Submitted:
27 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
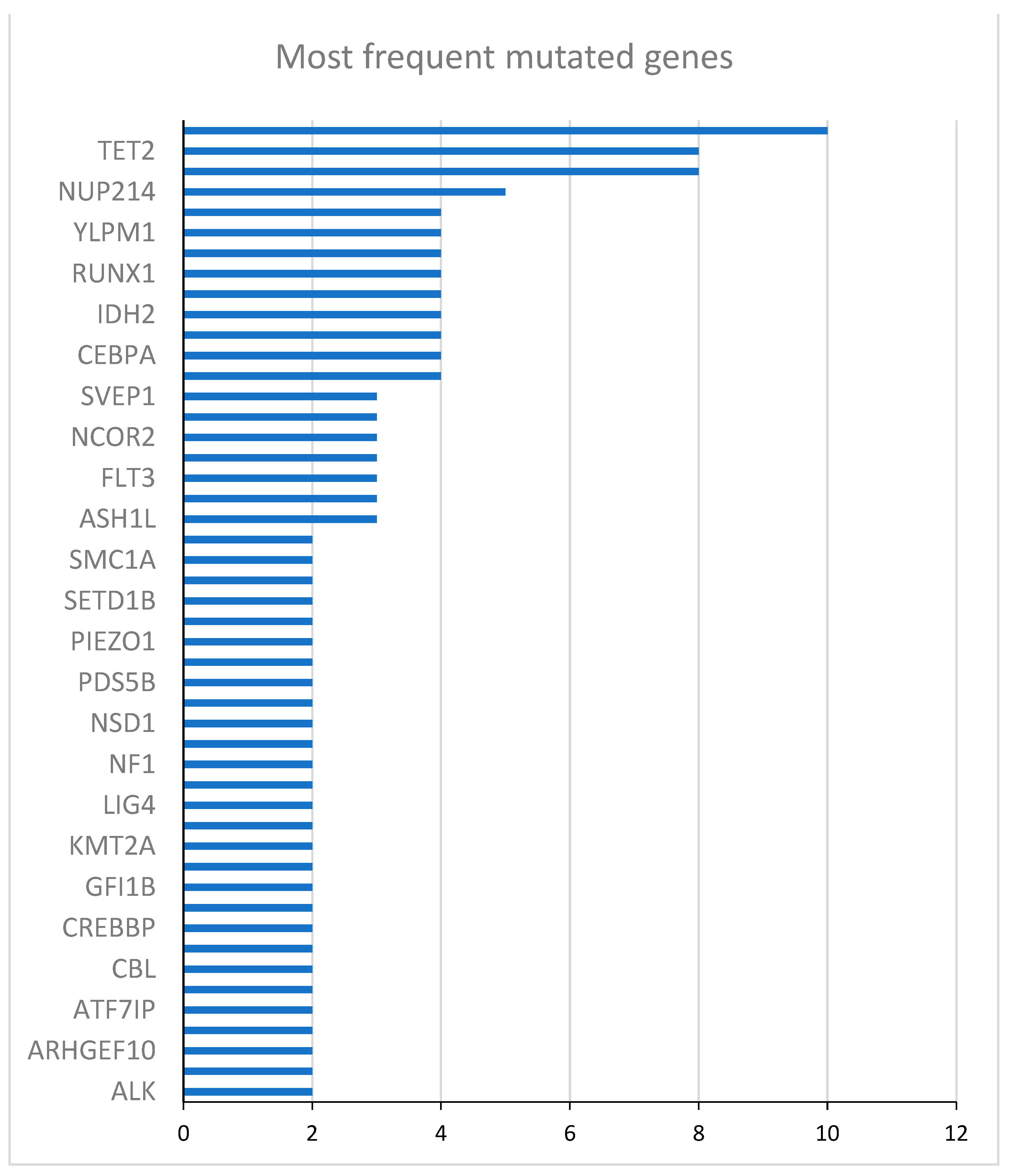
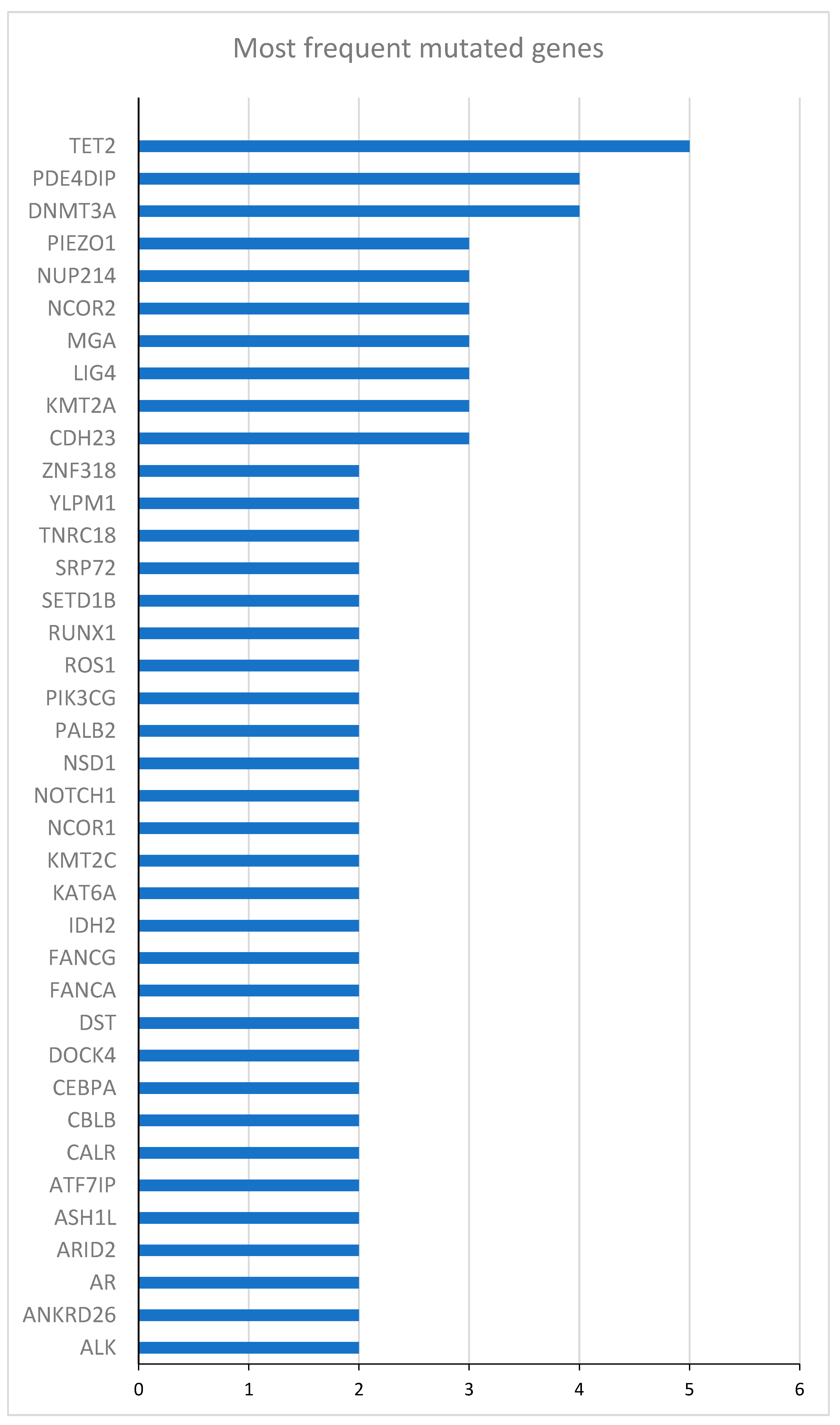
2.1. Description of the Study Cohort
2.2. Primary Endpoints
2.2.1. Effect Modifications by Gene Mutations at AML Diagnosis
2.2.2. Effect Modifications by Gene Mutations at Randomization
3. Discussion
3.1. Study Limitations
3.2. Conclusion
4. Materials and Methods
4.1. Study Design and Patient Population
4.2. Inclusion and Exclusion Criteria
4.3. Study Endpoints
4.4. Study Procedures
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [CrossRef]
- Hernandez-Valladares, M.; Aasebø, E.; Berven, F.; Selheim, F.; Bruserud, Ø. Biological Characteristics of Aging in Human Acute Myeloid Leukemia Cells: The Possible Importance of Aldehyde Dehydrogenase, the Cytoskeleton and Altered Transcriptional Regulation. Aging 2020, 12, 24734–24777. [CrossRef]
- Ferrara, F. Conventional Chemotherapy or Hypomethylating Agents for Older Patients with Acute Myeloid Leukaemia? Hematol. Oncol. 2014, 32, 1–9. [CrossRef]
- Webster, J.A.; Pratz, K.W. Acute Myeloid Leukemia in the Elderly: Therapeutic Options and Choice. Leuk. Lymphoma 2018, 59, 274–287. [CrossRef]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing Survival Outcomes with Post-Remission Therapy in Acute Myeloid Leukemia. Am. J. Hematol. 2019, 94, 803–811. [CrossRef]
- Molica, M.; Breccia, M.; Foa, R.; Jabbour, E.; Kadia, T.M. Maintenance Therapy in AML: The Past, the Present and the Future. Am. J. Hematol. 2019, 94, 1254–1265. [CrossRef]
- de Lima, M.; Roboz, G.J.; Platzbecker, U.; Craddock, C.; Ossenkoppele, G. AML and the Art of Remission Maintenance. Blood Rev. 2021, 49, 100829. [CrossRef]
- Reville, P.K.; Kadia, T.M. Maintenance Therapy in AML. Front. Oncol. 2020, 10. [CrossRef]
- Ramos, F.; Thépot, S.; Pleyer, L.; Maurillo, L.; Itzykson, R.; Bargay, J.; Stauder, R.; Venditti, A.; Seegers, V.; Martínez-Robles, V.; et al. Azacitidine Frontline Therapy for Unfit Acute Myeloid Leukemia Patients: Clinical Use and Outcome Prediction. Leuk. Res. 2015, 39, 296–306. [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood 2015, 126, 291–299. [CrossRef]
- Molica, M.; Mazzone, C.; Niscola, P.; Carmosino, I.; Di Veroli, A.; De Gregoris, C.; Bonanni, F.; Perrone, S.; Cenfra, N.; Fianchi, L.; et al. Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers 2022, 14, 4897. [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [CrossRef]
- Garcia-Manero, G.; Döhner, H.; Wei, A.H.; Torre, I.L.; Skikne, B.; Beach, C.L.; Santini, V. Oral Azacitidine (CC-486) for the Treatment of Myeloid Malignancies. Clin. Lymphoma Myeloma Leuk. 2022, 22, 236–250. [CrossRef]
- Huls, G.; Chitu, D.A.; Havelange, V.; Jongen-Lavrencic, M.; van de Loosdrecht, A.A.; Biemond, B.J.; Sinnige, H.; Hodossy, B.; Graux, C.; Kooy, R. van M.; et al. Azacitidine Maintenance after Intensive Chemotherapy Improves DFS in Older AML Patients. Blood 2019, 133, 1457–1464. [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [CrossRef]
- Oliva, E.N.; Candoni, A.; Salutari, P.; Palumbo, G.A.; Reda, G.; Iannì, G.; Tripepi, G.; Cuzzola, M.; Capelli, D.; Mammì, C.; et al. Azacitidine Post-Remission Therapy for Elderly Patients with AML: A Randomized Phase-3 Trial (QoLESS AZA-AMLE). Cancers 2023, 15, 2441. [CrossRef]
- Sauerer, T.; Velázquez, G.F.; Schmid, C. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Immune Escape Mechanisms and Current Implications for Therapy. Mol. Cancer 2023, 22, 180. [CrossRef]
- Mina, A.; Greenberg, P.L.; Deeg, H.J. How I Reduce and Treat Posttransplant Relapse of MDS. Blood 2024, 143, 1344–1354. [CrossRef]
- Rageul, J.; Kim, H. Fanconi Anemia and the Underlying Causes of Genomic Instability. Environ. Mol. Mutagen. 2020, 61, 693–708. [CrossRef]
- Pawlikowska, P.; Delestré, L.; Gregoricchio, S.; Oppezzo, A.; Esposito, M.; Diop, M.B.; Rosselli, F.; Guillouf, C. FANCA Deficiency Promotes Leukaemic Progression by Allowing the Emergence of Cells Carrying Oncogenic Driver Mutations. Oncogene 2023, 42, 2764–2775. [CrossRef]
- D’Andrea, A.D. Susceptibility Pathways in Fanconi’s Anemia and Breast Cancer. N. Engl. J. Med. 2010, 362, 1909–1919. [CrossRef]
- Voso, M.T.; Fabiani, E.; Zang, Z.; Fianchi, L.; Falconi, G.; Padella, A.; Martini, M.; Li Zhang, S.; Santangelo, R.; Larocca, L.M.; et al. Fanconi Anemia Gene Variants in Therapy-Related Myeloid Neoplasms. Blood Cancer J. 2015, 5, e323. [CrossRef]
- Chang, L.; Zhang, L.; Zhao, B.; Cheng, X.; Wan, Y.; Zhang, R.; Yuan, W.; Gao, X.; Zhu, X. Mutation Spectrum, Expression Profiling, and Prognosis Evaluation of Fanconi Anemia Signaling Pathway Genes for 4259 Patients with Myelodysplastic Syndromes or Acute Myeloid Leukemia. BMC Med. Genomics 2023, 16, 290. [CrossRef]
- Steinberg-Shemer, O.; Goldberg, T.A.; Yacobovich, J.; Levin, C.; Koren, A.; Revel-Vilk, S.; Ben-Ami, T.; Kuperman, A.A.; Zemer, V.S.; Toren, A.; et al. Characterization and Genotype-Phenotype Correlation of Patients with Fanconi Anemia in a Multi-Ethnic Population. Haematologica 2020, 105, 1825–1834. [CrossRef]
- Woo, J.; Howard, N.P.; Storer, B.E.; Fang, M.; Yeung, C.C.; Scott, B.L.; Deeg, H.J. Mutational Analysis in Serial Marrow Samples during Azacitidine Treatment in Patients with Post-Transplant Relapse of Acute Myeloid Leukemia or Myelodysplastic Syndromes. Haematologica 2017, 102, e216–e218. [CrossRef]
- Nagata, Y.; Makishima, H.; Kerr, C.M.; Przychodzen, B.P.; Aly, M.; Goyal, A.; Awada, H.; Asad, M.F.; Kuzmanovic, T.; Suzuki, H.; et al. Invariant Patterns of Clonal Succession Determine Specific Clinical Features of Myelodysplastic Syndromes. Nat. Commun. 2019, 10, 5386. [CrossRef]
| Characteristics | N= 53 |
|---|---|
| Age, median years (IQR) | 71 (66 - 74) |
| Male, n (%) | 28 (52.8) |
| AML de novo, n (%) | 46 (86.8) |
| Hemoglobin (Hb), mean g/dL (±SD) | 9.4±1.6 |
| White blood cell (WBC) × 103, median (IQR) | 15.6 (2.8 – 37.8) |
| Platelet (PLT) × 103, median (IQR) | 61.5 (27.0 – 85.0) |
| WHO Classification, n (%) | |
| AML with minimal differentiation | 8 (15.1) |
| Acute myelomonocytic leukemia | 9 (17.0) |
| AML with myelodysplasia-related changes | 9 (17.0) |
| AML with maturation | 12 (22.6) |
| Acute monoblastic and monocytic leukemia | 5 (9.4) |
| AML without maturation | 5 (9.4) |
| AML with recurrent genetic abnormalities | 3 (5.7) |
| Therapy-related myeloid neoplasms | 1 (1.9) |
| Acute erythroid leukemia | 1 (1.9) |
| Cytogenetic risk profile, n (%) | |
| Intermediate | 40 (75.5) |
| Poor | 7 (13.2) |
| Not evaluable | 6 (12.3) |
| Characteristics | 5-Aza (n=11) |
BSC (n=13) |
All patients (n=24) |
|---|---|---|---|
| Age, median years (IQR) | 70 (66-75) | 73 (65-74) | 71 (65-74) |
| Male, n (%) | 6 (54.5) | 6 (46) | 12 (50) |
| AML de novo, n (%) | 9 (82) | 13 (100.0) | 22 (92) |
| Hemoglobin (Hb), mean g/dL (±SD) | 9.3±0.9 | 9.4±1.4 | 9.4±1.2 |
| White blood cell (WBC) × 103, median (IQR) | 3.1 (1.7 - 40.2) | 17.1 (2.7 - 25.1) | 15.6 (1.8 - 28.9) |
| Platelet (PLT) × 103, median (IQR) | 43 (26 - 63) | 29 (22-71) | 41 (24 - 65) |
| WHO Classification, n (%) | |||
| AML with minimal differentiation | 1 (9.1) | 2 (15.4) | 3 (12.5) |
| Acute myelomonocytic leukemia | 3 (27.3) | 2 (15.4) | 5 (20.8) |
| AML with myelodysplasia-related changes | 2 (18.2) | 1 (7.7) | 3 (12.5) |
| AML with maturation | 2 (18.2) | 3 (23.1) | 5 (20.8) |
| Acute monoblastic and monocytic leukemia | 1 (9.1) | 3 (23.1) | 4 (16.6) |
| AML without maturation | 1 (9.1) | - | 1 (4.2) |
| AML with recurrent genetic abnormalities | - | 1 (7.7) | 1 (4.2) |
| Therapy-related myeloid neoplasms | - | 1 (7.7) | 1 (4.2) |
| Acute erythroid leukemia | 1 (9.1) | - | 1 (4.2) |
| Cytogenetic risk profile, n (%) | |||
| Intermediate | 8 (72.7) | 11(84.6) | 19 (79.2) |
| Poor | 1 (9.1) | 2 (15.4) | 3 (12.5) |
| Not evaluable | 2 (18.2) | - | 2 (8.3) |
| Gene | Mutated patients (N) | Unmutated patients (N) | HR (95% CI) | p-value |
|---|---|---|---|---|
| DNMT3A | 10 | 14 | 0.45 (0.15 - 1.30) | 0.14 |
| TET2 | 8 | 16 | 1.20 (0.44 - 3.27) | 0.73 |
| NPM1 | 8 | 16 | 0.47 (0.15 - 1.48) | 0.17 |
| NUP214 | 5 | 19 | 0.72 (0.23 - 2.23) | 0.56 |
| ZNF318 | 4 | 20 | 1.92 (0.62 - 5.95) | 0.28 |
| YLPM1 | 4 | 20 | 0.37 (0.85 - 1.65) | 0.19 |
| WT1 | 4 | 20 | 1.39 (0.39 - 4.88) | 0.61 |
| RUNX1 | 4 | 20 | 0.34 (0.15 - 2.85) | 0.57 |
| NCOR1 | 4 | 20 | 0.34 (0.08 - 1.50) | 0.15 |
| IDH2 | 4 | 20 | 0.52 (0.12 - 2.31) | 0.39 |
| FANCA | 4 | 20 | 4.96 (1.34 - 18.35) | 0.02 |
| CEBPA | 4 | 20 | 1.01 (0.29 - 3.53) | 0.98 |
| BCOR | 4 | 20 | 1.29 (0.29 - 5.74) | 0.74 |
| Gene | Mutated patients (N) | Unmutated patients (N) | HR (95% CI) | p-value |
|---|---|---|---|---|
| DNMT3A | 10 | 14 | 0.53 (0.19 - 1.43) | 0.21 |
| TET2 | 8 | 16 | 1.38 (0.53 - 3.57) | 0.52 |
| NPM1 | 8 | 16 | 0.53 (0.19 -1.62) | 0.28 |
| NUP214 | 5 | 19 | 0.69 (0.22 - 2.11) | 0.51 |
| ZNF318 | 4 | 20 | 1.92 (0.62 - 5.95) | 0.26 |
| YLPM1 | 4 | 20 | 0.58 (0.16 - 2.01) | 0.39 |
| WT1 | 4 | 20 | 1.26 (0.36 -4.38) | 0.72 |
| RUNX1 | 4 | 20 | 0.60 (0.14 - 2.63) | 0.50 |
| NCOR1 | 4 | 20 | 0.49 (0.14 - 1.74) | 0.27 |
| IDH2 | 4 | 20 | 0.49 (0.11 - 2.15) | 0.34 |
| FANCA | 4 | 20 | 4.96 (1.34 - 18.35) | 0.02 |
| CEBPA | 4 | 20 | 0.93 (0.27 - 3.24) | 0.91 |
| BCOR | 4 | 20 | 0.74 (0.29 - 5.74) | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





