Submitted:
30 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Dengue Virus (DENV)
1.2. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
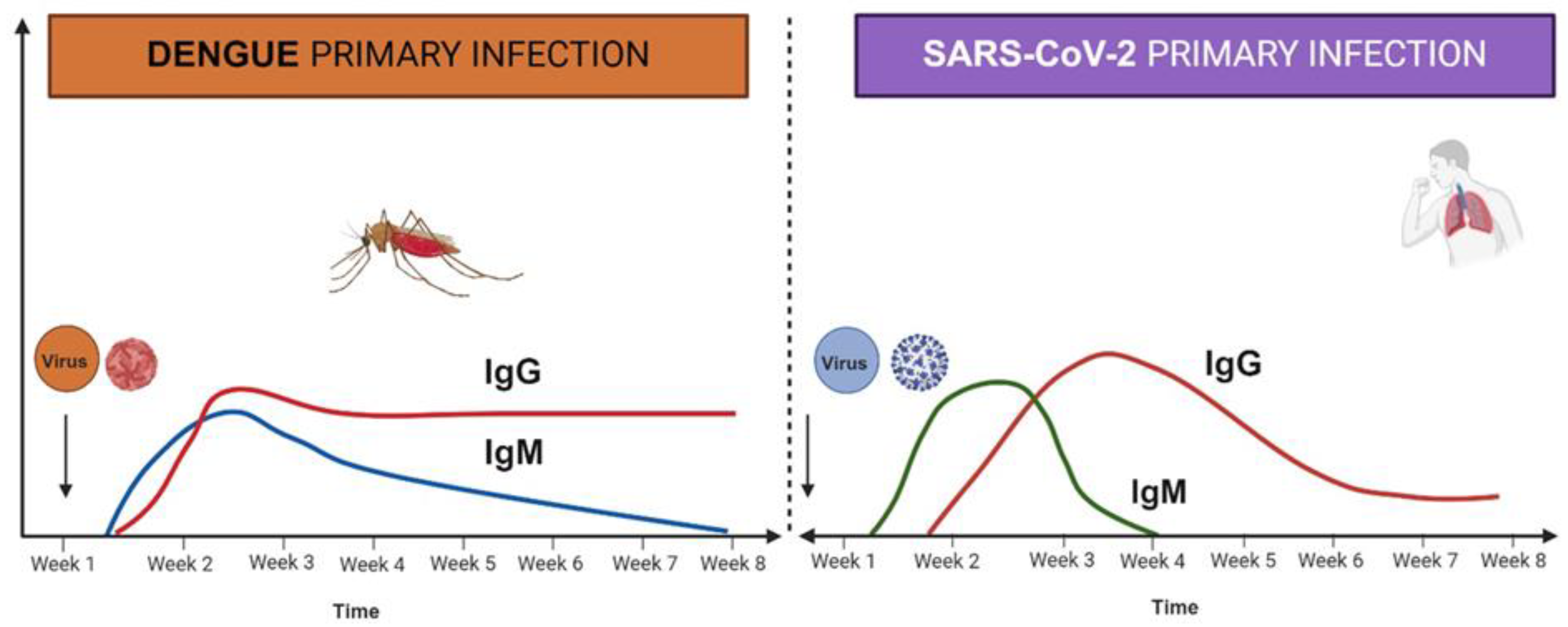
2. Role of Cytokines in DENV and SARS-CoV-2 Infection
2.1. Interferon Signal Transduction and miRNA
2.2. Cytokines in Viral Infection and Cytokine Storm
3. -Humoral Responses in Dengue and SARS-CoV-2 Infections
3.1. Antibodies in Dengue and SARS-CoV-2 Infection
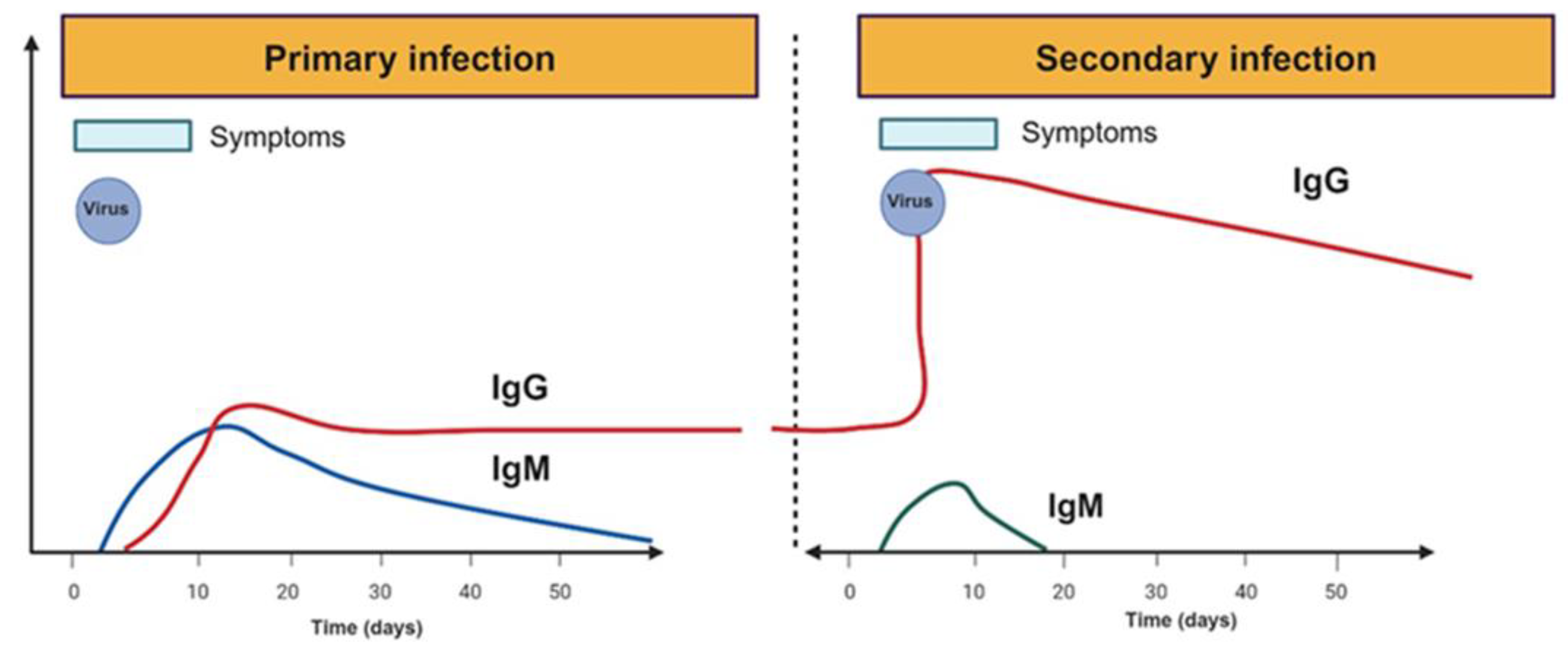
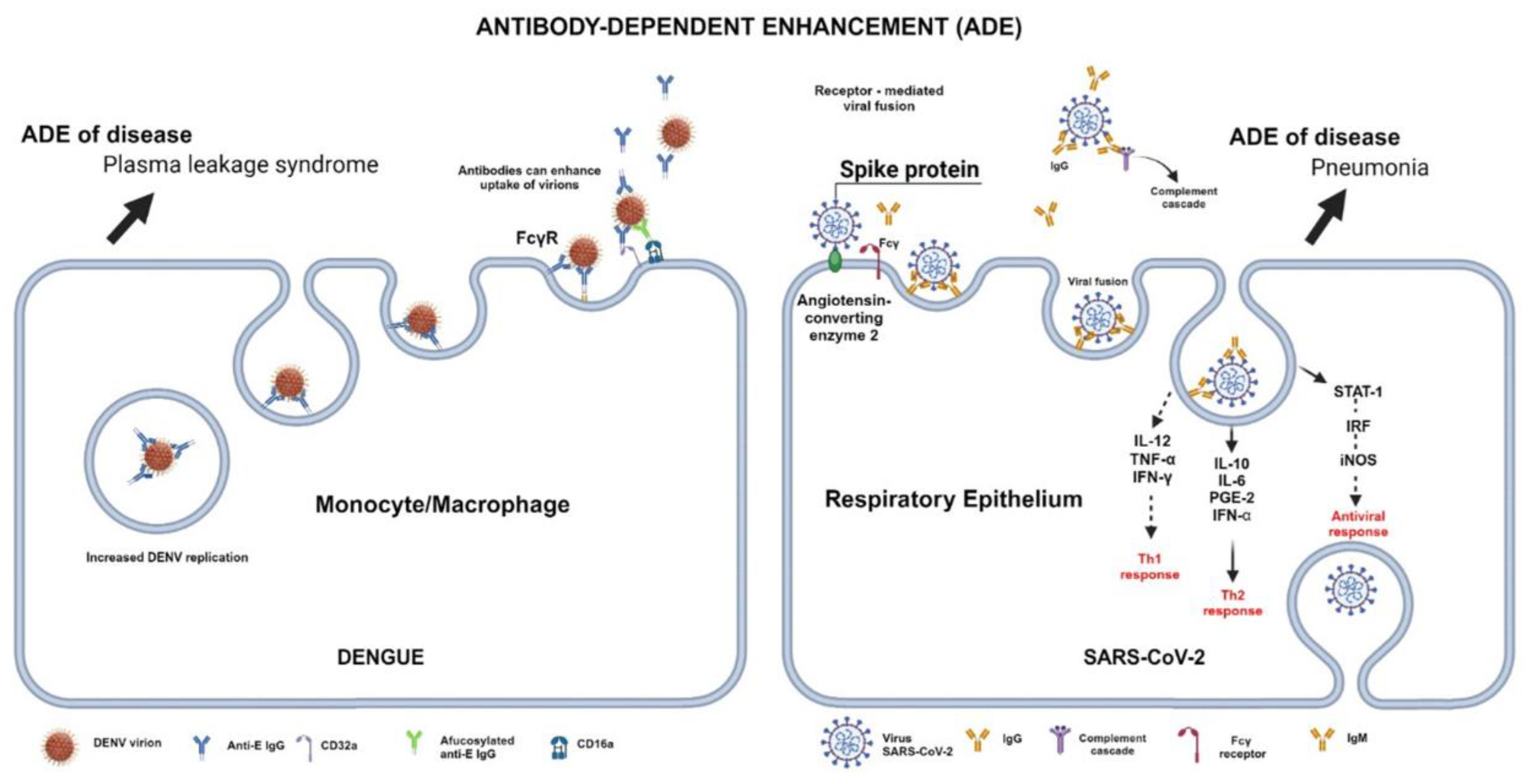
3.2. Autoantibodies after Dengue and SARS-CoV-2 Infection
3.3. Thrombocytopenia in Dengue and SARS-CoV-2 Infections
4. Long-Term Alterations after SARS-CoV-2 Infection
5. Vaccines
5.1. Dengue Vaccines
5.2. COVID-19 Vaccines
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Acknowledgments
References
- World Health Organization (WHO). Dengue and Severe Dengue: World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 09 September 2024).
- World Health Organization (WHO). Coronavirus disease (COVID-19). https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 09 September 2024).
- Hung, Y. P.; Lee, C. C.; Chen, Y. W.; Lee, J. C.; Chiu, C. W.; Hsueh, P. R.; Ko, W. C. Incidence and co-infection with COVID-19 of dengue during the COVID-19 pandemic. Formos Med Assoc 2024 Jun 14: S0929-6646(24)00283-3. [CrossRef]
- Bukhari, M. H.; Annan, E.; Haque, U.; Arango, P.; Falconar, A. K. I.; Romero-Vivas, C. M. Pre-or co-SARS-CoV-2 Infections Significantly Increase Severe Dengue Virus Disease Criteria: Implications for Clinicians. Pathogens (Basel, Switzerland), 2024; 13(7), 573. [CrossRef]
- Tang, N.; Lim, J. T.; Dickens, B.; Chiew, C.; Ng, L. C.; Chia, P. Y.; Leo, Y. S.; Lye, D. C.; Tan, K. B.; Wee, L. E. Effects of Recent Prior Dengue Infection on Risk and Severity of Subsequent SARS-CoV-2 Infection: A Retrospective Cohort Study. Open forum infectious diseases, 2024; 11(8), ofae397. [CrossRef]
- Cheng, Y. L.; Chao, C. H.; Lai, Y. C.; Hsieh, K. H.;, Wang, J. R.;, Wan, S. W.; Huang, H. J.; Chuang, Y. C.; Chuang, W. J.; Yeh, T. M. Antibodies against the SARS-CoV-2 S1-RBD cross-react with dengue virus and hinder dengue pathogenesis. Front Immunol, 2024; 13, 941923. [CrossRef]
- Pajor, M. J.; Long, B;, Liang, S. Y. Dengue: A focused review for the emergency clinician. The Am J Emerg Med 2024, 82, 82–87. [CrossRef]
- León-Figueroa, D.A.; Abanto-Urbano, S.; Olarte-Durand, M.; Nuñez-Lupaca, J.N.; Barboza, J.J.; Bonilla-Aldana, D.K.; Yrene-Cubas, R.A.; Rodriguez-Morales, A.J. COVID-19 and dengue coinfection in Latin America: A systematic review. New Microbes, New Infect. 2022 Nov-Dec; 49:101041. [CrossRef]
- Harapan, H.; Ryan, M.; Yohan, B.; Abidin, R.S.; Nainu, F.; Rakib, A.; Jahan, I.; Emran, T.B.; Ullah, I.; Panta, K.; Dhama, K.; Sasmono, R.T. COVID-19 and dengue: Double punches for dengue-endemic countries in Asia. Rev Med Virol. 2021 Mar;31(2):e2161. [CrossRef]
- Khan, M. B.; Yang, Z. S.; Lin, C. Y.; Hsu, M. C.; Urbina, A. N.; Assavalapsakul, W.; Wang, W. H.; Chen, Y. H.; Wang, S. F. Dengue overview: An updated systemic review. J Infect Public Health. 2023; 16(10), 1625–1642. [CrossRef]
- Li, H.H.; Su, M.P.; Wu, S.C.; Tsou, H.H.; Chang, M.C.; Cheng, Y.C.; Tsai, K.N.; Wang, H.W.; Chen, G.H.; et alTang CK, Chung PJ, Tsai WT, Huang LR, Yueh YA, Chen HW, Pan CY, Akbari OS, Chang HH, Yu GY, Marshall JM, Chen CH. Mechanical transmission of dengue virus by Aedes aegypti may influence disease transmission dynamics during outbreaks. EBioMedicine. 2023 Aug; 94:104723. [CrossRef]
- Safadi, D.E.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses. 2023 Jan 27;15(2):364. [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Hurtado-Monzón, A.M.; Farfan-Morales, C.N.; Cervantes-Salazar, M.; et al. Isolation and characterization of exosomes released from mosquito cells infected with dengue virus. Virus Res. 2019 Jun; 266:1-14. [CrossRef]
- Vedpathak, S.; Sharma, A.; Palkar, S.; Bhatt, V.R.; Patil, V.C.; Kakrani, A.L. et al. Platelet-derived exosomes disrupt endothelial cell monolayer integrity and enhance vascular inflammation in dengue patients. Front Immunol. 2024 Jan 3; 14:1285162. [CrossRef]
- Khanam, A.; Gutiérrez-Barbosa, H.; Lyke, K.E.; Chua, J.V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses. 2022 Nov 21;14(11):2575. [CrossRef]
- Wahala, W.M.P.B.; De Silva, A.M. The Human Antibody Response to Dengue Virus Infection. Viruses 2011, 3, 2374-2395. [CrossRef]
- Imrie, A.; Meeks, J.; Gurary, A.; Sukhbaatar, M.; Truong, T. T.; Cropp, C. B.; Effler, P. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 2007 Dec;20(4):672-5.
- St. John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat Rev Immunol 2019; 19, 218–230. [CrossRef]
- Wu, Q.; Jing, Q.; Wang, X.; Yang, L.; Li, Y.; Chen, Z.; Ma, M.; Yang, Z. Kinetics of IgG Antibodies in Previous Cases of Dengue Fever-A Longitudinal Serological Survey. Int J Environ Res Public Health. 2020 Sep 9;17(18):6580.
- Sawant, J.; Patil, A.; Kurle, S. A Review: Understanding Molecular Mechanisms of Antibody-Dependent Enhancement in Viral Infections. Vaccines (Basel). 2023 Jul 14;11(7):1240. [CrossRef]
- Narayan, R.; Tripathi, S. Intrinsic ADE: The Dark Side of Antibody-Dependent Enhancement During Dengue Infection. Front Cell Infect Microbiol. 2020 Oct 2; 10:580096. [CrossRef]
- Schmid, M.A.; Diamond, M.S.; Harris, E. Dendritic cells in dengue virus infection: targets of virus replication and mediators of immunity. Front Immunol. 2014 Dec 17; 5:647. [CrossRef]
- Sinha, S., Singh, K., Ravi Kumar, Y. S., Roy, R., Phadnis, S., Meena, V., Bhattacharyya, S., Verma, B. Dengue virus pathogenesis and host molecular machineries. J Biomed Sci 2024; 31, 43. [CrossRef]
- Cabezas, S.; Bracho, G.; Aloia, A.L.; Adamson, P.J.; Bonder, C.S.; Smith, J.R.; Gordon, D.L.; Carr, J.M. Dengue Virus Induces Increased Activity of the Complement Alternative Pathway in Infected Cells. J Virol. 2018 Jun 29;92(14): e00633-18. [CrossRef]
- Jayathilaka, D.; Gomes, L.; Jeewandara, C.; Jayarathna, G.S.B.; Herath, D.; Perera, P.A.; Fernando, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; Malavige, G.N. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat Commun. 2018 Dec 7;9(1):5242. [CrossRef]
- Singh, A.; Bisht, P.; Bhattacharya, S.; Guchhait, P. Role of Platelet Cytokines in Dengue Virus Infection. Front Cell Infect Microbiol. 2020 Sep 30; 10:561366. [CrossRef]
- Muller, D.A.; Depelsenaire, A.C.; Young, P.R. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J Infect Dis. 2017 Mar 1;215(suppl_2):S89-S95. [CrossRef]
- Glasner DR, Ratnasiri K, Puerta-Guardo H, Espinosa DA, Beatty PR, Harris E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017 Nov 9;13(11):e1006673. [CrossRef]
- Chao, C.H.; Wu, W.C.; Lai, Y.C.; Tsai, P.J.; Perng, G.C.; Lin, Y.S.; Yeh, T.M. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019 Apr 22;15(4): e1007625. [CrossRef]
- Choi, Y.; Saron, W.A.; O'Neill, A.; Senanayake, M.; Wilder-Smith, A.; Rathore, A.P.; St John, A.L. NKT cells promote Th1 immune bias to dengue virus that governs long-term protective antibody dynamics. J Clin Invest. 2024 Aug 1;134(18):e169251. [CrossRef]
- Hilligan, K.L.; Namasivayam, S.; Sher. A. BCG mediated protection of the lung against experimental SARS-CoV-2 infection. Front Immunol. 2023 Sep 8;14:1232764. [CrossRef]
- Puc, I.; Jain, H.; Odat, R.M.; Hussein, A.M.; Dey, D.; Ahmed, M.; Jain, J.; Goyal, A.; Ratnani, T.; Idrees, M.; Prajjwal, P.; Passey, S.; Yadav, R. Efficacy and outcomes of BCG re-vaccination in COVID-19: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Ann Med Surg (Lond). 2024 Jul 17;86(9):5439-5446. [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020 Mar 16; 24:91-98. [CrossRef]
- Yan, X.; Zhao, X.; Du, Y.; Wang, H.; Liu, L.; Wang, Q.; Liu, J.; Wei, S. Dynamics of anti-SARS-CoV-2 IgG antibody responses following breakthrough infection and the predicted protective efficacy: A longitudinal community-based population study in China. Int J Infect Dis. 2024 Aug; 145:107075. [CrossRef]
- Movsisyan, M.; Truzyan, N.; Kasparova, I.; Chopikyan, A.; Sawaqed, R.; et al. Tracking the evolution of anti-SARS-CoV-2 antibodies and long-term humoral immunity within 2 years after COVID-19 infection. Sci Rep. 2024 Jun 11;14(1):13417. [CrossRef]
- Swadźba, J.; Panek, A.; Wąsowicz, P.; Anyszek, T.; Martin, E. High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus. Vaccines 2024, 12, 471. [CrossRef]
- De Sanctis, J.B.; García, A.H.; Moreno, D.; Hajduch, M. Coronavirus infection: An immunologists' perspective. Scand J Immunol. 2021 Jun;93(6):e13043. [CrossRef]
- Jackson, C.B., Farzan, M., Chen, B. et al. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022; 23, 3–20. [CrossRef]
- Alla, D.; Alla, S.S.M.; Vempati, R.; Bhatt, H.; Sultana, Q.; Bhatt, S.; Mohsin, T.; Siddiqua, A. Dengue & COVID-19: A Comparison and the Challenges at Hand. Cureus. 2022 Nov 25;14(11):e31877. [CrossRef]
- Malavige, G.N.; Jeewandara, C.; Ogg, G.S. Dengue and COVID-19: two sides of the same coin. J Biomed Sci 2022; 29, 48 . [CrossRef]
- Wang, X.; Tang, G.; Liu, Y.; Zhang, L.; Chen, B.; Han, Y.; et al. The role of IL-6 in coronavirus, especially in COVID-19. Front Pharmacol. 2022 Nov 23; 13:1033674. [CrossRef]
- Garmendia, J.V.; García, A.H.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Autoimmunity and Immunodeficiency in Severe SARS-CoV-2 Infection and Prolonged COVID-19. Curr Issues Mol Biol. 2022 Dec 21;45(1):33-50. [CrossRef]
- Shurrab, F.M.; Al-Sadeq, D.W.; Amanullah, F.H.; Al-Absi, E.S.; Qotba, H.; Yassine, H.M.; Abu-Raddad, L.J.; Nasrallah, G.K. Low Risk of Serological Cross-Reactivity between the Dengue Virus and SARS-CoV-2-IgG Antibodies Using Advanced Detection Assays. Intervirology. 2022;65(4):224-229. [CrossRef]
- Silvestre, O.M.; Costa, L.R.; Lopes, B.V.R.; Barbosa, M.R.; Botelho, K.K.P.; et al. Previous Dengue Infection and Mortality in Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2021 Sep 7;73(5):e1219-e1221. [CrossRef]
- Castillo Ramirez, J.A.; Urcuqui-Inchima, S. Dengue Virus Control of Type I IFN Responses: A History of Manipulation and Control. J Interferon Cytokine Res. 2015 Jun;35(6):421-30. [CrossRef]
- Hoang, H.D.; Naeli, P.; Alain, T.; Jafarnejad, S.M. Mechanisms of impairment of interferon production by SARS-CoV-2. Biochem Soc Trans. 2023 Jun 28;51(3):1047-1056. [CrossRef]
- Brzoska, J.; von Eick, H.; Hündgen, M. (). Interferons in COVID-19: missed opportunities to prove efficacy in clinical phase III trials? Front. Med. 2023; 10, 1198576. [CrossRef]
- Su, Y.; Lin, T.; Liu, C.; Cheng, C.; Han, X., Jiang, X. microRNAs, the Link Between Dengue Virus and the Host Genome. Front Microbiol. 2021 Aug 11;12:714409. [CrossRef]
- Limothai, U.; Jantarangsi, N.; Suphavejkornkij, N.; Tachaboon, S.; Dinhuzen, J.; Chaisuriyong, W.; Trongkamolchai, S.; Wanpaisitkul, M.; Chulapornsiri, C.; Tiawilai, A.; Tiawilai, T.; Tantawichien, T.; Thisyakorn, U.; Srisawat, N. Discovery and validation of circulating miRNAs for the clinical prognosis of severe dengue. PLoS Negl Trop Dis. 2022 Oct 17;16(10):e0010836. [CrossRef]
- De Sanctis, J.B.; García, A.; Garmendia, J. V.; Moreno, D.; Hajduch, M.; Radzioch, D. Importance of miRNA in SARS-CoV-2 infection. Gac. Med. Caracas 2020; 128 (Suppl 1) S17-S22. [CrossRef]
- Ergün, S.; Sankaranarayanan, R.; Petrović, N. Clinically informative microRNAs for SARS-CoV-2 infection. Epigenomics. 2023 Jul;15(13):705-716. [CrossRef]
- Ho, T.C.; Yen, K.L.; Vats, A.; Tsai, J.J.; Chen, P.L.; Chien, Y.W.; Lo, Y.C.; Perng, G.C. Cytokine Signature of Dengue Patients at Different Severity of the Disease. Int J Mol Sci. 2021 Mar 12;22(6):2879. [CrossRef]
- Bonny, T.S.; Patel, E.U.; Zhu, X.; Bloch, E.M.; Grabowski, M.K.; Abraham, A.G.; Abraham, A.G.; Littlefield, K.; Shrestha, R.; Benner, S.E., et. Al. Cytokine and Chemokine Levels in Coronavirus Disease 2019 Convalescent Plasma. Open Forum Infect Dis. 2020 Nov 26;8(2): ofaa574. [CrossRef]
- Batista MA, Calvo-Fortes F, Silveira-Nunes G, Camatta GC, Speziali E, Turroni S, Teixeira-Carvalho A, Martins-Filho OA, Neretti N, Maioli TU, Santos RR, Brigidi P, Franceschi C, Faria AMC. Inflammaging in Endemic Areas for Infectious Diseases. Front Immunol. 2020 Nov 12;11:579972. [CrossRef]
- Ubah, C.S.; Kearney, G.D.; Pokhrel, L.R. Asthma May Not be a Potential Risk Factor for Severe COVID-19 Illness: A Scoping Review. Environ Health Insights. 2024 Jan 3; 18:11786302231221925. [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L. et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021 Jul;6:100122. [CrossRef]
- Dayarathna, S.; Jeewandara, C.; Gomes, L.; Somathilaka, G.; Jayathilaka, D. et al. Similarities and differences between the 'cytokine storms' in acute dengue and COVID-19. Sci Rep. 2020 Nov 16;10(1):19839. [CrossRef]
- Bhatt, P.; Varma, M.; Sood, V.; Ambikan, A.; Jayaram, A.; Babu, N.; Gupta, S.; Mukhopadhyay, C.; Neogi, U. Temporal cytokine storm dynamics in dengue infection predicts severity. Virus research, 2024; 341, 199306. [CrossRef]
- Zhang, J. Immune responses in COVID-19 patients: Insights into cytokine storms and adaptive immunity kinetics. Heliyon, 2024; 10(14), e34577. [CrossRef]
- Reis, G.; Moreira Silva, E. A. S.; Medeiros Silva, D. C.; Thabane, L.; Campos, V. H. S.; Ferreira, T. S.; Santos, C. V. Q.; Nogueira, A. M. R.; Almeida, A. P. F. G.; et al. Early Treatment with Pegylated Interferon Lambda for Covid-19. The New England J Med, 2023; 388(6), 518–528. [CrossRef]
- Palma-Ocampo, H. K.; Flores-Alonso, J. C.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Flores-Mendoza, L.; Herrera-Camacho, I.; Rosas-Murrieta, N. H.; Santos-López, G. Interferon lambda inhibits dengue virus replication in epithelial cells. Virology journal, 2015; 12, 150. [CrossRef]
- Sanchez-Vargas, L.A.; Anderson, K.B.; Srikiatkhachorn, A.; Currier, J.R.; Friberg, H.; Endy, T.P.; Fernandez, S.; Mathew, A.; Rothman, AL. Longitudinal Analysis of Dengue Virus-Specific Memory T Cell Responses and Their Association With Clinical Outcome in Subsequent DENV Infection. Front Immunol. 2021 Jul 28;12:710300. [CrossRef]
- Sánchez-Vargas, L. A.; Kounlavouth, S.; Smith, M. L.; Anderson, K. B.; Srikiatkhachorn, A.; Ellison, D. W.; Currier, J. R.; Endy, T. P.; Mathew, A.; Rothman, A. L. Longitudinal Analysis of Memory B and T Cell Responses to Dengue Virus in a 5-Year Prospective Cohort Study in Thailand. Front Immunol, 2019; 10, 1359. [CrossRef]
- Ramu, S. T.; Dissanayake, M.; Jeewandara, C.; Bary, F.; Harvie, M.; Gomes, L.; Wijesinghe, A.; Ariyaratne, D.; Ogg, G. S.; Malavige, G. N. Antibody and memory B cell responses to the dengue virus NS1 antigen in individuals with varying severity of past infection. Immunology, 2023; 170(1), 47–59. [CrossRef]
- Nakayama, E.E.; Shioda, T. SARS-CoV-2 Related Antibody-Dependent Enhancement Phenomena In Vitro and In Vivo. Microorganisms 2023, 11, 1015. [CrossRef]
- Wang, S.; Wang, J.; Yu, X.; Jiang, W.; Chen, S.; Wang, R.; Wang, M.; Jiao, S.; Yang, Y.; Wang, W, et al. Antibody-dependent enhancement (ADE) of SARS-CoV-2 pseudoviral infection requires FcγRIIB and virus-antibody complex with bivalent interaction. Commun Biol 2022; 5, 262. [CrossRef]
- Thomas, S.; Smatti, M. K.; Alsulaiti, H.; Zedan, H. T.; Eid, A. H.; Hssain, A. A.; Abu Raddad, L. J.; Gentilcore, G.; Ouhtit, A.; Althani, A. A. et al. Antibody-dependent enhancement (ADE) of SARS-CoV-2 in patients exposed to MERS-CoV and SARS-CoV-2 antigens. J Med Virol, 2024; 96(5), e29628. [CrossRef]
- Lechuga, G. C.; Temerozo, J. R.; Napoleão-Pêgo, P.; Carvalho, J. P. R. S.; Gomes, L. R.; Bou-Habib, D. C., Morel, C. M., Provance, D. W., Jr, Souza, T. M. L., & De-Simone, S. G. (2024). Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein. International journal of molecular sciences, 25(15), 8180. [CrossRef]
- Ghorai, T.; Sarkar, A.; Roy, A.; Bhowmick, B.; Nayak, D.; Das, S. Role of autoantibodies in the mechanisms of dengue pathogenesis and its progression: a comprehensive review. Arch Microbiol 2024; 206, 214. [CrossRef]
- Shih, H.I.; Chi, C.Y.; Tsai, P.F.; Wang, Y.P.; Chien, Y.W. Re-examination of the risk of autoimmune diseases after dengue virus infection: A population-based cohort study. PLoS Negl Trop Dis. 2023 Mar 7;17(3):e0011127. [CrossRef]
- Chuang, Y.C.; Lin, Y.S.; Liu, H.S.; Yeh, T.M. Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis. J Virol. 2014 Dec;88(23):13759-68. [CrossRef]
- Bastard, P.; Rosen, L. B.; Zhang, Q.; Michailidis, E.; Hoffmann, H. H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, N.Y.), 2020; 370(6515), eabd4585. [CrossRef]
- Crow, Y. J.; Casanova, J. L. Human life within a narrow range: The lethal ups and downs of type I interferons. Science immunology, 2024; 9(97), eadm8185. [CrossRef]
- Zhang, Q.; Kisand, K.; Feng, Y.; Rinchai, D.; Jouanguy, E.; Cobat, A.; Casanova, J. L.; Zhang, S. Y. In search of a function for human type III interferons: insights from inherited and acquired deficits. Curr Opin Immunol, 2024; 87, 102427. [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Signorini, S.G.; Serana, F.; Tiecco, G.; Imberti, L. Autoantibodies to Interferons in Infectious Diseases. Viruses. 2023 May 22;15(5):1215. [CrossRef]
- Busnadiego, I.; Abela, I. A.; Frey, P. M.; Hofmaenner, D. A.; Scheier, T. C.; Schuepbach, R. A.; Buehler, P. K.; Brugger, S. D.; Hale, B. G. Critically ill COVID-19 patients with neutralizing autoantibodies against type I interferons have increased risk of herpesvirus disease. PLoS biology, 2022; 20(7), e3001709. [CrossRef]
- Achleitner, M.; Mair, N. K.; Dänhardt, J.; Kardashi, R.; Puhan, M. A.; Abela, I. A.; Toepfner, N.; de With, K.; Kanczkowski, W.; Jarzebska, N.; et al. Absence of Type I Interferon Autoantibodies or Significant Interferon Signature Alterations in Adults With Post-COVID-19 Syndrome. Open forum infectious diseases, 2023; 11(1), ofad641. [CrossRef]
- Fernbach, S.; Mair, N. K.; Abela, I. A.; Groen, K.; Kuratli, R.; Lork, M.; Thorball, C. W.; Bernasconi, E.; Filippidis, P.; Leuzinger, K.; et al. Loss of tolerance precedes triggering and lifelong persistence of pathogenic type I interferon autoantibodies. J. Exp. Med, 2024; 221(9), e20240365. [CrossRef]
- Muri, J.; Cecchinato, V.; Cavalli, A.; Shanbhag, A. A.; Matkovic, M.; Biggiogero, M.; Maida, P. A.; Moritz, J.; Toscano, C.; Ghovehoud, E.; et al. Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course. Nature Immunol., 2023; 24(4), 604–611. [CrossRef]
- Chen, P.K.; Yeo, K.J.; Chang, S.H.; Liao, T.L.; Chou, C.H.; Lan, J.L.; Chang, C.K.; Chen, D.Y. The detectable anti-interferon-γ autoantibodies in COVID-19 patients may be associated with disease severity. Virol J. 2023 Feb 21;20(1):33. [CrossRef]
- Akbari, A.; Hadizadeh, A.; Amiri, M.; Najafi, N. N.; Shahriari, Z.; Jamialahmadi, T.; Sahebkar, A. Role of autoantibodies targeting interferon type 1 in COVID-19 severity: A systematic review and meta-analysis. J Trans Autoimm, 2023; 7, 100219. [CrossRef]
- Shih, H.P.; Ding, J.Y.; Sotolongo Bellón, J.; Lo, Y.F.; Chung, P.H.; Ting, H.T.; Peng, J.J.; Wu, T.Y.; Lin, C.H.; Lo, C.C.; et al. Pathogenic autoantibodies to IFN-γ act through the impedance of receptor assembly and Fc-mediated response. J Exp Med. 2022 Sep 5;219(9):e20212126. [CrossRef]
- Quirino-Teixeira, A. C.; Andrade, F. B.; Pinheiro, M. B. M.; Rozini, S. V.; Hottz, E. D. Platelets in dengue infection: more than a numbers game. Platelets, 2022; 33(2), 176–183. [CrossRef]
- Tsai, C.L.; Sun, D.S.; Su, M.T.; Lien, T.S.; Chen, Y.H.; Lin, C.Y.; Huang, C.H.; King, C.C.; Li, C.R.; Chen, T.H.; Chiu, Y.H.; Lu, C.C.; Chang, H.H. Suppressed humoral immunity is associated with dengue nonstructural protein NS1-elicited anti-death receptor antibody fractions in mice. Sci Rep. 2020 Apr 14;10(1):6294. [CrossRef]
- Vo, H.T.M.; Duong, V.; Ly, S.; Li, Q.Z.; Dussart, P.; Cantaert, T. Autoantibody Profiling in Plasma of Dengue Virus-Infected Individuals. Pathogens. 2020 Dec 18;9(12):1060. [CrossRef]
- Sanchez-Vargas, L. A.; Mathew, A.; Salje, H.; Sousa, D.; Casale, N. A.; Farmer, A.; Buddhari, D.; Anderson, K.; Iamsirithaworn, S.; Kaewhiran, S.; Friberg, H.; Currier, J. R.; Rothman, A. L. (). Protective Role of NS1-Specific Antibodies in the Immune Response to Dengue Virus through Antibody-Dependent Cellular Cytotoxicity. The J Inf. Dis. 2024; jiae137. Advanced online publication. [CrossRef]
- Fagyas. M.; Nagy, B. Jr.; Ráduly, A.P.; Mányiné, I.S.; Mártha, L.; Erdősi, G.; Sipka, S. Jr.; Enyedi, E.; Szabó, A.Á.; et al. The majority of severe COVID-19 patients develop anti-cardiac autoantibodies. Geroscience. 2022 Oct;44(5):2347-2360. [CrossRef]
- Hallmann, E.; Sikora, D.; Poniedziałek, B.; Szymański, K.; Kondratiuk, K.; Żurawski, J.; Brydak, L.; Rzymski, P. IgG autoantibodies against ACE2 in SARS-CoV-2 infected patients. J Med Virol. 2023 Jan;95(1):e28273. [CrossRef]
- Liu, Q.; Miao, H.; Li, S.; Zhang, P.; Gerber, G.F.; Follmann, D.; Ji, H.; Zeger, S.L.; Chertow, D.S.; Quinn, T.C.; et al. Anti-PF4 antibodies associated with disease severity in COVID-19. Proc Natl Acad Sci U S A. 2022 Nov 22;119(47):e2213361119. [CrossRef]
- Burbelo, P.D.; Castagnoli, R.; Shimizu, C.; Delmonte, O.M.; Dobbs, K.; Discepolo, V.; Lo Vecchio, A.; Guarino, A.; Licciardi, F.; et al. Autoantibodies Against Proteins Previously Associated With Autoimmunity in Adult and Pediatric Patients With COVID-19 and Children With MIS-C. Front Immunol. 2022 Mar 11;13:841126. [CrossRef]
- Sinnberg, T.; Lichtensteiger, C.; Ali, O.H.; Pop, O.T.; Jochum, A.K.; Risch, L.; Brugger, S.D.; Velic, A.; Bomze, D.; Kohler, P.; et al. Pulmonary Surfactant Proteins Are Inhibited by Immunoglobulin A Autoantibodies in Severe COVID-19. Am J Respir Crit Care Med. 2023 Jan 1;207(1):38-49. [CrossRef]
- Tang, K.T.; Hsu, B.C.; Chen, D.Y. Autoimmune and Rheumatic Manifestations Associated With COVID-19 in Adults: An Updated Systematic Review. Front Immunol. 2021 Mar 12;12:645013. [CrossRef]
- Hileman, C. O.; Malakooti, S. K.; Patil, N.; Singer, N. G.; McComsey, G. A. New-onset autoimmune disease after COVID-19. Front Immunol, 2024; 15, 1337406. [CrossRef]
- Damoiseaux, J.; Dotan, A.; Fritzler, M.J.; Bogdanos, D.P.; Meroni, P.L.; et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun Rev. 2022 Mar;21(3):103012. [CrossRef]
- Noordermeer, T.; Schutgens, R. E. G.; Visser, C.; Rademaker, E.; de Maat, M. P. M.; Jansen, A. J. G.; Limper, M.; et al. Lupus anticoagulant associates with thrombosis in patients with COVID-19 admitted to intensive care units: A retrospective cohort study. Res Pract Thromb Haemos, 2022; 6(6), e12809. [CrossRef]
- Emmenegger, M.; Kumar, S.S.; Emmenegger, V.; Malinauskas, T.; Buettner, T.; Rose, L.; et al. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins. PLoS Pathog. 2021 Dec 3;17(12):e1010118. [CrossRef]
- Cabral-Marques, O.; Halpert, G.; Schimke, L. F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G. C.; Freire, P. P.; Filgueiras, I. S.; et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nature Comm., 2022; 13(1), 1220. [CrossRef]
- Rossini, A.; Cassibba, S.; Perticone, F.; Benatti, S.V.; Venturelli, S.; Carioli, G.; Ghirardi, A.; Rizzi, M.; Barbui, T.; Trevisan, R.; Ippolito, S. Increased prevalence of autoimmune thyroid disease after COVID-19: A single-center, prospective study. Front Endocrinol (Lausanne). 2023 Mar 8;14:1126683. [CrossRef]
- Islam, A.; Cockcroft, C.; Elshazly, S.; Ahmed, J.; Joyce, K.; Mahfuz, H.; Islam, T.; Rashid, H.; Laher, I. Coagulopathy of Dengue and COVID-19: Clinical Considerations. Tropical medicine and infectious disease, 2022; 7(9), 210. [CrossRef]
- Obeagu, E.I.; Obeagu, G.U.; Aja, P.M.; Okoroiwu, G.I.A.; Ubosi. N.I.; Pius, T.; Ashiru, M.; Akaba, K.; Adias, T.C. Soluble platelet selectin and platelets in COVID-19: a multifaceted connection. Ann Med Surg (Lond). 2024 Jun 20;86(8):4634-4642. [CrossRef]
- Modhiran, N.; Watterson, D.; Blumenthal, A.; Baxter, A.G.; Young, P.R.; Stacey, K.J. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol Cell Biol. 2017 May;95(5):491-495. [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia Is Associated with Severe Coronavirus Disease 2019 (COVID-19) Infections: A Meta-Analysis. Clin. Chim. Acta. 2020; 506:145–148. [CrossRef]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020 Jun;99(6):1205-1208. [CrossRef]
- de Azeredo, E.L.; Monteiro, R.Q.; de-Oliveira Pinto, L.M. Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediators Inflamm. 2015; 2015:313842. [CrossRef]
- Putintseva, E.; Vega, G.; Fernández, L. Alterations in thrombopoiesis in patients with thrombocytopenia produced by dengue hemorrhagic fever. Nouv Rev Fr Hematol 1978. 1986;28(5):269-73.
- Guo, L.; Zhang, Q.; Gu, X.; Ren, L.; Huang, T.; Li, Y.; Zhang, H.; et al. Durability and cross-reactive immune memory to SARS-CoV-2 in individuals 2 years after recovery from COVID-19: a longitudinal cohort study. Lancet Microbe. 2024 Jan;5(1): e24-e33. [CrossRef]
- Carabelli, A. M.; Peacock, T. P.; Thorne, L. G.; Harvey, W. T.; Hughes, J.; COVID-19 Genomics UK Consortium, Peacock, S. J.; Barclay, W. S.; de Silva, T. I.; Towers, G. J.; Robertson, D. L. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nature Rev Microbiol, 2023, 21(3), 162–177. [CrossRef]
- Sharma, C.; Bayry, J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol 2023; 19, 399–400. [CrossRef]
- Onofrio, L.I.; Marin, C.; Dutto, J.; Brugo, M.B.; Baigorri, R.E.; Bossio, S.N.; et al. COVID-19 patients display changes in lymphocyte subsets with a higher frequency of dysfunctional CD8lo T cells associated with disease severity. Front Immunol. 2023 Sep 21; 14:1223730. [CrossRef]
- Bobcakova, A.; Barnova, M.; Vysehradsky, R.; Petriskova, J.; Kocan, I.; Diamant, Z.; Jesenak, M. Activated CD8+CD38+ Cells Are Associated with Worse Clinical Outcome in Hospitalized COVID-19 Patients. Front Immunol. 2022 Mar 14; 13:861666. [CrossRef]
- Gil-Bescós, R.; Ostiz, A.; Zalba, S.; Tamayo, I.; Bandrés, E.; Rojas-de-Miguel, E.; Redondo, M.; Zabalza, A.; Ramírez, N. Potency assessment of IFNγ-producing SARS-CoV-2-specific T cells from COVID-19 convalescent subjects. Life Sci Alliance. 2023 Mar 20;6(6):e202201759. [CrossRef]
- Chen, M.; Venturi, V.; Munier, C. M. L. Dissecting the Protective Effect of CD8+ T Cells in Response to SARS-CoV-2 mRNA Vaccination and the Potential Link with Lymph Node CD8+ T Cells. Biology, 2023; 12(7), 1035. [CrossRef]
- Du, J.; Wei, L.; Li, G.; Hua, M.; Sun, Y.; Wang, D.; et al. Persistent High Percentage of HLA-DR+CD38high CD8+ T Cells Associated With Immune Disorder and Disease Severity of COVID-19. Front Immunol. 2021 Sep 9; 12:735125. [CrossRef]
- Fan, X.; Song, J. W.; Cao, W. J.; Zhou, M. J.; Yang, T.; et al. T-Cell Epitope Mapping of SARS-CoV-2 Reveals Coordinated IFN-γ Production and Clonal Expansion of T Cells Facilitates Recovery from COVID-19. Viruses, 2024; 16(7), 1006. [CrossRef]
- Gonçalves Pereira, M. H.; Figueiredo, M. M.; Queiroz, C. P.; Magalhães, T. V. B.; et al. T-cells producing multiple combinations of IFNγ, TNF and IL10 are associated with mild forms of dengue infection. Immunology, 2020; 160(1), 90–102. [CrossRef]
- Zhao, J.; Xu, X.; Gao, Y.; Yu, Y.; Li, C. Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19. Int J Mol Sci. 2023 Sep 15;24(18):14133. [CrossRef]
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023 Mar;21(3):133-146. [CrossRef]
- da Silva, R.; Vallinoto, A. C. R.; Dos Santos, E. J. M. The Silent Syndrome of Long COVID and Gaps in Scientific Knowledge: A Narrative Review. Viruses, 2024; 16(8), 1256. [CrossRef]
- Tully, D.; Griffiths, C.L. Dengvaxia: the world's first vaccine for prevention of secondary dengue. Ther Adv Vaccines Immunother. 2021 May 17; 9:25151355211015839. [CrossRef]
- Thomas, S.J. Is new dengue vaccine efficacy data a relief or cause for concern? NPJ Vaccines. 2023 Apr 15;8(1):55. [CrossRef]
- López-Medina, E.; Biswal, S.; Saez-Llorens, X.; Borja-Tabora, C.; et al. Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. J Infect Dis. 2022 May 4;225(9):1521-1532. [CrossRef]
- Flacco, M.E.; Bianconi, A.; Cioni, G.; Fiore, M.; Calò, G.L.; Imperiali, G.; Orazi, V.; Tiseo, M.; Troia, A.; Rosso, A.; et al. Immunogenicity, Safety and Efficacy of the Dengue Vaccine TAK-003: A Meta-Analysis. Vaccines 2024, 12, 770. [CrossRef]
- Angelin, M.; Sjölin, J.; Kahn, F.; Ljunghill Hedberg, A.; et al. (). Qdenga® - A promising dengue fever vaccine; can it be recommended to non-immune travelers?. Travel med infec dis, 2023; 54, 102598. [CrossRef]
- Kariyawasam, R.; Lachman, M.; Mansuri, S.; Chakrabarti, S.; Boggild, A.K. A dengue vaccine whirlwind update. Ther Adv Infect Dis. 2023 Apr 20; 10:20499361231167274. [CrossRef]
- Young, A. T cells in SARS-CoV-2 infection and vaccination. Ther Adv Vaccines Immunother. 2022 Aug 24; 10:25151355221115011. [CrossRef]
- Najimi, N.; Kadi, C.; Elmtili, N.; Seghrouchni, F.; Bakri, Y. Unravelling humoral immunity in SARS-CoV-2: Insights from infection and vaccination. Human antibodies, 2024; 32(3), 85–106. [CrossRef]
- Kim W. Germinal Center Response to mRNA Vaccination and Impact of Immunological Imprinting on Subsequent Vaccination. Immune network, 2024; 24(4), e28. [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021 Jun 10;384(23):2202-2211. [CrossRef]
- Ikewaki, N.; Kurosawa, G.; Levy, G.A.; Preethy, S.; Abraham, S.J.K. Antibody-dependent disease enhancement (ADE) after COVID-19 vaccination and beta-glucans as a safer strategy in management. Vaccine. 2023 Apr 6;41(15):2427-2429. [CrossRef]
| DENGUE | SARS-CoV-2 |
|---|---|
| Anti-type-I-IFN autoantibodies [75]. Other anti-cytokine autoantibodies have not been analyzed. | Anti-type-I-IFN [72,76] and anti-type II IFN autoantibodies [80] in 5 % of the population. Autoantibodies against chemokines [79]. |
| Antibodies against platelets [83]. Antibodies against endothelial cells [58]. | Autoantibodies against components of the cardiovascular system IgM and IgG [87]. Autoantibodies against ACE2 [88] and anti-heparin factor 4 [89]. |
| Antibodies against receptors expressed on B cells TACI, BCMA, and BAFFR [84]. | Autoantibodies against the lung antigen KCNRG [90] and IgA autoantibodies against pulmonary surfactant proteins B and C decrease mucus secretion [91]. |
| IgG autoantibodies against several complement pathway components, such as Factor P and Complement C4 [85]. | Autoantibodies detected: antinuclear antibodies, P-ANCA, C-ANA, anti-Ro52, anti-Ro60, rheumatoid factor, anti-citrullinated autoantibodies, SLE-associated Smith-D3 protein [92,93,94], lupus anticoagulant [95]. |
| Autoantibodies against prothrombin were correlated with platelet counts in DHF patients [85]. | Autoantibody against prothrombin [96]. |
| Autoantibodies against KU(P70/P80), histone H-3 and H-4, M2, vitronectin, MPO, Sm/RNP [85]. | Antibodies against G-protein coupled receptors and RAS [97]. |
| Protective autoantibodies against viral non-structural protein 1 (NS1) [86]. | Autoantibodies are against thyroid hormone [98]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





