1. Introduction
Cervical cancer, the third leading cause of female mortality, is preceded by cervical intraepithelial neoplasia grade 3 (CIN3). Although cervical intraepithelial neoplasia grade 2 (CIN2) which is a less reproducible indicator, is generally considered a safety threshold for management [
1,
2]. Histologically confirmed CIN2 or more (CIN2+) lesions are often treated with excisional procedures like the large loop excision of the transformation zone (LLETZ) or the loop electrosurgical excision procedure (LEEP), particularly in high-resource settings, to mitigate the risk of cervical cancer progression [
3]. Excisional treatment aims to remove the transformation zone (TZ) of the cervix and the squamocolumnar junction (SCJ), taking into consideration the lesion size, location, and patient age [
4]. According to the guidelines of the 2011 International Federation of Cervical Pathology and Colposcopy (IFCPC), there are three excision types (1, 2, and 3) aligned with the different types of the TZ 1 to 3. A type 3 TZ can be performed with a single or double split (top-hat) [
5]. Moreover, the UK National Health Service (NHS) cervical screening program recommends an excision length of < 10 mm for a type 1 excision, 10-15 mm for a type 2 excision, and 15-25 mm for a type 3 excision [
6].
However, following the initial excisional treatment, 4 to 18% of immunocompetent patients experience a follow-up detection of CIN2+ that is generally labelled a persistent/recurrent lesion. Persistent lesions usually suggest undertreatment that often occurs in involved margins within 2 years post-treatment [
7,
8]. Additionally, CIN2+ or even cervical cancer can be detected 20 years post-treatment, possibly from new lesions or slow progression [
2,
9]. Long-term follow-up studies often assess cervical cancer risk instead of CIN2+ outcomes post-LLETZ [
10,
11]. Our prior study of 242 patients with CIN2+ treated with LLETZ and followed for up to 20 years identified high-risk human papillomavirus (HR-HPV) and involved margins as significant predictors of CIN2+ detection at follow-up [
12].
Clinicians aim for clear margins while balancing the amount of tissue volume removed and potential side effects with minimizing the risk of new or recurrent precancerous lesions [
13]. Further research is needed to compare the long-term role of HR-HPV, margins, and LLETZ characteristics in the risk of new CIN2+, thereby lowering patient anxiety and medical burden. This study aimed to reassess the association of HR-HPV, margin status, along with excision types (1, 2, or 3), and cone volume/dimensions (length, thickness, and circumference) with a follow-up detection of CIN2+ in a larger series spanning 25 years, compared with our previous report [
12].
2. Materials and Methods
2.1. Study Design
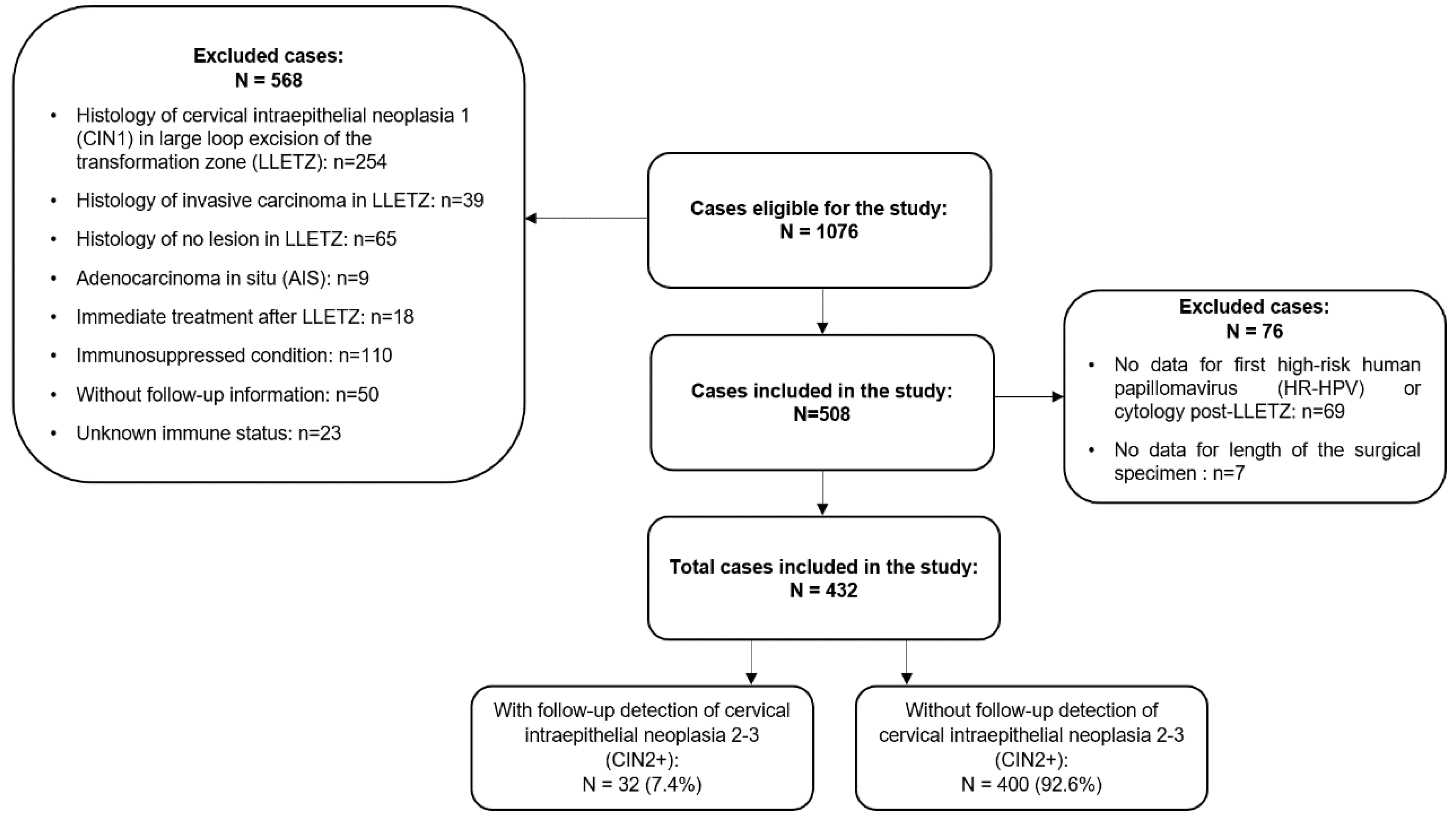
This is a retrospective observational study conducted in women with a diagnosis of CIN who underwent LLETZ at the Department of Gynecology at Hospital Bellvitge in Barcelona, Spain. The hospital is a regional reference center for cervical lesion treatment in the Baix Llobregat health district. Follow-up information was retrieved from the reviews of the hospital and primary healthcare records of the registered women. We enrolled 1076 adult women who had undergone LLETZ for low-grade cervical intraepithelial neoplasia (CIN1), CIN2+, adenocarcinoma in situ (AIS), cervical squamous cell carcinoma, and other cervical pathologies including an endocervical or ectocervical polyp and cervical nabothian cysts from June 1996 to December 2020. The collection of follow-up data ended in October 2021, leading to a maximum follow-up period of 25 years. Ultimately, 432 (40%) women treated with LLETZ for a diagnosis of CIN2+ with at least one follow-up visit (16.20%) post-LLETZ were included in the study. Prior to treatment, informed written consent was obtained from the patients. The project was approved by the research ethics committee.
Figure 1 shows the inclusion and exclusion criteria of the study.
2.2. Surgical Procedure
The excisional procedure for primary CIN2+ lesions was performed by LLETZ through a paracervical local anesthetic in all cases. Previously, Lugol's solution was applied to demarcate the area of abnormality. The diathermic loop size was selected according to the size of the lesion. Electrocoagulation for hemostasis was used and excised specimens were oriented anatomically with a stitch for pathological studies.
2.3. Type of Excision and Cone Volume/Dimensions
Data on the LLETZ excision types and dimensions were obtained from histology reports. After excision, the specimen was sent to the pathology unit for weighing and measurements to enable classification based on IFCPC and NHS criteria. The excision type was based on the TZ type per the IFCPC 2011 guidelines [
5]. Length was the distance from the external to the internal margins, thickness was the distance from the stromal margins to the surface of the excised specimen, and circumference was the perimeter of the excised specimen [
5].
The excision type category followed NHS 2016 guidelines [
6]. Cone volume was calculated using the formulas of Carcopino and Phadnis, with the Carcopino formula being "volume = (1/2) (4/3) x π x length x (circumference / 2π) x thickness" [
14] and the Phadnis formula being "volume = 1/2 (4/3) π (a / 2) (b / 2) c (a: transverse diameter, b: longitudinal diameter, c: depth)” [
15].
2.4. Cytology
After the LLETZ treatment, patients underwent conventional cytology at 6 months, which was replaced by liquid-based cytology in 2012. In the conventional method, ecto- and endocervical samples were obtained and the cytology slides were stained using the Papanicolaou method. Liquid-based cytology used a cyto-brush for cell collection and the ThinPrep liquid-based medium for transport. The cytology findings were classified according to the 1989 or 2001 Bethesda system [
16].
2.5. HR-HPV Determination
The presence of HR-HPV at 6 months post-LLETZ was tested using the Hybrid Capture 2 (HC2) assay and specimens were collected with the Digene sample conversion kit (Digene, Gaithersburg, MD, USA) [
17]. In 2019, the assay was replaced with the PCR
Cobas® 4800 HPV test. It provided individual results for HPV-16 and HPV-18, as well as a grouped result for 12 other HR-HPV genotypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) in a single analysis [
18].
2.6. Colposcopy
Colposcopy was conducted using a Carl Zeiss binocular colposcope (Jena, Germany) or an Optomic colposcope (Optomic, Spain), using the IFCPC terminology from 1990, 2002, and 2011 [
5]. During colposcopy, abnormal areas were visualized after applying 5% acetic acid or Lugol’s iodine solution. Endocervical curettage was performed for TZ type 3 or endocervical cases. Biopsies were taken for abnormal cytology results (e.g., atypical squamous cells of undetermined significance (ASCUS) or worse), positive HR-HPV tests post-LLETZ, or for those with limited SCJ visualization.
2.7. Follow-up
Follow-up appointments were set for 6 and 12 months post-LLETZ, including Pap smears and colposcopies, with HR-HPV testing performed at 6 months. If surgical margins were positive, the first visit occurred at 3 months post-LLETZ. If CIN2+ was confirmed by biopsy, the patients had to undergo a second LLETZ. If insufficient cervical tissue prevented a repeat LLETZ, a hysterectomy was performed. Patients returned to regular routine screening if two consecutive normal cytology results and normal colposcopies were obtained during follow-up post-LLETZ.
2.8. Criteria for The Follow-Up Detection of CIN2+
A follow-up detection of CIN2+ was defined as a cervical biopsy diagnosing CIN2+ after an initial LLETZ treatment, indicating a second LLETZ or a hysterectomy, as required. Due to the anticipated small number of cases, CIN2 and CIN3 were analyzed together. To avoid a presumption of a recurrent or incident nature of a new diagnosis of CIN2+ during follow-up, we refer to these lesions as a follow-up detection of CIN2+.
2.9. Statistical Methods
Data were extracted from electronic medical records into a Microsoft ACCESS form for prospective data collection and were retrospectively retrieved into a Microsoft Excel database. Descriptive statistics included counts and percentages for categorical variables and the median (min-max) with interquartile range (IQR) for continuous variables. Two-sided p-value <0.05 were considered to indicate a significant difference. Univariate and multivariate Cox proportional hazards models assessed associations with a follow-up detection of CIN2+ as well as hazard ratios (HR) and 95% confidence intervals (CI). The chi-square test or Kruskal-Wallis test analyzed the associations between surgical margins, type of excision, and cone volume/dimensions. Multiple imputation by chained equations (MICE) addressed the missing data regarding the type of excision. Treatment failure was calculated from the initial LLETZ to CIN2+ detection. The cumulative risk of a follow-up detection of CIN2+ was estimated using the Kaplan-Meier approach. Statistical analysis was performed using R version 4.1.0 for Windows (R Core Team, 2021) and IBM SPSS version 25.0 (IBM Corp, 2017, Armonk, NY, USA)
3. Results
3.1. Study Population
From a total of 1076 women treated with LLETZ, 432 cases with CIN2+ at baseline and follow-up information were included in the study, extending the study follow-up from the year 2006 to 2021 [
12]. Verification of the data until the year 2024 confirmed no new CIN2+ detections during follow-up in recent LLETZ cases. Following the inclusion-exclusion criteria, the present study featured a cohort of 258 new cases compared to our earlier report [
12]. Treatment success was observed in 92.6% of the total cases during the follow-up period, while 32 CIN2+ cases (7.4%) were detected after the initial LLETZ procedure. Among the 32 CIN2+ cases, histology reports indicated 3 cases of CIN2 (9.3%), 25 cases of CIN3 (78.1%), and 4 cases of CIN2-3 (12.5%).
3.2. Descriptive Characteristics Of The Study Cohort
The median time for the follow-up detection of CIN2+ was 11.5 months (interquartile range 3.8-27.9). CIN2+ was diagnosed in 18 cases (56.2%) within the first 12 months post-LLETZ, with margins involved in 13 (72%) of these cases. CIN2+ was detected in 5 cases (15.6%) between 12 and 24 months post-LLETZ. Furthermore, 2 cases (6.2%) were diagnosed between 24 and 29 months and 7 cases (21.9%) were observed after 30 months post-LLETZ. The longest time to detect a CIN2+ case was up to 198.2 months post-LLETZ. Therefore, 14 (43.7%) CIN2+ cases were detected after 12 months post-LLETZ, with margins involved in 4 (28%) cases (3 endocervical and 1 ectocervical). In total, we detected 90.6% of the CIN2+ cases within 5 years post-LLETZ. Among all the 32 CIN2+ cases detected in the follow-up, 22 underwent a repeat LLETZ procedure (68.7%), 9 underwent a hysterectomy (28.1%), and one was lost to follow-up (3.1%).
During the study, three cases of cervical cancer, one HPV-related vaginal cancer, and one HPV-related oropharyngeal cancer were detected. During follow-up, 65 cases (15%) were diagnosed with CIN1, of whom 31 (47.7%) received an LLETZ and 34 (52.3%) were monitored. One monitored case, a 52-year-old woman, developed CIN3 and then cervical cancer before undergoing a hysterectomy.
Table 1 shows the characteristics of the 432 women included in the final analysis. The median follow-up time of the study was 70.3 months (interquartile range 17.9-141), with 75% of the patients followed for over 141 months. The HR-HPV test post-treatment was positive in 100 women (23.1%), of whom 20 (20%) had a subsequent diagnosis of CIN2+. Among 332 (76.9%) cases of negative HR-HPV post-LLETZ, only 12 (3.6%) cases experienced a follow-up detection of CIN2+ with the most prolonged case up to 143 months after treatment.
Table 2 shows the characteristics of the surgical specimens, including different margin status, type of excision, cone volume/dimensions, and number of involved quadrants. Surgical margins were involved in 157 (36.3%) cases, uncertain in 43 cases (10.0%), and clear in 232 cases (53.7%). Overall, involved endocervical margins were more likely to be associated with a CIN2+ diagnosis during follow-up than the absence of endocervical involvement (p < 0.01) (
Table S1). The overall negative predictive value (NPV) of HR-HPV was 96.4% and of clear margins was 96.6%. Both combined showed the NPV of 98.7% at the end of the follow-up.
3.3. Surgical margins, type of excision, and cone volume/dimensions
Table 3 shows the association between surgical margins with age, type of excision, and cone volume/dimensions. Clear margins were more likely to be observed in women < 35 years in age (61.9%) compared to women aged ≥ 35 years (47.8%) (p < 0.001). Furthermore, the type of excision was also associated with margin status (p-value = 0.035). The proportion of clear margins was 46.1% for type 1 excisions, 53.8% for type 2 excisions, and 65.2% for type 3 excisions. However, there were no significant differences between excision type and ecto- and endocervical margin involvement (
Table S2). Among the different cone dimensions, a longer length of the surgical specimen was significantly associated with an increased presence of clear margins (p-value = 0.010). The median length was 12 mm among those with a clear margin, 10 mm for involved margins, and 8 mm for cases with uncertain margins.
3.4. Predictors of a follow-up detection of CIN2+ by multivariate analysis
Table 4 shows the multivariate Cox regression adjusted for the first HR-HPV positive result post-LLETZ, margins, and age, with all variables associated with a follow-up detection of CIN2+. Previously in
Figure S1, we observed that a positive HR-HPV result post-LLETZ, involved margins, and older age were associated with more and earlier follow-up detections of CIN2+. In multivariate analysis, HR-HPV detection post-LLETZ was the strongest predictor of a follow-up detection of CIN2+ compared to other factors (HR = 7.36, 95% CI = 3.55-15.26). There was almost a 4-fold increase in the HR among women with involved margins (HR = 3.9) or uncertain margins (HR = 4.4) compared to those with clear margins. An age ≥ 35 years predicted a HR of 2.9 in contrast to that observed for younger women. Adding treatment characteristics did not improve the models.
4. Discussion
4.1. Main Findings
Follow-up detection of CIN2+ occurred in 7.4% of our cohort compared to 5.7% in our previous report [
12]. A 6-month negative HR-HPV test resulted in just 3.6% new CIN2+ cases at follow-up, with one CIN2+ case after 143 months, emphasizing the importance of negative HR-HPV test post-treatment. Adding margins as a predictive factor to negative HR-HPV had a NPV of 98.7%. After LLETZ, 56.2% of the cases were detected within the first 12 months, likely due to insufficient initial treatment, with 72% of these cases showing margin involvement. The rest of the cases, including 3 cervical cancer cases, were detected after 12 months. Over 90% of the CIN2+ cases were detected within 5 years post-LLETZ. Positive HR-HPV result post-LLETZ, involved margins, uncertain margins, and an age ≥ 35 years were significant predictors of subsequent CIN2+ in the multivariate analysis, consistent with our previous report [
12]. The association of the type of excision and cone dimensions with CIN2+ detection at follow-up were overwritten by the margin status, probably due to the limited statistical power. However, as expected, the longest excision type, type 3 excision, and a length of approximately 12 mm were both linked to clear margins. Women aged ≥ 35 years were less likely to have clear margins. Overall, involved endocervical margins showed the relevance of the endocervical canal tissue in predicting further disease, which is consistent with other reports [
7].
4.2. Strengths and Limitations
Our comprehensive search at the primary health level, our hospital's main referral activities, an extended follow-up, and a sample size nearly twice that of our previous study [
12] provide deeper insights into long-term lesion detection and cervical cancer risk. We investigated the joint effect of post-treatment HR-HPV results and surgical margins, a novel approach in prior studies. We also analyzed margin distribution across excision types and cone dimensions, performed volume comparisons, and conducted imputation strategies to minimize bias. However, besides the retrospective nature of our study, uncertain margins and wide confidence intervals in certain categories posed challenges. The absence of type-specific HPV data made it difficult to distinguish between treatment failures and new cases, thereby affecting long-term cancer risk assessment.
4.3. Interpretation
The follow-up detection of CIN2+ cases up to 7.4%, aligns with the global average [
7,
19] and is lower than the 12.8% found in a recent study [
20]. Contrary to our previous report and existing literature [
8,
12,
19] the present study spanning a median follow-up period of 70 months found that over 40% of the CIN2+ cases were diagnosed after the first year post-LLETZ, underscoring the benefit of active follow-up for five years.
The 2019 guidelines of the American Society for Colposcopy and Cervical Pathology (ASCCP) recommend HPV-based testing at 6 months post-treatment, annual testing until three negative results, and then surveillance every 3 years for 25 years [
21]. Furthermore, the Spanish Association of Cervical Pathology and Colposcopy (AEPCC) suggest routine screening every 5 years after three negative co-tests following CIN2+ treatment [
22]. These recommendations may potentially reduce the risk of new CIN2+ cases post-treatment. The elevated rate of CIN2+ cases through the 5-year post-LLETZ period in our study may be attributed to irregular co-testing potentially contributing to an increased rate of CIN2+ detection at follow-up. Our results support a strict five-year follow-up post-treatment. Despite lacking regular co-testing, our study offers a basis for comparing outcomes with increased HR-HPV testing in follow-up. We observed that a negative HR-HPV at 6-months post-LLETZ combined with clear margins showed greater reassurance than other factors in predicting new CIN2+ in a long follow-up of 25 years. Knowledge of HPV type and regular HR-HPV tests could improve treatment insight.
Our current study, with nearly double the population compared to our previous report [
12], reaffirmed that the original predictive factors for a follow-up detection of CIN2+ remain consistent. While HR-HPV is the strongest risk factor, age and margins add additional predictive value. We also observed that women with both a positive HR-HPV result and involved margins had higher and earlier follow-up detections of CIN2+, which is consistent with the findings of our prior report emphasizing the need for personalized management based on HR-HPV status and margins [
12].
In our study, excision type did not affect CIN2+ detection at follow-up and, contrary to previous reports, we found no correlation between cone length and CIN2+ detection [
23]. A recent study by Foggiatto et al. noted that an excised endocervical canal length under 1.25 cm increased recurrence by 2.5 times [
20]. In our study, a low incidence of new CIN2+ cases post-LLETZ did not allow us to draw definitive conclusions. However, we found that a 12-mm length was associated with clear margins. Compared to the study of Foggiatto et al., we had a higher rate of involved margins. However, Foggiatto et al. observed more endocervical margin involvement, likely due to a shorter removed canal length that can lead to recurrence.
Our findings showed that the type of excision, especially type 3, was significantly linked to clearer margins. This is in line with a recent study showing that negative endocervical margins were observed in 86% of type 2 excisions compared to 78% of type 1 excisions. That study did not assess type 3 excisions [
24]. We observed no differences in ecto- and endocervical margin involvement across various excision types, probably due to the very low number of ecto- and endocervical margin involvement with each excision type.
Additionally, our study suggests a length of 12 mm as an oncological safe limit for achieving clear margins. This finding is consistent with previous reports [
13,
25]. Lengths over 10 mm may increase the preterm delivery risk [
26]. Prioritizing a greater length, particularly type 3 excisions, in older women is crucial to obtain clear margins, however it might arise the risk of cervical stenosis [
27]. Moreover, some studies show minor decline in sexual satisfaction after LLETZ, while others not [
28,
29]. Further research is needed on improved treatments when the SCJ is not fully visible, ensuring deeper lesions are addressed in surgery without unnecessary stroma removal to avoid additional side effects [
30]. Furthermore, our recent understanding is that the islands of reserve cells may remain in the endocervical canal giving rise to potential dysplastic lesions [
31].
Despite using two different formulas to calculate cone volume, we found no correlation between volume and a follow-up detection of CIN2+ or margin status, which is in accordance with previous findings [
32]. Length may be more influential, particularly for clearing endocervical glands, which can be as deep as 5.22 mm from the surface of the cervix [
6]. Thus, increasing tissue volume does not guarantee lesion removal from the endocervical glands.
5. Conclusions
Negative HR-HPV post-LLETZ and clear margins provide long-term reassurance in predicting a follow-up detection of CIN2+. Tailoring excision type and length, especially in women aged ≥ 35 years, can reduce margin involvement and optimize patient outcomes. Strict follow-up for 5 years post-LLETZ remains necessary, together with periodic HR-HPV testing.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: (A) Kaplan-Meier curve for follow-up detection of CIN2+ by HR-HPV post-LLETZ (p log-rank < 0.001). (B) Kaplan-Meier curve for follow-up detection of CIN2+ by margin status (p log-rank < 0.001). (C) Kaplan-Meier curve for follow-up detection of CIN2+ by margin status for positive HR-HPV post-LLETZ (p log-rank < 0.001). (D) Kaplan-Meier curve for follow-up detection of CIN2+ by margin status for negative HR-HPV post-LLETZ (p log-rank = 0.122). (E) Kaplan-Meier curve for follow-up detection of CIN2+ by age of the patients (p log-rank = 0.013). (F) Kaplan-Meier curve for follow-up detection of CIN2+ by age for positive HR-HPV post- LLETZ (p log-rank < 0.001). (G) Kaplan-Meier curve for follow-up detection of CIN2+ by age for negative HR-HPV post-LLETZ (p log-rank = 0.636); Table S1: Univariate analysis of predictive factors associated with follow-up detection of CIN2+ after large loop excision of the transformation zone (LLETZ); Table S2: Association between type of excision, length of the cone, age of the women, and first HR-HPV post -LLETZ with ectocervical and endocervical margins of the surgical specimen in women treated with large loop excision of the transformation zone (LLETZ) for cervical intraepithelial neoplasia 2-3 (CIN2+).
Author Contributions
This work was designed and planned by M-EF-M, SS, and FH. FH collected the data, wrote the manuscript, developed the tables, and performed some statistical analysis with support from M-EF-M and SS. M-EF-M and SS developed the theory and supervised the project. Statistical analysis was carried out by JP-M. All the authors contributed to the interpretation of the results. The final content of the manuscript was read and approved by all the authors.
Funding
This research received no funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of Bellvitge University Hospital (Protocol Code Reference PR137/21) (26 May 2021).
Informed Consent Statement
Patient consent was waived as this observational study consists of a retrospective analysis of our usual healthcare practice.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the localization of the database in the intranet of Bellvitge Hospital.
Conflicts of Interest
There are no conflicts of interest to be reported.
References
- CaCantor SB, Ph D, Atkinson EN, Ph D, Cardenas-turanzas M, et al. Natural History of Cervical Intraepithelial Neoplasia A Meta-analysis. Acta Cytol. 2005, 49, 405–415.
- McCredie MR, Sharples KJ, Paul C; et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro P, Arduino1 B, Borgo1 M, et al. Loop Electrosurgical Excision Procedure versus Cryotherapy in the Treatment of Cervical Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gynecol Minim Invasive Ther. 2018;7:145-151.
- Basu P, Taghavi K, Hu S, et al. Management of cervical premalignant lesions. Curr Probl Cancer. 2018;42:129–36.
- Bornstein J, Bentley J, Bösze P, et al. 2011 Colposcopic Terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet Gynecol. 2012;120:166–72.
- NHS Cervical Screening Programme 2016. Available online: https://pathlabs.rlbuht.nhs.uk/colp_and_programme_mgment_2016.pdf (accessed on 5 June 2024).
- Arbyn M, Redman CWE, Verdoodt F, et al. Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta-analysis. Lancet Oncol. 2017;18:1665–79.
- Kocken M, Helmerhorst TJM, Berkhof J, et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: A long-term multi-cohort study. Lancet Oncol. 2011;12:441–50.
- Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006;118:2048–55.
- Strander B, Andersson-Ellström A, Milsom I, et al. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: Population based cohort study. Br Med J. 2007;335:1077–80.
- Melnikow J, Mcgahan C, Sawaya GF, et al. Cervical Intraepithelial Neoplasia Outcomes After Treatment : Long-term Follow-up From the British Columbia Cohort Study. J Natl Cancer Inst. 2009;101:721–8.
- Fernández-Montolí ME, Tous S, Medina G, et al. Long-term predictors of residual or recurrent cervical intraepithelial neoplasia 2–3 after treatment with a large loop excision of the transformation zone: a retrospective study. BJOG An Int J Obstet Gynaecol. 2020;127:377–87.
- Papoutsis D, Rodolakis A, Mesogitis S, et al. Appropriate cone dimensions to achieve negative excision margins after large loop excision of transformation zone in the uterine cervix for cervical intraepithelial neoplasia. Gynecol Obstet Invest. 2013;75:163–8.
- Carcopino X, Mancini J, Prendiville W, et al. The Accuracy of Large Loop Excision of the Transformation Zone Specimen Dimensions in Determining Volume: A Multicentric Prospective Observational Study. J Low Genit Tract Dis. 2017;21:120–124.
- Phadnis S V, Atilade A, Young MPA, et al. The volume perspective : a comparison of two excisional treatments for cervical intraepithelial neoplasia ( laser versus LLETZ ). BJOG. 2010;117:615-9.
- Apgar BS, Zoschnick L, Wright TC. The 2001 Bethesda System Terminology. Am Fam Physician. 2003;68:1992–8.
- Schiffman MH, Kiviat NB, Burk RD, et al. Accuracy and Interlaboratory Reliability of Human Papillomavirus DNA Testing by Hybrid Capture. J Clin Microbiol. 1995;33:545–50.
- Martin IW, Steinmetz HB, Lefferts CL, et al. Evaluation of the Cobas 4800 HPV Test for Detecting High-Risk Human Papilloma-Virus in Cervical Cytology Specimens. Pathogens. 2012;1:30-6.
- Stasinou SM, Valasoulis G, Kyrgiou M, et al. Large Loop Excision of the Transformation Zone and Cervical Intraepithelial Neoplasia : A 22-Year Experience. Anticancer Res. 2012;32:4141–5.
- Foggiatto AI, De Carvalho NS, Fonseca FV, et al. Recurrence in Cervical High-Grade Squamous Intraepithelial Lesion: The Role of the Excised Endocervical Canal Length - Analysis of 2,427 Patients. J Low Genit Tract Dis. 2023;27:1–6.
- Cheung LC, Egemen D, Chen X, et al. 2019 ASCCP Risk-Based Management Consensus Guidelines: Methods for Risk Estimation, Recommended Management, and Validation. J Low Genit Tract Dis. 2020;24:90–101.
- Secondary Prevention of Cervical Cancer, 2022. Clinical Management of Abnormal Screening Results. Available online: https://www.aepcc.org/wp-content/uploads/2022/11/AEPCC-guidelines_Secondary-Prevention-2022.pdf (accessed on 21 March 2024).
- Papoutsis D, Rodolakis A, Antonakou A, et al. Cervical cone measurements and residual disease in LLETZ conisation for cervical intraepithelial neoplasia. In Vivo. 2011;25:691-5.
- Mirandez CC, Yoneda JY, Gertrudes LN, et al. The value of the endocervical margin status in LEEP: analysis of 610 cases. Arch Gynecol Obstet. 2022;306:851–6.
- Kawano K, Tsuda N, Nishio S; et al. Identification of appropriate cone length to avoid positive cone margin in high grade cervical intraepithelial neoplasia. J Gynecol Oncol. 2016, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ. 2016;354:i3633.
- Baldauf JJ, Dreyfus M, Ritter J, et al. Risk of cervical stenosis after large loop excision or laser conization. J Obstet Gynecol. 1996; 88:933-8.
- Sparić R, Papoutsis D, Kadija S, et al. Psychosexual outcomes in women of reproductive age at more than two-years from excisional cervical treatment–a cross-sectional study. J Psychosom Obstet Gynecol. 2019;40:128–37.
- Serati M, Salvatore S, Cattoni E, et al. The impact of the loop electrosurgical excisional procedure for cervical intraepithelial lesions on female sexual function. J Sex Med. 2010;7:2267-2272.
- Desai KT, de Sanjosé S, Schiffman M. Treatment of Cervical Precancers is the Major Remaining Challenge in Cervical Screening Research. Cancer Prev Res. 2023;16:649–52.
- Reich O, Regauer S, Lara Gutierrez A; et al. Copy number profiling implicates thin high-grade squamous intraepithelial lesions as a true precursor of cervical human papillomavirus-induced squamous cell cancer. Lab Investig 2024, 104, 102108. [Google Scholar] [CrossRef] [PubMed]
- Kitson SJ, Greig E, Michael E; et al. European Journal of Obstetrics & Gynecology and Reproductive Biology Predictive value of volume of cervical tissue removed during LLETZ on subsequent preterm delivery : a cohort study. Eur J Obstet Gynecol Reprod Biol. 2014, 180, 51–55. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).