Submitted:
17 September 2024
Posted:
18 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Japanese Quail (Coturnix coturnix japonica)
3. Influence of Heat Stress on Japanese Quail Production
4. Phytase Enzyme and the Hydrolysis of the Phytate Molecule
5. Phytase Overdose
6. Role of Phytase in Reducing Heat Stress
7. Calcium and Phosphorus in the Diet of Laying Quails (Coturnix coturnix japônica)
8. Calcium (Ca) Absorption
9. Transepithelial Calcium Transport Mediated by TRPV6 and Calbindin-D28K
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, T.C.; Gates, R.S.; Tinôco, I.F.F.; Zolnier, S.; Baêta, F.C. Behavior of Japanese quail in different air velocities and air temperatures. Pesquisa Agropecuária Brasileira 2017, 52, 344-354. [CrossRef]
- Truong, L.; Miller, M.R.; Sainz, R.D.; King, A.J. Changes in Japanese quail (Coturnix coturnix japonica) blood gases and electrolytes in response to multigenerational heat stress. PlosClimate 2023, 2, e0000144. [CrossRef]
- Moraes, L.R.; Delicato, M.E.A.; Cruz, A.S.; Silva, H.T.F.N.P.; Alves, C.V.B.V.; Campos, D.B.; Saraiva, E.P.; Costa, F.G.P.; Guerra, R.R. Methionine supplementing effects on intestine, liver and uterus morphology, and on positivity and expression of Calbindin-D28k and TRPV6 epithelial calcium carriers in laying quail in thermoneutral conditions and under thermal stress. PlosOne 2021, 16, e0245615. [CrossRef]
- Niu, Z.Y.; Liu, F.Z.; Yan, Q.L.; Li, W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poultry Science 2009, 88: 2101–2107. [CrossRef]
- Mehaisen, G.M.K.; Ibrahim, R.M.; Desoky, A.A.; Safaa, H.M.; El-Sayed, O.A.; Abass, A.O. The importance of propolis in alleviating the negative physiological effects of heat stress in quail chicks. PlosOne 2017, 12, 1–17. [CrossRef]
- Mehaisen, G.M.K.; Desoky, A.A.; Sakr, O.G.; Sallam, W.; Abass, A.O. Propolis alleviates the negative effects of heat stress on egg production, egg quality, physiological and immunological aspects of laying Japanese quail. PlosOne 2019, 14, e0214839. [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine aspects of the response to stress. Metabolism 2002, 51, 5–10. [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens. Poultry Science 2004, 83, 889–894. [CrossRef]
- Sahin, K.; Sahin, N.; Onderci, M. Vitamin E supplementation can alleviate negative effects of heat stress on egg production, egg quality, digestibility of nutrients and egg yolk mineral concentrations of Japanese quails. Research in Veterinary Science 2002, 73, 307–312. [CrossRef]
- Vercese, F.; Garcia, E.A.; Sartori, J.R.; Silva, A.P.; Faitarone, A.B.G.; Berto, D.A.; Molino, A.B.; Pelícia, K. Performance and egg quality of Japanese quails submitted to cyclic heat stress. Brazilian Journal of Poultry Science 2012, 14, 37-41. [CrossRef]
- Akdemir, F.; Sahin, N.; Orhan, C.; Tuzcu, M.; Sahin, K.; Hayirli, A. Chromium-histidinate ameliorates productivity in heat-stressed Japanese quails through reducing oxidative stress and inhibiting heat-shock protein expression. British Poultry Science 2015, 56: 247–254. [CrossRef]
- Patra, T.; Pati, P.K.; Mohapatra, A.K. Study on carcass quality of coloured broiler chicks supplemented with vitamin E and C during summer stress. SAARC Agricultural Information Centre (SAIC) 2011, 9, 123–132. Available at: https://www.cabdirect.org/cabdirect/abstract/20123050083.
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poultry Science 2012, 91: 575–582. [CrossRef]
- Sandikci, M.; Eren, U.; Onol, A.G.; Kum, S. The effect of heat stress and the use of Saccharomyces cerevisiae or (and) bacitracin zinc against heat stress on the intestinal mucosa in quails. Revue de Medecine Veterinaire 2004, 11, 552–556. Available at: https://www.researchgate.net/publication/289759420_The_effect_of_heat_stress_and_the_use_of_Saccharomyces_cerevisiae_or_and_bacitracin_zinc_against_heat_stress_on_the_intestinal_mucosa_in_quails.
- Sahin, N.; Orhan, C.; Tuzcu, M.; Sahin, K.; Kucuk, O. The Effects of Tomato Powder Supplementation on Performance and Lipid Peroxidation in Quail. Poultry Science 2008, 87, 276–283. [CrossRef]
- 16. Akdemir F, Köseman A; Şeker I. Alchemilla vulgaris effects on egg production and quality expressed by heat-stressed quail during the late laying period. South African Journal of Animal Science 2019, 49, 1-12. [CrossRef]
- Farias, M.R.S.; Leite, S.C.B.; Silva, H.P.; Pacheco, D.B.; Alves, G.C.; Abreu, C.G.; Freitas, E.R. Superdosing Phytases in the Diets of Light Laying Hens: Productive Performance and Bone Quality. Brazilian Journal of Poultry Science 2021, 23, 001-010. [CrossRef]
- Hirvonen, J.; Liljavirta, J.; Saarinen, M.T.; Lehtinen, M.J.; Ahonen, I.; Nurminen, P.I. Effect of Phytase on in Vitro Hydrolysis of Phytate and the Formation of myo-Inositol Phosphate Esters in Various Feed Materials. Journal of Agricultural and Food Chemistry 2019, 67, 11396−11402. [CrossRef]
- Sena, T.L.; Leite, S.C.B.; Vasconcelos, A.M.; Bezerra, M.M.R.; Abreu, C.G.; Farias, M.R.S.; Silveira, R.M.F. Does dietary supplementation with phytases affect the thermoregulatory and behavioral responses of pullets in a tropical environment? Journal of Thermal Biology 2020a, 88: 102499. [CrossRef]
- Pirgozliev, V.; Bedford, M.R.; Acamovic, T.; Allymehr, M. The effects of supplementary bacterial phytase on dietary energy and total tract amino acid digestibility when fed to young chickens. British Poultry Science 2011, 52, 245-254. [CrossRef]
- Martínez-Vallespín, B.; Männer, K.; Ader, P.; Zentek, J. Evaluation of high doses of phytase in a low-phosphorus diet in comparison to a phytate-free diet on performance, apparent ileal digestibility of nutrients, bone mineralization, intestinal morphology, and immune traits in 21-day-old broiler chickens. Animals 2022, 12, 1955. [CrossRef]
- Rojas, I.Y.M.; González, E.A.; Menocal, J.A.; Santos, T.T.; Arguello, J.R.; Coello, C.L. Assessment of a phytase included with lactic acid on productive parameters and on deposition of phosphorus, calcium, and zinc in laying hens fed with sorghum-soybean-meal-based diets. Journal of Applied Animal Research 2017, 46, 314-321. [CrossRef]
- Manobhavan, M.; Elangovan, A.V.; Sridhar, M.; Shet, D.; Ajith, S.; Pal, D.T.; Gowda, N.K.S. Effect of super dosing of phytase on growth performance, ileal digestibility and bone characteristics in broilers fed corn-soya-based diets. Journal of Animal Physiology and Animal Nutrition 2016, 100, 93-100. [CrossRef]
- Pinto, R.; Ferreira, A.S.; Albino, L.F.T.; Gomes, P.C.; Vargas Júnior, J.G. Protein and Energy Levels for Laying Japanese Quails. Revista Brasileira de Zootecnia 2002, 31, 1761-1770. [CrossRef]
- Vogado, G.M.S.; Silva, L.P. Características anatômicas e fatores genéticos ligados ao desenvolvimento reprodutivo de codornas de corte. In: I CONGEB. Editora Agron Science. 2023. p.119-133. [CrossRef]
- Lukanov, H.; Pavlova, I. Domestication changes in Japanese quail (Coturnix japonica): a review. World’s Poultry Science Journal 2020, 76, 787–801. [CrossRef]
- Lukanov, H. Domestic quail (Coturnix japonica domestica), is there such farm animal? World’s Poultry Science Journal 2019, 75, 547–558. [CrossRef]
- Pastore, S.M.; Oliveira, W.P.; Muniz, J.C.L. Panorama Da Coturnicultura No Brasil. Revista Eletrônica Nutritime 2012, 9, 2041–2049. Available at: https://portalidea.com.br/cursos/25e421f08de4aab6d494d4a76b957d11.pdf.
- Almeida, T.J.O.; Araújo, V.V.; Silva, A.V.; Silva, R.F.; Santos, N.A.; Santana, M.D.; Oliveira, V.P. Evolução Da Produção De Codornas Para Abate E Postura No Brasil. XIII Jornada de Ensino, Pesquisa e Extensão – JEPEX - UFRPE: Recife, Anais 2013. Available at: https://portalidea.com.br/cursos/6409d8df3ed7101311dc5da38592c83c.pdf.
- Silva, J.H.V.; Jordão Filho, J.; Costa, F.G.P.; Lacerda, P.B.; Vargas, D.G.V.; Lima, M.R. Exigências nutricionais de codornas. Revista Brasileira de Saúde e Produção Animal 2012, 13, 775–790. Available at: https://www.scielo.br/j/rbspa/a/kJDrRVLb6cMr7p6hskmZKzj/?format=pdf&lang=pt.
- Vieira, S.S. Desempenho e qualidade dos ovos de codornas japonesas (coturnix coturnix japônica) alimentadas com dietas contendo diferentes níveis óleo de palma. Diessertação (Mestre em Produção Animal), Universidade Federal Rural da Amazônia, Belém, Amazônia, Brasil, 2014. Available at: http://repositorio.ufra.edu.br/jspui/handle/123456789/754.
- Nasar, A.; Rahman, A.; Hoque, N.; Kumar Talukder, A.; Das, Z.C. A survey of Japanese quail (Coturnix coturnix japonica) farming in selected areas of Bangladesh. Veterinary World 2016, 9(9): 940-947. [CrossRef]
- Matos Júnior, J.J.L.; Furtado, D.A.; Ribeiro, N.L.; Marques, J.I.; Leite, P.G.; Nascimento, J.W.B.; Rodrigues, V.P.; Lopes Neto, J.P.; Rodrigues, L.R.; Santos, S.G.C.G.; Oliveira, A.G.; Silva, R.S. Productive performance, egg quality and the morphometry of the organs of Japanese quails (Cotournix cotournix japônica) kept at different temperatures. Food Science and Technology 2023, 43, 117822. [CrossRef]
- Sarcinelli, M.F.; Venturini, K.S.; Silva, L.C. Características dos ovos. Boletim Técnico, Universidade Federal do Espirito Santo. 2007. Available at: https://www.agais.com/telomc/b00707_caracteristicas_ovos.pdf.
- Carvalho, F.B.; Stringhini, J.H.; Jardim Filho, R.M.; Leandro, N.S.M.; Café, M.B.; Deus, H.A.S.B. Qualidade interna e de casca para ovos de poedeiras comerciais de diferentes linhagens e idades. Ciência Animal Brasileira 2007, 8(1): 25-30. 2007. https://www.revistas.ufg.br/vet/article/view/1155.
- Mota, A.S.B.; Lima, P.M.S.; Silva, D.S.; Abreu, V.K.G.; Freitas, E.R.; Pereira, A.L.F. Internal quality of eggs coated with cassava and yam starches. Revista Brasileira de Ciências Agrárias 2017, 12, 47-50. [CrossRef]
- NRC. Nutrient Requirements of Poultry. 9th ed. National Academics Press, Washington, DC, 1994. Available at: https://books.google.com.br/books?hl=pt-BR&lr=&id=bbV1FUqRcM0C&oi=fnd&pg=PT13&dq=NRC+1994+Nutrient+Requirements+of+Poultry.+9th+ed.+National+Academics+Press,+Washington&ots=IleM4AkqTs&sig=2Ll0iW3NGbzHsQc19UoHD17wtxM#v=onepage&q&f=false.
- INRA - Institut National de la Recherche Agronomique. Alimentação dos animais monogástricos: Suínos, Coelhos e Aves. 2.ed. São Paulo: Roca, 1999. 245p.
- Silva, J.H.V.; Costa, F.G.P. Tabelas para codornas japonesas e europeias. 2.ed. Jaboticabal: FUNEP, 2009. 107p.
- Rostagno, H.S.; Albino, L.F.T.; Hannas, M.I.; Donzele, J.L.; Sakomura, N.K.; Perazzo, F.G.; Brito, C.O. Brazilian tables for poultry and swine : composition of feedstuffs and nutritional requirements, 4 ed. Viçosa, MG. 2017. 488p.
- Rostagno, H.S.; Albino, L.F.T.; Calderano, A.A.; et al. Tabelas Brasileiras para aves e suínos. 5th edn. (Universidade Federal de Viçosa: Viçosa). 2024. 576p.
- Silva, R.C. Trocas de calor e desempenho de codornas japonesas confinadas em ambiente termoneutro e sob estresse térmico. Tese (Doutor em Engenharia Agrícola), Programa de Pós- Graduação em Engenharia Agrícola do Centro de Tecnologia e Recursos Naturais da Universidade Federal de Campina Grande, Campina Grande - Paraíba, Brasil. 2017. 108p. Available at: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/28065.
- Kim, D.H.; Kim, Y.B.; Lee, S.H.; Lee, Y.K.; Lee, S.D.; Lee, K.W. Identical thermal stress coupled with different temperature and humidity combinations affects nutrient digestibility and gut metabolites of laying hens. Brazilian Journal of Animal Science 2023, 52, e20220067. [CrossRef]
- Teyssier, J.R.; Brugaletta, G.; Sirri, F.; Dridi, S.; Samuel, J.; Rochell, S.J. A review of heat stress in chickens. Part II: Insights into protein and energy utilization and feeding. Frontiers in Physiology 2022, 13. [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [CrossRef]
- Linsley, J.G.; Burger, R.E. Respiratory and cardiovascular responses in the hyperthermic domestic cock. Poultry Science 1964, 43, 291-305. [CrossRef]
- Vercese, F. Efeito da temperatura sobre o desempenho e a qualidade dos ovos de codornas japonesas. Dissertação (Mestre em Zootecnia), Programa de Pós-Graduação em Zootecnia da universidade Estadual Paulista - Faculdade de Medicina Veterinária e Zotecnia, Botucatu - São Paulo. 2010. 70p. Available at: https://www.fmvz.unesp.br/Home/ensino/pos-graduacao768/zootecnia/dissertacoeseteses/francine-vercese.pdf.
- Furlan, R.L.; Macari, M.; Moraes, V.M.B. Malheiros, R.D.; Malheiros, E.B.; Secato, E.R. Alterações hematológicas e gasométricas em diferentes linhagens de frangos de corte submetidos ao estresse calórico agudo. Revista Brasileira de Ciência Avícola 1999, 1, 77-84.
- Ruzal, M.; Shinder, D.; Malka, I.; Yahav, S. Ventilation plays an important role in hens’ egg production at high ambient temperature. Poultry Science 2011, 90, 856-862. [CrossRef]
- Abdulkadir, A.; Reddy, D. A scoping review of the impact of heat stress on the organs of the Japanese quail (Coturnix japonica). The Journal of Basic and Applied Zoology 2023, 84. [CrossRef]
- Lesson, S.; Summers, J. D. Commercial poultry nutrition, Nottingham. Guelph: University Books, 1991. 283 p.
- Mongin, P. Role of acid-base balance in the physiology of egg-shell formation. World’s Poultry Science Journal 1968, 24, 200–230. [CrossRef]
- Campos, E.J. Avicultura: razões fatos e divergências. Belo Horizonte: Editora FEPMVZ; 2000. 311p.
- Ebeid, T.A.; Suzuki, T.; Sugiyama, T. High ambient temperature influences eggshell quality and calbindin-D28k localization of eggshell gland and all intestinal segments of laying hens. Poultry Science 2012, 91, 2282–2287. [CrossRef]
- Sahin, K.; Sahin, N.; Kucuk, O.; Hayirli, A.; Prasad, A.S. Role of dietary zinc in heat-stressed poultry: A review. Poultry Science 2009, 88, 2176-2183. [CrossRef]
- Melo, A.S.; Fernandes, R.T.V.; Marinho, J.B.M.; Arruda, A.M.V.; Figueirêdo, L.C.; Fernandes, R.T.V. Relação temperatura e nutrição sobre o desempenho de galinhas poedeiras. PUBVET 2016, 10, 855-860. [CrossRef]
- Kumar, S.; Anand, R. Effect of Germination and Temperature on Phytic Acid Content of Cereals. International Journal of Research in Agricultural Sciences 2021, 8, 1–13. Available at: https://ijras.org/administrator/components/com_jresearch/files/publications/IJRAS_932_FINAL.pdf.
- Kim, D.H.; Lee, Y.K.; Lee, S.D.; Kim, S.H.; Lee, S.R.; Lee, H.G.; Lee, K.W. Changes in Production Parameters, Egg Qualities, Fecal Volatile Fatty Acids, Nutrient Digestibility, and Plasma Parameters in Laying Hens Exposed to Ambient Temperature. Frontiers in Veterinary Science 2020, 7, 412. [CrossRef]
- Lehrfeld, J. High-performance Liquid Chromatography Analysis of Phytic Acid on a pH-stable, Macroporous Polymer Column. Cereal Chemistry 1989, 66, 510–515. Available at: https://www.cerealsgrains.org/publications/cc/backissues/1989/Documents/66_510.pdf.
- Bloot, A.P.M.; Kalschne, D.L.; Amaral, J.A.S.; Baraldi, I.J.; Canan, C. A Review of Phytic Acid Sources, Obtention, and Applications. Food Reviews International 2021, 39, 73-92. [CrossRef]
- Lolas, G.; Palamidis, N.; Markakis, P. Phytic Acid Total Phosphorus Relationship Relationship in Barley, Oats, Soybeans and Wheat. Cereal Chemistry 1976, 53, 867–871. Available at: https://www.cerealsgrains.org/publications/cc/backissues/1976/Documents/chem53_867.pdf.
- Stein, H.H. Analyzed values for P and phytate in feed ingredients. Monogastric Nutrition Laboratory, 2023. Available at: https://nutrition.ansci.illinois.edu/node/1753.
- Ravindran, S.; Ravindran, V.; Sivalogan, G. Total and Phytate Phosphorus Contents of Various Foods and Feedstuffs of Plant Origin. Food Chemistry 1994, 50, 133–136. [CrossRef]
- Frossard, E.; Bucher, M.; Mächler, F.; Mozafar, A.; Hurrell, R. Potential for Increasing the Content and Bioavailability of Fe, Zn and Ca in Plants for Human Nutrition. Journal of the Science of Food and Agriculture 2000, 80, 861–879. [CrossRef]
- Banaszkiewicz, T. Nutritional Value of Soybean Meal. Soybean and Nutrition. InTech. 2011. [CrossRef]
- Kasim, B.; Edwards,; H.M. The Analysis of Inositol Phosphate Forms in Feed Ingredients. Journal of the Science of Food and Agriculture 1998, 76, 1–9. [CrossRef]
- Canan, C.; Cruz, F.T.L.; Delaroza, F.; Casagrande, R.; Sarmento, C.P.M.; Shimokomaki, M.; Ida, E.I. Studies on the Extraction and Purification of Phytic Acid from Rice Bran. Journal of Food Composition and Analysis 2011, 24, 1057–1063. [CrossRef]
- Garcı́a-Estepa, R.; García-Estepa, R. M.; Guerra-Hernández, E.; García-Villanova, B. Phytic Acid Content in Milled Cereal Products and Breads. Food Research International 1999, 32, 217–221. [CrossRef]
- Hu, Y.X.; Van Harn, J.; Hendriks, W.H.; Van Baal, J.; Dijkslag, M.A.; Van Krimpen, M.M.; Bikker, P. Low-calcium diets increase duodenal mRNA expression of calcium and phosphorus transporters and claudins but compromise growth performance irrespective of microbial phytase inclusion in broilers. Poultry Science 2021, 100, 101488. [CrossRef]
- Figueirêdo, A.V.; Fialho, E.T.; Vitti, D.M.S.S.; Lopes, J.B.; Silva Filho, J.C.; Teixeira, A.S.; Lima, J.A.F. Ação da Fitase sobre a Disponibilidade Biológica do Fósforo, por Intermédio da Técnica de Diluição Isotópica, em Dietas com Farelo de Arroz Integral para Suínos. Revista Brasileira de Zootecnia 2000, 29, 177–182. [CrossRef]
- Payne, R.L.; Lavergne, T.K.; Southern, L.L. A comparison of two sources of phytase in liquid and dry forms in broilers. Poultry Science 2005, 84, 265–272. [CrossRef]
- Woyengo, T.A.; Nyachoti, C.M. Review: Supplementation of phytase and carbohydrases to diets for poultry. Canadian Journal of Animal Science 2011, 91, 177–192. [CrossRef]
- Costello, A.J.R.; Glonek, T.; Myers, T.C. 31P Nuclear Magnetic Resonance-pH Titrations of Myo-Inositol Hexaphosphate. CarbohyrIrate Research 1976, 46, 159-171. [CrossRef]
- Vasconcelos, D.M. Diferentes níveis nutricionais e de fitase nas dietas para codornas japonesas. Dissertação (Mestre em Zootecnia) Centro de Ciências Agrárias da Universidade Federal da Paraíba-UFPB, Areia, Paraíba – Brasil, 2018. Available at: https://repositorio.ufpb.br/jspui/bitstream/123456789/14997/1/DZ329.pdf.
- Gautier, A.E.; Walk, C.L.; Dilger, R.N. Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poultry Science 2017, 97, 211–218. [CrossRef]
- Alves, N.M.; Guimarães, L.H.S.; Piccoli, R.H.; Cardoso, P.G. Production and Partial Characterizationof an Extracellular Phytase Produced by Muscodor sp. under SubmergedFermentation. Advances in Microbiology 2016, 6, 23-32. [CrossRef]
- Rezaeipour, V.; Barsalani, A.; Abdullahpour, R. Effects of phytase supplementation on growth performance, jejunum morphology, liver health, and serum metabolites of Japanese quails fed sesame (Sesamum indicum) meal-based diets containing graded levels of protein. Tropical Animal Health and Production 2016, 48, 1141–1146. [CrossRef]
- Lelis, G.R.; Albino, L.F.T.; Silva, C.R.; Rostagno, H.S.; Gomes, P.G.; Borsatto, CG. Suplementação dietética de fitase sobre o metabolismo de nutrientes de frangos de corte. Revista Brasileira de Zootecnia 2010. 39, 1768-1773. [CrossRef]
- Jatuwong, K.; Suwannarach, N.; Kumla, J.; Penkhrue, W.; Kakumyan, P.; Lumyong, S. Bioprocess for Production, Characteristics, and Biotechnological Applications of Fungal Phytases. Frontiers in Microbiology 2020, 11, 188. [CrossRef]
- Sena, T.L.; Leite, S.C.B.; Farias, M.R.S.; Abreu, C.G.; Freitas, E.R.; Costa, A.C. Phytase Superdosing in the Diet of Lightweight Replacement Pullets: Performance, Organ Biometry and Bone Characteristics. Brazilian Journal of Poultry Science 2020b, 22, 001-008. [CrossRef]
- Kriseldi, R.; Walk, C.L.; Bedford, M.R.; Dozier, W.A. Inositol and gradient phytase supplementation in broiler diets during a 6-week production period: 2. Effects on phytate degradation and inositol liberation in gizzard and ileal digesta contentes. Poultry Science 2021, 100, 100899. [CrossRef]
- Vats, P.; Banerjee, U.C. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): an overview. Enzyme and Microbial Technology 2004, 35, 3-14. [CrossRef]
- Sato, V.S.; Jorge, J.A.; Oliveira, W.P.; Souza, C.R.F.; Guimarães, L.H.S. Phytase production by Rhizopus microsporus var. microsporus biofilm: characterization of enzymatic activity after spray drying in presence of carbohydrates and nonconventional adjuvants. Journal of Microbiology and Biotechnology 2014, 24, 177-87. [CrossRef]
- Dailin, D.J.; Hanapi, S.Z.; Elsayed, E.A.; Sukmawati, D.; Azelee, N.I.W.; Eyahmalay, J.; Siwapiragam, V.; El Enshasy, H. Fungal Phytases: Biotechnological Applications in Food and Feed Industries. Recent Advancement in White Biotechnology Through Fungi 2019, 65-99. [CrossRef]
- Greiner, R.; Konietzny, U. Phytases: Biochemistry, enzymology and characteristics relevant to animal feed use. In: Enzymes in farm animal nutrition, Bedford MR, Partridge GG. (eds). CAB Intl. Publishing, Oxfordshire, UK: 2010; pp.96–128. https://www.researchgate.net/publication/286044581_Phytases_Biochemistry_Enzymology_and_Characteristics_Relevant_to_Animal_Feed_Use.
- Bhavsar, K.; Khire, J.M. Current research and future perspectives of phytase bioprocessing. Royal Society of Chemistry advances 2014, 4, 26677–26691. [CrossRef]
- Santos, K.O.; Costa-Filho, J.; Riet, J.; Spagnol, K.L.; Nornberg, B.F.; Kütter, M.T.; Tesser, M.B.; Marins, L.F. Probiotic expressing heterologous phytase improves the immune system and attenuates inflammatory response in zebrafish fed with a diet rich in soybean meal. Fish Shellfish Immunol 2019, 93, 652-658. [CrossRef]
- Greiner, R.; Alminger, M.L. Carlsson, N.G. Stereospecificity of myo-Inositol Hexakisphosphate Dephosphorylation by a Phytate-Degrading Enzyme of Baker’s Yeast. Journal of Agricultural and Food Chemistry 2001, 49, 2228-2233. [CrossRef]
- Naves, L.P.; Corrêa, A.D.; Bertechini, A.G.; Gomide, E.M.; Santos, C.D. Effect of ph and Temperature on the Activity of Phytase Products Used in Broiler Nutrition. Brazilian Journal of Poultry Science 2012, 14, 159-232. [CrossRef]
- Delmaschio, I.B. Produção de fitases por fermentação em estado sólido e imobilização das enzimas por spray drying. Dissertação (Mestre em Ciências da Microbiologia) Programa de Pós-Graduação em Microbiologia, Área de Concentração – Microbiologia Industrial e Aplicada, do Instituto de Biociências, Letras e Ciências Exatas da Universidade Estadual Paulista (UNESP) “Júlio de Mesquita Filho”, Campus de São José do Rio Preto – Brasil. 2014. 115p. https://bdtd.ibict.br/vufind/Record/UNSP_5ea0a9a9549bebefeae1fbf3090e0ce6.
- Nezhad, N.G.; Rahman, R.N.Z.R.A.; Normi, Y.M.; Oslan, S.N.; Shari, F.M.; Leow, T.C. Integrative Structural and Computational Biology of Phytases for the Animal Feed Industry. Catalysts 2020, 10, 844. [CrossRef]
- Dallmann, H.M.; Avila, V.S.; Krabbe, E.L.; Surek, D.; Bedendo, G.C.; Toledo, T.S.; Dallmann, P.R.; Roll, A.A.P.; Roll, V.F.B.; Rutz, F. Different phytase levels and energy densities in broiler diets on performance, nutrient digestibility, and bone integrity from 28 to 35 days of age. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2023, 75, 280-292. [CrossRef]
- Cowieson, A.J.; Wilcock, P.; Bedford, M.R. Super-dosing effects of phytase in poultry and other monogastrics. World’s Poultry Science Journal 2011, 67, 225 – 236. [CrossRef]
- Nelson, T.S.; Shieh, T.R.; Wodzinski, R.J.; Ware, J.H. Effect of supplemental phytase on the utilization of phytate phosphorus by chicks. The Journal of Nutrition 1971, 101, 1289-1294. [CrossRef]
- Walk, C.L.; Bedford, M.R.; Santos, T.S.; Paiva, D.; Bradley, J.R.; Wladecki, H.; Honaker, C.; McElroy, A.P. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poultry Science 2013, 92, 719–725. [CrossRef]
- Fernandes, J.I.M.; Horn, D.; Ronconi, E.J.; Buzim, R.; Lima, F.K.; Pazdiora, D.A. Effects of Phytase Superdosing on Digestibility and Bone Integrity of Broilers. Journal of Applied Poultry Research 2019, 28, 390–398. [CrossRef]
- Leyva-Jimenez, H.; Alsadwi, A.M.; Gardner, K.; Voltura, E.; Bailey, C.A. Evaluation of high dietary phytase supplementation on performance, bone mineralization, and apparent ileal digestible energy of growing broilers. Poultry Science 2019. 98, 811-819. [CrossRef]
- Kim, J.H.; Pitargue, F.M.; Jung, H.; Han, G.P.; Choi, H.S.; Kil, D.Y. Effect of superdosing phytase on productive performance and egg quality in laying hens. Asian-Australasian Journal of Animal Sciences 2017, 30, 994-998. [CrossRef]
- Ribeiro, A.G.; Silva, R.S.; Costa, F.S.; Silva, E.G.; Santos Ribeiro, J.E.; Saraiva, E.P.; Costa, F.G.P.; Guerra, R.R. (2024) Phytase super-dosing modulates bone parameters and the concentration of the calcium epithelial carrier Calbindin-D28k in quails (Coturnix coturnix japonica) under thermal stress. Animal Production Science 2024, AN24057. [CrossRef]
- Saeed, M.; Abbas, G.; Alagawany, M.; Ali Kamboh, A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. Journal of Thermal Biology 2019, 84, 414-425. [CrossRef]
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. Journal of Thermal Biology 2018, 76, 101-106. [CrossRef]
- Farias, M.R.S.; Leite, S.C.B.; Vasconcelos, A.M.; Silva, T.A.G.; Leitão, A.M.F.; Sena, T.L.; Pacheco, D.B.; Abreu, C.G.; Silveira, R.M.F. Thermoregulatory, behavioral and productive responses of laying hens supplemented with different types and dosages of phytases raised in a hot environment: An integrative approach. Journal of Thermal Biology 2020, 94, 102773. [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. Journal of the Science of Food and Agriculture 2014, 95, 878-896. [CrossRef]
- Freeland-Graves, J.H.; Sanjeevi, N.; Lee, j.j. Global perspectives on trace element requirements. Journal of Trace Elements in Medicine and Biology 2015, 31, 135-141. [CrossRef]
- Borges, G.C.S. Peroxidação lipídica, desempenho e características de carcaça de frangos de corte estressados pelo calor e suplementados com zinco e selênio. Dissertação (Mestrado em Ciências Veterinárias). Faculdade de Medicina Veterinária, Uberlândia, Minas Gerais, Brasil. 2008. 61p. Available at: https://repositorio.ufu.br/handle/123456789/12987.
- Hu, P.; Li, K.; Peng, X.; Yao, T.; Zhu, C.; Gu, H.; Liu, H-Y.; Sun, M.; Hu, Y.; Ennab, W.; Luo, X.; Cai, D. Zinc intake ameliorates intestinal morphology and oxidative stress of broiler chickens under heat stress. Frontiers in Immunology 2023, 14, 1308907. [CrossRef]
- Kazim, S.; Omer, K. Zinc Supplementation Alleviates Heat Stress in Laying Japanese Quail. The Journal of Nutrition 2003, 133, 2808-2811. [CrossRef]
- Prasad, A.S.; Kucuk, O. Zinc in cancer prevention. Cancer and Metastasis Reviews 2002, 21, 291-295. [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The Role of Metallothionein in Oxidative Stress. International Journal of Molecular Science 2013, 14 6044–6066. [CrossRef]
- Zhang, Y.N.; Zhang, H.J.; Wang, J.; Yue, H.Y.; Qi, X.L.; Wu, S.G.; Qi, G.H. Effect of dietary supplementation of organic or inorganic zinc on carbonic anhydrase activity in eggshell formation and quality of aged laying hens. Poultry Science 2017, 96, 2176-2183. [CrossRef]
- Benesch, R.; Barron, N.S.; Mawson, C.A. Carbonic anhydrase, sulphonamides and shell formation in the domestic fowl, Nature 1944, 153, 138-139. [CrossRef]
- Nys, Y.; Hincke, M.; Arias, J.; Garcia-Ruiz, J.; Solomon, S. Avian eggshell mineralization. Poultry and Avian Biology Reviews 1999, 10, 143-166. Available at: https://www.researchgate.net/profile/Yves-Nys/publication/279562431_Avian_Eggshell_Mineralization/links/5630e38208ae0530378cf614/Avian-Eggshell-Mineralization.pdf.
- Holm, L.; Blomqvist, A.; Brandt, I.; Brunström, B.; Ridderstråle, Y.; Berg, C. Embryonic exposure to o, p’-DDT causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen. Environmental Toxicology and Chemistry 2006, 25, 2787-2793. [CrossRef]
- Mohebbifar, A.; Torki, M.; Ghasemi, H.A. Effect of phytase supplementation of diets with different levels of rice bran and non-phytate phosphorus on productive performance, egg quality traits, leukocytes profile and serum lipids of laying hens reared indoor under high environmental temperatures. Animal Feed Science and Technology 2015, 207, 222-233. [CrossRef]
- Hezaveh, M.S.S.; Ghasemi, H.A.; Hajkhodadadi, I.; Moradi, M.H. Single and combined effects of phytase and citric acid on growth performance, nutrient digestibility, bone characteristics, intestinal morphology, and blood components in meat-type quails fed low-phosphorous diets. Animal Feed Science and Technology 2020, 269, 114677. [CrossRef]
- Lima, H.J.D.A.; Barreto, S.L.T.; Donzele, J.L.; Valeriano, M.H.; Vieira, P.A.F.; Costa, C.H.R. Dietary phytase levels on performance and egg quality of Japanese quails. Revista Brasileira de Zootecnia 2011, 40, 129-134. [CrossRef]
- Gouveia, A.B.V.S.; Paulo, L.M.; Dias, A.G.F.; Batista, J.M.; Brasileiro, J.C.L.; Minafra, C.S.; Stringhini, J.H. Fitase na nutrição de aves de corte e postura: revisão de literatura. II Congresso Brasileiro de Produção Animal e Vegetal: “Produção Animal e Vegetal: Inovações e Atualidades 2022. 2, 984. [CrossRef]
- Ribeiro, C.L.N.; Barreto, S.L.T.; Reis, R.S.; Muniz, J.C.L.; Viana, G.S.; Ribeiro Junior, V.; Mendonça, M.O.; Ferreira, R.C.; DeGroot, A.A. The Effect of Calcium and Available Phosphorus Levels on Performance, Egg Quality and Bone Characteristics of Japanese Quails at End of the Egg-Production Phase. Brazilian Journal of Poultry Science 2016, 18, 033-040. [CrossRef]
- Stanquevis, C.E.; Furlan, A.C.; Marcato, S.M.; Oliveira-Bruxel, T.M.; Perine, T.P.; Finco, E.M.; Grecco, E.T.; Benites, M.I.; Zancanela, V.T. Calcium and available phosphorus requirements of Japanese quails in early egg-laying stage. Poultry Science 2021, 100, 147-158. [CrossRef]
- Souza, C.S.; Barreto, S.L.T.; Vieites, F.M.; Calderano, A.A.; Moraes, G.H.K.; Oliveira, M.G.A. Cálcio e fósforo na nutrição de codornas japonesas em postura. Science and Animal Health 2017, 5, 260-281. Available at: https://periodicos.ufpel.edu.br/index.php/veterinaria/article/view/9166/8350.
- Lautrou, M.; Pomar, C.; Dourmad, J.-Y.; Narcy, A.; Schmidely, P.; Létourneau-Montminy, M.P. Phosphorus and calcium requirements for bone mineralisation of growing pigs predicted by mechanistic modelling. Animal 2020, 14, s313–s322. [CrossRef]
- David, L.S.; Anwar, M.N.; Abdollahi, M.R.; Bedford, M.R.; Ravindran, V. Calcium Nutrition of Broilers: Current Perspectives and Challenges. Animals 2023, 13, 1590. [CrossRef]
- Pelicia, K.; Garcia, E.A.; Faitarone, A.B.G.; Silva, A.P.; Berto, D.A.; Molino, A.B.; Vercese, F. Calcium and Available Phosphorus Levels for Laying Hens in Second Production Cycle. Brazilian Journal of Poultry Science 2009, 11, 39 - 49. Available at: https://www.scielo.br/j/rbca/a/VgmDHbKHdXPv4vKMXvb9qhk/?format=pdf&lang=en.
- Carvalho, L.S.S.; Fernandes, E.A. Formation and eggshell quality laying and breeding hens. Medicina Veterinária, Recife 2013, 7, 35-44. Available at: https://www.journals.ufrpe.br/index.php/medicinaveterinaria/article/view/604.
- Mello, J. F. Influência dos níveis de cálcio e fósforo na dieta de matrizes de codornas japonesas, no desempenho produtivo e no desenvolvimento ósseo embrionário da progênie. Dissertação (Mestrado em Zootecnia), Programa de Pós-Graduação em Zootecnia, Universidade Estadual de Maringá, Paraná. 2015. 82p. Available at: http://repositorio.uem.br:8080/jspui/bitstream/1/1764/1/000220522.pdf.
- Chaves Hernández, A.J. Poultry and Avian Diseases. Encyclopedia of Agriculture and Food Systems. 2014; pp.504–20. [CrossRef]
- Zhao, S.C.; Teng, X.Q.; Xu, D.L.; Chi, X.; Ge, M.; Xu, S.W. Influences of low level of dietary calcium on bone characters in laying hens. Poultry Science 2020, 99, 7084-7091. [CrossRef]
- Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. [CrossRef]
- Adedokun, S.A.; Adeola, O. Calcium and phosphorus digestibility: metabolic limits. The Journal of Applied Poultry Research 2013, 22, 600-608. [CrossRef]
- IFP - Inorganic Feed phosphates. Phosphorus: a vital source of animal nutrition. 2024. Available at: https://www.feedphosphates.org/index.php/guides/11-guides/11-phosphorus-a-vital-source-of-animal-nutrition.
- Sinclair-Black, M.; Garcia-Mejia, R.A.; Blair, L.R.; Angel, R.; Arbe, X.; Cavero, D.; Ellestad, L.E. Circadian regulation of calcium and phosphorus homeostasis during the oviposition cycle in laying hens. Poultry Science 2024, 103, 103209. [CrossRef]
- Mazzuco, H. Osteoporose em poedeiras comerciais: uma doença metabólica multifatorial. Circular Técnica, n. 43, Concórdia: Embrapa Aves e Suínos, 2005; pp.1-8. Available at: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/57891/1/CUsersPiazzonDocumentsCIT-43.pdf.
- Miranda, C.C. Formas inorgânicas e orgânicas de minerais e Temperatura ambiente sobre o desempenho, Imunidade e parâmetros sanguíneos em frangos de Corte. Dissertação (Mestre em Zootecnia), Programa de Pós-Graduação em Zootecnia da Universidade Estadual Paulista, Faculdade de Medicina Veterinária e Zootecnia, Campus de Botucatu, Botucatu - SP, 2010. 59p. Available at: https://www.fmvz.unesp.br/Home/ensino/pos-graduacao768/zootecnia/dissertacoeseteses/carolina-carvalho-de-miranda.pdf.
- Bertechini, A. G. Metabolismo dos minerais. In: Bertechini, A. G. Nutrição de monogástricos. Lavras: Editora UFLA, 2012. p. 207-255.
- Silva, A.P. Níveis de cálcio e fósforo na dieta de codornas Japonesas (coturnix coturnix japonica) em diferentes fases do ciclo de produção e seus efeitos sobre desempenho Produtivo e qualidade dos ovos. Dissertação (Mestre em Zootecnia), Programa de Pós-Graduação em Zootecnia da Universidade Estadual Paulista, Faculdade de Medicina Veterinária e Zootecnia, Campus de Botucatu, Botucatu - SP, 2011. 58p. Available at: https://repositorio.unesp.br/server/api/core/bitstreams/d866ecc9-3f1e-4417-a647-48ead5a8c2f5/content.
- Albino, L.F.T.; Barreto, S.L.T. Criação de codornas para produção de ovos e carne. Viçosa: Aprenda Fácil, 2003. 268p.
- Pedroso, A.A.; Moraes, V.M.B.; Ariki, J.; Kronka, S.N. Níveis de cálcio e fósforo na ração sobre o desempenho e qualidade dos ovos de codornas japonesas. Ars Veterinaria 1999, 15, 135 –139.
- Masukawa, Y.; Fernandes, E.; Moraes, V.; Ariki, J.; Bruno, L. Níveis de cálcio da dieta sobre o desempenho e a qualidade da casca de ovos de codornas japonesas. Ars Veterinária 2001, 17, 144-148.
- Brandão, P.A.; Costa, F.G.P.; Silva, J.H.V.; Brandão, J.S.; Nobre, J.G.S.; Goulart, C.C. Exigência de cálcio para codornas japonesas (coturnix coturnix japonica) em postura. Acta Scientiarum. Animal Sciences 2007, 29, 17-21. Available at: https://www.redalyc.org/pdf/3031/303126486001.pdf.
- Costa, C.H.R.; Barreto, S.L.T.; Moura, W.C.O.; Reis, R.S.; Leite, C.D.S.; Maia, G.V.C. Níveis de fósforo e cálcio em dietas para codornas japonesas em postura. Revista Brasileira de Zootecnia 2007, 36, 2037-2046. [CrossRef]
- Costa, C.H.R.; Barreto, S.L.T.; Umigi, R.T.; Lima, H.J.D.A.; Araujo, M.S.; Medina, P. Balanço de cálcio e fósforo e estudo dos níveis desses minerais em dietas para codornas japonesas (45 a 57 semanas de idade). Revista Brasileira de Zootecnia 2010a, 39, 1748-1755. [CrossRef]
- Costa, C.H.R.; Barreto, S.L.T.; Gomes, P.C.; Maia, G.V.C.; Lipari, C.A.; Hosoda, L.H. Teores de cálcio em dietas para codornas japonesas no terço final de postura (45 a 57 semanas de idade). Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2010b, 62, 1225-1231. [CrossRef]
- Amoah, J.K.; Martin, E.A.; Barroga, A.J.; Garillo, E.P.; Domingo, I. Calcium and phosphorus requirements of Japanese quail layers. Journal of Applied Biosciences 2012, 54, 3892-3900. Available at: https://m.elewa.org/JABS/2012/54/5.pdf.
- Vieira, D.V.G.; Barreto, S.L.T.; Valeriano, M.H.; Jesus, L.F.D.; Silva, L.F.F.; Mencalha, R.; Barbosa, K.S.; Mendes, R.K.V.; Cassuce, M.R.; Melo, T.S. Exigências de cálcio e de fósforo disponível para codornas japonesas de 26 a 38 semanas de idade. Revista Brasileira de Saúde e Produção Animal 2012, 13, 204-213. Available at: https://www.scielo.br/j/rbspa/a/NvXwgKTH5RX5gJQmx4DkHsp/?format=pdf&lang=pt.
- Nascimento, M.Q. Níveis de cálcio e de fósforo em dietas para codornas japonesas utilizando fosfato bicálcico com duas granulometrias. Dissertação (Mestrado em Ciências Veterinárias), Universidade Federal do Espírito Santo, Alegre-ES, 2013. 84p. Available at: https://repositorio.ufes.br/server/api/core/bitstreams/52bfca24-9ea6-4806-87f3-a81e761c8309/content.
- Aguda, A.Y.; Sekoni, A.A.; Omage, J.J. Requirement of calcium and available phosphorus for laying Japanese quail birds (Coturnix coturnix japonica) in Nigeria. Journal of Animal and Poultry Sciences 2015, 4, 31-38. Available at: https://www.researchgate.net/publication/313387866_Requirement_of_Calcium_and_available_Phosphorus_for_Laying_Japanese_quail_birds_Coturnix_coturnix_japonica_in_Nigeria.
- Nascimento, M.Q.; Vargas Junior, J.G.; Pinto, C.E.L.; Demuner, L.F.; Petrucci, F.B.; Vieites, F.M. Optimal Available Phosphorus Levels in Diets Containing Different Dicalcium Phosphate Particle Sizes for Japanese Quails. Brazilian Journal of Poultry Science 2019, 21, 001-008. [CrossRef]
- Yang, J.H.; Hou, J.F.; Farquharson, C.; Zhou, Z.L.; Deng, Y.F.; Wang, L.; Yu, Y. Localisation and expression of TRPV6 in all intestinal segments and kidney of laying hens. British Poultry Science 2011, 52, 507–516. [CrossRef]
- Yu, E.; Sharma, S. Physiology, Calcium. StatPearls, 2023. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482128/.
- Bianco, S.D.C.; Peng, J.-B.; Takanaga, H.; Suzuki, Y.; Crescenzi, A.; Kos, C.H.; Zhuang, L.; Freeman, M.R.; Gouveia, C.H.A.; Wu, J.; Luo, H.; Mauro, T.; Brown, E.M.; Hediger, M.A. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. Journal of Bone and Mineral Research 2007, 22, 274–285, . [CrossRef]
- Hwang, C.-S.; Shemorry, A.; Varshavsky, A. Two proteolytic pathways regulate DNA repair by cotargeting the Mgt1 alkylguanine transferase. Proceedings of the National Academy of Sciences 2009, 106, 2142–2147. [CrossRef]
- Sugiyama, T.; Kikuchi, H.; Hiyama, S.; Nishizawa, K.; Kusuhara, S. Expression and localisation of calbindin D28k in all intestinal segments of the laying hen. British Poultry Science 2007, 48, 233–238. [CrossRef]
- Chang, W.; Tu, C.; Chen, T-H.; Bikle, D.; Shoback, D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Science Signaling 2008, 1, 1-23. [CrossRef]
- Schoulten, N.A.; Teixeira, A.S.; Freitas, R.T.F.; Bertechini, A.G.; Conte, A.J.; Silva, H.O. Níveis de cálcio em rações de Frangos de Corte na Fase Inicial Suplementadas com Fitase. Revista Brasileira de Zootecnia 2003, 32, 1190-1197. https://www.scielo.br/j/rbz/a/3YD6bYvWGrv8n6mW5YpyQxM/?format=pdf&lang=pt.
- Gobesso, A.A.O.; Wajnsztejn, H.; Ribeiro, R.M.; Bastos, F.L.; Etchichury, M.; Araújo Júnior, A.M.C. Comparison between different sources of minerals in horses with nutritional secondary hyperparathyroidism. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2021, 73, 73 – 81. [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Current Opinion in Pharmacology 2015, 22, 41–50. [CrossRef]
- Moe, S.M. Disorders Involving Calcium, Phosphorus, and Magnesium. Primary Care: Clinics in Office Practice 2008, 35, 215-237. [CrossRef]
- McDowell, L.R. Calcium and Phosphorus. In: Minerals in Animal and Human Nutrition. 2nd Edition, Elsevier Science BV, Amsterdam, 2003; 144p.
- Pinheiro, S.R.F. Niveis De Fósforo, De Cálcio E De Cloreto De Sódio Para Aves De Linhagens De Crescimento Lento Criadas Em Sistema Semi-Confinado. Tese (Doutor em Zootecnia) Universidade Estadual Paulista – UNESP, “Júlio de Mesquita Filho”, Jaboticabal – São Paulo – Brasil. 2009. https://repositorio.unesp.br/server/api/core/bitstreams/d5d73a2d-e13f-472a-8597-0e30913775cb/content.
- Underwood, E. J., & Suttle, N.F. The mineral nutrition of livestock 3rd edition. 3rd edn. CAB International, Wallingford, UK. 1999. Available at: https://www.cabidigitallibrary.org/doi/book/10.1079/9780851991283.0000.
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiological Reviews 2019, 99, 781-805. [CrossRef]
- Cross, H.S.; Peterlik, M. Calcium and inorganic phosphate transport in embryonic chick intestine: Triiodothyronine enhances the genomic action of 1,25-dihydroxycholecalciferol. Journal of Nutrition 1988, 118, 1529–1534. [CrossRef]
- Sugiyama, T.; Kusuhara, S. Avian calcium metabolismo and bone function. Asian-Australasian Journal of Animal Sciences 2001, 14, 82–90. https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/7351228.
- Bronner, F. Mechanisms of intestinal calcium absorption. Journal of Cellular Biochemistry 2003, 88, 387–393. [CrossRef]
- Van Abel, M.; Hoenderop, J.G.J.; Bindels, R.J.M. The epithelial calcium channels TRPV5 and TRPV6: Regulation and implications for disease. Naunyn-Schmiedeberg’s Archives of Pharmacology 2005, 371, 295–306. [CrossRef]
- Hirnet, D.; Olausson, J.; Fecher-Trost, C.; Bödding, M.; Nastainczyk, W.; Wissenbach, U.; Flockerzi, V.; Freichel, M. The TRPV6 gene, cDNA and protein. Cell Calcium 2003, 33, 509–518. [CrossRef]
- Belkacemi, L.; Bédard, I.; Simoneau, L.; Lafond, J. Calcium channels, transporters and exchangers in placenta: A review. Cell Calcium 2005, 37, 1–8. [CrossRef]
- Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. Calcium Absorption Across Epithelia. Physiological Reviews 2005, 85, 373-422. [CrossRef]
- Kim, H.J.; Lee, G.S.; Ji, Y.K.; Choi, K.C.; Jeung, E.B. Differential expression of uterine calcium transporter 1 and plasma membrane Ca 2+ ATPase 1b during rat estrous cycle. American Journal of Physiology-Endocrinology and Metabolism 2006, 291, E234–E241. [CrossRef]
- Suzuki, Y.; Kovacs, C.S.; Takanaga, H.; Peng, J.B.; Landowski, C.P.; Hediger, M.A. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. Journal of Bone and Mineral Research 2008, 23, 1249–1256. [CrossRef]
- Lee, G.S.; Jeung, E.B. Uterine TRPV6 expression during the estrous cycle and pregnancy in a mouse model. American Journal of Physiology - Endocrinology and Metabolism 2007, 293, 132–139. [CrossRef]
- Lambers, T.T.; Bindels, R.J.M.; Hoenderop, J.G.J. Coordinated control of renal Ca2+ handling. Kidney International 2006, 69, 650–654. [CrossRef]
- Burley, R.W.; Vadhera, D.V. The Avian Egg. Chemistry and Biology. John Wiley & Sons Co. New York. 1989; 472p.
- Yamamoto, K.R. Steroid receptor regulated transcription of specific genes and gene networks. Annual review of genetics 1985, 19, 209–252. [CrossRef]
- Ali, A.; Tan, H.Y.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction With the Microbiota in Food Allergy. Frontiers Immunology 2020, 11, 604054. [CrossRef]
- Tang, V.W.; Goodenough, D.A. Paracellular ion channel at the tight junction. Biophysical Journal 2003, 84, 1660 –1673. [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Villanueva-Tobaldo, C.V.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Epithelial Transport in Disease: An Overview of Pathophysiology and Treatment. Cells 2023, 12, 2455. [CrossRef]
- Friedman, P.A.; Gesek, F.A. Cellular calcium transport in renal epithelia: measurement, mechanisms, and regulation. Physiological Reviews 1995, 75, 429 – 471. [CrossRef]
- Peng, J.B.; Suzuki, Y.; Gyimesi, G.; Hediger, M.A. TRPV5 and TRPV6 Calcium-Selective Channels. In: Kozak JA, Putney JW Jr., editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 13. Available at: https://www.ncbi.nlm.nih.gov/books/NBK531440/.
- Nijenhuis, T.; Hoenderop, J.G.J.; Van der Kemp, A.W.C.M.; Bindels, R.J.M. Localization and Regulation of the Epithelial Ca 2+ Channel TRPV6 in the Kidney. Journal of the American Society of Nephrology 2003, 14, 2731–2740. [CrossRef]
- Brown, A.J.; Krits, I.; Armbrecht, H.J. Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Archives of Biochemistry and Biophysics 2005, 437, 51–58. [CrossRef]
- Bar, A. Calcium transport in strongly calcifying laying birds: Mechanisms and regulation. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology 2009, 152, 447–469. [CrossRef]
- Bianco, S.D.; Peng, J.B.; Takanaga, H.; Kos, C.H.; Crescenzi, A.; Brown, E.M.; Hediger, M.A. Mice lacking the epithelial calcium channel CaT1 (TRPV6) show a deficiency in intestinal calcium absorption depite high plasma levels of 1,25-dihydroxy vitamin D. FASEB Journal 2004, 18, A706. https://eurekamag.com/research/035/305/035305455.php.
- Hurwitz, S.; Harrison, H.C.; Harrison, H.E. Effect of vitamin D3 on the in vitro transport of calcium by the chick intestine. The Journal of nutrition 1967, 91, 319–323. [CrossRef]
- Peng, J.B.; Chen, X.Z.; Berger, U.V.; Weremowicz, S.; Morton, C.C.; Vassilev, P.M.; Brown, E.M.; Hediger, M.A. Human calcium transport protein cat1. Biochemical and Biophysical Research Communications 2000, 278, 326–332. [CrossRef]
- Wilkens, M.R.; Kunert-Keil, C.; Brinkmeier, H.; Schröder, B. Expression of calcium channel TRPV6 in ovine epithelial tissue. The Veterinary Journal 2009, 182, 294–300. [CrossRef]
- Wasserman, R.H.; Taylor, A.N. Vitamin D3-induced calcium-binding protein in chick intestinal mucosa. Science 1966, 152, 794–796. [CrossRef]
- Qin, X.; Klandorf, H.; Porter, D.W.; Holt, S.B.; Martin, W.G. Estrogen Enhancement of Ca-, Mg-, and Ca-Mg-Stimulated Adenosine Triphosphatase Activity in the Chick Shell Gland. General and Comparative Endocrinology 1993a, 89, 4–10. [CrossRef]
- Qin, X.; Klandorf, H. Effect of Estrogen in Relation to Dietary Vitamin D3 and Calcium on Activity of Intestinal Alkaline Phosphatase and Ca-ATPase in Immature Chicks. General and Comparative Endocrinology 1993b, 90, 318–327. [CrossRef]
- Morrissey, R.; Wasserman, R. Calcium absorption and calcium-binding protein in chicks on differing calcium and phosphorus intakes. American Journal of Physiology-Legacy Content 1971, 220, 1509–1515. [CrossRef]
- Wu, J.C.Y.; Smith, M.W.; Mitchell, M.A.; Peacock, M.A.; Turvey, A.; Keable, S.J. Enterocyte expression of calbindin, calbindin mRNA and calcium transport increases in jejunal tissue during onset of egg production in the fowl (Gallus domesticus). Comparative Biochemistry and Physiology -- Part A: Physiology 1993, 106, 263–269. [CrossRef]
- Cai, Q.; Chandler, J.S.; Wasserman, R.H.; Kumar, R.; Penniston, J.T. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proceedings of the National Academy of Sciences of the United States of America 1993, 90, 1345–1349. [CrossRef]
- McCormick, C.C. Passive diffusion does not play a major role in the absorption of dietary calcium in normal adults. Journal of Nutrition 2002, 132, 3428–3430. [CrossRef]
- Fleet, J.C.; Schoch, R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Critical Reviews in Clinical Laboratory Sciences 2010, 47, 181–195. [CrossRef]
- Corradino, R.A.; Wasserman, R.H.; Pubols, M.H.; Chang, S.I. Vitamin D3 induction of a calcium-binding protein in the uterus of the laying hen. Archives of Biochemistry and Biophysics 1968, 125, 378–380. [CrossRef]
- Wasserman, R.H.; Smith, C.A.; Smith, C.M.; Brindak, M.E.; Fullmer, C.S.; Krook, L.; Penniston, J.T.; Kumar, R. Immunohistochemical localization of a calcium pump and calbindin-D28k in the oviduct of the laying hen. Histochemistry 1991, 96, 413-8. [CrossRef]
- Bar, A.; Cohen, A.; Eisner, U.; Risenfeld, G.; Hurwitz, S. Differential Response of Calcium Transport Systems in Laying Hens to Exogenous and Endogenous Changes in Vitamin D Status. The Journal of Nutrition 1978, 108, 1322–1328. [CrossRef]
- Nys, Y.; Parkes, C.O.; Thomasset, M. Effects of suppression and resumption of shell formation and parathyroid hormone on uterine calcium-binding protein, carbonic anhydrase activity, and intestinal calcium absorption in hens. General and Comparative Endocrinology 1986, 64, 293–299. [CrossRef]
- Corradino, R.A.; Smith, C.A.; Krook, L.P.; Fullmer, C.S. Tissue-specific regulation of shell gland calbindin D28K biosynthesis by estradiol in precociously matured, vitamin D-depleted chicks. Endocrinology 1993, 132, 193–198. [CrossRef]
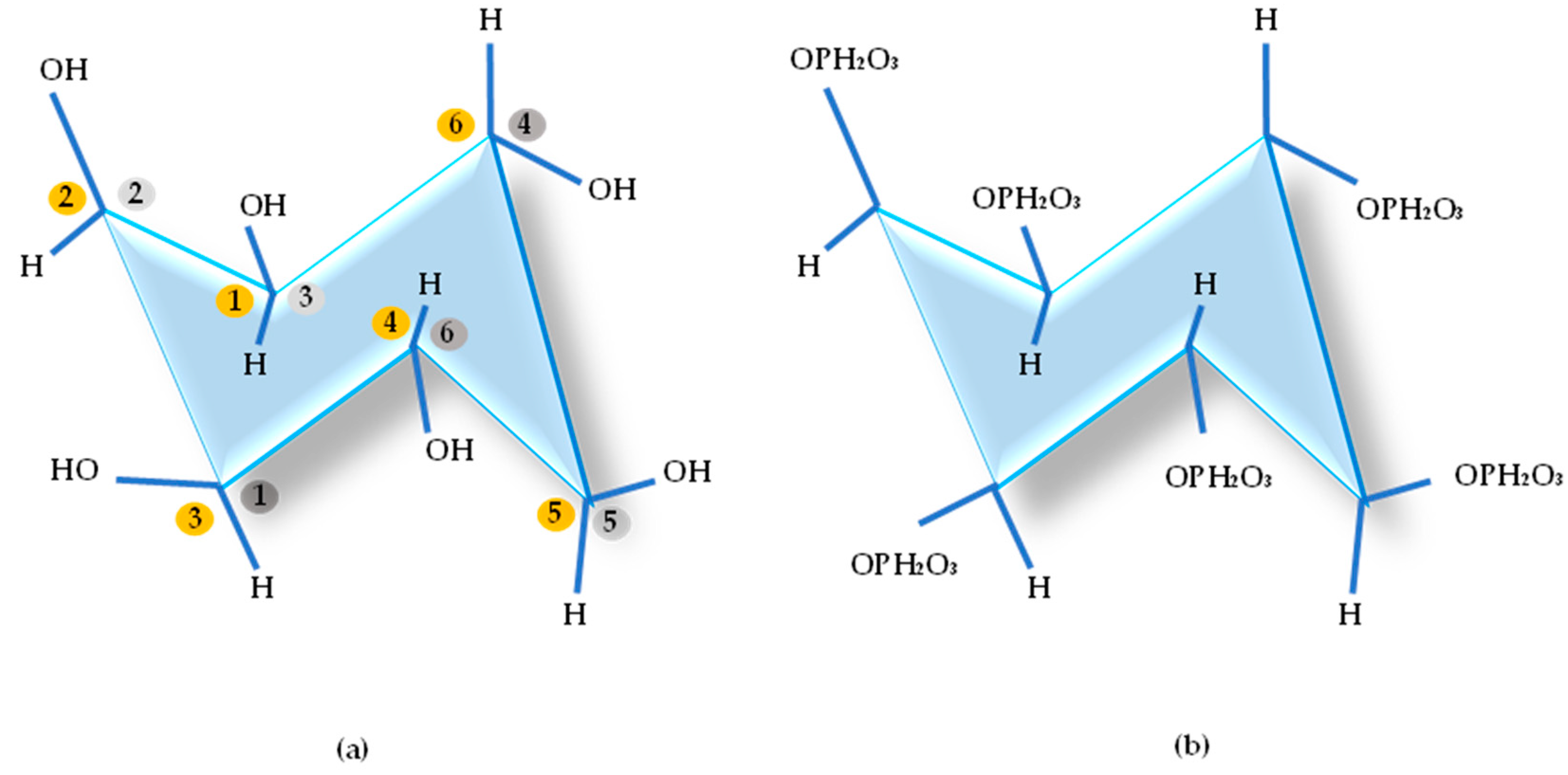
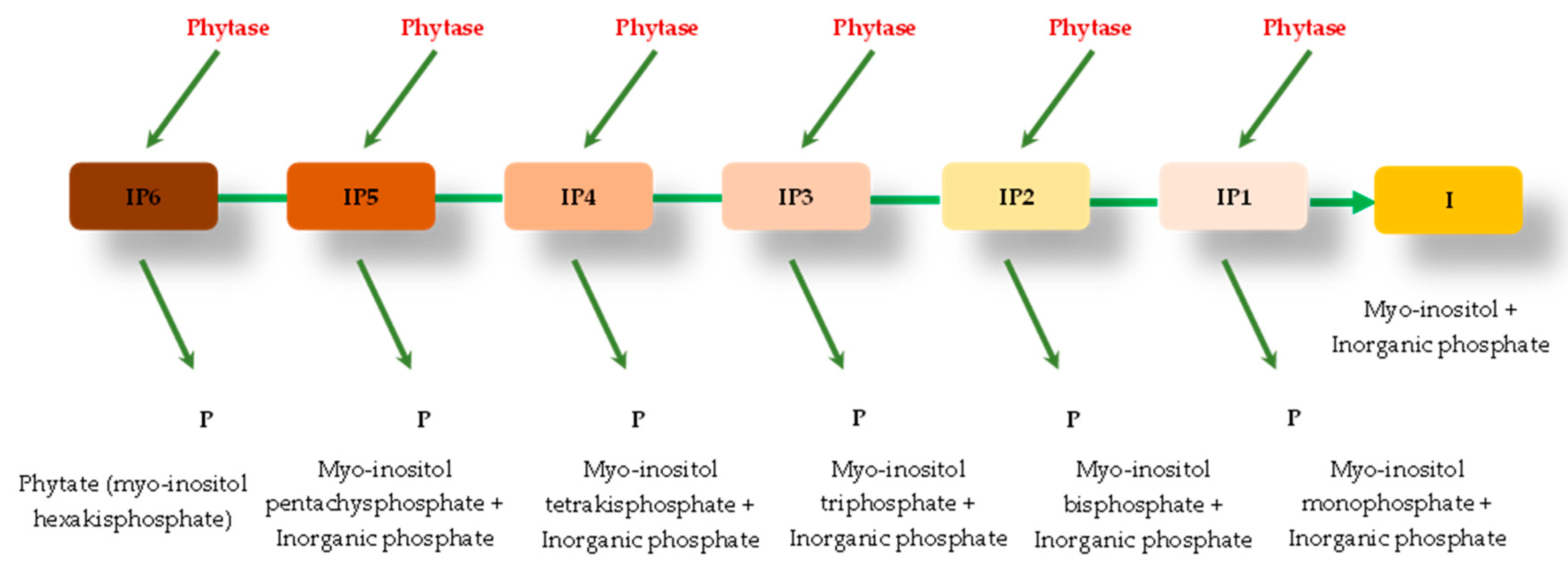
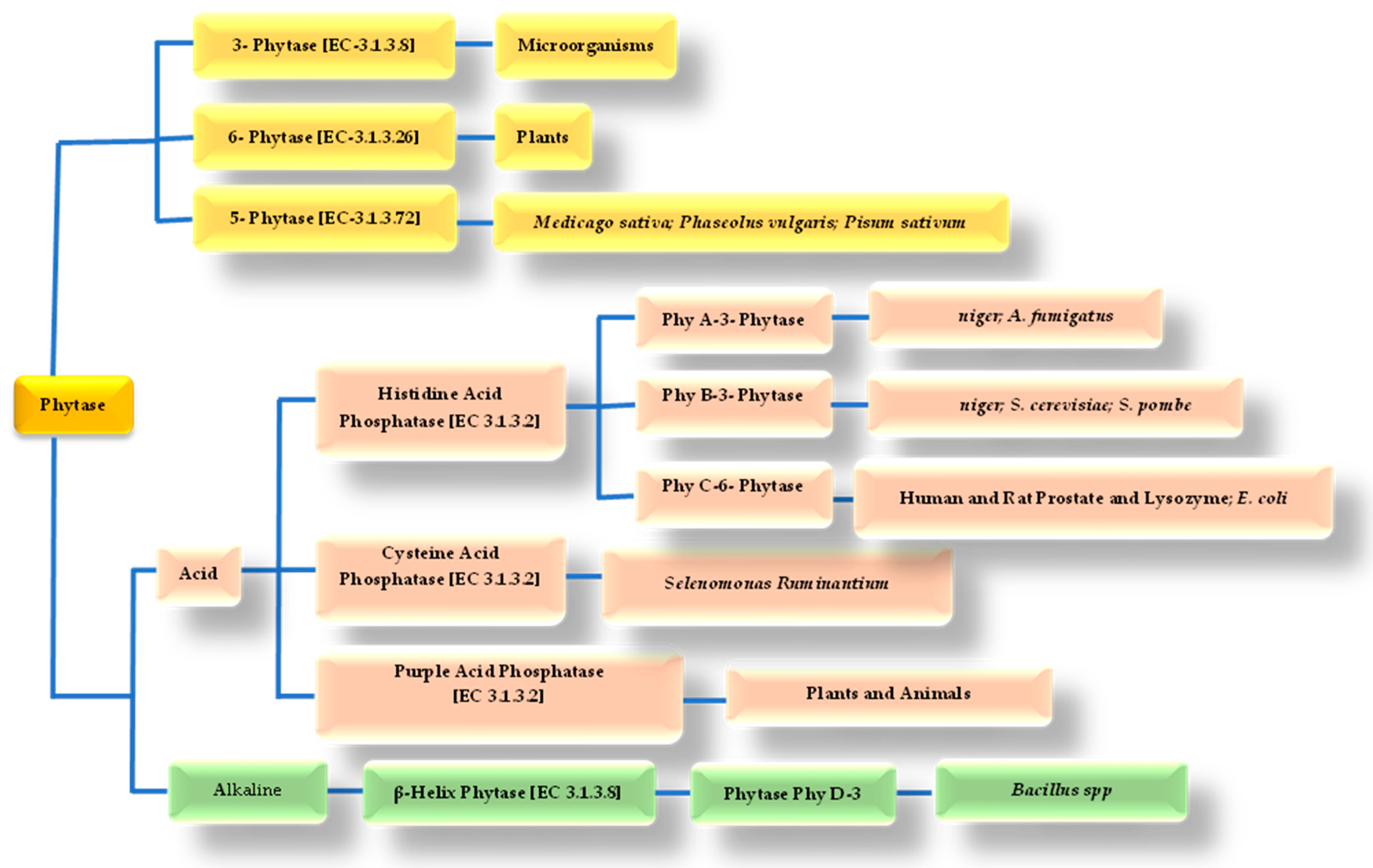
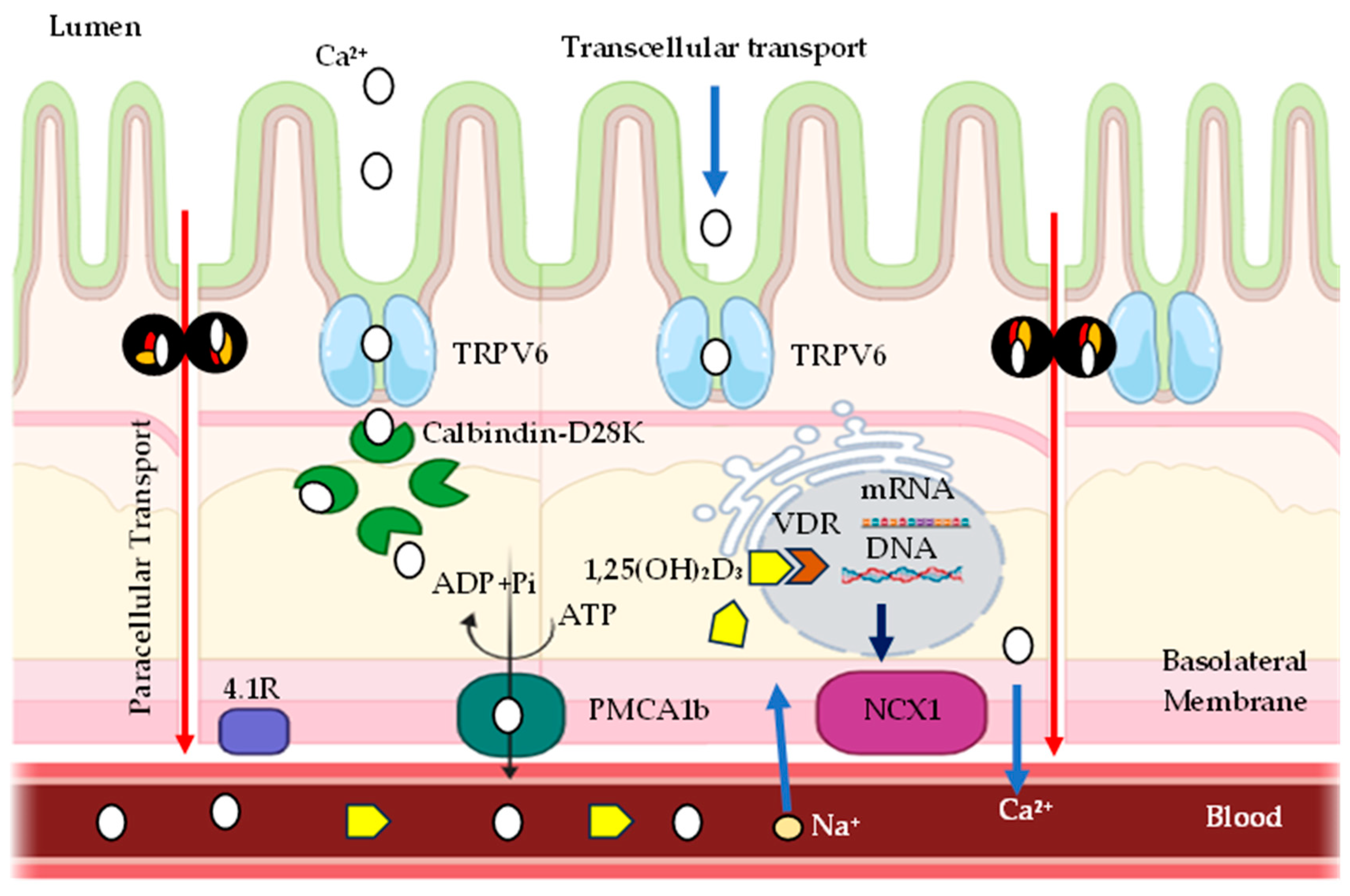
| Products | % Phytate | Reference |
| Corn | 0.78–1.05 | [59,60] |
| Soybean | 1.01–1.47 | [60,61] |
| Sorghum | 0.80 | [62] |
| Cottonseed meal | 2.65 | [62] |
| Corn germ | 2.97 | [62] |
| Polished rice | 0.60 | [60,63] |
| Oats | 0.79–1.01 | [60,61] |
| Wheat | 0.39–1.35 | [60,64] |
| Soybean bran | 1.0-1.5 | [65] |
| Rice bran | 5.90–6.48 | [66,67] |
| Wheat bran | 5.38 | [59,60] |
| Whole wheat flour | 2.22 | [60,68] |
| White wheat flour | 0.404 | [60,68] |
| Age - weeks | Ca (%) | P (%) | Literature |
| 6-16 | 3.50 | 0.45 | [137] |
| 6-29 | 2.00-3.05 | - | [138] |
| 6-19 | 3.51 | - | [139] |
| 8-21 | 2.50 | 0.31 | [140] |
| 45-57 | 3.50 | 0.15 | [141] |
| 45-57 | 3.80 | - | [142] |
| 7-54 | 2.50 | 0.25 | [135] |
| 12-42 | 2.50 | 0.35 | [143] |
| 26-38 | 2.00 | 0.31 | [144] |
| 21-36 | 3.10 | 0.32 | [145] |
| 7-20 | 2.50 | 0.35 | [146] |
| 8-56 | 2.99 | 0.31 | [40] |
| 6-57 | 2.0-3.8 | 0.15-0.45 | [120] |
| 20-32 | - | 0.39-0.44 | [147] |
| 9-24 | 2.68 | 0.38 | [119] |
| 8-56 | 3.01-3.04 | 0.31-0.80 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





