Submitted:
01 October 2024
Posted:
01 October 2024
Read the latest preprint version here
Abstract

Keywords:
1. Introduction
2. Mangrove and Associate Species with Anticancer Properties
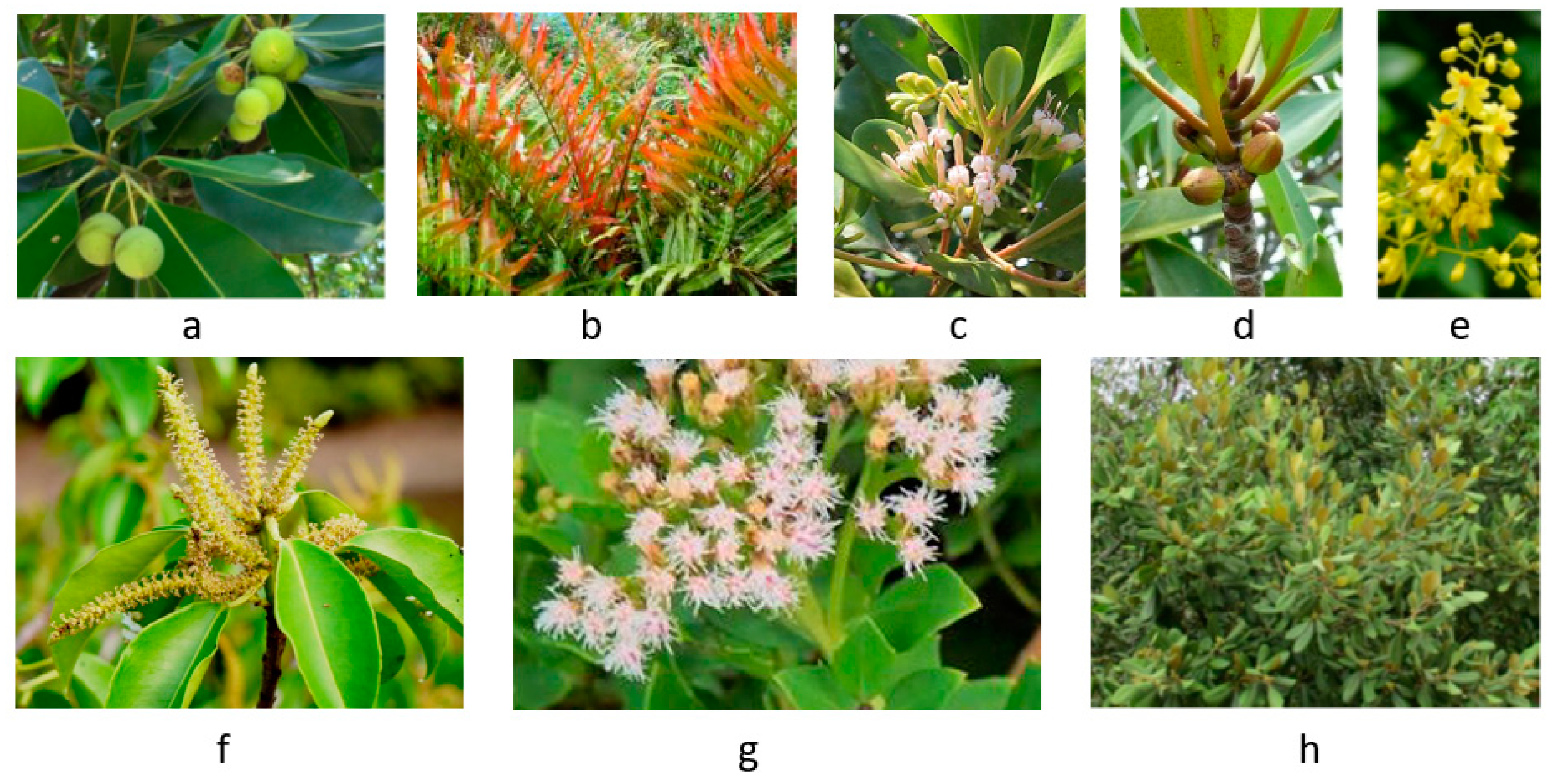
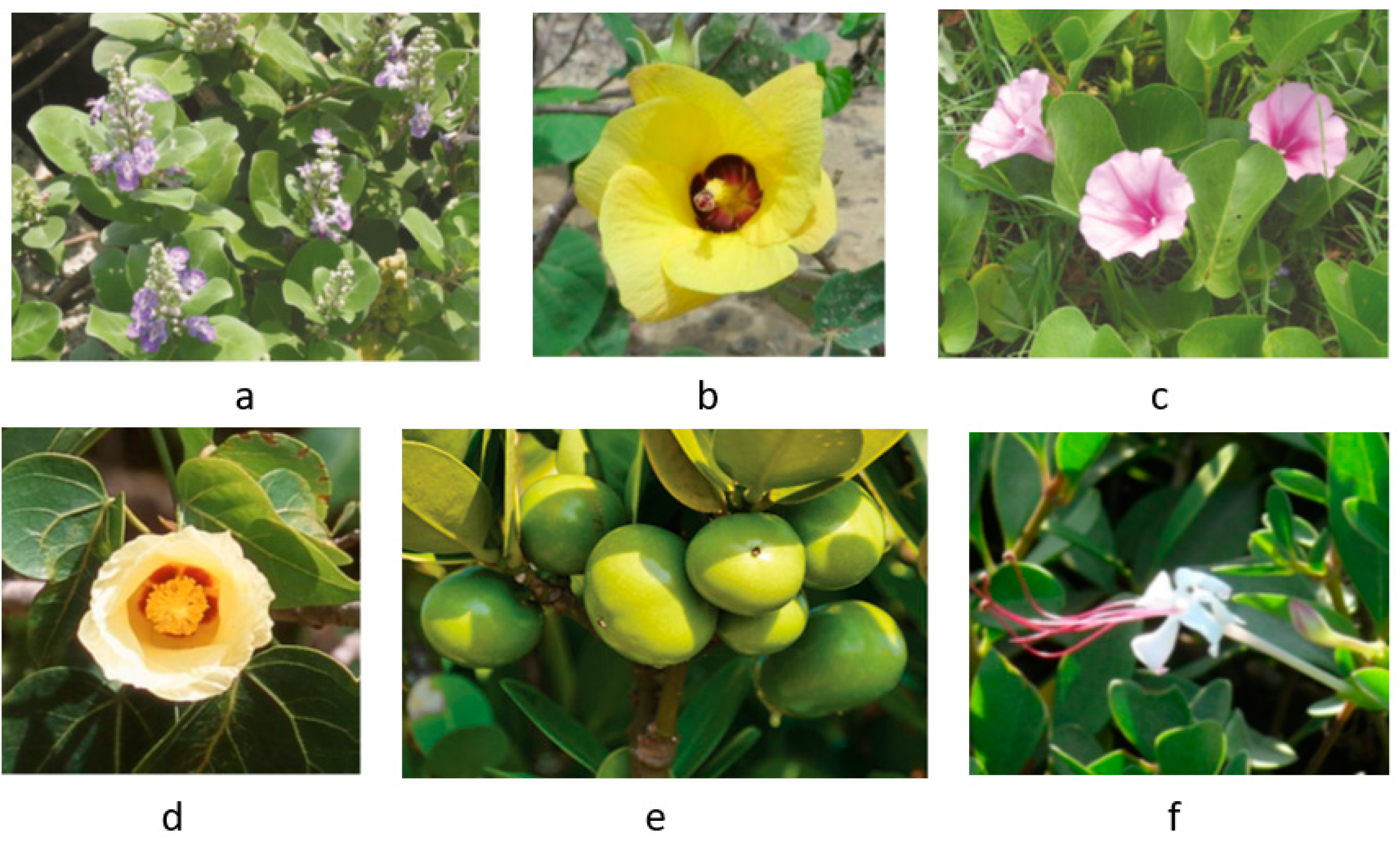
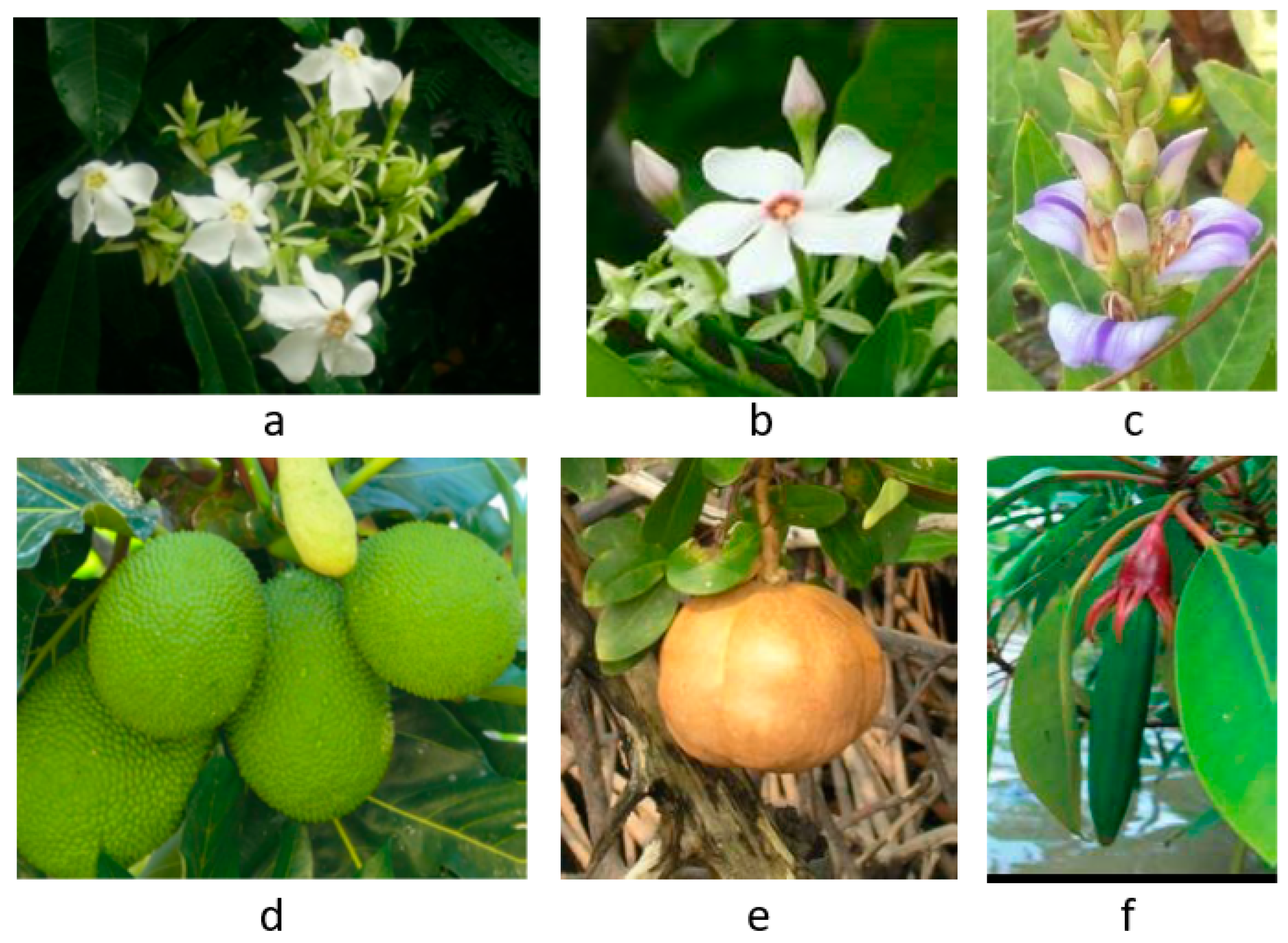
3. Anticancer Effects and Mechanisms of Mangrove Species
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Mu, M.; Li, X.; Lin, P.; Wang, W. Differentiation between true mangroves and mangrove associates based on leaf traits and salt contents. J. Plant Ecol. 2011, 4, 292−301. [CrossRef]
- Baba, S.; Chan, H.T.; Aksornkoae, S. Useful Products from Mangrove and other Coastal Plants; ISME Mangrove Educational Book Series No. 3., International Society for Mangrove Ecosystems (ISME) and International Tropical Timber Organization (ITTO), 2013; 99p.
- Bunting, P.; Rosenqvist, A.; Hilarides, L.; Lucas, R.M.; Thomas, N.; Tadono, T.; Worthington, T.A.; Spalding, M.; Murray, N.J.; Rebelo, L.M. Global mangrove extent change 1996–2020: Global mangrove watch version 3.0. Remote Sens. 2022, 14, 3657. [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; Earthscan, London, UK and Washington, DC, USA, 2010, 319p.
- Giesen, W.; Wulfraat, S.; Zieren, M.; Scholten, L. Mangrove Guidebook for Southeast Asia; FAO, Bangkok, Thailand and Wetlands International, Wageningen, Netherlands, 2007; 769p.
- Inoue, T.; Kohzu, A.; Akaji, Y.; Miura, S.; Baba, S.; Oshiro, N.; Kezuka, M.; Kainuma, M.; Tokuoka, H.; Naruse, T. Mangroves of Japan. In: Mangroves: Biodiversity, Livelihoods and Conservation; Chandra Das, S.; Pullaiah, T.; Ashton, E.C., eds., 2022; 463−487, Springer.
- Horstman, E.M.; Lundquist, C.J.; Bryan, K.R.; Bulmer, R.H.; Mullarney, J.C.; Stokes, D.J. The dynamics of expanding mangroves in New Zealand. In: Threats to Mangrove Forests: Hazards, Vulnerability, and Management 2018, 23−51, Springer.
- Maxwell, G.S.; Lai, C. Avicennia marina foliage as a salt enrichment nutrient for New Zealand dairy cattle. ISME/GLOMIS Electron. J. 2012, 10, 22−24.
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecol. Manag. 2002, 10, 421−452. [CrossRef]
- Das, G.; Gouda, S.; Mohanta, Y.K.; Patra, J.K. Mangrove plants: A potential source for anticancer drugs. Indian J. Geo-Mar. Sci. 2015, 44, 666−672.
- Mitra, S.; Naskar, N.; Chaudhuri, P. A review on potential bioactive phytochemicals for novel therapeutic applications with special emphasis on mangrove species. Phytomed. Plus. 2021, 1, 100107. [CrossRef]
- Dahibhate, N.L.; Saddhe, A.A.; Kumar, K. Mangrove plants as a source of bioactive compounds: A review. Nat. Prod. J. 2019, 9, 86−97. [CrossRef]
- Kimura, N.; Kainuma, M.; Inoue, T.; Chan, E.W.C.; Tangah, J.; Baba, K.; Oshiro, N.; Okamoto, C. Botany, uses, chemistry and bioactivities of mangrove plants V: Acrostichum aureum and A. speciosum. ISME/GLOMIS Electron. J. 2017, 15, 1−6.
- Akinwumi, K.A.; Abam, E.O.; Oloyede, S.T.; Adeduro, M.N.; Adeogun, Y.A.; Uwagboe, J.E. Acrostichium aureum Linn: Traditional use, phytochemistry and biological activity. Clin. Phytosci. 2022, 8, 1−18. [CrossRef]
- Kar, D.R.; Farhad, M.S.; Sahu, P.K. A review on pharmacological profiles of ethno-medicinal plant: Avicennia alba Blume. Int. J. Pharmatech Res. 2015, 7, 370−373.
- Baba, S.; Chan, H.T.; Oshiro, N.; Maxwell, G.S.; Inoue, T.; Chan, E.W.C. Botany, uses, chemistry and bioactivities of mangrove plants IV: Avicennia marina. ISME/GLOMIS Electron. J. 2016, 14, 5−10.
- Chan, E.W.C.; Tangah, J.; Kezuka, M.; Chan, H.T. Botany, distribution, phytochemistry and bioactivities of mangrove plants VI: Avicennia rumphiana. ISME/GLOMIS Electron. J. 2022, 20, 13−16.
- Chan, H.T.; Baba, S. Manual on Guidelines for Rehabilitation of Coastal Forests damaged by Natural Hazards in the Asia-Pacific Region. International Society for Mangrove Ecosystems (ISME) and International Tropical Timber Organization (ITTO), 2009; 66 pp.
- Chan, E.W.C.; Tangah, J.; Kezuka, M.; Hoan, H.D.; Binh, C.H. Botany, uses, chemistry and bioactivities of mangrove plants II: Ceriops tagal. ISME/GLOMIS Electron. J. 2015, 13, 39−43.
- Chan, E.W.C.; Oshiro, N.; Kezuka, M.; Kimura, N.; Baba, K.; Chan, H.T. Pharmacological potentials and toxicity effects of Excoecaria agallocha. J Appl Pharm Sci. 2018, 8, 166−173. [CrossRef]
- Chan, E.W.C.; Lim, W.Y.; Wong, C.W.; Ng, Y.K. Some notable bioactivities of Rhizophora apiculata and Sonneratia alba. ISME/GLOMIS Electron. J. 2022, 20, 23−26.
- Ng, W.L.; Chan, H.T. Survey of Rhizophora stylosa populations in Peninsular Malaysia. ISME/GLOMIS Electron J. 2012, 10, 4−6.
- Kainuma, M.; Kezuka, M.; Inoue, T.; Chan, E.W.C.; Tangah, J.; Baba, S.; Chan, H.T. Botany, uses, chemistry and bioactivities of mangrove plants I: Rhizophora stylosa. ISME/GLOMIS Electron. J. 2015, 13, 12−17.
- Baba, S.; Chan, H.T.; Kainuma, M.; Kezuka, M.; Chan, E.W.C.; Tangah, J. Botany, uses, chemistry and bioactivities of mangrove plants III: Xylocarpus granatum. ISME/GLOMIS Electron. J. 2016, 14, 1−4.
- Dey, D.; Quispe, C.; Hossain, R.; Jain, D.; Khan, R.A.; Janmeda, P.; Islam, M.T.; Suleria, H.A.R.; Martorell, M.; Daştan, S.D.; et al. Ethnomedicinal use, phytochemistry, and pharmacology of Xylocarpus granatum J. Koenig. Evid-Based Complement. Altern. Med. 2021, 2021, 8922196. [CrossRef]
- Meepol, W.; Maxwell, G.S.; Havanond, S. Aglaia cucullata: A little-known mangrove with big potential for research. ISME/GLOMIS Electron. J. 2020, 18, 4−9.
- Chan, E.W.C.; Baba, S.; Chan, H.T.; Kainuma, M.; Inoue, T.; Wong, S.K. Ulam herbs: A review on the medicinal properties of Anacardium occidentale and Barringtonia racemosa. J. Appl. Pharm. Sci. 2017, 7, 241−247. [CrossRef]
- Baba, S.; Chan, H.T.; Kezuka, M.; Inoue, T.; Chan, E.W.C. Artocarpus altilis and Pandanus tectorius: Two important fruits of Oceania with medicinal values. Emir. J. Food Agric. 2016, 28, 531−539. [CrossRef]
- Chan, E.W.C.; Tangah, J.; Baba, S.; Chan, H.T.; Kainuma, M.; Inoue, T. Caesalpinia crista: A coastal woody climber with promising therapeutic values. J. Appl. Pharm. Sci. 2018, 8, 133−140. [CrossRef]
- Kainuma, M.; Baba, S.; Chan, H.T.; Inoue, T.; Tangah, J.; Chan, E.W.C. Medicinal plants of sandy shores: A short review on Calophyllum inophyllum and Thespesia populnea. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 2056−2062.
- Chan, E.W.C.; Wong, S.K.; Chan, H.T.; Baba, S.; Kezuka, M. Cerbera are coastal trees with promising anticancer properties but lethal toxicity: A short review. J. Chin. Pharm. Sci. 2016, 25, 161−169. [CrossRef]
- Chan, E.W.C.; Tangah, J.; Inoue, T.; Kainuma, M.; Baba, K.; Oshiro, N.; Kezuka, M.; Kimura, N. Botany, uses, chemistry and pharmacology of Ficus microcarpa: A short review. Syst. Rev. Pharm. 2017, 8, 103−111.
- Inoue, T.; Kainuma, M.; Baba, S.; Oshiro, N.; Kimura, N.; Chan, E.W.C. Garcinia subelliptica Merr. (Fukugi): A multi-purpose coastal tree with promising medicinal properties. J. Intercult. Ethnopharmacol. 2017, 6, 121−127. [CrossRef]
- Chan, E.W.C.; Baba, S.; Chan, H.T.; Kainuma, M.; Tangah, J. Medicinal plants of sandy shores: A short review on Vitex trifolia L. and Ipomoea pes-caprae (L.) R. Br. Indian J. Nat. Prod. Resour. 2016, 7, 107−115. [CrossRef]
- Alam, F.; Rahman, M.S.; Alam, M.S.; Hossain, M.K.; Hossain, M.A.; Rashid, M.A. Phytochemical and biological investigations of Phoenix paludosa Roxb. Dhaka Univ. J. Pharm. Sci. 2009, 8, 7−10.
- Chan, E.W.C.; Ng, Y.K.; Wong, S.K.; Chan, H.T. Pluchea indica: An updated review of its botany, uses, bioactive compounds and pharmacological properties. Pharm. Sci. Asia 2022, 49, 77−85. [CrossRef]
- Balekar, N.; Nakpheng, T.; Srichana, T. Wedelia trilobata L: A phytochemical and pharmacological review. Chiang Mai J. Sci. 2014, 41, 590−605.
- Chen S, Phillips SM. Spinifex. Flora China 2006, 22, 553−554.
- Wong, S.K.; Chan, E.W.C. Antioxidant properties coastal and inland populations of Hibiscus tiliaceus. ISME/GLOMIS Electron. J. 2010, 8, 1−2.
- Wong, S.K.; Chan, E.W.C. Botany, uses, phytochemistry and bioactivities of mangrove associates I: Hibiscus tiliaceus. ISME/GLOMIS Electron. J. 2022, 20, 17−22.
- Chan EWC, Wong SK, Chan HT. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 47−152. https://doi. org/10.1016/j.joim.2018.03.001.
- Chan EWC, Wong SK, Chan HT. Volkameria inermis: An overview of its chemical constituents and pharmacological properties, notably the amelioration of motor tics. J. Herbmed Pharmacol. 2023, 12, 76−186. [CrossRef]
- Chowdhury, R, Bhuia, MS, Al Hasan, MS, Hossain Snigdha, S, Afrin, S, Büsselberg, D, Habtemariam, S, Sönmez Gürer, E, Sharifi-Rad, J, Ahmed Aldahish, A.; et al. Anticancer potential of phytochemicals derived from mangrove plants: Comprehensive mechanistic insights. Food Sci. Nutr. 2024, 1−32. [CrossRef]
- Saengha, W.; Karirat, T.; Buranrat, B.; Katisart, T.; Loutchanwoot, P.; Albaser, A.A.; Luang-In, V. Anticancer effects of Rhinacanthus nasutus and Acanthus ebracteatus extracts against human cervical cancer cells. Not. Bot. Horti. Agrobot. 2023, 51, 3035−3050. [CrossRef]
- Babu, B.H.; Shylesh, B.S.; Padikkala, J. Tumour reducing and anticarcinogenic activity of Acanthus ilicifolius in mice. J. Ethnopharmacol. 2002, 79, 27−33. [CrossRef]
- Zhao, D.; Xie, L.; Yu, L.; An, N.; Na, W.; Chen, F.; Li, Y.; Tan, Y.; Zhang, X. New 2-Benzoxazolinone derivatives with cytotoxic activities from the roots of Acanthus ilicifolius. Chem. Pharm. Bull. 2015, 63, 1087−1090. [CrossRef]
- Paul, T.; Ramasubbu, S. The antioxidant, anticancer and anticoagulant activities of Acanthus ilicifolius L. roots and Lumnitzera racemosa Willd. leaves, from southeast coast of India. J. Appl. Pharm. Sci. 2017, 7, 81−87. [CrossRef]
- Dai, H.F.; Mei, W.L.; Hong, K.; Zeng, Y.B.; Zhuang, L. Screening of the tumour cytotoxic activity of sixteen species of mangrove plants in Hainan. Chin. J. Mar. Drugs 2005, 69, 44−46.
- Uddin, S.J.; Grice, D.; Tiralongo, E. Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid-Based Complement. Altern. Med. 2011, Article ID 578092: 7 pp. https://doi. org/10.1093/ecam/nep111.
- Uddin, S.J.; Jason, T.L.; Beattie, K.D.; Grice, I.D.; Tiralongo, E. (2S,3S)-Sulfated pterosin C, a cytotoxic sesquiterpene from the Bangladeshi mangrove fern Acrostichum aureum. J. Nat. Prod. 2011, 74, 2010−2013. [CrossRef]
- Li, Y.; Dong, C.; Xu, M.J.; Lin, W.H. New alkylated benzoquinones from mangrove plant Aegiceras corniculatum with anticancer activity. J. Asian Nat. Prod. Res. 2020, 22, 121−130. [CrossRef]
- Arbiastutie, Y.; Diba, F.; Masriani, M. Cytotoxicity activity of several medicinal plants grow in mangrove forest against human cervical (HeLa), breast (T47D), and colorectal (WiDr) cancer cell lines. Int. J. Nutr. Pharmacol. Neurol. Dis. 2022, 12, 46−50. [CrossRef]
- Rahman, N.H.; Vigneswari, S.; Ahmad, A.Z.; Mohamad, H.A.; Muhammad, T.S. Cytotoxic effects and evidence of apoptosis from Avicennia alba extracts on human breast cancer cell line (MCF-7). J. Sustain. Sci. Manag. 2017, 12, 80−88.
- Han, L.; Huang, X.; Dahse, H.M.; Moellmann, U.; Fu, H.; Grabley, S.; Sattler, I.; Lin, W. Unusual naphthoquinone derivatives from the twigs of Avicennia marina. J. Nat. Prod. 2007, 70, 923−927. [CrossRef]
- Karami, L.; Majd, A.; Mehrabian, S.; Nabiuni, M.; Salehi, M.; Irian, S. Antimutagenic and anticancer effects of Avicennia marina leaf extract on Salmonella typhimurium TA100 bacterium and human promyelocytic leukaemia HL-60 cells. Sci. Asia 2012, 38, 349−355. [CrossRef]
- Huang, C.; Lu, C.K.; Tu, M.C.; Chang, J.H.; Chen, Y.J.; Tu, Y.H.; Huang, H.C. Polyphenol-rich Avicennia marina leaf extracts induce apoptosis in human breast and liver cancer cells and in a nude mouse xenograft model. Oncotarget 2016, 7, 35874. https://doi. org/10.18632/oncotarget.8624.
- Illian, D.N.; Basyuni, M.; Wati, R.; Hasibuan, P.A.Z. Polyisoprenoids from Avicennia marina and Avicennia lanata inhibit WiDr cells proliferation. Pharmacogn. Mag. 2018, 14, 513−518. [CrossRef]
- Eldohaji, L.M.; Fayed, B.; Hamoda, A.M.; Ershaid, M.; Abdin, S.; Alhamidi, T.B.; Mohammad, M.G.; Omar, H.A.; Soliman, S.S. Potential targeting of Hep3B liver cancer cells by lupeol isolated from Avicennia marina. Arch. Pharm. 2021, 354, 2100120. [CrossRef]
- Rahman, N.H.; Sevakumaran, V.; Ahmad, A.; Mohamad, H.; Zafar, M.N.; Sung, Y.Y.; Muhammad, T.S. Induction of cytotoxicity by Bruguiera gymnorrhiza in human breast carcinoma (MCF-7) cell line via activation of the intrinsic pathway. J. Adv. Pharm. Technol. Res. 2020, 11, 233−237. [CrossRef]
- Qayoom, H.; Alkhanani, M.; Almilaibary, A.; Alsagaby, S.A.; Mir, M.A. A network pharmacology-based investigation of brugine reveals its multi-target molecular mechanism against breast cancer. Med. Oncol. 2023, 40, 202. [CrossRef]
- He, L,; Wang, Y.S.; Wang, Q.J. In vitro antitumor activity of triterpenes from Ceriops tagal. Nat. Prod. Res. 2007, 21, 1228−1233. [CrossRef]
- Yang, Y.; Zhang, Y.; Liu, D.; Li-Weber, M.; Shao, B.; Lin, W. Dolabrane-type diterpenes from the mangrove plant Ceriops tagal with antitumor activities. Fitoterapia 2015, 103, 277−282. [CrossRef]
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Wati, R. Cytotoxic effect of polyisoprenoids from Rhizophora mucronata and Ceriops tagal leaves against WiDr colon cancer cell lines. Sains Malays. 2018, 47, 1953−1959. [CrossRef]
- Istiqomah, M.A.; Hasibuan, P.A.Z.; Nuryawan, A.; Sumaiyah, S.; Siregar, E.S.; Basyuni, M. The anticancer compound dolichol from Ceriops tagal and Rhizophora mucronata leaves regulates gene expressions in WiDr colon cancer. Sains Malays. 2021, 50, 181−189. [CrossRef]
- Patil, R.C.; Manohar, S.M.; Upadhye, M.V.; Katchi, V.I.; Rao, A.J.; Mule, A.; Moghe, A.S. Anti-reverse transcriptase and anticancer activity of stem ethanol extracts of Excoecaria agallocha (Euphorbiaceae). Ceylon J Sci. (Biol. Sci.) 2012, 40, 147−155. [CrossRef]
- Rifai, Y.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. New hedgehog/GLI signaling inhibitors from Excoecaria agallocha. Bioorg. Med. Chem. Lett. 2011, 21, 718−722. [CrossRef]
- Patra, J.K.; Thatoi, H. Anticancer activity and chromatography characterization of methanol extract of Heritiera fomes Buch. Ham., a mangrove plant from Bhitarkanika, India. Orient Pharm. Exper. Med. 2013, 13, 133−42. [CrossRef]
- Thao, N.P.; Luyen, B.T.; Diep, C.N.; Tai, B.H.; Kim, E.J.; Kang, H.K.; Lee, S.H.; Jang, H.D.; Cuong, N.T.; Thanh, N.V.; et al. In vitro evaluation of the antioxidant and cytotoxic activities of constituents of the mangrove Lumnitzera racemosa Willd. Arch. Pharm. Res. 2015, 38, 446−455. [CrossRef]
- Eswaraiah, G.; Peele, K.A.; Krupanidhi, S.; Indira, M.; Kumar, R.B.; Venkateswarulu, T.C. GC–MS analysis for compound identification in leaf extract of Lumnitzera racemosa and evaluation of its in vitro anticancer effect against MCF7 and HeLa cell lines. J. King Saud. Univ-Sci. 2020, 32, 780−783. [CrossRef]
- Sari, D.P.; Basyuni, M.; Hasibuan, P.A.Z.; Wati, R. The inhibition of polyisoprenoids from Nypa fruticans leaves on cyclooxygenase 2 expression of WiDr colon cancer cells. Asian J. Pharm. Clin. Res. 2018, 11, 154−157. [CrossRef]
- Istiqomah, M.A.; Hasibuan, P.A.; Sumaiyah, S.; Yusraini, E.; Oku, H.; Basyuni, M. Anticancer effects of polyisoprenoid from Nypa fruticans leaves by controlling expression of p53, EGFR, PI3K, AKT1, and mTOR genes in colon cancer (WiDr) cells. Nat. Prod. Commun. 2020, 15, 1−8. [CrossRef]
- Thao, N.P.; Linh, K.T.; Quan, N.H.; Trung, V.T.; Binh, P.T.; Cuong, N.T.; Nam, N.H.; Van Thanh, N. Cytotoxic metabolites from the leaves of the mangrove Rhizophora apiculata. Phytochem. Lett. 2022, 47, 51−55. [CrossRef]
- Prabhu, V.V.; Guruvayoorappan, C. Inhibition of metastatic lung cancer in C57BL/6 mice by marine mangrove Rhizophora apiculata. Asia Pac. J. Cancer Prev. 2013, 14, 1833−1840. [CrossRef]
- Youssef, A.M.; Maaty, D.A.; Al-Saraireh, Y.M. Phytochemistry and anticancer effects of mangrove (Rhizophora mucronata Lam.) leaves and stems extract against different cancer cell lines. Pharmaceuticals 2023, 16, 1−16. [CrossRef]
- Yang, X.H.; Li, H.B.; Chen, H.; Li, P.; Ye, B.P. Chemical constituents in the leave of Rhizophora stylosa L and their biological activities. Acta Pharm. Sin. 2008, 43, 974−978.
- Samarakoon, S.R.; Fernando, N.; Ediriweera, M.K.; Adhikari, A.; Wijayabandara, L.; de Silva, E.D.; Tennekoon, K. Isolation of hopenone-I from the leaves of mangrove plant Scyphiphora hydrophyllacea and its cytotoxic properties. Br. J. Pharm. Res. 2016, 10, 1−6. [CrossRef]
- Samarakoon, S.R.; Ediriweera, M.K.; Wijayabandara, L.; Fernando, N.; Tharmarajah, L.; Tennekoon, K.H.; Piyathilaka, P.; Adhikari, A. Isolation of cytotoxic triterpenes from the mangrove plant, Scyphiphora hydrophyllacea CF Gaertn (Rubiaceae). Trop. J. Pharm. Res. 2018, 17, 475−481. [CrossRef]
- Jing, L.; Feng, L.; Zhou, Z.; Shi, S.; Deng, R.; Wang, Z.; Liu, Y. Limonoid compounds from Xylocarpus granatum and their anticancer activity against esophageal cancer cells. Thorac. Cancer 2020, 11, 1817−1826. [CrossRef]
- Sahai R, Bhattacharjee A, Shukla VN, Yadav P, Hasanain M, Sarkar J, Narender T, Mitra K. Gedunin isolated from the mangrove plant Xylocarpus granatum exerts its anti-proliferative activity in ovarian cancer cells through G2/M-phase arrest and oxidative stress-mediated intrinsic apoptosis. Apoptosis. 2020, 25, 481−499. [CrossRef]
- Darmadi, J.; Batubara, R.R.; Himawan, S.; Azizah, N.N.; Audah, H.K.; Arsianti, A.; Kurniawaty, E.; Ismail, I.S.; Batubara, I.; Audah, K.A. Evaluation of Indonesian mangrove Xylocarpus granatum leaves ethyl acetate extract as potential anticancer drug. Sci. Rep. 2021, 11, 6080−6098. [CrossRef]
- Zhang, M.; Shi, Z.; Liu, J.; Shen, L.; Wu, J. New 30-ketophragmalins with anti-breast cancer activity against MDA-MB-453 cells from the Godavari mangrove, Xylocarpus moluccensis (Lam.) M. Roem. Phytochem. Lett. 2018, 26, 143−148. [CrossRef]
- Chaudhry, G.E.; Sohimi, N.K.; Mohamad, H.; Zafar, M.N.; Ahmed, A.; Sung, Y.Y.; Muhammad, T.S. Xylocarpus moluccensis induces cytotoxicity in human hepatocellular carcinoma HepG2 cell line via activation of the extrinsic pathway. Asian Pac. J. Cancer Prev. 2021, 22, 17−24. [CrossRef]
- Chumkaew, P.; Kato, S.; Chantrapromma, K. Potent cytotoxic rocaglamide derivatives from the fruits of Amoora cucullata. Chem. Pharm. Bull. 2006, 54, 1344−1346. [CrossRef]
- Ahmed, F.; Toume, K.; Sadhu, S.K.; Ohtsuki, T.; Arai, M.A.; Ishibashi, M. Constituents of Amoora cucullata with TRAIL resistance-overcoming activity. Org. Biomol. Chem. 2010, 8, 3696−3703. [CrossRef]
- Costa, A.R.; de Lima Silva, J.R.; de Oliveira, T.J.; da Silva, T.G.; Pereira, P.S.; de Oliveira Borba, E.F.; de Brito, E.S.; Ribeiro, P.R.; Almeida-Bezerra, J.W.; Júnior, J.T.; et al. Phytochemical profile of Anacardium occidentale L. (cashew tree) and the cytotoxic and toxicological evaluation of its bark and leaf extracts. S. Afr. J. Bot. 2020, 135, 355−364. [CrossRef]
- Taiwo, B.J.; Fatokun, A.A.; Olubiyi, O.O.; Bamigboye-Taiwo, O.T.; van Heerden, F.R.; Wright, C.W. Identification of compounds with cytotoxic activity from the leaf of the Nigerian medicinal plant, Anacardium occidentale L. (Anacardiaceae). Bioorg. Med. Chem. 2017, 25, 2327−2335. [CrossRef]
- Taiwo, B.J.; Popoola, T.D.; van Heerden, F.R.; Fatokun, A.A. Pentagalloylglucose, isolated from the leaf extract of Anacardium occidentale L., could elicit rapid and selective cytotoxicity in cancer cells. BMC Complement. Med. Ther. 2020, 20, 1−9. [CrossRef]
- Arung, E.T.; Wicaksono, B.D.; Handoko, Y.A.; Kusuma, I.W.; Yulia, D.; Sandra, F. Anticancer properties of diethylether extract of wood from sukun (Artocarpus altilis) in human breast cancer (T47D) cells. Trop. J. Pharm. Res. 2009, 8, 317−324. [CrossRef]
- Jeon, Y.J.; Jung, S.N.; Chang, H.; Yun, J.; Lee, C.W.; Lee, J.; Choi, S.; Nash, O.; Han, D.C.; Kwon, B.M. Artocarpus altilis (Parkinson) Fosberg extracts and geranyl dihydrochalcone inhibit STAT3 activity in prostate cancer DU145 cells. Phytother. Res. 2015, 29, 749−756. [CrossRef]
- Aswathy, M.; Parama, D.; Hegde, M.; Sherin, D.R.; Lankalapalli, R.S.; Radhakrishnan, K.V.; Kunnumakkara, A.B. Natural prenylflavones from the stem bark of Artocarpus altilis: Promising anticancer agents for oral squamous cell carcinoma targeting the Akt/mTOR/STAT-3 signaling pathway. ACS Omega 2024, 9, 24252−24267. [CrossRef]
- Ragasa, C.Y.; Espineli, D.L.; Shen, C.C. Cytotoxic triterpene from Barringtonia asiatica. Pharm. Chem. J. 2014, 48, 529−533. [CrossRef]
- Villanueva, N.S.; Vicera, C.V.; Leonida, S.F.; Paderog, M.J.; Sabido, E.M.; Saludes, J.P.; Dalisay, D.S. Barringtonia asiatica seed extract induces G1 cell cycle arrest in Saccharomyces cerevisiae and exhibits cytotoxicity in A2780 human ovarian cancer cells. J. Pharm. Res. Int. 2021, 33, 372−387. [CrossRef]
- Amran, N.; Rani, A.N.; Mahmud, R.; Yin, K.B. Antioxidant and cytotoxic effect of Barringtonia racemosa and Hibiscus sabdariffa fruit extracts in MCF-7 human breast cancer cell line. Pharmacogn. Res. 2016, 8, 66−70. [CrossRef]
- Das, B.; Srinivas, Y.; Sudhakar, C.; Mahender, I.; Laxminarayana, K.; Reddy, P.R.; Raju, T.V.; Jakka, N.M.; Rao, J.V. New diterpenoids from Caesalpinia species and their cytotoxic activity. Bioorg. Med. Chem. Lett. 2010, 20, 2847−2850. [CrossRef]
- Itoigawa, M.; Ito, C.; Tan, H.T.; Kuchide, M.; Tokuda, H.; Nishino, H.; Furukawa, H. Cancer chemopreventive agents, 4-phenylcoumarins from Calophyllum inophyllum. Cancer Lett. 2001, 169, 15−19. [CrossRef]
- Yimdjo, M.C.; Azebaze, A.G.; Nkengfack, A.E.; Meyer, A.M.; Bodo, B.; Fomum, Z.T. Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry 2004, 65, 2789−2795. [CrossRef]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Chantrapromma, K. New cytotoxic cardenolide glycoside from the seeds of Cerbera manghas. Chem. Pharm. Bull. 2004, 52, 1023−1025. [CrossRef]
- Zhao, Q.; Guo, Y.; Feng, B.; Li, L.; Huang, C.; Jiao, B. Neriifolin from seeds of Cerbera manghas L. induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Fitoterapia 2011, 82, 735−741. [CrossRef]
- Tsai, J.C.; Liu, W.S.; Tseng, Y.T.; Lam, H.I.; Chen, S.Y.; Fang, C.L.; Tong, T.S.; Lai, Y.J. Extracts of Cerbera manghas L. effectively inhibit the viability of glioblastoma cell lines and their cancer stemloids in vitro and in mouse xenograft model. J. Funct. Foods 2018, 48, 283−296. [CrossRef]
- Laphookhieo, S.; Cheenpracha, S.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Chantrapromma, K. Cytotoxic cardenolide glycoside from the seeds of Cerbera odollam. Phytochemistry 2004, 65, 507−510. [CrossRef]
- Nurhanan, N.Y.; Osman, A.; Jauri, M.H.; Sallehudin, N.J.; Mutalip, S.S. The in vitro anticancer activities of 17βH-neriifolin isolated from Cerbera odollam and its binding activity on Na+, K+-ATPase. Curr. Pharm. Biotechnol. 2020, 21, 37−44. [CrossRef]
- Haryoto, M.; Indrayudha, P.; Azizah, T.; Suhendi, A.; Haryoto, M.; Peni Indrayudha, T.A. Aktivitas sitotoksik ekstrak etanol tumbuhan sala (Cynometra ramiflora Linn) terhadap sel HeLa, T47D dan WiDR. J. Sci. Publ. 2013, 18, 21−28.
- Chiang, Y.M.; Chang, J.Y.; Kuo, C.C.; Chang, C.Y.; Kuo, Y.H. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry 2005, 66, 495−501. [CrossRef]
- Kim, K.H.; Lee, J.Y.; Li, W.Y.; Lee, S.; Jeong, H.S.; Choi, J.Y.; Joo, M. The ethanol extract of Garcinia subelliptica Merr. induces autophagy. BMC Complement. Med. Ther. 2021, 21, 280−291. [CrossRef]
- Yun, Y.; Shioura, M.; Hitotsuyanagi, Y.; Yotsumoto, S.; Takahashi, Y.; Aoyagi, Y.; Kinoshita, T.; Takeya, K.; Inoue, H. Garcinielliptone G from Garcinia subelliptica induces apoptosis in acute leukemia cells. Molecules 2021, 26, 2422−2431. [CrossRef]
- Yu, B.W.; Luo, J.G.; Wang, J.S.; Zhang, D.M.; Yu, S.S.; Kong, L.Y. Pentasaccharide resin glycosides from Ipomoea pes-caprae. J. Nat. Prod. 2011, 74, 620−628. [CrossRef]
- Chee, C.W.; Zamakshshari, N.H.; Lee, V.S.; Abdullah, I.; Othman, R.; Lee, Y.K.; Mohd Hashim, N.; Nor Rashid, N. Morindone from Morinda citrifolia as a potential anti-proliferative agent against colorectal cancer cell lines. PloS One 2022, 17, e0270970. https: //doi.org/10.1371/journal.pone.0270970.
- Samarakoon, S.R.; Shanmuganathan, C.; Ediriweera, M.K.; Tennekoon, K.H.; Piyathilaka, P.; Thabrew, I.; de Silva, E.D. In vitro cytotoxic and antioxidant activity of leaf extracts of mangrove plant, Phoenix paludosa Roxb. Trop. J. Pharm. Res. 2016, 15, 127−132. [CrossRef]
- Chen, H.Y.; Guh, J.H.; Chan, S.H.; Lee, S.S. Cytotoxic protobassic acid glycosides from Planchonella obovata leaf. Phytochem Lett. 2015, 11, 229−235. [CrossRef]
- Cho, J.J.; Cho, C.L.; Kao, C.L.; Chen, C.M.; Tseng, C.N.; Lee, Y.Z.; Liao, L.J.; Hong, Y.R. Crude aqueous extracts of Pluchea indica (L.) Less. inhibit proliferation and migration of cancer cells through induction of p53-dependent cell death. BMC Complement. Altern Med. 2012, 12, 265−276. [CrossRef]
- Kao, C.L.; Cho, J.; Lee, Y.Z.; Cheng, Y.B.; Chien, C.Y.; Hwang, C.F.; Hong, Y.R.; Tseng, C.N.; Cho, C.L. Ethanolic extracts of Pluchea indica induce apoptosis and antiproliferation effects in human nasopharyngeal carcinoma cells. Molecules 2015, 20, 11508−11523. [CrossRef]
- Cho, C.L.; Lee, Y.Z.; Tseng, C.N.; Cho, J.; Cheng, YB.; Wang, K.W.; Chen, H.J.; Chiou, S.J.; Chou, C.H.; Hong, Y.R. Hexane fraction of Pluchea indica root extract inhibits proliferation and induces autophagy in human glioblastoma cells. Biomed. Rep. 2017, 7, 416−422. [CrossRef]
- Chen, G.; Zhou, D.; Li, X.Z.; Jiang, Z.; Tan, C.; Wei, X.Y.; Ling, J.; Jing, J.; Liu, F.; Li, N. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Sci. Rep. 2017, 7, 10729. [CrossRef]
- Roy, R.; Pal, D.; Sur, S.; Mandal, S.; Saha, P.; Panda, C.K. Pongapin and karanjin, furanoflavonoids of Pongamia pinnata, induce G2/M arrest and apoptosis in cervical cancer cells by differential reactive oxygen species modulation, DNA damage, and nuclear factor kappa-light-chain-enhancer of activated B cell signaling. Phytother. Res. 2019, 33, 1084−1094. [CrossRef]
- Saraphon, C.; Boonloh, K.; Kukongviriyapan, V.; Yenjai, C. Cytotoxic flavonoids from the fruits of Derris indica. J. Asian Nat. Prod. Res. 2017, 19, 1198−1203. [CrossRef]
- Thu, N.T.; Ha, L.T.; Nga, V.T.; Tuyen, P.N.; Quang, T.T.; Danielle, F.R.; Pratt, L.M.; Phung, N.K. Six new phenolic glycosides and a new ceramide from the flowers of Wedelia biflora and their cytotoxicity against some cancer cell lines. Nat. Prod. Commun. 2013, 8, 367−372. [CrossRef]
- Huyen, P.T.; Van Loc, T.; Van Sung, T.; Thao, T.T. Chemical constituents of Spinifex littoreus collected from the coast of Quang Nam Province, Vietnam. Chem. Nat. Compd. 2019, 55, 141−143. [CrossRef]
- Chen, J.J.; Huang, S.Y.; Duh, C.Y.; Chen, I.S.; Wang, T.C.; Fang, H.Y. A new cytotoxic amide from the stem wood of Hibiscus tiliaceus. Planta Med. 2006, 72, 935−938. [CrossRef]
- Cheng, C.L.; Wang, Z.Z.; Li, P.L.; Zhang, X.W.; Wu, R.C.; Zhu, H.Y.; Tang, X.L.; Li, G.Q. Tetracyclic triterpenoids isolated from semi-mangrove plant Hibiscus tiliaceus. Chin. Chem. Lett. 2013, 24, 1080−1082. [CrossRef]
- Chen, D.L.; Ma, G.X.; Yang, E.L.; Yang, Y.; Wang, C.H.; Sun, Z.C.; Liang, H.Q.; Xu, X.D.; Wei, J.H. Cadinane-type sesquiterpenoid dimeric diastereomers hibisceusones A−C from infected stems of Hibiscus tiliaceus with cytotoxic activity against triple-negative breast cancer cells. Bioorg. Chem. 2022, 127, 105982. [CrossRef]
- Chen, D.L.; Chen, M.Y.; Hou, Y.; Wang, C.H.; Sun, Z.C.; Yang, Y.; Liang, H.Q.; Ma, G.X.; Xu, X.D.; Wei, J.H. Cadinane-type sesquiterpenoids with cytotoxic activity from the infected stems of the semi-mangrove Hibiscus tiliaceus. J. Nat. Prod. 2022, 85, 127−135. [CrossRef]
- Chu, S.C.; Yang, S.F.; Liu, S.J.; Kuo, W.H.; Chang, Y.Z.; Hsieh, Y.S. In vitro and in vivo anti-metastatic effects of Terminalia catappa L. leaves on lung cancer cells. Food Chem. Toxicol. 2007, 45, 1194−1201. [CrossRef]
- Lee, C.Y.; Yang, S.F.; Wang, P.H.; Su, C.W.; Hsu, H.F.; Tsai, H.T.; Hsiao, Y.H. Anti-metastatic effects of Terminalia catappa leaf extracts on cervical cancer through the inhibition of matrix metalloprotein-9 and MAPK pathway. Environ. Toxicol. 2019, 34, 60−66. [CrossRef]
- Boonsri, S.; Karalai, C.; Ponglimanont, C.; Chantrapromma, S.; Kanjana-Opas, A. Cytotoxic and antibacterial sesquiterpenes from Thespesia populnea. J. Nat. Prod. 2008, 71, 1173−1177. [CrossRef]
- Hernandez, M.M.; Heraso, C.; Villarreal, M.L.; Vargas-Arispuro, I.; Aranda, E. Biological activities of crude plant extracts from Vitex trifolia L. (Verbenaceae). J. Ethnopharmacol. 1999, 67, 37−44. [CrossRef]
- Aweng, E.R.; Hanisah, N.; Mohd. Nawi, M.A.; Nurhanan, M.Y.; Shamsul, M. Antioxidant activity and phenolic compounds of Vitex trifolia var. simplicifolia associated with anti-cancer. ISCA J. Biol. Sci. 2012, 1, 65−68.
- Kalavathi, R.; Sagayagiri, R. Anticancer activity of ethanolic leaf extract of Clerodendrum inerme against lung adenocarcinoma epithelial cell line. Eur. J. Mol. Biol. Biochem. 2016, 3, 69−72.
- Ba Vinh, L.; Thi Minh Nguyet, N.; Young Yang, S.; Hoon Kim, J.; Thi Vien, L.; Thi Thanh Huong, P.; Van Thanh, N.; Xuan Cuong, N.; Hoai Nam, N.; Van Minh, C.; et al. A new rearranged abietane diterpene from Clerodendrum inerme with antioxidant and cytotoxic activities. Nat. Prod. Res. 2018, 32, 2001−2007. [CrossRef]
- Voss, C.; Eyol, E.; Berger, M.R. Identification of potent anticancer activity in Ximenia americana aqueous extracts used by African traditional medicine. Toxicol. Appl. Pharmacol. 2006, 211, 177−187. [CrossRef]
- Maxted N. Ex situ and in situ conservation. Encyclopaedia of Biodiversity, Vol. 2, 2001, 683−695.
- Mahanayak B. Ex-situ and in-situ conservation of wildlife. World J. Biol. Pharm. Health Sci. 2024, 18, 277−282. [CrossRef]
- Chan, E.W.C.; Ng, S.M.; Sim, B.W.; Tan, H.C.; Lo, Z.L. Antioxidant, anti-tyrosinase and anti-quorum sensing activities of four mangrove tree species vs. green tea. J. Appl. Pharm. Sci. 2017, 7, 225−229. [CrossRef]
- Ashton, E.C. Threats to Mangroves and Conservation Strategies. In: Mangroves: Biodiversity, Livelihoods and Conservation; Chandra Das, S.; Pullaiah, T.; Ashton, E.C., eds., 2022, 217−230, Springer. [CrossRef]
- Laksanacom, A.; Seenprachawong, U. Valuing the attribute enhancements of urban park: A case of the King Rama IX International Mangrove Botanical Garden, Thailand. J. Appl. Econ. Manag. Strategy. 2023, 10, 47−65.
- Macintosh, D.J.; Ashton, E.C.; Havanon, S. Mangrove rehabilitation and intertidal biodiversity: A study in the Ranong mangrove ecosystem, Thailand. Estuar. Coast. Shelf Sci. 2002, 55, 331−345. [CrossRef]
- Putz, F.E.; Chan, H.T. Tree growth, dynamics, and productivity in a mature mangrove forest in Malaysia. For. Ecol. Manag. 1986, 17, 211−230. [CrossRef]
- Tangah, J.; Chung, A.Y.C.; Baba, S.; Chan, H.T.; Kezuka, M. Rehabilitation of Mangroves in Sabah: The SFD-ISME Collaboration (2014-2019). Sabah Forestry Department, International Society for Mangrove Ecosystems; Tokio Marine & Nichido Fire Insurance Co., Ltd. 2020, 58p.
- Chan, H.T. A note on tree species and productivity of a natural dryland mangrove forest in Matang, Peninsular Malaysia. J. Trop. For. Sci. 1989, 1, 399−400.
- Basyuni, M.; Bimantara, Y.; Siagian, M.; Wati, R.; Slamet, B.; Sulistiyono, N.; Nuryawan, A.; Leidonad, R. Developing community-based mangrove management through eco-tourism in North Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012109. [CrossRef]
- Basyuni, M.; Yani, P.; Hartini, K.S. Evaluation of mangrove management through community-based silvofishery in North Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012109. [CrossRef]
- Lan, B.T.H.; Phuong, T.T.L.; Dat, T.T.; Truong, D.D. Payment for urban mangrove forest conservation in Vietnam: A community case study of Can Gio Biosphere Reserve, Ho Chi Minh City. Sustainability. 2023, 15, 10299. [CrossRef]
- Aye, W.N.; Tong, X.; Tun, A.W. Species diversity, biomass and carbon stock assessment of Kanhlyashay natural mangrove forest. Forests. 2022, 13, 1013. [CrossRef]
| No. | Species (Synonym) | Common/Vernacular Name | Family | Life-Form | Ref. |
|---|---|---|---|---|---|
| Mangrove Species | |||||
| 1 | Acanthus ebracteatus | Sea holly, Jeruju hitam | Acanthaceae | Shrub | [5] |
| 2 | Acanthus ilicifolius | Mangrove holly, Jeruju putih | Acanthaceae | Shrub | [5] |
| 3 | Acrostichum aureum | Mangrove fern, Piai raya | Pteridaceae | Fern | [13,14] |
| 4 | Aegiceras corniculatum | River mangrove, Kacang-kacang | Primulaceae | Shrub | [5] |
| 5 | Avicennia alba | Api-api putih | Avicenniaceae | Tree | [15] |
| 6 | Avicennia marina | Grey or white mangrove | Avicenniaceae | Tree | [16] |
| 7 | Avicennia rumphiana | Velvety mangrove, Api-api bulu | Avicenniaceae | Tree | [17] |
| 8 | Bruguiera gymnorhiza | Oriental mangrove, Tumu merah | Rhizophoraceae | Tree | [18] |
| 9 | Bruguiera sexangula | Upriver orange mangrove | Rhizophoraceae | Tree | [5] |
| 10 | Ceriops tagal | Indian mangrove, Tengar | Rhizophoraceae | Tree | [19] |
| 11 | Excoecaria agallocha | Milky mangrove, Bebuta | Euphorbiaceae | Tree | [20] |
| 12 | Heritiera fomes | Sundari, Sundri, Sunder | Malvaceae | Tree | [5] |
| 13 | Lumnitzera racemosa | Black mangrove, Terentum putih | Combretaceae | Tree | [5] |
| 14 | Nypa fruticans | Nipa palm, Golpata | Arecaceae | Palm | [18] |
| 15 | Rhizophora apiculata | Tall stilt mangrove, Bakau minyak | Rhizophoraceae | Tree | [18,21] |
| 16 | Rhizophora mucronata | Loop stilt mangrove, Bakau kurap | Rhizophoraceae | Tree | [18] |
| 17 | Rhizophora stylosa | Long-style stilt mangrove | Rhizophoraceae | Tree | [22,23] |
| 18 | Syphiphora hydrophyllacea | Chingam, Nilad | Rubiaceae | Shrub | [5] |
| 19 | Xylocarpus granatum | Cannon ball mangrove | Meliaceae | Tree | [24,25] |
| 20 | Xylocarpus moluccensis | Cedar mangrove, Nyireh batu | Meliaceae | Tree | [5] |
| Associate species | |||||
| 1 |
Aglaia cucullata (Amoora cucullata) |
Pacific maple | Meliaceae | Tree | [26] |
| 2 | Anacardium occidentale | Cashew nut | Anacardiaceae | Tree | [27] |
| 3 | Artocarpus altilis | Breadfruit | Moraceae | Tree | [28] |
| 4 | Barringtonia asiatica | Sea poison tree, Putat laut | Lecythidaceae | Tree | [5] |
| 5 | Barringtonia racemosa | Powder puff tree, Common putat | Lecythidaceae | Tree | [5] |
| 6 | Caesalpinia crista | Squirrel’s claws, Kuku tupai | Fabaceae | Tree | [29] |
| 7 | Calophyllum inophyllum | Beach calophyllum, Penaga laut | Clusiaceae | Tree | [30] |
| 8 | Cerbera manghas | Sea mango, Bintaro | Apocynaceae | Tree | [31] |
| 9 | Cerbera odollam | Pong-pong, Suicide tree | Apocynaceae | Tree | [31] |
| 10 | Cynometra ramiflora | Wrinkle pod mangrove | Fabaceae | Tree | [5] |
| 11 | Ficus microcarpa | Chinese banyan, Curtain fig | Moraceae | Tree | [32] |
| 12 | Garcinia subelliptica | Happiness tree, Fukugi | Clusiaceae | Tree | [33] |
| 13 | Ipomoea pes-caprae | Beach morning glory, Goat’s foot | Convolvulaceae | Creeper | [34] |
| 14 | Morinda citrifolia | Indian mulberry, Mengkudu | Rubiaceae | Tree | [5] |
| 15 | Phoenix paludosa | Mangrove date palm | Arecaceae | Tree | [35] |
| 16 | Planchonella obovata | Sea gutta, Menasi | Sapotaceae | Tree | [5] |
| 17 | Pluchea indica | Marsh fleabane, Camphorweed | Asteraceae | Shrub | [36] |
| 18 |
Pongamia pinnata (Derris indica) |
Pongam, Indian beech | Fabaceae | Tree | [5] |
| 19 |
Sphagneticola triloba (Wedelia triloba) |
Yellow creeping daisy | Asteraceae | Herb | [37] |
| 20 | Spinifex littoreus | Hairy spinifex, Beach spinifex | Poaceae | Grass | [38] |
| 21 |
Talipariti tiliaceum (Hibiscus tiliaceus) |
Sea hibiscus, Chinese hibiscus | Malvaceae | Tree | [39,40] |
| 22 | Terminalia catappa | Sea almond, Ketapang | Combretaceae | Tree | [18] |
| 23 | Thespesia populnea | Portia tree, Milo, Bebaru | Malvaceae | Tree | [30] |
| 24 |
Vitex trifolia (V. ovata, V. rotundifolia) |
Common blue vitex, Legundi | Lamiaceae | Shrub | [34,41] |
| 25 | Volkameria inermis (Clerodendrum inerme) | Wild jasmine, Bengali | Lamiaceae | Shrub | [42] |
| 26 | Ximenia americana | Sea lime, Sea lemon | Olacaceae | Shrub | [5] |
| No. | Species | Effect and Mechanism | Ref. |
|---|---|---|---|
| 1 |
Acanthus ebracteatus |
The ethyl acetate leaf extract displayed cytotoxicity and antiproliferative activity and induced apoptosis against HeLa cervical cancer cells with an IC50 value of 34.4 µg/mL. | [44] |
| 2 |
Acanthus ilicifolius |
Administration of the ethanol leaf extract (500 mg/kg) was found to reduce EAT in mice. The maximum increase in life span of EAT mice was 75%. | [45] |
| Alkaloids (acanthosides A−D) from the ethanol root extract were cytotoxic to HepG2 liver, HeLa cervical and A549 lung cancer cells. Acanthosides A and B were the most cytotoxic to HepG2 liver cancer cells with IC50 values of 7.8 and 9.6 µM, respectively. | [46] | ||
| The aqueous root extract inhibited the growth and induced apoptosis of HepG2 liver cancer cells with an IC50 value of 39.8 μg/mL. | [47] | ||
| 3 |
Acrostichum aureum |
The ethyl acetate leaf extract displayed cytotoxic and antiproliferative activity against HeLa cervical cancer cells with an IC50 value of 6.3 μg/mL. | [48] |
| The methanol root extract exhibited cytotoxicity against AGS gastric cancer cells with an IC50 value of 1.0 mg/mL. | [49] | ||
| Pterosin C, a sulphated sesquiterpene from the methanol leaf extract, was cytotoxic to AGS gastric cancer cells with an IC50 value of 23.9 μM. | [50] | ||
| 4 |
Aegiceras corniculatum |
The methanol bark extract showed cytotoxicity against HT29 colon and MDA-MB-435 breast cancer cells with IC50 values were 0.33 and 0.66 mg/mL, respectively. | [49] |
| Five alkylated benzoquinones from the petroleum ether extract of stem and twig displayed cytotoxic activity towards HepG2 liver, BGC-823 gastric and A2780 ovarian cancer cells, and HL-60 leukemia cells. Strongest cytotoxicity was 5-O-methylrapanone with an IC50 value of 7.6 µM against HL-60 cells. | [51] | ||
| The methanol leaf extract inhibited HeLa cervical, T47D breast and WiDr colon cancer cells with IC50 values of 49.4, 78.1 and 45.6 μg/mL. | [52] | ||
| 5 |
Avicennia alba |
The methanol leaf extract inhibited HeLa cervical, T47D breast and WiDr colon cancer cells with IC50 values of 74.7, 50.8 and 73.2 μg/mL. | [52] |
| Against MCF-7 breast cancer cells, the diethyl ether leaf extract displayed the strongest cytotoxicity, followed by butanol and methanol leaf extracts with IC50 values of 25.1, 27.1 and 28.9 μg/mL, respectively. | [53] | ||
| 6 |
Avicennia marina |
Stenocarpoquinone B and avicequinone C from the methanol twig extract showed strong antiproliferative activities against HeLa cervical cancer cells (4.3 and 0.2 μg/mL) and K562 leukemia cells (3.2 and 1.1. μg/mL), respectively. | [54] |
| The ethanol leaf extract exhibited weak cytotoxicity in HL-60 leukemia cells with IC50 values of 600, 400 and 280 μg/mL after 24, 48 and 72 h, respectively. |
[55] | ||
| The ethyl acetate leaf extract induced apoptosis and inhibited migration of breast (AU565, MDA-MB-231 and BT483) and liver (HepG2 and Huh7) cancer cells. Antitumor activity was also observed in a xenograft mouse model of MDA-MB-231. | [56] | ||
| From the chloroform : methanol (2:1) leaf extract, isolated PIP exhibited weak anticancer activity against WiDr cells with an IC50 value of 155 μg/mL. Mechanisms of the anticancer activity involved inhibition of cell cycle and induction of apoptosis. | [57] | ||
| Lupeol, a pentacyclic triterpene, isolated from the hexane stem extract suppressed the growth of Hep3B liver cancer cells via induction of apoptosis, triggering the apoptotic pathway and down-regulating of BCL-2 expression. | [58] | ||
| 7 |
Avicennia rumphiana |
PIP isolated from the chloroform : methanol (2:1) leaf extract exhibited anticancer activity against WiDr cells with an IC50 value of 306 μg/mL. Mechanisms of the anticancer activity involved inhibition of cell cycle and induction of apoptosis. | [57] |
| 8 |
Bruguiera gymnorhiza |
Butanol, diethyl ether and methanol leaf extracts exhibited cytotoxicity against the MCF-7 breast cancer cells with IC50 values of 3.4, 16.2 and 37.1 µg/mL. Mechanisms included the induction of apoptosis and up-regulation of caspases. | [59] |
| 9 |
Bruguiera sexangula |
From the ethanol and chloroform stem and bark extracts, brugine, a sulphur-containing alkaloid, exerted anticancer activity against effects against sarcoma 180 and Lewis lung carcinoma cell via the calcium, cAMP and PI3K-Akt signaling pathways. | [60] |
| 10 |
Ceriops tagal |
From the ethanol extract of the embryo, betulin and 3-epi-betulinic acid acetate inhibited H-7402 liver and HeLa cervical cancer cells with IC50 values of 14.4 and 11.8 µg/mL, and 10.0 and 11.3 µg/mL, respectively. | [61] |
| Dolabrane-type diterpenes tagalenes A−F isolated from the ethanol extract of twig and leaf exhibited potent inhibition against a panel of tumor cell lines. Tagalene C was most potent with IC50 values of 3.7, 6.3 and 5.5 µM against HCT-8 colon, Bel-7402 liver and A2780 ovarian cancer cells, respectively. | [62] | ||
| PIP from the chloroform : methanol (2:1) leaf extract exhibited weak cytotoxicity against WiDr colon cancer cells with IC50 value of 276 µg/mL. Mechanisms involved apoptosis, cell cycle arrest, and decreased expression of Bcl-2 and cyclin D1. | [63] | ||
| Dolichol, a PIP isolated from the chloroform : methanol (2:1) leaf extract, exhibited cytotoxicity against WiDr colon cancer cells by reducing G0/G1 growth cycle, up-regulation of p53 expression, and down-regulation of EGFR, PI3K, Akt and mTOR expression. | [64] | ||
| 11 |
Excoecaria agallocha |
The ethanol stem extract exhibited potent cytotoxicity against capan-1 and miapaca-2 pancreatic cancer cells with IC50 values of 4.0 and 7.0 μg/mL, respectively. | [65] |
| Two flavonoid glycosides isolated from the methanol leaf extract displayed HH inhibition with IC50 values of 0.5 and 2.0 μM. Cytotoxicity was IC50 values of 0.7 and 1.8 μM against PANC1 pancreatic, and 0.8 and 2.4 μM against DU145 prostate cancer cells. | [66] | ||
| 12 |
Heritiera fomes |
Methanol leaf and stem extracts possessed anticancer properties with 40% inhibition against B16 mouse melanoma and against Swiss albino mice with EAT. | [67] |
| 13 |
Lumnitzera racemosa |
The aqueous leaf extract inhibited HepG2 liver cancer cells with an IC50 value of 26.0 μg/mL. Viability of cancer cells was 40% at 100 μg/mL of extract. | [47] |
| 1,5,6-Trihydroxy-3-methoxyxanthone and polygalatenoside E isolated from the methanol leaf extract were cytotoxic to HL-60 leukemia cells with IC50 values of 0.15 and 0.60 μM, respectively. | [68] | ||
| The methanol leaf extract exerted moderate cytotoxicity against MCF-7 breast and HeLa cervical cancer cells with IC50 values of 46.1 and 59.5 mg/mL, respectively. | [69] | ||
| 14 |
Nypa fruticans |
PIP from the chloroform : methanol (2:1) leaf extract exhibited anticancer activity towards WiDr colon cancer cells with IC50 value of 180 μg/mL via inhibition of COX-2. |
[70] |
| PIP from the chloroform : methanol (2:1) leaf extract inhibited WiDr colon cancer cells by modulating the expression of p53, EGFR, PI3K, AKT1 and mTOR. | [71] | ||
| 15 |
Rhizophora apiculata |
2,6-Dimethoxy-1,4-benzoquinone isolated from the methanol leaf extract exhibited inhibitory effects against SK-LU-1 lung, HepG2 liver and MCF-7 breast cancer cells with IC50 values of 13.1, 14.8 and 8.3 μM, respectively. | [72] |
| The methanol leaf extract inhibited metastasis in B16F-10 melanoma bearing mice by inhibiting pulmonary tumor nodule formation and increasing the survival rate of mice. | [73] | ||
| 16 |
Rhizophora mucronata |
PIP from the chloroform : methanol (2:1) leaf extract, exhibited weak exhibited cytotoxicity against WiDr colon cancer cells with IC50 value of 278 µg/mL. Mechanisms are by inducing apoptosis, cell cycle arrest, and decreasing the expression of Bcl-2 and cyclin D1. | [63] |
| Dolichol, a PIP from the chloroform : methanol (2:1) leaf extract, reduced the G0/G1 growth cycle of WiDr colon cancer cells by 82%. Mechanisms were via up-regulation of p53 expression, and down-regulation of EGFR, PI3K, Akt and mTOR expression. | [64] | ||
| The methanol leaf extract and stem extract exhibited weak anticancer effects. Their IC50 values were 127 and 107 µg/mL for CaCo-2 colon cancer cells, and 158 and 138 µg/mL for MCF-7 breast cancer cells. Against A549 lung cancer cells, the anticancer effect of the stem extract was 2.4 times stronger than the leaf extract. | [74] | ||
| 17 |
Rhizophora stylosa |
Of the compounds isolated from the leaves, taraxerol inhibited HeLa cervical and BGC-823 gastric cancer cells, both with an IC50 value of 73.4 μmol/L. Cis-careaborin inhibited BGC-823 cancer cells with an IC50 value of 45.9 μmol/L. | [75] |
| 18 |
Syphiphora hydrophyllacea |
Hopenone-I, a triterpenoid isolated from the hexane leaf extract, was cytotoxic against MCF-7 breast, HepG2 liver and AN3CA endometrial cancer cells with IC50 values of 7.8, 11.6 and 5.0 μM, respectively. |
[76] |
| UA and EA from hexane and chloroform extracts of leaves showed strong cytotoxic effects. IC50 values of UA were 8.5 and 7.8 μg/mL against MCF-7 breast cancer cells, and IC50 values of EA were 8.9 and 10.1 μg/mL against NCI-H292 lung cancer cells. IC50 values of paclitaxel used as a positive control were 4.3 and 10 μg/mL. | [77] | ||
| 19 |
Xylocarpus granatum |
Four limonoid compounds from the fruit strongly, inhibited the proliferation of Eca109 esophageal cancer cells, with xylogranatin C having the strongest activity (IC50 value of 9.5 μmol/L). Mechanism involved apoptosis via the DR and ER pathways. | [78] |
| Gedunin isolated from the aqueous ethanol fruit extract strongly inhibited the anti-proliferative activity of PA-1 ovarian cancer cells with IC50 value of 8.1 µM via G2/M-phase arrest and oxidative stress-mediated intrinsic apoptosis. | [79] |
||
| The ethyl acetate extract of leaves inhibited HeLa cervical and MCF-7 breast cancer cells by 92.9% and 96.6%, respectively. Inhibition by Dox used as a positive control was 95.7% and 94.0%. | [80] | ||
| 20 |
Xylocarpus moluccensis |
The methanol extract of pneumatophores showed cytotoxicity against AGS gastric and MDA-MB-435S melanoma cancer cells with IC50 values of 0.6 and 1.1 mg/mL, respectively. | [49] |
| Two novel 30-ketophragmalins (limonoids) isolated from the ethanol seed extract exhibited anticancer activity against MDA-MB-453 breast cancer cells with IC50 values of 2.1 and 9.0 µM. The anticancer activity of 30-ketophragmalins against breast cancer cells has been reported for the first time. | [81] | ||
| The diethyl ether leaf extract displayed strong cytotoxicity with an IC50 value of 0.22 μg/mL. The methanol leaf extract was much weaker at <30 μg/mL. Mechanisms involved induction of apoptosis and activation of caspases. | [82] |
| No. | Specie | Effect and Mechanism | Ref. |
|---|---|---|---|
| 1 |
Aglaia cucullata |
Two rocaglamide derivatives isolated from the successive hexane and DCM fruit extract exhibited potent cytotoxicity against KB oral and BC breast cancer cells with IC50 values of 0.002 and 0.005 µg/mL, and 0.06 and 0.002 µg/mL, respectively. | [83] |
| 1-O-Formylrocagloic acid from the methanol leaf extract displayed potent cytotoxic activity in TRAIL-resistant AGS gastric cancer cells by activating caspase-3/7, enhancing apoptosis, and expressing DR4 and DR5 mRNA. | [84] | ||
| 2 |
Anacardium occidentale |
The ethanol leaf and bark extracts exhibited cytotoxic activity against HL-60 leukemia (79% and 85%) and HCT116 colon (89% and 91%) cancer cells, respectively. The inhibition of extracts was comparable with curcumin (both 92%) used as a positive control. | [85] |
| Zoapatanolide A, a sesquiterpene lactone isolated from the ethanol leaf extract, exhibited anticancer effects on HeLa cervical cancer cells with an IC50 value of 36.2 μM. | [86] | ||
| PGG isolated from the ethanol leaf extract exerted cytotoxic activity against HeLa cervical and MRC5-SV2 lung cancer cells with IC50 values of 71.4 and 52.2 μg/mL, respectively. Cytotoxicity of PGG was due to the generation of ROS and to oxidative stress in the cancer cells. | [87] | ||
| 3 |
Artocarpus altilis |
The diethyl ether wood extract inhibited the growth of T47D breast cancer cells with an IC50 value of 6.2 μg/mL by inducing sub-G1 apoptosis. | [88] |
| The leaf and stem methanol extract, and the isolated GD inhibited the growth of DU145 prostate cancer cells with IC50 values of 20 μg/mL and 20 μM by inducing apoptosis via caspase-3 and PARP degradation. GD also inhibited in vivo tumor growth in DU145 cell xenograft mouse model. |
[89] | ||
| Isolated from the acetone stem bark extract, artonin E and artobiloxanthone inhibited SAS oral and T.Tn. esophageal cancer cells with IC50 values of 6.0 and 8.0 μM, and 11 and 22 μM, respectively. Mechanisms involved cell cycle arrest, apoptosis, and inhibition of invasion and migration of cancer cells. | [90] | ||
| 4 |
Barringtonia asiatica |
A mixture of betulinic acid and 22-O-tigloylcamelliagenin A from the DCM bark extract inhibited HCT116 colon and A549 lung cancer cells with IC50 values of 8.0 and 6.0 μg/mL, respectively. | [91] |
| The methanol seed extract induced cell cycle arrest in yeast cells and inhibited A2780 ovarian carcinoma cells with an IC50 value of 35 μg/mL. | [92] | ||
| 5 |
Barringtonia racemosa |
The methanol fruit extract exhibited cytotoxicity and inhibited the growth of MCF-7 breast cancer cells with an IC50 value of 57.6 μg/mL. | [93] |
| 6 |
Caesalpinia crista |
Two cassane diterpenoids isolated from the CHCl3 : methanol (1:1) extract of aerial parts displayed moderate cytotoxic activity towards HL-60 leukemia cells and HeLa cervical cancer cells, with IC50 values of 17.4 and 33.4 µM, and 19.8 and 33.9 µM, respectively. | [94] |
| 7 |
Calophyllum inophyllum |
Among ten 4-phenylcoumarins isolated from the DMSO aerial part extract and screened for inhibition of EBV-EA activation in TPA-activated Raji cells, eight exhibited inhibitory activity. Calocoumarin A was the most potent and it also markedly inhibited mouse skin carcinogenesis. | [95] |
| From the chloroform : methanol (2:1) root bark and fruit extract, calophyllolide, caloxanthone A and inophylloidic acid inhibited KB nasopharynx cancer cells with IC50 values of 3.5, 7.4 and 9.7 μg/mL, respectively. Inoxanthone and macluraxanthone were devoid of cytotoxic activity. | [96] | ||
| 8 |
Cerbera manghas |
Cardenolide glycosides from the CH2Cl2 extract of seeds, 7,8-dehydrocerberin, deacetyltanghinin and tanghinin were cytotoxic towards KB oral, BC breast and NCI-H187 lung cancer cells. Most cytotoxic were 7,8-dehydrocerberin against BC cells (0.001 μg/mL), deacetyltanghinin against BC cells (0.77 μg/mL) and tanghinin against KB cells (0.05 μg/mL). | [97] |
| Neriifolin from the methanol seed extract induced cell cycle arrest and apoptosis in HepG2 liver cancer cells with an IC50 value of 0.15 μg/mL. Apoptosis was induced via the activation of caspases, and the up-regulation of Fas and FasL expression. | [98] | ||
| Ethanol extracts of stems and fruits, and isolated neriifolin effectively inhibited the viability of glioblastoma cells and in mouse xenograft model. Cytotoxicity of extracts was 8.0 and 0.38 µg/mL against U251-MG cells, and 46 and 2.7 µg/mL against U373-MG cells, respectively. Cytotoxicity of the fruit extract was stronger than the stem extract. | [99] | ||
| 9 |
Cerbera odollam |
Cardenolide glycosides from the CH2Cl2 seed extract, exerted potent cytotoxicity towards KB oral, BC breast and NCI-H187 lung cancer cells. IC50 values were 17α-neriifolin (0.08, 0.05 and 0.03 µg/mL) and 17β-neriifolin (0.02, 0.05 and 0.08 µg/mL), respectively. | [100] |
| 17βH-neriifolin, a cardenolide glycoside, isolated from the hexane leaf extract, displayed potent anticancer properties against a panel of MCF-7 breast, T47D breast, HT-29 colon, A2780 ovarian, SKOV-3 ovarian and A375 skin cancer cells. IC50 values ranged from 0.02−0.03 μM. | [101] | ||
| 10 |
Cynometra ramiflora |
Cytotoxicity of the ethanol stem bark extract was strongest against T47D breast cancer cells (0.9 µg/mL) while that of the leaf extract was strongest against WiDr colon cancer cells (0.4 µg/mL). | [102] |
| 11 |
Ficus microcarpa |
Triterpenes from the methanol extract of aerial roots possessed significant cytotoxic activities against HONE-1 nasopharyngeal, KB oral epidermoid and HT29 colon cancer cells with IC50 values of 4.0−9.4 μM. Ursonic acid was the most potent. | [103] |
| 12 |
Garcinia subelliptica |
The ethanol leaf extract elicited cytotoxicity but not apoptosis in A549 and SNU2292 lung cancer cells. Mechanisms are by inducing autophagy, activating AMPK and suppressing mTOR pathways. | [104] |
| The methanol leaf extract was cytotoxic towards THP-1 and Jurkat leukemia cells. Garcinielliptone G, a benzylphloroglucinol, inhibited these cancer cells by inducing apoptosis, and activation of caspase-3 and PARP. |
[105] | ||
| 13 |
Ipomoea pes-caprae |
Pescapreins from the ethanol extract of aerial parts modulated multi-drug resistance in MCF-7/ADR adriamycin resistant breast cancer cells. The combined use of these compounds at 5 µg/mL increased the cytotoxicity of doxorubicin (anticancer drug) by 1.5−3.7 times. | [106] |
| 14 |
Morinda citrifolia |
Morindone, an anthraquinone from the root bark, extracted with four different solvents, inhibited a panel of HCT116, LS174T and HT29 colon cancer cells with IC50 values of 10.7, 20.5 and 19.2 µM, respectively. | [107] |
| 15 |
Phoenix paludosa |
Against MCF-7, MDA-MB-231 and SK-BR-3 breast, HEK-293 renal and ACHN kidney cancer cells, the methanol leaf extract displayed the stronger cytotoxic effects than hexane, chloroform and ethyl acetate extracts. Activity was however weaker than paclitaxel, used as a positive control. | [108] |
| 16 |
Planchonella obovata |
Three triterpenoid glycosides (6β-hydroxy-conyzasaponin N, mi-saponin A and ursolic acid) from the ethanol leaf extract showed moderate inhibitory activities against HL-60 leukemia cells with IC50 values of 16.9, 15.5 and 12.7 µM, respectively. | [109] |
| 17 |
Pluchea indica |
The aqueous extract of leaves and roots are cytotoxic to GBM8401 glioblastoma and HeLa cervical cancer cells with 75% and 70% inhibition. Mechanisms included promotion of apoptosis, and suppression of cell proliferation, viability and migration. | [110] |
| The ethanol root extract induced apoptosis, anti-proliferation, and migration of NPC-TW 01 and NPC-TW 04 nasopharyngeal carcinoma cells. Cytotoxicity was moderate with IC50 values of 108 and 93.2 μg/mL, respectively. Mechanisms are by inducing apoptosis, up-regulating the level of p53 and Bax, and down-regulating the level of Bcl-2. | [111] | ||
| The hexane fraction of the ethanol root extract weakly inhibited proliferation and induced autophagy of U87 and GBM glioblastoma cells. IC50 values were 353 and 334 μg/mL, respectively. The fraction suppressed the proliferation of GBM cells by inducing cell cycle arrest and autophagy. | [112] | ||
| 18 |
Pongamia pinnata |
LC, a natural chalcone isolated from the root, exhibited inhibition against H292 lung cancer cells with an IC50 value of 10 μM. Cytotoxic activity involved reduction of proliferation by inducing apoptosis, and by modulating caspase-3/-9 pathway. LC also inhibited tumor growth in S180-bearing mice. | [113] |
| Pongapin and karanjin, furanoflavanoids from the seed extract, inhibited cell growth against MDA-MB-231 breast, HeLa cervical, NCI H460 and HepG2 liver cancer cells with IC50 values of 16.9−39.3 μg/mL and 47.0−63.0 μg/mL, respectively. | [114] | ||
| Pinnatin, a flavonoid isolated from the ethyl acetate fruit extract, displayed potent cytotoxicity against KKU-100 bile duct and HepG2 liver cancer cells with IC50 values of 6.0 and 9.0 μg/mL, respectively. | [115] | ||
| 19 |
Sphagneticola triloba |
Wedebicosides A–C and E, new phenolic glycosides isolated from the ethanol flower extract, inhibited MCF-7 breast, HeLa cervical and NCI-H460 lung cancer cells. IC50 of wedebicoside C was 27.2, 42.4 and 27.5 μg/mL, respectively. | [116] |
| 20 |
Spinifex littoreus |
Hexane extract of flowers moderately inhibited MCF7 breast and HepG2 liver cancer cells with IC50 values of 45.1 and 70.4 μg/mL, respectively. | [117] |
| 21 |
Talipariti tiliaceum |
HA and HI were isolated from the methanol stem wood extract. HA displayed cytotoxic activity against P388 murine leukemia and HT-29 colon cancer cells with IC50 values of 1.7 and 3.8 μg/mL, respectively. HI was only cytotoxic to P388 cells (10.2 μg/mL). | [118] |
| Among three tetracyclic triterpenoids isolated from the methanol leaf and branch extracts, the analogue of tiliacol A had potent cytotoxicity against P388 murine leukemia, K562 leukemia and HeLa cervical cancer cells with IC50 values of 11.2, 13.5 and 11.5 mmol/L, respectively. | [119] | ||
| HO A−C from the DCM stem extract exhibited cytotoxic activity against MDA-MB-231 breast, HepG2 liver and Huh-7 liver cancer cells with IC50 values ranging from 3.1−10.7 µM, 3.5−8.4 µM and 4.9−10.7 µM, respectively. | [120] | ||
| Five cadinane sesquiterpenoids isolated from the DCM stem extract displayed cytotoxic activity against HepG2 and Huh7 liver cancer cells with IC50 values ranging from 3.5−6.8 μM. | [121] | ||
| 22 |
Terminalia catappa |
The ethanol leaf extract exerted anti-metastatic effects on A549 and LLC lung cancer cells by inhibiting the expression of MMP-2 and PAI-1. Cytotoxicity against A549 and LLC cells was not significant and 14.5 µg/mL, respectively. The extract also inhibited tumor growth in LLC-bearing mice. | [122] |
| The DMSO leaf extract exerted very low anti-metastatic effects on HeLa and SiHa cervical cancer cells by inhibiting the expression of MMP-9 via the ERK1/2 pathway. | [123] | ||
| 23 |
Thespesia populnea |
Among sesquiterpenoids isolated from the DCM extract of wood and heartwood, mansonone E exhibited significant anticancer activities against MCF-7 breast (0.05 µg/mL) and HT-29 colon (0.18 µg/mL) cancer cells. (+)-Gossypol was strongly cytotoxic to HeLa cervical and KB oral squamous cancer cells with IC50 values of 0.08 and 0.04 µg/mL, respectively. | [124] |
| 24 |
Vitex trifolia |
Hexane and DCM extracts of leaf and stem displayed cytotoxicity. Strongest cytotoxic activity was DCM leaf extract against KB prostate cancer cells (1.9 µg/mL), DCM stem extract against HCT-15 colon cancer cells (1.9 µg/mL), hexane leaf extract against HCT-15 colon cancer cells (3.6 µg/mL) and hexane stem extract against HCT-15 colon cancer cells (2.8 µg/mL). | [125] |
| Against MCF-7 breast and HT-29 colon cancer cells, the methanol leaf extract showed cytotoxic activities with IC50 values of 78.8 and 77.5 µg/mL. Against WRL-68 normal liver cells, cytotoxicity of the extract was 78.3 µg/mL. | [126] | ||
| 25 |
Volkameria inermis |
Against A549 lung cancer cells, the ethanol leaf extract displayed cytotoxicity with an IC50 value of 15.6 μg/mL. | [127] |
| HWA from the methanol extract of leaves was strongly cytotoxic to HCT116 colon cancer cells with an IC50 value of 3.5 μM. | [128] | ||
| 26 |
Ximenia americana |
The aqueous leaf extract strongly inhibited MCF-7 breast and CC531 colon cancer cells, including BV173 leukemia cells with IC50 values of 1.7, 3.3 and 1.8 µg/mL, respectively. No cytotoxicity was observed against MCF-10 non-tumorigenic epithelial cells. | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




