1. Introduction
Congenital insensitivity to pain with anhidrosis (CIPA), also known as hereditary sensory and autonomic neuropathy type IV (HSAN IV), is an extremely rare autosomal recessive disorder characterized by the inability to perceive pain, absence of sweating (anhidrosis), and various neurological abnormalities [
1]. First described in 1963 by Swanson, the syndrome has since been reported in scattered cases worldwide, the prevalence of disorder in Japan is estimated to be 1 in 600,000–950,000 [
2].
CIPA poses significant challenges to affected individuals due to their inability to sense pain, which often leads to repeated injuries, infections, and self-mutilation, particularly during infancy and childhood. Furthermore, the absence of sweating predisposes individuals to hyperthermia, as the body's thermoregulatory mechanism is impaired [
3], notably, there is no impact on salivation, lacrimation, or touch perception.
A nerve biopsy, genetic testing, and clinical assessment all contribute to the diagnosis, to detect the precise mutation and validate the diagnosis, neurotrophic receptor tyrosine kinase 1 (NTRK1) gene genetic analysis is required [
4].
The underlying cause of CIPA lies in mutations affecting the function of nerve fibers responsible for transmitting pain, temperature, and sweat sensations. These mutations typically involve the NTRK1 gene, which encodes the high-affinity nerve growth factor receptor tropomyosin receptor kinase A (TrkA). TrkA is crucial for the development and survival of sensory and sympathetic neurons, and its dysfunction results in the characteristic features of CIPA [
5].
The main goals of CIPA treatment are symptom relief and avoidance of consequences. Interventions are meant to improve the quality of life for those affected because there is no known cure, among strategies are proactive steps to prevent hyperthermia, pain avoidance education, and attentive wound care. Potential therapeutic approaches, such as gene therapy and pharmaceutical therapies that target neurodevelopment pathways, are being investigated in ongoing research [
6].
2. Case Report
2.1. First Hospitalization at Children’s Clinical University Hospital (CCUH)
A 3-year-old female patient was acutely hospitalized at the Children's Clinical University Hospital (CCUH) in Latvia from a regional hospital, due to suspicion of sepsis and acute osteomyelitis. It is known from the anamnesis that the patient five weeks before this hospitalization episode suffered a domestic injury (fell in a children's playground) in Dublin, Ireland.
The patient in Ireland was genetically confirmed to have CIPA with two mutations in the NTRK1 gene (ICD-10 code: G60.8; ORPHA:642; OMIM:256800), she does not feel pain and does not sweat. The patient is a child of nonconsanguineous parents. From the age of two months, the girl increasingly tears the skin from her fingers and damages her oral cavity and tongue with her teeth. The patient’s mother noted that the patient’s abrasions usually do not heal well, and her body temperature is often elevated.
Until now, the patient's parents denied any bone fractures. The girl has been noted to have a delay in language development, her vocabulary includes words in both English and Latvian, but she does not form sentences. The parents noted that the patient learns a new word every week and speech therapy classes are planned. Hearing was tested to be normal. The patient is sensitive to bright light, often requiring sunglasses, but has not been to an eye doctor.
Objectively, the patient has dry skin, especially on the hands. Distal phalanges with thickened, lichenified skin, in places without nails (nail dystrophy) (
Figure 1). Signs of unintentional self-harm in places on the body such as hematomas and scratches. The patient refused to show her teeth and oral cavities. The patient already had osteomyelitis of the right-hand finger in the anamnesis and the patient received antibacterial therapy for six weeks. The patient often has skin infections and usually receives antibacterial treatment for about 10 days.
Considering that the patient does not feel pain, the patient's parents sought medical attention late after the fall, only when the soft tissues around the fracture site showed visual signs of inflammation (swelling, redness, heat). As a result, the patient received conservative treatment for her tibial fracture in Dublin, Ireland. The patient's left leg was immobilized (the third day after a traumatic event) with a circular cast for five weeks in total.
Two days before hospitalization at CCUH, the patient developed febrile temperature rises (around 40° Celsius). X-ray, computed tomography (CT) scan, and blood tests were performed at a regional hospital before the patient was transferred to CCUH. The patient was found to have elevated inflammation indicators: white blood count (leucocytes) of 20.20 x 109/L (Ref. range 6.00-16.00 x109/L), C-reactive protein of 150.33 mg/L (Ref. range < 5 mg/L), procalcitonin of 19.11 ng/mL (Ref. range 0.00-0.50 ng/mL), and interleukin-6 of 94.4 pg/mL (Ref. range 0-5.9 pg/mL). Blood cultures were aerobically and anaerobically negative. The patient's fecal material was negative for
extended-spectrum beta-lactamase (ESBL) screening and negative for
vancomycin-resistant Enterococcus faecium (VRE) screening. The X-ray revealed a fracture with a pronounced hump (
Figure 2). A CT scan was additionally done and revealed the possibility of acute osteomyelitis.
Objectively, relatively pronounced oedema was observed in the front and medial, as well as the back surface of the lower leg and palpatory soreness. Once admitted to CCUH, the patient received intravenous antibacterial therapy with Clindamycin 200mg three times a day. The patient still had a febrile body temperature. On the second day, one dose of intravenous Ceftriaxone 1400mg was prescribed to the patient. On the third day, Clindamycin was changed to intravenous Vancomycin 290mg three times a day. Considering the results of X-ray and CT, as well as blood tests, three days after hospitalization, it was decided in favor of surgery - partial resection of the left tibia, cavity repair, and application of an external fixation device (
Figure 3).
The patient was transferred to the intensive care unit after the operation due to the development of sepsis. A central venous catheter and a nasogastric tube were placed in the patient. During the surgery, it was discovered that the cavity was filled with purulent contents, so the patient was connected to a wound vacuum assisted closure (VAC) device, followed by regular wound dressings under sedation and anesthesia (
Figure 4).
Staphylococcus aureus, which was resistant to Penicillin, was detected in a sample of pus and bone taken from the focus of osteomyelitis. The conclusion of the histological examination of the biopsies for the bone fragment confirmed the diagnosis of acute osteomyelitis. Antibacterial therapy with intravenous Oxacillin 1000mg six times a day was started. Two weeks after the partial resection surgery, the lower limb wound had signs of granulation, therefore, it was closed with rotated gastrocnemius and soleus musculocutaneous flaps. After the closure surgery, antibacterial therapy was continued (intravenous Oxacillin 1000mg, four times a day), as well as regular wound dressings under sedation. The patient developed inflammation in the perineum. She used an ointment containing zinc, the mother noted that this had occurred previously during long antibiotic durations.
A week before the patient's discharge from the hospital, the antibacterial therapy was changed to Clindamycin (intravenous 300mg, three times a day) and it had to be continued at home (Clindamycin oral suspension 100mg, three times a day for 10 days, and Clindamycin oral tablets 150mg, two times a day for 10 days). In a compensated satisfactory condition, the patient was discharged for outpatient therapy with a diagnosis of hematogenous osteomyelitis of the left proximal tibia.
2.2. Second Hospitalization at Children’s Clinical University Hospital (CCUH)
One week after discharge, the patient was admitted acutely at the CCUH in Latvia due to complaints about the instability of the external fixation device and discharge from the wounds. It was known that the patient caught the external fixation device while getting off the sofa. During examination, the external fixation device was unstable in the proximal part (
Figure 5) and purulent discharge was coming from the wounds.
As a result, the evacuation of the rods of the external fixation device from the bone was indicated, as well as obtaining culture swabs from the wounds. The culture of biological material from the wound and the focus of osteomyelitis was negative. The result of the histological examination of the bone fragments corresponded to residual phenomena after infected osteomyelitis. No signs of active inflammation were observed. C-reactive protein was 16.7 mg/L (Ref. range 0-5 mg/L). Other inflammatory markers were slightly elevated.
Wound cleaning and plaster immobilization were performed. Antibacterial therapy was started with intravenous Clindamycin 200mg three times a day. The patient rebounded well and was discharged from the hospital on the third postoperative day with recommendations to complete the antibacterial therapy at home, as well as to follow-up at CCUH for a planned surgery in three weeks.
2.3. Third Hospitalization at Children’s Clinical University Hospital (CCUH)
A month later, the patient was admitted for planned surgery - lower leg reconstruction. The patient underwent resection of the tibial pseudarthrosis, plasty with a vascularized fibular graft, and osteosynthesis with a locking plate (
Figure 6).
In the postoperative period, the patient received intravenous Cefazolin 500mg three times a day, as well as intravenous Clindamycin 200mg three times a day. The drain was removed on the third postoperative day and primary wound healing was present. Prescribed with recommendations to continue antibacterial therapy with Clindamycin oral tablets 150mg for another 10 days, and oral suspension of Cefuroxime 150mg for another 14 days. Instructions to continue plaster cast immobilization for another eight weeks.
2.4. Fourth Hospitalization at Children’s Clinical University Hospital (CCUH)
The patient was hospitalized due to an acute exacerbation of chronic osteomyelitis. Three months before this hospitalization episode, she returned to Ireland and injured her left lower leg and fractured the lateral and medial malleolus. The patient again fell in the children's playground, after a few days she refused to walk, and her parents noticed extensive swelling in her left ankle. 2 weeks later the patient's knee increased in size, and fluid accumulated in the knee joint (
Figure 7).
She developed septic symptoms and was admitted to a hospital in Dublin, Ireland, where repeated interventions were done to drain the excess fluid. She also received antibacterial therapy (Rifampicin and Cefazolin). Radiological examinations were performed (X-ray, CT, magnetic resonance imaging (MRI)), showing extensive changes from the distal third of the thigh to the distal third of the calf. The inflammatory process was visible in the distal femur and proximal tibial growth zone. Wide areas of destruction were also observed, which included the ossifications forming around the left knee joint. Formation of massive ossification around the diaphysis of the tibia with no signs of convalescence in the bone itself (
Figure 8 and
Figure 9). MRI examination of the left knee was performed due to knee joint effusion, where an abnormal amount of fluid in the joint and signs of inflammation were determined.
The patient was diagnosed with a new focus of osteomyelitis in the distal part of the left femur. After her condition was stabilized and compensated, she arrived in Latvia to be admitted in CCUH. There was a wound on the lateral surface of the left thigh discharging serous fluid, (patient arrived at CCUH with a VAC device attached) (
Figure 10).
The patient had an elevated erythrocyte sedimentation rate of 96 mm/h (Ref. range 0-20 mm/h), C-reactive protein 13.27 mg/L (Ref. range 0-5 mg/L). Blood culture for aerobic and anaerobic flora was negative. Staphylococcus epidermidis was determined in the wound. Methicillin-resistant Staphylococcus aureus (MRSA) was not found in the skin swab. Antibacterial therapy was started with intravenous Clindamycin 250mg three times a day, which was changed to intravenous Amikacin 150mg four times a day after two weeks.
Considering the nature and extent of the changes, the complexity of the condition (
Figure 11,
Figure 12 and
Figure 13), the patient was discussed at the council of surgeons and radiologists, where it was decided that reconstructive surgery was impossible and recommended considering the possibility of amputation for parents.
The patient was discharged with recommendations for continuing antibacterial therapy with oral Trimethoprim/Sulfamethoxazole 384mg two times a day for one month and outpatient follow-up.
Figure 14 depicts at the one-month follow-up, she has valgus of the left knee, and flexion in the hip joints.
After consultation with a technical orthopedist, it was determined that the passive range of motion in the ankle joint is normal, the range of the foot is increased, and the range of the knee joint is increased. Pronounced valgisation of the knee during walking causes repeated inflammation. The patient required a gait knee-ankle-foot orthosis to reduce knee valgus and to prevent or reduce the risk of recurrent inflammation. To ensure sufficient torsional control of the orthosis against the external lateral joint during walking, it is necessary to make a prosthesis from a laminate that will simultaneously ensure sufficient elasticity and lightness of the material, as well as resistance to torsional loads. It was recommended to make individual orthopedic shoes to be able to use the orthosis.
2.5. Fifth Hospitalization at Children’s Clinical University Hospital (CCUH)
3 months after the previous hospitalization, the patient was hospitalized again as an acute patient at the Children's Clinical University Hospital, Riga, with complaints of subfebrile body temperature and discomfort in the left hip joint, refusing to lean on the left leg. Objectively, the general condition of the patient was relatively compensated, SIRS positive. A wound was localized in the distal third of the left thigh, from which serous secretions were released. The wound has not completely healed from the last hospitalization. Full range of motion was determined in the left hip joint.
The patient was found to have elevated inflammation indicators: C-reactive protein of 106.31 mg/L (Ref. range < 5 mg/L) and interleukin-6 of 31.8 pg/mL (Ref. range 0-5.9 pg/mL). Blood cultures were aerobically and anaerobically negative. Wound swab was negative. An additional X-ray examination was carried out for patients’ left hip joint, femur and knee joint (
Figure 15).
Antibacterial therapy with intravenous Vancomycin 300mg 3 times a day, Cefotaxime 750mg 3 times a day and Oxacillin 550mg 4 times a day was initiated. A technical orthopedist was consulted, and a hard orthosis was created for the knee joint (
Figure 16).
The patient's condition gradually improved after receiving 6-day antibacterial therapy, and no new foci of osteomyelitis were found. The patient resumed leaning on her left leg. The patient was discharged from the hospital under the further care of a family doctor with recommendations to use a hard orthosis in addition to stabilizing the joint and to continue regular bandaging of the chronic wound.
3. Discussion
This case report describes a young female with Congenital Insensitivity to Pain with Anhidrosis (CIPA), a rare genetic disorder causing a lack of pain, temperature sensation, and the inability to sweat. This leads to self-inflicted injuries and severe complications like osteomyelitis and sepsis. The patient's frequent hospitalizations highlight the difficulties in managing CIPA.
Clinical presentation, diagnostic challenges and multidisciplinary approach: The patient's history of multiple fractures, chronic osteomyelitis, and recurrent infections reflects the complexity of managing CIPA. The absence of pain delays recognition of injuries, as seen in this case where a tibial fracture progressed to osteomyelitis without typical symptoms like pain or swelling. Radiological imaging was key for detecting osteolytic changes, but laboratory markers like C-reactive protein and ESR were sometimes unreliable [
7]. Initial conservative treatment in Dublin followed by surgical interventions in Latvia emphasizes the need for vigilant, proactive care.
This case highlights the need for a multidisciplinary approach to manage CIPA and chronic osteomyelitis, where the absence of pain and typical inflammatory signs complicates early intervention. Given the rarity and complexity of CIPA, a team involving orthopedic surgeons, pediatricians, pain specialists, psychologists, and rehabilitation experts is essential. Tailored diagnostic strategies and prolonged antibiotic therapy, often guided by culture results, are crucial for managing recurrent infections.
Osteomyelitis: Frequent secondary complication in CIPA patients, usually due to unnoticed fractures or prolonged pressure ulcers. Nabiyev et al. (2016) documented several cases of osteomyelitis in CIPA patients, highlighting the difficulty of early diagnosis and prolonged treatment due to delayed detection [
8]. Zhang and Haga (2014) reported that 65% of CIPA patients experience skeletal complications, with 91% of fractures occurring in the lower limbs. Fractures are most common between ages 1 and 7, while other bone disorders show no age pattern, with minor trauma (e.g., short falls) as the main cause [
9]. In this case, the patient initially developed osteomyelitis in the lower leg, but over time it spread to the distal end of the femur. There were also fractures in the ankles and toes.
Long-Term Outcomes and Prognosis: CIPA's chronic nature necessitates careful long-term prognosis evaluation, focusing on orthopedic issues, potential complications from amputation, and the patient's quality of life. Over nine months and five hospitalizations, the infection in our patient spread significantly, highlighting the need for parents to be well-informed about the rapid disease progression. HU et al., (2006) reported a case where the patient was followed up for 7 years, resulting in progressive acroosteolysis, which suggests that patients with CIPA syndrome should be under the supervision of specialists for a long time (all their lives) because the disease is progressive, increasing the development of various complications [
10].
Decision-Making Process for Amputation: Amputation decisions in CIPA patients involve careful consideration of the patient's and family’s preferences. Discussions should address the rationale, alternatives, and expected outcomes, providing support throughout. In this case, the patient's parents initially preferred conservative measures like orthotics. Risk-benefit analysis is essential, weighing benefits such as improved mobility against surgical and psychological risks. Literature shows parents often resist amputation, though it may become necessary as the disease progresses [
11,
12]. Auto-amputation due to self-harm has been reported by Shin et al. (2016), Das et al. (2020), and Cho et al. (2024) [
13,
14,
15].
Future Directions and Considerations: Further research into orthopedic management strategies, such as innovative surgical techniques and advanced implants, is warranted. Alternative pain management approaches, including nonpharmacological interventions, should be explored to improve quality of life. Enhanced genetic counseling and early screening can aid in monitoring and managing complications. Longitudinal studies are needed to track the progression of orthopedic issues and evaluate interventions, with standardized outcome measures to assess functional results, pain, and quality of life. Collaboration among researchers, clinicians, and patient advocacy groups is essential to advance knowledge. Multicenter and international collaborations can accelerate research and develop consensus guidelines for managing CIPA. Ethical dilemmas in invasive interventions like amputation should continue to be explored, and psychosocial support should be integrated into care plans to address the emotional and social impact of living with a rare genetic disorder.
This report provides valuable insights into CIPA's clinical features and management strategies. It underscores the importance of a multidisciplinary approach and serves as an educational resource for healthcare professionals. However, the findings are based on a single patient, limiting their generalizability and the understanding of CIPA's full spectrum. The report lacks long-term follow-up and focuses mainly on orthopedic issues, potentially missing other complications. While it mentions ongoing research, it doesn’t provide new insights into therapeutic interventions. The case is specific to Latvia, which may affect the applicability of the findings in other regions. The patient's treatment took place in 2 hospitals (Latvia and Dublin), which limits a fully detailed review of the treatment.
4. Conclusions
Early diagnosis of CIPA syndrome is crucial to prevent complications, as pathogenetic treatments are not available and surgical interventions are often challenging, not always achieving the desired results. Chronic osteomyelitis is a severe orthopedic complication that requires a robust, multidisciplinary approach. Future research should aim to develop early diagnostic markers and explore new treatments to enhance outcomes and quality of life for CIPA patients.
Author Contributions
Conceptualization, L.G., A.V., DZ.O., M.K., M.M.B., U.B.; methodology, L.G.; validation, A.V., DZ.O., M.K., U.B.; writing—original draft preparation, L.G..; writing—review and editing, A.V., DZ.O., M.K., M.M.B.; visualization, L.G..; supervision, U.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Riga Stradins University, Latvia, and Children’s Clinical University Hospital, Latvia.
Informed Consent Statement
Written informed consent has been obtained from the patient’s parents to publish this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rosemberg S, Marie SKN, Kliemann S: Congenital insensitivity to pain with anhidrosis (hereditary sensory and autonomic neuropathy type IV). Pediatric Neurology. 1994, 11:50-6. [CrossRef]
- Isogawa, N., Ito, K., Lee, J., Ikeda, M., Ishikawa, M., & Baba, Y. (2017). A case of congenital insensitivity to pain with anhidrosis with sensitivity reactions to the electric pulp test. Pediatric Dental Journal, 27(2), 101–104. [CrossRef]
- Cascella M, Muzio MR, Monaco F, Nocerino D, Ottaiano A, Perri F, Innamorato MA: Pathophysiology of nociception and rare genetic disorders with increased pain threshold or pain insensitivity. Pathophysiology. 2022, 29:435-52. [CrossRef]
- Lee ST, Lee J, Lee M, Kim JW, Ki CS: Clinical and genetic analysis of Korean patients with congenital insensitivity to pain with anhidrosis. Muscle Nerve. 2009, 40:85 5-9. [CrossRef]
- Indo Y: Nerve growth factor and the physiology of pain: lessons from congenital insensitivity to pain with anhidrosis. Clin Genet. 2012, 82:341-50. [CrossRef]
- Kubota, M., Hoshino, H., Indo, Y., Ikeda, K., Baba, N., Nozaki, M., Tanaka, H., Haga, N., Kubodera, T., Fukuoka, S., Tomioka, T., Shirakawa, K., Hamabe, F., & Tanaka, C. (2018). Guidelines for the comprehensive treatment and care of congenital insensitivi-ty to pain with anhidrosis (2nd ed.). Health and Labor Sciences Research Grants for Research on Intractable Diseases, Ministry of Health, Labor, and Welfare.
- Mifsud M, Spiteri M, Camilleri K, Bonello M, Azzopardi T, Abela M. The Orthopedic Manifestations of Congenital Insensitivity to Pain: A Population-based Study. Indian J Orthop. 2019 Sep-Oct;53(5):665-673. PMID: 31488938; PMCID: PMC6699213. [CrossRef]
- Nabiyev, V., Kara, A. & Aksoy, M.C. Multidisciplinary assessment of congenital insensitivity to pain syndrome. Childs Nerv Syst 32, 1741–1744 (2016). [CrossRef]
- Zhang, Y., Haga, N. Skeletal complications in congenital insensitivity to pain with anhidrosis: a case series of 14 patients and review of articles published in Japanese. J Orthop Sci 19, 827–831 (2014). [CrossRef]
- HU, Jun; ZHANG, Ai-bin; LIN, Zhen; ZHOU, Jiang-nan. Congenital insensitivity to pain with anhidrosis and progressing acro-osteolysis: a case report with 7-year follow-up. Chinese Medical Journal 119(24):p 2134-2137, December 2006.
- Özkaya AK, Güler E, Arık E, Namlı AR, Cevizli D, Güngör O. A case of congenital insensitivity to pain with anhidrosis. Turk Pediatri Ars. 2014 Jun 1;49(2):177-9. PMID: 26078659; PMCID: PMC4462290. [CrossRef]
- Nabyev, V. N., Seneran, H., & Aksoy, M. C. (2018, August 1). Congenital Insensitivity to Pain Syndrome with Anhidrosis. Review of Literature. https://www.pediatricsresearchjournal.com/articles/congenital-insensitivity-to-pain-syndrome-withanhidrosis-review-of-literature.html.
- Shin JY, Kim SW, Roh SG, Lee NH, Yang KM. Congenital Insensitivity to Pain and Anhidrosis. Arch Plast Surg. 2016 Jan;43(1):95-7. Epub 2016 Jan 15. PMID: 26848454; PMCID: PMC4738137. [CrossRef]
- Das, A., Rajbansh, P., Yadav, M., & Kumar, P. (2020). Congenital insensitivity to pain with anhidrosis: A rare entity. Indian Dermatology Online Journal, 11(2), 274. [CrossRef]
- Cho JH, Hwang S, Kwak YH, Yum MS, Seo GH, Koh JY, Ju YS, Yoon JH, Kang M, Do HS, Kim S, Kim GH, Bae H, Lee BH. Clini-cal and genetic characteristics of three patients with congenital insensitivity to pain with anhidrosis: Case reports and a review of the literature. Mol Genet Genomic Med. 2024 Apr;12(4):e2430. PMID: 38581121; PMCID: PMC10997844. [CrossRef]
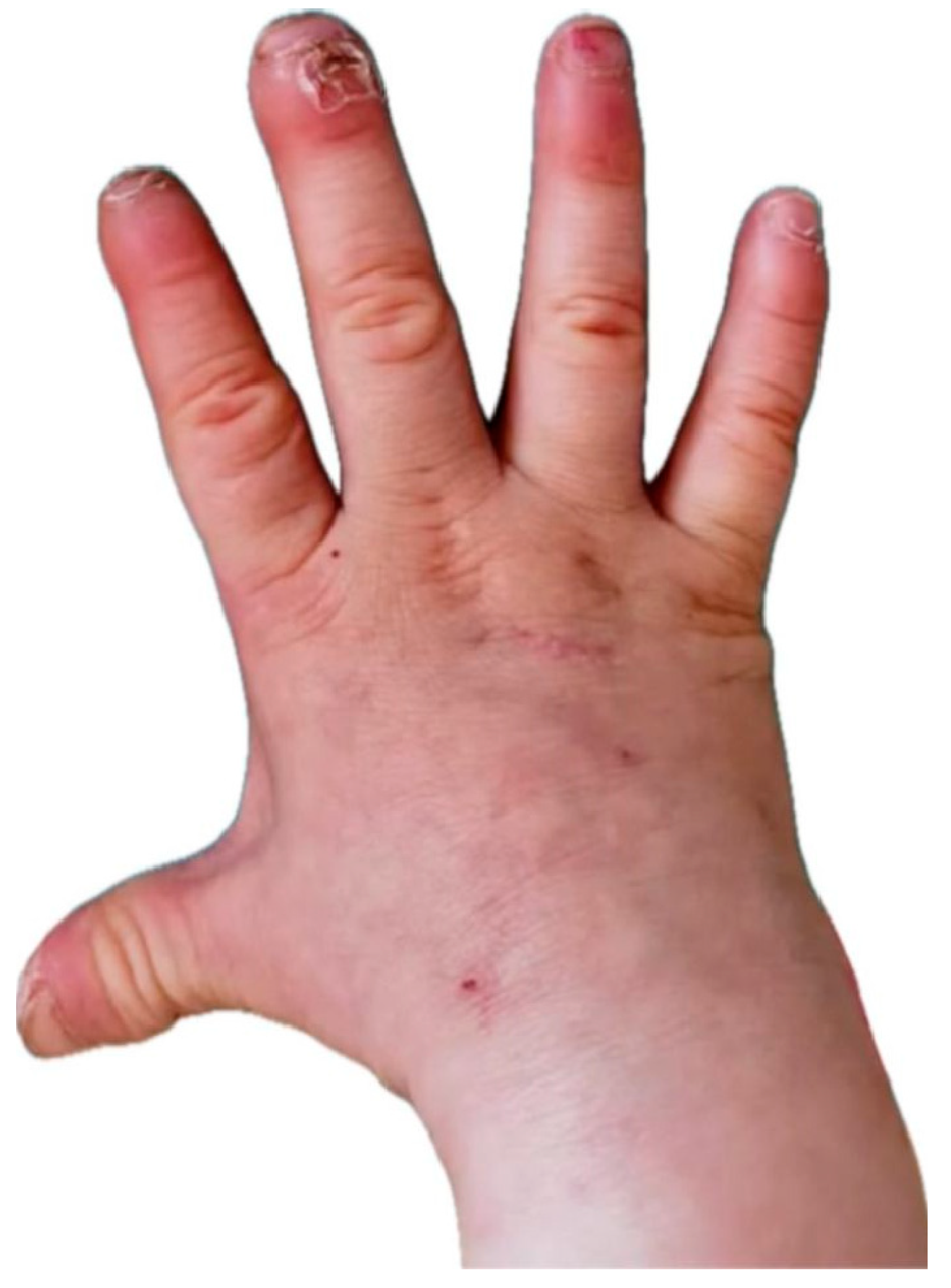
Figure 1.
The appearance of the patient's right hand with signs of dry skin, distal phalanges with thickened, lichenified skin, and places without nails (nail dystrophy).
Figure 1.
The appearance of the patient's right hand with signs of dry skin, distal phalanges with thickened, lichenified skin, and places without nails (nail dystrophy).
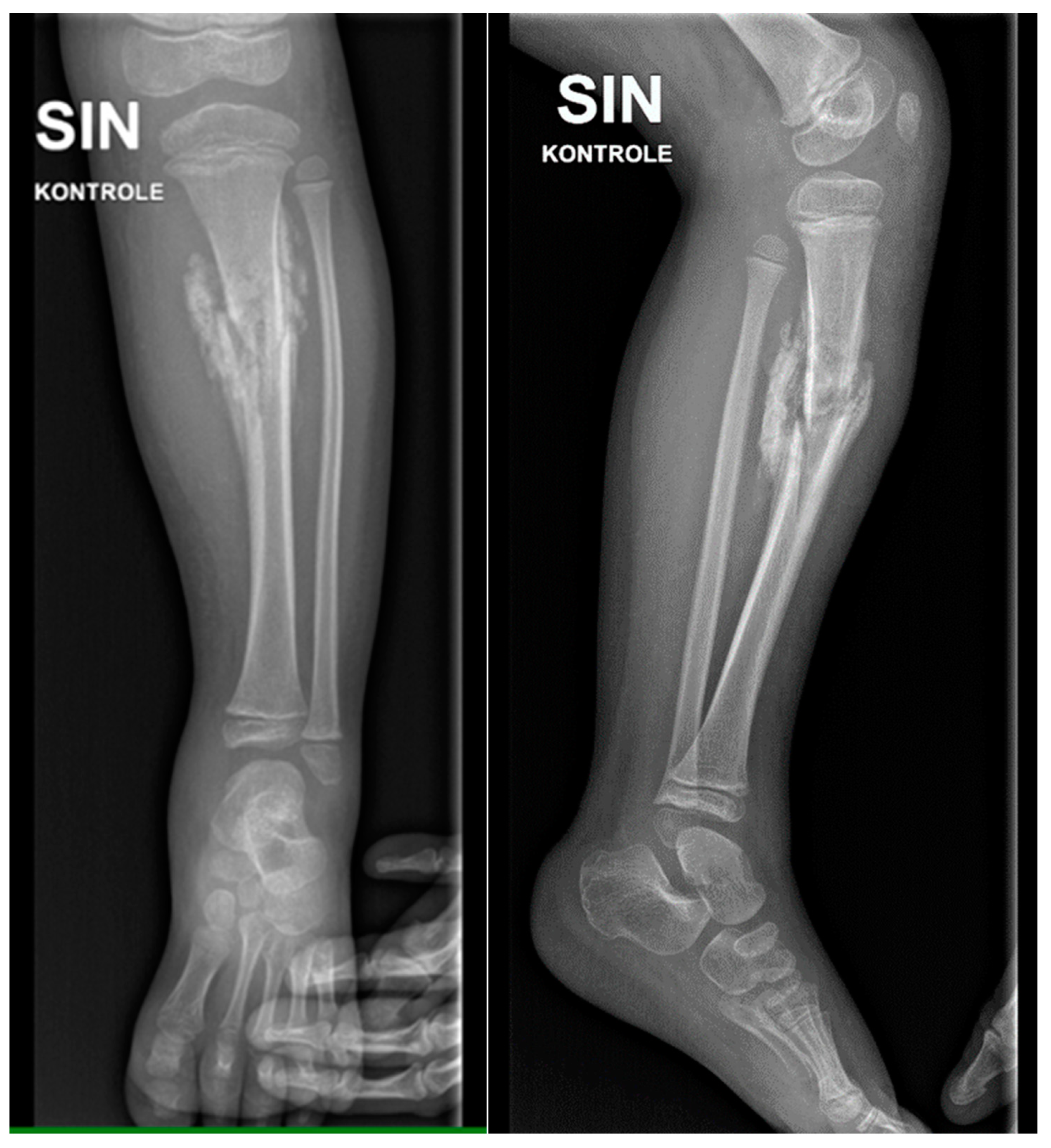
Figure 2.
X-ray examination of the left lower leg anterior-posterior (AP) and lateral projections shows marked hyperostosis in soft tissue, moderate angular deformation, and a deformation angle of approximately 15 degrees, with sclerotic hyperostosis type changes around the fracture zone.
Figure 2.
X-ray examination of the left lower leg anterior-posterior (AP) and lateral projections shows marked hyperostosis in soft tissue, moderate angular deformation, and a deformation angle of approximately 15 degrees, with sclerotic hyperostosis type changes around the fracture zone.
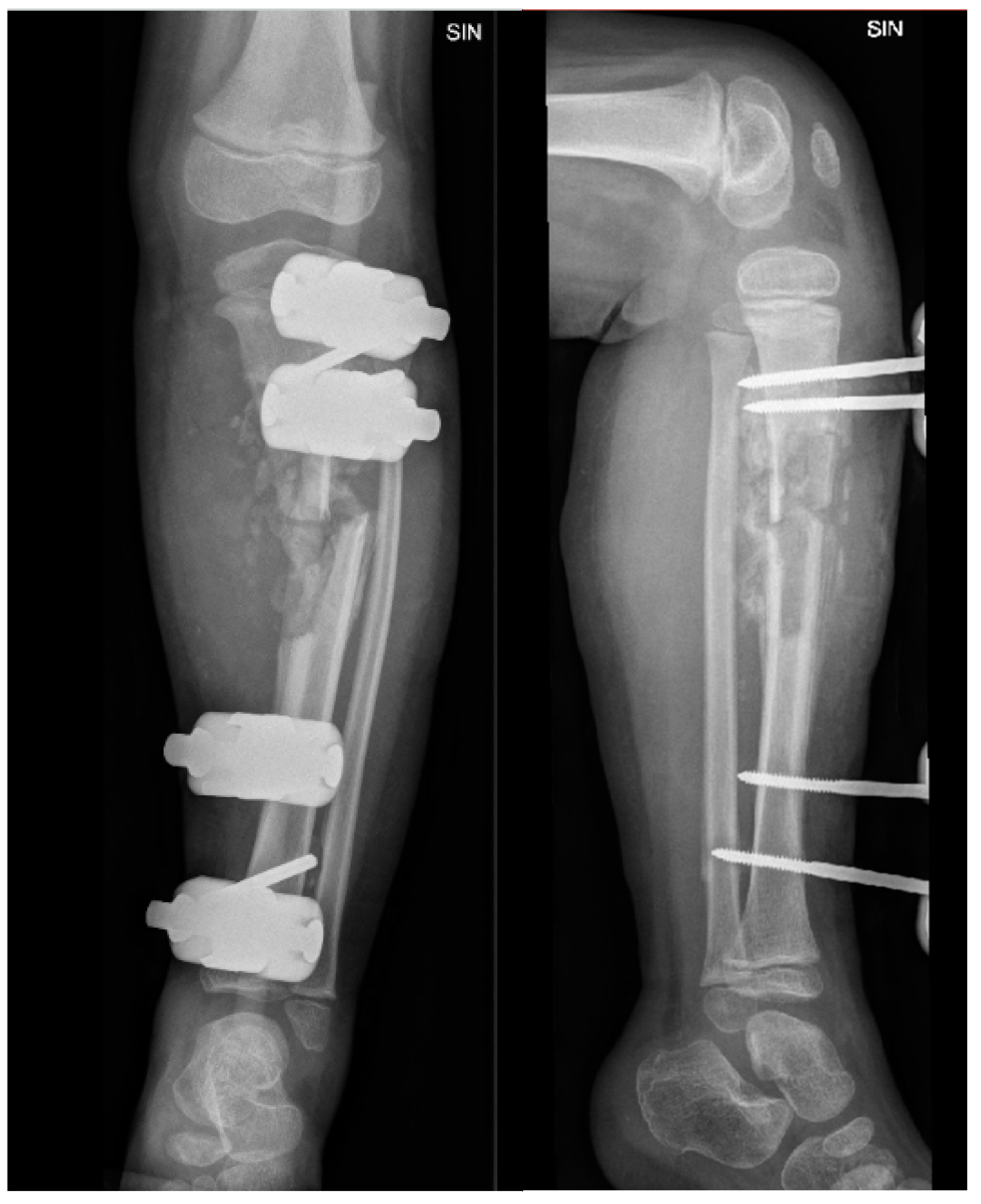
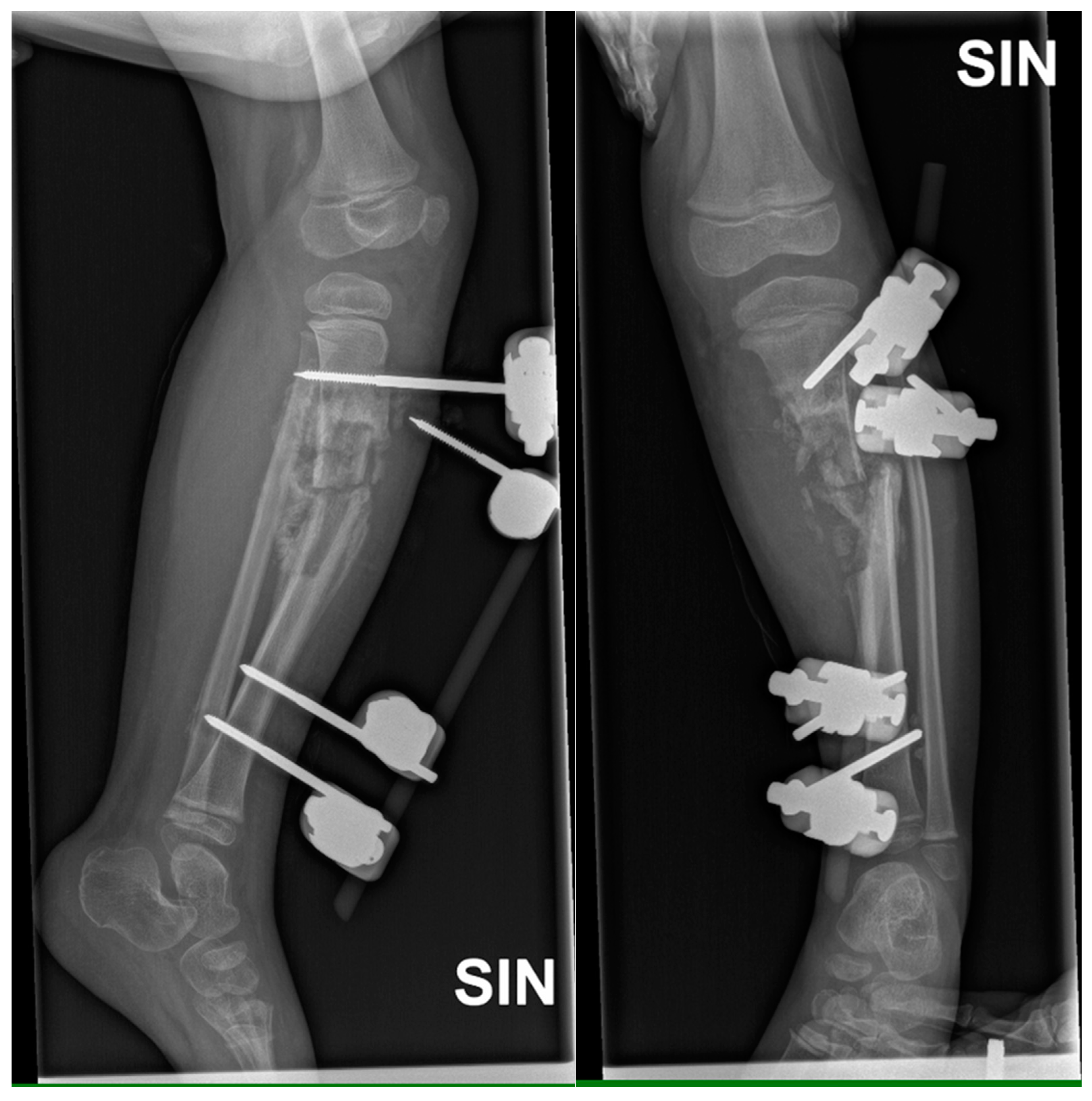
Figure 3.
X-ray examination of left lower leg in AP and lateral projections shows posttraumatic chronic osteomyelitis of left tibial diaphysis, opening 13 degrees medially after external fixation device application. A cavity (5.8 cm) and soft tissue calcifications are visible.
Figure 3.
X-ray examination of left lower leg in AP and lateral projections shows posttraumatic chronic osteomyelitis of left tibial diaphysis, opening 13 degrees medially after external fixation device application. A cavity (5.8 cm) and soft tissue calcifications are visible.
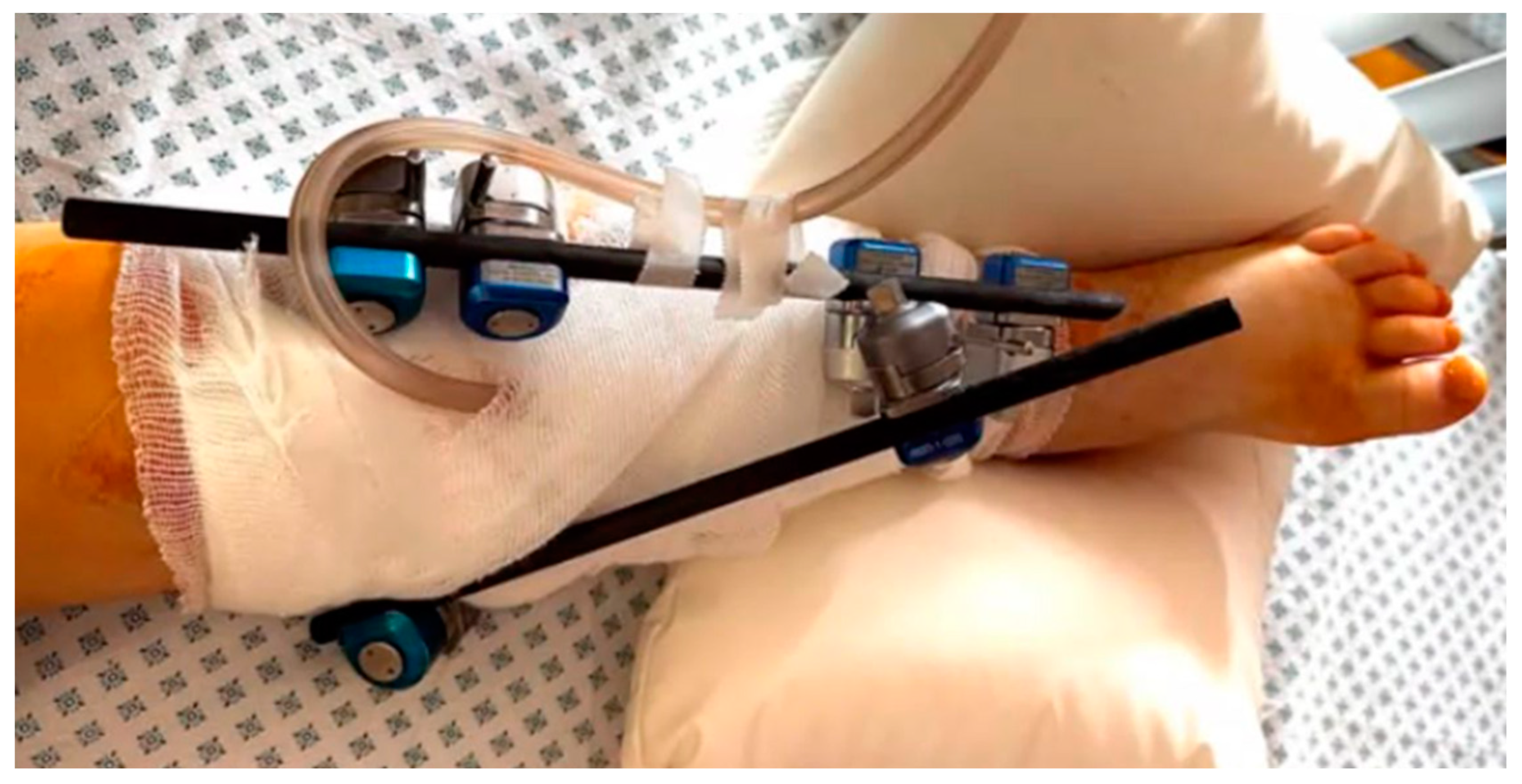
Figure 4.
The appearance of patient's lower limb after partial resection of the left tibia, cavity repair, application of an external fixation device, and vacuum assisted closure (VAC) device.
Figure 4.
The appearance of patient's lower limb after partial resection of the left tibia, cavity repair, application of an external fixation device, and vacuum assisted closure (VAC) device.
Figure 5.
X-ray examination of the left lower leg in AP and lateral projections after the patient fell from the sofa.
Figure 5.
X-ray examination of the left lower leg in AP and lateral projections after the patient fell from the sofa.
Figure 6.
X-ray examination of left lower leg in AP and lateral projections shows posttraumatic chronic osteomyelitis of left tibial diaphysis. After fixation with a locking plate and implantation of resected fibula diaphysis fragment, consolidation forms near implanted bone, with slightly increased soft tissue calcifications and sclerosis.
Figure 6.
X-ray examination of left lower leg in AP and lateral projections shows posttraumatic chronic osteomyelitis of left tibial diaphysis. After fixation with a locking plate and implantation of resected fibula diaphysis fragment, consolidation forms near implanted bone, with slightly increased soft tissue calcifications and sclerosis.
Figure 7.
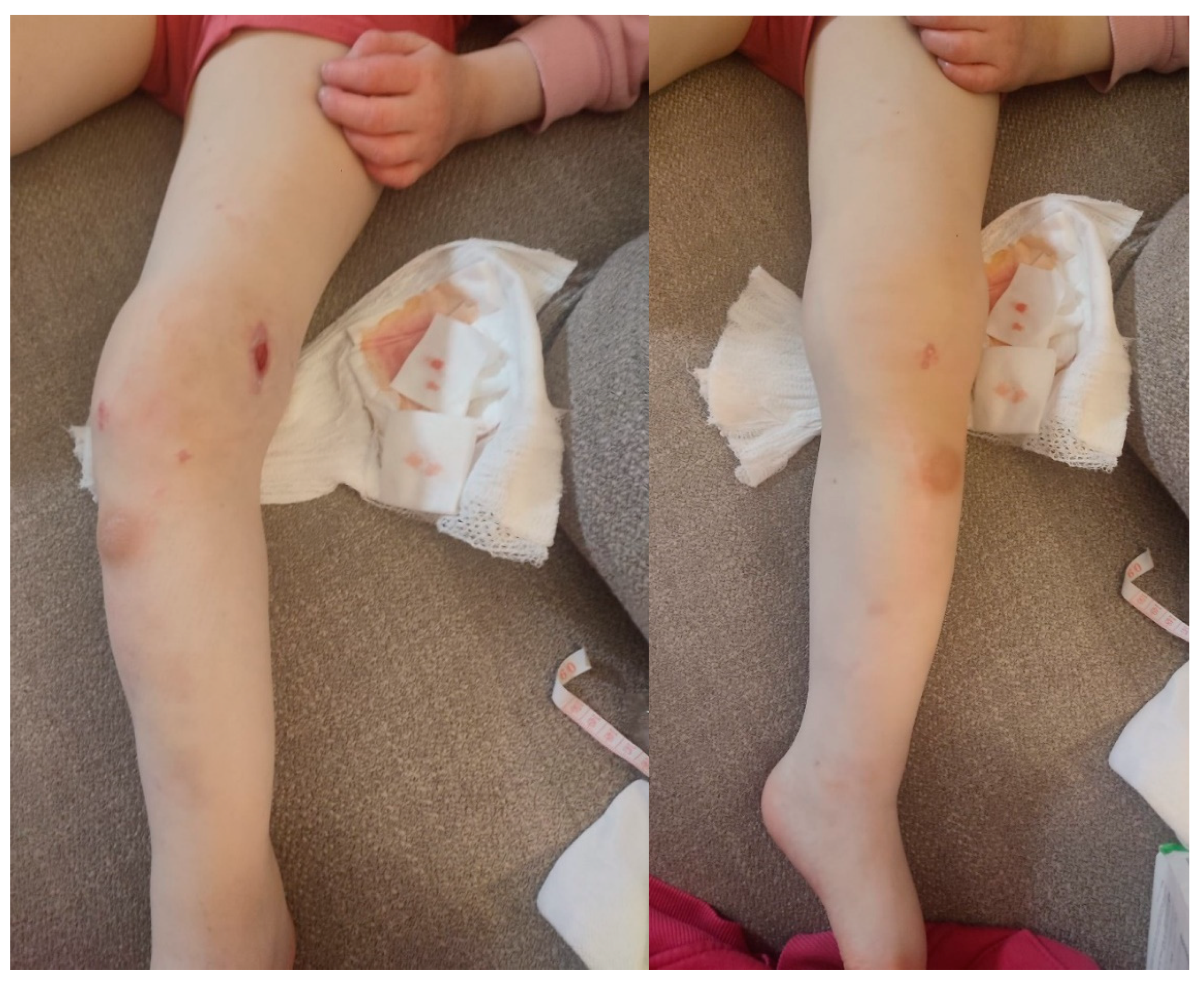
The appearance of the patient's leg 2 weeks after the diagnosis of the fracture of both malleolus of the left leg. Swelling in the left knee area, as well as a significant increase in the size of the left leg compared to the right leg.
Figure 7.
The appearance of the patient's leg 2 weeks after the diagnosis of the fracture of both malleolus of the left leg. Swelling in the left knee area, as well as a significant increase in the size of the left leg compared to the right leg.
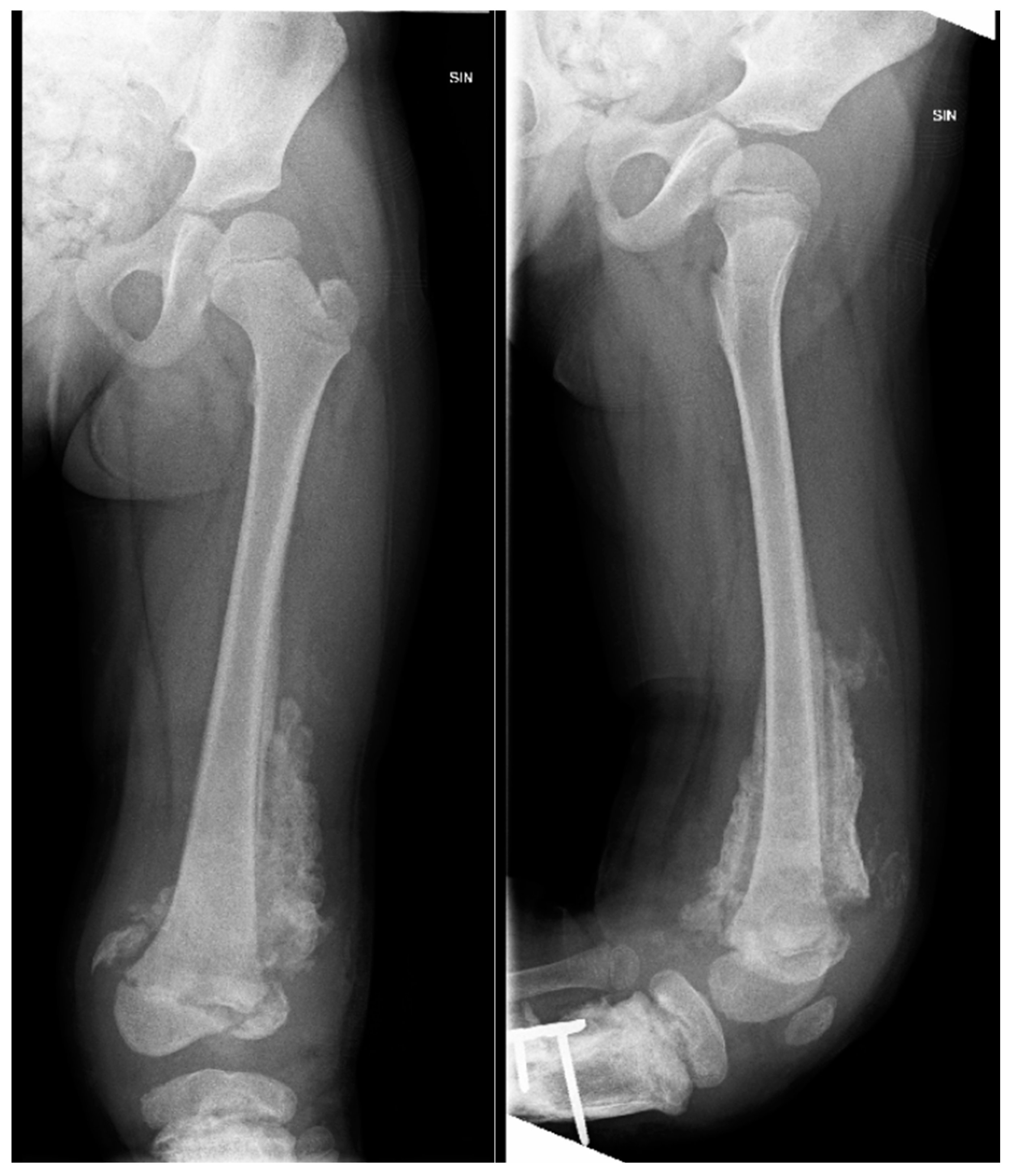
Figure 8.
The X-ray examination of the distal third of the thigh to the lower leg in AP and lateral projections reveals an inflammatory process in the distal femur and proximal tibial growth zone, wide areas of destruction, and massive ossification around the tibia diaphysis.
Figure 8.
The X-ray examination of the distal third of the thigh to the lower leg in AP and lateral projections reveals an inflammatory process in the distal femur and proximal tibial growth zone, wide areas of destruction, and massive ossification around the tibia diaphysis.
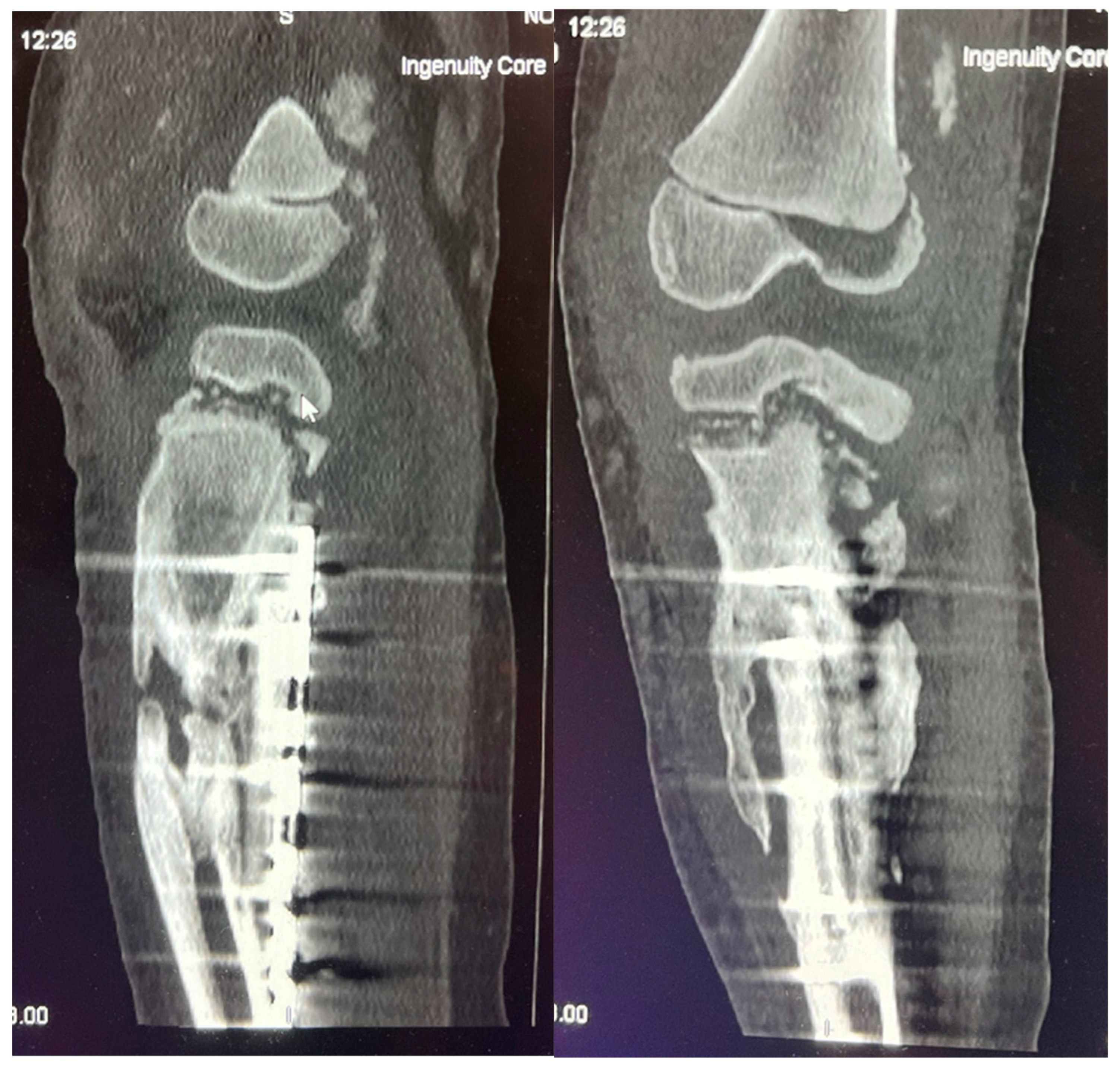
Figure 9.
CT imaging shows an inflammatory process in the distal femur and proximal tibial growth zone, with wide areas of destruction, including the left knee joint bones, and massive ossification around the tibia diaphysis, without signs of convalescence.
Figure 9.
CT imaging shows an inflammatory process in the distal femur and proximal tibial growth zone, with wide areas of destruction, including the left knee joint bones, and massive ossification around the tibia diaphysis, without signs of convalescence.
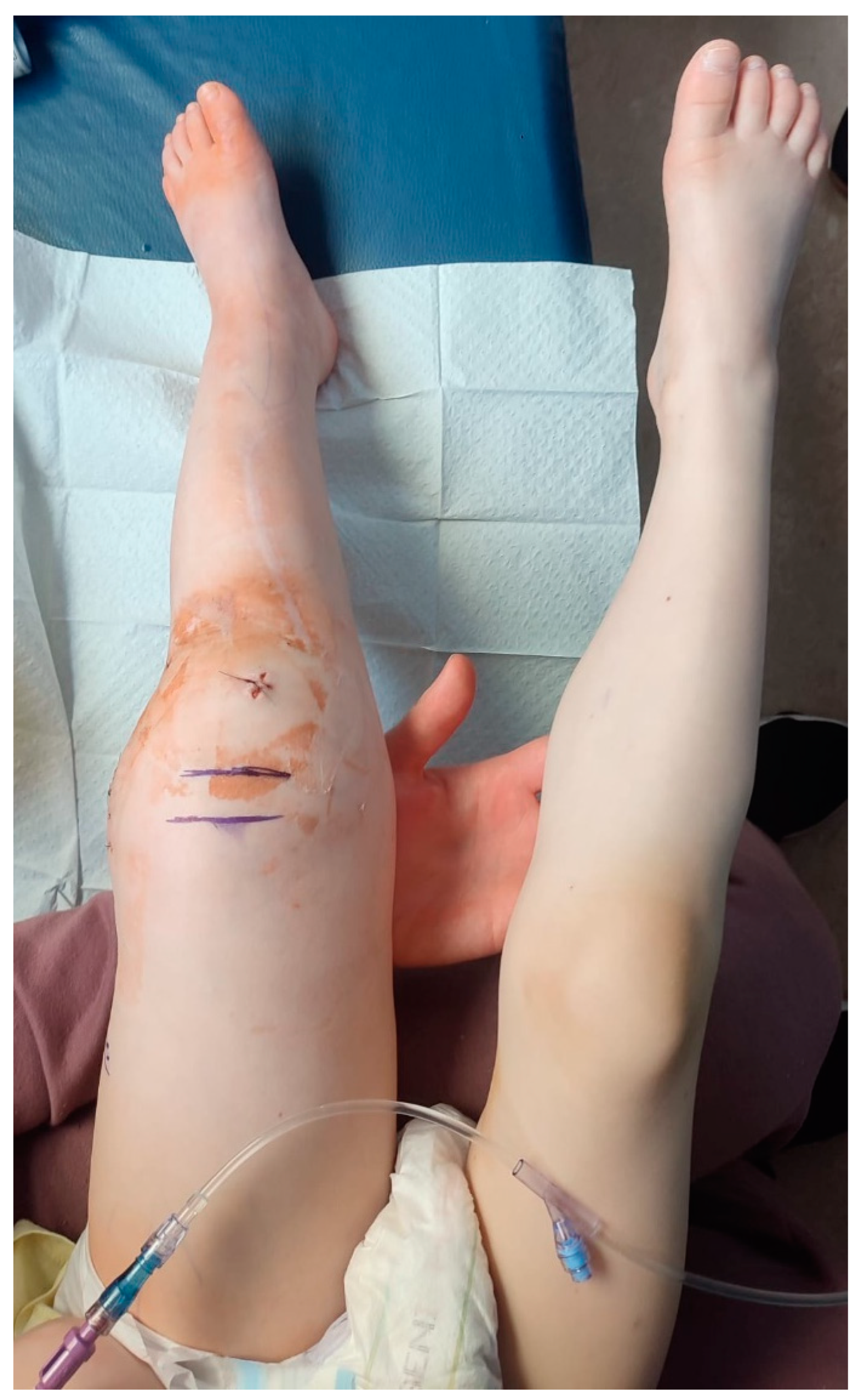
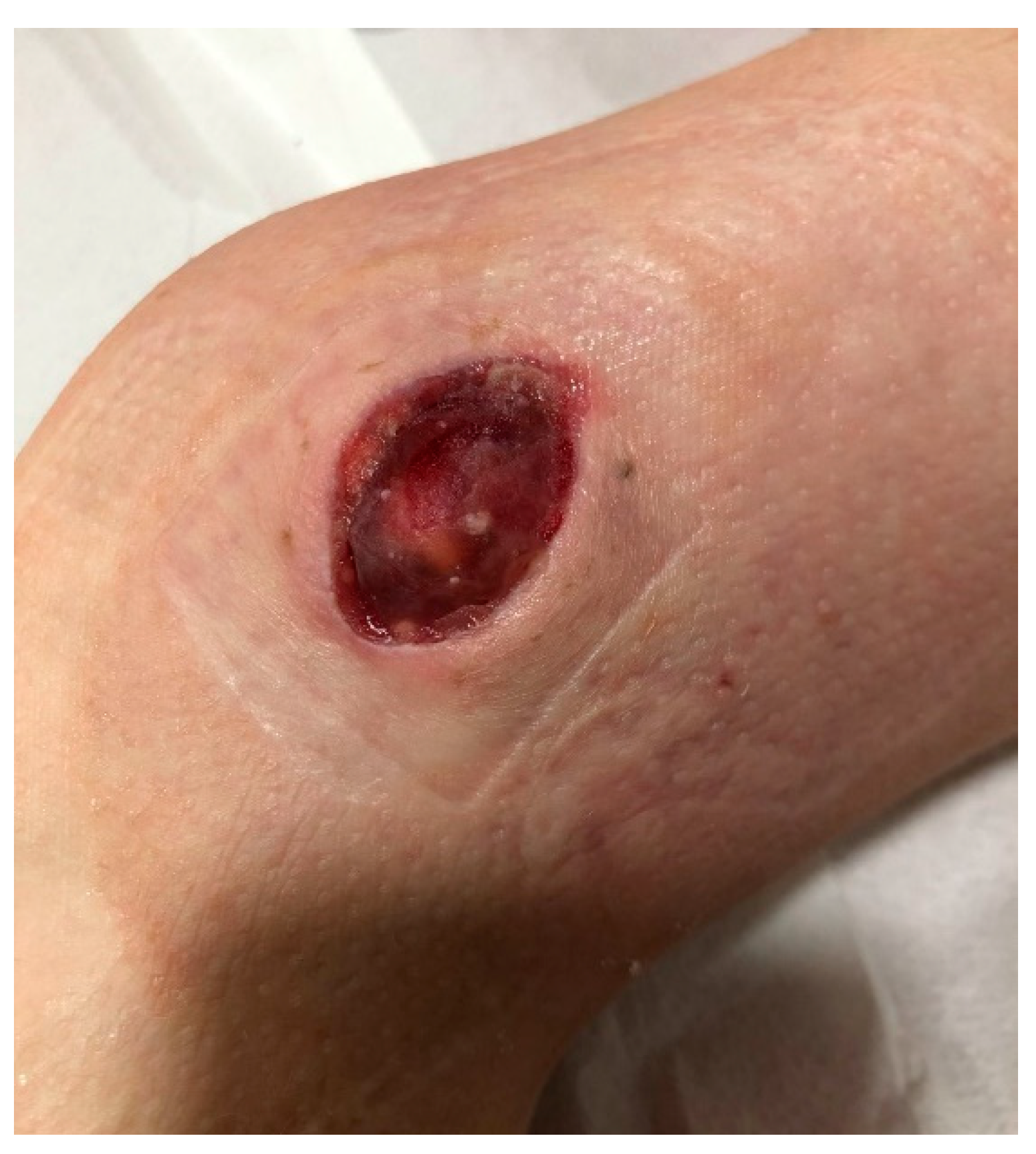
Figure 10.
The wound on the lateral surface of the left thigh providing serous discharge, the appearance of the wound after the removal of the VAC device on CCUH admittance.
Figure 10.
The wound on the lateral surface of the left thigh providing serous discharge, the appearance of the wound after the removal of the VAC device on CCUH admittance.
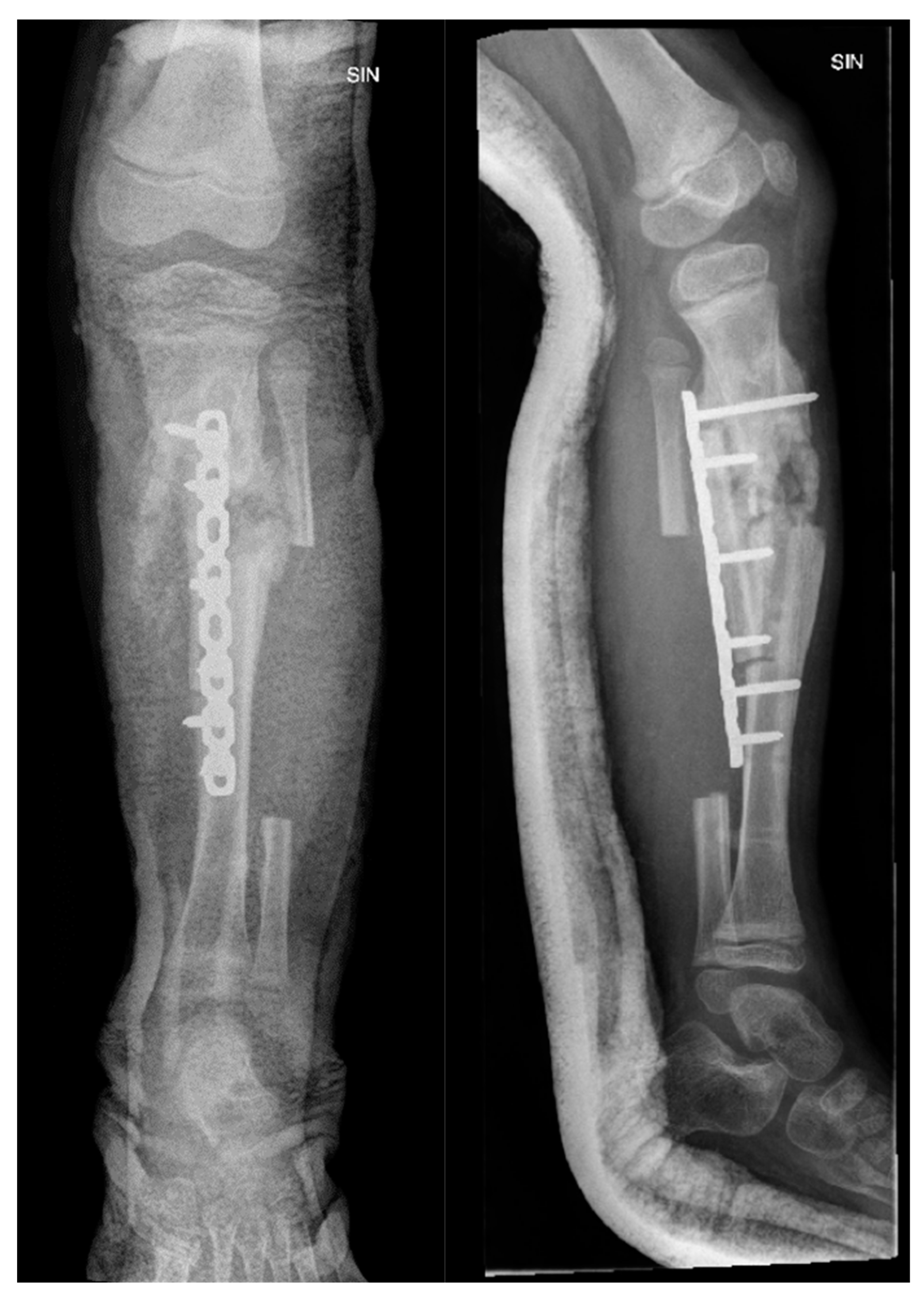
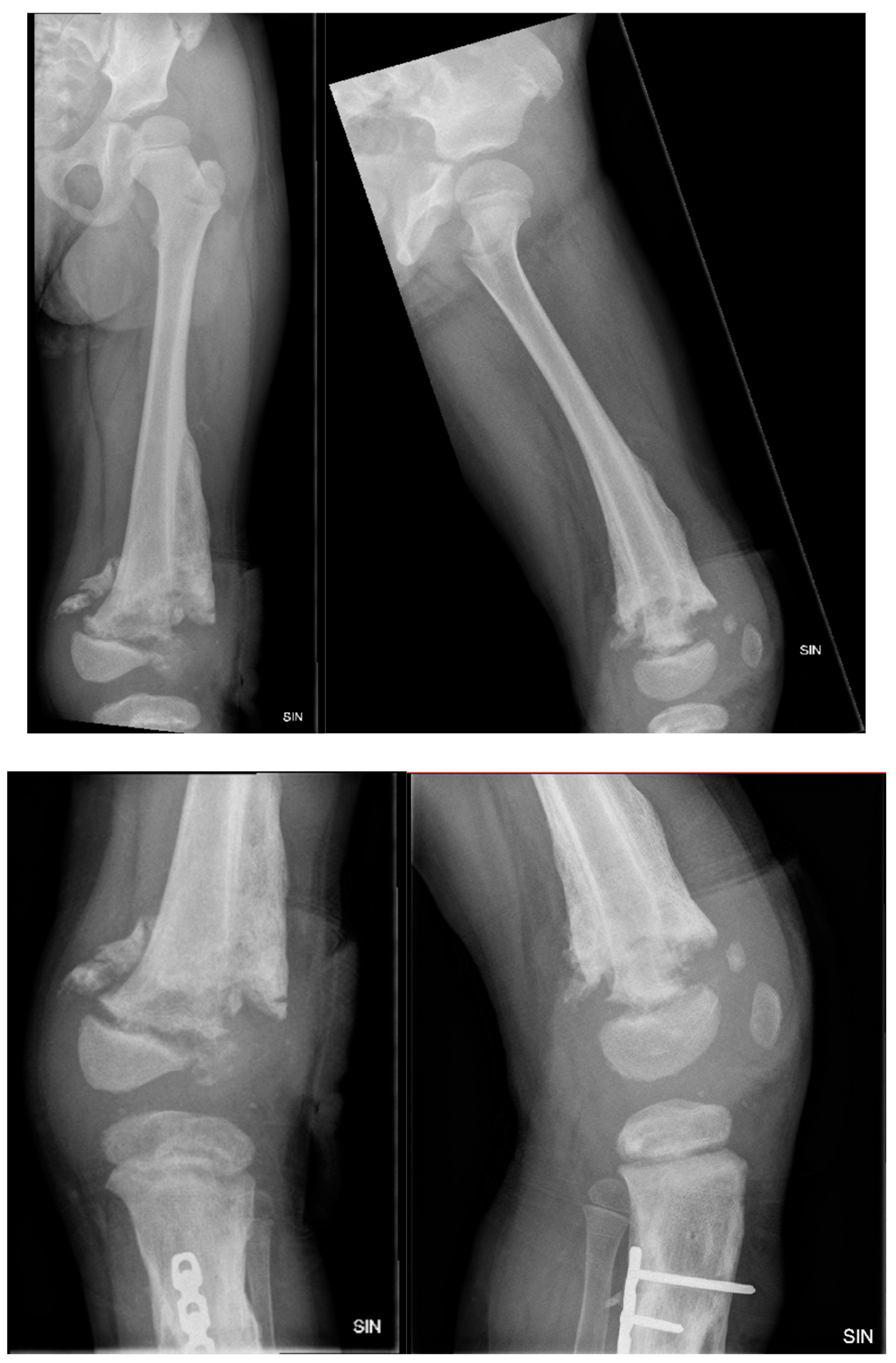
Figure 11.
The X-ray examination of the distal third of the thigh in AP and lateral projections shows a condition after posttraumatic chronic osteomyelitis of the left tibial diaphysis. For the left thigh - the picture corresponds to the condition after an epiphyseal fracture in the anamnesis, the lateral condyle of the femur is of small volume, heterogeneous structure. Massive calcification paraosseous to diaphysis of femur.
Figure 11.
The X-ray examination of the distal third of the thigh in AP and lateral projections shows a condition after posttraumatic chronic osteomyelitis of the left tibial diaphysis. For the left thigh - the picture corresponds to the condition after an epiphyseal fracture in the anamnesis, the lateral condyle of the femur is of small volume, heterogeneous structure. Massive calcification paraosseous to diaphysis of femur.
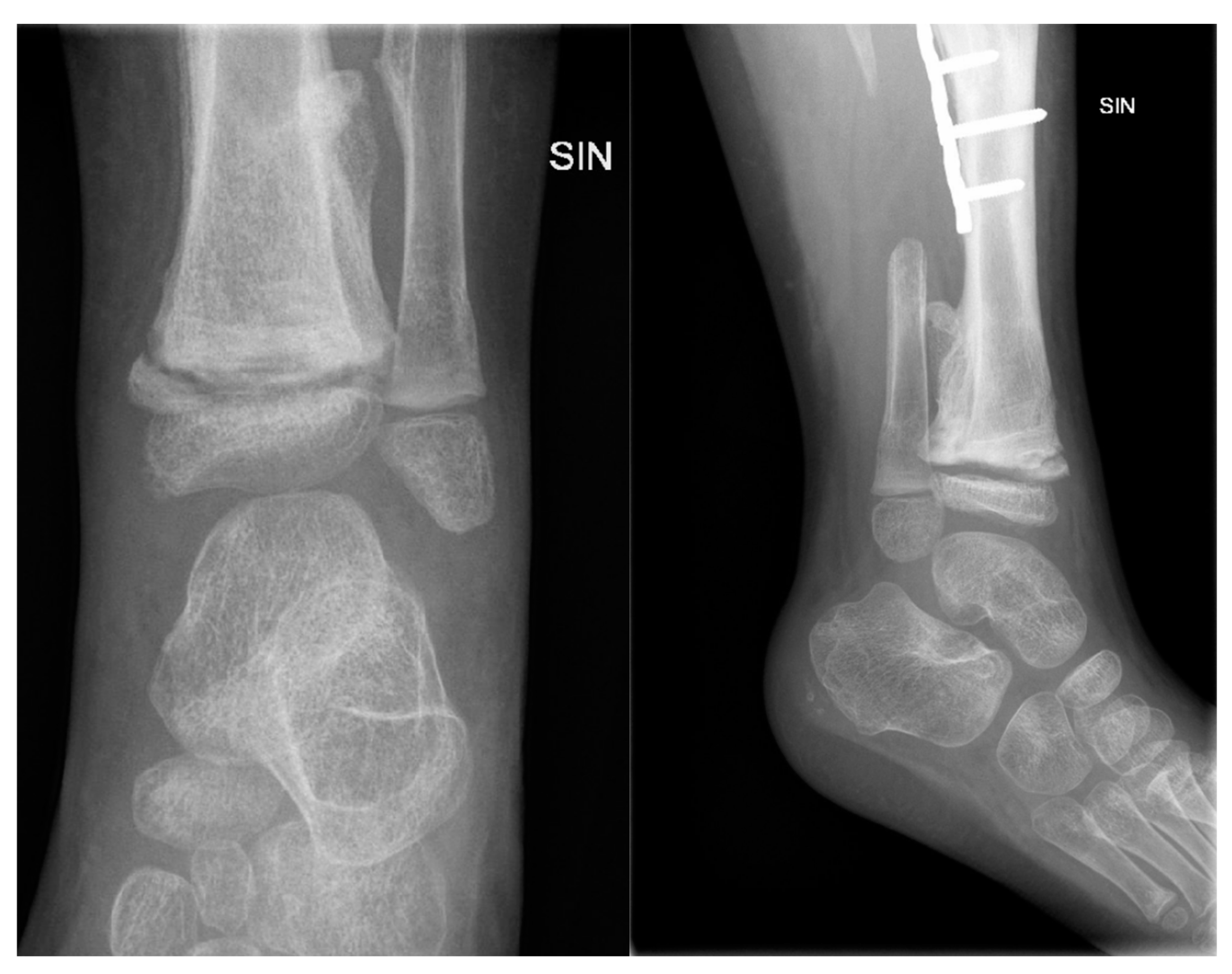
Figure 12.
X-ray examination of the proximal part of the left foot and the distal third of the lower leg in AP and lateral projections shows a condition in the left foot and lower leg after a plate fixation and implantation of a resected fibula diaphysis fragment. The area is not fully covered, and intense humming has developed after distal tibial osteoepiphysiolysis. Slight deformation in the metaphysis of the fibula remnant is also present.
Figure 12.
X-ray examination of the proximal part of the left foot and the distal third of the lower leg in AP and lateral projections shows a condition in the left foot and lower leg after a plate fixation and implantation of a resected fibula diaphysis fragment. The area is not fully covered, and intense humming has developed after distal tibial osteoepiphysiolysis. Slight deformation in the metaphysis of the fibula remnant is also present.
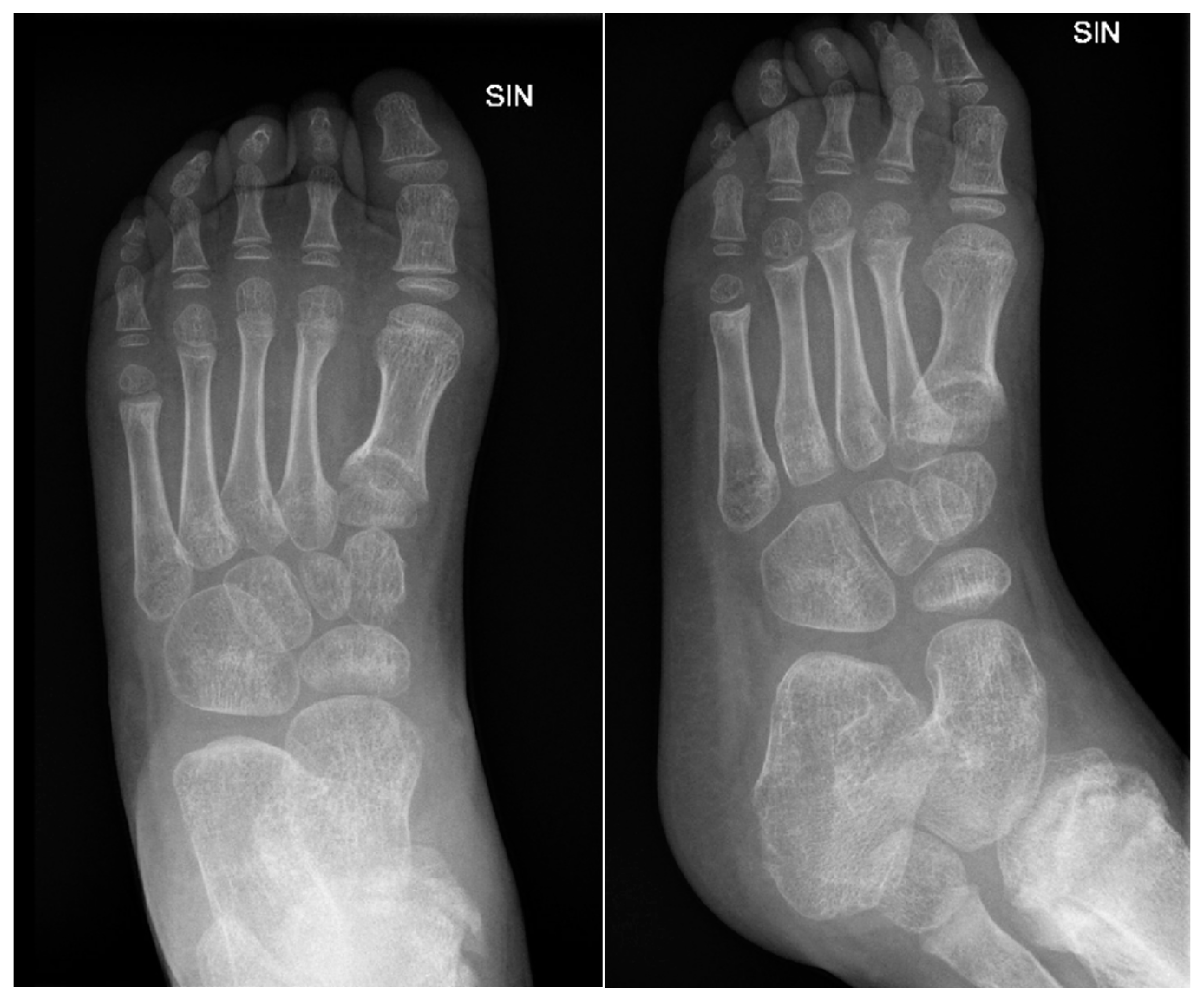
Figure 13.
X-ray examination of the left foot in AP and lateral projections. A fracture of the distal metaphysis of the second metatarsal bone of the left foot is visible without dislocation.
Figure 13.
X-ray examination of the left foot in AP and lateral projections. A fracture of the distal metaphysis of the second metatarsal bone of the left foot is visible without dislocation.
Figure 14.
The appearance of the patient's left leg four weeks after discharge from the hospital, she has pronounced valgus of the left knee, flexion in the hip joints, and a small wound on the lateral surface of the left thigh.
Figure 14.
The appearance of the patient's left leg four weeks after discharge from the hospital, she has pronounced valgus of the left knee, flexion in the hip joints, and a small wound on the lateral surface of the left thigh.
Figure 15.
The X-ray examination of the left hip joint, femur and knee joint in AP and lateral projections shows a small, heterogeneous lateral condyle of the femur, a lytic zone in the metaphysis, massive calcification in the mid-diaphysis, normal hip joint gap, smooth surfaces, and no pathologies in the hip joint bones.
Figure 15.
The X-ray examination of the left hip joint, femur and knee joint in AP and lateral projections shows a small, heterogeneous lateral condyle of the femur, a lytic zone in the metaphysis, massive calcification in the mid-diaphysis, normal hip joint gap, smooth surfaces, and no pathologies in the hip joint bones.
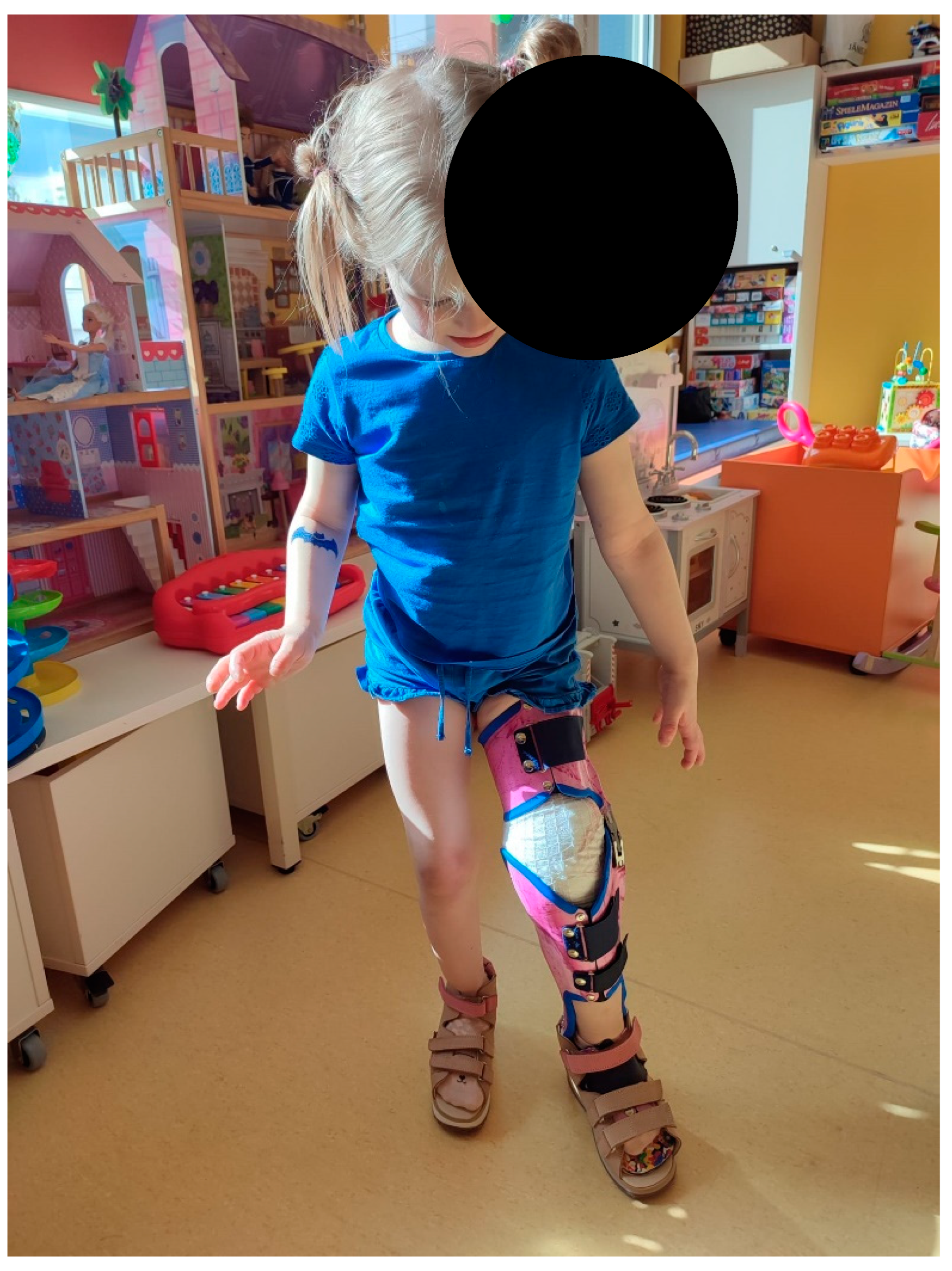
Figure 16.
The patient with the previously made orthopedic shoes and the hard orthosis for the knee joint created during this hospitalization.
Figure 16.
The patient with the previously made orthopedic shoes and the hard orthosis for the knee joint created during this hospitalization.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).