Submitted:
28 September 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Statistical Analysis
2.2. Ethical Approval
3. Results
3.1. First Nations Australian Patients
3.3. Screening for Infection Prior to Initiation of b/tsDMARD Therapy
3.4. Incidence and Timing of Infection
3.5. Site and Aetiology of Infection
3.6. Clinical Course
4. Discussion
5. Conclusions
Supplementary Files
References
- Sparks, J.A.; Harrold, L.R.; Simon, T.A.; Wittstock, K.; Kelly, S.; Lozenski, K.; et al. Comparative effectiveness of treatments for rheumatoid arthritis in clinical practice: A systematic review. Semin Arthritis Rheum. 2023, 62, 152249. [Google Scholar] [PubMed]
- Singh, J.A.; Wells, G.A.; Christensen, R.; Tanjong Ghogomu, E.; Maxwell, L.; Macdonald, J.K.; Filippini, G.; Skoetz, N.; Francis, D.; Lopes, L.C.; et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011, 2011, CD008794. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L. Infections and biologic therapy in rheumatoid arthritis: our changing understanding of risk and prevention. Rheum Dis Clin North Am 2012, 38, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Cameron, C.; Noorbaloochi, S.; Cullis, T.; Tucker, M.; Christensen, R.; Ghogomu, E.T.; Coyle, D.; Clifford, T.; Tugwell, P.; et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015, 386, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Primary prophylaxis in immunocompromised adults without HIV infection Availabe online: https://www.tg.org.au (accessed on 1 December).
- McDougall, C.; Hurd, K.; Barnabe, C. Systematic review of rheumatic disease epidemiology in the indigenous populations of Canada, the United States, Australia, and New Zealand. Semin Arthritis Rheum 2017, 46, 675–686. [Google Scholar] [CrossRef]
- Davis, J.S.; Currie, B.J.; Fisher, D.A.; Huffam, S.E.; Anstey, N.M.; Price, R.N.; Krause, V.L.; Zweck, N.; Lawton, P.D.; Snelling, P.L.; et al. Prevention of opportunistic infections in immunosuppressed patients in the tropical top end of the Northern Territory. Commun Dis Intell Q Rep 2003, 27, 526–532. [Google Scholar]
- Prinsloo, C.; Smith, S.; Law, M.; Hanson, J. The Epidemiological, Clinical, and Microbiological Features of Patients with Burkholderia pseudomallei Bacteraemia-Implications for Clinical Management. Trop Med Infect Dis 2023, 8. [Google Scholar] [CrossRef]
- Smith, S.; Kennedy, B.J.; Dermedgoglou, A.; Poulgrain, S.S.; Paavola, M.P.; Minto, T.L.; Luc, M.; Liu, Y.H.; Hanson, J. A simple score to predict severe leptospirosis. PLoS Negl Trop Dis 2019, 13, e0007205. [Google Scholar] [CrossRef]
- Stewart, A.G.A.; Smith, S.; Binotto, E.; McBride, W.J.H.; Hanson, J. The epidemiology and clinical features of rickettsial diseases in North Queensland, Australia: Implications for patient identification and management. PLoS Negl Trop Dis 2019, 13, e0007583. [Google Scholar] [CrossRef]
- Paltridge, M.; Smith, S.; Traves, A.; McDermott, R.; Fang, X.; Blake, C.; Milligan, B.; D'Addona, A.; Hanson, J. Rapid Progress toward Elimination of Strongyloidiasis in North Queensland, Tropical Australia, 2000-2018. Am J Trop Med Hyg 2020, 102, 339–345. [Google Scholar] [CrossRef]
- Smith, S.; Phillips, G.E.; McBride, W.J.H.; Hanson, J. Case Report: Endemic Amebiasis in Australia: Implications for Residents, Travelers, and Clinicians. Am J Trop Med Hyg 2017, 97, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.Z.; Conway, L.; Smith, L.K.; Fairhead, L.; Der, Y.S.; Payne, L.; Binotto, E.; Smith, S.; Hanson, J. The aetiology and clinical characteristics of cryptococcal infections in Far North Queensland, tropical Australia. PLoS One 2022, 17, e0265739. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Weston, J.; Mullen, A.; Knight, T.; Simpson, G. Tuberculosis in Far North Queensland, Australia: a retrospective clinical audit. Intern Med J 2019, 49, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.M.; Smith, M.; McBride, W.J. Mycobacterium ulcerans infection in North Queensland: the 'Daintree ulcer'. ANZ J Surg 2010, 80, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Hempenstall, A.; Smith, S.; Hanson, J. Leprosy in Far North Queensland: almost gone, but not to be forgotten. Med J Aust 2019, 211, 182–183. [Google Scholar] [CrossRef]
- Fairhead, L.J.; Smith, S.; Sim, B.Z.; Stewart, A.G.A.; Stewart, J.D.; Binotto, E.; Law, M.; Hanson, J. The seasonality of infections in tropical Far North Queensland, Australia: A 21-year retrospective evaluation of the seasonal patterns of six endemic pathogens. PLOS Global Public Health 2022, 2, e0000506. [Google Scholar] [CrossRef]
- Nguyen, A.D.K.; Smith, S.; Davis, T.J.; Yarwood, T.; Hanson, J. The efficacy and safety of a shortened duration of antimicrobial therapy for Group A Streptococcus bacteraemia. Int J Infect Dis, 2022. [Google Scholar] [CrossRef]
- Gora, H.; Smith, S.; Wilson, I.; Preston-Thomas, A.; Ramsamy, N.; Hanson, J. The epidemiology and outcomes of central nervous system infections in Far North Queensland, tropical Australia; 2000-2019. PLoS One 2022, 17, e0265410. [Google Scholar] [CrossRef]
- Horwood, P.F.; McBryde, E.S.; Peniyamina, D.; Ritchie, S.A. The Indo-Papuan conduit: a biosecurity challenge for Northern Australia. Aust N Z J Public Health 2018, 42, 434–436. [Google Scholar] [CrossRef]
- Socio-Economic Indexes for Areas (SEIFA), Australia. Availabe online: https://www.abs.gov.au/statistics/people/people-and-communities/socio-economic-indexes-areas-seifa-australia/latest-release (accessed on August 30).
- Kang, K.; Chau, K.W.T.; Howell, E.; Anderson, M.; Smith, S.; Davis, T.J.; Starmer, G.; Hanson, J. The temporospatial epidemiology of rheumatic heart disease in Far North Queensland, tropical Australia 1997-2017; impact of socioeconomic status on disease burden, severity and access to care. PLoS Negl Trop Dis 2021, 15, e0008990. [Google Scholar] [CrossRef]
- Hanson, J.; Smith, S.; Stewart, J.; Horne, P.; Ramsamy, N. Melioidosis-a disease of socioeconomic disadvantage. PLoS Negl Trop Dis 2021, 15, e0009544. [Google Scholar] [CrossRef]
- Vardanega, J.; Smith, L.K.; Smith, S.; Hanson, J. Animal bite wounds and their management in tropical Australia. Int J Infect Dis 2022, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Fox, M.; Anderson, A.; Fox, P.; Webster, K.; Williams, C.; Nield, B.; Bagshaw, R.; Hempenstall, A.; Smith, S.; et al. Chronic hepatitis B in remote, tropical Australia; successes and challenges. PLoS One 2020, 15, e0238719. [Google Scholar] [CrossRef] [PubMed]
- Guthridge, I.; Smith, S.; Horne, P.; Hanson, J. Increasing prevalence of methicillin-resistant Staphylococcus aureus in remote Australian communities: implications for patients and clinicians. Pathology 2019, 51, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Smith, S.; Brooks, J.; Groch, T.; Sivalingam, S.; Curnow, V.; Carter, A.; Hargovan, S. The applicability of commonly used predictive scoring systems in Indigenous Australians with sepsis: An observational study. PLoS One 2020, 15, e0236339. [Google Scholar] [CrossRef] [PubMed]
- Basaglia, A.; Kang, K.; Wilcox, R.; Lau, A.; McKenna, K.; Smith, S.; Chau, K.W.T.; Hanson, J. The aetiology and incidence of infective endocarditis in people living with rheumatic heart disease in tropical Australia. Eur J Clin Microbiol Infect Dis 2023, 42, 1115–1123. [Google Scholar] [CrossRef]
- Taunton, C.; Hawthorn, L.; Matysek, R.; Coates, M.; Pickering, E.; Neville, J.; Hanson, J.; Smith, S.; Hempenstall, A. A low burden of severe illness: the COVID-19 Omicron outbreak in the remote Torres and Cape region of Far North Queensland. Communicable Diseases Intelligence 2023. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Zumla, A.; Ustianowski, A. Tropical diseases: definition, geographic distribution, transmission, and classification. Infect Dis Clin North Am 2012, 26, 195–205. [Google Scholar] [CrossRef]
- Fairhead, L.; Vardanega, J.; Pandey, R.; Smith, S. Polymicrobial community-acquired Acinetobacter baumannii and Burkholderia pseudomallei bacteremia: opportunistic infections with similar risk factors in northern Australia. IDCases 2020, 21, e00833. [Google Scholar] [CrossRef]
- Strangfeld, A.; Eveslage, M.; Schneider, M.; Bergerhausen, H.J.; Klopsch, T.; Zink, A.; Listing, J. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis 2011, 70, 1914–1920. [Google Scholar] [CrossRef]
- Thyagarajan, V.; Norman, H.; Alexander, K.A.; Napalkov, P.; Enger, C. Risk of mortality, fatal infection, and fatal malignancy related to use of anti-tumor necrosis factor-alpha biologics by rheumatoid arthritis patients. Semin Arthritis Rheum 2012, 42, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Salaveria, K.; Smith, S.; Liu, Y.H.; Bagshaw, R.; Ott, M.; Stewart, A.; Law, M.; Carter, A.; Hanson, J. The Applicability of Commonly Used Severity of Illness Scores to Tropical Infections in Australia. Am J Trop Med Hyg 2021, 106, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Determinants of health for First Nations people. Availabe online: https://www.aihw.gov.au/reports/australias-health/social-determinants-and-indigenous-health (accessed on 17 September).
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.M.; Martin, G.S. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med 2006, 34, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Aboriginal and Torres Strait Islander Health Performance Framework: summary report August 2024. Australian Institute of Health and Welfare: Australian Government: Canberra, 2024.
- Quinn, E.K.; Massey, P.D.; Speare, R. Communicable diseases in rural and remote Australia: the need for improved understanding and action. Rural Remote Health 2015, 15, 3371. [Google Scholar] [CrossRef]
- Doran, M.F.; Crowson, C.S.; Pond, G.R.; O'Fallon, W.M.; Gabriel, S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002, 46, 2287–2293. [Google Scholar] [CrossRef]
- Thomas, K.; Vassilopoulos, D. Infections in Patients with Rheumatoid Arthritis in the Era of Targeted Synthetic Therapies. Mediterr J Rheumatol 2020, 31, 129–136. [Google Scholar] [CrossRef]
- Galloway, J.B.; Hyrich, K.L.; Mercer, L.K.; Dixon, W.G.; Fu, B.; Ustianowski, A.P.; Watson, K.D.; Lunt, M.; Symmons, D.P.; Consortium, B.C.C.; et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011, 50, 124–131. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Chen, L.; Delzell, E.; Baddley, J.W.; Beukelman, T.; Winthrop, K.L.; Griffin, M.R.; Herrinton, L.J.; Liu, L.; Ouellet-Hellstrom, R.; et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011, 306, 2331–2339. [Google Scholar] [CrossRef]
- Bellan, M.; Scotti, L.; Ferrante, D.; Calzaducca, E.; Manfredi, G.F.; Sainaghi, P.P.; Barone-Adesi, F. Risk of Severe Infection among Rheumatoid Arthritis Patients on Biological DMARDs: A Population-Based Cohort Study. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Dixon, W.G.; Abrahamowicz, M.; Beauchamp, M.E.; Ray, D.W.; Bernatsky, S.; Suissa, S.; Sylvestre, M.P. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis 2012, 71, 1128–1133. [Google Scholar] [CrossRef]
- de Almeida, A.L.B.; Guimaraes, M.; da Costa Pinto, M.R.; Pereira, L.R.; Reis, A.; Bonfiglioli, K.R.; Louzada-Junior, P.; Giorgi, R.D.N.; de Castro, G.R.W.; Radominski, S.C.; et al. Predictors of serious infections in rheumatoid arthritis-a prospective Brazilian cohort. Adv Rheumatol 2024, 64, 23. [Google Scholar] [CrossRef] [PubMed]
- Au, K.; Reed, G.; Curtis, J.R.; Kremer, J.M.; Greenberg, J.D.; Strand, V.; Furst, D.E.; Investigators, C. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 2011, 70, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Celkys, K.; Ly, J.; Soden, M. SAT0581 Serious Infection Rates With Biological Disease Modifying Anti-Rheumatic Agents (bDMARDs) and Presdisposing Factors: A 5-Year Retrospective Review. Annals of the Rheumatic Diseases 2020, 79, 1249–1250. [Google Scholar] [CrossRef]
- Rutherford, A.I.; Subesinghe, S.; Hyrich, K.L.; Galloway, J.B. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018, 77, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Varley, C.D.; Winthrop, K.L. Long-Term Safety of Rituximab (Risks of Viral and Opportunistic Infections). Curr Rheumatol Rep 2021, 23, 74. [Google Scholar] [CrossRef]
- Gron, K.L.; Arkema, E.V.; Glintborg, B.; Mehnert, F.; Ostergaard, M.; Dreyer, L.; Norgaard, M.; Krogh, N.S.; Askling, J.; Hetland, M.L.; et al. Risk of serious infections in patients with rheumatoid arthritis treated in routine care with abatacept, rituximab and tocilizumab in Denmark and Sweden. Ann Rheum Dis 2019, 78, 320–327. [Google Scholar] [CrossRef]
- Tudesq, J.J.; Cartron, G.; Riviere, S.; Morquin, D.; Iordache, L.; Mahr, A.; Pourcher, V.; Klouche, K.; Cerutti, D.; Le Quellec, A.; et al. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun Rev 2018, 17, 115–124. [Google Scholar] [CrossRef]
- Thomson, R.; Donnan, E.; Konstantinos, A. Notification of Nontuberculous Mycobacteria: An Australian Perspective. Ann Am Thorac Soc 2017, 14, 318–323. [Google Scholar] [CrossRef]
- Hempenstall, A.; Pilot, P.; McDonald, M.; Smith, S.; Hanson, J. Community antibiotic management of skin infections in the Torres Strait. Aust J Prim Health 2023, 29, 91–98. [Google Scholar] [CrossRef]
- Townend, R.; Smith, S.; Hanson, J. Microbiological and clinical features of patients with cellulitis in tropical Australia; disease severity assessment and implications for clinical management. Am J Trop Med Hyg In press. 2024. [Google Scholar]
- Nofz, L.; Koppen, J.; De Alwis, N.; Smith, S.; Hanson, J. The microbiology of ear cultures in a high-burden setting in tropical Australia: Implications for clinicians. Clin Otolaryngol 2019, 44, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.Z.; Aaron, L.; Coulter, C.; Parkes-Smith, J.; Badrick, T.; May, K.; Armstrong, M.; Hendry, S.; Sundac, L.; Dang, L.; et al. A multi-centre retrospective study of Nocardia speciation and antimicrobial susceptibility in Queensland, Australia. Eur J Clin Microbiol Infect Dis 2023, 42, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Crump, J.A.; Stafford, R.; Glass, K.; Howden, B.P.; Kirk, M.D. Increasing incidence of invasive nontyphoidal Salmonella infections in Queensland, Australia, 2007-2016. PLoS Negl Trop Dis 2019, 13, e0007187. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, D.; Sinclair, R. Tropical and exotic dermatoses and ulcers. Aust Fam Physician 2014, 43, 604–609. [Google Scholar] [PubMed]
- Kirk, M.; Ford, L.; Glass, K.; Hall, G. Foodborne illness, Australia, circa 2000 and circa 2010. Emerg Infect Dis 2014, 20, 1857–1864. [Google Scholar] [CrossRef]
- Review of the prevention and control of Legionella pneumophila infection in Queensland; Chief Health Officer’s report; Brisbane, 2013.
- Lau, G.; Yu, M.L.; Wong, G.; Thompson, A.; Ghazinian, H.; Hou, J.L.; Piratvisuth, T.; Jia, J.D.; Mizokami, M.; Cheng, G.; et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int 2021, 15, 1031–1048. [Google Scholar] [CrossRef]
- Rodriguez, M.; Fishman, J.A. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev 2004, 17, 770–782. [Google Scholar] [CrossRef]
- Birrell, J.M.; Boyd, R.; Currie, B.J.; Anstey, N.M.; Abeyaratne, A.; Majoni, S.W.; Krause, V.L. Invasive group A streptococcal disease in the Northern Territory and the impact of melioidosis antibiotic prophylaxis. Med J Aust 2022, 217, 544–545. [Google Scholar] [CrossRef]
- Majoni, S.W.; Hughes, J.T.; Heron, B.; Currie, B.J. Trimethoprim+Sulfamethoxazole Reduces Rates of Melioidosis in High-Risk Hemodialysis Patients. Kidney Int Rep 2018, 3, 160–167. [Google Scholar] [CrossRef]
- Bryce, A.; Davison, S.; Currie, B.J.; Birrell, J.M.; Baird, R.W.; Abeyaratne, A.; Majoni, S.W.; Brewster-O'Brien, T.; Tong, S.Y.C. Lower Rates of Staphylococcus aureus Bloodstream Infection in Patients on Hemodialysis Receiving Trimethoprim-Sulfamethoxazole Melioidosis Prophylaxis. Open Forum Infect Dis 2024, 11, ofae431. [Google Scholar] [CrossRef]
- Choquet-Kastylevsky, G.; Vial, T.; Descotes, J. Allergic adverse reactions to sulfonamides. Curr Allergy Asthma Rep 2002, 2, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, H.; Kato, T.; Nishikawa, J.; Tanaka, T.; Fujimoto, N. Adverse effects of trimethoprim-sulfamethoxazole for the prophylaxis of Pneumocystis pneumonia in dermatology. J Dermatol 2021, 48, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Curtis, J.R.; Kim, M.J.; Lee, H.; Song, Y.W.; Lee, E.B. Pneumocystis pneumonia in patients with rheumatic diseases receiving prolonged, non-high-dose steroids-clinical implication of primary prophylaxis using trimethoprim-sulfamethoxazole. Arthritis Res Ther 2019, 21, 207. [Google Scholar] [CrossRef]
- Al-Quteimat, O.M.; Al-Badaineh, M.A. Methotrexate and trimethoprim-sulphamethoxazole: extremely serious and life-threatening combination. J Clin Pharm Ther 2013, 38, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Horne, P.; Rubenach, S.; Gair, R.; Stewart, J.; Fairhead, L.; Hanson, J. Increased Incidence of Melioidosis in Far North Queensland, Queensland, Australia, 1998-2019. Emerg Infect Dis 2021, 27, 3119–3123. [Google Scholar] [CrossRef]
- Herbert, D.R.; Stoltzfus, J.D.C.; Rossi, H.L.; Abraham, D. Is Strongyloides stercoralis hyperinfection induced by glucocorticoids a result of both suppressed host immunity and altered parasite genetics? Mol Biochem Parasitol 2022, 251, 111511. [Google Scholar] [CrossRef]
- Buonfrate, D.; Salas-Coronas, J.; Munoz, J.; Maruri, B.T.; Rodari, P.; Castelli, F.; Zammarchi, L.; Bianchi, L.; Gobbi, F.; Cabezas-Fernandez, T.; et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect Dis 2019, 19, 1181–1190. [Google Scholar] [CrossRef]
- Listing, J.; Strangfeld, A.; Kary, S.; Rau, R.; von Hinueber, U.; Stoyanova-Scholz, M.; Gromnica-Ihle, E.; Antoni, C.; Herzer, P.; Kekow, J.; et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005, 52, 3403–3412. [Google Scholar] [CrossRef]
- Dixon, W.G.; Symmons, D.P.; Lunt, M.; Watson, K.D.; Hyrich, K.L.; British Society for Rheumatology Biologics Register Control Centre, C.; Silman, A.J.; British Society for Rheumatology Biologics, R. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum 2007, 56, 2896–2904. [CrossRef]
- Favalli, E.G.; Maioli, G.; Caporali, R. Biologics or Janus Kinase Inhibitors in Rheumatoid Arthritis Patients Who are Insufficient Responders to Conventional Anti-Rheumatic Drugs. Drugs 2024, 84, 877–894. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Tanaka, Y.; Lee, E.B.; Wollenhaupt, J.; Al Enizi, A.; Azevedo, V.F.; Curtis, J.R. Prevention and management of herpes zoster in patients with rheumatoid arthritis and psoriatic arthritis: a clinical review. Clin Exp Rheumatol 2022, 40, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.W.T.; Smith, S.; Kang, K.; Dheda, S.; Hanson, J. Antibiotic Prophylaxis for Melioidosis in Patients Receiving Hemodialysis in the Tropics? One Size Does Not Fit All. Am J Trop Med Hyg 2018, 99, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Arauz, A.B.; Papineni, P. Histoplasmosis. Infect Dis Clin North Am 2021, 35, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, S.; Dat, V.Q.; Thanh, N.T.; Ly, V.T.; Chan, J.F.; Yuen, K.Y.; Ning, C.; Liang, H.; Li, L.; Chowdhary, A.; et al. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Glob Health 2021, 9, e1618–e1622. [Google Scholar] [CrossRef]
- Bergstrom, L.; Yocum, D.E.; Ampel, N.M.; Villanueva, I.; Lisse, J.; Gluck, O.; Tesser, J.; Posever, J.; Miller, M.; Araujo, J.; et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum 2004, 50, 1959–1966. [Google Scholar] [CrossRef]
| All n=310 | No serious infection n=236 |
Any serious infection n=74 |
Hazard ratio (95% CI) | p | |
|---|---|---|---|---|---|
| Age a | 56 (47-63) | 54 (45-63) | 58 (53-65) | 1.04 (1.01-1.06) | 0.001 b |
| Female Gender | 225 (73%) | 169 (72%) | 56 (76%) | 1.20 (0.70-2.04) | 0.51 |
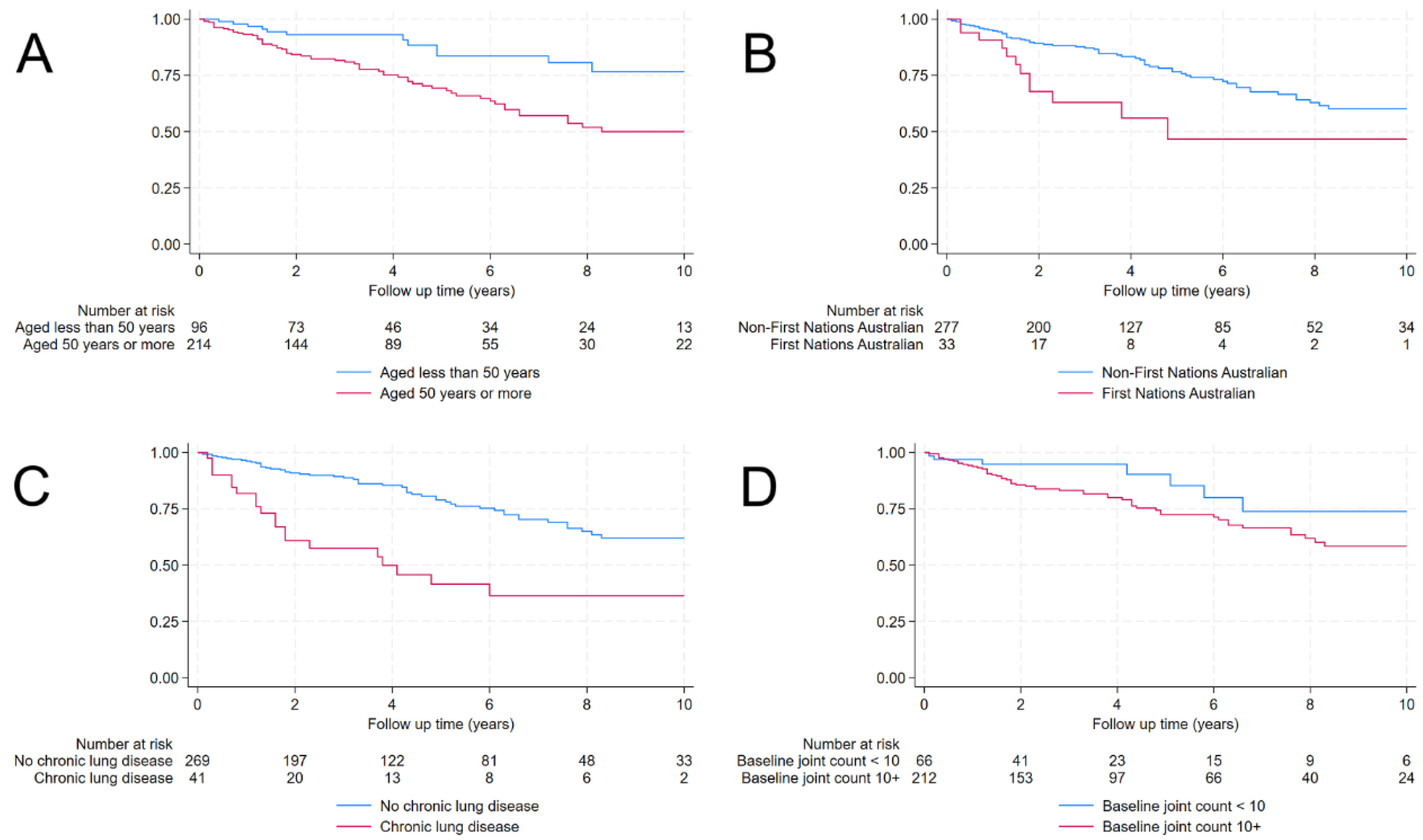
| First Nations Australian | 33 (11%) | 21 (9%) | 12 (16%) | 2.43 (1.30-4.54) | 0.005 b |
| Urban address | 180 (56%) | 141 (60%) | 39 (53%) | 0.79 (0.50-1.25) | 0.32 |
| Remote address | 59 (19%) | 41 (17%) | 18 (24%) | 1.49 (0.88-2.54) | 0.12 |
| Charlson Comorbidity Index | 2 (1-3) | 1 (1-2) | 2 (1-4) | 1.14 (1.04-1.26) | 0.005 b |
| Severe Comorbidity (≥5) | 31 (10%) | 18 (8%) | 13 (18%) | 1.73 (0.95-3.15) | 0.07 |
| Cardiac disease | 29 (9%) | 17 (7%) | 12 (16%) | 1.86 (1.00-3.46) | 0.049 b |
| Lung disease | 41 (13%) | 21 (9%) | 20 (27%) | 3.28 (1.96-5.48) | <0.001 b |
| Diabetes mellitus | 19 (6%) | 13 (6%) | 6 (8%) | 1.80 (0.78-4.15) | 0.17 |
| Neurological disease | 11 (4%) | 8 (3%) | 3 (4%) | 1.02 (0.32-3.23) | 0.98 |
| Renal disease | 3 (1%) | 2 (1%) | 1 (1%) | 1.02 (0.14-7.34) | 0.99 |
| Liver disease | 4 (1%) | 3 (1%) | 1 (1%) | 1.12 (0.16-8.07) | 0.91 |
| Seropositive disease c | 226/292 (77%) | 175/224 (78%) | 51/68 (75%) | 0.93 (0.53-1.61) | 0.79 |
| Joint count c | 24 (10-28) | 23 (8-27) | 26 (22-31) | 1.04 (1.01-1.07) | 0.005 b |
| Corticosteroids at baseline c | 119/284 (42%) | 99/220 (45%) | 20/64 (31%) | 0.85 (0.50-1.44) | 0.54 |
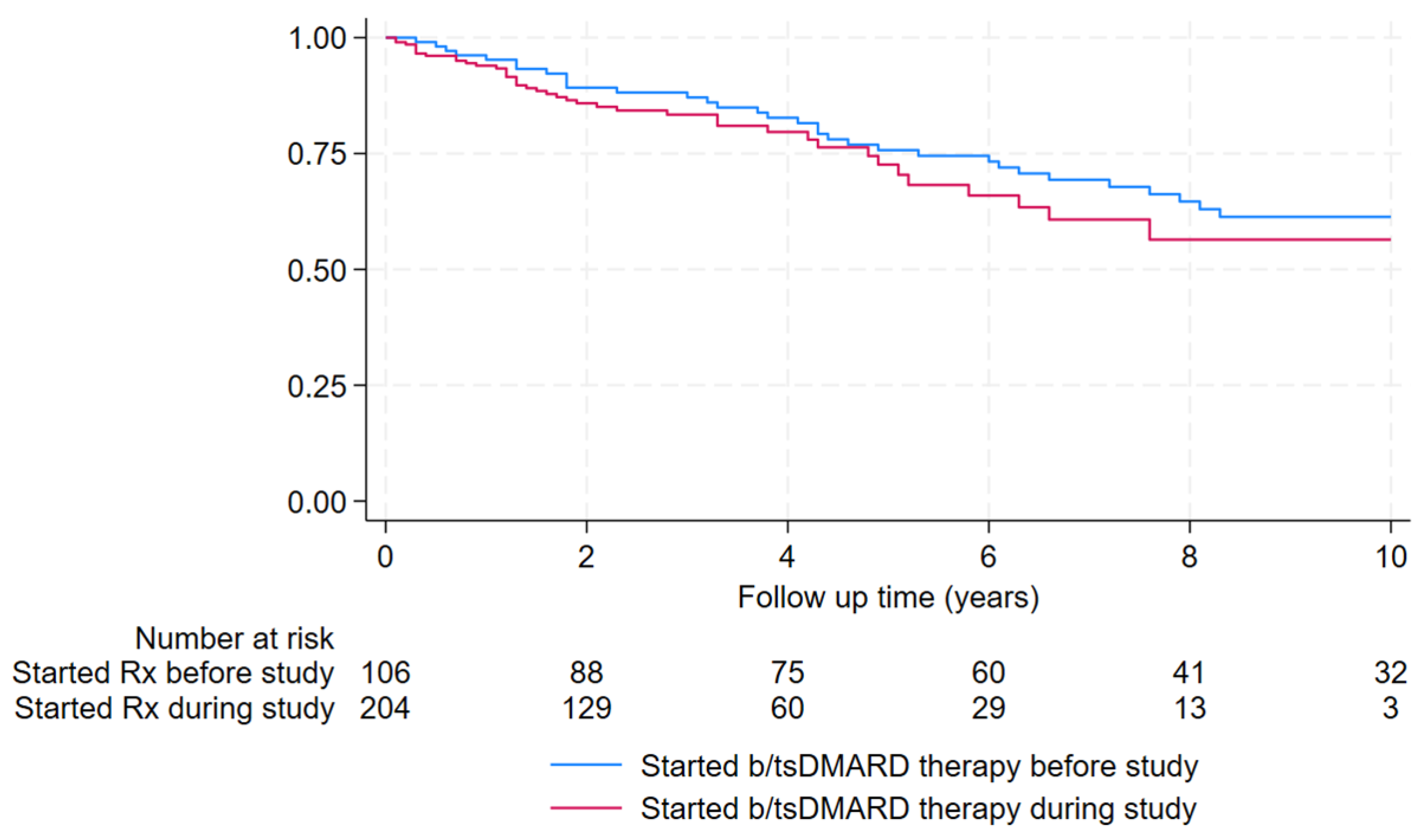
| Started b/tsDMARD during the study | 204 (66%) | 163 (69%) | 41 (55%) | 1.27 (0.79-2.05) | 0.33 |
| TNFi ever | 281 (91%) | 214 (91%) | 67 (91%) | 1.20 (0.55-2.63) | 0.64 |
| Adalimumab ever | 163 (53%) | 121 (51%) | 42 (57%) | 0.94 (0.59-1.49) | 0.78 |
| Golimumab ever | 45 (15%) | 32 (14%) | 13 (18%) | 1.49 (0.82-2.72) | 0.19 |
| Etanercept ever | 126 (41%) | 99 (42%) | 27 (36%) | 0.71 (0.44-1.14) | 0.15 |
| Certolizumab ever | 35 (11%) | 27 (11%) | 8 (11%) | 1.09 (0.52-2.28) | 0.81 |
| Infliximab ever | 17 (5%) | 12 (5%) | 5 (7%) | 0.93 (0.37-2.30) | 0.87 |
| JAKi ever | 17 (5%) | 14 (6%) | 3 (4%) | 0.80 (0.25-2.53) | 0.70 |
| Baricitinib ever | 2 (1%) | 2 (1%) | 0 | - | - |
| Tofacitinib ever | 16 (5%) | 13 (6%) | 3 (4%) | 0.84 (0.26-2.65) | 0.76 |
| Abatacept ever | 35 (11%) | 25 (11%) | 10 (14%) | 0.86 (0.44-1.68) | 0.67 |
| Tocilizumab ever | 98 (32%) | 68 (29%) | 30 (41%) | 1.10 (0.69-1.75) | 0.69 |
| Rituximab ever | 57 (18%) | 35 (15%) | 22 (30%) | 1.50 (0.91-2.47) | 0.12 |
| Age, sex | First Nations Australian | Remote Residence | Charlson comorbidity index | Clinical syndrome | Microbiological isolate | Other immunosuppression at time of admission | ICU admission | B/tsDMARD at time of admission | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 46 F | No | No | 3 | Cellulitis leading to bacteraemia and systemic infection | MRSA | HCQ & prednisone | Yes | Adalimumab | Died |
| 72 F | No | No | 6 | Cellulitis | None | LFL, HCQ & prednisone | No | Etanercept | Died |
| 66 F | No | No | 4 | IE COPD | None | Prednisone | No | Adalimumab | Died |
| 67 M | No | No | 4 | Neutropenic sepsis | None | Prednisone | No | Rituximab | Died |
| 61 M | Yes | No | 3 | Community acquired pneumonia & osteomyelitis | Burkholderia pseudomallei & Acinetobacter baumannii | MTX, HCQ | Yes | Etanercept | Survived |
| 61 F | Yes | No | 6 | Septic shock with unclear source | None | LFL & prednisone | Yes | Infliximab | Survived |
| 61 F | Yes | No | 6 | Septic shock with unclear source | None | LFL & prednisone | Yes | Certolizumab | Survived |
| 61 F | No | No | 4 | Community acquired pneumonia | MTX & prednisone | Yes | Tocilizumab | Survived | |
| 57 F | Yes | Yes | 8 | IE COPD | Streptococcus pneumoniae | MTX, HCQ & prednisone | Yes | Etanercept | Survived |
| 57 F | Yes | Yes | 8 | Urinary tract infection | Escherichia coli | MTX | Yes | Etanercept | Survived |
| 60 F | No | Yes | 7 | Hospital acquired pneumonia | Influenza A | None | Yes | Tocilizumab | Survived |
| 62 F | No | No | 4 | Colitis | Clostridioides difficile | HCQ | Yes | Tocilizumab | Survived |
| 71 F | No | No | 5 | Diverticulitis | None | HCQ & prednisone | Yes | Tocilizumab | Survived |
| 64 F | No | No | 4 | Community acquired pneumonia | Streptococcus pneumoniae | prednisone | Yes | Adalimumab | Survived |
| 47 F | No | No | 4 | Prosthetic joint infection | MSSA | MTX & prednisone | Yes | Rituximab | Survived |
| 69 F | No | No | 4 | Cholecystitis | Polymicrobial | MTX & HCQ | Yes | Tocilizumab | Survived |
| All n=147 | No ICU/death n=131 |
ICU/death n=16 |
P | |
|---|---|---|---|---|
| Age at admission | 61 (56-69) | 61 (56-69) | 61 (58-67) | 0.77 |
| Female gender | 112 (76%) | 98 (75%) | 14 (88%) | 0.36 |
| First Nations Australian | 50 (34%) | 45 (34%) | 5 (31%) | 1.0 |
| Urban address | 57 (39%) | 49 (37%) | 8 (50%) | 0.42 |
| Remote address | 53 (36%) | 50 (38%) | 3 (19%) | 0.17 |
| Seropositive rheumatoid arthritis | 106/122 (87%) | 94/110 (85%) | 12/12 (100%) | 0.36 |
| Charlson Comorbidity Index | 3 (2-5) | 3 (2-4) | 4 (4-6) | <0.001 |
| Severe comorbidity (≥5) | 38 (26%) | 31 (24%) | 7 (44%) | 0.13 |
| Other contributing factors a | 81 (55%) | 71 (54%) | 10 (63%) | 0.60 |
| On adalimumab at admission | 39 (27%) | 36 (27%) | 3 (19%) | 0.56 |
| Golimumab at admission | 8 (5%) | 8 (6%) | 0 | 0.60 |
| Etanercept at admission | 27 (18%) | 23 (18%) | 4 (25%) | 0.50 |
| Certolizumab at admission | 7 (5%) | 6 (5%) | 1 (6%) | 0.56 |
| Infliximab at admission | 4 (3%) | 3 (2%) | 1 (6%) | 0.37 |
| Tofacitinib at admission | 3 (2%) | 3 (2%) | 0 | 1.0 |
| Abatacept at admission | 2 (1%) | 2 (2%) | 0 | 1.0 |
| Tocilizumab at admission | 38 (26%) | 33 (25%) | 5 (31%) | 0.56 |
| Rituximab previously | 24 (16%) | 20 (15%) | 4 (25%) | 0.30 |
| Methotrexate at admission | 77 (52%) | 71 (54%) | 6 (38%) | 0.29 |
| Leflunomide at admission | 24 (16%) | 20 (15%) | 4 (25%) | 0.30 |
| Sulfasalazine at admission | 3 (2%) | 3 (2%) | 0 | 1.0 |
| Hydroxychloroquine at admission | 51 (35%) | 44 (34%) | 7 (44%) | 0.42 |
| Corticosteroids at admission | 44 (30%) | 33 (25%) | 11 (69%) | 0.001 |
| Prednisone dose (milligrams) at admission | 0 (0-4) | 0 (0-1) | 4 (0-5) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





