1. Introduction
Anemia is a widespread global health issue, defined by a reduction in the number of red blood cells (RBCs) or a decrease in hemoglobin concentration within the cells, falling below normal levels.[
1] According to the World Health Organization (WHO), in 2019, anemia affected 30% (571 million) of women aged 15-49 years, 37% (32 million) of pregnant women, and 40% (269 million) of children aged 6-59 months.[
2] Additionally, over 20% of individuals aged 85 years and older suffer from anemia.[
3] It is a common condition across different nations and age groups. Anemia can be triggered by various acute and chronic conditions, surgeries, menstrual blood loss, gastrointestinal bleeding, infections, immune-mediated disorders, and inherited diseases, causing fatigue, cognitive impairment, multi-organ dysfunction, irreversible complications, and even life-threatening conditions.[
4]
Anemia typically presents with nonspecific symptoms, and a wide range of signs may suggest its presence.[
4] As a result, diagnosing anemia based on physical examination alone is challenging. Historically, anemia diagnosis has relied on laboratory assessments of hemoglobin concentration, which are generally conducted through blood tests. While this approach is highly accurate, it is invasive, costly, and dependent on specialized equipment and trained professionals.[
5] These resources may not always be accessible, especially in remote or underdeveloped healthcare settings.
Anemia leads to insufficient oxygen delivery due to a lack of adequate hemoglobin, resulting in a series of cardiovascular changes that ultimately cause left ventricular hemodynamic alterations.[
6,
7] Chest X-ray (CXR) is a commonly used tool in hospitals due to its low cost and the availability of extensive databases. However, because anatomical structures are superimposed along the projection direction, interpreting chest X-rays can be challenging. This effect makes detecting subtle abnormalities at specific locations difficult.[
8] For human radiologists, it is nearly impossible to interpret anemia from CXR images alone. However, by evaluating signs like the "aortic ring sign" and the "interventricular septum sign," radiologists can use computed tomography (CT) to assess anemia.[
9] Recent studies have explored the use of electrocardiogram (EKG)-based deep learning models (DLM) to predict anemia by analyzing the cardiovascular changes that result in corresponding electrical changes in the heart.[
10] Compared to EKG, CXR is theoretically a more intuitive method for observing anemia-related volume and ventricular changes. Additionally, DLM has been widely used in analyzing CXR images for conditions like tuberculosis, lung cancer, COVID-19, and cardiomegaly, although none of these studies have focused on anemia detection.[
11] Recent research has also successfully used CXR-based DLM to predict cardiovascular age, further suggesting that DLM can assess cardiovascular status from CXR images.[
12] Given the success of EKG-based DLM in predicting anemia and prior studies demonstrating the capability of CXR-based DLM in assessing cardiovascular conditions, we hypothesize that DLM could predict anemia through CXR.
Based on the aforementioned discussion, it is evident that CXR has the potential to capture cardiovascular changes, and its application within DLM has already been demonstrated in various medical conditions. Building on this foundation, we conducted a retrospective cohort study and developed a DLM to predict anemia in order to test this hypothesis. Additionally, we performed an in-depth analysis of subgroups in an effort to identify factors that may affect predictive accuracy and explored the associations between relevant characteristics. Finally, we performed a comparative analysis between the predictive capabilities of the DLM and traditional clinical data. Specifically, we sought to determine whether the DLM, when combined with imaging data from CXR, could outperform predictions made using conventional clinical information alone. Our goal was to assess whether the integration of machine learning with imaging techniques provides an advantage for clinical anemia scenario.
2. Method
2.1 Data source and Population
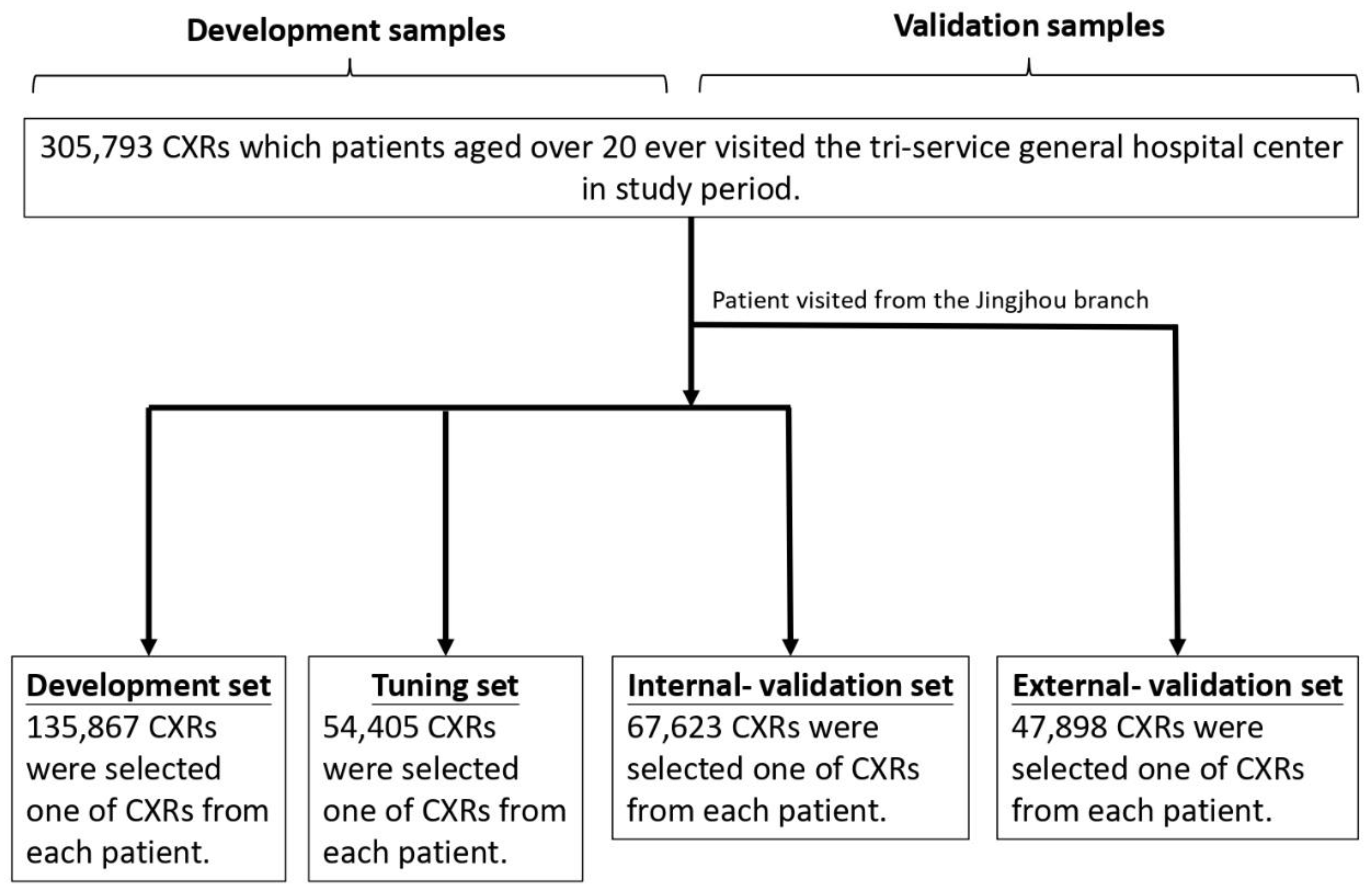
The database utilized for this research was sourced from Tri-Service General Hospital in Taipei, Taiwan, and the study received ethical approval from the hospital’s institutional review board (IRB No. C202305019). The CXRs were selected according to the following criteria: (1) Data collection occurred between June 2016 and February 2022; (2) Patients younger than 20 years old were excluded; and (3) The CXRs originated from the emergency department (ER), outpatient department (OPD), or inpatient department (IPD). Each patient contributed only one CXR, chosen at a randomized point in time to ensure a representative sample, as previously applied in other studies. This approach was chosen to provide a more representative sample, as opposed to using only the most recent X-ray, and better simulates the scenario where a patient may present at an outpatient clinic at any given time. Additionally, previous studies have confirmed that despite the randomness in selection, the model's performance remains consistent.[
13]
The database was divided into four subsets: development, tuning, internal validation, and external validation sets. Their baseline characteristics and image features are presented in
Table 1, with the process of data separation illustrated in
Figure 1. A total of 305,793 CXRs met the inclusion criteria, and the corresponding demographic details were analyzed. To facilitate the separation process, 47,898 CXRs from the Jingjhou branch were assigned to the external validation set, ensuring that no data from this branch participated in the model training phase, allowing for independent validation, which was also widely used in previous study.[
14] For model development, 135,867 CXRs from branches outside Jingjhou were assigned to the development set, 54,405 to the tuning set for parameter optimization, and 67,623 to the internal validation set. The development and tuning sets are both involved in the training process. The development set serves as the primary dataset for training, while the tuning set is used to fine-tune the model and adjust its training trajectory. Since both of them are already part of the training process, it cannot be considered for independent case predictions and, therefore, will not be included in the result presentation.
All the P values of the characteristics were less than 0.001, except anemia. The mean age ranged from 50.9 to 52.2 years.
2.2 Observation Variables
Our main observational variables included both disease-related and basic patient characteristics.
Disease characteristics included heart failure (HF), diabetes mellitus (DM), chronic kidney disease (CKD), coronary artery disease (CAD), hypertension (HTN), Hyperlipidemia (HLP), and Chronic Obstructive Pulmonary Disease (COPD).
Basic patient characteristics included age, sex, acquisition location (e.g., IPD, OPD, or ER), posteroanterior (PA) or anteroposterior (AP) view, and hemoglobin level. Anemia was defined as a hemoglobin concentration of less than 10 g/dL. Hemoglobin values were collected within one day before or after the CXR.
The definitions of the other variables were described in Supplemental material.
2.3 The CXR Report Analysis
To gain a deeper understanding of the relationship between lung structure and anemia, we analyzed radiologist-typed CXR reports. The reports were preprocessed to standardize the content, and key phrases identified by the radiologist were used for keyword extraction and analysis.
2.4. The Implementation of the DLM
The details of the DLM are referred and revised from previous research.[
12]
The methods used in this article will be described in the following, which including the data preprocessing, deep learning network structure and related parameters.
The CXR data were recorded in DICOM format with a resolution of more than 3000 × 3000 pixels, with each linking to a digital label about age. To standardize picture size, we scaled down the short side to 256 pixels in proportion and randomly cropped 224×224 pixels with a 50-50 chance for horizontal inversion as input. After that, data were generated to 10 subdata by 10-crop evaluation based on a previous study.[
15] The average of these 10 probabilities is the output of our data given by the DLM.
The major feature extraction architecture is based on a 50-layer SE-ResNeXt, which won the ImageNet Large Scale Visual Recognition Challenge (ILSVRC) in 2017.[
16] We used transfer learning by ImageNet data and preserved the final layer parameter for our DLM initial parameter. The output data from SE-ResNeXt passed through a subsequent global pooling layer and a fully connected layer with 1 digital output with a softmax function. The final output is the age at which the DLM predicted if patient has anemia.
Each parameter of the CXR-age DLM was updated to minimize the mean-square error loss, while CXR-sex by cross-entropy loss, and the oversampling process was implemented based on the weights computed on the prevalence of that class in the development set. We used 32 batch sizes for training, and all parameters were trained by Adam (adaptive moment estimation), with an initial learning rate of 0.001 and a decay of 10 times when the loss plateaued after an epoch. The loss calculated by the tuning set was recorded for each epoch, and we chose the epoch with minimal loss to avoid DLM overfitting to the development set. The only regularization method for avoiding overfitting was a weight decay of 10-4 in this study. The above details were implemented by the package MXNet (R package version 1.3.0).
2.5. Outcome and Statistical Analysis
The details were described in Supplemental Material.
3. Results
3.1. Baseline Characteristics
Patient characteristics in the development, tuning, internal validation and external validation sets are shown in
Table 1. All the P values for the characteristics were less than 0.001, except for the anemia rate. This discrepancy may be attributed to differences in population composition between the internal and external validation sets. The mean age ranged from 50.9 to 52.2 years.
3.2. Performance of the DLM to Predict Anemia
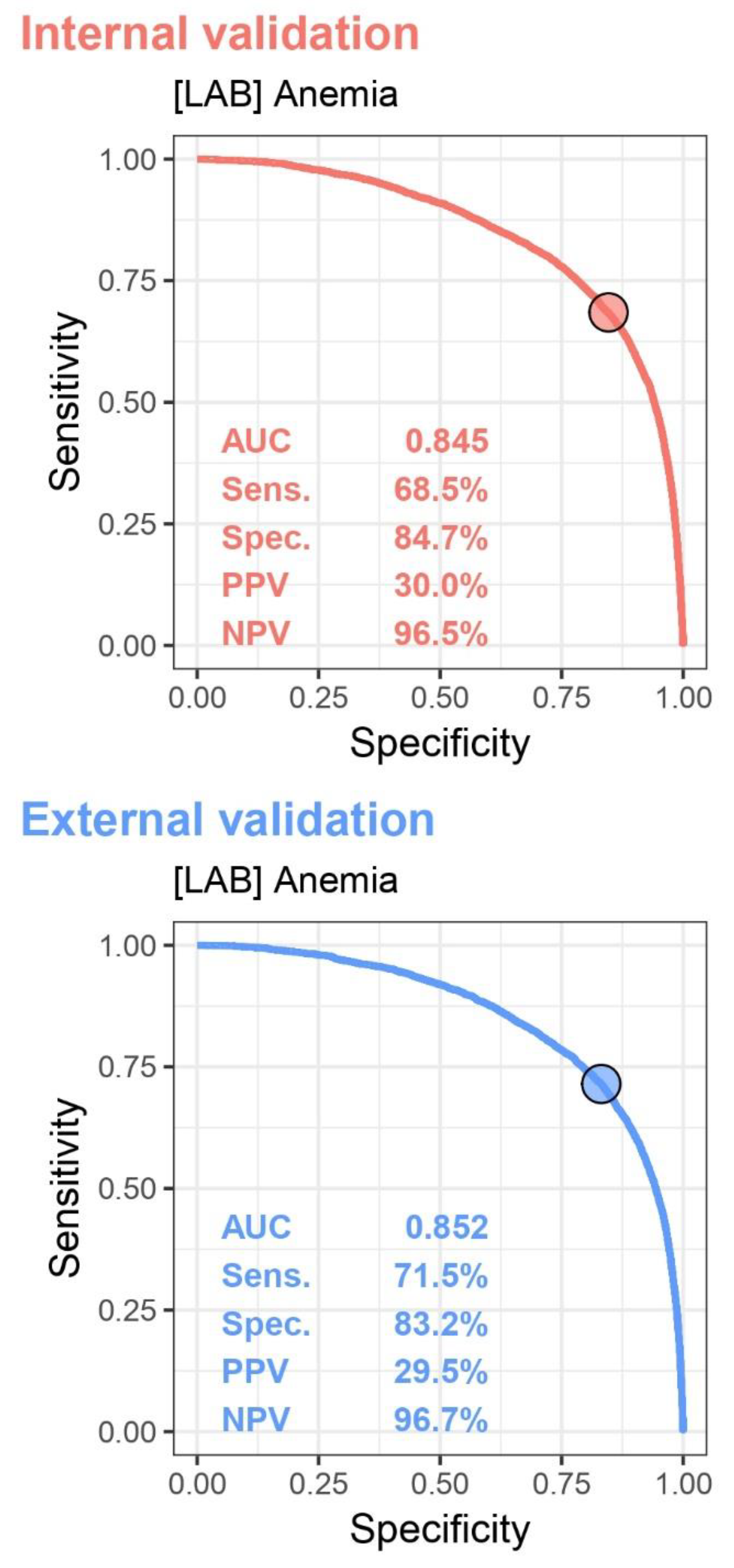
The Receiver Operator Characteristic (ROC) Curve and the related variances, which are presented in
Figure 2, are used to calculate the Area Under Curve (AUC) and identify an optimal cutoff point for distinguishing anemia.
In the internal validation set, the AUC curve was 0.845, with a sensitivity of 68.5%, specificity of 84.7%, positive predictive value of 30.0%, and negative predictive value of 96.5%. In the external validation set, the AUC curve was 0.852, with a sensitivity of 71.5%, specificity of 83.2%, positive predictive value of 29.5%, and negative predictive value of 96.7%.
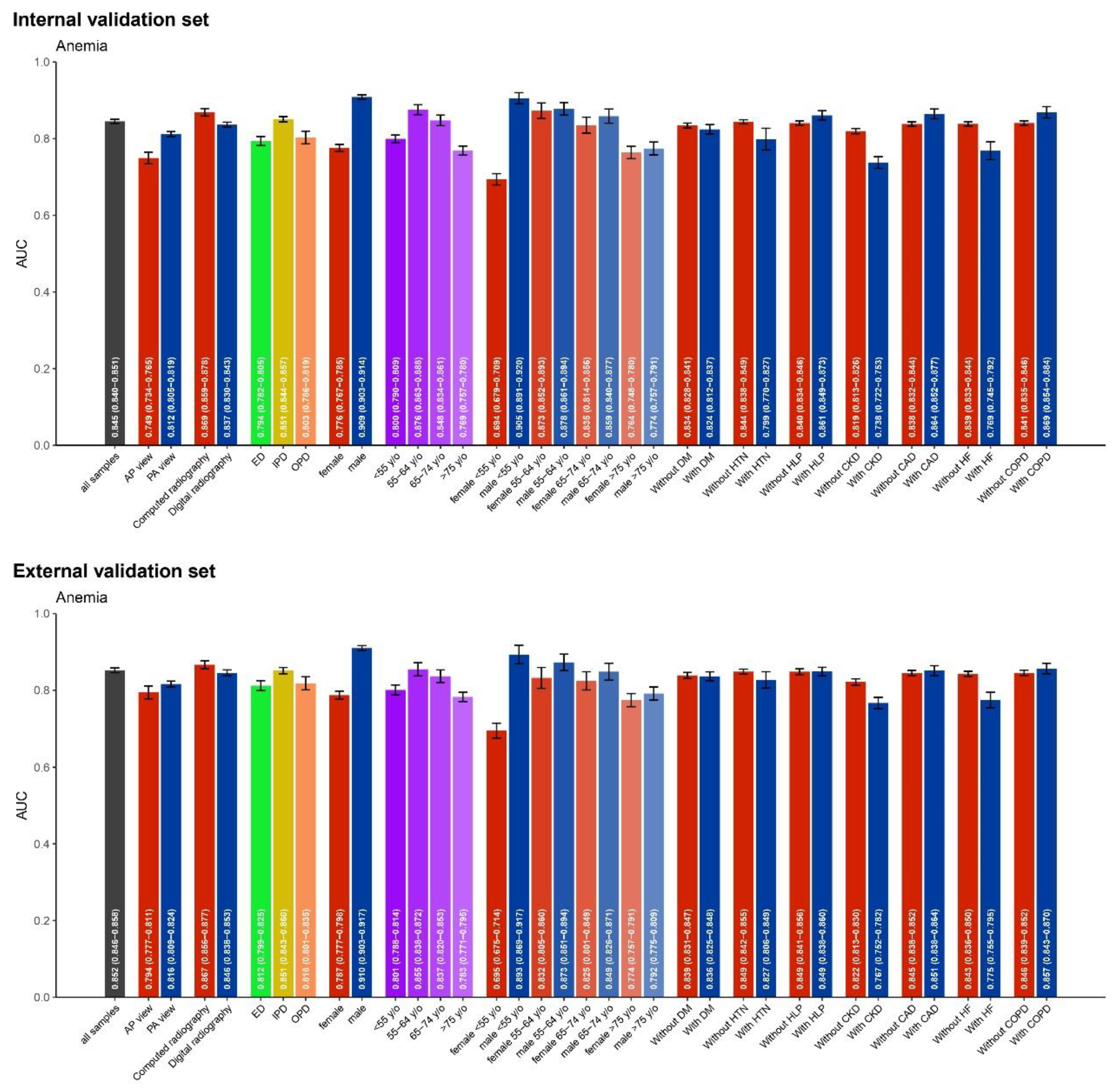
3.3. AUC values for Anemia Detection Across Various Subgroups
We analyzed the AUC values for anemia detection across various subgroups in both internal and external validation datasets, presented in
Figure 3. Factors considered included age, gender, diagnostic method (e.g., computed radiography, digital radiography), clinical settings (e.g., ER, IPD, OPD), and underlying clinical conditions. Overall, performance varied across these dimensions, with notable differences between the internal and external validation results.
In the internal validation, computed radiography (AUC 0.869, 95% CI: 0.859−0.878) and PA view (AUC 0.812, 95% CI: 0.805−0.819) showed better diagnostic performance. The inpatient department (AUC 0.851, 95% CI: 0.844−0.857) performed better than the outpatient and emergency departments. Regarding age and gender, males (AUC 0.909, 95% CI: 0.903−0.914) and individuals aged 55 to 64 years (AUC 0.876, 95% CI: 0.863−0.888) demonstrated superior diagnostic ability, although a significant decline was observed in younger females under 55 years. For underlying clinical conditions, patients with hypertension (AUC 0.794, 95% CI: 0.782−0.805), chronic kidney disease (AUC 0.764, 95% CI: 0.748−0.780), and heart failure (AUC 0.767, 95% CI: 0.752−0.782) exhibited lower diagnostic performance compared to those without these conditions.
In the external validation, similar patterns emerged. Computed radiography (AUC 0.867, 95% CI: 0.856−0.877) again showed high performance, but there was no statistical difference between AP and PA views (AUC 0.816, 95% CI: 0.809−0.824). The IPD (AUC 0.855, 95% CI: 0.838−0.872) outperformed the OPD and ER. In terms of demographic factors, males (AUC 0.910, 95% CI: 0.903−0.917) and individuals aged 55 to 64 years (AUC 0.876, 95% CI: 0.863−0.888) showed better performance, but detection ability was reduced in younger females. Additionally, patients with chronic kidney disease (AUC 0.774, 95% CI: 0.757−0.791) and heart failure (AUC 0.775, 95% CI: 0.755−0.795) demonstrated lower diagnostic accuracy compared to those without these conditions.
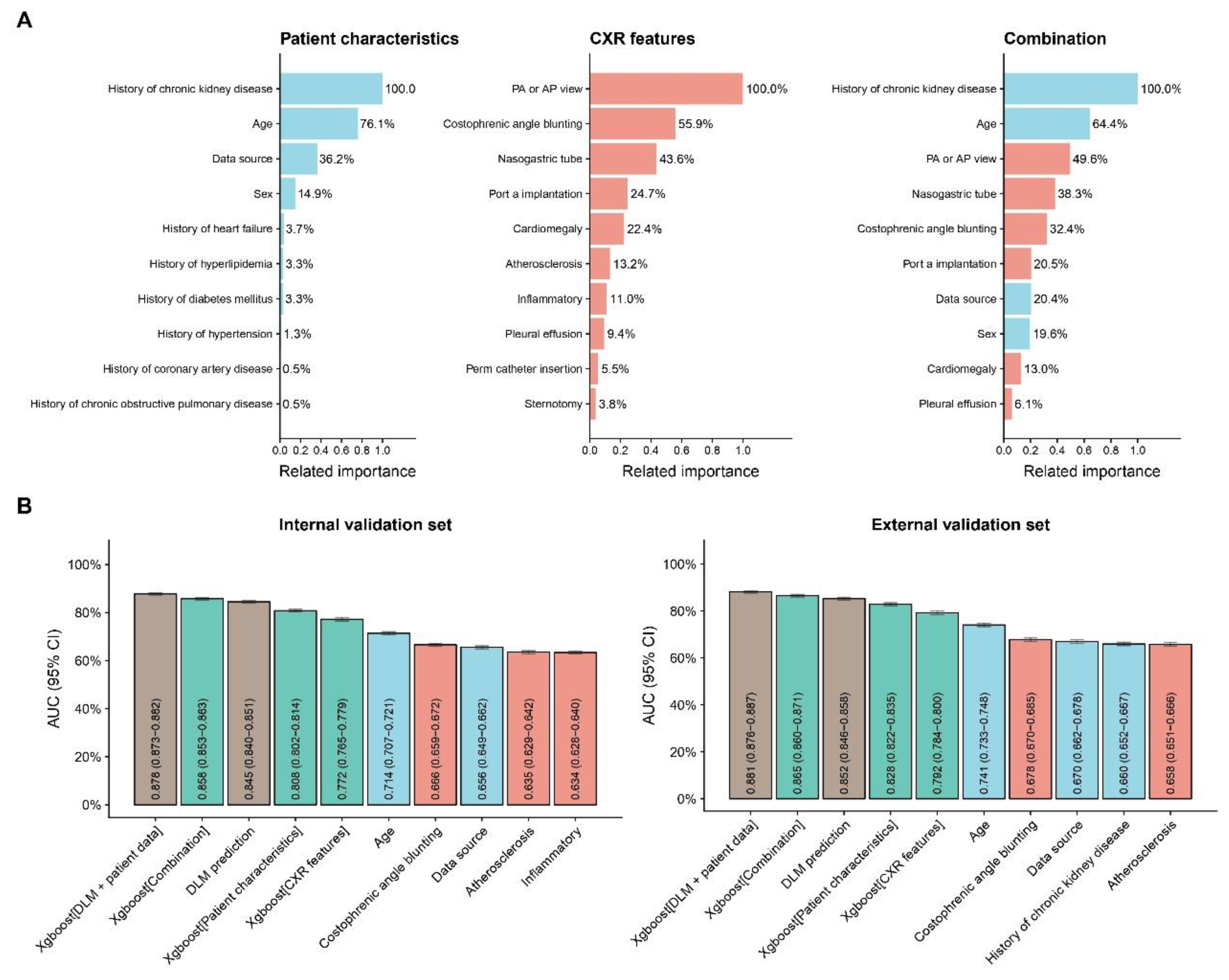
3.4. Related Important Features With Anemia
In
Figure 4A, the subgroup with a history of chronic kidney disease had the highest relative importance in predicting anemia. Other important features included age (64.4% relative importance compared to the chronic kidney disease subgroup), PA or AP view (49.6%), nasogastric tube (38.3%), and costophrenic angle blunting (32.4%).
3.5. Comparison with other Prediction Models
In
Figure 4B, the deep learning model was compared with several other models using clinically relevant characteristics. In the internal validation set, the combination of the DLM with patient data showed the highest AUC. Following that, the models combining all information and the deep learning model alone also performed well, demonstrating DLM's advantage in predicting anemia. The external validation set exhibited a similar trend, further supporting the model’s robustness.
4. Discussion
In this retrospective cohort study, we developed a CXR-based DLM to detect anemia. The ROC curve revealed AUC values of 0.845 for internal validation and 0.852 for external validation, with both sets displaying high negative predictive values and moderate sensitivity and specificity in distinguishing anemia. Subgroup analysis showed that in the internal validation, computed radiography and the inpatient setting performed the best, with males and individuals aged 55-64 achieving the highest diagnostic accuracy. In contrast, younger females and patients with hypertension, chronic kidney disease, and heart failure showed lower diagnostic performance, a trend also observed in the external validation set. Further analysis identified several key features associated with anemia prediction, with a history of chronic kidney disease having the highest relative importance. Other notable factors included age, PA or AP view, nasogastric tube placement, and costophrenic angle blunting. Comparisons of various models demonstrated that the DLM combined with patient data had the highest AUC in the internal validation set, while other models, including those using all available information or the DLM alone, also performed well. A similar trend was seen in the external validation, further underscoring the model’s predictive strength.
Our study demonstrated that the CXR-based DLM has excellent ability in distinguishing anemia. According to previous studies and our own findings, anemia can induce noticeable changes in chest imaging.[
9,
17,
18,
19] From a physiological standpoint, when hemoglobin (Hb) levels are greater than 10 g/dL, non-hemodynamic mechanisms, such as elevated 2,3-diphosphoglycerate (2,3-DPG), enable a rightward shift in the oxygen dissociation curve, which typically compensates for hemoglobin deficits.[
20] However, in non-resting conditions or when hemoglobin falls below 10 g/dL, cardiac output begins compensating for tissue hypoxia.[
6] Several mechanisms are responsible for this compensatory response. First, afterload decreases due to reduced systemic vascular resistance, potentially caused by hypoxic vasodilation induced by hypoxia-generated metabolites and flow-mediated vasodilation driven by increased blood flow. This vasodilation effect is mediated by endothelial cells and endothelium-derived relaxing factors.[
21] Secondly, preload increases due to enhanced venous return.[
6] Finally, and most importantly, anemia increases preload through the Frank-Starling mechanism and augments inotropic response via sympathetic activation, leading to changes in left ventricular hemodynamic performance.[
7,
22] However, these changes are difficult for human interpretation on CXR alone. Nevertheless, previous research on unenhanced chest CT has shown that the aortic ring sign is sensitive (82.5%) and specific (87%) in detecting anemia, while the interventricular septum sign is less sensitive (32%) but highly specific (99.2%). These results suggest that anemia can be detected using unenhanced chest CT scans.[
9] Moreover, Studies using multidetector computed tomography showed that the aortic ring sign had higher sensitivity (84%) and specificity (92%) compared to the interventricular septum sign (sensitivity 72%, specificity 100%).[
17] The objective analysis data revealed a strong correlation between blood pool attenuation at different anatomical sites and serum hemoglobin levels.[
17] A significant correlation (r = 0.60) was specifically observed between aortic CT attenuation and hemoglobin levels, with 84% sensitivity, 94% specificity, and an AUC of 0.89 for anemia detection.[
17] Thus, it can be concluded that anemia indeed causes cardiovascular changes detectable on chest imaging. Several studies also support this conclusion.[
18,
19] Our study also corroborates this evidence, indicating that anemia produces specific alterations in chest imaging, which can be detected and differentiated by the DLM.
Several factors may influence the accuracy of detecting anemia. In our subanalysis, better image quality was associated with higher AUC values, as seen in the PA view and computed radiography. Additionally, the overall predictive accuracy tends to decrease with age. However, females under 55 years old were an exception, likely due to regular blood loss from menstruation, a common cause of anemia in women.[
23] Furthermore, subgroups with CKD and HF showed lower accuracy in detecting anemia. This may be due to several factors, such as hemodynamic instability during dialysis in CKD patients or heart failure-related cardiac remodeling, which affects left ventricular systolic and diastolic function,[
24] potentially obscuring the aortic ring and other possible anemia sign on imaging. Nonetheless, the overall AUC remained relatively stable across the different subgroups.
Several key predictive features were identified. According to previous data, anemia is associated with various clinical scenarios such as hemorrhage, gastrointestinal bleeding, infections, and inflammation.[
4] In our study, a history of CKD showed the highest predictive importance. Several factors contributed to this outcome. First, although anemia is associated with many factors such as age, underlying disease history, various interventions, and imaging characteristics (e.g., consolidation change, pneumonia, pulmonary edema), patients with a history of CKD (37.4%) had a 6.8-fold higher prevalence of anemia compared to those without CKD (5.5%) [Supplementary table 1]. The prevalence of anemia in CKD reported in the United States—stage 3: 17.4%, stage 4: 50.3%, stage 5: 53.4%—corresponds with our findings.[
25] Several mechanisms contribute to anemia in CKD, including reduced erythropoietin (EPO) production, iron deficiency, chronic inflammation, reduced red blood cell lifespan due to uremia, and blood loss from dialysis.[
26] The second most important factor is age. It is well-established that anemia can be caused by various physiological factors, with age being a significant contributor.[
4] The third key factor is the AP view, which is often associated with patients too weak to complete an PA view. These factors are strongly associated with anemia detection and contribute to the DLM's predictive capabilities.
To the best of our knowledge, this is the first study to use a CXR-based DLM to predict anemia. Previous studies have explored the use of CXR-based models for various clinical predictions, but none have focused on anemia detection.[
11] Several DLMs have attempted to detect anemia through the use of EKG, facial images, and images of the palpebral conjunctiva.[
10,
27,
28] However, these methods face several limitations, such as the difficulty of obtaining images compared to CXR, lack of a standardized clinical protocol, the need for additional patient consent, and the absence of a large database and comprehensive external validation. Our results align with studies that demonstrate the efficacy of integrating patient data with imaging techniques to improve diagnostic accuracy. We also analyzed the performance of our DLM, and the results showed that the model outperformed predictions based solely on routine clinical data, especially when combined with patient data. This represents a significant advancement in predictive modeling for anemia compared to traditional methods.
The CXR-based DLM provides an accessible tool for early anemia detection, especially during initial screenings using passive electronic medical records. Because CXR is a routine and preliminary examination that is typically performed in emergency rooms, outpatient clinics, and during hospitalization, the DLM can be effectively utilized across different clinical settings. One of the key advantages of the DLM is its ability to leverage widely available chest X-rays, offering significant utility in resource-limited settings. Even a single portable X-ray can provide sufficient information, making it a valuable tool where advanced laboratory diagnostics are not readily accessible, assisting in the initial screening of individuals with anemia and guiding doctors in further evaluation and lifestyle recommendations. Importantly, the DLM does not require new interventions but rather analyzes existing data from routine examinations, enabling physicians to obtain a general understanding of whether anemia is present without increasing workload.
Figure 1.
Development, tuning, internal validation and external validation set generation. This diagram outlines the dataset creation and analysis strategy, designed to ensure a robust and reliable dataset for training, validating, and testing the network. Each patient’s data was assigned to one specific set—development, tuning, or validation—and once assigned, the data remained in that set to prevent any overlap or "cross-contamination." The external validation set was created using data exclusively from patients who visited the Jingzhou branch, while other patients contributed to different datasets. Further details on the flow and specific use of each dataset can be found in the Methods section.
Figure 1.
Development, tuning, internal validation and external validation set generation. This diagram outlines the dataset creation and analysis strategy, designed to ensure a robust and reliable dataset for training, validating, and testing the network. Each patient’s data was assigned to one specific set—development, tuning, or validation—and once assigned, the data remained in that set to prevent any overlap or "cross-contamination." The external validation set was created using data exclusively from patients who visited the Jingzhou branch, while other patients contributed to different datasets. Further details on the flow and specific use of each dataset can be found in the Methods section.
Figure 2.
Receiver Operating Characteristic (ROC) curves for distinguishing anemia. The Area Under the Curve (AUC) values are 0.845 for internal validation and 0.852 for external validation. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are indicated for both sets. The figure illustrates the performance of the model in identifying an optimal cutoff point for anemia diagnosis across internal and external validation datasets.
Figure 2.
Receiver Operating Characteristic (ROC) curves for distinguishing anemia. The Area Under the Curve (AUC) values are 0.845 for internal validation and 0.852 for external validation. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are indicated for both sets. The figure illustrates the performance of the model in identifying an optimal cutoff point for anemia diagnosis across internal and external validation datasets.
Figure 3.
AUC values for anemia detection across various subgroups in both internal and external validation sets. This figure illustrates the performance of the model in different patient populations, including variations by age, gender, diagnostic method (e.g., computed radiography, digital radiography), clinical settings (e.g., emergency department, inpatient, outpatient), with and without clinical underlying conditions, such as diabetes (DM), hypertension (HTN), hyperlipidemia (HLP), chronic kidney disease (CKD), coronary artery disease (CAD), heart failure (HF), and chronic obstructive pulmonary disease (COPD).
Figure 3.
AUC values for anemia detection across various subgroups in both internal and external validation sets. This figure illustrates the performance of the model in different patient populations, including variations by age, gender, diagnostic method (e.g., computed radiography, digital radiography), clinical settings (e.g., emergency department, inpatient, outpatient), with and without clinical underlying conditions, such as diabetes (DM), hypertension (HTN), hyperlipidemia (HLP), chronic kidney disease (CKD), coronary artery disease (CAD), heart failure (HF), and chronic obstructive pulmonary disease (COPD).
Figure 4.
The related important about patient characteristics and CXR features in predicting anemia, along with AUC comparisons between several models for internal and external validation sets. (A) The relative importance of various patient characteristics (e.g., history of chronic kidney disease, age, history of heart failure) and chest X-ray (CXR) features (e.g., PA or AP view, costophrenic angle blunting, nasogastric tube) are displayed. A combination of these factors was also evaluated for their impact on the prediction model. (B) Area Under the Curve (AUC) values with 95% confidence intervals (CI) are presented for different models in both the internal and external validation sets.
Figure 4.
The related important about patient characteristics and CXR features in predicting anemia, along with AUC comparisons between several models for internal and external validation sets. (A) The relative importance of various patient characteristics (e.g., history of chronic kidney disease, age, history of heart failure) and chest X-ray (CXR) features (e.g., PA or AP view, costophrenic angle blunting, nasogastric tube) are displayed. A combination of these factors was also evaluated for their impact on the prediction model. (B) Area Under the Curve (AUC) values with 95% confidence intervals (CI) are presented for different models in both the internal and external validation sets.
Table 1.
Patient characteristics and laboratory results in the development, tuning, internal validation and external validation sets.
Table 1.
Patient characteristics and laboratory results in the development, tuning, internal validation and external validation sets.
| Variable |
Development set
(n = 135,867) |
Tuning set
(n = 54,405) |
Internal
validation set
(n = 67,623) |
External
validation set
(n = 47,898) |
p-value |
| Main variable |
|
|
|
|
|
| Anemia |
12348(9.1%) |
4954(9.1%) |
5939(8.8%) |
4294(9.0%) |
0.112 |
| Basic Demographics |
|
|
|
|
|
| Age |
51.4±19.1 |
51.5±19.1 |
50.9±18.9 |
52.2±20.8 |
<0.001 |
| Gender(male) |
71323(52.5%) |
28493(52.4%) |
34806(51.5%) |
24436(51.0%) |
<0.001 |
| PA view |
127729(94.0%) |
51195(94.1%) |
63805(94.4%) |
45255(94.5%) |
<0.001 |
| |
|
|
|
|
|
| Acquisition location |
|
|
|
|
<0.001 |
| Emergency Room |
41238(30.4%) |
16613(30.5%) |
20769(30.7%) |
17583(36.7%) |
|
| Inpatient Department |
50797(37.4%) |
20526(37.7%) |
25233(37.3%) |
15173(31.7%) |
|
| Outpatient Department |
43832(32.3%) |
17266(31.7%) |
21621(32.0%) |
15123(31.6%) |
|
| |
|
|
|
|
|
| Disease history |
|
|
|
|
|
| Diabetes Mellitus |
13689(10.1%) |
5472(10.1%) |
6177(9.1%) |
7231(15.1%) |
<0.001 |
| Hypertension |
2784(2.0%) |
1101(2.0%) |
1248(1.8%) |
1856(3.9%) |
<0.001 |
| Hyperlipidemia |
17918(13.2%) |
7204(13.2%) |
7603(11.2%) |
10821(22.6%) |
<0.001 |
| Chronic Kidney Disease |
8686(6.4%) |
3511(6.5%) |
4017(5.9%) |
4016(8.4%) |
<0.001 |
| Heart failure |
3926(2.9%) |
1585(2.9%) |
1658(2.5%) |
2280(4.8%) |
<0.001 |
| Coronary Artery Disease |
11188(8.2%) |
4518(8.3%) |
4980(7.4%) |
5993(12.5%) |
<0.001 |
| Chronic Obstructive Pulmonary Disease |
8688(6.4%) |
3477(6.4%) |
3215(4.8%) |
5018(10.5%) |
<0.001 |