Introduction
Urogenital infections (UGI)are among the most common complications of Diabetes mellitus (DM) [
1]. The UGIs are caused commonly by microbial infectants include bacteria such as Escherichia coli, Klebsiella pneumoniae, and fungi such as Candida species [
2,
3] The prevalence of UGIs among Indian population is reported to be 1.3% for UTI and 5.3% for mycotic infections. [
4].
Among several therapeutic Oral Hypoglycaemic agents (OHA / OHGA), Sodium-glucose cotransporter 2 (SGLT2) inhibitors (ATC code A10BK) are a relatively recent and novel class particularly used in Type 2 DM. The SGLT2is have demonstrated substantial efficacy in glycaemic control, and in improving cardiovascular and renal outcomes in T2DM. [
5] However, considering the pharmacological action of SGLT2 inhibitors that promotes excretory elimination of blood sugar to reduce glycaemia, glycosuria develops and thereby increase the risk of urogenital infections(UGI) [
6]. This association of increased UGI risk is considered to be more prominent with increased dose of SGLT2is, probably pertaining to increased glycosuria [
5]. Various clinical trials on SGLT2i has reported urogenital infections among the frequent adverse events. [
6] These adverse effects have contributed to drug discontinuation and hospitalization in severe cases. This is particularly relevant as SGLT2 inhibitors exert their therapeutic effects by promoting glycosuria, thereby certainly increasing the risk of genital mycotic infections and urinary tract infections (UTIs). [
7]
Previous research has reported an association between SGLT2 inhibitors and UGIs. However, comprehensive studies aiming these effects in specific populations remain limited [
8]. Furthermore, several studies exploring the relationship between SGLT2 inhibitors and urogenital infections, there remains a paucity of comprehensive, region-specific research in this area [
8]. Therefore, the necessity to comprehensively assess the incidence and risk factors associated with urogenital infections in patients receiving SGLT2 inhibitors is significant [
9]. This study addresses these gaps by conducting a comprehensive analysis over a six-month period, contributing valuable insights into the epidemiology and clinical implications of urogenital infections associated with SGLT2 inhibitor therapy [
9].
This prospective observational study is designed to provide real-world data on this association, to enhance clinical understanding and patient care. The study aims to specify the region-specific epidemiology of SGLT2 inhibitors associated UGIs and identify and mitigate gaps in the literature particularly for Southern Indian population. [
10]
Materials and Methods
Study Population
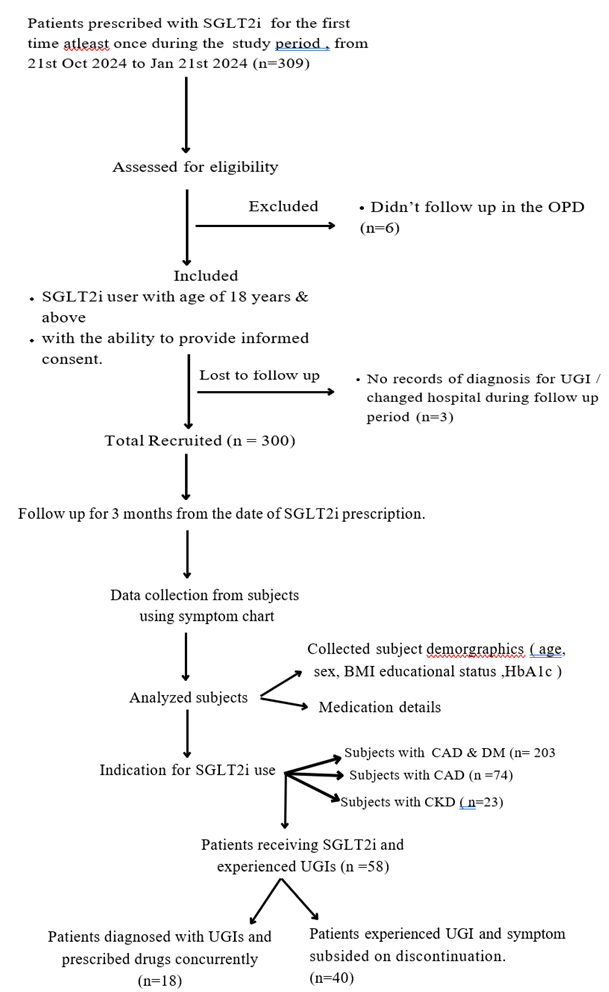
This prospective observational study was conducted at a tertiary care hospital, Malankara Orthodox Syrian Church Medical College Hospital in Kerala, India. The study included all patients aged ≥ 18 years who were prescribed SGLT2 inhibitors particularly dapagliflozin for the first time and to be continued as a regular medication. Patients required to provide written informed consent. The patients with a past history of recurrent urinary tract infections, subjects who were anticipated to have limitations in completing the study or those who refused to sign informed consent or participate were excluded from the study. All the patients received SGLT2i group medicine, Dapagliflozin (ATC code A10BK01). The study was designed to be an epidemiological observational study, ensuring availability of uninterfered observations of incidents and progression of UGIs, and the real-world data of the risk factors and clinical outcomes.
Sample Size Determination and Data Source
The patients attending the OP department receiving SGLT2i for therapy of T2DM, for the first time from October 21, 2023, to January 21, 2024, were enrolled and were followed up them for the next 3 months from initiation. The data included data on exposure (SGLT2 inhibitor use) and outcomes (occurrence of genital mycotic infections and urinary tract infections (UTIs)) in and the study objective was proved using temporal relationships and determine relative risk.
The exposure was defined as time of consumption of SGLT2 inhibitor therapy. The exposure was calculated using difference between date of outcome and date of start of treatment. represented by the variable SGLT2 inhibitor (specifically dapagliflozin) exposure. The outcome was defined as clinical development of urogenital infections or 90 days after the therapy, whichever was earlier. The positive outcome alias urogenital infections (UGIs) were defined as a clinical manifestation and symptoms either described by the patient or diagnosed by the clinician, which reveal a positive response to antifungal or antimicrobial medications. Any symptoms of urogenital infections that developed with SGLT2i consumption, followed by their resolution upon discontinuation, were considered as a potential association.
The epidemiological sample was calculated using Cochran’s formula.
where IE is incident of UGIs in SGLT2i-exposed population and INE is incident of UGIs of unexposed population. In this 4% is the assumed margin of error.
where IE is incident of UGIs in SGLT2i-exposed population and INE is incident of UGIs of unexposed population. In this 4% is the assumed margin of error.
where IEn is incident of UGIs in lower dose of SGLT2i-exposed population and IEn+1 is incident of UGIs sequentially higher dose of SGLT2i-exposed population.
The baseline study data was collected from hospital medical records and laboratory tests. All the eligible patients were contacted and the data was prospectively collected for development of UGIs for three months from the day of start of SGLT2i therapy. Symptom chart was prepared and used as a checklist.
Exposure and Outcome
The exposure variable was the use of SGLT2 inhibitors or dapagliflozin and the outcome variable was urogenital infections. We defined urogenital infections as clinical manifestations and symptoms either described by the patient or diagnosed by the clinician, which show a positive response to anti-fungal or anti-microbial medications. Any symptoms of urogenital infections that developed with SGLT2 inhibitors consumption, followed by their resolution upon discontinuation, were considered as a potential association.
Statistical Analysis
Data analysis was performed using IBM SPSS Statistics version 27. Descriptive statistics including means, frequencies, and percentages were used to summarize the data. Inferential statistical tests, such as chi-square tests and logistic regression, were used to analyse the data. The level of significance was set at p value < 0.05, and the confidence intervals were set at 95%.
Results
Demographics
The study population exhibited a range of demographic and clinical characteristics. Over half (53%) of the participants were aged 60 years or older, with the next largest age group being those aged 51-60 years (27.3%). Males comprised the majority (64.3%) of the study population. In terms of education level, most participants had completed secondary education (51%), followed by those with an undergraduate degree (25%), primary education (19.3%), and postgraduate education (4.7%). The majority of participants were overweight, with 53.7% having a body mass index (BMI) between 24-30, while 37.7% fell within the normal BMI range of 18-24. Regarding medication dosage, 69.33% of participants were prescribed a 10 mg dose, while 30.66% received a 5 mg dose. (
Table 1)
Symptomology
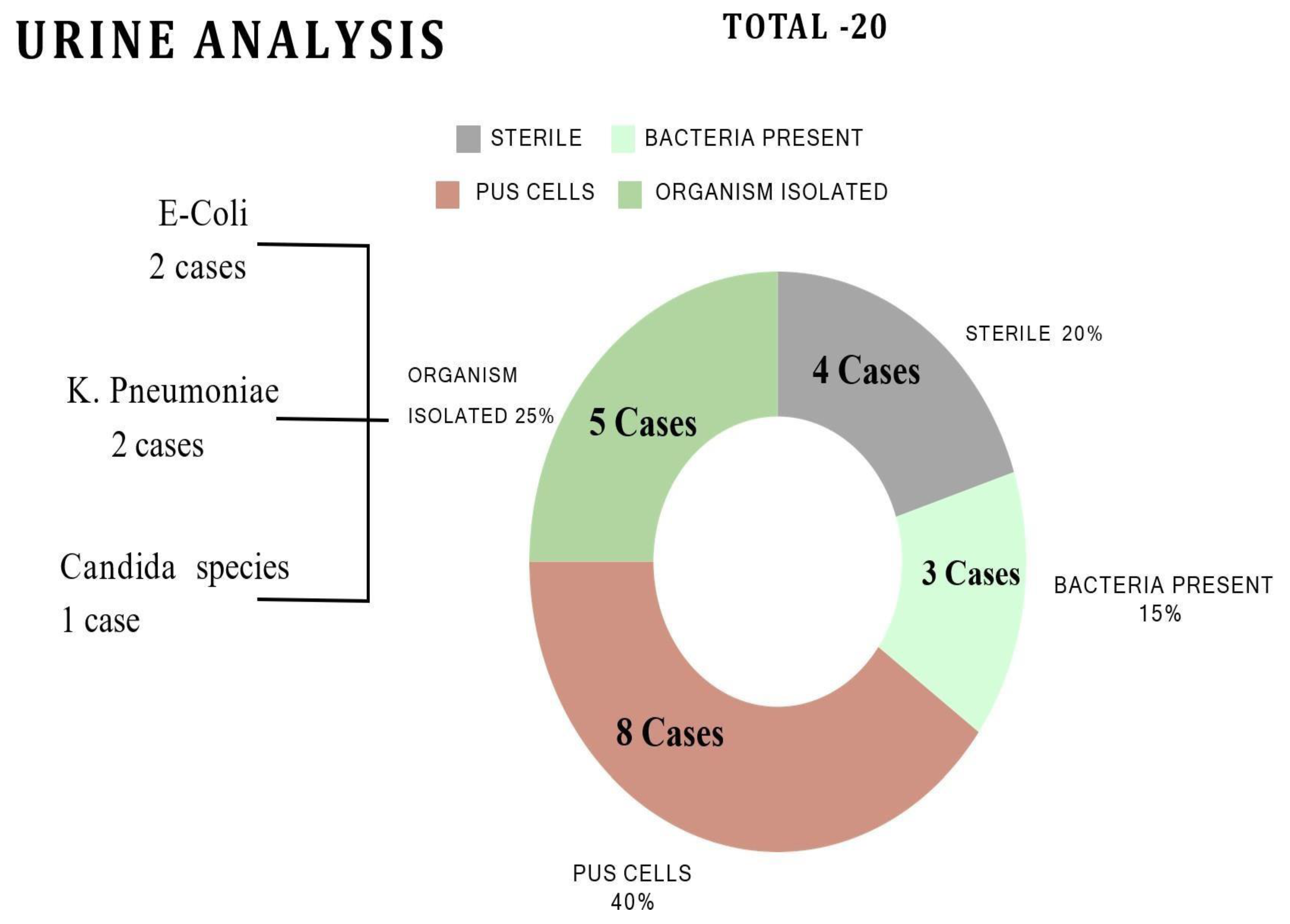
Common symptoms in males included abdominal pain (62%, 21/34), burning sensation (44%, 15/34), and painful urination (38%, 13/34), while females experienced itching (58%, 14/24), painful urination (46%, 11/24), and yellowish-white discharge (33%, 8/24). Microbiological analysis identified Escherichia coli, Klebsiella pneumoniae and Candida species (
Figure 1).
Analysis of Exposure
Among the 309 SGLT2i initiators, 300 users were followed up. The mean age of the study population was 60.55 years (SD = 10.2), and the participants aged 60 years and above were 53%. Males accounted for 64.3% (193/300) of the sample, while females accounted for 35.7% (107/300). Mean BMI was 24.97 (SD = 3.5), and mean HbA1c was 8.05% (SD = 1.2).
Incidence of Urogenital Infections
No significant association was found between HbA1c levels and number of infected patients (p > 0.05). The incidence of urogenital infections (UGI) was 19.3% (58/300), with a 95% confidence interval (CI) of 14.5-24.1%. Bacterial and fungal infections occurred in 16% (48/300) and 3% (9/300) of the patients, respectively(
Table 2). Males had a slightly lower incidence rate (18%, 35/193) than females 21%, (23/107), although this difference was not statistically significant (p = 0.874).
Primary Hypothesis Testing
Hypothesis: The use of SGLT2 inhibitors is associated with an increased risk of urogenital infections (UGIs) in patients attending a tertiary care hospital.
Null Hypothesis (H0): There is no significant association between SGLT2 inhibitors and UGIs.
Alternative Hypothesis (H1): There is a significant association between SGLT2 inhibitors and UGIs.
Testing the Hypothesis: The association between SGLT2 inhibitors and UGIs was tested using logistic regression analysis.
Results: The results showed a statistically significant association between SGLT2 inhibitors and UGIs (p < 0.05). The incidence of UGIs was 19.3% (58/300), with a 95% confidence interval (CI) of 14.5-24.1%.
Conclusion: The primary hypothesis was accepted, indicating that SGLT2 inhibitors are associated with an increased risk of UGIs.
Secondary and Exploratory Analysis - Dose-Response and Demographic Factors : (Table 6)
A significant dose-response relationship was observed with patients receiving 10 mg daily dose having a higher incidence of infections (55.2%, 32/58) than those receiving the 5 mg daily dose (44.8%, 26/58) (p < 0.05). No significant associations were found between the duration of SGLT2 inhibitor use, age, sex, BMI, educational status , and the occurrence of urogenital infections. Logistic regression analysis revealed that only dose was significantly associated with infection risk (OR = 1.43, 95% CI = 1.03-1.98, p < 0.05).
Table 4.
BACTERIAL SYMPTOMS IN MALES AND FEMALES.
Table 4.
BACTERIAL SYMPTOMS IN MALES AND FEMALES.
| SYMPTOMS |
MALE |
SYMPTOMS |
FEMALE |
Abdominal pain
/Pressure or cramping in the lowest abdomen |
6 |
Abdominal pain /Pressure or cramping in the lowest abdomen |
6 |
| Back pain or discomfort |
3 |
Back pain or discomfort |
5 |
| Burning sensation |
9 |
Burning sensation |
2 |
|
Cloudy urine / frothy urine |
3 |
Cloudy urine / frothy urine |
3 |
| Decreased urine output |
2 |
Decreased urine output |
1 |
| Dysuria |
6 |
Dysuria |
6 |
| Epididymo-orchitis |
1 |
Fever/ chills |
4 |
| Fever/ chills |
3 |
Feeling of incomplete emptying of the bladder |
1 |
|
Feeling of incomplete emptying of the bladder |
1 |
Itching |
12 |
| Itching |
12 |
Inability to start urine stream |
3 |
| Inability to start urine stream |
3 |
Increased frequency of urination |
4 |
| Increased frequency of urination |
6 |
Nausea |
1 |
| Redness |
5 |
Redness |
2 |
| Swelling |
1 |
Polydypsia |
1 |
| Tenderness |
2 |
Polyuria |
6 |
| Polydypsia |
1 |
Pyelonephritis |
1 |
| Polyuria |
1 |
Painful urination |
11 |
| Pyelonephritis |
1 |
Urgency to urinate |
4 |
| Prostatic abscess |
1 |
Vomiting |
1 |
| Painful urination |
9 |
Urosepsis |
1 |
|
Pinpoint pain |
2 |
|
| Urgency to urinate |
1 |
|
| Vomiting |
1 |
|
| Urosepsis |
1 |
|
Table 5.
SYMPTOMS OF FUNGAL INFECTIONS IN MALES.
Table 5.
SYMPTOMS OF FUNGAL INFECTIONS IN MALES.
| SYMPTOMS |
MALES |
FEMALES |
| Itching |
12 |
12 |
| Balanitis/ Balanoposthitis |
10 |
|
| Burning sensation |
7 |
|
| Soreness |
8 |
|
| Tenderness |
6 |
|
| Redness |
13 |
|
| Swelling |
9 |
|
| Vulvovaginal candidiasis |
|
1 |
| Yellowish-white discharge |
|
7 |
Table 6.
Dose - Response Relationship.
Table 6.
Dose - Response Relationship.
| SGLT2i Dose Level |
Number of Infected |
Total |
Pearson Chi-Square |
p value |
| No |
Yes |
| 5 mg |
86 (28.7%) |
6 (2%) |
92 (30.7%) |
13.965 |
<0.001 |
| 10 mg |
156 (52%) |
52 (17.3%) |
208 (69.3%) |
| Total |
242 (80.7%) |
58 (19.3%) |
300 (100%) |
Table 7.
Pearson Correlation for Onset of symptoms in Infected Males and Females.
Table 7.
Pearson Correlation for Onset of symptoms in Infected Males and Females.
| ONSET OF SYMPTOMS |
TOTAL |
MALES |
FEMALES |
Chi- square Value |
p value |
| <30 DAYS |
14 |
8 |
6 |
0.933 |
0.626 |
| 31-60 DAYS |
22 |
12 |
10 |
| 61-90 DAYS |
22 |
15 |
7 |
Table 8.
Patient Population with Infection based on HbA1c.
Table 8.
Patient Population with Infection based on HbA1c.
| HbA1c |
Total |
Males |
Females |
Males
(infected)
|
Females
(infected) |
Chi-square
Value |
p value |
| <8 |
155 |
100 |
55 |
16 |
11 |
0.024 |
0.874 |
| >8 |
145 |
93 |
52 |
19 |
12 |
Table 9.
Wald Test for Demographic Factors Associated with Urogenital Infections.
Table 9.
Wald Test for Demographic Factors Associated with Urogenital Infections.
| |
B |
S.E. |
Wald |
df |
p value |
Exp(B) |
| Constant |
-1.597 |
1.904 |
0.703 |
1 |
0.402 |
0.202 |
| Age |
-0.004 |
0.014 |
0.093 |
1 |
0.760 |
0.996 |
| Sex (Male) |
-0.232 |
0.307 |
0.572 |
1 |
0.450 |
0.793 |
| BMI |
-0.021 |
0.042 |
0.247 |
1 |
0.619 |
0.979 |
| HbA1C |
-0.007 |
0.083 |
0.006 |
1 |
0.938 |
0.994 |
Educational
Status |
|
|
1.664 |
3 |
0.645 |
|
| Educational Status (Primary) |
1.352 |
1.142 |
1.402 |
1 |
0.236 |
3.865 |
| Educational Status (Secondary) |
1.125 |
1.100 |
1.046 |
1 |
0.306 |
3.082 |
| Educational Status (UG) |
1.268 |
1.094 |
1.343 |
1 |
0.246 |
3.553 |
Discussion
This observational study identified the risk of urogenital infections in SGLT2 users with an incidence rate of 19.3 % in the population. Identified risk of both UTI and mycotic infections.
The findings of Lega.et.al comprises of increased risk of genital mycotic infections. [
11] The study of Ghosh et.al reported 25.2% polyuria and 9.4%genital mycotic infections in their follow up study of 12 months [
12]. Studies reported the similar increase of risk in men and women. In this study, mycotic infections are common in men that showed balanoposthitis and epididymo orchitis. Mostly these symptoms were seen after 30 days of exposure. These infections were treated with antifungal medications that further increased the burden to the patients. [
12]
Ghosh et.al reported 5 severe UTI and one acute AKI. [
12] While the study of Lega et.al did not find an increased risk of UTI. [
11]
From the study of Dave et.al there were 89 cases of severe UTI corresponding to an incidence rate of 1.86 cases per 1000 person-years of follow up, which doesn’t show increase in risk of UTI events among SGLT2I initiators. [
13,
14] Though the results are inconsistent to the known risk of UTI our study identified incidence rate of 15.6 cases per 100 persons in the 6 months study. The bacterial UTI symptoms include pyelonephritis and urosepsis. Ghosh et.al reported 5 severe UTI and one acute AKI. While the study of Lega et.al did not find an increased risk of UTI.
Uitrakul et.al reports on the increased UTI risk focusing on the individual SGLT2I, dapagliflozin, which is also highlighted in other significant studies. [
10] And other factors age, sex, BMI, HbA1c were not associated with the incidence of UTI. [
10] This study also does not signify the association of demographic factors with UGI. Patients taking 10 mg of dapagliflozin had higher incidence than 5 mg user with a p value of <0.001.
The findings of this study validate the hypothesis that SGLT2 inhibitors are associated with a risk of urogenital infections, with a notable dose-response relationship. The lack of association between demographic factors such as BMI, age, and HbA1c with infection risk suggests that the primary determinant of UGI is due to glycosuria rather than patient-specific factors.
11The clinical relevance of this study lies in its emphasis on real-world applicability. As healthcare providers increasingly prescribe SGLT2 inhibitors for their systemic benefits, it becomes imperative to recognize and mitigate potential side effects, including UGIs. This study demonstrates the importance of vigilant monitoring and patient education, particularly regarding personal hygiene and symptom recognition. [
15]
While numerous studies have reported similar findings, this study’s prospective observational design offers unique insights into the temporal relationship between SGLT2 inhibitor use and the onset of infections. Additionally, the regional specificity of this study provides data that is directly relevant to local clinical practices in India, where factors such as climate, healthcare infrastructure, and patient behaviors may influence the incidence of UGIs [
15].
However, it is essential to recognize the inherent limitations that may affect the interpretation and generalizability of our findings.
14 Addressing these limitations through meticulous study design, short study period, data collection and analysis represents a vital step in advancing our understanding of this complex relationship. Further research in this area is warranted to enhance patient care and outcomes [
13].
Conclusion
In this observational study addressing the association between SGLT2 inhibitors and urogenital infection provides generally higher incidence rates of infection. The risk of genital mycotic infections were higher in males. The study points out the need of patient hygiene and counselling for preventing urogenital infections (UGI) [
13]. Patients receiving SGLT2 inhibitors should be educated about proper genital hygiene practices. In addition, healthcare providers should emphasize the importance of seeking medical attention expediently if symptoms of urogenital infections occur [
14].
Funding
This study received no specific funding.
Conflict of Interest
The authors declare no conflicts of interest. This research was conducted without any financial or personal relationships that could have influenced the study outcomes.
Ethical Consideration and Informed Consent
Written informed consent was obtained from all participants before enrolment in the study. Consent was obtained in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board. Protocol number: MOSC/IEC/788/2023/2023.
References
- Roglic, Gojka. WHO Global report on diabetes: A summary. International Journal of Noncommunicable Diseases 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Varshney N, Billups SJ, Saseen JJ, Fixen CW. Sodium-glucose cotransporter-2 inhibitors and risk for genitourinary infections in older adults with type 2 diabetes. Ther Adv Drug Saf. 2021, 12, 2042098621997703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou YC, Zheng CM, Yen TH, Lu KC. Molecular mechanisms of SGLT2 inhibitors on cardiorenal protection. Int J Mol Sci 2020, 21, 7833. [Google Scholar] [CrossRef]
- Ferwani P, Maldar A, Shah N. Prevalence of Bacterial Urinary Tract Infection Among Patients With Type 2 Diabetes Mellitus on Sodium-Glucose Cotransporter-2 Inhibitors: A Prospective Real-World Setting Study. J ASEAN Fed Endocr Soc. 2022;37(2):5-8. doi: 10.15605/jafes.037.02.04. Epub 2022 Jul 25. PMID: 36578886; PMCID: PMC9758558.Fonseca-Correa JI, Correa-Rotter R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front Med (Lausanne) 2021, 8, 777861. [Google Scholar] [CrossRef]
- Thong KY, Yadagiri M, Barnes DJ, Morris DS, Chowdhury TA, Chuah LL, Robinson AM, Bain SC, Adamson KA, Ryder REJ; ABCD Nationwide Dapagliflozin Audit contributors. Clinical risk factors predicting genital fungal infections with sodiumglucose cotransporter 2 inhibitor treatment: The ABCD nationwide dapagliflozin audit. Prim Care Diabetes. 2018, 12, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013 21. Das B, Sheikh A, Ahmed B, Islam N. Clinical outcomes of sodium-glucose cotransporter-2 inhibitors in patients with type 2 Diabetes Mellitus: An observational study from Pakistan. Pak J Med Sci. 2021, 37, 1342–1346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo L, Wang J, Li L, Yuan L, Chen S, Wang H, Li T, Qi L, Yang H. A multicentre, prospective, non-interventional study evaluating the safety of dapagliflozin in patients with type 2 diabetes in routine clinical practice in China (DONATE). BMC Med. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko S, Kim H, Shinn J, Byeon SJ, Choi JH, Kim HS. Estimation of sodium-glucose cotransporter 2 inhibitor-related genital and urinary tract infections via electronic medical record-based common data model. J Clin Pharm Ther. 2021, 46, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Filippatos TD, Liberopoulos EN, Elisaf MS. Dapagliflozin in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015, 6, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Uitrakul S, Aksonnam K, Srivichai P, Wicheannarat S, Incomenoy S. The Incidence and Risk Factors of Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Using SGLT2 Inhibitors: A Real-World Observational Study. Medicines (Basel). 2022, 9, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lega IC, Bronskill SE, Campitelli MA, Guan J, Stall NM, Lam K, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019, 21, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Ghosh A, Gupta R, Singh P, Dutta A, Misra A. Sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes in North India: A 12-month prospective study in real-world setting. International Journal of Clinical Practice. 2018, 72, e13237. [Google Scholar] [CrossRef]
- Dave CV, Schneeweiss S, Kim D, Fralick M, Tong A, Patorno E. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Severe Urinary Tract Infections: A Population-Based Cohort Study. Ann Intern Med. 2019, 171, 248–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019, 21, 434–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saijo Y, Okada H, Hata S, Nakajima H, Kitagawa N, Okamura T, et al. Reasons for discontinuing treatment with sodium-glucose cotransporter 2 inhibitors in patients with diabetes in real-world settings: the KAMOGAWA-A study. J Clin Med. 2023, 12, 6993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).