Submitted:
18 October 2024
Posted:
21 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Details
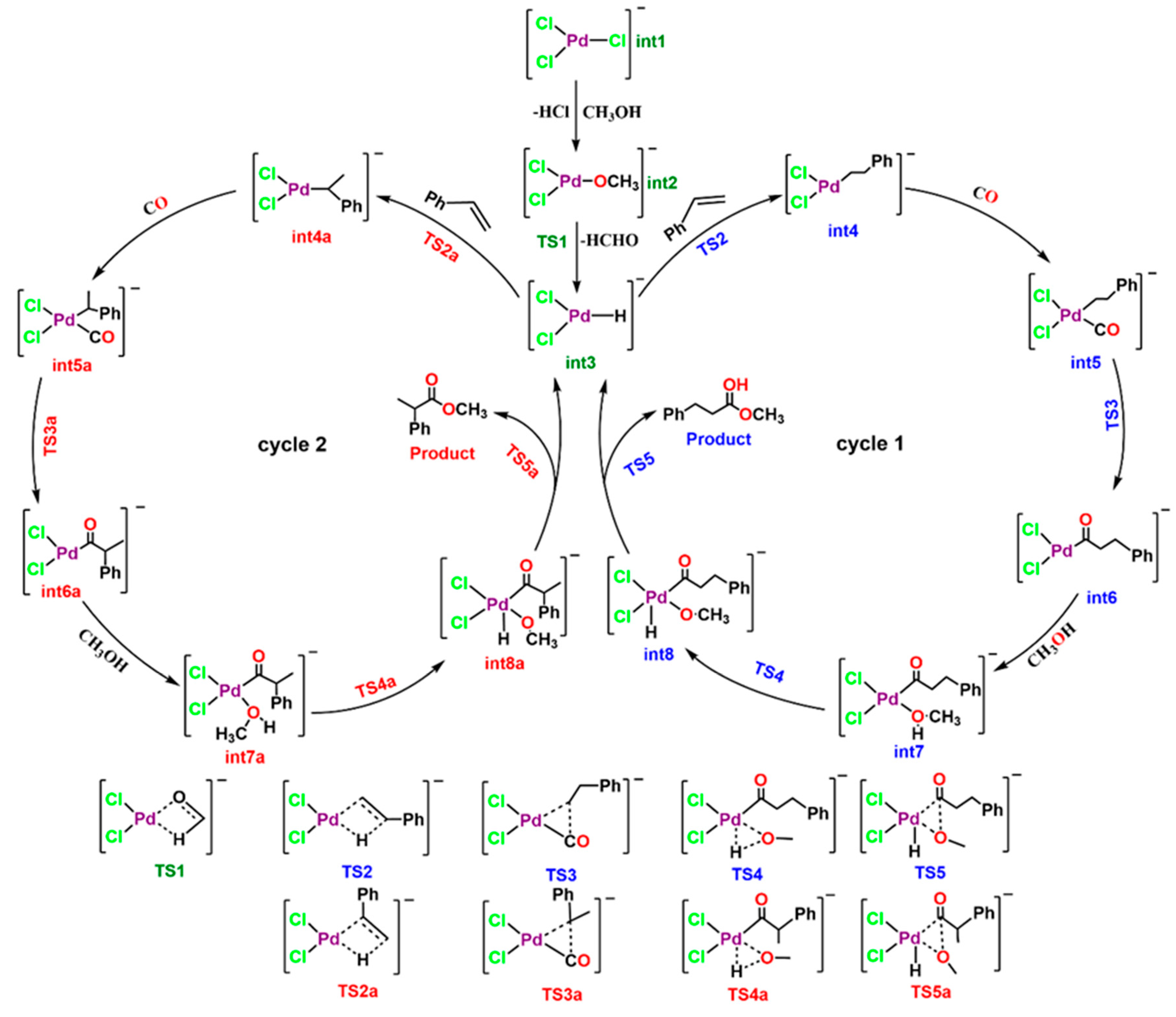
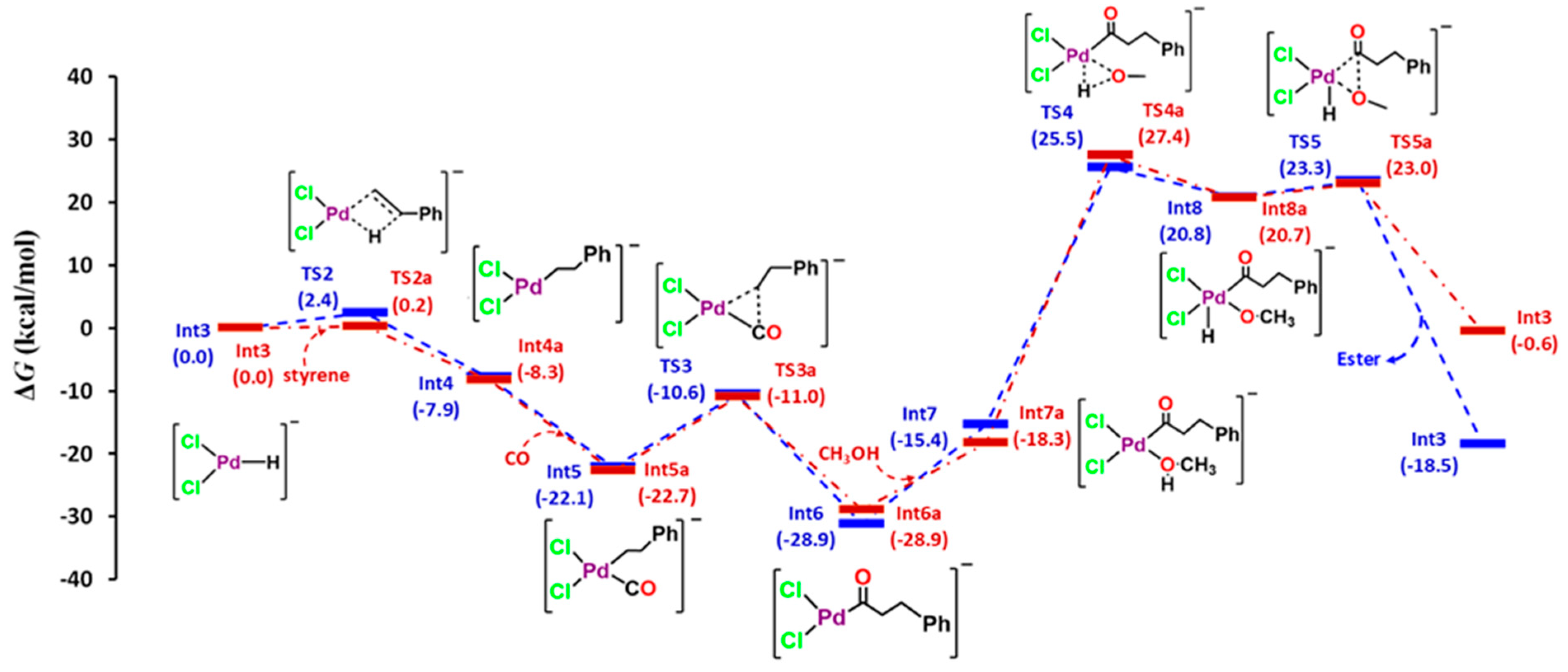
3. Results and Discussion
3.1. Energy Decomposition Analysis (EDA) and Natural Orbital for Chemical Valence (NOCV) Analysis
| TS | NOCV pair 1 | NOCV pair 2 |
|---|---|---|
| TS1 | 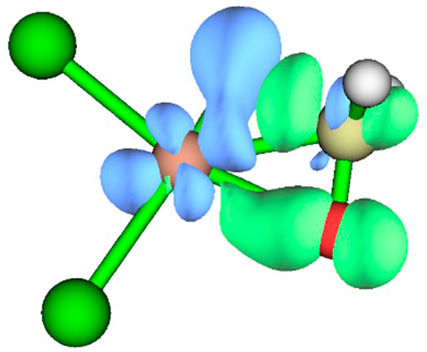 |
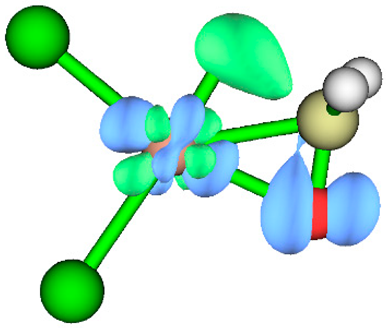 |
| TS2 | 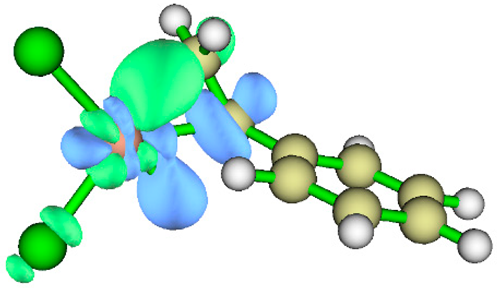 |
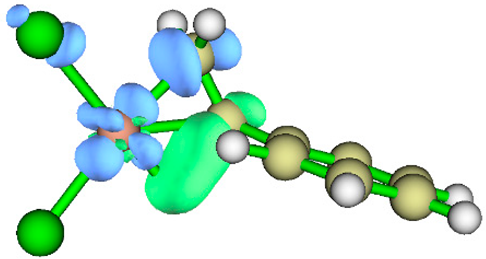 |
| TS3 | 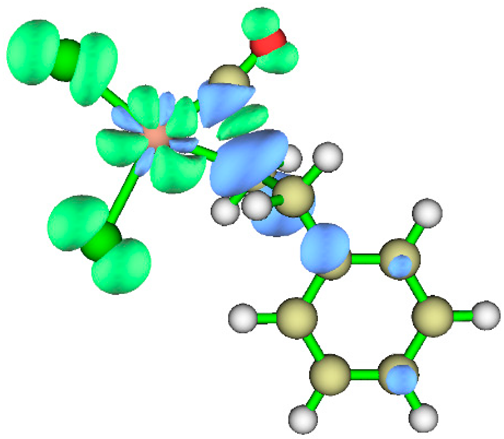 |
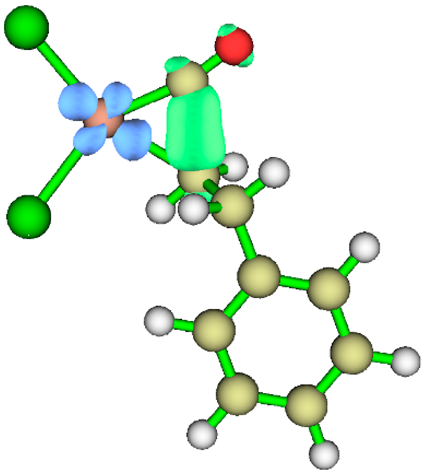 |
| TS4 | 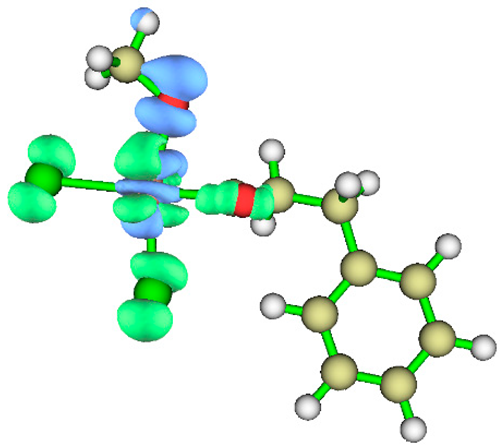 |
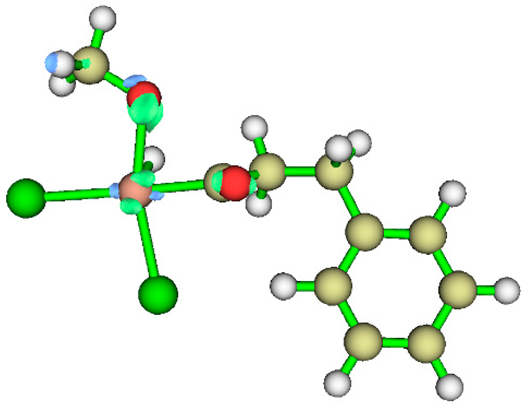 |
| TS5 | 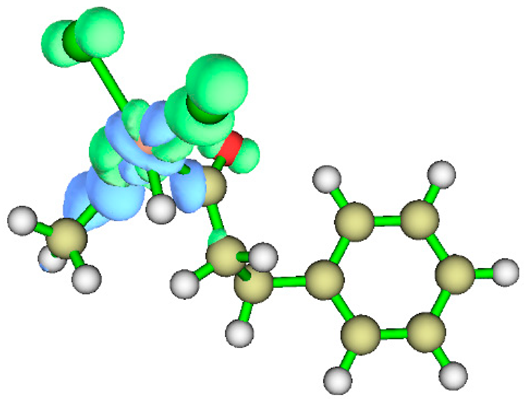 |
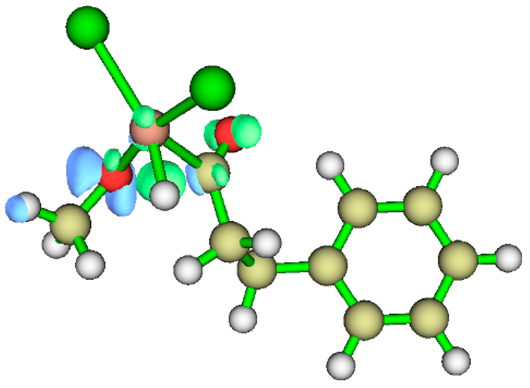 |
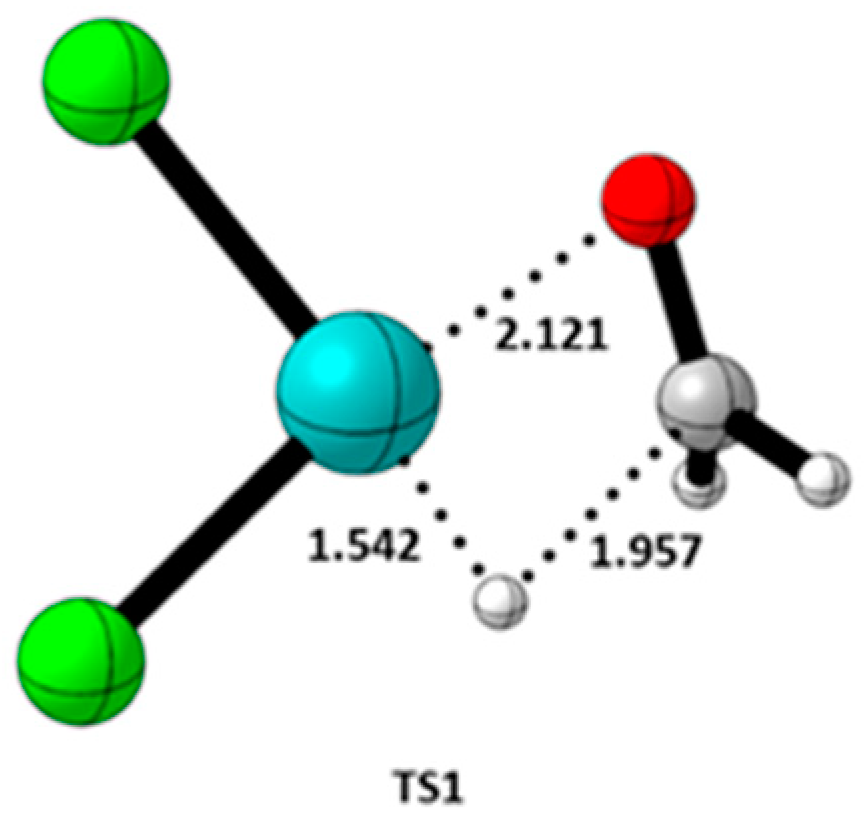
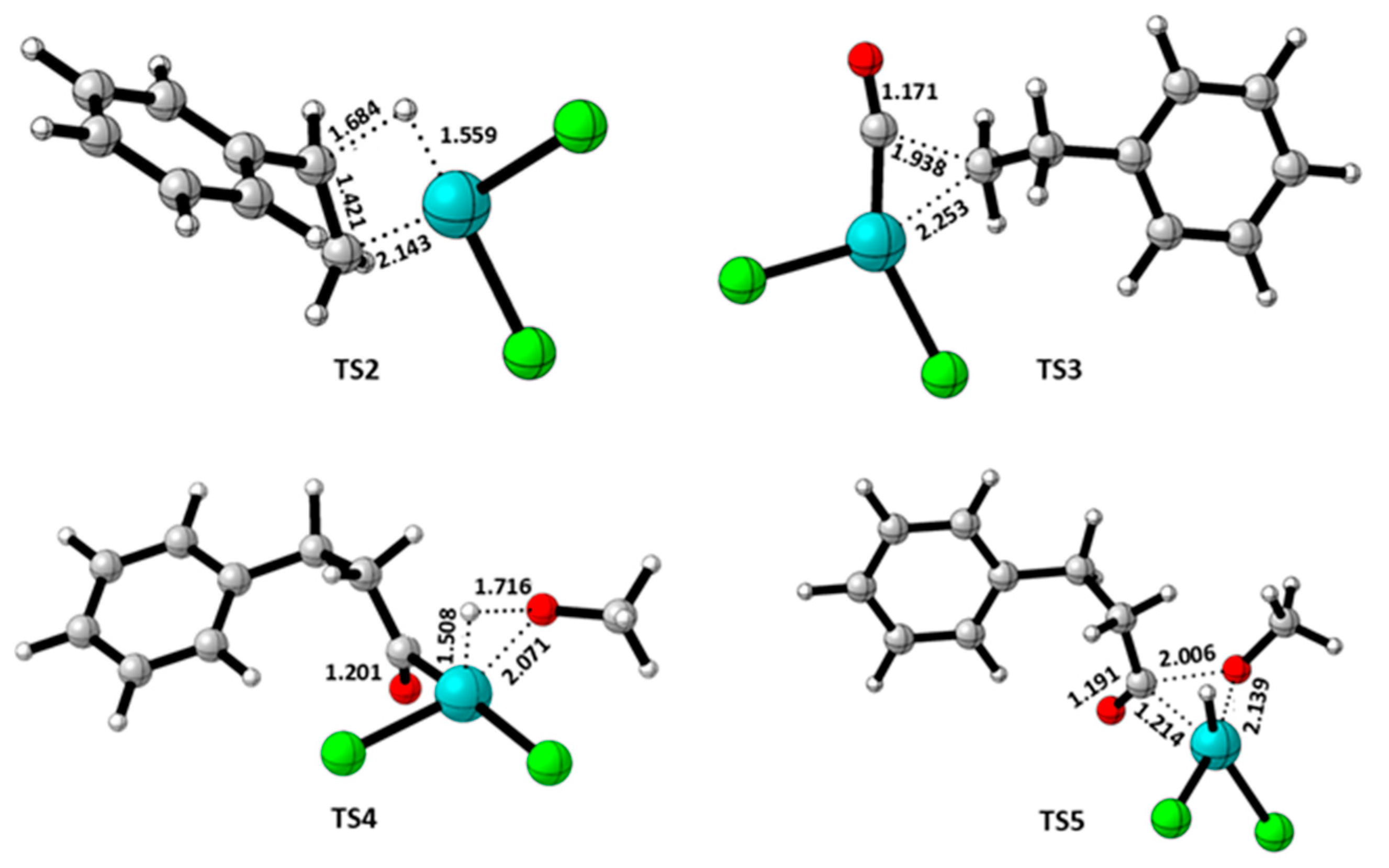
3.2. Quantum Theory of Atom in Molecule (QTAIM) Analysis
| TS | Distance (Å) | bond | ρ(rc) | ∇2ρ(rc) | G(rc) | V(rc) | H(rc) | ELF | λ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TS1 | 1.957 | C-H | 0.3008 | 0.1226 | 0.1235 | -0.2163 | -0.0928 | 0.6343 | -0.3008 | ||
| TS1 | 1.542 | Pd-H | 0.1369 | 0.0699 | 0.0985 | -0.1795 | -0.0810 | 0.5295 | -0.1369 | ||
|
TS2/ TS2a |
1.683/ 1.704 |
C-H | 0.3091/ 0.0823 |
0.0907/ 0.0419 |
0.1239 0.0359 |
-0.2253/ -0.0613 |
-0.1013/ -0.0254 |
0.6532/ 0.6076 |
-0.3091/ -0.0823 |
||
|
TS2/ TS2a |
1.558 1.553 |
Pd-H | 0.1418/ 0.1442 |
0.0625/ 0.0513 |
0.1022/ 0.1020 |
-0.1889/ -0.1912 |
-0.8664/ -0.0892 |
0.5397/ 0.5548 |
-0.1418/ -0.1442 |
||
|
TS2/ TS2a |
1.421 1.417 |
C-C | 0.2896/ 0.2923 |
-0.7424 -0.7560 |
0.0984/ 0.0981 |
-0.3825 -0.3855 |
-0.2840 -0.2873 |
0.9317 0.9341 |
-0.2896 -0.2923 |
||
| TS2a | 2.164 | Pd-C | 0.0890 | 0.2540 | 0.0862 | -0.1090 | -0.0227 | 0.2586 | -0.0890 | ||
|
TS2/ TS2a |
2.076 2.122 |
Pd-C | 0.1045/ 0.0947 |
0.2010/ 0.2143 |
0.0865/ 0.0825 |
-0.1229/ -0.1114 |
-0.0363/ -0.0289 |
0.3713/ 0.3197 |
-0.1045/ -0.0947 |
||
| TS3 | 3.321 | Cl-H(Ph) | 0.0456 | 0.11813 | 0.0241 | -0.0187 | 0.01962 | 0.1460 | -0.0456 | ||
|
TS3/ TS3a |
1.911/ 1.951 |
C-C | 0.0405/ 0.1026 |
-0.0663/ -0.1231 |
0.1452/ 0.1335 |
-0.3070/ -0.2754 |
-0.1618/ -0.1419 |
0.7724/ 0.7599 |
-0.1102/ -0.1026 |
||
|
TS4 TS4a |
2.801/ 2.847 |
Cl-H(Ph) | 0.1091/ 0.0520 |
0.0843/ 0.2001 |
0.0628/ 0.0433 |
-0.0482/ -0.1345 |
0.0145/ 0.0245 |
0.1421/ 0.1438 |
-0.1091/ -0.0520 |
||
|
TS4 TS4a |
2.820/ 2.852 |
Cl-H(CH3) | 0.0377/ 0.1131 |
0.1351/ 0.0987 |
0.1008/ 0.0730 |
-0.0777/ -0.0555 |
0.0231/ 0.0175 |
0.1230/ 0.1193 |
-0.0377/ -0.1131 |
||
| TS4a | 2.315 | H-O | 0.0377 | 0.1413 | 0.1052 | -0.0808 | 0.0243 | 0.1130 | -0.0377 | ||
|
TS5 TS5a |
2.343/ 2.561 |
O-H(Ph) | 0.0444 0.1228 |
0.1488 0.1277 |
0.1179 0.0972 |
-0.0993 -0.0774 |
0.0185 0.0198 |
0.1541 0.0893 |
-0.0445 -0.1228 |
||
|
TS5 TS5a |
2.011 2.031 |
H-H | 0.0419 0.0429 |
0.1381 0.1381 |
0.1063 0.1064 |
-0.0859 -0.0862 |
0.0204 0.0202 |
0.1553 0.1670 |
-0.0419 -0.0429 |
||
|
TS5 TS5a |
2.006/ 2.011 |
C-O | 0.2567 0.2537 |
0.1706 0.1724 |
0.1907 0.1905 |
-0.2245 -0.2223 |
-0.1237 -0.1168 |
0.3001 0.2924 |
-0.2567 -0.2537 |
||
4. Conclusion
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chem 2019, 5, 526–552. [Google Scholar] [CrossRef]
- Jurado, L.; Posada-Pérez, S.; Axet, M.R. Carbonylation Reactions Using Single-Atom Catalysts. ChemCatChem 2024. [Google Scholar] [CrossRef]
- Ma, K.; Martin, B.S.; Yin, X.; Dai, M. Natural product syntheses via carbonylative cyclizations. Nat. Prod. Rep. 2019, 36, 174–219. [Google Scholar] [CrossRef]
- Wu, X.-F.; Fang, X.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. Transition-Metal-Catalyzed Carbonylation Reactions of Olefins and Alkynes: A Personal Account. Acc. Chem. Res. 2014, 47, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Wu, X.-F. Transition Metal Catalyzed Carbonylation Reactions; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; ISBN 978-3-642-39015-9. [Google Scholar]
- Wei, W.-M.; Dong, F.-Q.; Zheng, R.-H.; Liu, Y.-Y.; Zhao, T.-T.; Fang, W.-J.; Qin, Y.-D. Theoretical study of the mechanism of palladium-catalyzed hydroaminocarbonylation of styrene with ammonium chloride. Comput. Theor. Chem. 2020, 1191, 113040. [Google Scholar] [CrossRef]
- Cernak, T.A.; Lambert, T.H. Multicatalytic Synthesis of α-Pyrrolidinyl Ketones via a Tandem Palladium(II)/Indium(III)-Catalyzed Aminochlorocarbonylation/Friedel−Crafts Acylation Reaction. J. Am. Chem. Soc. 2009, 131, 3124–3125. [Google Scholar] [CrossRef]
- Malkov, A.V.; Barłóg, M.; Miller-Potucká, L.; Kabeshov, M.A.; Farrugia, L.J.; Kočovský, P. Stereoselective Palladium-Catalyzed Functionalization of Homoallylic Alcohols: A Convenient Synthesis of Di- and Trisubstituted Isoxazolidines and β-Amino-δ-Hydroxy Esters. Chem.—A Eur. J. 2012, 18, 6873–6884. [Google Scholar] [CrossRef]
- Malkov, A.V.; Lee, D.S.; Barłóg, M.; Elsegood, M.R.J.; Kočovský, P. Palladium-Catalyzed Stereoselective Intramolecular Oxidative Amidation of Alkenes in the Synthesis of 1,3- and 1,4-Amino Alcohols and 1,3-Diamines. Chem.—A Eur. J. 2014, 20, 4901–4905. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef]
- Kočovský, P.; Bäckvall, J. The syn/anti-Dichotomy in the Palladium-Catalyzed Addition of Nucleophiles to Alkenes. Chem.—A Eur. J. 2015, 21, 36–56. [Google Scholar] [CrossRef]
- Shen, C.; Dong, K. Assembling Chiral Center of Heterocycles by Palladium-Catalyzed Asymmetric Hydrocarbonylation. Synlett 2022, 33, 815–821. [Google Scholar] [CrossRef]
- Gallarati, S.; Dingwall, P.; Fuentes, J.A.; Bühl, M.; Clarke, M.L. Understanding Catalyst Structure–Selectivity Relationships in Pd-Catalyzed Enantioselective Methoxycarbonylation of Styrene. Organometallics 2020, 39, 4544–4556. [Google Scholar] [CrossRef]
- Jing, T.-H.; Zhuang, Y.-Y.; Zhang, X.-X.; Qian, J.-G.; Zhao, X.-L.; Lu, Y.; Wang, H.-J.; Liu, Y. Pinwheel-like tridentate phosphines for controlling divergent regioselectivity in Pd-catalyzed alkoxycarbonylation of alkenes. J. Catal. 2024, 432, 115406. [Google Scholar] [CrossRef]
- Xu, T.; Sha, F.; Alper, H. Highly Ligand-Controlled Regioselective Pd-Catalyzed Aminocarbonylation of Styrenes with Aminophenols. J. Am. Chem. Soc. 2016, 138, 6629–6635. [Google Scholar] [CrossRef]
- Li, H.; Dong, K.; Jiao, H.; Neumann, H.; Jackstell, R.; Beller, M. The scope and mechanism of palladium-catalysed Markovnikov alkoxycarbonylation of alkenes. Nat. Chem. 2016, 8, 1159–1166. [Google Scholar] [CrossRef]
- Aguirre, P.A.; Lagos, C.A.; Moya, S.A.; Zúñiga, C.; Vera-Oyarce, C.; Sola, E.; Peris, G.; Bayón, J.C. Methoxycarbonylation of olefins catalyzed by palladium complexes bearing P,N-donor ligands. Dalt. Trans. 2007, 5419. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wang, C.; Dong, G.; Xiang, J.; Luo, T.; Liang, B.; Chen, J.; Yang, Z. Development of Thiourea-Based Ligands for the Palladium-Catalyzed Bis(methoxycarbonylation) of Terminal Olefins. European J. Org. Chem. 2003, 2003, 4346–4348. [Google Scholar] [CrossRef]
- Fekri, S.; Mansoori, Y. Supported Bis-(NHC)-Pd(II) complex: A phosphine-free catalyst supported on magnetic mesoporous silica for the CO-free carbonylation of aryl halides. J. Organomet. Chem. 2024, 1014, 123193. [Google Scholar] [CrossRef]
- Klingshirn, M.A.; Rogers, R.D.; Shaughnessy, K.H. Palladium-catalyzed hydroesterification of styrene derivatives in the presence of ionic liquids. J. Organomet. Chem. 2005, 690, 3620–3626. [Google Scholar] [CrossRef]
- Sims, H.S.; Dai, M. Palladium-Catalyzed Carbonylations: Application in Complex Natural Product Total Synthesis and Recent Developments. J. Org. Chem. 2023, 88, 4925–4941. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, H.; Liu, Y.; Deng, Z.; Douthwaite, M.; Dummer, N.F.; Lewis, R.J.; Liu, X.; Luan, S.; Dong, M.; et al. Ambient-pressure alkoxycarbonylation for sustainable synthesis of ester. Nat. Commun. 2024, 15, 7837. [Google Scholar] [CrossRef] [PubMed]
- Schelwies, M.; Paciello, R.; Pelzer, R.; Siegel, W.; Breuer, M. Palladium-Catalyzed Low Pressure Carbonylation of Allylic Alcohols by Catalytic Anhydride Activation. Chem.—A Eur. J. 2021, 27, 9263–9266. [Google Scholar] [CrossRef] [PubMed]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem. Soc. Rev. 2011, 40, 5151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-A.; Ye, Z.-S.; Duan, Y.; Zhou, Y.-G. Homogeneous palladium-catalyzed asymmetric hydrogenation. Chem. Soc. Rev. 2013, 42, 497–511. [Google Scholar] [CrossRef]
- Roberts, G.M.; Pierce, P.J.; Woo, L.K. Palladium Complexes with N-Heterocyclic Carbene Ligands As Catalysts for the Alkoxycarbonylation of Olefins. Organometallics 2013, 32, 2033–2036. [Google Scholar] [CrossRef]
- Demir, S.; Özdemir, İ.; Arslan, H.; VanDerveer, D. Butylene linked palladium N-heterocyclic carbene complexes: Synthesis and catalytic properties. J. Organomet. Chem. 2011, 696, 2589–2593. [Google Scholar] [CrossRef]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chemie Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef]
- Flahaut, A.; Roland, S.; Mangeney, P. Allylic alkylation and amination using mixed (NHC) (phosphine) palladium complexes under biphasic conditions. J. Organomet. Chem. 2007, 692, 5754–5762. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Lothschütz, C.; Döpp, R.; Rudolph, M.; Ramamurthi, T.D.; Rominger, F. Gold and Palladium Combined for Cross-Coupling. Angew. Chemie Int. Ed. 2009, 48, 8243–8246. [Google Scholar] [CrossRef]
- Amatore, C.; Carre, E.; Jutand, A.; M’Barki, M.A. Rates and Mechanism of the Formation of Zerovalent Palladium Complexes from Mixtures of Pd(OAc)2 and Tertiary Phosphines and Their Reactivity in Oxidative Additions. Organometallics 1995, 14, 1818–1826. [Google Scholar] [CrossRef]
- Tooze, R.P.; Whiston, K.; Malyan, A.P.; Taylor, M.J.; Wilson, N.W. Evidence for the hydride mechanism in the methoxycarbonylation of ethene catalysed by palladium–triphenylphosphine complexes. J. Chem. Soc. Dalt. Trans. 2000, 3441–3444. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Laurenczy, G.; Urrutigoïty, M.; Kalck, P. Hydride Route for the Palladium-Catalysed Cyclocarbonylation of Monoterpenes. Eur. J. Inorg. Chem. 2005, 2005, 4215–4225. [Google Scholar] [CrossRef]
- Mehara, J.; Anania, M.; Kočovský, P.; Roithová, J. Competing Mechanisms in Palladium-Catalyzed Alkoxycarbonylation of Styrene. ACS Catal. 2024, 14, 5710–5719. [Google Scholar] [CrossRef] [PubMed]
- Seayad, A.; Jayasree, S.; Damodaran, K.; Toniolo, L.; Chaudhari, R.V. On the mechanism of hydroesterification of styrene using an in situ-formed cationic palladium complex. J. Organomet. Chem. 2000, 601, 100–107. [Google Scholar] [CrossRef]
- Eastham, G.R.; Tooze, R.P.; Heaton, B.T.; Iggo, J.A.; Whyman, R.; Zacchini, S. Synthesis and spectroscopic characterisation of all the intermediates in the Pd-catalysed methoxycarbonylation of ethene. Chem. Commun. 2000, 609–610. [Google Scholar] [CrossRef]
- Muñoz, B.K.; Santos Garcia, E.; Godard, C.; Zangrando, E.; Bo, C.; Ruiz, A.; Claver, C. HP-NMR Study of the Pd-Catalyzed Methoxycarbonylation of Styrene Using Monodentate and Bidentate Phosphane-Modified Systems. Eur. J. Inorg. Chem. 2008, 2008, 4625–4637. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Parisel, S.; Gusev, O.V.; Kal’sin, A.M.; Vologdin, N.V.; Dolgushin, F.M. Methoxycarbonylation of styrene to methyl arylpropanoates catalyzed by palladium(II) precursors with 1,1′-bis(diphenylphosphino)metallocenes. J. Mol. Catal. A Chem. 2004, 224, 35–49. [Google Scholar] [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R.; Zacchini, S. Characterization and Dynamics of [Pd(L−L)H(solv)]+, [Pd(L−L)(CH2CH3)]+, and [Pd(L−L)(C(O)Et)(THF)]+ (L−L = 1,2-(CH2PBut2)2C6H4): Key Intermediates in the Catalytic Methoxycarbonylation of Ethene to Methylpropanoate. Organometallics 2002, 21, 1832–1840. [Google Scholar] [CrossRef]
- Naigre, R.; Chenal, T.; Ciprés, I.; Kalck, P.; Daran, J.-C.; Vaissermann, J. Carbon monoxide as a building block in organic synthesis. Part V. Involvement of palladium-hydride species in carbonylation reactions of monoterpenes. X-ray crystal structure of [Ph3PCH2CHCHPh]4[PdCl6][SnCl6]. J. Organomet. Chem. 1994, 480, 91–102. [Google Scholar] [CrossRef]
- Eastham, G.R.; Tooze, R.P.; Kilner, M.; Foster, D.F.; Cole-Hamilton, D.J. Deuterium labelling evidence for a hydride mechanism in the formation of methyl propanoate from carbon monoxide, ethene and methanol catalysed by a palladium complex. J. Chem. Soc. Dalt. Trans. 2002, 1613–1617. [Google Scholar] [CrossRef]
- Liu, J.; Heaton, B.T.; Iggo, J.A.; Whyman, R.; Bickley, J.F.; Steiner, A. The Mechanism of the Hydroalkoxycarbonylation of Ethene and Alkene–CO Copolymerization Catalyzed by Pd II–Diphosphine Cations. Chem.—A Eur. J. 2006, 12, 4417–4430. [Google Scholar] [CrossRef] [PubMed]
- Amadio, E.; Cavinato, G.; Dolmella, A.; Toniolo, L. Catalytic Properties of [Pd(COOMe)nX2−n(PPh3)2](n = 0, 1, 2; X = Cl, NO2, ONO2, OAc and OTs) in the Oxidative Carbonylation of MeOH. Inorg. Chem. 2010, 49, 3721–3729. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.V.; Derrien, N.; Barłóg, M.; Kočovský, P. Palladium-Catalyzed Alkoxycarbonylation of Terminal Alkenes To Produce α,β-Unsaturated Esters: The Key Role of Acetonitrile as a Ligand. Chem.—A Eur. J. 2014, 20, 4542–4547. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Bühl, M. Computational modelling of Pd-catalysed alkoxycarbonylation of alkenes and alkynes. Phys. Chem. Chem. Phys. 2021, 23, 15869–15880. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M.; Zuideveld, M.A.; Swennenhuis, B.H.G.; Freixa, Z.; Kamer, P.C.J.; Goubitz, K.; Fraanje, J.; Lutz, M.; Spek, A.L. Alcoholysis of Acylpalladium(II) Complexes Relevant to the Alternating Copolymerization of Ethene and Carbon Monoxide and the Alkoxycarbonylation of Alkenes: the Importance of Cis-Coordinating Phosphines. J. Am. Chem. Soc. 2003, 125, 5523–5539. [Google Scholar] [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R.; Zacchini, S. Synthesis and reactivity of palladium hydrido-solvento complexes, including a key intermediate in the catalytic methoxycarbonylation of ethene to methyl propanoate. J. Chem. Soc. Dalt. Trans. 2002, 3300–3308. [Google Scholar] [CrossRef]
- Ahmad, S.; Crawford, L.E.; Bühl, M. Palladium-catalysed methoxycarbonylation of ethene with bidentate diphosphine ligands: a density functional theory study. Phys. Chem. Chem. Phys. 2020, 22, 24330–24336. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.V.; Bloino, J.; et al. Gaussian 16, Revision C.01 2016.
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Preuss, H.; Pitzer, R.M. Relativistic and correlation effects for element 105 (hahnium, Ha): a comparative study of M and MO (M = Nb, Ta, Ha) using energy-adjusted ab initio pseudopotentials. J. Phys. Chem. 1993, 97, 5852–5859. [Google Scholar] [CrossRef]
- Bergner, A.; Dolg, M.; Küchle, W.; Stoll, H.; Preuß, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Martin, R.L.; Hay, P.J.; Pratt, L.R. Hydrolysis of Ferric Ion in Water and Conformational Equilibrium. J. Phys. Chem. A 1998, 102, 3565–3573. [Google Scholar] [CrossRef]
- Sparta, M.; Riplinger, C.; Neese, F. Mechanism of Olefin Asymmetric Hydrogenation Catalyzed by Iridium Phosphino-Oxazoline: A Pair Natural Orbital Coupled Cluster Study. J. Chem. Theory Comput. 2014, 10, 1099–1108. [Google Scholar] [CrossRef]
- Fantuzzi, F.; Nascimento, M.A.C.; Ginovska, B.; Bullock, R.M.; Raugei, S. Splitting of multiple hydrogen molecules by bioinspired diniobium metal complexes: a DFT study. Dalt. Trans. 2021, 50, 840–849. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Simple, Efficient, and Universal Energy Decomposition Analysis Method Based on Dispersion-Corrected Density Functional Theory. J. Phys. Chem. A 2023, 127, 7023–7035. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Knöpke, L.R.; Rabeah, J.; Chęciński, M.P.; Jiao, H.; Bentrup, U.; Brückner, A.; Martin, A.; Köckritz, A. From sunflower oil toward 1,19-diester: Mechanistic elucidation. J. Catal. 2013, 297, 44–55. [Google Scholar] [CrossRef]
- Roesle, P.; Caporaso, L.; Schnitte, M.; Goldbach, V.; Cavallo, L.; Mecking, S. A Comprehensive Mechanistic Picture of the Isomerizing Alkoxycarbonylation of Plant Oils. J. Am. Chem. Soc. 2014, 136, 16871–16881. [Google Scholar] [CrossRef]
- Dong, K.; Sang, R.; Wei, Z.; Liu, J.; Dühren, R.; Spannenberg, A.; Jiao, H.; Neumann, H.; Jackstell, R.; Franke, R.; et al. Cooperative catalytic methoxycarbonylation of alkenes: uncovering the role of palladium complexes with hemilabile ligands. Chem. Sci. 2018, 9, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Gopal Patra, S.; Kumar Chattaraj, P. Three coordinated first row-transition metal complexes, [M{N(SiMe3)2}3]−1/0: Structure, bonding, and magnetic properties. Polyhedron 2024, 256, 116990. [Google Scholar] [CrossRef]
- Pal, R.; Patra, S.G.; Chattaraj, P.K. Can a chemical bond be exclusively covalent or ionic? J. Chem. Sci. 2022, 134, 108. [Google Scholar] [CrossRef]
- Patra, S.G.; Drew, M.G.B.; Datta, D. δ-Acidity of benzene in [(benzene)RuII(N-N)Cl]+. Crystal structures, nuclear magnetic resonance spectra and nucleus independent chemical shifts. Inorganica Chim. Acta 2018, 471, 228–233. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, 1990. [Google Scholar]
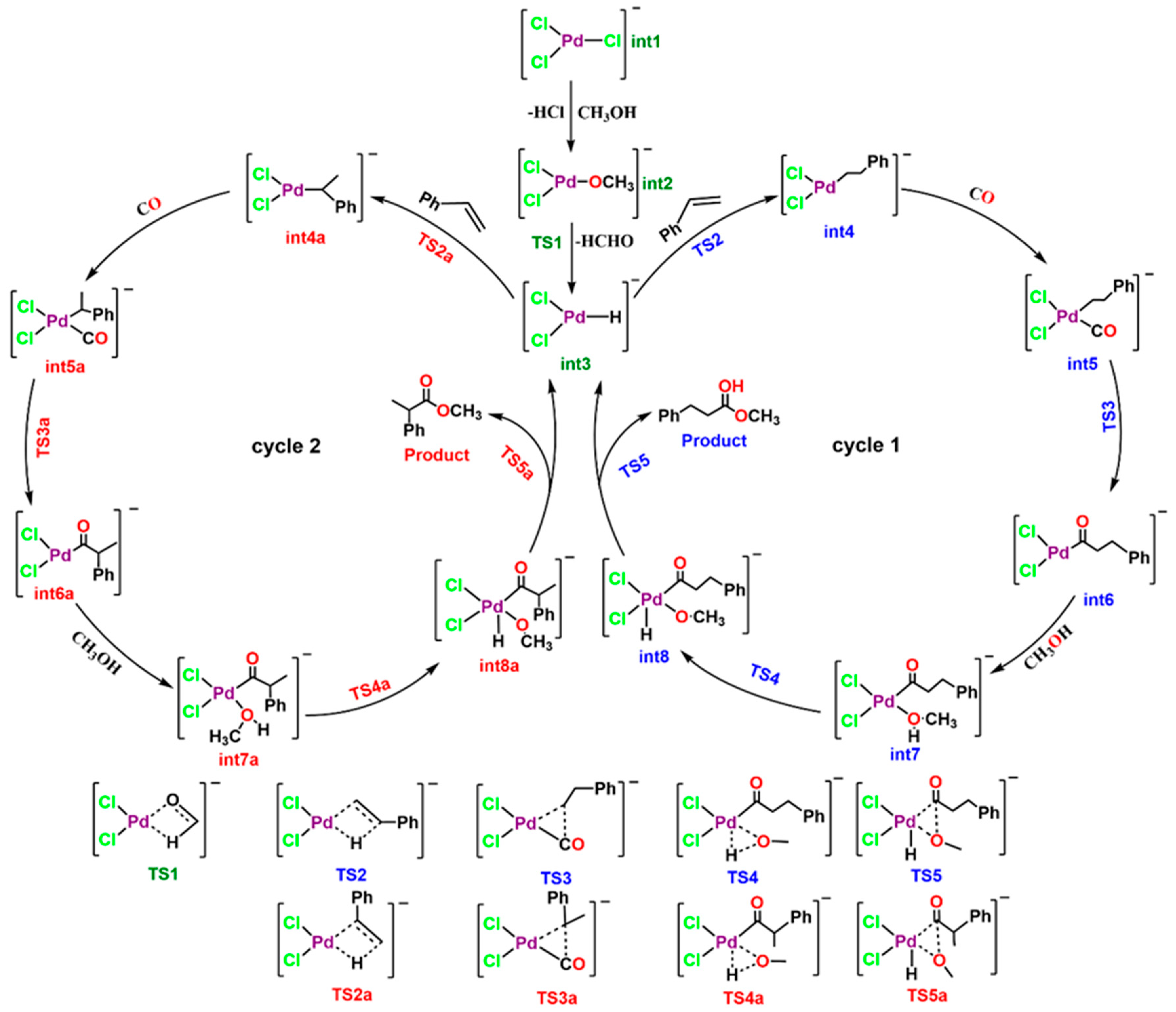
| TS | fragment1 (charge, multiplicity) | fragment2 (charge, multiplicity) |
|---|---|---|
| TS1 | HCHO (0, 1) | [PdCl2H]− (-1, 1) |
| TS2 | styrene (0, 1) | [PdCl2H]− (-1, 1) |
| TS3 | PhCH2CH2 (-1, 1) | [PdCl2CO] (1, 1) |
| TS3a | PhCH3CH (-1, 1) | [PdCl2CO] (1, 1) |
| TS4 | CH3O (-1, 1) | [PdCl2H(PhCH2CH2CO)] (-1, 1) |
| TS4a | CH3O (-1, 1) | [PdCl2H(PhCH2CH2CO)] (0, 1) |
| TS5 | CH3O (-1, 1) | [PdCl2H(PhCH2CH2CO)] (0, 1) |
| TS5a | CH3O (-1, 1) | [PdCl2H(Ph(CH3)CHCO)] (0, 1) |
| Species | ∆Eels | ∆Eex | ∆Epauli | ∆Eorb | ∆Ecor | ∆Edisp | ∆Etot |
|---|---|---|---|---|---|---|---|
| TS1 | -80.69 (15.94) |
-74.23 (14.66) |
237.05 (46.83) |
-96.98 (19.16) |
-13.96 (2.76) |
-3.32 (0.66) |
-32.13 |
| TS2 | -114.29 (16.19) |
-110.50 (15.66) |
334.23 (47.36) |
-120.89 (17.13) |
-18.41 (2.61) |
-7.44 (1.05) |
-37.30 |
| TS2a | -139.13 (16.96) |
-115.37 (14.06) |
339.21 (41.34) |
-198.12 (24.15) |
-20.95 (2.55) |
-7.67 (0.93) |
-142.04 |
| TS3 | -192.66 (19.43) |
-127.78 (12.88) |
428.82 (43.24) |
-217.37 (21.92) |
-17.76 (1.79) |
-7.35 (0.74) |
-134.10 |
| TS3a | -150.09 (18.45) |
-104.53 (12.85) |
349.35 (42.94) |
-182.29 (22.41) |
-17.34 (2.13) |
-9.98 (1.23) |
-114.89 |
| TS4 | -117.12 (20.96) |
-74.31 (13.30) |
224.62 (40.20) |
-123.04 (22.02) |
-14.60 (2.61) |
-5.10 (0.91) |
-109.55 |
| TS4a | -118.40 (20.60) |
-77.01 (13.40) |
232.37 (40.42) |
-125.94 (21.91) |
-15.45 (2.69) |
-5.67 (0.99) |
-110.12 |
| TS5 | -132.38 (21.63) |
-81.59 (13.33) |
253.06 (41.35) |
-122.41 (20.00) |
-16.50 (2.70) |
-6.02 (0.98) |
-105.83 |
| TS5a | -130.04 (21.36) |
-81.38 (13.37) |
252.20 (41.43) |
-121.77 (20.01) |
-16.83 (2.76) |
-6.46 (1.06) |
-104.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





