Submitted:
26 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
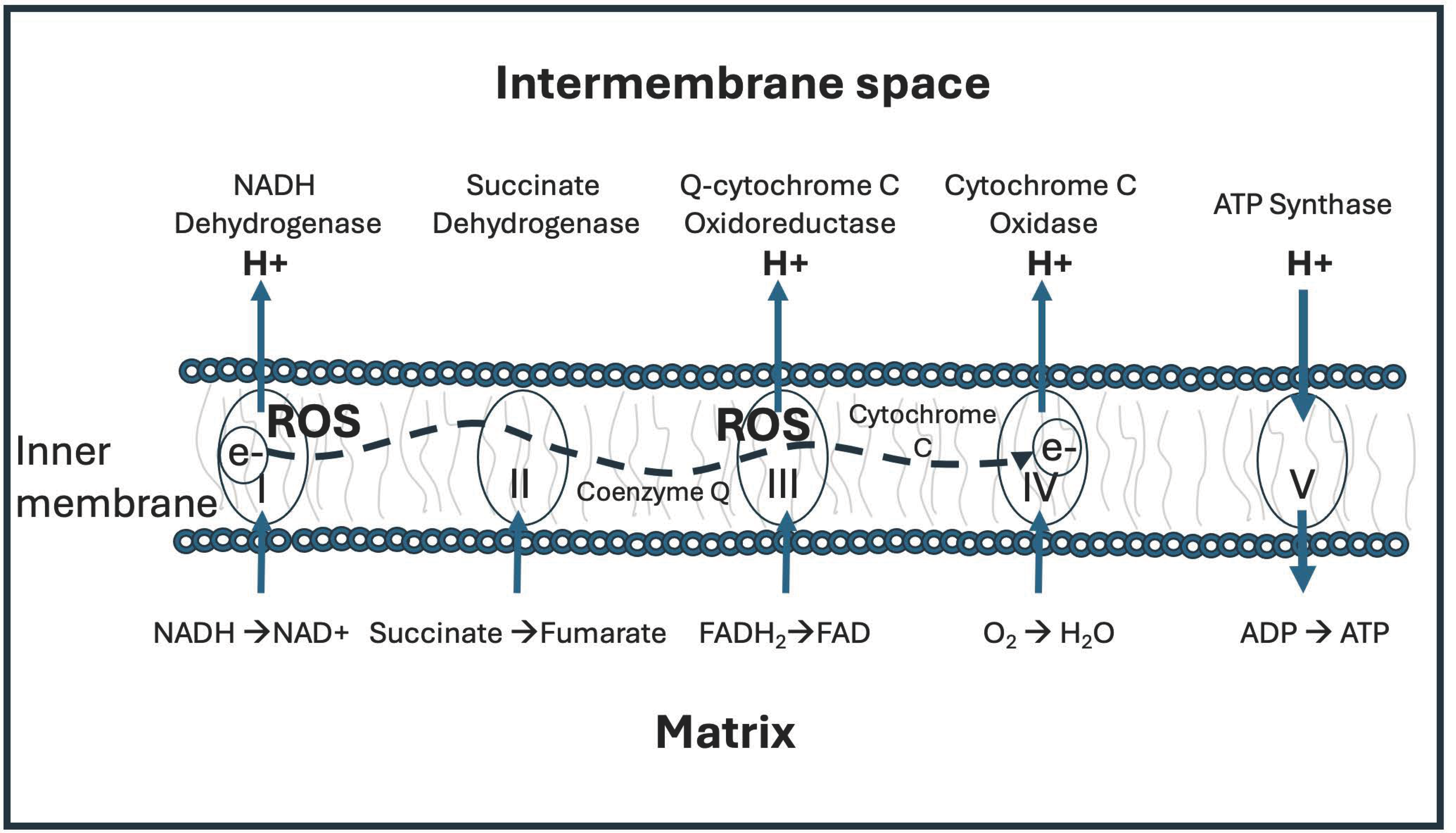
2. Mitochondrial ATPase Depends on Deupleted Protons
3. Deuterium Kinetic Isotope Effects
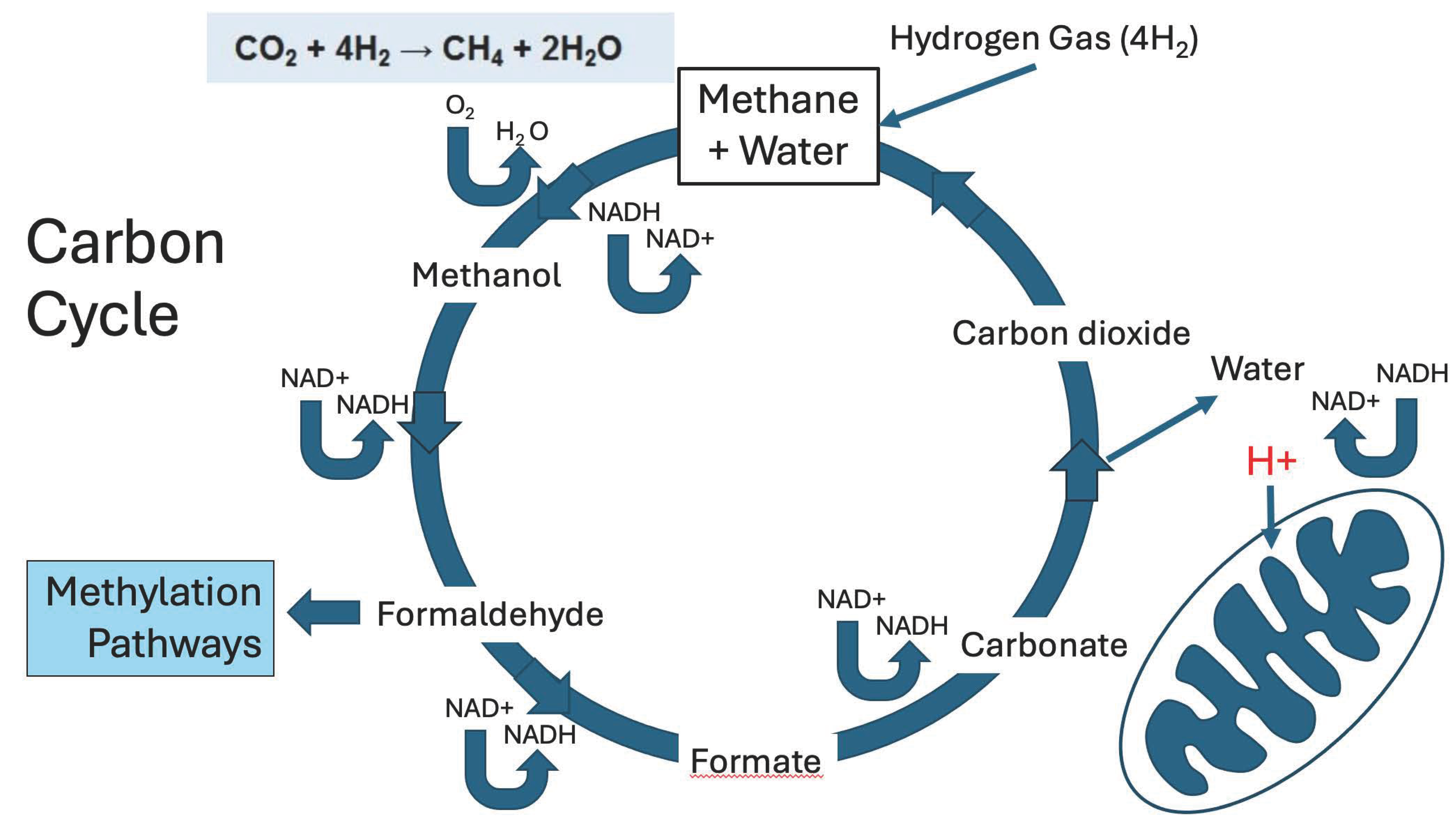
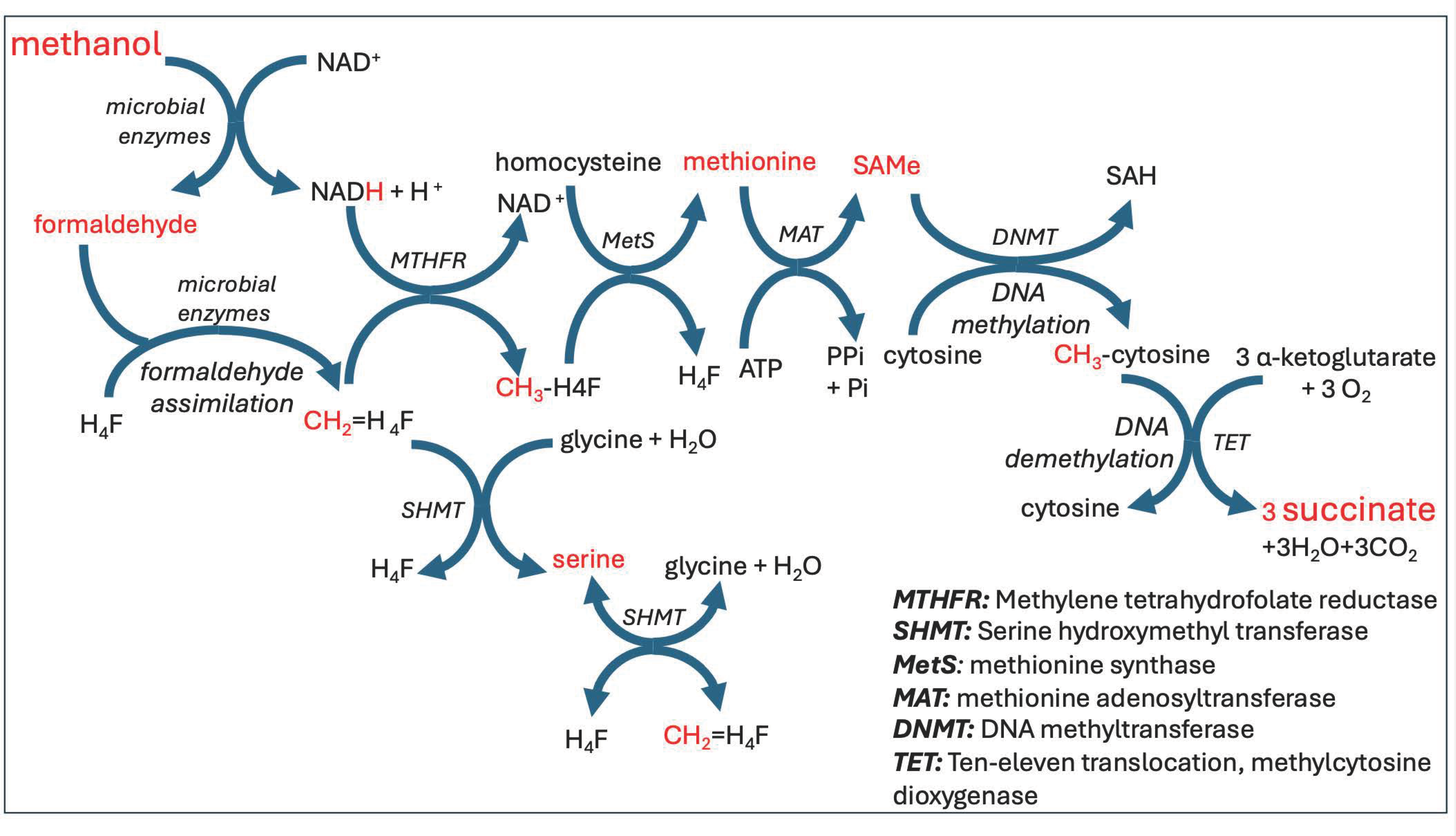
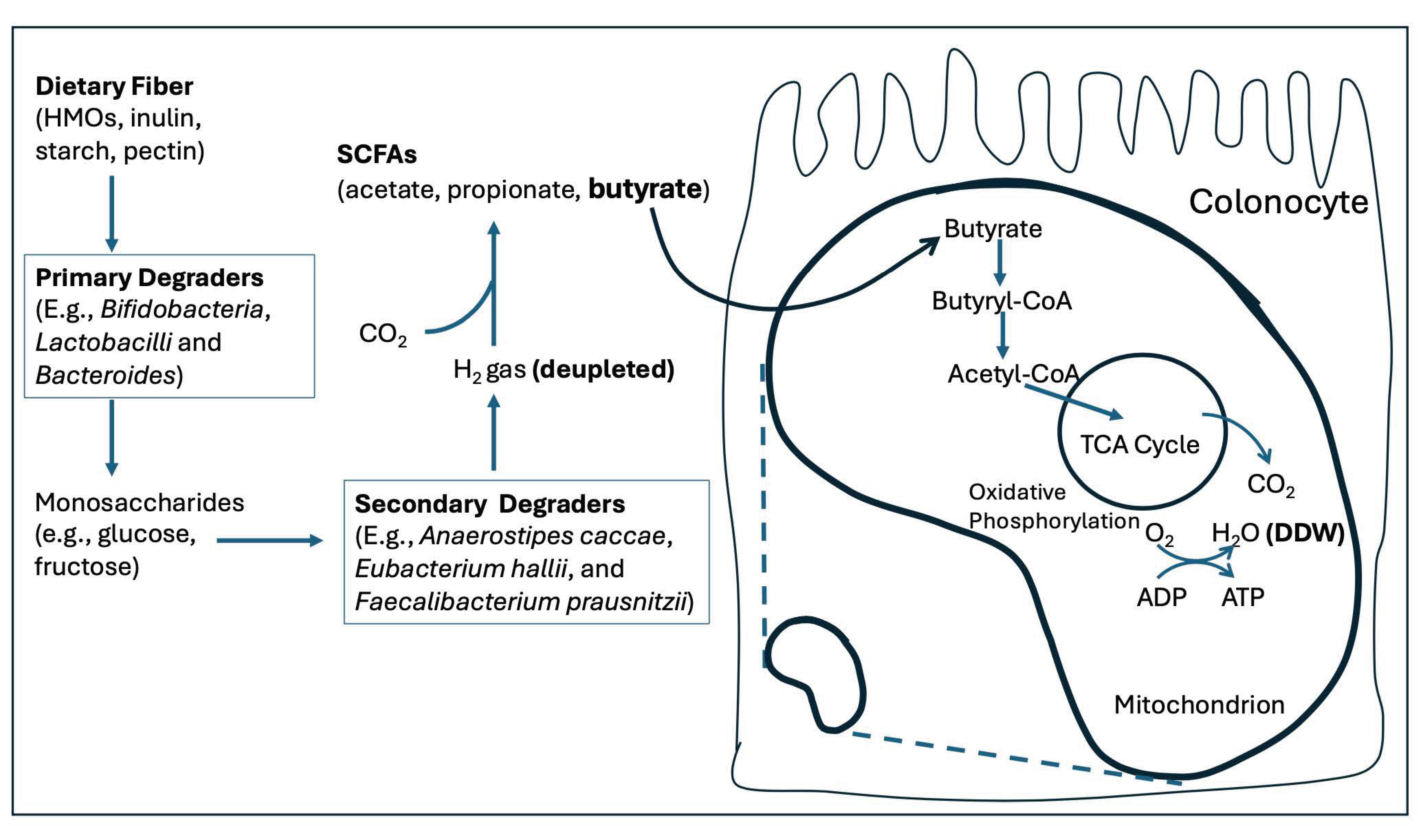
4. Microbes Supply Deupleted Nutrients to the Host
5. The Many Benefits of Butyrate to Human Health
6. Aldehyde Detoxification Pathways and Cancer
7. Choline, Cancer, and Cardiovascular Disease
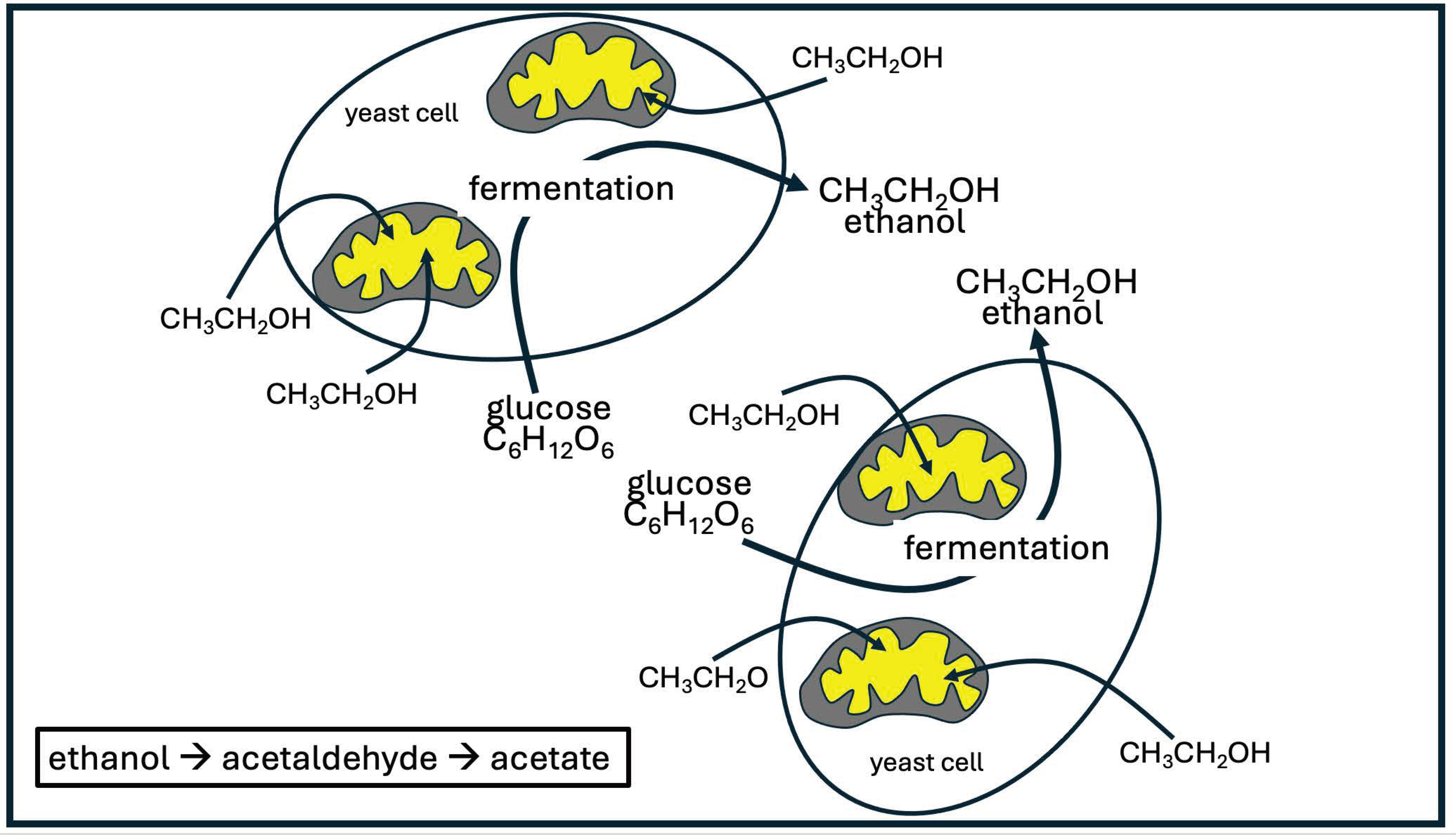
8. Yeast Overgrowth and Serum Ethanol
9. Yeast and Cancer cells have Much in Common
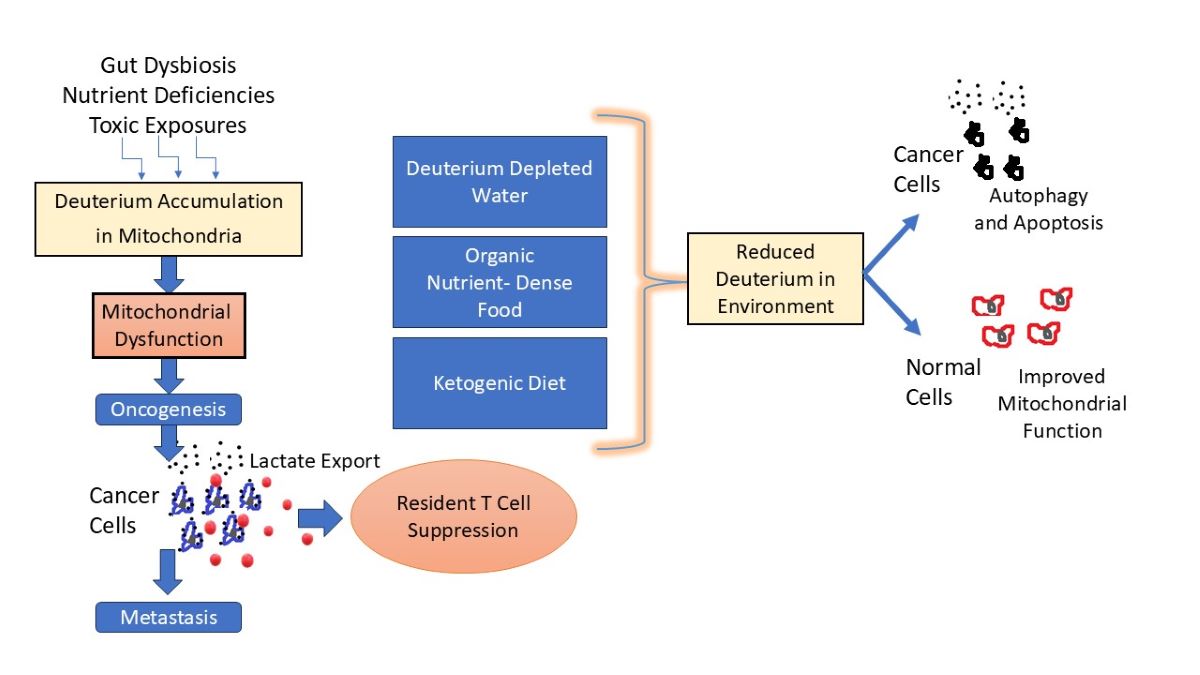
10. Explaining Cancer’s Unusual Metabolic Policies
10.1. V-ATPase and Microenvironment Proton Deupletion
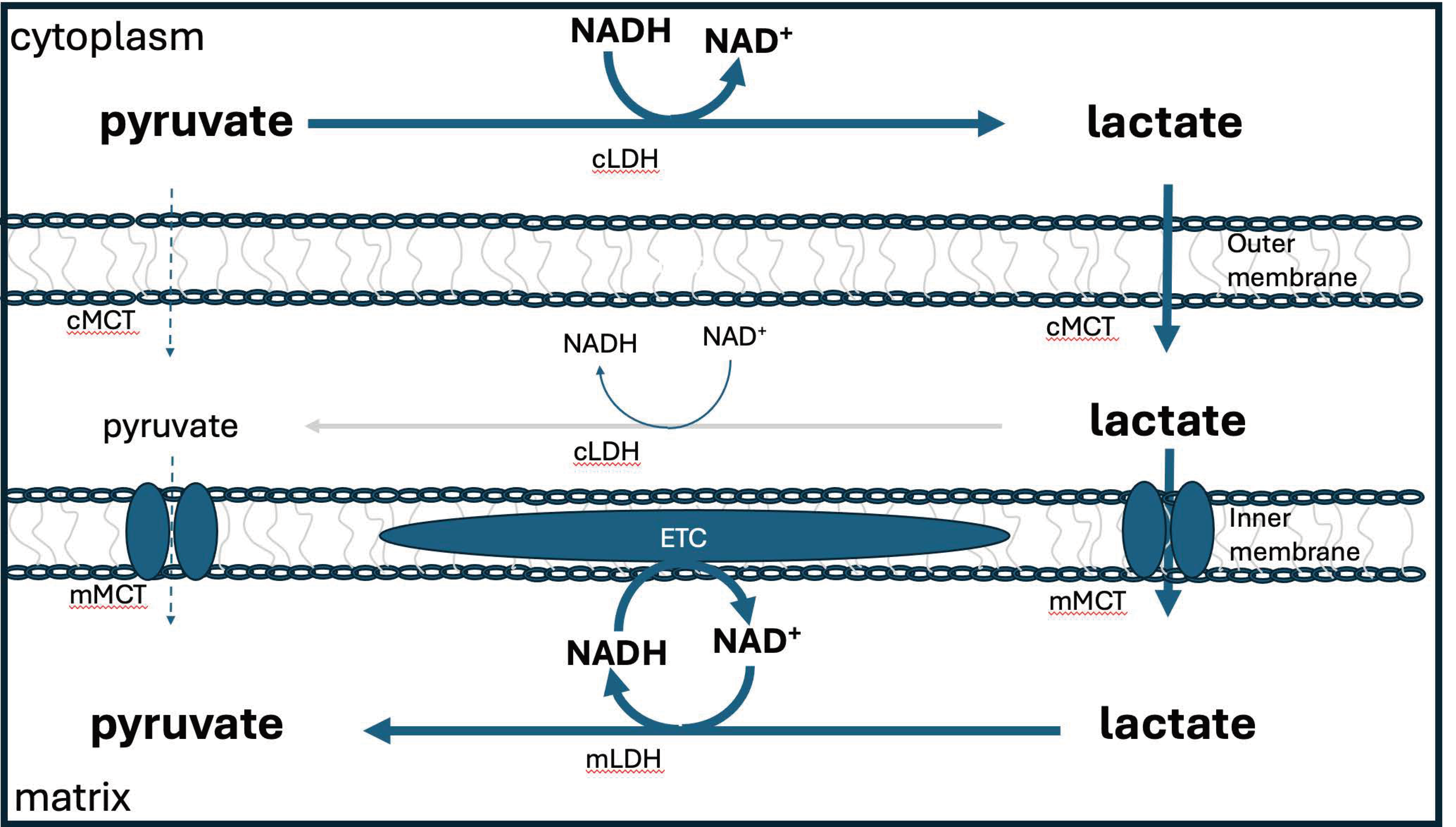
10.2. Lactate
10.3. Considerations Around Lactate, V-ATPase, Acidification, and M2 Macrophages
10.4. Formate Overflow and Cancer
11. Deuterium-depleted water (DDW) therapy as a treatment for cancer
11.1. Human Clinical Studies Show Promising Anti-Cancer Results with DDW Treatment
11.2. The Role of Cell Cycle Arrest
11.3. Understanding the Mechanism of Tumor Suppression by DDW
12. Discussion
12.1. DDW, Autophagy and Apoptosis
12.2. The Crosstalk between Senescence, Apoptosis and Cell Cycle Arrest Induced by DDW Treatment in Cancer
12.3. DDW and V-ATPase
12.4. Food as Medicine
13. Conclusions
Funding
Conflicts of Interest
References
- Yaglova, N.V.; Timokhina, E.P.; Obernikhin, S.S.; Yaglov, V.V. Emerging role of deuterium/protium disbalance in cell cycle and apoptosis. Int J Mol Sci. 2023, 24, 3107. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Seneff, S.; Tric, M.; Palcsuc, L.; Roman, A.; Zubarev, R.A. Active involvement of compartmental, inter- and intramolecular deuterium disequilibrium in adaptive biology. PNAS 2024, 121, e2412390121. [Google Scholar] [CrossRef]
- Bachner, P.; McKay, D.G.; Rittenberg, D. The pathologic anatomy of deuterium intoxication. PNAS 1964, 51, 464–471. [Google Scholar] [CrossRef]
- Xie, X.; Zubarev, R.A. Effects of low-level deuterium enrichment on bacterial growth. PLoS One. 2014, 17, 9–e102071. [Google Scholar] [CrossRef] [PubMed]
- Paliy, O.; Bloor, D.; Brockwell, D.; Gilbert, P.; Barber, J. Improved methods of cultivation and production of deuteriated proteins from E. coli strains grown on fully deuteriated minimal medium. J Appl Microbiol. 2003, 94, 580–6. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zubarev, R.A. Slight deuterium enrichment in water acts as an antioxidant: is deuterium a cell growth regulator? Mol Cell Proteomics. 2020, 19, 1790–1804. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Liu, C.; Fang, W.; Yang, H. Deuterium-depleted water selectively inhibits nasopharyngeal carcinoma cell proliferation in vitro. Nan Fang Yi Ke Da Xue Xue Bao. [article in Chinese]. 2021, 32, 1394-9. [Google Scholar]
- Stein, L.R.; Imai, S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012, 23, 420–8. [Google Scholar] [CrossRef]
- Boros, L.G.; Somlyai, I.; Kovács, B.Z.; Puskás, L.G.; Nagy, L.I.; Dux, L.; Farkas, G.; Somlyai, G. Deuterium depletion inhibits cell proliferation, RNA and nuclear membrane turnover to enhance survival in pancreatic cancer. Cancer Control. 2021, 28, 1073274821999655. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Wang, L.; Lu, F. Interaction between heavy water and single-strand DNA: A SERS study. Molecules. 2022, 27d, :6023. [CrossRef]
- Zlatska, A.; Gordiienko, I.; Vasyliev, R.; Zubov, D.; Gubar, O.; Rodnichenko, A.; Syroeshkin, A.; Zlatskiy, I. In vitro study of deuterium effect on biological properties of human cultured adipose-derived stem cells. Scientific World Journal. 2018, 2018, 5454367. [Google Scholar] [CrossRef]
- Olgun, A. Biological effects of deuteronation: ATP synthase as an example. Theor Biol Med Model 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Murcia Rios, A.; Vahidi, S.; Dunn, S.D.; Konermann, L. Evidence for a partially stalled γ rotor in F1-ATPase from hydrogen-deuterium exchange experiments and molecular dynamics simulations. J Am Chem Soc. 2018, 140, 14860–14869. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.P.; Haddad, M.Kh.; Fecht, H.-J. Light effect on water viscosity: Implication for ATP biosynthesis. Sci Rep 2015, 5, 12029. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Zhang, K.; Arakawa, H. Recent advances and new perspectives in mitochondrial dysfunction. Sci Rep 2023, 13, 7977. [Google Scholar] [CrossRef]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med (Encinitas). 2014, 13, 35–43. [Google Scholar]
- Luo, Y.; Ma, J.; Lu, W. The significance of mitochondrial dysfunction in cancer. Int J Mol Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Navratil, A.R.; Shchepinov, M.S.; Dennis, E.A. Lipidomics reveals dramatic physiological kinetic isotope effects during the enzymatic oxygenation of polyunsaturated fatty acids ex vivo. J Am Chem Soc. 2018, 140, 235–243. [Google Scholar] [CrossRef]
- Schleucher, J.; Vanderveer, P.; Markley, J.L.; Sharkey, T.D. Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant, Cell & Environment 1999, 22, 525–533. [Google Scholar] [CrossRef]
- Leadlay, P.F.; Albery, W.J.; Knowles, J.R. Energetics of triosephosphate isomerase: deuterium isotope effects in the enzyme-catalyzed reaction. Biochemistry. 1976, 15, 5617–20. [Google Scholar] [CrossRef]
- Henkel, S.; Ertelt, M.; Sander, W. Deuterium and hydrogen tunneling in the hydrogenation of 4-oxocyclohexa-2,5-dienylidene. Chemistry. 2014, 20, 7585–8. [Google Scholar] [CrossRef]
- Sutcliffe, M.J.; Scrutton, N.S. A new conceptual framework for enzyme catalysis. Hydrogen tunnelling coupled to enzyme dynamics in flavoprotein and quinoprotein enzymes. Eur J Biochem. 2002, 269, 3096–102. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.; Pudney, C.R.; Scrutton, N.S. Structural and mechanistic aspects of flavoproteins: probes of hydrogen tunnelling. FEBS J. 2009, 276, 3930–41. [Google Scholar] [CrossRef] [PubMed]
- Ballou, S.; Singh, P.; Nee, J.; Rangan, V.; Iturrino, J.; Geeganage, G.; Lwe, B.; Bangdiwala, S.I.; Palsson, O.S.; Sperber, AD.; et al. Prevalence and associated factors of bloating: Results from the Rome Foundation global epidemiology study. Gastroenterology 2023, 165, 647-655.e4. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.; Chimborazo, O.; László, E.; Temovski, M.; Palcsu, L. Rainwater isotopic composition in the Ecuadorian Andes and Amazon reflects cross-equatorial flow seasonality. Water 2022, 14, 2121. [Google Scholar] [CrossRef]
- Petit, J.R.; White, W.C.; Young, N.W.; Jouzel, J.; Korotkevich, Y.S. Deuterium excess in recent Antarctic snow. J Geophys Res. 1991, 96(D3), 5113–5122. [Google Scholar] [CrossRef]
- Ciais, P.; White, J.W.C.; Jouzel, J.; Petit, J.R. The origin of present-day Antarctic precipitation from surface snow deuterium excess data. J Geophys Res. 1985, 100, 1891718927. [Google Scholar]
- Sokol, K.P.; Robinson, W.E.; Oliveira, A.R.; Zacarias, S.; Lee, C.Y.; Madden, C.; Bassegoda, A.; Hirst, J.; Pereira, I.A.C.; Reisner, E. Reversible and selective interconversion of hydrogen and carbon dioxide into formate by a semiartificial formate hydrogenlyase mimic. J Am Chem Soc. 2019, 141, 17498–17502. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011, 17, 2241–52. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Yamamoto, H.; Hirano, S.I.; Sato, B.; Takefuji, Y.; Satoh, F. The overlooked benefits of hydrogen-producing bacteria. Med Gas Res. 2023, 13, 108–111. [Google Scholar] [CrossRef]
- Krichevsky, M.I.; Friedman, I.; Newell, M.F.; Sisler, F.D. Deuterium fractionation during molecular hydrogen formation in marine pseudomonad. J Biol Chem. 1961, 236, 2520–5. [Google Scholar] [CrossRef]
- Klein, V.J.; Irla, M.; Gil López, M.; Brautaset, T.; Fernandes Brito, L. Unravelling formaldehyde metabolism in bacteria: Road towards synthetic methylotrophy. Microorganisms. 2022, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.J.; Van Dien, S.J.; Lidstrom, M.E. Flux analysis uncovers key role of functional redundancy in formaldehyde meftabolism. PLoS Biol. 2005, 3, e16. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Chouchani, E.T. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat Chem Biol. 2022, 18, 461–469. [Google Scholar] [CrossRef]
- Gerecke, C.; Egea Rodrigues, C.; Homann, T.; Kleuser, B. The role of ten-eleven translocation proteins in inflammation. Front Immunol. 2022, 13, 861351. [Google Scholar] [CrossRef]
- Torao, E.G.; Petrus, S.; Fernandez, A.F.; Fraga, M.l.F. Global DNA hypomethylation in cancer: review of validated methods and clinical significance. Clin Chem Lab Med. 2012, 50, 1733-42. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Zeng, F.; Li, W.; He, Z.; Chen, W.; Zhu, W.; Zhang, B. The prognostic value of global DNA hypomethylation in cancer: a meta-analysis. PLoS One. 2014, 9, e106290. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.H.; Roy, N.C.; McNabb, W.C. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes. 2019, 10, 270–288. [Google Scholar] [CrossRef]
- Lajoie, S.F.; Bank, S.; Miller, T.L.; Wolin, M.J. Acetate production from hydrogen and [13C]carbon dioxide by the microflora of human feces. Appl Environ Microbiol. 1988, 54, 2723–7. [Google Scholar] [CrossRef] [PubMed]
- Bernalier, A.; Lelait, M.; Rochet, V.; Grivet, J.-P.; Gibson, G.R. Durand M. Acetogenesis from H, and CO, by methane- and non-methane-producing human colonic bacterial communities. FEMS Microbiology Ecology. 1996, 19, 193–202. [Google Scholar] [CrossRef]
- Bernalier, A.; Rochet, V.; Leclerc, M.; Dor, J.; Pochart, P. Diversity of H2/ CO2-utilizing acetogenic bacteria from feces of non-methane-producing humans. Curr Microbiol. 1996, 33, 94–9. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut microbial metabolite butyrate and its therapeutic role in inflammatory bowel disease: A literature review. Nutrients. 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Layec, S.; Auger, S.; Juste, C.; Henry, C.; Charif, S.; Jaszczyszyn, Y.; Sokol, H.; Beney, L.; Langella, P.; et al. E. Faecalibacterium duncaniae A2-165 regulates the expression of butyrate synthesis, ferrous iron uptake, and stress-response genes based on acetate consumption. Sci Rep. 2024, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004, 91, 915–23. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int J Oncol. 2024, 64, 44. [Google Scholar] [CrossRef] [PubMed]
- Son, M.Y.; Cho, H.S. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J Microbiol Biotechnol. 2023, 33, 849–856. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S2493S. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Glozak, M.A.; Seto, E. Histone deacetylases and cancer. Oncogene. 2007, 26, 5420–32. [Google Scholar] [CrossRef]
- Di Gennaro, E.; Bruzzese, F.; Caraglia, M.; Abruzzese, A.; Budillon, A. Acetylation of proteins as novel target for antitumor therapy: review article. Amino Acids. 2004, 26, 435–41. [Google Scholar] [CrossRef]
- Shin, H.; Lee, Y.S.; Lee, Y.C. Sodium butyrate-induced DAPK-mediated apoptosis in human gastric cancer cells. Oncol Rep. 2012, 27, 1111–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients. 2019, 11, 1026. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.X.; Xu, W.; Huang, L.; Zhao, J.Y.; Zhao, S.M. Butyrate induces apoptosis by activating PDC and inhibiting complex I through SIRT3 inactivation. Signal Transduct Target Ther. 2017, 2, 16035. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 2020, 11, 25. [Google Scholar] [CrossRef]
- Oka, Y.; Nakazawa, Y.; Shimada, M.; Ogi, T. Endogenous aldehyde-induced DNA-protein crosslinks are resolved by transcription-coupled repair. Nat Cell Biol. 2024, 26, 784–796. [Google Scholar] [CrossRef]
- Barnett, S.D.; Buxton, I.L.O. The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy. Crit Rev Biochem Mol Biol. 2017, 52, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Umansky, C.; Morellato, A.E.; Rieckher, M.; Scheidegger, M.A.; Martinefski, M.R.; Fernndez, G.A.; Pak, O.; Kolesnikova, K.; Reingruber, H.; Bollini, M.; et al. Endogenous formaldehyde scavenges cellular glutathione resulting in redox disruption and cytotoxicity. Nat Commun. 2022, 13, 745. [Google Scholar] [CrossRef]
- Chang, J.S.; Hsiao, JR.; Chen, CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci. 2017, 24, 19. [Google Scholar] [CrossRef]
- Wu, M.; Chang, S.C.; Kampman, E.; Yang, J.; Wang, X.S.; Gu, X.P.; Han, R.Q.; Liu, A.M.; Wallar, G.; Zhou, J.Y.; et al. Single nucleotide polymorphisms of ADH1B, ADH1C and ALDH2 genes and esophageal cancer: a population-based case-control study in China. Int J Cancer. 2013, 132, 1868–77. [Google Scholar] [CrossRef]
- Alter, B.P. Fanconi anemia and the development of leukemia. Best Pract Res Clin Haematol. 2014, 27, 214-21. [Google Scholar] [CrossRef]
- Oka, Y.; Hamada, M.; Nakazawa, Y.; Muramatsu, H.; Okuno, Y.; Higasa, K.; Shimada, M.; Takeshima, H.; Hanada, K.; Hirano, T.; et al. Digenic mutations in ALDH2 and ADH5 impair formaldehyde clearance and cause a multisystem disorder, AMeD syndrome. Sci Adv. 2020, 6, eabd7197. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Liscovitch, M.; Richardson, U.I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A. 1987, 84, 5474–7. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Davis, C.D.; Uthus, E.O. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood). 2004, 229, 988–95. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, K.A.; Kalmár, A.; Galamb, O.; Valcz, G.; Barták, B.K.; Nagy, Z.B.; Zsigrai, S.; Felletr, I.; V. Patai, A.; Micsik, T.; et al. Global DNA hypomethylation of colorectal tumours detected in tissue and liquid biopsies may be related to decreased methyl-donor content. BMC Cancer 2022, 22, 605. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; Fang, Y.J.; Pan, Z.Z.; Zhong, X.; Zheng, M.C.; Chen, Y.M.; Zhang, C.X. Choline and betaine intake and colorectal cancer risk in Chinese population: a case-control study. PLoS One. 2015, 10, e0118661. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.X.; Lv, E.H.; Wen, P.B.; Liu, X.; Wang, Y.T.; Cai, X.C.; Tian, J.Q.; et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol (Lausanne). 2023, 14, 1085041. [Google Scholar] [CrossRef] [PubMed]
- Dridi, B.; Fardeau, M.L.; Ollivier, B.; Raoult, D.; Drancourt, M. Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. Int J Syst Evol Microbiol. 2012, 62(Pt 8), 1902-1907. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Gaur, A.; Singhal, V.; Parakh, N.; Bhargava, B.; Sharma, A. The complex metabolism of trimethylamine in humans: endogenous and exogenous sources. Expert Rev Mol Med. 2016, 18, e19. [Google Scholar] [CrossRef]
- Brugre, J.F.; Borrel, G.; Gaci, N.; Tottey, W.; O’Toole, P.W.; Malpuech-Brugre, C. Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes. 2014, 5, 5–10. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Spector, T.D.; Youngblut, N.D.; Ley, R.E. Genomic insights into adaptations of trimethylamine-utilizing methanogens to diverse habitats, including the human gut. mSystems. 2021, 6, e00939–20. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of trimethylamine N-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients. 2018, 10, 1398. [Google Scholar] [CrossRef]
- Ramezani, A.; Nolin, T.D.; Barrows, I.R.; Serrano, M.G.; Buck, G.A.; Regunathan-Shenk, R.; West, R.E. 3rd.; Latham, P.S.; Amdur, R.; Raj, D.S. Gut colonization with methanogenic archaea lowers plasma trimethylamine N-oxide concentrations in apolipoprotein E-/- mice. Sci Rep. 2018, 8, 14752. [Google Scholar] [CrossRef]
- Ahmed, G.Y.; Osman, A.A.; Mukhtar, A. Acetylcholinesterase enzyme among cancer patients a potential diagnostic and prognostic indicator a multicenter case-control study. Sci Rep. 2024, 14, 5127. [Google Scholar] [CrossRef]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358-371.e9. [Google Scholar] [CrossRef]
- Zewude, R.T.; Croitoru, K.; Das, R.; Goldman, B.; Bogoch, II. Auto-brewery syndrome in a 50-year-old woman. CMAJ 2024, 196, E724–E727. [Google Scholar] [CrossRef]
- Tameez Ud Din, A.; Alam, F.; Tameez-Ud-Din, A.; Chaudhary, F.M.D. Auto-brewery syndrome: a clinical dilemma. Cureus 2020, 12, e10983. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäc, S. Lactate: the ugly duckling of energy metabolism. Nat Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Xiao, T.; Khan, A.; Shen, Y.; Chen, L.; Rabinowitz, J.D. Glucose feeds the tricarboxylic acid cycle via excreted ethanol in fermenting yeast. Nat Chem Biol 2022, 18, 1380–1387. [Google Scholar] [CrossRef]
- Somlyai, G.; Gyöngyi, Z.; Somlyai, I.; Boros, L.G. Pre-clinical and clinical data confirm the anticancer effect of deuterium depletion. Eur. J. Integr. Med. 2016, 8, 28. [Google Scholar] [CrossRef]
- Somlyai, G.; Kovács, B.Z.; Papp, A.; Somlyai, I. A preliminary study indicating improvement in the median survival time of glioblastoma multiforme patients by the application of deuterium depletion in combination with conventional therapy. Biomedicines. 2023, 13, 11–1989. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z. The research progress in the interaction between Candida albicans and cancers. Front Microbiol. 2022, 13, 988734. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wu, W.; Wu, S.; Young, A.; Yan, Z. Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol Res. 2023, 272, 127370. [Google Scholar] [CrossRef]
- Seo, W.; Gao, Y.; He, Y.; Sun, J.; Xu, H.; Feng, D.; Park, S.H.; Cho, Y.E.; Guillot, A.; Ren, T.; et al. ALDH2 deficiency promotes alcohol-associated liver cancer by activating oncogenic pathways via oxidized DNA-enriched extracellular vesicles. J Hepatol. 2019, 71, 1000–1011. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 2010, 5, 121–8. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807-3822.e12. [Google Scholar] [CrossRef]
- Teoh, F.; Pavelka, N. How Chemotherapy increases the risk of systemic Candidiasis in cancer patients: current paradigm and future directions. Pathogens. 2016, 5, 6. [Google Scholar] [CrossRef]
- Li, X.; Snyder, M.P. Yeast longevity promoted by reversing aging-associated decline in heavy isotope content. NPJ Aging Mech Dis. 2016, 2, 16004. [Google Scholar] [CrossRef]
- Bhat, T.A.; Kumar, S.; Chaudhary, A.K.; Yadav, N.; Chandra, D. Restoration of mitochondria function as a target for cancer therapy. Drug Discov Today. 2015, 20, 635–43. [Google Scholar] [CrossRef]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer metabolism: Phenotype, signaling and therapeutic targets. Cells. 2020, 9, 2308. [Google Scholar] [CrossRef]
- Stransky, L.; Cotter, K.; Forgac, M. The function of V-ATPases in cancer. Physiol Rev. 2016, 96, 1071–91. [Google Scholar] [CrossRef]
- Whitton, B.; Okamoto, H.; Packham, G.; Crabb, S.J. Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer. Cancer Med. 2018, 7, 3800–3811. [Google Scholar] [CrossRef]
- Perzov, N.; Padler-Karavani, V.; Nelson, H.; Nelson, N. Features of V-ATPases that distinguish them from F-ATPases. FEBS Lett. 2001, 504, 223–8. [Google Scholar] [CrossRef]
- Kotyk, A.; Dvorkov, M.; Koryta, J. Deuterons cannot replace protons in active transport processes in yeast. FEBS Lett. 1990, 264, 203–5. [Google Scholar] [CrossRef]
- Sennoune, S.R.; Bakunts, K.; Martínez, G.M.; Chua-Tuan, J.L.; Kebir, Y.; Attaya, M.N.; Martínez-Zaguiln, R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 2004, 286, C1443–52. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Kovács, B.Z.; Puskás, L.G.; Nagy, L.I.; Papp, A.; Gyöngyi, Z.; Fórizs, I.; Czuppon, G.; Somlyai, I.; Somlyai, G. Blocking the Increase of Intracellular Deuterium Concentration Prevents the Expression of Cancer-Related Genes, Tumor Development, and Tumor Recurrence in Cancer Patients. Cancer Control. 2022, 29, 10732748211068963. [Google Scholar] [CrossRef]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem Soc Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate is a metabolic mediator that shapes immune cell fate and function. Front Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A. 1999, 96, 1129–34. [Google Scholar] [CrossRef]
- Grimshaw, C.E.; Cleland, W.W. Deuterium isotope effects on lactate dehydrogenase using L-2-hydroxysuccinamate and effect of an inhibitor in the variable substrate on observed isotope effects. Biochemistry. 1980, 19, 3153–7. [Google Scholar] [CrossRef]
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers (Basel). 2020, 12, 3244. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Ther. 2022, 29, 1529–1541. [Google Scholar] [CrossRef]
- Apostolova, P.; Pearce, E.L. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022, 43, 969–977. [Google Scholar] [CrossRef]
- Barbieri, L.; Velia, P.; Gameiro, P.A.; Cunha, P.P.; Foskolou, I.P.; Rullman, E.; Bargiela, D.; Johnson, R.S.; Rundqvist, H. Lactate exposure shapes the metabolic and transcriptomic profile of CD8+ T cells. Front Immunol. 2023, 14, 1101433. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y. The role of metabolic reprogramming of tumor-associated macrophages in shaping the immunosuppressive tumor microenvironment. Biomed Pharmacother. 2023, 161, 114504. [Google Scholar] [CrossRef]
- Noe, J.T.; Rendon, B.E.; Geller, A.E.; Conroy, L.R.; Morrissey, S.M.; Young, L.E.A.; Bruntz, R.C.; Kim, E.J.; Wise-Mitchell, A.; Barbosa de Souza Rizzo, M.; et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci Adv. 2021, 7, eabi8602. [Google Scholar] [CrossRef] [PubMed]
- Nechipurenko, Y.D.; Semyonov, D.A.; Lavrinenko, I.A.; Lagutkin, D.A.; Generalov, E.A.; Zaitceva, A.Y.; Matveeva, O.V.; Yegorov, Y.E. The Role of Acidosis in the Pathogenesis of Severe Forms of COVID-19. Biology (Basel). 2021, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, A.M.; Seneff, S. Pathology deterioration in a pure β-zero thalassemia heterozygote after mRNA COVID-19 vaccination: A case report and literature review. IJVTPR 2024, 3, 1316–1344. [Google Scholar] [CrossRef]
- Hayes, C.; Donohoe, C.L.; Davern, M.; Donlon, N.E. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 2021, 500, 75–86. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Gatenbee, C.; Robertson-Tessi, M.; Bravo, R.; Dhillon, J.; Balagurunathan, Y.; Berglund, A.; Vishvakarma, N.; Ibrahim-Hashim, A.; Choi, J.; et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br J Cancer. 2019, 121, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Qian, B.Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O.C.; Tazzyman, S.; Danson, S.; Addison, C.; Clemons, M.; et al. Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015, 75, 3479–91. [Google Scholar] [CrossRef]
- Khan, F.; Lin, Y.; Ali, H.; Pang, L.; Dunterman, M.; Hsu, W.-H.; Frenis, K.; Rowe, R.G.; Wainwright, D.A.; McCortney, K.; et al. Lactate dehydrogenase A regulates tumor-macrophage symbiosis to promote glioblastoma progression. Nat Commun. 2024, 15, 1987. [Google Scholar] [CrossRef]
- Geistlinger, K.; Schmidt, J.D.R.; Beitz, E. Human monocarboxylate transporters accept and relay protons via the bound substrate for selectivity and activity at physiological pH. PNAS Nexus. 2023, 2, pgad007. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, E.P.; Schramm, V.L. Triple Isotope Effects Support Concerted Hydride and Proton Transfer and Promoting Vibrations in Human Heart Lactate Dehydrogenase. J Am Chem Soc. 2016, 16, 138–15004. [Google Scholar] [CrossRef] [PubMed]
- Meiser, J.; Tumanov, S.; Maddocks, O.; Labuschagne, C.F.; Athineos, D.; Van Den Broek, N.; Mackay, G.M.; Gottlieb, E.; Blyth, K.; Vousden, K.; et al. Serine one-carbon catabolism with formate overflow. Sci Adv. 2016, 2, e1601273. [Google Scholar] [CrossRef] [PubMed]
- Meiser, J. , Schuster, A., Pietzke, M. et al. Increased formate overflow is a hallmark of oxidative cancer. Nat Commun. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Delbrouck, C.; Pozdeev, V.I.; Oudin, A.; Grzyb, K.; Neises, L.; Kiweler, N.; Skupin, A.; Letellier, E.; Niclou, S.P.; Meiser, J. FSMP-09. Formate promotes cancer cell invasion and metastasis via calcium signaling. Neurooncol Adv. 2021, 3 (Suppl 1), i18. [Google Scholar] [CrossRef]
- Pietzke. M.; Meiser, J.; Vazquez, A. Formate metabolism in health and disease. Mol Metab. 2020, 33, 23–37. [Google Scholar] [CrossRef]
- Delbrouck, C.; Kiweler, N.; Chen, O.; Pozdeev, V.I.; Haase, L.; Neises, L.; Oudin, A.; Fouquier d’Hroul, A.; Shen, R.; Schlicker, L.; et al. Formate promotes invasion and metastasis in reliance on lipid metabolism. Cell Rep. 2023, 42, 113034. [Google Scholar] [CrossRef]
- Ye, J.; Fan, J.; Venneti, S.; Wan, Y.W.; Pawel, B.R.; Zhang, J.; Finley, L.W.; Lu, C.; Lindsten, T.; Cross, J.R.; et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014, 4, 1406–17. [Google Scholar] [CrossRef]
- Guerra, B.; Recio, C.; Aranda-Tavo, H.; Guerra-Rodríguez, M.; Garca-Castellano, J.M.; Fernndez-Pérez, L. The mevalonate pathway, a metabolic target in cancer therapy. Front Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Juarez, D.; Fruman, D.A. Targeting the Mevalonate Pathway in Cancer. Trends Cancer. 2021, 7, 525–540. [Google Scholar] [CrossRef]
- Jongen, V.H.; Hollema, H.; Van Der Zee, A.G.; Heineman, M.J. Aromatase in the context of breast and endometrial cancer. A review. Minerva Endocrinol. 2006, 31, 47–60. [Google Scholar]
- Fowler, K.A.; Gill, K.; Kirma, N.; Dillehay, D.L.; Tekmal, R.R. Overexpression of aromatase leads to development of testicular leydig cell tumors : an in vivo model for hormone-mediated testicular cancer. Am J Pathol. 2000, 156, 347–53. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Falcone, M.; Huerta Uribe, A.; Zhang, T.; Athineos, D.; Pietzke, M.; Vazquez, A.; Blyth, K.; Maddocks, O.D.K. Immune-regulated IDO1-dependent tryptophan metabolism is source of one-carbon units for pancreatic cancer and stellate cells. Mol Cell. 2021, 81, 2290-2302.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Somlyai, G.; Kovács, B.Z.; Papp, A.; Somlyai, I. A preliminary study indicating improvement in the median survival time of glioblastoma multiforme patients by the application of deuterium depletion in combination with conventional therapy. Biomedicines 2023, 11, 1989. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic cancer: A review of risk factors, diagnosis, and treatment. Technol Cancer Res Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Kovács, A.; Guller, I.; Krempels, K.; Somlyai, I.; Jnosi, I.; Gyöngyi, Z.; Szab, I.; Ember, I.; Somlyai, G. Deuterium depletion may delay the progression of prostate cancer. J Cancer Therapy 2011, 2, 548–556. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, H. Deuterium-depleted water in cancer therapy: A systematic review of clinical and experimental trials. Nutrients 2024, 16, 1397. [Google Scholar] [CrossRef]
- Dorgan, L.J.; Schuster, S.M. The effect of nitration and D2O on the kinetics of beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1981, 256, 3910–6. [Google Scholar] [CrossRef]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ 2020, 27, 843857. [Google Scholar] [CrossRef]
- Smirnov, A.Y.; Sulaberidze, G.A. Production of water with reduced content of deuterium for water supply system with desalination installation. J. Phys. Conf. Ser. 2018, 1099, 012035. [Google Scholar] [CrossRef]
- Lajos, R.; Braicu, C.; Jurj, A.; Chira, S.; Cojocneanu-Petric, R.; Pileczki, V.; Berindan-Neagoe, I. A miRNAs profile evolution of triple negative breast cancer cells in the presence of a possible adjuvant therapy and senescence inducer. J BUON. 2018, 23, 692–705. [Google Scholar] [PubMed]
- Chira, S.; Raduly, L.; Braicu, C.; Jurj, A.; Cojocneanu-Petric, R.; Pop, L.; Pileczki, V.; Ionescu, C.; Berindan-Neagoe, I. Premature senescence activation in DLD-1 colorectal cancer cells through adjuvant therapy to induce a miRNA profile modulating cellular death. Exp Ther Med. 2018, 16, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Yavari, K.; Kooshesh, L. Deuterium depleted water inhibits the proliferation of human MCF7 breast cancer cell lines by inducing cell cycle arrest. Nutr Cancer 2019, 71, 1019–1029. [Google Scholar] [CrossRef]
- Haseli, R.; Honarvar, M.; Yavari, K.; Ghavami, M. Synergistic anticancer effects of crocin combined with deuterium-depleted water on HT-29 cells. Anticancer Drugs. 2023, 34, 1162–1170. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; He, Z.; Fu, H.; Dai, Z.; Huang, G.; Li, B.; Qin, D.; Zhang, X.; Tian, L.; Fang, W.; Yang, H. Deuterium-depleted water (DDW) inhibits the proliferation and migration of nasopharyngeal carcinoma cells in vitro. Biomed Pharmacother. 2013, 67, 489–96. [Google Scholar] [CrossRef]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer effect of deuterium depleted water -- redox disbalance leads to oxidative stress. Mol Cell Proteomics 2019, 18, 2373–2387. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, M.; Li, S.; Li, M.; Cheng, B.; Ma, T.; Zhou, Z. The emerging potential role of p62 in cancer treatment by regulating metabolism. Trends Endocrinol Metab. 2023, 34, 474–488. [Google Scholar] [CrossRef]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic regulation of p62 is critical for cancer therapy. Int J Mol Sci. 2018, 8, 19–1405. [Google Scholar] [CrossRef]
- Carroll, B.; Otten, E.G.; Manni, D.; Stefanatos, R.; Menzies, F.M.; Smith, G.R.; Jurk, D.; Kenneth, N.; Wilkinson, S.; Passos, J.F.; et al. Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. Nat Commun. 2018, 9, 256. [Google Scholar] [CrossRef]
- Yi, H.; Talmon, G.; Wang, J. Glutamate in cancers: from metabolism to signaling. J Biomed Res. 2019, 34, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Koochekpour, S.; Majumdar, S.; Azabdaftari, G.; Attwood, K.; Scioneaux, R.; Subramani, D.; Manhardt, C.; Lorusso, G.D.; Willard, S.S.; Thompson, H.; et al. Serum glutamate levels correlate with Gleason score and glutamate blockade decreases proliferation, migration, and invasion and induces apoptosis in prostate cancer cells. Clin Cancer Res. 2012, 18, 5888–901. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.C. Design and synthesis of inhibitors of folate-dependent enzymes as antitumor agents. Adv Exp Med Biol. 1993, 338, 387–408. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Crovella, S.; Celsi, F. Mevalonate pathway blockade, mitochondrial dysfunction and autophagy: A possible link. Int J Mol Sci. 2015, 16, 16067–84. [Google Scholar] [CrossRef]
- De Giorgi, M.; Jarrett, K.E.; Burton, J.C.; Doerfler, A.M.; Hurley, A.; Li, A.; Hsu, R.H.; Furgurson, M.; Patel, K.R.; Han, J.; et al. Depletion of essential isoprenoids and ER stress induction following acute liver-specific deletion of HMG-CoA reductase. J Lipid Res. 2020, 61, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int J Mol Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Rao, S.; Tortola, L.; Perlot, T.; Wirnsberger, G.; Novatchkova, M.; Nitsch, R.; Sykacek, P.; Frank, L.; Schramek, D.; Komnenovic, V.; et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014, 5, 3056. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ 2022, 29, 946960. [Google Scholar] [CrossRef]
- Mathiassen, S.G.; De Zio, D.; Cecconi, F. Autophagy and the cell cycle: A complex landscape. Front Oncol. 2017, 7, 51. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of p53 in cell death and human cancers. Cancers (Basel) 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyi, Z.; Somlyai, G. Deuterium depletion can decrease the expression of C-myc Ha-ras and p53 gene in carcinogen-treated mice. In Vivo 2000, 14, 437–9. [Google Scholar]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer effect of deuterium depleted water - redox disbalance leads to oxidative stress. Mol Cell Proteomics 2019, 18, 2373–2387. [Google Scholar] [CrossRef]
- Kovács, B.Z.; Pusks, L.G.; Nagy, L.I.; Papp, A.; Gyöngyi, Z.; Frizs, I.; Czuppon, G.; Somlyai, I.; Somlyai, G. Blocking the increase of intracellular deuterium concentration prevents the expression of cancer-related genes, tumor development, and tumor recurrence in cancer patients. Cancer Control 2022, 29, 10732748211068963. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Basov, A.; Drobotenko, M.; Svidlov, A.; Gerasimenko, E.; Malyshko, V.; Elkina, A.; Baryshev, M.; Dzhimak, S. Inequality in the Frequency of the Open States Occurrence Depends on Single 2H/1H Replacement in DNA. Molecules 2020, 25, 3753. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, C.; Zhu, W.; Zheng, X.; Huang, Y.; Lu, Z. A facile strategy for the construction of purely organic optical sensors capable of distinguishing D2O from H2O. Angew Chem Int Ed Engl. 2019, 58, 6280–6284. [Google Scholar] [CrossRef]
- De Milito, A.; Canese, R.; Marino, M.L.; Borghi, M.; Iero, M.; Villa, A.; Venturi, G.; Lozuponem, F.; Iessi, E.; Logozzi, M.; et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010, 127, 207–19. [Google Scholar] [CrossRef]
- Das, A.; Sinha, S.; Acharya, B.R.; Paul, P.; Bhattacharyya, B.; Chakrabarti, G. Deuterium oxide stabilizes conformation of tubulin: A biophysical and biochemical study. BMB Rep. 2008, 41, 62–7. [Google Scholar] [CrossRef]
- Lopes, D.; Maiato, H. The tubulin code in mitosis and cancer. Cells 2020, 9, 2356. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients. 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
| Enzyme | Significance |
|---|---|
| ALDH4A1 | Mitochondrial NAD-dependent dehydrogenase; Essential role in pathway converting proline to glutamate |
| FDXR | Mitochondrial flavoprotein that converts NADP+ to NADPH |
| H6PD | A dehydrogenase that converts NADP+ to NADPH |
| DHFR | Regenerates FH$_4$ by reducing NADPH to NADP+ |
| GPX4 | Oxidizes glutathione, important antioxidant enzyme in mitochondria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





