while the first breath sample was collected on average on the 7th day of ventilation in this group of patients. The most prevalent pathogens observed in this pilot study were Acinetobacter baumannii (28%), followed by Pseudomonas aeruginosa (19%). Both microorganisms were also relatively often found to be the single causative pathogen, whereas the remaining microorganisms co-existed in most cases. The observed high mortality rate (83%) in the control group was the consequence of multi-organ failure not related to the bacterial infection. The inflammatory parameters differed substantially within the VAP cohort at the days of breath sampling, as exemplified by C-Reactive Protein reaching its maximum value of 18.54 mg/dL for a patient with Escherichia coli + Klebsiella pneumoniae + Pseudomonas aeruginosa coinfection and the maximum value of 497.96 mg/dL for a patient infected with a sole Pseudomonas aeruginosa. A similarly broad range of the results was observed for procalcitonin, reaching its maximum value of 0.06 ng/mL for a patient infected with Staphylococcus aureus and 177,06 ng/mL for a patient infected with Acinetobacter baumannii. This data confirms the urgent need for markers of bacterial infection in clinical settings.
Given the dynamics of inflammatory parameters and the likely change in pathogens causing VAP over a short time, the breath samples collected in multiple days capture distinct physiological conditions. On the one hand, this approach reflects different stages of the disease (e.g., early, mid, or late infection), providing meaningful information for the statistical model. On the other hand, changing conditions reduce the concern about sample duplication as well as bias related to patient-specific patterns and prevent sole sampling at the highest CRP/PCT values (which, as exemplified above, do not necessarily reflect the status of bacterial infection). Bearing this in mind, breath samples were collected from patients multiple times on different days if this did not disturb or interfere with routine clinical practice. Consequently, seventy-seven breath samples were collected from thirty-two VAP patients, and seventeen breath samples were measured from six control individuals whose microbiological cultures of collected BAL specimens remained sterile.
Only the in vitro confirmed metabolites (given in previous sections) were considered in the breath data analysis to ensure as high biological relevance of the results as possible. Amongst 39 VOCs significantly metabolized in vitro, 2-pentene was never found in the in vivo settings. The non-parametric Wilcoxon rank-sum test was performed for the remaining 38 VOCs detected in breath gas, revealing significantly different levels of 13 bacterial metabolites in breath samples collected from VAP and control (sterile) individuals. Another two metabolites, dimethyl sulfide and methacrolein, exhibit p-values just slightly exceeding the threshold for significance (0.059 and 0.077, respectively). Given their low p-values and the production of both compounds by bacteria investigated in vitro, we decided to further verify the usefulness of these two substances in the breath model. The compounds with significant p-values from the Wilcoxon rank-sum test (plus dimethyl sulfide and methacrolein) were then arranged according to their AUC values, calculated for discrimination of VAP from control patients in ROC analysis. Next, the prevalence of a given compound in the breath samples was considered, prioritizing at least 50% in one of the compared groups of patients so the candidate for a biomarker could be broadly applicable. The value of 50% was not considered a cut-off threshold since the relatively low occurrence of metabolites in the group of VAP patients may be related to a particular pathogen and not subject to population-specific variations. In turn, to avoid the pitfalls of multicollinearity between compounds included in a ROC analysis, the LASSO modeling was used, whereby the frequency value of >70% was considered to indicate the compounds with strong predictive power (a reliable feature for the model) and the value of <30% suggests that the compound might be prone to noise or exhibit high collinearity with other features. Therefore, the LASSO frequency value equal to 30% was the lowest acceptable for including a compound in the ROC model, but on the condition that this substance has proven to have very high biological relevance. The number of bacteria species producing a particular compound is thus another factor respected in reducing candidate biomarkers to avoid the overfitting of the ROC model, prioritizing the metabolites released by multiple bacteria. The detailed parameters for each metabolite are given in
Table 3.
According to the results of ROC analysis for individual features (
Table 3), the following compounds with a significant p-value of <0.05 for Wilcoxon rank-sum test do not reach the inclusion criteria for the multivariate model due to the LASSO penalization: 3-methyl-1-butene (frequency value of 0%), ethyl octanoate (10%), 1-octene (10%), 2-butene (20%), 2-nonanone (20%). Amongst the other three compounds reaching a required threshold LASSO frequency value of 30%, pentanal was excluded from further considerations as it was released by only one bacteria (
Escherichia coli), whereas 1-butanol and dimethyl sulfide passed further as they were released by three and all four tested bacteria, respectively. Ultimately, the following nine compounds were included in the multivariate ROC model differentiating VAP and control individuals: ethyl acetate, 3-methyl-1-butanol,
n-heptane, dimethyl disulfide, decanal, 1-butanol, ethyl methyl sulfide, dimethyl sulfide, and methacrolein. The performance of the ROC model composed of these nine bacterial metabolites is summarized in
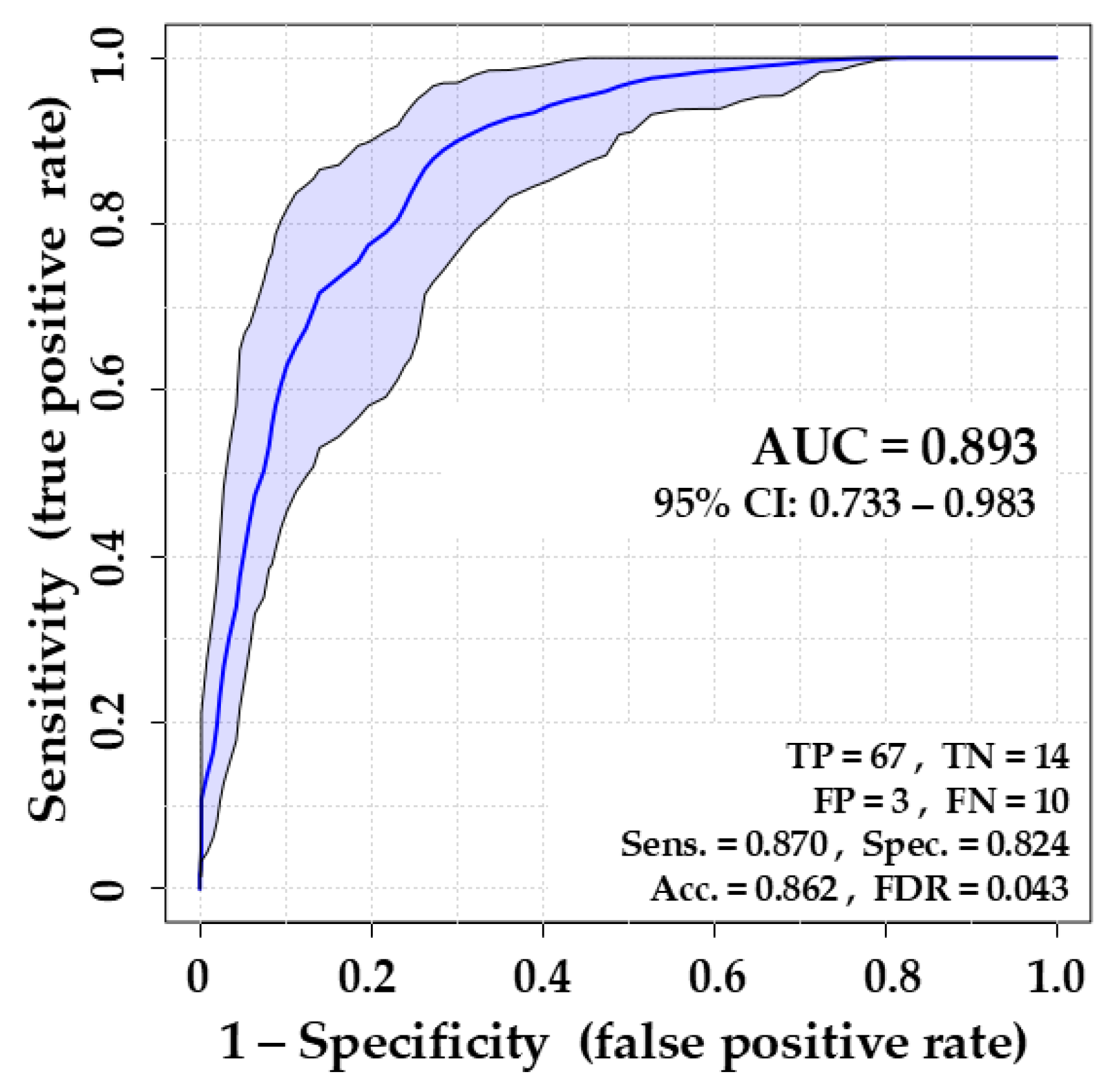
Figure 5.
The overall ability of the created ROC model based on nine bacterial metabolites to distinguish between positive (VAP) and negative (sterile) cases proved to be generally good, as the Area Under Curve reached the value of 0.893. The sensitivity (true positive rate) is 87%, allowing the correct identification of 67 out of 77 breath samples from VAP patients (leaving ten missed positive cases). It is an acceptable value, especially when supported with a solid specificity of 82% and an accuracy of 86%
3.2.3. VOCs Related to Underlying Pathogen
When focusing on the distinction between causative pathogens of VAP, one should assume that each species may have a unique metabolic signature or interact differently with the host. The volatile metabolites used to differentiate bacteria through exhaled breath analysis would need to reflect these pathogen-specific metabolic processes. For this purpose, it is reasonable to include the samples originating only from patients with a single-pathogen infection in the statistical calculation and exclude the cases with unknown contributions of co-existing mixed pathogens. Since this results in a small number of breath samples within the sub-groups (split according to the pathogen), we do not rely solely on PLS-DA for selecting discriminating metabolites. Instead, the following approach was undertaken. First, the metabolites reaching high values of variable importance in projection (VIP-scores) from PLS-DA were identified. Then, the non-parametric univariate ANOVA was performed to confirm that these metabolites are statistically significant across pathogen groups.
Focusing only on four bacteria investigated in this study, namely Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, altogether 13 VOC reaching VIP>1 could be found, including dimethyl disulfide, 1-undecene, 2-propanol, dimethyl sulfide, 1-propanol, ethanol, benzaldehyde, isoprene, propanal, acetaldehyde, 2-butanone, 2,3-butanedione, and methanethiol (Supplementary Figure SF1). The one-way non-parametric ANOVA (including correction for false discovery rate) proved the statistical significance for all of them except methanethiol (p=0.05097). A more detailed comparison of significance for the exhaled metabolites mentioned above between different pathogen types was performed using the non-parametric Kruskal-Wallis test for multiple (two-sided) comparisons. The results revealed statistical significance for 11 out of 13 mentioned VOCs when compared between breath samples from patients infected with sole Acinetobacter baumannii and sole Klebsiella pneumoniae (the only two non-significant for this pair of pathogens were ethanol and methanethiol). Six bacterial metabolites were found at significantly different levels in breath gas from VAP patients infected with Acinetobacter baumannii and Escherichia coli (dimethyl sulfide, isoprene, benzaldehyde, acetaldehyde, propanal, 2,3/butanedione). The exhaled levels of three metabolites (1-undecene, dimethyl sulfide, 1-propanol) reached statistical significance between patients infected with sole Klebsiella pneumoniae and sole Pseudomonas aeruginosa. For the remaining comparisons, no statistically significant differences were noted (except ethanol for Acinetobacter baumannii vs. Pseudomonas aeruginosa). The p-values of pair-wise comparison by non-parametric Kruskal-Wallis test are listed in Supplementary Table ST3.
Nevertheless, although the tested PLS-DA model passed the permutation test with p=0.003 from 1000 simulations (which suggests that the model's separation is better than random chance), it failed to pass the cross-validation, whereby both parameters evaluating the model were below the required value of 0.6, namely R2 = 0.384 (representing that the model does not fit well the training) and Q2 = 0.277 (reflecting the weak predictive ability of the model when applied to unseen data).
4. Discussion
Four microorganisms belonging to the most prevalent and dangerous pathogens in VAP patients were investigated in vitro regarding the metabolism of volatile organic compounds. The results confirm that two out of the reported 39 bacterial compounds, namely ethyl acetate and dimethyl sulfide, were produced by all four species, and the other nine compounds by three different pathogens, including methanethiol, ethyl methyl sulfide, dimethyl disulfide, acetaldehyde, isoprene, n-heptane, ethanol, 3-methyl-1-butanol, and 2-nonanone. On the contrary, four aldehydes, including methacrolein, 2-methylpropanal, 2-methyl-2-butenal, and benzaldehyde, were taken up by at least three pathogens under in vitro conditions. Consequently, the in vitro experiments presented here provide essential information in selecting bacteria-related volatiles that are highly potent for further in vivo studies.
The authors are aware that focusing only on 39 VOCs given in
Table 1 in the analysis of breath samples collected from ventilated patients may strongly limit this study, but we aim to ensure as high biological relevance as possible in selecting the candidates for biomarkers for VAP diagnosis. Using metabolites with confirmed origin, which are produced by at least one of the most prevalent bacteria in ICU settings, should not only increase the probability of correctly classifying the VAP patient but also allow the detection of metabolically active bacteria that are involved in biological pathways relevant to disease onset or progression, and not their inactive remnants (what is the risk of highly sensitive DNA-based analysis, such as PCR or LAMP).
The initially selected volatile metabolites of bacterial origin were carefuly verified, according to their values of AUC from ROC analysis, high LASSO frequency, significant p<0.05 for non-parametric ANOVA test with FDR correction, occurrence in breath samples and prevalence in the in vitro studies with bacteria cultures. All these activities ensure that metabolites chosen to the final ROC diagnostic model truly represent the active bacterial infection in the patients.
Concerning the here applied approach of multiple sampling from the same patient, it is commonly accepted and even recommended that when considering the kinetics of biomarkers, the assessment of serial measurements over time is more informative than a single value [
20]. By covering the broader timeframe, breath samples collected from a patient over multiple days could capture the metabolite changes linked to the progression of the disease, improving the ROC diagnostic model to be more reflective of real-world clinical dynamics.
The performance of the here presented diagnostic test is promising, especially considering the challenging nature of diagnosing VAP in critically ill patients due to the overlap of clinical symptoms and results of diagnostic methods with the other common pathologies, e.g., such as pulmonary contusions, atelectasis, pulmonary edema, and particularly ARDS.
In this regard, the AUC of 0.893 indicates very good discriminative ability, differentiating between VAP and non-VAP patients in approximately 89% of cases. The AUC value reached in this study is higher than that reported for CPIS, ranging from 0.67 to 0.76 [
7]. The AUC value of 89% is also superior to other recently reported studies on breath tests for VAP diagnosis [
12,
13].
A sensitivity of 87% is also very good, especially for life-threatening conditions like VAP, where missing a diagnosis could lead to severe consequences. Currently used clinical scores (CPIS) tend to have moderate sensitivity, often in the 60-80% range, depending on the population and specific criteria [
7]. In turn, the microbiological testing reaches sensitivities of 71% for BAL and 61% for PSB. Compared to other breath studies focused on VAP diagnosis, the present sensitivity of 87% is lower than reported 98% [
13] and 95% [
12].
A specificity of 82% is comparable with those for microbiological testing of BAL (80%) and PSB (76%) [
6] and higher than for CPIS score of 66% [
7] or other breath-based test with 49% [
13] and the range from 6% to 29% depending on individual substance [
12].
An accuracy of 86.2% is generally considered good, indicating that the breath test correctly classifies patients in over 86% of cases. In the context of VAP diagnostics, an accuracy above 80% is typically regarded as strong, especially given the challenges in diagnosing VAP.
A false Discovery Rate (FDR) of 4.3% is very good, as it indicates a low proportion of false positives among the positive results. This reduces the likelihood of unnecessarily treating patients who do not have VAP, which is important in ICU settings where overuse of antibiotics can lead to escalation of antibiotic resistance and the development of multidrug-resistant pathogens.
The evaluated performance of the proposed breath test, characterized by an AUC of 0.893, Sensitivity of 0.870, and Specificity of 0.824, suggests it is a promising diagnostic tool, comparable to other methods used in ICU settings for VAP diagnosis. Authors feel the necessity to underline that any breath test – at the current stage of knowledge – should not replace or compete with the existing diagnostic methods routinely used in clinical settings but complement the entire diagnostic procedure, particularly given the life-threatening nature of VAP and the high morbidity associated with missed diagnoses. Further validation in larger patient cohorts and comparison with current standards (e.g., microbiological confirmation) would be necessary to fully assess its clinical applicability.
5. Challenges and Study Limitations
The conceptual challenge in this study is the nature of breath analysis in general, as the concentrations of exhaled VOCs detected in the breath of mechanically ventilated patients can be affected by several factors, including ventilation settings and patient physiology. The influence of those parameters still needs to be evaluated to ensure reproducibility and manage variability, which is crucial to estimating the robustness of the proposed breath test.
Although focusing on VOCs with proven bacterial origin, identifying the specific microorganisms causing VAP by analyzing exhaled VOCs still requires further refinement, as the model presented here failed. Most probably, the small population size of patients enrolled in this study (when divided respectively to underlying pathogen) was insufficient to approach this problem accurately, indicating the main limitation of this study. Nevertheless, the indication of bacterial lung infection achieved in this study, although without identifying the underlying pathogen, is a significant clinical advantage, as it intensifies clinicians’ efforts for the particular patient.
6. Conclusions
The non-invasive nature of monitoring VOCs in breath gas and the unlimited accessibility of these samples are the obvious advantages over currently used BAL sampling for VAP diagnosis. Typically, non-invasive methods like clinical scoring systems or biomarkers (e.g., procalcitonin) often exhibit a moderate level of performance (sensitivity and accuracy ranging from 70-80%). The presented diagnostic breath-based test is competitive with most existing clinical methods for diagnosing VAP, reaching performance at 80-90% (AUC = 0.893, sensitivity = 0.870, and specificity = 0.824). Notably, only the volatile compounds linked to bacterial metabolism, reflecting the pathogen’s presence and ongoing infection, are directly targeted in the proposed breath-based test, improving its biological relevance. Altogether, the gathered results suggest this test might be a promising tool for VAP diagnosis, where high sensitivity is particularly important to prevent the development of life-threatening lung infections associated with high morbidity. However, further validation in larger patient cohorts in multicenter studies and comparison with current standards (e.g., microbiological confirmation) would be necessary to solidify its clinical utility in critical care settings.
Supplementary Materials
This manuscript has the following supplementary materials: Table ST1: proliferation rate of bacteria grown in vitro, Table ST2: VOCs emission from medical Respiratory Devices, Table ST3: Non-parametric Kruskal-Wallis test for significance of exhaled VOCs in patients infected with different pathogens, and Figure SF1: VIP scores for compounds included in PLS-DA model differentiation pathogen types by breath gas analysis.
Author Contributions
Conceptualization, W.F. and R.W.; methodology, W.F.; software, W.F. and K.Z.; validation, W.F.; formal analysis, W.F.; investigation, W.F., R.W., A.T., M.W., D.D., M.M., D.D., T.B.; resources, W.F., R.W., D.D., T.B.; data curation, W.F., R.W. and K.Z.; writing—original draft preparation, W.F.; writing—review and editing, W.F., R.W., T.B.; visualization, W.F.; supervision, W.F., R.W.; project administration, W.F.; funding acquisition, W.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NATIONAL SCIENCE CENTRE, POLAND, grant number 2017/26/D/NZ6/00136
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Bioethics Committee of the Nicolaus Copernicus University in Toru´n at the Ludwik Rydygier Collegium Medicum in Bydgoszcz under the number KB-218/2018.
Informed Consent Statement
Not applicable
Data Availability Statement
The dataset used and analyzed during the current study is available from the corresponding author upon reasonable request.
Acknowledgments
The authors are very thankful to Joanna Sierzputowska from the Department of Medical Analytics of the 10th Military Research Hospital and Polyclinic, Bydgoszcz, Poland, who kindly provided all clinical BAL specimens isolated from VAP patients and performed microbiological identification of detected strains.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Papazian, L.; Klompas, M.; Luyt, C. E. , Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xing, R.; Wang, C. , The effect of ventilator-associated pneumonia on the prognosis of intensive care unit patients within 90 days and 180 days. BMC Infect Dis 2021, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Mergulhao, P.; Pereira, J. G.; Fernandes, A. V.; Krystopchuk, A.; Ribeiro, J. M.; Miranda, D.; Castro, H.; Eira, C.; Morais, J.; Lameirao, C.; Gomes, S.; Leal, D.; Duarte, J.; Passaro, L.; Froes, F.; Martin-Loeches, I. , Epidemiology and Burden of Ventilator-Associated Pneumonia among Adult Intensive Care Unit Patients: A Portuguese, Multicenter, Retrospective Study (eVAP-PT Study). Antibiotics (Basel) 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Howroyd, F.; Chacko, C.; MacDuff, A.; Gautam, N.; Pouchet, B.; Tunnicliffe, B.; Weblin, J.; Gao-Smith, F.; Ahmed, Z.; Duggal, N. A.; Veenith, T. , Ventilator-associated pneumonia: pathobiological heterogeneity and diagnostic challenges. Nat Commun 2024, 15, 6447. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.; Hellyer, T. P.; Baudouin, S. V.; Conway Morris, A.; Linnett, V.; McAuley, D. F.; Perkins, G. D.; Simpson, A. J. , A national survey of the diagnosis and management of suspected ventilator-associated pneumonia. BMJ Open Respir Res 2014, 1, e000066. [Google Scholar] [CrossRef] [PubMed]
- Ego, A.; Preiser, J. C.; Vincent, J. L. , Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest 2015, 147, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.; Martin-Loeches, I.; Povoa, P.; Rodriguez, A.; Salluh, J.; Duhamel, A.; Nseir, S.; group, T. A. s. , Accuracy of the clinical pulmonary infection score to differentiate ventilator-associated tracheobronchitis from ventilator-associated pneumonia. Ann Intensive Care 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Beer, R.; Sponring, A.; Filipiak, A.; Ager, C.; Schiefecker, A.; Lanthaler, S.; Helbok, R.; Nagl, M.; Troppmair, J.; Amann, A. , Breath analysis for in vivo detection of pathogens related to ventilator-associated pneumonia in intensive care patients: a prospective pilot study. J Breath Res 2015, 9, 016004. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Fijten, R.; Smolinska, A.; Dallinga, J.; Boumans, M. L.; Stobberingh, E.; Boots, A.; Roekaerts, P.; Bergmans, D.; van Schooten, F. J. , Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci Rep 2015, 5, 17179. [Google Scholar] [CrossRef] [PubMed]
- van Oort, P. M.; de Bruin, S.; Weda, H.; Knobel, H. H.; Schultz, M. J.; Bos, L. D.; On Behalf Of The Mars, C. , Exhaled Breath Metabolomics for the Diagnosis of Pneumonia in Intubated and Mechanically-Ventilated Intensive Care Unit (ICU)-Patients. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Schmidt, M.; Oertel, P.; Obermeier, J.; Brock, B.; Kamysek, S.; Dunkl, J.; Zimmermann, R.; Schubert, J. K.; Miekisch, W. , Continuous real time breath gas monitoring in the clinical environment by proton-transfer-reaction-time-of-flight-mass spectrometry. Anal Chem 2013, 85, 10321–9. [Google Scholar] [CrossRef] [PubMed]
- Felton, T. W.; Ahmed, W.; White, I. R.; van Oort, P.; Rattray, N. J. W.; Docherty, C.; Bannard-Smith, J.; Morton, B.; Welters, I.; McMullan, R.; Roberts, S. A.; Goodacre, R.; Dark, P. M.; Fowler, S. J. , Analysis of exhaled breath to identify critically ill patients with ventilator-associated pneumonia. Anaesthesia 2023, 78, 712–721. [Google Scholar] [CrossRef] [PubMed]
- van Oort, P. M.; Nijsen, T. M.; White, I. R.; Knobel, H. H.; Felton, T.; Rattray, N.; Lawal, O.; Bulut, M.; Ahmed, W.; Artigas, A.; Povoa, P. R.; Martin-Loeches, I.; Weda, H.; Goodacre, R.; Schultz, M. J.; Dark, P. M.; Fowler, S. J.; Bos, L. D.; BreathDx, C. , Untargeted Molecular Analysis of Exhaled Breath as a Diagnostic Test for Ventilator-Associated Lower Respiratory Tract Infections (BreathDx). Thorax 2022, 77, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J. P. , 3rd, Hospital-acquired pneumonia: risk factors, microbiology, and treatment. Chest 2001, 119 (Suppl. 2), 373S–384S. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M. S.; Kamble, E. E.; Kumkar, S. N.; Tawre, M. S.; Pardesi, K. R. , Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front Microbiol 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Delle Rose, D.; Pezzotti, P.; Fortunato, E.; Sordillo, P.; Gini, S.; Boros, S.; Meledandri, M.; Gallo, M. T.; Prignano, G.; Caccese, R.; D'Ambrosio, M.; Citterio, G.; Rocco, M.; Leonardis, F.; Natoli, S.; Fontana, C.; Favaro, M.; Celeste, M. G.; Franci, T.; Testore, G. P.; Andreoni, M.; Sarmati, L. , Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: a retrospective analysis in six Italian hospitals. Eur J Clin Microbiol Infect Dis 2016, 35, 1531–9. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, T. P.; Morris, A. C.; McAuley, D. F.; Walsh, T. S.; Anderson, N. H.; Singh, S.; Dark, P.; Roy, A. I.; Baudouin, S. V.; Wright, S. E.; Perkins, G. D.; Kefala, K.; Jeffels, M.; McMullan, R.; O'Kane, C. M.; Spencer, C.; Laha, S.; Robin, N.; Gossain, S.; Gould, K.; Ruchaud-Sparagano, M. H.; Scott, J.; Browne, E. M.; MacFarlane, J. G.; Wiscombe, S.; Widdrington, J. D.; Dimmick, I.; Laurenson, I. F.; Nauwelaers, F.; Simpson, A. J. , Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia. Thorax 2015, 70, 41–7. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Wenzel, M.; Ager, C.; Mayhew, C. A.; Bogiel, T.; Wlodarski, R.; Nagl, M. , Molecular Analysis of Volatile Metabolites Synthesized by Candida albicans and Staphylococcus aureus in In Vitro Cultures and Bronchoalveolar Lavage Specimens Reflecting Single- or Duo-Factor Pneumonia. Biomolecules 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Zuchowska, K.; Filipiak, W. , Modern approaches for detection of volatile organic compounds in metabolic studies focusing on pathogenic bacteria: Current state of the art. J Pharm Anal 2024, 14, 100898. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P.; Coelho, L.; Cidade, J. P.; Ceccato, A.; Morris, A. C.; Salluh, J.; Nobre, V.; Nseir, S.; Martin-Loeches, I.; Lisboa, T.; Ramirez, P.; Rouze, A.; Sweeney, D. A.; Kalil, A. C. , Biomarkers in pulmonary infections: a clinical approach. Ann Intensive Care 2024, 14, 113. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Growth curves of bacteria cultivated in vitro: (A) Acinetobacter baumannii, (B) Escherichia coli, (C) Klebsiella pneumonia, (D) Pseudomonas aeruginosa. Colony Forming Units (CFU/ml) are plotted after logarithmic transformation in the function of incubation time.
Figure 1.
Growth curves of bacteria cultivated in vitro: (A) Acinetobacter baumannii, (B) Escherichia coli, (C) Klebsiella pneumonia, (D) Pseudomonas aeruginosa. Colony Forming Units (CFU/ml) are plotted after logarithmic transformation in the function of incubation time.
Figure 2.
Comparison of time-dependent profiles for production of ethyl acetate from (A) Acinetobacter baumannii, (B) Escherichia coli, (C) Klebsiella pneumoniae and (D) Pseudomonas aeruginosa.
Figure 2.
Comparison of time-dependent profiles for production of ethyl acetate from (A) Acinetobacter baumannii, (B) Escherichia coli, (C) Klebsiella pneumoniae and (D) Pseudomonas aeruginosa.
Figure 3.
Comparison of time-dependent profiles for production of dimethyl sulfide from (A) Acinetobacter baumannii, (B) Escherichia coli, (C) Klebsiella pneumoniae and (D) Pseudomonas aeruginosa.
Figure 3.
Comparison of time-dependent profiles for production of dimethyl sulfide from (A) Acinetobacter baumannii, (B) Escherichia coli, (C) Klebsiella pneumoniae and (D) Pseudomonas aeruginosa.
Figure 4.
Emission from the medical respiratory device parts (endotracheal tube with disposable catheter mount) for exemplary VOCs: (A) p-xylene, (B) ethyl acetate, (C) 1-butanol and (D) dimethyl sulfide.
Figure 4.
Emission from the medical respiratory device parts (endotracheal tube with disposable catheter mount) for exemplary VOCs: (A) p-xylene, (B) ethyl acetate, (C) 1-butanol and (D) dimethyl sulfide.
Figure 5.
Performance of the Receiver Operating Characteristic (ROC) model composed of nine bacterial metabolites (ethyl acetate, 3-methyl-1-butanol, n-heptane, dimethyl disulfide, decanal, 1-butanol, ethyl methyl sulfide, dimethyl sulfide, and methacrolein) for discrimination of breath samples of VAP patients and uninfected controls. AUC: Area Under Curve, CI: Confidence Intervals, TP: True Positive, TN: True Negative, FP: False positive, FN: False Negative, Sens.: Sensitivity = TP/(TP+FN), Spec.: Specificity = TN/(TN+FP), Acc.: Accuracy = (TP+TN)/(P+N), FDR: False Discovery Rate = FP/(FP+TP).
Figure 5.
Performance of the Receiver Operating Characteristic (ROC) model composed of nine bacterial metabolites (ethyl acetate, 3-methyl-1-butanol, n-heptane, dimethyl disulfide, decanal, 1-butanol, ethyl methyl sulfide, dimethyl sulfide, and methacrolein) for discrimination of breath samples of VAP patients and uninfected controls. AUC: Area Under Curve, CI: Confidence Intervals, TP: True Positive, TN: True Negative, FP: False positive, FN: False Negative, Sens.: Sensitivity = TP/(TP+FN), Spec.: Specificity = TN/(TN+FP), Acc.: Accuracy = (TP+TN)/(P+N), FDR: False Discovery Rate = FP/(FP+TP).
Table 1.
VOCs metabolized by Acinetobacter baumannii (ABA), Escherichia coli (ECO), Klebsiella pneumoniae (KPN) and Pseudomonas aeruginosa (PAE). The upward arrow (↑) indicates the release of a respective metabolite either with the amount proportional to the bacteria load (RPB) or release with the temporary maximum (RTM), after which the abundance of metabolites decreases. The downward arrow (↓) indicates an uptake (UPT) of a respective metabolite from the TSB medium.
Table 1.
VOCs metabolized by Acinetobacter baumannii (ABA), Escherichia coli (ECO), Klebsiella pneumoniae (KPN) and Pseudomonas aeruginosa (PAE). The upward arrow (↑) indicates the release of a respective metabolite either with the amount proportional to the bacteria load (RPB) or release with the temporary maximum (RTM), after which the abundance of metabolites decreases. The downward arrow (↓) indicates an uptake (UPT) of a respective metabolite from the TSB medium.
| VOCs |
CAS |
tR [min] |
target ion [m/z] |
Reference ions [m/z] |
ABA |
ECO |
KPN |
PAE |
| 2-Butene |
107-01-7 |
12.766 |
41 |
56, 39, 55 |
↑RTM |
↓UPT |
- |
↑RPB |
| Isoprene |
78-79-5 |
24.927 |
67 |
68, 53, 39 |
↑RPB |
↑RTM |
↑RTM |
- |
| 3-methyl-1-butene |
563-45-1 |
24.148 |
55 |
70, 41, 42 |
- |
- |
↑RPB |
↑RPB |
| (E)-2-Pentene |
109-68-2 |
25.680 |
55 |
70, 53, 56 |
↑RPB |
- |
↑RTM |
- |
| n-Heptane |
142-82-5 |
51.257 |
71 |
57, 43, 100 |
↑RTM |
↑RTM |
- |
↑RPB |
| 1-Octene |
111-66-0 |
58.528 |
55 |
70, 41, 83 |
- |
↑RPB |
- |
- |
| 1-Undecene |
821-95-4 |
73.600 |
55 |
70, 83, 97, 111 |
↑RPB |
↑RPB |
- |
↑RPB |
| Acetaldehyde |
75-07-0 |
9.644 |
43 |
44, 42, 41 |
↑RTM |
↑RPB |
↑RPB |
- |
| Propanal |
123-38-6 |
18.263 |
57 |
55, 39, 37 |
↑RTM |
↑RPB |
- |
- |
| Methacrolein |
78-85-3 |
28.261 |
70 |
41, 39, 42 |
↑RTM |
↓UPT |
↓UPT |
↓UPT |
| 2-Methylpropanal |
78-84-2 |
28.588 |
72 |
41, 43, 39 |
↑RTM |
↓UPT |
↓UPT |
↓UPT |
| Butanal |
123-72-8 |
31.169 |
44 |
72, 57, 41 |
- |
- |
- |
↓UPT |
| 2-Butenal |
4170-30-3 |
35.863 |
70 |
41, 39, 69 |
- |
↑RTM |
- |
- |
| 2-Methyl-2-Butenal |
1115-11-3 |
47.964 |
84 |
55, 39, 41 |
- |
↓UPT |
↓UPT |
↓UPT |
| 2-Ethylacrolein |
922-63-4 |
41.552 |
55 |
84, 56, 39 |
- |
↓UPT |
- |
↓UPT |
| 3-Methylbutanal |
590-86-3 |
42.417 |
58 |
71, 41, 86 |
- |
↓UPT |
↑RTM |
↓UPT |
| Pentanal |
110-62-3 |
45.598 |
44 |
58, 41, 57 |
↓UPT |
- |
- |
- |
| Benzaldehyde |
100-52-7 |
65.584 |
106 |
105, 77, 51 |
↓UPT |
↓UPT |
↓UPT |
↓UPT |
| Octanal |
124-13-0 |
70.320 |
84 |
69, 56, 100 |
↓UPT |
- |
- |
- |
| Decanal |
112-31-2 |
76.452 |
57 |
43, 55, 82 |
- |
↑RTM |
- |
- |
| Ethanol |
64-17-5 |
14.000 |
45 |
46, 43 |
- |
↑RPB |
↑RPB |
↑RPB |
| 1-Propanol |
71-23-8 |
26.439 |
59 |
42, 60, 41 |
- |
↑RPB |
↑RPB |
- |
| 2-Propanol |
67-63-0 |
22.209 |
45 |
43, 41, 59 |
- |
- |
- |
↑RPB |
| 2-Methyl-1-Propanol |
78-83-1 |
36.500 |
43 |
41, 56, 74 |
- |
↑RPB |
↑RPB |
- |
| 1-Butanol |
71-36-3 |
39.800 |
56 |
45, 41, 43 |
↑RTM |
- |
↑RTM |
- |
| 3-Methyl-1-Butanol |
123-51-3 |
50.395 |
55 |
70, 42, 41 |
↑RPB |
↑RPB |
↑RPB |
- |
| Ethyl Acetate |
141-78-6 |
33.382 |
43 |
70, 61, 45, 88 |
↑RTM |
↑RPB |
↑RPB |
↑RPB |
| Ethyl Octanoate |
106-32-1 |
75.589 |
88 |
57, 101, 127 |
- |
↑RTM |
↑RTM |
- |
| 2-Butanone |
78-93-3 |
31.539 |
72 |
43, 57, 42 |
- |
- |
↑RPB |
- |
| 2,3-Butanedione |
431-03-8 |
31.663 |
86 |
43, 42, 41 |
- |
- |
↑RTM |
- |
| 3-Penten-2-on |
625-33-2 |
48.521 |
69 |
84, 55, 41 |
- |
↑RTM |
- |
- |
| 2-Pentanone |
107-87-9 |
44.911 |
43 |
86, 71, 58 |
- |
- |
↑RPB |
↑RPB |
| 2-Heptanone |
110-43-0 |
63.400 |
58 |
43, 71, 114 |
- |
- |
↑RPB |
↑RTM |
| 2-Nonanone |
821-55-6 |
73.080 |
58 |
43, 71, 142 |
↑RPB |
- |
↑RPB |
↑RPB |
| Methanethiol |
74-93-1 |
10.900 |
47 |
48, 45, 46 |
↑RTM |
- |
↑RPB |
↑RTM |
| Dimethyl Sulfide |
75-18-3 |
20.432 |
62 |
47, 45, 61 |
↑RPB |
↑RTM |
↑RTM |
↑RPB |
| Ethyl Methyl Sulfide |
624-89-5 |
32.634 |
61 |
76, 48, 47 |
- |
↑RTM |
↑RTM |
↑RPB |
| Dimethyl Disulfide |
624-92-0 |
47.136 |
94 |
43, 79, 61 |
- |
↑RPB |
↑RPB |
↑RPB |
| 2-Methylfuran |
534-22-5 |
30.814 |
82 |
53, 81, 39 |
- |
- |
- |
↓UPT |
Table 3.
Results of ROC analysis discriminating breath samples collected from VAP (n=77) and control (n=17) individuals. AUC was calculated using the Linear Supported Vector Machine algorithm, which is robust for compounds with low occurrence in the tested population.
Table 3.
Results of ROC analysis discriminating breath samples collected from VAP (n=77) and control (n=17) individuals. AUC was calculated using the Linear Supported Vector Machine algorithm, which is robust for compounds with low occurrence in the tested population.
| VOCs |
VAP n>0 |
VAP |
Sterile n>0 |
Steril |
Wilcoxon Rank-Sum |
LASSO freq. [%] |
AUC |
| Ethyl Acetate |
77 |
100% |
14 |
82% |
0,00001 |
100 |
0.824 |
| 3-Methyl-1-Butanol |
51 |
66% |
3 |
18% |
0,00010 |
100 |
0.762 |
|
n-Heptane |
75 |
97% |
13 |
76% |
0,00014 |
100 |
0.753 |
| Dimethyl Disulfide |
25 |
32% |
0 |
0% |
0,00740 |
100 |
0.573 |
| 1-Octene |
75 |
97% |
13 |
76% |
0,00093 |
10 |
0.568 |
| Decanal |
49 |
64% |
3 |
18% |
0,00638 |
100 |
0.56 |
| Pentanal |
54 |
70% |
6 |
35% |
0,01730 |
30 |
0.533 |
| 1-Butanol |
19 |
25% |
0 |
0% |
0,02416 |
30 |
0.492 |
| 2-Nonanone |
3 |
4% |
3 |
18% |
0,04134 |
20 |
0.488 |
| 2-Butene |
77 |
100% |
17 |
100% |
0,02641 |
20 |
0.486 |
| Ethyl Methyl Sulfide |
0 |
0% |
4 |
24% |
0,00002 |
80 |
0.467 |
| 3-methyl-1-butene |
76 |
99% |
17 |
100% |
0,01744 |
0 |
0.467 |
| Ethyl Octanoate |
0 |
0% |
3 |
18% |
0,00021 |
10 |
0.459 |
| Dimethyl Sulfide |
75 |
97% |
14 |
82% |
0,05927 |
30 |
0.452 |
| Methacrolein |
77 |
100% |
16 |
94% |
0,07703 |
90 |
0.432 |
| 2,3-Butanedione |
75 |
97% |
12 |
71% |
0,12534 |
50 |
0.434 |
| 3-Methylbutanal |
24 |
31% |
7 |
41% |
0,13259 |
60 |
0.466 |
| 2-Butenal |
20 |
26% |
7 |
41% |
0,14670 |
90 |
0.448 |
| 2-Heptanone |
10 |
13% |
4 |
24% |
0,16042 |
60 |
0.466 |
| Benzaldehyde |
77 |
100% |
13 |
76% |
0,17835 |
40 |
0.432 |
| 2-Methylpropanal |
50 |
65% |
5 |
29% |
0,21740 |
100 |
0.475 |
| 2-Propanol |
71 |
92% |
17 |
100% |
0,23453 |
100 |
0.437 |
| 2-Butanone |
77 |
100% |
16 |
94% |
0,32112 |
10 |
0.447 |
| 3-Penten-2-on |
45 |
58% |
6 |
35% |
0,32642 |
0 |
0.55 |
| 2-Methyl-2-Butenal |
8 |
10% |
3 |
18% |
0,34197 |
10 |
0.480 |
| 2-Ethylacrolein |
12 |
16% |
1 |
6% |
0,34245 |
100 |
0.498 |
| Methanethiol |
70 |
91% |
14 |
82% |
0,34789 |
60 |
0.493 |
| Isoprene |
77 |
100% |
17 |
100% |
0,38197 |
100 |
0.485 |
| 1-Undecene |
40 |
52% |
9 |
53% |
0,41677 |
20 |
0.481 |
| 2-Methyl-1-Propanol |
9 |
12% |
1 |
6% |
0,44076 |
40 |
0.492 |
| 2-Methylfuran |
77 |
100% |
17 |
100% |
0,46127 |
0 |
0.476 |
| Ethanol |
73 |
95% |
17 |
100% |
0,53599 |
40 |
0.511 |
| Acetaldehyde |
77 |
100% |
17 |
100% |
0,70894 |
0 |
0.514 |
| 2-Pentanone |
74 |
96% |
17 |
100% |
0,71625 |
70 |
0.518 |
| Octanal |
55 |
71% |
9 |
53% |
0,76448 |
100 |
0.531 |
| 1-Propanol |
56 |
73% |
17 |
100% |
0,95274 |
80 |
0.507 |
| Propanal |
75 |
97% |
16 |
94% |
0,96866 |
90 |
0.522 |
| Butanal |
77 |
100% |
16 |
94% |
1,00000 |
10 |
0.553 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).